1. Introduction
The kiwifruit [(
Actinidia deliciosa (A. Chev.) C. F. Liang et A. R. Ferguson var.
deliciosa)] is a species of noteworthy agricultural and economic importance. Native to China, it is now grown globally because of its high nutritional content, excellent flavour, and health benefits [
1]. With an estimated value of 3 billion dollars worldwide, kiwifruit production has a relevant economic impact on the primary industry of the main producers. Italy is the third global producer, with a production of 523,120 tonnes harvested from 24,850 productive hectares [
2]. Half of the Italian production is concentrated in the Latium region with an area of 8,888 ha and 250,252 tons of fruit harvested in 2023 [
3]. As for many other fresh fruits and vegetables, the kiwifruit crop is affected by several pests and diseases that can potentially pose a risk to production sustainability. Due to its root anatomy and development [
4], kiwifruit is particularly susceptible to high temperatures [
5] and anaerobic conditions, particularly, waterlogging [
6,
7]. Early reports of damages caused by oomycetes proliferating in water-excess conditions have been documented [
8,
9,
10,
11]. Very recently, a new disorder of kiwifruit, named Kiwifruit Vine Decline Syndrome (KVDS) has been reported in Italy. Firstly reported in some orchards in Veneto region in 2012 [
12], it has quickly become of major concern in several kiwifruit areas along the country, causing significant yield reduction and even crop mortality [
13]. To the best of our knowledge, the disease remains confined to Italy, though similar vine decline disorders likely attributable to KVDS and linked to damage caused by oomycetes thriving in waterlogged conditions [
8,
9,
10,
11] have been recently reported in Turkey [
14,
15,
16,
17] and China [
18].
The symptomatology of KVDS is primarily evident in the root system of affected plants, showing widespread browning and large rotten portions with the presence of typical rat tails. The lack of absorbent roots leads to the loss of root functions, which can then impair the epigeal part of the plant, resulting in centripetal leaves drying, leaf drop, fruit fall, and ultimately plant death [
19]. Several oomycetes (e.g.,
Phytophthora spp.,
Pythium spp.,
Phytopythium spp.), fungi (e.g.,
Cylindrocarpon spp.,
Fusarium spp.,
Pyrenochaeta spp.) and bacteria (
Clostridium spp.,
Erwinia spp.), or their secondary metabolites produced, have been often associated with, or isolated from KVDS affected roots, but their role as the main cause of the disease has not yet been clearly demonstrated [
20,
21].
There is an intimate interaction between crops and soil microbial communities [
22,
23] which influences soil health. Soil microbiota, including bacteria, fungi, archaea, and other microorganisms, play a key role in soil functions, providing nutrients for plants, forming soil aggregates, decomposing organic matter, suppressing diseases, and mitigating climate change [
24,
25,
26,
27]. Soil microbial community diversity and abundance can lead to changes in nutrient cycling and soil organic matter dynamics [
28]. In the present work, we aimed to evaluate the impact of KVDS on soil health indicators and microbial communities in symptomatic and asymptomatic kiwifruit plants, by comparing seasonal differences in soil physical and biochemical parameters and their effect on the associated microbiota, including variations in biomass, activity, and composition.
2. Materials and Methods
2.1. Experimental Design and Soil Sampling
This study was conducted in two orchards located in Latium, Italy. Both orchards were subjected to integrated management, with similar farming techniques. Orchard 1 (O1) was in Lanuvio (south of the province of Rome, 41°37'14.2"N 12°43'06.0", O1S - 41°37'11.8"N 12°43'09.8"E) and Orchard 2 (O2) in Aprilia (province of Latina, 41°33'33.9"N 12°43'45.2"E; O2S - 41°33'31.1"N 12°43'49.7"E). Soil from Orchard 1 was classified as Haplic Phaeozems (25-50%); Luvic Phaeozems (10-25%); and Cambic Endoleptic Phaeozems (<10%). The surrounding landform was described as slopes and eroded plateau surfaces on pyroclastic products mainly consolidated (tuffs). Those from Orchard 2 were classified as Haplic Luvisols (25-50%); and Protovertic Endogleyic Cambisols (25-50%). The surrounding landform was described as “high” Pontine plain surfaces on prevailing fluvial deposits [
29]. In each orchard, both asymptomatic (A) and symptomatic (S) areas were identified (O1A and O1S for Orchard 1, O2A and O2S for Orchard 2) using a severity scoring scale as described in paragraph 2.2. Four plants were randomly selected (replicates, n = 4) within each symptomatic and asymptomatic area of each orchard. For each plant, five sub-samples of rhizosphere soil were collected around the trunk, about 50 cm far from the collar, to create a composite soil sample (ca. 3kg). Soil sampling was conducted twice per year (spring and autumn) to evaluate the possible influence of phenology and crop season. Soil samples were air-dried at room temperature in the lab and sieved to < 2 mm before storage at 4 °C for chemical and biochemical analyses, and at −20 °C for phospholipid fatty acid (PLFAs) and molecular analysis.
2.2. Severity Score Disease
A scoring scale from 0 to 3 for disease severity for both epigeal and hypogeal symptoms was set up, to better distinguish between symptomatic and asymptomatic plants and to accurately categorize them into different classes. For epigeal symptoms: 0 = no symptoms, healthy plant; 1 = mild symptoms (plant decay sometimes identifiable by declining new shoots and the appearance of leaf chlorosis, few new shoots, reduced leaf size, widespread leaf chlorosis); 2 = severe symptoms (phylloptosis, carpoptosis, and reduced fruit size, (if present), vine decline); 3 = dead plant. For hypogeal symptoms: 0 = no symptoms, healthy roots; 1 = mild symptoms (reduced adsorbent root and widespread necrosis); 2 = severe symptoms (some primary roots and almost all secondary roots rot and loss of cortical tissues begins, showing the rat’s tails and almost disappearing absorbent roots); 3 = dead plant.
2.3. Soil Physicochemical Properties
The water content of the soil was measured gravimetrically. Soil pH, electrical conductivity (EC), the water-soluble content of carbon (WSC), and the water-soluble nitrogen content (WSN) were measured in a soil:distilled water extract (1:5, w-v). Soil pH was analysed using a pH meter (9615S–10D; HORIBA Scientific, Piscataway, NJ, USA). EC was analysed using a conductivity cell (9382–10D, HORIBA Scientific, Piscataway, NJ, USA). WSC and WSN were analysed using a C/N analyser for liquid samples (Multi N/C 3100, Analytic Jena, Germany). Ammonium (NH4
+) was analysed by colorimetric determination in KCl extracts following the method by Kandeler and Gerber [
30]. Soil macro and micro-nutrient contents were measured using an ICP-OES spectrometer (ICAP 6500 DUO from Thermo-Scientific, Waltham, MA, USA) after a process of nitric-perchloric acid digestion. Total carbon (TC), total nitrogen (TN), and soil organic carbon (SOC) content were analysed using an Elemental Analyser (C/N Flash EA 112 Series-Leco Truspec).
2.4. Soil Microbial Respiration and Enzyme Activities
Basal soil respiration (BSR) (mg CO
2-C kg
-1 soil day
-1) was measured by placing 20 g of soil, at 50 % of its water holding capacity (WHC), in hermetically sealed flasks and incubated for 40 days at 28 ºC in the darkness. Released CO
2 was measured periodically with an infrared CO
2 analyser (CheckMate 3O2 (Zr) CO2–100%; MOCON Europe A/S, Ringsted, Denmark). Beta-glucosidase and alkaline phosphatase activities were analysed using the methods of Eivazi and Tabatabai [
31] and Tabatabai and Bremner [
32], respectively. Both activities were expressed in units of micromoles of p-nitrophenol (PNP) produced per gram of dry soil per hour (μmol PNP g
−1 h
−1). Urease activity was determined using the buffered method of Kandeler and Gerber [
30] and was expressed in units of micromoles of ammonium-N produced per gram of dry soil per hour (μmolNH
4+-N g
−1 h
−1).
2.5. Phospholipid Fatty Acid (PLFAs) Analysis
Soil microbial biomass was determined by phospholipids fatty acids analysis (PLFAs). Fatty acids were extracted from soil using a chloroform:methanol:citrate buffer (1:2:0.8 v/v/v) according to Bligh and Dyer [
33]. Fatty acids were then fractionated to obtain the phospholipidic fraction [
34] and then transformed into fatty acid methyl esters [
35]. The PLFAs were analysed with a gas chromatograph (8860 GC System, Agilent Technologies, Santa Clara, USA) equipped with a flame ionization detector (FID), using a DB–Fast FAME capillary column (30 m × 0.25 mm ID × 0.25 μm film) (Agilent Technologies), with helium as the carrier gas. The conditions used were: an initial temperature of 80 °C for 1 min 30 sec, then an increase to 160 °C with a ramp of 40 °C/min, then to 167 °C at 0.5 °C/min, then to 200 °C at 30 °C/min, and finally to 230 °C at 4 °C/min. All fatty acids mentioned in this article are described according to the standard nomenclature of Vestal and White [
36]. The fatty acids i15:0, a15:0, i16:0, i17:0, 16:1ω7, cy17:0, cy19:0, 10Me16:0, and 10Me18:0 are considered representative of bacterial biomass [
34,
37] and the fatty acids 18:2ω6,9t and 18:2ω6,9c are considered representative of fungal biomass [
38,
39]. The fatty acids i15:0,a15:0, i16:0, i17:0, 10Me16:0, and 10Me18:0 are considered representative of Gram-positive (Gram+) biomass, while fatty acids 16:1ω7, cy17:0, and cy19:0 are considered representative of Gram-negative (Gram-) biomass [
34,
37]. The actinobacterial representative fatty acids are 10Me16:0 and 10Me18:0 [
37].
2.6. DNA Extraction
DNA extraction from 250 mg of soil from each sample was performed using a DNeasy PowerSoil Pro Kit (QIAGEN) following the manufacturer’s instructions with a modified homogenization step using the FastPrep Instrument (MP Biomedicals, SantaAna, CA, USA) instead of vortexing. DNAs were quantified and quality checked using the DeNovix dsDNA High Sensitivity Assay kit (DeNovix) and the DeNovix DS-11 FX+ Spectrophotometer/Fluorometer (DeNovix Inc., USA) respectively. DNA concentration for each extraction was standardized to 20 ng/μL−1, and only 3 low-concentrated samples were standardized at 5 ng/μL−1.
