1. Introduction
Plants of the genus
Ajuga are not officially recognized in main pharmacopoeias but are widely used in traditional medicine across many countries. According to the World Flora, the genus
Ajuga in the Lamiaceae family includes approximately 300 species, of which 91 species are recognized as independent taxa, 192 have synonymous names, and 10 species remain undefined [
1]. The International Plant Names Index list 261 species of the genus
Ajuga [
2]. The Ukrainian Plant Name Reference includes 10 species of the genus
Ajuga [
3]. Nine species of this genus grow in Ukraine, the most common being
Ajuga reptans L.,
A. genevensis L., and
A. laxmannii (Murray) Benth.
The aerial parts of
Ajuga plants contain various biologically active substances, including flavonoids, hydroxycinnamic acids, essential oils, alkaloids, tannins, organic acids, and others [
4,
5]. The chemical composition of
A. reptans is relatively understudied. It contains volatile oils, iridoids (such as 8-O-acetyl-harpagide) [
6,
7], flavonoids (including isoquercitrin), tannins, and traces of alkaloids [
8,
9]. Phytoecdysteroids have also been isolated from
A. reptans, including 20-hydroxyecdysone, 29-norcyasterone, 5,20-dihydroexidizone, sengosterone, ajugalactone, and ajugosterone [
10,
11]. The herb also contains macro- and microelements [
12] as well as fatty acids [
13].
Species of the genus
Ajuga are widely used in traditional medicine. These plants are recognized for their diaphoretic, antiseptic, hemostatic, and anti-inflammatory properties [
5,
14]. Most species of this genus are used to treat viral diseases, colds, rheumatism, stomach diseases, and gallstone disease. Some species are also used in the treatment of malaria and oncology [
15,
16,
17,
18]. The herb of
A. reptans is used in traditional Austrian medicine as a tea to treat respiratory disorders [
19]. In traditional Bulgarian medicine, the plant is regarded as a remedy that enhances metabolism and is also used for gastrointestinal diseases [
20]. In Polish folk medicine,
A. reptans is used for its laxative, analgesic, and astringent properties and is known for its anti-edematous, anti-hemorrhagic, and anti-inflammatory effects [
21].
A. reptans is cultivated in the Botanical Garden of the renowned cosmetic brand Yves Rocher as a plant that enriches the "Vegetal" lifting line. In 1959, Yves Rocher's plant cosmetics experts studied
A. reptans and discovered its high collagen content, which has a pronounced lifting effect. The company’s scientists successfully extracted and patented a collagen concentrate, subsequently developing a new facial care line that restores skin structure using a biotechnological process [
22]. Additionally, the phenylpropanoid glycoside from
A. reptans has been used to create a composition for the prevention and treatment of androgenic alopecia and telogen effluvium [
23].
Moreover, species of the genus
Ajuga are used as ornamental plants due to their vibrant flower colours and prolonged blooming periods. These plants are appreciated for their varied leaf shapes, textures, and colours. Ornamental varieties of
A. reptans, such as ‘Atropurpurea’, ‘Burgundy Glow’, and ‘Multicolor’, are commonly cultivated [
24].
A literature review indicates that the genus Ajuga plants are valuable medicinal species that have been used in traditional medicine across various countries for a long time. They contain a complex array of biologically active compounds and exhibit diverse pharmacological activities. However, Ajuga species have been scarcely studied, and the available data on their distribution and medical use suggest promising opportunities for further research and the development of new medicinal products for clinical and pharmaceutical applications.
The purpose of the study was to conduct phytochemical and pharmacological research on A. reptans L. herb extracts to establish their potential for use in medical and pharmaceutical practice.
2. Results
The soft extracts (AR) obtained from the A. reptans herb are viscous masses of dark brown colour with a faint specific odour. The yield of extracts was as follows: AR1 (extractant water) 26.2% ± 0.18%, AR2 (50% ethanol) 28.4% ± 0.21%, and AR3 (70% ethanol) 28.9% ± 0.24%.
Using commonly accepted qualitative chemical reactions and chromatographic methods [
25,
26,
27], the presence of organic acids, amino acids, phenolic compounds (including hydroxycinnamic acids, coumarins, flavonoids, and tannins), iridoids, terpenoids, saponins, phytosterols, and vitamin K in the extracts of
A. reptans herb was confirmed. The quantitative content of the main groups of biologically active substances (BAS) in the studied extracts was determined (
Table 1).
