Submitted:
07 October 2024
Posted:
08 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Functional Traits – From Field to Remotely Sensed Observations
3. Functional Diversity and Ecological Function
4. Diversity, Landsliding, and Mountainscapes
4.1. Plant traits, Montane Ecosystems, and Landsliding
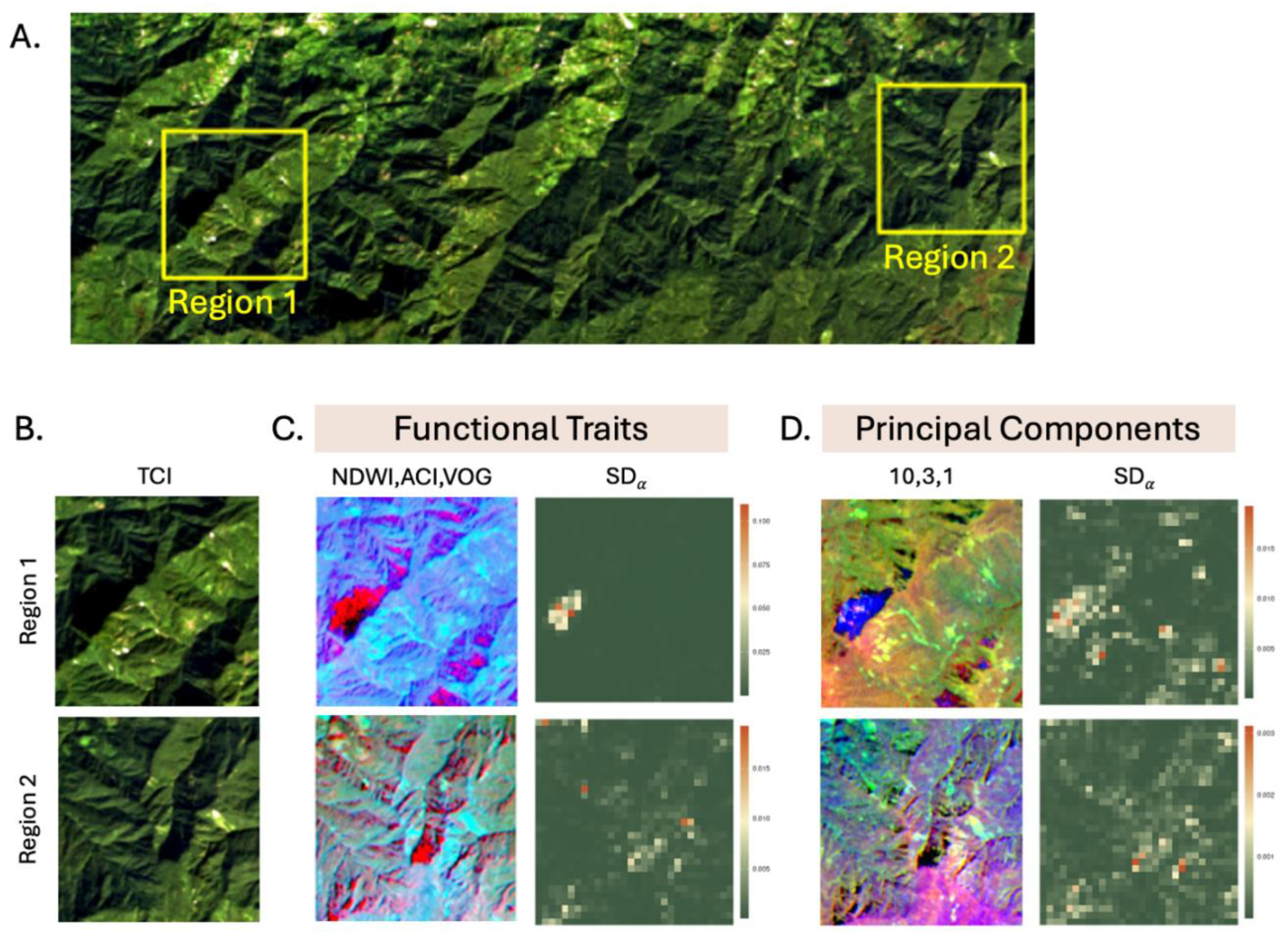
4.2. Soil and Lithology Attributes, Montane Ecosystems, and Landsliding
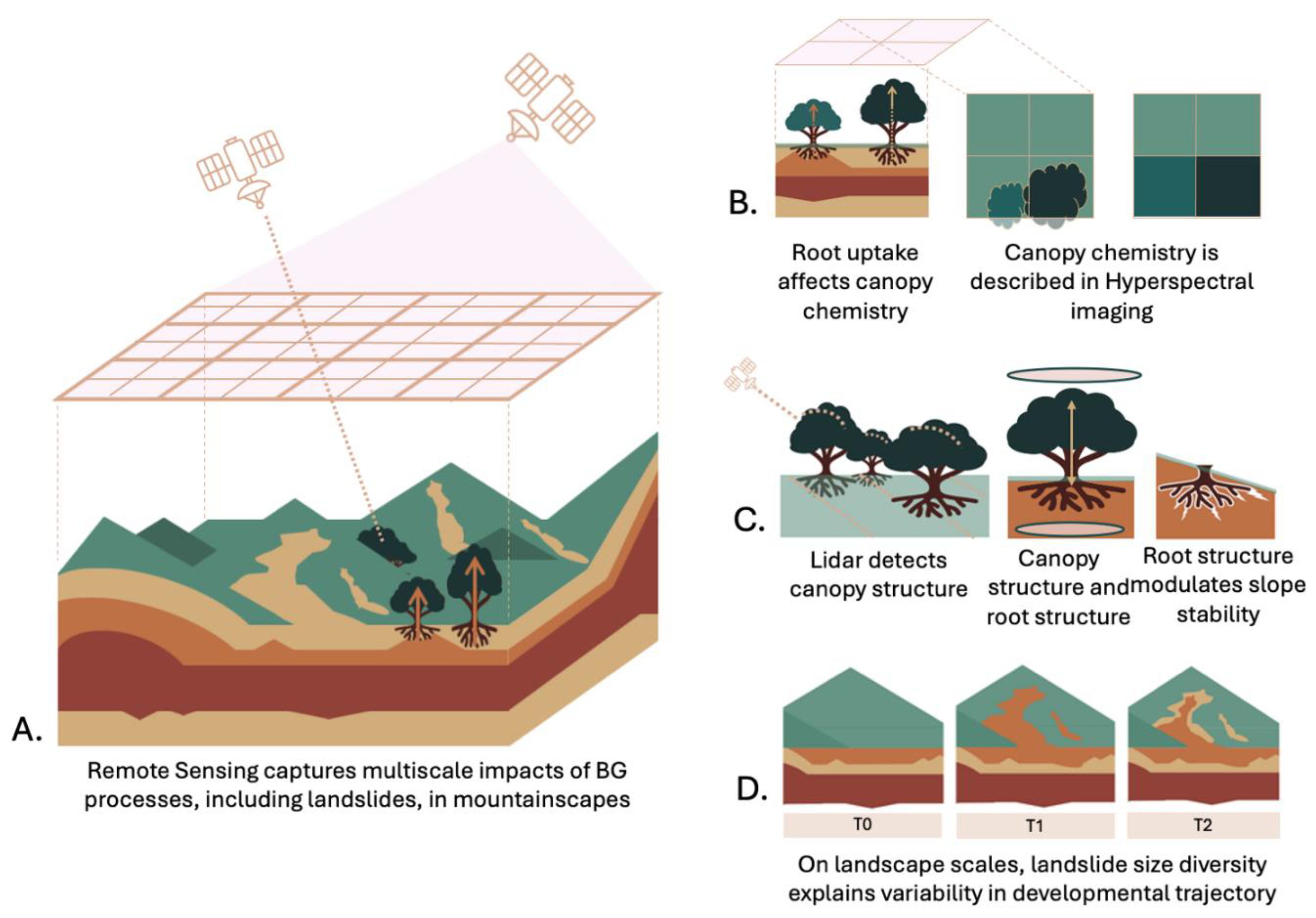
5. Hyperspectral Remote Sensing Can Integrate Plant Traits, Soil-Rock Attributes, and Landslide Studies to Understand the Diversity, Functioning, and Dynamics of Mountainscapes
6. Conclusion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Chapin, F.S., 3rd; Carpenter, S.R.; Kofinas, G.P.; Folke, C.; Abel, N.; Clark, W.C.; Olsson, P.; Smith, D.M.; Walker, B.; Young, O.R. , et al. Ecosystem stewardship: Sustainability strategies for a rapidly changing planet. Trends Ecol. Evol. 2010, 25, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.C. The Post-2020 Global Biodiversity Framework: How did we get here, and where do we go next? Integr. Conserv. 2023, 2, 1–9. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Schneider, F.D.; Santos, M.J.; Armstrong, A.; Carnaval, A.; Dahlin, K.M.; Fatoyinbo, L.; Hurtt, G.C.; Schimel, D.; Townsend, P.A. , et al. Integrating remote sensing with ecology and evolution to advance biodiversity conservation. Nat. Ecol. Evol. 2022, 6, 506–519. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity: back to basics and looking forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Gutiérrez, J.; Rifai, S.; Shenkin, A.; Oliveras, I.; Bentley, L.P.; Svátek, M.; Girardin, C.A.J.; Both, S.; Riutta, T.; Berenguer, E. , et al. Pantropical modelling of canopy functional traits using Sentinel-2 remote sensing data. Remote Sens. Environ. 2021, 252, 112122. [Google Scholar] [CrossRef]
- Maxwell, A.E.; Wilson, B.T.; Holgerson, J.J.; Bester, M.S. Comparing harmonic regression and GLAD Phenology metrics for estimation of forest community types and aboveground live biomass within forest inventory and analysis plots. Int. J. Appl. Earth Obs. 2023, 122, 103435. [Google Scholar] [CrossRef]
- Neinavaz, E.; Schlerf, M.; Darvishzadeh, R.; Gerhards, M.; Skidmore, A.K. Thermal infrared remote sensing of vegetation: Current status and perspectives. Int. J. Appl. Earth Obs. 2021, 102, 102415. [Google Scholar] [CrossRef]
- Skidmore, A.K.; Pettorelli, N. Agree on biodiversity metrics to track from space. Nature 2015, 523, 504–512. [Google Scholar] [CrossRef]
- Wallis, C.I.B.; Homeier, J.; Peña, J.; Brandl, R.; Farwig, N.; Bendix, J. Modeling tropical montane forest biomass, productivity and canopy traits with multispectral remote sensing data. Remote Sens. Environ. 2019, 225, 77–92. [Google Scholar] [CrossRef]
- Xiong, X.; Butler, J.J. MODIS and VIIRS calibration history and future outlook. Remote Sens. 2020, 12, 2523. [Google Scholar] [CrossRef]
- Helmer, E.H.; Goodwin, N.R.; Gond, V.; Souza, Jr.; C.M., *!!! REPLACE !!!*; Asner, G.P. Characterizing tropical forests with multispectral imagery. In Land Resources: Monitoring, Modeling and Mapping; Thenkabail, P.S., Ed.; Taylor & Francis Group: Boca Raton, Florida, USA, 2015; pp. 367–396. [Google Scholar]
- Asner, G.P.; Martin, R.E.; Knapp, D.E.; Tupayachi, R.; Anderson, C.B.; Sinca, F.; Vaughn, N.R.; Llactayo, W. Airborne laser-guided imaging spectroscopy to map forest trait diversity and guide conservation. Science 2017, 355, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Kokaly, R.F.; Asner, G.P.; Ollinger, S.V.; Martin, M.E.; Wessman, C.A. Characterizing canopy biochemistry from imaging spectroscopy and its application to ecosystem studies. Remote Sens. Environ. 2009, 113, S78–S91. [Google Scholar] [CrossRef]
- Pascual-Venteo, A.B.; Portalés, E.; Berger, K.; Tagliabue, G.; Garcia, J.L.; Pérez-Suay, A.; Rivera-Caicedo, J.P.; Verrelst, J. Prototyping crop traits retrieval models for CHIME: Dimensionality reduction strategies applied to PRISMA data. Remote Sens. 2022, 14, 2448. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.D.; Morsdorf, F.; Schmid, B.; Petchey, O.L.; Hueni, A.; Schimel, D.S.; Schaepman, M.E. Mapping functional diversity from remotely sensed morphological and physiological forest traits. Nat. Commun. 2017, 8, 1441. [Google Scholar] [CrossRef] [PubMed]
- Ustin, S.L.; Roberts, D.A.; Gamon, J.A.; Asner, G.P.; Green, R.O. Using imaging spectroscopy to study ecosystem processes and properties. BioScience 2004, 54, 523–534. [Google Scholar] [CrossRef]
- Abrams, M.; Yamaguchi, Y. Twenty Years of ASTER Contributions to Lithologic Mapping and Mineral Exploration. Remote Sens. 2019, 11, 1394. [Google Scholar] [CrossRef]
- Misbah, K.; Laamrani, A.; Khechba, K.; Dhiba, D.; Chehbouni, A. Multi-sensors remote sensing applications for assessing, monitoring, and mapping NPK content in soil and crops in African agricultural land. Remote Sens. 2022, 14, 81. [Google Scholar] [CrossRef]
- Novais, J.J.; Poppiel, R.R.; Lacerda, M.P.C.; Oliveira, M.P.; Demattê, J.A.M. Spectral Mixture Modeling of an ASTER Bare Soil Synthetic Image Using a Representative Spectral Library to Map Soils in Central-Brazil. AgriEngineering 2023, 5, 156–172. [Google Scholar] [CrossRef]
- Helfenstein, I.S.; Schneider, F.D.; Schaepman, M.E.; Morsdorf, F. Assessing biodiversity from space: Impact of spatial and spectral resolution on trait-based functional diversity. Remote Sens. Environ. 2022, 275, 113024. [Google Scholar] [CrossRef]
- Lechner, A.M.; Foody, G.M.; Boyd, D.S. Applications in remote sensing to forest ecology and management. One Earth 2020, 2, 405–412. [Google Scholar] [CrossRef]
- Pettorelli, N.; Schulte to Bühne, H.; Tulloch, A.; Dubois, G.; Macinnis-Ng, C.; Queirós, A.M.; Keith, D.A.; Wegmann, M.; Schrodt, F.; Stellmes, M. , et al. Satellite remote sensing of ecosystem functions: opportunities, challenges and way forward. Remote Sens. Ecol. Conserv. 2018, 4, 71–93. [Google Scholar] [CrossRef]
- Ma, X.; Mahecha, M.D.; Migliavacca, M.; van der Plas, F.; Benavides, R.; Ratcliffe, S.; Kattge, J.; Richter, R.; Musavi, T.; Baeten, L. , et al. Inferring plant functional diversity from space: the potential of Sentinel-2. Remote Sens. Environ. 2019, 233, 111368. [Google Scholar] [CrossRef]
- Zheng, Z.; Schmid, B.; Zeng, Y.; Schuman, M.C.; Zhao, D.; Schaepman, M.E.; Morsdorf, F. Remotely sensed functional diversity and its association with productivity in a subtropical forest. Remote Sens. Environ. 2023, 290, 113530. [Google Scholar]
- Fernández-García, V.; Marcos, E.; Fernández-Guisuraga, J.M.; Fernández-Manso, A.; Quintano, C.; Suárez-Seoane, S.; Calvo, L. Multiple endmember spectral mixture analysis (MESMA) applied to the study of habitat diversity in the fine-grained landscapes of the Cantabrian Mountains. Remote Sens. 2021, 13. [Google Scholar] [CrossRef]
