1. Introduction
Vitamin D deficiency has traditionally been associated with bone health disorders. In recent years, however, it has received attention due to the association between low levels of this vitamin and cardiovascular risk, as well as inflammatory, infectious, and neoplastic processes [
1].
Current guidelines recommend that vitamin D status should be monitored by measuring 25 OH vitamin D (calcidiol) levels. While calcidiol offers several advantages, including a long half-life (2-3 weeks) and greater stability, there are other considerations: on the one hand, there is significant methodological variability in its measurement; on the other hand, the association between vitamin D status and bone health markers, as well as other extra-skeletal functions, is not particularly strong in paediatric populations and is subject to variation according to specific factors, including age, race, obesity, medication use, genetic factors, and chronic diseases. For these reasons, efforts have been made in recent years to advance the understanding of new markers, including free vitamin D.
Vitamin D and its metabolites are closely related to serum proteins, with the vitamin D-binding protein (VDBP) representing the most significant of these (accounting for 85-90% of the total). Approximately 10-15% is bound to albumin, with less than 0.1% circulating freely [
2,
3]. VDBP is a protein produced by the liver with a large number of polymorphisms. Three main haplotypes have been described (Gc1F, Gc1S, and Gc2), the frequency of which varies according to breed and geographic distribution [
4,
5,
6]. The combination of different haplotypes determines the concentration of VDBP, its affinity for 25 OH vitamin D and, consequently, the levels of free vitamin D. These findings could explain the lack of correlation between total 25 OH vitamin D levels and clinical manifestations [
7].
Furthermore, VDBP and albumin levels can vary in patients with certain conditions. For instance, they tend to decrease in cases of cirrhosis or in elderly patients with multiple comorbidities. Conversely, they increase in the last months of pregnancy. In such circumstances, both calcidiol and free vitamin D levels may fluctuate, prompting the suggestion that the latter may more accurately reflect vitamin D status [
8,
9,
10].
Given that cystic fibrosis (CF) is a disease characterized by a state of chronic inflammation and alterations in oxidative stress alterations, with pulmonary exacerbations, poor intake, and pancreatic insufficiency often resulting in hypoproteinemia and malnutrition, it could be hypothesized that the determination of free vitamin D may be of particular interest in this condition.
The main objective of our study was to analyze the genotype and quantify the levels of vitamin D-binding protein (VDBP) and free vitamin D in a sample of clinically stable cystic fibrosis patients.
2. Materials and Methods
2.1. Study
This is a multicentre, cross-sectional, and prospective study involving patients with CF, exocrine pancreatic insufficiency who are clinically stable. The study examined levels of vitamin D (total and free), as well as the VDBP different haplotypes. Approval for the study was granted by the clinical research ethics committee of Hospital Universitario Central de Asturias (HUCA) (number 67/19) and the committee of the rest of the participating hospitals also gave their approval. All participants between 12-18 years gave their consent to participate in the study, and adult patients and parents of minors signed informed consent forms to participate in the study.
2.2. Subjects
Patients diagnosed with CF in a clinically stable condition, defined by clinical criteria: absence of cough, fever, expectoration, or hemoptysis, and must no have undergone any oral or intravenous antibiotic treatment in the 2 weeks prior to their inclusion in the study.
CF patients with exocrine pancreatic insufficiency defined by a fecal elastase determination <200 mcg/g of stool.
Platelet count < 50,000/mm³
Significant liver function impairment, severe cholestasis, or renal insufficiency
Hospitalization or administration of oral or intravenous antibiotics within the 2 weeks prior to the start of the study.
2.3. Study Development and Response Evaluation
All analytical determinations were collected at hospitals of origin, stored at appropriate temperatures, and subsequently analyzed in a centralized manner.
Genotyping of the Vitamin D-Binding Protein (VDBP): Blood samples were collected in 9 ml EDTA tubes and frozen at -20ºC until shipment. Upon receipt at the destination laboratory, the blood, once thawed, was subjected to the salt-based genomic DNA extraction method. The areas of the GC gene (encoding VDBP), which contain the SNPs rs7041 and rs4588, were amplified by polymerase chain reaction (PCR) using the specific primers: 5' TGTAAAAGATCTGAAATGG 3' and 3' CATAATGGCATCTCAATAA 5' (annealing of 48º) following the thermocycling protocol used by Cillero et al. (Ann Clin Biochem. 2018 1: 4563218780135).
Amplicons were purified and sequenced by automated Sanger sequencing using the same primers individually. The reading of the electropherograms for the detection of SNPs was carried out with the Sequencher 5.4 software (Gene Codes Corporation).
Table 1.
Correlation between SNPs rs7041 and rs4588 and kind of GC gene allele.
Table 1.
Correlation between SNPs rs7041 and rs4588 and kind of GC gene allele.
SNP rs7041
NM_000583.4
(GC): c.1296T>G; p.D432E
|
SNP rs4588
NM_000583.4 (GC): c.1307C>A; p.T436K
|
SNP-based allele |
| Nucleotide |
Amino Acid |
Nucleotide |
Amino Acid |
| GAT
|
D |
ACG |
T |
1F |
| GAG
|
E |
ACG |
T |
1S |
| GAT
|
E |
AAG |
K |
2 |
Total Vitamin D levels (25OH Vitamin D, Calcidiol): Serum total 25OHD concentration was measured by an electro-chemiluminescence assay (Roche Diagnostics GmbH, Mannheim, Germany). For serum 25OHD, the inter-assay coefficient of variation (CV) determined with PreciControl VitDT 1 and 2 ranged from 3.76% to 10.2%. Vitamin D (calcidiol) levels were considered deficient when below 20 ng/ml, insufficient between 20 and 30 ng/ml, and sufficient when above 30 ng/ml [
11].
Free vitamin D levels: Direct measurement of free 25OHD concentration was performed by a competitive ELISA assay (KARF1991, DiaSource, Louvain-la-Nueve, Belgium), which detects the free fraction. The analytical sensitivity obtained ranged from 2.4 to 3.9 pg/mL.
