1. Introduction
Non-melanoma skin cancers (NMSCs), which include basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) [
1], constitute approximately one- third of malignancies diagnosed globally [
2], are the most-diagnosed cancers in the United States [
3], and are a cause of significant morbidity [
4,
5]. Incidences of BCC and SCC were 525 and 262 per 100,000 persons, respectively, in the US in 2019 [
6] and NMSC incidence is thought to be increasing by around 2% annually [
3,
5,
7]. Approximately 2,000 people in the US and 65,000 globally die from NMSC each year [
4,
8,
9]. NMSCs originate from epidermal cells via multifactorial pathogenesis, including exposure to ultraviolet and ionizing radiation, human papillomavirus, certain genetic diseases, and profound immune suppression, and are highly diverse in both clinical presentation and biological evolution [
1].
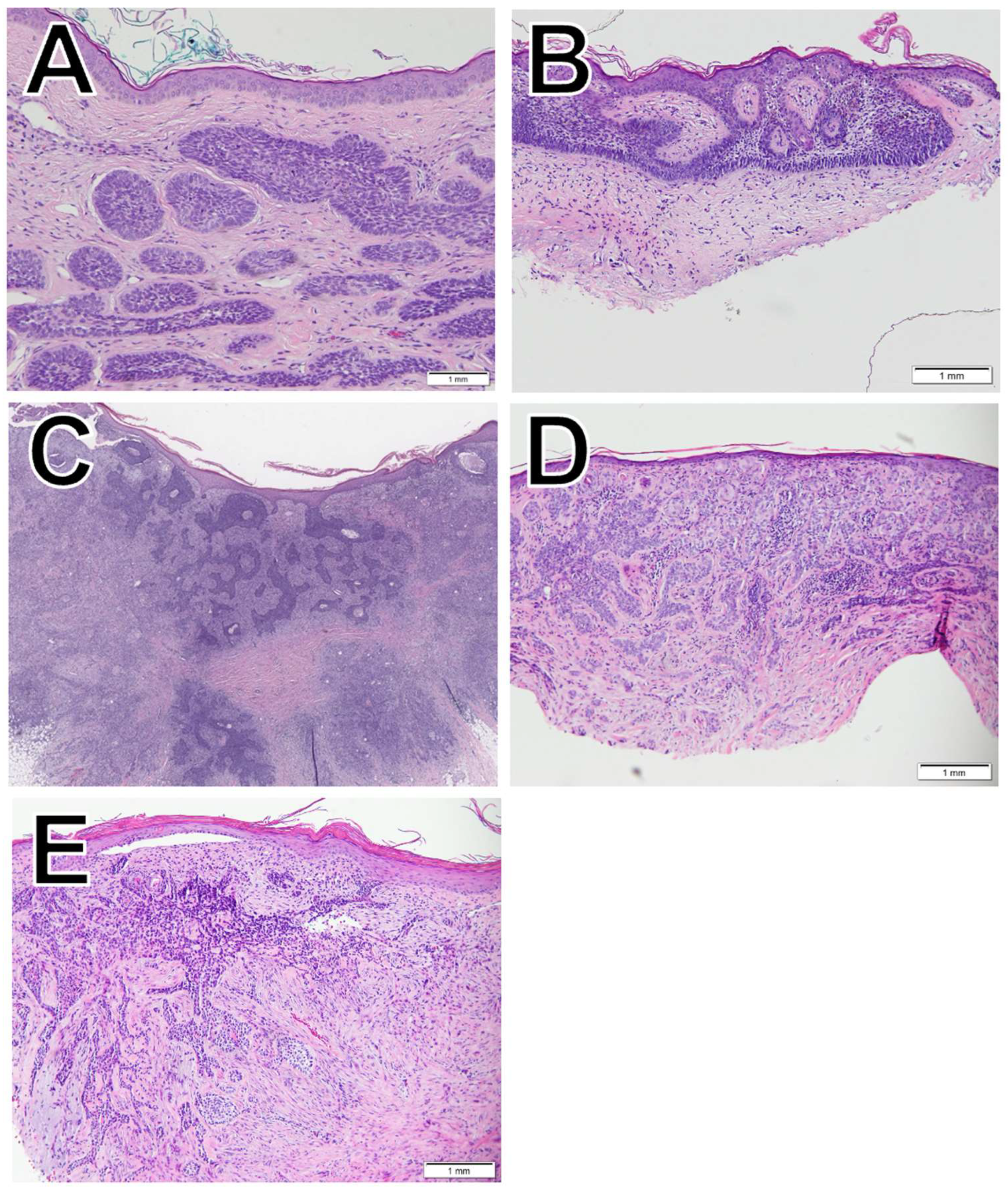
BCC (
Figure 1), the most common type of skin cancer, originates in the basal cells, which are at the base of the epidermis [
10]. While it is rare for BCC to metastasize and mortality is therefore low, if left untreated, BCC can result in high morbidity via local invasion and tissue destruction [
1]. There are over 25 morphological subtypes of BCC [
11], and in this study, we evaluate five of them: nodular (
Figure 1A), superficial (
Figure 1B), squamous differentiation (
Figure 1C), infiltrative (
Figure 1D), and morpheaform (
Figure 1E).
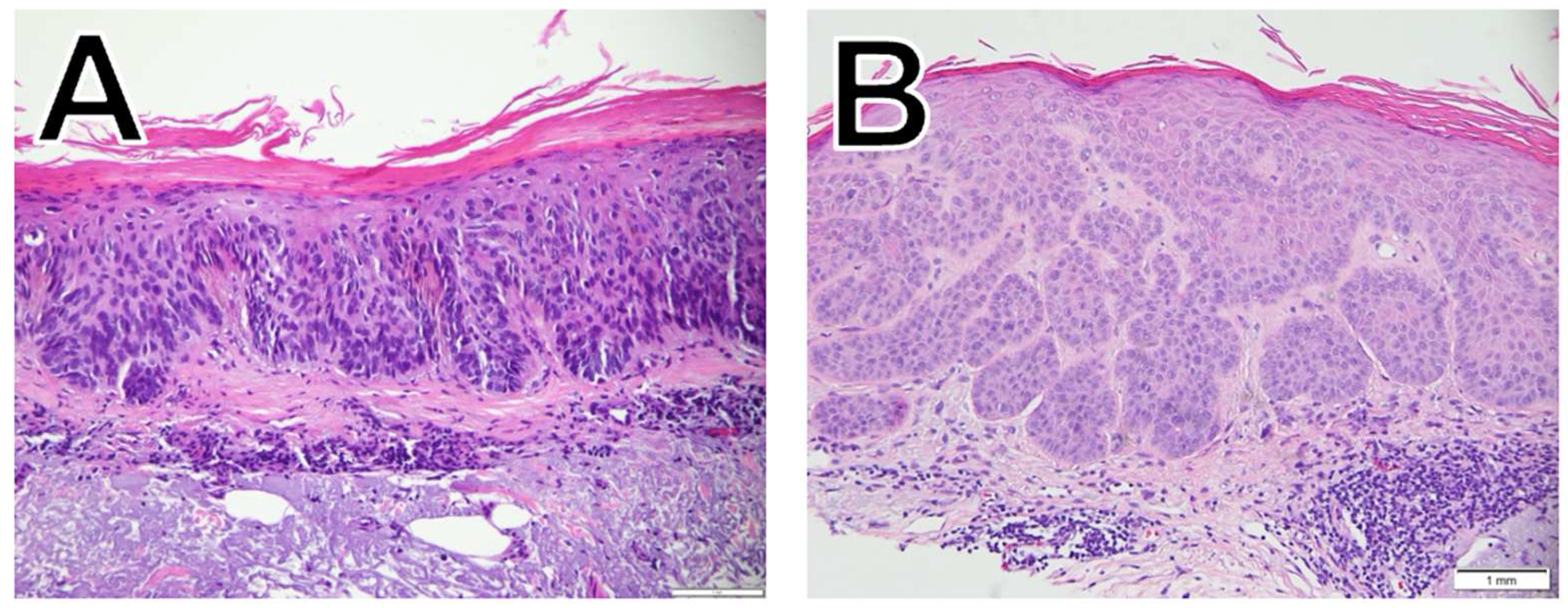
In contrast to BCC, SCC (
Figure 2) originates in the squamous cells, the flat cells in the superficial part of the epidermis. SCC subtypes range from slow growing to aggressive, invasive tumors with a higher risk of metastasis [
1,
12]. The SCC subtypes included in this study are squamous cell carcinoma in situ (SCCIS;
Figure 2A), and well-differentiated SCC (
Figure 2B). SCC tumor characteristics such as site, thickness, and ability to spread and patient characteristics such as older age, male sex, prior treatment with B-Raf enzyme (BRAF) inhibitors, and concomitant immunosuppressive conditions are all associated with increased mortality [
1]. SCCIS is the earliest form of SCC with the cancer cells confined to the epidermis and is not usually symptomatic but is characterized by large, red, scaly/crusted patches [
10]. However, SCCIS can progress to invasive SCC, and early treatment is therefore recommended [
10].
