Submitted:
07 October 2024
Posted:
07 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Polyurethanes (PUs)
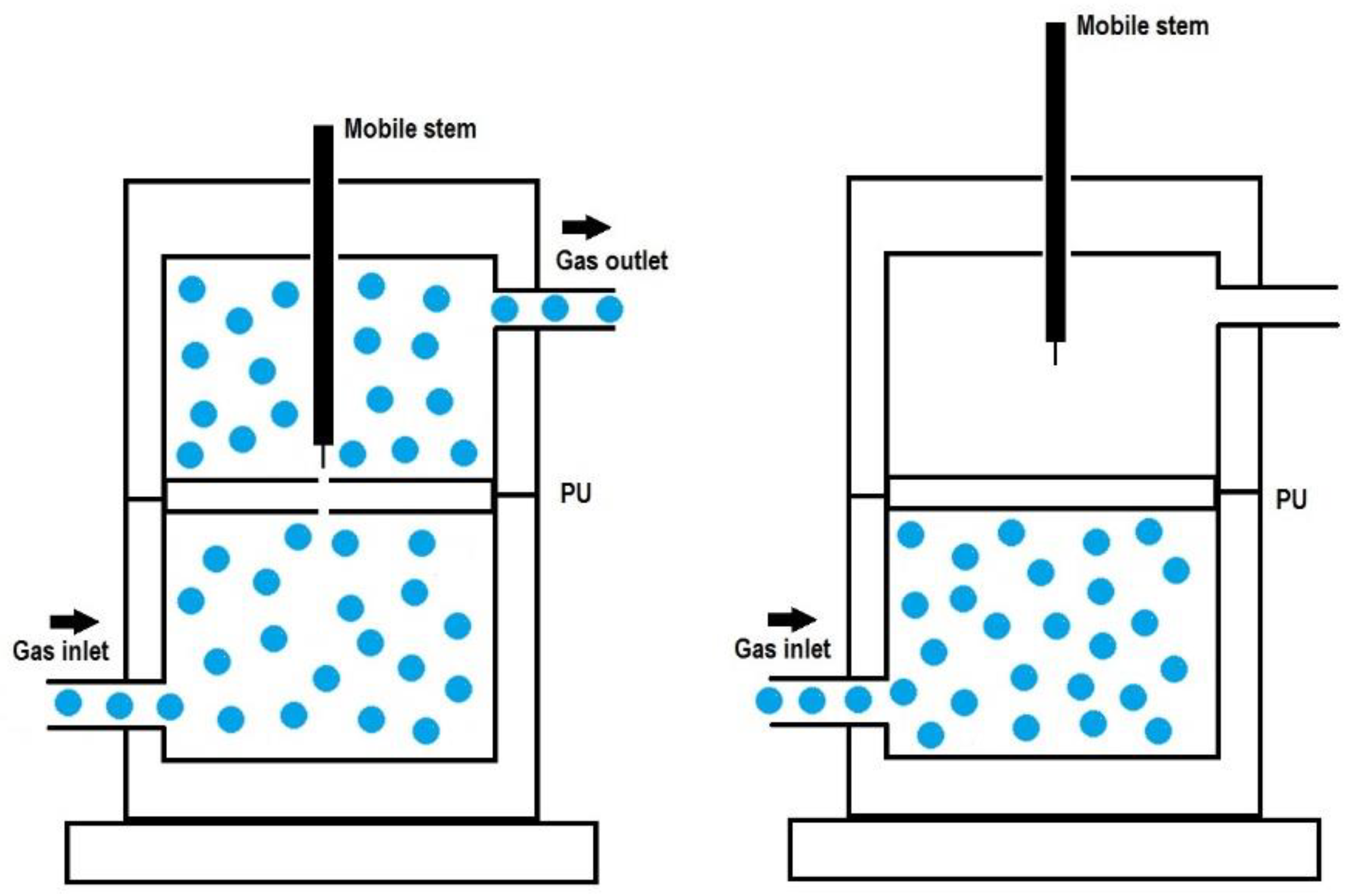
2.3. Experimental Techniques
3. Results and Discussion
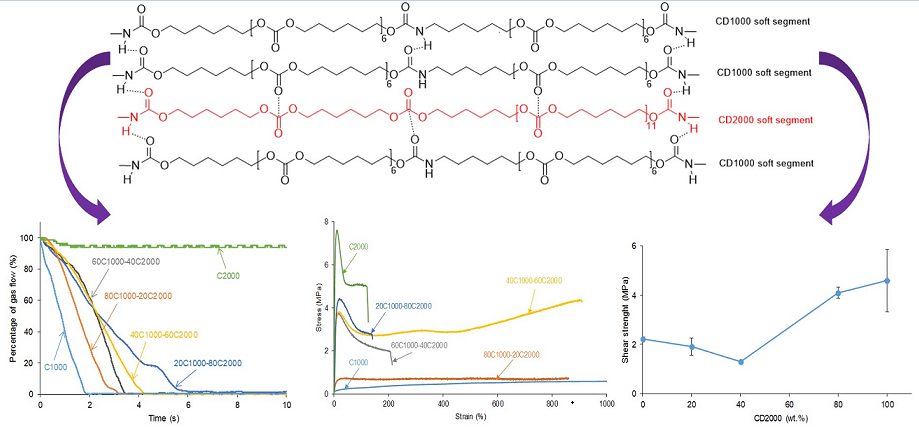
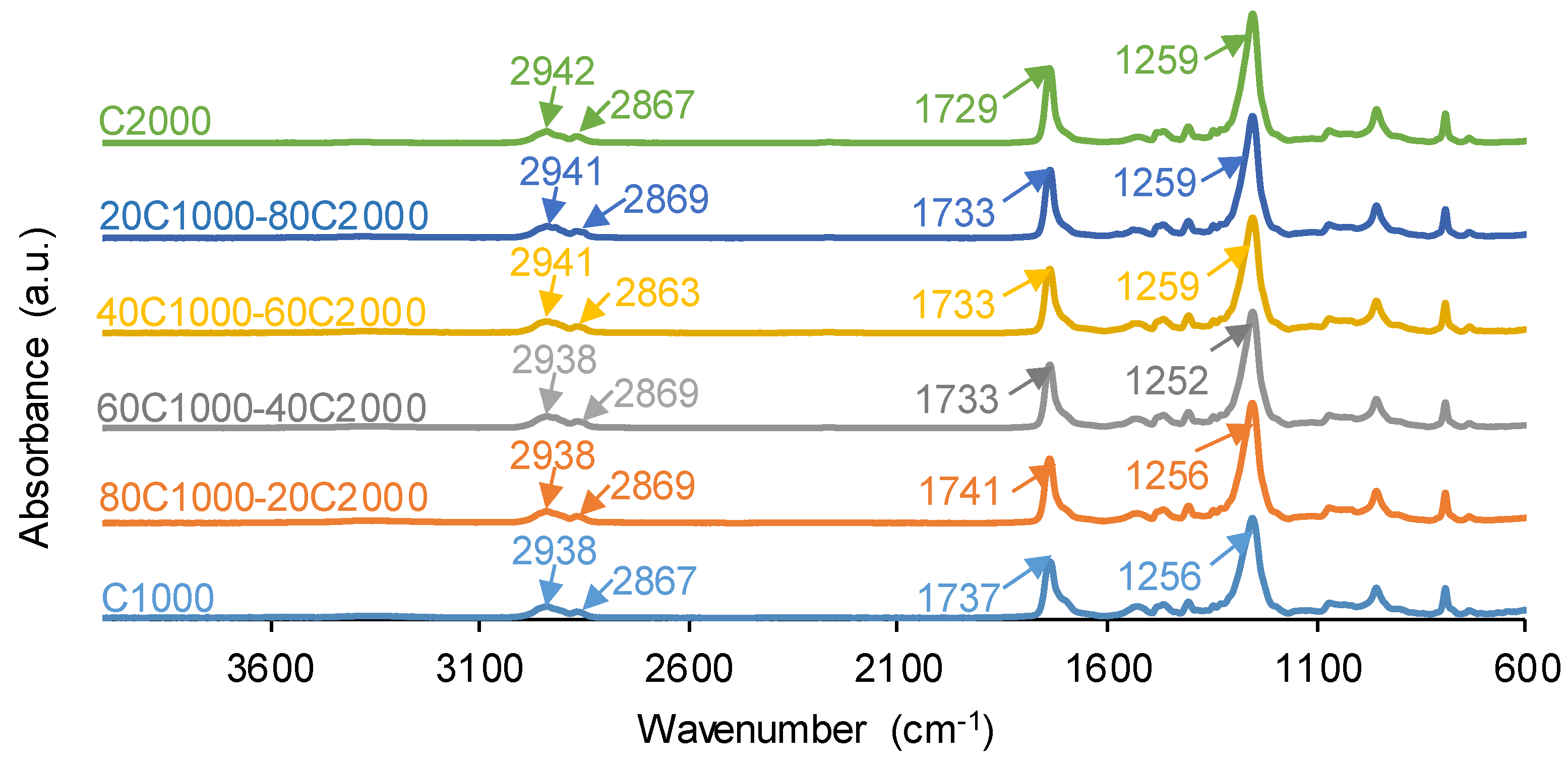
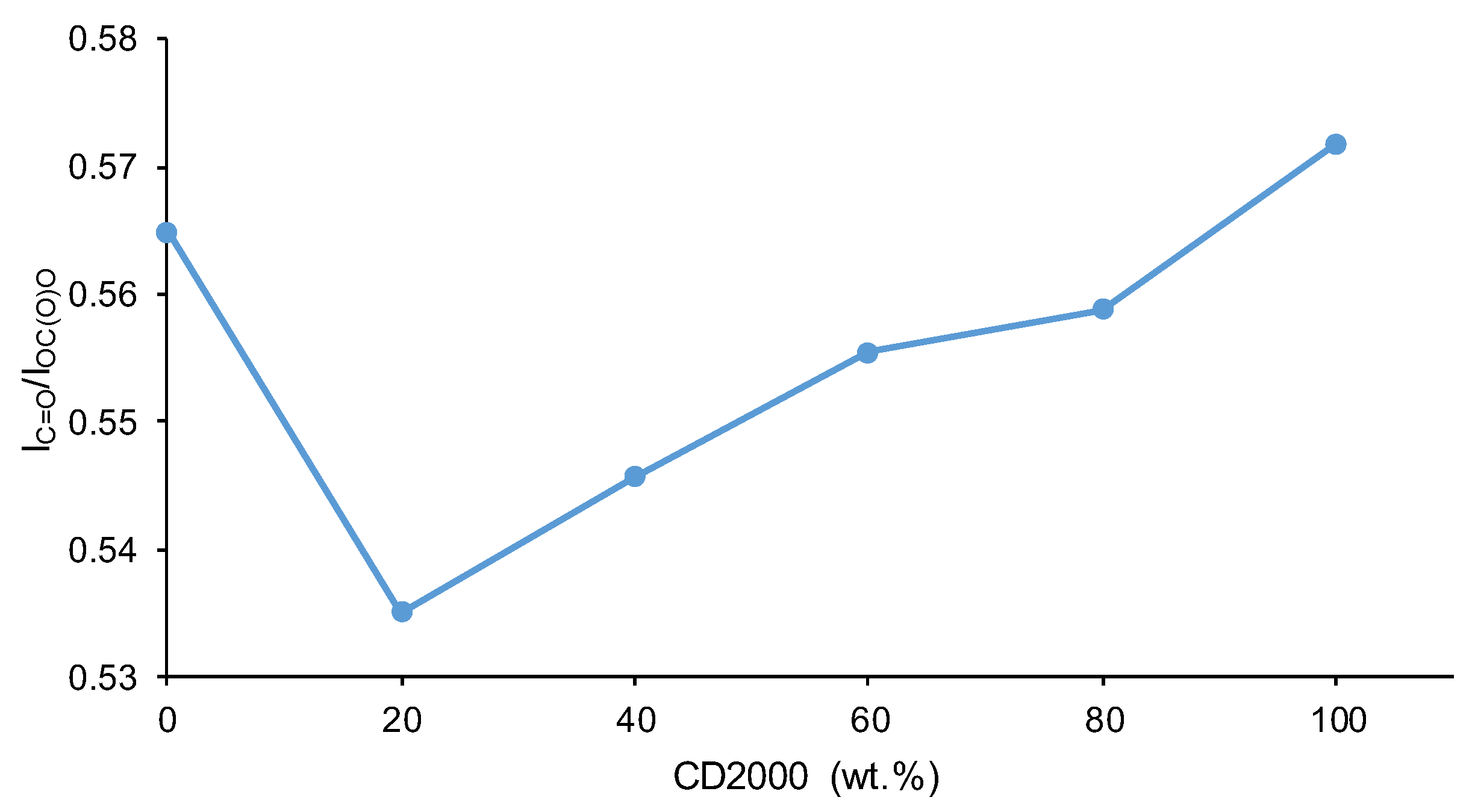
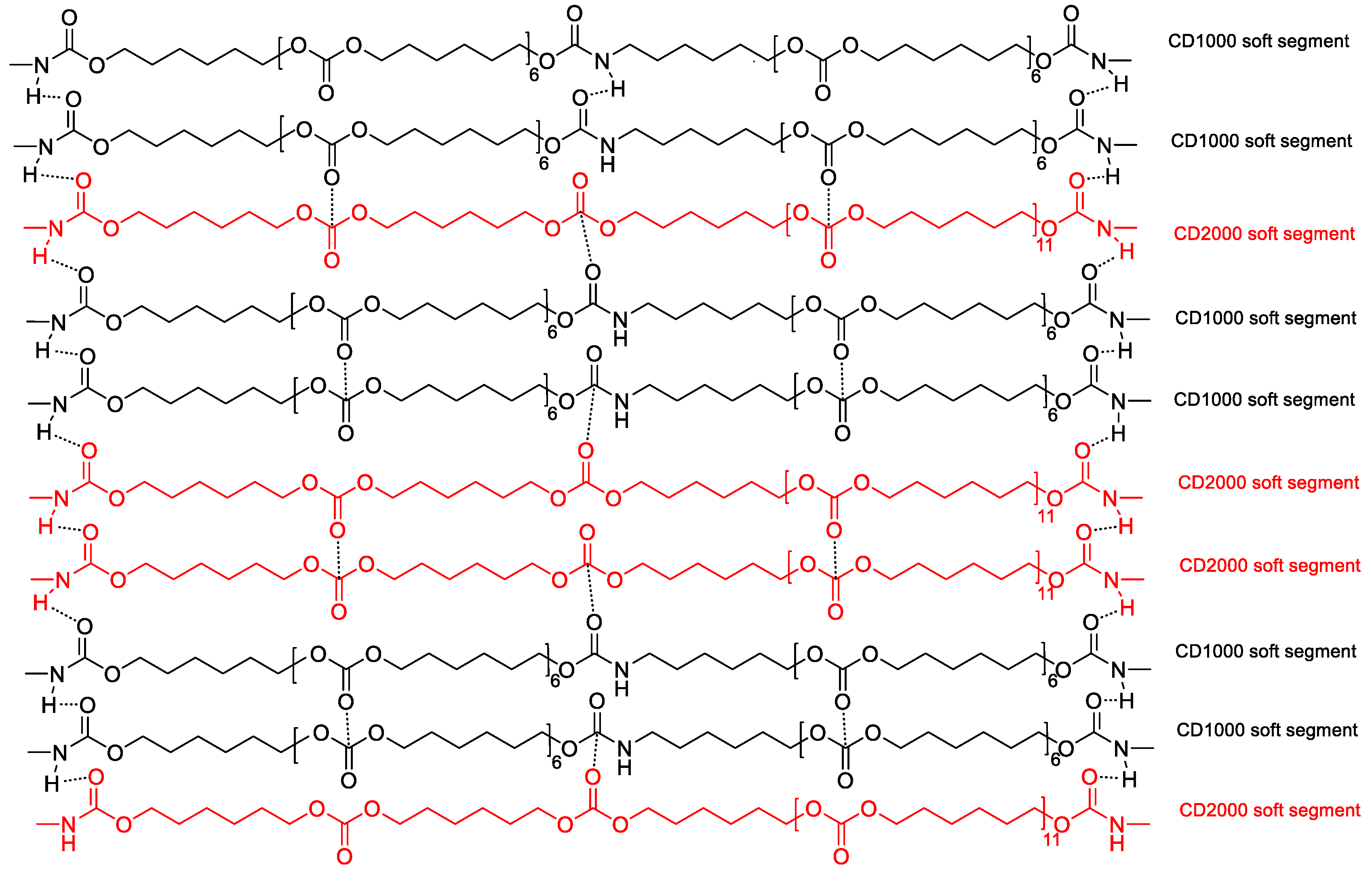
3.1. Structural Characterization and Degree of Phase Separation of PUs
| PU | tan delta | Ttan delta (°C) |
| C1000 | 0.38 | 10 |
| 80C1000-20C2000 | 0.27 | 30 |
| 60C1000-40C2000 | 0.21 | 26 |
| 40C1000-60C2000 | 0.20 | 26 |
| 20C1000-80C2000 | 0.17 | 24 |
| C2000 | 0.16 | 17 |
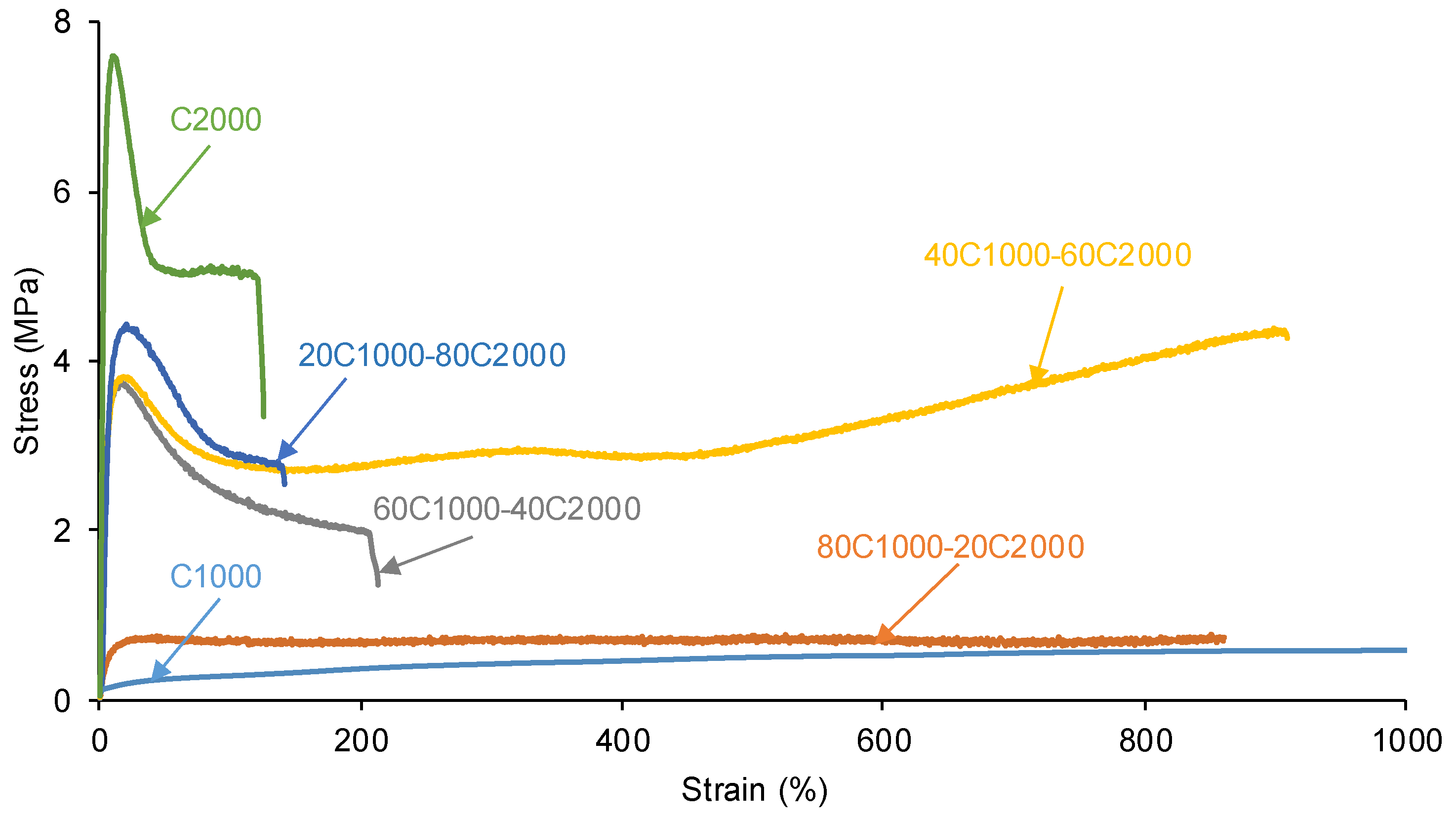
3.2. Mechanical Properties of the PUs
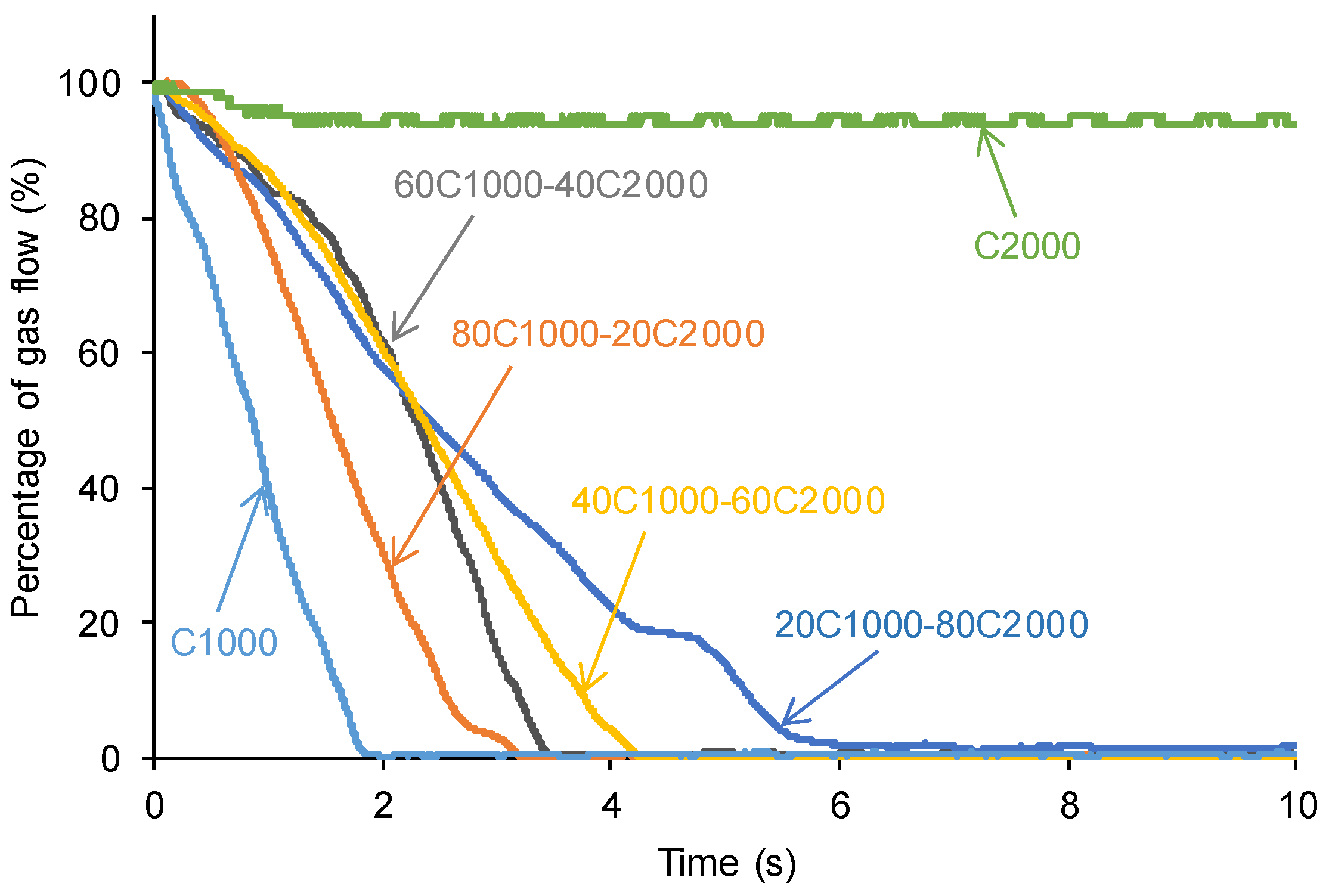
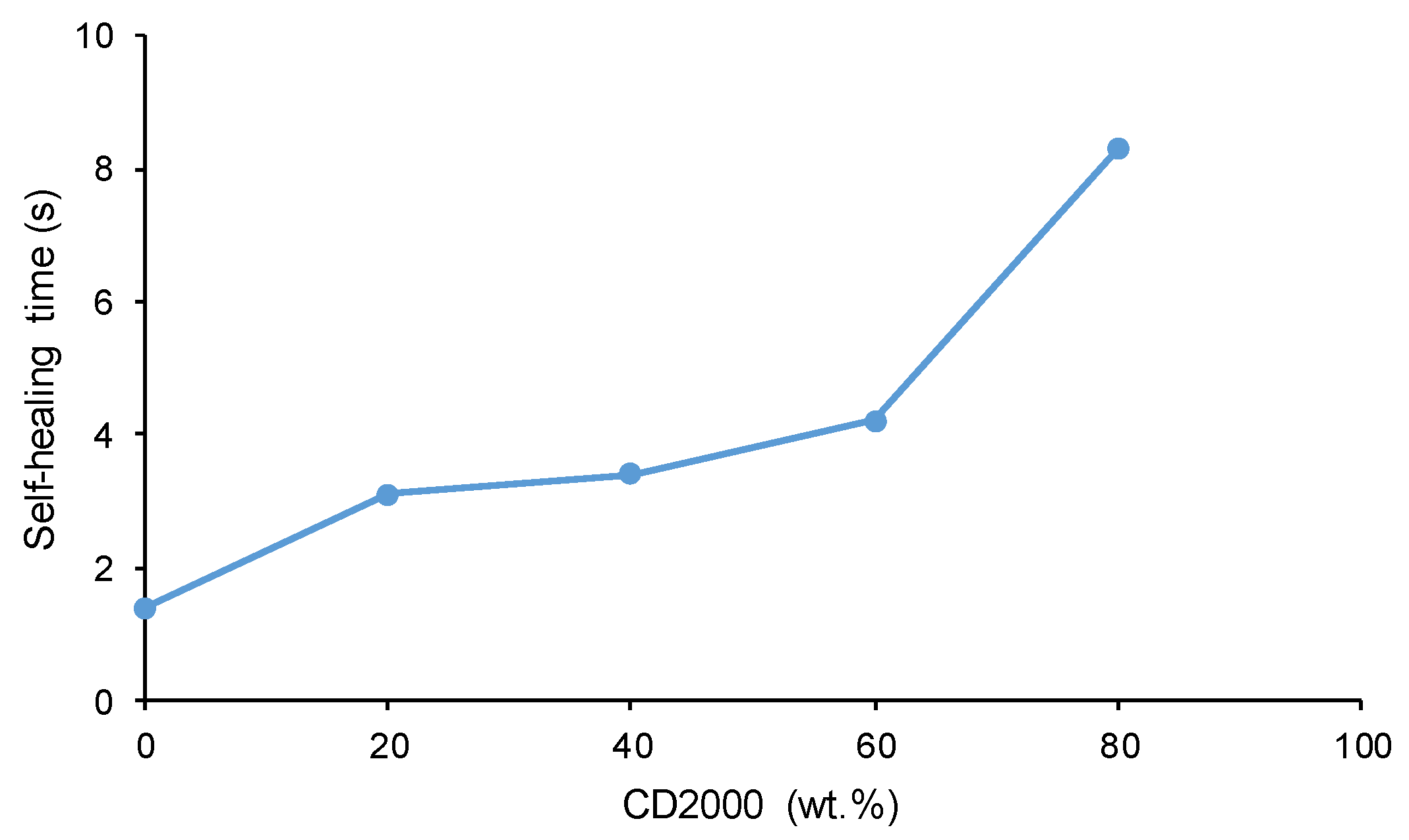
3.3. Assessment of Self-Healing at 20 °C of the PUs
3.4. Adhesion of Stainless Steel/PU Adhesive/Stainless Steel Joints
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matos-Pérez, C.R.; White, J.D.; Wilker, J.J. Polymer composition and substrate influences on the adhesive bonding of a biomimetic, cross-linking polymer. J. Am. Chem. Soc. 2012, 134, 9498–9505. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, W.; Hu, J.; Xie, D.; Gerhard, E.; Nisic, M.; Shan, D.; Qian, G.; Zheng, S.; Yang, J. Synthesis and characterization of anti-bacterial and anti-fungal citrate-based mussel-inspired bioadhesives. Biomaterials. 2016, 85, 204–217. [Google Scholar] [CrossRef]
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P.J. Adhesion of polymers. Prog. Polym. Sci. 2009, 34, 948–968. [Google Scholar] [CrossRef]
- Engels, H.W.; Pirkl, H.G.; Albers, R.; Albach, R.W.; Krause, J.; Hoffmann, A.; Casselmann, H.; Dormish, J. Polyurethanes: Versatile materials and sustainable problem solvers for today’s challenges. Angew. Chem. Int. Ed. 2013, 52, 9422–9441. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Z.; Zhang, L.; Guo, Y.; Song, J.; Lou, J.; Guan, Q.; He, C.; You, Z. Strong, detachable, and self-healing dynamic crosslinked hot melt polyurethane adhesive. Mater. Chem. Front. 2019, 3, 1833–1839. [Google Scholar] [CrossRef]
- Han, F.; Shah, S.A. A.; Liu, X.; Zhao, F.; Xu, B.; Zhang, J.; Cheng, J. Preparation and properties of self-healing cross-linked polyurethanes based on blocking and deblocking reaction. React. Funct. Polym. 2019, 144, 104347. [Google Scholar] [CrossRef]
