1.Introduction
Endometrial cancer (EC) is the most common gynecological malignancy in developed countries and the sixth most common cancer in women worldwide. Reflecting the trends seen in other Eastern European countries, the incidence of endometrial cancer has been on the rise in Romania. [
1] Globocan reported 1,539 new cases of EC cancer in 2012, with a 48% increase by 2022 [
1], coinciding with a current obesity prevalence of up to 36%, which poses a significant public health challenge.
Factors such as urbanization, lifestyle changes, and increasing obesity rates contribute to the rise in endometrial cancer cases, as patients often present with obesity, diabetes, and hypertension.
Endometrial cancer was the first type of cancer identified as associated with obesity [
2], a condition that not only promotes cancer development but also negatively impacts treatment outcomes. Women with severe obesity who develop endometrial cancer face a significantly higher risk of mortality from both the cancer and related co-morbidities compared to their lean counterparts.
Obesity has also been linked to lower quality of life among endometrial cancer survivors, as well as a higher body mass index (BMI).[
2] This association has been well established and follows a dose-response relationship, with the incidence of endometrial cancer increasing as body mass index (BMI) increases.[
2] A meta-analysis of 26 research papers conducted by the American Institute for Cancer Research indicated that with each increment of five BMI units, the probability of acquiring endometrial cancer escalated by 50%.[
3]
The main treatment for endometrial cancer patients is surgery (total abdominal hysterectomy and bilateral salpingo – oophorectomy) with or without pelvic lymph node dissection or sentinel lymph node sampling followed by adjuvant treatment. The recommendations for adjuvant therapy in early-stage endometrial cancer are based on FIGO staging (The International Federation of Gynecology and Obstetrics) in regard to the presence or absence of various risk factors, including older age, extensive myometrial invasion, high tumor grade, large tumor size, and lymphovascular space invasion (LVSI). [
4,
5,
6]
The classification of early-stage uterine cancer into low-risk, intermediate-risk, and high-risk categories relies on a combination of these risk factors; however, definitions can vary among researchers and studies. In early-stage endometrial cancer, the vaginal cuff is the most common site for recurrence when adjuvant treatment is not used. Most recurrences take place in this location or in the proximal third of the vagina, with only about 10% occurring in the middle and distal thirds.[
7] Depending on the surgical technique, the scar region may cover 1cm or even more, and the potentially affected vaginal wall has a thickness of 2-5mm, depending on the patient age. Since the Vaginal cuff is the most common site of relapse for this type of cancer, based on clinical evidence, adjuvant brachytherapy is widely considered the standard treatment to decrease vaginal recurrence in patients with intermediate-risk EC after surgery.
The Postoperative Radiation Therapy in Endometrial Carcinoma PORTEC-2 trial was initiated to determine if vaginal brachytherapy (VBT) would be as effective as external beam radiation therapy (EBRT) in reducing vaginal recurrence, while also causing fewer treatment-related side effects and enhancing quality of life.Patients were randomized after surgery to adjuvant radiation with either pelvic EBRT (External Beam Radiation Therapy) or VCBT (Vaginal Cuff Brachytherapy) and demonstrated no difference in vaginal cuff recurrences or overall survival between the two modalities. The results of this randomised trial established that vaginal cuff brachytherapy (VCBT) is highly effective at achieving local control and minimizing the risk of vaginal recurrence, which is the most common site of disease recurrence in patients with high-intermediate risk endometrial carcinoma. VCBT provides excellent vaginal control and rates of locoregional recurrence, with overall survival and disease-free survival rates comparable to external beam radiation therapy (EBRT). Additionally, VCBT significantly improves quality of life and reduces gastrointestinal toxicity. Therefore, VBT should be the preferred adjuvant treatment for patients with high-intermediate risk endometrial carcinoma. [
5] The result of this study has been translated into increasing utilization of high-dose-rate (HDR) VCBT as treatment of choice in high intermediate risk patients based on various society-specific guidelines for the management of patients with EC.
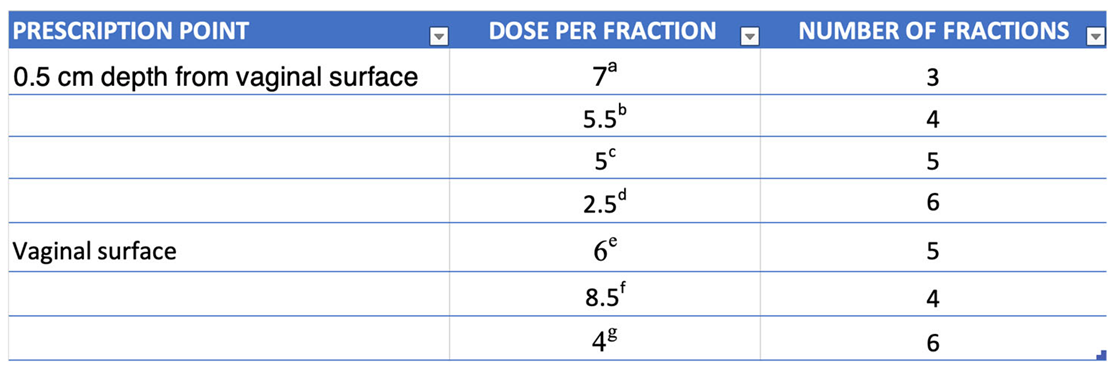
There is various fractionation schemes used clinically with no general consensus about the superiority of one regimen over other. The American Brachytherapy Society (ABS) does not recommend one fractionation scheme over the other in their report [
8]
The latest survey of vaginal brachytherapy practices [
8] revealed that the most frequently utilized fractionation scheme is 7 Gy x 3 fractions, prescribed to a depth of 0.5 cm, followed by 6 Gy x 5 fractions prescribed to the vaginal surface. The next most common schemes were 5.5 Gy x 4 fractions and 5 Gy x 5 fractions, both prescribed to a depth of 0.5 cm, with 7.5 Gy x 5 fractions prescribed to the vaginal surface coming last, the total dose prescribed being in the 30 Gy margin. It is important to note that there is significant variability in the dose-fractionation schemes used in practice for vaginal cuff brachytherapy.[
9] All these regimens appear effective based on institutional reports. Acceptable and frequently utilized fractionation schemes are presented in
Table 1.
