Submitted:
06 October 2024
Posted:
07 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Computational Method
3. Results and Discussion
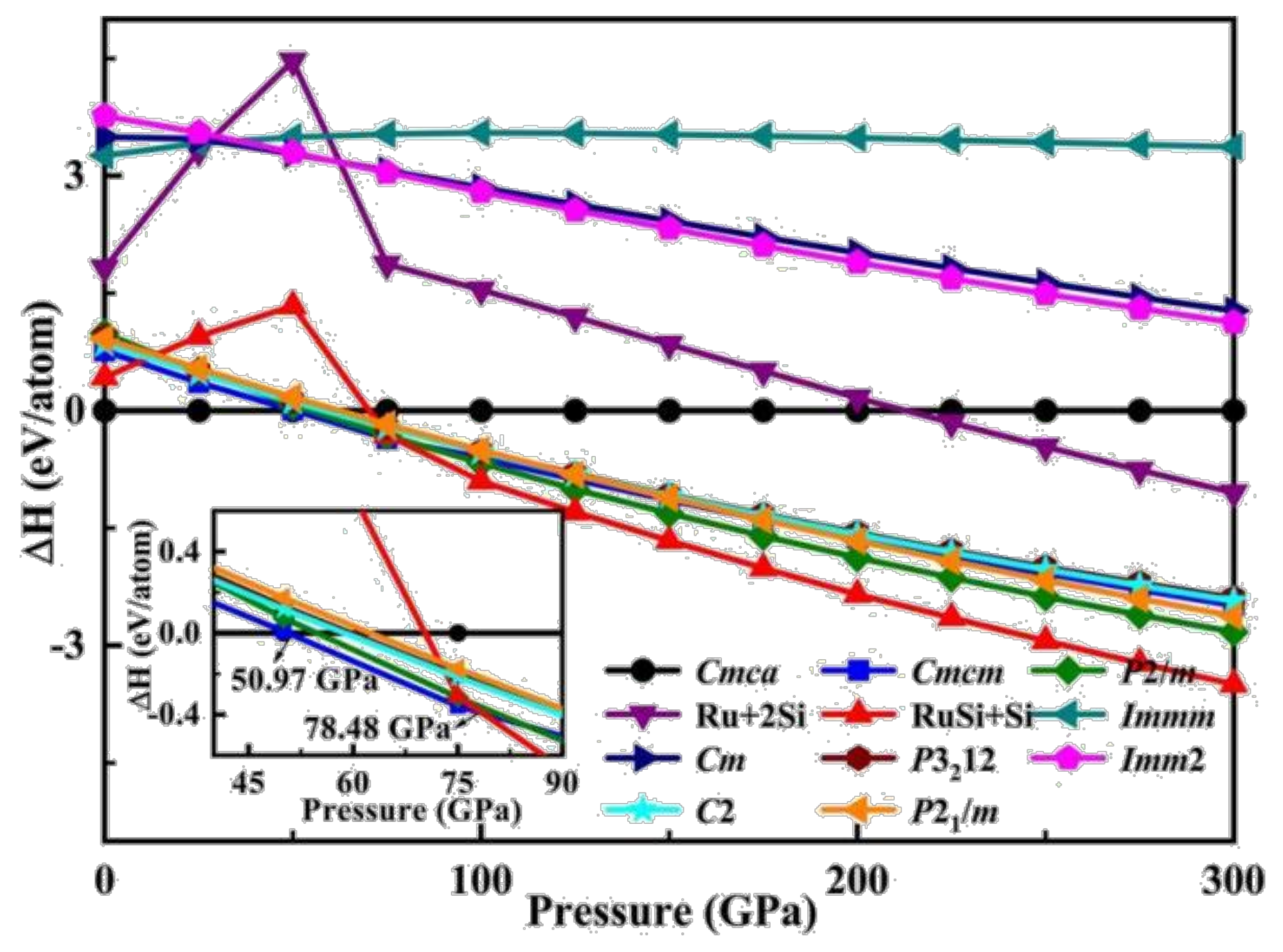
3.1. Structural Transition and Dynamical Stability
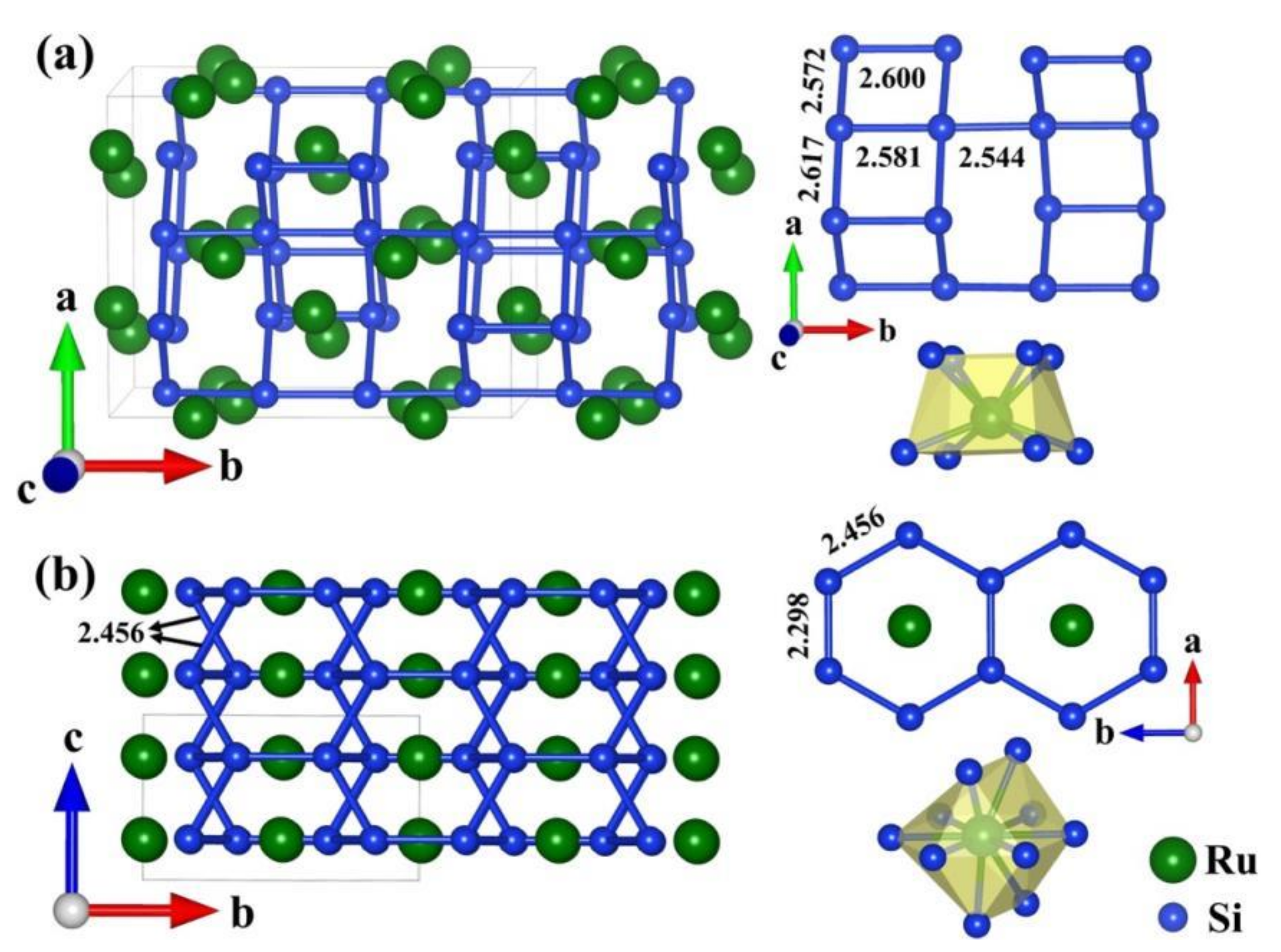
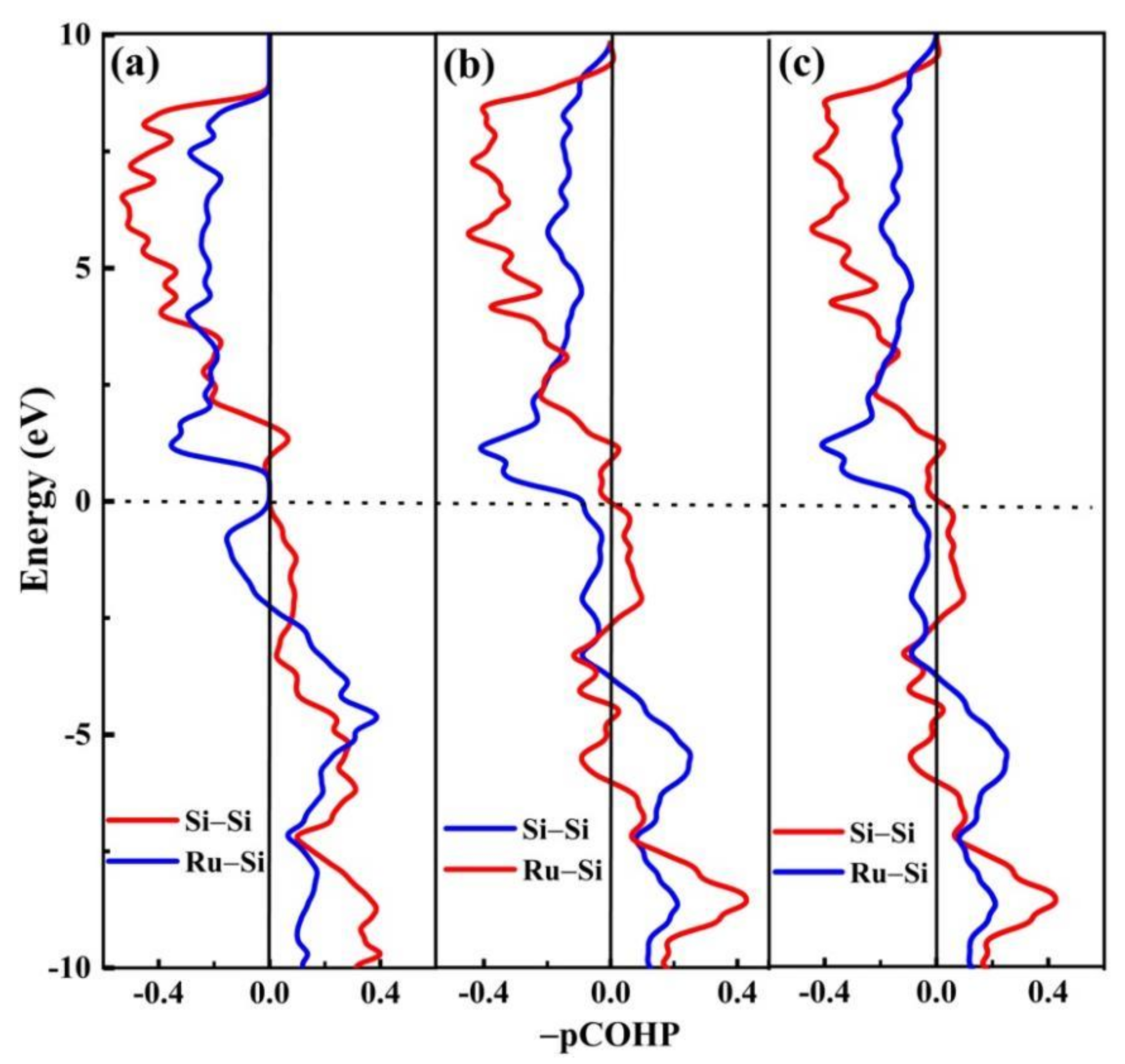
3.2. Structural Character and Bonding Essence
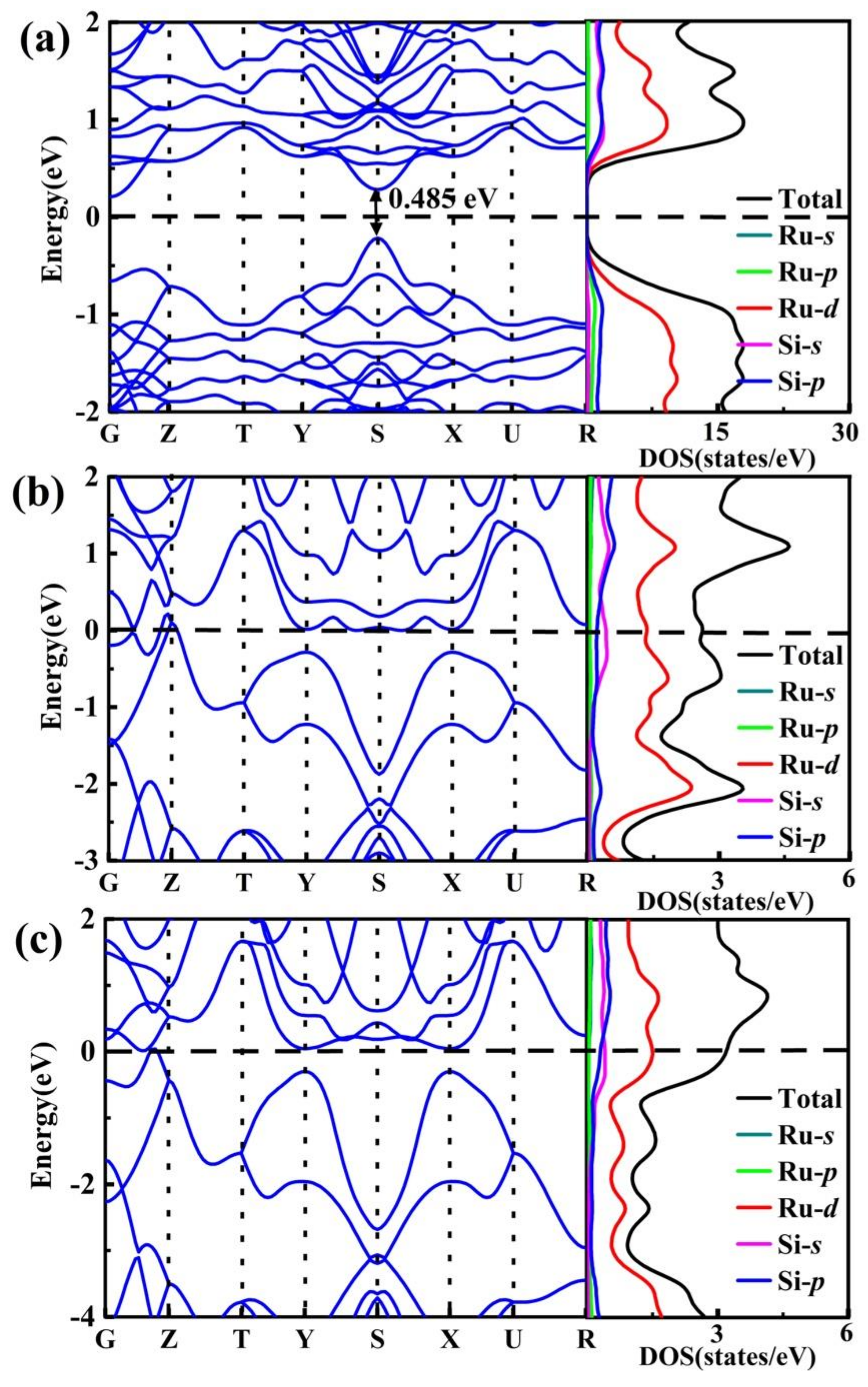
3.3. Electronic Structure and Superconductive Property
3.4. Mechanical Property and Hardness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Tse, J.S.; Tanaka, K.; Iitaka, T.; Ma, Y. Superconductive sodalite-like clathrate calcium hydride at high pressures. Proc. Natl. Acad. Sci. USA 2012, 109, 6463–6466. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, K.; Xie, Y.; Yang, X.; Wang, Y.; Zhou, M.; Liu, H.; Yu, X.; Zhao, Y.; Wang, H.; Liu, G.; Ma, Y. High-temperature superconducting phase in clathrate calcium hydride CaH6 up to 215 K at a pressure of 172 GPa. Phys. Rev. Lett. 2022, 128, 167001. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Li, Z.; Duan, D.; Cui, T. Superconducting phases of YH9 under pressure. Phys. Rev. B 2023, 108, 174507. [Google Scholar] [CrossRef]
- Kong, P.; Minkov, V.S.; Kuzovnikov, M.A.; Drozdov, A.P.; Besedin, S.P.; Mozaffari, S.; Balicas, L.; Balakirev, F.F.; Prakapenka, V.B.; Chariton, S.; Knyazev, D.A.; Greenberg, E.; Eremets, M.I. Superconductivity up to 243 K in the yttrium-hydrogen system under high pressure. Nat. Commun. 2021, 12, 5075. [Google Scholar] [CrossRef]
- Errea, I.; Belli, F.; Monacelli, L.; Sanna, A.; Koretsune, T.; Tadano, T.; Bianco, R.; Calandra, M.; Arita, R.; Mauri, F.; Flores-Livas, J.A. Quantum crystal structure in the 250-kelvin superconducting lanthanum hydride. Nature 2020, 578, 66–69. [Google Scholar] [CrossRef]
- Geballe, Z.M.; Liu, H.; Mishra, A.K.; Ahart, M.; Somayazulu, M.; Meng, Y.; Baldini, M.; Hemley, R.J. Synthesis and stability of lanthanum superhydrides. Angew. Chem. Int. Ed. 2018, 57, 688–692. [Google Scholar] [CrossRef]
