1. Introduction
Endophytes are a group of microorganisms (bacteria, actinobacteria, or fungi) that reside in the internal tissues of plants in symbiotic associations without causing any disease symptoms [
1,
2,
3]. Among endophytes, endophytic fungi are among the important components of plant-microorganism interaction systems. Endophytic fungi include Chytridiomycota, Zygomycota, Ascomycota, and Basidiomycota, which occur in diverse environments [
4]. On the basis of their lifestyle, functional diversity, biology and mode of transmission, endophytic fungi are classified as systemic/true endophytes (mutualistic for the entirety of their lifespan) or transient/nonsystemic endophytes (adapting transient modes of lifestyles, mutualistic and parasitic being the two most common modes, during different life cycle stages) [
5]. The mutualistic relationships of these endophytic fungi with host plants are well known to have profound beneficial impacts on plant communities, such as improving plant fitness, increasing tolerance to biotic and abiotic stresses, and increasing plant biomass. In turn, the host plant provides fungal endophytes with refuge and nutrients and allows them to survive within the next generation of host plants.
The association of a plant with endophytic fungi is, therefore, a supreme beneficial relationship for both partners, especially the plant. Fungal endophytes can stimulate the growth of plants directly or indirectly by triggering the solubilization of phosphorus, potassium, and zinc [
6,
7]. Endophytic fungi also confer resistance against plant pathogens through enhanced production of plant secondary metabolites, endophyte-specific metabolite synthesis, induction of systemic or local immune responses or competitive elimination of pathogens [
8]. Plants are subjected to various abiotic stresses, such as drought stress, temperature stress, salinity stress and heavy metal toxicity stress, during their life cycle [
9]. In addition to their intrinsic mechanism of adaptation, endophytes also enhance the alleviation of the negative effects of stress conditions faced by host plants through two mechanisms: (a) activation of the stress response after host exposure and (b) synthesis of antistress biochemicals by endophytes [
10].
Endophytic fungi have similar important ecological functions in invasive ecosystems, and the presence or absence of mutualist fungi may partially explain why some alien plants become invasive [
11]. First, endophytic fungi can directly act on invasive plants to improve their growth, reproduction, defense against pathogens and herbivores and environmental tolerance. For example, Aschehoug, E. T.; Metlen, K. L.; Callaway, R. M. and Newcombe, G. [
12] reported that inoculation with the endophyte
Alternaria (CID120) could directly promote the growth of the invasive plant
Centaurea stoebe. Endophytic fungi can improve the reproductive success of invasive plants by increasing germination [
13] or facilitating the allocation of plant resources to adopt more efficient reproductive strategies [
14]. For example, endophytic infection of
Cyperus rotundus plants reduces investment in expensive sexual reproductive structures (inflorescence) but increases investment in the primary mode of reproduction (asexual tub production). This trade-off may increase population growth and thus establishment success [
14]. The invasive annual
Anthemis cotula leaves have endophytic microorganisms with plant growth-promoting traits, such as ammonia production, indole acetic acid (IAA) production, phosphate dissolution and biocontrol of active substances [
15]. Currie et al. reported that foliar endophytic fungi (
Colletotrichum acutatum,
Alternaria alternata and
Cladosporium oxysporum) can effectively reduce the pathogenicity of
Puccinia komarovii, a rust pathogen, to the invasive plant
Impatiens glandulifera [
16]. The invasive grass
Cirsium arvense was inoculated with the endophyte
Chaetomium cochliodes to produce oxolipin and jasmonate metabolites, which induced the production of plant defense metabolites for systemic resistance [
17]. Rudgers and Clay reported that
Neotyphodium coenophialum significantly reduced the abundance and diversity of herbivorous insects in the invasive
Lolium arundinaceum [
18]. The survival rate of
Phragmites australis seedlings under salt stress could be improved by inoculation with endophytes from the dark septum (DSE). When exposed to salt stress,
P. australis can establish reciprocal relationships with DSEs [
19].
Second, by interfering with native plants, endophytic fungi can also indirectly increase the competitive advantage of invasive plants; for example, they can change the soil nutrient content and soil through the cycle of endophytic fungal species to inhibit the growth of native plants and indirectly promote the development of their own species [
20]. AMF can also reduce the dependence of host plants on symbiosis with AMF without changing their biomass and indirectly promote the establishment of host grass and subsequent invasion [
21].
Ageratina adenophora (Sprengel) R.M. King & H. Robinson is a perennial herb of Asteraceae native to the Mexican area of America. This plant has invaded more than 40 tropical to temperate regions worldwide, causing severe ecological impacts and economic losses [
20]. Since the invasion of Yunnan Province from the China–Myanmar border in the 1940s,
A. adenophora has been widely distributed in Yunnan Province and neighboring Sichuan, Guizhou, Guangxi, Xizang and other provinces [
22] and is one of the 18 most severely invasive plants in China [
23]. The
A. adenophora can accumulate beneficial microorganisms (such as nitrogen-fixing bacteria) around its roots to gain a sustained growth advantage [
24,
25]. Moreover, some promoting bacteria have a more significant growth-promoting effect on
A. adenophora than on local plants do [
26].
A. adenophora can also accumulate large amounts of native plant pathogenic fungi in its leaf tissue in the form of endophytic fungi, which may help its successful invasion [
27]; these dominant genera of foliar endophytic fungi include
Colletotrichum,
Nemania,
Phomopsis, and
Xylaria [
28].
Previously, some
Colletotrichum endophytes were shown to have a weak negative impact on the growth of
A. adenophora planted in agricultural soil and forest soil but can increase the pathogenicity of the foliar pathogen
Diaporthe helianthi and slightly reduce the herbivory of
A. adenophora seedlings in the wild [
29]. Species of
Colletotrichum are commonly found in many plant hosts as pathogens, endophytes and occasionally saprobes. It has been reported that
Colletotrichum, an endophyte, exists in
Dendrobium orchids [
30],
Taxus mairei [
31],
Citrus grandis cv. “Tomentosa” [
32], tea plants [
33], and wild banana plants (
Musa acuminata) [
34].