2.7. Bacterial V4 and Fungal ITS2 Amplicon Sequencing
The V4 region of the bacterial 16S rRNA gene was amplified using barcoded primers 515F and 806R [
40]. The PCR amplification of DNA of the fungal ITS2 region was performed using barcoded primers gITS7 and ITS4 [
41], in three PCR reactions per sample, as described by [ifčáková,etal.[
42]. Both primers were composed of the barcode (4–6 nucleotides), a spacer (2 nucleotides) absent in all GenBank sequences at this position to avoid preferential amplification of some targets [
43], and the specific primer. Both forward and reverse primers were barcoded to make sure that barcode-switching did not affect the results, and to avoid problems with Caporaso primers [
40]. The PCR products were purified using a MiniElute Kit (Qiagen) and the concentrations were measured with a Qubit 4 Fluorometer (ThermoFisher Scientific). Afterward, the library was prepared according to Vera et al. (2021) [
44] and sequencing of microbial amplicons was performed on Illumina MiSeq (in a paired-end 2 x 300 base pair (bp) run). Bioinformatic processing of the sequences was conducted using the USEARCH pipeline and UPARSE-OTU algorithm [
45]. The paired-end (PE) sequences were first merged with the command -fastq_mergepairs. Then, PE reads were quality-filtered allowing a maximum e-value of 1.0, trimmed (to 240 and 250 bp for prokaryotic and fungal libraries, respectively), dereplicated, and sorted by abundance (removing singletons), prior chimaera detection and determination of OTUs (operational taxonomic units) at 97% sequence identity. Finally, the original sequences were mapped to OTUs at the 97% identity threshold to obtain one OTU table for the prokaryotic community and one OTU table for the fungal community. The taxonomic affiliation of each OTU was obtained using the -syntax algorithm against the RDP 16S rRNA training set for 16s rRNA gene sequences [
46] and UNITE for ITS2 sequences [
47] with an 80% confidence threshold in both cases. The sequencing depth across libraries was normalized and, normalized OTU tables were used for downstream analyses. Diversity indicators (Shannon-Wiener index -H- and richness -R-) were calculated for the bacterial community (16S rRNA sequences) and the fungal community (ITS sequences) using the command -alpha_div. The DNA sequences have been submitted to the NCBI SRA with the assigned accession number PRJNA1156594.
2.8. Statistical Analysis
The data normality and homoscedasticity were checked with the Kolmogorov-Smirnov and Levene tests, respectively. To assess the effect of the factor “plant’s status”, data, including diversity indicators (Shannon-Wiener index -H- and richness -R-) from each orchard at each sampling time, were subjected to a one-way ANOVA. The data were further subjected to a two-way repeated measures analysis of variance (ANOVA) to test the interactive effects of the “plant’s status” (Symptomatic, Asymptomatic) and “orchard” (O1, O2) within the same sampling period. The effects of the factor “plant’s status” and its interaction with the other independent factors on seasonal variations were tested in a three-way repeated measures ANOVA. The ANOVA test was followed by post hoc Tukey’s significant difference test. Differences at P ≤ 0.05 were considered statistically significant.
To assess variations in soil microbial structure (bacterial and fungal communities) between asymptomatic and symptomatic areas in each of the two orchards, a non-metric multidimensional scaling (NMDS) analysis was conducted using Bray-Curtis dissimilarities at the OTU level. The significance of the differences was assessed via PerMANOVA with 9999 permutations using the adonis2 function in R version 4.2.1 with the "vegan" package. Indicator Species Analysis (ISA) was used to link relative abundance at the OTU level with orchard status running the “indicspecies” package in R version 4.2.1. A threshold level of indicator value with 95% significance (p-value ≤ 0.05) was chosen as cut off for identifying indicator species [
48].
3. Results
3.1. Severity Scale Disease
The use of a disease assessment scale has been used to better characterize the plant disease status in all studied areas and to correlate the results of the soil parameters with the severity of KVDS symptoms. The severity of KVDS in the symptomatic and asymptomatic areas of both orchards (O1 and O2) in the two seasons is reported in
Table 1. The scoring scale of the epigeal part defined the correct asymptomatic and symptomatic status in both orchards and seasons. Despite the absence of epigeal symptoms, the hypogeal scoring indicated an initial disease symptom in asymptomatic plants in Orchard 2 from spring onwards.
Table 1.
Average score of the epigeal and root symptoms of 4 kiwifruit plants of the 2 orchards collected in spring and autumn. O: Orchards; A: Asymptomatic; S: Symptomatic.
Table 1.
Average score of the epigeal and root symptoms of 4 kiwifruit plants of the 2 orchards collected in spring and autumn. O: Orchards; A: Asymptomatic; S: Symptomatic.
| Description |
Orchard 1 (O1) |
Orchard 2 (O2) |
| Asymptomatic |
Symptomatic |
Asymptomatic |
Symptomatic |
| Epigeal |
Spring |
0 |
2 |
0 |
2 |
| Autumn |
0 |
2 |
0 |
2 |
| Ipogeal |
Spring |
0 |
1,75 |
0,25 |
1,25 |
| Autumn |
0 |
1,75 |
0,75 |
2 |
| |
|
|
|
|
|
Table 2.
