1. Introduction
Inspired by “genomics,” various “-omics” fields like transcriptomics, proteomics, metabolomics, glycomics, and lipidomics have emerged over the past two decades [
1,
2]. Traditional biochemical methods are inefficient, while omics technologies use high-throughput methods like microarrays and mass spectrometry to generate extensive datasets [
1,
3,
4]. Supported by bioinformatics, these advancements offer significant insights into biological mechanisms, forming a comprehensive framework for modern life science [
5,
6,
7,
8].
The postgenomic era has driven omics technologies in biomedical [
2,
6,
9,
10,
11] and pharmaceutical research [
8,
12], enabling efficient exploration of genomes, transcriptomes, and proteomes with high sensitivity and resolution [
13]. Omics technologies revolutionize biological research by examining collective interactions within cellular systems or biochemical processes, rather than isolated components. Omics represents the evolution of collective thought and data, forming a crucial part of systems biology [
2,
12]. These technologies facilitate identifying and validating novel therapeutic targets, assessing drug toxicity and safety, exploring pharmacology, and establishing molecular-based diagnoses and prognoses, thus paving the way for personalized healthcare [
7,
9].
Systems immunology offers a comprehensive understanding of the immune system by examining single immunological components and pathways as interconnected networks[
14]. This holistic approach contrasts with methods that focus on individual parts, aiming instead to elucidate how these parts interact and function together, a complex task requiring specialized methodologies [
15].
Over the past century, experimental strategies have defined cell types and states within the immune system, revealing key molecular and functional components and establishing causal relationships in transcriptional and functional cascades driving immune activation [
16]. High-throughput, high-resolution technologies from the omics field have revolutionized immunology in the last two decades, enabling the simultaneous assessment of numerous cellular, functional, and molecular parameters [
15,
17]. These advancements allow the simultaneous evaluation of hundreds to thousands of cellular, functional, and molecular parameters with increasing efficiency and speed. Immunomics, intersecting immunology and genomics, is crucial in studying implant-based capsular fibrosis, particularly with SMIs [
18,
19,
20]. The complex interplay between the immune system and foreign materials leads to adverse reactions and fibrotic responses [
21,
22,
23,
24,
25,
26,
27,
28,
29,
30].
Silicone, widely used in medical implants, triggers foreign body responses, leading to the formation of a fibrous capsule and impaired tissue function [
31,
32,
33,
34,
35]. While SMIs have transformed breast augmentation and reconstruction [
36,
37,
38], their clinical utility is often hampered by capsular contracture, characterized by excessive extracellular matrix (ECM) accumulation around the implant [
39]. This fibrotic reaction, driven by the host immune response, presents a significant clinical challenge [
40].
Fibrosis initiation involves an inflammatory phase mediated by innate and adaptive immune cells [
28,
29,
30,
41]. Macrophages, neutrophils, and mast cells activate fibroblasts to produce ECM proteins [
18,
20,
28,
29,
30,
41,
42,
43,
44,
45]. Silicone binds nonspecifically to blood proteins, leading to inflammation and protein adsorption on implant surfaces [
30,
41,
46,
47,
48,
49,
50]. Macrophages and other immune cells uptake silicone debris, contributing to fibrotic responses [
48,
51,
52,
53,
54,
55]. SMIs exemplify foreign body-induced fibrotic diseases relevant to other silicone-based medical devices [
30,
31,
32,
41,
55,
56,
57].
In recent years, there has been a growing interest in elucidating the molecular mechanisms underlying SMI capsular fibrosis [
28,
29,
45,
58], with a particular focus on the intricate interplay between the immune system and implanted materials [
34,
41,
54,
55,
56,
57,
59,
60,
61,
62,
63]. Understanding these immune-mediated processes is crucial for developing targeted therapeutic strategies to mitigate or prevent capsular fibrosis and improve patient outcomes following breast implant surgery.
In this review, we aim to provide a comprehensive overview of the immune-mediated mechanisms involved in SMI associated capsular fibrosis, focusing on molecular insights and potential therapeutic interventions. We synthesize current knowledge and recent advancements in the field, exploring the molecular pathways and cellular responses that drive fibrosis in response to SMI implantation. Additionally, we discuss the clinical implications of capsular fibrosis, including its impact on patient outcomes, diagnostic challenges, and current treatment modalities.
This review evaluates preclinical breakthroughs and innovative research strategies aimed at understanding SMI-induced fibrosis, highlighting their translational potential for developing targeted therapies to prevent or mitigate capsular fibrosis in clinical practice. Our goal is to provide clinicians, researchers, and healthcare professionals with a thorough understanding of SMI capsular fibrosis and its immune-mediated pathogenesis, identifying novel therapeutic avenues to improve patient outcomes in breast implant surgery.
Immunomics offers significant promise for understanding the immunological basis of implant-based capsular fibrosis (IBCF) and devising innovative diagnostic and treatment strategies. By modulating immune responses and inflammation pathways identified through immunomics, tailored interventions can prevent or alleviate capsular contracture. Through interdisciplinary collaboration and cutting-edge technologies, immunomics has the potential to revolutionize the management of capsular fibrosis in implant-based surgeries.
2. Molecular Mechanisms of SMI Capsular Fibrosis
Breast augmentation with SMIs is a widely practiced procedure, with approximately 2,2 million surgeries performed in 2022 alone [
64]. Despite its popularity, peri-SMI capsular contracture remains a significant complication, with reported incidence rates ranging from 0.5% to 50% [
65,
66,
67,
68]. This condition involves the formation of a fibrous capsule around the implant, leading to pain, aesthetic issues, and potential functional impairment, necessitating revision surgery or even implant removal.
In contrast, breast reconstruction is often performed after a mastectomy and typically employs an immediate two-stage expander-based approach. his method involves the use of an inflatable implant known as a tissue expander, which allows for the gradual expansion of the mastectomy skin flap before placing a permanent implant [
69,
70,
71]. The choice of tissue expander, influenced by surface roughness, plays a critical role in determining final aesthetic outcomes and the likelihood of fibrotic capsule formation [
39,
55,
72,
73,
74,
75,
76]. Histopathological studies underscore that the type of tissue expander used can imprint characteristics onto the capsule, influencing long-term results [
39,
41,
57,
62].
Capsular fibrosis results from a complex interplay of factors, primarily driven by the foreign body response (FBR) initiated upon implantation [
28]. This cascade involves excessive deposition of collagenous and non-collagenous ECM components by activated fibroblasts and myofibroblasts. Chronic inflammatory reactions triggered by stimuli such as infections, autoimmune reactions, and tissue injury further exacerbate fibrosis through pathways including TGF-β, Smad, NF-κB, and MAPK signaling [
19,
21,
22,
23,
24,
25,
26,
27,
34].
Research on silicone-induced fibrosis, particularly with SMIs, serves as a model for understanding foreign body-induced fibrotic diseases.
SMIsprovoke a multifaceted immune response and subsequent fibrosis [
30,
41]. This process is critical in understanding the body’s reaction to such implants, which can significantly impact the success and longevity of the implant [
39]. The immune response to SMIs involves various cellular and molecular mechanisms, including inflammation, immune cell activation, and fibrosis formation.
The process of capsular fibrosis around SMIsis characterized by a complex sequence of biological responses, including immediate immune activation, early-stage inflammatory and fibrotic changes, and chronic inflammation leading to capsular contracture (
Figure 1).
When an SMI is implanted, the body recognizes it as a foreign object, triggering an immediate immune response (
Figure 1). This response begins with the activation of the innate immune system, which includes the recruitment of immune cells such as neutrophils, macrophages, and dendritic cells to the site of SMI implantation. These cells release cytokines and chemokines, signaling molecules that mediate inflammation and recruit additional immune cells to the area [
28,
29,
30,
41,
77,
78].
During the acute phase of the immune response, which occurs within the first few days post-implantation, there is a surge in systemic inflammatory mediators. This includes a rapid increase in cytokines such as IFN-γ, IL-1β, and TNF-α, which are associated with a Th1-type immune response [
28,
29,
41]. These cytokines promote inflammation and prepare the site for subsequent wound healing and fibrosis formation phases [
30].
Macrophages play a crucial role in the immune response to SMIs (
Figure 1). They can adopt different functional phenotypes, including the pro-inflammatory M1 phenotype and the anti-inflammatory, tissue-remodeling M2 phenotype. The balance between these phenotypes influences the extent of inflammation and fibrosis around the implant [
30,
41,
73].
M1 macrophages, which are predominant during the early inflammatory response, produce pro-inflammatory cytokines that exacerbate inflammation. In contrast, M2 macrophages, which appear later, secrete anti-inflammatory cytokines and growth factors that promote tissue repair and fibrosis [
41,
79]. The persistence of M1 macrophages and a delayed transition to the M2 phenotype can lead to chronic inflammation and excessive fibrosis [
41,
73,
79].
Fibrosis around SMIs is a result of the body’s attempt to isolate and protect itself from the foreign material. This process involves the deposition of ECM components (
Figure 1), such as collagen, which form a fibrous capsule around the implant [
30,
78,
80,
81]. The degree of fibrosis is influenced by various factors, including the extent of the initial inflammatory response and the duration of chronic inflammation [
30,
41,
78,
80,
81].
Key mediators of fibrosis include transforming growth factor-beta (TGF-β), which stimulates the production of ECM components and inhibits their degradation [
28,
29,
30,
41,
78]. The balance between matrix metalloproteinases (MMPs), which degrade ECM, and their tissue inhibitors (TIMPs) is also crucial in regulating fibrosis [
28,
29,
30,
41,
78]. An imbalance favoring TIMPs over MMPs can lead to excessive ECM deposition and fibrosis [
41,
80,
81].
Chronic inflammation is a significant factor in the long-term response to SMIs (
Figure 1c). Persistent inflammation can result in ongoing tissue remodeling and fibrosis, leading to the formation of a thick, fibrous capsule around the implant. This capsule can contract over time, causing capsular contracture, which is a common complication of breast implants [
32,
39,
77,
82,
83].
Markers of chronic inflammation, such as S100 proteins, are often upregulated in response to SMIs. These proteins play a role in sustaining the inflammatory response and promoting fibrosis [
30,
41,
84,
85,
86,
87]. The accumulation of pro-inflammatory and pro-fibrotic cytokines, such as IL-17 and TGF-β, further contributes to chronic inflammation and fibrosis [
30,
41,
73,
79].
The extent of capsular contracture varies based on factors such as implant type and placement [
88,
89,
90,
91]. Clinical studies have provided compelling evidence that implant surfaces with smoother textures, specifically those with reduced roughness profiles like Ra 4 µm, elicit reduced levels of inflammation and fibrosis compared to their rougher counterparts [
30,
39,
41,
55,
59,
73,
92,
93]. These findings underscore the pivotal role of surface microtopography in modulating tissue responses post-implantation. Smoother surfaces are associated with diminished immune activation, characterized by lower levels of pro-inflammatory cytokines and a decelerated pro-fibrotic response [
41,
55,
73]. This effect is attributed to altered protein adsorption patterns and reduced cell adhesion on smoother surfaces, which collectively contribute to a more favorable tissue integration environment [
30,
41,
56,
59,
61]. By minimizing the inflammatory cascade and promoting a balanced cytokine profile, smoother surfaces have the potential to enhance implant biocompatibility and mitigate complications such as capsular contracture [
39,
41]. These insights highlight the significance of surface engineering strategies aimed at optimizing implant design to improve patient outcomes across diverse clinical settings. The impact of the expander used in the initial reconstruction stage on long-term outcomes is significant. Studies show that using different surface topographies in tissue expanders affects the final reconstructive result by influencing tissue imprinting [
39,
94].
3. Genomics and Proteomics in SMI Capsular Fibrosis
3.1. Genomic Insights into SMI Capsular Fibrosis
Genomic profiling techniques play a pivotal role in unraveling the molecular underpinnings of capsular fibrosis, a vexing complication following breast implantation surgeries. Investigating the genetic underpinnings of SMI capsular fibrosis, a recent genome-wide study stands as a pioneering effort in unraveling the molecular landscape of this complication.
3.1.1. Genomic Profiling of Capsular Fibrosis
In their seminal study, Kyle et al. employed whole genome transcriptome analysis of capsular tissue to explore the dysregulated genetic landscape associated with breast fibrotic capsule formation, shedding light on potential biomarkers that govern this pathological process [
95]. By leveraging microarray technology, the researchers identified 257 significantly dysregulated genes in contracted breast capsules compared to controls. From these, six genes were scrutinized further based on their biological relevance and degree of dysregulation: aggrecan (ACAN), interleukin 8 (IL8), matrix metallopeptidase 12 (MMP12), serum amyloid A 1 (SAA1), tissue inhibitor of metalloproteinase 4 (TIMP4), and tumor necrosis factor superfamily member 11 (TNFSF11). The findings underscored distinct patterns of gene expression in contracted capsules, revealing up-regulation of IL8, MMP12, and SAA1, and down-regulation of ACAN, TIMP4, and TNFSF11. Validation through quantitative reverse transcriptase polymerase chain reaction (QRT-PCR) corroborated these findings, confirming significant up-regulation of IL8 and down-regulation of ACAN, TIMP4, and TNFSF11 in contracted capsules. Immunohistochemistry (IHC) further supported these results, demonstrating increased protein expression of IL8 and MMP12, alongside decreased expression of TIMP4 in contracted tissues [
95].
As detailed in
Figure 2, genome-wide studies represent a pivotal advancement in the exploration of SMI capsular fibrosis, providing insights into the intricate molecular mechanisms underlying this complex condition.
The study’s [
95] approach not only highlighted the pivotal role of inflammatory responses (IL8) and extracellular matrix remodeling (MMP12) in capsular fibrosis but also emphasized the potential diagnostic and therapeutic implications of these findings. IL8, known for its role in acute inflammation and fibrotic conditions, emerged as a key mediator in perpetuating the foreign body response around breast implants. Conversely, TIMP4’s diminished expression suggested a dysregulated matrix remodeling environment, conducive to fibrotic encapsulation.
However, while RT-qPCR offers high sensitivity and specificity in quantifying gene expression, its scope is often limited to known targets and predefined pathways, potentially overlooking novel genes crucial to the disease process [
95,
96]. In contrast, RNA Seq presents a promising alternative by enabling unbiased, genome-wide profiling of gene expression, thus facilitating the discovery of novel biomarkers and pathways implicated in SMI capsular fibrosis as already demonstrated for other types of inflammatory fibrotic diseases affecting soft tissues including lung, liver, kidney and skin [
97].
This methodological shift from targeted approaches like RT-qPCR to RNA Seq not only enhances the breadth of data captured but also supports the identification of previously unrecognized molecular signatures driving disease progression. Despite these advantages, RNA Seq is not without challenges, including the need for substantial bioinformatics expertise and computational resources for data analysis, which may pose barriers to widespread adoption in clinical settings.
3.1.2. Challenges and Future Directions
Applying genomic profiling techniques provides a comprehensive understanding of the molecular mechanisms underpinning breast capsular contracture formation. By identifying novel biomarkers and unraveling intricate gene expression patterns, this research establishes a foundational basis for developing future diagnostic tools and targeted therapeutic strategies to reduce the occurrence and severity of this prevalent post-surgical complication. It is important to note that a significant challenge in advancing genomic research on SMI capsular fibrosis is the acquisition of comprehensive patient material. Variability in disease presentation, patient demographics, and disease severity necessitate large-scale, well-characterized cohorts for robust statistical analyses and generalizability of findings [
98,
99,
100,
101].
3.2. Proteomic Insights into SMI Capsular Fibrosis
Proteomics differs from genomic studies by directly investigating the protein composition, modifications, and interactions that define the pathophysiology of capsular fibrosis. This capability is essential for identifying specific biomarkers reflecting disease progression and treatment response, enhancing diagnostic accuracy and prognostic assessment in clinical settings. By uncovering novel protein biomarkers and pathways associated with SMI capsular fibrosis, proteomics not only deepens our understanding of disease mechanisms but also establishes the groundwork for personalized therapeutic strategies aimed at improving patient care and outcomes. Analyzing SMI capsular fibrosis through proteomics, rather than genomics, provides distinct advantages due to its ability to capture dynamic protein interactions at the implant interface and surrounding tissues, which play crucial roles in inflammation, immune responses, and fibrotic changes.
3.2.1. Proteomic Studies on Protein Adsorption and Immune Responses
Protein adsorption to the surface of silicon mammary implants (SMI) has been extensively studied, both by incubation with serum proteins [
56,
61,
62,
102] and post-operatively by stripping off the proteome in animal models [
73]. The use of silicone-linked immuno-sorbent assay (SILISA) facilitated an understanding of how proteins adhere to silicone implants, inducing adverse immunological reactions and fibrotic responses [
56,
61]. Statistical analyses of proteins like fibronectin, C-reactive protein (CRP), immunoglobulin G (IgG), and heat shock protein 60 (HSP 60) revealed their significant correlation with fibrotic reactions.
In another study, researchers investigated serological parameters in 143 individuals, including 93 with SMIs and 50 controls, to evaluate systemic effects associated with these implants [
62]. Patients with SMIs exhibited elevated levels of circulating immune complexes (CIC), anti-polymer antibodies (APA), procollagen III, and soluble intercellular adhesion molecule-1 (sICAM-1) compared to controls. These differences correlated with the severity of capsular fibrosis and the duration of implantation, indicating a connection between serological abnormalities and fibrotic complications surrounding SMIs. The study underscored the potential clinical utility of these serological markers in predicting and monitoring adverse outcomes in SMI patients, advocating for thorough clinical and serologic monitoring in this population.
Of note, the serum is not the only origin of foreign body response towards implants. Tissue injury, after surgical insertion of SMI, immediately activates the innate immune system setting in motion a local inflammatory response and proinflammatory mediation that includes the recruitment of inflammatory cells from the circulation [
28,
29]. Nowadays, wound fluid is commonly used for protein profiling and analysis. However, the correct method of sample collection is crucial in highly sensitive proteomic analyses [
103,
104].
