Introduction
Cervical cancer (CC) is the fourth leading cause of cancer-associated deaths in women, with an estimated 3,42,000 deaths and 6,04,000 new cases worldwide in 2020 (World Health Organization, 2021). With a survival rate of only 50%, the severity of the disease is a major concern for the global population (Xu et al., 2020). The leading cause of CC is the Human papillomavirus (HPV), a sexually transmitted disease that infects the epithelial cells through microscale lesions (Smith et al., 2019). Based on their ability to induce oncogenicity, HPVs are categorized into high-risk HPVs (hrHPV) and low-risk HPVs (lrHPV) (Brown et al., 2021).
Around 90% of CC infections caused by hrHPVs are temporary and regress spontaneously, pointing to the involvement of HPVs in triggering CC, despite the influence of other elements on the formation, development, and progression of the disease (World Health Organization, 2021). The natural immune response of the body is usually sufficient to control the infection and prevent the formation of lesions or tumors (Xu et al., 2020). However, host-related factors, such as sexual behavior, hormonal contraceptives, and individual immunity, also play a role in CC (Smith et al., 2019).
The vaginal microbiome (VMB) may also have a significant impact on the progression of CC. Contemporary researchers have explored the potential correlation between the VMB and CC, and the VMB profile with a certain dominant species may impact the individual immune response, oncogenesis, and HPV clearance (Brown et al., 2021).
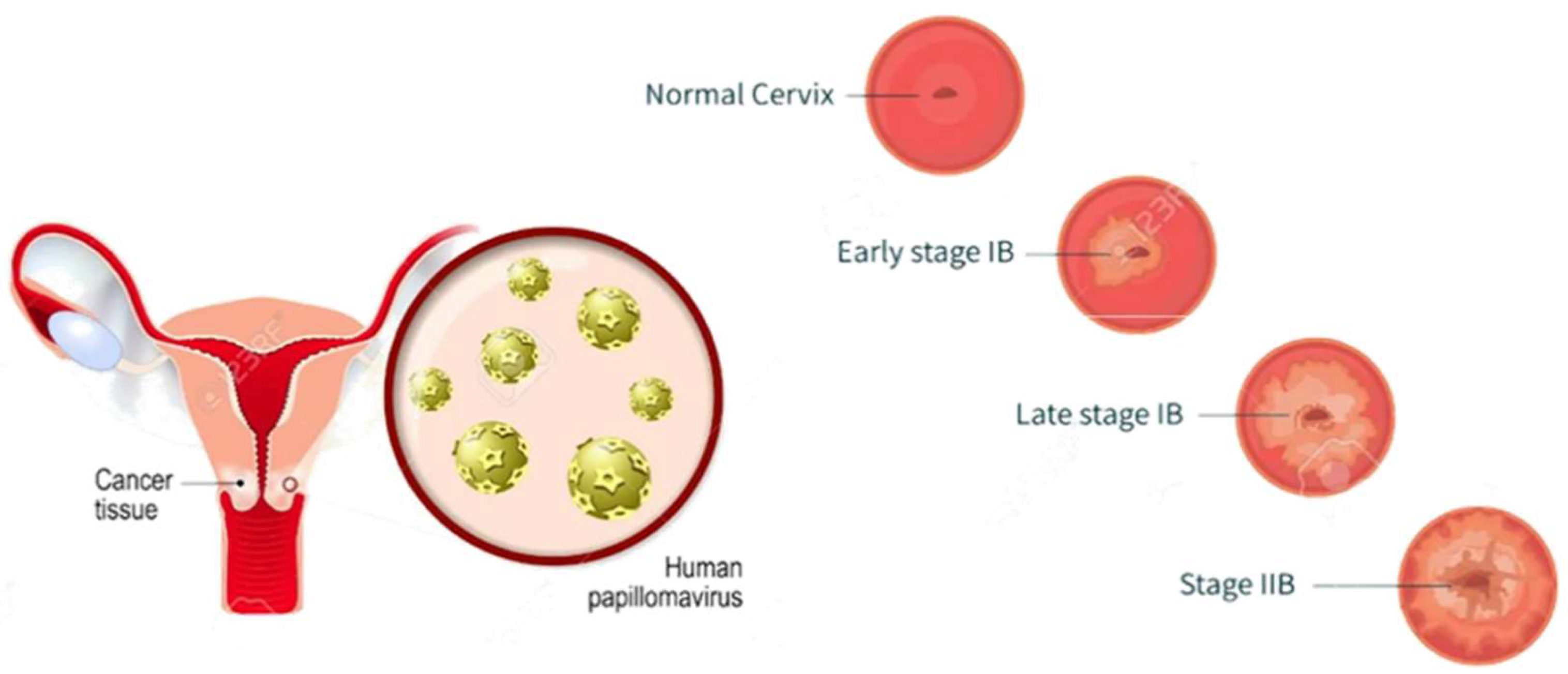
Figure 1.