Identification and epidemiological data: Identification by codes assigned to the hospital and the patient, date of birth, sex, clinical form, genetics.
Concomitant medication: Probiotics, corticosteroids, potentiators, modulators, pancreatic enzymes...
Anthropometry: The weight and height of each patient were directly obtained, with the patient barefoot and in underwear, using instruments with a precision of 50 g and 0.5 cm, respectively. Calculation of anthropometric parameters, was performed using the nutritional tool of the
Spanish Society of Pediatric Gastroenterology, Hepatology and Nutrition (SEGHNP;
www.seghnp.org). Body mass index (BMI) was calculated and expressed by percentile and z-score using the World Health Organization 2006 (WHO;
https://www.who.int) reference values for children under 6 years of age and Carrascosa 2010 reference values [
12] for children over 6 years. The nutritional status of each patient was classified according to the criteria agreed upon by the
North American and European CF societies (based on BMI in adults and percentiled BMI in children) as follows: undernourished (<18.5 kg/m²; <P10), at nutritional risk (18.5-21.9 kg/m²; P10-P49), well-nourished (22-24.9 kg/m²; P50-P84), overweight (25-29.9 kg/m², P85-P94), and obese (≥30 kg/m²; ≥P95).
2.4. Statistical Analysis
Study data were collected and managed using REDCap [
13] electronic data capture tools hosted at SEGHNP (
www.seghnp.org). Technical support was provided by the AEG REDCap Support Unit, shared with the Spanish Association of Gastroenterology (AEG). REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing an intuitive interface for validated data entry, audit trails for tracking data manipulation and export procedures, automated export procedures for seamless data downloads to common statistical packages, and procedures for importing data from external sources. Statistical analysis was performed using the Statistical Package (STATA), version 13.0.
Basic statistical techniques of descriptive analysis were used in the study. Kolmogorov-Smirnov tests were used to assess normality. Pearson and Spearman correlation tests were used to analyze the joint behaviour of quantitative variables. Two-tailed t-tests were used to compare means between 2 groups. Chi-square tests were used for comparison of proportions. Non-parametric tests (Kruskall-Wallis for comparison of means between three or more groups) were used for parameters that did not meet statistical normality criteria. Differences were considered statistically significant when p-values were less than 0.05.
3. Results
3.1. Sample Description
A sample of 48 patients was obtained. 52% of the sample were male. The median age was 13.75 years (range 6-46). Only one-third of the sample had been diagnosed with CF through neonatal screening. All patients had pancreatic insufficiency, and only 11% had a history of meconium ileus. 56.25% were homozygous for the delta F508 mutation, 39.6% were heterozygous, and only 2 patients (4.15%) were non-carriers of this mutation. 28% were receiving corticosteroid treatment and 31% were on CFTR modulators. 23% had liver involvement without cirrhosis, and 21% had glucose metabolism disorders.
The characteristics of the sample are summarized below (
Table 2):
3.2. Haplotype Description
The most common allele was Gc1s, present in 72% of patients, followed by Gc2, present in 52%, and the least common allele was Gc1F, present in 27%. Regarding the combination of haplotypes, the most frequent was Gc1/Gc1 (48%), followed by Gc1/Gc2 (39.5%) and Gc2/Gc2 (12.5%). Considering the six possible haplotype combinations, the most frequent was Gc1s/Gc2, present in one-third of the sample (33%), followed by Gc1s/Gc1s (27%), Gc1s/Gc1f (12.5%), Gc2/Gc2 (12.5%), Gc1f/Gc1f (8.5%), and Gc1f/Gc2 (6.5%).
3.3. Description of Vitamin D Levels and Dosages
All patients received vitamin D supplementation. Doses ranged between 500 and 7000 IU per day (mean 2646 IU, SD 1602). The median total vitamin D (calcidiol) level of the patients included in the sample was 21.2 ng/ml (interquartile range 15.25 - 26.85). Nine patients (18.7%) had levels within the sufficiency range. The remaining 81.3% had levels within the insufficiency range: 23 patients (48%) had levels below 20 ng/ml (deficiency) and 16 (33.3%) had levels between 20 and 30 ng/ml. The median free vitamin D level was 4.245 pg/ml (interquartile range 3.9 – 5.57). 21 patients in the sample (43.75%) had levels below 3.9 pg/ml. A positive correlation was observed between calcidiol and free vitamin D levels (r=0.8709; p<0.0001).
3.4. Description of Calcidiol and Free Vitamin D Levels According to Haplotypes (Table 3, Table 4 and Table 5)
Table 3 describes the vitamin D and free vitamin D levels according to the Gc1f-Gc1s or Gc2 haplotype. Patients with the Gc2 polymorphism exhibited a higher proportion of total vitamin D levels within the insufficiency range: 92% vs 69.5% in patients without this polymorphism (p=0.047). After adjusting for potential confounding factors, including season, vitamin D supplement dosage, sex, and CF-related diabetes diagnosis, the OR was 13.46 (95% CI 1.35 – 133; p=0.027).
Table 3.
Comparison of total and free vitamin d levels according to the presence of the 3 existing haplotypes.
Table 3.