Mean value of the soil physicochemical properties, pH, electrical conductivity (dS m-1), water-soluble C (mg kg-1 soil) WSC, water-soluble N (mg kg-1 soil) WSN, NH4+, total N, total C, soil organic carbon SOC, Calcium carbonate content CaCO3, total carbon:nitrogen ratio C/N, β-glucosidase (μmol PNF g-1 soil h-1), alkaline phosphatase (μmol PNF g-1 soil h-1), urease activity (μmol NH4+ g-1 soil h-1), and Basal Soil Respiration (mg CO2 kg-1 soil day-1), and the abundance (biomass in nmol g-1 soil h-1) of the microbial community divided into Fungi, Bacteria, Gram- and Gram+ Bacteria, Actinobacteria, and Total PLFAs of Orchard 1 and 2 in Spring and Autumn. β-glucos.: β-glucosidase activity, Alk. Phos.: alkaline phosphatase activity, Urease: urease activity, BSR: basal soil respiration; the letters A and S indicate Asymptomatic and Symptomatic, respectively. For the One-Way ANOVA, the significance levels are shown as * P ≤ 0.05, * * P ≤ 0.01, and ***P ≤ 0.001.
Table 2.
Mean value of the soil physicochemical properties, pH, electrical conductivity (dS m-1), water-soluble C (mg kg-1 soil) WSC, water-soluble N (mg kg-1 soil) WSN, NH4+, total N, total C, soil organic carbon SOC, Calcium carbonate content CaCO3, total carbon:nitrogen ratio C/N, β-glucosidase (μmol PNF g-1 soil h-1), alkaline phosphatase (μmol PNF g-1 soil h-1), urease activity (μmol NH4+ g-1 soil h-1), and Basal Soil Respiration (mg CO2 kg-1 soil day-1), and the abundance (biomass in nmol g-1 soil h-1) of the microbial community divided into Fungi, Bacteria, Gram- and Gram+ Bacteria, Actinobacteria, and Total PLFAs of Orchard 1 and 2 in Spring and Autumn. β-glucos.: β-glucosidase activity, Alk. Phos.: alkaline phosphatase activity, Urease: urease activity, BSR: basal soil respiration; the letters A and S indicate Asymptomatic and Symptomatic, respectively. For the One-Way ANOVA, the significance levels are shown as * P ≤ 0.05, * * P ≤ 0.01, and ***P ≤ 0.001.
| |
Orchard 1 (O1) |
Orchard 2 (O2) |
| |
Spring |
Autumn |
Spring |
Autumn |
| Variables |
Asymptomatic |
Symptomatic |
Asymptomatic |
Symptomatic |
Asymptomatic |
Symptomatic |
Asymptomatic |
Symptomatic |
| pH |
7.4 |
8.0*** |
7.5 |
8.1** |
7.2 |
7.1 |
7.5 |
7.4 |
| EC |
150.7 |
293.8*** |
100.2 |
126.1* |
259.8 |
190.9* |
150.9 |
159.6 |
| WSC |
58.7 |
72.8 |
248.7 |
182.5*** |
39.3 |
48.3 |
262.3 |
328.6** |
| WSN |
12.5 |
32.2*** |
20.2 |
24.1 |
34.6 |
13.9*** |
40.6 |
47.2 |
| NH4 + |
2.7 |
1.2 |
1.1 |
1.6 |
1.4 |
1.7 |
1.9 |
1.9 |
| Total N |
0.1 |
0.2** |
0.2 |
0.2 |
0.3 |
0.3 |
0.3 |
0.4* |
| Total C |
1.4 |
2.1*** |
2.0 |
2.4 |
2.5 |
2.3 |
2.9 |
3.5* |
| SOC |
1.3 |
1.9 *** |
1.5 |
2.1 |
2.3 |
2.3 |
2.5 |
3.2* |
| CaCO3 |
1.1 |
1.9 |
2.6 |
2.8 |
1.8 |
1.8 |
2.7 |
4.0 |
| C/N |
9.5 |
11.1* |
8.7 |
9.7 |
9.7 |
9.6 |
8.8 |
9.0 |
| β-glucos. |
0.54 |
1.44** |
0.94 |
1.64*** |
1.49 |
1.17 |
1.8 |
2.5 |
| Alk. Phos. |
2.57 |
5.00*** |
4.55 |
4.49 |
4.03 |
4.72 |
8.52 |
11.1 |
| Urease |
1.69 |
1.56 |
1.39 |
0.99 |
1.36 |
1.19 |
0.74 |
0.93* |
| BSR |
2.64 |
7.92*** |
5.73 |
5.96 |
3.51 |
5.05* |
8.32 |
13.78*** |
| Fungi |
0.8 |
2.3 *** |
1.6 |
1.2 |
1.5 |
2.6 * |
1.1 |
1.4 |
| Bacteria |
10.7 |
20.6 *** |
18.9 |
18.7 |
22.6 |
22.4 |
20.7 |
27.1 ** |
| Gram - |
3.6 |
7.5 *** |
6.7 |
6.5 |
7.4 |
6.7 |
6.6 |
8.9 ** |
| Gram + |
7 |
13.1 *** |
12.2 |
11.7 |
15.2 |
17.4 |
14.1 |
18.0 ** |
| Actinobac. |
0.6 |
1.0 *** |
0.7 |
0.6 |
0.9 |
1.0 |
0.5 |
0.7 |
| Total PLFAs |
18.5 |
39.6 *** |
31.0 |
31.9 |
40.5 |
43.5 |
35.7 |
42.7 |
| Gram+/Gram- |
1.9 |
1.8 |
2.0 |
2.0 |
2.0 |
2.6 |
2.1 |
2.1 |
| Fung/Bac |
0.07 |
0.11 ** |
0.07 |
0.07 |
0.07 |
0.11 ** |
0.05 |
0.06 |
3.2. Soil Physicochemical Properties
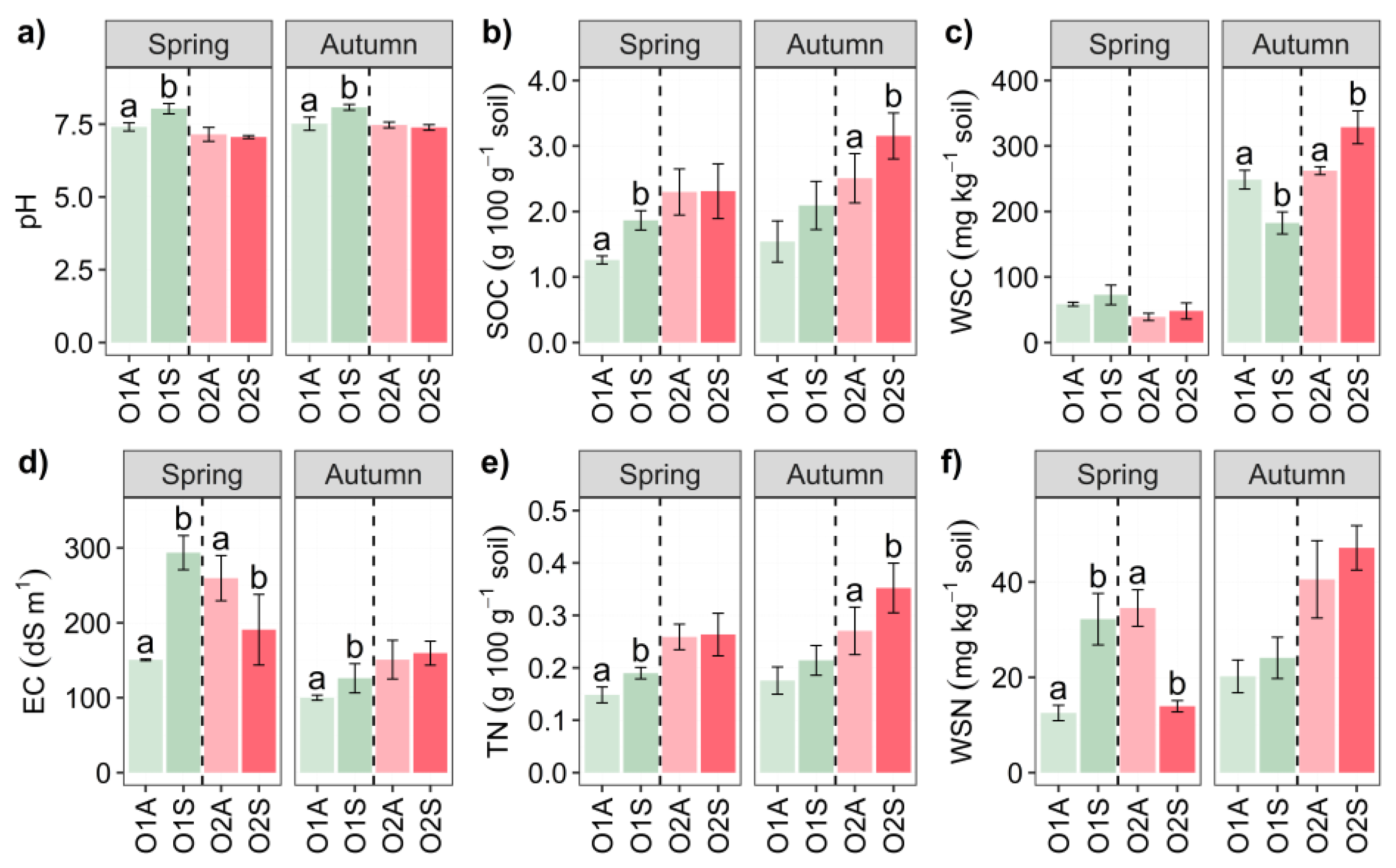
No significant differences in soil microelements content were found between the two orchard types and symptomatologic status (data not shown). The results of the One-Way ANOVA for pH, EC, WSC, and WSN are shown in
Figure 1. Among them, significantly higher pH values were only found in O1 symptomatic soils in both seasons compared to asymptomatic ones. Higher EC values were observed in O1 symptomatic soils in both seasons (P < 0.05) whereas they were lower in O2 symptomatic soils only in spring. In spring, no significant differences in WSC were recorded between symptomatic and asymptomatic soils for both O1 and O2 soil samples. However, in autumn, WSC was lower in the soil of O1 from symptomatic plants, whereas it was higher in O2 plants (P < 0.05). WSN values were significantly different only in spring, higher for O1 symptomatic plants and lower for O2 symptomatic plants. Two-way ANOVA results of the spring sampling (
Table S1) showed significant O × S interactions for pH, EC, and WSN, whereas in autumn this interaction was significant only for pH and WSC. The triple O × S × t interaction of the measured parameters was significant for all parameters, except pH (
Table S3). Significantly higher values of SOC and TN were recorded in spring for O1 and in autumn for O2.
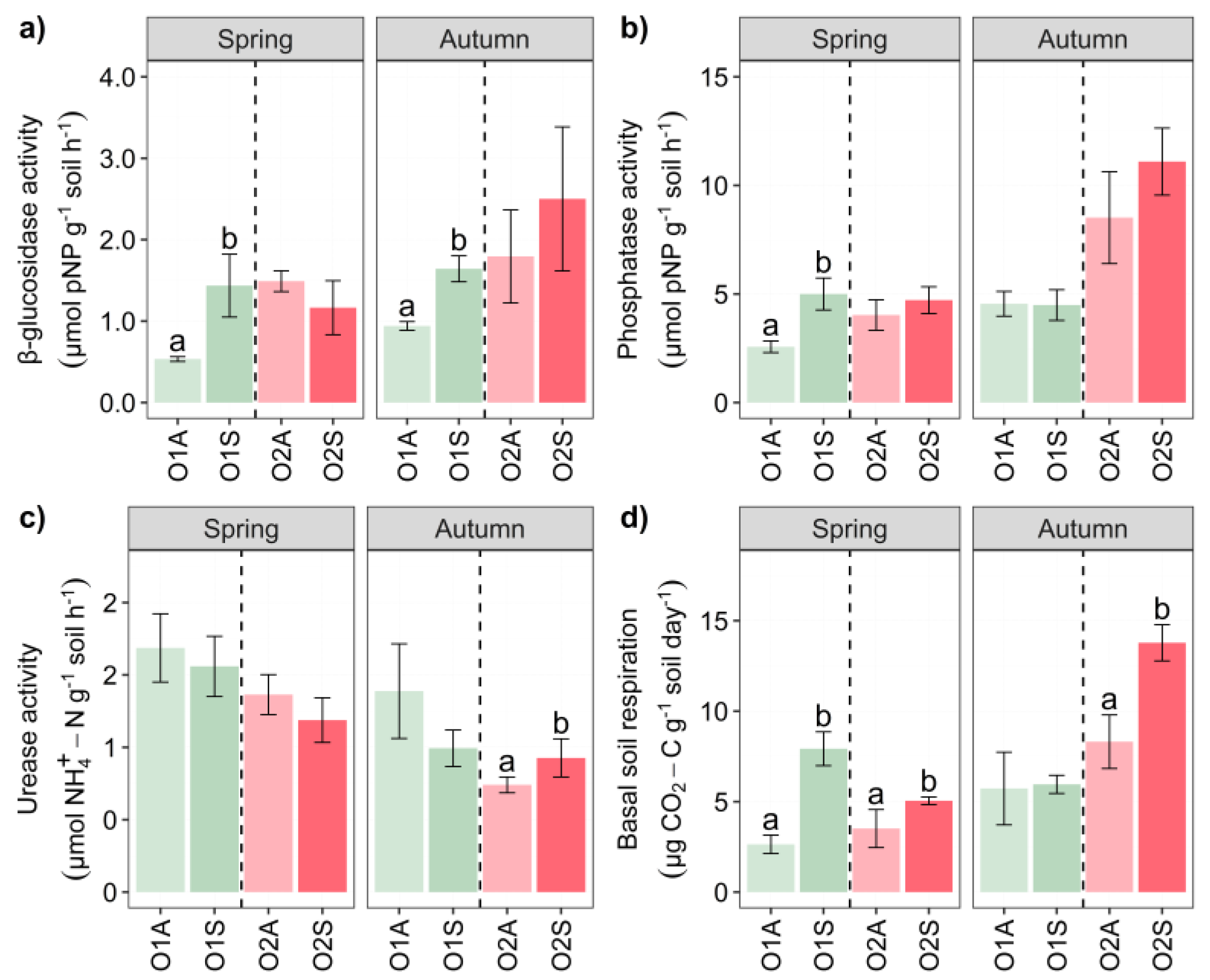
3.3. Soil Basal Respiration and Enzyme Activities
The enzyme activities showed overall distinct patterns. In both seasons, β-glucosidase activity was significantly higher in O1 symptomatic soils than in the corresponding asymptomatic areas (
Figure 2a). Regarding alkaline phosphatase, the soils in the symptomatic areas in spring (O1) showed significantly higher activity than those in the corresponding asymptomatic areas (
Figure 2b).
Urease activity was slightly higher in symptomatic soils collected in O2 in autumn (
Figure 2c) than in asymptomatic soils. With the only exception of O1 in autumn, a general trend of significantly increased basal respiration of the symptomatic soils was observed (
Figure 2d). Two-way ANOVA O × S interaction was highly significant for β-glucosidase and BSR in spring, whereas it was significant for Urease and BSR in autumn. Three-way ANOVA showed significant O × S × t interactions for all four parameters measured (
Table S3).