Current National Comprehensive Cancer Network guidelines for the treatment of localized, high- and low-risk BCCs and SCCs include surgical excision or Mohs micrographic surgery (MMS) [
13,
14]. MMS is a precise, tissue-sparing method of removing skin cancer that allows for microscopic evaluation of the entire tumor margin [
15]. In a network meta-analysis of 40 randomized trials and 5 nonrandomized studies, MMS had a 3.8% rate of recurrence, which was found to be similar to recurrence rates following excision (3.3%), curettage and diathermy (5.9%), and external-beam radiation therapy (3.2%) [
16]. Importantly, follow-up duration of included studies ranged from 1 month to 10 years, and patients included in the studies in this meta-analysis were largely older adults (mean age range 63-66 years), were mostly male (median 61%), and had primarily histologically low-risk superficial or nodular BCC with a mean lesion diameter range of 5-13 mm.
Radiation therapy is an option for patients who are poor surgical candidates or for those who prefer a nonsurgical approach in cosmetically sensitive areas [
13,
14]. Image-guided superficial radiation therapy (IGSRT), a newer treatment modality cleared by the United States Food and Drug Administration in 2015, uses an integrated high-resolution dermal ultrasound technology to improve lesion visualization. This allows for more precise radiation targeting owing to a more accurate assessment of the tumor depth and width, allowing clinicians to provide adaptive radiation treatment planning. In IGSRT, an ultrasound set to a frequency of ~22 MHz, the optimal frequency for evaluating a skin layer with a depth of 0–6 mm, is used to determine the extent of the lesion beyond clinical visibility [
17]. IGSRT has demonstrated a 99.3% rate of local tumor control in 2,917 NMSC lesions with a median 14.5 month follow up [
17]. Similarly, an analysis of 1,899 NMSC lesions treated with IGSRT found a 99.7% absolute lesion control with an average of 7.5 weeks of treatment, a stable control rate of 99.6% with follow-up >12 months, and a 60 month local control of 99.4% [
18]. Retrospective cohort studies have also shown that IGSRT for early-stage NMSCs is clinically equivalent to MMS and statistically significantly superior to traditional non-image-guided SRT and other radiation therapy technologies at 2 years’ follow up [
19,
20]. Specifically, in comparison with traditional SRT, IGSRT has demonstrated statistically superior 2-year recurrence rates (0.7% overall, 1.1% for BCC, 0.8% for SCC, and 0.0% for SCCIS) in a retrospective cohort study of 2,880 lesions [
20]. Furthermore, meta-analyses of 2 studies evaluating IGSRT and 4 studies evaluating traditional SRT found that local control of early-stage, high- and low-risk NMSCs was statistically superior with IGSRT compared with traditional SRT overall and in all cancer subtypes when stratified by histology [
21,
22].
There is now a need for larger cohort studies to evaluate freedom from recurrence following IGSRT stratified by patient and disease characteristics, such as patient age, sex, socioeconomic status, tumor location, and lesion histology. This will aid in identifying the most suitable tumor types and subtypes for IGSRT. Therefore, the objective of this large retrospective cohort study was to determine the effect of histology (BCC, SCC, or SCCIS) and NMSC subtypes on freedom from recurrence rates in patients with NMSC treated with IGSRT.
2. Materials and Methods
2.1. IGSRT Treatment Methodology and Energy/Dose Selection Process
The treatment methodology has been previously described in detail [
17,
18] and follows a general guideline (the Ladd-Yu protocol) [
17] for treatment dose, energy, fractionation, and therapeutic biologic effect. A standardized protocol with a total of ~20 fractions using single energy or a sequential combination of 50kVp, 70kVp, or 100kVp energy X-ray treatment is generally delivered 2–4 times per week with pre-treatment, daily high-resolution dermal ultrasound (HRDUS) prior to “beam-on” to assess/confirm tumor configuration/location and detect changes which may indicate a prescription change as necessary, with adaptive radiation treatment planning. HRDUS is also performed during initial simulation for treatment-planning purposes and at follow-up evaluations after treatment course completion to evaluate response.
2.2. Tumor Configuration and Depth Determination
HRDUS uses a non-invasive 20–22 MHz ultrasound with Doppler component probe that is intrinsic to the IGSRT unit (Sensus SRT-100 Vision), which allows visualization of 0–10 mm into the skin structure, including visualization of the epithelium, papillary layer, and sometimes down to the reticular layer depending on anatomic location and skin thickness. This high-resolution/high-frequency ultrasound allows clear visualization of normal skin anatomy and the disrupting tumor, which occupies a black space is hypoechoic without Doppler color speckles, and allows for precise visualization, measurement, and capture of the exact depth of penetration allowing the clinician to perform adaptive radiation therapy planning during a course of care, analogous to how surgeons can assess efficacy and adapt their approach between individual stages of resection during MMS. The width and configuration of the tumor can also be easily discerned with HRDUS and is integral to localization and treatment planning, reducing the risk of anatomical miss and misadministration.
