1. Introduction
It is currently estimated that approximately 1.5 billion adults are overweight globally, and out of these, nearly 500 million are obese [
1]. This is one of the main causes of the development of metabolic syndrome and comorbidities such as type 2 diabetes mellitus, non-alcoholic fatty liver disease (NAFLD), hypertension, and cardiovascular diseases, among others. In fact, NAFLD was reported as the main chronic liver pathology worldwide, impacting more than 30% of the world’s population [
2]. Recently, the nomenclature for NAFLD has been updated to metabolic dysfunction-associated steatotic liver disease (MASLD), because the latter is more accurate to the pathology and less stigmatizing [
2]. The prevalence of MASLD runs in parallel to the increase in obesity.
MASLD is characterized by at least 5 % lipid accumulation in the liver in the absence of other known causes of fatty liver, such as alcohol or drug use. This disease has been shown to be the leading cause of chronic liver disease and a major cause of liver mortality and morbidity [
3]. If the disease is not detected and treated early, it can progress to metabolic dysfunction-associated steato-hepatitis (MASH), with inflammatory infiltrates in the hepatic lobes, apoptotic bodies, and fibrosis [
4]. In the most severe cases, with prolonged fibrosis, this disease can lead to cirrhosis, which results in a liver unable to carry out its normal activities, or even further, to the possible development of liver carcinoma. In both cases, the only possible treatment is a liver transplant.
The progression from MASLD to metabolic dysfunction-associated steato-hepatitis (MASH) and, finally, to cirrhosis can be explained through the double-impact theory [
5], which suggests a first attack that involves insulin resistance, generating lipogenesis and the accumulation of fatty acids (mainly oleic and palmitic) in the liver. Since the accumulation of fatty acids is toxic, they are processed by hepatocytes in the mitochondria and peroxisomes through β-oxidation or packaged into vesicles in the cytoplasm and exported outside of the liver [
6]. But, if the rate of vesicle elimination is lower than the rate of formation, steatosis develops. The second attack is caused by the constant oxidative stress generated by the high production of reactive oxygen species (ROS) in mitochondrial beta-oxidation, causing cellular damage. ROS are neutralized through the antioxidant defense mechanisms of the cells, but the constant release of ROS can deplete these defenses, inducing cell apoptosis and fibrosis and leading to cirrhosis. To avoid the harmful effects of ROS, two different systems are used: intrinsic antioxidant systems, such as antioxidant enzymes, and dietary antioxidant molecules, such as polyphenols derived from plant sources, including flavonoids. When any of these systems fail, a state of oxidative stress can arise [
7]. Particularly in MASLD, oxidative stress is generated by an excessive influx of fatty acids into the liver, generating the accumulation of lipids called lipotoxic lipids [
8].
Despite the worldwide prevalence of MASLD, there are currently no pharmacological treatments, so once diagnosed, it is treated in the same way as obesity [
9]. Therefore, new approaches are necessary [
10]. One option is antioxidant therapy using N-acetylcysteine [
11]. Therefore, the use of extracts obtained from some plants that demonstrate antioxidant capacity can be evaluated. Alcoholic extracts obtained from
Sycyos angulatus leaves showed a capacity to inhibit lipid accumulation in NAFLD—mainly those with high flavonoid content, especially flavone glycosides [
12].
The central composite rotatable design (CCRD) has been established as a powerful strategy for maximizing the extraction of bioactive compounds from various plants [
13]. In this study, we employed CCRD to identify extraction conditions that maximize the antioxidant activity of
Citrus sinensis (CS) extracts within a determined set of variables. The best conditions obtained for CS were then applied to prepare
Baccharis articulata (BA) extracts. Subsequently, the effects of both extracts on an in vitro human model for metabolic dysfunction-associated steatotic liver disease (MASLD) were evaluated.
2. Results
2.1. Extraction Conditions for Maximum Antioxidant Capacity in Infusions
This study employed a Central Composite RotableDesign (CCRD) to establish the extraction conditions that maximize the antioxidant capacity (AC) of extracts from Citrus sinensis (CS). The effects of three independent variables – extraction temperature, incubation time and extract concentrationon the AC were evaluated. The chosen ranges for these variables (
Table 1) were selected to reflect conditions typically used by consumers when preparing infusions [
14].
Table 1 presents the studied variables, their corresponding experimental levels, and the Antioxidant Capacity (AC) values of the infusions prepared under the CCRD. The AC values ranged from 10% to 49% in these infusions. To evaluate the factors influencing these variations in AC, we performed a statistical analysis using a quadratic model. The regression for the quadratic model was significant at the 95% confidence level (Adjusted R²:0,8265). The ANOVA (
Table 2) results indicated that the quadratic model showed no lack of fit for the data (p-value: 0.8805).
The generated regression coefficients of the model of the AC response are shown in
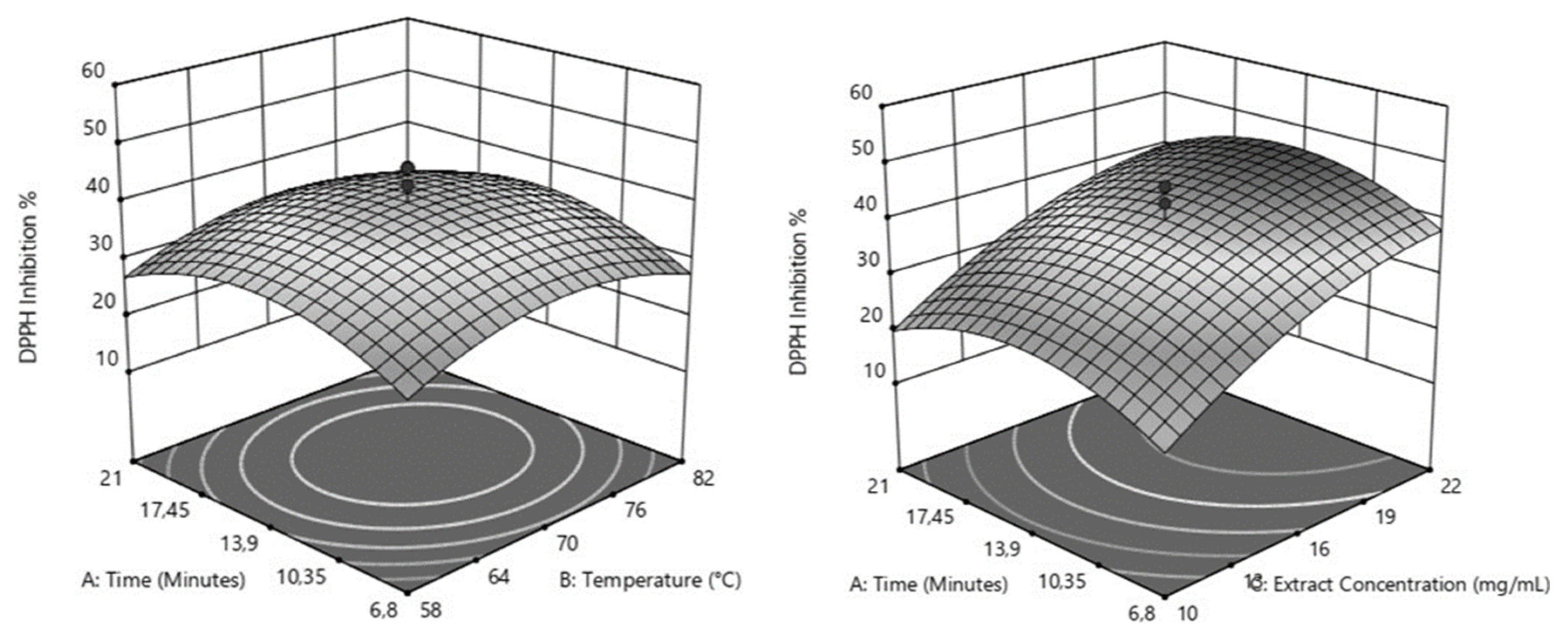
Table 3. The analysis of variance (ANOVA) revealed that extract concentration was the most significant factor influencing AC of Citrus sinensis leaf infusions (p < 0.0001). Within the studied range, the effects of incubation time (p = 0.5309) and extraction temperature (p = 0.4013) were not statistically significant when considered independently. However, their quadratic effect suggests an optimal range for maximizing AC. The negative quadratic effects obtained for incubation time (p=0.0010) and temperature (p = 0.0035) suggest that increasing these variables favored the antioxidant capacity (AC) of the infusions up to a maximum level, after which there was a decrease. These estimated peak values for AC were observed at approximately 14 minutes and 70 °C (as shown in
Figure 1a). On the other hand, the quadratic effect of extract concentration is also significant (p = 0.0248), although it appears negative. As observed in
Figure 1b, the AC of the infusions increases with increasing extract concentration and incubation time until it reaches a maximum, followed by a deceleration in growth. Notably, the maximum AC is achieved at an incubation time of approximately 14 minutes across the entire range of extract concentrations evaluated. Additionally, these behaviors are most pronounced at an incubation temperature of 70°C. Our first results suggested that maximum AC was achieved at 70°C incubation for 14 min. Additionally, higher extract concentrations were found to be crucial for maximizing AC. Consequently, we further investigated AC at higher extract concentrations up to 80mg/mL, 70°C incubation temperature, and an incubation time of 14 minutes. Maximum AC was determined with 30 mg/mL of Citrus sinensis.