- Asner, G.P.; Anderson, C.B.; Martin, R.E.; Tupayachi, R.; Knapp, D.E.; Sinca, F. Landscape biogeochemistry reflected in shifting distributions of chemical traits in the Amazon forest canopy. Nat. Geosci. 2015, 8, 567–573. [Google Scholar] [CrossRef]
- Asner, G.P.; Knapp, D.E.; Anderson, C.B.; Martin, R.E.; Vaughn, N. Large-scale climatic and geophysical controls on the leaf economics spectrum. Proc. Natl. Acad. Sci. U S A 2016, 113, E4043–E4051. [Google Scholar] [CrossRef] [PubMed]
- Zini, S.; Barbato, M.P.; Piccoli, F.; Napoletano, P. Deep learning hyperspectral pansharpening on large scale PRISMA dataset. Remote Sens. 2024, 16, 2079. [Google Scholar] [CrossRef]
- Bogan, S.A.; Antonarakis, A.S.; Moorcroft, P.R. Imaging spectrometry-derived estimates of regional ecosystem composition for the Sierra Nevada, California. Remote Sens. Environ. 2019, 228, 14–30. [Google Scholar] [CrossRef]
- Hacker, P.W.; Coops, N.C.; Laliberté, E.; Michaletz, S.T. Variations in accuracy of leaf functional trait prediction due to spectral mixing. Ecol. Indic. 2022, 136, 108687. [Google Scholar]
- Quintano, C.; Fernández-Manso, A.; Fernández-Guisuraga, J.M.; Calvo, L. Unmixing PRISMA hyperspectral images by multiple endmember spectral mixture analysis (MESMA) to assess fire severity in Mediterranean forest ecosystems. Imaging Spectrometry XXVI: Applications; Sensors, and Processing, 2023; p. 126880I. [Google Scholar]
- Waser, L.T.; Rüetschi, M.; Psomas, A.; Small, D.; Rehush, N. Mapping dominant leaf type based on combined Sentinel-1/-2 data – Challenges for mountainous countries. ISPRS J. Photogramm. Rem. Sens. 2021, 180, 209–226. [Google Scholar]
- Weiss, D.J.; Walsh, S.J. Remote Sensing of Mountain Environments. Geogr. Comp. 2009, 3, 1–21. [Google Scholar] [CrossRef]
- Brown, J.H.; Gupta, V.K.; Li, B.-L.; Milne, B.T.; Restrepo, C.; West, G.B. The fractal nature of nature: power laws, ecological complexity and biodiversity. Philosophical Transactions of the Royal Society of London Series B Biological Sciences 2002, 357, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Medwedeff, W.G.; Clark, M.K.; Zekkos, D.; West, A.J. Characteristic landslide distributions: An investigation of landscape controls on landslide size. Earth Planet. Sc. Lett. 2020, 539, 116203. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E.; Anderson, C.B.; Knapp, D.E. Quantifying forest canopy traits: Imaging spectroscopy versus field survey. Remote Sens. Environ. 2015, 158, 15–27. [Google Scholar]
- Bertoldi, L.; Massironi, M.; Visonà, D.; Carosi, R.; Montomoli, C.; Gubert, F.; Naletto, G.; Pelizzo, M.G. Mapping the Buraburi granite in the Himalaya of Western Nepal: Remote sensing analysis in a collisional belt with vegetation cover and extreme variation of topography. Remote Sens. Environ. 2011, 115, 1129–1144. [Google Scholar] [CrossRef]
- Loizzo, R.; Ananasso, C.; Guarini, R.; Lopinto, E.; Candela, L.; Pisani, A.R. The PRISMA Hyperspectral Mission. In Living Planet Symposium 2016, 9-13 May 2016, Prague, Czech Republic; 13 May 2016. [Google Scholar]
- Storch, T.; Honold, H.-P.; Chabrillat, S.; Habermeyer, M.; Tucker, P.; Brell, M.; Ohndorf, A.; Wirth, K.; Betz, M.; Kuchler, M. , et al. The EnMAP imaging spectroscopy mission towards operations. Remote Sens. Environ. 2023, 294, 113632. [Google Scholar]
- Schneider, F.D.; Ferraz, A.; Schimel, D. Watching Earth’s interconnected systems at work. Eos 2019, 100. [Google Scholar] [CrossRef]
- Stavros, E.N.; Chrone, J.; Cawse-Nicholson, K.; Freeman, A.; Glenn, N.F.; Guild, L.; Kokaly, R.; Lee, C.; Luvall, J.; Pavlick, R. , et al. Designing an Observing System to Study the Surface Biology and Geology (SBG) of the Earth in the 2020s. Journal of Geophysical Research: Biogeosciences 2023, 128, e2021JG006471. [Google Scholar]
- Nieke, J.; Rast, M. Status: Copernicus Hyperspectral Imaging Mission for the Environment (CHIME). IGARSS 2019, 4609–4611. [Google Scholar]
- Planets Lab PBC. Tanager-1 Is Ready For Launch: Planet’s First Hyperspectral Satellite. Available online: https://www.planet.com/pulse/tanager-1-is-ready-for-launch-planets-first-hyperspectral-satellite/.
- Asner, G.P.; Martin, R.E.; Knapp, D.E.; Tupayachi, R.; Anderson, C.; Carranza, L.; Martinez, P.; Houcheime, M.; Sinca, F.; Weiss, P. Spectroscopy of canopy chemicals in humid tropical forests. Remote Sens. Environ. 2011, 115, 3587–3598. [Google Scholar] [CrossRef]
- Reich, P.B.; Wright, I.J.; Cavender-Bares, J.; Craine, J.M.; Oleksyn, J.; Westoby, M.; Walters, M.B. The evolution of plant functional variation: Traits, spectra, and strategies. Int. J. Plant Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Díaz, S.; Cabido, M. Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Diaz, S.; Purvis, A.; Cornelissen, J.H.; Mace, G.M.; Donoghue, M.J.; Ewers, R.M.; Jordano, P.; Pearse, W.D. Functional traits, the phylogeny of function, and ecosystem service vulnerability. Ecol. Evol. 2013, 3, 2958–2975. [Google Scholar] [CrossRef]
- Lavorel, S.; Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Naeem, S.; Wright, J.P. Disentangling biodiversity effects on ecosystem functioning: deriving solutions to a seemingly insurmountable problem. Ecol. Lett. 2003, 6, 567–579. [Google Scholar]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Bruelheide, H.; Dengler, J.; Purschke, O.; Lenoir, J.; Jiménez-Alfaro, B.; Hennekens, S.M.; Botta-Dukát, Z.; Chytrý, M.; Field, R.; Jansen, F. , et al. Global trait–environment relationships of plant communities. Nat. Ecol. Evol. 2018, 2, 1906–1917. [Google Scholar] [CrossRef] [PubMed]
- Šímová, I.; Violle, C.; Svenning, J.-C.; Kattge, J.; Engemann, K.; Sandel, B.; Peet, R.K.; Wiser, S.K.; Blonder, B.; McGill, B.J. , et al. Spatial patterns and climate relationships of major plant traits in the New World differ between woody and herbaceous species. J. Biogeogr. 2018, 45, 895–916. [Google Scholar] [CrossRef]
- Laliberte, E.; Schweiger, A.K.; Legendre, P. Partitioning plant spectral diversity into alpha and beta components. Ecol Lett 2020, 23, 370–380. [Google Scholar]
- Rossi, C.; Kneubühler, M.; Schütz, M.; Schaepman, M.E.; Haller, R.M.; Risch, A.C. Remote sensing of spectral diversity: A new methodological approach to account for spatio-temporal dissimilarities between plant communities. Ecol. Indic. 2021, 130, 108106. [Google Scholar] [CrossRef]
- Curran, P.J. Remote sensing of foliar chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Gholizadeh, H.; Friedman, M.S.; McMillan, N.A.; Hammond, W.M.; Hassani, K.; Sams, A.V.; Charles, M.D.; Garrett, D.R.; Joshi, O.; Hamilton, R.G. , et al. Mapping invasive alien species in grassland ecosystems using airborne imaging spectroscopy and remotely observable vegetation functional traits. Remote Sens. Environ. 2022, 271, 112887. [Google Scholar] [CrossRef]