Colletotrichum plays both pathogenic and endophytic roles depending on the interaction between
Colletotrichum and the specific host, as well as biotic and abiotic factors in the environment [
35]. Because
A. adenophora can be infected frequently by diverse foliar pathogens in its invaded range, particularly members of the family Didymellaceae [
36], it is unclear whether the widely occurring foliar
Colletotrichum endophytes also increase the pathogenicity of the dominant foliar pathogens from the family Didymellaceae. Moreover, although some
Colletotrichum endophytes have a weak negative impact on the growth of
A. adenophora [
29], it is necessary to determine whether they can increase host plant resistance to environmental stressors such as drought stress or nutrient stress because
A. adenophora occurs in the wild in harsh environments (roadsides and railway embankments), which are characterized by drought and low nutrient availability [
20].
In this study, we planted sterile seedlings of A. adenophora, inoculated them with endophytic fungal strains of Colletotrichum with distinct genetic backgrounds and compared their role in the resistance of invasive A. adenophora to the pathogenicity of three foliar pathogen members from the Didymellaceae family, as well as to abiotic stress, including drought and nutrient stress. We expected that, compared with plants inoculated with A. adenophora without endophytes, those inoculated with Colletotrichum would (1) have greater biomass, (2) decrease the pathogenicity of the pathogen on their leaves, and (3) alleviate the effects of drought stress and nutrient stress on plant growth.
4. Discussion
Understanding the function of fungal endophytes associated with invasive plants has become an increasing concern of invasion biologists. In general, recently introduced fungal endophytes can directly help invasive plants improve growth, reproduction, defense against pathogens and herbivores and environmental tolerance [
12,
13,
14,
16]. Previously, a high abundance of the endophytic fungus Colletotrichum was found to be enriched in the leaves of
A. adenophora [
28]. In this study, we further tested the effects of these endophytic fungi on the growth of
A. adenophora under pathogen and environmental stress conditions. (1) The host
A. adenophora did not grow well after asymptomatic infection with the endophytic fungus
Colletotrichum, as indicated by reduced plant photosynthesis-related physiological indices (chlorophyll content and soluble sugar content), increased plant resistance-related physiological indices [total phenolic (TP) and peroxidase (POD) activity], and decreased biomass. (2) Endophytic
Colletotrichum fungi had some positive effects on the leaves of
A. adenophora, but genetically distinct endophytic fungi presented different responses to different pathogens. (3) Under drought or nutrient stress, the endophyte
Colletotrichum promoted the growth of
A. adenophora, especially in terms of increasing stem length and aboveground biomass and regulating the leaf chlorophyll content.
In contrast to expectation 1, although the leaf of the host
A. adenophora was not affected by inoculation with any of the strains of
Colletotrichum inoculated via spore suspension (
Figure 4a) or by wounding and inoculation via agar discs (
Figure 4b), inoculation with
Colletotrichum had adverse effects on
A. adenophora growth because all the strains reduced the
A. adenophora biomass (
Figure 2). This adverse effect also reduced plant photosynthetic efficiency by reducing the contents of chlorophyll and soluble sugars (
Figure 1ab). Photosynthesis is considered one of the main driving forces of plant growth, and leaf pathogens affect one of the most important physiological processes. Many studies have shown that pathogen infection results in a decrease in the photosynthetic rate and photosynthetic unit changes, and these changes may be due to cutting or damage to the photosynthetic apparatus [
42]. Photosynthesis decreases in bean plants infected with
Colletotrichum lindemuthianum [
43], and photosynthesis decreases in acai leaves infected with
Colletotrichum [
42]. Moreover, it was also demonstrated that
Monographella albescens was able to impair the photosynthetic process in both symptomatic and asymptomatic leaf areas [
44]. In fact, many pathogens may impair photosynthesis in asymptomatic, although colonized, tissues, i.e., often called virtual lesions [
45,
46,
47]. Therefore, inoculation with the endophytic fungus
Colletotrichum inhibited photosynthesis in
A. adenophora to some extent, leading to a further reduction in biomass, which also indicated that these endophytic
Colletotrichum fungi in
A. adenophora behave physiologically like pathogens. Previous studies have shown that beneficial microbes, like pathogens, can elicit a plant immune response (Wippel 2023), e.g., the induction of the plant hypersensitive response, including the formation of reactive oxygen species (ROS), the induction of salicylic acid (SA), jasmonic acid (JA) signalling, the upregulation of pathogenesis-related (PR) genes and plant defensin 1.2 (PDF1.2) [
48,
49]. It is valuable to determine whether the expression of immune-related genes such as PR1/2/5 and PDF1.2 is elicited in
A. adenophora by the colonization of these endophytes in the future.
As expected 2, endophytic Colletotrichum fungi had some positive effects on the dominant pathogen Didymellaceae when inoculated on the leaves of
A. adenophora (
Figure 5cd). Didymellaceae are the dominant foliar pathogens. These pathogens occur widely on
A. adenophora in the invaded range, and some members exhibit high virulence and a narrow host range [
36]. In particular, their virulence evolves with invasion history [
50]. Therefore, these pathogens may have important impacts on the expansion of
A. adenophora along with a delayed invasion history. Our findings indicated that the
Colletotrichum-Didymellaceae interaction may be important for understanding the role of foliar fungi in plant invasions. Similarly, many studies have verified that foliar fungi can help invasive host plants resist pathogens; for example, foliar endophytic fungi of the invasive plant
Impatiens glandulifera appear to be antagonistic to rust fungus (
Puccinia komarovii) [
16].
Cryptosporiopsis quercina isolated from
Tripterygium wilfordii, an endophytic fungus, produces a special active peptide, cyclohexenone, which inhibits the growth of the plant pathogens
Sclerotinia sclerotiorum and
Botrytis cinerea [
51].
Nonetheless, our findings suggest that the mode of action of endophytic fungi against pathogens is much more complex and depends on the different genetic backgrounds of both endophytic fungi and the specific pathogen being investigated. For example, endophytes of different genotypes presented different resistances to pathogens, with the endophyte AX198 being more active against the pathogens G56 and Y122 than AX39 and AX115 (
Figure 5abc). The resistance of endophytic fungi to different pathogen genotypes also differs, with the endogenous fungi AX39 and AX115 promoting infection by the pathogens
M. ageratinae G56 and Y122 but weakening infection by the pathogen
M. speciosa S188 (
Figure 5). These results suggested that the resistance of the AX198 strain differed from that of the AX39 and AX115 strains to pathogens, which reflected the differences among strains from different complexes. Second, the results also indicated that AX198 resulted in different LMA responses (
Figure 3) and different physiological responses (
Figure 1) in
A. adenophora. Because most
Colletotrichum strains inhibit the growth of Didymellaceae strains both in vitro and in vivo (unpublished data), the resistance of these endophytic
Colletotrichum strains may occur through competitive exclusion of the Didymellaceae pathogen. On the other hand, our results also suggest a synergistic interaction between
Colletotrichum and other pathogens, which results in greater virulence against
A. adenophora. Similar to our previous study, the foliar fungus
Colletotrichum sp. promoted the pathogenicity of
Diaporthe helianthi on
A. adenophora leaves [
29]. Coinfections are common in nature and usually increase the pathogenicity of pathogens to the host [
52]. For example, compared with inoculation with either of the two pathogens separately, coinfection with
Verticillium dahliae or
Colletotrichum coccodes causes more severe foliar disease symptoms and crown rot in potato (Nicola) [
53].
