Submitted:
06 October 2024
Posted:
08 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
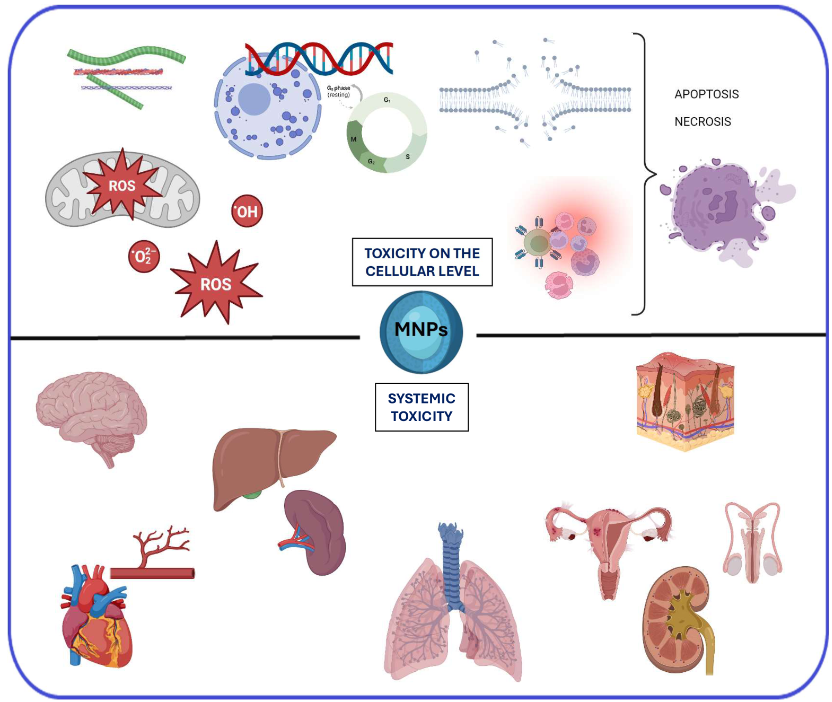
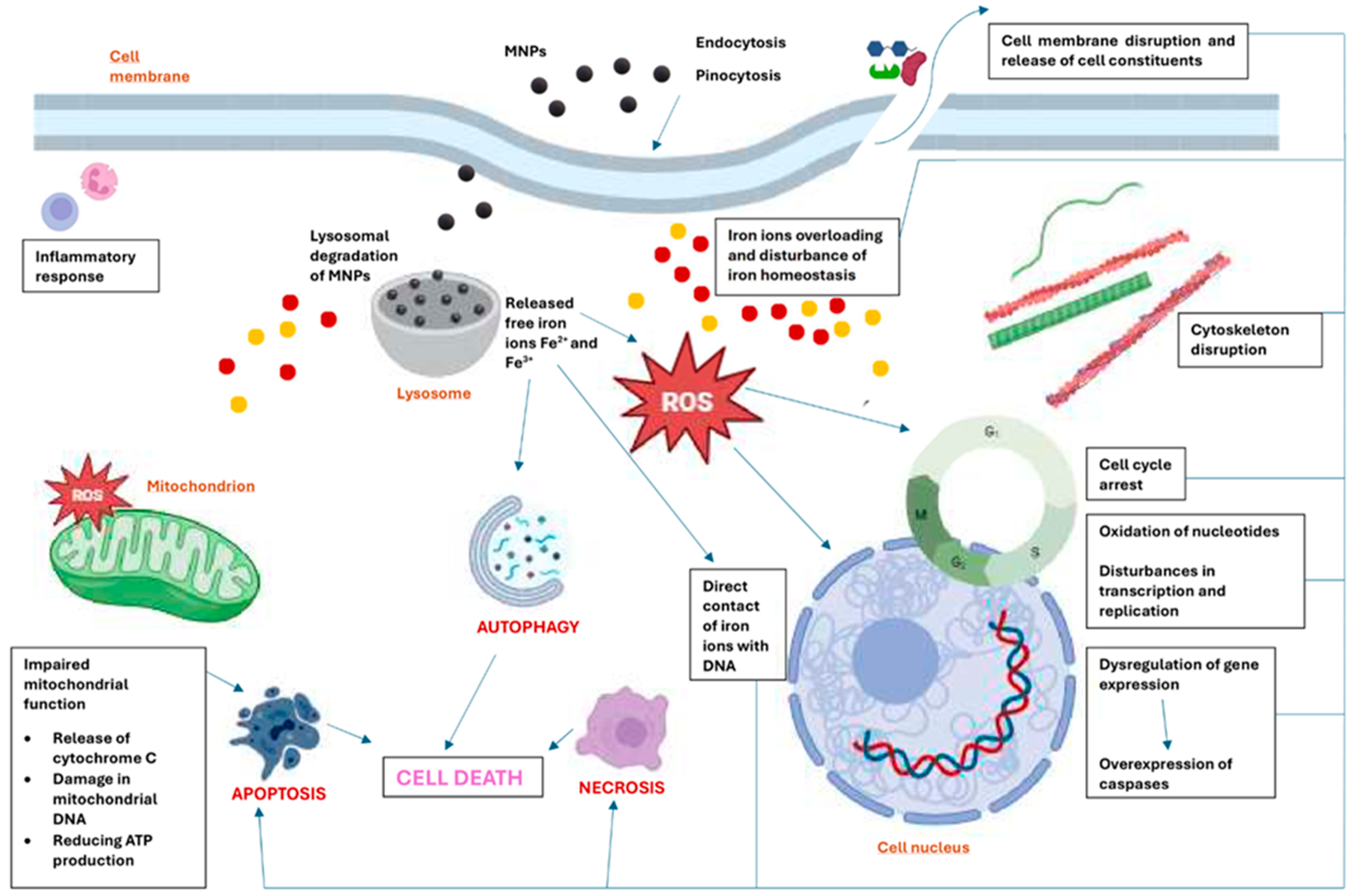
2. The primary mechanisms of toxicity of MNPs at the cellular level
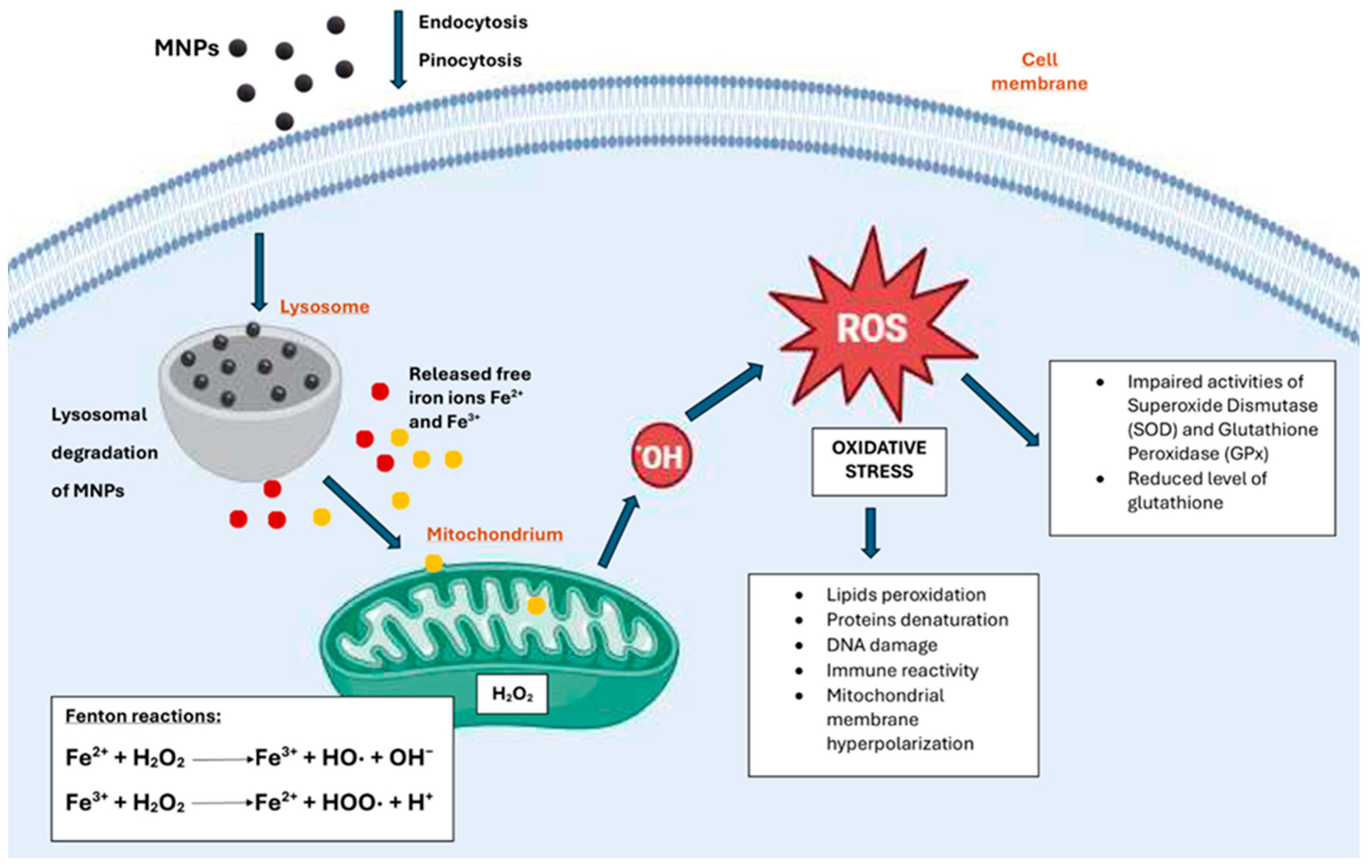
2.1. Oxidative Stress
2.2. Genotoxic effects
2.3. Cytoskeleton Disruption
2.4. Cell Membrane Disruption
2.5. Changes in the Cell Cycle
2.6. Dysregulation of Gene Expression
2.7. Inflammatory Response
2.8. Disturbance in Iron Homeostasis
2.9. Disturbance of Cell Migration and Mobility
3. Systemic and Organ-Toxic Effects of MNPs
3.1. Brain and Nervous System
3.2. Heart and Circulatory System
3.3. Liver, Spleen, and Lymph nodes
3.4. Respiratory System
3.5. Urinary system
3.6. Reproductive system
3.7. Skin
6. Conclusions and Future Perspectives
Funding
Consent for publication
Availability of data and materials
Competing interests
Authors' contributions
Ethics approval and consent to participate
Abbreviations
| AC – amino cellulose. |
| AC- alternating current. |
| ALP- alkaline phosphatase. |
| ALT- alkaline aminotransferase. |
| AMF- alternating magnetic field. |
| ANT- adenine nucleotide translocase. |
| APTES- (3-aminopropyl)triethoxysilane. |
| APTMS- (3-aminopropyl)trimethoxysilane. |
| BBB- blood-brain barrier. |
| BCECs- brain capillary endothelial cells. |
| BSA – bovine serum albumin. |
| CAT- catalase. |
| CF – cobalt ferrite. |
| CMC- N-carboxymethyl chitosan. |
| CPK-MB- creatine phosphokinase-MB. |
| CS – chitosan. |
| CSF- cerebrospinal fluid. |
| DEAP- 3-(diethylamino)-propyl amine. |
| DEX- dextran. |
| DMSA- dimercaptosuccinic acid. |
| DOX- doxorubicin. |
| EGFR- epidermal growth factor receptor. |
| ETC- electron transfer chain. |
| GGT- gamma-glutamyl. |
| GPx – glutathione peroxidase. |
| GSH- glutathione reductase. |
| HUVECs- human umbilical vein endothelial cells. |
| ICG- indocyanine green. |
| LDH- lactate dehydrogenase. |
| LPS- lipopolysaccharide. |
| MDA- malondialdehyde. |
| MNPs – magnetic nanoparticles. |
| MPTP – mitochondrial permeability transition pore. |
| MRI- magnetic resonance imaging. |
| MTD- magneto-thermodynamic. |
| MTT- 3-(4:5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. |
| MTX- methotrexate. |
| NAC- N-acetylcysteine. |
| NSCLC- non-small cell lung cancer. |
| NSCs- neural stem cells. |
| PAA- polyacrylic acid. |
| PAH – poly(allylamine hydrochloride). |
| PAMAM- polyamidoamine. |
| PDADMAC – poly(diallyl dimethylammonium chloride). |
| PEG – poly(ethylene amine). |
| PEI – poly(ethylene imine). |
| PLL- poly-L-lysine. |
| PVA- polyvinyl alcohol. |
| ROS – reactive oxygen species. |
| SOD – superoxide dismutase. |
| TAC- tacrolimus. |
| TEOS- tetraethoxysilane. |
| TLR4- Toll-like receptor 4. |
| VAN- vancomycin. |
| VDAC – voltage-dependent anion channel. |
References
- Avval, Z.M.; Malekpour, L.; Raeisi, F.; Babapoor, A.; Mousavi, S.M.; Hashemi, S.A.; Salari, M. Introduction of Magnetic and Supermagnetic Nanoparticles in New Approach of Targeting Drug Delivery and Cancer Therapy Application. Drug Metabolism Reviews 2020, 52, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M. Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery. Int J Mol Sci 2015, 16, 8070–8101. [Google Scholar] [CrossRef]
- Foy, S.P.; Manthe, R.L.; Foy, S.T.; Dimitrijevic, S.; Krishnamurthy, N.; Labhasetwar, V. Optical Imaging and Magnetic Field Targeting of Magnetic Nanoparticles in Tumors. ACS Nano 2010, 4, 5217–5224. [Google Scholar] [CrossRef] [PubMed]
- Stanicki, D.; Vangijzegem, T.; Ternad, I.; Laurent, S. An Update on the Applications and Characteristics of Magnetic Iron Oxide Nanoparticles for Drug Delivery. Expert Opin Drug Deliv 2022, 19, 321–335. [Google Scholar] [CrossRef]
- Palzer, J.; Eckstein, L.; Slabu, I.; Reisen, O.; Neumann, U.P.; Roeth, A.A. Iron Oxide Nanoparticle-Based Hyperthermia as a Treatment Option in Various Gastrointestinal Malignancies. Nanomaterials 2021, 11, 3013. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Narayanaswamy, V.; Alaabed, S.; Sambasivam, S.; Muralee Gopi, C.V.V. Principles of Magnetic Hyperthermia: A Focus on Using Multifunctional Hybrid Magnetic Nanoparticles. Magnetochemistry 2019, 5, 67. [Google Scholar] [CrossRef]
- Mu, X.; Li, J.; Yan, S.; Zhang, H.; Zhang, W.; Zhang, F.; Jiang, J. siRNA Delivery with Stem Cell Membrane-Coated Magnetic Nanoparticles for Imaging-Guided Photothermal Therapy and Gene Therapy. ACS Biomater Sci Eng 2018, 4, 3895–3905. [Google Scholar] [CrossRef]
- Russell, E.; Dunne, V.; Russell, B.; Mohamud, H.; Ghita, M.; McMahon, S.J.; Butterworth, K.T.; Schettino, G.; McGarry, C.K.; Prise, K.M. Impact of Superparamagnetic Iron Oxide Nanoparticles on in Vitro and in Vivo Radiosensitisation of Cancer Cells. Radiat Oncol 2021, 16, 104. [Google Scholar] [CrossRef]
- De Toledo, L.D.A.S.; Rosseto, H.C.; Bruschi, M.L. Iron Oxide Magnetic Nanoparticles as Antimicrobials for Therapeutics. Pharm Dev Technol 2018, 23, 316–323. [Google Scholar] [CrossRef]
- Rodrigues, G.R.; López-Abarrategui, C.; De La Serna Gómez, I.; Dias, S.C.; Otero-González, A.J.; Franco, O.L. Antimicrobial Magnetic Nanoparticles Based-Therapies for Controlling Infectious Diseases. Int J Pharm 2019, 555, 356–367. [Google Scholar] [CrossRef]
- Li, Z.; Xue, L.; Wang, P.; Ren, X.; Zhang, Y.; Wang, C.; Sun, J. Biological Scaffolds Assembled with Magnetic Nanoparticles for Bone Tissue Engineering: A Review. Materials 2023, 16, 1429. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Iversen, T.-G.; Sandvig, K. New Metal-Based Nanoparticles for Intravenous Use: Requirements for Clinical Success with Focus on Medical Imaging. Nanomedicine 2010, 6, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.F. The Regulation of Iron Absorption and Homeostasis. Clin Biochem Rev 2016, 37, 51–62. [Google Scholar]
- Mai, T.; Hilt, J.Z. Magnetic Nanoparticles: Reactive Oxygen Species Generation and Potential Therapeutic Applications. J Nanopart Res 2017, 19, 253. [Google Scholar] [CrossRef]
- Ahmad, A.; Ansari, Md.M.; Kumar, A.; Vyawahare, A.; Mishra, R.K.; Jayamurugan, G.; Raza, S.S.; Khan, R. Comparative Acute Intravenous Toxicity Study of Triple Polymer-Layered Magnetic Nanoparticles with Bare Magnetic Nanoparticles in Swiss Albino Mice. Nanotoxicology 2020, 14, 1362–1380. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Laurent, S.; Shokrgozar, M.A.; Hosseinkhani, M. Toxicity Evaluations of Superparamagnetic Iron Oxide Nanoparticles: Cell “Vision” versus Physicochemical Properties of Nanoparticles. ACS Nano 2011, 5, 7263–7276. [Google Scholar] [CrossRef]
- Malvindi, M.A.; De Matteis, V.; Galeone, A.; Brunetti, V.; Anyfantis, G.C.; Athanassiou, A.; Cingolani, R.; Pompa, P.P. Toxicity Assessment of Silica Coated Iron Oxide Nanoparticles and Biocompatibility Improvement by Surface Engineering. PLoS ONE 2014, 9, e85835. [Google Scholar] [CrossRef]
- Shukla, S.; Jadaun, A.; Arora, V.; Sinha, R.K.; Biyani, N.; Jain, V.K. In Vitro Toxicity Assessment of Chitosan Oligosaccharide Coated Iron Oxide Nanoparticles. Toxicol Rep 2015, 2, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Abakumov, M.A.; Semkina, A.S.; Skorikov, A.S.; Vishnevskiy, D.A.; Ivanova, A.V.; Mironova, E.; Davydova, G.A.; Majouga, A.G.; Chekhonin, V.P. Toxicity of Iron Oxide Nanoparticles: Size and Coating Effects. J Biochem Mol Toxicol 2018, 32, e22225. [Google Scholar] [CrossRef]
- Yang, W.; Lee, J.; Hong, S.; Lee, J.; Lee, J.; Han, D.-W. Difference between Toxicities of Iron Oxide Magnetic Nanoparticles with Various Surface-Functional Groups against Human Normal Fibroblasts and Fibrosarcoma Cells. Materials 2013, 6, 4689–4706. [Google Scholar] [CrossRef]
- Vonarbourg, A.; Passirani, C.; Saulnier, P.; Benoit, J.-P. Parameters Influencing the Stealthiness of Colloidal Drug Delivery Systems. Biomaterials 2006, 27, 4356–4373. [Google Scholar] [CrossRef] [PubMed]
- Owensiii, D.; Peppas, N. Opsonization, Biodistribution, and Pharmacokinetics of Polymeric Nanoparticles. Int J Pharm 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, D.; Ceña, V. Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell. Pharmaceutics 2020, 12, 371. [Google Scholar] [CrossRef]