- Chadwick, K.D.; Asner, G.P. Geomorphic transience moderates topographic controls on tropical canopy foliar traits. Ecol. Lett. 2020, 23, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Balzotti, C.S.; Asner, G.P.; Taylor, P.G.; Cleveland, C.C.; Cole, R.; Martin, R.E.; Nasto, M.; Osborne, B.B.; Porder, S.; Townsend, A.R. Environmental controls on canopy foliar nitrogen distributions in a Neotropical lowland forest. Ecol. Appl. 2016, 26, 2449–2462. [Google Scholar] [PubMed]
- Osborne, B.B.; Nasto, M.K.; Asner, G.P.; Balzotti, C.S.; Cleveland, C.C.; Sullivan, B.W.; Taylor, P.G.; Townsend, A.R.; Porder, S. Climate, topography, and canopy chemistry exert hierarchical control over soil N cycling in a Neotropical lowland forest. Ecosystems 2017, 20, 1089–1103. [Google Scholar]
- Miraglio, T.; Coops, N.C.; Wallis, C.I.B.; Crofts, A.L.; Kalacska, M.; Vellend, M.; Serbin, S.P.; Arroyo-Mora, J.P.; Laliberté, E. Mapping canopy traits over Québec using airborne and spaceborne imaging spectroscopy. Sci. Rep. 2023, 13, 17179. [Google Scholar]
- Asner, G.P.; Vitousek, P.M. Remote analysis of biological invasion and biogeochemical change. Proc. Natl. Acad. Sci. U S A 2005, 102, 4383–4386. [Google Scholar]
- Hall, S.J.; Asner, G.P. Biological invasion alters regional nitrogen-oxide emissions from tropical rainforests. Glob. Change Biol. 2007, 13, 2143–2160. [Google Scholar] [CrossRef]
- Asner, G.P.; Nepstad, D.; Cardinot, G.; Ray, D. Drought stress and carbon uptake in an Amazon forest measured with spaceborne imaging spectroscopy. Proc. Natl. Acad. Sci. U S A 2004, 101, 6039–6044. [Google Scholar] [CrossRef]
- Balzotti, C.S.; Asner, G.P. Biotic and abiotic controls over canopy function and structure in humid Hawaiian forests. Ecosystems 2018, 21, 331–348. [Google Scholar]
- Asner, G.P. Hyperspectral remote sensing of canopy chemistry, physiology and diversity in tropical rainforests - Chapter 12. In Hyperspectral Remote Sensing of tTopical and Subtropical Forests; Kalacska, M., Sanchez-Azofeifa, G.A., Eds.; Taylor and Francis Group: Boca Raton, Florida, US, 2008; p. 352. [Google Scholar]
- Gamon, J.A.; Rahman, A.F.; Dungan, J.L.; Schildhauer, M.; Huemmrich, K.F. Spectral Network (SpecNet)—What is it and why do we need it? Remote Sens. Environ. 2006, 103, 227–235. [Google Scholar] [CrossRef]
- Kattenborn, T. Linking canopy reflectance and plant functioning through radiative transfer models. Dr. rer. nat. Thesis, Karlsruher Institut für Technologie, Karlsruhe, Germany, 2018. [Google Scholar]
- Wang, Z.; Chlus, A.; Geygan, R.; Ye, Z.; Zheng, T.; Singh, A.; Couture, J.J.; Cavender-Bares, J.; Kruger, E.L.; Townsend, P.A. Foliar functional traits from imaging spectroscopy across biomes in eastern North America. New Phytol. 2020, 228, 494–511. [Google Scholar] [CrossRef] [PubMed]
- Asner, G.P.; Martin, R.E.; Ford, A.J.; Metcalfe, D.J.; Liddell, M.J. Leaf chemical and spectral diversity in Australian tropical forests. Ecol. Appl. 2009, 19, 236–253. [Google Scholar] [CrossRef]
- Chadwick, K.; Asner, G. Organismic-scale remote sensing of canopy foliar traits in lowland tropical forests. Remote Sens. 2016, 8, 87. [Google Scholar] [CrossRef]
- Matheny, A.M.; Mirfenderesgi, G.; Bohrer, G. Trait-based representation of hydrological functional properties of plants in weather and ecosystem models. Plant Divers. 2017, 39, 1–12. [Google Scholar] [CrossRef]
- Asner, G.P.; Haxo, B.; Knapp, D.E. Computing for analysis and modeling of hyperspectral imagery. In High Performance Computing for Remote Sensing; Plaza, S., Chang, C.I., Eds.; Chapman and Hall Press: New York, New York, US, 2007; pp. 110–130. [Google Scholar]
- Doughty, C.E.; Asner, G.P.; Martin, R.E. Predicting tropical plant physiology from leaf and canopy spectroscopy. Oecologia 2011, 165, 289–299. [Google Scholar]
- Féret, J.-B.; François, C.; Gitelson, A.; Asner, G.P.; Barry, K.M.; Panigada, C.; Richardson, A.D.; Jacquemoud, S. Optimizing spectral indices and chemometric analysis of leaf chemical properties using radiative transfer modeling. Remote Sens. Environ. 2011, 115, 2742–2750. [Google Scholar] [CrossRef]
- Serbin, S.P.; Townsend, P.A. Scaling functional traits from leaves to canopies. Chapter 3. In Remote Sensing of Plant Biodiversity; Cavender-Bares, J., Gamon, J.A., Townsend, P.A., Eds.; Springer Open, 2020; pp. 43–82. [Google Scholar]
- Verrelst, J.; Camps-Valls, G.; Muñoz-Marí, J.; Rivera, J.P.; Veroustraete, F.; Clevers, J.G.P.W.; Moreno, J. Optical remote sensing and the retrieval of terrestrial vegetation bio-geophysical properties – A review. ISPRS J. Photogramm. Rem. Sens. 2015, 108, 273–290. [Google Scholar] [CrossRef]
- Bannari, A.; Morin, D.; Bonn, F.; Huete, A.R. A review of vegetation indices. Remote Sens. Rev. 1995, 13, 95–120. [Google Scholar] [CrossRef]
- Beauchemin, M.; Fung, K.B. On statistical band selection for image visualization. Phogrammetric Engineering and Remote Sensing 2001, 67, 571–574. [Google Scholar]
- Ollinger, S.V. Sources of variability in canopy reflectance and the convergent properties of plants. New Phytol. 2011, 189, 375–394. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.A.; Asner, G.P.; Martin, R.E.; Knapp, D.E.; Anderson, C.; Kennedy-Bowdoin, T.; Saenz, R.; Aguilar, A.; Joseph Wright, S. Linking imaging spectroscopy and LiDAR with floristic composition and forest structure in Panama. Remote Sens. Environ. 2014, 154, 358–367. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E.; Carranza-Jimenez, L.; Sinca, F.; Tupayachi, R.; Anderson, C.B.; Martinez, P. Functional and biological diversity of foliar spectra in tree canopies throughout the Andes to Amazon region. New Phytol. 2014, 204, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.C.; Anderson, J.; Davidson, K.J.; Ely, K.S.; Lamour, J.; Li, Q.; Morrison, B.D.; Yang, D.; Rogers, A.; Serbin, S.P. A best-practice guide to predicting plant traits from leaf-level hyperspectral data using partial least squares regression. J. Exp. Biol. 2021, 72, 6175–6189. [Google Scholar] [CrossRef]
- Feilhauer, H.; Asner, G.P.; Martin, R.E.; Schmidtlein, S. Brightness-normalized Partial Least Squares Regression for hyperspectral data. J. Quant. Spectrosc. Ra. 2010, 111, 1947–1957. [Google Scholar] [CrossRef]
- Féret, J.-B.; François, C.; Asner, G.P.; Gitelson, A.A.; Martin, R.E.; Bidel, L.P.R.; Ustin, S.L.; le Maire, G.; Jacquemoud, S. PROSPECT-4 and 5: Advances in the leaf optical properties model separating photosynthetic pigments. Remote Sens. Environ. 2008, 112, 3030–3043. [Google Scholar] [CrossRef]