The interactions between endophytic microorganisms and plants are influenced by environmental factors, including nutrient conditions [
54] and drought conditions [
55]. As expected 3, inoculation with the endophyte
Colletotrichum could alleviate the effects of drought stress and nutrient stress on
A. adenophora by increasing several growth indicators of
A. adenophora, including the aboveground biomass, stem length and chlorophyll content. The response intensity of
A. adenophora under drought or nutrient stress also varied with the genetic background of these endophytes. Relatively, the strain AX198 promoted the growth of
A. adenophora more strongly than the other two strains did (
Figure 6 and
Figure 7). This result is consistent with that of strain AX198 being more active against the pathogens G56 and Y122 than AX39 and AX115 (
Figure 5abc). Moreover, the greater LMA after AX198 inoculation was consistent with these results (
Figure 3). Interestingly, compared with those under normal hydrothermal and nutrient conditions, both AX39 and AX115 resulted in longer stem lengths (see
Figure 2d), which reflects a complex response to abiotic stress and depends on the different genetic backgrounds of endophytic fungi. More physiological indicators for stress resistance, including superoxide dismutase (SOD) and catalase (CAT) activity, membrane relative permeability, and malondialdehyde (MDA) content [
56,
57,
58,
59,
60], are valuable for understanding the detailed physiological changes induced by genetically distinct endophytes in
A. adenophora under stress conditions.
Furthermore, the effects of the endophyte
Colletotrichum on plant
A. adenophora may be dose-dependent of environmental factor. For example, Zhang et al. studied the effects of endophytic fungal infection on the germination and seedling growth of perennial ryegrass under different salt stress levels [
61]. They reported that at low concentrations of NaCl, infection with endophytic fungi had little effect on seedling growth, but at high concentrations, the fungus had a significant promoting effect on seedling growth. Cheplick et al. reported that inoculation with endophytic fungi reduced the number of tillers in perennial ryegrass under drought stress, but the number of tillers increased after the recovery period [
62]. The endophytic fungus
Colletotrichum tofieldiae Ct0861, which is a symbiotic fungus of Arabidopsis thaliana, promotes plant growth and reproduction only under phosphate starvation conditions [
63,
64]. In soils with different water-holding capacities, the effects of fungal endophytes on the growth of perennial ryegrass differ because of their different effects under drought conditions [
65]. Therefore, it is necessary to explore the interactions between endophytic microorganisms and plants under different drought and nutrient stresses (including different nutrient element stresses).
In general, our findings suggested the high abundance of the leaf endophytic fungus
Colletotrichum did not significantly promote the growth of
A. adenophora under normal hydrothermal and nutrient conditions; however, these endophytic
Colletotrichum strains could help
A. adenophora resist foliar fungal pathogens and improve the response to drought or nutrient stress, depending on the genetic background of the endophytic fungi. Our results provide novel clues to explain why
A. adenophora contains a high abundance of
Colletotrichum endophytes [
27], but some
Colletotrichum endophytes have a weak negative impact on the growth of
A. adenophora [
29]. Nonetheless, as plant mutualists, microbiomes are not a single organism but a community of species with complex interactions among microbial taxa and between microbes and their shared host. The presence of an individual strain is detrimental to the host, but multiple strains can be beneficial [
66]. Therefore, the effects of multiple
Colletotrichum inoculations on the stress resistance of
Colletotrichum are also worth studying in the future.
Figure 1.
Physiological indices of A. adenophora inoculated with endophytic Colletotrichum strains. (a) Chlorophyll content, (b) soluble sugar content, (c) total phenol content, and (d) peroxidase activity. Dots with different colours represent the raw data of each sample inoculated with the Colletotrichum AX39, AX115 and AX198 strains. The RI represents the response index, where the negative RI in panels (a) and (b) indicates reduced chlorophyll content and soluble sugar content in the treatment with Colletotrichum spp. infection compared with the control without Colletotrichum spp. infection. The positive RIs in panels (c) and (d) indicate increased total phenol content and peroxidase POD activity in the treatment with Colletotrichum spp. infection compared with the control without Colletotrichum spp. infection. Nonparametric Mann-Whitney U tests or independent sample T tests were used to identify the differences between each treatment group and the control group (* < 0.05, ** < 0.01, *** <0.001). Post hoc comparisons were performed via Duncan’s test for equal variance and Dunnett’s test for unequal variance (T3 test) to determine whether the difference in the RI was significant among the treatments inoculated with AX39, AX115 and AX198, with different letters indicating significant differences. The error bars are the standard errors.
Figure 1.
Physiological indices of A. adenophora inoculated with endophytic Colletotrichum strains. (a) Chlorophyll content, (b) soluble sugar content, (c) total phenol content, and (d) peroxidase activity. Dots with different colours represent the raw data of each sample inoculated with the Colletotrichum AX39, AX115 and AX198 strains. The RI represents the response index, where the negative RI in panels (a) and (b) indicates reduced chlorophyll content and soluble sugar content in the treatment with Colletotrichum spp. infection compared with the control without Colletotrichum spp. infection. The positive RIs in panels (c) and (d) indicate increased total phenol content and peroxidase POD activity in the treatment with Colletotrichum spp. infection compared with the control without Colletotrichum spp. infection. Nonparametric Mann-Whitney U tests or independent sample T tests were used to identify the differences between each treatment group and the control group (* < 0.05, ** < 0.01, *** <0.001). Post hoc comparisons were performed via Duncan’s test for equal variance and Dunnett’s test for unequal variance (T3 test) to determine whether the difference in the RI was significant among the treatments inoculated with AX39, AX115 and AX198, with different letters indicating significant differences. The error bars are the standard errors.
