Submitted:
07 October 2024
Posted:
08 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethics Committee Approval
2.2. Study Design
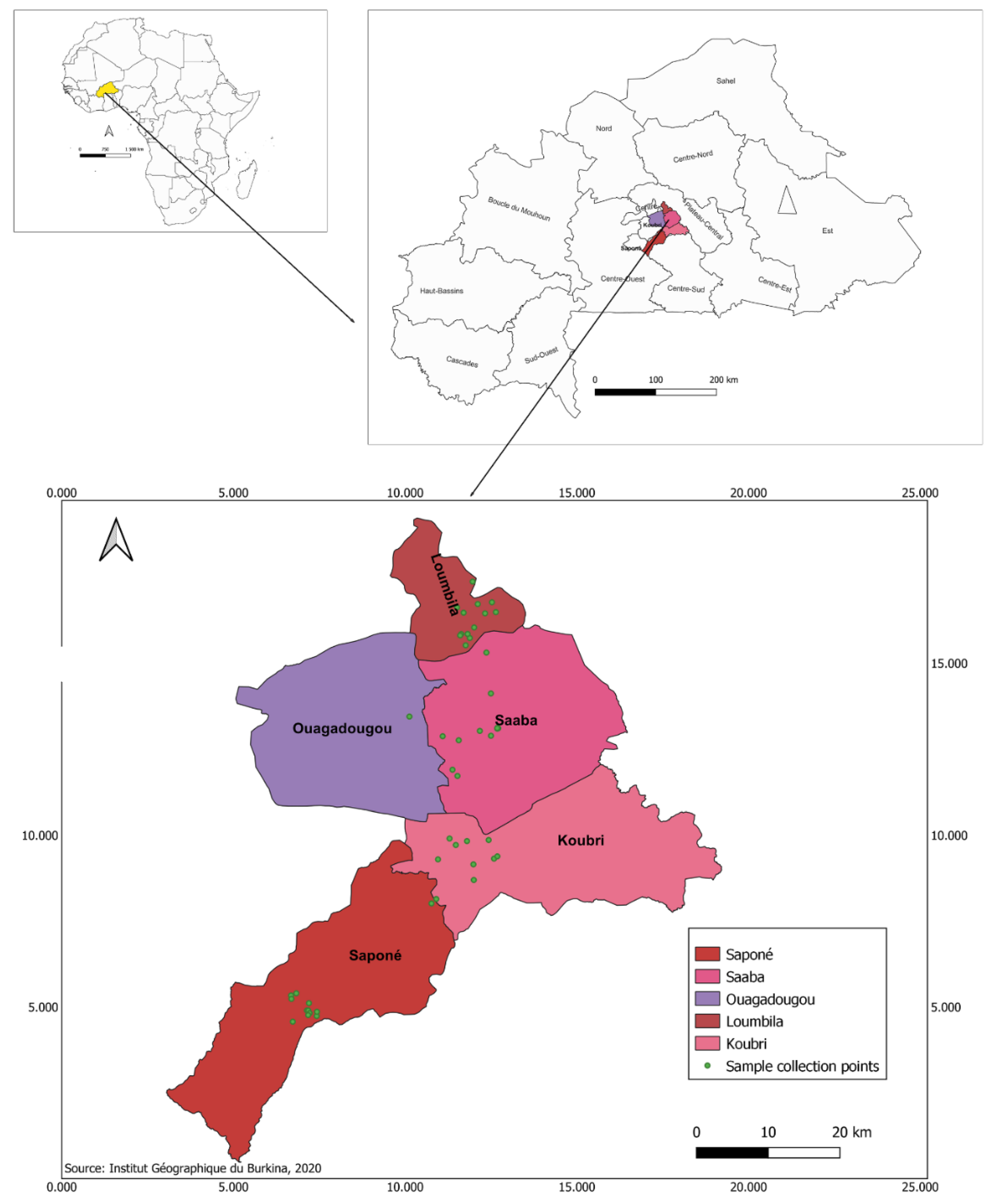
2.3. Sampling
2.4. Bacterial Isolation and Identification
2.5. Antibiotic Sensitivity Testing