- Kötz, B.; Schaepman, M.; Morsdorf, F.; Bowyer, P.; Itten, K.; Allgöwer, B. Radiative transfer modeling within a heterogeneous canopy for estimation of forest fire fuel properties. Remote Sens. Environ. 2004, 92, 332–344. [Google Scholar] [CrossRef]
- Svendsen, D.H.; Hernández-Lobato, D.; Martino, L.; Laparra, V.; Moreno-Martínez, Á.; Camps-Valls, G. Inference over radiative transfer models using variational and expectation maximization methods. Machine Learning 2023, 112, 921–937. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Noland, T.L.; Mohammed, G.H.; Sampson, P.H. Scaling-up and model inversion methods with narrowband optical indices for chlorophyll content estimation in closed forest canopies with hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1491–1507. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E. Spectral and chemical analysis of tropical forests: Scaling from leaf to canopy levels. Remote Sens. Environ. 2008, 112, 3958–3970. [Google Scholar] [CrossRef]
- Pacheco-Labrador, J.; de Bello, F.; Migliavacca, M.; Ma, X.; Carvalhais, N.; Wirth, C. A generalizable normalization for assessing plant functional diversity metrics across scales from remote sensing. Methods Ecol. Evol. 2023, 14, 2123–2136. [Google Scholar] [CrossRef]
- Verrelst, J.; Rivera-Caicedo, J.P.; Reyes-Muñoz, P.; Morata, M.; Amin, E.; Tagliabue, G.; Panigada, C.; Hank, T.; Berger, K. Mapping landscape canopy nitrogen content from space using PRISMA data. ISPRS J. Photogramm. Rem. Sens. 2021, 178, 382–395. [Google Scholar]
- Asner, G.P.; Knapp, D.E.; Boardman, J.; Green, R.O.; Kennedy-Bowdoin, T.; Eastwood, M.; Martin, R.E.; Anderson, C.; Field, C.B. Carnegie Airborne Observatory-2: Increasing science data dimensionality via high-fidelity multi-sensor fusion. Remote Sens. Environ. 2012, 124, 454–465. [Google Scholar] [CrossRef]
- Lee, C.M.; Cable, M.L.; Hook, S.J.; Green, R.O.; Ustin, S.L.; Mandl, D.J.; Middleton, E.M. An introduction to the NASA Hyperspectral InfraRed Imager (HyspIRI) mission and preparatory activities. Remote Sens. Environ. 2015, 167, 6–19. [Google Scholar]
- McCorkel, J.; Kuester, M.; Johnson, B.R.; Kampe, T.U. NEON ground validation capabilities for airborne and space-based imagers. Proceedings SPIE, 2011; p. 81530Z. [Google Scholar]
- Dechant, B.; Kattge, J.; Pavlick, R.; Schneider, F.D.; Sabatini, F.; Moreno, A.; Butler, E.; Bodegom, P.; Vallicrosa, H.; Kattenborn, T. , et al. Intercomparison of global foliar trait maps reveals fundamental differences and limitations of upscaling approaches. Remote Sens. Environ. 2024, 311, 114276. [Google Scholar] [CrossRef]
- Wocher, M. Unlocking the benefits of spaceborne imaging spectroscopy for sustainabile agriculture. Dr. rer. nat. Thesis, Universität at München, München, 2022. [Google Scholar]
- Baret, F.; Guyot, G. Potentials and limits of vegetation indices for LAI and APAR assessment. Remote Sens. Environ. 1991, 35, 161–173. [Google Scholar] [CrossRef]
- Martin, R.E.; Chadwick, K.D.; Brodrick, P.G.; Carranza-Jimenez, L.; Vaughn, N.R.; Asner, G.P. An approach for foliar trait retrieval from airborne imaging spectroscopy of tropical forests. Remote Sens. 2018, 10, 199. [Google Scholar] [CrossRef]
- Zheng, Z.; Zeng, Y.; Schneider, F.D.; Zhao, Y.; Zhao, D.; Schmid, B.; Schaepman, M.E.; Morsdorf, F. Mapping functional diversity using individual tree-based morphological and physiological traits in a subtropical forest. Remote Sens. Environ. 2021, 252, 112170. [Google Scholar]
- Knox, N.M.; Skidmore, A.K.; Prins, H.H.T.; Asner, G.P.; van der Werff, H.M.A.; de Boer, W.F.; van der Waal, C.; de Knegt, H.J.; Kohi, E.M.; Slotow, R. , et al. Dry season mapping of savanna forage quality, using the hyperspectral Carnegie Airborne Observatory sensor. Remote Sens. Environ. 2011, 115, 1478–1488. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; Chapin, F.S.; Tecco, P.A.; Gurvich, D.E.; Grigulis, K. Functional Diversity — at the Crossroads between Ecosystem Functioning and Environmental Filters. In Terrestrial Ecosystems in a Changing World; Canadell, J.G., Pataki, D.E., Pitelka, L.F., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2007; pp. 91–91. [Google Scholar]
- Tilman, D. Functional Diversity. In Encyclopedia of Biodiversity; 2001; Volume 3, pp. 109–120. [Google Scholar]
- Ali, A. Biodiversity–ecosystem functioning research: Brief history, major trends and perspectives. Biol. Conserv. 2023, 285, 110210. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin Iii, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S. , et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and Ecosystem Functioning. Annu. Rev. Ecol. Syst 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Biswas, S.R.; Mallik, A.U. Disturbance effects on species diversity and functional diversity in riparian and upland plant communities. Ecology 2010, 91, 28–35. [Google Scholar] [CrossRef]
- Cursach, J.; Rita, J.; Gómez-Martínez, C.; Cardona, C.; Capó, M.; Lázaro, A. The role of landscape composition and heterogeneity on the taxonomical and functional diversity of Mediterranean plant communities in agricultural landscapes. PLOS ONE 2020, 15, e0238222. [Google Scholar] [CrossRef]
- de Bello, F.; Lavorel, S.; Hallett, L.M.; Valencia, E.; Garnier, E.; Roscher, C.; Conti, L.; Galland, T.; Goberna, M.; Májeková, M. , et al. Functional trait effects on ecosystem stability: assembling the jigsaw puzzle. Trends Ecol. Evol. 2021, 36, 822–836. [Google Scholar] [CrossRef] [PubMed]
- Loreau, M.; Barbier, M.; Filotas, E.; Gravel, D.; Isbell, F.; Miller, S.J.; Montoya, J.M.; Wang, S.; Aussenac, R.; Germain, R. , et al. Biodiversity as insurance: From concept to measurement and application. Biol. Rev. 2021, 96, 2333–2354. [Google Scholar] [CrossRef]
- McWilliam, M.; Hoogenboom, M.O.; Baird, A.H.; Hughes, T.P. Biogeographical disparity in the functional diversity and redundancy of corals. Proc. Natl. Acad. Sci. U S A 2018, 115, 3084–3089. [Google Scholar] [CrossRef]
- Peterson, G.; Allen, C.R.; Holling, C.S. Ecological Resilience, Biodiversity, and Scale. Ecosystems 1998, 1, 6–18. [Google Scholar] [CrossRef]
- Restrepo, C.; Renjifo, L.M.; Marples, P. Frugivorous birds in fragmented neotropical montane forests: Landscape pattern and body mass distribution. In Tropical Forest Remnants: Ecology, Management and Conservation of Fragmented Ecosystems; Laurance, W.F., Bierregaard, R.O., Eds.; University of Chicago Press: Chicago, 1997; pp. 171–189. [Google Scholar]
- Castro Sánchez-Bermejo, P.; deCastro-Arrazola, I.; Cuesta, E.; Davis, A.L.V.; Moreno, C.E.; Sánchez-Piñero, F.; Hortal, J. Aridity drives the loss of dung beetle taxonomic and functional diversity in three contrasting deserts. J. Biogeogr. 2022, 49, 2243–2255. [Google Scholar] [CrossRef]