- Longfang, R.; Congcong, L.; Taotao, Q. Preparation of boroxine-based self-healing polyurethane with repeatable adhesive property under mild conditions. Mater. Today Chem. 2022, 26, 101126. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, J.; Zhang, X.; Du, Y.; Li, K.; Liu, Y.; Yu, G.; Jia, Y.; Song, S. Dual dynamic bonds self-healing polyurethane with superior mechanical properties over a wide temperature range. Eur. Polym. J. 2022, 163, 110934. [Google Scholar] [CrossRef]
- Urdl, K.; Kandelbauer, A.; Kern, W.; Müller, U.; Thebault, M.; Zikulnig-Rusch, E. Self-healing of densely crosslinked thermoset polymers—a critical review. Prog. Org. Coat. 2017, 104, 232–249. [Google Scholar] [CrossRef]
- Thakur, V.K.; Kessler, M.R. Self-healing polymer nanocomposite materials: A review. Polymer. 2015, 69, 369–383. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, L.; Pan, C.; Li, D. Review of recent achievements in self-healing conductive materials and their applications. J. Mater. Sci. 2018, 53, 27–46. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, J.; Gan, L.; Long, M. Research progress in bio-based self-healing materials. Eur. Polym. J. 2020, 129, 109651. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Sun, A.; Jing, M.; Liu, X.; Wei, L.; Wu, K.; Fu, Q. A self-reinforcing and self-healing elastomer with high strength, unprecedented toughness and room-temperature reparability. Mater. Horiz. 2021, 8, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Aguirresarobe, R.H.; Nevejans, S.; Reck, B.; Irusta, L.; Sardon, H.; Asua, J.M.; Ballard, N. Healable and self-healing polyurethanes using dynamic chemistry. Prog. Polym. Sci. 2021, 114, 101362. [Google Scholar] [CrossRef]
- Wong, C.S.; Hassan, N.I.; Su’ait, M.S.; Serra, M.A. P.; Gonzalez, J.A. M.; Granda, L.A.; Badri, K.H. Photo-activated self-healing bio-based polyurethanes. Ind. Crops Prod. 2019, 140, 111613. [Google Scholar] [CrossRef]
- Durand-Silva, A.; Cortés-Guzmán, K.P.; Johnson, R.M.; Perera, S.D.; Diwakara, S.D.; Smaldone, R.A. Balancing self-healing and shape stability in dynamic covalent photoresins for stereolithography 3D printing. ACS Macro Lett. 2021, 10, 486–491. [Google Scholar] [CrossRef]
- Kuhl, N.; Bode, S.; Bose, R.K.; Vitz, J.; Seifert, A.; Hoeppener, S.; Garcia, S.J.; Spange, S.; van der Zwaag, S.; Hager, M.D.; Schubert, U.S. Acylhydrazones as reversible covalent crosslinkers for self-healing polymers. Adv. Funct. Mater. 2015, 25, 3295–3301. [Google Scholar] [CrossRef]
