1. Introduction
Intracranial aneurysms (IAs) are pathological dilations of the brain-supplying arteries. Although IAs can often remain asymptomatic for many years, they pose a significant risk of rupture. The estimated incidence of subarachnoid hemorrhage (SAH) is 6.7 per 100,000 persons per year, resulting in approximately 500,000 cases globally each year [
1]. Despite advanced intensive care, SAH has a 30-day mortality rate of about 42%, with 10-25% of patients dying before reaching the hospital [
2,
3,
4]. Additionally, up to 50% of survivors suffer from long-term impairments, requiring ongoing assistance in daily life [
4,
5,
6,
7].
The mechanisms leading to aneurysm rupture are not fully understood. Numerous extrinsic and intrinsic factors are associated with aneurysm rupture, including aneurysm size, location, configuration, surface characteristics, and hemodynamic properties [
8,
9]. Additionally, known cardiovascular risk factors such as arterial hypertension, nicotine abuse, and alcohol consumption contribute to aneurysm progression and eventual rupture [
8]. These influencing factors are partially modifiable and include the following: heavy alcohol consumption, smoking, obesity, arterial hypertension, presence of diabetes mellitus, and hypercholesterolemia [
10,
11,
12,
13,
14,
15]. However, the specific impact of these risk factors remains unclear. While there is an agreement regarding an increased risk associated with alcohol consumption [
10,
13], smoking [
11,
12,
13], and arterial hypertension [
11,
13,
14,
15], the data regarding the influence of overweight, diabetes, and hypercholesterolemia are inconsistent. Notably, some studies have identified a higher BMI as a protective factor, particularly in women [
12,
16]. Feigin demonstrated the protective effect of diabetes and hypercholesterolemia, which was corroborated by further studies [
12,
13,
17].
On the other hand, the role of these risk factors in the development of cardiovascular diseases is well-documented [
18,
19]. The Heart Disease and Stroke Statistics 2017 Update highlights key trends in cardiovascular health. Notably, mean LDL cholesterol levels among US adults have decreased, but the use of cholesterol-lowering treatments, including statins, has significantly increased, indicating a widespread incidence of this issue that is effectively managed with medication. Hypertension remains widespread, affecting 34% of US adults aged 20 and older. Additionally, around 23.4 million US adults have diagnosed diabetes, with another 7.6 million undiagnosed cases and 81.6 million people with prediabetes. Obesity rates among US adults and youth have risen markedly, and 15.2% of US adults used tobacco in 2015 [
18]. Similar trends are observed in Germany, where the prevalence of overweight, obesity, and high blood pressure has increased. Smoking rates among women have risen by about 8%, particularly among younger women, while high cholesterol levels have shown a slight decrease [
19]. This increase in modifiable lifestyle risk factors and cardiovascular diseases is largely attributed to the growing prevalence of unhealthy lifestyles, characterized by poor diet, physical inactivity, excessive alcohol consumption, and smoking. Consequently, these factors may also play a significant role in the pathophysiology of IA formation and rupture, as well as in the treatment of patients with IA.
In this study, we aimed to establish a stereotypical patient model, named “MARVIN” (Metabolic and Adverse Risk Factors and Vices influencing Intracranial aneurysms), who represents individuals with an unhealthy lifestyle and associated vascular diseases. This model underscores the typical risk factors and characteristics of patients whose lifestyles significantly contribute to vascular complications, emphasizing the importance of targeted lifestyle modifications in this population. This study furthermore aimed to investigate the extent to which modifiable lifestyle-related risk factors (such as smoking, heavy alcohol consumption, and obesity) and cardiovascular risk factors (including arterial hypertension, diabetes mellitus, hyperlipidemia, and vascular diseases), which are often associated with an unhealthy lifestyle, influence the rupture of IA and the clinical outcomes of patients with IA.
2. Materials and Methods
To conduct this study, we examined a previously collected database of patients at the Department of Neurosurgery of the Otto-von-Guericke University Hospital Magdeburg. The database included 303 patients who had been diagnosed with 517 saccular IAs and who had been presented to our department during the period from 2007 to 2020. IA were diagnosed using computed tomography angiography, magnetic resonance angiography, or digital subtraction angiography. We conducted a retrospective analysis and applied the following inclusion criteria: Presence of complete data sets regarding the following lifestyle risk factors: arterial hypertension, diabetes type 2, hypercholesterolemia, vascular diseases of various organ systems, nicotine abuse, alcohol abuse and obesity; Availability of information regarding the rupture status and the clinical outcome.
A total of 77 patients was excluded because of incomplete data on lifestyle-related risk factors. Vascular diseases were defined as peripheral arterial occlusive disease, stroke, myocardial infarction, and coronary artery stenosis. Furthermore, we excluded one patient due to missing information on rupture status and five patients due to the absence of clinical outcome data (
Figure 1).
2.1. Data Acquisition
The clinical data for this study were obtained retrospectively through a comprehensive analysis of patient medical records, anamneses, medication registries, and existing diagnostic imaging. The evaluation of patient medical histories included the assessment of prevalent cardiovascular diseases, associated risk factors, and other critical health conditions, such as malignant neoplasms or significant autoimmune disorders requiring immunosuppressive therapy. Regarding the natural progression and management of IAs, extensive data were collected from the results of diagnostic imaging, aneurysm-specific risk factors, and assessments of patient clinical outcomes following aneurysm treatment or rupture. The relevant parameters are outlined and defined as follows:
The collected epidemiological data included the age of the patient at the time of diagnosis and their biological sex. Pre-existing conditions and risk factors were also assessed, including hypertension, defined by a documented diagnosis or the use of antihypertensive medications [
14], and diabetes mellitus, diagnosed as type 2 diabetes or the use of oral antidiabetic drugs or insulin. Hyperlipidemia was documented either by diagnosis or through the use of medication to lower blood lipid or cholesterol levels. Other vascular diseases, such as peripheral arterial disease, stroke, myocardial infarction, and coronary artery stenosis, were included in the analysis. Obesity was defined as a body mass index greater than 30 kg/m², while nicotine use encompassed both current and former users [
13,
14]. Alcohol use was classified as the consumption of more than 50 grams of alcohol per week [
13].
Aneurysm-specific factors were considered, including aneurysm rupture, which was determined based on intra-operative findings, imaging results, or CT evidence of hemorrhage patterns. Aneurysm multiplicity was defined as the presence of two or more intracranial aneurysms [
20], and aneurysm localization was assessed through angiographic evaluation. Clinical scores used in the study included the WFNS (World Federation of Neurosurgical Societies) score, which classifies SAH based on the Glasgow Coma Scale and the presence of focal neurological deficits [
21]. The Fisher Grade was employed to classify the severity of SAH based on CT scan findings, ranging from no visible blood to large intracerebral or intraventricular clots [
22]. Finally, the Modified Rankin Scale at discharge was used to assess neurological and functional impairment, with scores ranging from no symptoms to death [
23].
2.2. Statistical Analysis
In this study, we considered patient age, sex, initial WFNS score, and various lifestyle-related factors - hypertension, nicotine abuse, diabetes type 2, hypercholesterolemia, obesity, alcohol abuse, and a history of vascular diseases - as independent variables. The dependent variables were the patient outcomes, measured by the mRS score, and the occurrence of IA rupture. The mRS was categorized into three groups: favorable outcome (mRS = 0–2), poor outcome (mRS = 3–5), and death (mRS = 6).
To ensure data plausibility and avoid collinearities, we examined the relationships between the dependent variables both statistically and visually. Dichotomous variables were tested for independence using the χ² test, while the relationship between dichotomous and continuous variables was assessed using the t-test.
We modeled the influence of independent variables on rupture using logistic regression, and on patient outcomes using proportional odds logistic regression (POLR). Initially, simple regressions were performed for each dependent variable. Subsequently, variables that were found to be significant were included in multiple regression analyses.