- Frainer, A.; Primicerio, R.; Dolgov, A.; Fossheim, M.; Johannesen, E.; Lind, S.; Aschan, M. Increased functional diversity warns of ecological transition in the Arctic. Proceedings of the Royal Society B Biological Sciences 2021, 288, 202110054. [Google Scholar] [CrossRef]
- Lavorel, S.; Grigulis, K.; McIntyre, S.; Williams, N.S.G.; Garden, D.; Dorrough, J.; Berman, S.; Quétier, F.; Thébault, A.; Bonis, A. Assessing functional diversity in the field – methodology matters! Funct. Ecol. 2008, 22, 134–147. [Google Scholar] [CrossRef]
- Spasojevic, M.J.; Suding, K.N. Inferring community assembly mechanisms from functional diversity patterns: the importance of multiple assembly processes. J. Ecol. 2012, 100, 652–661. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Liu, S.; Chai, Y.; Dang, H.; Yue, M.; Liu, X.; Guo, Y. Patterns of diversity and community assembly change across local to regional scales: An evidence of deterministic assembly processes along resource availability gradient at temperate forest. Ecol. Indic. 2021, 132, 108261. [Google Scholar] [CrossRef]
- Carmona, C.P.; de Bello, F.; Mason, N.W.H.; Lepš, J. Traits without borders: Integrating functional diversity across scales. Trends Ecol. Evol. 2016, 31, 382–394. [Google Scholar] [CrossRef] [PubMed]
- de Bello, F.; Thuiller, W.; Lepš, J.; Choler, P.; Clément, J.-C.; Macek, P.; Sebastià, M.-T.; Lavorel, S. Partitioning of functional diversity reveals the scale and extent of trait convergence and divergence. J. Veg. Sci. 2009, 20, 475–486. [Google Scholar] [CrossRef]
- Villéger, S.; Grenouillet, G.; Brosse, S. Decomposing functional β-diversity reveals that low functional β-diversity is driven by low functional turnover in European fish assemblages. Global Ecol. Biogeogr. 2013, 22, 671–681. [Google Scholar] [CrossRef]
- Petchey, O.L.; O’Gorman, E.J.; Flynn, D.F.B. A functional guide to functional diversity measures - Chapter 4. In Biodiversity, Ecosystem Functioning, and Human Wellbeing: An Ecological and Economic Perspective; Naeem, S., Bunker, D.E., Hector, A., Loreau, M., Perrings, C., Eds.; Oxford University Press: UK, 2009; pp. 49–59. [Google Scholar]
- Cardinale, B.J.; Srivastava, D.S.; Duffy, J.E.; Wright, J.P.; Downing, A.L.; Sankaran, M.; Jouseau, C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 2006, 443, 989–992. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and Its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Holling, C.S. Cross-Scale Morphology, Geometry, and Dynamics of Ecosystems. Ecol. Monogr. 1992, 62, 447–502. [Google Scholar]
- MacArthur, R.; Wilson, E.O. The Theory of Island Biogeography. Princeton University Press: Princeton, New Jersey, US, 1967; p. 203. [Google Scholar]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. Leaf Life-Span in Relation to Leaf, Plant, and Stand Characteristics among Diverse Ecosystems. Ecol. Monogr. 1992, 62, 365–392. [Google Scholar] [CrossRef]
- Ustin, S.L.; Gamon, J.A. Remote sensing of plant functional types. New Phytol. 2010, 186, 795–816. [Google Scholar] [CrossRef]
- Mason, N., W. H. ; Mouillot, D.; Lee, W.G.; Wilson, J.B.; Setälä, H. Functional Richness, Functional Evenness and Functional Divergence: The Primary Components of Functional Diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Mouchet, M.A.; Villéger, S.; Mason, N.W.H.; Mouillot, D. Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- Schleuter, D.; Daufresne, M.; Massol, F.; Argillier, C. A user's guide to functional diversity indices. Ecol. Monogr. 2010, 80, 469–484. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a mutlifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef]
- Cimoli, E.; Lucieer, A.; Malenovský, Z.; Woodgate, W.; Janoutová, R.; Turner, D.; Haynes, R.S.; Phinn, S. Mapping functional diversity of canopy physiological traits using UAS imaging spectroscopy. Remote Sens. Environ. 2024, 302, 113958. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E. Spectranomics: Emerging science and conservation opportunities at the interface of biodiversity and remote sensing. GECCO 2016, 8, 212–219. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Gamon, J.A.; Hobbie, S.E.; Madritch, M.D.; Meireles, J.E.; Schweiger, A.K.; Townsend, P.A. Harnessing plant spectra to integrate the biodiversity sciences across biological and spatial scales. Am. J. Bot. 2017, 104, 966–969. [Google Scholar] [CrossRef]
- Schweiger, A.K.; Cavender-Bares, J.; Townsend, P.A.; Hobbie, S.E.; Madritch, M.D.; Wang, R.; Tilman, D.; Gamon, J.A. Plant spectral diversity integrates functional and phylogenetic components of biodiversity and predicts ecosystem function. Nat. Ecol. Evol. 2018, 2, 976–982. [Google Scholar] [CrossRef]
- Wang, R.; Gamon, J.A. Remote sensing of terrestrial plant biodiversity. Remote Sens. Environ. 2019, 231, 111218. [Google Scholar] [CrossRef]
- Rocchini, D.; Santos, M.J.; Ustin, S.L.; Feret, J.B.; Asner, G.P.; Beierkuhnlein, C.; Dalponte, M.; Feilhauer, H.; Foody, G.M.; Geller, G.N. , et al. The spectral species concept in living color. J Geophys Res Biogeosci 2022, 127, e2022JG007026. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.D.; Ferraz, A.; Hancock, S.; Duncanson, L.I.; Dubayah, R.O.; Pavlick, R.P.; Schimel, D.S. Towards mapping the diversity of canopy structure from space with GEDI. Env. Res. Lett. 2020, 15, 115006. [Google Scholar] [CrossRef]
- Schweiger, A.K.; Laliberte, E. Plant beta-diversity across biomes captured by imaging spectroscopy. Nat. Commun. 2022, 13, 2767. [Google Scholar] [CrossRef]
- Eichel, J.; Stoffel, M.; Wipf, S. Go or grow? Feedbacks between moving slopes and shifting plants in high mountain environments. Progress in Physical Geography: Earth and Environment 2023, 47, 967–985. [Google Scholar] [CrossRef]
- Restrepo, C.; Walker, L.R.; Shiels, A.B.; Bussmann, R.; Claessens, L.; Fisch, S.; Lozano, P.; Negi, G.; Paolini, L.; Poveda, G. , et al. Landsliding and Its Multiscale Influence on Mountainscapes. BioScience 2009, 59, 685–698. [Google Scholar] [CrossRef]
- Walker, L.R.; Shiels, A.B. Landslide Ecology. Cambridge University Press: Cambridge, 2013. [Google Scholar]
- Myster, R.W.; Fernandez, D.S. Spatial gradients and patch structure on two Puerto Rican landslides. Biotropica 1995, 27, 149–159. [Google Scholar] [CrossRef]
- Tanyaş, H.; Allstadt, K.E.; van Westen, C.J. An updated method for estimating landslide-event magnitude. Earth Surf. Processes 2018, 43, 1836–1847. [Google Scholar] [CrossRef]
- Samia, J.; Temme, A.; Bregt, A.; Wallinga, J.; Guzzetti, F.; Ardizzone, F.; Rossi, M. Do landslides follow landslides? Insights in path dependency from a multi-temporal landslide inventory. Landslides 2017, 14, 547–558. [Google Scholar] [CrossRef]