2.2. Infusion
2.2.1. Infusion Characterization
The first parameter evaluated was infusion antioxidant capacity using 30 mg/mL of Citrus sinensis infusion or Baccharis articulate infusion (
Table 4). N-acetylcyteine (NAC) was used as a positive antioxidant capacity control (Scavenging activity against DPPH of 5 mM NAC: 96.11 ± 0.63 %). Concentration protein, polyphenols and flavonoid were determined. Regarding them, Baccharis articulate showed higher content of flavonoids and proteins compared to Citrus sinensis (
Table 4), while no significant difference was observed in polyphenol content.
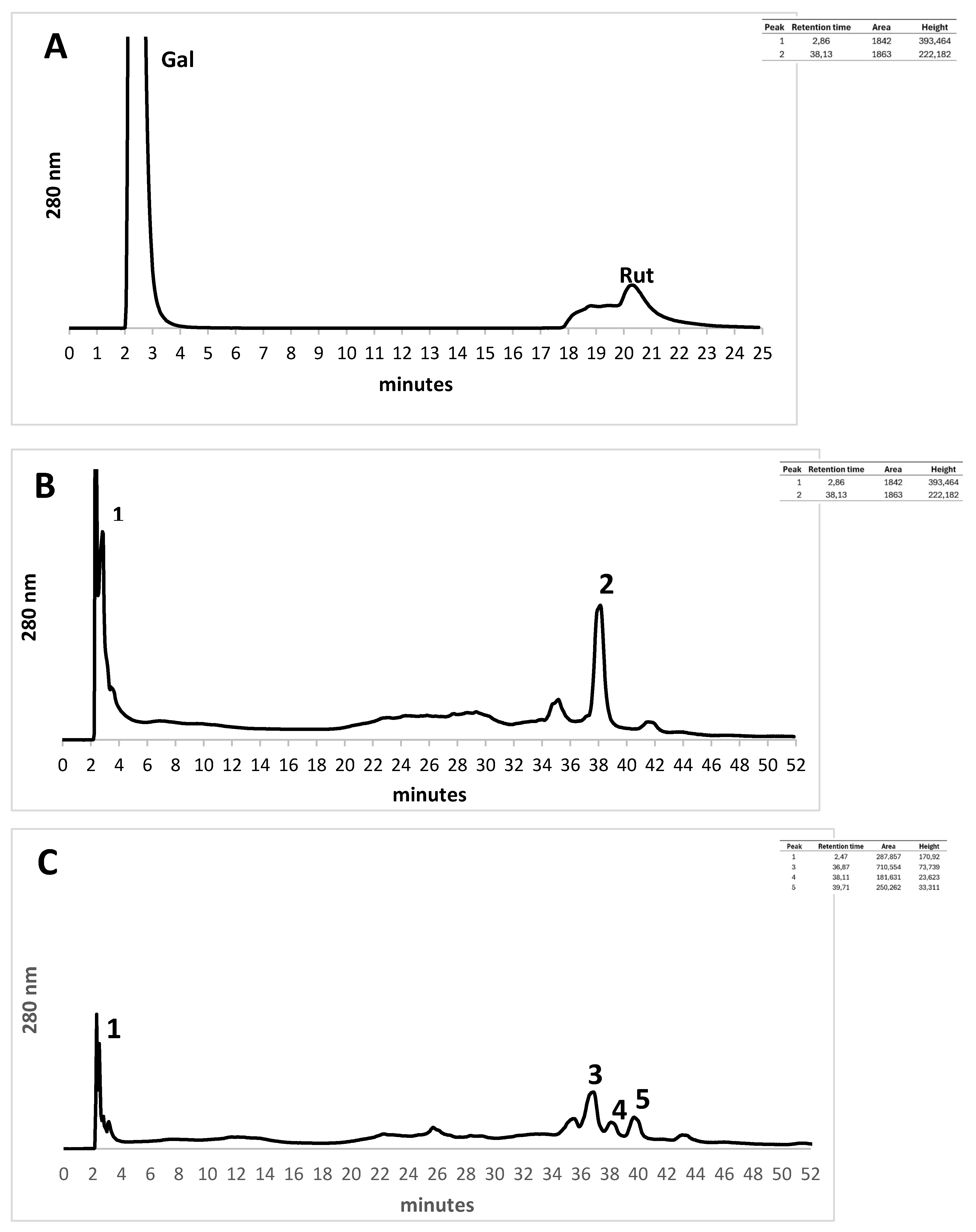
As shown in the
Figure 2 both infusions present gallic acid in high concentration. The other components are lower than galic acid. No rutin was observed in these samples.
Inserted in figure: table with peak retention times, area and height of compounds present in each analyzed infusion.