- Swanson, J.A.; Baer, S.C. Phagocytosis by Zippers and Triggers. Trends Cell Biol 1995, 5, 89–93. [Google Scholar] [CrossRef]
- Wu, H.; Yin, J.-J.; Wamer, W.G.; Zeng, M.; Lo, Y.M. Reactive Oxygen Species-Related Activities of Nano-Iron Metal and Nano-Iron Oxides. J Food Drug Anal 2014, 22, 86–94. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of Metal and Metal Oxide Nanoparticles: A Review. Environ Chem Lett 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Kiliç, G.; Costa, C.; Fernández-Bertólez, N.; Pásaro, E.; Teixeira, J.P.; Laffon, B. Effects of Iron Oxide Nanoparticles: Cytotoxicity, Genotoxicity, Developmental Toxicity, and Neurotoxicity: Effects of Iron Oxide Nanoparticles. Environ Mol Mutagen 2015, 56, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K. Integration of ER Stress, Oxidative Stress and the Inflammatory Response in Health and Disease. Int J Clin Exp Med 2010, 3, 33–40. [Google Scholar]
- Pongrac, I.M.; Pavičić, I.; Milić, M.; Brkić Ahmed, L.; Babič, M.; Horák, D.; Vinković Vrček, I.; Gajović, S. Oxidative Stress Response in Neural Stem Cells Exposed to Different Superparamagnetic Iron Oxide Nanoparticles. Int J Nanomedicine 2016, 11, 1701–1715. [Google Scholar] [CrossRef]
- Wu, L.; Wen, W.; Wang, X.; Huang, D.; Cao, J.; Qi, X.; Shen, S. Ultrasmall Iron Oxide Nanoparticles Cause Significant Toxicity by Specifically Inducing Acute Oxidative Stress to Multiple Organs. Part Fibre Toxicol 2022, 19, 24. [Google Scholar] [CrossRef]
- Ying, H.; Ruan, Y.; Zeng, Z.; Bai, Y.; Xu, J.; Chen, S. Iron Oxide Nanoparticles Size-Dependently Activate Mouse Primary Macrophages via Oxidative Stress and Endoplasmic Reticulum Stress. Int Immunopharm 2022, 105, 108533. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.O.; Parveen, N.; Ahmad, M.F.; Wani, A.L.; Afrin, S.; Rahman, Y.; Jameel, S.; Khan, Y.A.; Siddique, H.R.; Tabish, M.; et al. Evaluation of DNA Interaction, Genotoxicity and Oxidative Stress Induced by Iron Oxide Nanoparticles Both in Vitro and in Vivo: Attenuation by Thymoquinone. Sci Rep 2019, 9, 6912. [Google Scholar] [CrossRef] [PubMed]
- Kenzaoui, B.H.; Bernasconi, C.C.; Hofmann, H.; Juillerat-Jeanneret, L. Evaluation of Uptake and Transport of Ultrasmall Superparamagnetic Iron Oxide Nanoparticles by Human Brain-Derived Endothelial Cells. Nanomedicine 2012, 7, 39–53. [Google Scholar] [CrossRef]
- Zhang, T.; Qian, L.; Tang, M.; Xue, Y.; Kong, L.; Zhang, S.; Pu, Y. Evaluation on Cytotoxicity and Genotoxicity of the L-Glutamic Acid Coated Iron Oxide Nanoparticles. J Nanosci Nanotechnol 2012, 12, 2866–2873. [Google Scholar] [CrossRef]
- Du, S.; Li, J.; Du, C.; Huang, Z.; Chen, G.; Yan, W. Overendocytosis of Superparamagnetic Iron Oxide Particles Increases Apoptosis and Triggers Autophagic Cell Death in Human Osteosarcoma Cell under a Spinning Magnetic Field. Oncotarget 2017, 8, 9410–9424. [Google Scholar] [CrossRef] [PubMed]
- Petters, C.; Thiel, K.; Dringen, R. Lysosomal Iron Liberation Is Responsible for the Vulnerability of Brain Microglial Cells to Iron Oxide Nanoparticles: Comparison with Neurons and Astrocytes. Nanotoxicology 2016, 10, 332–342. [Google Scholar] [CrossRef]
- Hanot, C.C.; Choi, Y.S.; Anani, T.B.; Soundarrajan, D.; David, A.E. Effects of Iron-Oxide Nanoparticle Surface Chemistry on Uptake Kinetics and Cytotoxicity in CHO-K1 Cells. Int J Mol Sci 2015, 17, 54. [Google Scholar] [CrossRef]
- Watanabe, M.; Yoneda, M.; Morohashi, A.; Hori, Y.; Okamoto, D.; Sato, A.; Kurioka, D.; Nittami, T.; Hirokawa, Y.; Shiraishi, T.; et al. Effects of Fe3O4 Magnetic Nanoparticles on A549 Cells. Int J Mol Sci 2013, 14, 15546–15560. [Google Scholar] [CrossRef]
- Hohnholt, M.C.; Geppert, M.; Dringen, R. Treatment with Iron Oxide Nanoparticles Induces Ferritin Synthesis but Not Oxidative Stress in Oligodendroglial Cells. Acta Biomater 2011, 7, 3946–3954. [Google Scholar] [CrossRef]
- Lindemann, A.; Fraederich, B.M.; Pries, R.; Wollenberg, B.; Lüdtke-Buzug, K.; Graefe, K. Biological Impact of Superparamagnetic Iron Oxide Nanoparticles for Magnetic Particle Imaging of Head and Neck Cancer Cells. Int J Nanomedicine 2014, 5025. [Google Scholar] [CrossRef]
- Remya, N.S.; Syama, S.; Sabareeswaran, A.; Mohanan, P.V. Toxicity, Toxicokinetics and Biodistribution of Dextran Stabilized Iron Oxide Nanoparticles for Biomedical Applications. Int J Pharm 2016, 511, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Repar, N.; Jovičić, E.J.; Kump, A.; Birarda, G.; Vaccari, L.; Erman, A.; Kralj, S.; Nemec, S.; Petan, T.; Drobne, D. Oleic Acid Protects Endothelial Cells from Silica-Coated Superparamagnetic Iron Oxide Nanoparticles (SPIONs)-Induced Oxidative Stress and Cell Death. Int J Mol Sci 2022, 23, 6972. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Alhadlaq, H.A.; Khan, M.A.M.; Akhtar, Mohd.J. Selective Killing of Cancer Cells by Iron Oxide Nanoparticles Mediated through Reactive Oxygen Species via P53 Pathway. J Nanopart Res 2013, 15, 1225. [Google Scholar] [CrossRef]
- Flieger, J.; Franus, W.; Panek, R.; Szymańska-Chargot, M.; Flieger, W.; Flieger, M.; Kołodziej, P. Green Synthesis of Silver Nanoparticles Using Natural Extracts with Proven Antioxidant Activity. Molecules 2021, 26, 4986. [Google Scholar] [CrossRef]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Ram Singh, S.P.; Deenadhayalan, R.; Rajesh, V.V.; Padmanaban, S.; Radhakrishnan, K. Nanoparticles, a Double-Edged Sword with Oxidant as Well as Antioxidant Properties—A Review. Oxygen 2022, 2, 591–604. [Google Scholar] [CrossRef]
- Chavan, R.R.; Bhinge, S.D.; Bhutkar, M.A.; Randive, D.S.; Wadkar, G.H.; Todkar, S.S.; Urade, M.N. Characterization, Antioxidant, Antimicrobial and Cytotoxic Activities of Green Synthesized Silver and Iron Nanoparticles Using Alcoholic Blumea Eriantha DC Plant Extract. Mater Today Commun 2020, 24, 101320. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′ -Deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J Environ Sci Health C 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid Med Cell Longev 2014, 2014, 1–31. [Google Scholar] [CrossRef]
- Jahanbani, J.; Ghotbi, M.; Shahsavari, F.; Seydi, E.; Rahimi, S.; Pourahmad, J. Selective Anticancer Activity of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) against Oral Tongue Cancer Using in Vitro Methods: The Key Role of Oxidative Stress on Cancerous Mitochondria. J Biochem Mol Toxicol 2020, 34. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, J.; Liu, J.; Lin, G.; Xie, F.; Pang, X.; Pei, Y.; Cheng, Y.; Zhang, Y.; Lin, Z.; et al. Oxidative Stress-Driven DR5 Upregulation Restores TRAIL/Apo2L Sensitivity Induced by Iron Oxide Nanoparticles in Colorectal Cancer. Biomaterials 2020, 233, 119753. [Google Scholar] [CrossRef] [PubMed]
- Dan Dunn, J.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive Oxygen Species and Mitochondria: A Nexus of Cellular Homeostasis. Redox Biology 2015, 6, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Laffon, B.; Fernández-Bertólez, N.; Costa, C.; Brandão, F.; Teixeira, J.P.; Pásaro, E.; Valdiglesias, V. Cellular and Molecular Toxicity of Iron Oxide Nanoparticles. In Cellular and Molecular Toxicology of Nanoparticles; Saquib, Q., Faisal, M., Al-Khedhairy, A.A., Alatar, A.A., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, 2018; Vol. 1048, pp. 199–213. ISBN 978-3-319-72040-1. [Google Scholar]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron Oxide Nanoparticles May Damage to the Neural Tissue through Iron Accumulation, Oxidative Stress, and Protein Aggregation. BMC Neurosci 2017, 18, 51. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Q.; Zhao, D.; Lian, F.; Li, X.; Qi, W. The Impact of Oxidative Stress-Induced Mitochondrial Dysfunction on Diabetic Microvascular Complications. Front Endocrinol (Lausanne) 2023, 14, 1112363. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural Regen Res 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Mancuso, M.; Calsolaro, V.; Orsucci, D.; Carlesi, C.; Choub, A.; Piazza, S.; Siciliano, G. Mitochondria, Cognitive Impairment, and Alzheimer’s Disease. Int J Alzheimer’s Dis 2009, 2009, 1–8. [Google Scholar] [CrossRef]