3. Results
3.1. Cohort Overview
3.1.1. Rupture Analysis
The cohort for the rupture analysis consisted of 225 patients, with a mean age of 54.8 years. Among these patients, 33.3% were male (n = 75) and 66.7% were female (n = 150). Arterial hypertension was present in 73.3% of the patients, highlighting its significant prevalence. Nicotine abuse was also notably high, affecting 62.7% of the patients. Obesity was identified in 29.8% of the cohort, while hypercholesterolemia was noted in 20.0%. Diabetes type 2 affected 12.9% of the patients, and vascular diseases were reported in 11.6%. Alcohol abuse was observed in 16.0% of the patients (
Table 1).
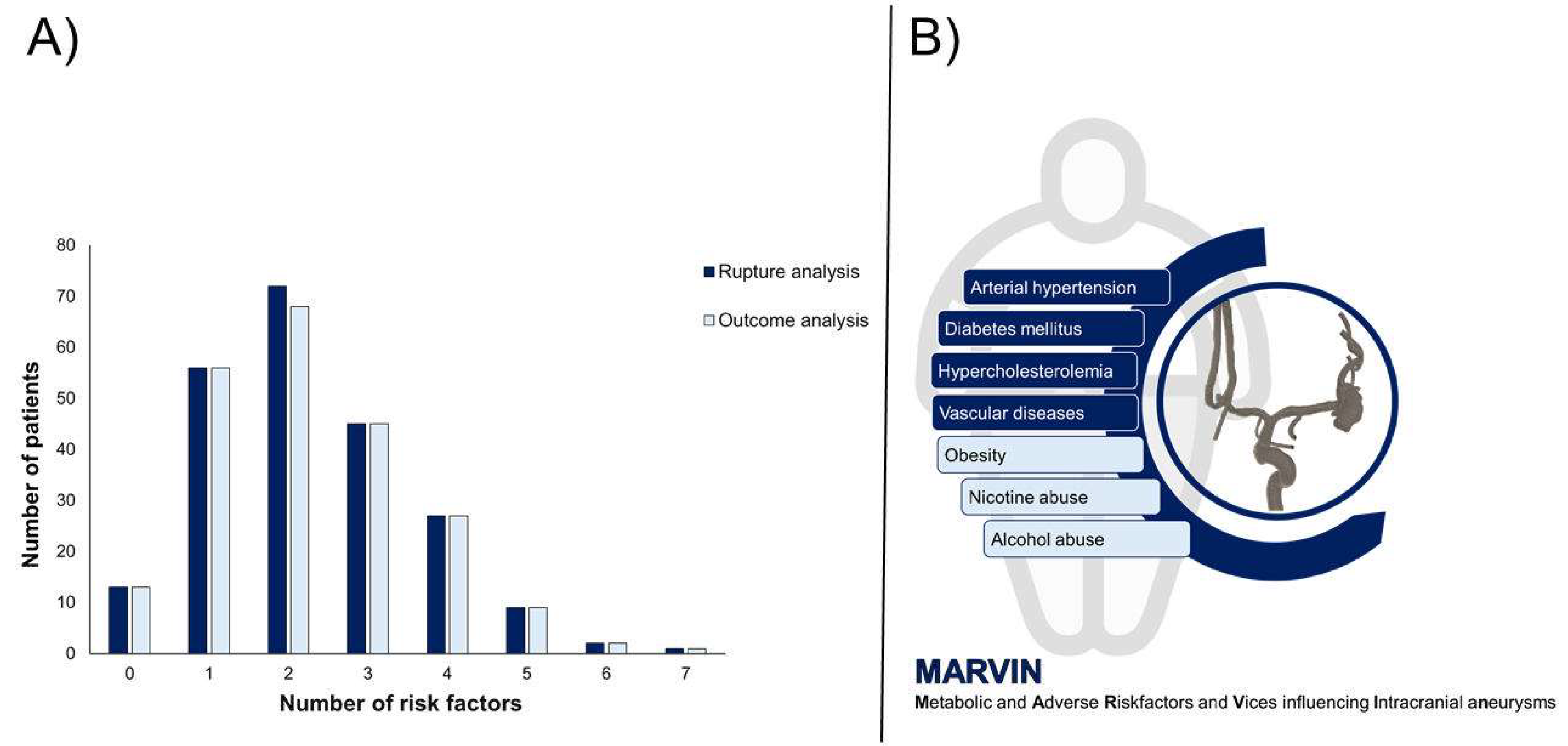
The distribution of the number of lifestyle-related risk factors among the patients was as follows. A small group of patients, 5.8%, had no risk factors. One risk factor was present in 24.9% of the patients, while 32.0% had two risk factors. A total of 20.0% of the patients had three risk factors, and 12.0% had four risk factors. Only 4.0% of the patients had five risk factors, and an even smaller percentage had six (0.9%) and seven (0.4%) risk factors (
Figure 2).
154 patients presented with SAH, 88 patients had multiple aneurysms. The majority of IAs were found in the anterior communicating artery (ACOM), affecting 46.7% of the patients. The middle cerebral artery (MCA) was the next most common site, with 20.0% of the patients having aneurysms in this location. Aneurysms in the internal carotid artery (ICA) were identified in 12.4% of the patients, while 11.1% had aneurysms in the basilar artery. Other locations included the posterior inferior cerebellar artery (PICA), with 3.1% of the patients, and the posterior communicating artery (PCOM), vertebral artery, superior cerebellar artery (SCA), each affecting 1.3% of the patients. Less frequently, aneurysms were located at the anterior cerebral artery (ACA), pericallosal artery, and anterior choroidal artery, each affecting 0.9% of the patients. The mean diameter of the IA in this cohort was 7.4mm (
Table 1).
The distribution of the WFNS scores among the analyzed ruptured cases was as follows: WFNS 1 in 86 cases (55.8%), WFNS 2 in 23 cases (14.9%), WFNS 3 in 4 cases (2.6%), WFNS 4 in 21 cases (13.6%), WFNS 5 in 18 cases (11.7%). Regarding the Fisher grades, 4 cases (2.6%) were classified as grade 1, 5 cases (3.3%) as grade 2, 55 cases (35.7%) as grade 3, and 89 cases (55.8%) as grade 4 (
Table 1).
3.1.2. Outcome Analysis
The cohort for the outcome analysis consisted of 221 patients, with a mean age of 54.9 years, including 145 females (65.6%) and 76 males (34.4%). Arterial hypertension was present in 73.8% of the patients, while 13.1% had diabetes type 2, and 20.4% had hypercholesterolemia. A history of vascular diseases was noted in 11.8% of the patients. Nicotine abuse was reported in 62.4%, alcohol abuse in 16.3%, and obesity in 29.0% of the cohort (
Table 1).
The distribution of the number of lifestyle-related risk factors per patient varied. Specifically, 5.9% of the patients had no risk factors, 25.3% had one, and 30.8% had two. Additionally, 20.3% of the patients had three risk factors, 12.2% had four, 4.1% had five, 0.9% had six, and 0.5% had seven risk factors (
Figure 2).
Among the 221 patients, 152 presented with SAH and 87 had multiple IA. The majority of IA were found at the ACOM, comprising 46.6% of the cases. The MCA was the next most common site, accounting for 20.4% of the cases. IA in the ICA were identified in 12.2% of patients, while 10.9% had IA in the basilar artery. IA were less frequently located in the posterior inferior cerebellar artery, the PCOM, the vertebral artery, ACA, pericallosal artery, anterior choroidal artery, and superior cerebellar artery (
Table 1). The mean diameter of the IA in this cohort was 7.4mm (
Table 1).
For the outcome analysis, in ruptured cases, the distribution of the WFNS score and the Fisher grade was as follows: WFNS scores included 86 cases (56.6%) with WFNS 1, 23 cases (15.1%) with WFNS 2, 4 cases (2.6%) with WFNS 3, 21 cases (14.8%) with WFNS 4, and 18 cases (11.8%) with WFNS 5. Regarding Fisher grades, the distribution was 4 cases (2.6%) with grade 1, 5 cases (3.3%) with grade 2, 54 cases (35.5%) with grade 3, and 89 cases (56.6%) with grade 4 (
Table 1).
