Submitted:
07 October 2024
Posted:
08 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Plasmids, Peptides, and Cell lines
1.2. Development of mCXCR5-Producing Hybridomas
1.3. ELISA
1.4. Flow Cytometric Analysis
2. Results
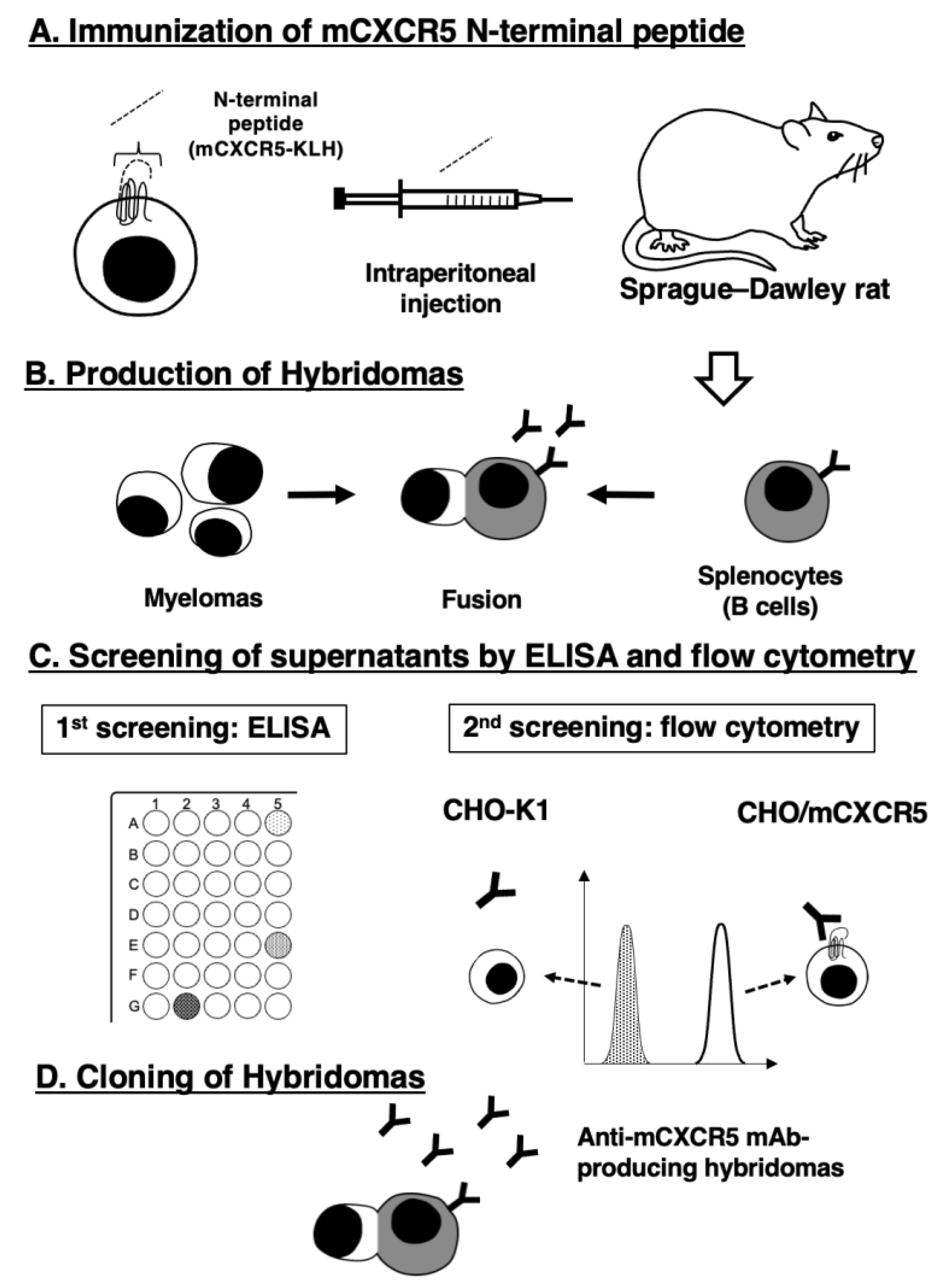
2.1. Development of Anti-mCXCR5 mAbs Using N-Terminal Peptide Immunization
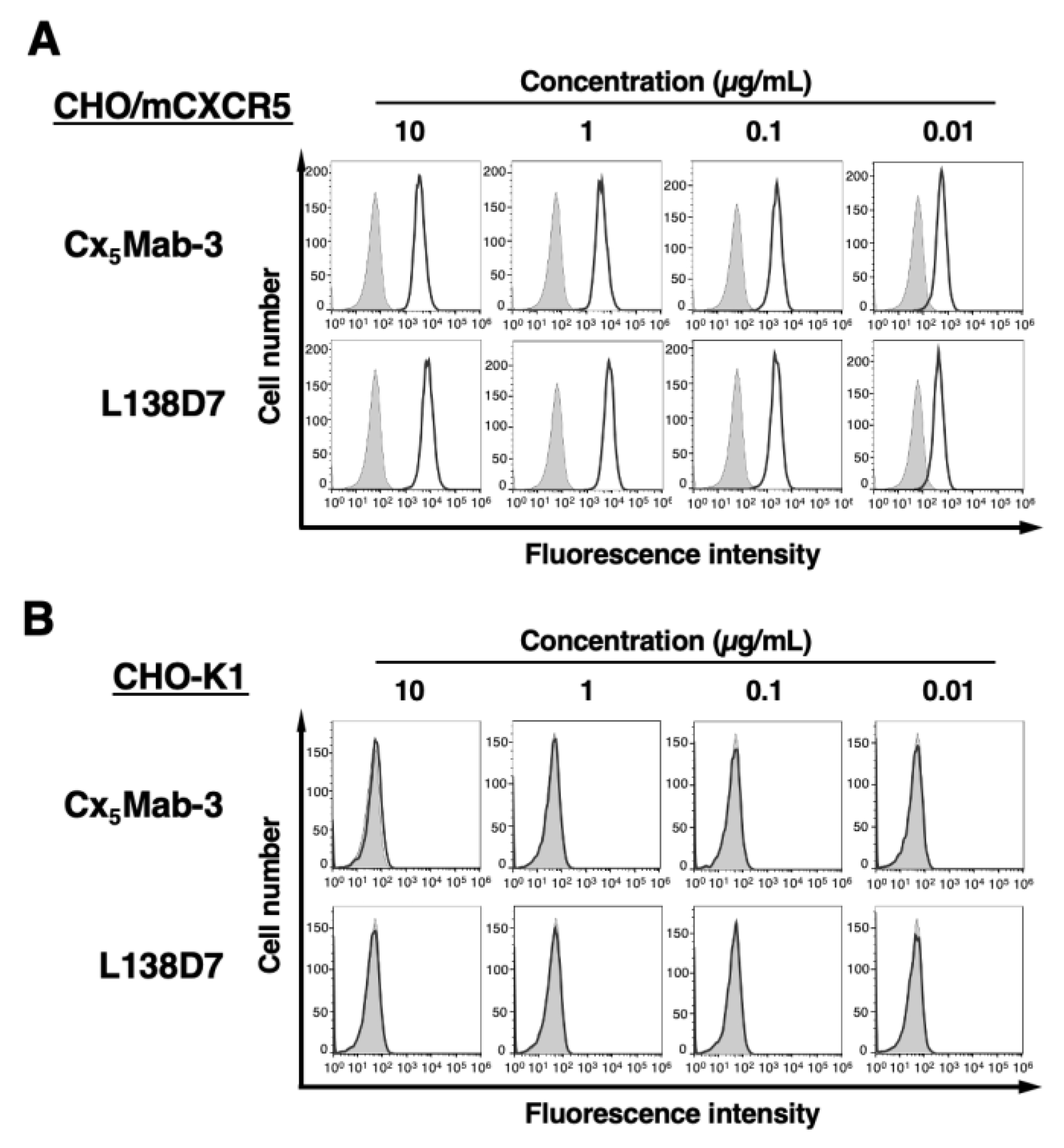
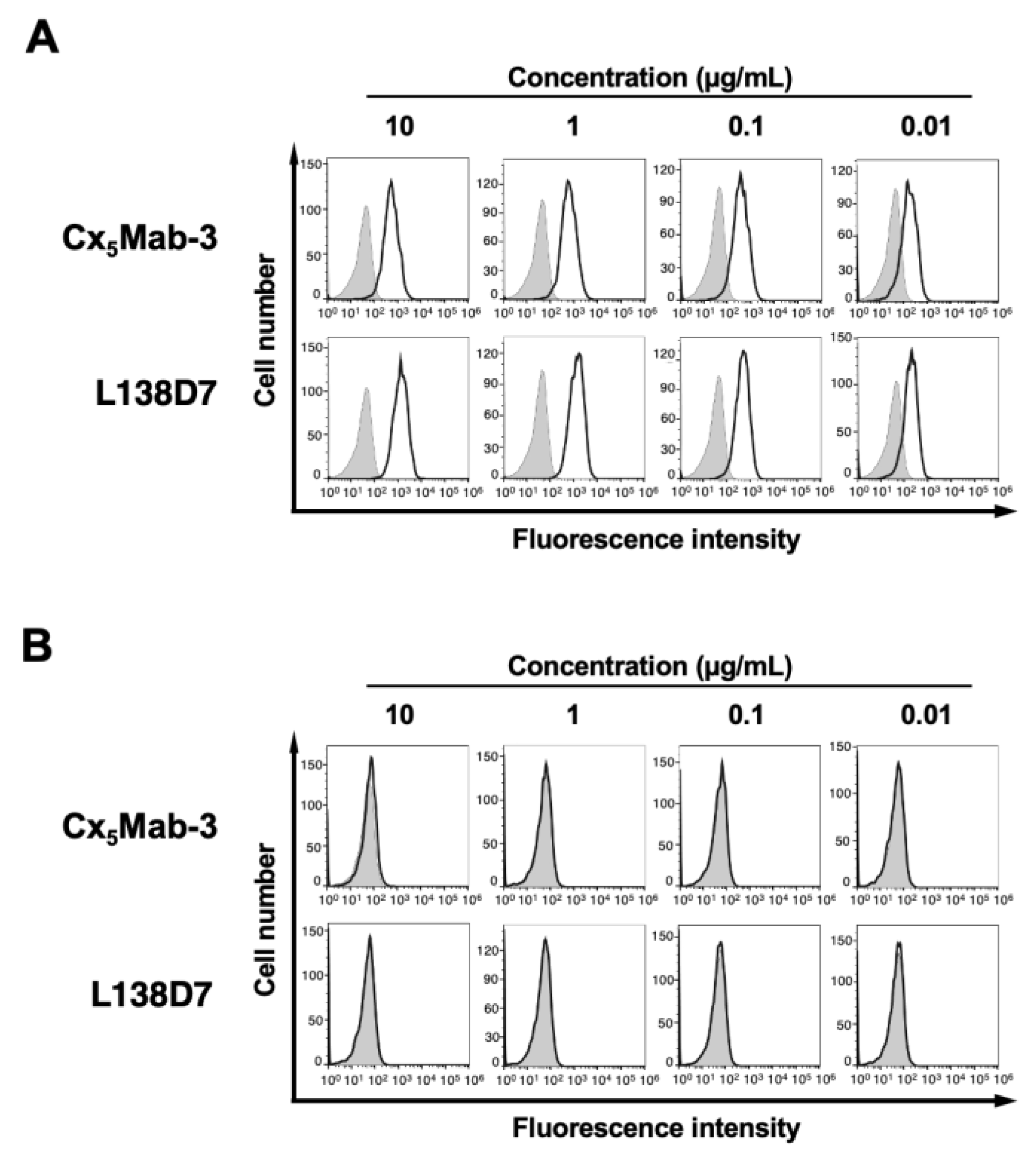
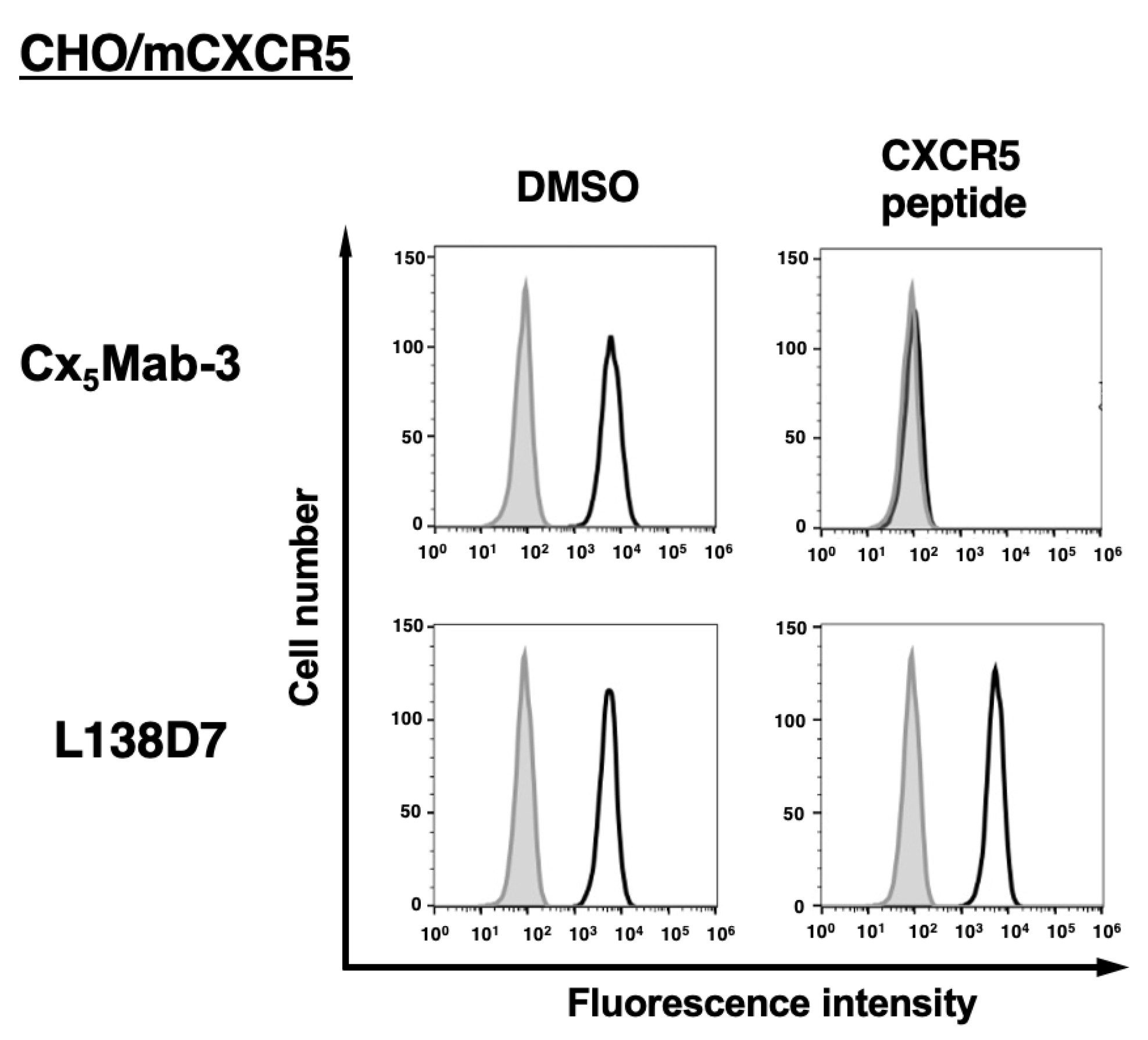
2.2. Flow Cytometric Analysis Using Cx5Mab-3
2.3. Determination of Dissociation Constant of Anti-mCXCR5 mAbs against CHO/mCXCR5 Cells
4. Discussion
Author Contributions
Conflicts of Interest
References
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. Febs j 2018;285(16): 2944-2971. [CrossRef]
- Luther, S.A.; Cyster, J.G. Chemokines as regulators of T cell differentiation. Nat Immunol 2001;2(2): 102-107. [CrossRef]
- Sokol, C.L.; Luster, A.D. The chemokine system in innate immunity. Cold Spring Harb Perspect Biol 2015;7(5). [CrossRef]