2.3. Data Collection
Data collection followed a similar process as described in published studies [
17,
18]. IGSRT treatment records of over 11,000 patients with 20,069 NMSC lesions treated at multiple institutions across the continental United States between 2016 and 2023 were retrospectively gathered. Exclusion criteria include cases that were not nonmelanoma skin cancers (i.e., keloids), missing pertinent documentation (i.e., treatment chart, simulation stats like time, dose, and fractionation). Lesions that were not treated to a curative dose were included in this intention-to-treat analysis. Patient characteristics and treatment parameters for these lesions were extracted manually and accessed electronically from written and electronic medical records (EMR) for all institutions. Additional data from the EMR, including race, ethnicity, past medical history, medications, follow-up dates, and mortality status/expiratory dates were collected with algorithmic programming conducted by Sympto Health, Inc.
2.4. Statistical Analysis
Detailed logs of NMSC recurrences were maintained by the dermatology practices’ radiation therapists. These recurrence logs were used to quantify recurrence events. Freedom from recurrence was estimated using the Kaplan-Meier method. Groups were compared with respect to freedom from recurrence using the log-rank test.
2.5. Ethics
The ethics committee/Institutional Review Board (IRB) of WIRB-Copernicus Group (WCG™) waived ethical approval for this work. The dataset was de-identified prior to analysis and all data personnel adhered to the Health Insurance Portability and Accountability Act (HIPAA) and ethical standards to protect patient information.
3. Results
3.1. Patient and Disease Characteristics
Demographic and disease characteristics are summarized in