- Zhong, X.; Sun, Y.; Iitaka, T.; Xu, M.; Liu, H.; Hemley, R.J.; Chen, C.; Ma, Y. Prediction of above-room-temperature superconductivity in lanthanide/actinide extreme superhydrides. J. Am. Chem. Soc. 2022, 144, 13394–13400. [Google Scholar] [CrossRef]
- Li, Z. ; He, X. ; Zhang, C. ; Lu, K. ; Min, B.; Zhang J.; Zhang, S.; ZhaoJ.; Shi, L.; Peng, Y.; Feng, S.; Deng, Z.; Song, J.; Liu, Q.; Wang, X.; Yu, R.; Wang, L.; Li, Y.; Bass, J.D.; Prakapenka, V.; Chariton, S.; Liu, H.; Jin, C.; Sci. Superconductivity above 70 K observed in lutetium polyhydrides. China Phys. Mech. 2023, 66, 267411.
- Yamanaka, S.; Enishi, E.; Fukuoka, H.; Yasukawa, M. High-Pressure synthesis of a new silicon clathrate superconductor, Ba8Si46. Inorg. Chem. 2000, 39, 56–58. [Google Scholar] [CrossRef]
- Imai, M.; Kikegawa, T. In situ observation of the formation of Si clathrate Ba8Si46 at high pressure and high temperature. Inorg. Chem. 2008, 47, 8881–8883. [Google Scholar] [CrossRef]
- Gao, K.; Cui, W.; Chen, J.; Wang, Q.; Hao, J.; Shi, J.; Liu, C.; Botti, S.; Marques, M.A.L.; Li, Y. Superconducting hydrogen tubes in hafnium hydrides at high pressure. Phys. Rev. B 2021, 104, 214511. [Google Scholar] [CrossRef]
- Liu, M.; Cui, W.; Shi, J.; Hao, J.; Li, Y. Superconducting H7 chain in gallium hydrides at high pressure. Phys. Chem. Chem. Phys. 2023, 25, 7223–7228. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, Z.; Zhang, X.; Yang, G. Layered hydride LiH4 with a pressure-insensitive superconductivity. Inorg. Chem. 2024, 63, 8257–8263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Jin, X.; Meng, X.; Bao, G.; Ma, Y.; Liu, B. and Cui, T.; Ab initio study revealing a layered structure in hydrogen-rich KH6 under high pressure. Phys. Rev. B. 2012, 86, 0147118. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Guo, J.; Huang, X.; Cui, T. Absence of superconductivity in I4/mmm-FeH5: experimental evidence. Phys. Chem. Chem. Phys. 2024, 26, 7371–7376. [Google Scholar] [CrossRef]