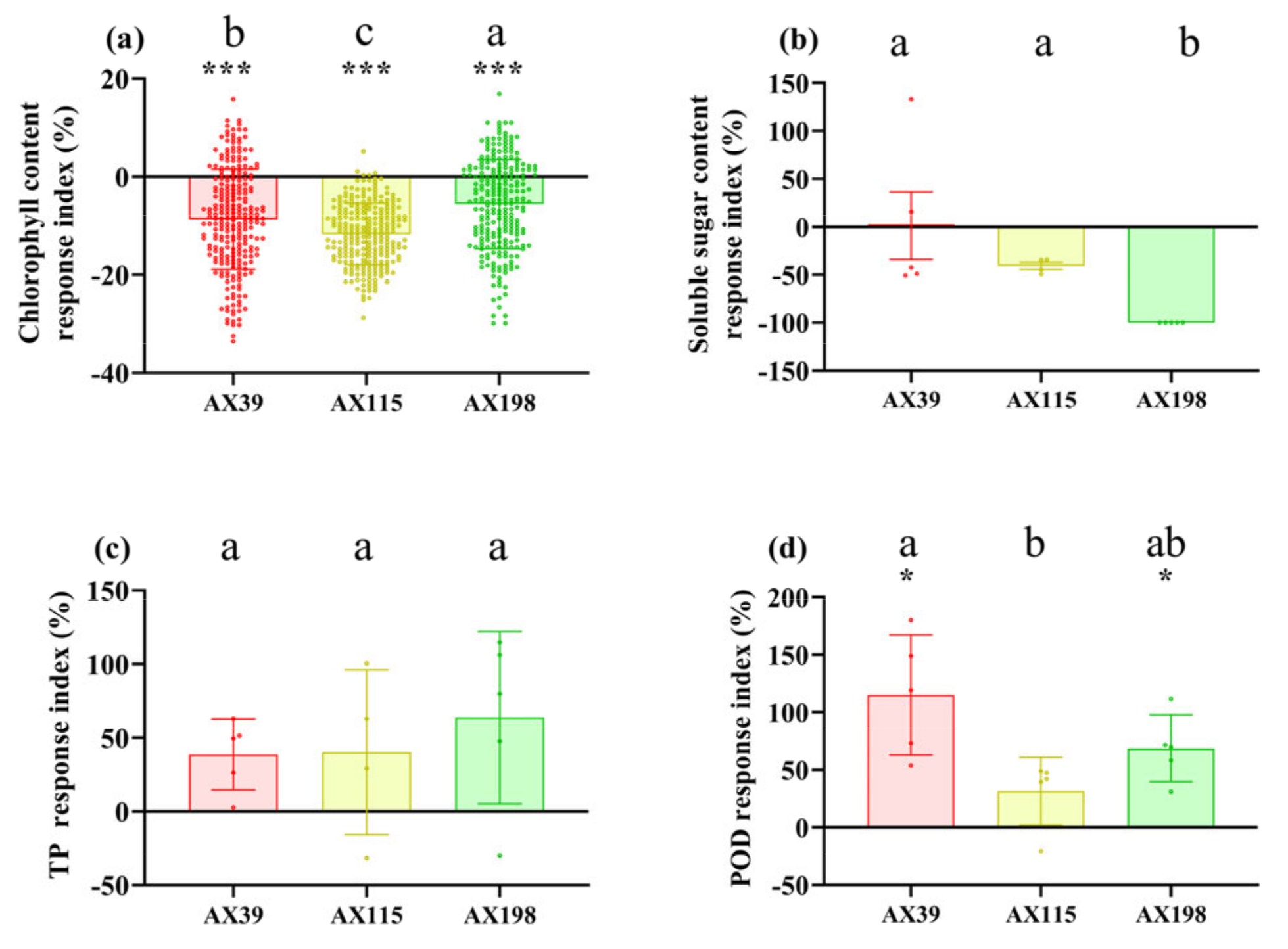
Figure 2.
Biomass of A. adenophora inoculated with endophytic Colletotrichum strains. (a) Aboveground parts, (b) underground parts, (c) branch number, (d) stem length, (e) root length, and (f) root shoot ratio. Dots with different colours represent the raw data of each sample inoculated with the Colletotrichum AX39, AX115 and AX198 strains. A negative RI indicates a reduced biomass of A. adenophora in the experimental treatment with Colletotrichum spp. infection compared with that in the control without Colletotrichum spp. infection. Nonparametric Mann-Whitney U tests or independent sample T tests were used to identify the differences between each treatment group and the control group (* < 0.05, ** < 0.01, *** <0.001). Post hoc comparisons were performed via Duncan’s test for equal variance and Dunnett’s test for unequal variance (T3 test) to determine whether the difference in the RI was significant among the treatments inoculated with AX39, AX115 and AX198, with different letters indicating significant differences. The error bars are the standard errors.
Figure 2.
Biomass of A. adenophora inoculated with endophytic Colletotrichum strains. (a) Aboveground parts, (b) underground parts, (c) branch number, (d) stem length, (e) root length, and (f) root shoot ratio. Dots with different colours represent the raw data of each sample inoculated with the Colletotrichum AX39, AX115 and AX198 strains. A negative RI indicates a reduced biomass of A. adenophora in the experimental treatment with Colletotrichum spp. infection compared with that in the control without Colletotrichum spp. infection. Nonparametric Mann-Whitney U tests or independent sample T tests were used to identify the differences between each treatment group and the control group (* < 0.05, ** < 0.01, *** <0.001). Post hoc comparisons were performed via Duncan’s test for equal variance and Dunnett’s test for unequal variance (T3 test) to determine whether the difference in the RI was significant among the treatments inoculated with AX39, AX115 and AX198, with different letters indicating significant differences. The error bars are the standard errors.