- D’Errico, M.; Parlanti, E.; Pascucci, B.; Filomeni, G.; Mastroberardino, P.G.; Dogliotti, E. The Interplay between Mitochondrial Functionality and Genome Integrity in the Prevention of Human Neurologic Diseases. Arch Biochem and Biophys 2021, 710, 108977. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J Affect Disord 2018, 62, 1403–1416. [Google Scholar] [CrossRef]
- Sharma, C.; Kim, S.; Nam, Y.; Jung, U.J.; Kim, S.R. Mitochondrial Dysfunction as a Driver of Cognitive Impairment in Alzheimer’s Disease. Int J Mol Sci 2021, 22, 4850. [Google Scholar] [CrossRef]
- Shukla, R.K.; Badiye, A.; Vajpayee, K.; Kapoor, N. Genotoxic Potential of Nanoparticles: Structural and Functional Modifications in DNA. Front Genet 2021, 12, 728250. [Google Scholar] [CrossRef]
- Bourrinet, P.; Bengele, H.H.; Bonnemain, B.; Dencausse, A.; Idee, J.-M.; Jacobs, P.M.; Lewis, J.M. Preclinical Safety and Pharmacokinetic Profile of Ferumoxtran-10, an Ultrasmall Superparamagnetic Iron Oxide Magnetic Resonance Contrast Agent. Invest Radiol 2006, 41, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Wang, X.; Zhang, L.; Wang, J. Iron Nanoparticles Significantly Affect the In Vitro and In Vivo Expression of Id Genes. Chem Res Toxicol 2015, 28, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, N.; Akbari, A.; Jamshidbeigi, M.; Falsafi, M. Functionalization of Fe3O4@SiO2 Magnetic Nanoparticles with Nicotinamide and in Vitro DNA Interaction. J Mol Liq 2016, 224, 227–233. [Google Scholar] [CrossRef]
- Robinson, J.M. Reactive Oxygen Species in Phagocytic Leukocytes. Histochem Cell Biol 2008, 130, 281. [Google Scholar] [CrossRef]
- Ndozangue-Touriguine, O.; Hamelin, J.; Bréard, J. Cytoskeleton and Apoptosis. Biochem Pharmacol 2008, 76, 11–18. [Google Scholar] [CrossRef]
- Askri, D.; Cunin, V.; Béal, D.; Berthier, S.; Chovelon, B.; Arnaud, J.; Rachidi, W.; Sakly, M.; Amara, S.; Sève, M.; et al. Investigating the Toxic Effects Induced by Iron Oxide Nanoparticles on Neuroblastoma Cell Line: An Integrative Study Combining Cytotoxic, Genotoxic and Proteomic Tools. Nanotoxicology 2019, 13, 1021–1040. [Google Scholar] [CrossRef]
- Master, A.M.; Williams, P.N.; Pothayee, N.; Pothayee, N.; Zhang, R.; Vishwasrao, H.M.; Golovin, Y.I.; Riffle, J.S.; Sokolsky, M.; Kabanov, A.V. Remote Actuation of Magnetic Nanoparticles For Cancer Cell Selective Treatment Through Cytoskeletal Disruption. Sci Rep 2016, 6, 33560. [Google Scholar] [CrossRef]
- Connord, V.; Clerc, P.; Hallali, N.; El Hajj Diab, D.; Fourmy, D.; Gigoux, V.; Carrey, J. Real-Time Analysis of Magnetic Hyperthermia Experiments on Living Cells under a Confocal Microscope. Small 2015, 11, 2437–2445. [Google Scholar] [CrossRef]
- Zhang, E.; Kircher, M.F.; Koch, M.; Eliasson, L.; Goldberg, S.N.; Renström, E. Dynamic Magnetic Fields Remote-Control Apoptosis via Nanoparticle Rotation. ACS Nano 2014, 8, 3192–3201. [Google Scholar] [CrossRef]
- Shin, E.H.; Li, Y.; Kumar, U.; Sureka, H.V.; Zhang, X.; Payne, C.K. Membrane Potential Mediates the Cellular Binding of Nanoparticles. Nanoscale 2013, 5, 5879–5886. [Google Scholar] [CrossRef]
- Pongrac, I.M.; Dobrivojević, M.; Ahmed, L.B.; Babič, M.; Šlouf, M.; Horák, D.; Gajović, S. Improved Biocompatibility and Efficient Labeling of Neural Stem Cells with Poly(L-Lysine)-Coated Maghemite Nanoparticles. Beilstein J Nanotechnol 2016, 7, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Gonnissen, D.; Qu, Y.; Langer, K.; Öztürk, C.; Zhao, Y.; Chen, C.; Seebohm, G.; Dufer, M.; Fuchs, H.; Galla, H.-J.; et al. Comparison of Cellular Effects of Starch-Coated SPIONs and Poly(Lactic-Co-Glycolic Acid) Matrix Nanoparticles on Human Monocytes. Int J Nanomedicine 2016, Volume 11, 5221–5236. [Google Scholar] [CrossRef]
- Yan, L.; Liu, X.; Liu, W.-X.; Tan, X.-Q.; Xiong, F.; Gu, N.; Hao, W.; Gao, X.; Cao, J.-M. Fe 2 O 3 Nanoparticles Suppress Kv1.3 Channels via Affecting the Redox Activity of Kv β 2 Subunit in Jurkat T Cells. Nanotechnology 2015, 26, 505103. [Google Scholar] [CrossRef] [PubMed]
- Gualdani, R.; Guerrini, A.; Fantechi, E.; Tadini-Buoninsegni, F.; Moncelli, M.R.; Sangregorio, C. Superparamagnetic Iron Oxide Nanoparticles (SPIONs) Modulate hERG Ion Channel Activity. Nanotoxicology 2019, 13, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Shokrollahi, F.; Salehzadeh, A.; Kafilzadeh, F.; Zaefizadeh, M. Evaluation of the Effect of Iron Oxide Nanoparticles Functionalized by Glucose and Conjugated with Coumarin (Fe3O4@Glu-Coumarin NPs) on the Expression of CASP8, CASP9, P53, mTOR1, and MAPK1 Genes in Liver Cancer Cell Line. Gene Reports 2023, 33, 101818. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Wahab, R.; Saquib, Q.; Ahmad, J.; Farshori, N.N.; Al-Sheddi, E.S.; Al-Oqail, M.M.; Al-Massarani, S.M.; Al-Khedhairy, A.A. Iron Oxide Nanoparticles Induced Cytotoxicity, Oxidative Stress, Cell Cycle Arrest, and DNA Damage in Human Umbilical Vein Endothelial Cells. J Trace Elem Med Biol 2023, 127302. [Google Scholar] [CrossRef]
- Hernandes, E.P.; Lazarin-Bidóia, D.; Bini, R.D.; Nakamura, C.V.; Cótica, L.F.; De Oliveira Silva Lautenschlager, S. Doxorubicin-Loaded Iron Oxide Nanoparticles Induce Oxidative Stress and Cell Cycle Arrest in Breast Cancer Cells. Antioxidants 2023, 12, 237. [Google Scholar] [CrossRef]
- Rostami, S.; Tafvizi, F.; Kheiri Manjili, H.R. High Efficacy of Tamoxifen-Loaded L-Lysine Coated Magnetic Iron Oxide Nanoparticles in Cell Cycle Arrest and Anti-Cancer Activity for Breast Cancer Therapy. Bioimpacts 2022, 12, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Majeed, S.; Mohd Rozi, N.A.B.; Danish, M.; Mohamad Ibrahim, M.N.; Joel, E.L. In Vitro Apoptosis and Molecular Response of Engineered Green Iron Oxide Nanoparticles with L-Arginine in MDA-MB-231 Breast Cancer Cells. J Drug Deliv Sci Technol 2023, 80, 104185. [Google Scholar] [CrossRef]
- Mesárošová, M.; Kozics, K.; Bábelová, A.; Regendová, E.; Pastorek, M.; Vnuková, D.; Buliaková, B.; Rázga, F.; Gábelová, A. The Role of Reactive Oxygen Species in the Genotoxicity of Surface-Modified Magnetite Nanoparticles. Toxicology Lett 2014, 226, 303–313. [Google Scholar] [CrossRef]
- Fernández-Bertólez, N.; Costa, C.; Brandão, F.; Duarte, J.A.; Teixeira, J.P.; Pásaro, E.; Valdiglesias, V.; Laffon, B. Evaluation of Cytotoxicity and Genotoxicity Induced by Oleic Acid-coated Iron Oxide Nanoparticles in Human Astrocytes. Environ and Mol Mutagen 2019, 60, 816–829. [Google Scholar] [CrossRef] [PubMed]
- Riedl, S.J.; Shi, Y. Molecular Mechanisms of Caspase Regulation during Apoptosis. Nat Rev Mol Cell Biol 2004, 5, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Fridman, J.S.; Lowe, S.W. Control of Apoptosis by P53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef]
- Grosse, S.; Stenvik, J.; Nilsen, A.M. Iron Oxide Nanoparticles Modulate Lipopolysaccharide-Induced Inflammatory Responses in Primary Human Monocytes. Int J Nanomedicine 2016, 11, 4625–4642. [Google Scholar] [CrossRef]
- Soares, J.-B.; Pimentel-Nunes, P.; Roncon-Albuquerque, R.; Leite-Moreira, A. The Role of Lipopolysaccharide/Toll-like Receptor 4 Signaling in Chronic Liver Diseases. Hepatol Int 2010, 4, 659–672. [Google Scholar] [CrossRef]
- Gerogianni, A.; Bal, M.; Mohlin, C.; Woodruff, T.M.; Lambris, J.D.; Mollnes, T.E.; Sjöström, D.J.; Nilsson, P.H. In Vitro Evaluation of Iron Oxide Nanoparticle-Induced Thromboinflammatory Response Using a Combined Human Whole Blood and Endothelial Cell Model. Front Immunol 2023, 14, 1101387. [Google Scholar] [CrossRef]
- Chauhan, A.; Anjaly, K.; Saini, A.; Kumar, R.; Kuanr, B.K.; Sharma, D. Vitamin K3-Loaded Magnetic Nanoparticle-Mediated Synergistic Magnetothermodynamic Therapy Evokes Massive ROS and Immune Modulation for Augmented Antitumor Potential. ACS Appl Mater Interfaces 2023, 15, 27515–27532. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Comin-Colet, J.; De Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R.; et al. Iron Deficiency across Chronic Inflammatory Conditions: International Expert Opinion on Definition, Diagnosis, and Management. American J Hematol 2017, 92, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Knutson, M.D. Non-Transferrin-Bound Iron Transporters. Free Radic Biol Med 2019, 133, 101–111. [Google Scholar] [CrossRef]