Table 1. A total of 19,988 lesions were included. Patients were mostly male (61.7%) and aged ≥65 years (84.2%); the median age was 74.9 years. Most lesions were located on the head or neck (63.7%), which are known high-risk BCC and SCC locations [
1], and most were categorized as stage 0 (23.4%) or stage 1 (65.7%). With regards to histology, lesions were diagnosed as BCC in 49.5% of lesions, SCC in 26.4%, SCCIS in 23.2%, and ≥2 NMSCs in 1.0%%.
3.2. Freedom from Recurrence Rates by Histology
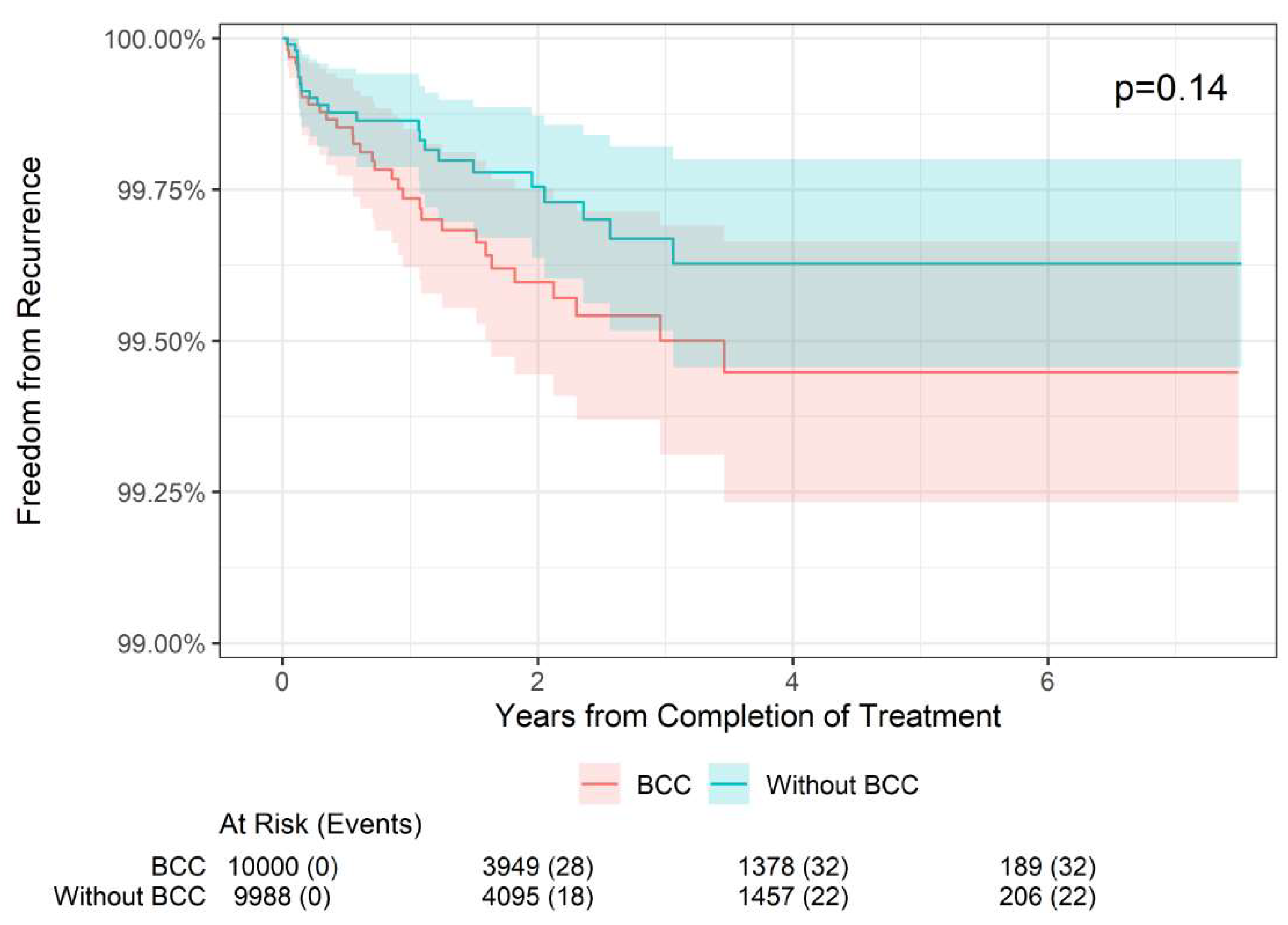
The 2-, 4-, and 6-year recurrence and freedom from recurrence rates are presented by histology in
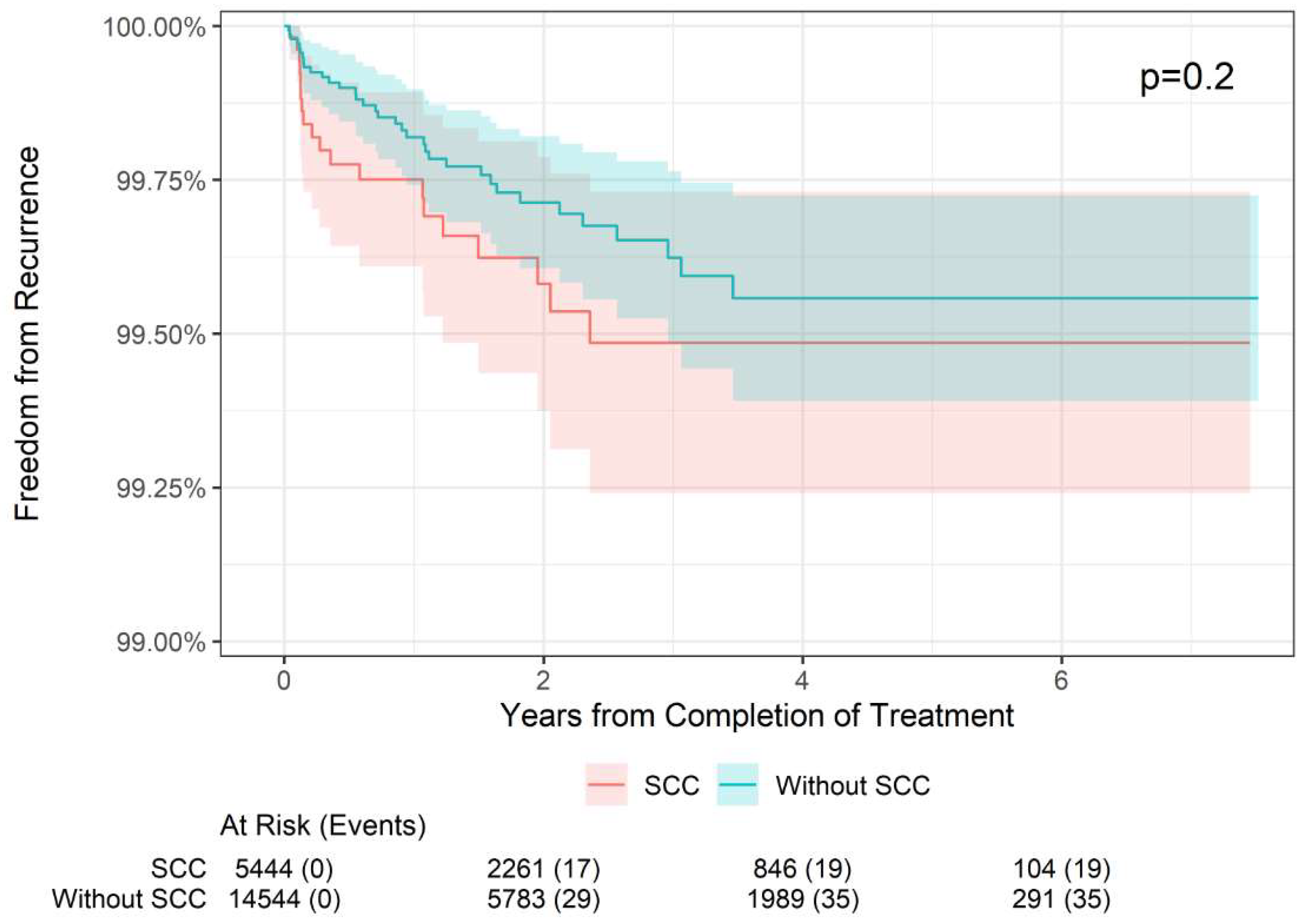
Table 2. In BCC lesions (n = 9,928), the 2-year freedom from recurrence rate was 99.60% compared with 99.75% in non-BCC lesions (i.e., SCC or SCCIS). The 4-year freedom from recurrence rate was 99.45% compared with 99.63% in non-BCC lesions, and the 6-year freedom from recurrence rate was 99.45% compared with 99.63% in non-BCC lesions. These differences were not statistically different (p = 0.14;
Figure 3). Likewise, in SCC lesions (n = 5,294), the 2-year freedom from recurrence rate was 99.58% compared with 99.71% in non-SCC lesions, the 4-year freedom from recurrence rate was 99.49% compared with 99.56% in non-SCC lesions, and the 6-year freedom from recurrence rate was 99.49% compared with 99.56% in non-SCC lesions; this was also not statistically significant (p = 0.2;
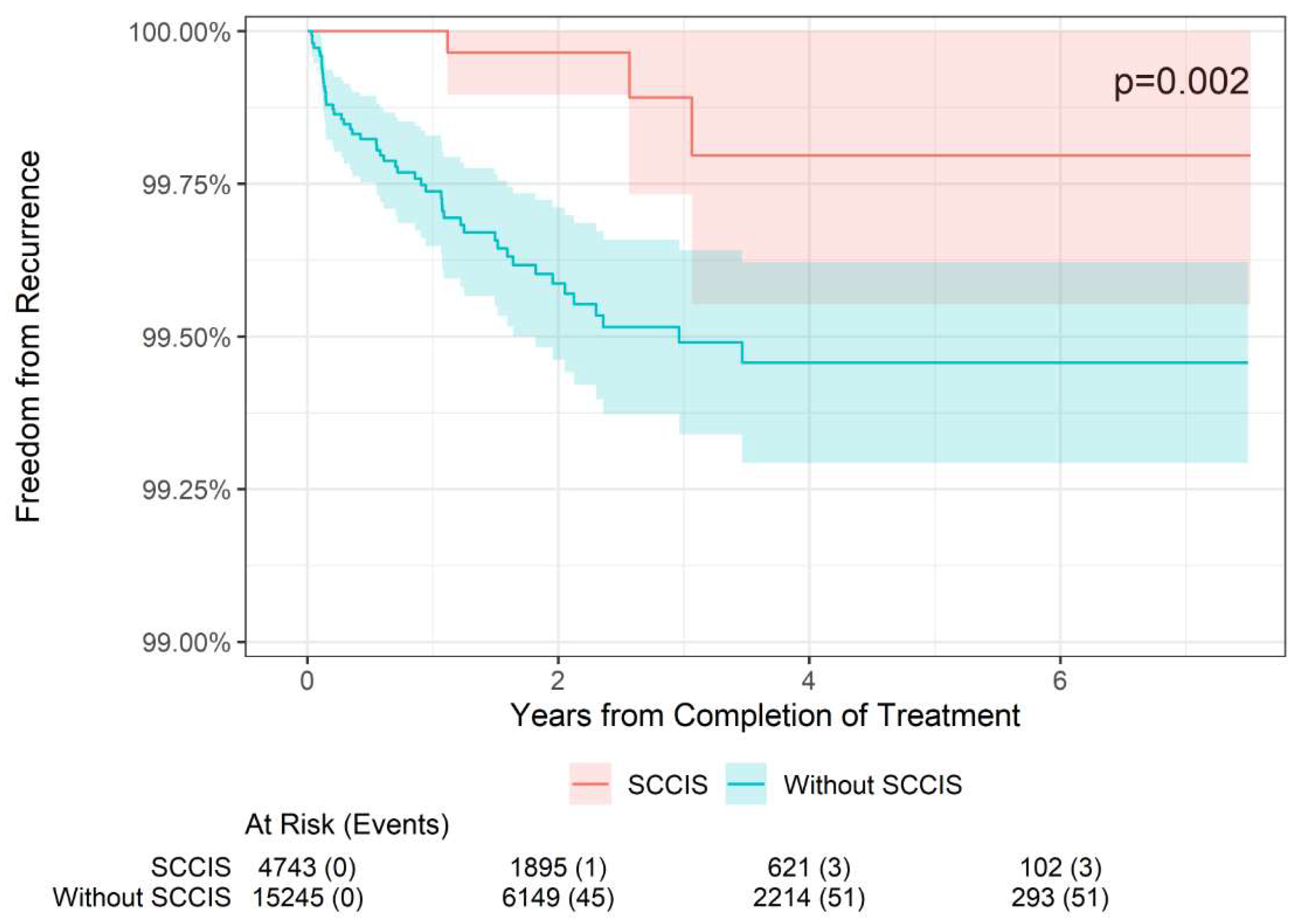
Figure 4). However, for SCCIS lesions (n = 4,648), the 2-, 4-, and 6-year freedom from recurrence rates were slightly higher than in non-SCCIS lesions, and this difference was statistically significant (p = 0.002;
Figure 5), which is concordant with the lower stage.