Another important problem regarding VCBT is the prescription depth of the dose. The common prescription depth of 5 mm from cylinder surface, though potentially more toxic than prescribing to the surface, can be justified by location of vaginal lymphatics. [
9,
14,
15] Ninety-five percent of the vaginal lymphatics lie within 3 mm of the vaginal surface, so ensuring adequate dose to at least this depth may be important.[
16]
It is important to establish the length of the vagina on physical exam prior to treatment in order to prescribe the dose to the correct length. In PORTEC-2, the upper half of the vagina was treated, while Sorbe et al. focused on treating the upper two-thirds of the vagina. The ABS guidelines recommend administering vaginal cuff brachytherapy (VCBT) with an active length (AL) of 3-5 cm after hysterectomy.[
16] Practices vary widely, but the most frequently used fixed length and fractional length prescriptions for endometrial cancer are 4 cm and the proximal half of the vagina, respectively.[
9] For clear cell and serous histologic features, treatment of the entire vagina should be considered. [
10]
Beside the different fractionation schedules, other risk factors can also influence the toxicities and the treatment outcomes, and since there is a shortage of research examining the impact of overweight on dosimetric factors regarding VCBT, our objective was to assess how BMI affects the radiation dose to organs at risk during exclusive post-surgery VCBT.
2. Materials and Methods
We collected brachytherapy data in a retrospective manner from patients treated in our department between February 2016 and May 2024. The analysis was performed on 242 patients who underwent surgical intervention for endometrial cancer and were treated with exclusive adjuvant VCBT using three-dimensional computed tomography (CT) based treatment plan.
Patients characteristics regarding age, staging, prescribed dose, cylinder diameter and prescribed length, D2cc (minimum dose within the 2 cm
3 volume receiving the highest dose) to organs at risk (OARs) and dose to target volume D90 HR_CTV (dose to 90% of high-risk clinical target volume) are shown in
Table 2.
To ensure optimal reproducibility and the lowest dose to organs at risk, all patients underwent an at-home bowel preparations and had a Foley balloon inserted at time of brachytherapy.
Applicator insertion was performed using the largest diameter cylinder that could comfortably fit into the vaginal vault, with the cylinder positioned to remain parallel to the cranio-caudal axis of the patient. We used three types of applicators from Varian Medical Systems, respectively the closed CT vaginal cylinder, the shielded cylinder and the universal stump cylinder. Applicators were chosen with regard to patient characteristics, availability and different diameter sizes.
For contouring of target volume, physicians included 5 mm volume from cylinder surface even in the tip, while the length of contour was based on patient’s characteristics of the vagina length, usually 3 to 5 cm. Organs at risk, respectively bladder, rectum, sigmoid and small bowel were contoured according to GEC-ESTRO guidelines.
With each insertion, additional factors that might have influenced individual dosimetry, such as cylinder diameter, active length and the dose regiments were documented.
Patients were then categorized into four groups based on their BMI at the time of hysterectomy according to World Health Organization (WHO) classification. [
17] Individual anthropometric data were gathered, and BMI was calculated using the standard formula: mass (kg) divided by height (m²).
2.1. CT Acquisition
All patients underwent a planning CT scan at first insertion, utilizing 2.5 mm thick slices while in supine position. At each fraction, Foley bladder catheter was employed to instill a dilute contrast medium (2 mL of Omnipaque350 mixed with 48 mL of saline solution) while the Foley balloon was filled with saline solution, to enhance bladder visibility during segmentation and to ensure volume reproducibility during treatment.
Holloway et al. conducted a retrospective study indicating that the inter-fraction variance for D2cc doses to the bladder and rectum is minimal, at 6-8%. [
18] They concluded that there is insufficient evidence to justify the need for recording doses to organs at risk (OARs) with each fraction. While the delivery of VCBT in multiple sessions based on a single plan raised concerns [
19], data indicated that a certain level of homogeneity between insertions was necessary [
20]. This led to the development of well-established pre-insertion protocols for rectum and bowel preparation, as well as bladder filling [
21].
2.2. Segmentation and Planning
CTs were transferred to Varian Medical Systems 3D Brachytherapy Planning (we used version 13.6 until 2019, version 16.0 until 2023 and version 17.0 ever since) for contouring and treatment planning. For calculations, we used the nominal source strength of 10 Ci 40,700 cGy cm2/h for a 192 Iridium source and we applied a TG-43 line-source dose calculation formalism. At the treatment console, the treatment time was recalculated automatically using the actual source strength at the time of treatment.
Also, for target volume and organs at risk dose evaluation we used the well-known EQD2 spread sheet from Nag S. [
22]
The entire bladder volume was outlined, and the rectum was defined as extending from 2 cm above the cylinder tip to 2 cm below the last target volume contoured slide.
After ensuring a minimal dose of 100% to D90 of HR-CTV, dose volume histograms were assessed for each fraction and data was collected for the D2cc of all OAR. Cylinder size, active length and dose prescription were also recorded.
2.3. Body Mass Index Assessment
At the time of hysterectomy BMI was documented for all patients included in the study.