- Xie, H.; Yao, Y.; Feng, X.; Duan, D.; Song, H.; Zhang, Z.; Jiang, S.; Redfern, S.A.T.; Kresin,V. Z.; Pickard, C. J.; Cui, T. Hydrogen pentagraphenelike structure stabilized by hafnium: a high-temperature conventional superconductor. Phys. Rev. Lett. 2020, 125, 217001. [CrossRef]
- Chen, Y.; Liu, Z.; Lin, Z.; Jiang, Q.; Du, M.; Zhang, Z.; Song, H.; Xie, H.; Cui, T.; Duan, D. High Tc superconductivity in layered hydrides XH15 (X = Ca, Sr, Y, La) under high pressures. Front. Phys. 2022, 17, 103–109. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, L.; Du, M.; Duan, D. A perspective on reducing stabilizing pressure for high-temperature superconductivity in hydrides. J. Phys.: Condens. Matter 2024, 36, 493002. [Google Scholar] [CrossRef]
- Song, J.; Curtin, W.A. Atomic mechanism and prediction of hydrogen embrittlement in iron curtin. Nat. Mater. 2013, 12, 145–151. [Google Scholar]
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen embrittlement in different materials: A review. Int. J. Hydrogen Energ. 2018, 43, 21603–21616. [Google Scholar] [CrossRef]
- Nagamatsu, J.; Nakagawa, N.; Muranaka, T.; Zenitani, Y.; Akimitsu, J. Superconductivity at 39 K in magnesium diboride. Nature 2001, 410, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Pei, C.; Zhang, J.; Wang, Q.; Zhao, Y.; Gao, L.; Gong, C.; Tian, S.; Luo, R.; Li, M.; Yang, W.; Lu, Z.Y.; Lei, H.; Liu, K.; Qi, Y. Pressure-induced superconductivity at 32 K in MoB2. Natl. Sci. Rev. 2023, 10, nwad034. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, K.; Liu, H.; Zhang, S. Superconductive sodium carbides with pentagon carbon at high pressures. J. Phys. Chem. Lett. 2021, 12, 5850–5856. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Lu, S.; Pickard, C.J.; Liu, H.; Redfern, S.A.T.; Ma, Y. Carbon network evolution from dimers to sheets in superconducting ytrrium dicarbide under pressure. Commun. Chem. 2018, 1, 85. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, Y.; Jiang, X ; Wang, B.; Zhao, J.; Mater, J. Unconventional stoichiometric two-dimensional potassium nitrides with anion-driven metallicity and superconductivity. Chem. C 2024, 12, 103–109.