3.2. Analysis of Clinical Outcome and Rupture
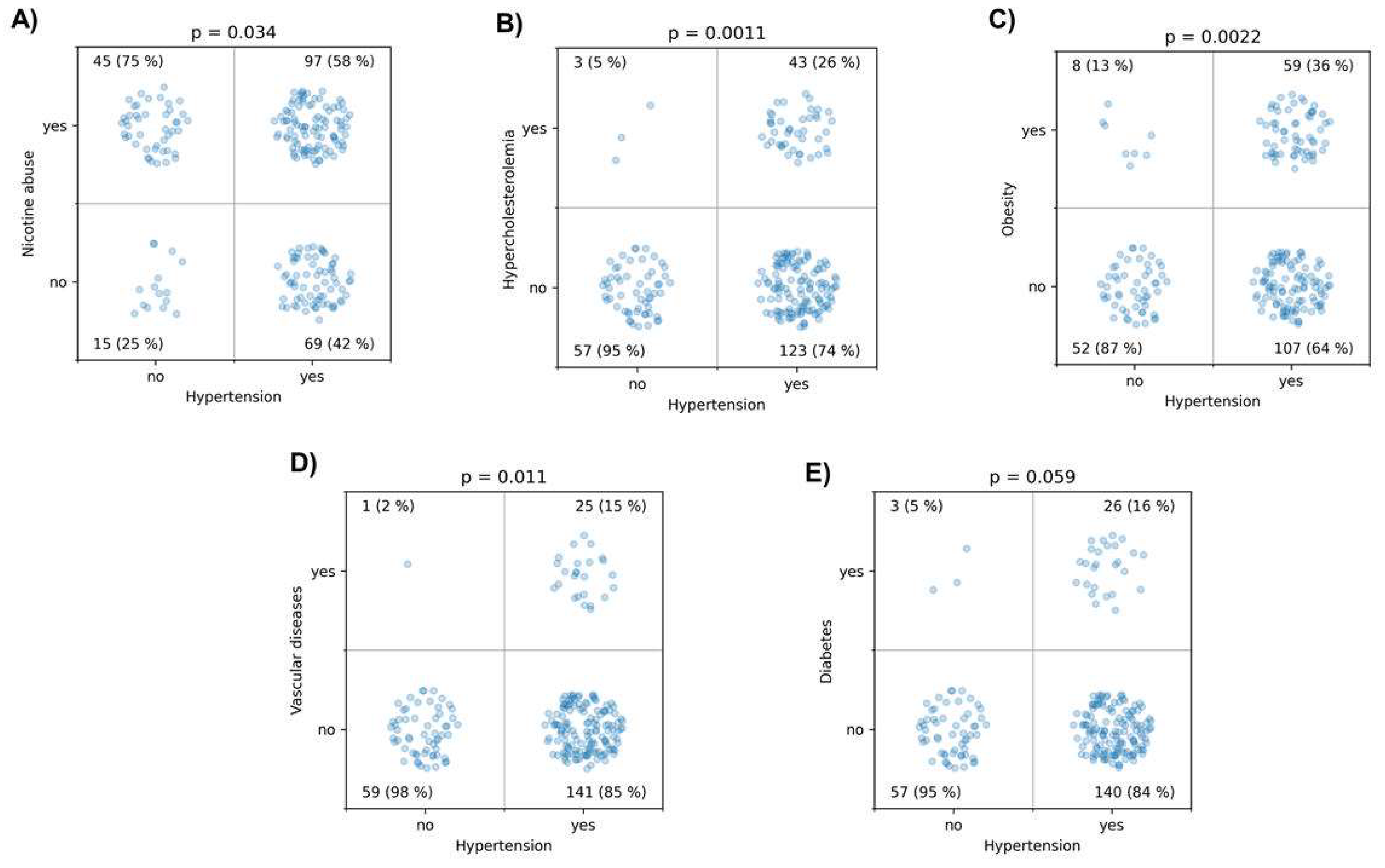
Initially, the independent variables were examined in detail for potential associations. Hypertension was associated with nicotine abuse (p = 0.034), hypercholesterolemia (p = 0.0011), obesity (p = 0.0022), history of vascular diseases (p = 0.011), and showed a trend with diabetes (p = 0.059) (
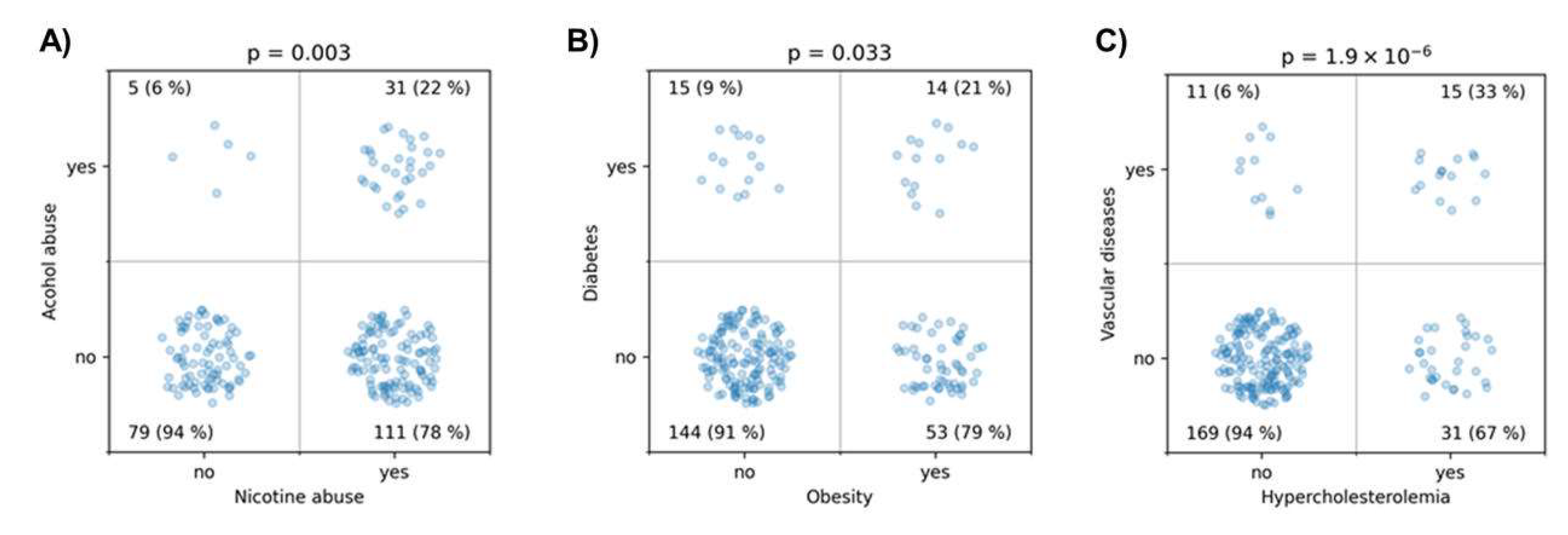
Figure 3). There was a strong association between nicotine and alcohol abuse (p = 0.003). Obesity was associated with diabetes (p = 0.033), and hypercholesterolemia was highly significantly associated with a history of vascular diseases (p < 10
-5) (
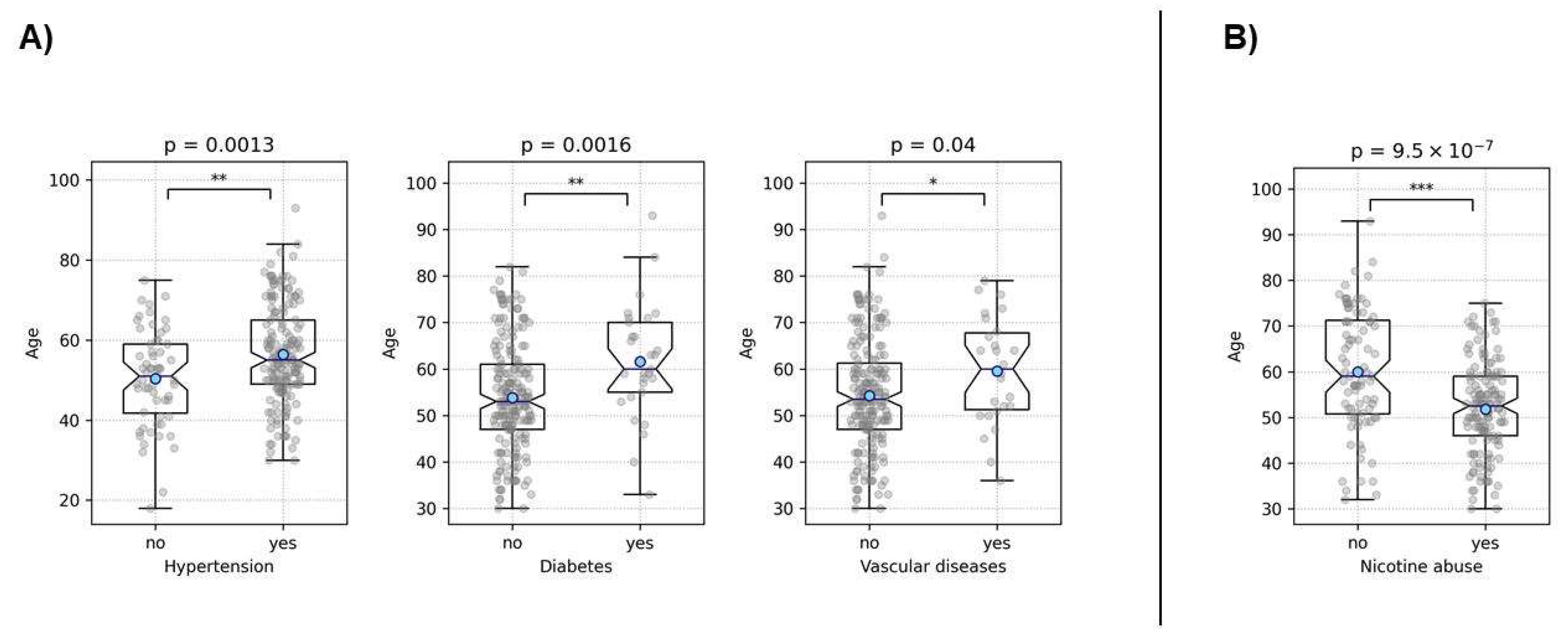
Figure 4). These findings demonstrate strong interrelationships among common lifestyle-associated vascular risk factors, supporting our hypothesis of a stereotypical unhealthy patient with multiple resultant vascular diseases and emphasizing the validity of our data. Furthermore, older patients were more likely to have hypertension (p = 0.0013), diabetes (p = 0.0016), and a history of vascular diseases (p = 0.04) (