3.3. Freedom from Recurrence Rates by Histologic Subtype
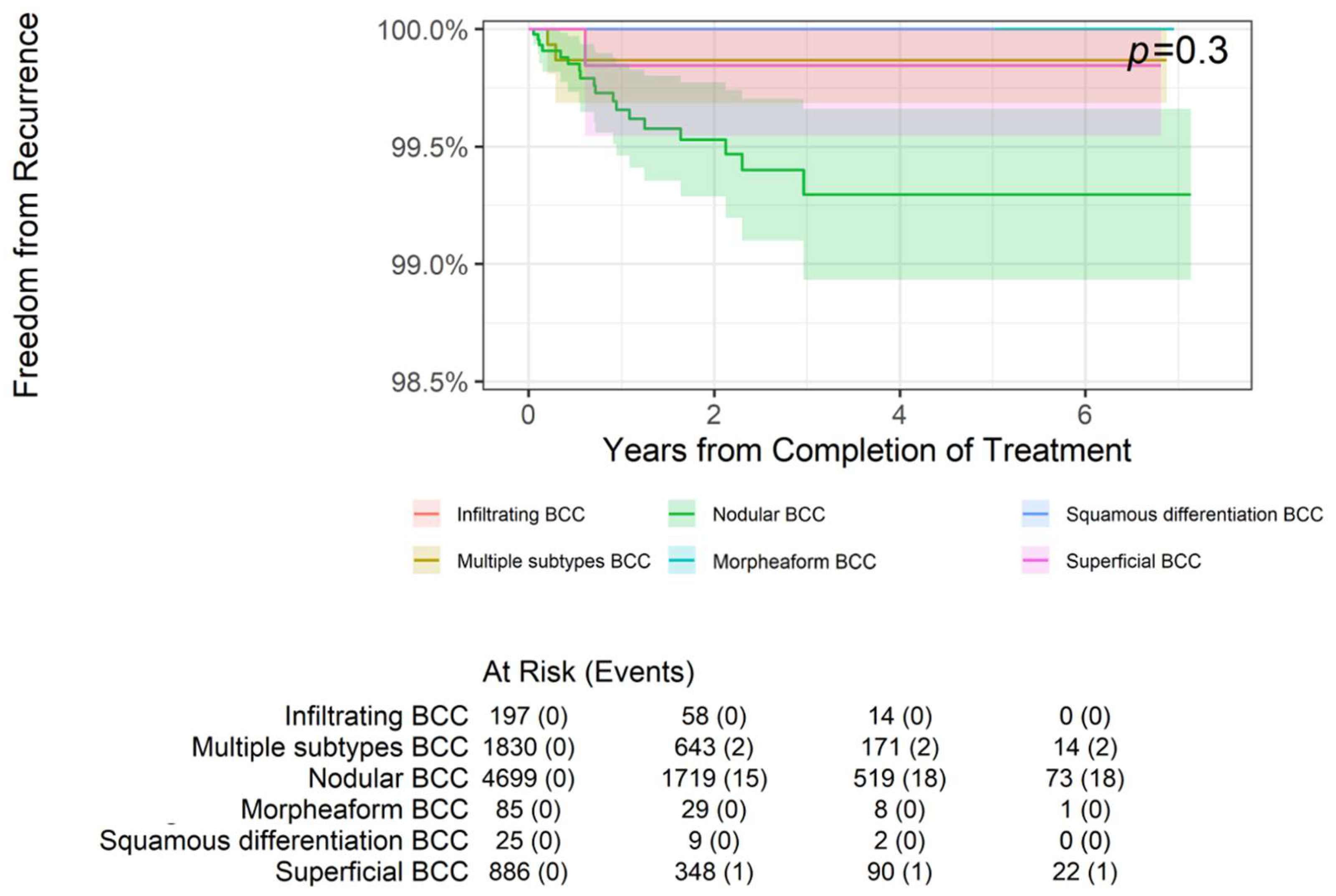
While these histologic groupings are relatively homogeneous, several specific histologic subtypes bear individual assessment (
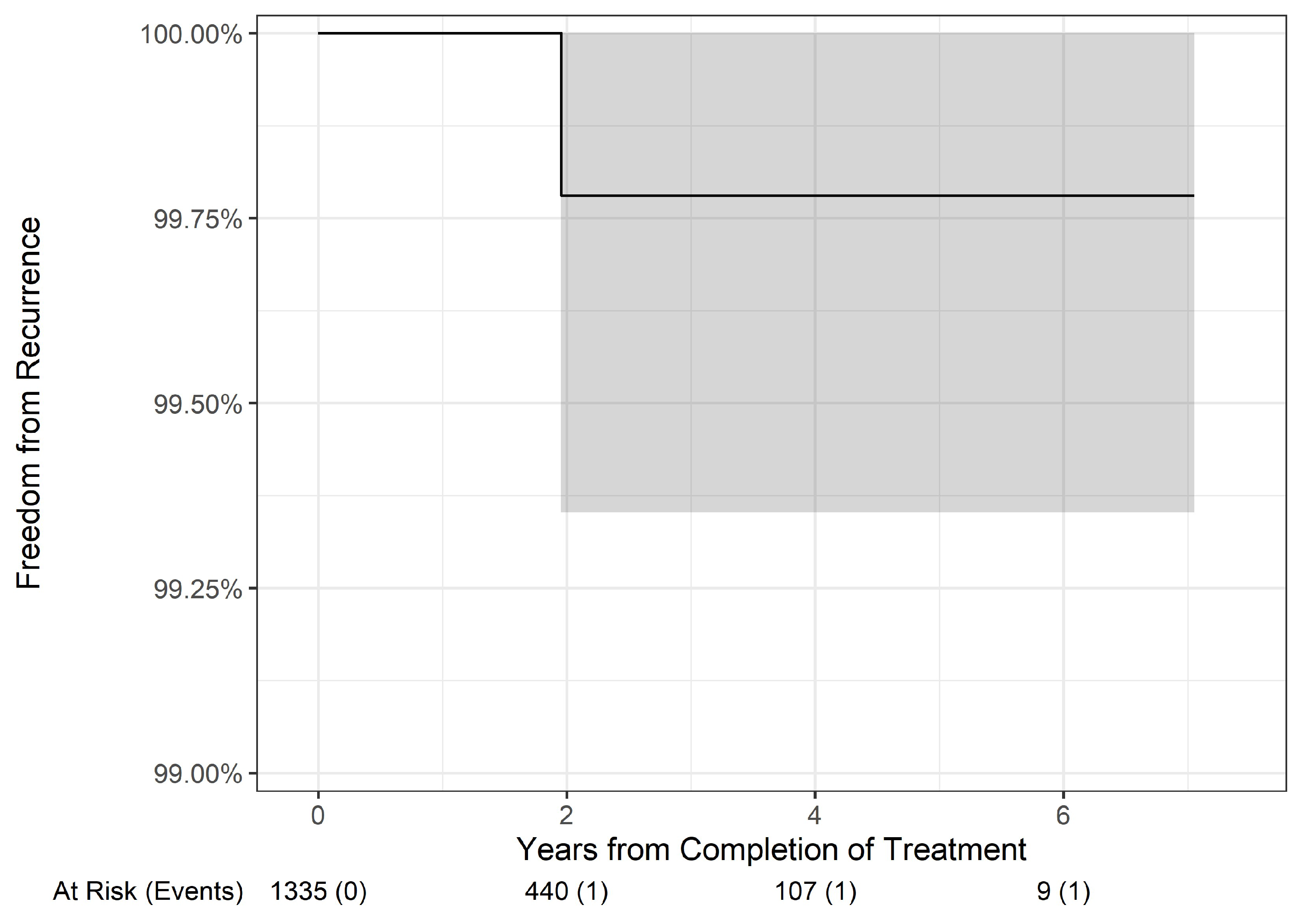
Table 3). The most common BCC subtype was nodular, with 4,699 lesions, and they experienced 2-, 4-, and 6-year freedom from recurrence rates of 99.53%, 99.29%, and 99.29%, respectively. Similarly, the 1,335 well-differentiated (WD) SCC lesions had 2-, 4-, and 6-year freedom from recurrence rates of 99.78% each. These subgroup results did not differ significantly from the larger groupings.