Chromatographic analysis of organic acids was carried out using the paper chromatography (PC) method with a mobile phase consisting of
n-butanol, formic acid, and water (75:15:10), compared against authentic samples of organic acids. The chromatograms were sprayed with a 0.05% alcohol solution of bromothymol blue and a 0.1% alcohol solution of sodium 2,6-dichlorophenolindophenolate. The appearance of pink spots on a blue background and yellow spots on a blue background, respectively, confirmed the presence of organic acids (
Figure 1). As a result of the chromatographic analysis, 8 organic acids: succinic, lactic, tartaric, oxalic, citric, ascorbic, malic, and benzoic acids were identified in
A. reptans herb extracts, and their total content was determined using the titrimetric method (
Table 1).
The high-performance thin-layer chromatography (HPTLC) method was used to identify phenolic compounds in the extracts. Chromatographic plates of MERCK Silica gel F254 and a solvent system consisting of formic acid anhydrous – water – ethyl acetate (10:10:80) were used for chromatography. A solution of 10 g/L diphenylboric acid aminoethyl ester in methanol and 50 g/L macrogol 400 P in methanol was used to develop the chromatograms. The results were evaluated by comparing the R
f values of the zones on the chromatogram of the test solution with those of the reference solution. The following reference substances were used for preparing the comparison solution: rutin, chlorogenic acid, hyperoside, apigenin-7-glucoside, ferulic acid, luteolin, apigenin, luteolin-7-glucoside, caffeic acid, and quercetin (
Figure 2) [
28,
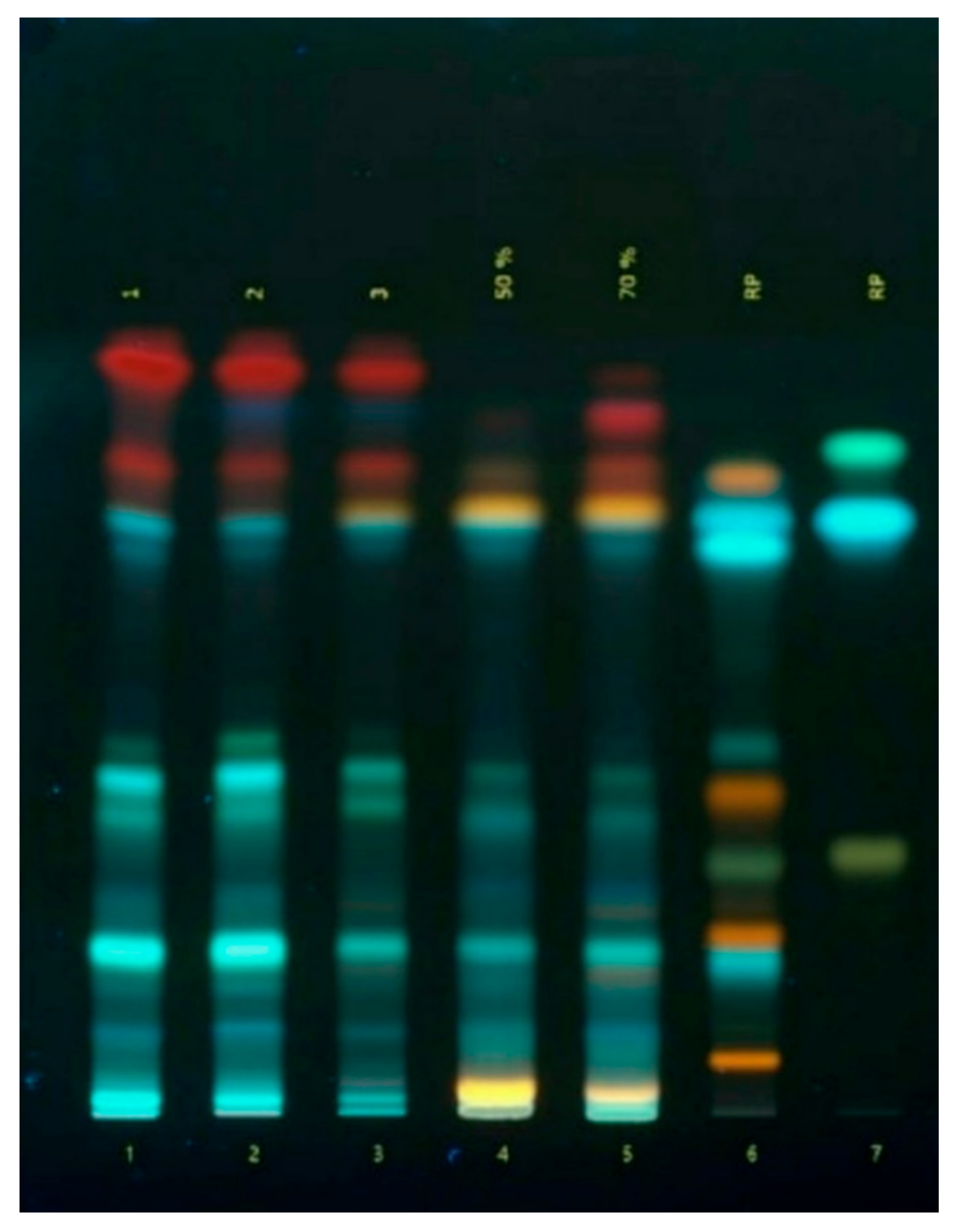
29]. As a result of the analysis using high-performance thin-layer chromatography (HPTLC), 13 to 15 phenolic compounds belonging to hydroxycinnamic acids, flavonoids, and coumarins were identified.