- Chen, Y.; Cai, X.; Wang, H.; Wang, H.; Wang, H. Novel triadius-like N4 specie of iron nitride compounds under high pressure. Sci. Rep. 2018, 8, 10670. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Kong, X.; Duan, D. Stability and superconductivity of K–P compounds under Pressure. Inorg. Chem. 2017, 56, 12529–12534. [Google Scholar] [CrossRef]
- Li, X.; Bergara, A.; Zhang, X.; Li, F.; Liu, Y.; Yang, G. Superconducting Ce2P3 and CeP2 with an interesting planar P layer. J. Mater. Chem. C 2024, 12, 2526–2533. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Yang, Z.; Liu, Y.; Yang, G. Pressure-stabilized graphene-like P layer in superconducting LaP2. Phys. Chem Chem. Phys. 2022, 24, 6469–6475. [Google Scholar] [CrossRef]
- Connétable, D.; Timoshevskii, V.; Masenelli, B.; Beille, J.; Marcus, J.; Barbara, B.; Saitta, A.M.; Rignanese, G.-M. Superconductivity in doped sp3 semiconductors: the case of the clathrates. Phys. Rev. Lett. 2003, 91, 247001. [Google Scholar] [CrossRef]
- Rachi, T.; Yoshino, H.; Kumashiro, R.; Kitajima, M.; Kobayashi, K.; Yokogawa, K.; Murata, K.; Kimura, N.; Aoki, H.; Fukuoka, H.; Yamanaka, S.; Shimotani, H.; Takenobu, T.; Iwasa, Y.; Sasaki, T.; Kobayashi, N.; Miyazaki, Y ; Saito, K.; Guo, F.; Kobayashi, K.; Osaka, K.; Kato, K.; Takata, M,; Tanigaki1, K. Superconductivity and physical properties of Ba24Si100 determined from electric transport, specific-heat capacity, and magnetic susceptibility measurements. Phys. Rev. B 2005, 72, 144504.
- Yamanaka, S.; Izumi, S.; Maekawa, S.; Umemoto, K. Phase diagram of the La–Si binary system under high pressure and the structures of superconducting LaSi5 and LaSi10. J. Solid. State. Chem. 2009, 182, 1991–2003. [Google Scholar] [CrossRef]
- Sung, H.-J.; Han, W.H.; Lee, I.-H.; Chang, K.J. Superconducting open-framework allotrope of silicon at ambient pressure. Phys. Rev. Lett. 2018, 120, 157001. [Google Scholar] [CrossRef] [PubMed]
- Flores-Livas, J.A; Sanna, A. Spintronics: Superconductivity in intercalated group-IV honeycomb structures. Phys. Rev. B. 2015, 91, 054508. [Google Scholar] [CrossRef]
- Huan, T.D. Spintronics: Pressure-stabilized binary compounds of magnesium and silicon. Phys. Rev. Mater. 2018, 2, 023803. [Google Scholar] [CrossRef]
- Zha, D.-B.; Jiang, P.; Huang, H.-M.; Li, Y.-L. Spintronics: Refined phase diagram and kagome-lattice superconductivity in Mg-Si system. Phys. Rev. Mater. 2023, 7, 114805. [Google Scholar] [CrossRef]
- Bordet, P.; Affronte, M.; Sanfilippo, S.; Nunez-Regueiro, M.; Laborde, O.; Olcese, G.L.; Palenzona, A.; LeFloch, S.; Levy, D.; Hanfland, M. Spintronics: Structural phase transitions in under high pressure. Phys. Rev. B. 2000, 62, 11392–11397. [Google Scholar] [CrossRef]
- Sanfilippo, S.; Elsinger, H.; Nunez-Regueiro, M.; Laborde, O.; LeFloch, S.; Affronte, M.; Olcese, G.L.; Palenzona, A. Spintronics: Superconducting high pressure CaSi2 phase with Tc up to 14 K. Phys. Rev. B. 2000, 61, R3800–R3803. [Google Scholar] [CrossRef]
- Imai, M.; Hirata, K.; Hirano, T. Spintronics: Superconductivity of trigonal BaSi2. Physica C 1995, 245, 12–14. [Google Scholar] [CrossRef]
- Satoh, T.; Asada, Y.J. Spintronics: Superconductivity of LaSi2. Phys. Soc. Japan. 1970, 28, 263–263. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Liu, H. Spintronics: Stable structures and superconductivity in a Y-Si system under high pressure. J. Phys. Chem. Lett. 2021, 12, 10388–10393. [Google Scholar] [CrossRef] [PubMed]
- Hilleke, K.P.; Zurek, E. Rational Design of Superconducting Metal Hydrides via Chemical Pressure Tuning. Angew. Chem. Int. Ed. 2022, 61, e202207589. [Google Scholar] [CrossRef] [PubMed]