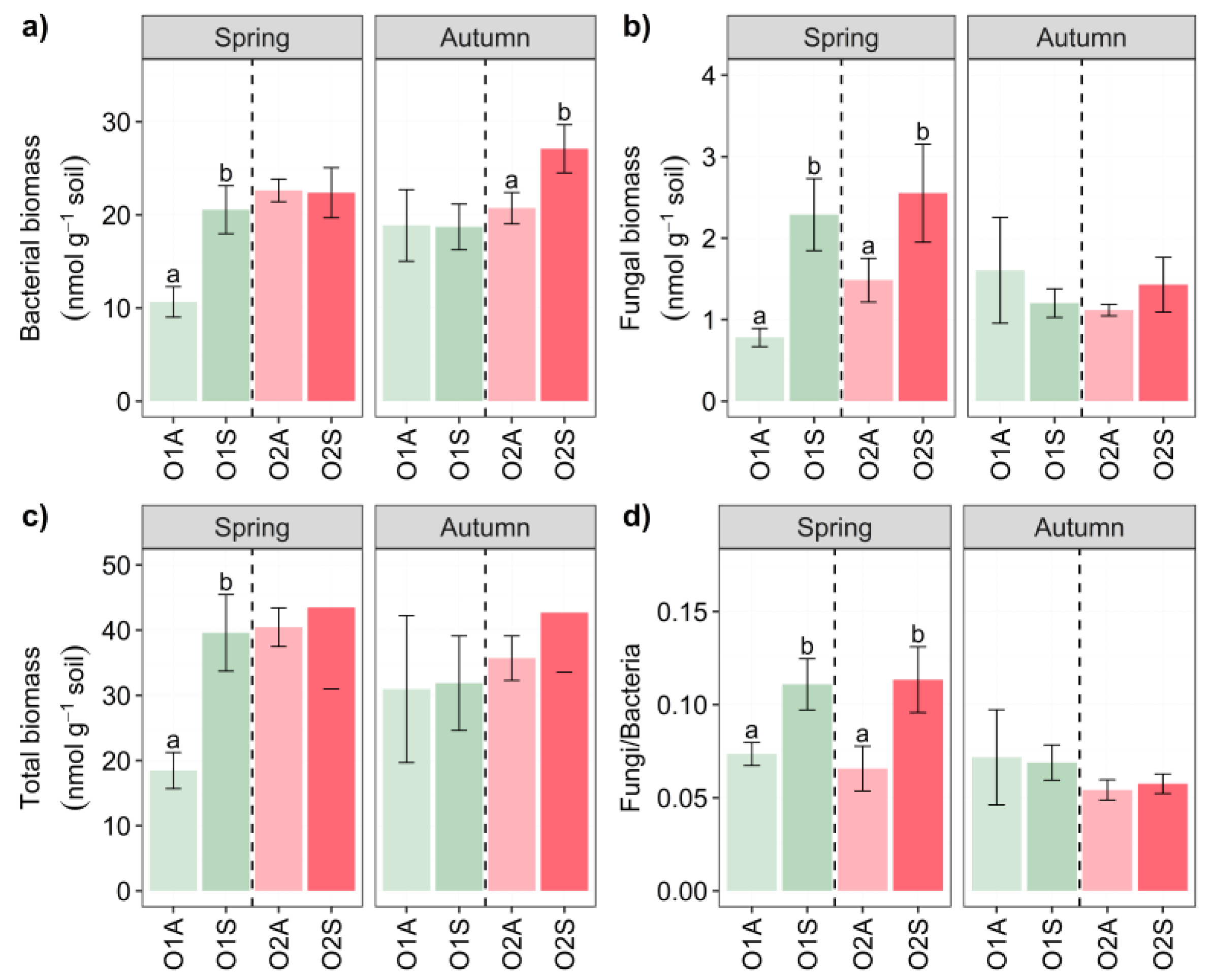
3.4. Phospholipid Fatty Acid (PLFAs) Analysis
The microbial biomass of the soil was assessed by the soil PLFA extraction, and the results are shown in
Figure 3. As a general trend, in spring, the O1 symptomatic soils showed always significantly higher total biomass, fungi, bacteria, and fungi:bacteria ratio, whereas in O2 it was only the case for the bacterial biomass and the fungi:bacteria ratio. Conversely, in autumn only the fungal and bacterial biomass was significantly higher in O2 symptomatic soils (
Figure 3a). The O × S interaction was significant for bacteria in both spring and autumn (
Table S1–S2), likewise to the triple O × S × t interaction. The latter was also significant for fungi (
Table S1–S3).
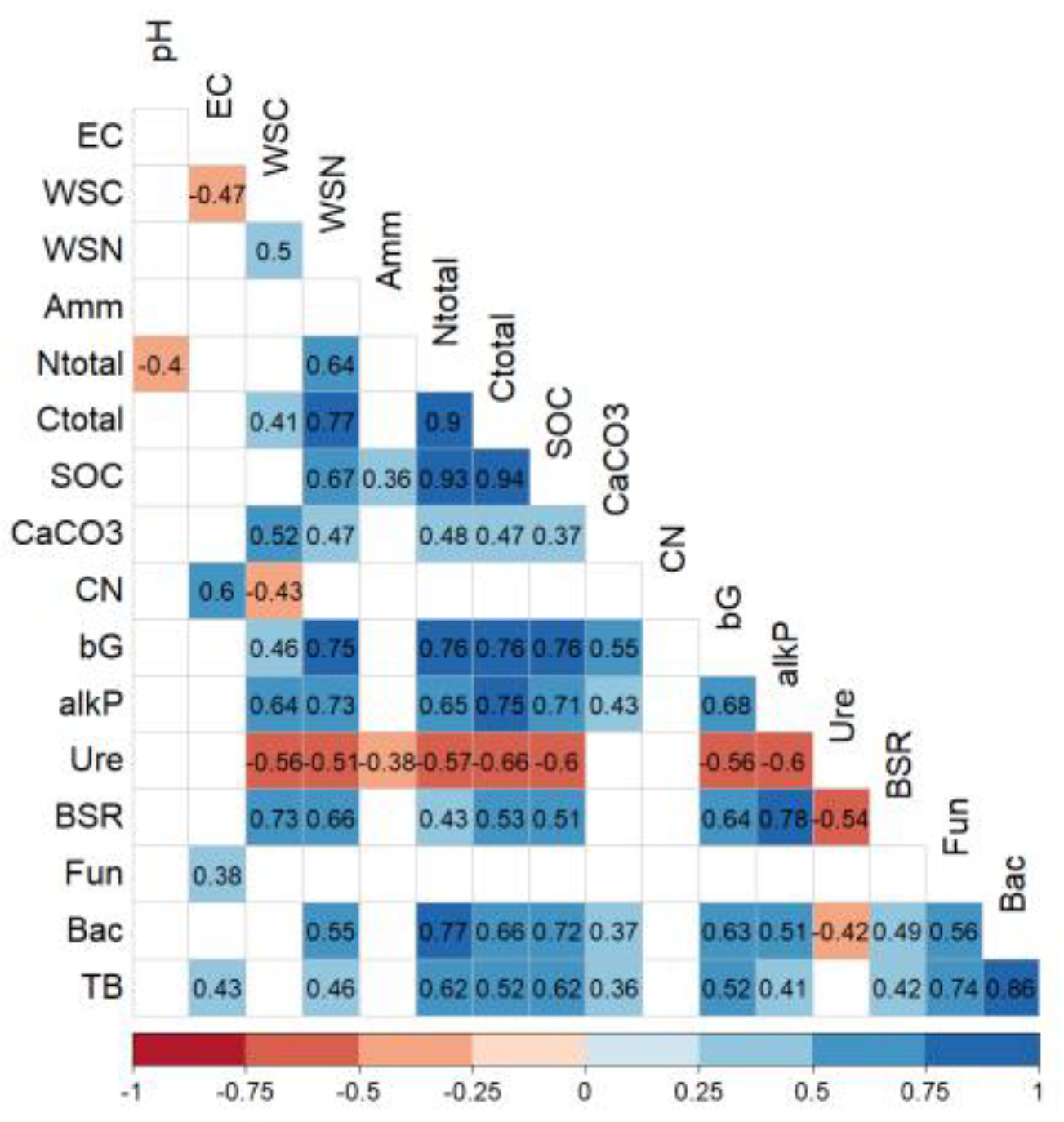
The analysis of the correlation between all the measured parameters is shown in
Figure 4. Worth mentioning are the positive correlations between fungi and bacteria, those of β-glucosidase activity with several physiochemical parameters (WSN, TC, TN, SOC), and some enzymatic activities (alkaline P and BSR). Very interesting positive correlations have been observed between BSR and, WSC, WSN, and alkaline P., while bacteria were highly correlated with N total and SOC. In addition, positive correlations were found with soil respiration and β-glucosidase and phosphatase activities, but no significant correlation with urease. In contrast, no significant correlation was found between fungal biomass and these soil parameters (
Figure 4).
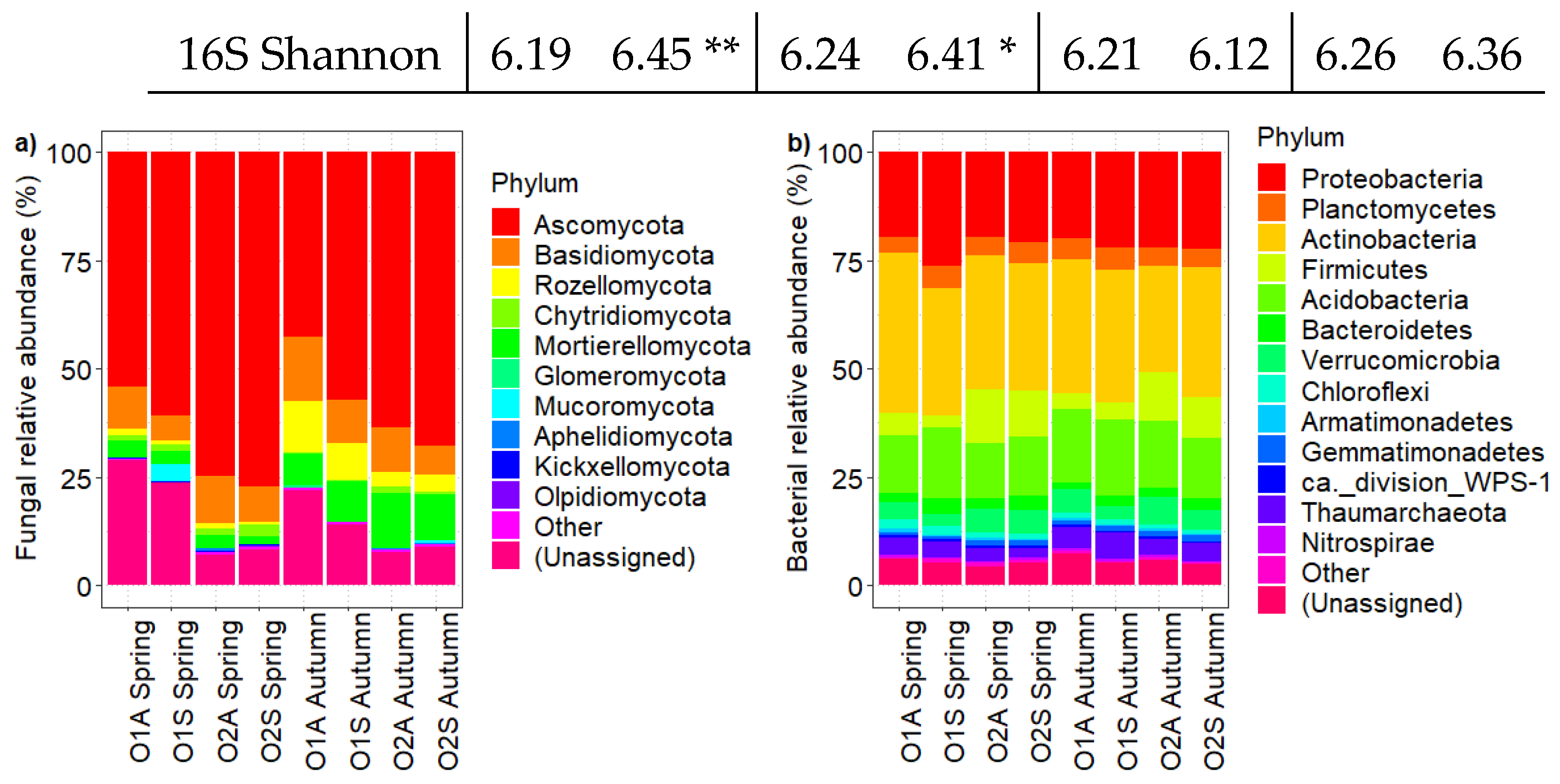
3.5. Fungal ITS and Bacterial 16Samplicon Sequencing
To compare the microbial communities of asymptomatic and symptomatic rhizosphere soil samples, we performed amplicon sequencing of fungal ITS (ITS 2) and bacterial 16S (V4) regions. There were no significant differences in fungal diversity (Shannon and richness) across seasons and plant status (
Table 3). About bacterial diversity, it was higher in O1S and O2S than in the corresponding asymptomatic plants in spring, but there were no significant differences in both orchards in autumn. The relative abundance of soil microbiota at the
phylum level for both Orchards in the two sampling seasons is reported in
Figure 5. As for fungi,
Ascomycota was the most abundant phylum, followed by
Basidiomycota,
Rozellomycota, and
Chytridiomycota. One-way ANOVA of the most abundant
phylum showed differences between the two symptomatologic areas only in Orchard 1 in both sampling times (
Table S6). In both seasons, the relative abundance of
Ascomycota was significantly higher in the soils of symptomatic than in asymptomatic trees in O1, and
Basidiomycota relative abundance was higher in the asymptomatic areas of the same orchard (
Table S6;
P ≤ 0.05). Among the bacterial phyla,
Proteobacteria,
Actinobacteria,
Firmicutes, and
Acidobacteria were the most prevalent. Their relative abundance varied significantly with the health status of trees in Orchard 1, particularly in spring, and all except
Proteobacteria showed similar trends in autumn. As in the case of fungi, the most noticeable differences between the bacterial phyla were observed in Orchard 1. The effect of the plant status on bacterial abundance shifted over time, with significant changes in
Proteobacteria and
Acidobacteria during spring (
Table S7; P ≤ 0.001), and in
Actinobacteria and
Planctomycetes during autumn (
Table S7; P ≤ 0.001), with the symptomatic areas showing the highest relative abundances.