- Shimokawa, E. A natural recovery process of vegetation on landslide scars and landslide periodicity in forested drainage basings. In Symposium on Effects of Forest Land use on Erosion and Slope Stabililty; O’Loughlin, C.L., Pearce, A.J., Eds.; Environment and Policy Institute East-West Center, University of Hawaii: Honolulu, Hawaii, United States, 1984; pp. 99–107. [Google Scholar]
- Alexandrowicz, Z.; Margielewski, W. Impact of mass movements on geo- and biodiversity in the Polish Outer (Flysch) Carpathians. Geomorphology 2010, 123, 290–304. [Google Scholar] [CrossRef]
- Freund, C.A.; Silman, M.R. Developing a more complete understanding of tropical montane forest disturbance ecology through landslide research. Front. For. Glob. Change 2023, 6, 1091387. [Google Scholar]
- Furusawa, J.; Makoto, K.; Utsumi, S. A large-scale field experiment of artificially caused landslides with replications revealed the response of the ground-dwelling beetle community to landslides. Ecol. Evol. 2023, 13, e9939. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.; Cardigos, P.; Oliveira, S.C.; Zêzere, J.L. Floristic and vegetation successional processes within landslides in a Mediterranean environment. Sci. Total Environ. 2017, 574, 969–981. [Google Scholar] [PubMed]
- He, J.-b.; Wu, Y.-h.; Bing, H.-j.; Zhu, H.; Zhou, J. Soil chronosequence derived from landslides on the upper reach of Minjiang River, western China. J. Mt. Sci. 2023, 20, 1282–1292. [Google Scholar] [CrossRef]
- Lasota, J.; Kraj, W.; Honkowicz, B.; Staszel, K.; Błońska, E. Nutrient status of tree seedlings in a site recovering from a landslide. Forests 2020, 11, 709. [Google Scholar] [CrossRef]
- Ramos Scharrón, C.E.; Castellanos, E.J.; Restrepo, C. The transfer of modern organic carbon by landslide activity in tropical montane ecosystems. Journal of Geophysical Research: Biogeosciences 2012, 117, G03016. [Google Scholar]
- Restrepo, C.; Vitousek, P.; Neville, P. Landslides significantly alter land cover and the distribution of biomass: an example from the Ninole ridges of Hawai'i. Plant Ecology 2003, 166, 131–143. [Google Scholar] [CrossRef]
- Dennison, P.E.; Roberts, D.A. Endmember selection for multiple endmember spectral mixture analysis using endmember average RMSE. Remote Sens. Environ. 2003, 87, 123–135. [Google Scholar] [CrossRef]
- Kluczek, M.; Zagajewski, B.; Kycko, M. Airborne HySpex hyperspectral versus multitemporal Sentinel-2 images for mountain plant communities mapping. Remote Sens. 2022, 14. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Despain, D.G.; Clark, R.N.; Livo, K.E. Mapping vegetation in Yellowstone National Park using spectral feature analysis of AVIRIS data. Remote Sens. Environ. 2003, 84, 437–456. [Google Scholar]
- Marcinkowska-Ochtyra, A.; Zagajewski, B.; Raczko, E.; Ochtyra, A.; Jarocińska, A. Classification of high-mountain vegetation communities within a diverse giant mountains ecosystem using airborne APEX hyperspectral imagery. Remote Sens. 2018, 10, 570. [Google Scholar] [CrossRef]
- Basnet, B.; Vodacek, A. Tracking land use/land cover dynamics in cloud prone areas using moderate resolution satellite data: A case study in Central Africa. Remote Sens. 2015, 7, 6683–6709. [Google Scholar] [CrossRef]
- Bicudo da Silva, R.F.; Millington, J.D.A.; Moran, E.F.; Batistella, M.; Liu, J. Three decades of land-use and land-cover change in mountain regions of the Brazilian Atlantic Forest. Landscape Urban Plan. 2020, 204, 103948. [Google Scholar] [CrossRef]
- Colby, J.D.; Keating, P.L. Land cover classification using Landsat TM imagery in the tropical highlands: The influence of anisotropic reflectance. Int. J. Remote Sens. 1998, 19, 1479–1500. [Google Scholar] [CrossRef]
- Peyre, G.; Osorio, D.; François, R.; Anthelme, F. Mapping the páramo land-cover in the Northern Andes. Int. J. Remote Sens. 2021, 42, 7777–7797. [Google Scholar] [CrossRef]
- Brandt, J.S.; Townsend, P.A. Land use – land cover conversion, regeneration and degradation in the high elevation Bolivian Andes. Landscape Ecol. 2006, 21, 607–623. [Google Scholar] [CrossRef]
- Chen, X.; Chen, W.; Xu, M. Remote-sensing monitoring of postfire vegetation dynamics in the Greater Hinggan Mountain Range Based on long time-series data: Analysis of the effects of six topographic and climatic factors. Remote Sens. 2022, 14. [Google Scholar] [CrossRef]
- Hamunyela, E.; Brandt, P.; Shirima, D.; Do, H.T.T.; Herold, M.; Roman-Cuesta, R.M. Space-time detection of deforestation, forest degradation and regeneration in montane forests of Eastern Tanzania. Int. J. Appl. Earth Obs. 2020, 88, 102063. [Google Scholar]
- Miletić, B.R.; Matović, B.; Orlović, S.; Gutalj, M.; Đorem, T.; Marinković, G.; Simović, S.; Dugalić, M.; Stojanović, D.B. Quantifying forest cover loss as a response to drought and dieback of Norway Spruce and evaluating sensitivity of various vegetation indices using remote sensing. Forests 2024, 15, 662. [Google Scholar] [CrossRef]
- Restrepo, C.; Alvarez, N. Landslides and Their Contribution to Land-cover Change in the Mountains of Mexico and Central America1. Biotropica 2006, 38, 446–457. [Google Scholar] [CrossRef]
- Novellino, A.; Pennington, C.; Leeming, K.; Taylor, S.; Alvarez, I.G.; McAllister, E.; Arnhardt, C.; Winson, A. Mapping landslides from space: A review. Landslides 2024, 21, 1041–1052. [Google Scholar]
- Chapman, D.S. Greater phenological sensitivity to temperature on higher Scottish mountains: new insights from remote sensing. Glob. Change Biol. 2013, 19, 3463–3471. [Google Scholar] [CrossRef]
- Cho, M.A.; Skidmore, A.K. Hyperspectral predictors for monitoring biomass production in Mediterranean mountain grasslands: Majella National Park, Italy. Int. J. Remote Sens. 2009, 30, 499–515. [Google Scholar] [CrossRef]
- Schino, G.; Borfecchia, F.; De Cecco, L.; Dibari, C.; Iannetta, M.; Martini, S.; Pedrotti, F. Satellite estimate of grass biomass in a mountainous range in central Italy. Agrofor. Syst. 2003, 59, 157–162. [Google Scholar] [CrossRef]
- Soenen, S.A.; Peddle, D.R.; Hall, R.J.; Coburn, C.A.; Hall, F.G. Estimating aboveground forest biomass from canopy reflectance model inversion in mountainous terrain. Remote Sens. Environ. 2010, 114, 1325–1337. [Google Scholar] [CrossRef]
- Uscanga, A.; Shuler, S.; Silva, L.C.R. Incorporating small-scale disturbances in models of forest structure and aboveground biomass of tropical mountains. Ecosphere 2024, 15, e4744. [Google Scholar] [CrossRef]
- Ollinger, S.V.; Smith, M.L.; Martin, M.E.; Hallett, R.A.; Goodale, C.L.; Aber, J.D. Regional variation in foliar chemistry and N cycling among forests of diverse history and composition. Ecology 2002, 83, 339–355. [Google Scholar]