2.6. Phenotypic Detection of ESBL Production
2.7. Data Analysis
3. Results
3.1. Prevalence of ESBL-Producing E. coli and Klebsiella spp. Isolates by Sample and Farm Type
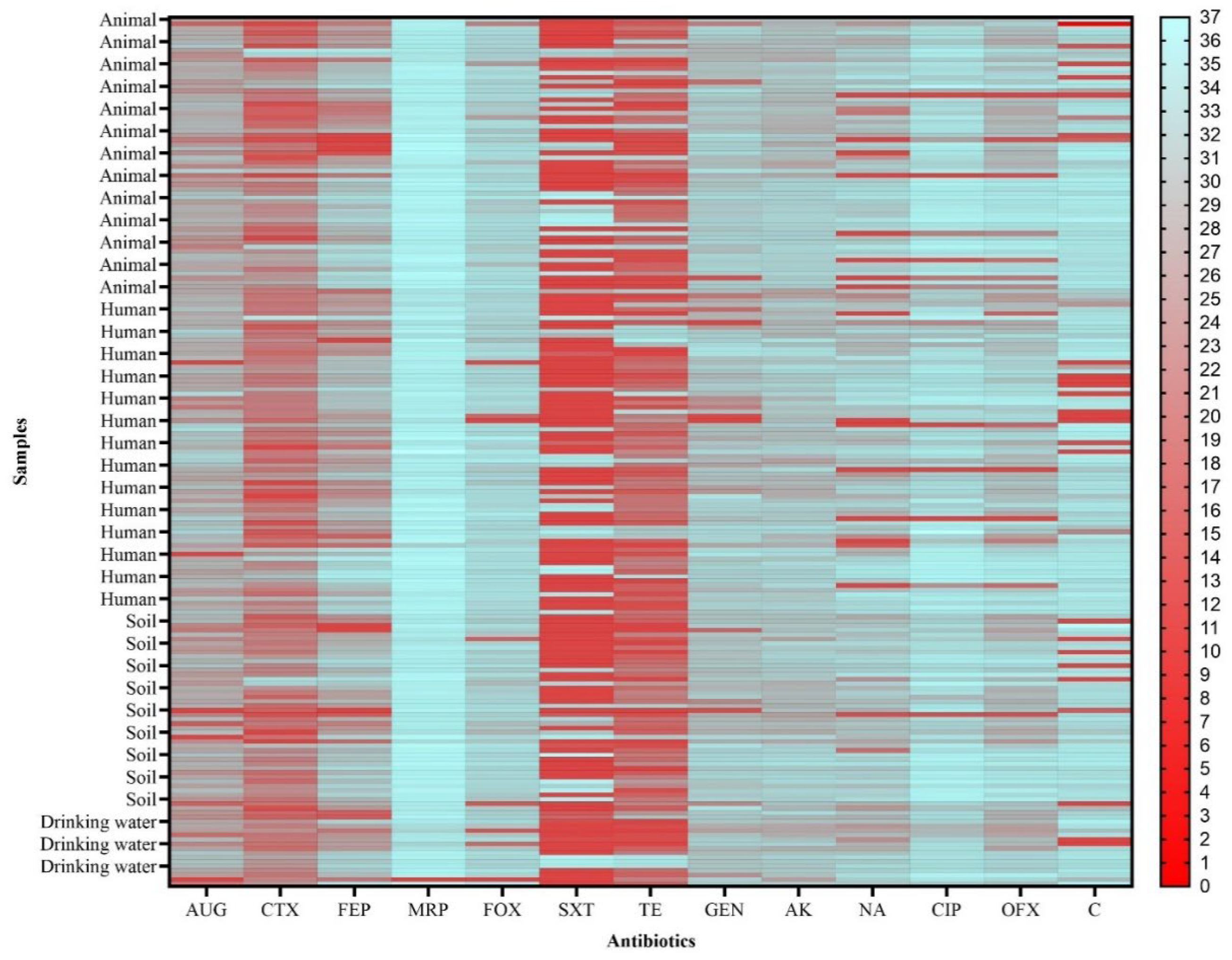
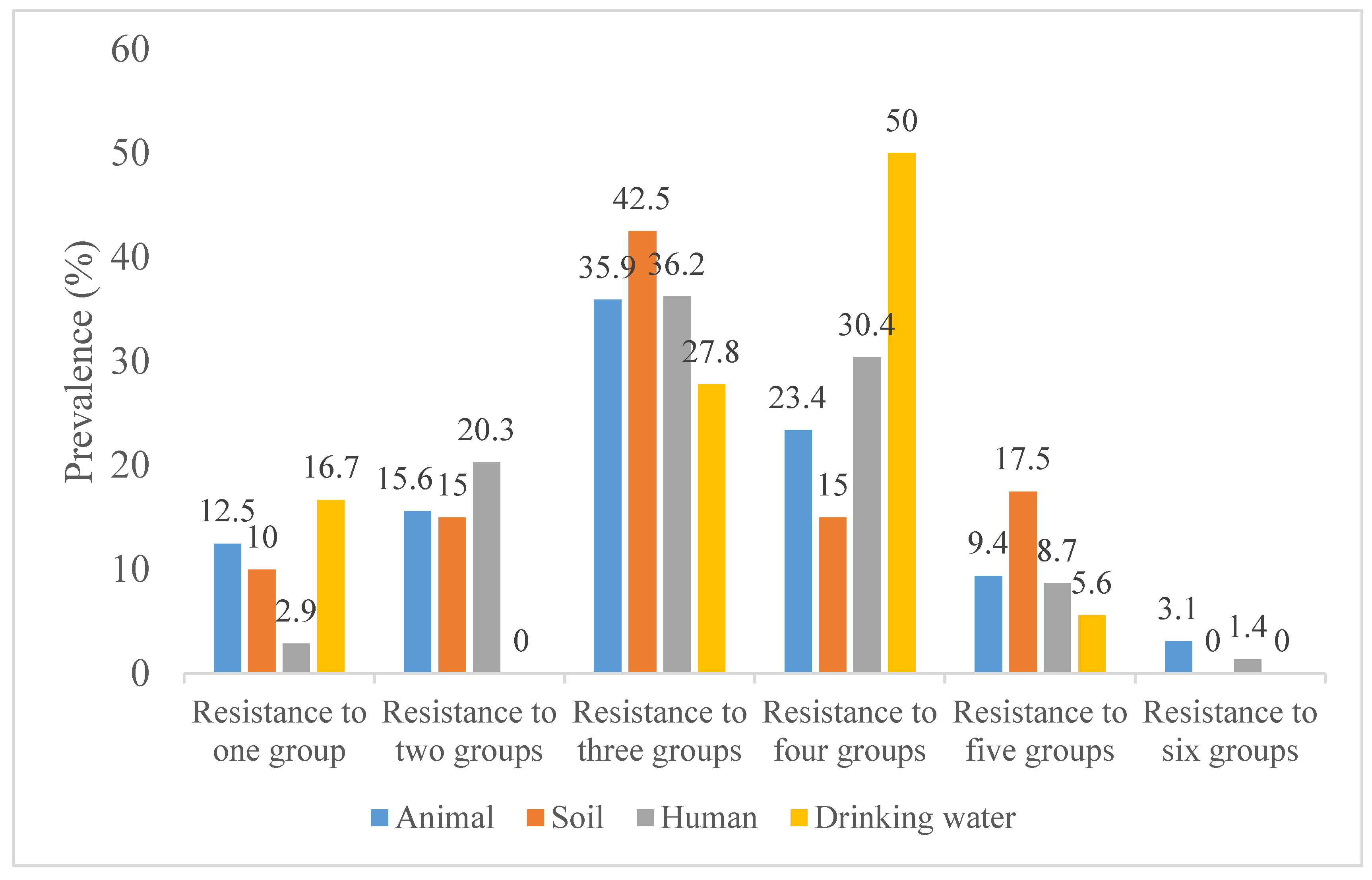
3.2. Antibiotic Resistance of ESBL-Producing E. coli and Klebsiella spp. Isolates
4. Discussion
5. Conclusion
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [CrossRef] [PubMed]
- World Health Organization New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis. 2019, 28–30.