Since it is unlikely that the disease has a bacterial origin [
20], an Indicator Species Analysis (ISA) was conducted on the fungal community to determine which OTUs were most associated with asymptomatic and symptomatic soil samples. Sixteen and twenty-three OTUs, mostly Ascomycota, were significantly associated with the soils of the symptomatic and asymptomatic areas, respectively, and were identified as potential indicator species. In more detail, OTUs to the family
Pyronemataceae and
Aspergillaceae were enriched in the soils of the asymptomatic areas, whilst fungal taxa assigned to the class
Leotiomycetes and the family
Periconiaceae were found to be the most statistically associated with the soils of the asymptomatic areas (
Table 4 and
Table 5).
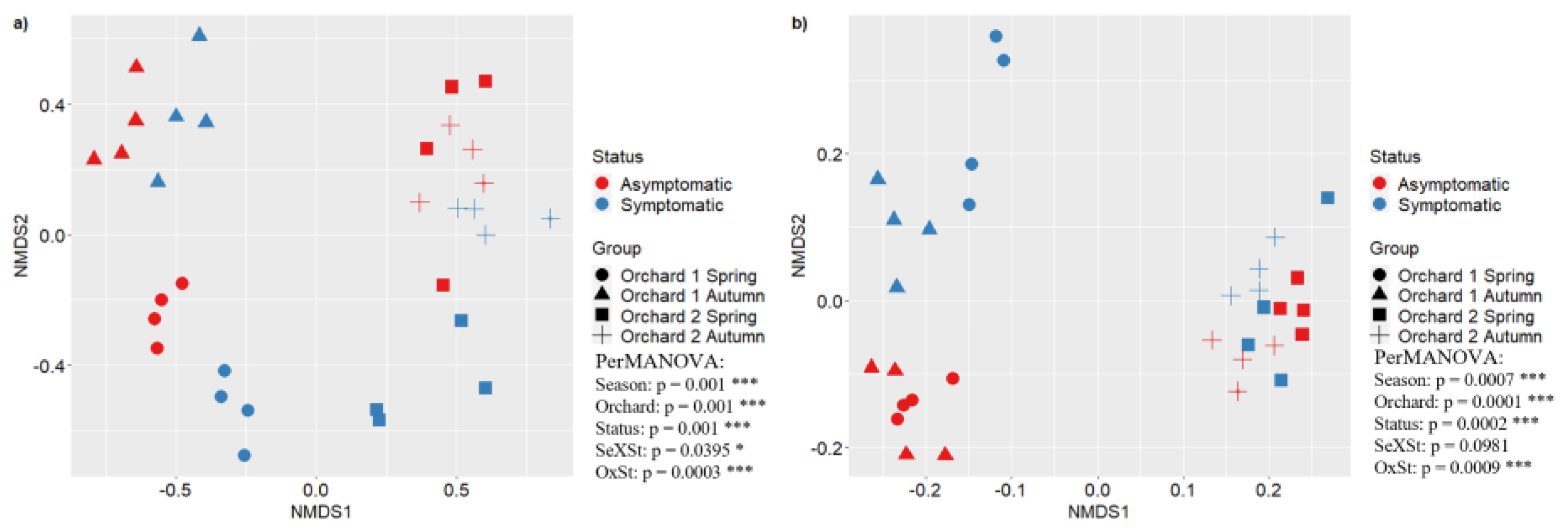
The structure of soil microbial communities was assessed by means of a non-metric multidimensional Scaling (NMDS) of bacterial and fungal OTUs in both orchards (O1 and O2). The NMDS analysis showed significant differences in fungal and bacterial community composition between orchards and disease status. The PerMANOVA test confirmed the differences in NMDS, indicating significant statistical differences as reported in
Figure 6.
4. Discussion
4.1. Severity Scale Disease
Since this study aimed to evaluate the impact of Kiwifruit Vine Decline Syndrome (KVDS) on soil health indicators and microbial communities, it was of primary importance to identify areas with and without KVDS by comparing symptomatic and asymptomatic kiwifruit trees in different orchards. Kiwifruit trees affected by KVDS may appear asymptomatic above ground while the same trees may already exhibit root symptoms. This pattern can be attributed to disease progression [
49,
50,
51]: mild symptoms typically emerge in early spring, and, if the syndrome persists, plants may deteriorate rapidly in summer or autumn, coinciding with fruit ripening when nutrient demand peaks, explaining the worsening of root symptoms in autumn. An easy-to-use severity scale, as described in paragraph 2.2, is useful for planning early interventions and mitigating the impacts of the disease [
52].
4.2. KVDS Impact on Soil Physicochemical Properties and Enzymatic Activities
The results of the comparative analysis indicated that orchard characteristics, symptomatologic plant status, and seasonal variations, significantly correlated with soil properties and enzyme activities, which are critical factors in understanding and managing KVDS. In Orchard 1 (O1), soil pH was higher in soils from symptomatic trees during both seasons, while Orchard 2 (O2) showed no significant differences in soil pH based on symptomatology. Other studies have found that soil pH variations, in particular pH > 7, significantly affect nutrient solubility and microbial activity, influencing plant health and disease resistance [
53,
54]. However, the higher pH level observed exclusively in symptomatic areas of Orchard 1 indicates that KVDS severity cannot be directly attributed to pH levels alone.
Enzyme activities have been widely used as indicators of soil health and quality [
55,
56]. Our findings indicated that enzyme activities and basal soil respiration were more strongly influenced by orchard type and seasonal variations than by the symptomatologic status of trees. Bastida et al. (2008) [
55] reported that higher enzyme activities are generally associated with healthier soils and better plant growth and, depending on local soil conditions, also with management practices. Conversely, our results showed that some enzyme activities and basal respiration in symptomatic areas were higher than in asymptomatic areas. The explanation of these results might reflect the activity of the pathogenic microbial community in KVDS symptomatic areas as well as the existence of decomposed root tissues in diseased plants that can generate substrate for greater microbial activity. Indeed, we found a link between β-glucosidase activity and root symptom severity, suggesting that this enzyme might be secreted by phytopathogenic Oomycetes and/or by other fungi to facilitate the penetration into the roots of plants. In Orchard 2 during spring, mild root symptoms in asymptomatic areas suggested a potential link with the β-glucosidase activity dynamics. Previous studies have reported some Oomycetes species as pathogenic to kiwifruit [
15,
57], and other works have reported high β-glucosidase activity in Oomycetes [
58] and fungi [
59]. In the same way, the higher BSR levels in symptomatic areas could be due to higher organic matter availability, as indicated by soil organic carbon levels and increased fungal biomass in soils with diseased plants, as outlined below.
4.3. KVDS Impacts on Soil Microbial Community
The symptomatic status of trees and the season influenced soil microbial biomass, with increased microbial abundance, in particular, fungal biomass, in both orchards in spring, when the KVDS symptoms were present. Similar results were obtained in other work assessed on other crops indicating an increase in fungal and bacterial abundance in diseased roots [
60,
61]. These results highlight that KVDS can impact microbial biomass which is crucial for soil health [
62], by influencing plant growth, nutrient cycling, and disease resistance [
63] through beneficial interactions with plant roots. Although it is challenging to identify a specific pathogen associated with the disease through metabarcoding, the distinct microbial groups found in asymptomatic rhizosphere soils highlighted the complex plant-microbe interactions, crucial to soil health and fertility [
64].
Metabarcoding confirmed differences in community composition between asymptomatic and symptomatic areas consistent with our hypothesis. Our results suggested an intense interaction between season and plant health status in shaping the level of fungal diversity. In spring, there was a consistent trend toward higher richness and Shannon index in asymptomatic O2 than in symptomatic O2, whereas in autumn, this distinction was less pronounced. In contrast, bacterial diversity did not exhibit a consistent trend across seasons and health states, suggesting the involvement of factors additional to seasonality and KVDS symptoms in modulating the level of bacterial diversity.
Further, the Indicators species analysis at the OTU level confirmed that
Ascomycota was more representative in symptomatic areas and underscored the importance of groups like
Glomeromycota and
Basidiomycota in asymptomatic rhizosphere soil, which are phyla known for positive role in plant interactions [
65]. When beneficial microorganisms dominate, they can suppress the growth and activity of pathogens, while saprophytes continue to recycle nutrients efficiently. An imbalance due to an overabundance of pathogens, such as
Phytopythium vexans known to cause root rots [
66,
67], or a depletion of beneficial microbes, can lead to poor plant health, reduced crop yields, and increased susceptibility to diseases. It has been reported that some fungal species could produce phytotoxic exudates such as
Dactylonectria spp. [
68] that could be involved in KVDS symptoms [
12,
69]. Here we found an increase in the relative abundance of pathogenic fungi like
Ilyonectria spp. reported as a causal agent of necrotic lesions on woody roots [
70]. In the context of KVDS, disruption of beneficial microbial populations, including those involved in nutrient cycling and pathogen suppression, appears to exacerbate KVDS symptoms [
71]. One factor contributing to the negative impact of these useful microbes is the excess of irrigation volumes over the years, leading to progressive soil compaction along planted rows in kiwifruit orchards. Moreover, this study allowed us to relate KVDS to changes in the bacterial community, namely changes in the abundance of important soil functional genera, like
Firmicutes which includes
Bacillus genera, less represented in symptomatic areas of kiwifruit orchards. Other works suggest that microorganisms, such as
Proteobacteria,
Firmicutes, and mycorrhizal fungi, can alleviate abiotic stresses such as drought, salinity, and temperature extremes, thereby enhancing plant resilience [
72]. Conversely, pathogenic bacteria can significantly reduce crop yields, leading to dysbiosis, in which pathogenic microbes dominate, out-competing and suppressing beneficial organisms.
