Submitted:
08 October 2024
Posted:
10 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Engineered Bacteria Experimental Studies
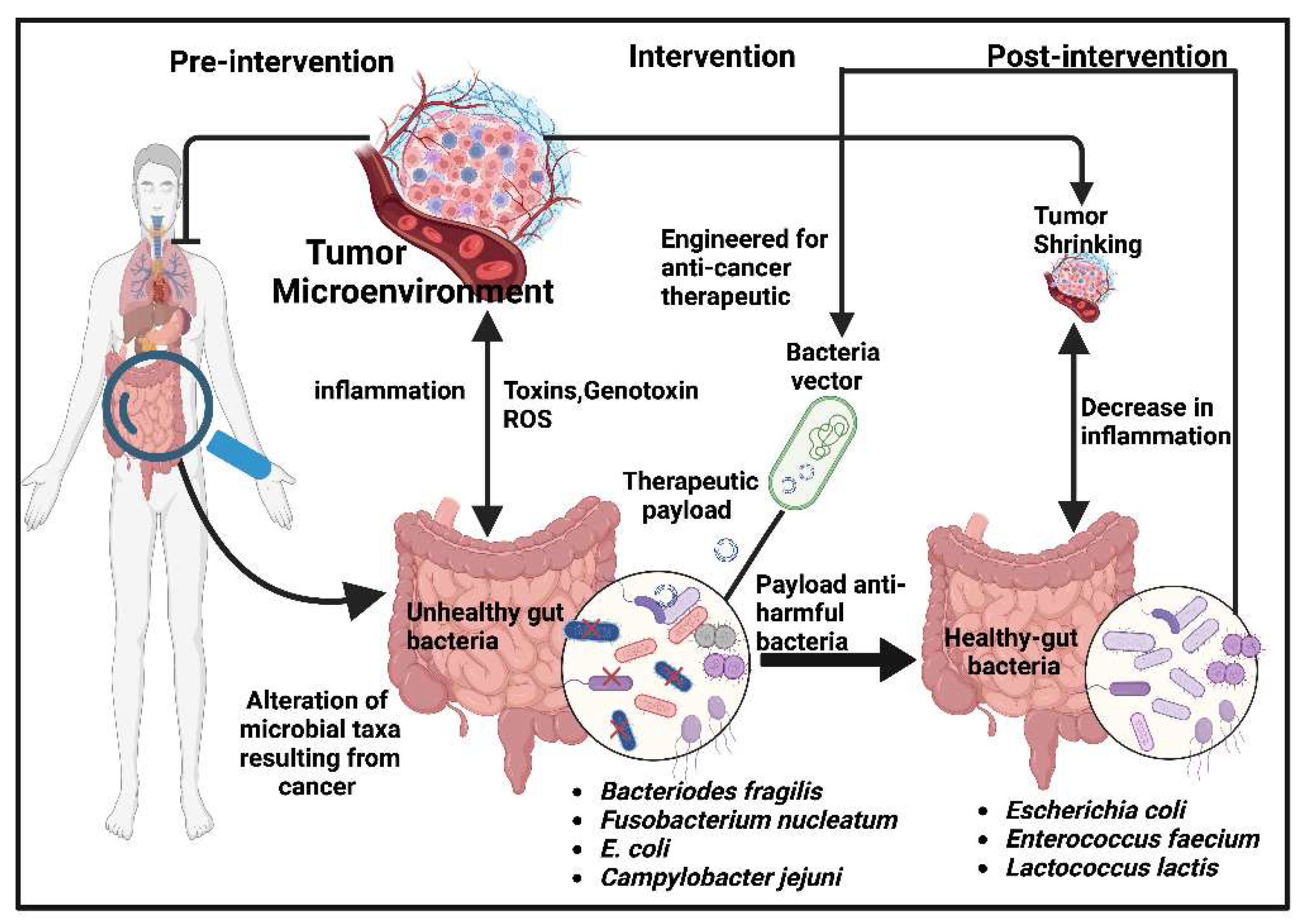
3. Gut Microbiome and Anti-Cancer Payload Delivery
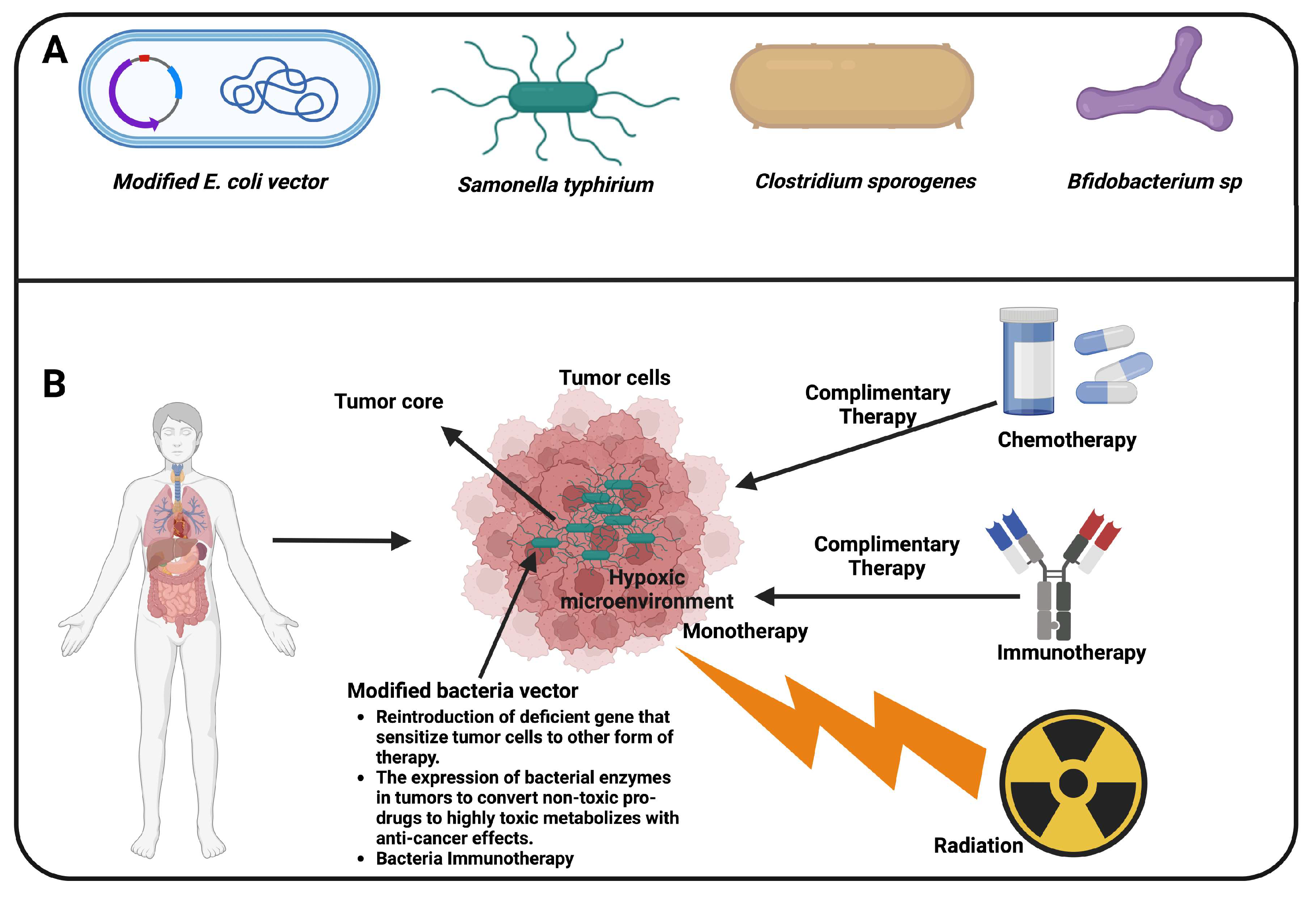
4. Strategies for the Application of Invasive Bacteria in Cancer Therapy
5. Perspective and Limitation, and Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- R. Gardlik, J.H. R. Gardlik, J.H. Fruehauf, Bacterial vectors and delivery systems in cancer therapy, IDrugs, 13 (2010) 701-706.