- Ouedraogo, A.S.; Sanou, M.; Kissou, A.; Sanou, S.; Solaré, H.; Kaboré, F.; Poda, A.; Aberkane, S.; Bouzinbi, N.; Sano, I.; et al. High Prevalence of Extended-Spectrum ß-Lactamase Producing Enterobacteriaceae among Clinical Isolates in Burkina Faso. BMC Infect. Dis. 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kpoda, D.S.; Ajayi, A.; Somda, M.; Traore, O.; Guessennd, N.; Ouattara, A.S.; Sangare, L.; Traore, A.S.; Dosso, M. Distribution of Resistance Genes Encoding ESBLs in Enterobacteriaceae Isolated from Biological Samples in Health Centers in Ouagadougou, Burkina Faso. BMC Res. Notes 2018, 11, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Boeckel, T.P. Van; Glennon, E.; Chen, D.; Gilbert, M.; Robinson, T.; Grenfell, B.; Levin, S.; Bonhoeffer, S.; Laxminarayan, R. Reducing Antimicrobial Use in Food Animals: Consider User Fees and Regulatory Caps on Veterinary Use. Science 2017, 351, 1350. [Google Scholar] [CrossRef] [PubMed]
- Henchion, M.; Moloney, A.P.; Hyland, J.; Zimmermann, J.; Mccarthy, S. Animal The International Journal of Animal Biosciences Review : Trends for Meat, Milk and Egg Consumption for the next Decades and the Role Played by Livestock Systems in the Global Production of Proteins. Animal 2021, 15, 100287. [Google Scholar] [CrossRef]
- Mshana, S.E.; Sindato, C.; Matee, M.I.; Mboera, L.E.G. Antimicrobial Use and Resistance in Agriculture and Food Production Systems in Africa: A Systematic Review. Antibiotics 2021, 10. [Google Scholar] [CrossRef]
- Ministère des ressources animales et halieutiques Analyse de La Chaîne de Valeurs Des Petits Ruminants Au Burkina Faso; 2017.
- Butcher, A.; Cañada, J.A.; Sariola, S. How to Make Noncoherent Problems More Productive: Towards an AMR Management Plan for Low Resource Livestock Sectors. Humanit. Soc. Sci. Commun. 2021, 8, 1–10. [Google Scholar] [CrossRef]
- Caudell, M.A.; Dorado-Garcia, A.; Eckford, S.; Creese, C.; Byarugaba, D.K.; Afakye, K.; Chansa-Kabali, T.; Fasina, F.O.; Kabali, E.; Kiambi, S.; et al. Towards a Bottom-up Understanding of Antimicrobial Use and Resistance on the Farm: A Knowledge, Attitudes, and Practices Survey across Livestock Systems in Five African Countries. PLoS One 2020, 15, 1–26. [Google Scholar] [CrossRef]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-Spectrum β-Lactamases: An Update on Their Characteristics, Epidemiology and Detection. JAC-Antimicrobial Resist. 2021, 3. [Google Scholar] [CrossRef]
- Braun, S.D.; Ahmed, M.F.E.; El-adawy, H.; Hotzel, H. Surveillance of Extended-Spectrum Escherichia Coli in Dairy Cattle Farms in the Nile Delta, Egypt. 2016, 7. [CrossRef]
- Gay, N.; Leclaire, A.; Laval, M.; Miltgen, G.; Jégo, M.; Stéphane, R.; Jaubert, J.; Belmonte, O.; Cardinale, E. Risk Factors of Extended-Spectrum β-Lactamase Producing Enterobacteriaceae Occurrence in Farms in Reunion, Madagascar and Mayotte Islands, 2016-2017. Vet. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Fashae, K.; Engelmann, I.; Monecke, S.; Braun, S.D.; Ehricht, R. Molecular Characterisation of Extended-Spectrum ß-Lactamase Producing Escherichia Coli in Wild Birds and Cattle, Ibadan, Nigeria. BMC Vet. Res. 2021, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Montso, K.P.; Dlamini, S.B.; Kumar, A.; Ateba, C.N.; Garcia-Perdomo, H.A. Antimicrobial Resistance Factors of Extended-Spectrum Beta-Lactamases Producing Escherichia Coli and Klebsiella Pneumoniae Isolated from Cattle Farms and Raw Beef in North-West Province, South Africa. Biomed Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Saidani, M.; Messadi, L.; Soudani, A.; Daaloul-Jedidi, M.; Châtre, P.; Ben Chehida, F.; Mamlouk, A.; Mahjoub, W.; Madec, J.Y.; Haenni, M. Epidemiology, Antimicrobial Resistance, and Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae in Clinical Bovine Mastitis in Tunisia. Microb. Drug Resist. 2018, 24, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Aworh, M.K.; Ekeng, E.; Nilsson, P.; Egyir, B.; Owusu-Nyantakyi, C.; Hendriksen, R.S. Extended-Spectrum ß-Lactamase-Producing Escherichia Coli Among Humans, Beef Cattle, and Abattoir Environments in Nigeria. Front. Cell. Infect. Microbiol. 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sanou, S.; Ouedraogo, A.S.; Lounnas, M.; Zougmore, A.; Pooda, A.; Zoungrana, J.; Ouedraogo, G.A.; Traore-Ouedraogo, R.; Ouchar, O.; Jean-Pierre, H.; et al. Epidemiology and Molecular Characterization of Enterobacteriaceae Producing Extended-Spectrum β-Lactamase in Intensive and Extensive Breeding Animals in Burkina Faso. PAMJ - One Heal. 2022, 8, 1–12. [Google Scholar] [CrossRef]
- Ouedraogo, A. .; Jean Pierre, H.; Banuls, A.L.; Oedraogo, R.; Godreuil, S. Émergence et Diffusion de La Résistance Aux Antibiotiques En Afrique de l’Ouest : Facteurs Favorisants et Évaluation de La Menace. Med. Sante Trop. 2017, 27, 147–154. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Fifteenth Informational Supplement M100-S15. CLSI, Wayne, PA, U. Clsi; 2021; ISBN 9781684401048.