3.2.2. Proteomic Profiling of SMI Surfaces
Protein adsorption to silicone surfaces was further investigated using an untargeted proteomics approach focused on identifying proteins critical for local immune reactions to silicone implants [
102]. The study utilized both, in vivo analyses of explanted silicone implants and in vitro models incubated with wound bed fluid. Differential analysis, mass spectrometry, database matching, and Western blotting were employed to identify the 30 most abundant proteins adhering to silicone (e.g., Actin, Fibronectin, Vitronectin, Fibrinogen, Collagen I, Laminin, MMP2). Structural proteins, host defense mediators, and transport-related proteins emerged as predominant components. Additionally, biochemical modifications of fibronectin, vitronectin, and HSP 60 were observed post-adhesion. These findings underscored the role of silicone surface properties in protein degradation and unfolding, potentially leading to immune responses and fibrotic processes. New targets for prognosis and therapy in silicone implant-related complications were identified. For untargeted proteomics and biomarker discovery studies, identification and measurement of large protein numbers simultaneously by mass spectrometry is favored, yet rare in implant-immunoreactivity research [
105].
3.2.3. Intraindividual Comparative Proteomic Profiling
Protein adsorption and its implications were further investigated in real time in patients through intraindividual comparative proteomic profiling of plasma, wound fluid, and adhesive peptidomes associated with silicone breast implants (SMIs) over an extended period post-SMI implantation (
Figure 3) [
30]. Pre-operative plasma samples were taken before surgery to understand baseline systemic protein profiles, while wound fluid from surgical drains was collected daily from day 1 to day 5 post-implantation to capture the acute phase of wound healing and inflammation. Additionally, proteins adhered to the SMI surface were analyzed approximately eight months after implantation to identify chronic inflammatory responses and potential biomarkers for capsular fibrosis. By using samples from the same individuals, the study minimized inter-individual variability and provided a comprehensive characterization of protein profile changes during different phases of wound healing and inflammatory reactions at the implant site. The study employed Tandem Mass Tags (TMT) based mass spectrometry to quantify the proteins from wound fluid and plasma samples. Additionally, proteins adhering to the silicone surface of the tissue expanders (inflatable SMI), removed approximately six to eight months post-SMI implantation, were also analyzed by nanoLC-MS/MS.
The intraindividual comparative proteomic profiling of plasma, wound fluid, and adhesive proteomes associated with SMIs provided a detailed insight into the dynamic protein changes during the post-operative healing process and subsequent fibrosis development. Pre-operative plasma samples established a systemic protein baseline, while post-operative wound fluid, collected daily from day 1 to day 5, captured the acute immune response to tissue injury and implant placement. The proteomic landscape during the early postoperative period showed elevated expression of proteins involved in oxidative stress, coagulation, and immediate wound healing, such as superoxide dismutase, catalase, and plasminogen activator inhibitor-1 (PAL-1), which are critical for controlling bleeding and clot formation. The wound fluid also revealed neutrophil-derived enzymes like matrix metalloproteinases (MMP8, MMP9), underscoring the onset of ECM remodeling—a process crucial for both normal wound healing and fibrosis progression.
At eight months post-implantation, the adhesive proteome on the SMI surface reflected a shift towards chronic inflammation, with the persistence of proteins linked to ECM turnover, such as various collagen types (COL1, COL3, COL6), and fibrosis drivers like S100A8 and S100A9, indicating an ongoing fibrotic response. Antimicrobial proteins such as PGLYRP1 and CAMP were also present, suggesting a sustained immune response contributing to chronic inflammation. Heat shock proteins (HSP60, HSP90) adhered to the implant surface, playing roles in both inflammatory regulation and fibrosis. These findings point to a prolonged immune reaction and ECM dysregulation, further driving fibrotic encapsulation around the implant.
In comparison with genomic profiling [
95], this method provided a more direct view of the actual protein activities and changes at the implant site over time. Genomic analyses tend to highlight potential signaling pathways and gene expressions, such as those mediated by TGF-β and interferon-γ, which modulate fibroblast activation and ECM deposition. However, proteomic profiling revealed the functional protein products and their interactions within the wound environment, offering a clearer picture of the real-time biological processes. While genomic data emphasize regulatory cytokines like IL-6 and TNF-α in the transition from inflammation to fibrosis, proteomic profiling directly captures the proteins driving ECM remodeling and chronic immune responses, offering potential biomarkers for diagnosing and managing capsular fibrosis in breast implant patients.
This method enabled a detailed investigation of the wound proteome’s response to different surface textures of SMI [
30,
41,
59].
3.3. Integration of Genomics and Proteomics for Comprehensive Understanding
3.3.1. Complementary Roles of Genomics and Proteomics
Genomic profiling techniques, such as RT-qPCR and RNA Seq have shed light on the molecular mechanisms underpinning conditions like capsular fibrosis, emphasizing critical pathways related to inflammation and ECM remodeling. By identifying key differentially expressed genes, these analyses elucidate how various cellular signals contribute to the fibrotic process [
95,
96,
97].
In contrast, proteomic approaches provide a real-time perspective by examining protein interactions directly at the implant interface [
30,
41,
61]. Techniques such as SILAC [
61] and mass spectrometry [
30,
41] allow for the detection of specific proteins involved in chronic inflammation and fibrosis development, offering a dynamic view of the biological landscape. These proteomic studies have identified novel biomarkers that could enhance diagnostic accuracy and inform therapeutic strategies for managing capsular fibrosis.
While genomic analyses focus on gene expression patterns, proteomic studies capture the temporal changes in protein expression and their contributions to inflammatory responses and the progression of fibrosis. Together, these findings illuminate the complex interplay between molecular and protein-level changes, highlighting potential biomarkers for early detection and identifying new therapeutic targets. This integrative approach promises to improve clinical outcomes for patients with silicone breast implants by providing a comprehensive understanding of the mechanisms driving fibrotic encapsulation.
3.3.2. Future Directions and Clinical Implications
Both genomic and proteomic approaches are indispensable for elucidating the complex molecular mechanisms underlying SMI capsular fibrosis. While genomics provide fundamental genetic insights, proteomics offers a more dynamic and clinically relevant perspective by directly analyzing protein interactions crucial to disease pathogenesis. While genomics provides foundational genetic insights, proteomics offers a more dynamic and clinically relevant perspective by directly analyzing protein interactions crucial to disease pathogenesis. This integrated approach not only enhances our understanding of disease mechanisms but also paves the way for improved patient care and outcomes through personalized medicine strategies.
4. Fibroblast Dynamics and Immune Interactions: Navigating Capsular Fibrosis in Silicone Implant Biocompatibility
4.1. Fibroblast Activation and Differentiation
Capsular fibrosis, a common complication following silicone implantation, involves complex interactions between biomaterial surfaces and host tissue responses, prominently featuring fibroblast activation and differentiation processes [
28]. Fibroblasts are crucial in wound healing and tissue repair, undergoing distinct activation states in response to signals from the implant microenvironment [
106].
4.1.1. Role of Fibroblasts in Wound Healing
Fibroblasts play a vital role in all three phases of wound healing (WH), orchestrating the repair process by producing regulatory molecules and interacting with other cell populations involved in healing mechanisms [
107,
108]. Injury triggers an inflammatory reaction via cytokines from platelet degranulation [
109]. Immune cells increase pro-inflammatory mediators, such as interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-12 (IL-12), tumor necrosis factor-α (TNF-α), and inducible nitric oxide synthase (iNOS), fueling inflammation and stimulating fibroblast recruitment and activation [
109].
4.1.2. Fibroblast Response to Silicone Implants
Silicone surfaces initiate a cascade of events triggering acute and chronic inflammatory responses [
28,
29,
30]. Initial acute responses involve immune cell recruitment and cytokine release, influencing fibroblast behavior [
28,
29,
30]. Immunohistochemical analysis of fibrous capsules from patients with silicone breast implants showed the presence of fibroblasts along with macrophages, dendritic cells, and activated CD4+ T-cells at the capsule/silicone implant interface [
57]. Activated fibroblasts transition into myofibroblasts, characterized by α-smooth muscle actin (α-SMA) expression and enhanced contractility [
110]. Fibroblasts accumulate around implants, correlating with contracture severity as per the Baker classification system [
111,
112]. This indicates fibroblasts play a crucial role in the formation and maintenance of the fibrotic capsule.
4.1.3. Crosstalk and Inflammatory Phase
During the inflammatory phase, activated fibroblasts produce pro-inflammatory cytokines like TNF-α, interferon-gamma (IFN-γ), IL-6, and IL-12, and release chemokines such as CXCL1, CX3CL1, and CCL2 to recruit immune cells [
109,
113]. They also interact via ICAM1 and CD40 expression to activate dendritic cells [
114], remodel the wound stroma through matrix metalloproteinases (MMPs) secretion, and respond to interstitial flow changes by modifying microenvironment properties [
115,
116]. Collectively, fibroblasts modulate immune cell recruitment, behavior, retention, and survival in damaged tissue, with fibroblast-macrophage crosstalk being particularly important for transitioning from the inflammatory to the proliferation phase, ensuring proper healing progression [
106,
117].
4.1.4. Influence of Implant Surface Properties
In implant-related fibrosis, the interplay among the inflammatory milieu, reactive oxygen species, and implant surface characteristics significantly impacts fibroblast behavior and the formation of fibrotic capsules [
48,
118]. Fibroblasts generate fibrous ECM around implants, rich in collagen I/III, fibronectin, and proteoglycans [
119]. Myofibroblasts, central to this process, form stress fibers and express α-SMA, exerting mechanical forces essential for ECM organization and crosslinking [
112,
120].
The surface topography of silicone implants profoundly influences fibroblast activity. Surface topography and texture impact fibroblast attachment, proliferation, migration, and differentiation, suggesting that implant surface physical properties modulate fibroblast responses and potentially influence fibrous capsule formation [
55].
Histological studies identified increased CD3+ T cells and macrophages in capsular biopsies from textured implants, indicating an interplay between T-cells and fibroblasts in the fibrotic response[
55]. The activation of T-cells promotes fibroblast activation and differentiation, contributing to fibrosis. Cytokine profiling of PBMC responses to silicone surfaces revealed matrix-specific differences, especially in IL-6 and TNF-α levels, which influence fibroblast activity. Quantitative RT-PCR analysis showed changes in monocyte/macrophage markers and related cytokines like TNF-α and IL-1β, further implicating the immune response in fibroblast activation and differentiation [
55].
Animal models (rabbits and mice) have demonstrated that implants with smoother surfaces provoke reduced immune responses, characterized by lower levels of inflammatory cytokines and fewer activated fibroblasts [
63]. Specifically, implants with an average roughness radius (Ra) of 4 µm exhibit minimal capsular tension lines and rippling, suggesting decreased fibrosis. Conversely, textured implants often show double capsule formation and higher levels of wear debris within capsules, indicating more severe immune reactions and fibrotic encapsulation [
63].
Recent clinical studies involving intra- and interindividual analyses in human patients have highlighted the profound influence of surface microstructure (SMI) on fibroblast behavior and subsequent fibrotic responses [
39,
41,
59]. Comparisons between SMIs with roughness levels of Ra 60 µm and Ra 4 µm have revealed that reducing surface roughness mitigates early pro-inflammatory responses, while rougher surfaces intensify immune reactions and increase capsular thickness in chronic stages. Rougher surfaces enhance inflammatory signaling pathways such as NF-κB, promoting fibrosis progression, whereas smoother surfaces attenuate inflammation, thereby reducing fibroblast activation and myofibroblast differentiation [
30,
39,
41,
55,
59,
63]. Proteomic analyses indicate that rougher surfaces favor the production of fibrosis-associated proteins, whereas smoother surfaces promote proteins associated with fibrosis resolution, thereby influencing ECM remodeling dynamics [
30,
41,
63]. Surface topography thus dictates initial immune responses and long-term fibrotic outcomes, underscoring its critical role in optimizing implant designs to manage fibrosis [
30,
39,
41,
55,
59,
63].
4.1.5. Sustained Injury and Myofibroblast Differentiation
Under sustained injury or prolonged inflammation, fibroblasts can differentiate into myofibroblasts, contractile cells contributing to tissue contraction and ECM stabilization [
121]. This transition is associated with persistent proliferation and resistance to apoptosis, leading to aberrant ECM deposition and tissue dysfunction [
122]. Factors like hypoxia, prevalent near avascular implants, upregulate HIF-1α, promoting fibrogenesis [
123]. Myofibroblasts, stimulated by TGF-β, which is abundant in fibrotic capsules, express α-SMA and other contractile proteins, facilitating wound contraction and ECM remodeling [
22,
23,
24,
26]. This process is integral to fibrotic capsule formation, characterized by dense collagen deposition and tissue contraction [
124,
125,
126,
127,
128].
4.1.6. ECM Remodeling
The ECM environment around silicone implants regulates fibroblast activity and differentiation [
28,
29,
57]. Histological studies reveal collagen fiber orientation and thickness alterations within capsule tissue, indicative of ECM remodeling orchestrated by fibroblasts [
129]. Activated fibroblasts remodel the ECM by producing components like collagen and fibronectin [
48,
124,
127,
128,
130]. ECM remodeling processes involve proteins like collagen and MMPs, contributing to capsular fibrosis development [
81,
131,
132].
Wound contraction, vascularization decline, ECM turnover, and tensile strength recovery mark the final phase, lasting over a year [
133]. Myofibroblasts regulate wound contraction and tissue remodeling, synthesizing ECM proteins and assuming a contractile phenotype [
134]. The fibroblast-myofibroblast transdifferentiation is regulated by TFG-β1 and ECM stiffness, with myofibroblasts incorporating α-SMA into stress fibers [
135]. Contractile activity causes wound contraction and increases ECM stiffness, inducing myofibroblast differentiation and persistence [
136]. ECM remodeling involves a balanced production of MMPs and ECM proteins, including collagen III being replaced by collagen I, resulting in increasing complexity, order, and tensile strength [
137]. Apoptosis affects immune, endothelial, and myofibroblast cells, reducing vascularization and transitioning granulation tissue to scar tissue [
128]. Chronic silicone implant presence perpetuates fibroblast activation and differentiation, leading to sustained ECM remodeling and fibrotic tissue formation [
28,
29,
30].
4.1.7. Implications
Proper timing for inflammation resolution is crucial for successful healing progression, as persistent fibroblast activation and excessive pro-inflammatory mediator production can lead to chronic wounds and fibrosis [
20,
121]. Excessive fibroblast activity can result in hypertrophic scarring and keloid formation [
112,
125,
138,
139,
140], while altered signaling pathways, apoptosis failure, and excessive mechanical stress can perpetuate myofibroblast activity [
133,
135,
138]. Myofibroblast dysfunctions can also cause delayed wound healing due to failure in ECM reconstitution [
135].
In conclusion, fibroblast activation and differentiation are critical processes in capsular fibrosis associated with silicone implants. Understanding the molecular mechanisms driving these processes, including the influence of surface topography and cytokine signaling, is essential for developing strategies to mitigate fibrotic complications and improve clinical outcomes for patients undergoing implant-based surgeries.
4.2. Immune Cell Interactions and Inflammatory Responses
Capsular fibrosis formation around silicone implants involves intricate interactions between biomaterial surfaces and immune cells, influencing inflammation and fibrotic outcomes. Understanding these immune responses at the molecular level is essential for developing targeted therapies and improving clinical outcomes.
4.2.1. Molecular Mechanisms of Immune Cell Activation
Medical device bioperformance and biocompatibility are both directly related to unwanted side effects such as foreign body response, inflammation, and cell adhesion [
141]. Immune cells, including macrophages, dendritic cells, and T cells, respond to silicone implantation by recognizing the biomaterial as a foreign body [
28]. Macrophages initiate the inflammatory cascade by releasing cytokines such as TNF-α, IL-1β, and IL-6, which stimulate fibroblast activation and collagen deposition around the implant [
28,
29,
41,
55,
57,
73]. Dendritic cells process and present antigens derived from the implant to T cells, initiating adaptive immune responses crucial for sustained inflammation and fibrosis [
57,
73,
114].
4.2.2. Role of T Cells in Fibrotic Encapsulation
CD4+ T cells play a pivotal role in regulating immune responses to silicone implants, influencing fibroblast behavior through cytokine secretion and direct cell-cell interactions [
57]. Studies have shown increased infiltration of CD4+ T cells in fibrous capsules, correlating with the severity of fibrosis and chronic inflammation [
55]. These T cells secrete cytokines such as IFN-γ and TGF-β, which modulate fibroblast activation and promote myofibroblast differentiation [
57,
73]. The interactions between T cells and other immune cells at the molecular level contribute significantly to the pathogenesis of capsular fibrosis.
Effective healing is usually characterized by a dominant CD4+ T helper 1 (Th1) cell response, whereas a predominant CD4+ T helper 2 (Th2) response and an increase in CD4+ T helper 17 (Th17) cells lead to chronic inflammation, which can ultimately result in fibrosis [
29]. Th1 cells mediate tissue damage responses by producing Th1-related pro-inflammatory cytokines (IFN-γ, IL-12) that suppress fibroblast-induced collagen synthesis and attenuate fibrosis [
142]. Conversely, Th2 cells mediate adaptive immune responses to injury by producing pro-fibrotic (anti-inflammatory) cytokines (e.g., IL-4, IL-13, IL-10) [
142]. As a commonly recognized opponent of Th1 cells, Th2 cells can alter Th1-associated IFN-γ expression levels and high levels of Th2 cytokines have been reported in several fibrotic diseases [
142].
Regulatory T cells (Tregs), another subset of CD4+ T cells, play a critical role in controlling immune responses to self and foreign antigens, thereby preventing autoimmune diseases [
143,
144,
145,
146,
147,
148,
149]. Tregs can be divided into two groups: “natural” Tregs (nTregs), produced by the thymus and characterized by the expression of interleukin-2 receptor (IL-2R) and the forkhead box P3 (Foxp3) transcription factor, exhibit suppressive regulatory activity. Peripheral-induced Tregs (iTregs) arise from the differentiation of naive T cells in the periphery and also secrete anti-inflammatory cytokines such as IL-10 and TGF-β1[
145,
147,
148,
149].