- Su, Z.; Chen, L.; Niu, Q.; Yang, B.; Huang, Z. Association of Gene Polymorphisms in CXC Chemokine Receptor 5 with Rheumatoid Arthritis Susceptibility. Iran J Allergy Asthma Immunol 2022;21(5): 537-548. [CrossRef]
- Shi, J.; Hou, S.; Fang, Q.; et al. PD-1 Controls Follicular T Helper Cell Positioning and Function. Immunity 2018;49(2): 264-274.e264. [CrossRef]
- Kazanietz, M.G.; Durando, M.; Cooke, M. CXCL13 and Its Receptor CXCR5 in Cancer: Inflammation, Immune Response, and Beyond. Front Endocrinol (Lausanne) 2019;10: 471. [CrossRef]
- Shiels, M.S.; Pfeiffer, R.M.; Hildesheim, A.; et al. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst 2013;105(24): 1871-1880. [CrossRef]
- Eide, H.A.; Halvorsen, A.R.; Sandhu, V.; et al. Non-small cell lung cancer is characterised by a distinct inflammatory signature in serum compared with chronic obstructive pulmonary disease. Clin Transl Immunology 2016;5(11): e109. [CrossRef]
- Hussain, M.; Adah, D.; Tariq, M.; et al. CXCL13/CXCR5 signaling axis in cancer. Life Sci 2019;227: 175-186. [CrossRef]
- Panse, J.; Friedrichs, K.; Marx, A.; et al. Chemokine CXCL13 is overexpressed in the tumour tissue and in the peripheral blood of breast cancer patients. Br J Cancer 2008;99(6): 930-938. [CrossRef]
- Singh, S.; Singh, R.; Sharma, P.K.; et al. Serum CXCL13 positively correlates with prostatic disease, prostate-specific antigen and mediates prostate cancer cell invasion, integrin clustering and cell adhesion. Cancer Lett 2009;283(1): 29-35. [CrossRef]
- https://www.biolegend.com/ja-jp/products/purified-anti-mouse-cd185-cxcr5-antibody-8454, accessed on 10 May 2024.
- Hsieh, C.H.; Jian, C.Z.; Lin, L.I.; et al. Potential Role of CXCL13/CXCR5 Signaling in Immune Checkpoint Inhibitor Treatment in Cancer. Cancers (Basel) 2022;14(2). [CrossRef]
- Crotty, S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol 2011;29: 621-663. [CrossRef]
- Im, S.J.; Hashimoto, M.; Gerner, M.Y.; et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 2016;537(7620): 417-421. [CrossRef]