2.2.2. Infusion In Vitro Toxicity
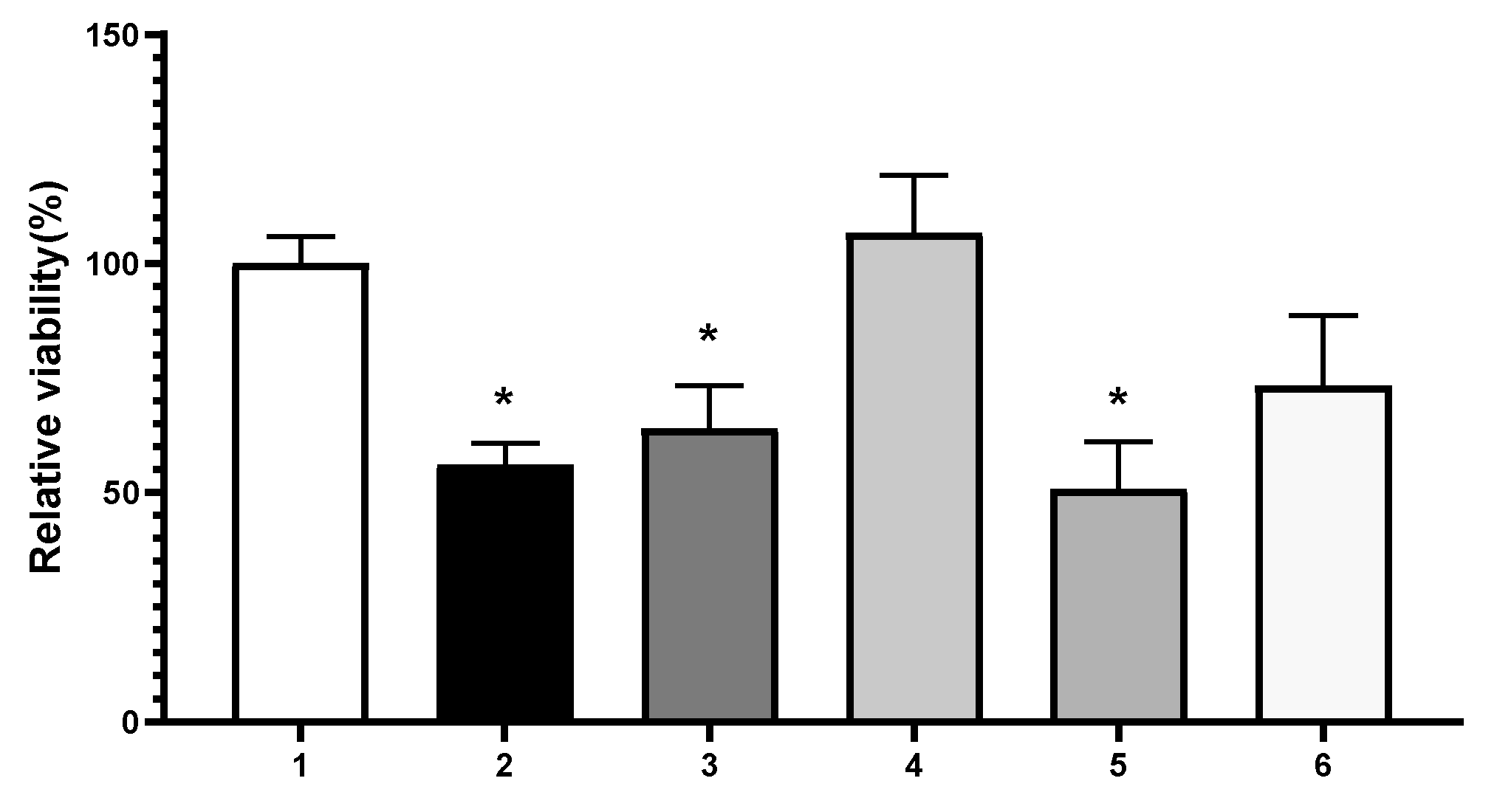
Toxicity was evaluated through MTT assay on Hep-G2 cells by adding different concentrations of the infusions for 48 h. Both original infusions were toxic (
Figure 3), while a 1:10 dilution showed no significant difference between no treated cells and treated cells (100 ± 6 [No treated cells]
vs 106.7 ± 12.5 [
Citrus sinensis, 14 µg RE/mL: CS infusion] or
vs 73.3 ± 15.5
Baccharis articulata, 43 µg RE/mL: BA infusion], both showed no significant difference). Therefore, all the experiments were performed with CS infusion and BA infusion
2.3. MASLD Model
2.3.1. Model Development
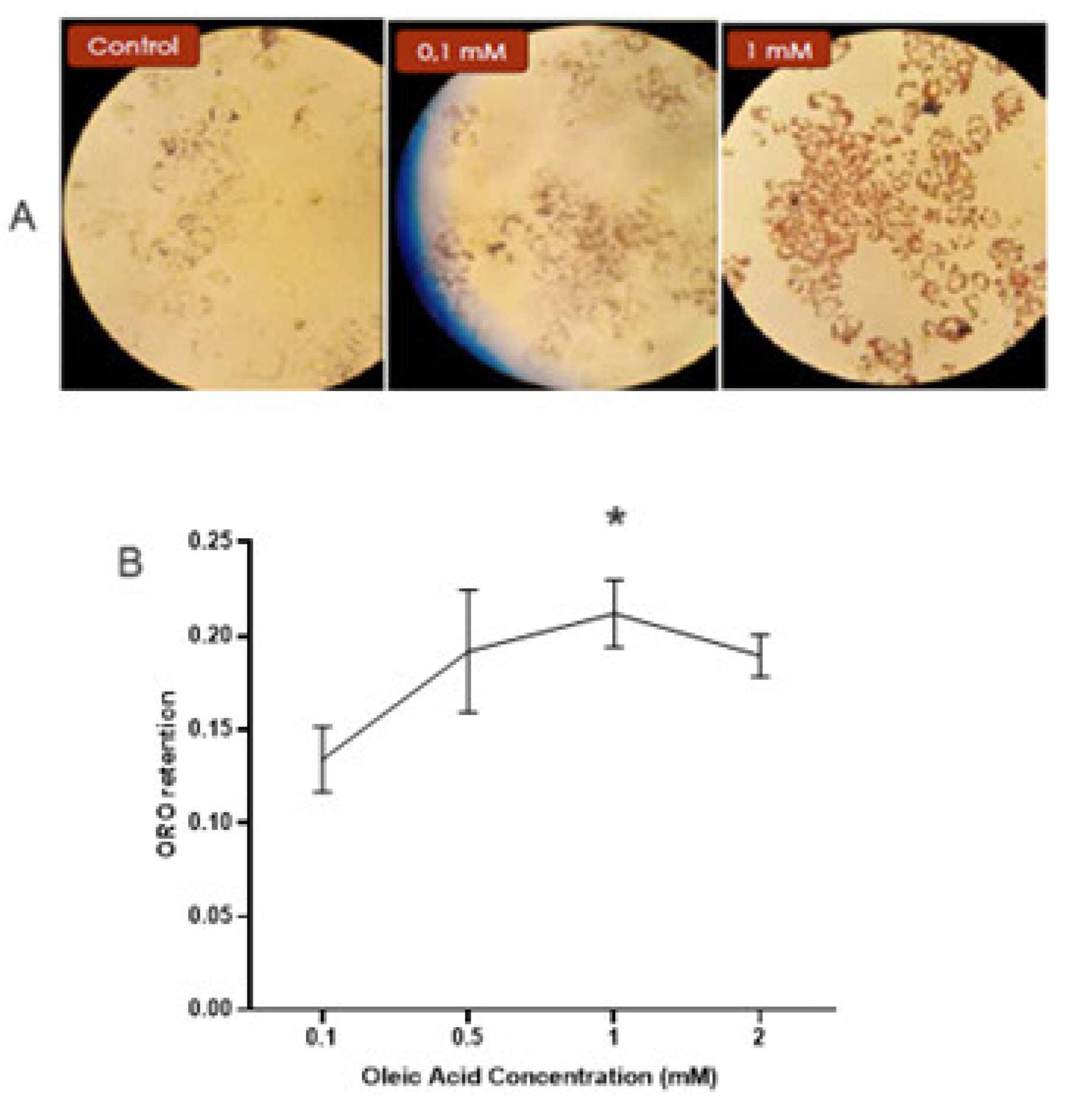
MASLD was developed by adding different oleic acid concentrations to Hep-G2 cells for 48 h. The neutral lipid content was assessed by staining the cells with Oil Red O and photographed (
Figure 4a). Afterward, colorant retention was determined by extracting it with isopropyl alcohol. No significant difference was observed between the control cells and the 0.1 mM Oleic Acid-treated cells (0.128 ± 0.019 [control] vs. 0.134 ± 0.017 [0.1 mM Oleic Acid]). The maximum lipid content was observed with 1mM Oleic Acid (0.212 ± 0.018 [1 mM] vs 0.134 ± 0.017 [0.1 mM], p <0.01), as shown in
Figure 4b.
