Submitted:
08 October 2024
Posted:
09 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
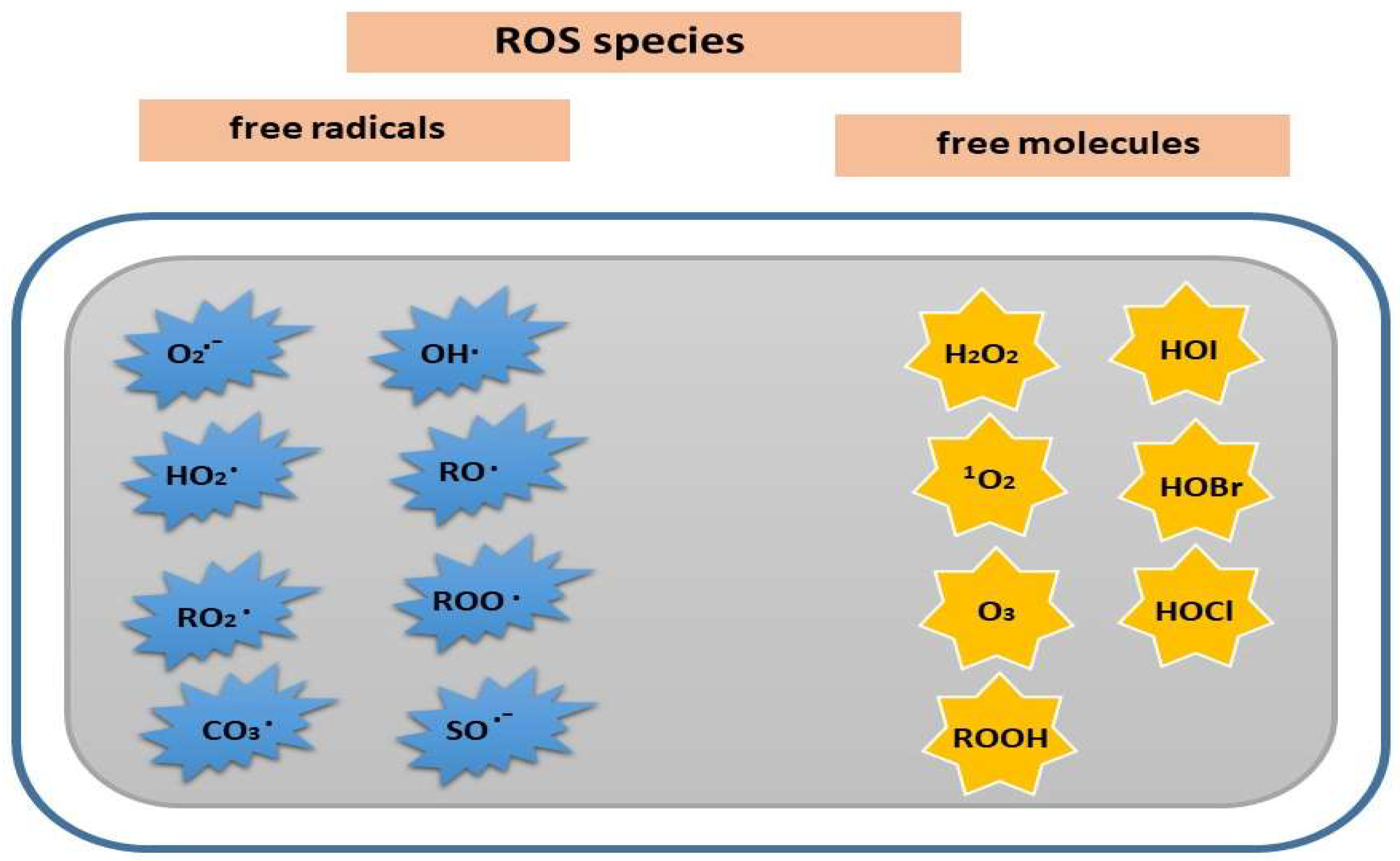
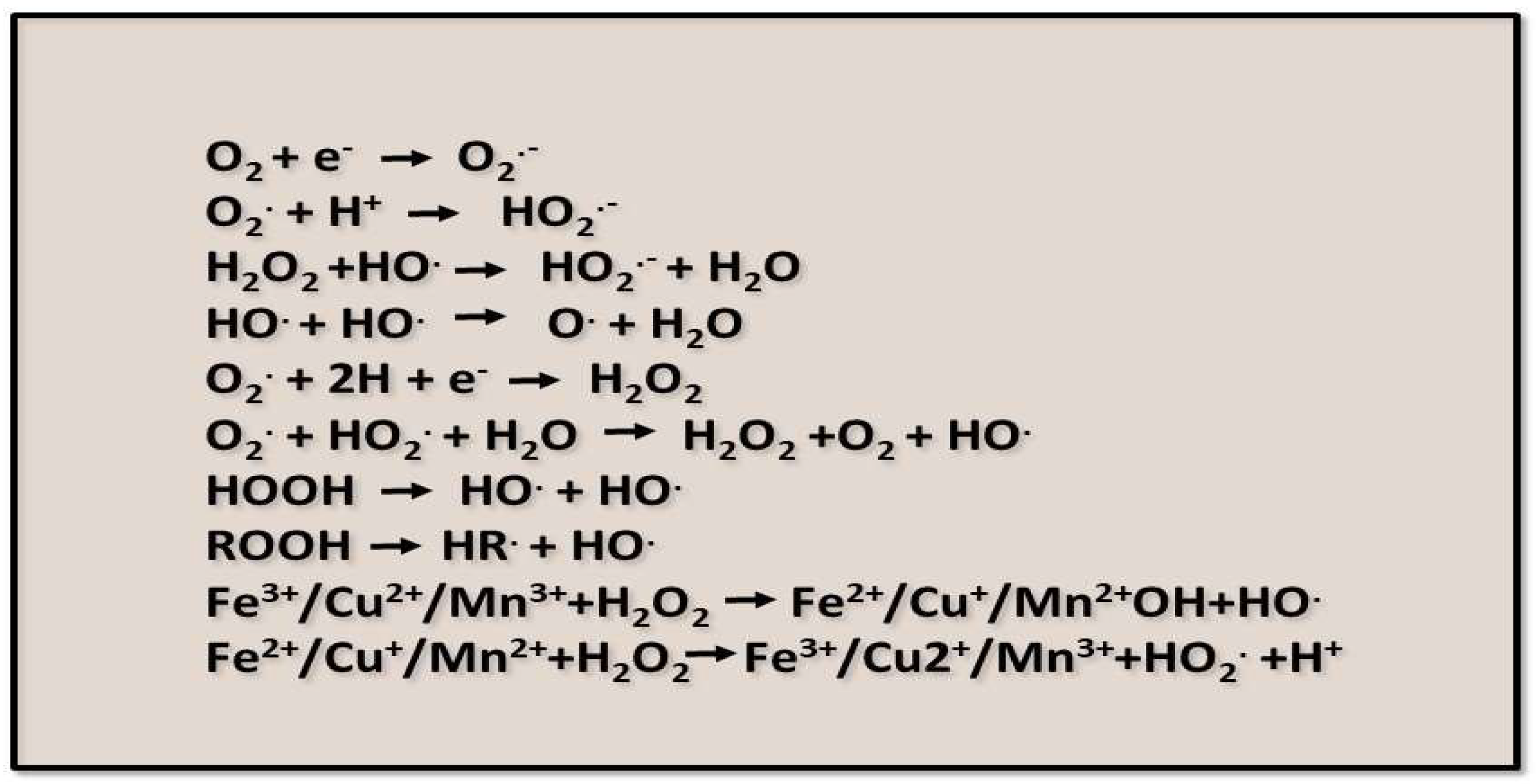
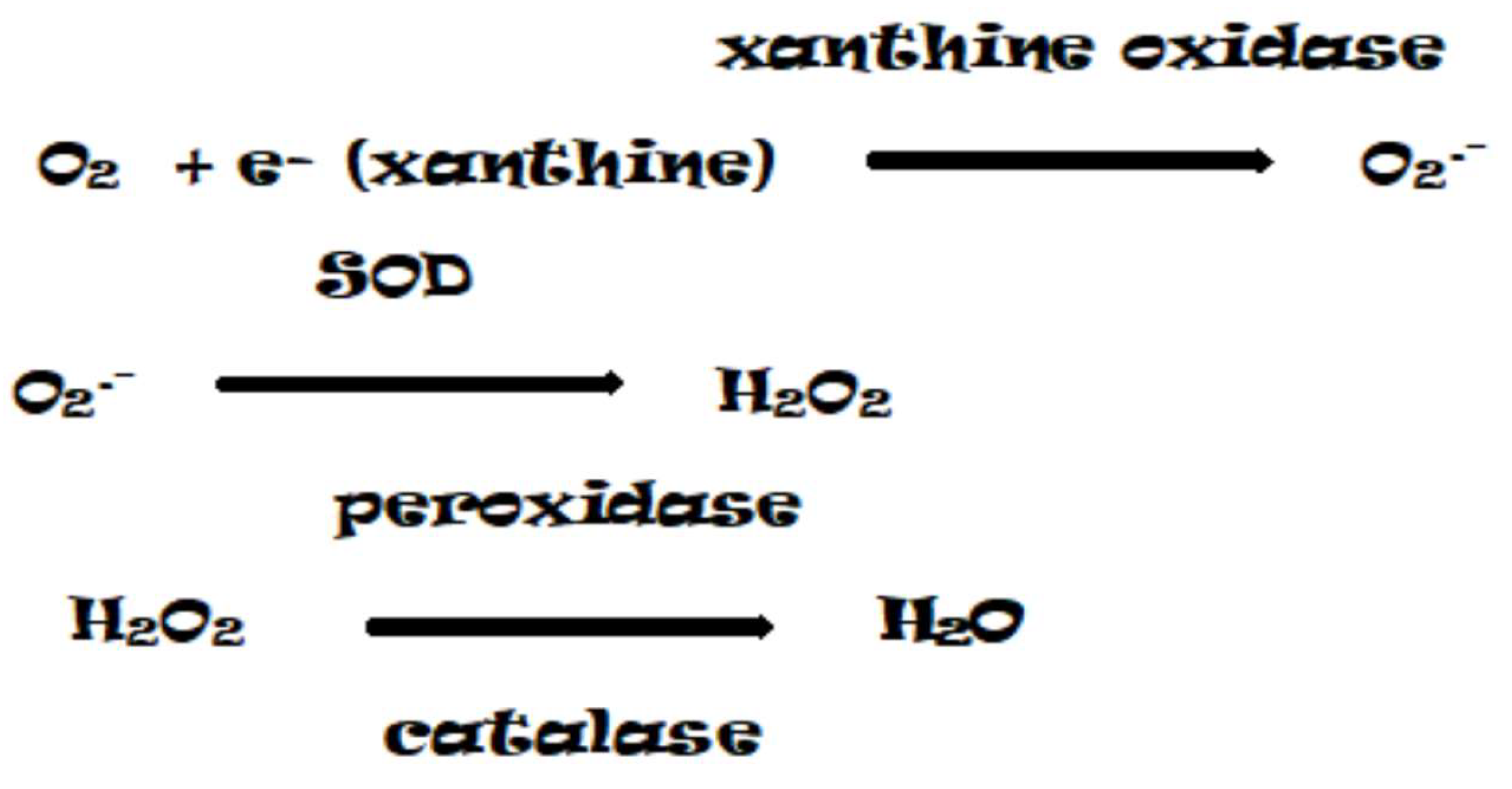
2. Reactive Oxygen Species (ROS) and Their Formation in Plant Cells
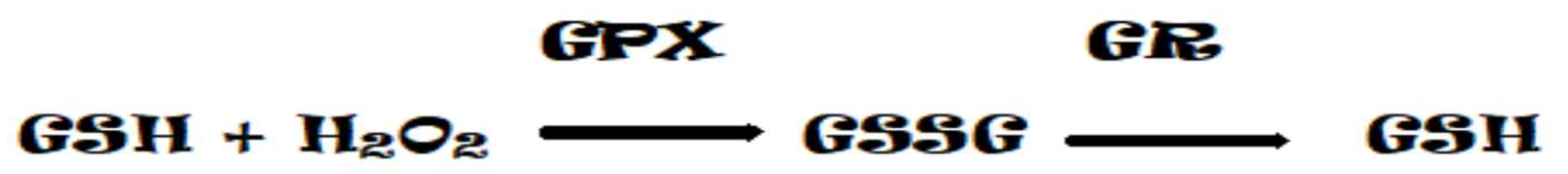
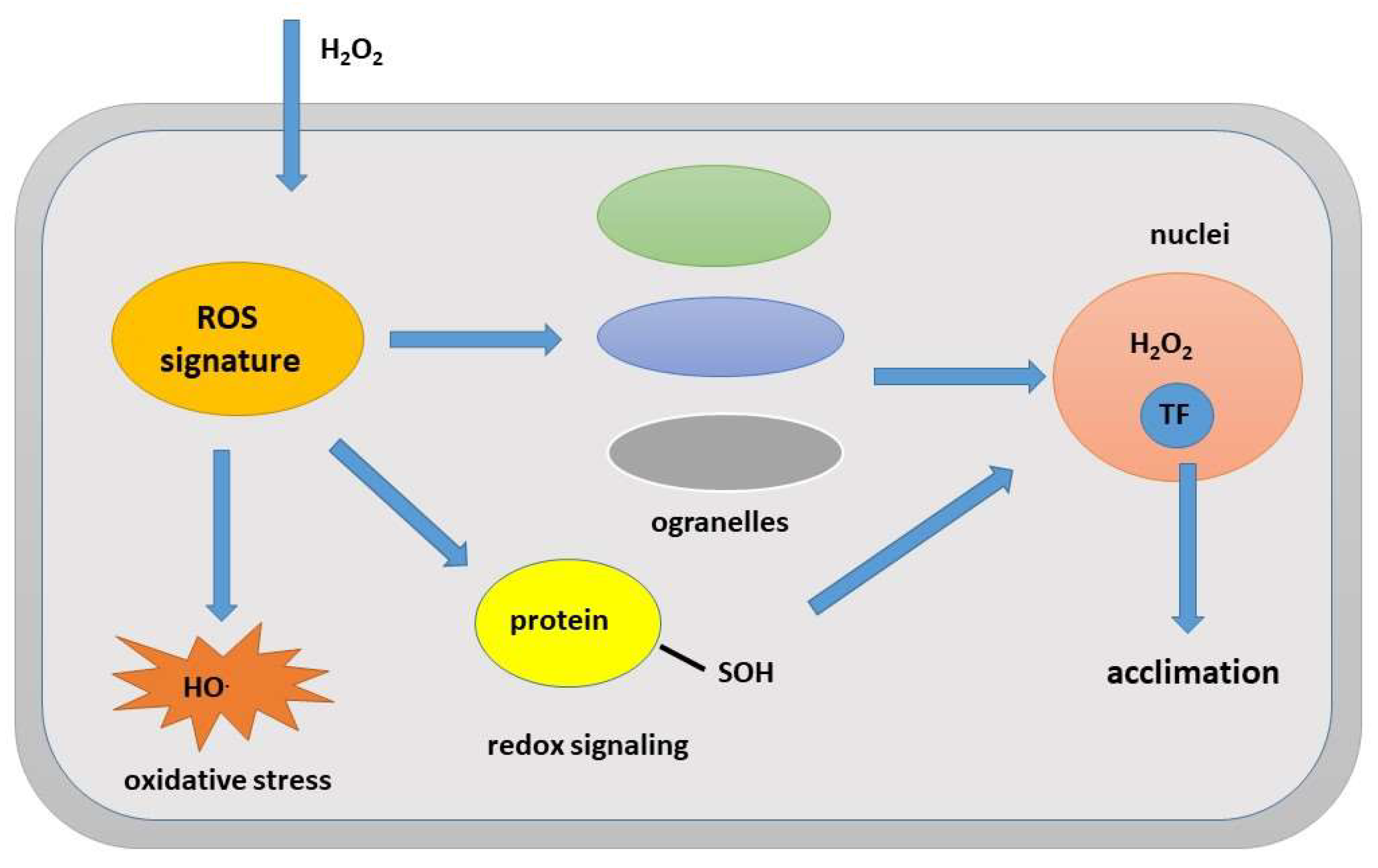
3. Oxidative Stress
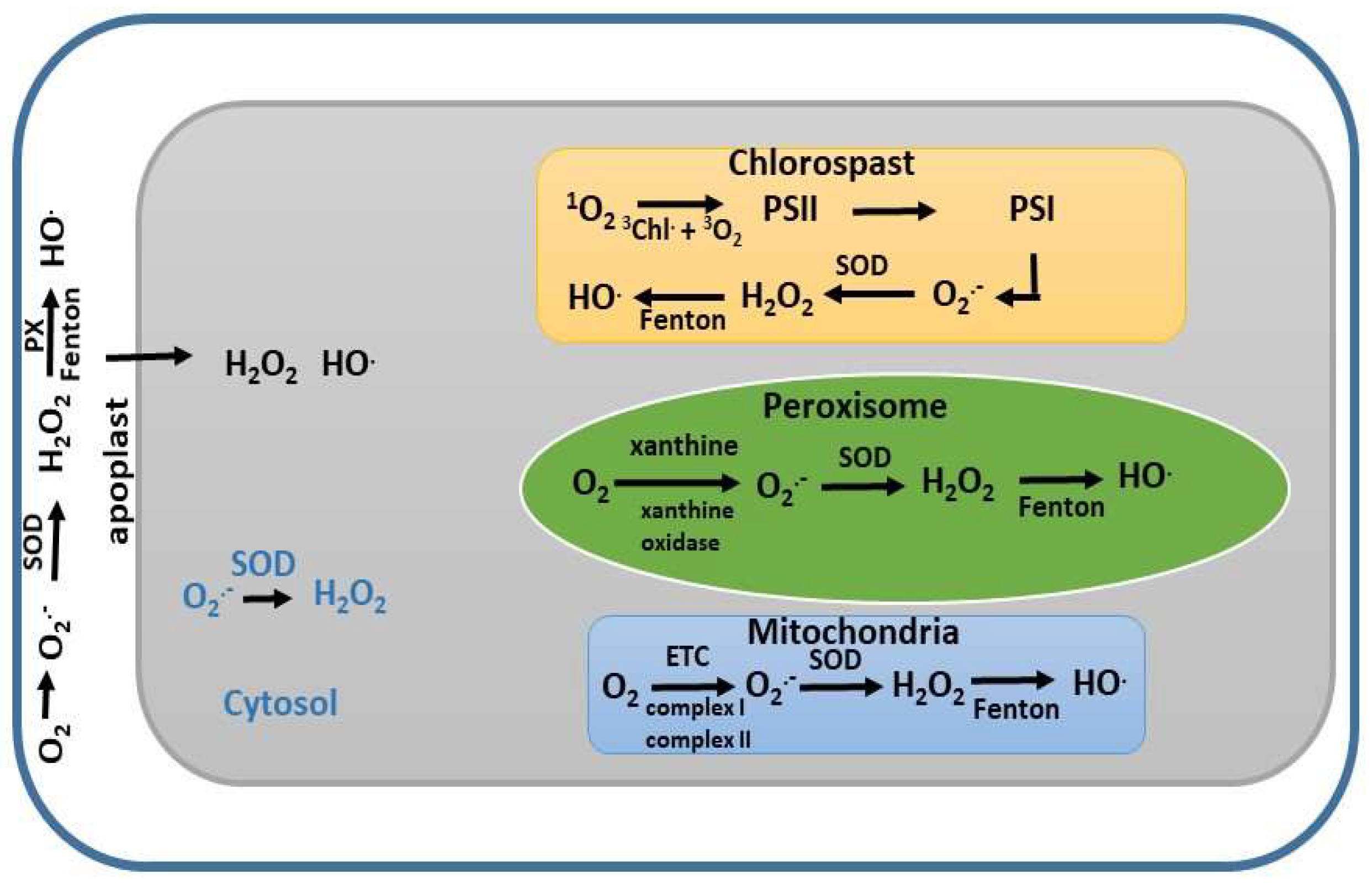
4. Regulation of ROS in Cellular Compartments
4.1. Regulation of ROS in Сhloroplasts
4.2. Regulation of ROS in Mitochondria
4.3. Regulation of ROS in Peroxisomes
4.4. Regulation of ROS in the Apoplast
4.5. Regulation of ROS in Cell Walls and Plasma Membranes
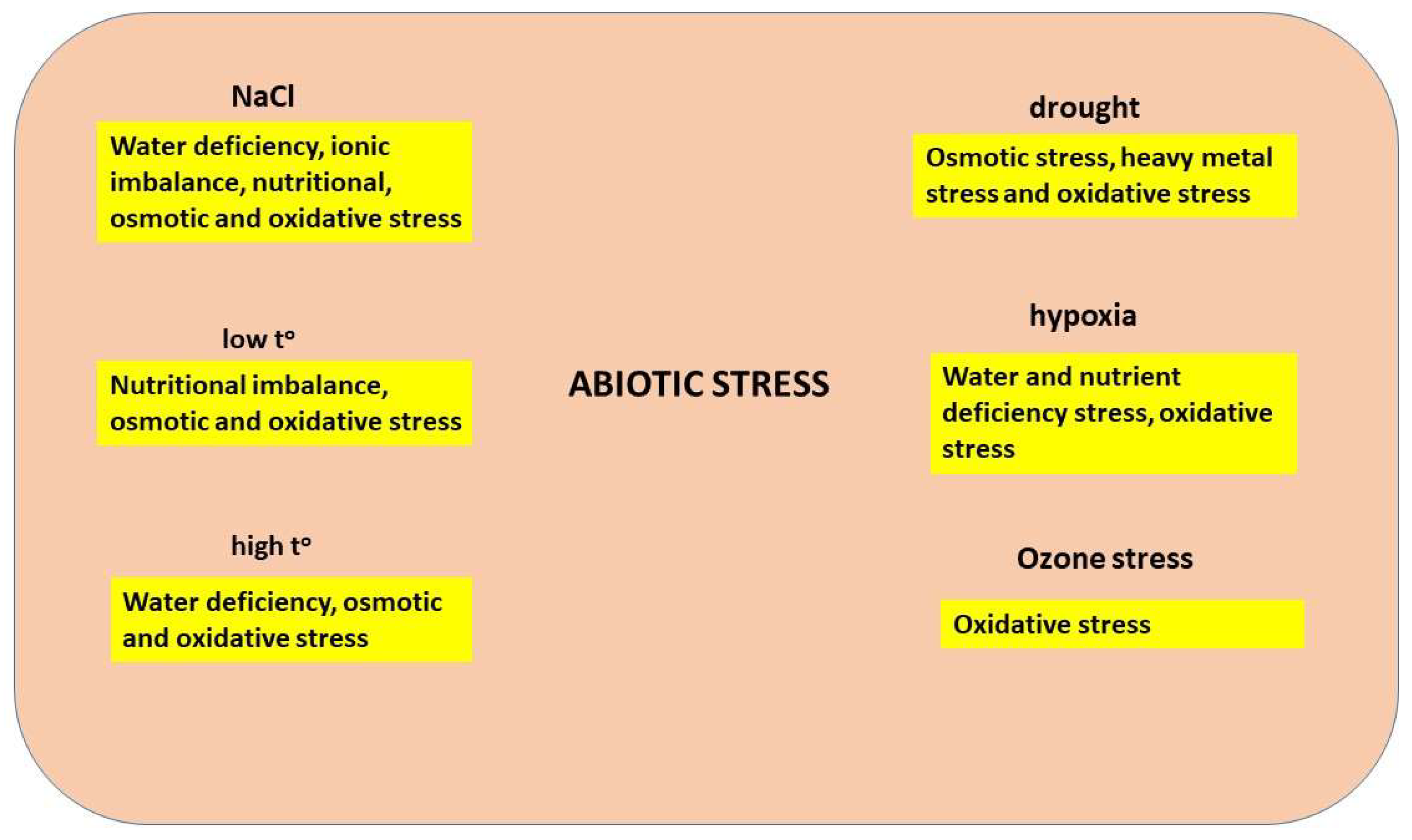
5. ROS under Abiotic Stress
6. Conclusion
Funding
Data Availability Statement
Conflicts of Interest
References
- Mittler, R.: Vanderauwera, S.: Suzuki, N.: Miller, G.: Tognett,i V.B.: Vandepoele, K.: Gollery, M.: Shulaev, V.: Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011. 16, 300–309. [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress. Ann Bot. 2003, 91, 179–194. [CrossRef]
- Foyer C.H., Noctor G. Redox signaling in plants. Antioxid. Redox Signal. 2013,18, 2087–2090. [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [CrossRef]
- Zeng, J.; Dong, Z.; Wu, H.; Tian, Z.; Zhao, Z. Redox regulation of plant stem cell fate. EMBO J. 2017, 36, 2844-2855. [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [CrossRef]
- Dangl, J.L; Jones, J.D. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826-833. [CrossRef]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [CrossRef]
- Chan, K.X.;, Mabbitt, P.D.; Phua, S.Y.; Mueller, J.W.; Nisar, N,; Gigolashvili, T.; Stroeher, E.; Grassl, J.; Arlt, W.; Estavillo, G.M.; Jackson, C.J.; Pogson, B.J. Sensing and signaling of oxidative stress in chloroplasts by inactivation of the SAL1 phosphoadenosine phosphatase. Proc Natl Acad Sci U S A 2016, 113, E4567-4576. [CrossRef]