- B.F.-L. Sieow, K.S. B.F.-L. Sieow, K.S. Wun, W.P. Yong, I.Y. Hwang, M.W. Chang, Tweak to treat: reprograming bacteria for cancer treatment, Trends in cancer, 7 (2021) 447-464.
- A. Heggie, T.L. A. Heggie, T.L. Thurston, T. Ellis, Microbial messengers: nucleic acid delivery by bacteria, Trends in Biotechnology, (2024).
- C.K. Baban, M. C.K. Baban, M. Cronin, D. O’Hanlon, G.C. O’Sullivan, M. Tangney, Bacteria as vectors for gene therapy of cancer, Bioengineered bugs, 1 (2010) 385-394.
- I. Fajac, S. I. Fajac, S. Grosse, J.-M. Collombet, G. Thevenot, S. Goussard, C. Danel, C. Grillot-Courvalin, Recombinant Escherichia coli as a gene delivery vector into airway epithelial cells, Journal of controlled release, 97 (2004) 371-381.
- Z. Wang, W. Z. Wang, W. Sun, R. Hua, Y. Wang, Y. Li, H. Zhang, Promising dawn in tumor microenvironment therapy: engineering oral bacteria, International Journal of Oral Science, 16 (2024) 24.
- K. Yazawa, M. K. Yazawa, M. Fujimori, J. Amano, Y. Kano, S.i. Taniguchi, Bifidobacterium longum as a delivery system for cancer gene therapy: selective localization and growth in hypoxic tumors, Cancer gene therapy, 7 (2000) 269-274.
- J.H. Zheng, J.-J. J.H. Zheng, J.-J. Min, Targeted cancer therapy using engineered Salmonella typhimurium, Chonnam medical journal, 52 (2016) 173-184.
- J. Pahle, L. J. Pahle, L. Menzel, N. Niesler, D. Kobelt, J. Aumann, M. Rivera, W. Walther, Rapid eradication of colon carcinoma by Clostridium perfringens Enterotoxin suicidal gene therapy, BMC cancer, 17 (2017) 1-14.
- M. Yang, F. M. Yang, F. Yang, W. Chen, S. Liu, L. Qiu, J. Chen, Bacteria-mediated cancer therapies: opportunities and challenges, Biomaterials Science, 9 (2021) 5732-5744.
- C.-H. Lee, Engineering bacteria toward tumor targeting for cancer treatment: current state and perspectives, Applied microbiology and biotechnology, 93 (2012) 517-523.
- J.M. Pawelek, K.B. J.M. Pawelek, K.B. Low, D. Bermudes, Bacteria as tumour-targeting vectors, The lancet oncology, 4 (2003) 548-556.
- S. Zhou, C. S. Zhou, C. Gravekamp, D. Bermudes, K. Liu, Tumour-targeting bacteria engineered to fight cancer, Nature Reviews Cancer, 18 (2018) 727-743.
- D. Lin, X. D. Lin, X. Feng, B. Mai, X. Li, F. Wang, J. Liu, X. Liu, K. Zhang, X. Wang, Bacterial-based cancer therapy: An emerging toolbox for targeted drug/gene delivery, Biomaterials, 277 (2021) 121124.
- I.Y. Lin, T.T.H. I.Y. Lin, T.T.H. Van, P.M. Smooker, Live-attenuated bacterial vectors: tools for vaccine and therapeutic agent delivery, Vaccines, 3 (2015) 940-972.
- R.B. Mokhtari, T.S. R.B. Mokhtari, T.S. Homayouni, N. Baluch, E. Morgatskaya, S. Kumar, B. Das, H. Yeger, Combination therapy in combating cancer, Oncotarget, 8 (2017) 38022.
- N.P. Minton, Clostridia in cancer therapy, Nature Reviews Microbiology, 1 (2003) 237-242.
- J.R. Möse, G. J.R. Möse, G. Möse, Oncolysis by clostridia. I. Activity of Clostridium butyricum (M-55) and other nonpathogenic clostridia against the Ehrlich carcinoma, Cancer research, 24 (1964) 212-216.
- A. Osahor, K. A. Osahor, K. Deekonda, C.-W. Lee, E.U.-H. Sim, A. Radu, K. Narayanan, Rapid preparation of adherent mammalian cells for basic scanning electron microscopy (SEM) analysis, Analytical biochemistry, 534 (2017) 46-48.