BCC subtypes (p = 0.3,
Figure 6) or WD SCC subtype (
Figure 7) lesions did not have statistically different overall freedom from recurrence rates.
3.4. Example Cases
A few cases of treatment results following IGSRT for NMSCs are highlighted below. Cases demonstrating complete responses, and a recurrence are included. All patients had no prior treatment history for their NMSCs undergoing IGSRT.
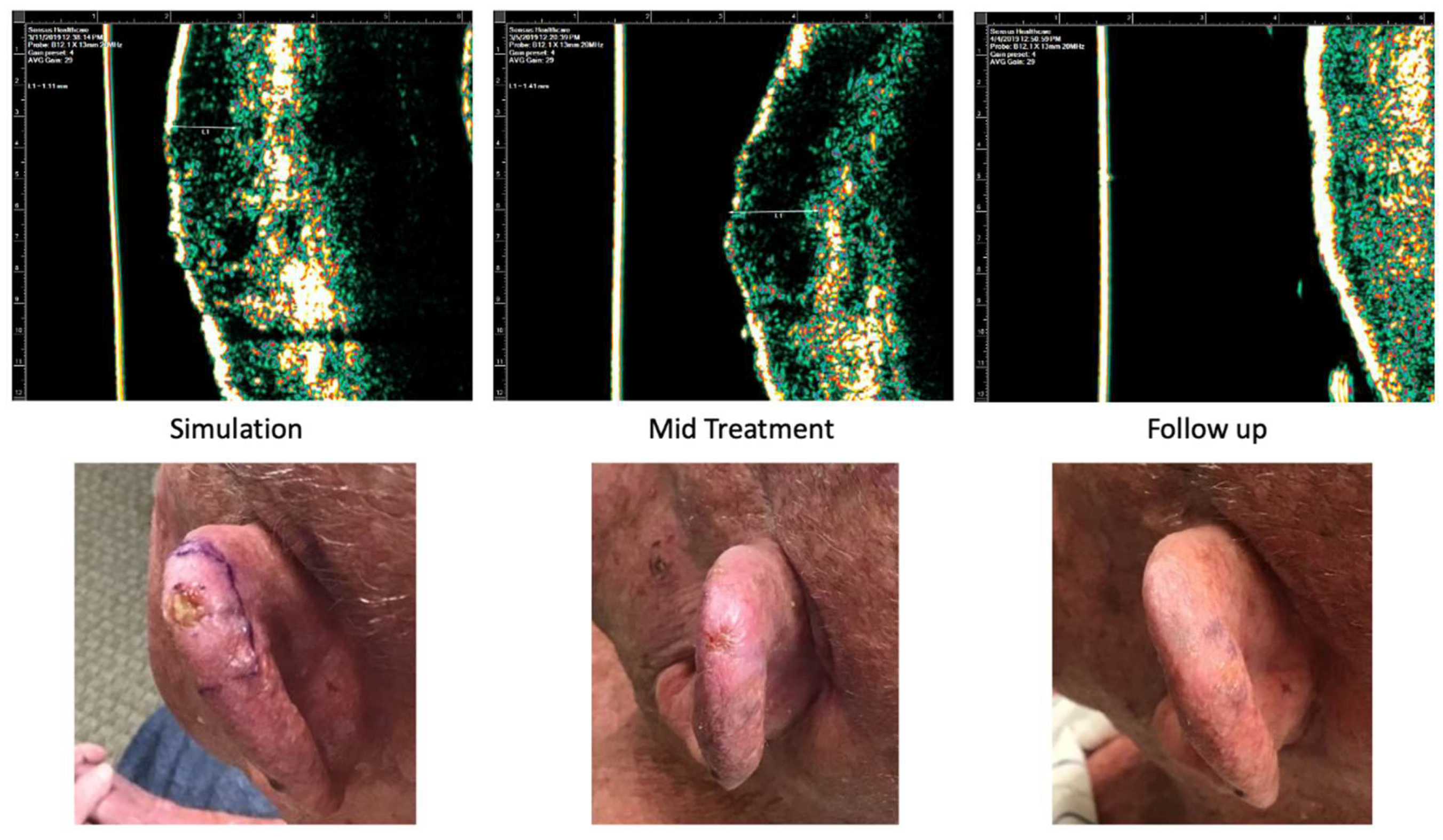
Case 1 is of an 81-year-old male who presented with a 0.5 cm nodular BCC on the left superior helix. He received a total dose of 55 Gy delivered in 20 fractions distributed in 3 fractions/week. The most severe side effects were a Radiation Therapy Oncology Group (RTOG) toxicity grade of 2. At 4 months follow up, the patient had no clinical evidence of disease.
Figure 8.
Case 1. Complete response of nodular basal cell carcinoma to IGSRT. Top panels demonstrate the ultrasound images of the IGSRT device before treatment (simulation), mid treatment, and at final follow up. The bottom panels demonstrate the clinical response at these same time points.
Figure 8.
Case 1. Complete response of nodular basal cell carcinoma to IGSRT. Top panels demonstrate the ultrasound images of the IGSRT device before treatment (simulation), mid treatment, and at final follow up. The bottom panels demonstrate the clinical response at these same time points.
