Submitted:
07 October 2024
Posted:
08 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Distinct Molecular Signatures of Stem Cell Markers in MTC Cell Lines
2.2. Validation Analysis Confirmed Higher Expression of Stemness Markers in MZ-CRC-1 Cells
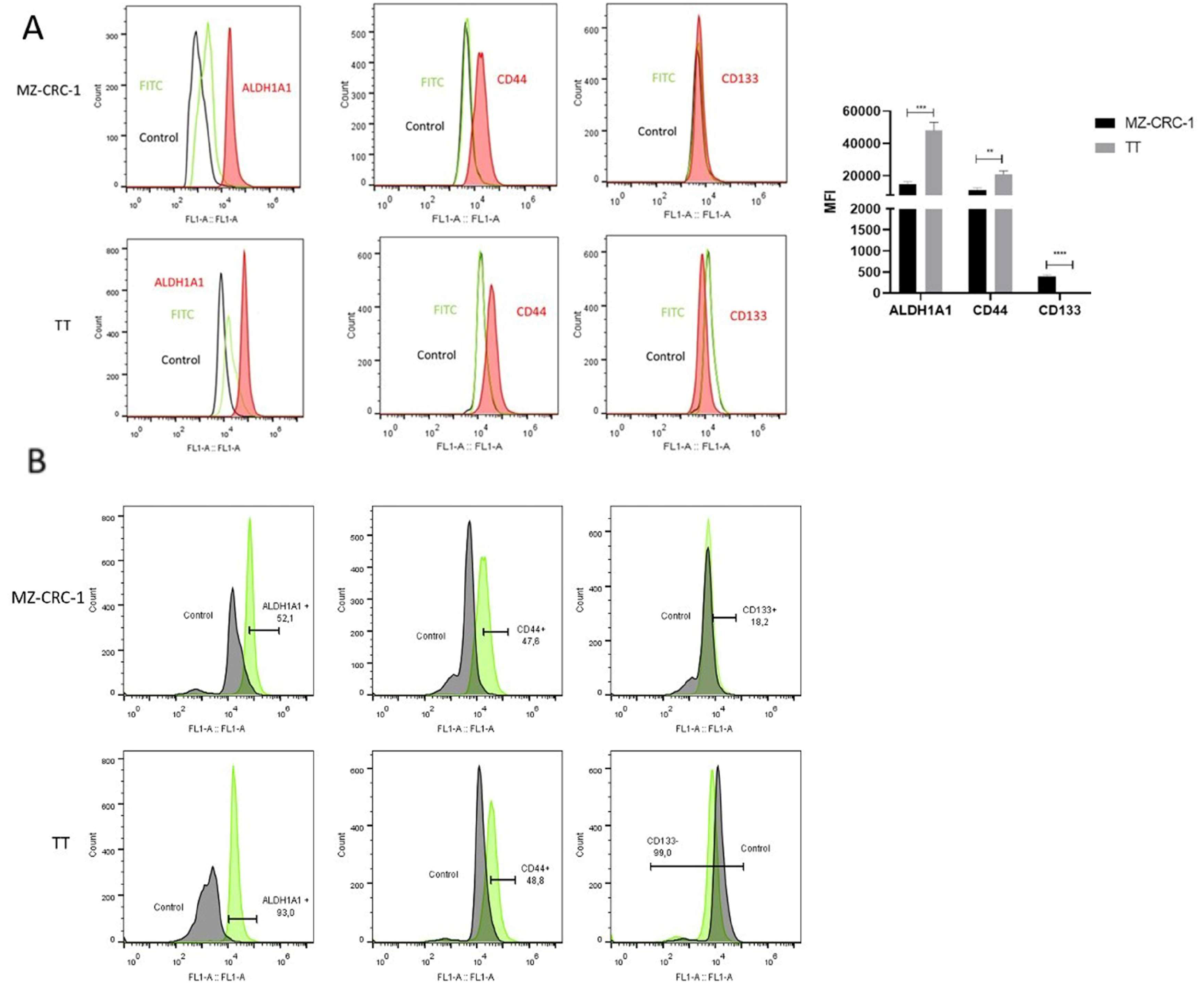
2.3. The Expression of Hypothetical Thyroid Cancer Stem Cell in MTC Cell Lines
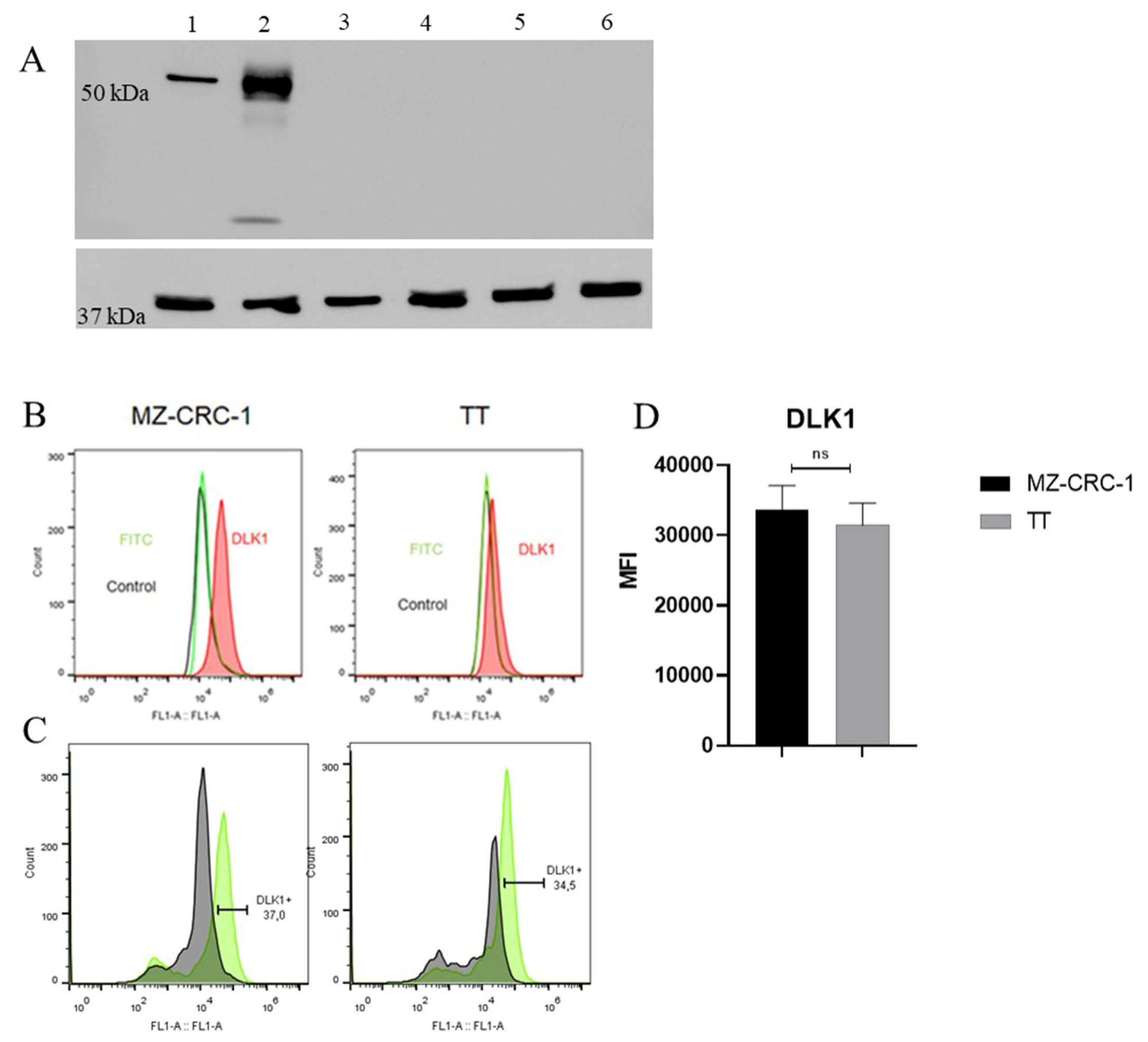
2.4. DLK1 Is Specifically Expressed in MTC Cell Lines
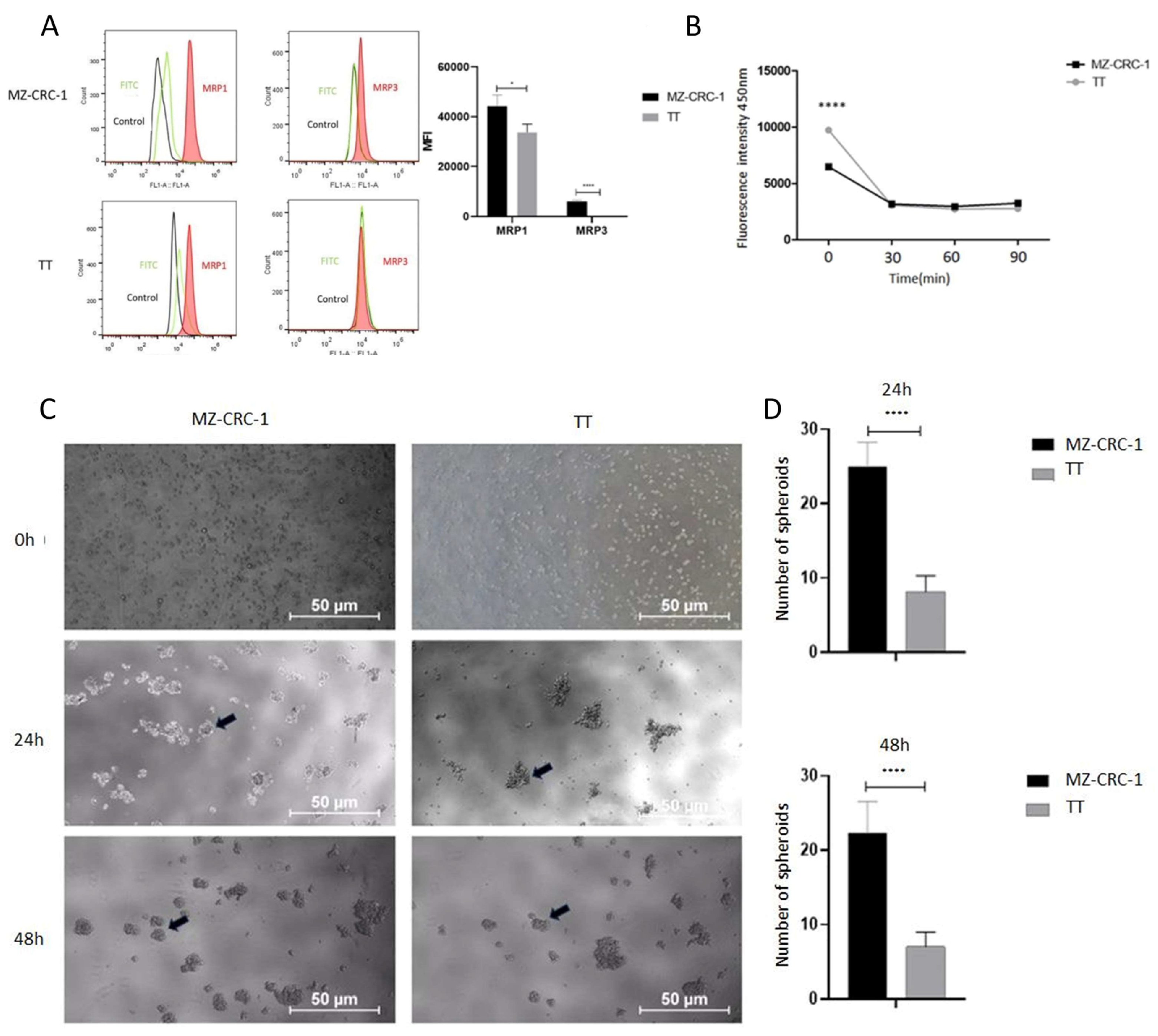
2.5. Medullary Thyroid Carcinoma Cells Exhibit Expression of Multidrug Resistance Proteins
2.6. Enhanced Spheroid Generation Ability in MZ-CRC-1 Cells
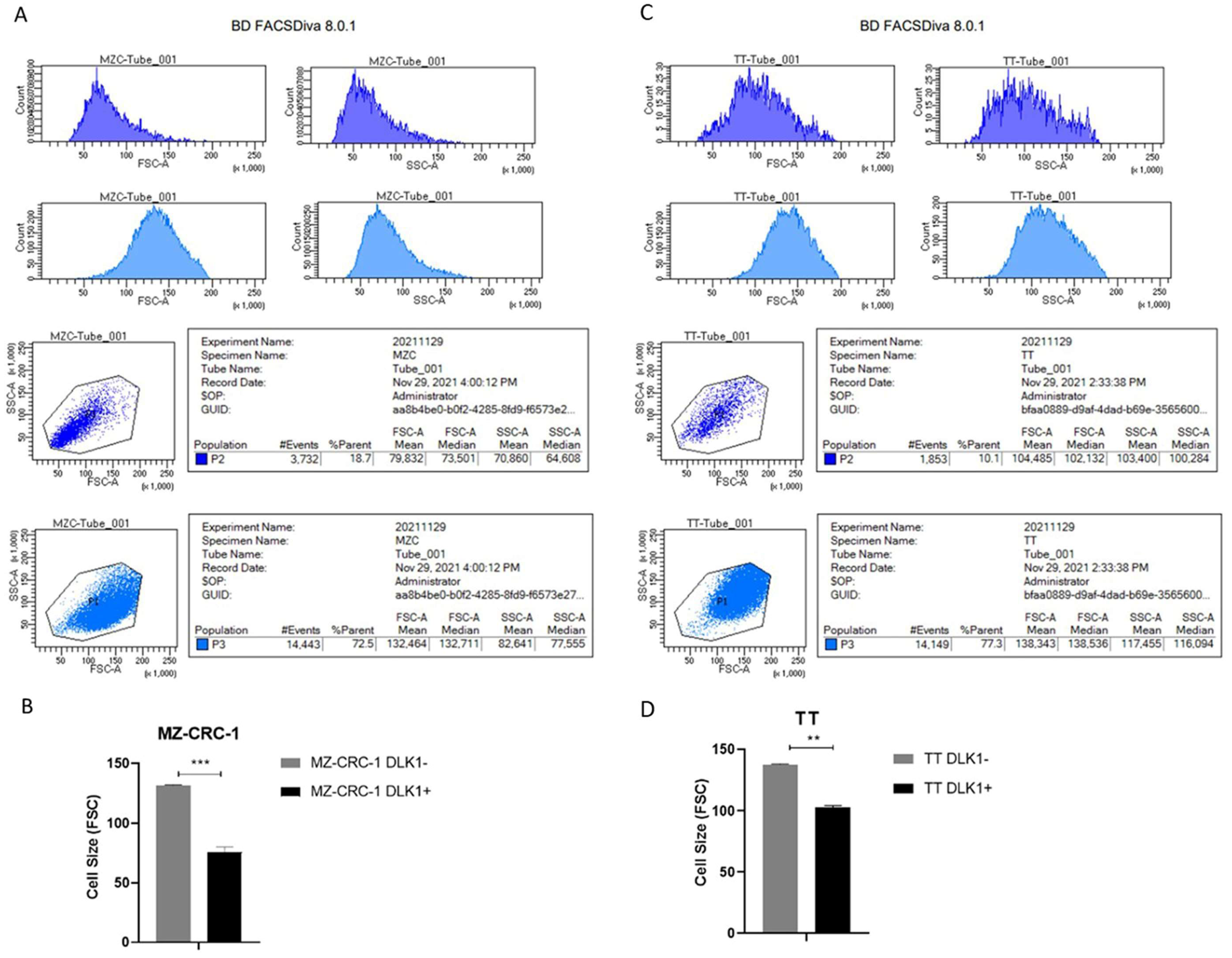
2.7. Cell Sorting and Characterization of DLK1-Positive Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Protein Array Analysis
4.3. Flow Cytometry Analysis
4.4. Hoechst 33342 Efflux Assay
4.6. Generation of Spheroids Cultures
4.7. Western Blot Analysis
4.8. Fluorescence-Activated Cell Sorting (FACS)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- American Thyroid Association Guidelines Task Force; Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib H, Moley JF, Pacini F, Ringel MD, Schlumberger M, Wells SA Jr. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009 Jun;19(6):565-612. Erratum in: Thyroid. 2009 Nov;19(11):1295. [CrossRef] [PubMed]
- Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robinson B, Rosenthal MS, Santoro M, Schlumberger M, Shah M, Waguespack SG; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015 Jun;25(6):567-610. [CrossRef] [PubMed]
- Cerutti JM, Maciel RM. An unusual genotype-phenotype correlation in MEN 2 patients: should screening for RET double germline mutations be performed to avoid misleading diagnosis and treatment? Clin Endocrinol (Oxf). 2013 Oct;79(4):591-2. Epub 2013 May 11. [CrossRef] [PubMed]
- Araujo AN, Moraes L, França MI, Hakonarson H, Li J, Pellegrino R, Maciel RM, Cerutti JM. Genome-wide copy number analysis in a family with p.G533C RET mutation and medullary thyroid carcinoma identified regions potentially associated with a higher predisposition to lymph node metastasis. J Clin Endocrinol Metab. 2014 Jun;99(6):E1104-12. Epub 2014 Mar 6. [CrossRef] [PubMed]
- Signorini PS, França MI, Camacho CP, Lindsey SC, Valente FO, Kasamatsu TS, Machado AL, Salim CP, Delcelo R, Hoff AO, Cerutti JM, Dias-da-Silva MR, Maciel RM. A ten-year clinical update of a large RET p.Gly533Cys kindred with medullary thyroid carcinoma emphasizes the need for an individualized assessment of affected relatives. Clin Endocrinol (Oxf). 2014 Feb;80(2):235-45. Epub 2013 Jun 28. [CrossRef] [PubMed]