- WHO WHO Bacterial Priority Pathogens List, 2024; 2024; ISBN 978-92-4-009346-1.
- European Food Safety Authority and European Centre for Disease Prevention and Control The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2010. EFSA J. 2012, 10. [CrossRef]
- Nossair, M.A.; Abd El Baqy, F.A.; Rizk, M.S.Y.; Elaadli, H.; Mansour, A.M.; El-Aziz, A.H.A.; Alkhedaide, A.; Soliman, M.M.; Ramadan, H.; Shukry, M.; et al. Prevalence and Molecular Characterization of Extended-Spectrum β-Lactamases and AmpC β-Lactamase-Producing Enterobacteriaceae among Human, Cattle, and Poultry. Pathogens 2022, 11. [Google Scholar] [CrossRef]
- Falgenhauer, L.; Imirzalioglu, C.; Oppong, K.; Akenten, C.W.; Hogan, B.; Krumkamp, R.; Poppert, S.; Levermann, V.; Schwengers, O.; Sarpong, N.; et al. Detection and Characterization of ESBL-Producing Escherichia Coli from Humans and Poultry in Ghana. Front. Microbiol. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Muleme, J.; Musoke, D.; Balugaba, B.E.; Kisaka, S.; Makumbi, F.E.; Buregyeya, E.; Isunju, J.B.; Wambi, R.; Mugambe, R.K.; Kankya, C.; et al. Epidemiology of Extended-Spectrum Beta-Lactamase-Producing Escherichia Coli at the Human-Animal-Environment Interface in a Farming Community of Central Uganda. PLOS Glob. Public Heal. 2023, 3, e0001344. [Google Scholar] [CrossRef]
- Geuther, N.; Mbarushimana, D.; Habarugira, F.; Buregeya, J.D.; Kollatzsch, M.; Pfüller, R.; Mugabowindekwe, M.; Ndoli, J.; Mockenhaupt, F.P. ESBL-Producing Enterobacteriaceae in a Rural Rwandan Community: Carriage among Community Members, Livestock, Farm Products and Environment. Trop. Med. Int. Heal. 2023, 28, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Subramanya, S.H.; Bairy, I.; Metok, Y.; Baral, B.P.; Gautam, D.; Nayak, N. Detection and Characterization of ESBL-Producing Enterobacteriaceae from the Gut of Subsistence Farmers, Their Livestock, and the Surrounding Environment in Rural Nepal. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.K.; Coulibaly, J.K.; Tiekoura, B.K.; Yapi, F.H.; Djaman, J.A. Molecular Characterisation of Extended-Spectrum Beta-Lactamase Producing Escherichia Coli Isolated from Cattle Faeces in Abidjan District, Ivory Coast. Microbiol. Res. J. Int. 2018, 25, 1–10. [Google Scholar] [CrossRef]
- Ohene Larbi, R.; Ofori, L.A.; Sylverken, A.A.; Ayim-Akonor, M.; Obiri-Danso, K. Antimicrobial Resistance of Escherichia Coli from Broilers, Pigs, and Cattle in the Greater Kumasi Metropolis, Ghana. Int. J. Microbiol. 2021, 2021. [Google Scholar] [CrossRef]
- Ouchar Mahamat, O.; Kempf, M.; Lounnas, M.; Tidjani, A.; Hide, M.; Benavides, J.A.; Carrière, C.; Bañuls, A.L.; Jean-Pierre, H.; Ouedraogo, A.S.; et al. Epidemiology and Prevalence of Extended-Spectrum β-Lactamase- and Carbapenemase-Producing Enterobacteriaceae in Humans, Animals and the Environment in West and Central Africa. Int. J. Antimicrob. Agents 2021, 57, 106203. [Google Scholar] [CrossRef]