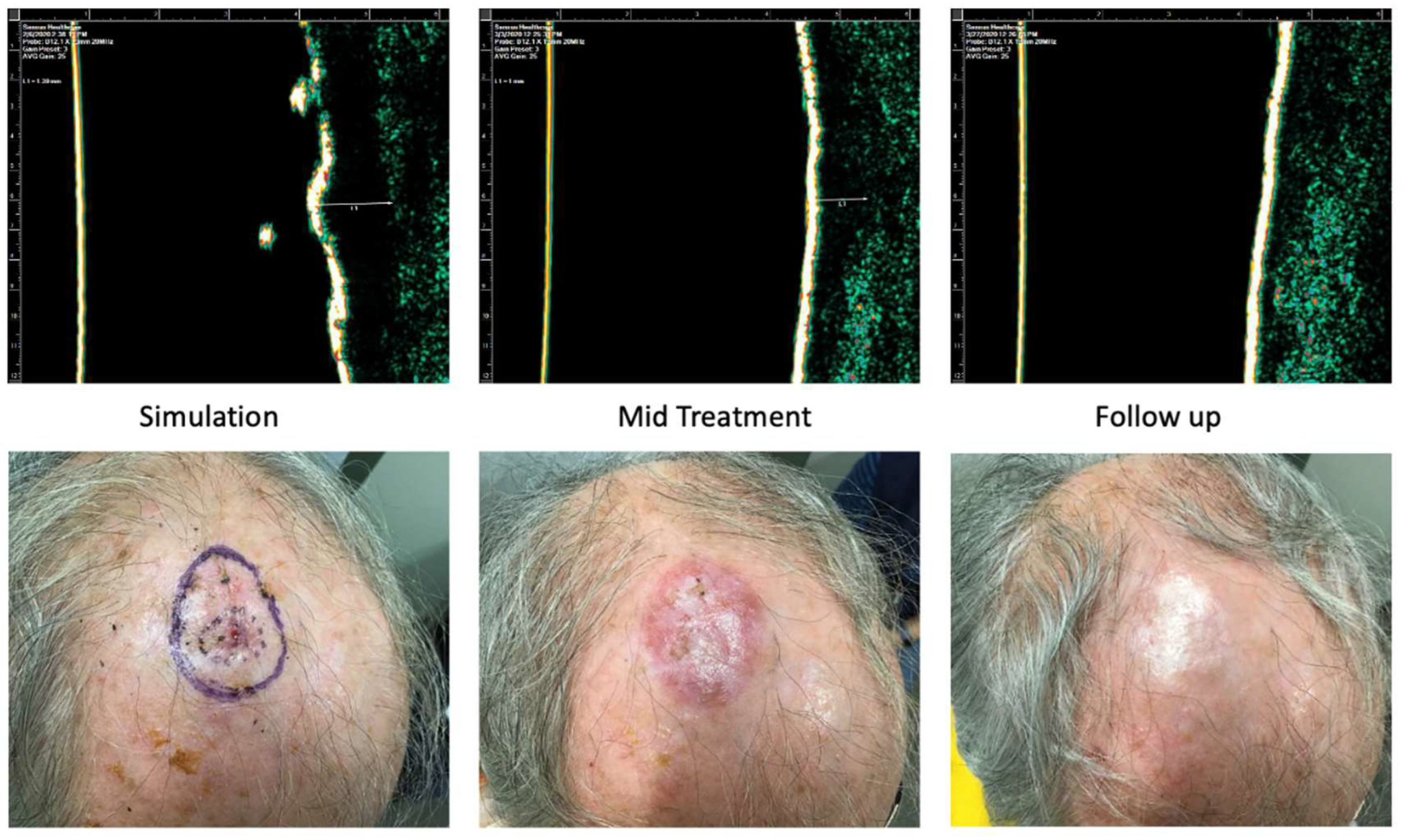
Case 2 is of an 83-year-old male with past medical history significant for diabetes mellitus and hypertension who presented with a 1.8 cm SCC on the scalp. He received a total dose of 54 Gy delivered in 20 fractions distributed in 3 fractions/week. The worst radiation toxicity was graded RTOG 2. At 3 weeks follow-up, the patient had no clinical evidence of disease.
Figure 9.
Case 2. Complete response of squamous cell carcinoma to IGSRT. Top panels demonstrate the ultrasound images of the IGSRT device before treatment (simulation), mid treatment, and at final follow up. The bottom panels demonstrate the clinical response at these same time points.
Figure 9.
Case 2. Complete response of squamous cell carcinoma to IGSRT. Top panels demonstrate the ultrasound images of the IGSRT device before treatment (simulation), mid treatment, and at final follow up. The bottom panels demonstrate the clinical response at these same time points.
Case 3 is of an 85-year-old female with past medical history significant for hypertension and dementia who presented with a 1.4 cm nodular BCC on the left malar cheek. She received a total dose of 55 Gy delivered in 20 fractions distributed in 3 fractions/week. The worst radiation toxicity was graded RTOG 3.
Figure 10 demonstrates the findings at 4 weeks follow up. This site was biopsied at 16 weeks post-IGSRT for concern of recurrence, and a superficial/nodular BCC was identified. The patient was referred to Mohs surgery for further treatment.
4. Discussion
The major finding from this intention-to-treat analysis of a large retrospective cohort study is that the effects of IGSRT on invasive NMSCs are not significantly impacted by tumor histology. Freedom from recurrence rates at 2, 4, and 6 years did not differ significantly between BCC vs. non-BCC or SCC vs. non-SCC lesions, suggesting that IGSRT is an equally viable option for either histology. A variety of BCC histologic subtypes and well-differentiated SCC also had similar freedom from recurrence rates. In contrast, and as was expected, freedom from recurrence rates were slightly higher in SCCIS lesions compared with invasive disease at 2, 4, and 6 years. Finally, freedom from recurrence was not affected by BCC or SCC histological subtype.
These findings demonstrate that IGSRT is viable therapeutic option for NMSC regardless of histology type or subtype and are supportive of previous research which found that IGSRT is a safe, well-tolerated therapy that demonstrates excellent local tumor control and absolute lesion control [
17] and confirm the previously reported superior recurrence rates compared with traditional non-image guided SRT [
19,
20].
IGSRT has several advantages as a treatment modality, including being well-tolerated, relatively quick to administer, and suitable for patients who have contraindications for surgery or who refuse surgery. Furthermore, IGSRT preserves function and provides favorable cosmetic outcomes, thereby supporting the primary goals of skin cancer treatment, “the complete removal of the tumor and the maximal preservation of function and cosmesis”[
13,
14,
19].
The most significant limitation of this study is its retrospective design, which allows for analysis of correlation but not causation. Future prospective trials would possibly improve the quality of the evidence available regarding the impact of individual patient and disease characteristics on the effects of IGSRT on freedom from recurrence in patients with NMSC.
Further retrospective analyses by other specific demographic and disease characteristics, such as age, tumor location, socioeconomic status, and comorbidities, are needed to gather insights into the potential effects of these characteristics on disease recurrence and to characterize the patient populations that might gain the most benefit from IGSRT. Additionally, further work to characterize personalized treatment strategies, including genomic [
23,
24] and imaging [
25] methods could facilitate better patient selection and more individualized radiation treatment plans, including plans for treatment with IGSRT.
5. Conclusions
In summary, this is the first large retrospective cohort study of 20,069 NMSC lesions treated with IGSRT to evaluate and compare freedom from recurrence by tumor histologic type. Overall, this study found that freedom from recurrence rates do not vary significantly among BCC or SCC lesions (or their subtypes) but are improved with SCCIS. In combination with previous findings demonstrating the safety profile and efficacy of IGSRT as well as cohort studies indicating the superiority of IGSRT over SRT, these results further suggest that IGSRT is a viable first-line therapeutic option for patients diagnosed with early-stage NMSCs, particularly for those who cannot tolerate or choose not to undergo surgical removal, regardless of their histology. It is important to offer patients as many effective treatment options as possible, and the results of this study support the use of IGSRT in a range of NMSC types.