- Ceolin L, Duval MADS, Benini AF, Ferreira CV, Maia AL. Medullary thyroid carcinoma beyond surgery: advances, challenges, and perspectives. Endocr Relat Cancer. 2019 Aug 1;26(9):R499-R518. [CrossRef] [PubMed]
- Maciel RMB, Camacho CP, Assumpção LVM, Bufalo NE, Carvalho AL, de Carvalho GA, Castroneves LA, de Castro FM, Ceolin L, Cerutti JM, Corbo R, Ferraz TMBL, Ferreira CV, França MIC, Galvão HCR, Germano-Neto F, Graf H, Jorge AAL, Kunii IS, Lauria MW, Leal VLG, Lindsey SC, Lourenço DM, Maciel LMZ, Magalhães PKR, Martins JRM, Martins-Costa MC, Mazeto GMFS, Impellizzeri AI, Nogueira CR, Palmero EI, Pessoa CHCN, Prada B, Siqueira DR, Sousa MSA, Toledo RA, Valente FOF, Vaisman F, Ward LS, Weber SS, Weiss RV, Yang JH, Dias-da-Silva MR, Hoff AO, Toledo SPA, Maia AL. Genotype and phenotype landscape of MEN2 in 554 medullary thyroid cancer patients: the BrasMEN study. Endocr Connect. 2019 Mar 1;8(3):289-298. [CrossRef] [PubMed]
- Moura MM, Cavaco BM, Pinto AE, Leite V. High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J Clin Endocrinol Metab. 2011 May;96(5):E863-8. Epub 2011 Feb 16. [CrossRef] [PubMed]
- Agrawal N, Jiao Y, Sausen M, Leary R, Bettegowda C, Roberts NJ, Bhan S, Ho AS, Khan Z, Bishop J, Westra WH, Wood LD, Hruban RH, Tufano RP, Robinson B, Dralle H, Toledo SP, Toledo RA, Morris LG, Ghossein RA, Fagin JA, Chan TA, Velculescu VE, Vogelstein B, Kinzler KW, Papadopoulos N, Nelkin BD, Ball DW. Exomic sequencing of medullary thyroid cancer reveals dominant and mutually exclusive oncogenic mutations in RET and RAS. J Clin Endocrinol Metab. 2013 Feb;98(2):E364-9. Epub 2012 Dec 21. [CrossRef] [PubMed]
- Oczko-Wojciechowska M, Czarniecka A, Gawlik T, Jarzab B, Krajewska J. Current status of the prognostic molecular markers in medullary thyroid carcinoma. Endocr Connect. 2020 Dec;9(12):R251-R263. [CrossRef] [PubMed]
- Yeh T, Yeung M, Sherman EJ, Tuttle RM, Sabra MM. Structural Doubling Time Predicts Overall Survival in Patients with Medullary Thyroid Cancer in Patients with Rapidly Progressive Metastatic Medullary Thyroid Cancer Treated with Molecular Targeted Therapies. Thyroid. 2020 Aug;30(8):1112-1119. Epub 2020 Apr 20. [CrossRef] [PubMed]
- Elisei R, Cosci B, Romei C, Bottici V, Renzini G, Molinaro E, Agate L, Vivaldi A, Faviana P, Basolo F, Miccoli P, Berti P, Pacini F, Pinchera A. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab. 2008 Mar;93(3):682-7. Epub 2007 Dec 11. [CrossRef] [PubMed]
- Moura MM, Cavaco BM, Leite V. RAS proto-oncogene in medullary thyroid carcinoma. Endocr Relat Cancer. 2015 Oct;22(5):R235-52. [CrossRef] [PubMed]
- Ciampi R, Romei C, Ramone T, Prete A, Tacito A, Cappagli V, Bottici V, Viola D, Torregrossa L, Ugolini C, Basolo F, Elisei R. Genetic Landscape of Somatic Mutations in a Large Cohort of Sporadic Medullary Thyroid Carcinomas Studied by Next-Generation Targeted Sequencing. iScience. 2019 Oct 25;20:324-336. Epub 2019 Sep 26. [CrossRef] [PubMed]