5. Conclusions
The overall results of the present study highlighted the seasonal dynamics of soil physicochemical properties, enzyme activities, and microbial biodiversity in kiwifruit orchards affected by KVDS. Significant differences in enzymatic activities and microbial biomass are associated with KVDS symptoms. Metabarcoding revealed distinct microbial communities in symptomatic versus asymptomatic areas. Β-glucosidase activity in symptomatic rhizospheres merits further investigation as a potential indicator of plant-soil interactions. The complex interplay between KVDS, soil health, and microbial communities highlights the need for integrated management strategies to mitigate the impact of the disease on kiwifruit crops. Further investigations will shed light on the aetiology of the disease to help the kiwifruit producers to reduce losses and preserve soil health and fertility. Interdisciplinary studies incorporating additional environmental and host-related variables are crucial to understanding the KVDS aetiology useful to improve sustainable soil management practices. The impact of KVDS on microbial communities, favouring pathogenic organisms, underscores the need for strategies to restore and maintain a healthy microbial balance in the rhizosphere.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1, Table S2, Table S3, Table S4, Table S5, Table S6, Table S7.
Author Contributions
Conceptualization, V.B., L.P., F.B., Data curation, V.B., A.V., J.S, R.L-M., Formal analysis, V.B., A.V., J.S, R.L-M., Investigation, V.B., A.V., J.S, R.L-M., Methodology, V.B., A.V., F.B., Funding acquisition, M.R., A.I., F.B., Supervision, L.P., L.L., S.V., M.R., A.I., F.B., Writing – original draft, V.B., A.V., L.P., J.S, A.I., F.B., Writing – review & editing V.B., A.V., L.P., L.L., S.V., M.R., A.I., F.B., All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-funded by the Italian Ministry of University and Research (MUR) under the “Programma Operativo Nazionale Ricerca e Innovazione” (PON R&I) 2014-2020 (FSE REACT-EU) (Grant agreement No. DOT1326JZS-23 to V. B.) and by Zespri Fresh Produce Italy Srl. This study is a part of the AGROALNEXT programme and was supported by MCIN with NextGeneration EU funding (PRTR-C17.I1) J.A.Siles acknowledges the support of grant IJC2018-034997-I funded by MICIU/AEI/10.13039/501100011033.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This work is dedicated to the memory of Dr. Luca Riccioni. We thank Apofruit Italia, Agrintesa, and the farmer for their kind permission to conduct the sampling.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pinto, T. Kiwifruit, a botany, chemical and sensory approach a review. Advances in Plants & Agriculture Research 2018, 8. [Google Scholar]
- FAOSTAT. Online database (available at https://www.fao.org/faostat/en/#home, accessed January 2024) 2024.
- ISTAT. Istituto nazionale di statistica data extraction 19 jan 2024, 11h00 utc (gmt) da i.Stat (available at http://dati.Istat.It/). 2024.
- Lemon, C.W.; Considine, J.A. Anatomy and histochemistry of the root system of the kiwifruit vine, actinidia deliciosa var. Deliciosa. Annals of Botany 1993, 71, 117–129. [Google Scholar] [CrossRef]
- Bardi, L.; Nari, L.; Morone, C.; Faga, M.G.; Malusà, E. Possible role of high temperature and soil biological fertility on kiwifruit early decline syndrome. Frontiers in Agronomy 2020, 2. [Google Scholar] [CrossRef]
- D'Ippolito, I.; Mang, S.M.; Elshafie, H.S.; Camele, I.; Scillitani, G.; Mastrodonato, M.; Sofo, A.; Mininni, A.N.; Xylogiannis, E. In Morpho-anatomical and microbiological analysis of kiwifruit roots with kvds symptoms, 2022; International Society for Horticultural Science (ISHS), Leuven, Belgium: pp 131-136.
- Smith, G.S.; Judd, M.J.; Miller, S.A.; Buwalda, J.G. Recovery of kiwifruit vines from transient waterlogging of the root system. New Phytologist 1990, 115, 325–333. [Google Scholar] [CrossRef]
- Baudry, A.; Morzieres, J.P.; Ellis, R. Effect of phytophthora spp. On kiwifruit in france. New Zealand Journal of Crop and Horticultural Science 1991, 19, 395–398. [Google Scholar] [CrossRef]
- Conn, K.; Gubler, W.; Mircetich, S.; Hasey, J. Pathogenicity and relative virulence of nine phytophthora spp. From kiwifruit. Phytopathology 1991, 81, 974–979. [Google Scholar] [CrossRef]
- Latorre, B.; Alvarez, C.; Ribeiro, O. Phytophthora root rot of kiwifruit in chile. Plant Disease 1991, 75, 949–952. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Jee, H.-J.; Cha, K.-H.; Ko, S.-J.; Park, K.-B. Occurrence of phytophthora root rot on kiwifruit in korea. The Plant Pathology Journal 2001, 17, 154–158. [Google Scholar]
- Tacconi, G.; Paltrinieri, S.; Mejia, J.F.; Fuentealba, S.P.; Bertaccini, A.; Tosi, L.; Giacopini, A.; Mazzucchi, U.; Favaron, F.; Sella, L.; et al. Vine decline in kiwifruit: Climate change and effect on waterlogging and phytophthora in north italy. 2015, 93-97.
- Savian, F.; Ginaldi, F.; Musetti, R.; Sandrin, N.; Tarquini, G.; Pagliari, L.; Firrao, G.; Martini, M.; Ermacora, P. Studies on the aetiology of kiwifruit decline: Interaction between soil-borne pathogens and waterlogging. Plant and Soil 2020, 456, 113–128. [Google Scholar] [CrossRef]
- Kurbetli, İ.; Ozan, S. Occurrence of hytophthora root and stem rot of kiwifruit in turkey. Journal of Phytopathology 2013, 161, 887–889. [Google Scholar] [CrossRef]
- Polat, Z.; Awan, Q.N.; Hussain, M.; Akgül, D.S. First report of phytopythium vexans causing root and collar rot of kiwifruit in turkey. Plant Disease 2017, 101, 1058. [Google Scholar] [CrossRef]
- Türkkan, M.; Özer, G.; Karaca, G.; Erper, İ.; Derviş, S. Characterization and pathogenicity of pythium-like species associated with root and collar rot of kiwifruit in turkey. Plant Disease 2021, 106, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Akilli, S.; Serçe, Ç.U.; Zekaİ Katircioğlu, Y.; Karakaya, A.; Maden, S. Involvement of phytophthora citrophthora in kiwifruit decline in turkey. Journal of Phytopathology 2011, 159, 579–581. [Google Scholar] [CrossRef]
- Wang, K.X.; Xie, Y.L.; Yuan, G.Q.; Li, Q.Q.; Lin, W. First report of root and collar rot caused by phytopythium helicoides on kiwifruit (actinidia chinensis). Plant Disease 2015, 99, 725. [Google Scholar]
- Donati, I.; Cellini, A.; Sangiorgio, D.; Caldera, E.; Sorrenti, G.; Spinelli, F. Pathogens associated to kiwifruit vine decline in italy. Agriculture 2020, 10, 119. [Google Scholar] [CrossRef]
- Bardi, L. Early kiwifruit decline: A soil-borne disease syndrome or a climate change effect on plant–soil relations? Frontiers in Agronomy 2020, 2. [Google Scholar] [CrossRef]
- Spigaglia, P.; Barbanti, F.; Marocchi, F.; Mastroleo, M.; Baretta, M.; Ferrante, P.; Caboni, E.; Lucioli, S.; Scortichini, M. Clostridium bifermentans and c. Subterminale are associated with kiwifruit vine decline, known as moria, in italy. Plant Pathology 2020, 69, 765–774. [Google Scholar] [CrossRef]
- García-Díaz, C.; Siles, J.A.; Moreno, J.L.; García, C.; Ruiz-Navarro, A.; Bastida, F. Phenological stages of wheat modulate effects of phosphorus fertilization in plant-soil microbial interactions. Plant and Soil 2024. [Google Scholar] [CrossRef]
- Barquero, M.B.; García-Díaz, C.; Dobbler, P.T.; Jehmlich, N.; Moreno, J.L.; López-Mondéjar, R.; Bastida, F. Contrasting fertilization and phenological stages shape microbial-mediated phosphorus cycling in a maize agroecosystem. Science of The Total Environment 2024, 951, 175571. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nature Reviews Microbiology 2023. [Google Scholar] [CrossRef]
- Dai, Z.; Guo, X.; Lin, J.; Wang, X.; He, D.; Zeng, R.; Meng, J.; Luo, J.; Delgado-Baquerizo, M.; Moreno-Jiménez, E.; et al. Metallic micronutrients are associated with the structure and function of the soil microbiome. Nature Communications 2023, 14, 8456. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Stirling, E.; Liu, Y.; Zhao, K.; Zhou, J.; Singh, B.K.; Tang, C.; Dahlgren, R.A.; Xu, J. Soil biogeochemical cycle couplings inferred from a function-taxon network. Research 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Madigan, M.T.; Bender, K.S.; Buckley, D.H.; Sattley, W.M.; Stahl, D.A. Brock biology of microorganisms. Pearson: 2018.
- Vera, A.; Moreno, J.L.; García, C.; Morais, D.; Bastida, F. Boron in soil: The impacts on the biomass, composition and activity of the soil microbial community. Science of The Total Environment 2019, 685, 564–573. [Google Scholar] [PubMed]
- Napoli, R. P.M., Di Ferdinando, S. (A cura di). Atlante dei suoli del lazio. ARSIAL Regione Lazio. ISBN 978-88-904841-2-4 2019.