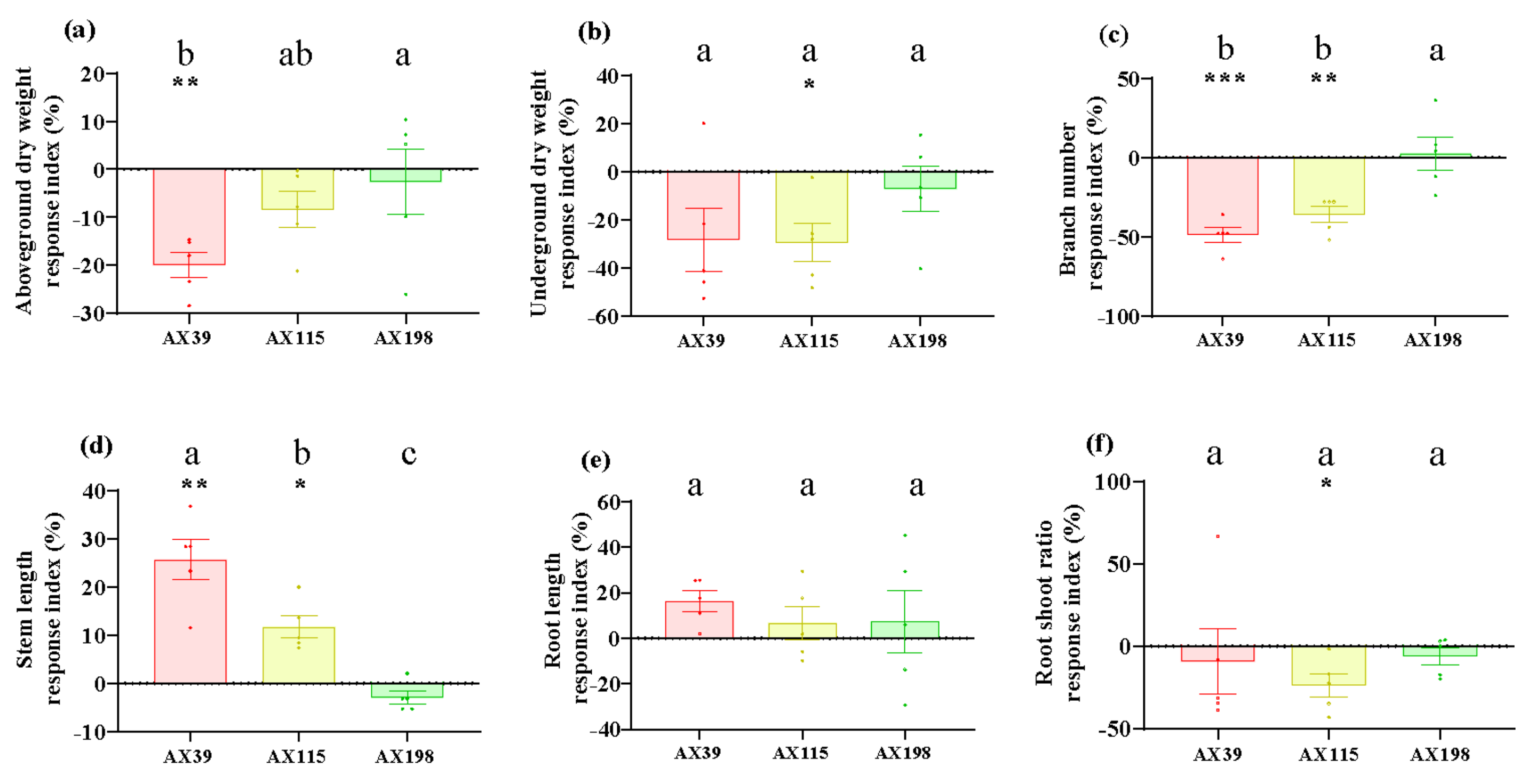
Figure 3.
LMA (dry weight per unit area) of A. adenophora inoculated with endophytic Colletotrichum strains. (a) The second pair of leaves, (b) the fifth pair of leaves. Dots with different colours represent the raw data of each sample inoculated with the Colletotrichum AX39, AX115 and AX198 strains. A negative RI indicates a reduced LMA of A. adenophora in the experimental treatment with Colletotrichum spp. infection compared with that in the control without Colletotrichum spp. infection. Nonparametric Mann-Whitney U tests or independent sample T tests were used to identify the differences between each treatment group and the control group (* < 0.05, ** < 0.01, *** <0.001). Post hoc comparisons were performed via Duncan’s test for equal variance and Dunnett’s test for unequal variance (T3 test) to determine whether the difference in the RI was significant among the different treatments inoculated with AX39, AX115 and AX198, with different letters indicating significant differences. The error bars are the standard errors.
Figure 3.
LMA (dry weight per unit area) of A. adenophora inoculated with endophytic Colletotrichum strains. (a) The second pair of leaves, (b) the fifth pair of leaves. Dots with different colours represent the raw data of each sample inoculated with the Colletotrichum AX39, AX115 and AX198 strains. A negative RI indicates a reduced LMA of A. adenophora in the experimental treatment with Colletotrichum spp. infection compared with that in the control without Colletotrichum spp. infection. Nonparametric Mann-Whitney U tests or independent sample T tests were used to identify the differences between each treatment group and the control group (* < 0.05, ** < 0.01, *** <0.001). Post hoc comparisons were performed via Duncan’s test for equal variance and Dunnett’s test for unequal variance (T3 test) to determine whether the difference in the RI was significant among the different treatments inoculated with AX39, AX115 and AX198, with different letters indicating significant differences. The error bars are the standard errors.
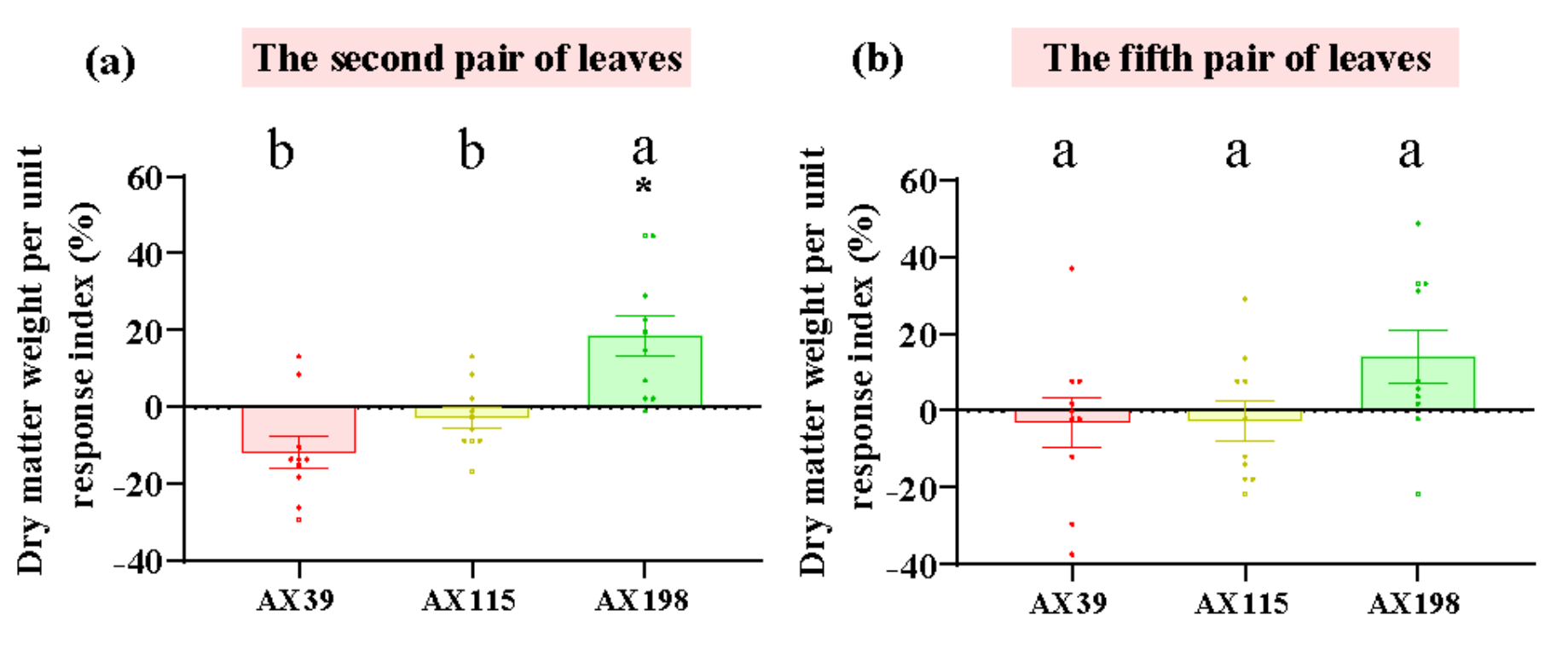
Figure 4.