BMI was calculated and classified according to the World Health Organization (WHO) definitions. [
6] The formula for BMI is weight in kilograms divided by the square of height in meters. The categories are as follows: underweight < 18.5 kg/m²; normal weight 18.5-24.9 kg/m²; overweight 25.0-29.9 kg/m²; obese class I 30.0-34.9 kg/m²; obese class II 35.0-39.9 kg/m²; and obese class III > 40.0 kg/m².
2.4. Statistical Analysis
Data collection was performed using Microsoft Office 365 Suite—Office Excel. Data
processing, statistical analysis and chart generation were performed using International Business Machines Corporation® Statistical Product and Service Solutions® v.26.0. We calculated correlation between BMI and all dosimetric endpoint using Spearman’s method. All tests were two-tailed. Correlation between BMI and age, FIGO staging, dose schedule, cylinder diameter, prescribed length, D2cc for OAR’s and D90 were assessed.
3. Results
In the selected period, we identified 242 endometrial cancer patients treated surgically with salpino-oforectomy with or without lymphnode staging and based on the surgical findings on the histopathological exam they underwent adjuvant exclusive vaginal cuff brachytherapy as a subsequent treatment based on our institution protocol. BMI at the time of hysterectomy was assessed.
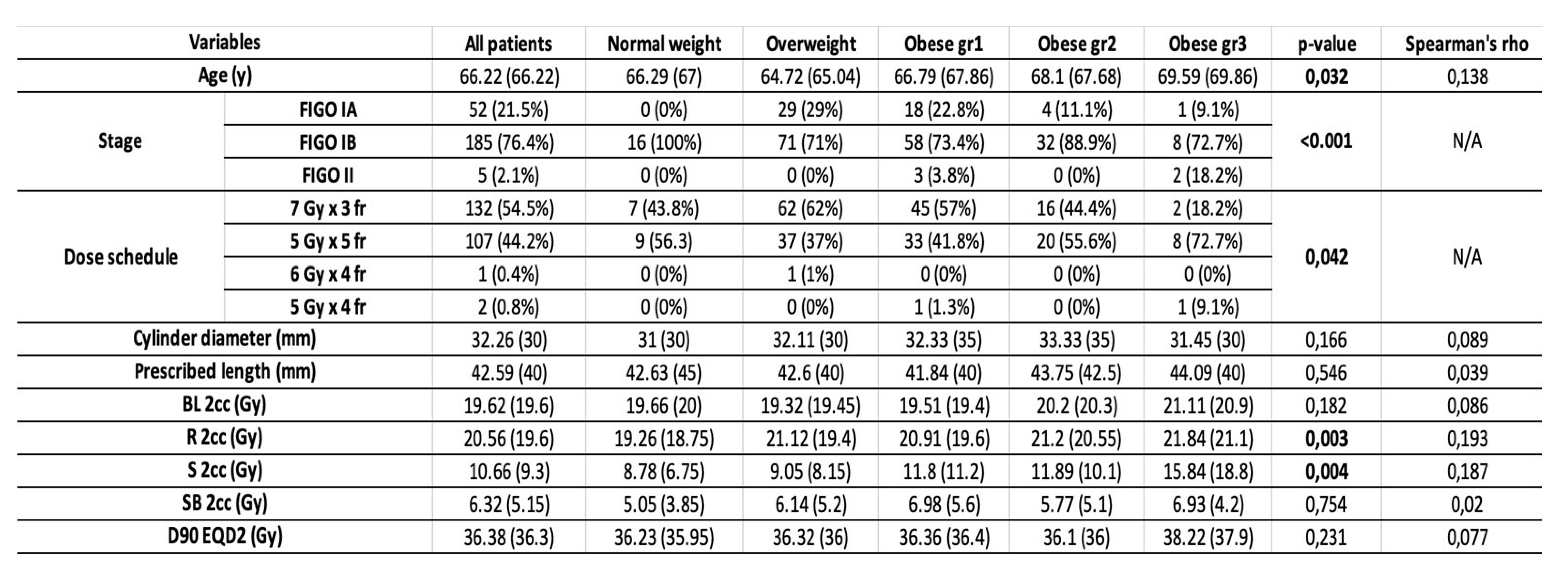
For all patients, mean height was 165 cm [150-188], mean weight 82 kg [47-135] and mean BMI was 30.27 [19.5-52.7]. We found significant weak positive relationships between BMI and age.
A total of 242 planning CT scan were assessed at first insertions that translated into 242 treatment plans for a total 945 insertions. Dose schedules were 7 Gray (Gy) x 3 fractions (fr), 5 Gy x 5 fr, 6 Gy x 4 fr and 5 Gy x 4 fr, with 54.5 % of all treatment being the 7 Gy x 3 fr schedule, 44.21% the 5 Gy x 5 fr leaving 1.2% for the other schedules. Dose regimens reflected a biological equivalent dose of ~30 Gy prescribed to tagert volume. All treatment plans had min dose % of 100, and according to planning calculations, the D90 (EQD2) dose to target volume ranged from 28.7 to 45,7 Gy with mean dose of 36.38 Gy.