- Mailloux, J.R. Application of mitochondria-targeted pharmaceuticals for the treatment of heart disease. Curr. Pharm. Des. 2016, 22, 4763–4779. [CrossRef]
- Elstner, E.F. Metabolism of activated oxygen species. In: Davies DD, ed. Biochemistry of plants, Vol. 11. London: Academic Press, 1987, 253–315. [CrossRef]
- Droge, W. Free radicals in the physiological control of cell function. Physiol Rev 2002, 82, 47–95. [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.; Zulfiqar, F.; Alam, M.; Fujita, M. Regulation of ROS metabolism in plants under environmental stress: A review of recent experimental evidence. Int. J. Mol. Sci. 2020, 21, 8695. [CrossRef]
- Bhattacharjee, S. . Reactive Oxygen Species in Plant Biology. Springer; New Delhi, India: ROS and oxidative stress: Origin and implication. 2019, 1–31. [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [CrossRef]
- Bolwell, G.P.; Wojtaszek, P. Mechanisms for generation of reactive oxygen species in plant defence--a broad perspective. Physiological and Molecular Plant Pathology 1997, 51, 347–366. [CrossRef]
- Pfister-Sieber, M.; Braendle, R. Aspects of plant behavior under anoxia and post-anoxia. Proceedings of the Royal Society of Edinburgh 1994, 102B, 313–324. [CrossRef]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Plant Molecular Biology 1997, 48, 251–275. [CrossRef]
- Dorion, S.; Ouellet, J.C.; Rivoal, J. Glutathione metabolism in plants under stress: beyond reactive oxygen species detoxification. Metabolites 2021, 11, 641. [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem 2010, 48, 909–930. [CrossRef]
- Kandhari, P. Generic differences in antioxidant concentration in the fruit tissues of four major cultivars of apples. Master Thesis, University of Maryland, College Park 2004.