- Scaramellini, N.; Fischer, D.; Agarvas, A.R.; Motta, I.; Muckenthaler, M.U.; Mertens, C. Interpreting Iron Homeostasis in Congenital and Acquired Disorders. Pharmaceuticals 2023, 16, 329. [Google Scholar] [CrossRef]
- Chrishtop, V.V.; Mironov, V.A.; Prilepskii, A.Y.; Nikonorova, V.G.; Vinogradov, V.V. Organ-Specific Toxicity of Magnetic Iron Oxide-Based Nanoparticles. Nanotoxicology 2021, 15, 167–204. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-X.; Tang, W.-L.; Wang, X.-X. Superparamagnetic Iron Oxide Nanoparticles May Affect Endothelial Progenitor Cell Migration Ability and Adhesion Capacity. Cytotherapy 2010, 12, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Mulens-Arias, V.; Rojas, J.M.; Sanz-Ortega, L.; Portilla, Y.; Pérez-Yagüe, S.; Barber, D.F. Polyethylenimine-Coated Superparamagnetic Iron Oxide Nanoparticles Impair in Vitro and in Vivo Angiogenesis. Nanomedicine 2019, 21, 102063. [Google Scholar] [CrossRef]
- Cromer Berman, S.M.; Kshitiz; Wang, C.J.; Orukari, I.; Levchenko, A.; Bulte, J.W.M.; Walczak, P. Cell Motility of Neural Stem Cells Is Reduced after SPIO-Labeling, Which Is Mitigated after Exocytosis: Inhibition of Cell Motility and SPIO Exocytosis. Magn Reson Med 2013, 69, 255–262. [Google Scholar] [CrossRef]
- Rojas, J.M.; Sanz-Ortega, L.; Mulens-Arias, V.; Gutiérrez, L.; Pérez-Yagüe, S.; Barber, D.F. Superparamagnetic Iron Oxide Nanoparticle Uptake Alters M2 Macrophage Phenotype, Iron Metabolism, Migration and Invasion. Nanomedicine 2016, 12, 1127–1138. [Google Scholar] [CrossRef]
- Mohsin, A.; Hussain, M.H.; Mohsin, M.Z.; Zaman, W.Q.; Aslam, M.S.; Shan, A.; Dai, Y.; Khan, I.M.; Niazi, S.; Zhuang, Y.; et al. Recent Advances of Magnetic Nanomaterials for Bioimaging, Drug Delivery, and Cell Therapy. ACS Appl Nano Mater 2022, 5, 10118–10136. [Google Scholar] [CrossRef]
- Petters, C.; Irrsack, E.; Koch, M.; Dringen, R. Uptake and Metabolism of Iron Oxide Nanoparticles in Brain Cells. Neurochem Res 2014, 39, 1648–1660. [Google Scholar] [CrossRef] [PubMed]
- D’Agata, F.; Ruffinatti, F.; Boschi, S.; Stura, I.; Rainero, I.; Abollino, O.; Cavalli, R.; Guiot, C. Magnetic Nanoparticles in the Central Nervous System: Targeting Principles, Applications and Safety Issues. Molecules 2017, 23, 9. [Google Scholar] [CrossRef]
- Thomsen, L.B.; Linemann, T.; Pondman, K.M.; Lichota, J.; Kim, K.S.; Pieters, R.J.; Visser, G.M.; Moos, T. Uptake and Transport of Superparamagnetic Iron Oxide Nanoparticles through Human Brain Capillary Endothelial Cells. ACS Chem Neurosci 2013, 4, 1352–1360. [Google Scholar] [CrossRef]
- Shin, T.H.; Lee, G. Reduced Lysosomal Activity and Increased Amyloid Beta Accumulation in Silica-Coated Magnetic Nanoparticles-Treated Microglia. Arch Toxicol 2024, 98, 121–134. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Aly, E.M.; Mohamed, F.F.; Noor, N.A.; Elsayed, A.A. Neurotoxicity of Green- Synthesized Magnetic Iron Oxide Nanoparticles in Different Brain Areas of Wistar Rats. NeuroToxicology 2020, 77, 80–93. [Google Scholar] [CrossRef]
- Kim, Y.; Kong, S.D.; Chen, L.-H.; Pisanic, T.R.; Jin, S.; Shubayev, V.I. In Vivo Nanoneurotoxicity Screening Using Oxidative Stress and Neuroinflammation Paradigms. Nanomedicine 2013, 9, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yoon, T.-J.; Yu, K.N.; Kim, B.G.; Park, S.J.; Kim, H.W.; Lee, K.H.; Park, S.B.; Lee, J.-K.; Cho, M.H. Toxicity and Tissue Distribution of Magnetic Nanoparticles in Mice. Toxicol Sci 2006, 89, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim Fouad, G.; El-Sayed, S.A.M.; Mabrouk, M.; Ahmed, K.A.; Beherei, H.H. Neuroprotective Potential of Intranasally Delivered Sulforaphane-Loaded Iron Oxide Nanoparticles Against Cisplatin-Induced Neurotoxicity. Neurotox Res 2022, 40, 1479–1498. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Gong, S.; Li, J.; Wang, Y.; Zhang, X.; Zheng, H.; Zhang, Q.; You, J.; Huang, Z.; Chen, Y. Co-Loading Antioxidant N-Acetylcysteine Attenuates Cytotoxicity of Iron Oxide Nanoparticles in Hypoxia/Reoxygenation Cardiomyocytes. Int J Nanomedicine 2019, 14, 6103–6115. [Google Scholar] [CrossRef]
- Kumfu, S.; Khamseekaew, J.; Palee, S.; Srichairatanakool, S.; Fucharoen, S.; Chattipakorn, S.C.; Chattipakorn, N. A Combination of an Iron Chelator with an Antioxidant Exerts Greater Efficacy on Cardioprotection than Monotherapy in Iron-Overload Thalassemic Mice. Free Radic Res 2018, 52, 70–79. [Google Scholar] [CrossRef]
- Wongjaikam, S.; Kumfu, S.; Khamseekaew, J.; Sripetchwandee, J.; Srichairatanakool, S.; Fucharoen, S.; Chattipakorn, S.C.; Chattipakorn, N. Combined Iron Chelator and Antioxidant Exerted Greater Efficacy on Cardioprotection Than Monotherapy in Iron-Overloaded Rats. PLoS ONE 2016, 11, e0159414. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Hofmann, H.; Rothen-Rutishauser, B.; Petri-Fink, A. Assessing the In Vitro and In Vivo Toxicity of Superparamagnetic Iron Oxide Nanoparticles. Chem Rev 2012, 112, 2323–2338. [Google Scholar] [CrossRef]
- Iversen, N.K.; Frische, S.; Thomsen, K.; Laustsen, C.; Pedersen, M.; Hansen, P.B.L.; Bie, P.; Fresnais, J.; Berret, J.-F.; Baatrup, E.; et al. Superparamagnetic Iron Oxide Polyacrylic Acid Coated γ-Fe2O3 Nanoparticles Do Not Affect Kidney Function but Cause Acute Effect on the Cardiovascular Function in Healthy Mice. Toxicol Appl Pharmacol 2013, 266, 276–288. [Google Scholar] [CrossRef]
- Edge, D.; Shortt, C.M.; Gobbo, O.L.; Teughels, S.; Prina-Mello, A.; Volkov, Y.; MacEneaney, P.; Radomski, M.W.; Markos, F. Pharmacokinetics and Bio-Distribution of Novel Super Paramagnetic Iron Oxide Nanoparticles (SPIONs) in the Anaesthetized Pig. Clin Exp Pharmacol Physiol 2016, 43, 319–326. [Google Scholar] [CrossRef]
- Manickam, V.; Periyasamy, M.; Dhakshinamoorthy, V.; Panneerselvam, L.; Perumal, E. Recurrent Exposure to Ferric Oxide Nanoparticles Alters Myocardial Oxidative Stress, Apoptosis and Necrotic Markers in Male Mice. Chem-Biol Interact 2017, 278, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Tariq, S.; Attoub, S.; Ali, B.H. Ultrasmall Superparamagnetic Iron Oxide Nanoparticles Acutely Promote Thrombosis and Cardiac Oxidative Stress and DNA Damage in Mice. Part Fibre Toxicol 2015, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Shetake, N.G.; Ali, M.; Kumar, A.; Bellare, J.; Pandey, B.N. Theranostic Magnetic Nanoparticles Enhance DNA Damage and Mitigate Doxorubicin-Induced Cardio-Toxicity for Effective Multi-Modal Tumor Therapy. Biomater Adv 2022, 142, 213147. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Prasad, S.V.N.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of Doxorubicin Is Mediated through Mitochondrial Iron Accumulation. J Clin Invest 2014, 124, 617–630. [Google Scholar] [CrossRef]
- Jain, D. Cardiotoxicity of Doxorubicin and Other Anthracycline Derivatives. J Nucl Cardiol 2000, 7, 53–62. [Google Scholar] [CrossRef]
- Namdari, M.; Eatemadi, A. Cardioprotective Effects of Curcumin-Loaded Magnetic Hydrogel Nanocomposite (Nanocurcumin) against Doxorubicin-Induced Cardiac Toxicity in Rat Cardiomyocyte Cell Lines. Artif Cells Nanomed Biotechnol 2017, 45, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Q.; Peng, J.; Su, J.; Lu, X.; Zhao, Y.; Qian, Z. Doxorubicin-Conjugated Heparin-Coated Superparamagnetic Iron Oxide Nanoparticles for Combined Anticancer Drug Delivery and Magnetic Resonance Imaging. J Biomed Nanotechnol 2016, 12, 1963–1974. [Google Scholar] [CrossRef]
- Xiong, F.; Wang, H.; Feng, Y.; Li, Y.; Hua, X.; Pang, X.; Zhang, S.; Song, L.; Zhang, Y.; Gu, N. Cardioprotective Activity of Iron Oxide Nanoparticles. Sci Rep 2015, 5, 8579. [Google Scholar] [CrossRef]
- Nowak-Jary, J.; Machnicka, B. In Vivo Biodistribution and Clearance of Magnetic Iron Oxide Nanoparticles for Medical Applications. Int J Nanomedicine 2023, Volume 18, 4067–4100. [Google Scholar] [CrossRef]