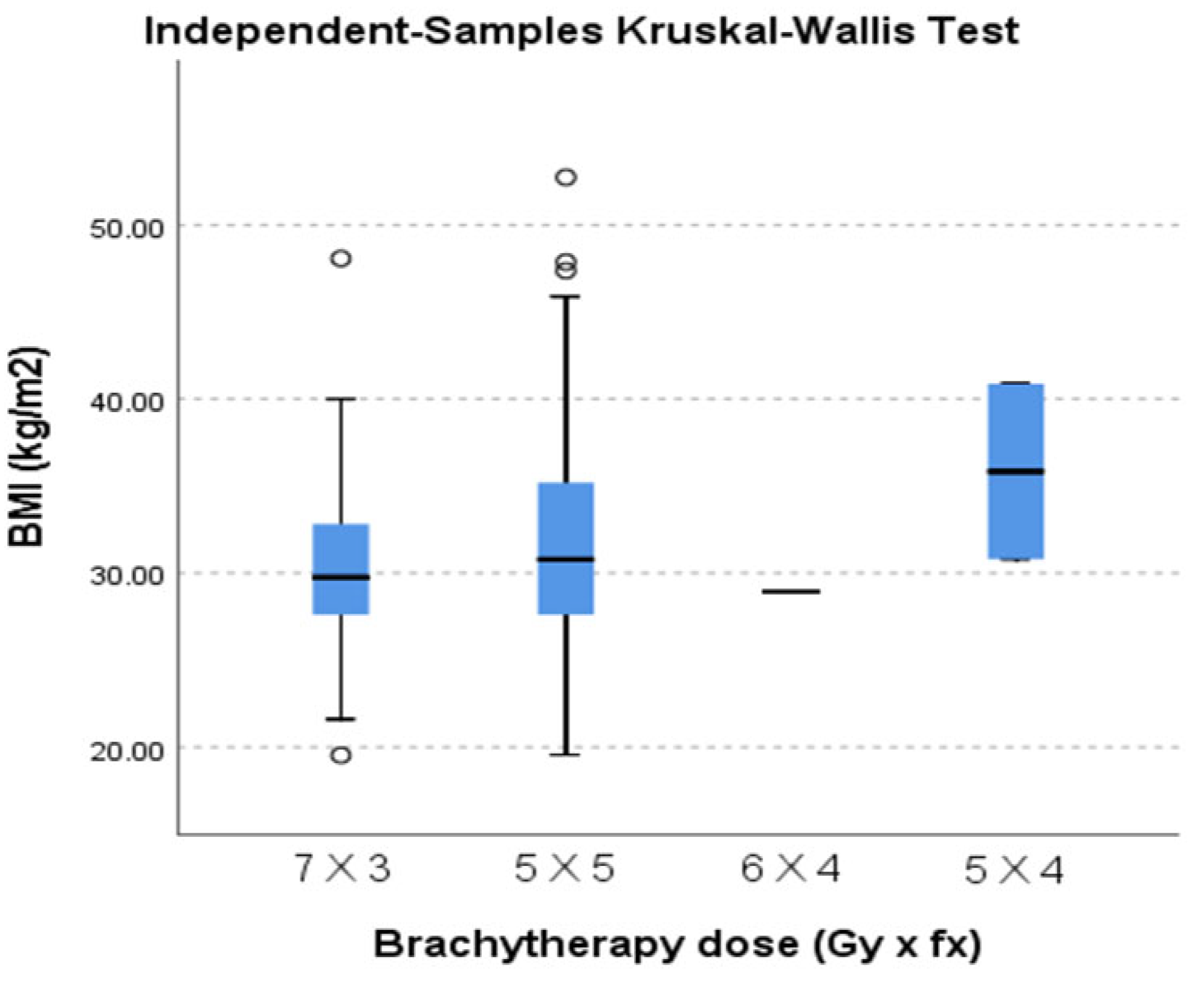
There was a significant variability between the different weight classes with regard to the brachytherapy dose regimens (
Figure 1) but no statistical significance.
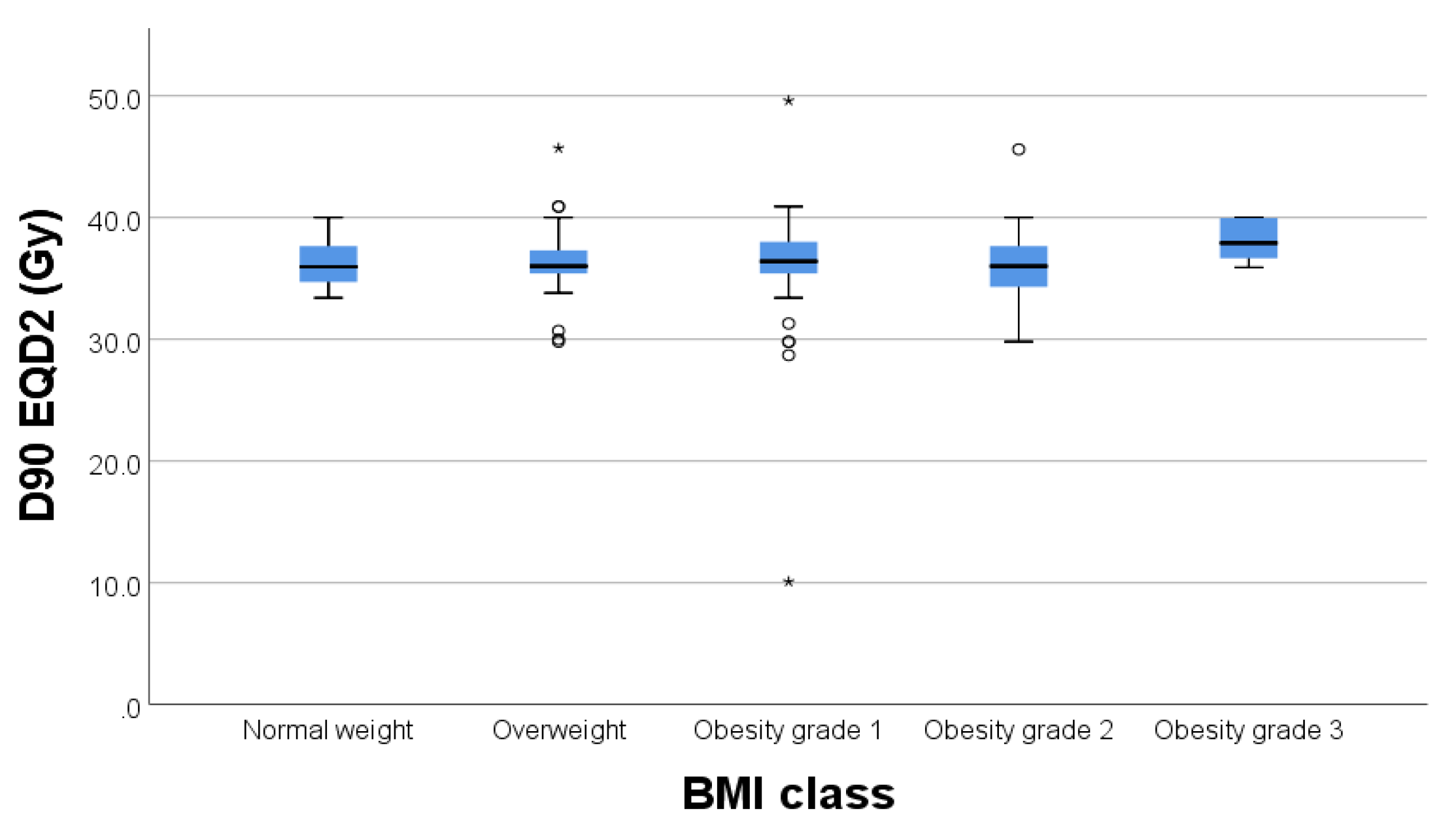
Regarding the correlation between BMI and D90 to target volume