- Kim M, Kim BH. Current Guidelines for Management of Medullary Thyroid Carcinoma. Endocrinol Metab (Seoul). 2021 Jun;36(3):514-524. Epub 2021 Jun 22. [CrossRef] [PubMed]
- Pezzani R, Bertazza L, Cavedon E, Censi S, Manso J, Watutantrige-Fernando S, Pennelli G, Galuppini F, Barollo S, Mian C. Novel Prognostic Factors Associated with Cell Cycle Control in Sporadic Medullary Thyroid Cancer Patients. Int J Endocrinol. 2019 Feb 18;2019:9421079. [CrossRef] [PubMed]
- Subbiah V, Gouda MA, Iorgulescu JB, Dadu R, Patel K, Sherman S, Cabanillas M, Hu M, Castellanos LE, Amini B, Meric-Bernstam F, Shen T, Wu J. Adaptive Darwinian off-target resistance mechanisms to selective RET inhibition in RET driven cancer. NPJ Precis Oncol. 2024 Mar 4;8(1):62. [CrossRef] [PubMed]
- Subbiah V, Shen T, Terzyan SS, Liu X, Hu X, Patel KP, Hu M, Cabanillas M, Behrang A, Meric-Bernstam F, Vo PTT, Mooers BHM, Wu J. Structural basis of acquired resistance to selpercatinib and pralsetinib mediated by non-gatekeeper RET mutations. Ann Oncol. 2021 Feb;32(2):261-268. Epub 2020 Nov 5. [CrossRef] [PubMed]
- Maliszewska A, Leandro-Garcia LJ, Castelblanco E, Macià A, de Cubas A, Goméz-López G, Inglada-Pérez L, Álvarez-Escolá C, De la Vega L, Letón R, Gómez-Graña Á, Landa I, Cascón A, Rodríguez-Antona C, Borrego S, Zane M, Schiavi F, Merante-Boschin I, Pelizzo MR, Pisano DG, Opocher G, Matias-Guiu X, Encinas M, Robledo M. Differential gene expression of medullary thyroid carcinoma reveals specific markers associated with genetic conditions. Am J Pathol. 2013 Feb;182(2):350-62. Epub 2012 Nov 28. [CrossRef] [PubMed]
- Bi Y, Meng Y, Wu H, Cui Q, Luo Y, Xue X. Expression of the potential cancer stem cell markers CD133 and CD44 in medullary thyroid carcinoma: A ten-year follow-up and prognostic analysis. J Surg Oncol. 2016 Feb;113(2):144-51. Epub 2016 Jan 12. [CrossRef] [PubMed]
- Cordero-Barreal A, Caleiras E, López de Maturana E, Monteagudo M, Martínez-Montes ÁM, Letón R, Gil E, Álvarez-Escolá C, Regojo RM, Andía V, Marazuela M, Guadalix S, Calatayud M, Robles-Díaz L, Aguirre M, Cano JM, Díaz JÁ, Saavedra P, Lamas C, Azriel S, Sastre J, Aller J, Leandro-García LJ, Calsina B, Roldán-Romero JM, Santos M, Lanillos J, Cascón A, Rodríguez-Antona C, Robledo M, Montero-Conde C. CD133 Expression in Medullary Thyroid Cancer Cells Identifies Patients with Poor Prognosis. J Clin Endocrinol Metab. 2020 Nov 1;105(11):dgaa527. [CrossRef] [PubMed]
- Vargas CVF, Ceolin L, Scheffel RS, Benini AF, Graudenz MS, Maia AL. The tissue expression pattern of CA 19.9 is associated with oncological features in medullary thyroid carcinoma. Endocrine. 2020 Dec;70(3):544-551. Epub 2020 Jun 13. [CrossRef] [PubMed]