Development stages of cervical cancer.
Figure 1.
Development stages of cervical cancer.
Vaginal Microbiome (VMB)
A group of microorganisms involved in a network of interactions with human tissues, referred to as the human microbiome, consists of trillions of symbiotic, commensal, or pathogenic microorganisms that interact and coexist with the host (Sender, Fuchs, & Milo, 2016). The vaginal microbiome (VMB) is a subset of the human microbiome and consists of a smaller diversity of microorganisms compared to the human gut microbiota (Zheng, Martin, & Lukacik, 2019). In a study by Marrazzo et al. (2015), the Lactobacillus was found to be a highly dominant species in the vaginal flora of 396 women.
Various factors such as hormonal changes, personal hygiene, ethnicity, and disease conditions like diabetes, lactation, and stress play a significant role in shaping the floral composition of the VMB. For example, changes in ethnicity can alter the VMB due to differences in the vaginal microbiota of different races, regions, and environments (Zheng et al., 2019). The changes in each of these factors can impact the entire VMB, as demonstrated by the connection between changes in the VMB and sexually transmitted infections related to bacterial vaginosis (Marrazzo et al., 2015).
Cervicovaginal Microbiomes and Host Defenses
According to recent studies, the homeostasis of the cervicovaginal microbiome and the local microenvironment is maintained by their interaction (Caron et al., 2020). However, any imbalance in this equilibrium can lead to chronic inflammation, genome instability, abnormal cellular proliferation, epithelial barrier breach, metabolic dysregulation, and angiogenesis (Caron et al., 2020). To protect the genital tract, the human body relies on the immune response, mucosal epithelial barrier, and lactic acid secretion (Caron et al., 2020).