we did not find any statistically significant correlations with a p=0.231. (
Figure 2.)
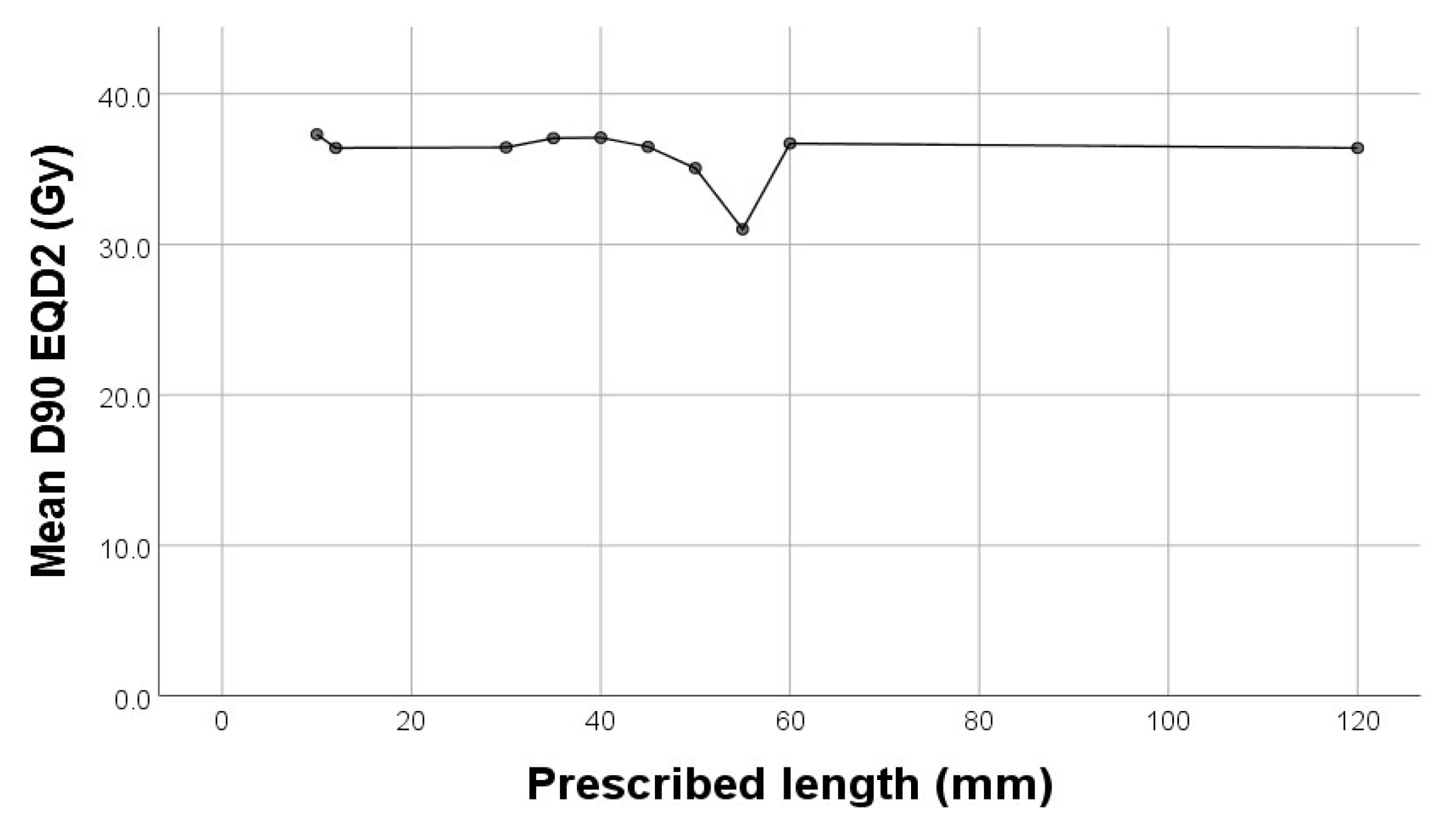
In our study there was a significant weak negative correlation between D90 (Gy) and cylinder diameter (p=0.013, rho=-0.159) and prescribed length (p<0.001, rho=-0.255) (
Figure 3).