- Pitoia F, Trimboli P, Abelleira E. Primary resistance to selpercatinib in a patient with advanced medullary thyroid cancer. Endocrine. 2024 May 27. Epub ahead of print. [CrossRef] [PubMed]
- Barbet J, Campion L, Kraeber-Bodéré F, Chatal JF; GTE Study Group. Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J Clin Endocrinol Metab. 2005 Nov;90(11):6077-84. Epub 2005 Aug 9. [CrossRef] [PubMed]
- Williams JF, Zhao M, Najdawi F, Ahmadi S, Hornick JL, Wong KS, Barletta JA. Grading of Medullary Thyroid Carcinoma: an Interobserver Reproducibility Study. Endocr Pathol. 2022 Sep;33(3):371-377. Epub 2022 May 13. [CrossRef] [PubMed]
- Araujo AN, Camacho CP, Mendes TB, Lindsey SC, Moraes L, Miyazawa M, Delcelo R, Pellegrino R, Mazzotti DR, Maciel RMB, Cerutti JM. Comprehensive Assessment of Copy Number Alterations Uncovers Recurrent AIFM3 and DLK1 Copy Gain in Medullary Thyroid Carcinoma. Cancers (Basel). 2021 Jan 9;13(2):218. [CrossRef] [PubMed]
- Helman LJ, Thiele CJ, Linehan WM, Nelkin BD, Baylin SB, Israel MA. Molecular markers of neuroendocrine development and evidence of environmental regulation. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2336-9. [CrossRef] [PubMed]
- Pittaway JFH, Lipsos C, Mariniello K, Guasti L. The role of delta-like non-canonical Notch ligand 1 (DLK1) in cancer. Endocr Relat Cancer. 2021 Oct 15;28(12):R271-R287. [CrossRef] [PubMed]
- Kim HS, Ahn SH, Kim HJ, Park JW, Han I. Delta-like Factor 1 as a Possible Therapeutic Target for Sarcomas. Clin Orthop Surg. 2020 Sep;12(3):404-412. Epub 2020 Jun 26. [CrossRef] [PubMed]
- Grassi ES, Pietras A. Emerging Roles of DLK1 in the Stem Cell Niche and Cancer Stemness. J Histochem Cytochem. 2022 Jan;70(1):17-28. Epub 2021 Oct 4. [CrossRef] [PubMed]
- Nagayama Y, Shimamura M, Mitsutake N. Cancer Stem Cells in the Thyroid. Front Endocrinol (Lausanne). 2016 Feb 29;7:20. [CrossRef] [PubMed]
- Hadoux J, Pacini F, Tuttle RM, Schlumberger M. Management of advanced medullary thyroid cancer. Lancet Diabetes Endocrinol. 2016 Jan;4(1):64-71. Epub 2015 Oct 23. [CrossRef] [PubMed]
- Angelousi A, Hayes AR, Chatzellis E, Kaltsas GA, Grossman AB. Metastatic medullary thyroid carcinoma: a new way forward. Endocr Relat Cancer. 2022 May 31;29(7):R85-R103. [CrossRef] [PubMed]
- Shimaoka K, Schoenfeld DA, DeWys WD, Creech RH, DeConti R. A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer. 1985 Nov 1;56(9):2155-60. [CrossRef] [PubMed]
- Pelizzo MR, Boschin IM, Bernante P, Toniato A, Piotto A, Pagetta C, Nibale O, Rampin L, Muzzio PC, Rubello D. Natural history, diagnosis, treatment and outcome of medullary thyroid cancer: 37 years experience on 157 patients. Eur J Surg Oncol. 2007 May;33(4):493-7. Epub 2006 Nov 27. [CrossRef] [PubMed]