- Ayala, A.; Muñoz, M.F.; Argüelles, S Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid Med Cell Longev. 2012, 2014, 360438. [CrossRef]
- Gupta, D.K.; Palma, J.M.; Corpas, F.J. Reactive Oxygen Species and Oxidative Damage in Plants under Stress; Springer: New York, NY, USA 2015. [CrossRef]
- Joshi, R.; Wani, S.H.; Singh, B.; Bohra, A.; Dar, Z.A.; Lone, A.A.; Pareek, A.; Singla-Pareek, S.L. Transcription factors and plants response to drought stress: Current understanding and future directions. Front. Plant Sci. 2016, 7, 1029. [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol. Trace Elem. Res. 2011, 143, 1758–1776. [CrossRef]
- Decros, G.; Baldet, P.; Beauvoit, B.; Stevens, R.; Flandin, A.; Colombié, S.; et al. Get the balance right: ROS homeostasis and redox signalling in fruit. Front. Plant Sci. 2019, 10, 1091. [CrossRef]
- Naviaux, R.K. Oxidative shielding or oxidative stress? J Pharmacol Exp Ther 2012, 342, 608–618. [CrossRef]
- Paciolla, C.; Paradiso, A.; de Pinto, M. “Cellular redox homeostasis as central modulator in plant stress response,” in Redox State as a Central Regulator of Plant-Cell Stress Responses, eds Gupta D., Palma J., Corpas F. (Berlin: Springer;) 2016, 1–23. [CrossRef]
- Bae, Y.S.; Oh, H.; Rhee, S.G.; Yoo, Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol Cells 2011, 32, 491–509. [CrossRef]
- Hernandez-Garcia, D.; Wood, C.D.; Castro-Obregon, S.; Covarrubias, L. Reactive oxygen species: a radical role in development? Free Radic Biol Med. 2010, 49, 130–143. [CrossRef]
- Foyer, C.H.; Noctor, G. Stress-triggered redox signalling: what’s in pROSpect? Plant, Cell Environ. 2016, 39, 951–964. [CrossRef]
- Dietz, K.J. Efficient high light acclimation involves rapid processes at multiple mechanistic levels. J. Exp. Bot. 2015, 66, 2401–2414. [CrossRef]