The asymptomatic leaves of A. adenophora plants inoculated with Colletotrichum spore mixture (a) and wounded and inoculated with agar discs of Colletotrichum (b). "CK" represents the control group without Colletotrichum inoculation.
Figure 4.
The asymptomatic leaves of A. adenophora plants inoculated with Colletotrichum spore mixture (a) and wounded and inoculated with agar discs of Colletotrichum (b). "CK" represents the control group without Colletotrichum inoculation.
Figure 5.
Pathogenicity effects of inoculating endophyte Colletotrichum strains on A. adenophora after challenge with the (a) pathogen G56, (b) pathogen Y122 and (c) pathogen S188. Dots with different colours represent the raw data of each sample inoculated with the Colletotrichum AX39, AX115 and AX198 strains. The specific leaf spot area and morphology are shown in (d); scale bar =10 mm, and "CK" represents the control group without Colletotrichum inoculation. Nonparametric Mann-Whitney U tests or independent sample T tests were used to identify the differences between each treatment group and the control group (* < 0.05, ** < 0.01, *** <0.001). Post hoc comparisons were performed via Duncan’s test for equal variance and Dunnett’s test for unequal variance (T3 test) to determine whether the difference in the RI was significant among the different treatments inoculated with AX39, AX115 and AX198, with different letters indicating significant differences. The error bars are the standard errors.
Figure 5.
Pathogenicity effects of inoculating endophyte Colletotrichum strains on A. adenophora after challenge with the (a) pathogen G56, (b) pathogen Y122 and (c) pathogen S188. Dots with different colours represent the raw data of each sample inoculated with the Colletotrichum AX39, AX115 and AX198 strains. The specific leaf spot area and