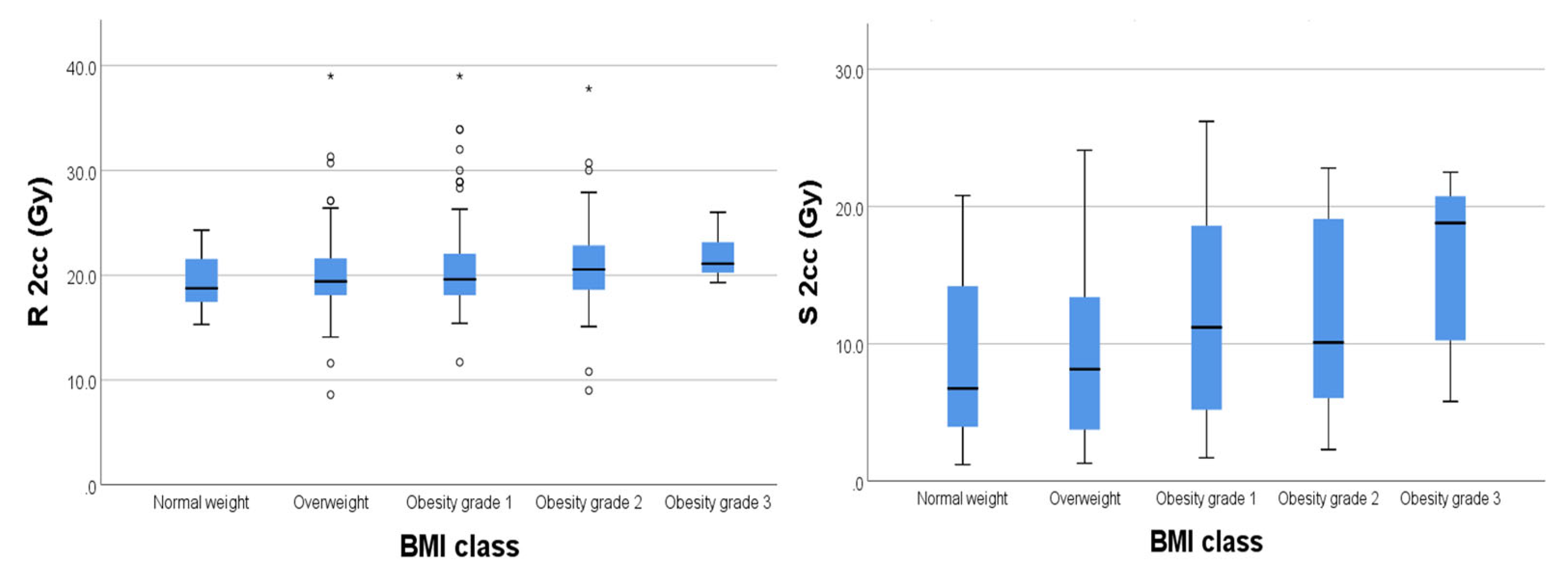
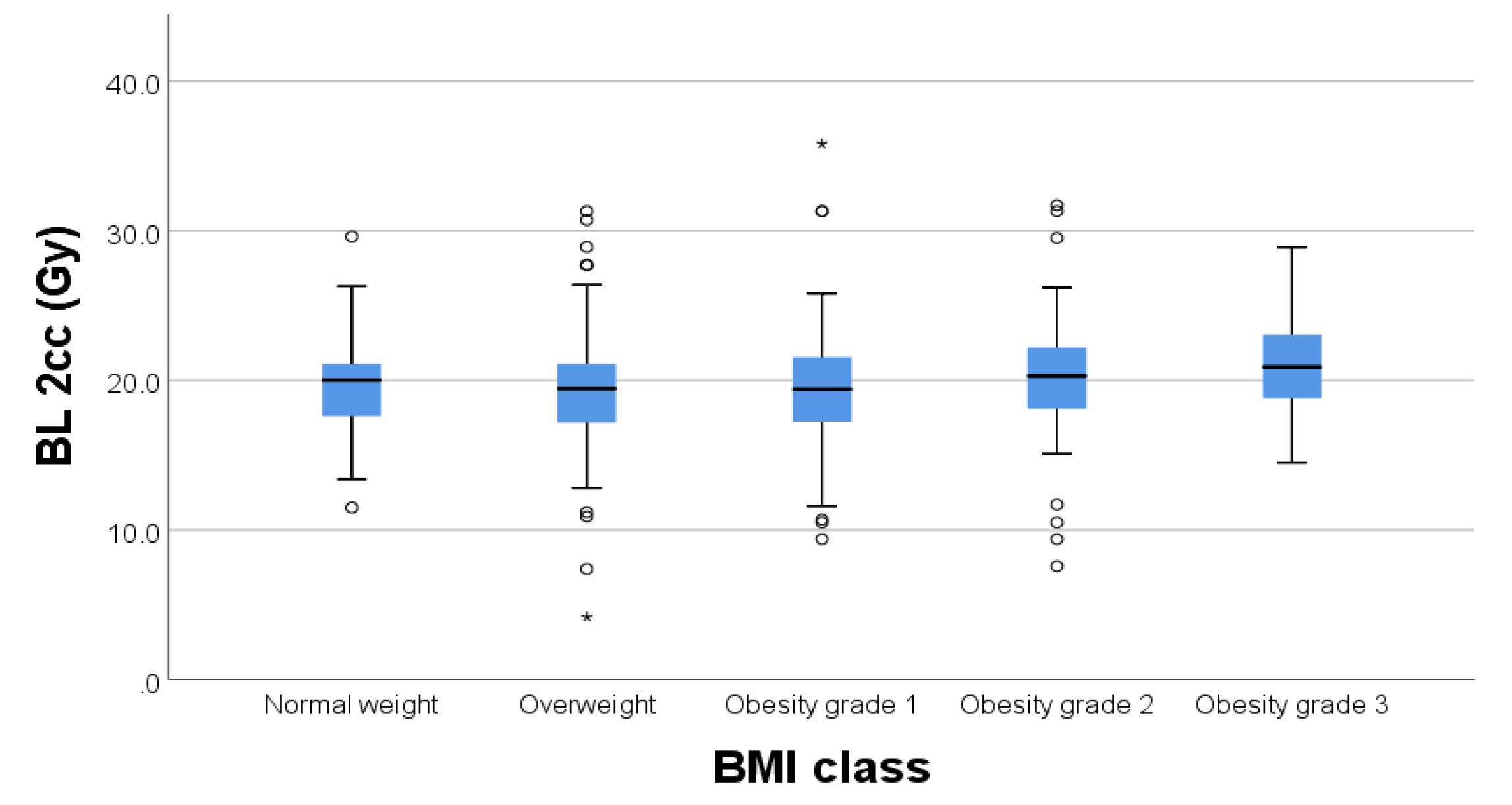
For organs at risk, we extracted drom the DVH the 2cc dose and used the EQD2 Nag S. spread sheet for dose calculations. As results we had mean D2cc for bladder 19.62 Gy [4.2-31.7], rectum 20.56 Gy [8.6-26.3], sigmoid 6.32 Gy [1.2-8.6] and small bowel 6.32 Gy [0.4-7.8].
We found significant weak positive relationships (
Figure 4) between BMI and D2cc for rectum (p=0.003) and D2cc for sigmoid (p=0.004) respectively.
Regarding the bladder there was no significant correlation between BMI and D2cc (p=0,182) (
Figure 5).
4. Discussions
Vaginal cuff Brachytherapy treatment planning is traditionally based on guidelines established by the International Comission on Radiation Units and Measurements [
19] and doses to OAR’s were limited to 2D points. Being that in the past 10 years accesibility to 3D imaging modalities has significantly increased, 3D CT based treatment planning gives us volumetric dose informations not only for the OAR’s but also for target volume.
Considering that 98.8% of the patients in our study were over 50 years old and that obesity is a national health concern, our findings indicate a statistically significant correlation between BMI and age (p=0.032). This correlation is weakly positive, suggesting that BMI tends to increase slightly with age.
Published data shows that women with low BMI tend to have less abdominal adipose tissue, thus subjecting the normal tissue to higher doses.[
23] In our data, patients having a high BMI show a non-significat correlation between BMI and dose to target volume, wich translates into an homogeneity in treatments amongst our patients. Though, we correletad a high BMI with slightly higher doses to OAR’s. For rectum and sigmoid we found a statistically significant weak positive relationship (p=0.003 for rectum and p=0.004 for sigmoid). This might be due to high mobility of the sigmoid colon, shown also by Boyle et al [
24].
In terms of the dose schedule, we recorded 2 patients who received 4 x 5 Gy. These patients had a treatment plan with total dose prescription prescribed of 5 x 5 Gy, but due to toxicity issues did not attend the final session of brachytherapy. Most of our patients underwent one of the thow most-known schedules for exclusive VCBT, respectively 3x7Gy or 5x5Gy; analized data showing a week but significat correlation between dose schedulle and BMI.