- Townsend, P.A. Application of imaging spectroscopy to mapping canopy nitrogen in the forests of the Central Appalachian mountains using Hyperion and AVIRIS. IEEE Trans. Geosci. Remote Sens. 2003, 41, 1347–1354. [Google Scholar] [CrossRef]
- Chadwick, K.D.; Asner, G.P. Landscape evolution and nutrient rejuvenation reflected in Amazon forest canopy chemistry. Ecol. Lett. 2018, 21, 978–988. [Google Scholar] [CrossRef]
- Schneider, F.D.; Longo, M.; Paul-Limoges, E.; Scholl, V.M.; Schmid, B.; Morsdorf, F.; Pavlick, R.P.; Schimel, D.S.; Schaepman, M.E.; Moorcroft, P.R. Remote sensing-based forest modeling reveals positive effects of functional diversity on productivity at local spatial scale. Journal of Geophysical Research: Biogeosciences 2023, 128, e2023JG007421. [Google Scholar] [CrossRef]
- Durán, S.M.; Martin, R.E.; Díaz, S.; Maitner, B.S.; Malhi, Y.; Salinas, N.; Shenkin, A.; Silman, M.R.; Wieczynski, D.J.; Asner, G.P. , et al. Informing trait-based ecology by assessing remotely sensed functional diversity across a broad tropical temperature gradient. Sci. Adv. 2019, 5, eaaw8114. [Google Scholar] [CrossRef] [PubMed]
- Freund, C.A.; Clark, K.E.; Curran, J.F.; Asner, G.P.; Silman, M.R. Landslide age, elevation and residual vegetation determine tropical montane forest canopy recovery and biomass accumulation after landslide disturbances in the Peruvian Andes. J. Ecol. 2021, 109, 3555–3571. [Google Scholar] [CrossRef]
- Li, M.; Ma, C.; Du, C.; Yang, W.; Lyu, L.; Wang, X. Landslide response to vegetation by example of July 25–26, 2013, extreme rainstorm, Tianshui, Gansu Province, China. B. Eng. Geol. Environ. 2021, 80, 751–764. [Google Scholar]
- Yunus, A.P.; Fan, X.; Tang, X.; Jie, D.; Xu, Q.; Huang, R. Decadal vegetation succession from MODIS reveals the spatio-temporal evolution of post-seismic landsliding after the 2008 Wenchuan earthquake. Remote Sens. Environ. 2020, 236, 111476. [Google Scholar]
- Jiao, Q.; Zhang, B.; Liu, L.; Li, Z.; Yue, Y.; Hu, Y. Assessment of spatio-temporal variations in vegetation recovery after the Wenchuan earthquake using Landsat data. Nat. Hazards 2014, 70, 1309–1326. [Google Scholar] [CrossRef]
- Saito, H.; Uchiyama, S.; Teshirogi, K. Rapid vegetation recovery at landslide scars detected by multitemporal high-resolution satellite imagery at Aso volcano, Japan. Geomorphology 2022, 398. [Google Scholar] [CrossRef]
- Asner, G.P.; Mascaro, J. Mapping tropical forest carbon: Calibrating plot estimates to a simple LiDAR metric. Remote Sens. Environ. 2014, 140, 614–624. [Google Scholar] [CrossRef]
- Nakata, Y.; Hayamizu, M.; Ishiyama, N. Assessing primary vegetation recovery from earthquake-induced landslide scars: A real-time kinematic unmanned aerial vehicle approach. Ecol. Eng. 2023, 193, 107019. [Google Scholar]
- Thapa, P.S.; Daimaru, H.; Yanai, S. Analyzing vegetation recovery and erosion status after a large Landslide at Mt. Hakusan, Central Japan. Ecol. Eng. 2024, 198, 107144. [Google Scholar] [CrossRef]
- Badía, D.; Martí, C.; Aznar, J.M.; León, J. Influence of slope and parent rock on soil genesis and classification in semiarid mountainous environments. Geoderma 2013, 193-194, 13–21. [Google Scholar] [CrossRef]
- Feldman, S.B.; Zelazny, L.W.; Baker, J.C. High-elevation forest soils of the Southern Appalachians: I. Distribution of parent materials and soil-landscape relationships. Soil Sci. Soc. Am. J. 1991, 55, 1629–1637. [Google Scholar] [CrossRef]
- Mage, S.M.; Porder, S. Parent material and topography determine soil phosphorus status in the Luquillo Mountains of Puerto Rico. Ecosystems 2013, 16, 284–294. [Google Scholar] [CrossRef]
- Wilson, S.G.; Dahlgren, R.A.; Margenot, A.J.; Rasmussen, C.; O'Geen, A.T. Expanding the Paradigm: The influence of climate and lithology on soil phosphorus. Geoderma 2022, 421, 115809. [Google Scholar] [CrossRef]
- Hahm, W.J.; Rempe, D.M.; Dralle, D.N.; Dawson, T.E.; Lovill, S.M.; Bryk, A.B.; Bish, D.L.; Schieber, J.; Dietrich, W.E. Lithologically controlled subsurface critical zone thickness and water storage capacity determine regional plant community composition. Water Resour. Res. 2019, 55, 3028–3055. [Google Scholar]
- Hahm, W.J.; Riebe, C.S.; Lukens, C.E.; Araki, S. Bedrock composition regulates mountain ecosystems and landcape evolution. Proc. Natl. Acad. Sci. U S A 2014, 111, 3338–3343. [Google Scholar] [CrossRef]
- Watt, M.S.; Pearse, G.D.; Dash, J.P.; Melia, N.; Leonardo, E.M.C. Application of remote sensing technologies to identify impacts of nutritional deficiencies on forests. ISPRS J. Photogramm. Rem. Sens. 2019, 149, 226–241. [Google Scholar]
- Chen, Y.; Wang, Y.; Zhang, F.; Dong, Y.; Song, Z.; Liu, G. Remote sensing for lithology mapping in vegetation-covered regions: Methods, challenges, and opportunities. Minerals 2023, 13, 1153. [Google Scholar] [CrossRef]
- Peyghambari, S.; Zhang, Y. Hyperspectral remote sensing in lithological mapping, mineral exploration, and environmental geology: An updated review. J. Appl. Remote Sens. 2021, 15, 031501. [Google Scholar] [CrossRef]
- Bai, S.; Zhao, J. A new strategy to fuse remote sensing data and geochemical data with different machine learning methods. Remote Sens. 2023, 15, 930. [Google Scholar] [CrossRef]
- Fan, Y.-H.; Wang, H. Application of remote sensing to identify Copper–Lead–Zinc deposits in the Heiqia area of the West Kunlun Mountains, Chinas. Sci. Rep. 2020, 10, 12309. [Google Scholar] [CrossRef]
- He, J.; Chen, D.; Zhan, Y.; Ren, X.; Li, Q. A retrieval model of surface geochemistry composition based on remotely sensed data. Open Geosci. 2023, 15, 20220514. [Google Scholar] [CrossRef]
- Riaza, A.; Strobl, P.; Beisl, U.; Hausold, A.; Müller, A. Spectral mapping of rock weathering degrees on granite using hyperspectral DAIS 7915 spectrometer data. Int. J. Appl. Earth Obs. 2001, 3, 345–354. [Google Scholar]
- Sousa, F.J.; Sousa, D.J. Spatial patterns of chemical weathering at the basal tertiary nonconformity in California from multispectral and hyperspectral optical remote sensing. Remote Sens. 2019, 11, 2528. [Google Scholar] [CrossRef]
- Thomas, M.; Walter, M.R. Application of hyperspectral infrared analysis of hydrothermal alteration on Earth and Mars. Astrobiology 2002, 2, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, T.; Chabrillat, S.; Pignatti, S.; Milewski, R.; Karyotis, K.; Brell, M.; Ruhtz, T.; Bochtis, D.; Zalidis, G. Evaluation of airborne HySpex and spaceborne PRISMA hyperspectral remote sensing data for soil organic matter and carbonates estimation. Remote Sens. 2023, 15, 1106. [Google Scholar] [CrossRef]
- Baptista, G.M.M.; Corrêa, R.S.; dos Santos, P.F.; Madeira Netto, J.S.; Meneses, P.R. Use of imaging spectroscopy for mapping and quantifying the weathering degree of tropical soils in Central Brazil. Appl. Environ. Soil Sci. (Online) 2011, 2011, 641328. [Google Scholar]