Since it has been described that oleic acid can produce a cytotoxic effect during MASLD model development, we assessed the extracellular concentrations of two of the main liver enzymes, alanine transaminase (ALT) and aspartate transaminase (AST), to evaluate a possible protective effect of the infusions against cellular damage. Oleic acid and the infusions (CS or BA) were added to the cells, and after 48 h, the conditioned medium was collected to determine the enzyme activities. The activity of the oleic acid treatment (model) was evaluated and set to 100. Citrus sinensis (CS infusion) was effective in decreasing the release of both enzymes (63.1 % [ALT] and 39.7 % [AST] compared to no-treated model) while Baccharis articulate (BA infusion) only showed a significant effect on AST (54.6% compared to no-treated model).
2.4.2. Infusion Effect on MASLD Model
After incubating the Hep-G2 cells with both Oleic Acid and infusions (CS or BA), the intracellular neutral lipid content was measured with Oil Red O staining, and then the photographs were analyzed using the image analysis software ImageJ (
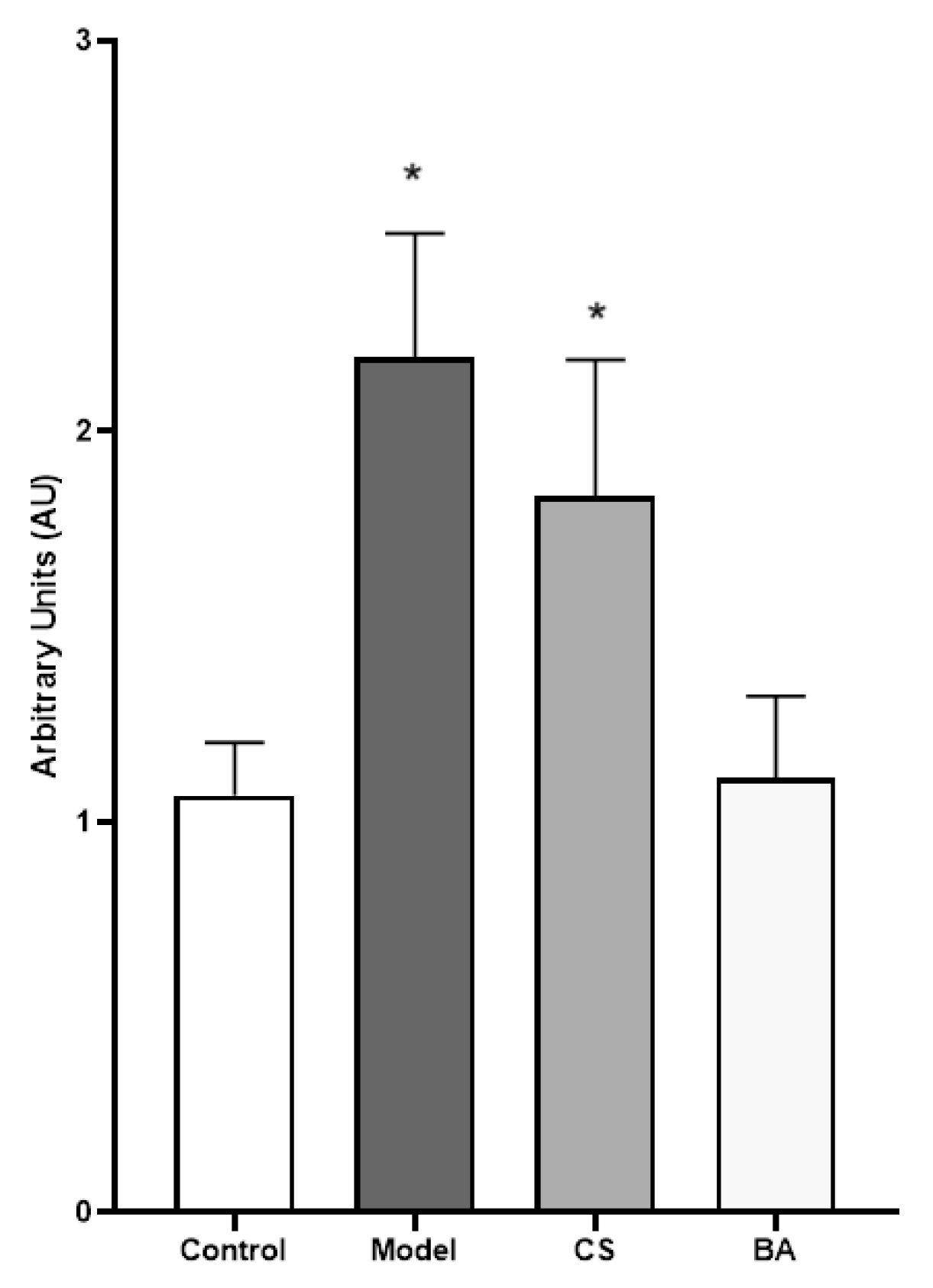
Figure 5). The model cells showed twice the lipid content than the control (1.07 ± 0.14 AU [control] vs. 2.19 ± 0.32 AU [model], p<0.05). The CS infusion did not show a significant difference from the model. On the other hand, the BA infusion inhibited almost 50% of the lipid droplet content (1.11 ± 0.21 AU [BA infusion] vs. 2.19 ± 0.32 AU [model], p < 0.05).
3. Discussion
As previously mentioned, there are currently no specific treatments for MASLD. However, due to the nature of this disease, where progress is mainly driven by cellular damage caused by oxidative stress, the use of antioxidants to treat it is a therapeutic alternative of current importance and interest [
7].
