1. The Human Microbiome: Basics and Beyond
“Microbiome” refers to the combined genetic material of all microbes within a specific niche, such as the human gut. The human body contains approximately 1.3 times more microbial cells (microbiota) than human cells and approximately 100 times more microbial genes than the human genome [
1]. These microbial genes (the microbiome) encode a variety of functions that affect the digestive system, as well as overall health, through interactions with various parts of our body. The human microbiome, particularly the gut microbiome, which consists of billions of microorganisms, is now recognized to play a critical role in maintaining gut health, contributing to the maturation and function of the immune system, and even influencing the function and homeostasis of other peripheral organs [
2]. Research shows that the microbiome influences various conditions, from metabolic diseases like obesity and diabetes to mental health issues; our understanding of its impact throughout the body is constantly expanding [
3,
4,
5].
1.1. Methodologies for Studying the Microbiome
The microbiomics field has made significant methodological advancements in recent years, and coinciding improvements in various technologies have underscored the field’s potential. The advent of next-generation sequencing (NGS) technologies and the development of advanced bioinformatics tools have revolutionized the study of microbiomes. These breakthroughs have, for instance, significantly reduced the time and cost required to analyze the entire gut microbiome, making it feasible to process the larger sample sizes necessary. As a result, large-scale cohort studies have become more accessible, leading to the discovery of associations between gut microbiota and various diseases, including brain disorders, obesity, diabetes, and inflammatory bowel disease. These advancements have greatly enhanced our understanding of the human gut microbiome and its impact on health [
6]. Also, specific microbiome signatures associated with diseases and drug responses can be discovered through metagenomics, the study of genetic materials from the microbiome. The predominant sequencing methodologies for microbiome analysis include 16S rRNA amplicon sequencing, internal transcribed spacer (ITS) amplicon sequencing, and shotgun metagenomics. 16S rRNA amplicon sequencing is primarily used for bacterial profiling, while ITS sequencing is specific to fungal communities. These amplicon-based approaches are advantageous for samples with low microbial load or high human DNA contamination, as they selectively amplify microbial-specific DNA regions. However, being PCR-dependent, they are susceptible to amplification-induced biases and artifacts, such as chimeric sequences and skewed microbial abundances. Additionally, these methods offer lower taxonomic resolution compared to shotgun metagenomics.
Shotgun metagenomics provides higher taxonomic resolution, which is crucial for identifying disease-associated microbial biomarkers. It also enables the characterization of functional genes within the microbial community and is less prone to PCR-induced artifacts. Nonetheless, this method is more sensitive to issues in low-biomass samples and contamination from human DNA, as it does not rely on targeted amplification. To mitigate this challenge, strategies such as human DNA depletion prior to library preparation can be employed to improve the accuracy and efficiency of shotgun metagenomics in such contexts.
Emerging technologies are poised to outdo even fully-optimized shotgun metagenomics. Metatranscriptomics allows the study of gene expression in gut microbes related to diseases, thereby identifying specific metabolic pathways involved. Additionally, multi-omics approaches enable the simultaneous analysis of a patient's gut microbiota and metabolome, a set of small molecules that exist in a sample. This can reveal the association of specific microbial communities with drug responses, providing critical information for developing personalized treatment strategies [
7,
8].
In the future, the continuous evolution of these technologies will further deepen microbiome research and significantly enhance our understanding of human health and diseases.
1.2. Composition and Function of the Human Microbiome
The human microbiome begins to develop immediately after birth, gradually stabilizing by the age of three [
7]. This established microbiome contributes to maintaining the body's homeostasis. It is estimated that approximately 50 different bacterial phyla exist in the gut microbiota, with six being major players: Actinobacteria, Proteobacteria, Fusobacteria, Verrucomicrobiota, Bacteroidetes, and Firmicutes; Bacteroidetes and Firmicutes comprise >90% of the total flora population in the gut [
9]. Disruption of the microbiome’s composition, termed dysbiosis, is increasingly recognized as a factor in various diseases. For example, compelling evidence links gut microbiota to cardiovascular health. Short-chain fatty acids (SCFAs) produced by beneficial bacteria like
Bifidobacteria and
Faecalibacterium prausnitzii regulate blood pressure and cholesterol, and a decrease in these bacteria is associated with increased risk of cardiovascular disease (CVD) [
10,
11]. The gut microbiome also influences energy metabolism. A high abundance of Firmicutes bacteria, known for their efficient energy extraction, is linked to obesity and type 2 diabetes. Conversely, a higher abundance of Bacteroidetes, which promote leanness, is associated with a healthier metabolic profile [
12]. Furthermore, dysbiosis in the gut microbiome plays a role in inflammatory bowel disease (IBD). A decrease in beneficial bacteria like
Bifidobacteria and
Lactobacillus, coupled with an increase in potentially harmful bacteria like
E. coli, can disrupt the gut epithelial barrier and trigger chronic inflammation [
13]. These relationships emphasize the critical role of the microbiome in human health. Understanding them opens new avenues for developing novel and tailored therapeutic strategies, such as fecal microbiota transplants (FMTs), to prevent and manage numerous diseases.
1.3. The Microbiome's Influence on the Immune System
The microbiome also plays an important role in immune system development and function at all stages of life. During pregnancy, metabolites and microbiome-induced antigens produced by the mother's microbiome can cross the placenta and reach the fetus. This exposure helps the fetus adapt its immune system to the mother's microbial environment, preparing it for birth, when the mother's microbiome will colonize the infant's gut [
14]. This exposure is not only tolerated, but also required for proper immune development [
15]. Throughout the first three years of life, the child's microbiome undergoes dramatic changes influenced by diverse factors, including diet, geographic location, medical care,
etc. As the innate immune system matures, the microbiome stabilizes [
15,
16,
17]. The microbiome continues to shape the immune system throughout life. It acts as a training ground for immune cells, educating them to differentiate between harmless microbes and potential pathogens. This delicate balance is crucial for preventing chronic inflammatory diseases and allergies [
18,
19]. Additionally, the microbiome produces beneficial metabolites that influence immune function and regulate inflammation. In essence, the microbiome acts as a key partner in the immune system's development and ongoing regulation [
20].
The introduction should briefly place the study in a broad context and highlight why it is important. It should define the purpose of the work and its significance. The current state of the research field should be carefully reviewed and key publications cited. Please highlight controversial and diverging hypotheses when necessary. Finally, briefly mention the main aim of the work and highlight the principal conclusions. As far as possible, please keep the introduction comprehensible to scientists outside your particular field of research. References should be numbered in order of appearance and indicated by a numeral or numerals in square brackets—e.g., [
1] or [
2,
3], or [
4,
5,
6]. See the end of the document for further details on references.
2. Rare Childhood Neurological Diseases
There are more than 10,000 unique rare diseases, which have environmental, infectious, cancer, and gene-based etiologies. Nearly half of all rare diseases are neurological, affecting the brain, spinal cord, and/or peripheral nerves and muscles. Among these, genetic etiologies predominate: 80-85% of rare neurological conditions can be traced to pathogenic variants in single genes that disrupt the functions of essential proteins [
21]. Rare diseases are defined by prevalence, usually averaging about 40 - 50 cases per 100,000 people. Despite the low prevalence, the cumulative impact of rare diseases is substantial, with more than 33 million people affected in the United States and more than 300 million people affected worldwide [
22].