- Chang, K.; Jia, H.; Gu, S.Y. A transparent, highly stretchable, self-healing polyurethane based on disulfide bonds. Eur. Polym. J. 2019, 112, 822–831. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, J.; Song, S. Synthesis and characterization of linear polyisoprene supramolecular elastomers based on quadruple hydrogen bonding. Polymers. 2020, 12, 110. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, H.; Chen, S. New zwitterionic polyurethanes containing pendant carboxyl-pyridinium with shape memory, shape reconfiguration, and self-healing properties. Polymer. 2019, 180, 121727. [Google Scholar] [CrossRef]
- Arévalo-Alquichire, S.; Morales-Gonzalez, M.; Navas-Gómez, K.; Diaz, L.E.; Gómez-Tejedor, J.A.; Serrano, M.A.; Valero, M.F. Influence of polyol/crosslinker blend composition on phase separation and thermo-mechanical properties of polyurethane thin films. Polymers. 2020, 12, 666. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Mo, F.; Yang, Y.; Stadler, F.J.; Chen, S.; Yang, H.; Ge, Z. Development of zwitterionic polyurethanes with multi-shape memory effects and self-healing properties. J. Mater. Chem. A. 2015, 3, 2924–2933. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, L.; Li, P.; Maitz, M.F.; Wang, K.; Shang, T.; Dai, S.; Fu, Y.; Zhao, Y.; Yang, Z.; Wang, J.; Li, X. Adhesive and self-healing polyurethanes with tunable multifunctionality. Research. 2022, 9795682. [Google Scholar] [CrossRef]
- Dehaghani, M.Z.; Kaffashi, B. , Haponiuk, J.T.; Piszczyk, L. Shape memory thin films of polyurethane: Does graphene content affect the recovery behavior of polyurethane nanocomposites? Polym. Compos. 2020, 41, 3376–3388. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Gao, G. Bioinspired adhesive hydrogels tackified by nucleobases. Adv. Funct. Mater. 2017, 27, 1703132. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; Wen, X.; Wang, S.; Wang, H. Mechanically strong, wet adhesive, and self-healing polyurethane ionogel enhanced with a semi-interpenetrating network for underwater motion detection. ACS Appl. Mater. Interfaces. 2022, 14, 54203–54214. [Google Scholar] [CrossRef]
- Li, T.; Xie, Z.; Xu, J.; Weng, Y.; Guo, B.H. Design of a self-healing cross-linked polyurea with dynamic cross-links based on disulfide bonds and hydrogen bonding. Eur. Polym. J. 2018, 107, 249–257. [Google Scholar] [CrossRef]