Figure 5). Interestingly, younger patients were more likely to be associated with nicotine abuse (p < 10
-6) (
Figure 5). The gender of the patients had no influence in any of the analyses.
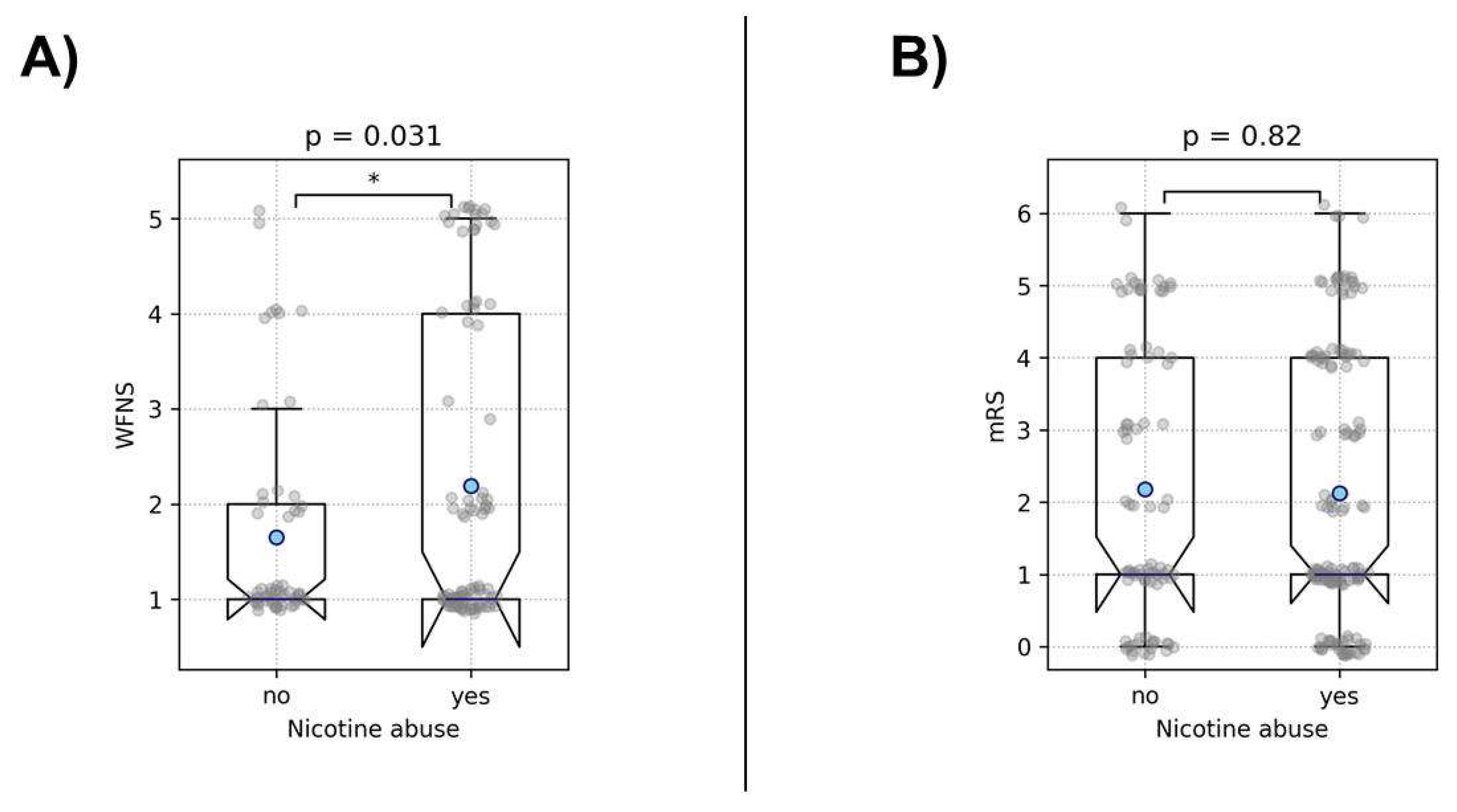
The analysis of the impact of the examined risk factors on the initial clinical presentation after aneurysm rupture revealed that smokers presenting with ruptured aneurysms were initially more severely affected, exhibiting WFNS scores approximately half a grade higher (p = 0.031). Interestingly, nicotine abuse did not correlate with the eventual patient outcome (trichotomized mRS, p = 0.8), indicating that despite the initially higher WFNS scores, the outcomes in our cohort were not worse compared to patients who were not smoking (
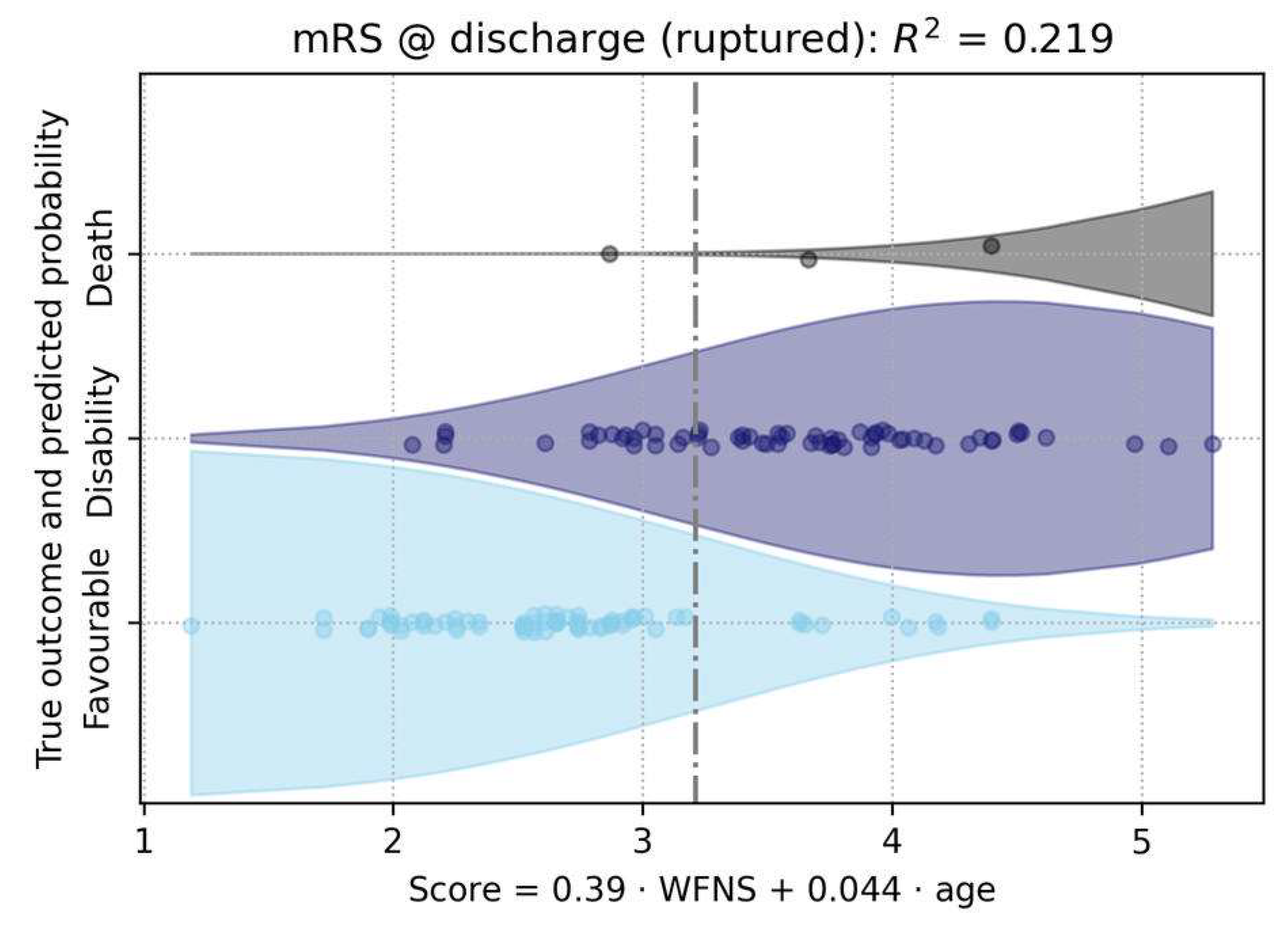
Figure 6). The only significant predictor for a worse outcome were the WFNS score (p < 10
-6) and age (p < 10