- Torresan, F., Armellin, C., & Iacobone, M. (2020). Management of medullary thyroid carcinoma. Annals Of Thyroid, 5. [CrossRef]
- Gild ML, Clifton-Bligh RJ, Wirth LJ, Robinson BG. Medullary Thyroid Cancer: Updates and Challenges. Endocr Rev. 2023 Sep 15;44(5):934-946. [CrossRef] [PubMed]
- Bruce JY, Bible KC, Chintakuntlawar AV. Emergence of Resistant Clones in Medullary Thyroid Cancer May Not Be Rescued by Subsequent Salvage Highly Selective Rearranged During Transfection-Inhibitor Therapy. Thyroid. 2021 Feb;31(2):332-333. Epub 2020 Aug 4. [CrossRef] [PubMed]
- Solomon BJ, Tan L, Lin JJ, Wong SQ, Hollizeck S, Ebata K, Tuch BB, Yoda S, Gainor JF, Sequist LV, Oxnard GR, Gautschi O, Drilon A, Subbiah V, Khoo C, Zhu EY, Nguyen M, Henry D, Condroski KR, Kolakowski GR, Gomez E, Ballard J, Metcalf AT, Blake JF, Dawson SJ, Blosser W, Stancato LF, Brandhuber BJ, Andrews S, Robinson BG, Rothenberg SM. RET Solvent Front Mutations Mediate Acquired Resistance to Selective RET Inhibition in RET-Driven Malignancies. J Thorac Oncol. 2020 Apr;15(4):541-549. Epub 2020 Jan 24. [CrossRef] [PubMed]
- Hadoux J, Elisei R, Brose MS, Hoff AO, Robinson BG, Gao M, Jarzab B, Isaev P, Kopeckova K, Wadsley J, Führer D, Keam B, Bardet S, Sherman EJ, Tahara M, Hu MI, Singh R, Lin Y, Soldatenkova V, Wright J, Lin B, Maeda P, Capdevila J, Wirth LJ; LIBRETTO-531 Trial Investigators. Phase 3 Trial of Selpercatinib in Advanced RET-Mutant Medullary Thyroid Cancer. N Engl J Med. 2023 Nov 16;389(20):1851-1861. Epub 2023 Oct 21. [CrossRef] [PubMed]
- Kim M, Kim BH. Current Guidelines for Management of Medullary Thyroid Carcinoma. Endocrinol Metab (Seoul). 2021 Jun;36(3):514-524. Epub 2021 Jun 22. [CrossRef] [PubMed]
- Guo M, Sun Y, Wei Y, Xu J, Zhang C. Advances in targeted therapy and biomarker research in thyroid cancer. Front Endocrinol (Lausanne). 2024 Mar 4;15:1372553. [CrossRef] [PubMed]
- Okafor C, Hogan J, Raygada M, Thomas BJ, Akshintala S, Glod JW, Del Rivero J. Update on Targeted Therapy in Medullary Thyroid Cancer. Front Endocrinol (Lausanne). 2021 Aug 19;12:708949. [CrossRef] [PubMed]
- Adams JM, Strasser A. Is tumor growth sustained by rare cancer stem cells or dominant clones? Cancer Res. 2008 Jun 1;68(11):4018-21. [CrossRef] [PubMed]
- Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011 Aug 1;71(15):5346-56. Epub 2011 Jun 13. [CrossRef] [PubMed]
- Vinogradov S, Wei X. Cancer stem cells and drug resistance: the potential of nanomedicine. Nanomedicine (Lond). 2012 Apr;7(4):597-615. [CrossRef] [PubMed]
- Mai Y, Su J, Yang C, Xia C, Fu L. The strategies to cure cancer patients by eradicating cancer stem-like cells. Mol Cancer. 2023 Oct 18;22(1):171. [CrossRef] [PubMed]