- Wolf, P.L. Biochemical Diagnosis of Liver Disease. Indian J Clin Biochem 1999, 14, 59–90. [Google Scholar] [CrossRef]
- Askri, D.; Ouni, S.; Galai, S.; Arnaud, J.; Chovelon, B.; Lehmann, S.G.; Sturm, N.; Sakly, M.; Sève, M.; Amara, S. Intranasal Instillation of Iron Oxide Nanoparticles Induces Inflammation and Perturbation of Trace Elements and Neurotransmitters, but Not Behavioral Impairment in Rats. Environ Sci Pollut Res 2018, 25, 16922–16932. [Google Scholar] [CrossRef] [PubMed]
- Kazemipour, N.; Nazifi, S.; Poor, M.H.H.; Esmailnezhad, Z.; Najafabadi, R.E.; Esmaeili, A. Hepatotoxicity and Nephrotoxicity of Quercetin, Iron Oxide Nanoparticles, and Quercetin Conjugated with Nanoparticles in Rats. Comp Clin Pathol 2018, 27, 1621–1628. [Google Scholar] [CrossRef]
- Salimi, M.; Sarkar, S.; Fathi, S.; Alizadeh, A.; Saber, R.; Moradi, F.; Delavari, H. Biodistribution, Pharmacokinetics, and Toxicity of Dendrimer-Coated Iron Oxide Nanoparticles in BALB/c Mice. Int J Nanomedicine 2018, 13, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Yaremenko, A.V.; Zelepukin, I.V.; Ivanov, I.N.; Melikov, R.O.; Pechnikova, N.A.; Dzhalilova, D.Sh.; Mirkasymov, A.B.; Bragina, V.A.; Nikitin, M.P.; Deyev, S.M.; et al. Influence of Magnetic Nanoparticle Biotransformation on Contrasting Efficiency and Iron Metabolism. J Nanobiotechnol 2022, 20, 535. [Google Scholar] [CrossRef] [PubMed]
- Paulini, F.; Marangon, A.R.M.; Azevedo, C.L.; Brito, J.L.M.; Lemos, M.S.; Sousa, M.H.; Veiga-Souza, F.H.; Souza, P.E.N.; Lucci, C.M.; Azevedo, R.B. In Vivo Evaluation of DMSA-Coated Magnetic Nanoparticle Toxicity and Biodistribution in Rats: A Long-Term Follow-Up. Nanomaterials 2022, 12, 3513. [Google Scholar] [CrossRef]
- Fakhri, Z.; Karimi, N.; Saba, F.; Zhaleh, M. Biocompatibility of Magnetic Nanoparticles Synthesized through Green Routed with a Focus on Hematological and Histological Analysis. Bioorg Chem 2023, 137, 106552. [Google Scholar] [CrossRef]
- Rahman, M. Magnetic Resonance Imaging and Iron-Oxide Nanoparticles in the Era of Personalized Medicine. Nanotheranostics 2023, 7, 424–449. [Google Scholar] [CrossRef]
- Zhou, K.; Li, Z.-Z.; Cai, Z.-M.; Zhong, N.-N.; Cao, L.-M.; Huo, F.-Y.; Liu, B.; Wu, Q.-J.; Bu, L.-L. Nanotheranostics in Cancer Lymph Node Metastasis: The Long Road Ahead. Pharmacol Res 2023, 198, 106989. [Google Scholar] [CrossRef]
- Vu-Quang, H.; Yoo, M.-K.; Jeong, H.-J.; Lee, H.-J.; Muthiah, M.; Rhee, J.H.; Lee, J.-H.; Cho, C.-S.; Jeong, Y.Y.; Park, I.-K. Targeted Delivery of Mannan-Coated Superparamagnetic Iron Oxide Nanoparticles to Antigen-Presenting Cells for Magnetic Resonance-Based Diagnosis of Metastatic Lymph Nodes in Vivo. Acta Biomater 2011, 7, 3935–3945. [Google Scholar] [CrossRef]
- Sekino, M.; Kuwahata, A.; Ookubo, T.; Shiozawa, M.; Ohashi, K.; Kaneko, M.; Saito, I.; Inoue, Y.; Ohsaki, H.; Takei, H.; et al. Handheld Magnetic Probe with Permanent Magnet and Hall Sensor for Identifying Sentinel Lymph Nodes in Breast Cancer Patients. Sci Rep 2018, 8, 1195. [Google Scholar] [CrossRef]
- Hu, H.; Fu, G.; Ding, Z.; Hu, Y.; Luo, G.; Yin, Z. Polyacrylic Acid-Modified Superparamagnetic Iron Oxide Nanoparticles Differentiate Between Hyperplastic and Metastatic Breast Cancer Lymph Nodes. J Biomed Nanotechnol 2023, 19, 2085–2092. [Google Scholar] [CrossRef]
- Kubovcikova, M.; Sobotova, R.; Zavisova, V.; Antal, I.; Khmara, I.; Lisnichuk, M.; Bednarikova, Z.; Jurikova, A.; Strbak, O.; Vojtova, J.; et al. N-Acetylcysteine-Loaded Magnetic Nanoparticles for Magnetic Resonance Imaging. Int J Mol Sci 2023, 24, 11414. [Google Scholar] [CrossRef] [PubMed]
- Tenório, M.C.D.S.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.D.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Moacă, E.-A.; Watz, C.; Faur, A.-C.; Lazăr, D.; Socoliuc, V.; Păcurariu, C.; Ianoș, R.; Rus, C.-I.; Minda, D.; Barbu-Tudoran, L.; et al. Biologic Impact of Green Synthetized Magnetic Iron Oxide Nanoparticles on Two Different Lung Tumorigenic Monolayers and a 3D Normal Bronchial Model—EpiAirwayTM Microtissue. Pharmaceutics 2022, 15, 2. [Google Scholar] [CrossRef]
- Sadhukha, T.; Wiedmann, T.S.; Panyam, J. Inhalable Magnetic Nanoparticles for Targeted Hyperthermia in Lung Cancer Therapy. Biomaterials 2013, 34, 5163–5171. [Google Scholar] [CrossRef]
- Abdelaziz, M.M.; Hefnawy, A.; Anter, A.; Abdellatif, M.M.; Khalil, M.A.F.; Khalil, I.A. Respirable Spray Dried Vancomycin Coated Magnetic Nanoparticles for Localized Lung Delivery. Int J Pharm 2022, 611, 121318. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Morais, P.C.; Bentes De Azevedo, R.; Lacava, Z.G.M.; Villanueva, A.; Del Puerto Morales, M. Magnetic Nanoparticles Coated with Dimercaptosuccinic Acid: Development, Characterization, and Application in Biomedicine. J Nanopart Res 2014, 16, 2589. [Google Scholar] [CrossRef]
- Chaves, S.B.; Silva, L.P.; Lacava, Z.G.M.; Morais, P.C.; Azevedo, R.B. Interleukin-1 and Interleukin-6 Production in Mice’s Lungs Induced by 2, 3 Meso-Dimercaptosuccinic-Coated Magnetic Nanoparticles. J Appl Phys 2005, 97, 10Q915. [Google Scholar] [CrossRef]
- Zhang, Q.; Yin, R.; Guan, G.; Liu, H.; Song, G. Renal Clearable Magnetic Nanoparticles for Magnetic Resonance Imaging and Guided Therapy. WIREs Nanomed Nanobiotechnol 2024, 16, e1929. [Google Scholar] [CrossRef]
- Choi, H.S.; Ipe, B.I.; Misra, P.; Lee, J.H.; Bawendi, M.G.; Frangioni, J.V. Tissue- and Organ-Selective Biodistribution of NIR Fluorescent Quantum Dots. Nano Lett 2009, 9, 2354–2359. [Google Scholar] [CrossRef]
- Zhou, H.; Ge, J.; Miao, Q.; Zhu, R.; Wen, L.; Zeng, J.; Gao, M. Biodegradable Inorganic Nanoparticles for Cancer Theranostics: Insights into the Degradation Behavior. Bioconjugate Chem 2020, 31, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Bruns, O.T.; Kaul, M.G.; Hansen, E.C.; Barch, M.; Wiśniowska, A.; Chen, O.; Chen, Y.; Li, N.; Okada, S.; et al. Exceedingly Small Iron Oxide Nanoparticles as Positive MRI Contrast Agents. Proc Natl Acad Sci USA 2017, 114, 2325–2330. [Google Scholar] [CrossRef]
- Liu, W.; Deng, G.; Wang, D.; Chen, M.; Zhou, Z.; Yang, H.; Yang, S. Renal-Clearable Zwitterionic Conjugated Hollow Ultrasmall Fe 3 O 4 Nanoparticles for T 1 -Weighted MR Imaging in Vivo. J Mater Chem B 2020, 8, 3087–3091. [Google Scholar] [CrossRef]
- Zhou, T.; Dong, Y.; Wang, X.; Liu, R.; Cheng, R.; Pan, J.; Zhang, X.; Sun, S. Highly Sensitive Early Diagnosis of Kidney Damage Using Renal Clearable Zwitterion-Coated Ferrite Nanoprobe via Magnetic Resonance Imaging In Vivo. Adv Healthcare Materials 2024, 2304577. [Google Scholar] [CrossRef]
- Al Alalaq, M.A.; Al–Hadedee, L.T.; Alrubeii, A.M.S. Effect of Iron Oxide Nanoparticles Prepared by Chemical Method on the Kidneys, Liver and Brain of Male Mice. IOP Conf Se.: Earth Environ Sci 2023, 1252, 012132. [Google Scholar] [CrossRef]
- Nadia Salem Alrawaiq; Azman Abdullah A Review of Flavonoid Quercetin: Metabolism, Bioactivity and Antioxidant Properties. Int J PharmTech Res 2014, 6, 933–941.
- Attia, H.R.; Thalij, K.M. Determination of the Effect of Oral Dosage of Labna Product Supplemented with Fe 3 O 4 Conjugated with Chitosan Nanoparticles on Growth Parameters, Liver and Kidney Parameters in Anaemic Rats Induced by Phenylhydrazine. IOP Conf Ser: Earth Environ Sci 2023, 1262, 062049. [Google Scholar] [CrossRef]
- Odhiambo, J.F.; DeJarnette, J.M.; Geary, T.W.; Kennedy, C.E.; Suarez, S.S.; Sutovsky, M.; Sutovsky, P. Increased Conception Rates in Beef Cattle Inseminated with Nanopurified Bull Semen1. Biol Reprod 2014, 91. [Google Scholar] [CrossRef] [PubMed]
- Feugang, J.M.; Rhoads, C.E.; Mustapha, P.A.; Tardif, S.; Parrish, J.J.; Willard, S.T.; Ryan, P.L. Treatment of Boar Sperm with Nanoparticles for Improved Fertility. Theriogenology 2019, 137, 75–81. [Google Scholar] [CrossRef]