- Dietz, K.J. Thiol-based peroxidases and ascorbate peroxidases: why plants rely on multiple peroxidase systems in the photosynthesizing chloroplast? Mol. Cells 2016, 39, 20–25. [CrossRef]
- Noctor, G.; Foyer, C.H. Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol. 2016, 171, 1581–1592. [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of subcellular ROS and NO metabolism in higher plants: Multifunctional signaling molecules. Antioxidants. 2019, 8, 641. [CrossRef]
- Podgorska, A.; Burian, M.; Szal, B. Extra-cellular but extra-ordinarily important for cells: Apoplastic reactive oxygen species metabolism. Front. Plant Sci. 2017, 8, 1353. [CrossRef]
- Janků, M.; Luhová, L.; Petřivalský, M. On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants. 2019, 8, 105. [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [CrossRef]
- Kumar, V.; Irfan, M.; Ghosh, S.; Chakraborty, N.; Chakraborty, S.; Datta, A. Fruit ripening mutants reveal cell metabolism and redox state during ripening. Protoplasma 2016, 253, 581–594. [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Breusegem, F.V. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663-679. [CrossRef]
- Gilbert, H.F. Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol 1990, 63, 69–172. [CrossRef]
- Rajasekaran, N.S.; Connell, P.; Christians, E.S.; Yan, L.J.; Taylor, R.P.; Orosz, A.; Zhang, X.Q.; Stevenson, T.J.; Peshock, R.M.; Leopold, J.A.; Barry, W.H.; Loscalzo, J.; Odelberg, S.J.; Benjamin, I.J. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell 2007, 130, 427–439. [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [CrossRef]
- Panieri, E.; Santoro, M.M. ROS signaling and redox biology in endothelial cells. Cell Mol. Life Sci. 2015, 72, 3281–3303. [CrossRef]