- Flores-Livas, J.A.; Debord, R.; Botti, S.; Miguel, A.S.; Pailhès, S.; Marques, M.A.L. Spintronics: Superconductivity in layered binary silicides: A density functional theory study. Phys. Rev. B. 2011, 84, 184503. [Google Scholar] [CrossRef]
- Buschinger, B.; Geibel, C.; Diehl, J.; Weiden, M.M.; Guth, W.; Wildbrett, A.; Horn, S.; Steglich, F. Spintronics: Preparation and low temperature properties of FeSi-type RuSi. J. Alloy. Compd. 1997, 256, 57–60. [Google Scholar] [CrossRef]
- Perring, L.; Bussy, F.; Gachon, J.C.; Feschotte, P. The ruthenium-silicon system. J. Alloy. Compd. 1999, 284, 198–205. [Google Scholar] [CrossRef]
- Opahle, I.; Madsen, G.K.H.; Drautz, R. High throughput density functional investigations of the stability, electronic structure and thermoelectric properties of binary silicides. Phys. Chem. Chem. Phys. 2012, 14, 16197–16202. [Google Scholar] [CrossRef]
- Wolf, W.; Bihlmayer, G.; Blügel, S. Electronic structure of the Nowotny chimney-ladder silicide Ru2Si3. Phys. Rev. B 1997, 55, 6918–6926. [Google Scholar] [CrossRef]
- Ivanenko, L.; Behr, G.; Spinella, C.R.; Borisenko, V.E. RuSi2: evidence of a new binary phase in the ruthenium-silicon system. J. Cryst. Growth 2002, 236, 572–576. [Google Scholar] [CrossRef]
- Zhang, C.; Kuang, X.; Jin, Y.; Lu, C.; Zhou, D.; Li, P.; Bao, G.; Hermann, A. Prediction of stable ruthenium silicides from first-principles calculations: stoichiometries, crystal structures, and physical properties. ACS Appl. Mater. Inter. 2015, 7, 26776–26782. [Google Scholar] [CrossRef]
- Xiong, Z.; Wang, X.; Cao, L.; Wang, J.; Yu, J.; Yin, H.; Wang, X.; Ma, J. Structural stability, phase transition, electronic, elastic and thermodynamic properties of RuX (X= Si, Ge, Sn) alloys under high pressure. J. Alloy. Compd. 2017, 693, 440–447. [Google Scholar] [CrossRef]
- Ivanenkoa, L.I.; Shaposhnikova, V.L.; Filonova, A.B.; Krivosheevaa, A.V.; Borisenkoa, V.E.; Migasb, D.B.; Migliob, L.; Behrc, G.; Schumannc, J. Electronic properties of semiconducting silicides: fundamentals and recent predictions. Thin solid films 2004, 461, 141–147. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Lv, J.; Ma, Y. Materials discovery at high pressures. Nat. Rev. Mater. 2017, 2, 1–16. [Google Scholar] [CrossRef]
- Miao, M.; Sun, Y.; Zurek, E.; Lin, H. Chemistry under high pressure. Nat. Rev. Chem. 2020, 4, 508–527. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, J.; Zhu, L.; Ma, Y. Crystal structure prediction via particle-swarm optimization. Phys. Rev. B 2010, 82, 094116. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, J.; Zhu, L.; Ma, Y. CALYPSO: A method for crystal structure prediction. Comput. Phys. Commun. 2012, 183, 2063–2070. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C.; Jin, Y.; Li, S.; Zhang, W.; Kong, P.; Xie, C.; Du, C.; Liu, Q.; Zhang, C. Structural, mechanical and electronic properties and hardness of ionic vanadium dihydrides under pressure from first-principles computations. Sci. Rep. 2020, 10, 8868. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Y.; Yang, F.; Zhang, J.; Zhang, C.; Kuang, F.; Ju, M.; Li, S.; Cheng, S. Prediction of potential high-temperature superconductivity in ternary Y-Hf-H compounds under high pressure. Sci. Rep. 2024, 14, 17670. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, W.; Zhang, J.; Li, S.; Cheng, S.; Sun, W.; Ju, M.; Zhang, C. An unexpectedly stable Y2B5 compound with the fractional stoichiometry under ambient pressure. Arab. J. Chem. 2023, 16, 104546. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Zhang, J.; Li, S.; Ju, M.; Sun, W.; Dou, X.; Jin, Y. A ten-fold coordinated high-pressure structure in hafnium dihydrogen with increasing superconducting transition temperature induced by enhancive. Chinese Phys. B 2023, 32, 527–534. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Savin, A.; Jepsen, O.; Flad, J.; Andersen, O.K.; Preuss, H.; Schnering, H.G. Electron localization in solid-state structures of the elements: the diamond structure. Angew. Chem., Int. Ed. Engl. 1992, 31, 187–188. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B. 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Dronskowski, R.; Bloechl, P.E. Crystal orbital Hamilton populations (COHP): energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 1993, 97, 8617–8624. [Google Scholar] [CrossRef]
- Maintz, S.; Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. LOBSTER: A tool to extract chemical bonding from plane-wave based DFT. J. Comput. Chem. 2016, 37, 1030–1035. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; Corso, A.D.; Gironcoli, S.; Fabris, S.; Fratesi, G.; Gebauer, R.; Gerstmann, U.; Gougoussis, C.; Kokalj, A.; Lazzeri, M.; Martin-Samos, L.; Marzari, N.; Mauri, F.; Mazzarello, R.; Paolini, S.; Pasquarello, A.; Paulatto, L.; Sbraccia, C.; Scandolo, S.; Sclauzero, G.; Seitsonen, A.P., Smogunov, A.; Umari, P.; Wentzcovitch, R.M. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys.: Condens. Matter 2009, 21, 395502.
- Opahle, I.; Parma, A.; McEnary, E.J.; Drautz, R.; Madsen, G.KH. High-throughput study of the structural stability and thermoelectric properties of transition metal silicides. New J. Phys. 2013, 15, 105010. [Google Scholar] [CrossRef]
- Siegrist, T.; Hulliger, F.; Travaglini, G. The crystal structure and some properties of ReSi2. J. Alloy. Compd. 1983, 92, 119–129. [Google Scholar] [CrossRef]
- Cynn, H.; Klepeis, J.E.; Yoo, C.S.; Young, D.A. Osmium has the lowest experimentally determined compressibility. Phys. Rev. Lett. 2002, 88, 135701. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Ikuta, D.; Sinogeikin, S.; Li, Q.; Zhang, Y.; Chen, C. Direct observation of a pressure-induced precursor lattice in silicon. Phys. Rev. Lett. 2012, 109, 205503. [Google Scholar] [CrossRef] [PubMed]
- Vocado, L.; Price, G.D.; Wood, I.G. Crystal structure, compressibility and possible phase transitions in Ɛ-FeSi studied by first-principles pseudopotential calculations. Acta Cryst. 1999, B55, 484–493. [Google Scholar] [CrossRef]
- Shaposhnikov, V.L.; Filonov, A.B.; Krivosheeva, A.V.; Ivanenko, L.I.; Borisenko, V.E. Structural, electronic and optical properties of a new binary phase–ruthenium disilicide. Phys. Stat. Sol. (b) 2005, 242, 2864–2871. [Google Scholar] [CrossRef]
- Kim, D.Y.; Stefanoski, S.; Kurakevych, O.O.; Strobel, T.A. Synthesis of an open-framework allotrope of silicon. Nat. Mater. 2015, 14, 169–173. [Google Scholar] [CrossRef]
- Pan, Y.; Jing, C.; Wu, Y. The structure, mechanical and electronic properties of WSi2 from first-principles investigations. Vacuum 2019, 167, 374–381. [Google Scholar] [CrossRef]
- Wang, J.; Kuang, X.; Jin, Y.; Lu, C.; Huang, X. Theoretical investigation on the structural phase transition, elastic properties and hardness of RhSi under high pressure. J. Alloy Compd. 2014, 592, 42–47. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Wei, J.; Zhong, T.; Zhu, L.; Zhang, X.; Liu, H.; Zhang, S. Hardness and superconductivity in tetragonal LiB4 and NaB4. J. Chem. Phys. 2023, 159, 234707. [Google Scholar] [CrossRef]
- Zhang, S.; Du, X.; Lin, J.; Bergara, A.; Chen, X.; Liu, X.; Zhang, X.; Yang, G. Superconducting boron allotropes. Phys. Rev. B. 2020, 101, 174507. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, G.; Wang, J.; Lu, C.; Jin, Y.; Kuang, X.; Hermann, A. Prediction of novel high-pressure structures of magnesium niobium dihydride. ACS Appl. Mater. Interfaces 2017, 9, 26169–26176. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Debessai, M.; Hamlin, J.J.; Gangopadhyay, A.K.; Schilling, J.S. Pressure-Induced superconductivity in CaLi2. Phys. Rev. Lett. 2008, 100, 197003. [Google Scholar] [CrossRef]