Comparison of total and free vitamin d levels according to the presence of the 3 existing haplotypes.
| |
Gc1f |
Gc1s |
Gc2 |
Si
(n-=13) |
No
(n-=35) |
p |
Si
(n-=35) |
No
(n-=13) |
P |
Si
(n-=25) |
No
(n-=28) |
P |
Total vitamin D
(ng/ml) |
25.5 ±10.3 |
20.5 ±8.6 |
0.095 |
22.1 ± 8.9
|
21.3 ±10.4 |
0.776 |
19.5 ± 7,4 |
24.4 ± 10.5 |
0.063 |
Free vitamina D
(pg/ml) |
5.4 ± 2.4 |
4.4 ± 1.5 |
0.124 |
4.7 ±1.6 |
4.8 ±2.4 |
0.846 |
4.2 ± 1.5 |
5.3 ± 2.0 |
0.033 |
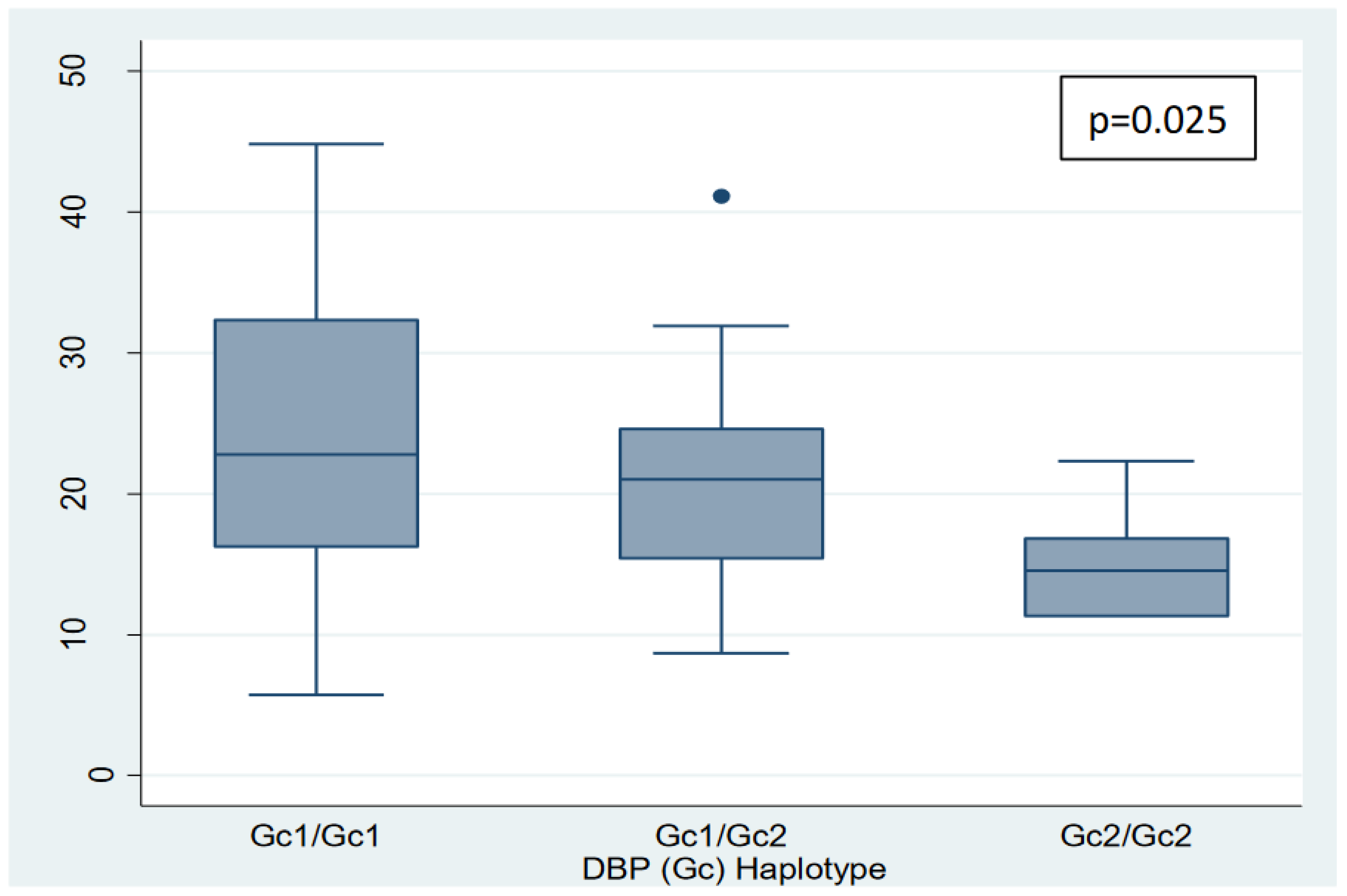
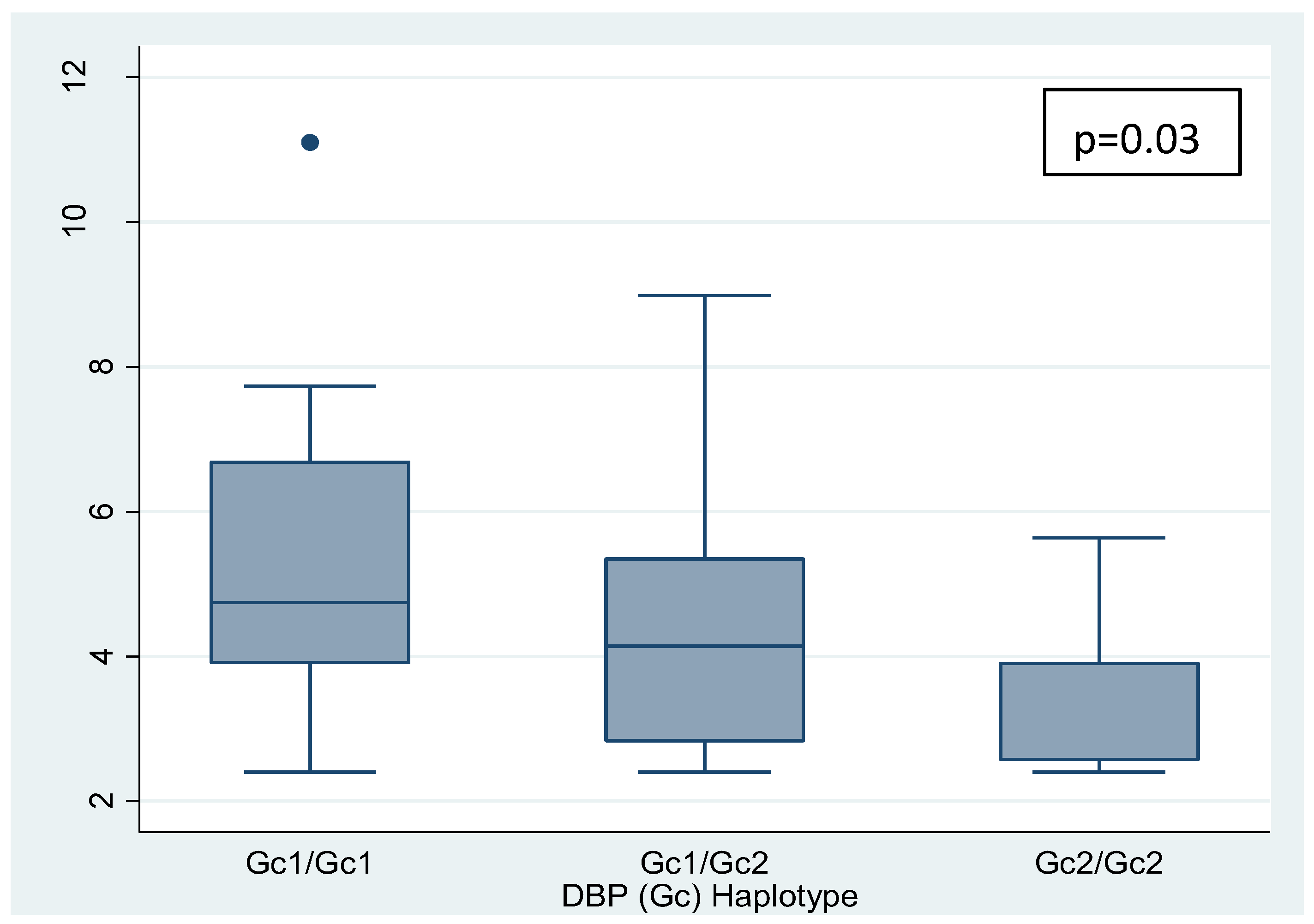
Table 4.
Vitamin D and Free Vitamin D levels according to haplotypes divided into 3 categories-.
Table 4.
Vitamin D and Free Vitamin D levels according to haplotypes divided into 3 categories-.
| Haplotypes |
Vitamin D |
P |
Free vitamin D |
P |
| Gc1/Gc1 |