Advancements in genomic technologies have improved diagnostic timelines and rates; yet individuals living with a rare disease typically spend more than five years searching for a diagnosis, and up to 50% remain undiagnosed [
23,
24,
25]. Furthermore, less than 5% of rare diseases have FDA-approved treatment options [
23]. The current standard-of-care for the majority of rare diseases is symptomatic management only. As a consequence, many patients live with life-long disabilities. Rare diseases disproportionately affect children and early mortality is tragically high, with 3 in 10 not living to see their fifth birthdays [
22,
25].
An accurate diagnosis is recognized as “central to the practice of medicine” and “essential for informed care and promoting patient and family well-being” [
23,
24]. Nevertheless, individuals and families with a rare neurological disease frequently encounter multiple challenges in getting an accurate diagnosis. This process is so commonplace that it is routinely referred to as a
diagnostic odyssey, and is usually filled with physical, mental, financial, and emotional stressors. Diagnosing and treating rare diseases poses unique challenges, to both families and physicians, not typically present when dealing with more common illnesses.
Considering the serious unmet medical needs and difficult circumstances faced by patients and their families, further research into various rare diseases is strongly encouraged [
26]. Because of the profound influence the gut microbiota has on the nervous system and overall health, it is imperative that we as a field explore this complex mechanism and its relation to rare childhood neurological disorders as a means to improve diagnostic rates and provide potential treatment options [
27].
3. The Microbiome and Neurological Health: Establishing the Connection
Over the past two decades, the fields of microbiology and neurology have come together to study an incredible aspect of human health: the gut-brain axis. This bi-directional communication pathway connecting the immune, endocrine, and central nervous (CNS) systems is essential for maintenance of normal body function and health. Dysbiosis of the microbiome can therefore harm other regions of the body, and numerous studies have demonstrated the influence of microbiota on the nervous system [
27,
28]. For instance, distinct differences are seen in the gut microbiota composition of children with autism spectrum disorder (ASD) compared to that of neurotypically-developing children. These differences are believed to influence neurodevelopment and behavior through the gut-brain axis [
29]. In an example of beneficial microbial influence, a fecal transplant study of children with ASD and their healthy siblings demonstrated significant improvement in ASD symptoms in the transplant recipients, suggesting that modifying the gut microbiota could benefit individuals with ASD–again, through the gut-brain axis [
30,
31].
A systematic review of gut microbes’ impact on neurodevelopment by Caputi
et al. examined alterations in the intestinal microbiota of individuals with neurodevelopmental disorders such as ASD, ADHD, and Rett syndrome. Reviewers highlighted the functional contributions of the gut microbiome, pointing to significant alterations that could cause or exacerbate the symptoms and challenges associated with these disorders [
32]. This phenomenon could be particularly critical in the earliest stages of life, as alterations in the microbiome during those periods have been linked to increased risk of developing neurodevelopmental disorders later in life. For example, antibiotic use in infancy has been associated with disrupted gut flora and a heightened risk of developing neuropsychiatric disorders later in life [
5,
33].
3.1. Epilepsy
Epilepsy is a debilitating, chronic, heterogeneous disease of the brain that afflicts nearly 70 million people worldwide with a considerable social and economic burden. Epilepsy is the most frequent chronic neurologic condition in childhood and affects 0.5% to 1% of children; it is characterized by transient clinical manifestations arising from synchronized, high-frequency, or excessive abnormal neuronal activity in the CNS, which results in temporary neurological dysfunction [
9,
34]. The prevalence of epilepsy does not differ by gender or age group [
35,
36].
Epilepsy leads to a diminished seizure threshold and the predisposition to have repeated seizures, which affect the “neurobiological, cognitive, psychological, and social consequences of this condition” [
35,
37]. Epilepsy is diagnosed when an individual has two unprovoked seizures occurring more than 24 hours apart, has a single unprovoked seizure (if recurrence risk is high), or has a prior diagnosis of any epilepsy syndrome [
38].
Seizures are required for the diagnosis of epilepsy, but not all people who experience a seizure have epilepsy. Seizures are episodes of abnormal, excessive, hypersynchronous neuronal activity in the brain that leads to uncontrolled alterations of neurologic function. They present as objective signs or subjective symptoms such as decreased awareness, involuntary muscle contractions, an unusual smell, involuntary laughing, and stiffening. Seizures develop when the excitability of cortical neurons exceeds a certain threshold [
37,
39,
40]. In 2017, the International League Against Epilepsy (ILAE) revised the classification of seizures into focal (seizures arising in one hemisphere of the brain), generalized (seizures originating in both hemispheres simultaneously), and unknown onset, with subcategories that include motor and non-motor. Focal seizures can be further categorized by consciousness status with retained or impaired awareness. The most common types of epilepsy are generalized and unknown, characterized by seizures of the same categories [
41].
The diverse pathophysiology of epilepsy corresponds to its equally diverse etiologies. Identifying the underlying etiology of epilepsy is vital for effective treatment and optimal prognosis [
35,
37]. In the ILAE classification, etiologies are divided into six categories: structural, genetic, infectious, metabolic, immune, and unknown. These groupings are not mutually exclusive, and many etiologies fall into more than one category. The relative prevalences of each category observed within a geographic region vary; for example, least developed countries (LDC) often have higher rates of infection-related epilepsy [
35,
37,
42].
3.2. The Microbiome-Epilepsy Link
The pathogenesis of epilepsy is multifactorial and the specific mechanisms are unknown. Until recently, it was believed that the etiology of most neurological disorders was related to abnormal brain function. Numerous animal and clinical studies now suggest that the microbiota via the gut-brain axis has a role in multiple neurological disorders including epilepsy [
9,
43]. Studies in animal models have shown multiple potential mechanisms that can influence epileptogenesis. The gut microbiome has a critical role in maintaining the integrity of the blood-brain barrier (BBB), which when compromised leads to neuroinflammation. SCFA produced by the GM have been shown to upregulate the expression of tight junction proteins which are important for BBB permeability. The GM also helps regulate the production of glutamine and GABA which is important in maintaining the proper balance in excitation/inhibition for neuronal synapses.
Akkermasia muciniphila and
Lactobacillus acidophilus can augment the endocannabinoid system in controlling neuronal excitability [
44].
Other studies of the gut microbiome have demonstrated that certain gut bacteria can produce compounds that affect the central nervous system, potentially influencing seizure susceptibility and frequency [
45]. Ketogenic diets, which alter the gut microbiome, have been used effectively to manage drug-resistant epilepsy (DRE), the “failure of adequate trials of two tolerated and appropriately chosen and used anti-epileptic drug schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom.”, in children, indicating a possible link between diet-modulated microbiome changes and seizure control [
9,
43,
46,
47]. These studies contribute to a growing body of evidence suggesting that the gut microbiome plays a crucial role in the development and manifestation of neurodevelopmental disorders including epilepsy. The microbiome's influence on the central nervous system, possibly through the gut-brain axis, points to new directions for research, prevention, and treatment strategies for these conditions.
Additionally, multiple population-based studies revealed differences in gut microbiota between healthy controls and patients with epilepsy [
9]. Xie
et al. reported that 14 infants with refractory epilepsy had a gut microbiome composition that differed significantly from that of healthy controls (n = 30), with lower alpha diversity and a significant increase in Firmicutes and Proteobacteria [
46]. Similarly, Peng
et al. reported that refractory epilepsy (n = 42) patients exhibited markedly different microbiota composition from that seen in people without the disease, including increased prevalence of typically rare phyla like Verrucomicrobia, and increased alpha diversity when compared with patients with non-refractory epilepsy (n = 49). Increased seizure frequency and lowered alpha diversity also correlated with decreased populations of
Bifidobacterium and
Lactobacillus [48].