Author Contributions
Conceptualization, E.M.M. and J.B.S.; methodology, E.M.M., J.B.S.; X.X., Y.Y. and Z.Z.; formal analysis, E.M.M.; investigation, E.M.M., C.J.C., S.H., E.S.M., B.N.K., J.W., J.B.S.; resources, J.B.S.; data curation, E.M.M.; writing—original draft preparation, E.M.M., C.J.C., S.H., E.S.M., B.N.K., J.W., J.B.S.; writing—review and editing, E.M.M., C.J.C., S.H., E.S.M., B.N.K., J.W., J.B.S.; visualization, E.M.M.; supervision, E.M.M., J.B.S.; project administration, E.M.M.; funding acquisition, J.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Dermatology Association of Radiation Therapy.
Institutional Review Board Statement
Ethical review and approval were waived for this study were waived by the WCG Institutional Review Board (IRB) because the dataset was de-identified prior to analysis and all data personnel adhered to the Health Insurance Portability and Accountability Act (HIPAA) and ethical standards to protect patient information.
Informed Consent Statement
Patient consent was waived by the WCG Institutional Review Board (IRB) because the dataset was de-identified prior to analysis and all data personnel adhered to the Health Insurance Portability and Accountability Act (HIPAA) and ethical standards to protect patient information.
Data Availability Statement
Data are not publicly available but are available upon reasonable request from the corresponding author.
Acknowledgments
The authors would like to acknowledge the following people who were instrumental in collecting and extracting data from various different locations, without whom this study would not be possible: Amanda Boatner, Berta Benzschawel, Beth Ann Marogi, Brianna Moore, Carolyn Mahoot, Charles Bingner, Cherie Hoppe, Diane Krause, Lio Yu, Eric Hill, Erica Kovaluskie, Isabella Moore, Jacob Britt, Jacqueline Contino, Jennilee Kovaluskie, Jenny Folden, JoAnne Porta, Karen McKenna, Katie Curtiss, Kim McLaugherty, Kimberly Wilke-Zuma, Krupal Patel, Lacy Smith, Leslie Walker, Lisa Gonzalez, Lisha Kelly, Macie Najera, Mairead Moloney, Mandi Brown, Michael Kaczmarski, Michelle Smith, Nick Natalizio, Olivia Desalvo, Paula Wright, Peter Kaczmarski, Prithvi Narasimhan, Rachel Lindley, Rachelle Moore, Samantha Sheehan, Sarah Moss, Soham More, Stacey Robinson, Tonya Johnson, and Yancy Boatner.
Conflicts of Interest
This study received funding from The Dermatology Association of Radiation Therapy (DART). No authors are employed by or affiliated with DART. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication. .
References
- Cives M, Mannavola F, Lospalluti L, et al. Non-Melanoma Skin Cancers: Biological and Clinical Features. Int J Mol Sci. 2020;21(15).
- Leigh, IM. Progress in skin cancer: the U.K. experience. Br J Dermatol. 2014;171(3):443-445.
- Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015;151(10):1081-1086.
- American Cancer Society. Key statistics for basal and squamous cell skin cancers. 31 October 2023.
- Ciążyńska M, Kamińska-Winciorek G, Lange D, et al. The incidence and clinical analysis of non-melanoma skin cancer. Scientific Reports. 2021;11(1):4337.
- Aggarwal P, Knabel P, Fleischer AB, Jr. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85(2):388-395.
- Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069-1080.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80(2):303-317.
- Fitzmaurice C, Abate D, Abbasi N, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5(12):1749-1768.
- American Cancer Society. What are basal and squamous cell skin cancers? 31 October 2023.
- McDaniel B, Badri T, Steele RB. Basal Cell Carcinoma. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright © 2024, StatPearls Publishing LLC.; 2024.
- Howell JY, Hadian Y, Ramsey ML. Squamous Cell Skin Cancer. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright © 2024, StatPearls Publishing LLC.; 2024.
- National Comprehensive Cancer Network. Basal cell skin cancer, NCCN Guidelines Version 3.2024. Internet: National Comprehensive Cancer Network;2024.
- National Comprehensive Cancer Network. Squamous cell skin cancer, NCCN Guidelines Version 1.2024. Internet: National Comprehensive Cancer Network;2024.
- Mohs micrographic surgery (updated 25 Jul 2023). StatPearls Publishing; 2024. https://www.ncbi.nlm.nih.gov/books/NBK441833/.
- Drucker AM, Adam GP, Rofeberg V, et al. Treatments of Primary Basal Cell Carcinoma of the Skin: A Systematic Review and Network Meta-analysis. Ann Intern Med. 2018;169(7):456-466.
- Yu L, Oh C, Shea CR. The Treatment of Non-Melanoma Skin Cancer with Image-Guided Superficial Radiation Therapy: An Analysis of 2917 Invasive and In Situ Keratinocytic Carcinoma Lesions. Oncol Ther. 2021;9(1):153-166.
- Tran A, Moloney M, Kaczmarski P, et al. Analysis of image-guided superficial radiation therapy (IGSRT) on the treatment of early-stage non-melanoma skin cancer (NMSC) in the outpatient dermatology setting. J Cancer Res Clin Oncol. 2023;149(9):6283-6291.
- McClure EM, Sedor G, Jin Y, Kattan MW. Image-guided superficial radiation therapy has superior 2-year recurrence probability to Mohs micrographic surgery. Clin Transl Radiat Oncol. 2023;43:100678.
- McClure E, Sedor G, Moloney M, Jin Y, Yu L, Kattan MW. Image guidance improves freedom from recurrence rate after superficial radiation therapy for non-melanoma skin cancer. Advances in Radiation Oncology. 2024:101463.
- Yu L, Moloney M, Tran A, Zheng S, Rogers J. Local control comparison of early-stage non-melanoma skin Cancer (NMSC) treated by superficial radiotherapy (SRT) and external beam radiotherapy (XRT) with and without dermal image guidance: a meta-analysis. Discov Oncol. 2022;13(1):129.
- Yu L, Moloney M, Zheng S, Rogers J. High resolution dermal ultrasound (US) combined with superficial radiation therapy (SRT) versus non-image guided SRT or external beam radiotherapy (XRT) in early-stage epithelial cancer: a comparison of studies. BMC Cancer. 2023;23(1):98.
- Scott JG, Berglund A, Schell MJ, et al. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol. 2017;18(2):202-211.
- Scott JG, Sedor G, Ellsworth P, et al. Pan-cancer prediction of radiotherapy benefit using genomic-adjusted radiation dose (GARD): a cohort-based pooled analysis. Lancet Oncol. 2021;22(9):1221-1229.
- Lou B, Doken S, Zhuang T, et al. An image-based deep learning framework for individualizing radiotherapy dose. Lancet Digit Health. 2019;1(3):e136-e147.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).