- Ouédraogo, A.S.; Sanou, S.; Kissou, A.; Poda, A.; Aberkane, S.; Bouzinbi, N.; Nacro, B.; Ouédraogo, R.; Van De Perre, P.; Carriere, C.; et al. Fecal Carriage of Enterobacteriaceae Producing Extended-Spectrum Beta-Lactamases in Hospitalized Patients and Healthy Community Volunteers in Burkina Faso. Microb. Drug Resist. 2017, 23, 63–70. [Google Scholar] [CrossRef]
- Soré, S.; Sanou, S.; Sawadogo, Y.; Béogo, S.; Dakouo, S.N.P.; Djamalladine, M.D.; lboudo, K.S.; Ouoba, B.; Zoungrana, J.; Poda, A.; et al. Faecal Carriage of Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae in Healthy Volunteers and Hospitalized Patients in Ouagadougou, Burkina Faso: Prevalence, Resistance Profile, and Associated Risk Factors. African J. Clin. Exp. Microbiol. 2021, 22, 157–163. [Google Scholar] [CrossRef]
- Aworh, M.K.; Abiodun-Adewusi, O.; Mba, N.; Helwigh, B.; Hendriksen, R.S. Prevalence and Risk Factors for Faecal Carriage of Multidrug Resistant Escherichia Coli among Slaughterhouse Workers. Sci. Rep. 2021, 11, 13362. [Google Scholar] [CrossRef]
- Aworh, M.K.; Kwaga, J.; Okolocha, E.; Mba, N.; Thakur, S. Prevalence and Risk Factors for Multi-Drug Resistant Escherichia Coli among Poultry Workers in the Federal Capital Territory, Abuja, Nigeria. PLoS One 2019, 14, 1–15. [Google Scholar] [CrossRef]
- Bezabih, Y.M.; Bezabih, A.; Dion, M.; Batard, E.; Teka, S.; Obole, A.; Dessalegn, N.; Enyew, A.; Roujeinikova, A.; Alamneh, E.; et al. Comparison of the Global Prevalence and Trend of Human Intestinal Carriage of ESBL-Producing Escherichia Coli between Healthcare and Community Settings: A Systematic Review and Meta-Analysis. JAC-Antimicrobial Resist. 2022, 4. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.; Allende, A.; Álvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. Transmission of Antimicrobial Resistance (AMR) during Animal Transport. EFSA J. 2022, 20. [Google Scholar] [CrossRef]
- Hammerum, A.M.; Larsen, J.; Andersen, V.D.; Lester, C.H.; Skytte, T.S.S.; Hansen, F.; Olsen, S.S.; Mordhorst, H.; Skov, R.L.; Aarestrup, F.M.; et al. Characterization of Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia Coli Obtained from Danish Pigs, Pig Farmers and Their Families from Farms with High or No Consumption of Third- or Fourth-Generation Cephalosporins. J. Antimicrob. Chemother. 2014, 69, 2650–2657. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.N.; Anderson, J.D.; Mumma, J.; Mahmud, Z.H.; Cumming, O. The Association between Domestic Animal Presence and Ownership and Household Drinking Water Contamination among Peri-Urban Communities of Kisumu, Kenya. PLoS One 2018, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Abera, B.; Kibret, M.; Mulu, W. Extended-Spectrum Beta (β)-Lactamases and Antibiogram in Enterobacteriaceae from Clinical and Drinking Water Sources from Bahir Dar City, Ethiopia. PLoS One 2016, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Odumosu, B. T. , and A. A.R. Occurrence of Extended-Spectrum Beta-Lactamase Producing Enterobacteriaceae Isolates in Communal Water Sources in Ogun State, NIGERIA. 2015, 16, 28–32. [Google Scholar]