Among the antioxidants that have been studied, one of the greatest clinical interests is N-acetylcisteine (NAC), a drug that has been previously demonstrated to inhibit lipid accumulation in adipocytes [
15,
16,
17,
18,
19]. It is a precursor in the synthesis of glutathione (GSH), one of the key molecules of ROS processing. It can be directly oxidized by several free radicals; NAC effects on liver diseases can be considered responsible for the degradation of ROS generated during the development of MASLD [
11]. Here, we used natural extracts obtained using the CCRD approach. It has recently been shown that an aqueous infusion from
Hura crepitans L had a better antioxidant capacity than extracts obtained with methanol [
20], which are generally used for extractions of compounds such as polyphenols or flavonoids present in raw material. Here, aqueous extractions were carried out at high temperatures, trying to mimic a possible use of these raw materials in the hands of consumers. It is noteworthy that, in our hands, the experimental temperatures were higher than 50°C and we did not observe significant differences in the antioxidant capacity in our extracts with different incubation temperatures. On the contrary, Vasallo et al. showed significant differences in the obtained extracts’ antioxidant capacity according to the treatment temperature between 20°C and 100°C, suggesting that lower temperatures are not convenient for extraction procedures. Considering the CCRD results, we decided to use 70°C as the temperature to obtain our aqueous infusions. Both infusions showed a high antioxidant capacity compared to the N-acetycysteine control and other reports. Since most compounds of
Citrus sinensis or
Baccharis articulata leaves can be extracted with alcohol solvents, few articles have evaluated aqueous extracts. Regarding
Citrus sinensis, one of them showed almost 14% scavenging activity for DPPH using one-third of the concentration employed in this study [
21]. Meanwhile, regarding
Baccharis trimera leaves, using more than twice the concentration that we used for aqueous extraction produced almost 70% DPPH radical scavenging activity [
22], suggesting that our approach achieved similar results to those previously published.
Although significant differences were observed in the protein and flavonoid contents between the infusions, no significant differences were observed in the polyphenol contents. HPLC showed that both infusions presented the phenolic compounds of gallic and benzoic acid, while only
Baccharis articulata showed flavonoid contents such as luteolin and quercetin, coinciding with a higher flavonoid content in this infusion compared to
Citrus sinensis. Other reports indicate that
Baccharis articulata extracts are mainly composed of flavonoids [
23], coinciding with our results. Several studies have shown that flavonoids have powerful antioxidant properties, which could be related to their chemical structure, giving them an excellent capacity to capture free radicals [
24,
25].
The MASLD model was developed using oleic acid as an inducer. Because of the lipid accumulation in the cells, oxidative stress can be expected, with cellular damage as a consequence. Therefore, both ALT and AST enzymes were released during the experiments. We observed that both infusions reverted an oleic effect on the release of hepatic enzymes, suggesting the hepatic protector effect of the infusions used here. Nevertheless, only the
Baccharis articulata infusion significantly decreased the lipid content in the model. It was recently reported that
Baccharis trimera leaf aqueous extract could suppress lipid accumulation in two different models [
23,
26]. Concerning the relationship between the antioxidant capacity of these infusions and their effects on the MASLD model, we speculate that flavonoids present in this infusion could be important in producing an inhibitory effect on lipid accumulation. Based on this, it has been reported that flavonoids, such as quercetin, could affect lipid metabolism by inhibiting lipogenesis and reducing oxidative stress in obesity animal models [
27]. It has also been reported that extracts with high concentrations of flavonoids improved dyslipidemia in patients with metabolic syndrome [
28].
We obtained Citrus sinensis and Baccharis articulata infusions with high antioxidant capacity, which mainly depends on the weight of the leaves used for incubation to obtain them. Only the Baccharis articulata infusion was effective in decreasing the lipid content in the model. We suggest that flavonoids could be the compounds related to the effect on liver cells. Our approach to developing aqueous incubation at a high temperature over a short duration could bring a possible consumer of these plants closer.
4. Materials and Methods
Reagents and Cells
Gallic acid and rutin were purchased from Sigma Aldrich, Inc., USA. The human Hep-G2 cell line was obtained from the American Type Culture Collection (MD, USA).
Infusions Preparation
Leaves from Citrus sinesis and Baccharis articulata were collected in Buenos Aires Province, Argentina (latitude 34°28’16´´S, longitude 58°45´29´´W), during the vegetative phase; they were identified as such by botanical faculty members. The plant material was air-dried and ground smaller than 500 µm. The obtained powder was used immediately for the extraction process. Extracts were prepared by incubating 0.08-0.8 g of powdered leaves suspended in 10mL of PBS (phosphate-buffered saline 50 mM, pH 7.4) at 50-90°C for 2-30 min. After the incubation, the infusions were filtered through a 22 µm membrane and stored at -20°C until use.
Experimental Design
In order to investigate the extraction conditions for maximizing the antioxidant capacity of Citrus sinensis infusions within a range that reflects consumer practices, a central composite rotatable design (CCRD) was employed. This design strategy was utilized with axial points at ±1.68 and included 17 experimental runs with 3 replicates at the center point. The CCRD allowed for a comprehensive evaluation of the effects of three independent variables: the incubation time (6.8-21 min), extraction temperature (58-82 °C), and extract concentration (the weight-to-volume ratio of the plant material, 10-22 mg of leaf powder/mL PBS).
To analyze the effects of the independent variables and their interactions on the dependent variable (antioxidant capacity), the response surfaces and regression analysis were generated using the Design-Expert Program (version 11). The significance of each variable and interaction was assessed using the p-value. To visualize the relationships among the variables, response surface plots were generated, offering a graphical representation of the complex interactions. The AC data were analyzed using an exponential regression model to estimate the ideal extract concentration in the Design-Expert Program (version 11).
Subsequently, the best conditions obtained for Citrus sinensis were applied to Baccharis articulata.
Antioxidant Capacity Evaluation
The infusion antioxidant capacity was determined using a 2,2-difenil-1 picrilhidrazil (DPPH) assay [
29], and the results were expressed as percentages of DPPH free radical scavenging by the samples. NAC was used as an antioxidant control.
Infusion Characterization
The obtained infusions were chemically characterized. The total protein concentration was quantified using the Bradford assay [
30]; the total flavonoid concentration was quantified using the aluminum chloride procedure [
31], using rutin (1mg/mL) as the standard solution; the total polyphenol content was quantified using the colorimetric Folin technique [
32], using gallic acid (0.1mg/mL) as the standard solution.
HPLC Determinations
The samples were analyzed using AKTA Purify Amersham Bioscience Limited, employing a 5µ C18 reverse-phase analytical column (250 x 4.6 mm), an injection volume of 100 µl, total flow of 1 ml/min, and a binary gradient elution of two solvents, H2O + trifluoro acetic acid 0.1% (solvent A) and acetonytril + trifluoro acetic acid 0.1% (solvent B).
The first step of the gradient elution program was 6%-10% of solvent B for 15 minutes. The second step of the gradient followed 10%-22% of solvent B over the next 17 minutes. In the third step, 22% of solvent B was isocratically held for 15 minutes. The HPLC elution was detected at a wavelength of 280 nm. The column was washed with 100% of solvent B to ensure no cross-contamination between the samples. The chromatographic data obtained were evaluated with UnicornTM system software. Peak integration was used to identify and measure the retention time, area, and height of each component.
Toxicity Assay
The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) cell viability assay was used to evaluate the toxicity of the infusions on Hep-G2 cells. This technique is based on the reduction of the MTT by mitochondrial enzymes present in viable cells; the product of this reduction generates a purple product that is measured at 550 nm [
34]. Briefly, the cell media were changed to media containing MTT (1 mg/mL), and the cells were incubated for 1h. Afterward, the media was removed, and the cells were treated with ethanol for 10 min to extract the remaining dye into the cells. Then, the absorbance was measured at 550 nm. The untreated cells and infusion-treated cells were evaluated. Then, 50mM of H
2O
2 was used as a toxic control.