- Rateb, S.A. Purification of Cryopreserved Camel Spermatozoa Following Protease-based Semen Liquefaction by Lectin-functionalized DNA-defrag Magnetic Nanoparticles. Reprod Domestic Animals 2021, 56, 183–192. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H.J.; Doo, S.H.; Yang, W.J.; Choi, D.; Kim, J.H.; Won, J.H.; Song, Y.S. Use of Nanoparticles to Monitor Human Mesenchymal Stem Cells Transplanted into Penile Cavernosum of Rats with Erectile Dysfunction. Korean J Urol 2015, 56, 280. [Google Scholar] [CrossRef] [PubMed]
- Sundarraj, K.; Manickam, V.; Raghunath, A.; Periyasamy, M.; Viswanathan, M.P.; Perumal, E. Repeated Exposure to Iron Oxide Nanoparticles Causes Testicular Toxicity in Mice. Environ Toxicol 2017, 32, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Noori A; Parivar K; Modaresi M; Messripour M; Yousefi MH; Amiri GR Effect of Magnetic Iron Oxide Nanoparticles on Pregnancy and Testicular Development of Mice. Afr J Biotechnol 2011, 10, 1221–1227. [CrossRef]
- Al-Shammari, M.S.; Al-Saaidi, J.A. Influence of Magnetic Iron Oxide Nanoparticles in Reproductive Efficiency of Adult Male Rats. Int J Vet Sci 2023, 37, 507–513. [Google Scholar] [CrossRef]
- Zhao, Y.; Ng, K.W. Nanotoxicology in the Skin: How Deep Is the Issue? Nano LIFE 2014, 04, 1440004. [Google Scholar] [CrossRef]
- Silva, S.A.M.E.; Michniak-Kohn, B.; Leonardi, G.R. An Overview about Oxidation in Clinical Practice of Skin Aging. An Bras Dermatol 2017, 92, 367–374. [Google Scholar] [CrossRef]
- Amin, R.M.; Abdelmonem, A.; Verwanger, T.; Elsherbini, E.; Krammer, B. Cytotoxicity of Magnetic Nanoparticles on Normal and Malignant Human Skin Cells. Nano LIFE 2014, 04, 1440002. [Google Scholar] [CrossRef]
- Duval, K.E.A.; Vernice, N.A.; Wagner, R.J.; Fiering, S.N.; Petryk, J.D.; Lowry, G.J.; Tau, S.S.; Yin, J.; Houde, G.R.; Chaudhry, A.S.; et al. Immunogenetic Effects of Low Dose (CEM43 30) Magnetic Nanoparticle Hyperthermia and Radiation in Melanoma Cells. Int J Hyperther 2019, 36, 37–46. [Google Scholar] [CrossRef]
- Amatya, R.; Kim, D.; Min, K.A.; Shin, M.C. Iron Oxide Nanoparticles-Loaded Hydrogels for Effective Topical Photothermal Treatment of Skin Cancer. J Pharm Investig 2022, 52, 775–785. [Google Scholar] [CrossRef]
- Buchman, J.T.; Hudson-Smith, N.V.; Landy, K.M.; Haynes, C.L. Understanding Nanoparticle Toxicity Mechanisms To Inform Redesign Strategies To Reduce Environmental Impact. Ac. Chem Res 2019, 52, 1632–1642. [Google Scholar] [CrossRef]
- Nowak-Jary J, Płóciennik A, Machnicka B. Functionalized Magnetic Fe3O4 Nanoparticles for Targeted Methotrexate Delivery in Ovarian Cancer Therapy. Int J Mol Sci 2024, 25, 9098. [CrossRef]
- Misra, S.K.; Dybowska, A.; Berhanu, D.; Luoma, S.N.; Valsami-Jones, E. The Complexity of Nanoparticle Dissolution and Its Importance in Nanotoxicological Studies. Sci Total Environ 2012, 438, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ucar, A.; Parlak, V.; Ozgeris, F.B.; Yeltekin, A.C.; Arslan, M.E.; Alak, G.; Turkez, H.; Kocaman, E.M.; Atamanalp, M. Magnetic Nanoparticles-Induced Neurotoxicity and Oxidative Stress in Brain of Rainbow Trout: Mitigation by Ulexite through Modulation of Antioxidant, Anti-Inflammatory, and Antiapoptotic Activities. Sci Total Environ 2022, 838, 155718. [Google Scholar] [CrossRef] [PubMed]
- Bardestani, A.; Ebrahimpour, S.; Esmaeili, A.; Esmaeili, A. Quercetin Attenuates Neurotoxicity Induced by Iron Oxide Nanoparticles. J Nanobiotechnol 2021, 19, 327. [Google Scholar] [CrossRef]
- Durdík, Š.; Vrbovská, H.; Olas, A.; Babincová, M. Influence of Naturally Occurring Antioxidants on Magnetic Nanoparticles: Risks, Benefits, and Possible Therapeutic Applications. Gen Physiol Biophys 2013, 32, 173–177. [Google Scholar] [CrossRef]
- Chen, L.; Wu, L.; Liu, F.; Qi, X.; Ge, Y.; Shen, S. Azo-Functionalized Fe 3 O 4 Nanoparticles: A near-infrared Light Triggered Drug Delivery System for Combined Therapy of Cancer with Low Toxicity. J Mater Chem B 2016, 4, 3660–3669. [Google Scholar] [CrossRef]
- Hohnholt, M.C.; Dringen, R. Iron-Dependent Formation of Reactive Oxygen Species and Glutathione Depletion after Accumulation of Magnetic Iron Oxide Nanoparticles by Oligodendroglial Cells. J Nanopart Res 2011, 13, 6761–6774. [Google Scholar] [CrossRef]
- Yang.; Kuang, H.; Zhang, W.; Aguilar, Z.P.; Xiong, Y.; Lai, W.; Xu, H.; Wei, H. Size Dependent Biodistribution and Toxicokinetics of Iron Oxide Magnetic Nanoparticles in Mice. Nanoscale 2015, 7, 625–636. [CrossRef] [PubMed]
- Wang, Q.; Shen, M.; Zhao, T.; Xu, Y.; Lin, J.; Duan, Y.; Gu, H. Low Toxicity and Long Circulation Time of Polyampholyte-Coated Magnetic Nanoparticles for Blood Pool Contrast Agents. Sci Rep 2015, 5, 7774. [Google Scholar] [CrossRef]
- Agotegaray, M.; Campelo, A.; Zysler, R.; Gumilar, F.; Bras, C.; Minetti, A.; Massheimer, V.; Lassalle, V. Influence of Chitosan Coating on Magnetic Nanoparticles in Endothelial Cells and Acute Tissue Biodistribution. J Biomater Sci Polym Ed 2016, 27, 1069–1085. [Google Scholar] [CrossRef]
- Bhandari, R.; Gupta, P.; Dziubla, T.; Hilt, J.Z. Single Step Synthesis, Characterization and Applications of Curcumin Functionalized Iron Oxide Magnetic Nanoparticles. Mater Sci Eng C Mater Biol Appl 2016, 67, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Iacovita, C.; Florea, A.; Dudric, R.; Pall, E.; Moldovan, A.; Tetean, R.; Stiufiuc, R.; Lucaciu, C. Small versus Large Iron Oxide Magnetic Nanoparticles: Hyperthermia and Cell Uptake Properties. Molecules 2016, 21, 1357. [Google Scholar] [CrossRef] [PubMed]
- Jarockyte, G.; Dangelaite, E.; Stasys, M.; Statkute, U.; Poderys, V.; Tseng, T.-C.; Hsu, S.-H.; Karabanovas, V.; Rotomskis, R. Accumulation and Toxicity of Superparamagnetic Iron Oxide Nanoparticles in Cells and Experimental Animals. Int J Mol Sci 2016, 17, 1193. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Miao, Y.; Chen, Z.; Qiang, P.; Cui, L.; Jing, H.; Guo, Y. Magnetic Ferroferric Oxide Nanoparticles Induce Vascular Endothelial Cell Dysfunction and Inflammation by Disturbing Autophagy. J Hazard Mater 2016, 304, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Agotegaray, M.A.; Campelo, A.E.; Zysler, R.D.; Gumilar, F.; Bras, C.; Gandini, A.; Minetti, A.; Massheimer, V.L.; Lassalle, V.L. Magnetic Nanoparticles for Drug Targeting: From Design to Insights into Systemic Toxicity. Preclinical Evaluation of Hematological, Vascular and Neurobehavioral Toxicology. Biomater Sci 2017, 5, 772–783. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, Distribution, Clearance, and Toxicity of Iron Oxide Nanoparticles with Different Sizes and Coatings. Sci Rep 2018, 8, 2082. [Google Scholar] [CrossRef]
- Marimon-Bolívar, W.; Tejeda-Benítez, L.P.; Núñez-Avilés, C.A.; De Léon-Pérez, D.D. Evaluation of the in Vivo Toxicity of Green Magnetic Nanoparticles Using Caenorhabditis Elegans as a Biological Model. Environ Nanotechnol Monit Manag 2019, 12, 100253. [Google Scholar] [CrossRef]
- Ayubi, M.; Karimi, M.; Abdpour, S.; Rostamizadeh, K.; Parsa, M.; Zamani, M.; Saedi, A. Magnetic Nanoparticles Decorated with PEGylated Curcumin as Dual Targeted Drug Delivery: Synthesis, Toxicity and Biocompatibility Study. Mater Sci Eng C 2019, 104, 109810. [Google Scholar] [CrossRef] [PubMed]
- Patsula, V.; Tulinska, J.; Trachtová, Š.; Kuricova, M.; Liskova, A.; Španová, A.; Ciampor, F.; Vavra, I.; Rittich Ursinyova, M.; Dusinska, M.; Ilavska, S.; Horvathova, M.; Masanova, V.; Uhnakova, I.; Horák, D. Toxicity Evaluation of Monodisperse PEGylated Magnetic Nanoparticles for Nanomedicine. Nanotoxicology 2019, 13, 510–526. [Google Scholar] [CrossRef]
- Caro, C.; Egea-Benavente, D.; Polvillo, R.; Royo, J.L.; Pernia Leal, M.; García-Martín, M.L. Comprehensive Toxicity Assessment of PEGylated Magnetic Nanoparticles for in Vivo Applications. Colloid Surface B 2019, 177, 253–259. [Google Scholar] [CrossRef]
- Nosrati, H.; Salehiabar, M.; Fridoni, M.; Abdollahifar, M.-A.; Kheiri Manjili, H.; Davaran, S.; Danafar, H. New Insight about Biocompatibility and Biodegradability of Iron Oxide Magnetic Nanoparticles: Stereological and In Vivo MRI Monitor. Sci Rep 2019, 9, 7173. [Google Scholar] [CrossRef] [PubMed]
- Farid, R.M.; Gaafar, P.M.E.; Hazzah, H.A.; Helmy, M.W.; Abdallah, O.Y. Chemotherapeutic Potential of L-Carnosine from Stimuli-Responsive Magnetic Nanoparticles against Breast Cancer Model. Nanomedicine 2020, 15, 891–911. [Google Scholar] [CrossRef] [PubMed]