Furthermore, Şafak
et al. compared 30 patients with focal epilepsy to 10 non-epileptic healthy controls using principal component analysis and demonstrated that the composition of gut bacteria clustered differently in patients with epilepsy. Proteobacteria were higher and Fusobacteria were lower in the epilepsy group [
49].
Studies by Dong
et al. reinforced these results, again uncovering significantly increased Fusobacteria. Dong
et al. also concurred with Xie
et al., reporting significant differences in the gut microbiota composition between epilepsy patients and non-epileptic healthy controls.
Fusobacterium mortiferum, Bacteroides fragilis, Ruminococcus gnavus, and
Fusobacterium spp. were identified as potential risk factors for developing epilepsy, with increased levels of
Fusobacterium in particular standing out as a potential diagnostic biomarker for epilepsy [
50,
51].
Ouyang and colleagues explored the potential causal relationship between the gut microbiota and epilepsy and identified specific microbe taxa that were associated with three subtypes of generalized epilepsy. They collected genetic variants from a previous genome-wide association study, gut microbiota, and metabolites from the gut microbiota to conduct a bi-directional Mendelian randomization study. They found that family Veillonellaceae (phylum Firmicutes) was associated with a higher risk of childhood absence epilepsy. Class Melainabacteria was coupled with a lower risk of generalized epilepsy with tonic-clonic seizures; and class Betaproteobacteria, along with order Burkholderials (phylum Proteobacteria), are potential protective factors in juvenile myoclonic epilepsy [
9,
52].
Studies of DRE by Peng
et al. and Lee
et al. showed similar results, with an increase in phylum Firmicutes. Previous studies of the phylum Proteobacteria in relation to epilepsy are less consistent [
48,
52,
53]. These inconsistencies may result from differences in study design, sample size, or the analysis method. Despite these discrepancies, a growing body of preclinical and clinical studies support the idea that patients with epilepsy often display alterations in their gut microbiome. Moreover, patients with DRE may experience even more changes in the composition of their microbiota when compared to non-epileptic controls and patients with drug sensitive epilepsy [
54]. The Materials and Methods should be described with sufficient details to allow others to replicate and build on the published results. Please note that the publication of your manuscript implicates that you must make all materials, data, computer code, and protocols associated with the publication available to readers. Please disclose at the submission stage any restrictions on the availability of materials or information. New methods and protocols should be described in detail while well-established methods can be briefly described and appropriately cited.
Research manuscripts reporting large datasets that are deposited in a publicly available database should specify where the data have been deposited and provide the relevant accession numbers. If the accession numbers have not yet been obtained at the time of submission, please state that they will be provided during review. They must be provided prior to publication.
Interventionary studies involving animals or humans, and other studies that require ethical approval, must list the authority that provided approval and the corresponding ethical approval code.
3.3. Potential Microbiome Mechanisms in Epilepsy Pathogenesis
The underlying mechanisms of how the gut microbiota is related to epilepsy are still not fully understood. The gut microbiota interacts with the brain primarily through the nervous, endocrine, and immune systems, and through metabolic signaling pathways. Furthermore, the influence of the gut microbiome on epilepsy may not rely on one specific pathway and instead on a combination of all these pathways [
52]
. As studies have shown, patients diagnosed with epilepsy experience an increase in the bacteria phyla Firmicutes, Proteobacteria, and Verrucomicrobiota, all of which are immune-harmful, whereas Bacteroidetes and Actinobacteria, which are immune-beneficial, are decreased. [
36,
45]
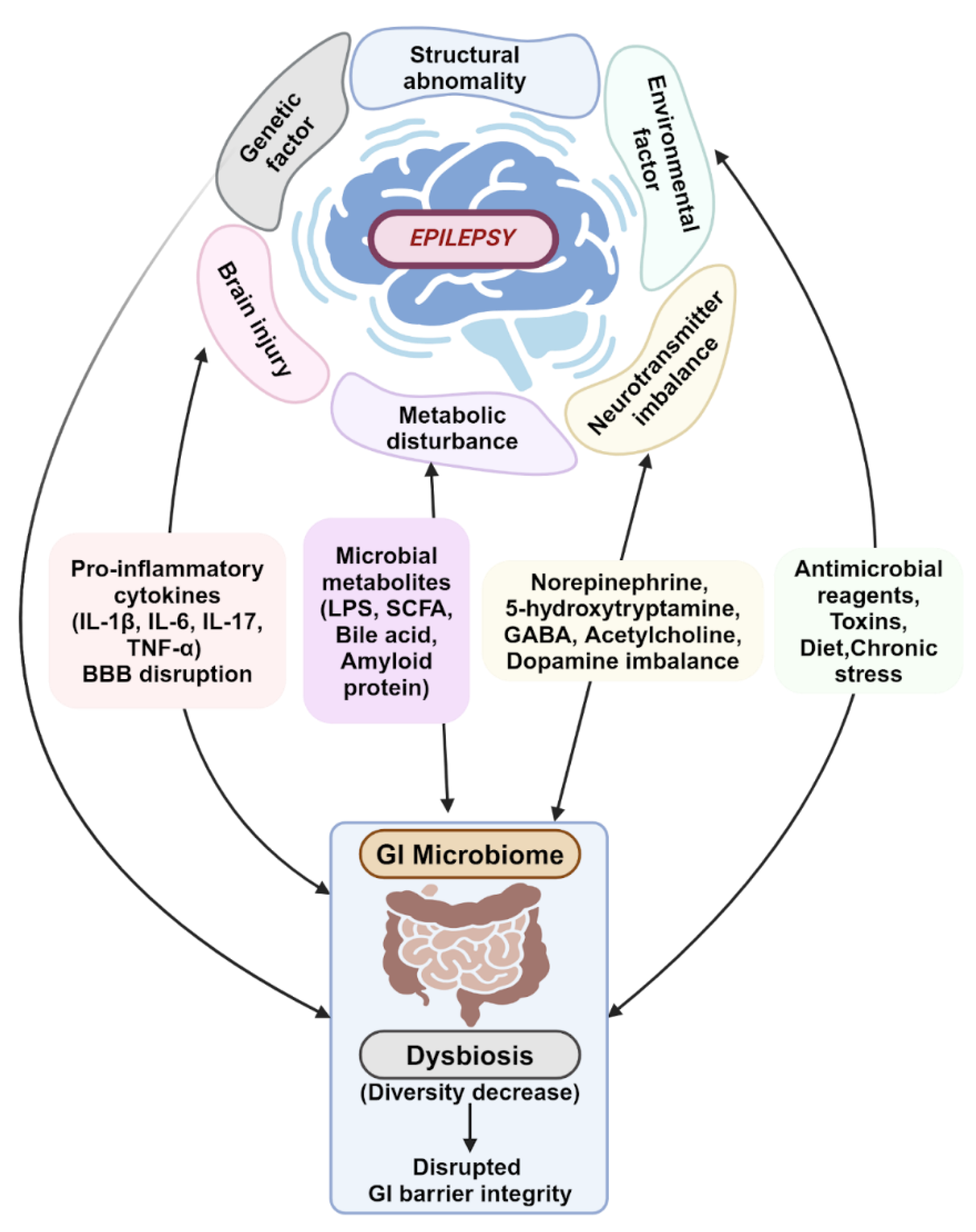
Epileptogenesis has been linked to neuroimmunity and neuroinflammation. The gut microbiota impacts the inflammatory and immune pathways by regulating the maturation of microglial cells and activating astrocytes (
Figure 1). These nerve cells are vital to the immune and inflammatory responses of the CNS [
36]. Astrocytes and microglia interact in a way that leads to increased pro-inflammatory cytokine production and BBB permeability. This causes the intrusion of peripheral blood immune cells and cytokines into the CNS, and subsequent chronic neuroinflammation [
36,
45].