- Hansen, J.M.; Go, Y.M.; Jones, D.P. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol 2006, 46, 215–234. [CrossRef]
- Leister, D. Piecing the puzzle together: the central role of reactive oxygen species and redox hubs in chloroplast retrograde signaling. Antioxid. Redox Signal 2019, 30, 1206–1219. [CrossRef]
- Chan, Z.; Yokawa, K.; Kim, W.Y.; Song, C.P. ROS regulation during plant abiotic stress responses. Front. Plant Sci. 2016, 7, 1536. [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141,391–396. [CrossRef]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory burst oxidases: the engines of ROS signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [CrossRef]
- Vaahtera, L.; Brosche, M.; Wrzaczek, M.; Kangasjarvi, J. Specificity in ROS signaling and transcript signatures. Antioxid. Redox Signal. 2014, 21, 1422–1441. [CrossRef]
- Gilroy, S.; Białasek, M.; Suzuki, N.; Górecka, M.; Devireddy, A.R.l Karpiński, S.; Mittler, R. ROS, Calcium, and Electric Signals: Key Mediators of Rapid Systemic Signaling in Plants. Plant Physiol. 2016, 171, 1606–1615. [CrossRef]
- Mignolet-Spruyt, L., Xu, E., Idanheimo, N., Hoeberichts, F.A., Muhlenbock, P., Brosche, M., Van Breusegem, F. and Kangasjarvi, J. Spreading the news: subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 2016, 67, 3831–3844. [CrossRef]
- Raja,V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [CrossRef]
- Shakirova, F.M.; Allagulova, C.R.; Maslennikova, D.R.; Klyuchnikova, E.O.; Avalbaev, A.M.; Bezrukova, M.V. Salicylic acid-induced protection against cadmium toxicity in wheat plants. Environ. Exp. Bot. 2016, 122, 19–28. [CrossRef]
- Singh, A.; Kumar, A.; Yadav, S.; Singh, I K. Reactive oxygen species-mediated signaling during abiotic stress. Plant Gene 2019, 18, 100–173. [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [CrossRef]