- Traoré O, Kpoda DS, Dembélé R, Saba CKS, Cairns J, Barro N, H.K. Microbiological and Physicochemical Quality of Groundwater and Risk Factors for Its Pollution in Ouagadougou, Burkina Faso. Water 2023, 15, 3734. [CrossRef]
- Adesoji, A.T.; Ogunjobi, A.A. Detection of Extended Spectrum Beta-Lactamases Resistance Genes among Bacteria Isolated from Selected Drinking Water Distribution Channels in Southwestern Nigeria. Biomed Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- de Boeck, H.; Miwanda, B.; Lunguya-Metila, O.; Muyembe-Tamfum, J.J.; Stobberingh, E.; Glupczynski, Y.; Jacobs, J. ESBL-Positive Enterobacteria Isolates in Drinking Water. Emerg. Infect. Dis. 2012, 18, 1019–1020. [Google Scholar] [CrossRef]
- Kagambèga, A.B.; Dembélé, R.; Traoré, O.; Wane, A.A.; Mohamed, A.H.; Coulibaly, H.; Fall, C.; Bientz, L.; M’Zali, F.; Mayonnove, L.; et al. Isolation and Characterization of Environmental Extended Spectrum β-Lactamase-Producing Escherichia Coli and Klebsiella Pneumoniae from Ouagadougou, Burkina Faso. Pharmaceuticals 2024, 17, 1–13. [Google Scholar] [CrossRef]
- Onuoha, S.C.; Okafor, C.O.O.; Eronmosele, B.O.; Ovia, K.N.; Nwosu, M.C.; Onwere, C.C.; Ude, I.U.; Ezeme-Nwafor, A.C.; Ani, P. Prevalence and Antibiotic Resistant Escherichia Coli Isolated from Abattoir and Aquaculture Environment in Ebonyi State, South East Nigeria. Res. J. Heal. Sci. 2023, 11, 128–137. [Google Scholar] [CrossRef]
- Waseem, H.; Williams, M.R.; Stedtfeld, R.D.; Hashsham, S.A. Antimicrobial Resistance in the Environment. Water Environ. Res. 2017, 89, 921–941. [Google Scholar] [CrossRef] [PubMed]
- Jesumirhewe, C.; Springer, B.; Allerberger, F.; Ruppitsch, W. Genetic Characterization of Antibiotic Resistant Enterobacteriaceae Isolates From Bovine Animals and the Environment in Nigeria. Front. Microbiol. 2022, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Seni, J.; Falgenhauer, L.; Simeo, N.; Mirambo, M.M.; Imirzalioglu, C.; Matee, M.; Rweyemamu, M.; Chakraborty, T.; Mshana, S.E. Multiple ESBL-Producing Escherichia Coli Sequence Types Carrying Quinolone and Aminoglycoside Resistance Genes Circulating in Companion and Domestic Farm Animals in Mwanza, Tanzania, Harbor Commonly Occurring Plasmids. Front. Microbiol. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ager, E.O.; Carvalho, T.; Silva, E.M.; Ricke, S.C.; Hite, J.L. Global Trends in Antimicrobial Resistance on Organic and Conventional Farms. Sci. Rep. 2023, 13, 1–13. [Google Scholar] [CrossRef]
- Abraham, S.; Wong, H.S.; Turnidge, J.; Johnson, J.R.; Trott, D.J. Carbapenemase-Producing Bacteria in Companion Animals: A Public Health Concern on the Horizon. J. Antimicrob. Chemother. 2014, 69, 1155–1157. [Google Scholar] [CrossRef]
- Ramírez-Castillo, F.Y.; Guerrero-Barrera, A.L.; Avelar-González, F.J. An Overview of Carbapenem-Resistant Organisms from Food-Producing Animals, Seafood, Aquaculture, Companion Animals, and Wildlife. Front. Vet. Sci. 2023, 10. [Google Scholar] [CrossRef]
| Number of samples/ farms analysed N |
Samples containing ESBL-Ec and/or ESBL-K spp. n (%) |