The gut microbiota is pivotal in maintaining the integrity of the BBB. The gut microbiota can induce glial cells, including microglia, to release pro-inflammatory cytokines that induce neuronal hyperexcitability and, eventually, seizures. The permeability of the BBB is also susceptible to inflammatory cytokines secreted by gut bacteria into systemic circulation; these activate peripheral immune cells, which can promote neuroinflammation and increase neuronal excitability, also ultimately resulting in seizures (
Figure 1) [
45,
50].
Short chain fatty acids (SCFA) produced by the gut microbiota can influence the immune system by stimulating the synthesis and release of immunoglobulins from plasma cells. It is theorized that SCFAs are also able to exert neuromodulatory effects, such as decreasing seizure intensity and increasing the threshold for seizures (
Figure 1). When dysbiosis occurs, decreased production of SCFA results, negating these neuroprotective effects [
45,
50].
Additionally, gut microbes may be able to regulate the metabolism of dietary tryptophan, which can lead to the release of pro-inflammatory cytokines and neurotoxic metabolites and the development of chronic neuroinflammation and seizures (
Figure 1). Neurotransmitter imbalance has been indicated as one of the major causes of epilepsy. Certain gut microbes secrete neurotransmitters like norepinephrine, 5-hydroxytryptamine, GABA, acetylcholine, and dopamine. These are important to the regulation of the immune response and crucial for homeostasis, and can directly or indirectly affect the excitability of neurons in the CNS, promoting seizures (
Figure 1) [
36,
45,
50].
It is known that stress can promote the induction of epilepsy. The hypothalamic-pituitary-adrenal (HPA) axis is a neuroendocrine system central to the stress response [
55]. Different hormones from the HPA evoke different seizure effects. Most deoxycorticosterones exert anti-seizure effects, while corticotropin-releasing hormone and corticosterone may promote seizures. Evidence has suggested that gut microbiota can influence the HPA, although the specific mechanism is not known. It has further been proposed that stress can upregulate glucocorticoids and heighten glutamatergic signaling to promote seizures. Cortisol receptors are expressed on many types of cells in the gut and could also affect the functionality of the intestines, thus altering the microbiota [
36,
56].
The microbiota can impact the CNS directly, through the vagus nerve, or indirectly by way of the enteric nervous system (ENS). Enteroendocrine cells (EECs) found in the lumen of the intestines are gut sensory epithelial cells capable of eliciting neurotransmission in response to external stimuli. These cells have an assortment of molecular receptors that can detect microbiota components, catabolites, and toxins. EECs can sense stimuli secreted by the GM which interact with various neurotransmitters and conduct signals through the vagus nerve, which can affect CNS excitability [
9,
57].
Neuropod cells are a specialized subtype of EECs that synapse with the vagus nerve and transduce sensory signals from the lumen of the intestines to the brainstem. Neuropod cells play a critical role in sensing metabolites produced by the gut microbiota, including SCFAs, bile acids, phenols, indoles, bioactive lipids, and neurotransmitters [
9,
58].
3.4. Microbiome-Related Epilepsy Treatment
The current standard of care for epilepsy is management using anti-epileptic drugs. This treatment modality is successful in treating this chronic condition for a majority of patients; but up to 30% of patients suffer from DRE [
43,
45,
59]. Surgery, vagus nerve stimulation, and the ketogenic diet (KD) are options for individuals with DRE. The KD has been a treatment option for epilepsy since the 1920s, with some studies reporting that more than half of patients adhering to the diet see a 50% reduction in seizures, one-third see 90% seizure reduction, and some patients achieve complete control of their seizures [
40,
43]. Clinical studies and animal models reveal the potential role the gut microbiota has in DRE. Patients with refractory epilepsy have a higher alpha diversity compared to drug-sensitive patients [
9]. Olson and colleagues performed studies in germ-free mice to better understand the mechanism for which the KD decreases seizures. Although some bacterial species were lost in response to the KD, the authors did find a decrease in alpha diversity and an increase in
Akkermansia muciniphila, Parabacterioides, Sutterella, and Erysipelotrichaceae. These studies altered the abundance of specific bacterial species and demonstrated that adherence to the KD altered the microbiome in such a way as to increase the GABA/glutamate ratio levels and thereby decrease seizures. Their research demonstrated that seizure improvement arising from the KD was induced by the augmentation of selective gut bacteria. The underlying mechanism hinges on microbial interactions that decrease bacterial glutamyltranspeptidase activity, which results in decreased gamma-glutamylated amino acids, which in turn affects the GABA/glutamate ratios in the hippocampus [
43].
4. Future Research Approaches
4.1. Emerging Technologies for Microbiome Research
Existing technologies used in microbiome research are being continually refined for improved accuracy and higher throughput. The difficulties associated with microbe identification via culturing in early research development spurred sequencing technology advancements and the identification of many bacterial species through genomic methods [
60]. The transition from traditional culturing techniques to first-generation DNA sequencing (utilizing the Sanger method) paved the way for the current use of second-generation sequencing. Divisions between the emerging third and fourth sequencing generations remain the subject of much discussion; so far, the implementation of single-molecule sequencing and nanopore technology, generally considered third-generation sequencing techniques, are becoming more widespread [
61,
62]. Both techniques offer long-read sequencing without concern for amplification biases [
63]. The long-read sequencing technique allows for more accurate assembly of microbial genomes and a better understanding of the overall structure of the genome, including complex repetitive sequences and structural variations. Long-read sequencing significantly improves the accuracy and resolution of taxonomic identification within the microbiome, which helps us identify the functional genes of microbes and better elucidate their metabolic pathways [
64]. This plays a crucial role in improving the prevention, diagnosis, and treatment strategies for various diseases. Nanopore technology, in particular, enables single-molecule sequencing (when charged biological molecules pass through nano-scale holes) [
62]. This combination of single-molecule and long-read sequencing strategies offers significant prospects as a genetic diagnostic tool. Beyond real-time targeted sequencing, nanopore technology can detect structural variants in genomes, which are commonly observed in cancer [
65]. Single-molecule sequencing, with an emphasis on nanopore technology, presents a prominent emerging toolkit that can greatly contribute to the diagnosis of genetic conditions, particularly those impacted by the microbiome and dysbiosis.
Cutting-edge advancements are not limited to sequencing technologies. Microbiome multi-omics strategies include culturomics, metatranscriptomics, and metabolomics, which are all primarily used to investigate samples with lower microbial biomass [
60]. As low-biomass organs are difficult to characterize in microbiome ecology, the use of high-throughput cell cultures, metagenomic mRNA analysis, and metabolite identification via multi-omics is invaluable in the identification and analysis of the small quantities of microbes present in these organs. After microbes are identified and their functionality hypothesized, investigations of microbiome changes are often completed
in vitro or within animal models. While these approaches remain largely reliable and serve an important role within microbiome analysis and understanding, the emerging use of organ-on-a-chip and host-microbiota module technology allows researchers to better assess human host responses independently of traditional models. One such gut-on-a-chip model is HuMiX, which is a modular, microfluidics-based, human-microbial co-culture model that incorporates a separatory nanoporous membrane between the human cells and bacteria [
66]. Effective models of the GI human-microbiome interface are essential to researchers’ efforts to elucidate the microbial mechanisms of disease, and groundbreaking technologies like HuMiX highlight the continuing progress of the microbiome field toward such goals. These advances in microbiome research technologies and methodologies have revealed the increasing relevance of the gut-brain axis and its potential role in personalized medicine.