Regarding staging, the distribution of FIGO stages varied significantly acoss different weight classes, with a slight trend towards higher stage being associated with higher BMI class.
In terms of corelation between dose to target volume in regards to applicator width respectively active lengts, our data shows a week negative correlation p=0.013 respectively p<0.001.
In regards to dose fractionation and doses to OAR’s we only found that doses to 2cc for sigmoid and small bowel were smaller for the 3x7Gy schedule.
5. Conclusions
Obesity remains a primary concern among endometrial cancer patients, as a higher body mass index (BMI) was associated with slightly increased radiation doses to the rectum and sigmoid. However, the D90, a key dosimetric parameter, was not significantly impacted by elevated BMI, indicating that treatment was delivered homogeneously. Future clinical studies are necessary to investigate the correlation between these dosimetric findings and late treatment-related toxicities to better understand the long-term implications and optimize therapeutic outcomes
Author Contributions
Conceptualization, A.T.K.-M. and D.C.P.; Methodology, A.T.K.-M., D.C.P.; Software, D.C.P. and A.T.; Validation, D.C.P.; Formal analysis, A.T.K.-M., D.C.P., A.T. and C.O, O.F.C; Investigation, A.T.K.-M., D.C.P., A.T, A.R.A, O.F.C and C.O.; Resources, A.T.K.-M., D.C.P., O.F.C and C.O.; Data curation, A.T.K.-M., D.C.P. and A.R.A.; Writing—original draft, A.T.K.-M., A.T. and D.C.P.; Writing—review & editing, A.T.K.-M., D.C.P.; Supervision. ,C.O, O.F.C A.I..; Project administration, A.T.K.-M and A.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Ct angiography is a standard procedure in our protocol. The protocol was validated by the institutional review board.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study, including the use of clinical data for scientific analysis.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patient privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [CrossRef].
- Lindemann K, Vatten LJ, Ellstrom-Engh M, Eskild A. Bodymass, diabetes and smoking, and endometrial cancer risk: a follow-up study. Br J Cancer 2008;98(9):1582e5.