Oil Red O Staining and Lipid Quantification
The Oil Red O technique was used as described [
35]. Briefly, cells were washed 3 times with PBS and then fixed with 4% formaldehyde for 30 min. The cells were washed with PBS and then stained with 0.4% Oil Red O (ORO) solution (Sigma Aldrich, Inc., USA) for 30 min at room temperature. After staining, the cells were washed with water. The stained lipid droplets were observed under a microscope and photographed. Droplet quantification was obtained from the photographs using ImageJ software (
http://imagej.nih.gov/ij). Then, the dye was extracted with isopropanol, and its absorbance was determined at 510 nm to quantify the stained lipids. Four independent experiments were evaluated.
The untreated cells, oleic acid-treated cells, and oleic acid plus infusion-treated cells were evaluated. The infusions’ effects on intracellular lipid accumulation were determined by incubating the cells with both Oleic Acid (1 mM) and infusions (CS or BA) for 48 h. CS: Citrus sinensis infusion (14 µg RE/mL); BA: Baccharis articulate infusion (43 µg RE/mL)
Hepatic Enzyme Determination
The enzyme release of alanine transaminase (ALT) and aspartate transaminase (AST) from the cells was determined using a commercial kit (Wiener SRL, Argentina). The conditioned media from the cells were collected, and the activity of the enzymes was measured. The conditioned media from the oleic acid-treated cells (model) and oleic acid plus infusion-treated cells were evaluated. The infusions’ effects were determined by incubating the cells with both Oleic Acid (1 mM) and infusions (CS or BA) for 48 h. CS: Citrus sinensis infusion (14 µg RE/mL); BA: Baccharis articulate infusion (43 µg RE/mL). The model (oleic acid treated cells) absorbance was considered as 100%.
Statistical Analysis
All the results are expressed as the mean ± standard deviation from 3 or 4 independent experiments. Statistical analysis was performed using one-way analysis of variance (ANOVA).
Author Contributions
Investigation and data curation, Francisco Gualdieri; investigation and methodology, Gabriela Rocha; investigation and data curation Ruben Iacono; funding acquisition, review the original draft, Mauricio De Marzi; conceptualization, methodology, funding acquisition, supervision and original draft preparation, Liliana N. Guerra. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: This research was funded by UNIVERSIDAD NACIONAL DE LUJAN, DEP. CIENCIAS BÁSICAS, DISPCD-CBLUJ 243-21 and UNIVERSIDAD NACIONAL DE LUJÁN, RESREC-LUJ 254-22
Institutional Review Board Statement
Not applicable
Data Availability Statement
Data are contained within the article
Acknowledgments
We appreciate Gonzalo Vicente for his participation in provision of plant sources.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Finucane, M. M.; Stevens, G. A.; Cowan, M. J.; Danaei, G.; Lin, J. K.; Paciorek, C. J.; Singh, G. M.; Gutierrez, H. R.; Lu, Y.; Bahalim, A. N.; Farzadfar, F.; Riley, L. M.; Ezzati, M. National, Regional, and Global Trends in Body-Mass Index since 1980: Systematic Analysis of Health Examination Surveys and Epidemiological Studies with 960 Country-Years and 9·1 Million Participants. The Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M. E.; Sookoian, S. From NAFLD to MASLD: Updated Naming and Diagnosis Criteria for Fatty Liver Disease. Journal of Lipid Research 2024, 65. [Google Scholar] [CrossRef] [PubMed]
- Whalley, S.; Puvanachandra, P.; Desai, A.; Kennedy, H. Hepatology Outpatient Service Provision in Secondary Care: A Study of Liver Disease Incidence and Resource Costs. Clinical Medicine 2007, 7, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A. J.; Brunt, E. M.; Kleiner, D. E.; Kowdley, K. V.; Chalasani, N.; Lavine, J. E.; Ratziu, V.; McCullough, A. Endpoints and Clinical Trial Design for Nonalcoholic Steatohepatitis. Hepatology 2011, 54, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Day, C. P.; James, O. F. W. Steatohepatitis: A Tale of Two “Hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Buqué, X.; Aspichueta, P.; Ochoa, B. Fundamento Molecular de La Esteatosis Hepática Asociada a La Obesidad. Rev. esp. enferm. dig. 2008, 100. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. IJMS 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B. A. Non-Alcoholic Fatty Liver Disease. BMC Med 2017, 15. [Google Scholar] [CrossRef]
- Polyzos, S. A.; Kountouras, J.; Mantzoros, C. S. Obesity and Nonalcoholic Fatty Liver Disease: From Pathophysiology to Therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef]
- Chan, W.-K.; Chuah, K.-H.; Rajaram, R. B.; Lim, L.-L.; Ratnasingam, J.; Vethakkan, S. R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. Journal of Obesity & Metabolic Syndrome 2023, 32, 197–213. [Google Scholar] [CrossRef]
- De Andrade, K.; Moura, F.; Dos Santos, J.; De Araújo, O.; De Farias Santos, J.; Goulart, M. Oxidative Stress and Inflammation in Hepatic Diseases: Therapeutic Possibilities of N-Acetylcysteine. IJMS 2015, 16, 30269–30308. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Dang, L.H; Ha, T.K.; Pham, H.T.; Lee, B.W.; Oh, W.K. Flavone Glycosides from Sicyos Angulatus and Their Inhibitory Effects on Hepatic Lipid Accumulation. Phytochem 2019, 157, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Sayuti, N.H.; Kamarudin, A.A; Saad, N.; Razak, N.A.; Esa, N.M. Optimized Green Extraction Conditions of Matcha Green Tea (Camellia Sinensis) Using Central Composite Design for Maximal Polyphenol and Antioxidant Contents. 2021, 16, 3255–3271. 16.