-5) (
Figure 7).
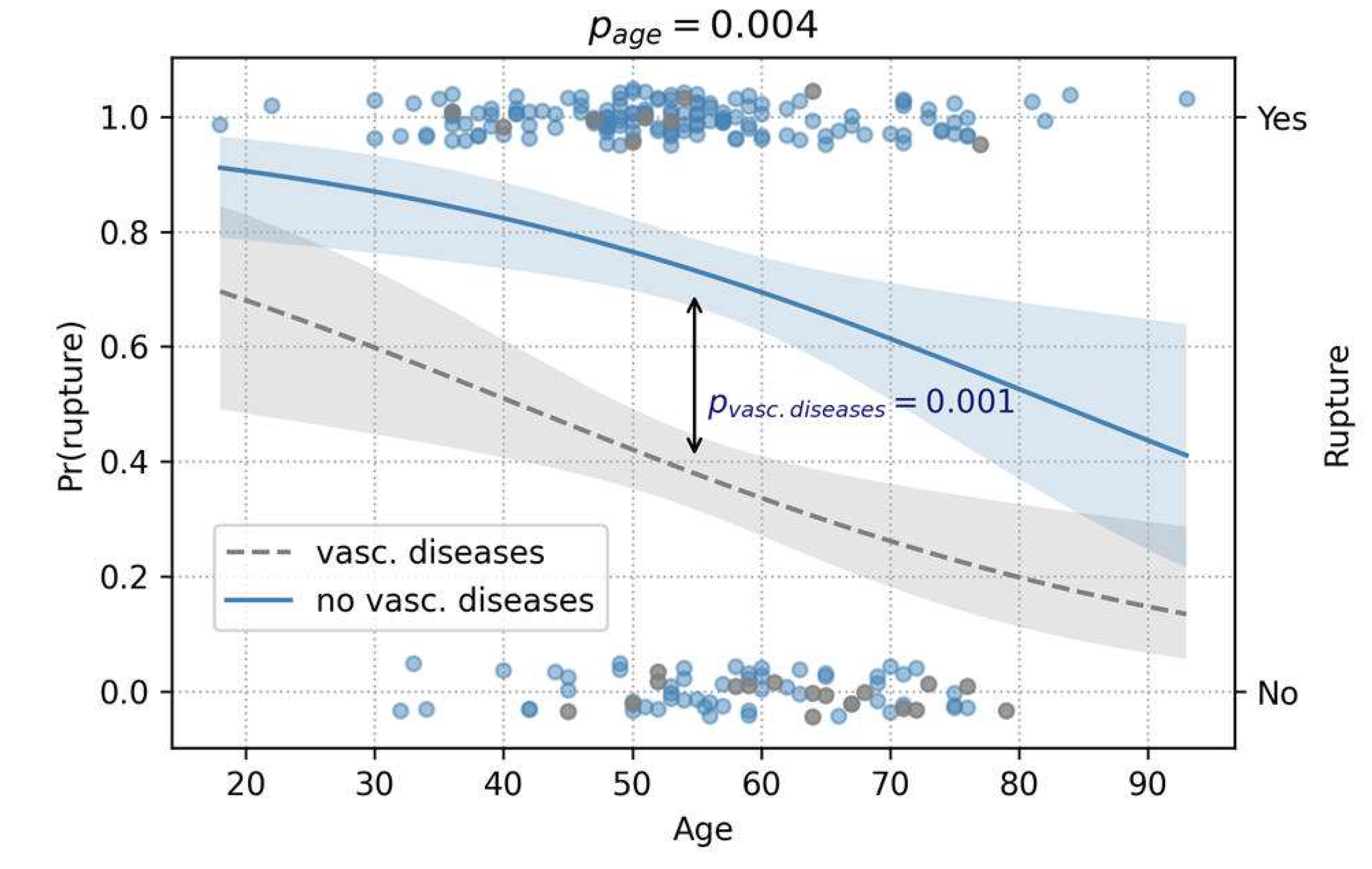
Regarding rupture, age and a history of vascular diseases were both significant predictors (p = 0.004 and p = 0.001, respectively). Counterintuitively, both acted protectively against rupture (
Figure 8).
4. Discussion
In this study, we aimed to investigate the extent to which modifiable lifestyle-related risk factors (such as smoking, heavy alcohol consumption, and obesity) and cardiovascular risk factors (including arterial hypertension, diabetes mellitus, hyperlipidemia, and vascular diseases) influence the rupture of IAs and clinical outcomes of affected patients. To visualize our hypothesis, we establish a stereotypical patient model named “MARVIN” to represent individuals with an unhealthy lifestyle and associated vascular diseases. This model highlights the typical risk factors and characteristics of patients whose lifestyles significantly contribute to vascular complications, emphasizing the importance of targeted lifestyle modifications.