Scarce data exist on specific local side effects (local immune response, activity of immune cells) focusing on lymphocytes isolated from fibrous capsules. The cellular composition of fibrous capsules formed around SMIs was characterized, revealing that macrophages and fibroblasts were the most predominant cell populations in the region abutting the silicone surface (designated as “pseudo synovium”). Moreover, significant numbers of activated CD4+ CD25+CD45RO memory T cells were present at this site and adjacent to the vessel [
57]. Notably, among T cells, Treg numbers in peri-SMI fibrotic capsules were inversely proportional to the degree of fibrosis (Baker scores I to IV). Particularly noteworthy was the observation that Tregs were reduced in capsules removed from patients with clinically severe symptoms of capsular contracture (Baker scores III to IV) [
57].
Tregs exhibit different transcriptional changes in response to regenerative or fibrogenic environmental cues [
150]. The controversy about the role of Tregs in fibrosis is corroborated by the facts that Th17 cells are relatively resistant to Treg suppression [
151], and notwithstanding that TGF-β1 is the most prominent profibrotic cytokine itself, it is also inducing differentiation of naïve T cells [
151].
Tregs isolated from capsules with high-grade fibrosis demonstrated the ability to suppress peripheral T effector cells but exhibited significantly less suppression potential when combined with intracapsular T effector cells [
54]. These findings suggest that in the early stages of fibrosis, Tregs play a crucial role in controlling capsular fibrosis by down-regulating Th1/Th17+ effector cells and reducing profibrotic cytokine production.
4.2.3. Influence of Implant Surface Properties on T Cell Immune Responses
The physical and chemical properties of silicone implant surfaces, such as texture and surface roughness, influence immune cell adhesion, cytokine secretion, and subsequent fibrotic responses [
30,
39,
41,
55,
59,
63]. Textured surfaces enhance immune cell activation compared to smoother surfaces, exacerbating capsular thickness and fibrotic severity clinically observed with different types of silicone implants [
39,
41,
73].
Silicone surfaces, when co-cultured in vitro with human peripheral blood mononuclear cells (PBMCs), did not induce T-cell proliferation or significantly modify the distribution of T-cell subsets [
55]. This suggests that silicone alone does not elicit a robust T-cell response, although it can influence the cytokine milieu. Variations in silicone surface textures prompted distinct cytokine responses, particularly in IL-6 and TNF-α levels. These cytokines are implicated in inflammation and fibrosis, suggesting that surface texture may impact the intensity of inflammatory reactions and subsequent fibrotic encapsulation. The study reaffirmed previous observations that textured implants tend to evoke stronger immune responses compared to smooth ones. Textured surfaces were associated with increased cytokine secretion, notably IL-6 and TNF-α, which could potentially contribute to elevated rates of capsular contracture. Adhesion and gene expression analyses indicated that textured surfaces could modify macrophage behavior, as indicated by alterations in CD14, CD68, and other markers [
55]. This interaction is pivotal for elucidating the underlying mechanisms of capsular contracture and fibrosis.
In vivo studies in patients examined the impact of reducing implant surface roughness from Ra 60 µm to Ra 4 µm on inflammatory tissue repair following implantation. Flow cytometric analysis revealed no significant difference in the distribution of CD4+ T cell subpopulations (including TH1, TH17, CM, and EM) between smoother (Ra 4 µm) and rougher (Ra 60 µm) SMI surfaces, indicating that surface topography did not directly influence the proliferation or distribution of these T cell subsets around implants. Despite this, cytokine secretion analysis showed a pronounced TH1 response characterized by increased secretion of IFNγ, IL-1b, and TNFα around both SMI types, suggesting robust TH1-mediated responses irrespective of surface roughness. Gene expression analysis indicated elevated levels of IFNγ and IL17 around rougher surfaces (Ra 60 µm) compared to smoother surfaces (Ra 4 µm), indicating a heightened proinflammatory and profibrotic T cell response around rougher implants. Correlation analysis further supported these findings, showing significant positive correlations between IL17A secretion and TH17 cells, as well as between IFNγ expression and TH1 cells specifically in wounds enclosed by SMI 60 µm, highlighting the interplay between T cell cytokine production and immune cell profiles influenced by implant surface roughness.
The study found a consistent macrophage response around both SMI types, indicating no significant difference in macrophage presence or distribution based on surface roughness. However, gene expression analysis revealed differences in macrophage polarization between surfaces. Fibrotic tissue encapsulating the rougher implant exhibited increased gene expression of M1 markers such as IFNγ and CCL2, indicative of a proinflammatory macrophage phenotype, whereas capsule enclosing the smoother implant showed elevated gene expression of M2 markers like IL4 and IL10, suggesting a shift towards an anti-inflammatory or pro-tissue repair macrophage phenotype. Immunohistological analysis of the intracapsular environment demonstrated enrichment of CD25+ and Foxp3+ immune cells around rougher SMI, associated with Treg cells, suggesting a regulatory role in dampening immune responses and potentially modulating tissue repair processes.
4.2.4. Macrophages in the Context of Breast Implants: Cellular Interactions and Inflammatory Responses
Macrophages play a pivotal role as early responders to surgically placed implants, accumulating at the implantation site for extended periods to phagocytose cellular debris and implant abrasion products [
152]. Their presence triggers upregulation of pro-inflammatory and pro-fibrotic cytokines such as IL-1, IL-8, MCP-1, CXCL13, and MIP, which further recruit macrophages and modulate their activity during the Foreign Body Response (FBR) [
153,
154,
155]. Despite their efforts, macrophages often fail to completely engulf large implants, leading to frustrated phagocytosis and chronic inflammation [
156]. This chronic inflammatory state contributes to the formation of Foreign Body Giant Cells (FBGC) and exerts trophic actions on vascular cells, adaptive immune cells, and fibroblasts, ultimately contributing to implant fibrosis [
157,
158,
159].
Studies using animal models have demonstrated that abolishing macrophage recruitment, through methods such as clodronate liposome-induced deletion, can significantly reduce monocyte infiltration, FBGC formation, neovascularization, and fibrosis around implants [
152,
157,
160]. Furthermore, targeting macrophage receptors like colony-stimulating factor-1 receptor (CSF-1R), which is upregulated post-implantation, has shown promising results in suppressing implant fibrosis [
152].
4.2.5. Strategies Targeting Macrophages through Implant Surface Modifications
The objectives of implant surface modifications targeting macrophages are twofold: (1) to suppress the formation of detrimental macrophage phenotypes while stimulating regenerative and resolving activation states [
161,
162,
163], and (2) to prevent the formation of FBGCs, which typically follow the chronic inflammatory phase of the FBR [
158].
Macrophages exhibit diverse activation states, broadly categorized into pro-inflammatory M1 and pro-regenerative (yet pro-fibrotic) M2 types [
162,
163]. M1 macrophages dominate the early phases of the FBR, producing cytokines like PDGF, TNF-α, IL-6, G-CSF, and GM-CSF, crucial for inflammation and tissue remodeling [
153,
164]. In contrast, M2 macrophages, induced by cytokines such as IL-4 and IL-13, play roles in tissue repair but can also promote fibrosis and FBGC formation [
165].
Surface chemistry and topography of implants significantly influence macrophage responses, including recruitment, adhesion, spreading, and activation [
166]. For instance, macrophages attach to implant surfaces via integrins and sense surface characteristics through Toll-like receptors and scavenger receptors [
167,
168,
169]. Modulating these interactions alters macrophage attachment, spreading, and fusion into FBGCs [
165,
170,
171].
Studies have highlighted that micro-roughness features within a specific range (e.g., 0.51–1.36 μm) promote M2-like phenotypes in macrophages, influencing their cytokine profiles and fusion into FBGCs [
172]. Similarly, nano-patterned surfaces with defined features influence macrophage behaviors, affecting their inflammatory profiles and phagocytic activities [
173,
174,
175]. Additionally, substrate stiffness plays a critical role, with stiffer materials promoting pro-inflammatory responses and FBGC formation in macrophages compared to softer substrates [
176,
177].
Reducing implant surface roughness from Ra 60 µm to Ra 4 µm, in vivo in human patients, revealed a consistent presence of macrophages around both types of surfaces, indicating no significant difference in macrophage distribution based on surface roughness. However, gene expression analysis uncovered distinct macrophage polarization patterns between the surfaces. Capsular tissue surrounding the rougher implants exhibited heightened expression of M1 markers such as IFNγ and CCL2, suggesting a proinflammatory macrophage phenotype. In contrast, capsules enveloping the smoother implants showed increased expression of M2 markers like IL4 and IL10, indicative of an anti-inflammatory or pro-tissue repair macrophage phenotype. Immunohistological analysis of the intracapsular environment further revealed an enrichment of CD25+ and Foxp3+ immune cells around the rougher surface implants, implicating T regulatory (Treg) cells in potentially attenuating immune responses and influencing tissue repair processes.
Understanding macrophage behaviors and responses to implant surfaces is crucial for developing strategies to mitigate implant fibrosis and enhance biocompatibility. Targeted modifications of surface properties can alter macrophage activation states, influencing their inflammatory profiles and their propensity to form FBGCs. Future research should continue to explore how specific surface modifications can effectively modulate macrophage interactions, thereby improving the long-term performance and safety of breast implants.
4.3. Implications for Clinical Practice
Cellular and tissue-level studies have elucidated critical insights into the mechanisms underlying capsular fibrosis associated with silicone breast implants. Key findings (
Table 1.) underscore the pivotal roles of fibroblasts, immune cells, particularly macrophages and T cells, and the influence of implant surface properties in driving inflammatory and fibrotic responses.
Understanding these cellular and molecular mechanisms is crucial for developing strategies to mitigate capsular fibrosis and improve clinical outcomes for patients undergoing implant-based surgeries. Surface modifications that promote an anti-fibroticmacrophage phenotype and modulate T cell responses could potentially reduce the incidence and severity of fibrotic encapsulation. Future research should focus on optimizing implant designs to balance biocompatibility with reduced inflammatory responses, thereby enhancing the longevity and safety of silicone breast implants.
In conclusion, comprehensive insights into fibroblast behavior, immune cell interactions, and the impact of implant surface properties provide a foundation for advancing therapeutic approaches aimed at minimizing complications associated with silicone breast implants. Continued research efforts are essential to refine our understanding and translate these findings into clinically effective strategies for enhancing patient outcomes in implant-based surgery.
5. Microbial Interactions and Biofilm Formation on Breast Implants: Implications for Capsular Contracture
5.1. The Race for the Surface: Host Cells vs. Bacteria
Upon the implantation of a breast implant, a “race for the surface” occurs in which host cells (such as macrophages, fibroblasts, and platelets) compete with bacteria to occupy the implant surface [
178]. Although implanted under specific sterilization and disinfection guidelines [
179,
180,
181], microbial colonization and biofilm formation on the implant surface can occur. Biofilms are communities of bacteria encased in a protective matrix, making them resistant to the immune response and antibiotics [
182]. The antimicrobial immune response may be activated as a part of the overall immune reaction [
29]. These biofilms act as bacterial reservoirs and are sources of chronic and/or sub-clinical infections [
183].
As bacteria approach the implant, they encounter Van der Waals, electrostatic, and hydrophobic interactions with the implant surface, leading to initial adherence [
184,
185]. This is followed by more permanent site-specific interactions, where bacterial pili and fimbriae form attachments with the biomaterial surface or its conditioning film [
186]. At this point, bacteria transition from their planktonic to sessile state. Biofilm formation proceeds with rapid bacterial proliferation, production of extracellular polymeric substances (EPS), and a phenotypic shift contributing to the resilient nature of biofilm growth [
187]. Within the biofilm, bacteria communicate via quorum sensing, releasing signaling molecules to coordinate gene expression [
188]. Biofilm bacteria can also detach and revert to their planktonic state, dispersing to colonize new surfaces [
189]. In the context of breast implants, this could involve other areas on the implant or different tissues and regions of the body.
Many bacterial species produce binding proteins specific to collagen and fibronectin, which form the fibrous capsule around the breast implant [
190,
191]. This facilitates bacterial attachment and adherence to the capsule, followed by proliferation and biofilm formation. Increased numbers of fibroblasts correlate with a higher incidence of capsular contracture, and more bacteria are found on contracted capsules compared to non-contracted ones [
192]. A porcine model by Tamboto et al. demonstrated that biofilm formation on and around breast implants is associated with a fourfold increased risk of developing capsular contracture [
193].
The most common bacterial species detected on breast implants are Staphylococcus spp., particularly
Staphylococcus epidermidis [
194]. These species are normal skin flora, suggesting contamination of the implant or site during surgery. In a rat model, Miller et al. reported that the hematogenous spread of
Staphylococcus aureus from a remote infection increased capsule thickness, myofibroblast numbers, and collagen density around implanted silicone blocks [
195].
5.2. Biofilm Formation and Ist Implications for Breast Implants
In a recent study, moving to an in vitro setting, the evaluation of SMI surface patches with diverse topographies revealed significant effects on microbial adhesion, growth, and colonization by
S. epidermidis and
S. aureus [
59]. Both bacterial species adhered and colonized all tested surfaces, but the presence of silicone markedly inhibited their growth and colonization compared to controls without silicone patches[
59]. The topography of the silicone patch significantly influenced bacterial growth, with increased texture correlating positively with enhanced bacterial colonization. Biofilm formation, a recognized virulence factor in biomaterial-related infections, was evident on textured surfaces after inoculation with
S. epidermidis or
S. aureus. The topography of the silicone patch significantly influenced bacterial growth and colonization, with enhanced texture correlating with increased bacterial growth [
59].
Antimicrobial immune responses refer to the body’s defense mechanisms against microbial invaders, such as bacteria. In the context of silicone breast implants, the immune response is typically triggered by the presence of foreign material (silicone) in the body that involves the activation of immune cells and processes aimed at removing or isolating the foreign material [
153,
196,
197]. The initial response to SMIs includes an inflammatory reaction. Inflammation is a part of the immune response and involves the recruitment of immune cells to the implant site to clear debris and potentially harmful substances [
29]. While silicone itself is not a microbial agent, the immune system may respond to the implant by releasing antimicrobial substances as a general defense mechanism.
A diverse array of proteins linked to immune response, inflammation, and wound healing was discovered in the vicinity of the SMI [
30]. In vivo analysis of SMI surface-associated proteome, including plasma, local tissue, and early fibrosis stages, revealed a significant inflammatory storm within the first five days post-implantation, with antimicrobial agents adhering to the SMI surface over the next 6 to 8 months [
30,
41]. Notably, 65 plasma-derived components were involved in the antimicrobial humoral response, with FLG2 exclusively associated with rougher SMI surfaces, indicating a chronic antimicrobial inflammatory response [
59]. Next-generation sequencing (NGS) analysis of wound-associated microbiomes revealed significant topography-specific variations, with higher microbial diversity and quantity on rougher surfaces [
59]. Skin microbiome assessment at incision sites identified only eight species, with
Staphylococcus hominis and
Staphylococcus epidermidis being prevalent This finding indicates that the types of bacteria present are related to the surgical environments where they were found. Staphylococci, identified in acute and chronic wounds and on SMI surfaces, emphasize the need to understand individual skin microbiomes in surgical contexts [
59].
5.3. Antimicrobial Immune Responses and Proteomic Insights in Capsular Fibrosis
To gain deeper insight into the mechanisms driving capsular fibrosis around SMIs, it is crucial to consider the significant role of antimicrobial strategies and inflammatory responses (
Figure 4). Key processes such as sterilization, disinfection, and surface modifications (
Figure 4a) of SMIs are essential in minimizing microbial contamination. Both immediate and chronic antimicrobial inflammatory responses play a role in this context, with persistent microbial contamination leading to ongoing inflammation and fibrous capsule formation (
Figure 4b). Furthermore, bacterial adhesion and biofilm formation are critical stages that influence the onset of capsular contracture (
Figure 4c). These considerations underscore how antimicrobial interventions and inflammatory dynamics are integral to the development of capsular fibrosis and highlight potential strategies for mitigating these challenges.
6. Clinical Translation and Future Directions
Advances in biomaterial science and molecular studies have illuminated new pathways for enhancing clinical outcomes in breast implant surgery.
One of the significant potentials lies in personalized risk assessment and management strategies. By deciphering individual variations in immune responses and microbial colonization patterns, clinicians can tailor surgical approaches to mitigate risks such as capsular contracture. This personalized approach may involve optimized implant selection and targeted antimicrobial prophylaxis. Such strategies aim to improve patient outcomes by reducing complications associated with immune reactions and biofilm formation.
Furthermore, the development of biomaterials and implant modifications stands at the forefront of innovation. Insights from molecular studies and preclinical models are driving the creation of next-generation implants designed to minimize biofilm formation and inflammatory responses. These advancements include the exploration of anti-biofilm coatings, immunomodulatory surfaces, and materials that mimic natural tissue interactions. By improving implant biocompatibility and longevity, these innovations hold the potential to enhance patient satisfaction and reduce the need for revision surgeries.
In parallel, the identification of novel therapeutic targets through molecular pathways offers promising avenues for targeted interventions. Potential therapies such as anti-inflammatory agents, quorum sensing inhibitors, and biofilm disruptors are being investigated to prevent or mitigate fibrotic reactions post-implantation. These targeted treatments aim to modulate immune responses and improve the overall biointegration of implants, thereby advancing the field towards more effective management of complications and improved long-term patient outcomes.
Moreover, advancements in surgical techniques and post-operative care protocols are being informed by molecular insights. Understanding the intricate mechanisms of wound healing and immune modulation guides refinements in surgical approaches. This includes strategies to minimize tissue trauma during implantation, optimize tissue integration around implants, and enhance infection prevention measures. By implementing evidence-based practices derived from molecular and preclinical research, clinicians can strive to optimize surgical outcomes and reduce the incidence of complications.