- M. Larsen, U. M. Larsen, U. Griesenbach, S. Goussard, D. Gruenert, D. Geddes, R.a. Scheule, S. Cheng, P. Courvalin, C. Grillot-Courvalin, E. Alton, Bactofection of lung epithelial cells in vitro and in vivo using a genetically modified Escherichia coli, Gene therapy, 15 (2008) 434-442.
- C. Grillot-Courvalin, S. C. Grillot-Courvalin, S. Goussard, P. Courvalin, Bacteria as gene delivery vectors for mammalian cells, Horizontal Gene Transfer, (2002) 261-265.
- K. Narayanan, P.E. K. Narayanan, P.E. Warburton, DNA modification and functional delivery into human cells using Escherichia coli DH10B, Nucleic acids research, 31 (2003) e51-e51.
- D. Morrissey, G.C. D. Morrissey, G.C. O’Sullivan, M. Tangney, Tumour targeting with systemically administered bacteria, Current gene therapy, 10 (2010) 3-14.
- K.S. Allemailem, Innovative approaches of engineering tumor-targeting bacteria with different therapeutic payloads to fight cancer: A smart strategy of disease management, International Journal of Nanomedicine, (2021) 8159-8184.
- R.O. Akinsola, M. R.O. Akinsola, M. Adewoyin, C.-W. Lee, E.U.-H. Sim, K. Narayanan, RFP-based method for real-time tracking of invasive bacteria in a heterogeneous population of cells, Analytical biochemistry, 634 (2021) 114432.
- I.A. Khalil, K. I.A. Khalil, K. Kogure, H. Akita, H. Harashima, Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery, Pharmacological reviews, 58 (2006) 32-45.
- K. Narayanan, C.W. K. Narayanan, C.W. Lee, A. Radu, E.U.H. Sim, Escherichia coli bactofection using Lipofectamine, Analytical biochemistry, 439 (2013) 142-144.
- R.O. Akinsola, C.W. R.O. Akinsola, C.W. Lee, E.U.H. Sim, K. Narayanan, Inhibition of lysosomal vacuolar proton pump down-regulates cellular acidification and enhances E. coli bactofection efficiency, Analytical biochemistry, 616 (2021) 114088.
- S. Dimitriadis, L. S. Dimitriadis, L. Dova, I. Kotsianidis, E. Hatzimichael, E. Kapsali, G.S. Markopoulos, Imaging Flow Cytometry: Development, Present Applications, and Future Challenges, Methods and Protocols, 7 (2024) 28.
- J.P. Robinson, R. J.P. Robinson, R. Ostafe, S.N. Iyengar, B. Rajwa, R. Fischer, Flow cytometry: the next revolution, Cells, 12 (2023) 1875.
- R.O. Akinsola, Investigating the E. coli vector trafficking through the endo-lysosomal and autophagy pathways to improve its efficiency, Monash University.
- C.R. Pangilinan, C.-H. C.R. Pangilinan, C.-H. Lee, Salmonella-based targeted cancer therapy: updates on a promising and innovative tumor immunotherapeutic strategy, Biomedicines, 7 (2019) 36.
- K.M. Broadway, B.E. K.M. Broadway, B.E. Scharf, Salmonella typhimurium as an anticancer therapy: recent advances and perspectives, Current Clinical Microbiology Reports, 6 (2019) 225-239.
- K. Liang, Q. K. Liang, Q. Liu, P. Li, H. Luo, H. Wang, Q. Kong, Genetically engineered Salmonella Typhimurium: Recent advances in cancer therapy, Cancer letters, 448 (2019) 168-181.
- E. Dekaboruah, M.V. E. Dekaboruah, M.V. Suryavanshi, D. Chettri, A.K. Verma, Human microbiome: an academic update on human body site specific surveillance and its possible role, Archives of microbiology, 202 (2020) 2147-2167.
- S. Runge, S.P. S. Runge, S.P. Rosshart, The mammalian metaorganism: a holistic view on how microbes of all kingdoms and niches shape local and systemic immunity, Frontiers in Immunology, 12 (2021) 702378.