morphology are shown in (d); scale bar =10 mm, and "CK" represents the control group without Colletotrichum inoculation. Nonparametric Mann-Whitney U tests or independent sample T tests were used to identify the differences between each treatment group and the control group (* < 0.05, ** < 0.01, *** <0.001). Post hoc comparisons were performed via Duncan’s test for equal variance and Dunnett’s test for unequal variance (T3 test) to determine whether the difference in the RI was significant among the different treatments inoculated with AX39, AX115 and AX198, with different letters indicating significant differences. The error bars are the standard errors.
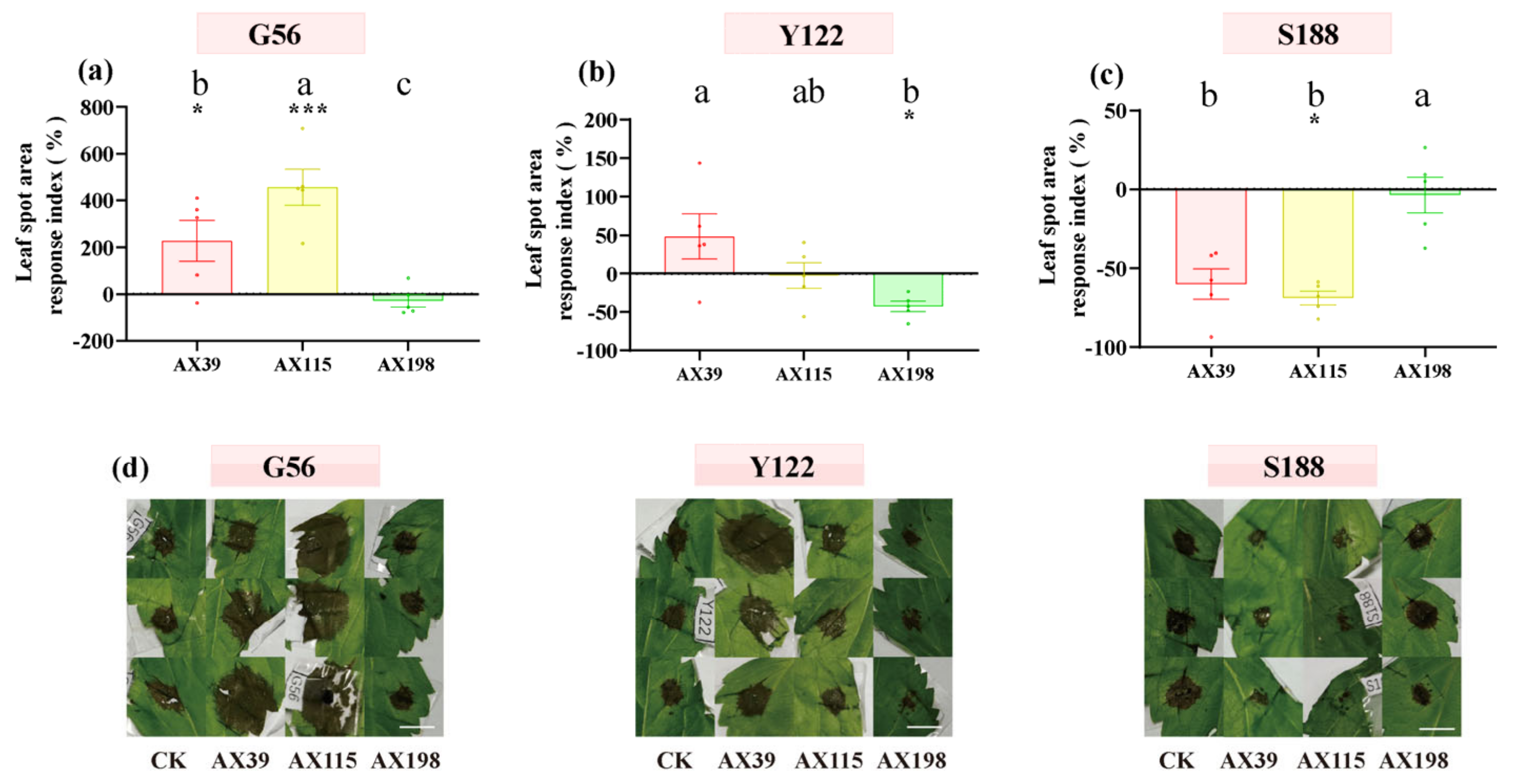
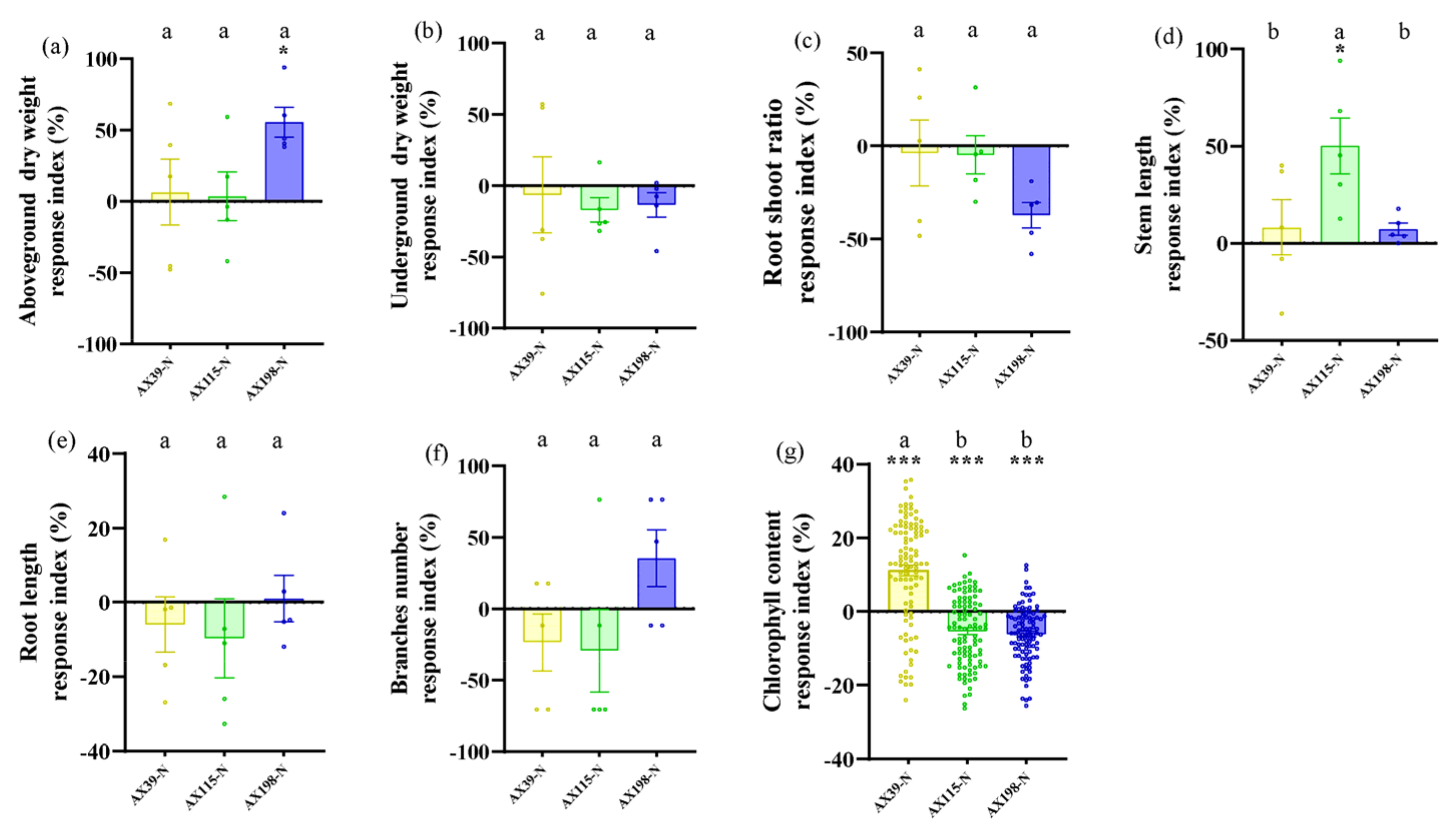
Figure 6.
Biomass and chlorophyll content of A. adenophora inoculated with endophyte Colletotrichum strains under normal treatment and drought stress. (a) Aboveground parts, (b) underground parts, (c) root shoot ratio, (d) stem length, (e) root length, (f) branch number, and (g) chlorophyll content. A positive RI indicates an increased biomass of A. adenophora in the drought stress (-W) treatment with Colletotrichum strain (AX39, AX115, or AX198) inoculation compared with that without Colletotrichum inoculation. Nonparametric Mann-Whitney U tests or independent sample T tests were used to identify the differences between each drought stress treatment group and the normal treatment group (* < 0.05, ** < 0.01, *** <0.001). Post hoc comparisons were performed via Duncan’s test for equal variance and Dunnett’s test for unequal variance (T3 test) to show that the differences in the RIs were significant among the treatments inoculated with strains AX39, AX115 and AX198, with different letters indicating significant differences. The error bars are the standard errors.
Figure 6.