22.8
(16.2 – 32.3) * |
0.0597
|
4.74
(3.9 – 6.68) |
0.5391 |
| Gc1/Gc2 |
21
(15.4 – 24.6) * |
4.14
(2.82 – 5.34) |
| Gc2/Gc2 |
14.5
(11.3 – 16.8) |
3.9
(2.57 – 3.9) |
Table 5.
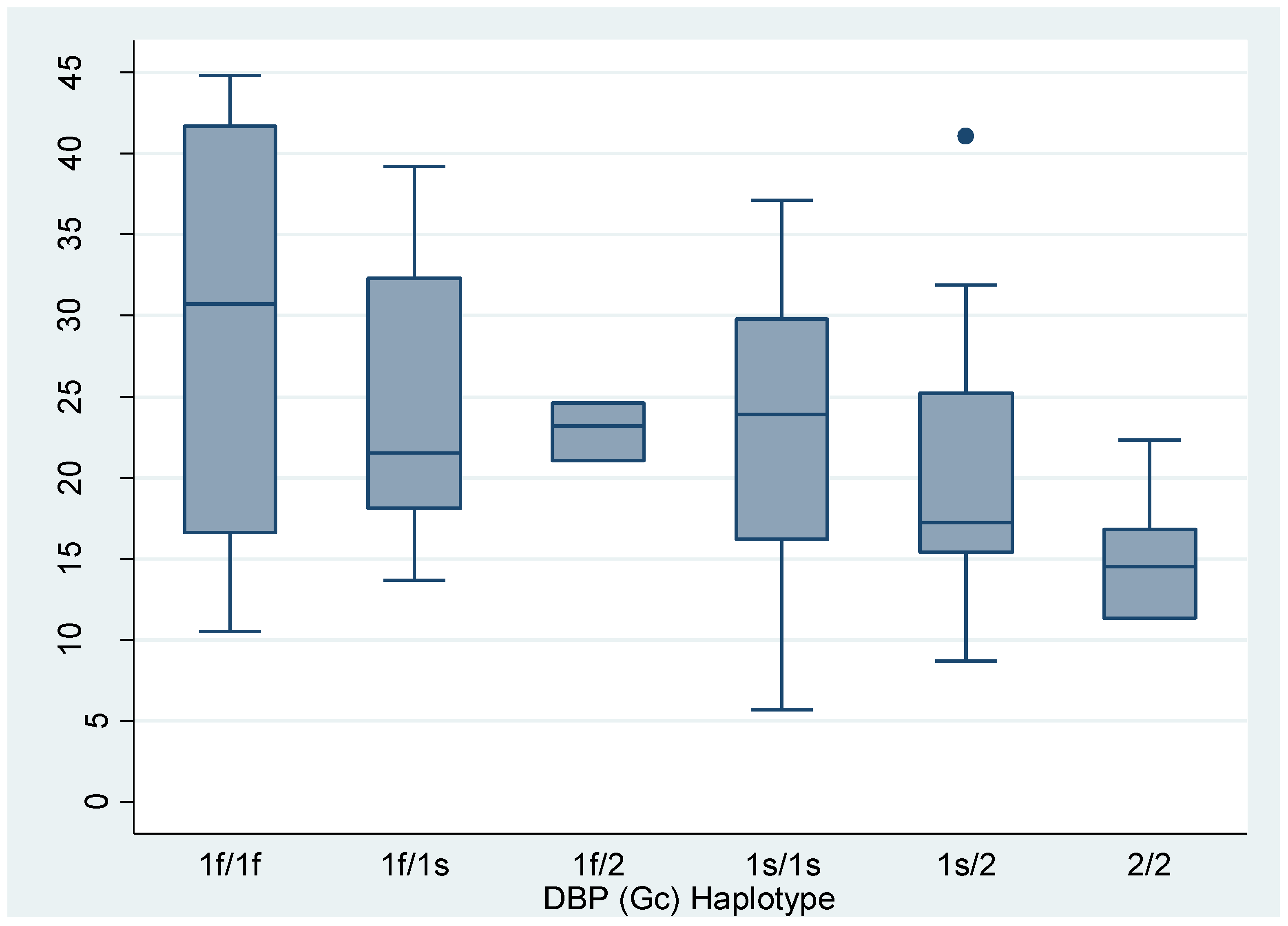
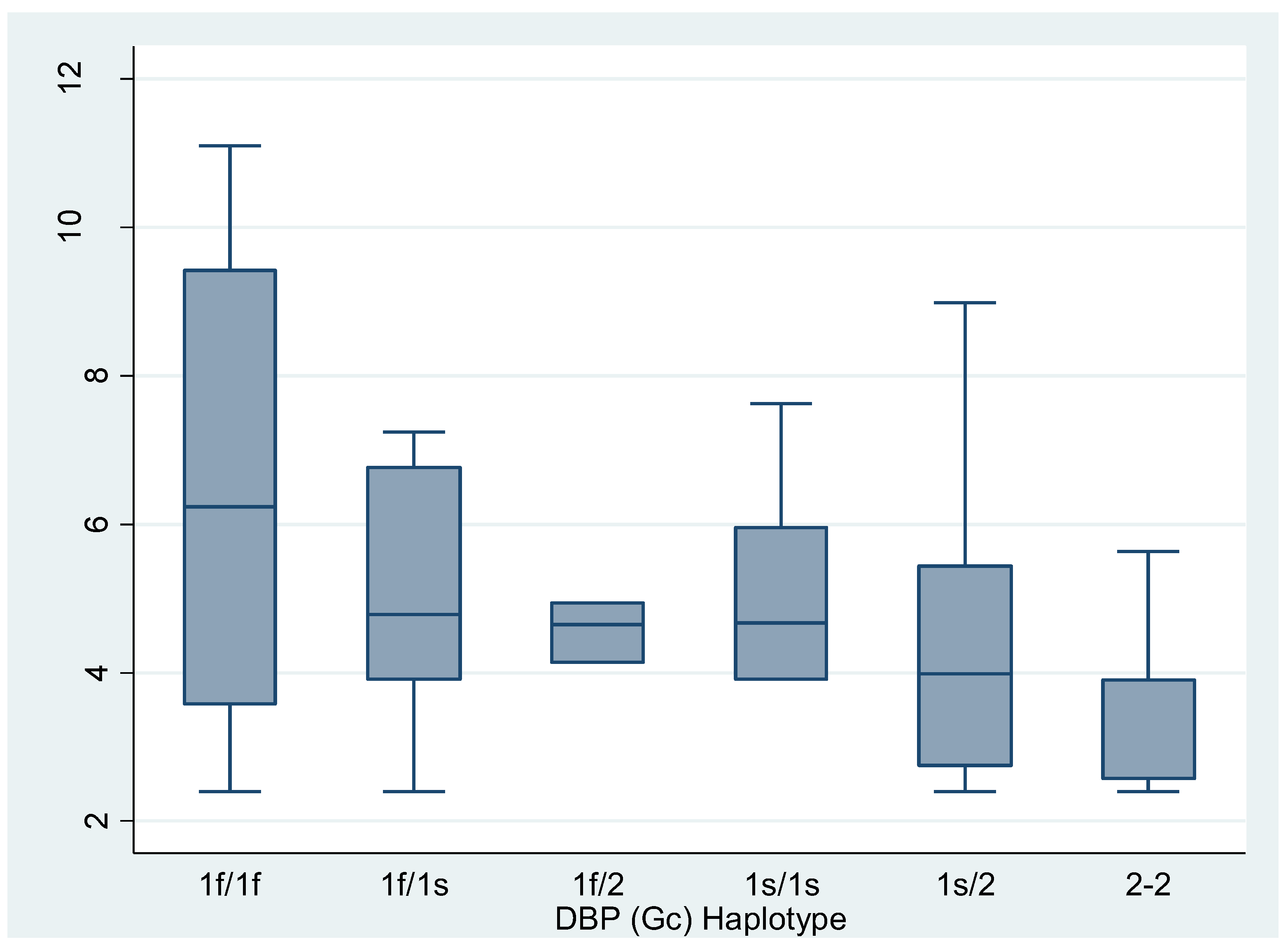
Vitamin D and Free Vitamin D Levels according to haplotypes divided into 6 categories. Data are expressed as median and interquartile range (Kruskall-Wallis).
Table 5.
Vitamin D and Free Vitamin D Levels according to haplotypes divided into 6 categories. Data are expressed as median and interquartile range (Kruskall-Wallis).
| Haplotypes |
Vitamin D |
P |
Free vitamin D libre |
P |
| Gc1f/Gc1f |
30.7
(16.65 – 41.7) |
0.2637 |
6.625
(3.57 – 9.41) |
0.3683 |
| Gc1f/Gc1s |
21.55
(18.1 – 32.3) |
4.78
(3.9 – 6.76) |
| Gc1f/Gc2 |
23.2
(21 – 24.6)* |
4.65
(4.14 – 4.94) |
| Gc1s/Gc1s |
23.9
(16.2 – 29.8) |
4.67
(3.9 – 5.95) |
| Gc1s/Gc2 |
17.25
(15.35 – 25.2) |
3.98
(2.75 – 5.435) |
| Gc2/Gc2 |
14.55
(11.3 – 16.8) |
3.9
(2.57 – 3.9) |
In contrast, in carriers of the Gc1f polymorphism, a lower proportion of patients with deficient vitamin D levels was observed, 23% vs 57% (p=0.036). After adjusting for potential confounding factors, including season, vitamin D supplement dosage, sex, and CF-related diabetes diagnosis, the OR was 0.22 (95% CI 0.05 – 0.99-, p=0.027). A lower proportion of patients with free vitamin D levels below 3.9 ng/ml was also observed in carriers of this haplotype: 23% vs 54.3% in patients without this polymorphism (p=0.054). After adjusting for the aforementioned factors, the OR was 0.25 (95% CI 0.06 – 1.11; p=0.070).