- Grande, A.M.; Bijleveld, J.C.; Garcia, S.J.; Van Der Zwaag, S. A combined fracture mechanical–rheological study to separate the contributions of hydrogen bonds and disulphide linkages to the healing of poly (urea-urethane) networks. Polymer. 2016, 96, 26–34. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Zeng, W.; Jin, H.; Shang, X.; Zhou, R. Bioinspired fast room-temperature self-healing, robust, adhesive, and AIE fluorescent waterborne polyurethane via hierarchical hydrogen bonds and use as a strain sensor. ACS Appl. Mater. Interfaces. 2023, 15, 35469–35482. [Google Scholar] [CrossRef]
- Li, J.; Celiz, A.D.; Yang, J.; Yang, Q.; Wamala, I.; Whyte, W.; Seo, B.R.; Vasilyev, N.V.; Vlassak, J.J.; Mooney, D.J. Tough adhesives for diverse wet surfaces. Science. 2017, 357, 378–381. [Google Scholar] [CrossRef] [PubMed]
- García-Pacios, V.; Costa, V.; Colera, M.; Martín-Martínez, J.M. Affect of polydispersity on the properties of waterborne polyurethane dispersions based on polycarbonate polyol. Int. J. Adhes. Adhes. 2010, 3, 456–465. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Chen, J.; Yao, M.; Liu, X.; Jiang, Z. Self-healing polycarbonate-based polyurethane with shape memory behavior. Macromol. Res. 2019, 27, 649–656. [Google Scholar] [CrossRef]
- Matějka, L.; Špírková, M.; Dybal, J.; Kredatusová, J.; Hodan, J.; Zhigunov, A.; Šlouf, M. Structure evolution during order–disorder transitions in aliphatic polycarbonate based polyurethanes. Self-healing polymer. Chem. Eng. J. 2019, 357, 611–624. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Chen, S.; Wang, G.; Han, S.; Guo, J.; Yang, L.; Hu, J. Physical cross-linked aliphatic polycarbonate with shape-memory and self-healing properties. J. Mol. Liq. 2023, 381, 121798. [Google Scholar] [CrossRef]
- Paez-Amieva, Y.; Martín-Martínez, J.M. Dynamic non-covalent exchange intrinsic self-healing at 20 °C mechanism of polyurethane induced by interactions among polycarbonate soft segments. Polymers. 2024, 16, 924. [Google Scholar] [CrossRef]
- Paez-Amieva, Y.; Martín-Martínez, J.M. Influence of the molecular weight of the polycarbonate polyol on the intrinsic self-healing at 20 °C of polyurethanes. Polymers. 2024, 16, 2724. [Google Scholar] [CrossRef]
- Paez-Amieva, Y.; Mateo-Oliveras, N.; Martín-Martínez, J.M. Polyurethanes made with blends of polyester and polycarbonate polyols - New evidences supporting the dynamic non-covalent exchange mechanism of intrinsic self-healing at 20 °C. Polymers.