Long-term monitoring and patient education are also pivotal aspects influenced by molecular studies. Comprehensive monitoring protocols informed by molecular biomarkers [
30] can facilitate early detection of complications and prompt intervention. Equally important is patient education, which empowers individuals with knowledge about their implants and potential risks. Educating patients on the importance of regular follow-up evaluations and self-monitoring can promote proactive management and early intervention, contributing to improved long-term implant success and patient satisfaction.
Lastly, collaborative research efforts and rigorous clinical trials are essential for translating scientific discoveries into clinical practice. Interdisciplinary collaboration between researchers, clinicians, and industry stakeholders accelerates the validation of emerging technologies and therapies. This collaborative approach ensures that advancements in breast implant surgery are evidence-based and patient-centered, fostering continuous improvement and innovation in the field of plastic and reconstructive surgery.
In conclusion, the clinical translation of insights from molecular studies and preclinical models holds immense promise for revolutionizing breast implant surgery. By integrating these advancements into clinical practice, clinicians can strive to enhance implant biocompatibility, reduce complications, and improve overall patient outcomes. Continued research, interdisciplinary collaboration, and evidence-based practice are crucial for realizing these goals and advancing the standard of care in breast implant surgery.
7. Challenges and Limitations
Despite significant advancements, several challenges and limitations persist in current research on breast implant surgery, particularly concerning immune responses and novel interventions.
One of the primary challenges is the variability in individual immune responses to implants. While molecular studies have elucidated key pathways involved in immune reactions, there remains a substantial variation among patients in how their immune systems react to implanted materials. This variability can influence the risk of complications such as capsular contracture and infection, making it challenging to predict outcomes and tailor personalized treatment strategies effectively.
Another unresolved question pertains to the long-term outcomes of novel interventions aimed at mitigating complications associated with breast implants. While preclinical models and early clinical studies show promise for therapies targeting biofilm formation, inflammation, and implant integration, the durability and effectiveness of these interventions over extended periods remain uncertain. Longitudinal studies are needed to assess the sustainability of therapeutic benefits and potential long-term risks associated with new biomaterials and treatments.
Furthermore, the complexity of biofilm-related infections poses a significant challenge in breast implant surgery. Biofilms are resilient communities of bacteria encased in an extracellular matrix, which makes them resistant to immune responses and conventional antibiotics. Despite advances in understanding biofilm formation and its impact on capsular contracture, effective strategies to prevent and eradicate biofilms on implants remain elusive. Future research efforts should focus on developing innovative anti-biofilm strategies that can be translated into clinical practice.
Moreover, the translational gap between benchtop research and clinical implementation presents a persistent limitation. While preclinical models provide valuable insights into biological mechanisms and therapeutic targets, translating these findings into safe and effective clinical interventions requires rigorous validation in human studies. Bridging this gap necessitates collaborative efforts among researchers, clinicians, and regulatory bodies to ensure that novel interventions meet stringent safety and efficacy standards before widespread adoption.
Ethical considerations also pose challenges in breast implant research, particularly regarding patient consent, long-term surveillance, and the communication of risks and benefits associated with implants. Ensuring comprehensive informed consent and empowering patients with accurate information about potential risks, including immune responses and long-term outcomes, is essential for promoting patient autonomy and shared decision-making in surgical settings.
In conclusion, while molecular insights and preclinical breakthroughs have significantly advanced our understanding of breast implant surgery, several challenges and unanswered questions remain (
Table 2). Addressing variability in immune responses, assessing the durability of novel interventions, combating biofilm-related infections, closing the translational gap, and navigating ethical considerations are critical priorities for future research. By addressing these challenges collaboratively and systematically, researchers and clinicians can strive to optimize patient outcomes and advance the field toward safer, more effective breast implant surgery practices.
8. Conclusion
In conclusion, the exploration of immune mechanisms underlying SMI capsular fibrosis represents a crucial frontier in aesthetic and reconstructive surgery. Through this review, we have delved into the intricate interplay of host responses and microbial interactions, highlighting both the progress made and the challenges that lie ahead.
The findings underscore the multifaceted nature of immune responses to breast implants, shaped by factors ranging from biomaterial properties to individual variations in immune profiles. Despite these complexities, advancements in molecular insights and preclinical models offer promising avenues for improving clinical outcomes. By elucidating key pathways involved in capsular fibrosis, such as inflammation, biofilm formation, and tissue integration, researchers are paving the way for targeted interventions that could mitigate complications and enhance the longevity of implants.
Looking forward, ongoing research holds the potential to catalyze transformative breakthroughs in treatment strategies for breast implant patients. Future studies focusing on personalized medicine approaches, innovative biomaterials, and novel anti-biofilm therapies are poised to redefine standards of care in plastic surgery. Moreover, the integration of patient-centric outcomes, ethical considerations, and regulatory frameworks will be pivotal in translating research findings into safe and effective clinical practices.
In essence, the journey toward unraveling the immune web of SMI capsular fibrosis is not just about understanding biological mechanisms; it is about improving quality of life. By harnessing the collective efforts of scientists, clinicians, and patients, we can envisage a future where complications are minimized, outcomes are optimized, and patient care in plastic and reconstructive surgery reaches new heights of excellence. As we continue to push the boundaries of knowledge and innovation, the promise of achieving these goals remains within our grasp.
Author Contributions
Writing—original draft preparation, I.S.; writing—review and editing, K.F., M.L., D.C.C.-H., A.A., A.I., S.S., and D.W.; data curation, I.S.; visualization, I.S.; supervision, I.S. D.W. acquired funding. All authors have read and agreed to the published version of the manuscript.
Funding
Research on this project was funded by Establishment Labs, Costa Rica (ID D152500-015-015) to D.W.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest
References
- Hasin, Yehudit, Marcus Seldin, and Aldons Lusis. 2017. Multi-omics approaches to disease. Genome Biol. 18. BioMed Central Ltd. [CrossRef]
- Barh, D, V Zambare, and V Azevedo. 2013. Omics: applications in biomedical, agricultural, and environmental sciences.
- Aderem, Alan. 2005. Systems biology: its practice and challenges. Cell 121. Elsevier B.V.: 511–513. [CrossRef]
- Yan, Shikai, Dale G. Nagle, Yu Dong Zhou, and Weidong Zhang. 2018. Application of Systems Biology in the Research of TCM Formulae. Systems Biology and Its Application in TCM Formulas Research. Elsevier: 31–67. [CrossRef]
- Miho, Enkelejda, Alexander Yermanos, Cédric R. Weber, Christoph T. Berger, Sai T. Reddy, and Victor Greiff. 2018. Computational Strategies for dissecting the high-dimensional complexity of adaptive immune repertoires. Front. Immunol. 9. Frontiers Media S.A.: 224. [CrossRef]
- Schussek, Sophie, Angela Trieu, and Denise L. Doolan. 2014. Genome- and proteome-wide screening strategies for antigen discovery and immunogen design. Biotechnology Advances 32. Elsevier Inc.: 403–414. [CrossRef]
- Singh, S, DK Sarma, V Verma, … R Nagpal - … and Biophysical Research, and undefined 2023. 2024. Unveiling the future of metabolic medicine: omics technologies driving personalized solutions for precision treatment of metabolic disorders. Elsevier. https://www.sciencedirect.com/science/article/pii/S0006291X23011002. Accessed June 10.
- Chavda, Vivek, Rajashri Bezbaruah, Disha Valu, Sanjay Desai, Nildip Chauhan, Swati Marwadi, Gitima Deka, and Zhiyong Ding. 2023. Clinical Applications of “Omics” Technology as a Bioinformatic Tool. Bioinformatics Tools for Pharmaceutical Drug Product Development. wiley: 117–145. [CrossRef]
- Targeting, IV Quiroz - Biotechnology and Drug Development for, and undefined 2024. 2024. Exploring the Intersection of Omics Technologies and Biotechnology in Drug Interaction Studies. books.google.com. https://books.google.com/books?hl=de&lr=&id=EEb9EAAAQBAJ&oi=fnd&pg=PA188&dq=application+of+omics+technologies+in+biomedical+and+pharmaceutical+research.&ots=u6sMBjDv5b&sig=H6rlgX4u3iryxa8SXaFFJAlzPtk. Accessed June 10.
- Stein, Catarina M., Ralf Weiskirchen, Frederik Damm, and Paulina M. Strzelecka. 2021. Single-cell omics: Overview, analysis, and application in biomedical science. Journal of Cellular Biochemistry 122. John Wiley and Sons Inc: 1571–1578. [CrossRef]
- Aga, AM, AA Woldesemayat - and Cellular Biomedical Sciences, and undefined 2024. 2024. Application of Omics and Bioinformatics Technologies in Response to COVID-19 Pandemic. cellbiopharm.com. [CrossRef]
- Bai, JPF, IN Melas, J Hur, E Guo - Expert opinion on drug discovery, and undefined 2018. 2017. Advances in omics for informed pharmaceutical research and development in the era of systems medicine. Taylor & Francis 13. Taylor and Francis Ltd.: 1–4. [CrossRef]
- Dai, Xiaofeng, and Li Shen. 2022. Advances and Trends in Omics Technology Development. Frontiers in Medicine 9. Frontiers Media S.A. [CrossRef]
- Davis, Mark M., Cristina M. Tato, and David Furman. 2017. Systems immunology: just getting started. Nature immunology 18. Nat Immunol: 725–732. [CrossRef]
- Bonaguro, Lorenzo, Jonas Schulte-Schrepping, Thomas Ulas, Anna C. Aschenbrenner, Marc Beyer, and Joachim L. Schultze. 2022. A guide to systems-level immunomics. Nature Immunology 2022 23:10 23. Nature Publishing Group: 1412–1423. [CrossRef]
- Pulendran, Bali, and Mark M. Davis. 2020. The science and medicine of human immunology. Science 369. American Association for the Advancement of Science. [CrossRef]
- Devenish, Liam P., Musa M. Mhlanga, and Yutaka Negishi. 2021. Immune Regulation in Time and Space: The Role of Local- and Long-Range Genomic Interactions in Regulating Immune Responses. Frontiers in Immunology 12. Frontiers Media S.A. [CrossRef]
- Wynn, Thomas A. 2007. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. Journal of Clinical Investigation. [CrossRef]
- Ji, Litong, Tie Wang, Lining Tian, Hongjiang Song, and Meizhuo Gao. 2020. Roxatidine inhibits fibrosis by inhibiting NF-κB and MAPK signaling in macrophages sensing breast implant surface materials. Molecular Medicine Reports 21. Spandidos Publications: 161–172. [CrossRef]
- Wynn, T. A. 2008. Cellular and molecular mechanisms of fibrosis. Journal of Pathology 214: 199–210. [CrossRef]
- Kuo, Yao Lung, I. Ming Jou, Seng Feng Jeng, Chun Hui Chu, Jhy Shrian Huang, Tai I. Hsu, Li Ren Chang, Po Wei Huang, Jian An Chen, and Ting Mao Chou. 2019. Hypoxia-induced epithelial-mesenchymal transition and fibrosis for the development of breast capsular contracture. Scientific Reports 9. Nature Publishing Group: 1–6. [CrossRef]
- Biernacka, Anna, Marcin Dobaczewski, and Nikolaos G. Frangogiannis. 2011. TGF-β signaling in fibrosis. Growth Factors 29: 196–202. [CrossRef]
- Chaikuad, Apirat, and Alex N. Bullock. 2016. Structural basis of intracellular TGF-β signaling: Receptors and smads. Cold Spring Harbor Perspectives in Biology 8. Cold Spring Harbor Laboratory Press. [CrossRef]
- Meng, Xiao Ming, David J. Nikolic-Paterson, and Hui Yao Lan. 2016. TGF-β: The master regulator of fibrosis. Nature Reviews Nephrology. Nature Publishing Group. [CrossRef]
- Lan, Hui Yao. 2011. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. International Journal of Biological Sciences. Ivyspring International Publisher. [CrossRef]
- Margadant, Coert, and Arnoud Sonnenberg. 2010. Integrin-TGF-Β crosstalk in fibrosis, cancer and wound healing. EMBO Reports. [CrossRef]
- Hu, He He, Dan Qian Chen, Yan Ni Wang, Ya Long Feng, Gang Cao, Nosratola D. Vaziri, and Ying Yong Zhao. 2018. New insights into TGF-β/Smad signaling in tissue fibrosis. Chemico-Biological Interactions. Elsevier Ireland Ltd. [CrossRef]
- Wick, Georg, Cecilia Grundtman, Christina Mayerl, Thomas-Florian Wimpissinger, Johann Feichtinger, Bettina Zelger, Roswitha Sgonc, and Dolores Wolfram. 2013. The immunology of fibrosis. Annual review of immunology 31: 107–35. [CrossRef]
- Wick, Georg, Aleksandar Backovic, Evelyn Rabensteiner, Nadine Plank, Christian Schwentner, and Roswitha Sgonc. 2010. The immunology of fibrosis: innate and adaptive responses. Trends in immunology 31. Europe PMC Funders: 110. [CrossRef]
- Schoberleitner, Ines, Klaus Faserl, Bettina Sarg, Daniel Egle, Christine Brunner, and Dolores Wolfram. 2023. Quantitative Proteomic Characterization of Foreign Body Response towards Silicone Breast Implants Identifies Chronological Disease-Relevant Biomarker Dynamics. Biomolecules 13. MDPI. [CrossRef]
- Siggelkow, Wulf, D. M. Gescher, A. Siggelkow, D. Klee, E. Malik, W. Rath, and A. Faridi. 2004. In vitro analysis of modified surfaces of silicone breast implants. The International journal of artificial organs 27. Int J Artif Organs: 1100–1108. [CrossRef]
- Siggelkow, Wulf, Andre Faridi, Katrin Spiritus, Uwe Klinge, Werner Rath, and Bernd Klosterhalfen. 2003. Histological analysis of silicone breast implant capsules and correlation with capsular contracture. Biomaterials 24. Elsevier: 1101–1109. [CrossRef]
- Handel, N., J. A. Jensen, Q. Black, J. R. Waisman, and M. J. Silverstein. 1995. The fate of breast implants: a critical analysis of complications and outcomes. Plastic and reconstructive surgery 96. Plast Reconstr Surg: 1521–1533. [CrossRef]
- Kuehlmann, Britta, Isabel Zucal, Clark Andrew Bonham, Lydia Marie Joubert, and Lukas Prantl. 2021. SEM and TEM for identification of capsular fibrosis and cellular behavior around breast implants – a descriptive analysis. BMC Molecular and Cell Biology 22. BioMed Central. [CrossRef]
- Prantl, Lukas, Stephan Schreml, Stefan Fichtner-Feigl, Nina Pöppl, Marita Eisenmann-Klein, Hartmut Schwarze, and Bernd Füchtmeier. 2007. Clinical and morphological conditions in capsular contracture formed around silicone breast implants. Plastic and reconstructive surgery 120. Plast Reconstr Surg: 275–284. [CrossRef]
- Colwell, Amy S., and Erin M. Taylor. 2020. Recent Advances in Implant-Based Breast Reconstruction. Plastic and reconstructive surgery 145. Plast Reconstr Surg: 421e–432e. [CrossRef]
- Frey, Jordan D., Ara A. Salibian, Nolan S. Karp, and Mihye Choi. 2019. Implant-Based Breast Reconstruction: Hot Topics, Controversies, and New Directions. Plastic and reconstructive surgery 143. Plast Reconstr Surg: 404e–416e. [CrossRef]
- Ricci, Joseph A., Sherise Epstein, Adeyiza O. Momoh, Samuel J. Lin, Dhruv Singhal, and Bernard T. Lee. 2017. A meta-analysis of implant-based breast reconstruction and timing of adjuvant radiation therapy. Journal of Surgical Research. [CrossRef]
- Schoberleitner, Ines, Angela Augustin, Daniel Egle, Christine Brunner, Birgit Amort, Bettina Zelger, Andrea Brunner, and Dolores Wolfram. 2023. Is It All about Surface Topography? An Intra-Individual Clinical Outcome Analysis of Two Different Implant Surfaces in Breast Reconstruction. Journal of clinical medicine 12. J Clin Med. [CrossRef]