- Kulesza J, Paluszkiewicz E, Augustin E. Cellular Effects of Selected Unsymmetrical Bisacridines on the Multicellular Tumor Spheroids of HCT116 Colon and A549 Lung Cancer Cells in Comparison to Monolayer Cultures. Int J Mol Sci. 2023 Oct 30;24(21):15780. [CrossRef] [PubMed]
- Gheytanchi E, Naseri M, Karimi-Busheri F, Atyabi F, Mirsharif ES, Bozorgmehr M, Ghods R, Madjd Z. Morphological and molecular characteristics of spheroid formation in HT-29 and Caco-2 colorectal cancer cell lines. Cancer Cell Int. 2021 Apr 13;21(1):204. [CrossRef] [PubMed]
- Lytle NK, Barber AG, Reya T. Stem cell fate in cancer growth, progression and therapy resistance. Nat Rev Cancer. 2018 Nov;18(11):669-680. [CrossRef] [PubMed]
- Huang JL, Oshi M, Endo I, Takabe K. Clinical relevance of stem cell surface markers CD133, CD24, and CD44 in colorectal cancer. Am J Cancer Res. 2021 Oct 15;11(10):5141-5154. [PubMed]
- Sun S, Yang Q, Jiang D, Zhang Y. Nanobiotechnology augmented cancer stem cell guided management of cancer: liquid-biopsy, imaging, and treatment. J Nanobiotechnology. 2024 Apr 12;22(1):176. [CrossRef] [PubMed]
- Lone SN, Nisar S, Masoodi T, Singh M, Rizwan A, Hashem S, El-Rifai W, Bedognetti D, Batra SK, Haris M, Bhat AA, Macha MA. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol Cancer. 2022 Mar 18;21(1):79. [CrossRef] [PubMed]
- Zito G, Richiusa P, Bommarito A, Carissimi E, Russo L, Coppola A, Zerilli M, Rodolico V, Criscimanna A, Amato M, Pizzolanti G, Galluzzo A, Giordano C. In vitro identification and characterization of CD133(pos) cancer stem-like cells in anaplastic thyroid carcinoma cell lines. PLoS One. 2008;3(10):e3544. Epub 2008 Oct 28. [CrossRef] [PubMed]
- Todaro M, Iovino F, Eterno V, Cammareri P, Gambara G, Espina V, Gulotta G, Dieli F, Giordano S, De Maria R, Stassi G. Tumorigenic and metastatic activity of human thyroid cancer stem cells. Cancer Res. 2010 Nov 1;70(21):8874-85Epub 2010 Oct 19. [CrossRef] [PubMed]
- Hardin H, Montemayor-Garcia C, Lloyd RV. Thyroid cancer stem-like cells and epithelial-mesenchymal transition in thyroid cancers. Hum Pathol. 2013 Sep;44(9):1707-13. Epub 2013 Mar 22. [CrossRef] [PubMed]
- Yasui K, Shimamura M, Mitsutake N, Nagayama Y. SNAIL induces epithelial-to-mesenchymal transition and cancer stem cell-like properties in aldehyde dehydroghenase-negative thyroid cancer cells. Thyroid. 2013 Aug;23(8):989-96. Epub 2013 Jul 20. [CrossRef] [PubMed]
- Ahn SH, Henderson YC, Williams MD, Lai SY, Clayman GL. Detection of thyroid cancer stem cells in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2014 Feb;99(2):536-44. Epub 2013 Dec 3. [CrossRef] [PubMed]