Our analysis revealed that vascular risk factors were highly prevalent in the patient cohort. Arterial hypertension was present in 73.3% of the patients, highlighting its significant prevalence. Among the modifiable risk factors, nicotine abuse stood out, affecting 62.7% of the patients, making it a significant target for prevention efforts. Additionally, obesity was present in 29.8% of the patients, hypercholesterolemia in 20.0%, type 2 diabetes in 12.9%, and vascular diseases, including coronary issues, in 11.6%. Alcohol abuse was identified in 16.0% of the cohort. Upon examining the independent variables, we found strong associations between several risk factors. Hypertension was commonly linked with nicotine abuse, hypercholesterolemia, obesity, and a history of vascular diseases. Nicotine and alcohol abuse showed a strong interrelationship. Obesity was frequently associated with diabetes, and hypercholesterolemia was significantly linked to a history of vascular diseases. These findings demonstrate the robust interrelationships among common lifestyle-associated vascular risk factors, further supporting our hypothesis of a stereotypical unhealthy patient with multiple resultant vascular diseases.
The significance of these risk factors is also evident in the numerous scores used to assess the risk of IA rupture. For example, the PHASES score awards an additional point to patients with arterial hypertension [
14]. The New Treatment score, published by Juvela, specifically highlights ongoing nicotine abuse as a critical risk factor [
24,
25]. In the UIATS, which balances the risk of treatment against the risk of spontaneous progression based on various criteria, smoking, alcohol abuse, and arterial hypertension are key considerations. According to UIATS, patients with these risk factors are more likely to be recommended for treatment [
6]. To emphasize the importance of these factors, smoking cessation and control of arterial hypertension are clearly advised upon the diagnosis of an incidental aneurysm [
8]. Conversely, the influence of risk factors such as overweight, diabetes, and hypercholesterolemia remains unclear, with some authors suggesting protective effects [
12,
13,
16,
17].
Besides lifestyle-related risk factors, patient age is also a significant risk factor, with numerous studies demonstrating a link between older age and increased rupture rates [
12,
14]. In our study, IA rupture was inversely associated with older age and the presence of vascular diseases, indicating that older patients and those with vascular diseases were less frequently affected by rupture. This finding contrasts with the results described in the literature. Due to the retrospective nature of this study, one possible explanation for the apparent protection conferred by cardiovascular diseases and older age is that these patients may undergo cranial imaging more frequently due to nonspecific symptoms or their known conditions. This leads to the detection and subsequent treatment of intracranial aneurysms before rupture, thus classifying them as
“unruptured
” in our analysis. The natural course without such interventions remains unclear in these cases. Interestingly, our study found no significant impact of lifestyle-associated risk factors on the likelihood of rupture. A noteworthy aspect of our findings is that smokers who experienced a rupture were initially more severely affected clinically. However, this did not reflect in the clinical outcome, indicating that despite higher initial WFNS scores, the final outcomes in our cohort were not worse compared to non-smoking patients. In our study, the clinical outcome was influenced only by the WFNS score and the patien
t’s age, with younger patients exhibiting better clinical outcomes.
Our findings further reveal that most patients (156 vs. 67) had more than one vascular risk factor, with most exhibiting two risk factors. This supports our “MARVIN” hypothesis, indicating that many patients harbor multiple risk factors and should be considered “vascularly unhealthy.” Conversely, there are “otherwise vascularly healthy” aneurysm patients who might require separate consideration.
Research on coronary heart disease (CHD) has already clarified the quantitative relationship between these lifestyle-related risk factors and CHD risk through the Framingham Heart Study and other investigations [
26,
27]. These studies have shown that the major risk factors for CHD are additive in their predictive power, meaning that the total risk for an individual can be calculated by summing the contributions of each major risk factor.
In response to the high prevalence of these risk factors, the American Heart Association (AHA) has identified seven key health metrics, or
“ideal health factors,
” to help reduce the future burden of cardiovascular disease [
28]. These metrics encompass regular physical activity, maintaining a normal BMI, adhering to a healthy diet, achieving low serum cholesterol levels, sustaining normal blood pressure, maintaining low fasting plasma glucose levels, and abstaining from smoking. Achieving these ideal health factors is associated with lower levels of biomarkers for subclinical atherosclerosis, such as carotid intima-media thickness [
29,
30], and is linearly linked to the risk of developing cardiovascular disease, as well as both all-cause and cardiovascular mortality [
31,
32,
33]. Attaining ideal levels of these key cardiovascular health factors is crucial for reducing the risk of premature cardiovascular-related death [
31]. Unfortunately, very few people, including children, achieve an ideal health profile [
18].
Given this background, a logical next step in understanding the natural course of patients with IA is to closely examine vascular risk factors and their specific impacts on risk. This approach could provide deeper insights into the progression and management of IA, potentially mirroring the comprehensive risk assessment models established for CHD. Considering the high prevalence of multiple vascular risk factors among IA patients, it is crucial to adopt a multifactorial risk assessment strategy. This strategy could help identify patients at higher risk and tailor individualized treatment plans, ultimately improving patient outcomes.
This study has several limitations that must be acknowledged. Firstly, the retrospective nature of our analysis introduces inherent biases, as it relies on previously collected data, which may not comprehensively capture the natural course of treated incidental aneurysms. The lack of prospective follow-up data limits our ability to observe the progression of these aneurysms over time, which is crucial for understanding the long-term impact of the identified risk factors. Secondly, due to the observational design of the study, we cannot draw direct causal relationships from our results. While associations between certain risk factors and aneurysm rupture were identified, causality cannot be established without more rigorous, controlled studies. Another limitation is the relatively small cohort size compared to other studies on aneurysm risk factors. The limited number of patients reduces the generalizability of our findings and may impact the statistical power of the analysis. Additionally, the intrinsic characteristics of aneurysms, such as size, are significant risk factors for rupture. To better isolate the impact of lifestyle and other factors on rupture, it would be necessary to control for these aneurysm-specific characteristics. However, due to the small sample size, propensity score matching was not feasible in this study. Future studies with larger cohorts should consider employing this method to more effectively eliminate the influence of aneurysm characteristics. Larger, multicenter studies are also needed to validate these results and provide more robust conclusions.
5. Conclusions
This study highlights the significant prevalence of modifiable lifestyle-related and cardiovascular risk factors among patients with IA. Arterial hypertension and nicotine abuse were particularly prevalent and most patients hat more than one risk factor, supporting the “MARVIN” model of a stereotypical patient with multiple risk factors. Despite strong associations with IA presence, these factors did not affect the likelihood of rupture. Interestingly, the inverse relationship between rupture and both older age and existing vascular diseases suggests that these groups may undergo more frequent incidental detection and treatment, classifying their aneurysms as “unruptured” in our analysis. The analysis of clinical outcomes in our cohort revealed that smokers presenting with ruptured IA were initially more severely affected, with higher WFNS scores, but their final outcomes were not worse compared to non-smokers. The only significant predictors of worse outcomes were the WFNS score and patient age. These findings emphasize the need for multifactorial risk assessment strategies to better identify and manage high-risk patients. Future prospective studies with larger cohorts are essential to validate these results and further explore the natural progression of untreated incidental IA.
Use of GenAI
We confirm that language editing of this manuscript was conducted using the AI-based tool, ChatGPT (OpenAI). The tool was used exclusively for improving language clarity and style. No content, scientific analysis, or data interpretation was altered during this process. The final version of the manuscript has been carefully reviewed and approved by all authors to ensure accuracy and consistency.
Author Contributions
Conceptualization, V.M.S., I.E.S. and B.N.; Data curation, V.M.S. and C.A.D.; Formal analysis, V.M.S., A.A. and H.S.; Investigation, V.M.S., A.A. and H.S.; Methodology, I.F. and R.K.; Project administration, B.N.; Resources, K.-P.S. and A.R.; Software, not applicable.; Supervision, I.E.S.; Validation, V.M.S. and B.N.; Visualization, V.M.S. and B.N.; Writing—original draft, V.M.S. and B.N.; Writing—review and editing, A.A., C.A.D., I.E.S, I.F., R.K.; Funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of the Medical Faculty of the Otto-von-Guericke University (protocol codes RENOVA 94/20, date of approval: 22 June 2020).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study, where all data were collected as part of routine patient care. No identifying patient data were published.