- V. Matson, C.S. V. Matson, C.S. Chervin, T.F. Gajewski, Cancer and the microbiome—influence of the commensal microbiota on cancer, immune responses, and immunotherapy, Gastroenterology, 160 (2021) 600-613.
- Y. Chen, J. Y. Chen, J. Zhou, L. Wang, Role and mechanism of gut microbiota in human disease, Frontiers in cellular and infection microbiology, 11 (2021) 625913.
- D. Zheng, T. D. Zheng, T. Liwinski, E. Elinav, Interaction between microbiota and immunity in health and disease, Cell research, 30 (2020) 492-506.
- L.J. Spielman, D.L. L.J. Spielman, D.L. Gibson, A. Klegeris, Unhealthy gut, unhealthy brain: The role of the intestinal microbiota in neurodegenerative diseases, Neurochemistry international, 120 (2018) 149-163.
- A.M. Barbosa, A. A.M. Barbosa, A. Gomes-Gonçalves, A.G. Castro, E. Torrado, Immune system efficiency in cancer and the microbiota influence, Pathobiology, 88 (2021) 170-186.
- P. Raoul, V. P. Raoul, V. De Gaetano, G. Sciaraffia, G. Ormea, M. Cintoni, C. Pozzo, A. Strippoli, A. Gasbarrini, M.C. Mele, E. Rinninella, Gastric Cancer, Immunotherapy, and Nutrition: The Role of Microbiota, Pathogens, 13 (2024) 357.
- E. Montassier, T. E. Montassier, T. Gastinne, P. Vangay, G. Al-Ghalith, S. Bruley des Varannes, S. Massart, P. Moreau, G. Potel, M. de La Cochetière, E. Batard, Chemotherapy-driven dysbiosis in the intestinal microbiome, Alimentary pharmacology & therapeutics, 42 (2015) 515-528.
- G. Goel, T. G. Goel, T. Requena, S. Bansal, Human-Gut Microbiome: Establishment and Interactions, Academic Press2022.
- C. Xing, Y. C. Xing, Y. Du, T. Duan, K. Nim, J. Chu, H.Y. Wang, R.-F. Wang, Interaction between microbiota and immunity and its implication in colorectal cancer, Frontiers in Immunology, 13 (2022) 963819.
- F. Taieb, C. F. Taieb, C. Petit, J.-P. Nougayrède, E. Oswald, The enterobacterial genotoxins: cytolethal distending toxin and colibactin, EcoSal plus, 7 (2016) 10.1128/ecosalplus. ESP-0008-2016.
- K. Hartl, M. K. Hartl, M. Sigal, Microbe-driven genotoxicity in gastrointestinal carcinogenesis, International Journal of Molecular Sciences, 21 (2020) 7439.
- S. Vivarelli, R. S. Vivarelli, R. Salemi, S. Candido, L. Falzone, M. Santagati, S. Stefani, F. Torino, G.L. Banna, G. Tonini, M. Libra, Gut microbiota and cancer: from pathogenesis to therapy, Cancers, 11 (2019) 38.
- H.H. Chen, M.T. H.H. Chen, M.T. Kuo, Improving radiotherapy in cancer treatment: Promises and challenges, Oncotarget, 8 (2017) 62742.
- A.R. Kumar, A.R. A.R. Kumar, A.R. Devan, B. Nair, B.S. Vinod, L.R. Nath, Harnessing the immune system against cancer: current immunotherapy approaches and therapeutic targets, Molecular biology reports, (2021) 1-21.
- W. Torres, V. W. Torres, V. Lameda, L.C. Olivar, C. Navarro, J. Fuenmayor, A. Pérez, A. Mindiola, M. Rojas, M.S. Martínez, M. Velasco, Bacteria in cancer therapy: beyond immunostimulation, J. Cancer Metastasis Treat, 4 (2018).
- P.S. Hegde, D.S. P.S. Hegde, D.S. Chen, Top 10 challenges in cancer immunotherapy, Immunity, 52 (2020) 17-35.
- R. Franzin, G.S. R. Franzin, G.S. Netti, F. Spadaccino, C. Porta, L. Gesualdo, G. Stallone, G. Castellano, E. Ranieri, The use of immune checkpoint inhibitors in oncology and the occurrence of AKI: where do we stand?, Frontiers in immunology, 11 (2020) 574271.