- Shimamura M, Nagayama Y, Matsuse M, Yamashita S, Mitsutake N. Analysis of multiple markers for cancer stem-like cells in human thyroid carcinoma cell lines. Endocr J. 2014;61(5):481-90. Epub 2014 Feb 15. [CrossRef] [PubMed]
- Gianì F, Vella V, Nicolosi ML, Fierabracci A, Lotta S, Malaguarnera R, Belfiore A, Vigneri R, Frasca F. Thyrospheres From Normal or Malignant Thyroid Tissue Have Different Biological, Functional, and Genetic Features. J Clin Endocrinol Metab. 2015 Sep;100(9):E1168-78. Epub 2015 Jul 7. [CrossRef] [PubMed]
- Zane M, Scavo E, Catalano V, Bonanno M, Todaro M, De Maria R, Stassi G. Normal vs cancer thyroid stem cells: the road to transformation. Oncogene. 2016 Feb 18;35(7):805-15. Epub 2015 May 11. [CrossRef] [PubMed]
- Takano, T. Fetal cell carcinogenesis of the thyroid: a modified theory based on recent evidence. Endocr J. 2014;61(4):311-20. Epub 2014 Jan 22. [CrossRef] [PubMed]
- Hardin H, Zhang R, Helein H, Buehler D, Guo Z, Lloyd RV. The evolving concept of cancer stem-like cells in thyroid cancer and other solid tumors. Lab Invest. 2017 Oct;97(10):1142-1151. Epub 2017 Apr 10. [CrossRef] [PubMed]
- Lloyd RV, Hardin H, Montemayor-Garcia C, Rotondo F, Syro LV, Horvath E, Kovacs K. Stem cells and cancer stem-like cells in endocrine tissues. Endocr Pathol. 2013 Mar;24(1):1-10. [CrossRef] [PubMed]
- Kucerova L, Feketeova L, Kozovska Z, Poturnajova M, Matuskova M, Nencka R, Babal P. In vivo 5FU-exposed human medullary thyroid carcinoma cells contain a chemoresistant CD133+ tumor-initiating cell subset. Thyroid. 2014 Mar;24(3):520-32. Epub 2013 Dec 13. [CrossRef] [PubMed]
- Turányi E, Dezso K, Paku S, Nagy P. DLK is a novel immunohistochemical marker for adrenal gland tumors. Virchows Arch. 2009 Sep;455(3):295-9. Epub 2009 Aug 14. [CrossRef] [PubMed]
- van Limpt V, Chan A, Caron H, Sluis PV, Boon K, Hermus MC, Versteeg R. SAGE analysis of neuroblastoma reveals a high expression of the human homologue of the Drosophila Delta gene. Med Pediatr Oncol. 2000 Dec;35(6):554-8. [CrossRef] [PubMed]
- Yin D, Xie D, Sakajiri S, Miller CW, Zhu H, Popoviciu ML, Said JW, Black KL, Koeffler HP. DLK1: increased expression in gliomas and associated with oncogenic activities. Oncogene. 2006 Mar 23;25(13):1852-61. [CrossRef] [PubMed]
- López-Terrada D, Gunaratne PH, Adesina AM, Pulliam J, Hoang DM, Nguyen Y, Mistretta TA, Margolin J, Finegold MJ. Histologic subtypes of hepatoblastoma are characterized by differential canonical Wnt and Notch pathway activation in DLK+ precursors. Hum Pathol. 2009 Jun;40(6):783-94. Epub 2009 Feb 5. [CrossRef] [PubMed]
- Grassi ES, Jeannot P, Pantazopoulou V, Berg TJ, Pietras A. Niche-derived soluble DLK1 promotes glioma growth. Neoplasia. 2020 Dec;22(12):689-701. Epub 2020 Oct 23. [CrossRef] [PubMed]