- Luvsannyam, Enkhmaa, Dhara Patel, Zaira Hassan, Swetha Nukala, Manoj R Somagutta, and Pousettef Hamid. 2020. Overview of Risk Factors and Prevention of Capsular Contracture Following Implant-Based Breast Reconstruction and Cosmetic Surgery: A Systematic Review. Cureus 12. Cureus, Inc. [CrossRef]
- Schoberleitner, Ines, Klaus Faserl, Christoph H Tripp, Elisabeth Judith Pechriggl, Stephan Sigl, Andrea Brunner, Bettina Zelger, et al. 2024. Silicone implant surface microtopography modulates inflammation and tissue repair in capsular fibrosis. Frontiers in Immunology 15. Frontiers: 1342895. [CrossRef]
- Lefkowitz, Doris L., and Stanley S. Lefkowitz. 2001. Macrophage-neutrophil interaction: A paradigm for chronic inflammation revisited. Immunology and Cell Biology 79. Immunol Cell Biol: 502–506. [CrossRef]
- Sugimoto, Michelle A., Juliana P. Vago, Mauro Perretti, and Mauro M. Teixeira. 2019. Mediators of the Resolution of the Inflammatory Response. Trends in Immunology. Elsevier Ltd. [CrossRef]
- Zhang, Mengjuan, and Song Zhang. 2020. T Cells in Fibrosis and Fibrotic Diseases. Frontiers in Immunology. Frontiers Media S.A. [CrossRef]
- Safran, Tyler, Hillary Nepon, Carrie K. Chu, Sebastian Winocour, Amanda M. Murphy, Peter G. Davison, Tassos Dionisopolos, and Joshua Vorstenbosch. 2021. Healing, Inflammation, and Fibrosis: Current Concepts in Capsular Contracture: Pathophysiology, Prevention, and Management. Seminars in Plastic Surgery 35. Thieme Medical Publishers: 189. [CrossRef]
- Kim, Jin Ku, Evan A. Scott, and Donald L. Elbert. 2005. Proteomic analysis of protein adsorption: Serum amyloid P adsorbs to materials and promotes leukocyte adhesion. Journal of Biomedical Materials Research - Part A 75: 199–209. [CrossRef]
- Swartzlander, Mark D., Christopher A. Barnes, Anna K. Blakney, Joel L. Kaar, Themis R. Kyriakides, and Stephanie J. Bryant. 2015. Linking the foreign body response and protein adsorption to PEG-based hydrogels using proteomics. Biomaterials 41. Elsevier Ltd.: 26–36. [CrossRef]
- Witherel, Claire E., Daniel Abebayehu, Thomas H. Barker, and Kara L. Spiller. 2019. Macrophage and Fibroblast Interactions in Biomaterial-Mediated Fibrosis. Advanced Healthcare Materials 8. Wiley-VCH Verlag: 1801451. [CrossRef]
- Greisler, Howard P. 1990. Interactions at the blood/material interface. Annals of vascular surgery 4. Ann Vasc Surg: 98–103. [CrossRef]
- Faruq, Omar, Pham Ngoc Chien, Nilsu Dönmez, Sun Young Nam, and Chan Yeong Heo. 2021. Functionalization of Silicone Surface with Drugs and Polymers for Regulation of Capsular Contracture. Polymers 13. Multidisciplinary Digital Publishing Institute (MDPI). [CrossRef]
- Atiyeh, Bishara, and Saif Emsieh. 2022. Effects of Silicone Breast Implants on Human Cell Types In Vitro: A Closer Look on Host and Implant. Aesthetic Plastic Surgery 46. Springer: 2609–2611. [CrossRef]
- Xing, S., J. P. Santerre, R. S. Labow, and E. L. Boynton. 2002. The effect of polyethylene particle phagocytosis on the viability of mature human macrophages. Journal of Biomedical Materials Research 61: 619–627. [CrossRef]
- Tavazzani, F., S. Xing, J. E. Waddell, D. Smith, and E. L. Boynton. 2005. In vitro interaction between silicone gel and human monocyte-macrophages. Journal of biomedical materials research. Part A 72. J Biomed Mater Res A: 161–167. [CrossRef]
- Wolfram, Dolores, Evelyn Rabensteiner, Cecilia Grundtman, Günther Böck, Christina Mayerl, Walther Parson, Giovanni Almanzar, Carlo Hasenöhrl, Hildegunde Piza-Katzer, and Georg Wick. 2012. T regulatory cells and TH17 cells in peri-silicone implant capsular fibrosis. Plastic and Reconstructive Surgery. [CrossRef]
- Cappellano, Giuseppe, Christian Ploner, Susanne Lobenwein, Sieghart Sopper, Paul Hoertnagl, Christina Mayerl, Nikolaus Wick, Gerhard Pierer, Georg Wick, and Dolores Wolfram. 2018. Immunophenotypic characterization of human T cells after in vitro exposure to different silicone breast implant surfaces. PLoS ONE. [CrossRef]
- Backovic, Aleksandar, Dolores Wolfram, Barbara Del-Frari, Hildegunde Piza, Lukas A. Huber, and Georg Wick. 2007. Simultaneous analysis of multiple serum proteins adhering to the surface of medical grade polydimethylsiloxane elastomers. Journal of Immunological Methods 328: 118–127. [CrossRef]
- Wolfram, Dolores, Christian Rainer, Harald Niederegger, Hildegunde Piza, and Georg Wick. 2004. Cellular and molecular composition of fibrous capsules formed around silicone breast implants with special focus on local immune reactions *. Journal of Autoimmunity 23. Academic Press: 81–91. [CrossRef]
- Kuehlmann, Britta, Rebekka Burkhardt, Nina Kosaric, and Lukas Prantl. 2018. Capsular fibrosis in aesthetic and reconstructive-cancer patients: A retrospective analysis of 319 cases. Clinical Hemorheology and Microcirculation 70. IOS Press: 191–200. [CrossRef]
- Schoberleitner, Ines, Leoni Baier, Michaela Lackner, Lisa-Maria Zenz, Débora C. Coraça-Huber, Wendy Ullmer, Annabelle Damerum, et al. 2024. Surface Topography, Microbial Adhesion, and Immune Responses in Silicone Mammary Implant-Associated Capsular Fibrosis. International Journal of Molecular Sciences 25. MDPI AG: 3163. [CrossRef]
- Lin, Alexandra J., Sarah J. Karinja, Jaime L. Bernstein, Julia Jin, Yoshiko Toyoda, Andrew J. Miller, Pat B. Zanzonico, and Jason A. Spector. 2018. In Search of a Murine Model of Radiation-Induced Periprosthetic Capsular Fibrosis. Annals of plastic surgery 80. NLM (Medline): S204–S210. [CrossRef]
- Backovic, Aleksandar, Hong Lei Huang, Barbara Del Frari, Hildegunde Piza, Lukas A. Huber, and Georg Wick. 2007. Identification and dynamics of proteins adhering to the surface of medical silicones in vivo and in vitro. Journal of Proteome Research 6. J Proteome Res: 376–381. [CrossRef]
- Wolfram, D., B. Oberreiter, C. Mayerl, E. Soelder, H. Ulmer, H. Piza-Katzer, G. Wick, and A. Backovic. 2008. Altered systemic serologic parameters in patients with silicone mammary implants. Immunology Letters 118. Immunol Lett: 96–100. [CrossRef]
- Doloff, Joshua C., Omid Veiseh, Roberto de Mezerville, Marcos Sforza, Tracy Ann Perry, Jennifer Haupt, Morgan Jamiel, et al. 2021. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nature Biomedical Engineering 5. Nature Research: 1115–1130. [CrossRef]
- Newswire, P R. 2023. The Latest Global Survey from ISAPS Reports a Significant Rise in Aesthetic Surgery Worldwide. ISAPS-surgery-survey.
- Alfano, Carmine, Marco Mazzocchi, and Nicolò Scuderi. 2004. Mammary compliance: An objective measurement of capsular contracture. Aesthetic Plastic Surgery 28. [CrossRef]
- Henriksen, Trine F., Jon P. Fryzek, Lisbet R. Hölmich, Joseph K. McLaughlin, Kim Kjøller, Annette Pernille Høyer, Jørgen H. Olsen, and Søren Friis. 2005. Surgical intervention and capsular contracture after breast augmentation: A prospective study of risk factors. Annals of Plastic Surgery 54. [CrossRef]
- Henriksen, Trine F., Lisbet R. Hölmich, Jon P. Fryzek, Søren Friis, Joseph K. McLaughlin, Annette Pernille Høyer, Kim Kjøller, and Jørgen H. Olsen. 2003. Incidence and Severity of Short-Term Complications after Breast Augmentation: Results from a Nationwide Breast Implant Registry. Annals of Plastic Surgery 51. [CrossRef]
- Handel, Neal, Tracy Cordray, Jaime Gutierrez, and J. Arthur Jensen. 2006. A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plastic and Reconstructive Surgery 117. Plast Reconstr Surg: 757–767. [CrossRef]
- Eriksen, Catharina, Emelie Nordstrand Lindgren, Jan Frisell, and Birgit Stark. 2012. A prospective randomized study comparing two different expander approaches in implant-based breast reconstruction: one stage versus two stages. Plastic and reconstructive surgery 130. Plast Reconstr Surg. [CrossRef]
- Fischer, John P., Jonas A. Nelson, Emily Cleveland, Brady Sieber, Jeff I. Rohrbach, Joseph M. Serletti, and Suhail Kanchwala. 2013. Breast reconstruction modality outcome study: a comparison of expander/implants and free flaps in select patients. Plastic and reconstructive surgery 131. Plast Reconstr Surg: 928–934. [CrossRef]
- Chisholm, E. M., Sheelagh Marr, J. Macfie, A. C. Broughton, and T. G. Brennan. 1986. Post-Mastectomy breast reconstruction using the inflatable tissue expander. British Journal of Surgery 73. Br J Surg: 817–820. [CrossRef]
- McLaughlin, Joseph K., Loren Lipworth, Diane K. Murphy, and Patricia S. Walker. 2007. The safety of silicone gel-filled breast implants: A review of the epidemiologic evidence. Annals of Plastic Surgery. [CrossRef]
- Doloff, Joshua C., Omid Veiseh, Roberto de Mezerville, Marcos Sforza, Tracy Ann Perry, Jennifer Haupt, Morgan Jamiel, et al. 2021. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nature Biomedical Engineering 2021 5:10 5. Nature Publishing Group: 1115–1130. [CrossRef]
- Chiu, Wen Kuan, Megan Fracol, Lauren N. Feld, Cecil S. Qiu, and John Y.S. Kim. 2021. Judging an Expander by Its Cover: A Propensity-Matched Analysis of the Impact of Tissue Expander Surface Texture on First-Stage Breast Reconstruction Outcomes. Plastic and reconstructive surgery 147. Plast Reconstr Surg: 1E-6E. [CrossRef]
- Fairchild, Berry, Warren Ellsworth, Jesse C. Selber, David P. Bogue, Dmitry Zavlin, Stephanie Nemir, Cristina M. Checka, and Mark W. Clemens. 2020. Safety and Efficacy of Smooth Surface Tissue Expander Breast Reconstruction. Aesthetic surgery journal 40. Aesthet Surg J: 53–62. [CrossRef]
- Lee, Kyeong Tae, Hae Yeon Park, Byung Joon Jeon, Goo Hyun Mun, Sa Ik Bang, and Jai Kyong Pyon. 2021. Does the Textured-Type Tissue Expander Affect the Outcomes of Two-Stage Prosthetic Breast Reconstruction? A Propensity Score Matching Analysis between Macrotextured and Microtextured Expanders. Plastic and reconstructive surgery 147. Plast Reconstr Surg: 545–555. [CrossRef]
- Mempin, Maria, Honghua Hu, Durdana Chowdhury, Anand Deva, and Karen Vickery. 2018. The A, B and C’s of silicone breast implants: Anaplastic large cell lymphoma, biofilm and capsular contracture. Materials. MDPI AG. [CrossRef]
- Zhang, Li, El Mustapha Haddouti, Kristian Welle, Christof Burger, Dieter C. Wirtz, Frank A. Schildberg, and Koroush Kabir. 2020. The Effects of Biomaterial Implant Wear Debris on Osteoblasts. Frontiers in Cell and Developmental Biology. [CrossRef]
- Groves, Angela M., Carl J. Johnston, Ravi S. Misra, Jacqueline P. Williams, and Jacob N. Finkelstein. 2016. Effects of IL-4 on Pulmonary Fibrosis and the Accumulation and Phenotype of Macrophage Subpopulations Following Thoracic Irradiation. International journal of radiation biology 92. NIH Public Access: 754. [CrossRef]
- Trojanek, Joanna B., Jacek Michałkiewicz, Renata Grzywa-Czuba, Wojciech Jańczyk, Lidia Gackowska, Izabela Kubiszewska, Anna Helmin-Basa, Aldona Wierzbicka-Rucińska, Mieczysław Szalecki, and Piotr Socha. 2020. Expression of Matrix Metalloproteinases and Their Tissue Inhibitors in Peripheral Blood Leukocytes and Plasma of Children with Nonalcoholic Fatty Liver Disease. Mediators of Inflammation 2020. Hindawi Limited. [CrossRef]
- Singh, Drishtant, Vikrant Rai, and Devendra K Agrawal. 2023. Regulation of Collagen I and Collagen III in Tissue Injury and Regeneration. Cardiology and cardiovascular medicine 7. NIH Public Access: 5. [CrossRef]
- Bachour, Yara, Stephan P. Verweij, Susan Gibbs, Johannes C.F. Ket, Marco J.P.F. Ritt, Frank B. Niessen, and Margriet G. Mullender. 2018. The aetiopathogenesis of capsular contracture: A systematic review of the literature. Journal of Plastic, Reconstructive and Aesthetic Surgery. Churchill Livingstone. [CrossRef]
- Spear, S L, and J L Baker. 1995. Classification of capsular contracture after prosthetic breast reconstruction. Plastic and reconstructive surgery 96: 1119–23; discussion 1124.
- Sreejit, Gopalkrishna, Michelle C. Flynn, Mallikarjun Patil, Prasanna Krishnamurthy, Andrew J. Murphy, and Prabhakara R. Nagareddy. 2020. S100 family proteins in inflammation and beyond. Advances in Clinical Chemistry 98. Elsevier: 173–231. [CrossRef]
- Singh, P ;, S A Ali, E Kalyuzhny, Parul Singh, and Syed Azmal Ali. 2022. Multifunctional Role of S100 Protein Family in the Immune System: An Update. Cells 2022, Vol. 11, Page 2274 11. Multidisciplinary Digital Publishing Institute: 2274. [CrossRef]
- Gonzalez, Laura L., Karin Garrie, and Mark D. Turner. 2020. Role of S100 proteins in health and disease. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1867. Elsevier: 118677. [CrossRef]
- Sattar, Zeeshan, Alnardo Lora, Bakr Jundi, Christopher Railwah, and Patrick Geraghty. 2021. The S100 Protein Family as Players and Therapeutic Targets in Pulmonary Diseases. Pulmonary Medicine 2021. Hindawi Limited. [CrossRef]
- Stevens, W. Grant, Salvatore J. Pacella, Andrew J.L. Gear, Mark E. Freeman, Celeste McWhorter, Marissa J. Tenenbaum, and David A. Stoker. 2008. Clinical Experience With a Fourth-Generation Textured Silicone Gel Breast Implant: A Review of 1012 Mentor MemoryGel Breast Implants. Aesthetic Surgery Journal 28. [CrossRef]
- Cole, Norman M. 2018. Consequences of the U.S. Food and Drug Administration-Directed Moratorium on Silicone Gel Breast Implants: 1992 to 2006. Plastic and Reconstructive Surgery 141. [CrossRef]
- Cohen, Benjamin E., Thomas M. Biggs, Ernest D. Cronin, and Donald R. Collins. 1997. Assessment and longevity of the silicone gel breast implant. Plastic and Reconstructive Surgery 99. [CrossRef]
- Lam, Martin C., Gisela Walgenbach-Brünagel, Alexey Pryalukhin, Jens Vorhold, Thomas Pech, Jörg C. Kalff, Glen Kristiansen, and Klaus J. Walgenbach. 2019. Management of Capsular Contracture in Cases of Silicone Gel Breast Implant Rupture with Use of Pulse Lavage and Open Capsulotomy. Aesthetic Plastic Surgery 43. [CrossRef]
- Atlan, Michael, Brian M. Kinney, and Tracy Ann Perry. 2020. Intra- and Inter-Shell Roughness Variability of Breast Implant Surfaces. Aesthetic Surgery Journal 40. Oxford Academic: NP324–NP326. [CrossRef]
- Barr, S., E. W. Hill, and A. Bayat. 2017. Functional biocompatibility testing of silicone breast implants and a novel classification system based on surface roughness. Journal of the Mechanical Behavior of Biomedical Materials 75. Elsevier: 75–81. [CrossRef]
- Zhang, Xin Rui, Pham Ngoc Chien, Xuan Tung Trinh, Sun Young Nam, and Chan Yeong Heo. 2022. Comparison of Formation of Capsule Among Different Breast Silicone Implants. In vivo (Athens, Greece) 36. In Vivo: 2756–2766. [CrossRef]
- Kyle, Daniel J.T., Alison G. Harvey, Barbara Shih, Kian T. Tan, Iskander H. Chaudhry, and Ardeshir Bayat. 2013. Identification of molecular phenotypic descriptors of breast capsular contracture formation using informatics analysis of the whole genome transcriptome. Wound Repair and Regeneration 21. John Wiley & Sons, Ltd.: 762–769. [CrossRef]
- Kuang, Jujiao, Xu Yan, Amanda J. Genders, Cesare Granata, and David J. Bishop. 2018. An overview of technical considerations when using quantitative real-time PCR analysis of gene expression in human exercise research. PLoS ONE 13. PLOS. [CrossRef]
- Ng, Michael T.H., Rowie Borst, Hamez Gacaferi, Sarah Davidson, Jessica E. Ackerman, Peter A. Johnson, Caio C. Machado, et al. 2024. A single cell atlas of frozen shoulder capsule identifies features associated with inflammatory fibrosis resolution. Nature Communications 2024 15:1 15. Nature Publishing Group: 1–21. [CrossRef]
- Pang, Shichao, Loic Yengo, Christopher P. Nelson, Felix Bourier, Lingyao Zeng, Ling Li, Thorsten Kessler, et al. 2023. Genetic and modifiable risk factors combine multiplicatively in common disease. Clin. Res. Cardiol. 112. Springer Science and Business Media Deutschland GmbH: 247–257. [CrossRef]
- McCarthy, Mark I., Gonçalo R. Abecasis, Lon R. Cardon, David B. Goldstein, Julian Little, John P.A. Ioannidis, and Joel N. Hirschhorn. 2008. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat. Rev. Genet 9: 356–369. [CrossRef]
- McClellan, Jon, and Mary Claire King. 2010. Genetic heterogeneity in human disease. Cell 141. Elsevier B.V.: 210–217. [CrossRef]
- Trastulla, Lucia, Georgii Dolgalev, Sylvain Moser, Laura T. Jiménez-Barrón, Till F. M. Andlauer, Moritz von Scheidt, Douglas M. Ruderfer, et al. 2024. Distinct genetic liability profiles define clinically relevant patient strata across common diseases. Nature Communications 2024 15:1 15. Nature Publishing Group: 1–28. [CrossRef]
- Barr, Simon Patrick, Ernie W Hill, and Ardeshir Bayat. 2018. Novel Proteomic Assay of Breast Implants Reveals Proteins With Significant Binding Differences: Implications for Surface Coating and Biocompatibility. Aesthetic Surgery Journal 38. Oxford University Press: 962–969. [CrossRef]
- Staiano-Coico, L., P. J. Higgins, S. B. Schwartz, A. J. Zimm, and J. Goncalves. 2000. Wound fluids: a reflection of the state of healing. Ostomy/wound Management 46: 85S-93S; quiz 94S.