The subgroup analysis revealed statistically significant differences between carriers of the Gc1/Gc1 haplotypes and Gc2/Gc2 carriers (p=0.0357) and between Gc1/Gc2 carriers and Gc2/Gc2 carriers (p=0.0483) (
Figure 1 and
Figure 2).
Furthermore, a negative linear trend was observed between the polymorphisms grouped into 3 categories (Gc1/Gc1, Gc1/Gc2, and Gc2/Gc2, in that order) and vitamin D and free vitamin D levels (p=0.025 and p=0.033, respectively) (nonparametric tests for trend: Cuzick's test) (
Figure 3 and
Figure 4).
4. Discussion
In the present study, we have analyzed the different VDBP haplotypes, as well as total and free vitamin D levels in a sample of pediatric and young adult patients with stable cystic fibrosis. Regarding calcidiol levels, it was observed that only a minority of patients exhibited values within the sufficiency range. This trend has already been identified in previous studies conducted in patients with CF [
14,
15,
16]. This may have implications not only for bone health but also for the anti-inflammatory and antibacterial roles, among others, of vitamin D, which in turn can significantly impact the clinical evolution and stability of patients with CF, given the characteristics of their disease [
15].
For several years now, there has been an effort to advance the understanding of new markers for assessing the nutritional status of vitamin D. The free vitamin D theory suggests that the free portion, unbound to proteins, is biologically active and could more accurately reflect nutritional status. In the present study, a robust linear correlation was observed between vitamin D and free vitamin D levels, a circumstance previously described by
Lee et al. [
16] and subsequently replicated in both healthy populations and patients without hypoalbuminemia or hypoproteinemia [
17,
18]. Historically, CF has been associated with an elevated risk of malnutrition. However, since the implementation of neonatal screening, the use of high-calorie diets, management in accredited units, and the introduction of CFTR modulators, there has been a progressive reduction in malnutrition and an improvement in albumin levels. In both the study conducted by
Lee et al [
16] and our own, over 80% of patients were well-nourished. This circumstance could partly explain the good correlation between the two markers. Our findings do not indicate that determining free vitamin D in patients with CF provides more valuable information or offers greater benefit in assessing vitamin D nutritional status compared to calcidiol.
The most prevalent VDBP haplotype was Gc1s, followed by Gc2, and subsequently Gc1f. While specific racial data were not available, the origin of the samples, allows for the reasonable assumption that the majority of patients were Caucasian. Our findings are consistent with those of previous studies investigating other pathologies [
19] and in healthy populations, where the most frequent haplotype in the Caucasian race is Gc1s, while the Gc1f haplotype is more frequent in the black and Asian races [
4]. Regarding haplotype combinations, the most frequent combination observed in other studies in the Caucasian race was Gc1s/Gc2, which is consistent with the results of the present study. The least frequent combination observed in other studies was Gc1F/Gc1F [
20], whereas in the present study, it was Gc1F/Gc2.
The relationship between VDBP polymorphisms and total and free vitamin D levels was investigated. VDBP polymorphisms determine the degree of affinity of vitamin D for VDBP as well as changes in calcidiol levels. In our study, higher levels of both vitamin D and free vitamin D were observed in those with greater affinity GC1-1>GC1-2>GC2-2, while those with at least one GC2 polymorphism were at higher risk of vitamin D deficiency, regardless of other factors. To the best of our knowledge, no studies on VDBP polymorphisms in CF patients have been conducted. Consequently, we are unable to make direct comparisons with studies in healthy populations and patients with other pathologies. In this regard, our data are consistent with the findings of the review by Bouillon et al. [
21], which describes a 5-15% reduction in calcidiol levels associated with the Gc2-2 haplotype, as observed in several studies conducted on diverse populations [
22,
23,
24]. Furthermore, patients carrying the Gc1f allele exhibited a reduced risk of vitamin D deficiency. Nevertheless, other studies, such as that conducted by Schwartz et al. [
25], did not identify a clear linear correlation between the variables under investigation, although differences between haplotypes were observed.
The results obtained reinforce the significance of genetic factors in elucidating the mechanisms of vitamin D metabolism [
26]. Our data suggest that the Gc1f polymorphism would be the most "favourable" for vitamin D and free vitamin D levels, particularly when present alongside another Gc1 f or s polymorphism. It is noteworthy that this polymorphism is precisely the least frequent in the Caucasian race, while Gc2 was most related to deficiency.
Recently, through whole-genome sequencing (WGS), various Polygenic Risk Scores (PRS) have been developed, which play a pivotal role in the different responses to vitamin D supplementation observed in CF patients. In the study by
Lai, Song et al. [
27], in which CF patients diagnosed through neonatal screening were studied and followed up during the first 3 years of life, a subset of the sample exhibited insufficient vitamin D levels despite receiving adequate supplementation doses. Whole-genome sequencing (WGS) was performed, and a PRS was established based on the observed polymorphisms. In the multivariate analysis, PRS was found to be the most important factor related to nutritional status. Conversely, polymorphisms in other genes including
GC, LIPC, CYP24A1, and
PDE3B were associated with patient responsiveness to supplementation. In another study with a similar methodology (WGS with subsequent PRS determination) conducted by
Braun et al. [
28] on a sample of 80 adult CF patients receiving adequate vitamin D supplementation, a significant correlation between PRS and total vitamin D levels was also observed, thereby providing further support for this theory.
It is important to note that, at the time of the study, the triple therapy or high-efficacy therapy comprising the combination of the three modulators of the cystic fibrosis transmembrane conductance regulator (CFTR) channel: Elexacaftor-Tezacaftor-Ivacaftor, had not yet been approved. Consequently none of the patients included were receiving this medication and its associated benefits [
29].