The content of vitamin K
1, recalculated as Vikasol, and other phenolic compounds in the extracts of
A. reptans herb, determined by the spectrophotometric method, are presented in
Table 1.
Additionally, using high-performance liquid chromatography (HPLC), 20 phenolic compounds and quinic acid were identified and quantified in the extracts of
A. reptans herb (
Table 1).
The research results presented in
Table 1 indicate that among the identified hydroxycinnamic compounds, the dominant ones are
p-coumaric and caffeic acids. Among the flavonoids, the predominant compounds are rutin and quercetin. There is also a tendency to accumulate condensed tannins, specifically their metabolites, including pyrocatechin, gallocatechin, epicatechin, and epicatechin gallate.
Using gas chromatography-mass spectrometry (GC-MS) [
30,
31], the qualitative composition and quantitative content of steroids, along with the composition of the volatile fraction in the extracts of
A. reptans herb, were studied (
Table 2). As the aqueous extract AR1 of
A. reptans herb contained traces of terpenoids, these results made no sense to be shown. In the volatile fraction of
A. reptans herb extracts, 10 compounds were identified with hexahydrofarnesyl acetone and squalene being the dominant compounds.
The qualitative composition and quantitative content of amino acids in the extracts of
A. reptans herb were studied (
Table 3).
When a single intragastric administration of the permissible doses of the studied extracts was administered, no animals died. Observations were conducted over 14 days, during which the extracts did not negatively affect the functioning of vital organs and systems, including the liver, kidneys, and blood. Pathological-anatomical examinations of the laboratory mice revealed that the organs of the animals receiving the studied extracts did not differ in shape, size, colour, or consistency from those of the control group. The serous tissues in the abdominal cavity remained unchanged. The obtained results indicate the absence of hepatotoxicity during the intake of A. reptans extracts. Therefore, the findings suggest that intragastric administration of A. reptans herb extracts at a dose of 5000 mg/kg did not result in animal mortality, indicating an absence of toxic effects at this dose and characterizing the extracts as practically non-toxic (toxicity class V, LD50 > 5000 mg/kg) according to the toxicity classification of substances.
The hepatoprotective activity of
A. reptans herb extracts was assessed using a model of acute carbon tetrachloride-induced hepatitis [
32,
33]. The results of the biochemical studies of blood serum and liver homogenate are presented in
Table 4.
The study of the anti-inflammatory activity of
A. reptans extracts was conducted using a model of acute aseptic inflammation – carrageenan-induced rat paw oedema. The data on the effect of
A. reptans herb extracts and comparison drugs on the development of rat paw oedema, as well as their anti-exudative activity, are presented in
Table 5.
The study results on the duration of bleeding (M ± m, seconds) in guinea pigs from a cut wound with the local application of the extracts and the "Rotokan" preparation are presented in
Table 6.
The results of the study on the dynamics of the wound healing process with the application of
A. reptans extracts are presented in
Table 7.