- World Cancer Research Fund/American Institute for Cancer Research: Continuous Update Project Report. Food: Nutrition, Physical Activity, and the Prevention of Endometrial Cancer. 2013.
- AlHilli MM, Podratz KC, Dowdy SC, et al. Risk-scoring system for the individualized prediction of lymphatic dissemination in patients with endometrioid endometrial cancer. Gynecol Oncol. 2013;131(1):103-108.
- Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spreadpatterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60(8 Suppl):2035-2041.
- Zaino RJ, Kurman RJ, Diana KL, Morrow CP. Pathologic models to predict outcome for women with endometrial adenocarcinoma: the importance of the distinction between surgical stage and clinical stage--aGynaecologic Oncology Group study. Cancer. 1996;77(6):1115-1121.
- Creutzberg CL, Nout RA, Lybeert ML, Wárlám-Rodenhuis CC, Jobsen JJ, Mens JW, Lutgens LC, Pras E, van de Poll-Franse LV, van Putten WL; PORTEC Study Group. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2011 Nov 15;81(4):e631-8. doi: 10.1016/j.ijrobp.2011.04.013. Epub 2011 Jun 2. PMID: 21640520.
- Harkenrider MM, Block AM, Alektiar KM, et al. American Brachy-therapy Task Group Report: Adjuvant vaginal brachytherapy forearly-stage endometrial cancer: A comprehensive review. Brachytherapy 2017;16:95e108.
- Harkenrider MM, Grover S, Erickson BA, et al. Vaginal brachytherapy for postoperative endometrial cancer: 2014 Survey of the American Brachytherapy Society. Brachytherapy 2016;15:23e29.
- Nag S, Erickson B, Parikh S, et al. The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the endometrium. Int J Radiat Oncol Biol Phys 2000 - 48:779e790.
- Jolly S, Vargas C, Kumar T, et al. Vaginal brachytherapy alone: an alternative to adjuvant whole pelvis radiation for early stage endometrial cancer. Gynecol Oncol 2005;97:887e892.
- Sorbe B, Straumits A, Karlsson L. Intravaginal high-dose-rate brachytherapy for stage I endometrial cancer: a randomized study of two dose-per-fraction levels. Int J Radiat Oncol Biol Phys 2005; 62:1385e1389.
- MacLeod C, Fowler A, Duval P, et al. High-dose-rate brachytherapy alone post-hysterectomy for endometrial cancer. Int J Radiat OncolBiol Phys 1998;42:1033e1039.
- Townamchai K, Lee L, Viswanathan AN. A novel low dose fractionation regimen for adjuvant vaginal brachytherapy in early stage endometrioid endometrial cancer. Gynecol Oncol 2012;127:351e355.
- Choo JJ, Scudiere J, Bitterman Pet al.. Vaginal lymphatic channel location and its implication for intracavitary brachytherapy radiation treatment. Brachytherapy 2005; 4: 236-240. [PubMed] [Google Scholar] [Ref list].
- Small W Jr., Beriwal S, Demanes DJet al.. American Brachytherapy Society consensus guidelines for adjuvant vaginal cuff brachytherapy after hysterectomy. Brachytherapy 2012; 11: 58-67. [PubMed] [Google Scholar] [Ref list].
- World Health Organization (WHO) Obesity: preventing and managing the global epidemic. Report on a WHO Consultation on Obesity, Geneva, 3-5 June, 1997. WHO/NUT/NCD/98.1. Technical Report Series Number 894World Health Organization, Geneva 2000.
- Holloway CL, Macklin EA, Cormack RA, et al. Should the organs at risk be contoured in vaginal cuff brachy therapy? Brachytherapy 2011;10:313e317.
- ICRU. Dose and volume specifications for reporting intracavitary therapy in gynecology. Report 38. 23. Bethesda, MD: ICRU; 1985. p. 1.
- Sabater S, Andres I, Sevillano M, Berenguer R, Machin-Hamalainen S, Arenas M. Dose accumulation during vaginal cuff brachytherapy based on rigid/deformable registration vs. single plan addition. Brachytherapy. 2014 Jul-Aug;13(4):343-51. doi: 10.1016/j.brachy.2013.11.006. Epub 2013 Dec 31. PMID: 24388615.
- SSabater S, Arenas M, Berenguer R, Andres I, Jimenez-Jimenez E, Martos A, Fernandez-Lopez J, Sevillano M, Rovirosa A. Body Mass Index and Doses at Organs at Risk in a Mediterranean Population Treated with Postoperative Vaginal Cuff Brachytherapy. Cancer Res Treat. 2015 Jul;47(3):473-9. doi: 10.4143/crt.2014.115. Epub 2014 Nov 24. PMID: 25672575; PMCID: PMC4506118.
- Nag S, Gupta, A simple method of obtaining equivalent doses for use in HDR.
- Mahantshetty U, Shetty S, Majumder D, Adurkar P, Swamidas J, Engineer R, Chopra S, Shrivastava S. Optimal bladder filling during high-dose-rate intracavitary brachytherapy for cervical cancer: a dosimetric study. J Contemp Brachytherapy. 2017 Apr;9(2):112-117. doi: 10.5114/jcb.2017.67502. Epub 2017 Apr 30. PMID: 28533798; PMCID: PMC5437088.
- Boyle JM, Craciunescu O, Steffey B, Cai J, Chino J. Body mass index, dose to organs at risk during vaginal brachytherapy, and the role of three-dimensional CT-based treatment planning. Brachytherapy. 2014 Jul-Aug;13(4):332-6. doi: 10.1016/j.brachy.2013.12.002. Epub 2014 Jan 16. PMID: 24439964; PMCID: PMC4532275.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).