Data Availability Statement
The data supporting the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
Acknowledgments
We acknowledge support by the Open Access Publication fund of medical faculty of the Otto-von-Guericke-University Magdeburg.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hughes, J.D.; Bond, K.M.; Mekary, R.A.; Dewan, M.C.; Rattani, A.; Baticulon, R.; et al. Estimating the Global Incidence of Aneurysmal Subarachnoid Hemorrhage: A Systematic Review for Central Nervous System Vascular Lesions and Meta-Analysis of Ruptured Aneurysms. World Neurosurg. 2018, 115, 430–447. [Google Scholar] [CrossRef]
- Ingall, T.; Asplund, K.; Mähönen, M.; Bonita, R. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke 2000, 31, 1054–61. [Google Scholar] [CrossRef]
- van Gijn, J.; Kerr, R.S.; Rinkel, G.J.E. Subarachnoid haemorrhage. Lancet 2007, 369, 306–18. [Google Scholar] [CrossRef]
- Nieuwkamp, D.J.; Setz, L.E.; Algra, A.; Linn, F.H.H.; de Rooij, N.K.; Rinkel, G.J.E. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol 2009, 8, 635–42. [Google Scholar] [CrossRef]
- Hop, J.W.; Rinkel, G.J.; Algra, A.; van Gijn, J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 1997, 28, 660–4. [Google Scholar] [CrossRef]
- Etminan, N.; Brown, R.D.; Beseoglu, K.; Juvela, S.; Raymond, J.; Morita, A.; et al. The unruptured intracranial aneurysm treatment score: a multidisciplinary consensus. Neurology 2015, 85, 881–9. [Google Scholar] [CrossRef]
- Alleyne, C.H. Aneurysmal subarachnoid hemorrhage: have outcomes really improved? Neurology 2010, 74, 1486–7. [Google Scholar] [CrossRef]
- Petridis, A.K.; Kamp, M.A.; Cornelius, J.F.; Beez, T.; Beseoglu, K.; Turowski, B.; et al. Aneurysmal Subarachnoid Hemorrhage. Dtsch. Arztebl. Int. 2017, 114, 226–36. [Google Scholar] [CrossRef]
- Cebral, J.R.; Mut, F.; Weir, J.; Putman, C.M. Association of Hemodynamic Characteristics and Cerebral Aneurysm Rupture. AJNR Am. J. Neuroradiol. 2011, 32, 264–70. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wallin, A.; Wolk, A.; Markus, H.S. Differing association of alcohol consumption with different stroke types: a systematic review and meta-analysis. BMC Med 2016, 14, 178. [Google Scholar] [CrossRef]
- Karhunen, V.; Bakker, M.K.; Ruigrok, Y.M.; Gill, D.; Larsson, S.C. Modifiable Risk Factors for Intracranial Aneurysm and Aneurysmal Subarachnoid Hemorrhage: A Mendelian Randomization Study. J. Am. Heart Assoc. 2021, 10, e022277. [Google Scholar] [CrossRef]
- Sundström, J.; Söderholm, M.; Söderberg, S.; Alfredsson, L.; Andersson, M.; Bellocco, R.; et al. Risk factors for subarachnoid haemorrhage: a nationwide cohort of 950 000 adults. Int. J. Epidemiol. 2019, 48, 2018–25. [Google Scholar] [CrossRef]
- Feigin, V.L.; Rinkel, G.J.E.; Lawes, C.M.M.; Algra, A.; Bennett, D.A.; van Gijn, J.; et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke 2005, 36, 2773–80. [Google Scholar] [CrossRef]
- Greving, J.P.; Wermer, M.J.H.; Brown, R.D.; Morita, A.; Juvela, S.; Yonekura, M.; et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014, 13, 59–66. [Google Scholar] [CrossRef]
- Rapsomaniki, E.; Timmis, A.; George, J.; Pujades-Rodriguez, M.; Shah, A.D.; Denaxas, S.; et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet 2014, 383, 1899–911. [Google Scholar] [CrossRef]
- Kroll, M.E.; Green, J.; Beral, V.; Sudlow, C.L.M.; Brown, A.; Kirichek, O.; et al. Adiposity and ischemic and hemorrhagic stroke: Prospective study in women and meta-analysis. Neurology 2016, 87, 1473–81. [Google Scholar] [CrossRef]
- Can, A.; Castro, V.M.; Dligach, D.; Finan, S.; Yu, S.; Gainer, V.; et al. Lipid-Lowering Agents and High HDL (High-Density Lipoprotein) Are Inversely Associated With Intracranial Aneurysm Rupture. Stroke 2018, 49, 1148–54. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–603. [Google Scholar] [CrossRef]
- Thefeld, W. Verbreitung der Herz-Kreislauf-Risikofaktoren Hypercholesterin�mie, �bergewicht, Hypertonie und Rauchen in der Bev�lkerung. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz 2000, 43, 415–23. [Google Scholar] [CrossRef]
- Jabbarli, R.; Dinger, T.F.; Darkwah Oppong, M.; Pierscianek, D.; Dammann, P.; Wrede, K.H.; et al. Risk Factors for and Clinical Consequences of Multiple Intracranial Aneurysms: A Systematic Review and Meta-Analysis. Stroke 2018, 49, 848–55. [Google Scholar] [CrossRef]
- Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. Journal of Neurosurgery 1988, 68. [CrossRef]
- Fisher, C.M.; Kistler, J.P.; Davis, J.M. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980, 6, 1–9. [Google Scholar] [CrossRef]
- Broderick, J.P.; Adeoye, O.; Elm, J. Evolution of the Modified Rankin Scale and Its Use in Future Stroke Trials. Stroke 2017, 48, 2007–12. [Google Scholar] [CrossRef]
- Juvela, S. Treatment Scoring of Unruptured Intracranial Aneurysms. Stroke 2019, 50, 2344–50. [Google Scholar] [CrossRef]
- Juvela, S. PHASES score and treatment scoring with cigarette smoking in the long-term prediction of rupturing of unruptured intracranial aneurysms. J. Neurosurg. 2022, 136, 156–62. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–47. [Google Scholar] [CrossRef]
- Grundy, S.M.; Pasternak, R.; Greenland, P.; Smith, S.; Fuster, V. Assessment of Cardiovascular Risk by Use of Multiple-Risk-Factor Assessment Equations: A Statement for Healthcare Professionals From the American Heart Association and the American College of Cardiology. Circulation 1999, 100, 1481–92. [Google Scholar] [CrossRef]
- Andersson, C.; Vasan, R.S. Epidemiology of cardiovascular disease in young individuals. Nat. Rev. Cardiol. 2018, 15, 230–40. [Google Scholar] [CrossRef]
- Xanthakis, V.; Enserro, D.M.; Murabito, J.M.; Polak, J.F.; Wollert, K.C.; Januzzi, J.L.; et al. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation 2014, 130, 1676–83. [Google Scholar] [CrossRef]
- Spring, B.; Moller, A.C.; Colangelo, L.A.; Siddique, J.; Roehrig, M.; Daviglus, M.L.; et al. Healthy lifestyle change and subclinical atherosclerosis in young adults: Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation 2014, 130, 10–7. [Google Scholar] [CrossRef]
- Yang, Q.; Cogswell, M.E.; Flanders, W.D.; Hong, Y.; Zhang, Z.; Loustalot, F.; et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA 2012, 307, 1273–83. [Google Scholar] [CrossRef]
- Fang, N.; Jiang, M.; Fan, Y. Ideal cardiovascular health metrics and risk of cardiovascular disease or mortality: A meta-analysis. Int. J. Cardiol. 2016, 214, 279–83. [Google Scholar] [CrossRef]
- Nayor, M.; Enserro, D.M.; Vasan, R.S.; Xanthakis, V. Cardiovascular Health Status and Incidence of Heart Failure in the Framingham Offspring Study. Circ. Heart Fail. 2016, 9, e002416. [Google Scholar] [CrossRef]
Figure 1.