- S. Tan, D. S. Tan, D. Li, X. Zhu, Cancer immunotherapy: Pros, cons and beyond, Biomedicine & Pharmacotherapy, 124 (2020) 109821.
- M.A. Perazella, A.C. M.A. Perazella, A.C. Shirali, Immune checkpoint inhibitor nephrotoxicity: what do we know and what should we do?, Kidney International, 97 (2020) 62-74.
- Y. Liu, L. Y. Liu, L. Niu, N. Li, Y. Wang, M. Liu, X. Su, X. Bao, B. Yin, S. Shen, Bacterial-Mediated Tumor Therapy: Old Treatment in a New Context, Advanced Science, 10 (2023) 2205641.
- I. Castagliuolo, E. I. Castagliuolo, E. Beggiao, P. Brun, L. Barzon, S. Goussard, R. Manganelli, C. Grillot-Courvalin, G. Palu, Engineered E. coli delivers therapeutic genes to the colonic mucosa, Gene Therapy, 12 (2005) 1070-1078.
- L. Mohr, S. L. Mohr, S. Shankara, S.K. Yoon, T.U. Krohne, M. Geissler, B. Roberts, H.E. Blum, J.R. Wands, Gene therapy of hepatocellular carcinoma in vitro and in vivo in nude mice by adenoviral transfer of the Escherichia coli purine nucleoside phosphorylase gene, Hepatology, 31 (2000) 606-614.
- C. Cheng, Y. C. Cheng, Y. Lu, K. Chuang, W. Hung, J. Shiea, Y. Su, C. Kao, B. Chen, S. Roffler, T. Cheng, Tumor-targeting prodrug-activating bacteria for cancer therapy, Cancer gene therapy, 15 (2008) 393-401.
- J. Nemunaitis, C. J. Nemunaitis, C. Cunningham, N. Senzer, J. Kuhn, J. Cramm, C. Litz, R. Cavagnolo, A. Cahill, C. Clairmont, M. Sznol, Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients, Cancer gene therapy, 10 (2003) 737-744.
- J. Jiang, Y. J. Jiang, Y. Huang, Z. Zeng, C. Zhao, Harnessing engineered immune cells and bacteria as drug carriers for cancer immunotherapy, ACS nano, 17 (2023) 843-884.
- J.M. Brown, W.R. J.M. Brown, W.R. Wilson, Exploiting tumour hypoxia in cancer treatment, Nature Reviews Cancer, 4 (2004) 437-447.
- D.-H. Nguyen, A. D.-H. Nguyen, A. Chong, Y. Hong, J.-J. Min, Bioengineering of bacteria for cancer immunotherapy, nature communications, 14 (2023) 3553.
- D.C. Singleton, A. D.C. Singleton, A. Macann, W.R. Wilson, Therapeutic targeting of the hypoxic tumour microenvironment, Nature reviews Clinical oncology, 18 (2021) 751-772.
- Z. Chen, F. Z. Chen, F. Han, Y. Du, H. Shi, W. Zhou, Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions, Signal transduction and targeted therapy, 8 (2023) 70.
- S. Chowdhury, S. S. Chowdhury, S. Castro, C. Coker, T.E. Hinchliffe, N. Arpaia, T. Danino, Programmable bacteria induce durable tumor regression and systemic antitumor immunity, Nature medicine, 25 (2019) 1057-1063.
- A. Elsherbeny, H. A. Elsherbeny, H. Bayraktutan, U.C. Oz, C. Moloney, J.C. Ashworth, A.M. Grabowska, C. Alexander, Responsive Nanomaterial Delivery Systems for Pancreatic Cancer Management, Advanced Therapeutics, 7 (2024) 2300330.
- X. Xie, Y. X. Xie, Y. Zhang, F. Li, T. Lv, Z. Li, H. Chen, L. Jia, Y. Gao, Challenges and opportunities from basic cancer biology for nanomedicine for targeted drug delivery, Current cancer drug targets, 19 (2019) 257-276.