4.2. The Potential of Microbiome Profiling and Personalized Medicine in Treating Rare Neurological Diseases
Commonalities in gut microbial compositions across individuals can help us to identify common disease indicators, especially when broad patterns of dysbiosis are present. However, detecting rare diseases often requires a more detailed and precise analysis of the gut microbiome, as microbial changes involved may be subtle or unique [
67]. There is a dire need for alternative diagnostic strategies, such as detailed analysis of the gut-brain axis and microbial mechanisms, to potentially uncover biomarkers that genomic sequencing missed [
23]. An understanding of the specific roles that differing strains of each microbe play in the pathogenesis and progression of disease is necessary to enable the identification of diseases which have heretofore evaded diagnosis. It may also be important to understand if and how an individual's underlying germline genetic changes affect their microbiome. For instance, seizures and severe constipation, which often leads to a shift in the balance of gut microbiome and metabolism, are major findings in Rett syndrome and CDKL5 deficiency disorder, caused by variants in the
MECP2 and
CDKL5 genes, respectively [
68]. These genetic mutations may influence both neurological and gut health, suggesting a potential bidirectional relationship between the host's genetic makeup and the composition of their microbiome. Identifying unique disease-associated microbiome profiles may assist in diagnosing currently undiagnosed individuals or solidify a diagnosis in individuals with variants of uncertain significance. Even seemingly minute differences in the gut microbiome may be underlying factors in rare diseases, and must be considered and amended. After diagnosis, therapies such as prebiotics, probiotics, postbiotics, symbiotics, and fecal microbiota transplantation can be implemented to directly address microbial dysbiosis. Personalized therapies have the potential to impact the treatment of rare diseases by targeting the differences in microbial presence and/or abundance that are particular to that individual. Due to the prominence of the gut-brain axis, the treatment of rare neurological diseases is an area of particular interest that can be targeted via the gut microbiome.
In a study by Su
et al., multikingdom (archaea, fungi, and viruses) and gut microbiota markers were used to help diagnose ASD [
69]. This study involved metagenomic sequencing of fecal samples from 709 children with ASD and 374 neurotypical children. Children with ASD were found to host decreased levels of
Streptococcus thermophilus and SCFA-producing bacteria (Bacteroides spp. PHL2737 and
Lawsonibacter asaccharolyticus). Using a matched cohort of an additional 602 individuals with and without ASD, the team demonstrated that their novel model of 31 microbial features can facilitate the diagnosis of ASD [
69]. Similar studies in epilepsy could provide valuable new tools for predicting therapeutic responses to anti-epileptic medications, and even assist clinicians in anticipating the possible development of refractory epilepsy in their patients.
4.3. Prospects for Microbiome-Based Interventions and Therapeutic Strategies in Epilepsy and Other Neurological Conditions
Approximately 70% of epilepsy patients have refractory seizures which cannot be controlled by anti-epileptic medications [
70]. While the precise mechanism of refractory epilepsy remains unknown, improvements in seizure symptoms are a promising step toward treating this neurological condition via the gut microbiome. Additional research into related treatments, such as fecal microbiota transplantation, attempts to more directly address the gut dysbiosis observed in epilepsy. As an example, in a study of patients with ASD, Kang
et al. reported a 45% decrease in ASD symptoms (affecting language, social interaction, and behavior) two years after undergoing Microbiota Transfer Therapy (MTT), a type of fecal transplant, from a donor without ASD [
71]. As microbiome research technologies evolve and our understanding of the gut-brain axis continues to improve, microbiome-based interventions can be anticipated to grow in prevalence and efficacy in the clinical setting.
5. Challenges and Considerations
5.1. Ethical, Technical, and Methodological Challenges in Microbiome Research
Microbiome research comes with unique ethical considerations. Unlike germline genetic studies in rare childhood neurological disorders, for which established regulations and professional etiquette exist, microbiome studies do not yet address the same concerns for patient safety and privacy. The gut microbiome is more dynamic than the nervous system, changes as we age, and is susceptible to a tremendous degree of natural variation, including intraindividual variation (variation between samples taken at different times from the same person). It is this ability to change that drives research into therapeutic interventions. This mutability could also fuel patient safety concerns in the future. Within the microbiomics field, identification of secondary findings and struggles with genetic insurance discrimination are minimal, if non-existent, at this time. However, if this technology evolves into a major means of diagnosis and treatment, the regulatory landscape will also need to evolve, requiring commensurate testing and reporting standards. Chuong
et al. suggested that microbiome results should be returned to patients if they are “analytically valid, reveal an established and substantial risk of a serious health condition, and are clinically actionable” [
72]. Without regulations or established guidelines, determining risk or clinical relevance complicates reporting [
73].
Determining the profile of a “healthy” reference microbiome, or identifying optimal control populations for microbiome studies, is another challenge. Larger studies have shown a substantial difference in the microbiome of healthy individuals, and the causes of such variance are currently unknown [
74]. What is known is that an individual’s profile is influenced by their genetics, environmental and lifestyle factors, ethnicity, geographic location, and diet. For instance, in nonindustrialized countries, gut microbiota is typically geared toward fiber degradation; in industrialized countries, it reflects a profile tailored to mucin degradation and exposure to medications and antibiotics [
74].
Through the gut-brain axis, the gut microbiome's influence on the nervous system may extend to mental and behavioral health. In a study by Zheng
et al., germ-free mice were colonized with pooled fecal samples from individuals with non-medicated major depressive disorder [
75]. Behavioral testing two weeks post-transplantation showed increased depression-like and anxiety-like behaviors when compared to animals with a “healthy microbiota” transplant. Additional studies in animal models have also seen similar findings [
76,
77]. While short-term adverse events, such as gastrointestinal issues of bloating, gaseousness, and changes in bowel habits, as well as long-term events, such as obesity and immune-mediated disorders, have been reported in FMT individuals, the potential benefits of such treatments mean that long-term analysis of behavioral and mental health effects in humans is warranted [
78].
5.2. Considerations for Patient Safety, Privacy, and the Regulatory Landscape
Due to the ever-growing popularity of personalized medicine and direct-to-consumer testing, over thirty companies worldwide now sell products designed to assess “gut health” [
79]. Many of these companies advertise the ability to identify imbalances in gut health and provide customized recommendations for diet, probiotics, prebiotics, and supplements. Several companies further tout the ability to promote weight loss and assist in optimizing blood sugar levels. Some argue that these tests require more regulation, as they lack clinical validation and can financially exploit the general public; results generated by many commercial tests are difficult for even doctors to interpret [
79,
80]. Furthermore, methods of collection, processing, analysis, and reporting are not standardized. In a study by Forry
et al., they compared 16S and whole genome sequencing results from 44 academic, commercial, and government labs using seven shared reference samples. They found that protocol choices have significant effects on results, and significant and systematic measurement bias was also present [
81].
While there are no FDA-approved clinical microbiome tests, over 1,200 actively-recruiting gut microbiome clinical trials are listed on clinicaltrials.gov (as of August 2024). Eight of these studies focus on epilepsy, including the effects of the ketogenic diet, probiotics, vagal nerve stimulation, and fecal microbiota transplantation; one focuses specifically on the genetic CDKL5 disorder [
82]. An international and multicenter study named GEMMA (Genome, Environment, Microbiome, and Metabolome in Autism) is currently investigating the microbiome of around 600 siblings of individuals diagnosed with ASD from birth through the first three years of life [
83]. The study hopes to identify biomarkers that will provide a better understanding of the development of ASD in children and enable early diagnosis and treatment of ASD and related gastrointestinal symptoms. Similar large-scale studies in epilepsy may be needed to have a significant impact in the field.