Biomass and chlorophyll content of A. adenophora inoculated with endophyte Colletotrichum strains under normal treatment and drought stress. (a) Aboveground parts, (b) underground parts, (c) root shoot ratio, (d) stem length, (e) root length, (f) branch number, and (g) chlorophyll content. A positive RI indicates an increased biomass of A. adenophora in the drought stress (-W) treatment with Colletotrichum strain (AX39, AX115, or AX198) inoculation compared with that without Colletotrichum inoculation. Nonparametric Mann-Whitney U tests or independent sample T tests were used to identify the differences between each drought stress treatment group and the normal treatment group (* < 0.05, ** < 0.01, *** <0.001). Post hoc comparisons were performed via Duncan’s test for equal variance and Dunnett’s test for unequal variance (T3 test) to show that the differences in the RIs were significant among the treatments inoculated with strains AX39, AX115 and AX198, with different letters indicating significant differences. The error bars are the standard errors.
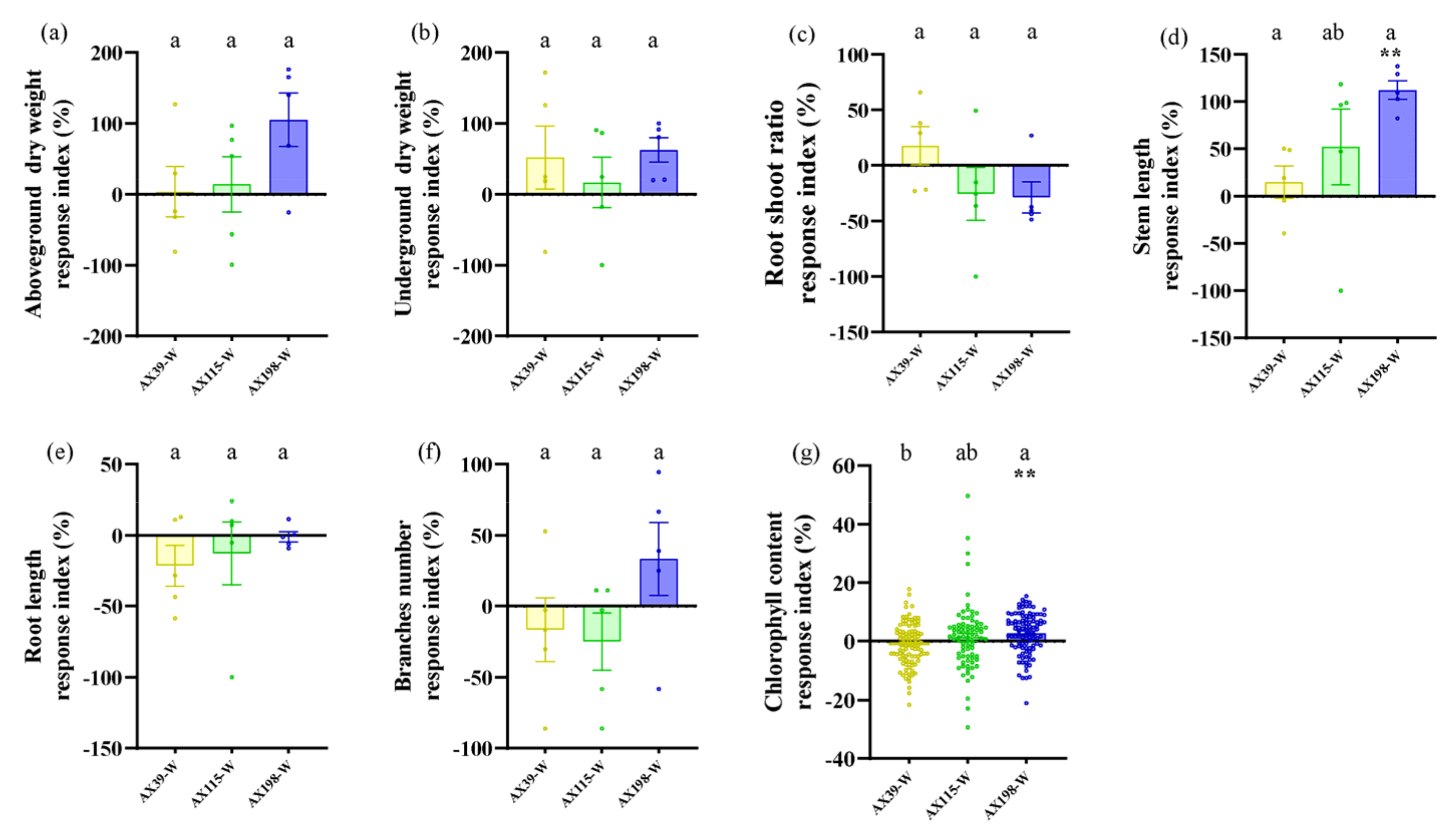
Figure 7.
Biomass and chlorophyll content of A. adenophora plants inoculated with endophytic Colletotrichum strains under normal conditions and nutrient stress conditions. (a) Aboveground parts, (b) underground parts, (c) root shoot ratio, (d) stem length, (e) root length, (f) branch number, and (g) chlorophyll content. A positive RI indicates an increased biomass of A. adenophora in the nutrient stress (-N) treatment with Colletotrichum strain (AX39, AX115, or AX198) inoculation compared with that without Colletotrichum inoculation. Nonparametric Mann-Whitney U tests or independent sample T tests were used to identify the differences between each nutrient stress treatment and the normal treatment (* < 0.05, ** < 0.01, *** <0.001). Post hoc comparisons were performed via Duncan’s test for equal variance and Dunnett’s test for unequal variance (T3 test) to show that the differences in the RIs were significant among the treatments inoculated with strains AX39, AX115 and AX198, with different letters indicating significant differences. The error bars are the standard errors.
Figure 7.
Biomass and chlorophyll content of A. adenophora plants inoculated with endophytic Colletotrichum strains under normal conditions and nutrient stress conditions. (a) Aboveground parts, (b) underground parts, (c) root shoot ratio, (d) stem length, (e) root length, (f) branch number, and (g) chlorophyll content. A positive RI indicates an increased biomass of A. adenophora in the nutrient stress (-N) treatment with Colletotrichum strain (AX39, AX115, or AX198) inoculation compared with that without Colletotrichum inoculation. Nonparametric Mann-Whitney U tests or independent sample T tests were used to identify the differences between each nutrient stress treatment and the normal treatment (* < 0.05, ** < 0.01, *** <0.001). Post hoc comparisons were performed via Duncan’s test for equal variance and Dunnett’s test for unequal variance (T3 test) to show that the differences in the RIs were significant among the treatments inoculated with strains AX39, AX115 and AX198, with different letters indicating significant differences. The error bars are the standard errors.