- Liu, N.; Zhao, Y.; Kang, M.; Wang, J.; Wang, X.; Feng, Y.; Yin, N.; Li, Q. The effects of the molecular weight and structure of polycarbonatediols on the properties of waterborne polyurethanes. Prog. Org. Coat. 2015, 82, 46–56. [Google Scholar] [CrossRef]
- García-Pacios, V.; Jofre-Reche, J.A.; Costa, V.; Colera, M.; Martín-Martínez, J.M. Coatings prepared from waterborne poly-urethane dispersions obtained with polycarbonates of 1, 6-hexanediol of different molecular weights. Prog. Org. Coat. 2013, 76, 1484–1493. [Google Scholar] [CrossRef]
- Niemczyk, A.; Piegat, A.; Olalla, Á.S.; El Fray, M. New approach to evaluate microphase separation in segmented polyurethanes containing carbonate macrodiol. Eur. Polym. J. 2017, 93, 182–191. [Google Scholar] [CrossRef]
- Wang, Y.; Balbuena, P.B. Associations of alkyl carbonates: Intermolecular C−H···O interactions. J. Phys. Chem. A. 2001, 43, 9972–9982. [Google Scholar] [CrossRef]
- Paez-Amieva, Y.; Martín-Martínez, J.M. Understanding the interactions between soft segments in polyurethanes: Structural synergies in blends of polyester and polycarbonate diol polyols. Polymers. 2023, 15, 4494. [Google Scholar] [CrossRef] [PubMed]
- Darla, V.R.; Ben, B.S.; Srinadh, K.V.S. Investigation on interfacial bonding strength between aluminum hub and CFRP bonded joints exposed to accelerated aging conditions. J. Adhes. Sci. Technol. 2024, 38, 31–43. [Google Scholar] [CrossRef]
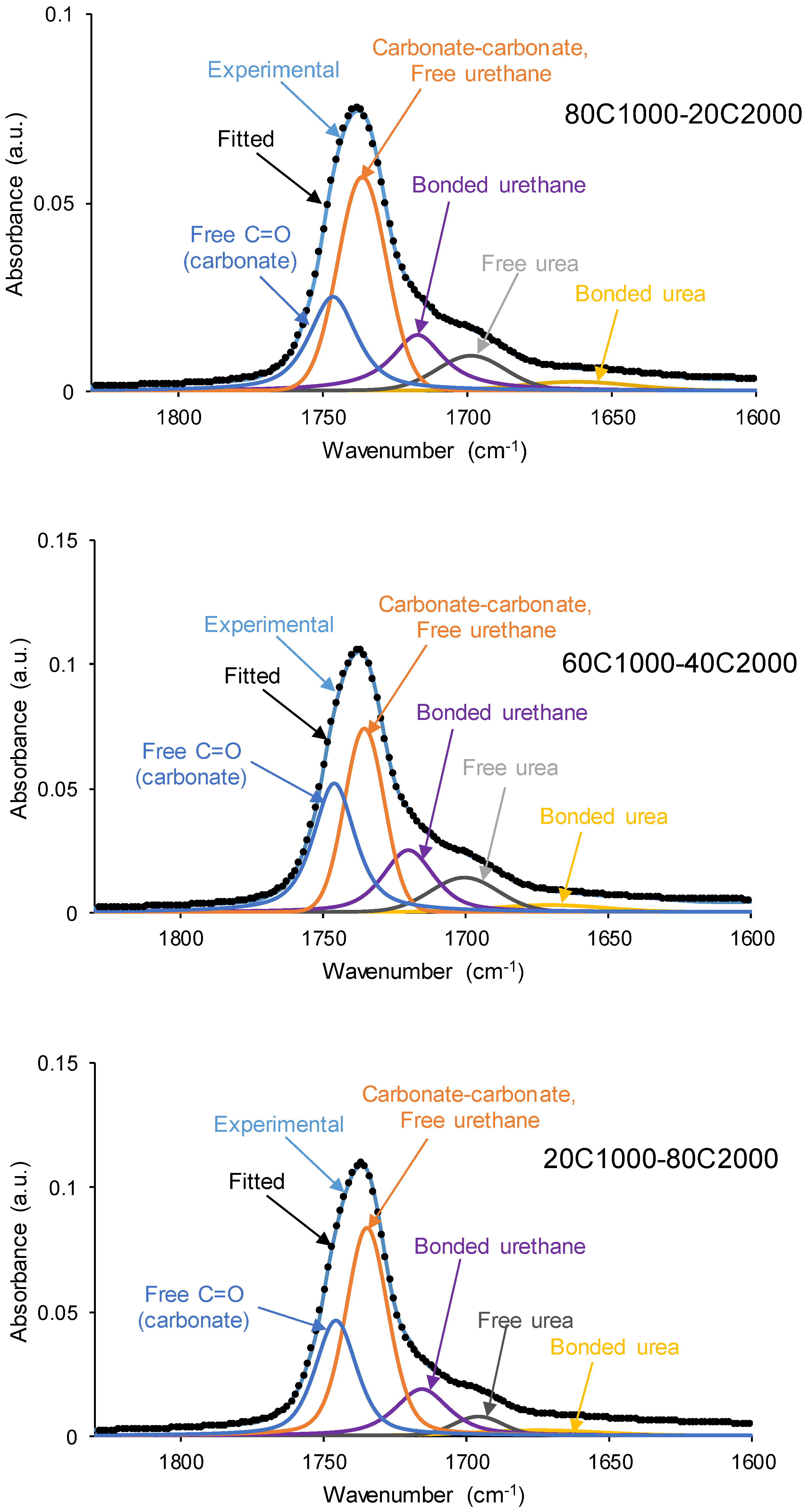
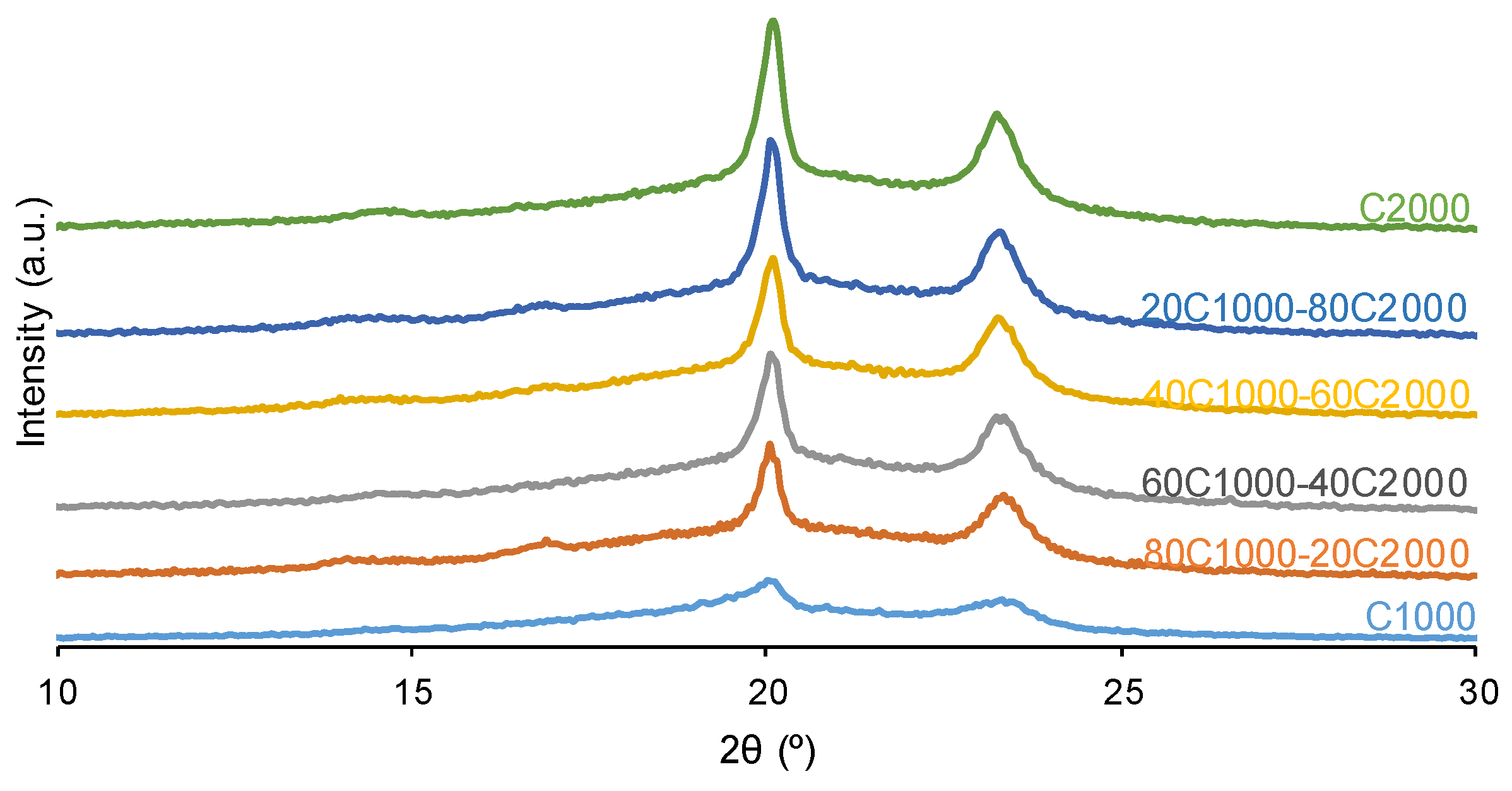
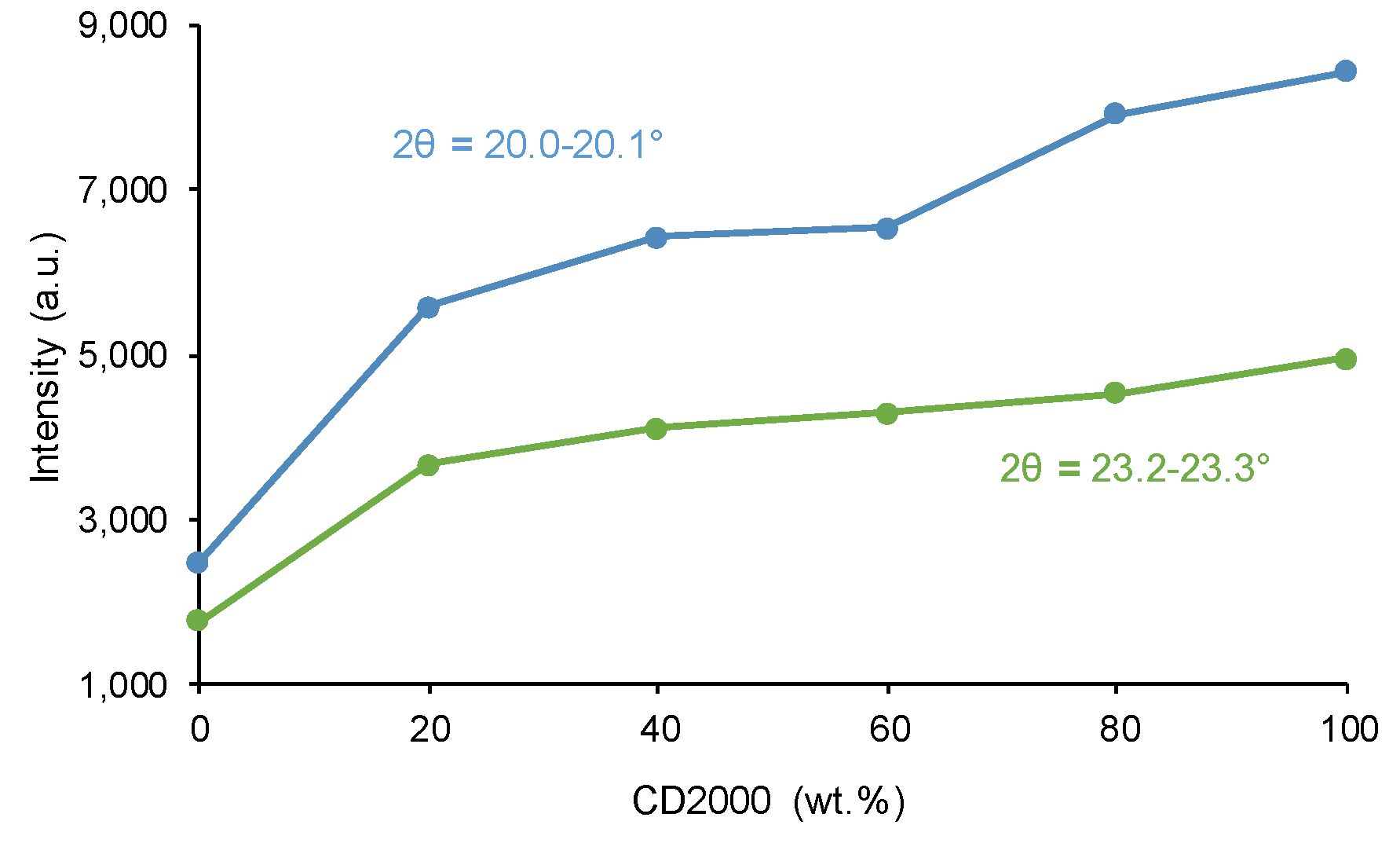
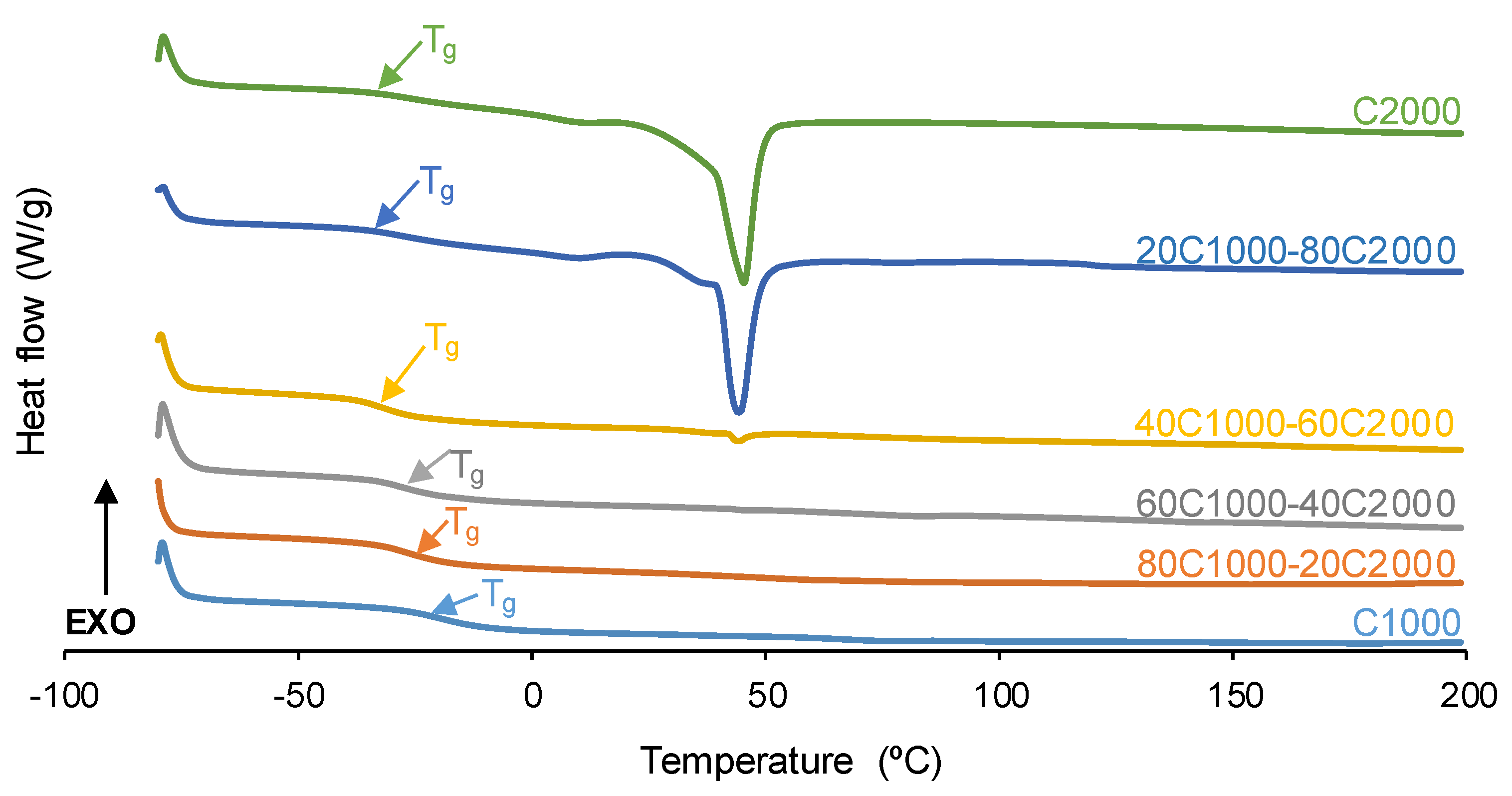