- Da Silveira, T. F. F.; Meinhart, A. D.; Ballus, C. A.; Godoy, H. T. The Effect of the Duration of Infusion, Temperature, and Water Volume on the Rutin Content in the Preparation of Mate Tea Beverages: An Optimization Study. Food Research International 2014, 60, 241–245. [Google Scholar] [CrossRef]
- Calzadilla, P.; Sapochnik, D.; Cosentino, S.; Diz, V.; Dicelio, L.; Calvo, J. C.; Guerra, L. N. N-Acetylcysteine Reduces Markers of Differentiation in 3T3-L1 Adipocytes. IJMS 2011, 12, 6936–6951. [Google Scholar] [CrossRef]
- Calzadilla, P.; Gómez-Serrano, M.; García-Santos, E.; Schiappacasse, A.; Abalde, Y.; Calvo, J. C.; Peral, B.; Guerra, L. N. N -Acetylcysteine Affects Obesity-Related Protein Expression in 3T3-L1 Adipocytes. Redox Report 2013, 18, 210–218. [Google Scholar] [CrossRef]
- Pieralisi, A.; Martini, C.; Soto, D.; Vila, M. C.; Calvo, J. C.; Guerra, L. N. N-Acetylcysteine Inhibits Lipid Accumulation in Mouse Embryonic Adipocytes. Redox Biology 2016, 9, 39–44. [Google Scholar] [CrossRef]
- Soto, D.; Gomez-Serrano, M.; Pieralisi, A.; Calvo, J. C.; Peral, B.; Guerra, L. N. N -Acetylcysteine Inhibits Kinase Phosphorylation during 3T3-L1 Adipocyte Differentiation. Redox Report 2017, 22, 265–271. [Google Scholar] [CrossRef]
- Soto, D.; Martini, C.; Frontera, E.; Montaldo, L.; Vila, M. C.; Calvo, J. C.; Guerra, L. N. N-Acetylcysteine Inhibits Lipids Production in Mature Adipocytes through the Inhibition of Peroxisome Proliferator-Activated Receptor γ. IJBcRR 2020, 17–29. [Google Scholar] [CrossRef]
- Vassallo, A.; Armentano, M. F.; Miglionico, R.; Caddeo, C.; Chirollo, C.; Gualtieri, M. J.; Ostuni, A.; Bisaccia, F.; Faraone, I.; Milella, L. Hura Crepitans L. Extract: Phytochemical Characterization, Antioxidant Activity, and Nanoformulation. Pharmaceutics 2020, 12. [Google Scholar] [CrossRef]
- Arrey Tarkang, P.; Nwachiban Atchan, A. P.; Kuiate, J.-R.; Okalebo, F. A.; Guantai, A. N.; Agbor, G. A. Antioxidant Potential of a Polyherbal Antimalarial as an Indicator of Its Therapeutic Value. Advances in Pharmacological Sciences 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Souza, F. R. M.; Silva, G. M. M.; Cadavid, C. O. M.; Lisboa, L. D. S.; Silva, M. M. C. L.; Paiva, W. S.; Ferreira, M. J. P.; De Paula Oliveira, R.; Rocha, H. A. O. Antioxidant Baccharis Trimera Leaf Extract Suppresses Lipid Accumulation in C. Elegans Dependent on Transcription Factor NHR-49. Antioxidants 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Simões-Pires, C. A.; Queiroz, E. F.; Henriques, A. T.; Hostettmann, K. Isolation and On-Line Identification of Anti-Oxidant Compounds from threeBaccharis Species by HPLC-UV-MS/MS with Post-Column Derivatisation. Phytochem. Anal. 2005, 16, 307–314. [Google Scholar] [CrossRef]
- Olszowy, M. What Is Responsible for Antioxidant Properties of Polyphenolic Compounds from Plants? Plant Physiology and Biochemistry 2019, 144, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Arce, E.; Saldías, M. Antioxidant Properties of Flavonoid Metal Complexes and Their Potential Inclusion in the Development of Novel Strategies for the Treatment against Neurodegenerative Diseases. Biomedicine & Pharmacotherapy 2021, 143, 112236. [Google Scholar] [CrossRef]
- De Souza Marinho Do Nascimento, D.; Oliveira, R.; Camara, R.; Gomes, D.; Monte, J.; Costa, M.; Fernandes, J.; Langassner, S.; Rocha, H. Baccharis Trimera (Less.) DC Exhibits an Anti-Adipogenic Effect by Inhibiting the Expression of Proteins Involved in Adipocyte Differentiation. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Van De Wier, B.; Koek, G. H.; Bast, A.; Haenen, G. R. M. M. The Potential of Flavonoids in the Treatment of Non-Alcoholic Fatty Liver Disease. Critical Reviews in Food Science and Nutrition 2017, 57, 834–855. [Google Scholar] [CrossRef]
- Tun, S.; Spainhower, C. J.; Cottrill, C. L.; Lakhani, H. V.; Pillai, S. S.; Dilip, A.; Chaudhry, H.; Shapiro, J. I.; Sodhi, K. Therapeutic Efficacy of Antioxidants in Ameliorating Obesity Phenotype and Associated Comorbidities. Front. Pharmacol. 2020, 11, 1234. [Google Scholar] [CrossRef]
- Sharma, O. P.; Bhat, T. K. DPPH Antioxidant Assay Revisited. Food Chemistry 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Bradford, M. M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. 1976, 72, 248–254.
- Magalhães, L. M.; Almeida, M. I. G. S.; Barreiros, L.; Reis, S.; Segundo, M. A. Automatic Aluminum Chloride Method for Routine Estimation of Total Flavonoids in Red Wines and Teas. Food Anal. Methods 2012, 5, 530–539. [Google Scholar] [CrossRef]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M. J. Rapid Determination of Polyphenols and Vitamin C in Plant-Derived Products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- Cui, W.; Chen, S. L.; Hu, K.-Q. Quantification and Mechanisms of Oleic Acid-Induced Steatosis in HepG2 Cells. Am J Transl Res 2010, 2, 95–104. [Google Scholar] [PubMed]
- Frontera, E.; Desimone, M. F.; Marzi, M. C. D.; Guerra, L. N. N-Acetylcysteine Delivery With Silica Nanoparticles Into 3T3-L1 Adipocytes. Ther. Deliv. 2021, 12, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Montaldo, L.; Gallo, A.; Rocha, G.; Csernoch, C.; Marzi, M. D.; Guerra, L. N. Anthocyanin-Enriched Extract from Ribes Nigrum Inhibits Triglyceride and Cholesterol Accumulation in Adipocytes. Ther. Deliv. 2023, 14, 675–687. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Response surfaces for Antioxidant Capacity of Citrus sinensis infusions (DPPH Inhibition%). 1a: Temperature (°C) x Time (minutes) at fixed extract concentration (16mg/ mL); 1b: Extract Concentration (mg/mL) x Time (minutes) at fixed Temperature (70°C).
Figure 1.
Response surfaces for Antioxidant Capacity of Citrus sinensis infusions (DPPH Inhibition%). 1a: Temperature (°C) x Time (minutes) at fixed extract concentration (16mg/ mL); 1b: Extract Concentration (mg/mL) x Time (minutes) at fixed Temperature (70°C).
Figure 2.
HPLC profiles at 280 nm. HPLC profiles at wavelength detection of 280 nm. C18 reverse-phase analytical column was employed with binary gradient mode (water-acetonitril), injection volume 100ul and total flow 1 ml/min. (A) Standard mixture with Gallic Acid (Gal) and Rutin (Rut), (B) Citrus sinensis infusion and (C) Baccharis articulata infusion are shown. Active compounds of the infusions were identified. Gallic acid (1), Benzoic acid (2 and 4), Luteolin (3), Quercetin (5).
Figure 2.
HPLC profiles at 280 nm. HPLC profiles at wavelength detection of 280 nm. C18 reverse-phase analytical column was employed with binary gradient mode (water-acetonitril), injection volume 100ul and total flow 1 ml/min. (A) Standard mixture with Gallic Acid (Gal) and Rutin (Rut), (B) Citrus sinensis infusion and (C) Baccharis articulata infusion are shown. Active compounds of the infusions were identified. Gallic acid (1), Benzoic acid (2 and 4), Luteolin (3), Quercetin (5).
Figure 3.