- Harvey, Joe, Kieran T. Mellody, Nicky Cullum, Rachel E.B. Watson, and Jo Dumville. 2022. Wound fluid sampling methods for proteomic studies: A scoping review. Wound Repair and Regeneration 30. John Wiley and Sons Inc: 317–333. [CrossRef]
- Hartman, Erik, Karl Wallblom, Mariena J.A. van der Plas, Jitka Petrlova, Jun Cai, Karim Saleh, Sven Kjellström, and Artur Schmidtchen. 2021. Bioinformatic Analysis of the Wound Peptidome Reveals Potential Biomarkers and Antimicrobial Peptides. Frontiers in Immunology 11. Frontiers Media S.A.: 3765. [CrossRef]
- Cialdai, Francesca, Chiara Risaliti, and Monica Monici. 2022. Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Frontiers in Bioengineering and Biotechnology 10. Frontiers Media SA. [CrossRef]
- Monaco, Jo An L., and W. Thomas Lawrence. 2003. Acute wound healing: An overview. Clinics in Plastic Surgery 30: 1–12. [CrossRef]
- Koh, Timothy J., and Luisa Ann DiPietro. 2011. Inflammation and wound healing: the role of the macrophage. Expert reviews in molecular medicine 13. [CrossRef]
- Bautista-Hernández, Luis Antonio, José Luis Gómez-Olivares, Beatriz Buentello-Volante, and Victor Manuel Bautista-de Lucio. 2017. Fibroblasts: the unknown sentinels eliciting immune responses against microorganisms. European Journal of Microbiology and Immunology 7. Akademiai Kiado Zrt.: 151–157. [CrossRef]
- Shinde, Arti V., Claudio Humeres, and Nikolaos G. Frangogiannis. 2017. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochimica et biophysica acta 1863. NIH Public Access: 298. [CrossRef]
- Brazin, Jacqueline, Stephanie Malliaris, Brittany Groh, Babak Mehrara, David Hidalgo, David Otterburn, Randi B. Silver, and Jason A. Spector. 2014. Mast cells in the periprosthetic breast capsule. Aesthetic Plast Surg. 38. Springer New York LLC: 592–601. [CrossRef]
- Noskovicova, Nina, Boris Hinz, and Pardis Pakshir. 2021. Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction. Cells 2021, Vol. 10, Page 1794 10. Multidisciplinary Digital Publishing Institute: 1794. [CrossRef]
- Saalbach, Anja, Claudia Klein, Jonathan Sleeman, Ulrich Sack, Friederike Kauer, Carl Gebhardt, Marco Averbeck, Ulf Anderegg, and Jan C. Simon. 2007. Dermal Fibroblasts Induce Maturation of Dendritic Cells. The Journal of Immunology 178. The American Association of Immunologists: 4966–4974. [CrossRef]
- Langevin, Helene M., Maiken Nedergaard, and Alan K. Howe. 2013. Cellular control of connective tissue matrix tension. Journal of Cellular Biochemistry 114: 1714–1719. [CrossRef]
- Correa-Gallegos, Donovan, Dongsheng Jiang, Simon Christ, Pushkar Ramesh, Haifeng Ye, Juliane Wannemacher, Shruthi Kalgudde Gopal, et al. 2019. Patch repair of deep wounds by mobilized fascia. Nature 576. Nature Research: 287–292. [CrossRef]
- Correa-Gallegos, Donovan, Dongsheng Jiang, and Yuval Rinkevich. 2021. Fibroblasts as confederates of the immune system. Immunological Reviews 302. John Wiley and Sons Inc: 147–162. [CrossRef]
- Mescher, Anthony L. 2017. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration 4. Wiley: 39–53. [CrossRef]
- Veiseh, Omid, and Arturo J. Vegas. 2019. Domesticating the foreign body response: Recent advances and applications. Advanced Drug Delivery Reviews 144. Elsevier B.V.: 148–161. [CrossRef]
- Akilbekova, Dana, and Kaitlin M. Bratlie. 2015. Quantitative Characterization of Collagen in the Fibrotic Capsule Surrounding Implanted Polymeric Microparticles through Second Harmonic Generation Imaging. PloS one 10. PLoS One. [CrossRef]
- Pakshir, Pardis, Nina Noskovicova, Monika Lodyga, Dong Ok Son, Ronen Schuster, Amanda Goodwin, Henna Karvonen, and Boris Hinz. 2020. The myofibroblast at a glance. Journal of Cell Science 133. Company of Biologists Ltd. [CrossRef]
- Wynn, Thomas A., and Thirumalai R. Ramalingam. 2012. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18: 1028–1040. [CrossRef]
- Lebleu, Valerie S., Gangadhar Taduri, Joyce O’Connell, Yingqi Teng, Vesselina G. Cooke, Craig Woda, Hikaru Sugimoto, and Raghu Kalluri. 2013. Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19: 1047–1053. [CrossRef]
- Higgins, DF. 2007. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 117: 3810–3820.
- Darby, Ian A., Betty Laverdet, Frédéric Bonté, and Alexis Desmoulière. 2014. Fibroblasts and myofibroblasts in wound healing. Clinical, Cosmetic and Investigational Dermatology 7. Dove Medical Press Ltd.: 301–311. [CrossRef]
- Hinz, B. 2016. The role of myofibroblasts in wound healing. Current Research in Translational Medicine 64. Elsevier Masson SAS: 171–177. [CrossRef]
- Sun, Yu Bo Yang, Xinli Qu, Georgina Caruana, and Jinhua Li. 2016. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation. 92. Elsevier Ltd.: 102–107. [CrossRef]
- Desjardins-Park, Heather E., Deshka S. Foster, and Michael T. Longaker. 2018. Fibroblasts and wound healing: An update. Regenerative Medicine 13. Future Medicine Ltd.: 491–495. [CrossRef]
- Bainbridge, P. 2013. Wound healing and the role of fibroblasts. Journal of Wound Care 22. MA Healthcare Ltd.: 407–412. [CrossRef]
- Moyer, Kurtis E., and H. Paul Ehrlich. 2013. Capsular contracture after breast reconstruction: collagen fiber orientation and organization. Plast Reconstr Surg 131: 680–685. [CrossRef]
- Darby, Ian A., and Tim D. Hewitson. 2007. Fibroblast Differentiation in Wound Healing and Fibrosis. International Review of Cytology 257: 143–179. [CrossRef]
- Mirastschijski, Ursula, Reinhild Schnabel, Juliane Claes, Wolfgang Schneider, Magnus S. Ågren, Carol Haaksma, and James J. Tomasek. 2010. Matrix metalloproteinase inhibition delays wound healing and blocks the latent transforming growth factor-β1-promoted myofibroblast formation and function. Wound Repair and Regeneration 18: 223–234. [CrossRef]
- Roeb, Elke. 2018. Matrix metalloproteinases and liver fibrosis (translational aspects). Matrix Biology 68–69. Elsevier: 463–473. [CrossRef]
- Wong, Victor W., Geoffrey C. Gurtner, and Michael T. Longaker. 2013. Wound healing: a paradigm for regeneration. Mayo Clinic proceedings 88. Mayo Clin Proc: 1022–1031. [CrossRef]
- Tomasek, James J., Giulio Gabbiani, Boris Hinz, Christine Chaponnier, and Robert A. Brown. 2002. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nature reviews. Molecular cell biology 3. Nat Rev Mol Cell Biol: 349–363. [CrossRef]
- Sawant, Mugdha, Boris Hinz, Katrin Schönborn, Isabel Zeinert, Beate Eckes, Thomas Krieg, and Ronen Schuster. 2021. A story of fibers and stress: Matrix-embedded signals for fibroblast activation in the skin. Wound Repair and Regeneration 29. John Wiley and Sons Inc: 515–530. [CrossRef]
- Grinnell, Frederick, and W. Matthew Petroll. 2010. Cell motility and mechanics in three-dimensional collagen matrices. Annual Review of Cell and Developmental Biology 26: 335–361. [CrossRef]
- Bernardo, Maria Ester, and Willem E. Fibbe. 2013. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 13. Cell Press: 392–402. [CrossRef]
- Hinz, Boris, and David Lagares. 2020. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nature Reviews Rheumatology 16. Nature Research: 11–31. [CrossRef]
- Hinz, Boris, Christopher A. McCulloch, and Nuno M. Coelho. 2019. Mechanical regulation of myofibroblast phenoconversion and collagen contraction. Experimental Cell Research 379. Elsevier Inc.: 119–128. [CrossRef]
- Klingberg, Franco, Boris Hinz, and Eric S. White. 2013. The myofibroblast matrix: implications for tissue repair and fibrosis. The Journal of Pathology 229. John Wiley & Sons, Ltd.: 298–309. [CrossRef]
- Kang, Sunah, Jungah Kim, Seulah Kim, Maierdanjiang Wufuer, Sohyun Park, Yumin Youngmin Kim, Dongkil Choi, et al. 2020. Efficient reduction of fibrous capsule formation around silicone breast implants densely grafted with 2-methacryloyloxyethyl phosphorylcholine (MPC) polymers by heat-induced polymerization. Biomaterials Science 8. Royal Society of Chemistry: 1580–1591. [CrossRef]
- Wynn, Thomas A. 2004. Fibrotic disease and the TH1/TH2 paradigm. Nature Reviews Immunology. Nature Publishing Group. [CrossRef]
- Plitas, George, and Alexander Y. Rudensky. 2020. Regulatory T Cells in Cancer. Annual Review of Cancer Biology 4. Annual Reviews Inc.: 459–477. [CrossRef]
- Zhang, Ximei, Nancy Olsen, and Song Guo Zheng. 2020. The progress and prospect of regulatory T cells in autoimmune diseases. Journal of Autoimmunity. Academic Press. [CrossRef]
- Sakaguchi, Shimon, Norihisa Mikami, James B. Wing, Atsushi Tanaka, Kenji Ichiyama, and Naganari Ohkura. 2020. Regulatory T Cells and Human Disease. Annual Review of Immunology 38. Annual Reviews Inc.: 541–566. [CrossRef]
- Motwani, Keshav, Leeana D. Peters, Willem H. Vliegen, Ahmed Gomaa El-sayed, Howard R. Seay, M. Cecilia Lopez, Henry V. Baker, et al. 2020. Human Regulatory T Cells From Umbilical Cord Blood Display Increased Repertoire Diversity and Lineage Stability Relative to Adult Peripheral Blood. Frontiers in Immunology 11. Frontiers Media S.A.: 611. [CrossRef]
- Roncarolo, Maria Grazia, Silvia Gregori, Rosa Bacchetta, Manuela Battaglia, and Nicola Gagliani. 2018. The Biology of T Regulatory Type 1 Cells and Their Therapeutic Application in Immune-Mediated Diseases. Immunity. Cell Press. [CrossRef]
- Savage, Peter A., David E.J. Klawon, and Christine H. Miller. 2020. Regulatory T Cell Development. Annual Review of Immunology 38. Annual Reviews Inc.: 421–453. [CrossRef]
- Shevach, Ethan M. 2011. Biological Functions of Regulatory T Cells. In Advances in Immunology, 112:137–176. Academic Press Inc. [CrossRef]
- Taylor, Amy E., Alexandra N. Carey, Ramesh Kudira, Celine S. Lages, Tiffany Shi, Simon Lam, Rebekah Karns, et al. 2018. Interleukin 2 Promotes Hepatic Regulatory T Cell Responses and Protects From Biliary Fibrosis in Murine Sclerosing Cholangitis. Hepatology 68. John Wiley and Sons Inc.: 1905–1921. [CrossRef]
- Lee, Gap Ryol. 2018. The balance of th17 versus treg cells in autoimmunity. International Journal of Molecular Sciences. MDPI AG. [CrossRef]
- Doloff, Joshua C., Omid Veiseh, Arturo J. Vegas, Hok Hei Tam, Shady Farah, Minglin Ma, Jie Li, et al. 2017. Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates. Nature materials 16. Nat Mater: 671–680. [CrossRef]
- Anderson, James M., Analiz Rodriguez, and David T. Chang. 2008. Foreign body reaction to biomaterials. Seminars in Immunology 20: 86–100. [CrossRef]
- Sussman, Eric M., Michelle C. Halpin, Jeanot Muster, Randall T. Moon, and Buddy D. Ratner. 2014. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Annals of biomedical engineering 42. Ann Biomed Eng: 1508–1516. [CrossRef]
- Wolf, Matthew T., Christopher L. Dearth, Christian A. Ranallo, Samuel T. LoPresti, Lisa E. Carey, Kerry A. Daly, Bryan N. Brown, and Stephen F. Badylak. 2014. Macrophage polarization in response to ECM coated polypropylene mesh. Biomaterials 35. Elsevier Ltd.: 6838–6849. [CrossRef]
- Klopfleisch, R., and F. Jung. 2017. The pathology of the foreign body reaction against biomaterials. Journal of Biomedical Materials Research - Part A 105. John Wiley and Sons Inc.: 927–940. [CrossRef]
- Dondossola, Eleonora, Boris M. Holzapfel, Stephanie Alexander, Stefano Filippini, Dietmar W. Hutmacher, and Peter Friedl. 2016. Examination of the foreign body response to biomaterials by nonlinear intravital microscopy. Nature biomedical engineering 1. Nat Biomed Eng. [CrossRef]
- Miron, Richard J., and Dieter D. Bosshardt. 2018. Multinucleated Giant Cells: Good Guys or Bad Guys? Tissue engineering. Part B, Reviews 24. Tissue Eng Part B Rev: 53–65. [CrossRef]
- Spiller, Kara L., Rachel R. Anfang, Krista J. Spiller, Johnathan Ng, Kenneth R. Nakazawa, Jeffrey W. Daulton, and Gordana Vunjak-Novakovic. 2014. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 35. Elsevier BV: 4477–4488. [CrossRef]
- Mooney, Jane E., Barbara E. Rolfe, Geoffrey W. Osborne, David P. Sester, Nico Van Rooijen, Gordon R. Campbell, David A. Hume, and Julie H. Campbell. 2010. Cellular plasticity of inflammatory myeloid cells in the peritoneal foreign body response. American Journal of Pathology 176. Elsevier Inc.: 369–380. [CrossRef]
- Smith, Tim D., Raji R. Nagalla, Esther Y. Chen, and Wendy F. Liu. 2017. Harnessing macrophage plasticity for tissue regeneration. Advanced Drug Delivery Reviews 114. Elsevier B.V.: 193–205. [CrossRef]
- Martin, Karen E., and Andrés J. García. 2021. Macrophage phenotypes in tissue repair and the foreign body response: Implications for biomaterial-based regenerative medicine strategies. Acta biomaterialia 133. Acta Biomater: 4–16. [CrossRef]
- Graney, Pamela L., Emily B. Lurier, and Kara L. Spiller. 2018. Biomaterials and Bioactive Factor Delivery Systems for the Control of Macrophage Activation in Regenerative Medicine. ACS biomaterials science & engineering 4. ACS Biomater Sci Eng: 1137–1148. [CrossRef]
- Bonner, James C. 2004. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine and Growth Factor Reviews 15. Elsevier BV: 255–273. [CrossRef]
- McNally, Amy K., and James M. Anderson. 2015. Phenotypic expression in human monocyte-derived interleukin-4-induced foreign body giant cells and macrophages in vitro: dependence on material surface properties. Journal of biomedical materials research. Part A 103. J Biomed Mater Res A: 1380–1390. [CrossRef]
- Abaricia, Jefferson O., Negin Farzad, Tyler J. Heath, Jamelle Simmons, Lais Morandini, and Rene Olivares-Navarrete. 2021. Control of innate immune response by biomaterial surface topography, energy, and stiffness. Acta Biomaterialia 133. Acta Materialia Inc: 58–73. [CrossRef]
- Love, Ryan J., and Kim S. Jones. 2013. The recognition of biomaterials: Pattern recognition of medical polymers and their adsorbed biomolecules. Journal of Biomedical Materials Research - Part A 101 A: 2740–2752. [CrossRef]
- Altieri, D. C., P. M. Mannucci, and A. M. Capitanio. 1986. Binding of fibrinogen to human monocytes. Journal of Clinical Investigation 78: 968–976. [CrossRef]
- Aiyelabegan, Hammed Tanimowo, and Esmaeil Sadroddiny. 2017. Fundamentals of protein and cell interactions in biomaterials. Biomedicine and Pharmacotherapy 88. Elsevier Masson SAS: 956–970. [CrossRef]
- Sheikh, Zeeshan, Patricia J. Brooks, Oriyah Barzilay, Noah Fine, and Michael Glogauer. 2015. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials 8. MDPI AG: 5671–5701. [CrossRef]
- Helming, Laura, and Siamon Gordon. 2009. Molecular mediators of macrophage fusion. Trends in cell biology 19. Trends Cell Biol: 514–522. [CrossRef]
- Zhang, Yang, Xian Cheng, John A. Jansen, Fang Yang, and Jeroen J.J.P. van den Beucken. 2019. Titanium surfaces characteristics modulate macrophage polarization. Materials Science and Engineering C 95. Elsevier Ltd.: 143–151. [CrossRef]
- Shayan, Mahdis, Jagannath Padmanabhan, Aaron H. Morris, Bettina Cheung, Ryan Smith, Jan Schroers, and Themis R. Kyriakides. 2018. Nanopatterned bulk metallic glass-based biomaterials modulate macrophage polarization. Acta Biomaterialia 75. Acta Materialia Inc: 427–438. [CrossRef]
- Mohiuddin, Mohammed, Hsu An Pan, Yao Ching Hung, and Guewha Steve Huang. 2012. Control of growth and inflammatory response of macrophages and foam cells with nanotopography. Nanoscale research letters 7. Nanoscale Res Lett. [CrossRef]
- Padmanabhan, Jagannath, Michael J. Augelli, Bettina Cheung, Emily R. Kinser, Barnett Cleary, Priyanka Kumar, Renhao Wang, et al. 2016. Regulation of cell-cell fusion by nanotopography. Scientific reports 6. Sci Rep. [CrossRef]
- Previtera, Michelle L., and Amitabha Sengupta. 2015. Substrate Stiffness Regulates Proinflammatory Mediator Production through TLR4 Activity in Macrophages. PloS one 10. PLoS One. [CrossRef]
- Irwin, E. F., K. Saha, M. Rosenbluth, L. J. Gamble, D. G. Castner, and K. E. Healy. 2008. Modulus-dependent macrophage adhesion and behavior. Journal of Biomaterials Science, Polymer Edition 19. VSP BV: 1363–1382. [CrossRef]
- Gristina, A.G., P. Naylor, and Q. Myrvik. 1998. Infections from biomaterials and implants: a race for the surface. Med Prog Technol 14: 205–224.