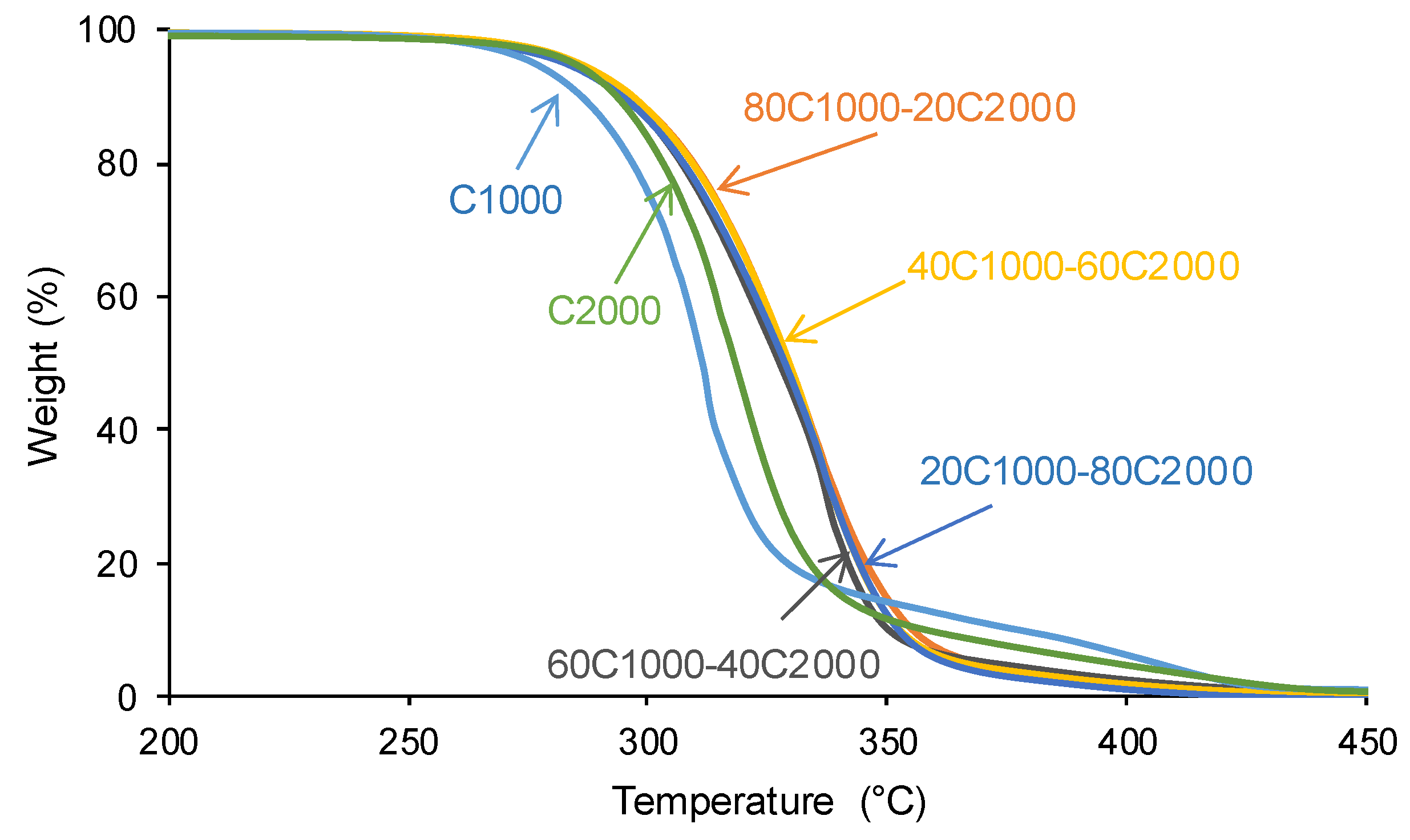
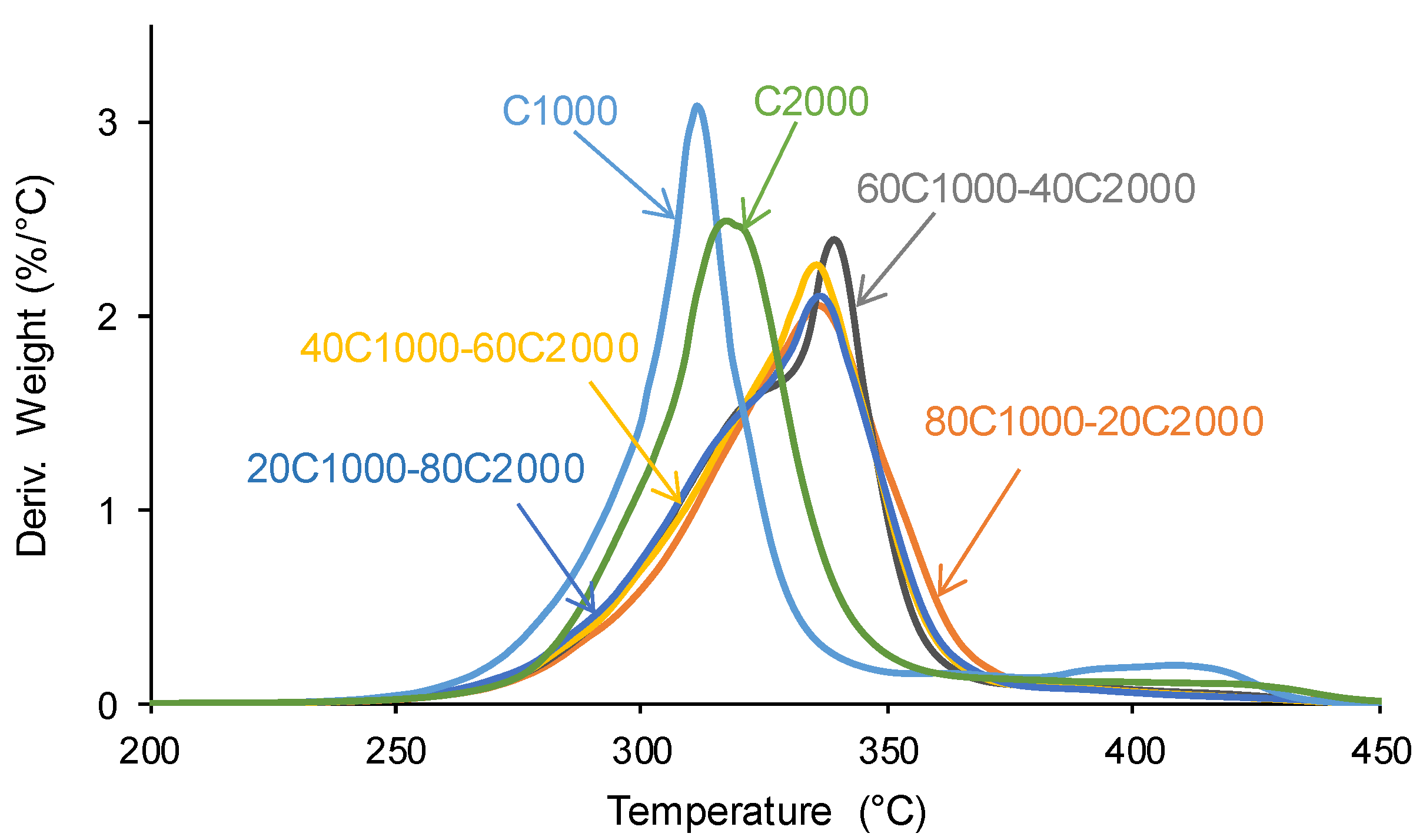
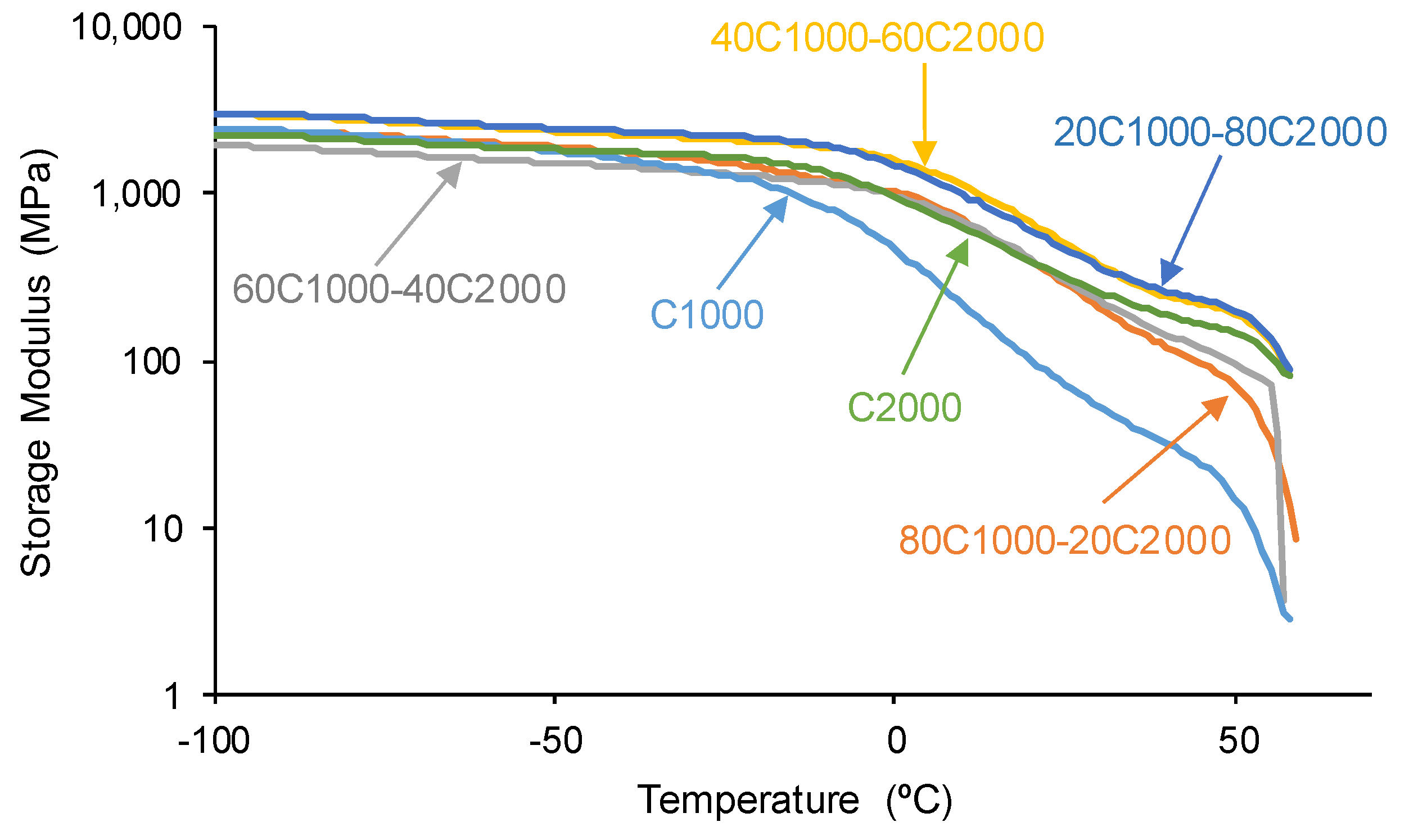
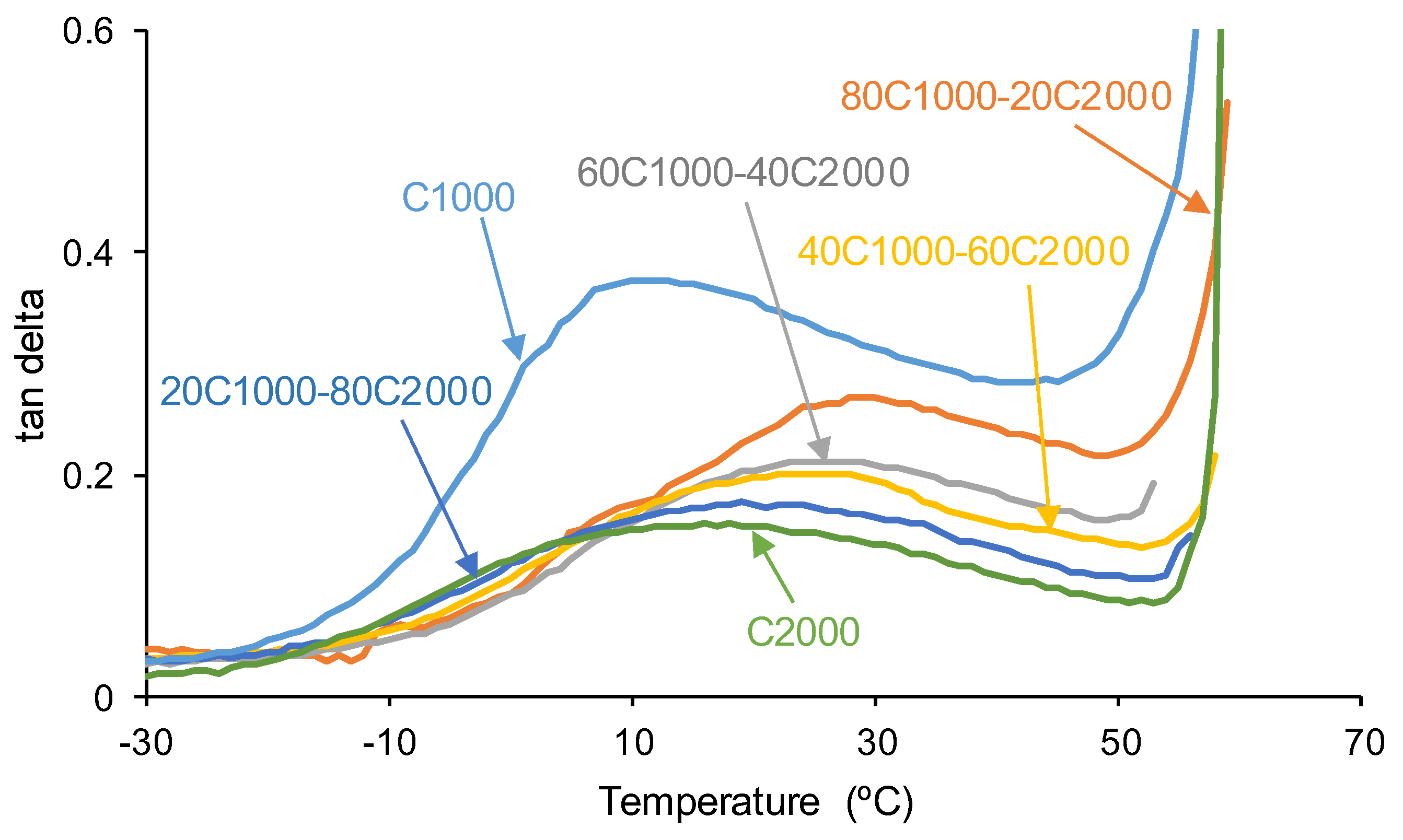
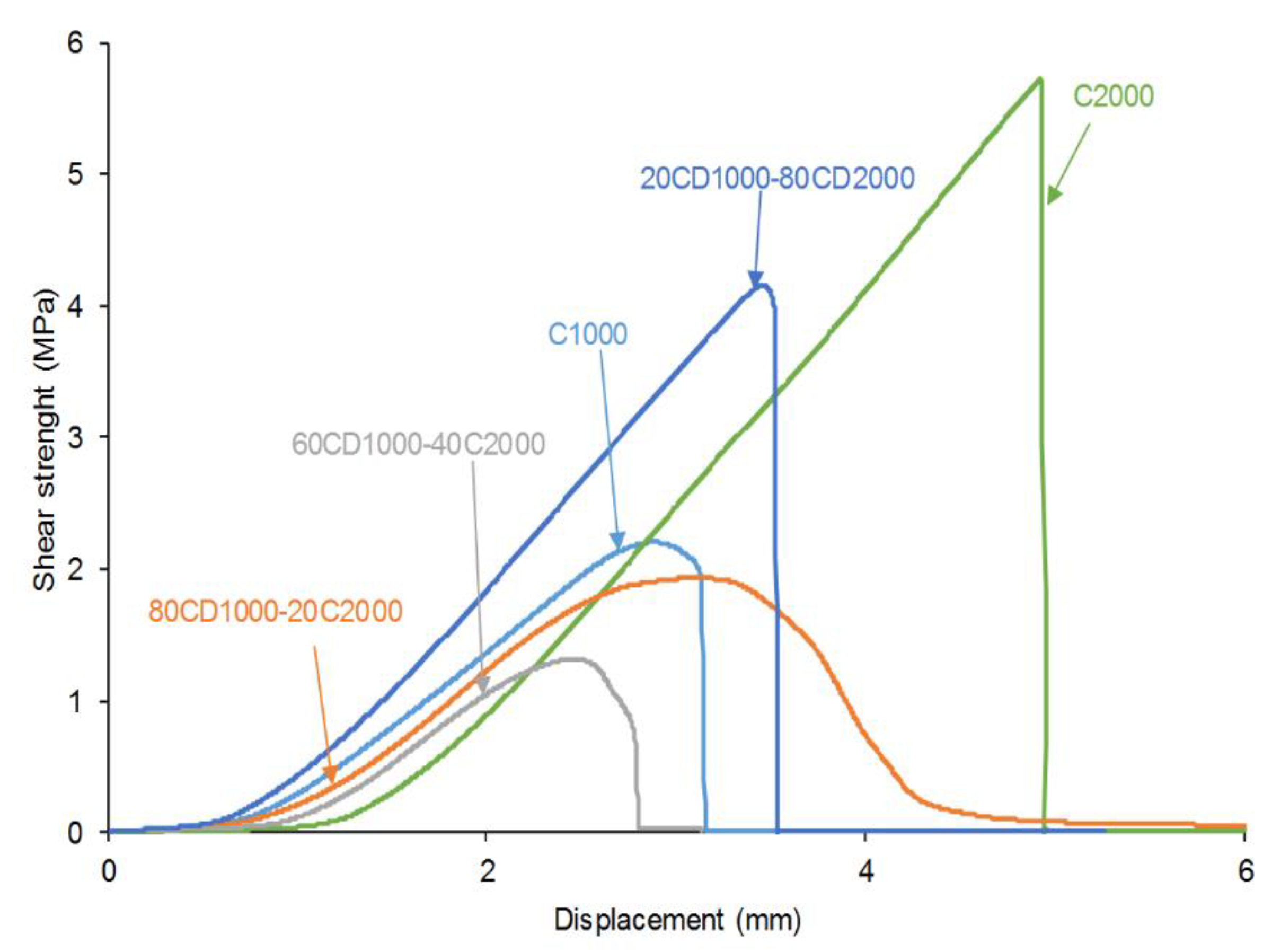
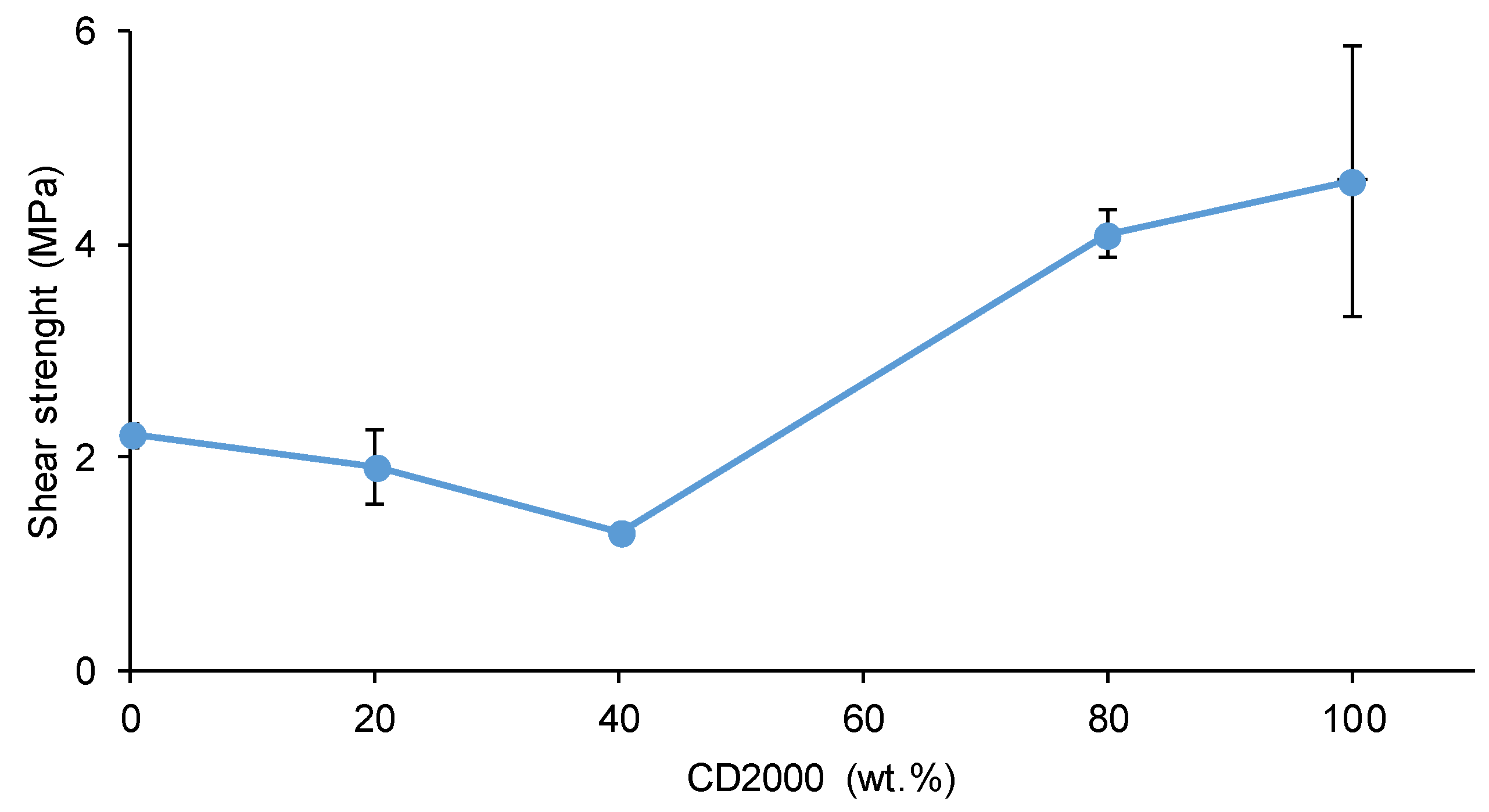
| PU | Polyols composition* | HS (wt%) |
| C1000 | CD1000 | 22 |
| 80C1000-20C2000 | 80 % CD1000+20 % CD2000 | 21 |
| 60C1000-40C2000 | 60 % CD1000+40 % CD2000 | 19 |
| 40C1000-60C2000 | 40 % CD1000+60 % CD2000 | 17 |
| 20C1000-80C2000 | 20 % CD1000+80 % CD2000 | 15 |
| C2000 | CD2000 | 13 |
| Wavenumber (cm-1) | Percentage (%) | Assignment | |||||
| C1000 | 80C1000-20C2000 | 60C1000-40C2000 | 40C1000-60C2000 | 20C1000-80C2000 | C2000 | ||
| 1654-1664 | 9 | 4 | 4 | 3 | 3 | 3 | Bonded urea |
| 1695-1697 | 15 | 10 | 11 | 12 | 4 | 12 | Free urea |