- Chen, X.-J.; Wang, J.-L.; Struzhkin, V.V.; Mao, H.; Hemley, R.J.; Lin, H.-Q. Superconducting behavior in compressed solid with a layered structure. Phys. Rev. Lett. 2008, 101, 077002. [Google Scholar] [CrossRef] [PubMed]
- Weller, T.E. Weller, T.E.; Ellerby, Mark.; Saxena, S.S.; Smith, R.P.; Skipper, N.T. Superconductivity in the intercalated graphite compounds C6Yb and C6Ca. Nat. Phys. 2005, 1, 39–41. [Google Scholar] [CrossRef]
- Yang, Q.P.; Zhang, H.M.; Zhao, J.J.; Jiang, X. Probing the superconductivity limit of Li-doped graphene. Adv. Funct. Mater. 2024, 2406023. [Google Scholar] [CrossRef]
- Liao, Z.W.; Yi, X.W.; You, J.Y.; Gu, B.; Su, G. Family of binary transition metal pnictide superconductors. Phys. Rev. B 2023, 108, 014501. [Google Scholar] [CrossRef]
- Kang, Y.-Gu.; Lee, I.-H.; Han, M.J.; Chang, K.J. Spintronics: Ab initio prediction of nontrivial topological band and superconductivity in stable metallic Si allotropes at ambient pressure. Phys. Rev. Mater. 2021, 5, 104802. [Google Scholar] [CrossRef]
- Wu, L.L.; Wan, B.; Liu, H.Y.; Gou, H.Y.; Yao, Y.S.; Li, Z.P.; Zhang, J.W.; Gao, F.M.; Mao, H-k. Spintronics: Coexistence of superconductivity and superhardness in beryllium hexaboride driven by inherent multicenter bonding. J. Phys. Chem. Lett. 2016, 7, 4898–4904. [CrossRef]
- Fu, Y.; Li, F.; Zhang, X.H.; Zhang, S.L.; Liu,Y.; Yang, G.C. Superconducting ScP4 with a novel phosphorus framework. Appl. Phys. A 2022, 128, 318. [CrossRef]
- Sananda, B.; Kreisel, A.; Valadkhani, A.; Dürrnagel, M.; Schwemmer, T.; Thomale, R.; Valentí, R.; Mazin, I.I. Hybrid s-wave superconductivity in CrB2. Phys. Rev. B 2023, L020501. [Google Scholar]
- Schilling, J.S. Studies in superconductivity at extreme pressures. Physica C 2007, 460, 182–185. [Google Scholar] [CrossRef]
- Hamlin, J.J. Superconductivity in the metallic elements at high pressures. Physica C 2015, 514, 59–76. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, Q.; Li, H.; Gao, B.; Wei, S.; Zhu, L.; Xu, H.; Liu, H.; Zhang, S. Superconductivity in dense scandium-based phosphides. Phys. Rev. B 2023, 108, 174513. [Google Scholar] [CrossRef]
- Kortus, J.; Mazin, I.I.; Belashchenko, K.D.; Antropov, V.P.; Boyer, L.L. Superconductivity of Metallic Boron in MgB2. Phys. Rev. Lett. 2001, 86, 4656–4659. [Google Scholar] [CrossRef]
- Lu, C.; Kuang, X.Y.; Wang, S.J.; Zhao, Y.R.; Tan, X.M. Theoretical investigation on the high-pressure structural transition and thermodynamic properties of cadmium. Europhys. Lett. 2010, 91, 16002. [Google Scholar] [CrossRef]
- Mouhat, F.; Coudert, F.X. Necessary and sufficient elastic stability conditions in various crystal systems. Phys. Rev. B 2014, 90, 224104. [Google Scholar] [CrossRef]
- Connétable, D.; Thomas, O. First-principles study of the structural, electronic, vibrational, and elastic properties of orthorhombic NiSi. Phys. Rev. B 2009, 79, 094101. [Google Scholar] [CrossRef]
- Shein, I.R.; Ivanovskii, A.L. Elastic properties of mono-and polycrystalline hexagonal AlB2-like diborides of s, p and d metals from first-principles calculations. J. Phys.: Condens. Matter 2008, 20, 415218. [Google Scholar] [CrossRef]
- Wu, Z.J.; Zhao, E.J.; Xiang, H.P.; Hao, X.F.; Liu, X.J.; Jian, M. Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles. Phys. Rev. B 2007, 76, 054115. [Google Scholar] [CrossRef]
- Haines, J.; Léger, J.M.; Bocquillon, G. Synthesis and design of superhard materials. Annu. Rev. Mater. Res. 2001, 31, 1–23. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, S.L.; Zhang, X.; Jia, L.H. First-principles investigation of new structure, mechanical and electronic properties of Mo-based silicides. Ceram. Int. 2018, 44, 1744–1750. [Google Scholar] [CrossRef]