- Rozhina, E.; Danilushkina, A.; Akhatova, F.; Fakhrullin, R.; Rozhin, A.; Batasheva, S. Biocompatibility of Magnetic Nanoparticles Coating with Polycations Using A549 Cells. J Biotechnology 2021, 325, 25–34. [Google Scholar] [CrossRef]
- Chandekar, K.V.; Shkir Mohd Alshahrani, T.; Ibrahim, E.H.; Kilany, M.; Ahmad, Z.; Manthrammel, M.A.; Al Faify, S.; Kateb, B.; Kaushik, A. One-Spot Fabrication and in-Vivo Toxicity Evaluation of Core-Shell Magnetic Nanoparticles. Mater Sci Eng C 2021, 122, 111898. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, J.; Jia, H.; Guo, X.; Yue, Y.; Yuan, Y.; Yue, T. Synthesis of Silver/Fe3O4@chitosan@polyvinyl Alcohol Magnetic Nanoparticles as an Antibacterial Agent for Accelerating Wound Healing. Int J Biol Macromol 2022, 221, 1404–1414. [Google Scholar] [CrossRef]
- Obireddy, S.R.; Lai, W.-F. ROS-Generating Amine-Functionalized Magnetic Nanoparticles Coupled with Carboxymethyl Chitosan for pH-Responsive Release of Doxorubicin. Int J Nanomedicine 2022, 17, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, X.; Lu, J.; Yin, C.; Li, G.; Bai, L.; Zhang, T.; Mo, J.; Wang, X.; Shi, Q.; Wang, T.; Zhou, Q. Development of ε-Poly(L-Lysine) Carbon Dots-Modified Magnetic Nanoparticles and Their Applications as Novel Antibacterial Agents. Front Chem 2023, 11, 1184592. [Google Scholar] [CrossRef]
- Do, X.-H.; Nguyen, T.D.; Le, T.T.H.; To, T.T.; Bui, T.V.K.; Pham, N.H.; Lam, K.; Hoang, T.M.N.; Ha, P.T. High Biocompatibility, MRI Enhancement, and Dual Chemo- and Thermal-Therapy of Curcumin-Encapsulated Alginate/Fe3O4 Nanoparticles. Pharmaceutics 2023, 15, 1523. [Google Scholar] [CrossRef]
| Size (TEM/DH) | Coating | Model | Dose | Significant methods for toxicity assessing | Toxicity | Ref |
|---|---|---|---|---|---|---|
| 212 nm/- | DOX1- 4, 4’-Azobis (4 - cyanovaleric acid) |
ICR mice | 200 μl of solution of free DOX and DOX-loaded MNPs at a dose normalized to be 3 mg/kg DOX equiv. | Histology | MNPs-Azo-DOX did not produce histopathological signs of cardiotoxicity like that observed for free DOX. |
[167] |
| -/50 nm | Rhodamine B isothiocyanate (RITC) within a silica shell | ICR mice | 100, 50, 25 mg/kg | Blood biochemistry, hematology and histology Neurotoxicity assays |
No significant changes were observed in the histological, hematological, and biochemistry tests. MNPs could penetrate the BBB without altering its function. |
[103] |
| 12 nm/- 26 nm/- |
Bare Silica-APTES2 |
HeLa and A549 cells | 0.5, 1, 2.5, 5 nM | WST-8 assay DCF fluorescence as a reporter of ROS generation |
Bare MNPs showed a substantial viability reduction at high concentrations (2.5, 5 nM) in both cell lines, whereas coated MNPs showed no sign of toxicity. A significant ROS generation was observed in cells treated with bare MNPs. Coated MNPs induced low levels of ROS. |
[17] |
| -/10 nm -/100 nm -/150 nm |
Bare APTMS3 TEOS4/APTMS |
HDFs and HT-1080 cells | 200–1000 μg/ml |
Cell Counting Kit-8 assay Comet assay |
In HDFs treated with increasing concentrations of each MNPs in a dose-dependent manner, a slight toxicity was observed. MNPs modified with APTMS resulted in significant dose-dependent genotoxicity against normal cells. Bare and TEOS/APTMS-coated MNPs resulted in neither extensive nor dose-dependent damage to the DNA stability in both cells. |
[20] |
| -/60 nm | DMSA5 | OLN-93 cells | 0.25, 1, 4 mM | LDH assay Staining with PI, H33342, and rhodamine 123 dyes |
No significant increase in the extracellular activity of the enzyme LDH was observed. Cultures incubated with 0.25 or 1 mM MNPs hardly contained any PI-positive cells despite the presence of many cells which was demonstrated by H33342 staining. Exposure to 0.25 mM MNPs did not increase ROS production, while many rhodamine 123-positive cells were present in cultures exposed to 1 or 4 mM MNPs. |
[168] |
| 6 nm/- 8 nm/- |
Bare Chitosan |
HeLa, A549 and HeK293 cells | 0.5, 2, 4 μg/μl | MTT assay and AO/EB staining | The toxic effect of chitosan-MNPs on A549 and HeLa cells was moderate compared to bare MNPs treatment, and this toxicity was found to be time—and dose-dependent. In the case of Hek293 cells, bare MNPs led to toxic effects, whereas chitosan-coated MNPs did not cause any significant toxicity. Chitosan-MNPs caused less apoptosis in healthy and cancer cell lines than bare MNPs. | [18] |
| 10, 20, 30, 40 nm/14, 25, 34, 43 nm | Oleic acid | Kunming mice | 20 mg/kg | Blood biochemistry |
The critical hepatic indicators were not significantly altered independent of the sizes of MNPs treated compared with the control. The kidney function indicators exhibited levels similar to those of the control group. | [169] |
| 4.5 nm/2-8 nm | PAA6-co-3- DEAPA7 |
HUVEC cells Mice |
Not available 0.2 ml of 0.138 mM solution |
MTT assay Histology |
No toxicity was observed. No abnormal changes were observed. |
[170] |
| 10 nm/ 369 nm (N1) 10 nm/ 238 nm (N2) |
Oleic acid-chitosan (N1) Oleic acid-chitosan and glutaraldehyde as cross-linker (N2) |
ECs cultures from Wistar rats Mice |
1, 10, 100 μg/ml 30 mg/kg |
MTT assay Measurement of NO production |
ECs treated with N1 nanoparticles for 6–24 h compared to control cells showed maximal cell viability. In contrast, a significant reduction in cell viability was evidenced in the treatment with the highest dose (100 μg/ml) after 36 h. The treatment with N2 MNPs did not affect cell viability in the whole range of doses and times explored. Endothelial NO production was not affected by the exposure to N1 or N2. |
[171] |
| 9.9 nm/ 406 nm |
Curcumin | HUVEC cells | 200 µl of medium at concentrations 1-1000 μg/ml | A calcein AM red-orange viability assay | Curcumin-coated MNPs showed less cell death relative to uncoated MNPs at variable concentrations. |
[172] |
| 34 nm/ 325 nm 270 nm/ 1100 nm |
PEG8 2000 Ethylene glycol |
D407, A548, MV35 and B12F10 cells | 0.05–0.2 mg/ml | MTT assay | No significant toxicity was observed. |
[173] |
| 10-50 nm (AFM) / 50 nm |
Bare | Mouse embryonic fibroblasts NIH3T3 | 32.5 ng/ml | XTT assay | No significant toxicity was observed. |
[174] |
| 10-20 nm/40-160 nm | Dextran | L929 fibroblast Albino rats (Wistar), Albino guinea pigs (Hartley), and Albino mice (Swiss) |
100- 800μg/ml 300-2000 mg/kg |
MTT assay Blood biochemistry and hematology Lymphocyte proliferation assay Detection of 8-OHdG by ELISA |
No proliferation inhibition in the whole range of MNPs concentrations was observed. The biochemical and hematological assessments following oral administration of MNPs were not significantly different from those in the control group. Seven days after exposure, a slight increase in cell number was observed in both T and B lymphocytes compared to the control. After 14 days and 21 days, the proliferation of T and B cells was reduced compared to day 0. The levels of 8-OHdG in mitochondrial DNA of MNPs exposed groups were comparable with that of control values. MNPs did not significantly affect the chromosome aberration frequencies in bone marrow cells or cell mitotic indices. |
[41] |
| 8,17,24 nm/32.2, 51.8, 84.4 nm | Human-like collagen (HLC) protein | BHK-21 cells |
100 μl of 12.5-100 μg/ml | WST-8 assay using Cell Counting Kit-8 (CCK-8) | No toxicity was observed for all concentration ranges and sizes, regardless of their incubation time. |
[175] |
| -/320 nm | Chitosan | ECs from Wistar rats Human blood F1 mice |
1-100 µg/ml 1, 10 and 100 µg/ml 30 mg/kg |
MTT assay NO production The erythrocyte sedimentation rate and hematology Histology |
No viability inhibition of cells was observed. The presence of the MNPs did not affect basal NO production. No significant differences in ESR in comparison to controls were observed. The hemolytic effect was not observed with any of the tested doses assayed. Histological examinations of the liver, stomach, intestine, lungs, and brain showed no changes at the end of the sub-acute exposure to MNPs in any of the mice after 28 days. The kidneys exhibited granular interstitial tissue, which was compatible with periarteriolar interstitial nephritis compared to the control. In the spleen, an increase in the presence of megakaryocytes was observed. |