- Brummelman, J.; Mazza, E.M.C.; Alvisi, G.; et al. High-dimensional single cell analysis identifies stem-like cytotoxic CD8(+) T cells infiltrating human tumors. J Exp Med 2018;215(10): 2520-2535. [CrossRef]
- He, R.; Hou, S.; Liu, C.; et al. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature 2016;537(7620): 412-428. [CrossRef]
- Kallies, A.; Zehn, D.; Utzschneider, D.T. Precursor exhausted T cells: key to successful immunotherapy? Nat Rev Immunol 2020;20(2): 128-136. [CrossRef]
- Thommen, D.S.; Koelzer, V.H.; Herzig, P.; et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med 2018;24(7): 994-1004. [CrossRef]
- de Chaisemartin, L.; Goc, J.; Damotte, D.; et al. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res 2011;71(20): 6391-6399. [CrossRef]
- Mitkin, N.A.; Hook, C.D.; Schwartz, A.M.; et al. p53-dependent expression of CXCR5 chemokine receptor in MCF-7 breast cancer cells. Sci Rep 2015;5: 9330. [CrossRef]
- Mitkin, N.A.; Muratova, A.M.; Sharonov, G.V.; et al. p63 and p73 repress CXCR5 chemokine receptor gene expression in p53-deficient MCF-7 breast cancer cells during genotoxic stress. Biochim Biophys Acta Gene Regul Mech 2017;1860(12): 1169-1178. [CrossRef]
- Takei, J.; Kaneko, M.K.; Ohishi, T.; et al. A defucosylated anti-CD44 monoclonal antibody 5-mG2a-f exerts antitumor effects in mouse xenograft models of oral squamous cell carcinoma. Oncol Rep 2020;44(5): 1949-1960. [CrossRef]
- Hosono, H.; Takei, J.; Ohishi, T.; et al. Anti-EGFR monoclonal antibody 134-mG2a exerts antitumor effects in mouse xenograft models of oral squamous cell carcinoma. Int J Mol Med 2020;46(4): 1443-1452. [CrossRef]
- Itai, S.; Ohishi, T.; Kaneko, M.K.; et al. Anti-podocalyxin antibody exerts antitumor effects via antibody-dependent cellular cytotoxicity in mouse xenograft models of oral squamous cell carcinoma. Oncotarget 2018;9(32): 22480-22497. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