6. Conclusion
The human microbiome is intricately and intrinsically linked to all body systems, playing a critical role in maintaining overall health. Its influence extends beyond the gut, reaching through complex interactions into the immune, endocrine, and nervous systems. As such, dysbiosis serves as a significant clue in understanding a myriad of diseases and disorders, including neurological conditions. This review has summarized the growing body of evidence that connects the microbiome to neurological diseases, ranging from common conditions like ASD to rare childhood disorders such as epilepsy.
The value of studying the microbiome in neurology, particularly in epilepsy, is becoming increasingly clear. New research suggests that specific microbial community profiles influence neurological development and seizure activity, providing potential avenues for novel microbiome-targeting therapies. As technological advances continue to improve our ability to analyze and understand these intricate microbial ecosystems, answers to longstanding questions about disease mechanisms and treatment options are becoming more and more accessible.
While the microbiome field as a whole is advancing rapidly, we are still only beginning to understand the full scope of the microbiome’s specific role in neurological health. The potential for future research is vast, but not without its challenges. Methodological limitations such as sample variability and the complexity of microbial community-host interactions are obstacles that urgently need to be overcome.
As research continues to delve into the microbiome’s influence on the brain, it is clear that this field holds great potential for unlocking novel insights into the pathogenesis and treatment of neurological disorders, particularly those affecting children. Ultimately, by integrating microbiome study with neurology, we may be able to offer new hope for early diagnosis, improved outcomes, and better quality of life for the patients and families impacted by these challenging conditions.
Data Availability Statement No new data were created.
Author Contributions
Conceptualization, K.L. and N.B.; writing—original draft preparation, N.B., K.R., L.N., H.H., and K.L.; writing—review and editing, N.B., K.R., S.C., M.H, and K.L.; visualization, K.L.; supervision, M.H. and K.L.; project administration, K.L.; funding acquisition, K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding. The submission fee is waived.
Acknowledgments
The figure image is created using BioRender.com. BioRender.com/v29e118.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Lathrop, S.K.; Bloom, S.M.; Rao, S.M.; Nutsch, K.; Lio, C.-W.; Santacruz, N.; Peterson, D.A.; Stappenbeck, T.S.; Hsieh, C.-S. Peripheral Education of the Immune System by Colonic Commensal Microbiota. Nature 2011, 478, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Romaní-Pérez, M.; Líebana-García, R.; Flor-Duro, A.; Bonillo-Jiménez, D.; Bullich-Vilarrubias, C.; Olivares, M.; Sanz, Y. Obesity and the Gut Microbiota: Implications of Neuroendocrine and Immune Signaling. FEBS J. 2024. [Google Scholar] [CrossRef] [PubMed]
- Tierney, B.T.; Tan, Y.; Kostic, A.D.; Patel, C.J. Gene-Level Metagenomic Architectures across Diseases Yield High-Resolution Microbiome Diagnostic Indicators. Nat. Commun. 2021, 12, 2907. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Syed, Y.A.; Khan, M.R. Understanding the Role of the Gut Microbiome in Brain Development and Its Association With Neurodevelopmental Psychiatric Disorders. Front Cell Dev Biol 2022, 10, 880544. [Google Scholar] [CrossRef]
- Gao, Y.; Li, D.; Liu, Y.-X. Microbiome Research Outlook: Past, Present, and Future. Protein Cell 2023, 14, 709–712. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct Target Ther 2022, 7, 135. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, W.; Li, Y. The Interplay between Microbiota and Brain-Gut Axis in Epilepsy Treatment. Front. Pharmacol. 2024, 15, 1276551. [Google Scholar] [CrossRef]
- Yang, F.; Chen, H.; Gao, Y.; An, N.; Li, X.; Pan, X.; Yang, X.; Tian, L.; Sun, J.; Xiong, X.; et al. Gut Microbiota-Derived Short-Chain Fatty Acids and Hypertension: Mechanism and Treatment. Biomed. Pharmacother. 2020, 130, 110503. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The Role of Short-Chain Fatty Acids in the Interplay between Gut Microbiota and Diet in Cardio-Metabolic Health. Gut Microbes 2021, 13, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, A.; Shahabi, S.; Bagheri, M.; Nabizadeh, E.; Jazani, N.H. The Protective Effect of Lactobacillus and Bifidobacterium as the Gut Microbiota Members against Chronic Urticaria. Int. Immunopharmacol. 2018, 59, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Gomez de Agüero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The Maternal Microbiota Drives Early Postnatal Innate Immune Development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef]
- Renz, H.; Adkins, B.D.; Bartfeld, S.; Blumberg, R.S.; Farber, D.L.; Garssen, J.; Ghazal, P.; Hackam, D.J.; Marsland, B.J.; McCoy, K.D.; et al. The Neonatal Window of Opportunity-Early Priming for Life. J. Allergy Clin. Immunol. 2018, 141, 1212–1214. [Google Scholar] [CrossRef]
- Donald, K.; Finlay, B.B. Early-Life Interactions between the Microbiota and Immune System: Impact on Immune System Development and Atopic Disease. Nat. Rev. Immunol. 2023, 23, 735–748. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, Birth Mode, and Diet Shape Microbiome Maturation during Early Life. Sci. Transl. Med. 2016, 8, 343ra82–ra343ra82. [Google Scholar] [CrossRef]
- Vijay, A.; Valdes, A.M. Role of the Gut Microbiome in Chronic Diseases: A Narrative Review. Eur. J. Clin. Nutr. 2021, 76, 489–501. [Google Scholar] [CrossRef]
- Zubeldia-Varela, E.; Barker-Tejeda, T.C.; Obeso, D.; Villaseñor, A.; Barber, D.; Pérez-Gordo, M. Microbiome and Allergy: New Insights and Perspectives. J. Investig. Allergol. Clin. Immunol. 2022, 32, 327–344. [Google Scholar] [CrossRef]
- Graham, D.B.; Xavier, R.J. Conditioning of the Immune System by the Microbiome. Trends Immunol. 2023, 44, 499–511. [Google Scholar] [CrossRef]
- The Lancet Neurology Rare Diseases: Maintaining Momentum. Lancet Neurol. 2022, 21, 203. [CrossRef] [PubMed]
- Schor, N.F.; Tamiz, A.P.; Koroshetz, W.J.; NINDS Ultra-Rare Gene-based Therapy (URGenT) Working Group; Broome, A. -M. NINDS Launches Network to Develop Treatments for Ultra-Rare Neurological Diseases. Nat. Biotechnol. 2021, 39, 1497–1499. [Google Scholar] [CrossRef] [PubMed]
- Sciascia, S.; Roccatello, D.; Salvatore, M.; Carta, C.; Cellai, L.L.; Ferrari, G.; Lumaka, A.; Groft, S.; Alanay, Y.; Azam, M.; et al. Unmet Needs in Countries Participating in the Undiagnosed Diseases Network International: An International Survey Considering National Health Care and Economic Indicators. Front Public Health 2023, 11, 1248260. [Google Scholar] [CrossRef] [PubMed]
- Bauskis, A.; Strange, C.; Molster, C.; Fisher, C. The Diagnostic Odyssey: Insights from Parents of Children Living with an Undiagnosed Condition. Orphanet J. Rare Dis. 2022, 17, 233. [Google Scholar] [CrossRef]
- RARE Disease Facts. Available online: https://globalgenes.org/rare-disease-facts/ (accessed on 26 June 2024).