| 1713-1725 | 14 | 18 | 18 | 15 | 16 | 11 | Bonded urethane |
| 1733-1735 | 38 | 43 | 34 | 40 | 48 | 40 | Carbonate-carbonate, free urethane |
| 1743-1745 | 24 | 24 | 33 | 29 | 29 | 34 | Free C=O (carbonate) |
| PU | Tg (°C) | ∆cp (J/G°C) | Tc (°C) | ΔHc (J/g) | Tm (°C) | ΔHm (J/g) |
| C1000 | -21 | 0.29 | - | - | - | - |
| 80C1000-20C2000 | -26 | 0.40 | - | - | - | - |
| 60C1000-40C2000 | -27 | 0.35 | - | - | - | - |
| 40C1000-60C2000 | -29 | 0.35 | - | - | 44 | 1 |
| 20C1000-80C2000 | -29 | 0.22 | 21 | 2 | 44 | 14 |
| C2000 | -29 | 0.20 | 20 | 1 | 45 | 23 |
| PU | Tss (°C) | Ths (°C) |
| C1000 | -18 | 236 |
| 80C1000-20C2000 | -23 | 231 |
| 60C1000-40C2000 | -27 | 232 |
| 40C1000-60C2000 | -31 | 235 |
| 20C1000-80C2000 | -32 | 238 |
| C2000 | -36 | 240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