Cell viability assay evaluation with infusions: (1) Untreated cells (maximum viability control), (2) 50 mM of hydrogen peroxide (toxicity control), (3) Citrus sinensis (140µg RE/mL), (4) Citrus sinensis (CS infusion: 14 µg RE/mL), (5) Baccharis articulate (430µg RE/mL), (6) Baccharis articulate (BA infusion: 43 µg RE/mL). The absorbance of the untreated cells was evaluated and set to 100. Each bar represents the mean ± SD of a triplicate experiment, and each result was compared to the untreated cells (1). SD: Standard deviation. * p < 0.05. RE: rutin equivalent.
Figure 3.
Cell viability assay evaluation with infusions: (1) Untreated cells (maximum viability control), (2) 50 mM of hydrogen peroxide (toxicity control), (3) Citrus sinensis (140µg RE/mL), (4) Citrus sinensis (CS infusion: 14 µg RE/mL), (5) Baccharis articulate (430µg RE/mL), (6) Baccharis articulate (BA infusion: 43 µg RE/mL). The absorbance of the untreated cells was evaluated and set to 100. Each bar represents the mean ± SD of a triplicate experiment, and each result was compared to the untreated cells (1). SD: Standard deviation. * p < 0.05. RE: rutin equivalent.
Figure 4.
Oil Red O-stained neutral lipid in Hep-G2 cells. The lipid accumulation was evaluated after applying different concentrations of oleic acid to the cells. (A) Lipid droplets were stained with Oil Red O and photographed; representative results from one of four experiments with similar results are shown. (B) The Oil Red O content was extracted from the cells, and the absorbance was determined at 510 nm. Each point represents the mean ± SD of a quadruplicate experiment, and each result was compared to 0.1 mM of oleic acid. SD: Standard deviation. p < 0.01.
Figure 4.
Oil Red O-stained neutral lipid in Hep-G2 cells. The lipid accumulation was evaluated after applying different concentrations of oleic acid to the cells. (A) Lipid droplets were stained with Oil Red O and photographed; representative results from one of four experiments with similar results are shown. (B) The Oil Red O content was extracted from the cells, and the absorbance was determined at 510 nm. Each point represents the mean ± SD of a quadruplicate experiment, and each result was compared to 0.1 mM of oleic acid. SD: Standard deviation. p < 0.01.
Figure 5.
Oil Red O-stained neutral lipids in Hep-G2 cells. The lipid accumulation after 48 h treatments was evaluated by staining the lipid droplets with Oil Red O: untreated cells (control), Oleic Acid treatment (model), Oleic Acid and CS infusion treatment (CS), Oleic Acid and BA infusion (BA). The absorbance of the control was evaluated and set to 100. Each bar represents the mean ± SD of a quadruplicate experiment, and each result was compared to the untreated cells (Control). SD: Standard deviation. * p < 0.05.
Figure 5.
Oil Red O-stained neutral lipids in Hep-G2 cells. The lipid accumulation after 48 h treatments was evaluated by staining the lipid droplets with Oil Red O: untreated cells (control), Oleic Acid treatment (model), Oleic Acid and CS infusion treatment (CS), Oleic Acid and BA infusion (BA). The absorbance of the control was evaluated and set to 100. Each bar represents the mean ± SD of a quadruplicate experiment, and each result was compared to the untreated cells (Control). SD: Standard deviation. * p < 0.05.
Table 1.
Central Composite Rotatable Design (CCRD) Matrix and its Relationship with the Experimental Response.
Table 1.
Central Composite Rotatable Design (CCRD) Matrix and its Relationship with the Experimental Response.
| Run |
Codified variables |
Decodified variables |
Response |
| |
A:Time |
B:Temperature |
C:Extract Concentration |
A |
B |
C |
AC: DPPH Inhibition |
| (minutes) |
(°C) |
(mg/mL) |
(minutes) |
(°C) |
(mg/mL) |
(%) |
| 1 |
1 |
1 |
-1 |
21 |
82 |
10 |
16 |
| 2 |
-1 |
1 |
1 |
6,8 |
82 |
22 |
37 |
| 3 |
1 |
-1 |
1 |
21 |
58 |
22 |
38 |
| 4 |
-1 |
1 |
-1 |
6,8 |
82 |
10 |
18 |
| 5 |
0 |
0 |
-1,68 |
13,9 |
70 |
5,9 |
10 |
| 6 |
0 |
0 |
0 |
13,9 |
70 |
16 |
43 |
| 7 |
1 |
1 |
1 |
21 |
82 |
22 |
39 |
| 8 |
0 |
-1,68 |
0 |
13,9 |
49,8 |
16 |
22 |
| 9 |
1,68 |
0 |
0 |
25,8 |
70 |
16 |
17 |
| 10 |
-1 |
-1 |
-1 |
6,8 |
58 |
10 |
10 |
| 11 |
0 |
0 |
1,68 |
13,9 |
70 |
26 |
42 |
| 12 |
0 |
0 |
0 |
13,9 |
70 |
16 |
46 |
| 13 |
-1,68 |
0 |
0 |
1,9 |
70 |
16 |
18 |
| 14 |
1 |
-1 |
-1 |
21 |
58 |
10 |
14 |
| 15 |
0 |
1,68 |
0 |
13,9 |
90,2 |
16 |
20 |
| 16 |
-1 |
-1 |
1 |
6,8 |
58 |
22 |
28 |
| 17 |
0 |
0 |
0 |
13,9 |
70 |
16 |
32 |
Table 2.
Analysis of variance of the values for Antioxidant capacity of the infusions prepared in the experimental conditions.
Table 2.
Analysis of variance of the values for Antioxidant capacity of the infusions prepared in the experimental conditions.
| Factor |
Sum of square |
DF |
Mean square |
F value |
p valuea
|
| Regression |
2168,52 |
6 |
361,42 |
24,59 |
0.0003 |
| Residual |
263,71 |
10 |
26,37 |
|
|
| Lack of fit |
155,04 |
8 |
19,38 |
0,3567 |
0,8805 |
| Pure error |
108,67 |
2 |
54,33 |
|
|
| Total SS |
243,24 |
16 |
|
|
|
Table 3.
Significant regression coefficients for the coded variables of the quadratic model of the Antioxidant Capacity response.
Table 3.
Significant regression coefficients for the coded variables of the quadratic model of the Antioxidant Capacity response.
| Parameters |
Coefficient |
Standard error |
pa
|
| M |
Mean |
40,03 |
2.96 |
0.0003 |
| A |
Time |
0,90 |
1,39 |
0,5309 |
| B |
Temperature |
1,22 |
1,39 |
0,4013 |
| C |
Extract concentration |
10,09 |
1,39 |
<0.0001 |
| A² |
Time2
|
-7,04 |
1,53 |
0.0010 |
| B² |
Temperature2
|
-5,80 |
1,53 |
0.0035 |
| C² |
Extract concentration 2
|
-4,04 |
1,53 |
0.0248 |
Table 4.
Characterization of infusions.
Table 4.
Characterization of infusions.
| Sample |
DPPH inhibition (%) |
Proteins (g/mL) |
Polyphenols (GAE mg/mL) |
Flavonoids (RE mg/mL) |
|
Citrus sinensis infusion |
71.65 ± 2.19 |
0.526 ± 0.115 |
0.542 ± 0.163 |
0.14 ± 0.04 |
|
Baccharis articulate infusion |
51.48 ± 1.34 |
9.947 ± 0.115 |
0.427 ± 0.048 |
0.43 ± 0.09 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).