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biology and Fertility of Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biology and Biochemistry 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biology and Biochemistry 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Canadian journal of biochemistry and physiology 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Frostegård, Å.; Bååth, E.; Tunlio, A. Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biology and Biochemistry 1993, 25, 723–730. [Google Scholar] [CrossRef]
- Guckert, J.B.; Antworth, C.P.; Nichols, P.D.; White, D.C. Phospholipid, ester-linked fatty acid profiles as reproducible assays for changes in prokaryotic community structure of estuarine sediments. FEMS Microbiology Letters 1985, 31, 147–158. [Google Scholar] [CrossRef]
- Vestal, J.R.; White, D.C. Lipid analysis in microbial ecology: Quantitative approaches to the study of microbial communities. BioScience 1989, 39, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Dungait, J.A.J.; Kemmitt, S.J.; Michallon, L.; Guo, S.; Wen, Q.; Brookes, P.C.; Evershed, R.P. Variable responses of the soil microbial biomass to trace concentrations of 13c-labelled glucose, using 13c-plfa analysis. European Journal of Soil Science 2011, 62, 117–126. [Google Scholar] [CrossRef]
- Brant, J.B.; Sulzman, E.W.; Myrold, D.D. Microbial community utilization of added carbon substrates in response to long-term carbon input manipulation. Soil Biology and Biochemistry 2006, 38, 2219–2232. [Google Scholar] [CrossRef]
- Rinnan, R.; Bååth, E. Differential utilization of carbon substrates by bacteria and fungi in tundra soil. Applied and Environmental Microbiology 2009, 75, 3611–3620. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16s rrna diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Ihrmark, K.; Bödeker, I.T.M.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandström-Durling, M.; Clemmensen, K.E.; et al. New primers to amplify the fungal its2 region – evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiology Ecology 2012, 82, 666–677. [Google Scholar] [CrossRef]
- Žifčáková, L.; Větrovský, T.; Howe, A.; Baldrian, P. Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter. Environmental Microbiology 2016, 18, 288–301. [Google Scholar] [CrossRef]
- Parameswaran, P.; Jalili, R.; Tao, L.; Shokralla, S.; Gharizadeh, B.; Ronaghi, M.; Fire, A.Z. A pyrosequencing-tailored nucleotide barcode design unveils opportunities for large-scale sample multiplexing. Nucleic Acids Research 2007, 35, e130–e130. [Google Scholar] [CrossRef] [PubMed]
- Vera, A.; Moreno, J.L.; Siles, J.A.; López-Mondejar, R.; Zhou, Y.; Li, Y.; García, C.; Nicolás, E.; Bastida, F. Interactive impacts of boron and organic amendments in plant-soil microbial relationships. Journal of Hazardous Materials 2021, 408, 124939. [Google Scholar] [CrossRef]
- Edgar, R.C. Uparse: Highly accurate otu sequences from microbial amplicon reads. Nature Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive bayesian classifier for rapid assignment of rrna sequences into the new bacterial taxonomy. Appl Environ Microbiol 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Abarenkov, K.Z. ; Allan; Piirmann, Timo; Pöhönen, Raivo; Ivanov, Filipp; Nilsson, R. Henrik; Kõljalg, Urmas. Unite usearch/utax release for eukaryotes. Version 04.04.2024. UNITE Community. 2024. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species:The need for a flexible asymmetrical approach. Ecological Monographs 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Campbell, C.L.; Neher, D.A. Estimating disease severity and incidence. In Epidemiology and management of root diseases, Campbell, C.L.; Benson, D.M., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 1994; pp. 117–147. [Google Scholar]
- Ploetz, R.C. Diseases of tropical fruit crops. Cabi Publishing: 2003.
- Agrios, G.N. Plant pathology. Elsevier: 2005.
- Gullino, M.L.; Albajes, R.; Nicot, P.C. Integrated pest and disease management in greenhouse crops. Springer International Publishing: New York, NY, USA, 2020; Vol. 9.
- Aulakh, M.S.; Malhi, S.S. Interactions of nitrogen with other nutrients and water: Effect on crop yield and quality, nutrient use efficiency, carbon sequestration, and environmental pollution. In Advances in agronomy, Academic Press: 2005; Vol. 86, pp 341-409.
- Naz, M.; Dai, Z.; Hussain, S.; Tariq, M.; Danish, S.; Khan, I.U.; Qi, S.; Du, D. The soil ph and heavy metals revealed their impact on soil microbial community. Journal of Environmental Management 2022, 321, 115770. [Google Scholar] [CrossRef] [PubMed]
- Bastida, F.; Zsolnay, A.; Hernández, T.; García, C. Past, present and future of soil quality indices: A biological perspective. Geoderma 2008, 147, 159–171. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biology and Biochemistry 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Prencipe, S.; Schiavon, G.; Rosati, M.; Nari, L.; Schena, L.; Spadaro, D. Characterization of phytopythium species involved in the establishment and development of kiwifruit vine decline syndrome. Microorganisms 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Brunner, F.; Wirtz, W.; Rose, J.K.C.; Darvill, A.G.; Govers, F.; Scheel, D.; Nürnberger, T. A β-glucosidase/xylosidase from the phytopathogenic oomycete, phytophthora infestans. Phytochemistry 2002, 59, 689–696. [Google Scholar] [CrossRef]
- Alconada, T.M.; Martínez, M.J. Purification and characterization of a β-glucosidase from the phytopathogenic fungus fusarium oxysporum f. Sp. Melonis. Letters in Applied Microbiology 1996, 22, 106–110. [Google Scholar] [CrossRef]
- Hossain, Z.; Hubbard, M.; Gan, Y.; Bainard, L.D. Root rot alters the root-associated microbiome of field pea in commercial crop production systems. Plant and Soil 2021, 460, 593–607. [Google Scholar] [CrossRef]
- Feng, Z.; Xiao, Y.; Li, N.; Gao, Q.; Wang, J.; Chen, S.-l.; Xing, R. Effects of root rot on microbial communities associated with goji berry (lycium barbarum) in the qaidam basin, china. European Journal of Plant Pathology 2023, 167, 853–866. [Google Scholar] [CrossRef]
- Wang, X.; Chi, Y.; Song, S. Important soil microbiota's effects on plants and soils: A comprehensive 30-year systematic literature review. Frontiers in Microbiology 2024, 15. [Google Scholar]
- Andreote, F.D.; Gumiere, T.; Durrer, A. Exploring interactions of plant microbiomes. Scientia Agricola 2014, 71. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition—current knowledge and future directions. Frontiers in Plant Science 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef] [PubMed]
- Savian, F.; Marroni, F.; Ermacora, P.; Firrao, G.; Martini, M. A metabarcoding approach to investigate fungal and oomycete communities associated with kiwifruit vine decline syndrome in italy. Phytobiomes Journal 2022, 6, 290–304. [Google Scholar] [CrossRef]
- Guaschino, M.; Garello, M.; Nari, L.; Zhimo, Y.V.; Droby, S.; Spadaro, D. Soil, rhizosphere, and root microbiome in kiwifruit vine decline, an emerging multifactorial disease. Frontiers in Microbiology 2024, 15. [Google Scholar] [CrossRef]
- Manici, L.M.; Caputo, F.; Luccioli, S.; Frattarelli, A.; Caboni, E. Phytotoxicity of dactylonectria spp. Exudates on kiwifruit vine and profile of secondary metabolites for understanding their relationship with the host plant. Rhizosphere 2024, 30, 100906. [Google Scholar] [CrossRef]
- Bergamaschi, V.; Pirone, L.; Valente, M.T.; Vitale, S.; Luongo, L.; Grottoli, A.; Marocchi, F.; Riccioni, L. Survey of kiwifruit vine decline syndrome in lazio region. Journal of Plant Pathology 2022, 104, 1207–1280. [Google Scholar]
- Tyson, J.L.; Donati, I.; Everett, K.R. Vine and fruit diseases. CABI 2023, 295–316. [Google Scholar]
- Manici, L.M.; Saccà, M.L.; Scotti, C.; Caputo, F. Quantitative reduction of soil bacteria and qualitative microbial changes: Biotic components associated to kiwifruit decline. Plant and Soil 2022, 477, 613–628. [Google Scholar] [CrossRef]
- Govindasamy, V.; George, P.; Raina, S.K.; Kumar, M.; Rane, J.; Annapurna, K. Plant-associated microbial interactions in the soil environment: Role of endophytes in imparting abiotic stress tolerance to crops. In Advances in crop environment interaction; Bal, S.K., Mukherjee, J., Choudhury, B.U., Dhawan, A.K., Eds.; Springer Singapore: Singapore, 2018; pp. 245–284. [Google Scholar]
Figure 1.
Bar chart of soil physicochemical properties of Orchards 1 and 2 in Spring and Autumn. pH, SOC: soil organic carbon content, WSC: soil water-soluble C, EC: electrical conductivity, Total N: total soil nitrogen content, WSN: soil water-soluble N, and the letters A and S indicate Asymptomatic and Symptomatic, respectively. One-way ANOVA significance is shown with different letters: a and b. Error bars represent the standard error of the mean (4 replicates).
Figure 1.
Bar chart of soil physicochemical properties of Orchards 1 and 2 in Spring and Autumn. pH, SOC: soil organic carbon content, WSC: soil water-soluble C, EC: electrical conductivity, Total N: total soil nitrogen content, WSN: soil water-soluble N, and the letters A and S indicate Asymptomatic and Symptomatic, respectively. One-way ANOVA significance is shown with different letters: a and b. Error bars represent the standard error of the mean (4 replicates).
Figure 2.
Bar chart of β-glucosidase (μmol PNF g-1 soil h-1), alkaline phosphatase (μmol PNF g-1 soil h-1), urease (μmol NH4+ g-1 soil h-1), and Basal Soil Respiration (BSR) (mg CO2 kg-1 soil day-1) of Orchard 1 and 2 in Spring and Autumn. The letters A and S indicate Asymptomatic and Symptomatic, respectively. One-Way ANOVA significance is shown with different letters: a and b. Error bars represent the standard error of the mean (4 replicates).
Figure 2.
Bar chart of β-glucosidase (μmol PNF g-1 soil h-1), alkaline phosphatase (μmol PNF g-1 soil h-1), urease (μmol NH4+ g-1 soil h-1), and Basal Soil Respiration (BSR) (mg CO2 kg-1 soil day-1) of Orchard 1 and 2 in Spring and Autumn. The letters A and S indicate Asymptomatic and Symptomatic, respectively. One-Way ANOVA significance is shown with different letters: a and b. Error bars represent the standard error of the mean (4 replicates).
Figure 3.
Bar chart of biomass abundance (nmol g-1 soil h-1) divided into Fungi, Bacteria, Gram- and Gram+ Bacteria, Actinobacteria, Total PLFAs, Fungi/Bacteria ratio, and Gram+/Gram- ratio of Orchard 1 and 2 in Spring and Autumn. The letters A and S indicate Asymptomatic and Symptomatic, respectively. One-way ANOVA significance is shown with different letters: a and b. Error bars represent the standard error of the mean (4 replicates).
Figure 3.
Bar chart of biomass abundance (nmol g-1 soil h-1) divided into Fungi, Bacteria, Gram- and Gram+ Bacteria, Actinobacteria, Total PLFAs, Fungi/Bacteria ratio, and Gram+/Gram- ratio of Orchard 1 and 2 in Spring and Autumn. The letters A and S indicate Asymptomatic and Symptomatic, respectively. One-way ANOVA significance is shown with different letters: a and b. Error bars represent the standard error of the mean (4 replicates).
Figure 4.
Heat map of Spearman’s correlation for soil and physiological parameters. Negative correlations and positive correlations are represented in blue and red, respectively. All correlations are significant at P < 0.05. EC: electrical conductivity, WSC: soil water-soluble C, WSN: soil water-soluble N, Amm: soil ammonium content, total N: total soil nitrogen content, total C: total soil carbon content, SOC: soil organic carbon, CaCO3: soil carbonate calcium content, CN: carbon:nitrogen ratio, bG: β-glucosidase activity, alkP: alkaline phosphatase activity, Ure: urease activity, BSR: basal soil respiration, Fun: soil fungal biomass, Bac: soil bacterial biomass, TB: total soil microbial biomass.
Figure 4.