- Triantaphylides, C.; Havaux, M. Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci. 2009, 14, 219–228. [CrossRef]
- Pospisil, P.; Arato, A.; Krieger-Liszkay, A.; Rutherford, A.W. Hydroxyl radical generation by photosystem II. Biochemistry 2004, 43, 6783–6792. [CrossRef]
- Pospisil, P. Production of reactive oxygen species by photosystem II. Biochim. Biophys. Acta. 2009, 1787. 1151–1160. [CrossRef]
- Pospisil, P. The role of metals in production and scavenging of reactive oxygen species in photosystem II. Plant Cell Physiol. 2014, 55, 1224–1232. [CrossRef]
- Pospisil, P. Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Front Plant Sci. 2016, 7, 1950. [CrossRef]
- Pospisil, P.; Yamamoto, Y. Damage to photosystem II by lipid peroxidation products. BBA Gen. Subj. 2017, 1861, 457–466. [CrossRef]
- Shumbe, L.; Chevalier, A.; Legeret, B.; Taconnat, L.; Monnet, F.; Havaux, M. Singlet oxygen-induced cell death in Arabidopsis under high-light stress is controlled by OXI1 kinase. Plant Physiol. 2016,. 170, 1757–1771. [CrossRef]
- Havaux, M.; Strasser, R.J.; Greppin, H. A theoretical and experimental analysis of the qP and q N coefficients of chlorophyll fluorescence quenching and their relation to photochemical and nonphotochemical events. Photosynth Res. 1991, 27, 41–55. [CrossRef]
- Susek, R.E.; Ausubel, F.M.; Chory, J. Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell. 1993, 74, 787–799. [CrossRef]
- Asada, K. The water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology 1999, 50, 601–639. [CrossRef]
- Rhoads, D.M.; Umbach, A.L.; Subbaiah, C.C.; Siedow, J.N. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006, 141, 357–366. [CrossRef]
- Mattos, L.; Moretti, C. Oxidative stress in plants under drought conditions and the role of different enzymes. Enzym. Eng. 2015, 5, 1–6.