- Lee, C.E.; Singleton, K.S.; Wallin, M.; Faundez, V. Rare Genetic Diseases: Nature’s Experiments on Human Development. iScience 2020, 23, 101123. [Google Scholar] [CrossRef]
- Gan, Y.; Chen, Y.; Zhong, H.; Liu, Z.; Geng, J.; Wang, H.; Wang, W. Gut Microbes in Central Nervous System Development and Related Disorders. Front. Immunol. 2023, 14, 1288256. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The Gut Microbiome in Neurological Disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Taniya, M.A.; Chung, H.-J.; Al Mamun, A.; Alam, S.; Aziz, M.A.; Emon, N.U.; Islam, M.M.; Hong, S.-T.S.; Podder, B.R.; Ara Mimi, A.; et al. Role of Gut Microbiome in Autism Spectrum Disorder and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 915701. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy Alters Gut Ecosystem and Improves Gastrointestinal and Autism Symptoms: An Open-Label Study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, G.; Wan, L.; Liang, Y.; Liu, X.; Yan, H.; Zhang, B.; Yang, G. Effect of Fecal Microbiota Transplantation in Children with Autism Spectrum Disorder: A Systematic Review. Front. Psychiatry 2023, 14, 1123658. [Google Scholar] [CrossRef]
- Caputi, V.; Hill, L.; Figueiredo, M.; Popov, J.; Hartung, E.; Margolis, K.G.; Baskaran, K.; Joharapurkar, P.; Moshkovich, M.; Pai, N. Functional Contribution of the Intestinal Microbiome in Autism Spectrum Disorder, Attention Deficit Hyperactivity Disorder, and Rett Syndrome: A Systematic Review of Pediatric and Adult Studies. Front. Neurosci. 2024, 18, 1341656. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, M.; D’Urso, F.; Piccininni, C.; Montagna, M.L.; Sardone, R.; Resta, E.; Dibello, V.; Daniele, A.; Giannelli, G.; Bellomo, A.; et al. The Relationship between Epigenetics and Microbiota in Neuropsychiatric Diseases. Epigenomics 2020, 12, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Aaberg, K.M.; Gunnes, N.; Bakken, I.J.; Lund Søraas, C.; Berntsen, A.; Magnus, P.; Lossius, M.I.; Stoltenberg, C.; Chin, R.; Surén, P. Incidence and Prevalence of Childhood Epilepsy: A Nationwide Cohort Study. Pediatrics 2017, 139. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, M.; Asuncion, R.M.D.; Al Khalili, Y. Idiopathic (Genetic) Generalized Epilepsy. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2024. [Google Scholar]
- Ding, M.; Lang, Y.; Shu, H.; Shao, J.; Cui, L. Microbiota-Gut-Brain Axis and Epilepsy: A Review on Mechanisms and Potential Therapeutics. Front. Immunol. 2021, 12, 742449. [Google Scholar] [CrossRef]
- Balestrini, S.; Arzimanoglou, A.; Blümcke, I.; Scheffer, I.E.; Wiebe, S.; Zelano, J.; Walker, M.C. The Aetiologies of Epilepsy. Epileptic Disord. 2021, 23, 1–16. [Google Scholar] [CrossRef]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE Official Report: A Practical Clinical Definition of Epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef]
- Beghi, E.; Berg, A.; Carpio, A.; Forsgren, L.; Hesdorffer, D.C.; Hauser, W.A.; Malmgren, K.; Shinnar, S.; Temkin, N.; Thurman, D.; et al. Comment on Epileptic Seizures and Epilepsy: Definitions Proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 1698–1699, author reply 1701–1702. [Google Scholar] [CrossRef]
- Devinsky, O.; Vezzani, A.; O’Brien, T.J.; Jette, N.; Scheffer, I.E.; de Curtis, M.; Perucca, P. Epilepsy. Nat Rev Dis Primers 2018, 4, 18024. [Google Scholar] [CrossRef]
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; Peltola, J.; Roulet Perez, E.; et al. Operational Classification of Seizure Types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef]
- Wirrell, E.C.; Nabbout, R.; Scheffer, I.E.; Alsaadi, T.; Bogacz, A.; French, J.A.; Hirsch, E.; Jain, S.; Kaneko, S.; Riney, K.; et al. Methodology for Classification and Definition of Epilepsy Syndromes with List of Syndromes: Report of the ILAE Task Force on Nosology and Definitions. Epilepsia 2022, 63, 1333–1348. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 174, 497. [Google Scholar] [CrossRef]
- Thambi, M.; Nathan, J.; Radhakrishnan, K. Can Change in Gut Microbiota Composition Be Used as a Surrogate Marker of Treatment Efficacy of Ketogenic Diet in Patients with Drug-Resistant Epilepsy? Epilepsy Behav. 2020, 113, 107444. [Google Scholar] [CrossRef] [PubMed]
- Arulsamy, A.; Shaikh, M.F. Epilepsy and Gut Microbiota. In Handbook of Neurodegenerative Disorders; Mohamed, E., Ed.; Springer Nature Singapore: Singapore, 2023; pp. 1–12. ISBN 9789811939495. [Google Scholar]
- Xie, G.; Zhou, Q.; Qiu, C.-Z.; Dai, W.-K.; Wang, H.-P.; Li, Y.-H.; Liao, J.-X.; Lu, X.-G.; Lin, S.-F.; Ye, J.-H.; et al. Ketogenic Diet Poses a Significant Effect on Imbalanced Gut Microbiota in Infants with Refractory Epilepsy. World J. Gastroenterol. 2017, 23, 6164–6171. [Google Scholar] [CrossRef] [PubMed]
- Lindefeldt, M.; Eng, A.; Darban, H.; Bjerkner, A.; Zetterström, C.K.; Allander, T.; Andersson, B.; Borenstein, E.; Dahlin, M.; Prast-Nielsen, S. The Ketogenic Diet Influences Taxonomic and Functional Composition of the Gut Microbiota in Children with Severe Epilepsy. NPJ Biofilms Microbiomes 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Qiu, X.; Lai, W.; Li, W.; Zhang, L.; Zhu, X.; He, S.; Duan, J.; Chen, L. Altered Composition of the Gut Microbiome in Patients with Drug-Resistant Epilepsy. Epilepsy Res. 2018, 147, 102–107. [Google Scholar] [CrossRef]
- Şafak, B.; Altunan, B.; Topçu, B.; Eren Topkaya, A. The Gut Microbiome in Epilepsy. Microb. Pathog. 2020, 139, 103853. [Google Scholar] [CrossRef]
- Dong, L.; Zheng, Q.; Cheng, Y.; Zhou, M.; Wang, M.; Xu, J.; Xu, Z.; Wu, G.; Yu, Y.; Ye, L.; et al. Gut Microbial Characteristics of Adult Patients With Epilepsy. Front. Neurosci. 2022, 16, 803538. [Google Scholar] [CrossRef]
- Pittman, Q.J. A Gut Feeling about the Ketogenic Diet in Epilepsy. Epilepsy Res. 2020, 166, 106409. [Google Scholar] [CrossRef]
- Ouyang, Y.; Chen, Y.; Wang, G.; Song, Y.; Zhao, H.; Xiao, B.; Yang, Z.; Long, L. Genetically Proxied Gut Microbiota, Gut Metabolites with Risk of Epilepsy and the Subtypes: A Bi-Directional Mendelian Randomization Study. Front. Mol. Neurosci. 2022, 15, 994270. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.; Lee, D.-H.; Kim, D.W. A Comparison of the Gut Microbiota among Adult Patients with Drug-Responsive and Drug-Resistant Epilepsy: An Exploratory Study. Epilepsy Res. 2021, 172, 106601. [Google Scholar] [CrossRef]
- Amlerova, J.; Šroubek, J.; Angelucci, F.; Hort, J. Evidences for a Role of Gut Microbiota in Pathogenesis and Management of Epilepsy. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Cano-López, I.; González-Bono, E. Cortisol Levels and Seizures in Adults with Epilepsy: A Systematic Review. Neurosci. Biobehav. Rev. 2019, 103, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Mengoli, M.; Conti, G.; Fabbrini, M.; Candela, M.; Brigidi, P.; Turroni, S.; Barone, M. Microbiota-Gut-Brain Axis and Ketogenic Diet: How Close Are We to Tackling Epilepsy? Microbiome Res. Rep. 2023, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.R.; Londregan, A.K.; Alexander, T.D.; Entezari, A.A.; Covarrubias, M.; Waldman, S.A. Enteroendocrine Cell Regulation of the Gut-Brain Axis. Front. Neurosci. 2023, 17, 1272955. [Google Scholar] [CrossRef]
- Kaelberer, M.M.; Rupprecht, L.E.; Liu, W.W.; Weng, P.; Bohórquez, D.V. Neuropod Cells: The Emerging Biology of Gut-Brain Sensory Transduction. Annu. Rev. Neurosci. 2020, 43, 337–353. [Google Scholar] [CrossRef]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of Drug Resistant Epilepsy: Consensus Proposal by the Ad Hoc Task Force of the ILAE Commission on Therapeutic Strategies: Definition of Drug Resistant Epilepsy. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Kwa, W.T.; Sundarajoo, S.; Toh, K.Y.; Lee, J. Application of Emerging Technologies for Gut Microbiome Research. Singapore Med. J. 2023, 64, 45–52. [Google Scholar] [CrossRef]
- Sobhani, N.; D’Angelo, A.; Conter, F.U.; Morris, R.; Li, Y. The Power of Whole Genomic Sequencing in Biomedical Research and Clinical Applications. In Comprehensive Precision Medicine; Elsevier, 2024; pp. 1–18 ISBN 9780128242568.