Heat map of Spearman’s correlation for soil and physiological parameters. Negative correlations and positive correlations are represented in blue and red, respectively. All correlations are significant at P < 0.05. EC: electrical conductivity, WSC: soil water-soluble C, WSN: soil water-soluble N, Amm: soil ammonium content, total N: total soil nitrogen content, total C: total soil carbon content, SOC: soil organic carbon, CaCO3: soil carbonate calcium content, CN: carbon:nitrogen ratio, bG: β-glucosidase activity, alkP: alkaline phosphatase activity, Ure: urease activity, BSR: basal soil respiration, Fun: soil fungal biomass, Bac: soil bacterial biomass, TB: total soil microbial biomass.
Figure 5.
Composition of fungal (a) and bacterial (b) abundance at phylum level of orchards 1 and 2 in Spring and Autumn.
Figure 5.
Composition of fungal (a) and bacterial (b) abundance at phylum level of orchards 1 and 2 in Spring and Autumn.
Figure 6.
NMDS analysis of fungal community (a) and bacterial community (b) of Orchard 1 in Spring (dot) and Autumn (triangle), Orchard 2 in Spring (square) and Autumn (cross). Reported asymptomatic and symptomatic samples in red and blue respectively. For the PerMANOVA test, the significance levels are shown at * P ≤ 0.05, ** P ≤ 0.01 and *** P ≤ 0.001. OxC means Orchard and Status interaction.
Figure 6.
NMDS analysis of fungal community (a) and bacterial community (b) of Orchard 1 in Spring (dot) and Autumn (triangle), Orchard 2 in Spring (square) and Autumn (cross). Reported asymptomatic and symptomatic samples in red and blue respectively. For the PerMANOVA test, the significance levels are shown at * P ≤ 0.05, ** P ≤ 0.01 and *** P ≤ 0.001. OxC means Orchard and Status interaction.
Table 3.
Mean of Richness and Shannon index of fungal (ITS2) and bacterial (16S) communities. * indicates significance P ≤ 0.05 and ** indicates significance P ≤ 0.01 for the One-Way ANOVA analysis.
Table 3.
Mean of Richness and Shannon index of fungal (ITS2) and bacterial (16S) communities. * indicates significance P ≤ 0.05 and ** indicates significance P ≤ 0.01 for the One-Way ANOVA analysis.
| |
Spring |
Autumn |
| Factor |
O1A |
O1S |
O2A |
O2S |
O1A |
O1S |
O2A |
O2S |
| ITS2 Richness |
348 |
366 |
427 |
396 |
379 |
400 |
458 |
448 |
| ITS2 Shannon |
4.03 |
3.98 |
4.69 |
4.35 |
4.25 |
4.50 |
4.72 |
4.75 |
| 16S Richness |
1261 |
1392 * |
1310 |
1439 * |
1311 |
1259 |
1354 |
1361 |
| 16S Shannon |
6.19 |
6.45 ** |
6.24 |
6.41 * |
6.21 |
6.12 |
6.26 |
6.36 |
Table 4.
OTUs representative of symptomatic samples obtained by indicator species analysis comparing the OTU Table of Symptomatic and Asymptomatic samples of both seasons. Reported the OTU number, Statistical Value (Stat), p-value, and identification (d: domain, p: phylum, c: class, o: order, f: family, g: genera, s: species).
Table 4.
OTUs representative of symptomatic samples obtained by indicator species analysis comparing the OTU Table of Symptomatic and Asymptomatic samples of both seasons. Reported the OTU number, Statistical Value (Stat), p-value, and identification (d: domain, p: phylum, c: class, o: order, f: family, g: genera, s: species).
| OTU Number |
Stat |
p-value |
Identification |
| Otu289 |
0.491 |
P ≤ 0.01 |
d: Fungi, p: Ascomycota, c: Leotiomycetes
|
| Otu112 |
0.487 |
P ≤ 0.01 |
d: Fungi, p: Ascomycota, c: Dothideomycetes, o: Pleosporales, f: Periconiaceae
|
| Otu200 |
0.463 |
P ≤ 0.01 |
d: Fungi, p: Ascomycota, c: Sordariomycetes, o: Hypocreales, f: Hypocreaceae
|
| Otu73 |
0.446 |
P ≤ 0.01 |
d: Fungi, p: Ascomycota, c: Sordariomycetes, o: Chaetosphaeriales, f: Chaetosphaeriaceae,g: Chloridium
|
| Otu74 |
0.436 |
P ≤ 0.01 |
d: Fungi, p: Ascomycota, c: Sordariomycetes, o: Glomerellales, f: Plectosphaerellaceae, g: Stachylidium, s: Stachylidium_bicolor
|
| Otu435 |
0.434 |
P ≤ 0.01 |
d: Fungi, p: Ascomycota, c: Eurotiomycetes, o: Eurotiales, f: Aspergillaceae, g: Aspergillus, s: Aspergillus_mangaliensis
|
| Otu207 |
0.429 |
P ≤ 0.01 |
d: Fungi |
| Otu520 |
0.423 |
P ≤ 0.01 |
d: Fungi, p: Ascomycota, c: Saccharomycetes, o: Saccharomycetales, f: Phaffomycetaceae, g: Wickerhamomyces
|
| Otu1916 |
0.417 |
P ≤ 0.01 |
d: Fungi, p: Ascomycota, c: Sordariomycetes, o: Chaetosphaeriales, f: Chaetosphaeriaceae
|
| Otu76 |
0.386 |
P ≤ 0.001 |
d: Fungi, p: Ascomycota
|
| Otu284 |
0.385 |
P ≤ 0.01 |
d: Fungi, p: Mucoromycota, c: Mucoromycetes, o: Mucorales, f: Mucoraceae, g: Mucor, s: Mucor_circinelloides
|
| Otu332 |
0.372 |
P ≤ 0.01 |
d: Fungi, p: Ascomycota, c: Dothideomycetes, o: Pleosporales
|
| Otu434 |
0.365 |
P ≤ 0.01 |
d: Fungi
|
| Otu177 |
0.353 |
P ≤ 0.01 |
d: Fungi, p: Ascomycota, c: Dothideomycetes, o: Pleosporales, f: Phaeosphaeriaceae, g: Phaeosphaeriopsis
|
| Otu191 |
0.329 |
P ≤ 0.01 |
d: Fungi, p: Ascomycota, c: Dothideomycetes, o: Pleosporales, f: Pleosporaceae, g: Alternaria
|
| Otu108 |
0.324 |
P ≤ 0.01 |
d: Fungi, p: Mortierellomycota, c: Mortierellomycetes, o: Mortierellales, f: Mortierellaceae, g: Mortierella
|
Table 5.
OTUs representative of asymptomatic samples obtained by indicator species analysis comparing the OTU Table of Symptomatic and Asymptomatic samples of both seasons. Reported the OTU number, Statistical Value (Stat), p-value, and identification (d: domain, p: phylum, c: class, o: order, f: family, g: genera, s: species).
Table 5.
OTUs representative of asymptomatic samples obtained by indicator species analysis comparing the OTU Table of Symptomatic and Asymptomatic samples of both seasons. Reported the OTU number, Statistical Value (Stat), p-value, and identification (d: domain, p: phylum, c: class, o: order, f: family, g: genera, s: species).
| OTU Number |
Stat |
p-value |
Identification |
| Otu258 |
0.506 |
P<0.001 |
d: Fungi, p: Ascomycota, c: Pezizomycetes, o: Pezizales, f: Pyronemataceae
|
| Otu201 |
0.502 |
P<0.01 |
d: Fungi, p: Ascomycota, c: Eurotiomycetes, o: Eurotiales, f: Aspergillaceae, g: Penicillium
|
| Otu806 |
0.490 |
P<0.01 |
d: Fungi, p: Ascomycota, c: Sordariomycetes, o: Hypocreales, f: Clavicipitaceae, g: i, s: Keithomyces_indicus
|
| Otu27 |
0.486 |
P<0.001 |
d: Fungi, p: Ascomycota, c: i, o: Sordariales, f: Chaetomiaceae
|
| Otu1041 |
0.484 |
P<0.01 |
d: Fungi, p: Ascomycota, c: Sordariomycetes, o: Hypocreales, f: Stachybotryaceae, g: Stachybotrys, s: Stachybotrys_limonisporus |
| Otu69 |
0.479 |
P<0.001 |
d: unidentified
|
| Otu675 |
0.469 |
P<0.01 |
d: Fungi, p: Rozellomycota
|
| Otu460 |
0.445 |
P<0.01 |
d: unidentified |
| Otu16 |
0.442 |
P<0.01 |
d: Fungi, p: Ascomycota, c: Sordariomycetes, o: Hypocreales, f: Nectriaceae, g: Fusarium
|
| Otu98 |
0.431 |
P<0.01 |
d: Fungi, p: Ascomycota, c: Dothideomycetes, o: Pleosporales, f: Massarinaceae, g: Stagonospora, s: Stagonospora_heteroderae |
| Otu107 |
0.423 |
P<0.01 |
d: Fungi, p: Ascomycota, c: Eurotiomycetes
|
| Otu391 |
0.418 |
P<0.01 |
d: Fungi, p: Ascomycota, c: Pezizomycetes, o: Pezizales, f: Pyronemataceae
|
| Otu173 |
0.407 |
P<0.01 |
d: Fungi, p: Ascomycota, c: Dothideomycetes, o: Tubeufiales, f: Tubeufiaceae, g: Helicoma
|
| Otu72 |
0.406 |
P<0.01 |
d: Fungi, p: Ascomycota, c: Sordariomycetes, o: Microascales, f: Halosphaeriaceae
|
| Otu104 |
0.403 |
P<0.01 |
d: Fungi, p: Basidiomycota, c: Agaricomycetes, o: Phallales, f: Phallaceae, g: Phallus, s: Phallus_hadriani |
| Otu105 |
0.402 |
P<0.01 |
d: Fungi, p: Ascomycota, c: Sordariomycetes
|
| Otu1389 |
0.382 |
P<0.01 |
d: Fungi, p: Ascomycota, c: Sordariomycetes, o: Sordariales, f: Chaetomiaceae, g: Chaetomium
|
| Otu591 |
0.355 |
P<0.01 |
d: Fungi, p: Ascomycota, c: Sordariomycetes, o: Coniochaetales
|
| Otu78 |
0.325 |
P<0.001 |
d: Fungi, p: Basidiomycota, c: Agaricomycetes, o: Phallales, f: Phallaceae, g: Phallus
|
| Otu274 |
0.299 |
P<0.01 |
d: Fungi, p: Ascomycota, c: Eurotiomycetes, o: Chaetothyriales, f: Herpotrichiellaceae, g: Cladophialophora
|
| Otu561 |
0.291 |
P<0.001 |
d: Fungi, p: Glomeromycota, c: Glomeromycetes, o: Glomerales, f: Glomeraceae
|
| Otu351 |
0.287 |
P<0.01 |
d: Fungi, p: Rozellomycota
|
| Otu243 |
0.220 |
P<0.01 |
d: Fungi, p: Basidiomycota, c: Agaricomycetes, o: Phallales, f: Clathraceae, g: Clathrus, s: Clathrus_ruber |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).