- Rasmusson, A.G.; Geisler, D.A.; Møller, I.M. The multiplicity of dehydrogenases in the electron transport chain of plant mitochondria. Mitochondrion 2008, 8, 47–60. [CrossRef]
- Braidot, E.; Petrussa, E.; Vianello, A.; Macri, F. Hydrogen peroxide generation by higher plant mitochondria oxidizing complex I of complex II substrates. FEBS Letters 1999, 451, 347–350. [CrossRef]
- Gille, L.; Nohl, H. The ubiquinol/bc1 redox couple regulates mitochondrial oxygen radical formation. Archives of Biochemistry and Biophysics 2001, 388, 34–38. [CrossRef]
- Möller, I.M. Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annual Review of Plant Physiology and Plant Molecular Biology 2001, 52, 561–91. [CrossRef]
- Blokhina, O.; Fagerstedt, K.V. Reactive oxygen species and nitric oxide in plant mitochondria: Origin and redundant regulatory systems. Physiol. Plant. 2010, 138, 447–462. [CrossRef]
- Giraud, E.; Ho, L.H.; Clifton, R.; Carroll, A.; Estavillo, G.; Tan, Y.F.; Howell, K.A.; Ivanova, A.; Pogson, B.J.; Millar, A.H. The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol. 2008, 147, 595–610. [CrossRef]
- Scialò, F.; Fernández-Ayala, D.J.; Sanz, A. Role of mitochondrial reverse electron transport in ROS signaling: Potential roles in health and disease. Front. Physiol. 2017, 8, 428. [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [CrossRef]
- Kerchev, P.; Waszczak, C.; Lewandowska, A.; Willems, P.; Shapiguzov, A.; Li, Z. Lack of GLYCOLATE OXIDASE1, but not GLYCOLATE OXIDASE2, attenuates the photorespiratory phenotype of CATALASE2- deficient Arabidopsis. Plant Physiol. 2016, 171, 1704–1719. [CrossRef]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2009, 11, 861–905. [CrossRef]
- Corpas, F.J.; del Río, L.A.; Palma, J.M. Impact of nitric oxide (NO) on the ROS metabolism of peroxisomes. Plants 2019, 8,37. [CrossRef]
- Reumann, S.; Chowdhary, G.; Lingner, T. Characterization, prediction and evolution of plant peroxisomal targeting signals type 1 (PTS1s). Biochim. Biophys. Acta 2016, 1863, 790–803. [CrossRef]
- Palma, J.M., Corpas, F.J., del Río, L.A. Proteome of plant peroxisomes: New perspectives on the role of these organelles in cell biology. Proteomics 2009, 9, 2301–2312. [CrossRef]
- Kostić, A.; Dimitrijević, D.S.; Stojanović, G.S.; Palić, I.R.; Đorđević, A.S.; Ickovski, J.D. Xanthine oxidase: Isolation, assays of activity, and inhibition. J. Chem. 2015, 2015, 1–8. [CrossRef]
- Schrader, M.; Fahimi, H.D. Peroxisomes and oxidative stress. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2006, 1763, 1755–1766. [CrossRef]
- Corpas, F.J.; Barroso, J.B.; Palma, J.M.; Rodriguez-Ruiz, M. Plant peroxisomes: A nitro-oxidative cocktail. Redox Biol. 2017, 11, 535–542. [CrossRef]
- Jimenez, A.; Hernandez, J.A.; del Río, L.A.; Sevilla, F. Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol. 1997, 114, 275–284. [CrossRef]
- Vandenabeele, S.; Vanderauwera, S.; Vuylsteke, M.; Rombauts, S.; Langebartels, C.; Seidlitz, H.K.; Zabeau, M.; Van Montagu, M.; Inzé, D.; Van Breusegem, F. Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J. 2004, 39, 45–58. [CrossRef]
- Gupta, K.; Sengupta, A.; Chakraborty, M.; Gupta, B. Hydrogen peroxide and polyamines act as double edged swords in plant abiotic stress responses. Front. Plant Sci. 2016, 7, 1343. [CrossRef]
- Rajab, H.; Khan, M S.; Malagoli, M.; Hell, R.; Wirtz, M. Sulfate-induced stomata closure requires the canonical ABA signal transduction machinery. Plants 2019, 8, 21. [CrossRef]
- Máthé, C.; Garda, T.; Freytag, C. The Role of Serine-threonine protein phosphatase PP2A in plant oxidative stress signaling—Facts and hypotheses. Int. J. Mol. Sci. 2019, 20, 3028. [CrossRef]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [CrossRef]
- Torres, M.A.; Dangl, J.L. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 2005, 8, 397–403. [CrossRef]
- Li, Y.; Xiang, J.; Wang, Y.; Zheng, L.; Fan, Y.; Li, Y.; Zhao, F. Analysis of antioxidant characteristics and related gene expression profiles of rice drought-tolerance lines derived from embryo-soaking with alternanthera philoxeroides DNA solution. J. Bot. Sci. 2015, 4, 30–36.
- Rietz, S.; Bernsdorff, F.E.; Cai, D. Members of the germin-like protein family in Brassica napus are candidates for the initiation of an oxidative burst that impedes pathogenesis of Sclerotinia sclerotiorum. J. Exp. Bot. 2012, 63, 5507–5519. [CrossRef]