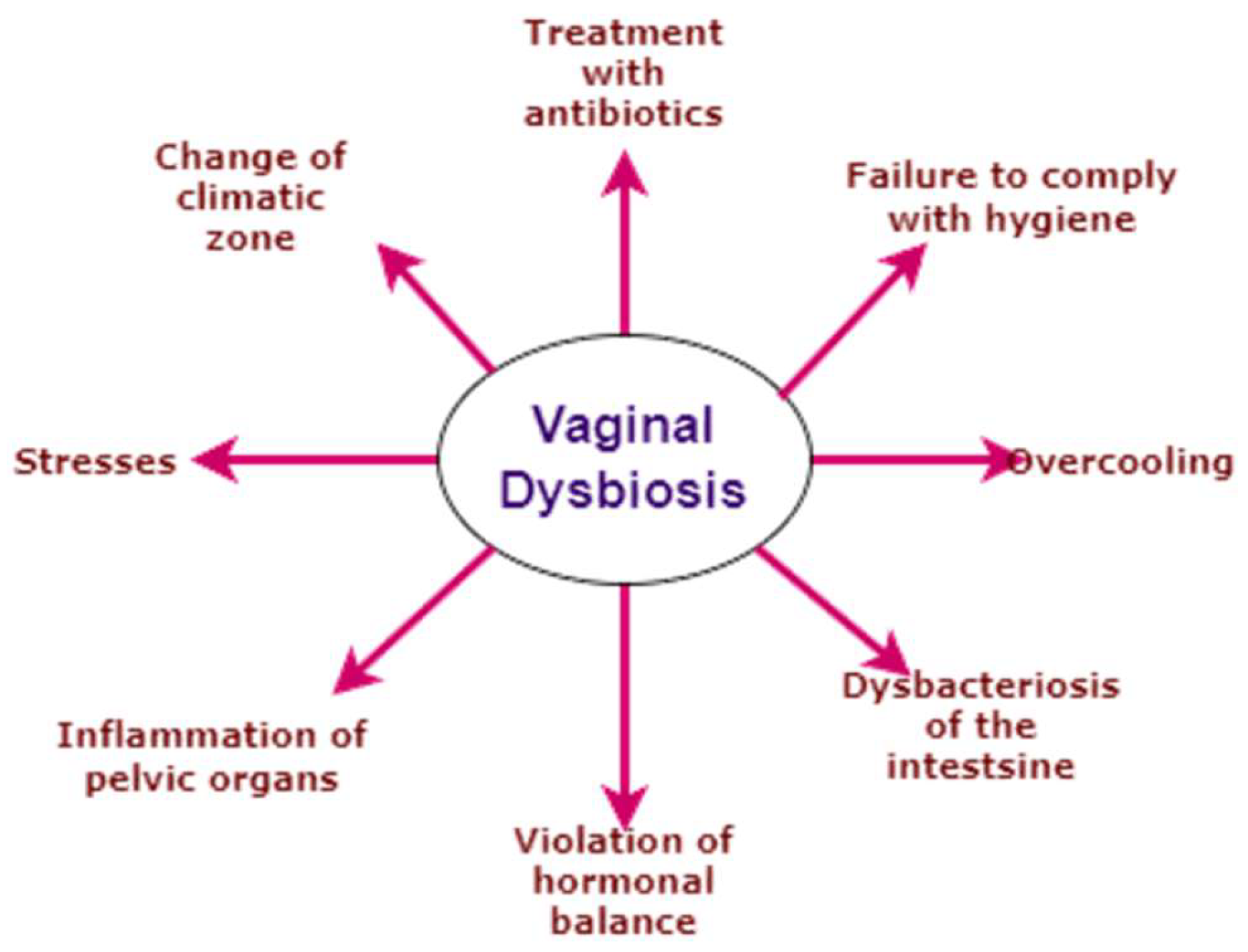
Figure 2.
Causes of Vaginal dysbiosis.
Figure 2.
Causes of Vaginal dysbiosis.
Lactic acid secretion is a vital defensive mechanism and is correlated with lactobacilli, which produce lactic acid and maintain a lower pH of 4.5, creating an unfavourable environment for invading pathogens (Caron et al., 2020). This environment serves as a barrier for sexually transmitted pathogens, prevents mucin degradation, and conserves the epithelial barrier by producing bacteriocins (Caron et al., 2020).
In a study, dextro lactic acid was found to provide a protective mechanism against vaginal dysbiosis (Caron et al., 2020). The progression of hrHPV-associated cervical wounds is directly correlated with lactic acid, as it inhibits the development of various sexually transmitted anaerobic agents (Caron et al., 2020). In the case of concurrent infections of both HPV and Chlamydia trachomatis along with the associated cervicovaginal microbiomes, a notable diversity in cervical microbial flora, including anaerobic species like A. vaginae and G. vaginalis, was observed, along with a decreased profile of Lactobacillus in infected women (Caron et al., 2020). In healthy women, a dominant profile was exhibited by Lactobacillus, with significantly less percentage of anaerobic bacteria (Caron et al., 2020). A solitary infection of Chlamydia trachomatis has a great variety of microbiota, while the lactobacillus level remains low (Caron et al., 2020).