- Belay, Ermias D., Lawrence B. Schonberger, Paul Brown, Suzette A. Priola, Bruce Chesebro, Robert G. Will, and David M. Asher. 2010. Disinfection and Sterilization of Prion-Contaminated Medical Instruments. Infection Control & Hospital Epidemiology 31. Cambridge University Press (CUP): 1304–1306. [CrossRef]
- Rutala, William A., and David J. Weber. 2001. New disinfection and sterilization methods. Emerging infectious diseases 7. Emerg Infect Dis: 348–353. [CrossRef]
- Rutala, William A., and David J. Weber. 2010. Guideline for Disinfection and Sterilization of Prion-Contaminated Medical Instruments. Infection Control & Hospital Epidemiology 31. Cambridge University Press (CUP): 107–117. [CrossRef]
- Donlan, Rodney M. 2002. Biofilms: Microbial Life on Surfaces. Emerging Infectious Diseases 8. Centers for Disease Control and Prevention: 881. [CrossRef]
- Vestby, L.K., T. Grønseth, R. Simm, and L.L. Nesse. 2020. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 9: 1–28.
- Boks, N.P., W. Norde, H.C. van der Mei, and H.J. Busscher. 2008. Forces involved in bacterial adhesion to hydrophilic and hydrophobic surfaces. Microbiology 154: 3122–3133.
- Malte, H. 1999. The DLVO theory in microbial adhesion. Colloids Surf B Biointerfaces 14: 105–119.
- Berne, C., C.K. Ellison, A. Ducret, and Y.V. Brun. 2018. Bacterial adhesion at the single-cell level. Nat Rev Microbiol 16: 616–627.
- Costerton, J.W. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284: 1318–1322.
- Fuqua, W.C., S.C. Winans, and E.P. Greenberg. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density- responsive transcriptional regulators. J Bacteriol 176: 269–275.
- Rumbaugh, K.P., and K. Sauer. 2020. Biofilm dispersion. Nat Rev Microbiol 18: 571–586.
- Josse, J., F. Laurent, and A. Diot. 2017. Staphylococcal adhesion and host cell invasion: fibronectin-binding and other mechanisms. Front Microbiol 8: 2433.
- Herman-Bausier, P., C. Valotteau, G. Pietrocola, and et al. 2016. Mechanical strength and inhibition of the Staphylococcus aureus collagen-binding protein cna. mBio 7: e01529–e01616.
- Walker, J.N., C.L. Pinkner, J.S. Pinkner, S.J. Hultgren, and T.M. Myckatyn. 2019. The detection of bacteria and matrix proteins on clinically benign and pathologic implants. Plast Reconstr Surg Glob Open 7: 1–9.
- Tamboto, H., K. Vickery, and A.K. Deva. 2010. Subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty. Plast Reconstr Surg 126: 835–842.
- Barbieri, R., M. Pesce, S. Franchelli, I. Baldelli, A. De Maria, and A. Marchese. 2015. Phenotypic and genotypic characterization of Staphylococci causing breast peri-implant infections in oncologic patients. BMC Microbiol 15: 1–10.
- Miller, K.E., B. Hontanilla, A. Cabello, D. Marre, L. Armendariz, and J. Leiva. 2016. The effect of late infection and antibiotic treatment on capsular contracture in silicone breast implants: a rat model. J Plast Reconstr Aesthet Surg 69: 70–76.
- Anderson, James M. 2008. Biocompatibility and Bioresponse to Biomaterials. Principles of Regenerative Medicine. Academic Press: 704–723. [CrossRef]
- Anderson, James M., and Amy K. McNally. 2011. Biocompatibility of implants: Lymphocyte/macrophage interactions. Seminars in Immunopathology 33: 221–233. [CrossRef]
Figure 1.
Stages of Capsular Fibrosis Around SMIs.Immediate Wound Response. During the acute wound healing phase immediately after SMI implantation, the implant is exposed to wound bed fluid. T cells are activated primarily due to microbial contamination, implant shedding or silicone bleeding, and protein adsorption onto the implant surface and differentiate into Th1 and Th17 responses, while the T regulatory (Treg) response is suppressed. Foreign Body Response. Innate immune cells, such as neutrophils, macrophages, and dendritic cells, are recruited to the implant site. Macrophages are activated and play a key role in fibrogenesis, contributing to the early stages of fibrosis. Early-Stage Fibrosis The extracellular matrix undergoes remodeling with significant collagen deposition. The fibrotic tissue encapsulating the implant undergoes neo-angiogenesis, leading to the formation of a fibrous capsule. Chronic Inflammation and Capsular Contracture. Chronic inflammation is perpetuated by the permanent adhesion of proteins to the SMI surface. Intracapsular silicone evasion and microbial infiltration further exacerbate the inflammatory response. This leads to excessive ECM remodeling and collagen deposition. The resulting thickened and contracted capsule causes discomfort and pain to the patient, potentially leading to implant displacement and necessitating revision surgery.
Figure 1.
Stages of Capsular Fibrosis Around SMIs.Immediate Wound Response. During the acute wound healing phase immediately after SMI implantation, the implant is exposed to wound bed fluid. T cells are activated primarily due to microbial contamination, implant shedding or silicone bleeding, and protein adsorption onto the implant surface and differentiate into Th1 and Th17 responses, while the T regulatory (Treg) response is suppressed. Foreign Body Response. Innate immune cells, such as neutrophils, macrophages, and dendritic cells, are recruited to the implant site. Macrophages are activated and play a key role in fibrogenesis, contributing to the early stages of fibrosis. Early-Stage Fibrosis The extracellular matrix undergoes remodeling with significant collagen deposition. The fibrotic tissue encapsulating the implant undergoes neo-angiogenesis, leading to the formation of a fibrous capsule. Chronic Inflammation and Capsular Contracture. Chronic inflammation is perpetuated by the permanent adhesion of proteins to the SMI surface. Intracapsular silicone evasion and microbial infiltration further exacerbate the inflammatory response. This leads to excessive ECM remodeling and collagen deposition. The resulting thickened and contracted capsule causes discomfort and pain to the patient, potentially leading to implant displacement and necessitating revision surgery.
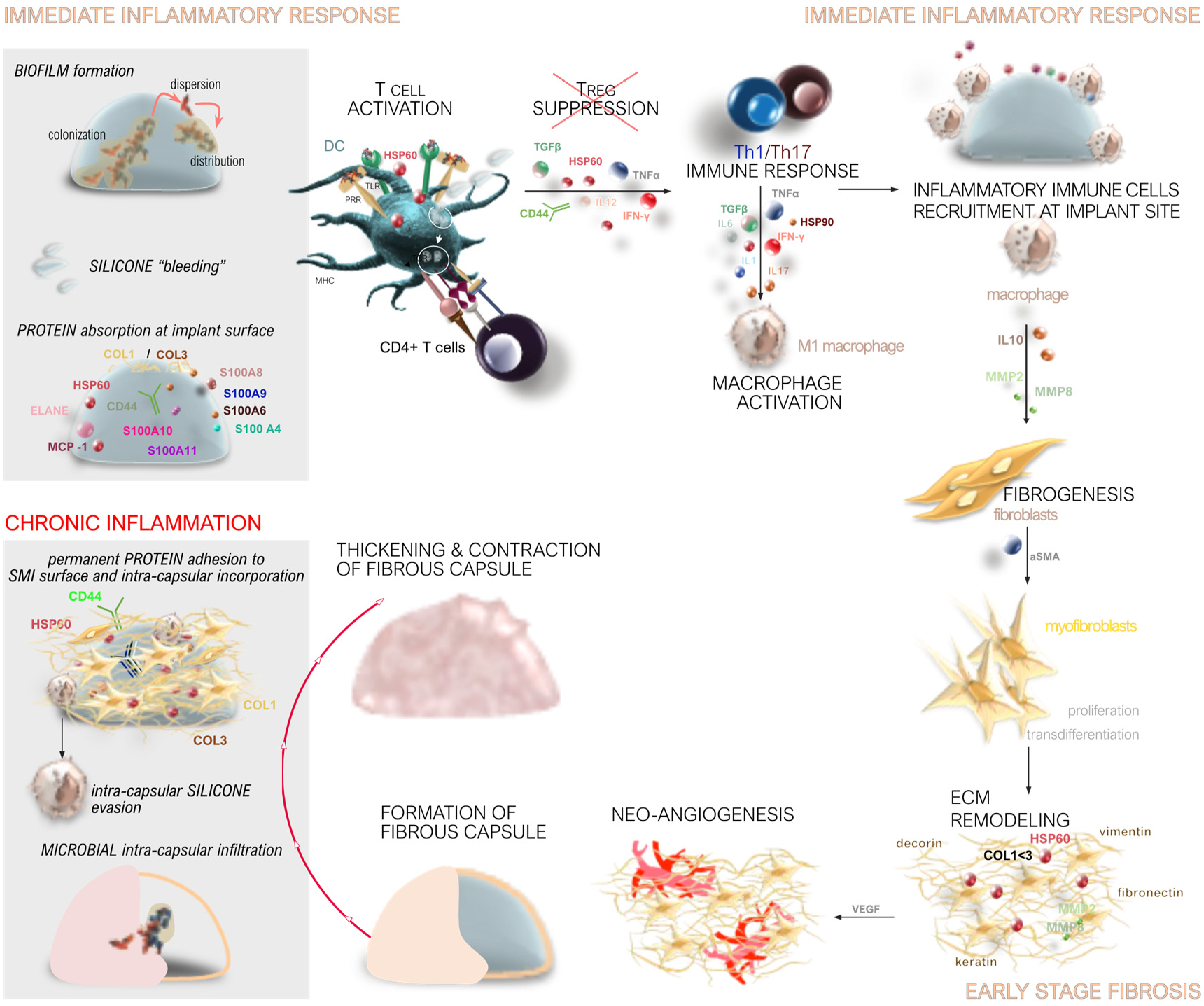
Figure 2.
Genomic Profiling of Capsular Fibrosis Around SMIs. Comprehensive profiling of capsular fibrosis around SMIs through genomic techniques, elucidating the underlying molecular mechanisms and potential clinical applications. Clinical Context: The study involved 18 female patients with 23 breast capsules, comparing control (Baker Grade I and II) and contracted (Baker Grade III and IV) capsules. Genomic Techniques: RNA samples underwent labeling with fluorescent dyes, reverse transcription, hybridization to microarray, and scanning to identify over-expressed (IL8, MMP12, SAA1) and under-expressed (ACAN, TIMP4, TNFSF11) genes. Validation: qPCR and sequential immunohistochemistry (IHC) validated the expression of key genes, with IL8 and MMP12 showing over-expression and ACAN, TIMP4, and TNFSF11 showing under-expression. Pro- and Anti-Inflammatory Genes: Highlighted genes included pro-inflammatory markers (IL8, MMP2) associated with ECM degradation and anti-inflammatory markers (ACAN, TIMP4) associated with ECM deposition.
Figure 2.
Genomic Profiling of Capsular Fibrosis Around SMIs. Comprehensive profiling of capsular fibrosis around SMIs through genomic techniques, elucidating the underlying molecular mechanisms and potential clinical applications. Clinical Context: The study involved 18 female patients with 23 breast capsules, comparing control (Baker Grade I and II) and contracted (Baker Grade III and IV) capsules. Genomic Techniques: RNA samples underwent labeling with fluorescent dyes, reverse transcription, hybridization to microarray, and scanning to identify over-expressed (IL8, MMP12, SAA1) and under-expressed (ACAN, TIMP4, TNFSF11) genes. Validation: qPCR and sequential immunohistochemistry (IHC) validated the expression of key genes, with IL8 and MMP12 showing over-expression and ACAN, TIMP4, and TNFSF11 showing under-expression. Pro- and Anti-Inflammatory Genes: Highlighted genes included pro-inflammatory markers (IL8, MMP2) associated with ECM degradation and anti-inflammatory markers (ACAN, TIMP4) associated with ECM deposition.
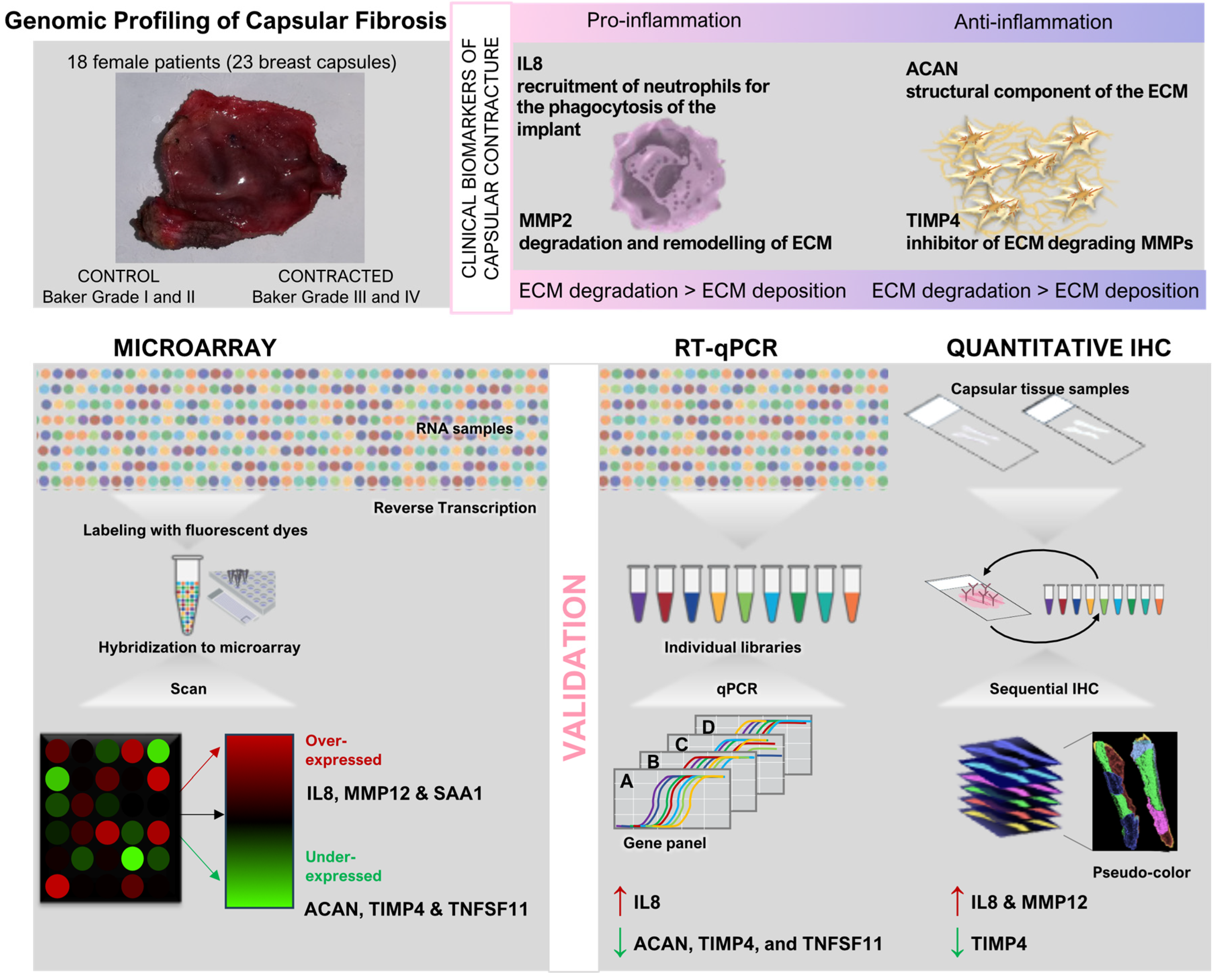
Figure 3.
Proteomic Profiling of Capsular Fibrosis Around SMIs. Comprehensive profiling of capsular fibrosis around SMIs through proteomic techniques, elucidating the underlying molecular mechanisms and potential clinical applications. Clinical context: this study comprised 10 female patients who underwent simultaneous prophylactic bilateral nipple-sparing mastectomy (NSME) and tissue expander-based breast reconstruction. Biological samples of wound bed fluid (referred to as WBF) were collected daily from day 1 to 5 following expander implantation. Wound drains, integral to the surgical procedure for patients undergoing expander-based reconstruction, were retained postoperatively. WBF was collected under sterile conditions. During reoperation, capsular tissue was harvested from two different implants. Proteomic Techniques: Time-dependent collection of wound bed fluid and resection of the implant surface followed by enzymatic protein digestion and peptide labeling with TMT tags. Multiplexed Proteomics: High pH fractionation, nano LC-MS analysis of peptide digests, and on-surface digestion of proteins into peptides revealed time-dependent protein expression and quantification post-surgery. Integration and Clinical Implications. Immediate Inflammatory Response: Depicted the dynamics of inflammatory response and wound healing post-implantation, including T cell activation, Th1/Th17 immune response, T regulatory (Treg) cell suppression, and macrophage activation. Protein Adsorption and Fibrosis Progression: Continuous protein adsorption to the implant surface and ECM remodeling contributed to capsular contracture and fibrosis. Biomarkers for Early Detection: Identified potential biomarkers such as HSPs and S100 proteins for early-stage fibrosis, offering diagnostic and therapeutic insights.