Presentation of the patient cohort according to the inclusion criteria of the study. Of 303 patients with IA, 226 had complete information on the lifestyle-associated risk factors recorded. Information on rupture status was available for 225 patients and on clinical outcome for 221.
Figure 1.
Presentation of the patient cohort according to the inclusion criteria of the study. Of 303 patients with IA, 226 had complete information on the lifestyle-associated risk factors recorded. Information on rupture status was available for 225 patients and on clinical outcome for 221.
Figure 2.
Part (A) of the figure shows the distribution of the number of risk factors for the rupture and outcome analyses, with the rupture analysis shown in dark blue and the outcome analysis in light blue. Part (B) of the figure schematically shows the concept of “MARVIN” which stands for “Metabolic and Adverse Risk Factors and Vices influencing Intracranial aneurysms” and shows the accumulation of the established seven risk factors in patients who lead an unhealthy lifestyle.
Figure 2.
Part (A) of the figure shows the distribution of the number of risk factors for the rupture and outcome analyses, with the rupture analysis shown in dark blue and the outcome analysis in light blue. Part (B) of the figure schematically shows the concept of “MARVIN” which stands for “Metabolic and Adverse Risk Factors and Vices influencing Intracranial aneurysms” and shows the accumulation of the established seven risk factors in patients who lead an unhealthy lifestyle.
Figure 3.
This figure illustrates the associations between various lifestyle-associated risk factors and hypertension, tested using the χ² test. (A) shows the association between hypertension and nicotine abuse, (B) between hypercholesterolemia and hypertension, (C) between obesity and hypertension, (D) between a history of vascular diseases and hypertension, and (E) between diabetes and hypertension.
Figure 3.
This figure illustrates the associations between various lifestyle-associated risk factors and hypertension, tested using the χ² test. (A) shows the association between hypertension and nicotine abuse, (B) between hypercholesterolemia and hypertension, (C) between obesity and hypertension, (D) between a history of vascular diseases and hypertension, and (E) between diabetes and hypertension.
Figure 4.
This figure illustrates the associations tested using the χ² test between (A) alcohol and nicotine abuse, (B) diabetes and obesity, and (C) history of vascular diseases and hypercholesterolemia.
Figure 4.
This figure illustrates the associations tested using the χ² test between (A) alcohol and nicotine abuse, (B) diabetes and obesity, and (C) history of vascular diseases and hypercholesterolemia.
Figure 5.
This figure illustrates the associations between age and various risk factors examined using a t-test. Part (A) depicts the association between older age and the increased likelihood of hypertension, diabetes, and a history of vascular diseases. Part (B) shows the association between younger age and a higher prevalence of nicotine abuse. Asterisks denote the level of statistical significance: * = p < 0.05, ** = p < 0.01, and *** = p < 0.001. Blue dots represent the mean values.
Figure 5.
This figure illustrates the associations between age and various risk factors examined using a t-test. Part (A) depicts the association between older age and the increased likelihood of hypertension, diabetes, and a history of vascular diseases. Part (B) shows the association between younger age and a higher prevalence of nicotine abuse. Asterisks denote the level of statistical significance: * = p < 0.05, ** = p < 0.01, and *** = p < 0.001. Blue dots represent the mean values.
Figure 6.
This figure illustrates the association between nicotine abuse and clinical outcomes in patients with ruptured IA, examined by a t-test. Part (A) shows that smokers presented with significantly higher WFNS scores at admission, indicating more severe initial clinical conditions. However, Part (B) demonstrates that nicotine abuse did not associate with a worse outcome at discharge, despite the more severe initial presentation. Asterisks denote the level of statistical significance: * = p < 0.05, ** = p < 0.01, and *** = p < 0.001. Blue dots represent the mean values.
Figure 6.
This figure illustrates the association between nicotine abuse and clinical outcomes in patients with ruptured IA, examined by a t-test. Part (A) shows that smokers presented with significantly higher WFNS scores at admission, indicating more severe initial clinical conditions. However, Part (B) demonstrates that nicotine abuse did not associate with a worse outcome at discharge, despite the more severe initial presentation. Asterisks denote the level of statistical significance: * = p < 0.05, ** = p < 0.01, and *** = p < 0.001. Blue dots represent the mean values.
Figure 7.
This figure illustrated the prediction of clinical outcome trichotomized in favorable outcome, disability or death (proportional odds logistic regression). Here, the only significant predictor for a worse outcome were the WFNS score and age. The two are combined into a composite score, as given by the regression model. The x-axis represents the composite score, which is calculated using the formula provided below the axis.
Figure 7.
This figure illustrated the prediction of clinical outcome trichotomized in favorable outcome, disability or death (proportional odds logistic regression). Here, the only significant predictor for a worse outcome were the WFNS score and age. The two are combined into a composite score, as given by the regression model. The x-axis represents the composite score, which is calculated using the formula provided below the axis.
Figure 8.
The prediction analysis of rupture, depicted in this figure, showed protective effects of higher age and history of vascular diseases against rupture (logistic regression). Pr(rupture) represents the conditional probability of rupture, based on the patient’s age and history of vascular diseases.
Figure 8.
The prediction analysis of rupture, depicted in this figure, showed protective effects of higher age and history of vascular diseases against rupture (logistic regression). Pr(rupture) represents the conditional probability of rupture, based on the patient’s age and history of vascular diseases.
Table 1.
Presentation of the demographic data for the analysis of the rupture and the clinical outcome.
Table 1.
Presentation of the demographic data for the analysis of the rupture and the clinical outcome.
| Parameter |
Rupture analysis (n = 225) |
Outcome analysis (n = 221) |
| Sex |
75 male; 150 female |
76 male; 145 female |
| Age at diagnosis (mean) |
54.8 (±12.5) years |
54.9 (±12.5) years |
| Arterial hypertension |
165 (73.3%) |
163 (73.8%) |
| Diabetes mellitus Type 2 |
29 (13%) |
29 (13.1%) |
| Hypercholesterolemia |
45 (20%) |
45 (20.4%) |
| Vascular diseases |
26 (11.6%) |
26 (11.8%) |
| Nicotine abuse |
141 (62.7%) |
138 (62.4%) |
| Alcohol abuse |
36 (16%) |
36 (16.3%) |
| Obesity |
67 (30%) |
64 (29.0%) |
| Ruptured aneurysms |
154 (68.4%) |
152 (68.8%) |
| Multiple aneurysms |
88 (39.1%) |
87 (39.4%) |
| Aneurysm size (mm, mean) |
7.4 (±3.0) |
7.4 (±3.0) |
| Aneurysm localization |
|
|
| ACA |
2 (0.9%) |
2 (0.8%) |
| ACOM |
105 (46.7%) |
103 (46.6%) |
| Pericallosal artery |
2 (0.9%) |
2 (0.8%) |
| MCA |
45 (20%) |
45 (20.4%) |
| ICA |
28 (12.4%) |
27 (12.2%) |
| PCOM |
3 (1.3%) |
3 (1.4%) |
| Anterior choroidal artery |
2 (0.9%) |
2 (0.8%) |
| Basilar artery |
25 (11.1%) |
24 (10.9%) |
| Vertebral artery |
3 (1.3%) |
3 (1.4%) |
| PICA |
7 (3.1%) |
7 (3.2%) |
| SCA |
3 (1.3%) |
3 (1.4%) |
| WFNS score |
|
|
| 1 |
86 (55.8%) |
86 (56.6%) |
| 2 |
23 (14.9%) |
23 (15.1%) |
| 3 |
4 (2.6%) |
4 (2.6%) |
| 4 |
21 (13.6%) |
21 (14.8%) |
| 5 |
18 (11.7%) |
18 (11.8%) |
| No information |
2 (1.3%) |
0 (0%) |
| Fisher grade |
|
|
| 1 |
4 (2.6%) |
4 (2.6%) |
| 2 |
5 (3.3%) |
5 (3.3%) |
| 3 |
55 (35.7%) |
54 (35.5%) |
| 4 |
89 (55.8%) |
89 (56.6%) |
| No information |
1 (0.7%) |
0 (0%) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).