Microbiome, Genital Infection, and Cancer
Many studies have discovered the connection between HPV and VMB. In a medical study on 169 infection-suspected women, the bacterial diversity is found to be high along with a reduction of lactobacilli. Bacterial communities known for immune dysregulation favor tumor proliferating ambiance. Bacterial vaginosis is related to increased HPV infections, and favoring the expansion of the difference in vaginal bacteria may aid in the tenacity of HPV infection. G. vaginalis secretes sialidase, an enzyme capable of cleaving the glycoproteins of vaginal mucus (eg. mucin). Histone acetylation regulating factor butyric acid is produced by the bacteria constituting bacterial vaginosis. The probable involvement of VMB in AIDS development and progression through the reactivation of HIV is reported.
The VBM of HPV-infected women was studied by Di Paola et al. after one year of infection. The study categorized BV into two, the first group was composed of high-profile anaerobic bacteria such as Atopobium, Sneathia, Gardnerella, Lactobacillus, and Prevotella species. In contrast, the second group consists of aerobic and anaerobic bacteria such as Brevibacterium, Pseudomonas, Enterococcus, Peptostreptococcus, Propionibacterium, Streptococcus, Shigella, and Bifidobacterium species. For women having stable HPV infection, anaerobic bacteria dominated in the VMB profiling with fewer lactobacilli, on the other hand, the VMB profiling of women with HPV infection clearance dominated with the second group of BV and a clear dominance of lactobacilli. In contrast with the observation regarding lactobacilli, L. iners secretes a cytotoxin, inerolysin. It exhibits a function similar to the protein vaginolysin from Gardnerella spp., which favors infections by aiding in the formation of vaginal epithelial pores. The study concluded with the distinctive roles of lactobacilli aligned with the risk of HPV infection, for e.g., a VMB with dominance displayed by L. iners is associated high risk of HPV, CC, and CIN infections, while a VMB with dominance displayed by L. crispatus associates lower risk of the same.
Role of Probiotics for HPV
“Probiotics are defined by the World Health Organization (WHO) as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (WHO, n.d.). Recent studies have shown that certain species of probiotics, such as Lactobacillus, Streptococcus, and Bifidobacterium, can improve the host’s inflammatory and immune response by altering the microbiota (Tensaouti et al., 2019).
One application of probiotics is in the treatment of urogenital infections, where Lactobacilli are commonly used (Wang et al., 2020). In particular, they have been employed to improve the vaginal flora through vaginal acidification, synergistic action, and prevention of bacterial adhesion (Mishra et al., 2018). Although the evidence of efficacy is not conclusive, probiotics have been used in the treatment of sexually transmitted diseases due to their low risk of side effects and ability to promote resistance (Palma et al., 2021). One study by Palma et al. (2021) found that the clearance of cytological abnormalities caused by HPV infection was twice as high in the probiotic group compared to the control group, however, further longitudinal studies are needed to confirm these findings.
Figure 3.
Microorganisms involved in probiotics.
Figure 3.
Microorganisms involved in probiotics.
Conclusion
“Cervical Cancer (CC) is a leading cause of female-oriented cancer and cancer-related deaths, primarily caused by HPV infection (Liu et al., 2020). The risk factors for CC progression, including personal hygiene, ethnicity, and disease conditions, may be influenced by the complex and dynamic composition of the vaginal microbiome (VMB) (Tensaouti et al., 2019). High-risk HPV infections are considered a major factor in the development of CC and vulvar and vaginal abnormalities (Wang et al., 2020).
Recent research has shown that the presence of certain bacterial species in the VMB may protect against HPV infection, aid in clearing the virus, and reduce lesions, ultimately lowering the risk of cancer proliferation (Mishra et al., 2018). Conversely, other bacterial species have been positively correlated with cancer progression and may play a significant role in its proliferation (Palma et al., 2021). Further extensive study of the VMB and its relationship with HPV infections is needed to develop alternative strategies for the treatment of HPV infections. Identifying specific microorganisms in the VMB could lead to the development of clinically relevant data for future treatment methods (Liu et al., 2020).