- Todai, M.; Hagihara, K.; Kishida, K.; Inui, H.; Nakano, T. Microstructure and fracture toughness in boron added NbSi2 (C40)/MoSi2 (C11b) duplex crystals. Scr. Mater. 2016, 113, 236–240. [Google Scholar] [CrossRef]
- Zhang, G.H.; Sun, G.D.; Chou, K.C. A novel process to prepare MoSi2 by reaction between MoS2 and Si. J. Alloy Compd. 2017, 694, 480–488. [Google Scholar] [CrossRef]
- Pu, D.L.; Pan, Y. Influence of high pressure on the structure, hardness and brittle-to-ductile transition of NbSi2 ceramics. Ceram. Int., 2021, 47, 2311–2318. [Google Scholar] [CrossRef]
- Ding, L.P.; Shao, P.; Zhang, F.H.; Lu, C.; Huang, X.F. Prediction of molybdenum nitride from first-principle calculations: crystal structures, electronic properties, and hardness. J. Phys. Chem. C 2018, 122, 21039–21046. [Google Scholar] [CrossRef]
- Gao, F.M.; He, J.L.; Wu, E.D.; Liu, S.M.; Yu, D.L.; Li, D.C.; Zhang, S.Y.; Tian, Y.J. Hardness of covalent crystals. Phys. Rev. Lett. 2003, 91, 015502. [Google Scholar] [CrossRef]
- Gao, F.M. Theoretical model of intrinsic hardness. Phys. Rev. B 2006, 73, 132104. [Google Scholar] [CrossRef]
- Andrievski, R.A. Superhard materials based on nanostructured high-melting point compounds: achievements and perspectives. Int. J. Refract. Met. Hard Mater. 2001, 19, 447–452. [Google Scholar] [CrossRef]
- Guo, X.J.; Li, L.; Liu, Z.Y.; Yu, D.L.; He, J.L.; Liu, R.P.; Xu, B.; Tian, Y.J.; Wang, H.T. Hardness of covalent compounds: Roles of metallic component and d valence electrons. J. Appl. Phys. 2008, 104, 023503. [Google Scholar] [CrossRef]
- Yan, H.Y.; Wei, Z.T.; Zhang, M.G.; Wei, Q. Exploration of stable stoichiometries, ground-state structures, and mechanical properties of the W-Si system. Ceram. Int. 2020, 46, 17034–17043. [Google Scholar] [CrossRef]

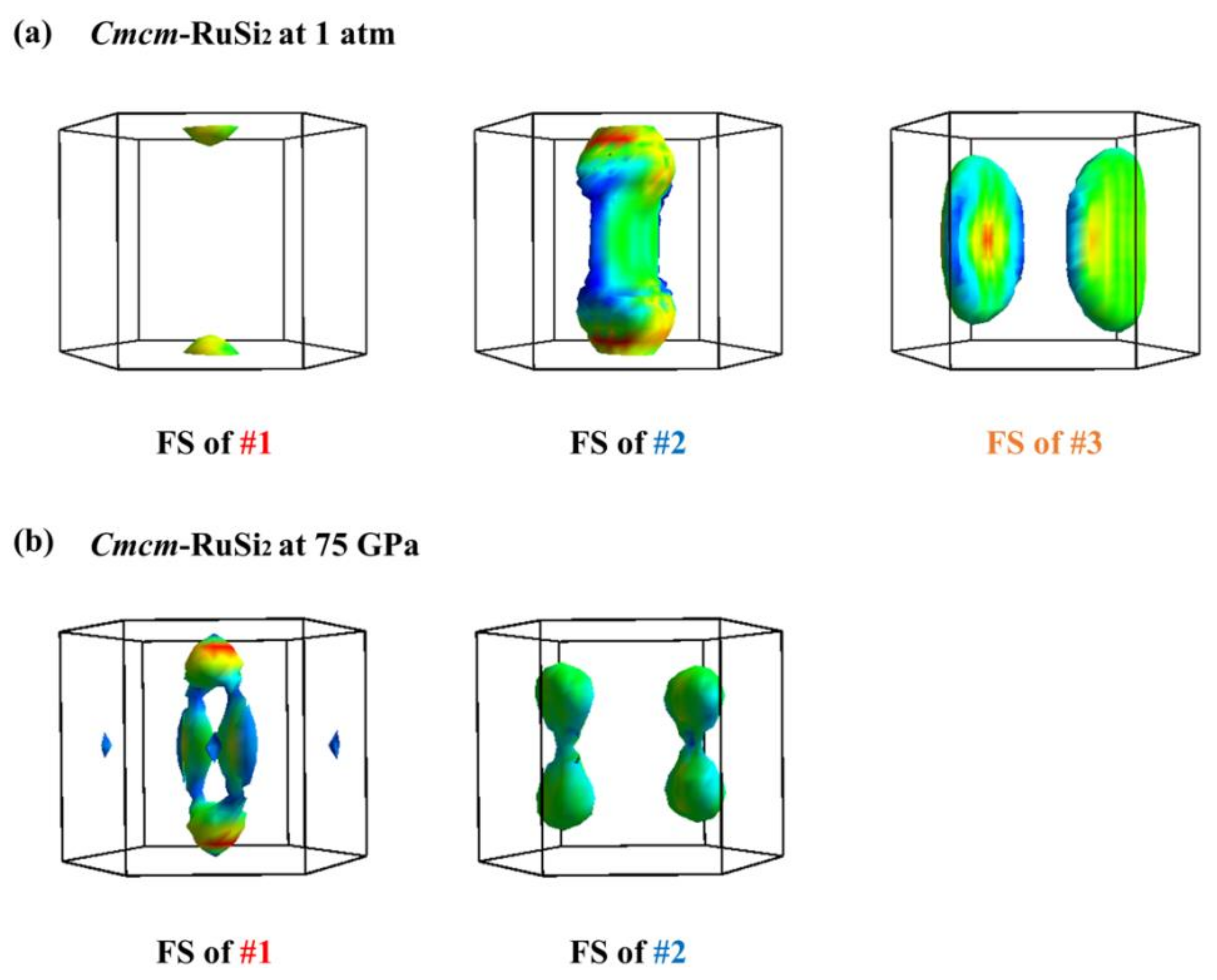
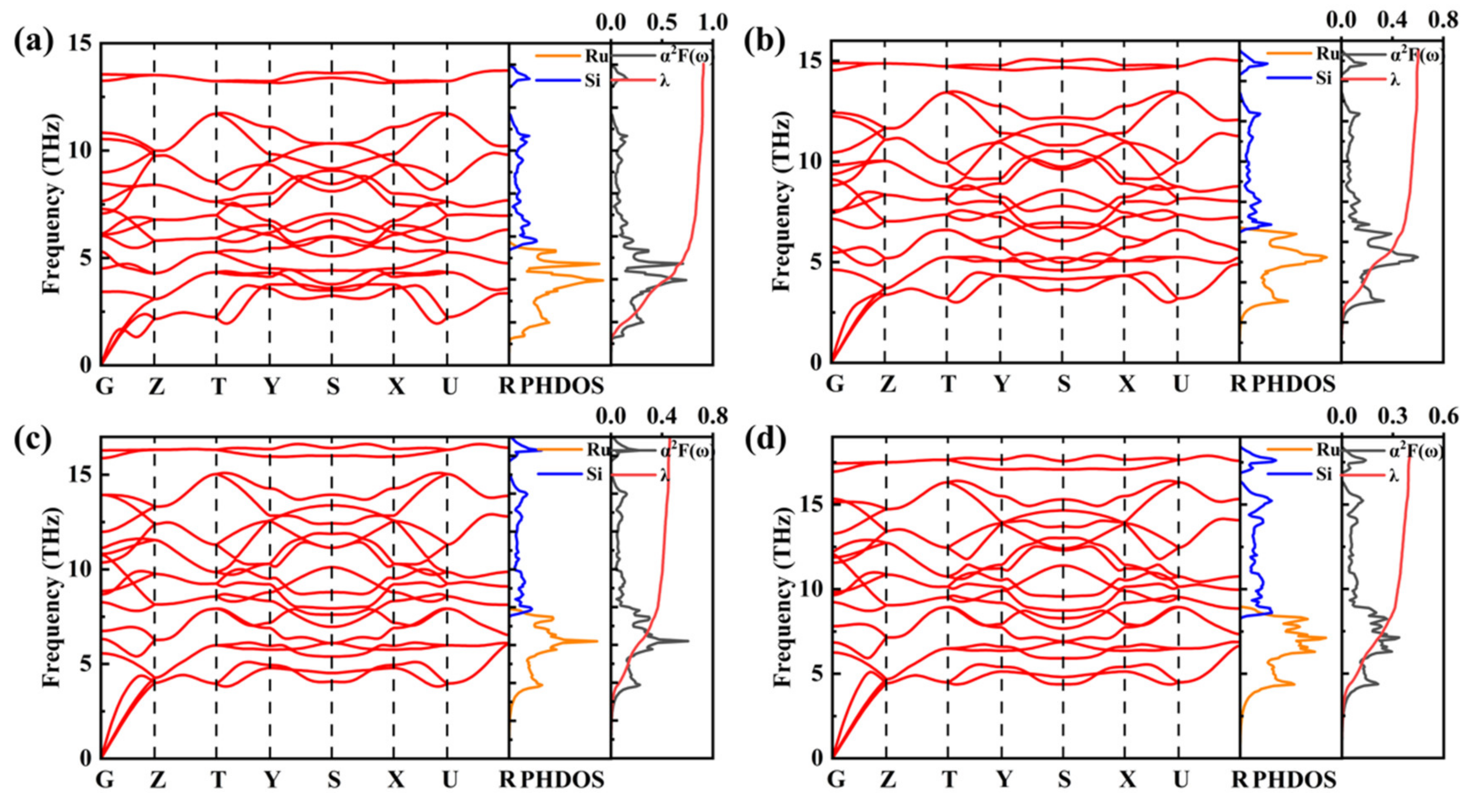
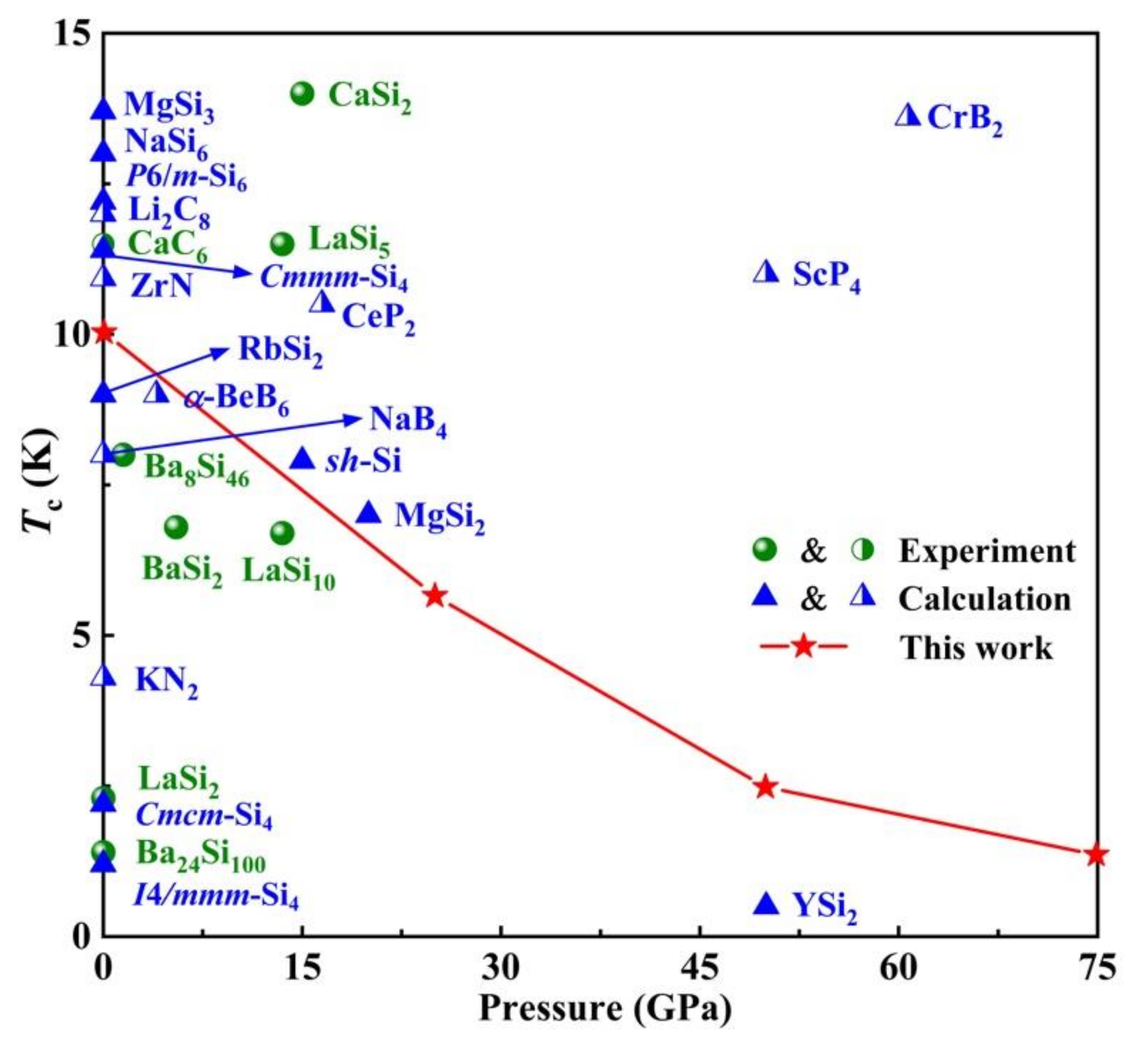
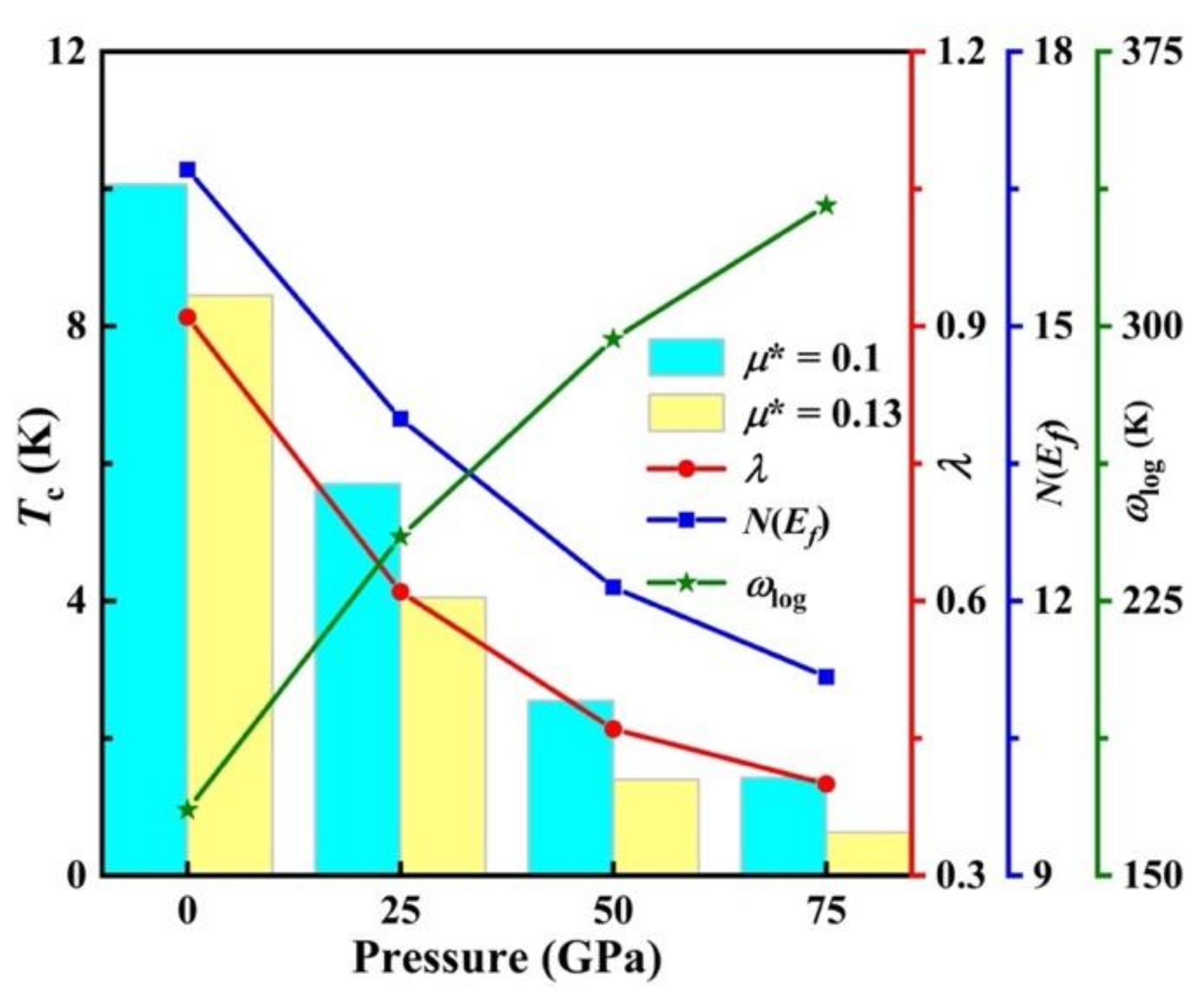
| Structure | Pressure | ωlog | N (Ef) | λ | Tc | |
| μ* = 0.10 | μ* = 0.13 | |||||
| Cmcm-RuSi2 | 1 atm | 167.97 | 16.71 | 0.91 | 10.07 | 8.45 |
| 25 GPa | 242.56 | 13.99 | 0.61 | 5.71 | 4.05 | |
| 50 GPa | 296.50 | 12.15 | 0.46 | 2.55 | 1.40 | |
| 75 GPa | 332.95 | 11.17 | 0.40 | 1.43 | 0.63 | |
| Pressure | Space group | C11 | C22 | C33 | C44 | C55 | C66 | C12 | C13 | C23 | G | B | B/G | E | ν |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 atm | Cmca | 271 | 336 | 327 | 103 | 111 | 122 | 115 | 90 | 112 | 107.99 | 173.53 | 1.61 | 268.30 | 0.24 |
| 1 atm | Cmcm | 389 | 288 | 292 | 85 | 90 | 69 | 96 | 112 | 128 | 89.28 | 181.20 | 2.03 | 230.05 | 0.29 |
| 75 GPa | Cmcm | 836 | 676 | 594 | 149 | 173 | 188 | 261 | 246 | 298 | 184.99 | 410.13 | 2.22 | 482.44 | 0.30 |
| bond | P | n | d (Å) | Vb (Å3) | Hv (GPa) | |
| Cmca | Ru-Si | 0.38 | 16 | 2.452 | 2.955 | 31.7 |
| 0.30 | 16 | 2.458 | 2.977 | 28a | ||
| 0.35 | 16 | 2.460 | 2.984 | |||
| 0.41 | 16 | 2.460 | 2.986 | |||
| 0.36 | 16 | 2.467 | 3.010 | |||
| 0.30 | 16 | 2.484 | 3.072 | |||
| 0.30 | 16 | 2.500 | 3.131 | |||
| 0.27 | 16 | 2.523 | 3.219 | |||
| Si-Si | 0.33 | 8 | 2.541 | 3.289 | ||
| 0.32 | 16 | 2.570 | 3.402 | |||
| 0.28 | 8 | 2.576 | 3.428 | |||
| 0.27 | 8 | 2.598 | 3.514 | |||
| 0.31 | 16 | 2.612 | 3.574 | |||
| 0.18 | 8 | 2.689 | 3.898 | |||
| 0.20 | 16 | 2.693 | 3.913 | |||
| Cmcm | Ru-Si | 2.23 | 8 | 2.226 | 6.367 | 26.8 |
| 0.32 | 4 | 2.242 | 6.513 | |||
| Si-Si | 0.37 | 4 | 2.293 | 6.967 | ||
| a Reference [49]. | 0.76 | 4 | 2.307 | 7.095 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