- Grassi ES, Pantazopoulou V, Pietras A. Hypoxia-induced release, nuclear translocation, and signaling activity of a DLK1 intracellular fragment in glioma. Oncogene. 2020 May;39(20):4028-4044. Epub 2020 Mar 24. [CrossRef] [PubMed]
- Moraes L, Zanchin NIT, Cerutti JM. ABI3, a component of the WAVE2 complex, is potentially regulated by PI3K/AKT pathway. Oncotarget. 2017 Jun 29;8(40):67769-67781. [CrossRef] [PubMed]
- Xu B, Fuchs TL, Ahmadi S, et al. International Medullary Thyroid Carcinoma Grading System: A Validated Grading System for Medullary Thyroid Carcinoma. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2022 Jan;40(1):96-104. [CrossRef] [PubMed]
- Zhu W, Hai T, Ye L, Cote GJ. Medullary thyroid carcinoma cell lines contain a self-renewing CD133+ population that is dependent on ret proto-oncogene activity. J Clin Endocrinol Metab. 2010 Jan;95(1):439-44. Epub 2009 Nov 6. [CrossRef] [PubMed]
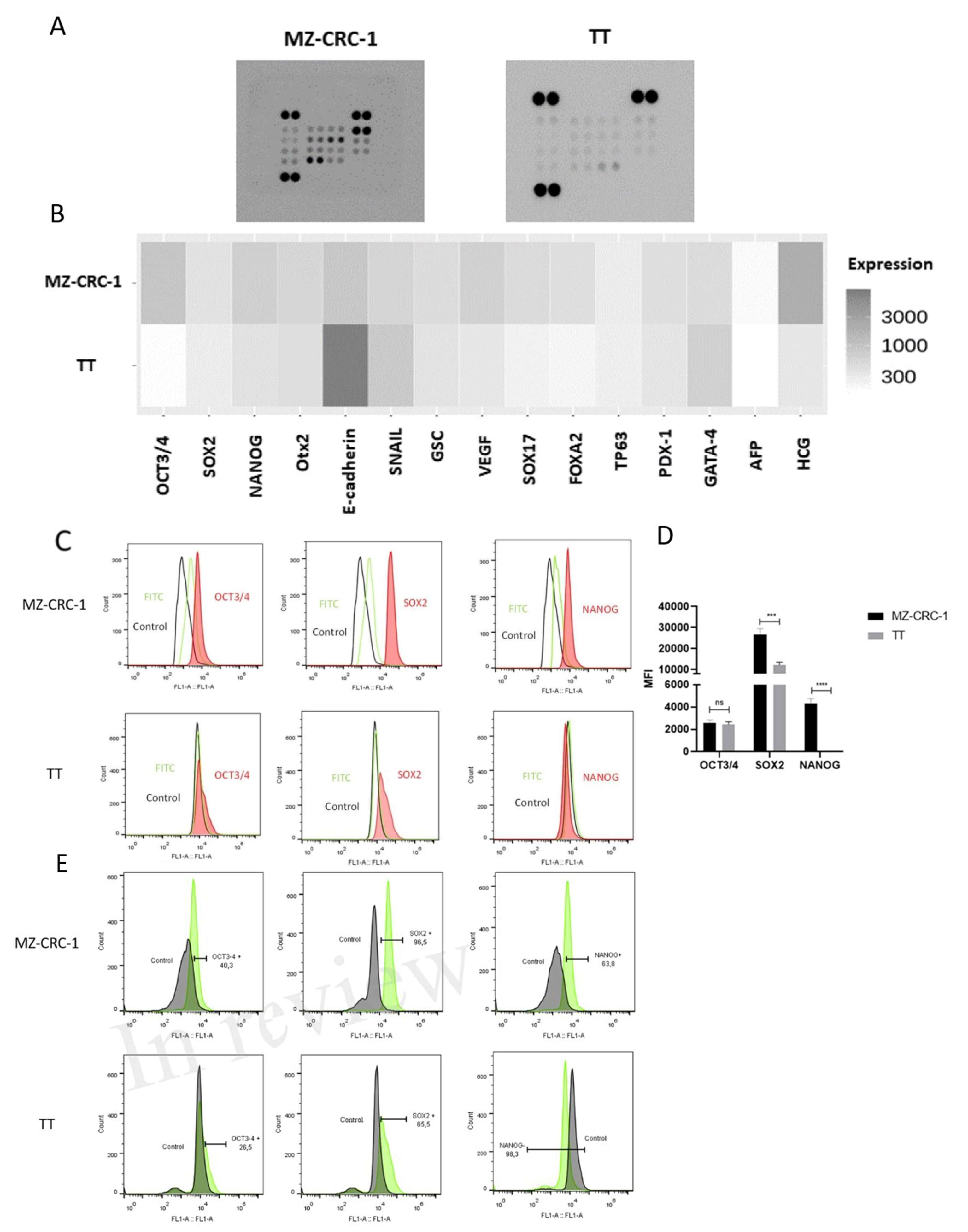
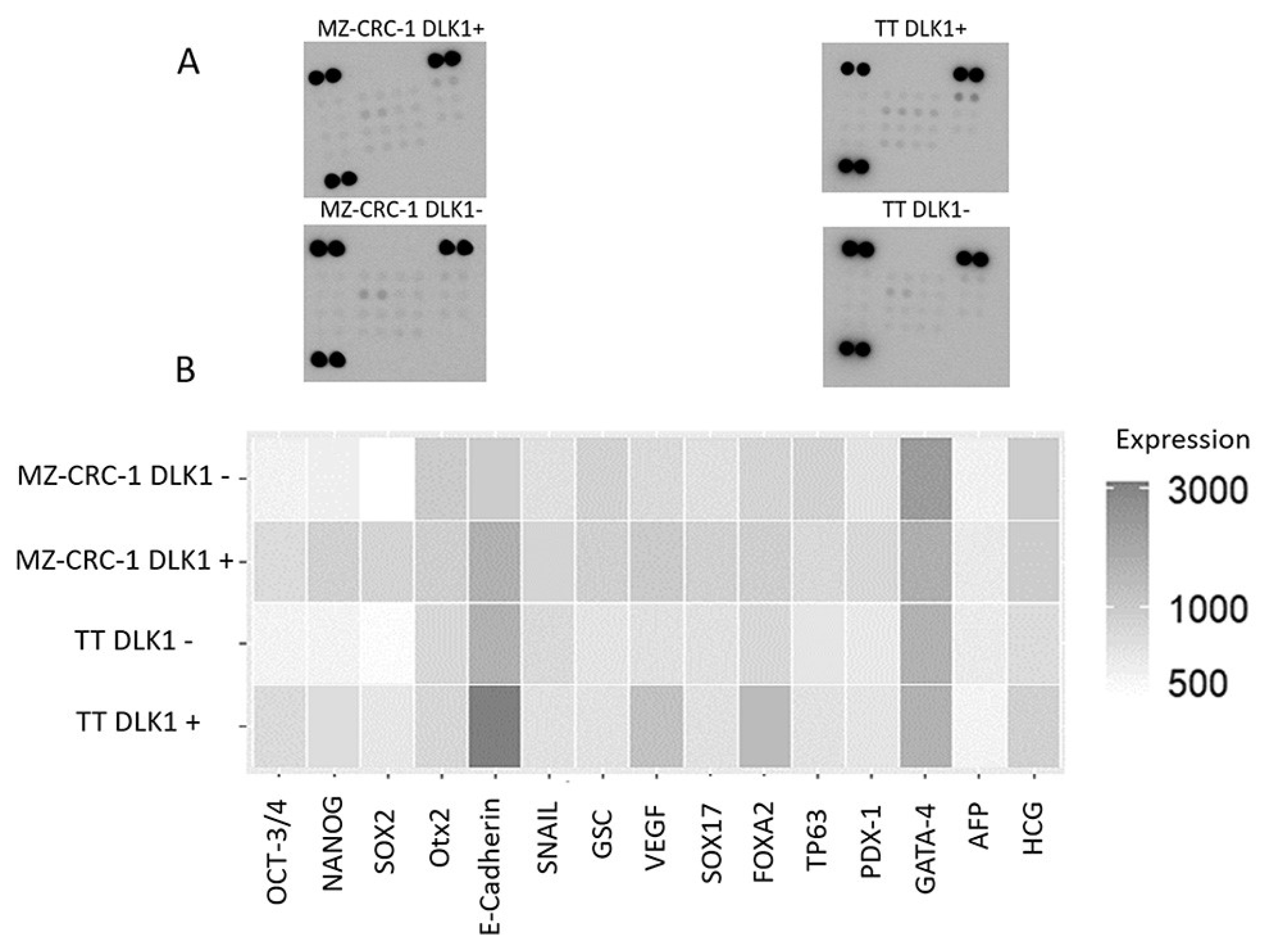
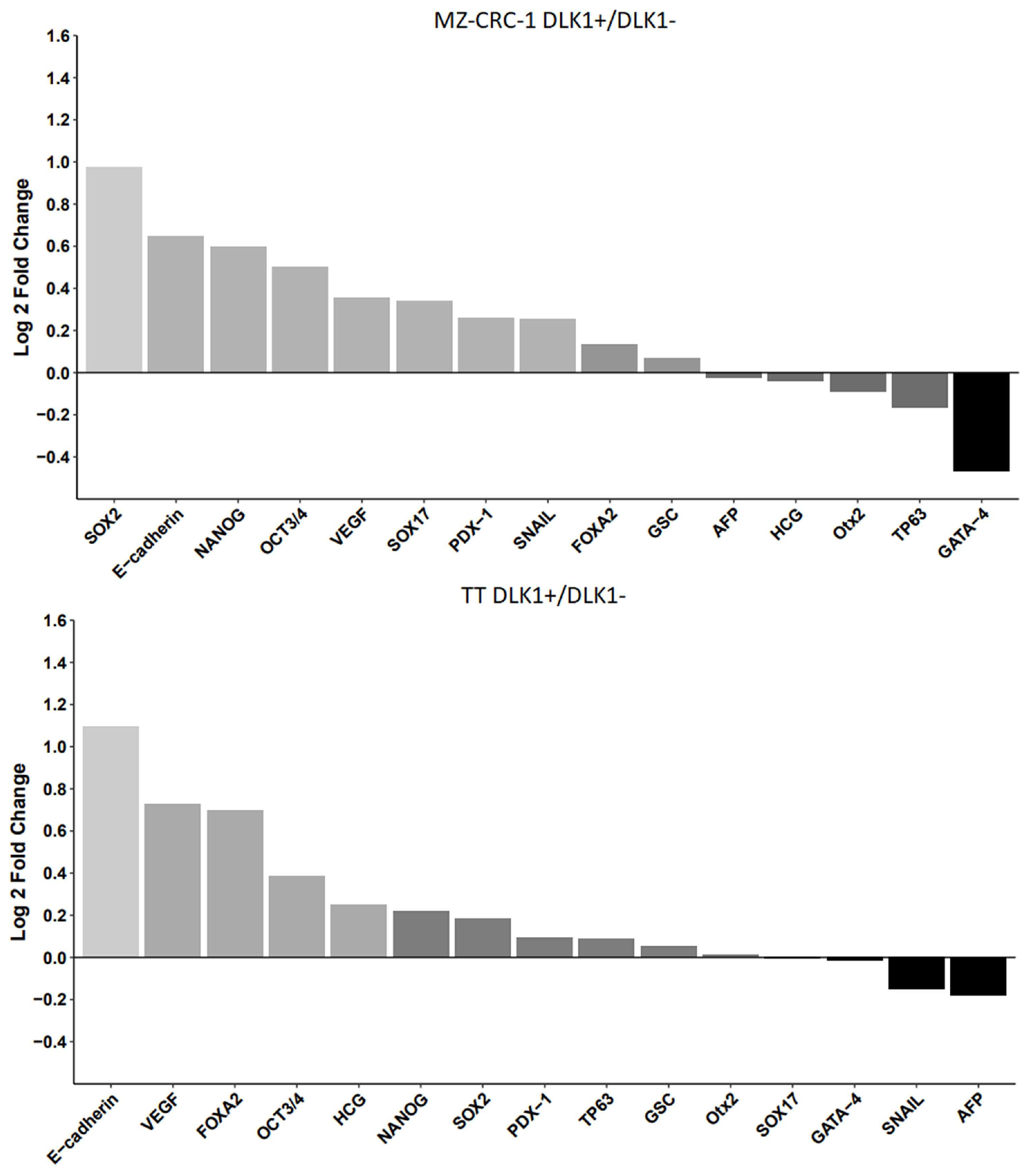
| Protein | Average intensity in TT cells | Average intensity in MZ-CRC-1 cells | Fold Change* | P-value |
|---|---|---|---|---|
| OCT3/4 | 173 | 941 | 5.439306 | 0,009 |
| SOX2 | 283 | 389 | 1.374558 | 0.0428 |
| NANOG | 398 | 786 | 1.974874 | 0.031 |
| OTX2 | 452 | 570 | 1.261062 | 0.0423 |
| E-Cadherin | 7956 | 893 | 0.112242 | 0.0017 |
| Snail | 846 | 589 | 0.696217 | 0.0059 |
| GSC | 430 | 479 | 1.113953 | NS |
| VEGF | 339 | 662 | 1.952802 | 0.0139 |
| SOX17 | 249 | 492 | 1.975904 | 0.0282 |
| FOXA2 | 218 | 435 | 1.995413 | NS |
| TP63 | 272 | 341 | 1.253676 | NS |
| PDX-1 | 359 | 434 | 1.208914 | 0.0247 |
| GATA-4 | 660 | 469 | 0.710606 | NS |
| AFP | 163 | 192 | 1.177914 | NS |
| HCG | 335 | 2048 | 6.113433 | 0.0035 |
| Protein | Average intensity in TT DLK1- cells | Average intensity in TT DLK1+ cells |
P-value | Average intensity in MZ-CRC-1 DLK1- cells |
Average intensity in MZ-CRC-1 DLK1+ cells |
P-value |
|---|---|---|---|---|---|---|
| OCT-3/4 | 528 | 689 | 0.0255 | 544 | 771 | 0.0041 |
| NANOG | 628 | 731 | 0.0119 | 569 | 859 | 0.0042 |
| SOX2 | 563 | 639 | 0.0241 | 440 | 864 | 0.013 |
| OTX2 | 810 | 818 | NS | 995 | 936 | NS |
| E-cadherin | 1495 | 3194 | 0.038 | 985 | 1544 | 0.011 |
| Snail | 781 | 703 | NS | 708 | 844 | 0.0091 |
| GSC | 669 | 695 | NS | 884 | 926 | NS |
| VEGF | 699 | 1156 | 0.0033 | 783 | 1002 | NS |
| SOX17 | 703 | 700 | NS | 716 | 907 | NS |
| FOXA2 | 803 | 1302 | 0.0245 | 856 | 941 | NS |
| TP63 | 650 | 691 | NS | 887 | 792 | NS |
| PDX-1 | 618 | 660 | NS | 679 | 811 | NS |
| GATA-4 | 1511 | 1495 | NS | 2192 | 1587 | 0.0105 |
| AFP | 603 | 533 | NS | 619 | 608 | NS |
| HCG | 757 | 901 | NS | 988 | 962 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