- Lin, B.; Hui, J.; Mao, H. Nanopore Technology and Its Applications in Gene Sequencing. Biosensors 2021, 11, 214. [Google Scholar] [CrossRef]
- Ardui, S.; Ameur, A.; Vermeesch, J.R.; Hestand, M.S. Single Molecule Real-Time (SMRT) Sequencing Comes of Age: Applications and Utilities for Medical Diagnostics. Nucleic Acids Res. 2018, 46, 2159–2168. [Google Scholar] [CrossRef]
- Marwaha, S.; Knowles, J.W.; Ashley, E.A. A Guide for the Diagnosis of Rare and Undiagnosed Disease: Beyond the Exome. Genome Med. 2022, 14, 23. [Google Scholar] [CrossRef]
- Jain, M.; Olsen, H.E.; Paten, B.; Akeson, M. The Oxford Nanopore MinION: Delivery of Nanopore Sequencing to the Genomics Community. Genome Biol. 2016, 17, 239. [Google Scholar]
- Shah, P.; Fritz, J.V.; Glaab, E.; Desai, M.S.; Greenhalgh, K.; Frachet, A.; Niegowska, M.; Estes, M.; Jäger, C.; Seguin-Devaux, C.; et al. A Microfluidics-Based in Vitro Model of the Gastrointestinal Human-Microbe Interface. Nat. Commun. 2016, 7, 11535. [Google Scholar] [CrossRef] [PubMed]
- Behrouzi, A.; Nafari, A.H.; Siadat, S.D. The Significance of Microbiome in Personalized Medicine. Clin. Transl. Med. 2019, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Christodoulou, J. MECP2 Disorders; University of Washington, Seattle, 2019;
- Su, Q.; Wong, O.W.H.; Lu, W.; Wan, Y.; Zhang, L.; Xu, W.; Li, M.K.T.; Liu, C.; Cheung, C.P.; Ching, J.Y.L.; et al. Multikingdom and Functional Gut Microbiota Markers for Autism Spectrum Disorder. Nat. Microbiol. 2024, 9, 2344–2355. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Dwivedi, R.; Bansal, M.; Tripathi, M.; Dada, R. Role of Gut Microbiota in Neurological Disorders and Its Therapeutic Significance. J. Clin. Med. Res. 2023, 12. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-Term Benefit of Microbiota Transfer Therapy on Autism Symptoms and Gut Microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef]
- Chuong, K.H.; Hwang, D.M.; Tullis, D.E.; Waters, V.J.; Yau, Y.C.W.; Guttman, D.S.; O’Doherty, K.C. Navigating Social and Ethical Challenges of Biobanking for Human Microbiome Research. BMC Med. Ethics 2017, 18, 1. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, H.; Lan, C.; Ren, J. Help, Hope and Hype: Ethical Considerations of Human Microbiome Research and Applications. Protein Cell 2018, 9, 404–415. [Google Scholar] [CrossRef]
- Shanahan, F.; Ghosh, T.S.; O’Toole, P.W. The Healthy Microbiome-What Is the Definition of a Healthy Gut Microbiome? Gastroenterology 2021, 160, 483–494. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut Microbiome Remodeling Induces Depressive-like Behaviors through a Pathway Mediated by the Host’s Metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’ Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the Blues: Depression-Associated Gut Microbiota Induces Neurobehavioural Changes in the Rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Pearson-Leary, J.; Zhao, C.; Bittinger, K.; Eacret, D.; Luz, S.; Vigderman, A.S.; Dayanim, G.; Bhatnagar, S. The Gut Microbiome Regulates the Increases in Depressive-Type Behaviors and in Inflammatory Processes in the Ventral Hippocampus of Stress Vulnerable Rats. Mol. Psychiatry 2020, 25, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Seo, G.S. Fecal Microbiota Transplantation: Is It Safe? Clin. Endosc. 2021, 54, 157–160. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Gastroenterology Hepatology Direct-to-Consumer Microbiome Testing Needs Regulation. Lancet Gastroenterol. Hepatol. 2024, 9, 583. [CrossRef]
- Hoffmann, D.E.; von Rosenvinge, E.C.; Roghmann, M.-C.; Palumbo, F.B.; McDonald, D.; Ravel, J. The DTC Microbiome Testing Industry Needs More Regulation. Science 2024, 383, 1176–1179. [Google Scholar] [CrossRef]
- Forry, S.P.; Servetas, S.L.; Kralj, J.G.; Soh, K.; Hadjithomas, M.; Cano, R.; Carlin, M.; Amorim, M.G. de; Auch, B.; Bakker, M.G.; et al. Variability and Bias in Microbiome Metagenomic Sequencing: An Interlaboratory Study Comparing Experimental Protocols. Sci. Rep. 2024, 14, 9785. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/search?term=gut%20microbiome%20epilepsy&aggFilters=status:rec&viewType=Table (accessed on 16 September 2024).
- Troisi, J.; Autio, R.; Beopoulos, T.; Bravaccio, C.; Carraturo, F.; Corrivetti, G.; Cunningham, S.; Devane, S.; Fallin, D.; Fetissov, S.; et al. Genome, Environment, Microbiome and Metabolome in Autism (GEMMA) Study Design: Biomarkers Identification for Precision Treatment and Primary Prevention of Autism Spectrum Disorders by an Integrated Multi-Omics Systems Biology Approach. Brain Sci. 2020, 10, 743. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).