Bactericidal and bacteriostatic activity of
A. reptans herb extracts against various microbial cultures were established. The bactericidal effect was indicated by forming distinct zones of complete inhibition of test culture growth around the wells containing the extracts. In contrast, the bacteriostatic action of the extracts was characterized by zones of partial inhibition of microorganism growth, which lacked clear edges, and atypical colonies formed within these zones (
Figure 3 and
Table 8).
3. Discussion
Among the phenolic compounds, 8 flavonoids were identified: rutin, quercetin, quercetin-3-glucoside, luteolin, apigenin, neohesperidin, naringin, and naringenin. Additionally, 4 tannin metabolites were identified: pyrocatechin, epicatechin, epicatechin gallate, and gallocatechin. 5 hydroxycinnamic acids were also identified: caffeic,
p-coumaric, sinapic, trans-cinnamic, and trans-ferulic acids. 3 phenol carboxylic acids were found: gallic, benzoic, and syringic acids. Quinic acid was also identified in the extracts. The predominant hydroxycinnamic acids in
A. reptans herb extracts were
p-coumaric, caffeic, and sinapic acids; among the flavonoids, rutin, quercetin, and neohesperidin were predominant; and among the tannin metabolites, epicatechin gallate and gallocatechin were the most significant. It was reported that in
A. reptans, raw materials from Turkey, caffeic and chlorogenic acids predominated among hydroxycinnamic acids and luteolin derivative - among flavonoids [
34]. In the other research isoquercitrin and ferulic acids were dominant phenolic compounds [
7]. Thus, Ukrainian raw materials have a similar qualitative composition but differ in dominant substances.
In the analysis of steroids, a total of 54 substances were detected, of which 9 steroids were identified: stigmasterol, pregna-5,17-dien-3-ol, ergosterol, 3β-steariloxy-ers-12-ene, stigmaster-5,22-dien-3-ol acetate, β-sitosterol, olean-12-ene, 3β-methoxy-5-cholestene, and traces of cholesterol. Stigmasterol was found to be the most concentrated. The steroid composition is quite well studied for this genus, so the results correspond to the previous research [
5].
As shown in
Table 2, 10 compounds were identified and quantified in the
A. reptans herb extracts, including 1 ketone, 2 alcohols, and 1 terpenoid compound. The alcohols and their derivatives included 3-heptanol and a-terpineol; the ketone identified was 2-heptanone. Among sesquiterpenes and their derivatives were α-bergamotene and hexahydrofarnesyl acetone; among aliphatic sesquiterpenes was farnesene; and among terpenoids was a-linalool. The extracts also contained acyclic triterpenes such as squalene and esters like methyl linoleate and linoleic acid. Other research has shown that teupolioside, martinoside, verbascoside, and isoverbascoside are the major chemical constituents among phenylproponoids, and teupolioside is the chemical marker of the
A. reptans extract, and major monoterpenoids include three iridoid glycosides: harpagide, 8-O-acetylharpagide and reptoside [
35]. Thus, our results extend the data on the chemical composition of the volatile fraction.
The qualitative composition and quantitative content of amino acids in the extracts of
A. reptans herb were studied for the first time. Sixteen amino acids were identified, including 7 essential amino acids: threonine, valine, methionine, isoleucine, leucine, phenylalanine, and lysine, as well as 1 conditionally essential amino acid, histidine. The identified amino acids included 10 monoaminomonocarboxylic acids: alanine, valine, glycine, isoleucine, leucine, methionine, serine, threonine, phenylalanine, and cysteine; 2 monoaminodicarboxylic acids: aspartic and glutamic acids; 2 diamino monocarboxylic acids: arginine and lysine; and 2 heterocyclic amino acids: histidine and tryptophan. According to experimental research data (
Table 3), glutamic acid, aspartic acid, arginine, leucine, serine, valine, and glycine predominated in
A. reptans herb extracts.
The extracts of
A. reptans herb are classified as practically non-toxic (toxicity class V) when administered intragastrically (LD
50 > 5000 mg/kg) according to the classification by K.K. Sidorov [
32].
The simultaneous administration of the extracts and the hepatotoxic poison led to a reduction (
Table 4) in the content of TBA-reactive substances by 1.58, 1.56, and 1.55 times, respectively, and a decrease in ALT activity by 1.52, 1.32, and 1.25 times in the blood serum of the experimental animals and by 1.88, 2.11, and 1.87 times, respectively, in the liver homogenate compared to the control group of animals.