It is important to highlight the key strengths of this study. It is the first to analyze VDBP polymorphisms in CF patients and relate them to total and free vitamin D levels. It is also a multicentre study with determinations centralized and performed using the same methodology, thereby ensuring reliability of the results. The calculation of free vitamin D using formulas, as has been traditionally done, does not appear to be an accurate approach. The ELISA technique, which was used in this work, appears to be a more precise method [
30].
As limitations, it should be noted that although vitamin D supplementation of the included patients is based on common criteria, being a multicenter study, the patients belong to very different geographical areas within the same country, with significantly varying sun exposure. This could affect total calcidiol and free vitamin D levels, which may in turn impact the results of the study. In the present study, patient adherence to prescribed vitamin D supplementation was not studied.
5. Conclusions
In CF, as in the general population, the most frequent VDBP polymorphism in the Caucasian race is Gc1s. VDBP polymorphisms influence serum vitamin D and free vitamin D levels in CF patients. There is a good correlation between free vitamin D and calcidiol levels, indicating that the determination of the latter in CF does not seem to provide additional benefits.
Author Contributions
Conceptualization, P.Q.C. and D.G.J.; methodology, P.Q.C. and D.G.J.; software, P.Q.C. and D.G.J.; validation, D.G:J..; formal analysis, D.G.J; investigation, N.A.L., R.G.R., M.G.G., M.A.B, A.I.R.D., A.E.F.L., H.G.P., C.G.A., C.G.G., S.V.S., L.P.Q., J.R.G.M., C.M.F and A.M.H; resources, N.A.L., R.G.R., M.G.G., M.A.B, A.I.R.D., A.E.F.L., H.G.P., C.G.A., C.G.G., S.V.S., L.P.Q., J.R.G.M., C.M.F and A.M.H.; data curation, D.G.J..; writing—original draft preparation, PQ.C.; writing—review and editing, P.Q.C., H.G.P., J.J.D.M. and D.G.J.; visualization, P.Q.C.; supervision, J.J.D.M. and D.G.J.; project administration, P.Q.C. and D.G.J.; funding acquisition, J.J.D.M. and D.G.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study received funding from grants obtained through the Ernesto Sanchez Villares Foundation, the Society of Pediatrics of Asturias, Cantabria, and Castilla y León (SCCALP) (2020/01 call), and the Spanish Society of Pediatric Gastroenterology Hepatology and Nutrition (SEGHNP) (2021 call).
Institutional Review Board Statement
Approval for the study was granted by the clinical research ethics committee of Hospital Universitario Central de Asturias (HUCA) (number 67/19) and the committee of the rest of the participating hospitals also gave their approval.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The dataset originated from the trial is available upon request to the corresponding author.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflicts of interest
References
- Autier P, Mullie P, Macacu A, Dragomir M, Boniol M, Coppens K, Pizot C, Boniol M. Effect of vitamin D suppemenatation on no-skeletal disorders: a systematic revies of meta-analiyses and randomized trials. Lancet Diabetes Endocrinol. 2017 ;5(12):986- 1004.
- D.D. Bikle, E. Gee, B. Halloran, M.A. Kowalski, E. Ryzen, J.G. Haddad, Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein, J. Clin. Endocrinol. Metab. 63 (4) (1986) 954–959.
- R. Bouillon, F.A. Vanassche, H. Vanbaelen, W. Heyns, P. Demoor, Influence of the vitamin D-binding protein on the serum concentration of 1,25- dihydroxyvitamin D3— significance of the free 1,25-dihydroxyvitamin D3 concentration, J. Clin. Invest. 67 (3) (1981) 589–596.
- Speeckaert M, Huang G, Delanghe JR, Taes YE.Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism.Clin Chim Acta. 2006 ;372(1-2):33-42.
- Yousefzadeh P, Shapses SA, Wang X.Vitamin D Binding Protein Impact on 25- Hydroxyvitamin D Levels under Different Physiologic and Pathologic Conditions.Int J Endocrinol. 2014;2014:981581.
- Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR, Thadhani R.Vitamin D-binding protein and vitamin D status of black Americans and white Americans.N Engl J Med. 2013 Nov 21;369(21):1991-2000.
- Cillero AI, Martinez-Morillo E, Mantecon L, Alonso MA, Gil-Peña H, Santos F, Alvarez FV.Phenotyping and relative quantification of vitamin D binding protein in a paediatric population by using liquid chomatography-tandem mass spectometry. Ann Clin Biochem. 2018 1: 4563218780135.
- D.D. Bikle, E. Gee, B. Halloran, J.G. Haddad, Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease, J. Clin. Invest. 74 (6) (1984) 1966–1971.
- J.B. Schwartz, J. Lai, B. Lizaola, L. Kane, S. Markova, P. Weyland, N.A. Terrault, N. Stotland, D. Bikle, A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations, J. Clin. Endocrinol. Metab. 99 (5) (2014) 1631–1637.
- Olmos-Ortiz, E. Avila, M. Durand-Carbajal, L. Diaz, Regulation of calcitriol biosynthesis and activity: focus on gestational vitamin D deficiency and adverse pregnancy outcomes, Nutrients 7 (1) (2015) 443–480.