[176] |
| -/80 nm -/40 nm -/40 nm |
BSA9 BSA BSA-PEG |
Human fibroblast cells Human glioblastoma U251 cells |
10−3 −10-7 M | MTT assay LDH assay Oxidative activity evaluated by a dichloro-dihydrofluorescein diacetate fluorescent dye. Comet assay |
After 48 hours, the highest concentration of BSA-IONP-80 and BSA-IONP-40 showed some cytotoxic effect, which was more robust in the case of BSA-MNP-40. BSA-MNPs-PEG toxicity was almost negligible in comparison to other types of MNPs. No significant change in the confluency area of U251 cells was observed. The measurements of LDH activity after 24 hours of incubation with MNPs have not shown any differences in cell membrane integrity for all samples in all concentrations tested. For 24 hours, all types of MNPs provided less ROS production than positive control. After 48 hours of HF-cells incubation, a noticeable increase in fluorescence level was observed. Signal intensity was almost equal to fluorescence intensity related to cells treated with a control solution of H2O2. As for U251 cells, a significantly lower fluorescence level was observed compared with the control solution of H2O2. 24 hours after incubation with different types of synthesized MNPs, there was no difference in DNA damage level between the control and experimental nanoparticles groups. An increase in DNA fragments was detected after 48 hours of HF-cells incubation with BSA-MNPs-40. In the case of the U251 cell line, no significant difference between DNA fragmentation in control and treated cells for all types and concentrations of MNPs used was observed. |
[19] |
| 10 nm /16.5 nm 30 nm/ 38.5 nm 10 nm/ 17.2 nm |
PEG PEG PEI10 |
RAW264.7 macrophage, SKOV-3 cancer cells BALB/c mice |
3.125 – 100 µg/ml 1.5 – 5 mg/kg |
MTS assay Hematology and blood biochemistry Histology |
SEI-10 induced dose-dependent cytotoxicity against both RAW264.7 macrophages and SKOV-3 cancer cells at the test concentrations, and SKOV-3 cells were relatively more susceptible to SEI-10 toxicity than RAW264.7 macrophages. No appreciable cytotoxic effects were observed for SMG-10 and SMG-30 at 25µg/mL; slight cytotoxicity was shown above 50µg/ml. The hematology and blood chemistry results on day seven post-injection showed that AST, total bilirubin, BUN, and creatinine were within the normal range, except that the level of ALT enzyme in mice treated with SMG-10 slightly increased compared to the PBS control. On day 14 post-injection, the increased ALT level in SMG-10-treated mice returned to normal. In mice treated with SMG-10 and SMG-30, slight mononuclear cell infiltration in the portal area of the liver was identified. Splenic plasmacytosis was noted in mice treated with SMG-30. |
[177] |
| -/60 nm | L-glutathione | Caenorhabditis elegans - non-parasitic nematodes | 10-200 mg/l | Mortality Growth Locomotion Fertility |
The presence of nanomaterial increased mortality without a specific relationship between the concentration and the number of dead nematodes. A slight decrease in the length of the worms was observed for the control. A decrease in locomotion was not significant. A decrease in the number of eggs placed was observed for each nematode by increasing the nanomaterial concentration in the medium. |
[178] |
| 24.33-34.24 nm/- | Curcumin-PEG | MCF7 cells Human red blood cells |
1-100 µg/ml 10 mg/ml |
MTT assay Hemolysis assay |
MNPs did not show any toxicity. Non hemolytic response was observed. |
[179] |
| 12 nm/ 40 nm (PA) 12 nm/ 72 nm (HA) |
PA-PEG phosphonic acid HA-PEG hydroxamic |
Primary human peripheral leucocytes | 0.12–75 µg/cm | Measurement of 3H-thymidine incorporation into DNA of cells |
No significant cytotoxic effect of PA-PEG@MNPs and HA-PEG@MNPs) was found after 24 and 72 hours of incubation. |
[180] |
| 3 nm/ 21 nm 14-20 nm/56 nm |
PEG | Mouse microglia cell line N13 Zebrafish embryos |
0.1-100 μg/ml 0.01-100 μg/ml |
MTT assay Evaluation of the hatching and survival rates of zebrafish embryos |
No significant cytotoxicity after 24 h of exposure to any MNPs was observed. Higher concentrations of MNPs (10 μg/ml and 100 μg/ml) showed an increased hatching rate compared to control non-exposed embryos. No mortality or malformations were observed in the embryos exposed to different doses of particles at 48 hours. |
[181] |
| 18 nm/ 230 nm |
PEG-Arginine | HFF2 and HEK293 cells Human red blood cells |
0.06-0.40 mg/ml 10 mg/ml |
MTT assay Hemolysis assay |
No significant cytotoxicity after exposure to any MNPs was observed. MNPs did not affect HRBCs of the blood. |
[182] |
| 9 nm/ 11.68 nm Nano-clusters of 100-150 nm/- |
Bare MNPs pPEG-AC11-poly(amidoamine- paraben-PEG |
Swiss albino mice | 5, 10, and 25 mg/kg | Blood biochemistry . Histology |
The highest dose of bare MNPs induced significant malfunctions in systemic biomarkers. In contrast, lower doses (5 and 10 mg/kg) of uncoated and all coated MNPs did not alter these biomarkers. All tissue sections, including liver, kidney, spleen, and heart, excluding lungs, treated with the highest dose (25 mg/kg) of bare MNPs demonstrated significant iron deposition. Lower doses (5 and 10 mg/kg) of uncoated MNPs and all coated NPs showed no iron accumulation. |
[15] |
| -/120 nm | L-carnosine | BALB/c mice |
Equivalent carnosine dose of 200 mg/kg/day | Blood biochemistry Histology |
A significant increase in the liver enzymes (ALT and AST) was observed. Iron accumulations were detected. No structural or histopathological changes were observed in the liver tissues, indicating no tissue damage. |
[183] |
| -/147 nm -/116.5 nm -/139 nm |
PEI PAH12 PDADMAC13 |
Human lung carcinoma cell line (A549). | 100 µg/ml | MTT assay Resazurin reduction assay |
The MTT test showed a slight decrease in the activity of cytosolic hydrogenases in all variants, most pronounced in the variant with MNPs-PEI. A test with resazurin reduction showed that incubation with MNPs-PEI slightly stimulated mitochondrial enzymes. |
[184] |
| 9.2 nm/ | PAA-CF14 | Splenic cells from rat Albino mice |
7.8 -1000 μg/ml Single dose of 100 μg/ml PAA@CF-MNPs |
Trypan blue dye exclusion method and MTT assay Blood biochemistry |
Non-significant cell growth stimulatory effects were observed at 1000, 62.5, 15.6, and 7.8 μg/ml and non-significant cell growth inhibitory effects at 500, 250, 125, and 31.25 μg/ml. The levels of ALT and AST showed a non-significant increase over the usual control group. The renal function parameters (serum urea and creatinine) were normal. |
[185] |
| Not available | Ag/Fe3O4-CS15-PVA16/Ag | HEK293 and LO2 cells Mice |
1.25-40 μg/ml Not available |
Cell counting kit-8 assay Hemolysis Histology |
No apparent toxic effects on cells were observed. Slight hemolysis was observed. No abnormal changes were observed. |
[186] |
| 46-57 nm/- | Carboxymethyl chitosan | MCF7 human breast cancer cells and 3T3 fibroblasts | 6.25-100 μg/ml | MTT assay | MNPs displayed toxic effects against MCF-7 cells. No toxicity towards 3T3 fibroblasts was observed. |
[187] |
| 2-5 nm/- | ε-Poly (L-lysine) carbon dots | Mouse MC3T3-E1 cells Human red blood cells |
0.1, 0.5, 1 mg/ml |
Cell counting kit-8 assay Homolysis |
No toxicity was observed. Low concentrations of MNPs (0.1 mg/ml) possessed acceptable hemocompatibility. |
[188] |
| 12-15 nm/98 nm | Curcumin/ Alginate |
Sarcoma 180 cancer cells Mice |
0.01-1000 µg/mL 80-120 mg/kg |
MTT assay Blood biochemistry Histology |
The cytotoxicity was observed only at high doses. Biochemical assay data indicated that AST and ALT values were higher in the treated mice than in the control mice, with a significant difference in AST values. In contrast, the levels of BUN and creatinine did not change significantly. The histological structures of livers changed compared to those in the control group, with the appearance of vacuolated hepatocytes. |
[189] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