Figure 3.
Proteomic Profiling of Capsular Fibrosis Around SMIs. Comprehensive profiling of capsular fibrosis around SMIs through proteomic techniques, elucidating the underlying molecular mechanisms and potential clinical applications. Clinical context: this study comprised 10 female patients who underwent simultaneous prophylactic bilateral nipple-sparing mastectomy (NSME) and tissue expander-based breast reconstruction. Biological samples of wound bed fluid (referred to as WBF) were collected daily from day 1 to 5 following expander implantation. Wound drains, integral to the surgical procedure for patients undergoing expander-based reconstruction, were retained postoperatively. WBF was collected under sterile conditions. During reoperation, capsular tissue was harvested from two different implants. Proteomic Techniques: Time-dependent collection of wound bed fluid and resection of the implant surface followed by enzymatic protein digestion and peptide labeling with TMT tags. Multiplexed Proteomics: High pH fractionation, nano LC-MS analysis of peptide digests, and on-surface digestion of proteins into peptides revealed time-dependent protein expression and quantification post-surgery. Integration and Clinical Implications. Immediate Inflammatory Response: Depicted the dynamics of inflammatory response and wound healing post-implantation, including T cell activation, Th1/Th17 immune response, T regulatory (Treg) cell suppression, and macrophage activation. Protein Adsorption and Fibrosis Progression: Continuous protein adsorption to the implant surface and ECM remodeling contributed to capsular contracture and fibrosis. Biomarkers for Early Detection: Identified potential biomarkers such as HSPs and S100 proteins for early-stage fibrosis, offering diagnostic and therapeutic insights.
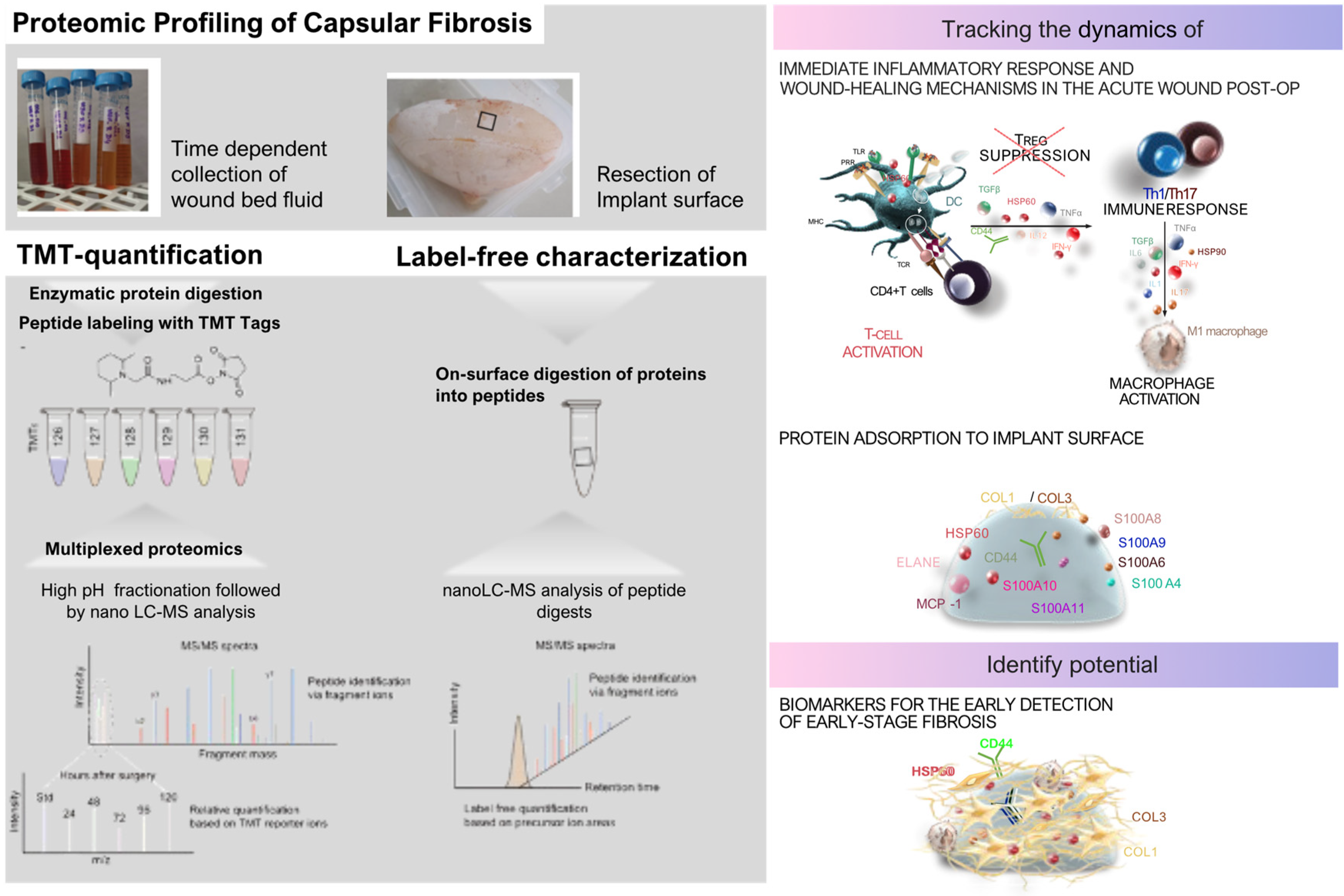
Figure 4.
Bacterial adhesion, biofilm formation on SMI surfaces, and clinical strategies for prevention. Bacterial colonization of SMI surfaces and the subsequent formation of biofilms are major contributors to chronic infection and the development of complications such as capsular contracture. Various clinical strategies are employed to minimize microbial contamination and biofilm formation at different stages of the surgical process. (a) Bacterial SMI surface adhesion, colonization, and biofilm formation. Reversible Phase: Planktonic bacteria initially adhere to the SMI surface through electrostatic and hydrophobic interactions. This phase is reversible, and bacteria can be removed if appropriate interventions are applied. Irreversible Phase: Over time, bacteria firmly attach to the SMI surface, proliferate, and form biofilms. The biofilm matrix protects bacteria from the immune response and antimicrobial agents, leading to persistent infection. Capsule Formation: The presence of bacterial biofilms and the continuous inflammatory response contribute to the formation and thickening of the fibrous capsule, leading to capsular contracture. (b) Flowchart illustrating the clinical strategies for preventing or reducing microbial contamination and biofilm formation on breast implants. Pre-operative strategies focus on assessing patient risk, preparing the surgical site through sterilization and disinfection, and choosing implants with surface modifications to reduce bacterial adhesion. Intra-operative practices emphasize maintaining a sterile environment, proper handling of the implant, and using antibiotic interventions to minimize contamination. Post-operative care includes monitoring the antimicrobial inflammatory response, administering antibiotics, and maintaining meticulous wound care to detect and prevent early signs of infection.
Figure 4.
Bacterial adhesion, biofilm formation on SMI surfaces, and clinical strategies for prevention. Bacterial colonization of SMI surfaces and the subsequent formation of biofilms are major contributors to chronic infection and the development of complications such as capsular contracture. Various clinical strategies are employed to minimize microbial contamination and biofilm formation at different stages of the surgical process. (a) Bacterial SMI surface adhesion, colonization, and biofilm formation. Reversible Phase: Planktonic bacteria initially adhere to the SMI surface through electrostatic and hydrophobic interactions. This phase is reversible, and bacteria can be removed if appropriate interventions are applied. Irreversible Phase: Over time, bacteria firmly attach to the SMI surface, proliferate, and form biofilms. The biofilm matrix protects bacteria from the immune response and antimicrobial agents, leading to persistent infection. Capsule Formation: The presence of bacterial biofilms and the continuous inflammatory response contribute to the formation and thickening of the fibrous capsule, leading to capsular contracture. (b) Flowchart illustrating the clinical strategies for preventing or reducing microbial contamination and biofilm formation on breast implants. Pre-operative strategies focus on assessing patient risk, preparing the surgical site through sterilization and disinfection, and choosing implants with surface modifications to reduce bacterial adhesion. Intra-operative practices emphasize maintaining a sterile environment, proper handling of the implant, and using antibiotic interventions to minimize contamination. Post-operative care includes monitoring the antimicrobial inflammatory response, administering antibiotics, and maintaining meticulous wound care to detect and prevent early signs of infection.
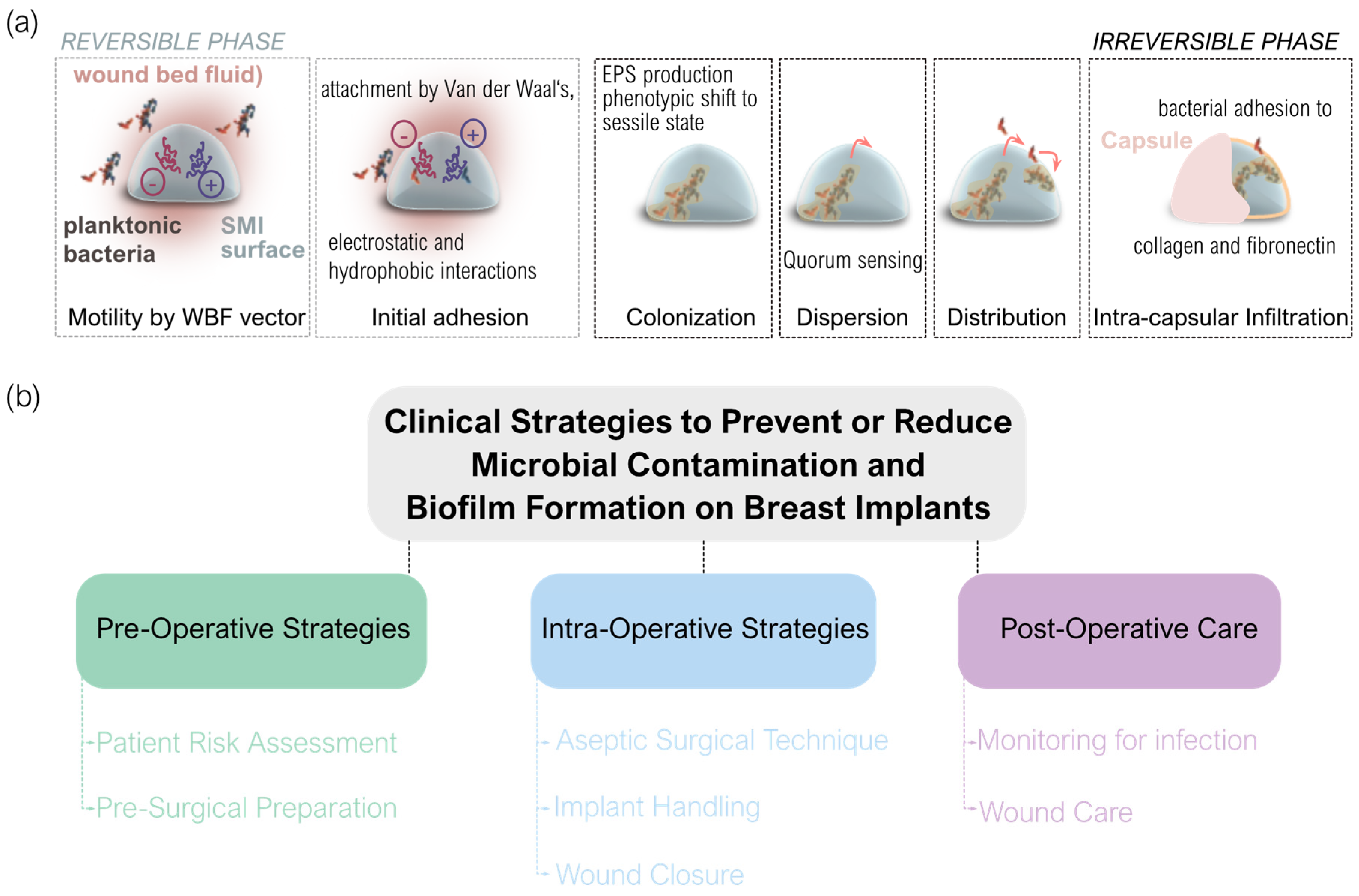
Table 1.
Key cellular and immune mechanisms in fibrosis around SMI.
Table 1.
Key cellular and immune mechanisms in fibrosis around SMI.
| Cellular/Immune Mechanism |
Role in Fibrosis and Implant Interaction |
Molecular Mechanisms and Key Markers |
Clinical Implications |
Fibroblast
Activation
|
Fibroblasts respond to the presence of silicone implants by activating into myofibroblasts, key cells in wound healing and fibrosis processes. |
TGF-β1, α-SMA, SMAD proteins: TGF-β1 signaling leads to SMAD protein activation, promoting α-SMA expression and fibroblast activation. |
Persistent activation can lead to excessive fibrosis, contributing to complications like capsular contracture. |
| Myofibroblast Differentiation |
Prolonged inflammation and mechanical stress trigger fibroblasts to differentiate into myofibroblasts, producing ECM components and driving tissue contraction. |
TGF-β1, α-SMA, Collagen I/III: TGF-β1 induces α-SMA and collagen production, facilitating myofibroblast differentiation and ECM contraction. |
Myofibroblast activity is a key driver of fibrotic capsule formation around implants, impacting implant outcomes. |
ECM
Remodeling
|
Fibroblasts and MMPs continuously remodel the ECM around silicone implants, balancing collagen deposition and degradation. |
MMPs, TIMPs, Collagen I/III: MMPs degrade ECM components, while TIMPs inhibit MMPs to regulate ECM remodeling. |
Dysregulated ECM remodeling can result in a stiffer, thicker fibrotic capsule, complicating implant removal or revision surgery. |
Immune Cell
Activation
|
Immune cells, including macrophages, dendritic cells, and T cells, are activated by silicone implants, releasing cytokines that further stimulate fibroblasts. |
IL-1β, TNF-α, IL-6, TGF-β: Pro-inflammatory cytokines like IL-1β and TNF-α activate fibroblasts and sustain chronic inflammation. |
Chronic immune activation can perpetuate fibrosis and contribute to implant-related complications like chronic inflammation. |
Macrophage
Polarization
|
Macrophages polarize into M1 (pro-inflammatory) or M2 (anti-inflammatory) phenotypes in response to implant properties, affecting fibrosis. |
CD86 (M1), CD206 (M2), IL-10, TGF-β: M1 macrophages express CD86 and produce IL-1β, while M2 express CD206 and secrete IL-10 and TGF-β. |
Targeting macrophage polarization through surface modifications could reduce fibrotic responses and improve implant biocompatibility. |
| T Cell-Mediated Responses |
CD4+ T cells, particularly Th1 and Th2 subsets, regulate fibrosis, with Th1 promoting and Th2 potentially reducing fibrotic responses. |
IFN-γ (Th1), IL-4, IL-13 (Th2): Th1 cells produce IFN-γ, driving fibrosis, while Th2 cells secrete IL-4 and IL-13, which may reduce fibrosis. |
Targeting T cell responses through specific immunomodulatory therapies, such as the use of monoclonal antibodies to block Th1 cytokines (e.g., IFN-γ) or promoting Th2 responses with IL-4 or IL-13, could help mitigate fibrosis around silicone implants. |
Regulatory
T Cells (Tregs)
|
Tregs modulate the immune response by suppressing excessive immune activation and maintaining immune tolerance around implants. |
FOXP3, IL-10, TGF-β: FOXP3 is a key marker for Tregs, which secrete IL-10 and TGF-β to suppress inflammation and fibrosis. |
Enhancing Treg activity could help in reducing chronic inflammation and fibrosis, improving implant biocompatibility. |
Table 2.
Clinical translation and future directions in SMI-based breast surgery.
Table 2.
Clinical translation and future directions in SMI-based breast surgery.
| Focus Area |
Advancements |
Clinical Translation |
Future Directions |
Personalized Risk
Management
|
- Tailored patient care by analyzing immune responses and microbial patterns (risk mitigation for capsular contracture). |
- Personalized implant selection, antimicrobial prophylaxis, and post-operative plans to reduce complications. |
- Further studies to better understand patient-specific immune responses to enable more precise and effective personalized care. |
Biomaterials & Implant
Modifications
|
- Development of advanced materials to reduce biofilm formation and inflammation (e.g., anti-biofilm coatings, immunomodulatory surfaces). |
- New biomaterials designed for biocompatibility, longevity, and lower complication rates, potentially reducing the need for revision surgeries. |
- Continued innovation in materials mimicking natural tissues and enhancing implant integration for better biocompatibility and reduced immune reactions. |
Targeted
Therapeutic
Approaches
|
- Exploration of therapies like anti-inflammatory agents, quorum sensing inhibitors, and biofilm disruptors to prevent fibrotic reactions. |
- Modulation of immune responses to improve biointegration and reduce fibrotic complications post-implantation. |
- Identifying and testing new therapeutic targets through molecular studies to prevent or mitigate complications. |
Surgical
Techniques & Post-op Care
|
- Improved techniques minimizing tissue trauma, optimizing tissue integration, and preventing infections through molecular insights. |
- Implementation of evidence-based surgical practices to enhance precision, minimize trauma, and improve recovery outcomes. |
- Further refinement of techniques based on molecular and preclinical data to reduce complications and improve healing. |
Patient
Monitoring & Education
|
- Use of molecular biomarkers for early detection of complications; emphasis on patient education regarding self-monitoring and follow-up care. |
- Development of monitoring protocols for early intervention, along with improved patient engagement for long-term implant success. |
|
| Personalized Medicine |
Generalized treatments may not consider individual patient factors, leading to suboptimal outcomes. |
Uniform antibiotic and antifibrotic regimens based on general risk profiles. |
Personalized treatments tailored to patient-specific factors (microbial flora, immune responses, genetic predisposition). |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).