- Wilschanski M, Munck A, Carrion E, Cipolli M, Collins S, Colombo C, Declercq D, Hatziagorou E, Hulst J, Kalnins D, Katsagoni CN, Mainz JG, Ribes-Koninckx C, Smith C, Smith T, Van Biervliet S, Chourdakis M. ESPEN-ESPGHAN-ECFS guideline on nutrition care for cystic fibrosis. Clin Nutr. 2024 Feb;43(2):413-445. Epub 2023 Dec 27. [CrossRef]
- Carrascosa A, Mesa J. Barcelona longitudinal growth study 1995-2017. Endocrinol Diabetes Nutr (Engl Ed). 2018 Jun-Jul;65(6):311-313. English, Spanish. [CrossRef]
- Cheng AC, Duda SN, Taylor R, Delacqua F, Lewis AA, Bosler T, Johnson KB, Harris PA. REDCap on FHIR: clinical data interoperability Services. J Biomed Inform. 2021 Sep;121:103871. Epub 2021 Jul 21. [CrossRef]
- Mangas-Sánchez C, Garriga-García M, Serrano-Nieto MJ, García-Romero R, Álvarez-Beltrán M, Crehuá-Gaudiza E, Muñoz-Codoceo R, Suárez-Cortina L, Vicente-Santamaría S, Martínez-Costa C, Díaz-Martin JJ, Bousoño-García C, González-Jiménez D. Vitamin D Status in Pediatric and Young Adult Cystic Fibrosis Patients. Are the New Recommendations Effective? Nutrients. 2021 Dec 9;13(12):4413. PMID: 34959965; PMCID: PMC8703649. [CrossRef]
- Daley T, Hughan K, Rayas M, Kelly A, Tangpricha V. Vitamin D deficiency and its treatment in cystic fibrosis. J Cyst Fibros. 2019 Oct;18 Suppl 2:S66-S73. PMID: 31679731. [CrossRef]
- Lee MJ, Kearns MD, Smith EM, Hao L, Ziegler TR, Alvarez JA, Tangpricha V.Free 25-Hydroxyvitamin D Concentrations in Cystic Fibrosis. Am J Med Sci. 2015 ;350(5):374-9.
- Mantecón L, Alonso MA, Moya V, Andrés AG, Avello N, Martínez-Morillo E ,et al.( 2018) Marker of vitamin D status in healthy children: Free or total 25-hydroxyvitamin D? PLoS ONE 13(8): e0202237. [CrossRef]
- Braegger C, Campoy C, Colomb V, Decsi T, Domellof M, Fewtrell M, et al. Vitamin D in the healthy European paediatric population. J Pediatr Gastroenterol Nutr. 2013; 56:692–701. PMID: 23708639. [CrossRef]
- Determination of Free 25(OH)D Concentrations and Their Relationships to Total 25(OH)D in Multiple Clinical Population Janice B. Schwartz,1 J. Christopher Gallagher,2 Rolf Jorde,3,4 Vivian Berg,4 Jennifer Walsh,5 Richard Eastell,5 Amy L. Evans,5 Simon Bowles,5 Kim E. Naylor,5 Kerry S. Jones,6 Inez Schoenmakers,6,7,8 Michael Holick,9 Eric Orwoll,10 Carrie Nielson,10 Martin Kaufmann,11 Glenville Jones,11 Roger Bouillon,12 Jennifer Lai,1 Davide Verotta,13 and Daniel Bikle.
- Cillero AI, Martínez-Morillo E, Mantecón L, et al. Phenotyping and relative quantification of vitamin D binding protein in a paediatric population by using liquid chromatography–tandem mass spectrometry. Annals of Clinical Biochemistry. 2019;56(1):56-63. [CrossRef]
- R. Bouillon, Genetic and Racial Differences in the Vitamin D Endocrine System, Endocrinol. Metab. Clin. North Am. 46 (2017) 1119–1135. [CrossRef]
- Lauridsen AL, Vestergaard P, Hermann AP, et al. Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the pheno- type of Gc (vitamin D-binding protein): a cross-sectional study on 595 early postmenopausal women. Calcif Tissue Int 2005;77(1):15–22.
- Pekkinen M, Saarnio E, Viljakainen HT, et al. Vitamin D binding protein genotype is associated with serum 25-hydroxyvitamin D and PTH concentrations, as well as bone health in children and adolescents in Finland. PLoS One 2014;9(1): e87292.
- Bu FX, Armas L, Lappe J, et al. Comprehensive association analysis of nine candidate genes with serum 25-hydroxy vitamin D levels among healthy Cauca- sian subjects. Hum Genet 2010;128(5):549–56.
- Schwartz JB, Gallagher JC, Jorde R, Berg V, Walsh J, Eastell R, Evans AL, Bowles S, Naylor KE, Jones KS, Schoenmakers I, Holick M, Orwoll E, Nielson C, Kaufmann M, Jones G, Bouillon R, Lai J, Verotta D, Bikle D. Determination of Free 25(OH)D Concentrations and Their Relationships to Total 25(OH)D in Multiple Clinical Populations. J Clin Endocrinol Metab. 2018 Sep 1;103(9):3278-3288. PMID: 29955795; PMCID: PMC6126881. [CrossRef]
- Usategui-Martín, R.; De Luis-Román, D.-A.; Fernández-Gómez, J.M.; Ruiz-Mambrilla, M.; Pérez-Castrillón, J.-L. Vitamin D Receptor (VDR) Gene Polymorphisms Modify the Response to Vitamin D Supplementation: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 360. [CrossRef]
- Lai HJ, Song J, Lu Q, Murali SG, Gajapathy M, Wilk BM, Brown DM, Worthey EA, Farrell PM; FIRST Study Group. Genetic factors help explain the variable responses of young children with cystic fibrosis to vitamin D supplements. Clin Nutr ESPEN. 2022 Oct;51:367-376. Epub 2022 Aug 8. PMID: 36184229. [CrossRef]
- Braun AT, Lai HJ, Laxova A, Biller JA, Hubertz EK, Zhao Z, Lu Q, Murali S, Brown DM, Worthey EA, Farrell PM. Vitamin D status and variable responses to supplements depend in part on genetic factors in adults with cystic fibrosis. J Cyst Fibros. 2024 Feb 20:S1569-1993(24)00020-1. Epub ahead of print. PMID: 38383231. [CrossRef]
- Barry PJ, Mall MA, Álvarez A, Colombo C, de Winter-de Groot KM, Fajac I, et al. Triple Therapy for Cystic Fibrosis Phe508del-Gating and Residual Function Genotypes. N Engl J Med. 2021; 385:815-25.
- J. B. Schwartz, J. Lai, B. Lizaola, L. Kane, S. Markova, P. Weyland, N. A. Terrault, N. Stotland, D. Bikle, A Comparison of Measured and Calculated Free 25(OH) Vitamin D Levels in Clinical Populations, The Journal of Clinical Endocrinology & Metabolism, Volume 99, Issue 5, 1 May 2014, Pages 1631–1637. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).