Samples containing ESBL-Ec n (%) | Samples containing ESBL-K n (%) |
|
|---|---|---|---|---|
| Sample type | ||||
| Cattle faeces | 68 | 58 (85.3)1 | 52 (76.5)2 | 17 (25.0) |
| Soil | 68 | 41 (60.3)1 | 32 (47.0)2 | 13 (19.1) |
| Human stools | 120 | 64 (53.3)1 | 47 (39.2)2 | 27 (22.5) |
| Drinking water | 66 | 25 (37.9)1 | 5 (7.6)2 | 22 (33.3) |
| Total | 322 | 188 (58.4) | 136 (42.2) | 79 (24.5) |
| Farm type | ||||
| Semi-intensive | 39 | 36 (92.3) | 21(53.8) | 15 (38.5) |
| Traditional | 28 | 24 (85.7) | 16 (57.1) | 8 (28.6) |
| Total | 67 | 60 (89.5) | 37 (55.2) | 23 (34.3) |
| Antibiotic group | Antibiotics (μg) | Cattle | Soil | Human | Water |
|---|---|---|---|---|---|
| N=52 (%) | N= 32 (%) | N= 47 (%) | N= 5 (%) | ||
| Beta-lactamins | Cefoxitin (30) | 1 (1.9) | 2 (6.3) | 1 (2.1) | 0 (0.0) |
| Cefotaxime (30) | 52 (100) | 30 (93.8) | 46 (97.9) | 5 (100) | |
| Cefepime (30) | 39 (75.0) | 27 (84.4) | 40 (85.1) | 3 (60.0) | |
| Meropenem (10) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Penicillin and inhibitors | Amoxicillin+ clavulanic acid (30) | 24 (46.2) | 20 (62.5) | 12 (25.5) | 4 (80.0) |
| Sulfonamides | Cotrimoxazole (25) | 30 (57.7) | 21 (65.6) | 32 (68.1) | 4 (80) |
| Quinolones, fluoroquinolones | Ciprofloxacin (5) | 5 (9.6) | 2 (6.3) | 5 (10.6) | 1 (20.0) |
| Ofloxacin (5) | 4 (7.7) | 5 (15.6) | 5 (10.6) | 0 (0.0) | |
| Nalidixic acid (30) | 13 (25.0) | 11 (34.4) | 12 (25.5) | 1 (20.0) | |
| Aminoglycosides | Amikacin (30) | 1 (1.9) | 3 (9.4) | 2 (4.3) | 0 (0.0) |
| Gentamicin (10) | 3 (5.8) | 3 (9.4) | 6 (12.8) | 0 (0.0) | |
| Phenicols | Chloramphenicol (30) | 6 (11.5) | 3 (9.4) | 6 (12.8) | 1 (20.0) |
| Cyclins | Tetracycline (30) | 42 (80.8) | 26 (81.3) | 41 (87.2) | 4 (80.0) |
| Multidrug resistance | 36 (69.2) | 19 (59.4) | 33 (70.2) | 4 (80.0) |
| Antibiotic group | Antibiotics (μg) | Cattle | Soil | Human | Water |
| N=17 (%) | N= 13 (%) | N= 27(%) | N= 22 (%) | ||
| Beta-lactamins | Cefoxitin (30) | 4 (23.5) | 0 (0.00) | 2 (7.4) | 2 (9.1) |
| Cefotaxime (30) | 14 (82.4) | 13 (100) | 24 (88.9) | 19 (86.4) | |
| Cefepime (30) | 14 (80.4) | 13 (100) | 23 (85.2) | 21 (95.5) | |
| Meropenem (10) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Penicillin and inhibitors | Amoxicillin+ clavulanic acid (30) | 9 (52.9) | 9 (69.2) | 16 (59.3) | 14 (63.6) |
| Sulphonamides | Cotrimoxazole (25) | 12 (70.6) | 11 (84.6) | 22 (81.5) | 17 (77.3) |
| Quinolones, fluoroquinolones | Ciprofloxacin (5) | 0 (0.0) | 2 (15.4) | 1 (3.7) | 0 (0.0) |
| Ofloxacin (5) | 0 (0.0) | 0 (0.0) | 1 (3.7) | 3 (13.6) | |
| Nalidixic acid (30) | 1 (5.9) | 2 (15.4) | 4 (14.8) | 5 (22.7) | |
| Aminoglycosides | Amikacin (30) | 0 (0.0) | 0 (0.0) | 1 (3.7) | 0 (0.0) |
| Gentamicin (10) | 2 (11.8) | 2 (15.4) | 4 (14.8) | 1 (4.6) | |
| Phenicol | Chloramphenicol (30) | 6 (35.3) | 3 (28.1) | 10 (37.0) | 1 (4.5) |
| Cyclins | Tetracycline (30) | 12 (70.6) | 13 (100) | 16 (59.3) | 16 (72.7) |
| Multidrug resistance | 14 (82.4) | 11 (84.6) | 21 (77.8) | 18 (81.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