- K. Mugwanda, S. K. Mugwanda, S. Hamese, W.F. Van Zyl, E. Prinsloo, M. Du Plessis, L.M. Dicks, D.B. Thimiri Govinda Raj, Recent advances in genetic tools for engineering probiotic lactic acid bacteria, Bioscience Reports, 43 (2023) BSR20211299.
- E. Skippington, M.A. E. Skippington, M.A. Ragan, Lateral genetic transfer and the construction of genetic exchange communities, FEMS microbiology reviews, 35 (2011) 707-735.
- A. Chabloz, J.V. A. Chabloz, J.V. Schaefer, I. Kozieradzki, S.J. Cronin, D. Strebinger, F. Macaluso, J. Wald, T.H. Rabbitts, A. Plückthun, T.C. Marlovits, Salmonella-based platform for efficient delivery of functional binding proteins to the cytosol, Communications biology, 3 (2020) 342.
- M.N. Dharmasena, C.M. M.N. Dharmasena, C.M. Feuille, C.E.C. Starke, A.A. Bhagwat, S. Stibitz, D.J. Kopecko, Development of an acid-resistant Salmonella Typhi Ty21a attenuated vector for improved oral vaccine delivery, PloS one, 11 (2016) e0163511.
- A. Phalipon, P. A. Phalipon, P. Sansonetti, Live AttenuatedShigella flexneriMutants as Vaccine Candidates Against Shigellosis and Vectors for Antigen Delivery, Biologicals, 23 (1995) 125-134.
- S. Bashiardes, T. S. Bashiardes, T. Tuganbaev, S. Federici, E. Elinav, The microbiome in anti-cancer therapy, Seminars in immunology, Elsevier, 2017, pp. 74-81.
- J.S. Desgrosellier, D.A. J.S. Desgrosellier, D.A. Cheresh, Integrins in cancer: biological implications and therapeutic opportunities, Nature Reviews Cancer, 10 (2010) 9.
- P. Majumder, Integrin-mediated delivery of drugs and nucleic acids for anti-angiogenic cancer therapy: Current landscape and remaining challenges, Bioengineering, 5 (2018) 76.
- J. Nip, H. J. Nip, H. Shibata, D.J. Loskutoff, D.A. Cheresh, P. Brodt, Human melanoma cells derived from lymphatic metastases use integrin alpha v beta 3 to adhere to lymph node vitronectin, The Journal of clinical investigation, 90 (1992) 1406-1413.
- J.K. Slack-Davis, K.A. J.K. Slack-Davis, K.A. Atkins, C. Harrer, E.D. Hershey, M. Conaway, Vascular cell adhesion molecule-1 is a regulator of ovarian cancer peritoneal metastasis, Cancer research, 69 (2009) 1469-1476.
- C.N. Landen, T.-J. C.N. Landen, T.-J. Kim, Y.G. Lin, W.M. Merritt, A.A. Kamat, L.Y. Han, W.A. Spannuth, A.M. Nick, N.B. Jennnings, M.S. Kinch, Tumor-selective response to antibody-mediated targeting of αvβ3 integrin in ovarian cancer, Neoplasia, 10 (2008) 1259-1267.
- M. Adachi, T. M. Adachi, T. Taki, M. Higashiyama, N. Kohno, H. Inufusa, M. Miyake, Significance of integrin α5 gene expression as a prognostic factor in node-negative non-small cell lung cancer, Clinical cancer research, 6 (2000) 96-101.
- T. Hillman, Bacteriobot Drug-Liposome Carriers: An Optimization of Cancer-Drug Delivery to the Colon by Manipulating the Gut Microbiome, Nanoparticle, 1 (2019) 1-10.
- A. Sousa, A.N. A. Sousa, A.N. Phung, N. Škalko-Basnet, S. Obuobi, Smart delivery systems for microbial biofilm therapy: dissecting design, drug release and toxicological features, Journal of Controlled Release, 354 (2023) 394-416.
- J. El Andari, D. J. El Andari, D. Grimm, Production, processing, and characterization of synthetic AAV gene therapy vectors, Biotechnology journal, 16 (2021) 2000025.
- T.A. Beacham, J.B. T.A. Beacham, J.B. Sweet, M.J. Allen, Large scale cultivation of genetically modified microalgae: a new era for environmental risk assessment, Algal Research, 25 (2017) 90-100.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