- Heyno, E.; Mary, V.; Schopfer, P.; Krieger-Liszkay, A. Oxygen activation at the plasma membrane: Relation between superoxide and hydroxyl radical production by isolated membranes. Planta 2011, 234, 35–45. [CrossRef]
- Bolwell, G.P.; Wojtaszek, P. Mechanisms for generation of reactive oxygen species in plant defence—a broad perspective. Physiological and Molecular Plant Pathology 1997, 51, 347–366. [CrossRef]
- Reggiani, R.; Bertani, A. Effect of decreasing oxygen concentration on polyamine metabolism in rice and wheat shoots. Journal of Plant Physiology 1989, 135, 375–377. [CrossRef]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global plant stress signaling: Reactive oxygen species at the cross-road. Front. Plant Sci. 2016, 7, 187. [CrossRef]
- Sharma, V.; Anderson, D.; Dhawan, A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis 2012, 17, 852–870. [CrossRef]
- Liu, J.H.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines function in stress tolerance: From synthesis to regulation. Front. Plant Sci. 2015, 6, 827. [CrossRef]
- Cuypers, A.; Hendrix, S.; Amaral dos Reis, R.; De Smet, S.; Deckers, J.; Gielen, H.; Jozefczak, M.; Loix, C.; Vercampt, H.; Vangronsveld, J. Hydrogen peroxide, signaling in disguise during metal phytotoxicity. Front. Plant Sci. 2016, 7, 470. [CrossRef]
- Kumar, S.P.J.; Prasad, R.S., Banerjee, R.; Thammineni, C. Seed birth to death: Dual functions of reactive oxygen species in seed physiology. Ann. Bot. 2015, 116, 663–668. [CrossRef]
- Van Breusegem, F.; Bailey-Serres, J.; Mittler, R. Unraveling the tapestry of networks involving reactive oxygen species in plants. Plant Physiol. 2008, 147, 978–984. [CrossRef]
- Yadav, S.; Modi, P.; Dave, A.; Vijapura, A.; Patel, D.; Patel, M. Effect of abiotic stress on crops. In: Hasanuzzaman M., Filho M.C.M.T., Fujita M., Nogueira T.A.R., editors. Sustainable Crop Production. IntechOpen; London, UK 2020, 3–24. [CrossRef]
- Kononenko, N.V.; Lazareva, E.M.; Fedoreyeva L.I.. Mechanisms of Antioxidant Resistance in Different Wheat Genotypes under Salt Stress and Hypoxia. Int J Mol Sci. 2023, 24,16878. [CrossRef]
- .Miller, G.A.D.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R.O.N. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [CrossRef]
- Gilroy, S.; Suzuki, N.; Miller, G.; Choi, W.G.; Toyota, M.; Devireddy, A.R.; Mittler, R. A tidal wave of signals: Calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 2014, 19, 623–630. [CrossRef]
- Tian, S.; Wang, X.; Li, P.; Wang, H.; Ji, H.; Xie, J.; Qiu, Q.; Shen, D.; Dong, H. Plant aquaporin AtPIP1;4 links apoplastic H2O2 induction to disease immunity pathways. Plant Physiol. 2016, 171, 1635–1650. [CrossRef]
- Huang, S.; VanAken, O.; Schwarzländer, M.; Belt, K.; Millar, A.H. The roles of mitochondrial reactive oxygen species in cellular signaling and stress responses in plants. Plant Physiol 2016, 171, 1551–1559. [CrossRef]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [CrossRef]
- Fichman, Y.; Mittler, R. Rapid systemic signaling during abiotic and biotic stresses: Is the ROS wave master of all trades? Plant J. 2020, 102, 887–896. [CrossRef]
- Suzuki, N.; Miller, G.; Salazar, C.; Mondal, H.A.; Shulaev, E.; Cortes, D.F.; Shuman, J.L.; Luo, X.; Shah, J.; Schlauch, K.; Shulaev, V.; Muttler, R. Temporal-Spatial Interaction between Reactive Oxygen Species and Abscisic Acid Regulates Rapid Systemic Acclimation in Plants. Plant Cell. 2013, 25, 3553–3569. [CrossRef]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [CrossRef]
- Zandalinas, S.I.; Fichman, Y.; Devireddy, A.R.; Sengupta, S.; Azad, R.K.; Mittler, R. Systemic signaling during abiotic stress combination in plants. Proc. Natl. Acad. Sci. USA. 2020, 117, 13810–13820. [CrossRef]
- Considine, M.; Sandalio, L.M.; Foyer, C.H. Unravelling how plants benefit from ROS and NO reactions, while resisting oxidative stress. Ann. Bot. 2015, 116, 469–473. [CrossRef]
- Davletova, S.; Rizhsky, L.; Liang, H.; Shengqiang, Z.; Oliver, D.J.; Coutu, J.; Shulaev, V.; Schlauch, K.; Mittler, R. Cytosolic Ascorbate Peroxidase 1 Is a Central Component of the Reactive Oxygen Gene Network of Arabidopsis. Plant Cell. 2005, 17, 268–281. [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [CrossRef]
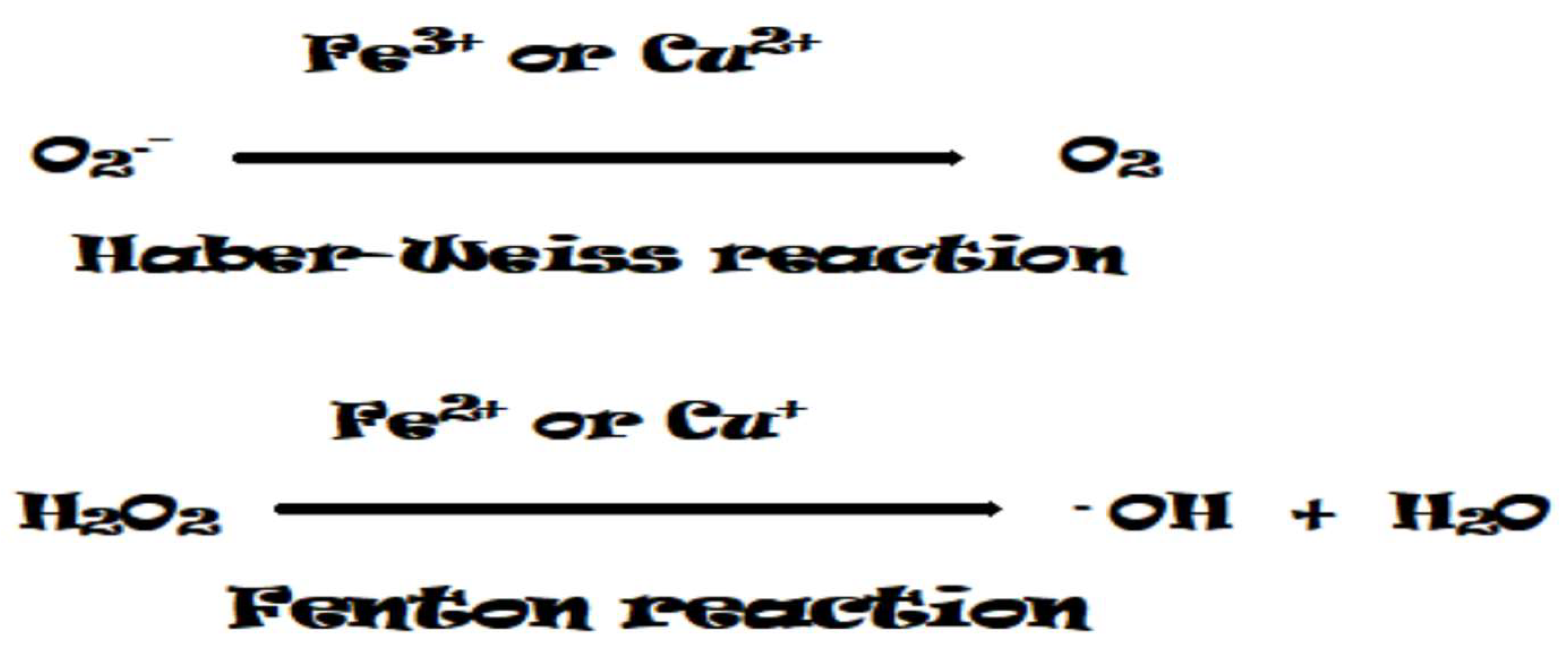
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