- Gomez, C.; Lagacherie, P.; Coulouma, G. Regional predictions of eight common soil properties and their spatial structures from hyperspectral Vis–NIR data. Geoderma 2012, 189-190, 176–185. [Google Scholar] [CrossRef]
- Leone, A.P.; Wright, G.G.; Corves, C. The application of satellite remote sensing for soil studies in upland areas of Southern Italy. Int. J. Remote Sens. 1995, 16, 1087–1105. [Google Scholar] [CrossRef]
- Palacios-Orueta, A.; Pinzón, J.E.; Ustin, S.L.; Roberts, D.A. Remote sensing of soils in the Santa Monica Mountains: II. Hierarchical foreground and background analysis. Remote Sens. Environ. 1999, 68, 138–151. [Google Scholar]
- Yu, H.; Kong, B.; Wang, G.; Du, R.; Qie, G. Prediction of soil properties using a hyperspectral remote sensing method. Arch. Acker Pfl. Boden. 2018, 64, 546–559. [Google Scholar]
- Zou, J.; Wei, Y.; Zhang, Y.; Liu, Z.; Gai, Y.; Chen, H.; Liu, P.; Song, Q. Remote sensing inversion of soil organic matter in cropland combining topographic factors with spectral parameters. Front. Environ. Sci. 2024, 12, 1420557. [Google Scholar] [CrossRef]
- Ge, Y.; Thomasson, J.A.; Sui, R. Remote sensing of soil properties in precision agriculture: A review. Front. Earth Sci. 2011, 5, 229–238. [Google Scholar] [CrossRef]
- Mulder, V.L.; de Bruin, S.; Schaepman, M.E.; Mayr, T.R. The use of remote sensing in soil and terrain mapping — A review. Geoderma 2011, 162, 1–19. [Google Scholar] [CrossRef]
- Park, J.; Kim, K. Quantification of rock mass weathering using spectral imaging. J. South. Afr. Inst. Min. Metall. 2019, 119, 1039–1046. [Google Scholar]
- van der Meer, F.D.; van der Werff, H.M.A.; van Ruitenbeek, F.J.A.; Hecker, C.A.; Bakker, W.H.; Noomen, M.F.; van der Meijde, M.; Carranza, E.J.M.; Smeth, J.B.d.; Woldai, T. Multi- and hyperspectral geologic remote sensing: A review. Int. J. Appl. Earth Obs. 2012, 14, 112–128. [Google Scholar] [CrossRef]
- Yu, H.; Kong, B.; Wang, Q.; Liu, X.; Liu, X. 14 - Hyperspectral remote sensing applications in soil: a review. In Hyperspectral Remote Sensing; Pandey, P.C., Srivastava, P.K., Balzter, H., Bhattacharya, B., Petropoulos, G.P., Eds.; Elsevier, 2020; pp. 269–291. [Google Scholar]
- Vitousek, P.; Asner, G.P.; Chadwick, O.A.; Hotchkiss, S. Landscape-level variation in forest structure and biogeochemistry across a substrate age gradient in Hawaii. Ecology 2009, 90, 3074–3086. [Google Scholar] [CrossRef] [PubMed]
- Porder, S.; Asner, G.P.; Vitousek, P.M. Ground-based and remotely sensed nutrient availability across a tropical landscape. Proc. Natl. Acad. Sci. U S A 2005, 102, 10909–10912. [Google Scholar] [CrossRef]
- Weintraub, S.R.; Taylor, P.G.; Porder, S.; Cleveland, C.C.; Asner, G.P.; Townsend, A.R. Topographic controls on soil nitrogen availability in a lowland tropical forest. Ecology 2015, 96, 1561–1574. [Google Scholar] [CrossRef]
- Chadwick, K.D.; Asner, G.P. Tropical soil nutrient distributions determined by biotic and hillslope processes. Biogeochemistry 2016, 127, 273–289. [Google Scholar] [CrossRef]
- Amaral, C.H.d.; Almeida, T.I.R.d.; Souza Filho, C.R.d.; Roberts, D.A.; Fraser, S.J.; Alves, M.N.; Botelho, M. Characterization of indicator tree species in neotropical environments and implications for geological mapping. Remote Sens. Environ. 2018, 216, 385–400. [Google Scholar] [CrossRef]
- Chakraborty, R.; Kereszturi, G.; Pullanagari, R.; Durance, P.; Ashraf, S.; Anderson, C. Mineral prospecting from biogeochemical and geological information using hyperspectral remote sensing - Feasibility and challenges. J. Geochem. Explor. 2022, 232, 106900. [Google Scholar]
- Collins, W.; Chang, S.-H.; Raines, G.L.; Canney, F.; Ashley, R. Airborne biogeophysical mapping of hidden mineral deposits. Econ. Geol. 1983, 78, 737–749. [Google Scholar] [CrossRef]
- Goetz, A.F.H.; Rock, B.N.; Rowan, L.C. Remote sensing for exploration: An overview. Econ. Geol. 1983, 78, 573–590. [Google Scholar] [CrossRef]
- Hede, A.N.H.; Kashiwaya, K.; Koike, K.; Sakurai, S. A new vegetation index for detecting vegetation anomalies due to mineral deposits with application to a tropical forest area. Remote Sens. Environ. 2015, 171, 83–97. [Google Scholar] [CrossRef]
- Hede, A.N.H.; Koike, K.; Kashiwaya, K.; Sakurai, S.; Yamada, R.; Singer, D.A. How can satellite imagery be used for mineral exploration in thick vegetation areas? Geochem. Geophy. Geosy. 2017, 18, 584–596. [Google Scholar] [CrossRef]
- Milton, N.M.; Collins, W.; Chang, S.-H.; Schmidt, R.G. Remote detection of metal anomalies on Pilot Mountain, Randolph County, North Carolina. Econ. Geol. 1983, 78, 605–617. [Google Scholar] [CrossRef]
- Rodriguez-Gomez, C.; Kereszturi, G.; Jeyakumar, P.; Pullanagari, R.; Reeves, R.; Rae, A.; Procter, J.N. Remote exploration and monitoring of geothermal sources: A novel method for foliar element mapping using hyperspectral (VNIR-SWIR) remote sensing. Geothermics 2023, 111, 102716. [Google Scholar]
- Rowan, L.C.; Crowley, J.K.; Schmidt, R.G.; Ager, C.M.; Mars, J.C. Mapping hydrothermally altered rocks by analyzing hyperspectral image (AVIRIS) data of forested areas in the Southeastern United States. J. Geochem. Explor. 2000, 68, 145–166. [Google Scholar] [CrossRef]
- Sabins, F.F. Remote sensing for mineral exploration. Ore Geol. Rev. 1999, 14, 157–183. [Google Scholar] [CrossRef]
- Schellekens, J., H.; Gilbes, F.; Rivera, G.D.; Yuri, C.Y.; Chardón, S.; Fong, Y. Reflectance spectra of tropical vegetation as a response to metal enrichment in the substrate of west-central Puerto rico. Caribb. J. Earth Sci. 2005, 39, 9–12. [Google Scholar]
- Shin, J.H.; Yu, J.; Wang, L.; Kim, J.; Koh, S.-M. Investigation of spectral variation of pine needles as an indicator of arsenic content in soils. Minerals 2019, 9, 498. [Google Scholar] [CrossRef]
- Alekseenko, V.A.; Shvydkaya, N.V.; Alekseenko, A.V.; Machevariani, M.M.; Bech, J.; Pashkevich, M.A.; Puzanov, A.V.; Nastavkin, A.V.; Roca, N. Element accumulation patterns of native plant species under the natural geochemical stress. Plants 2021, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.R.; Dunn, C.E.; Hall, G.E.M. Biological Systems in Mineral Exploration and Processing; Ellis Horwood: New York, United States, 1995. [Google Scholar]
- Dunn, C.E. Biogeochemistry in Mineral Exploration; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Ufimtseva, M.D. The patterns in accumulation of chemical elements by higher plants and their responses in biogeochemical provinces. Geochem. Int. 2015, 53, 441–455. [Google Scholar] [CrossRef]
- Hardiman, B.S.; Gough, C.M.; Butnor, J.R.; Bohrer, G.; Detto, M.; Curtis, P.S. Coupling Fine-Scale Root and Canopy Structure Using Ground-Based Remote Sensing. Remote Sens. 2017, 9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