The destruction of cell membrane components (
Table 4) resulted in the development of a pronounced cytolytic syndrome, as indicated by a 5.7-fold increase in ALT activity in the blood serum. The development of acute toxic hepatitis was characterized by an intensification of peroxide catabolic transformations, as evidenced by a 1.7 and 2.3-fold increase in the content of TBA-reactive substances in the blood serum and liver homogenate of the control group animals, respectively, compared to the indicators of intact animals.
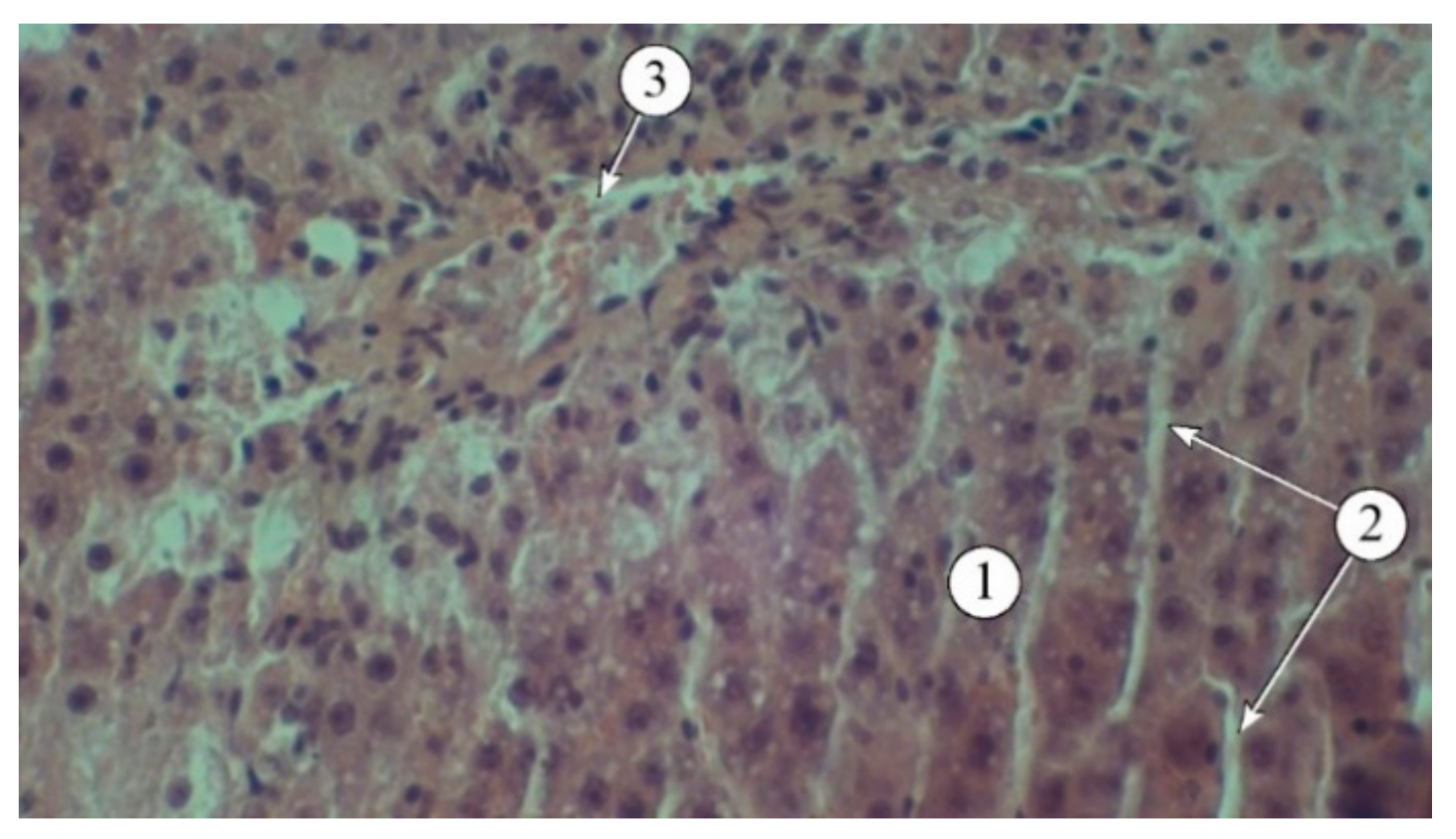
The results of the studies (
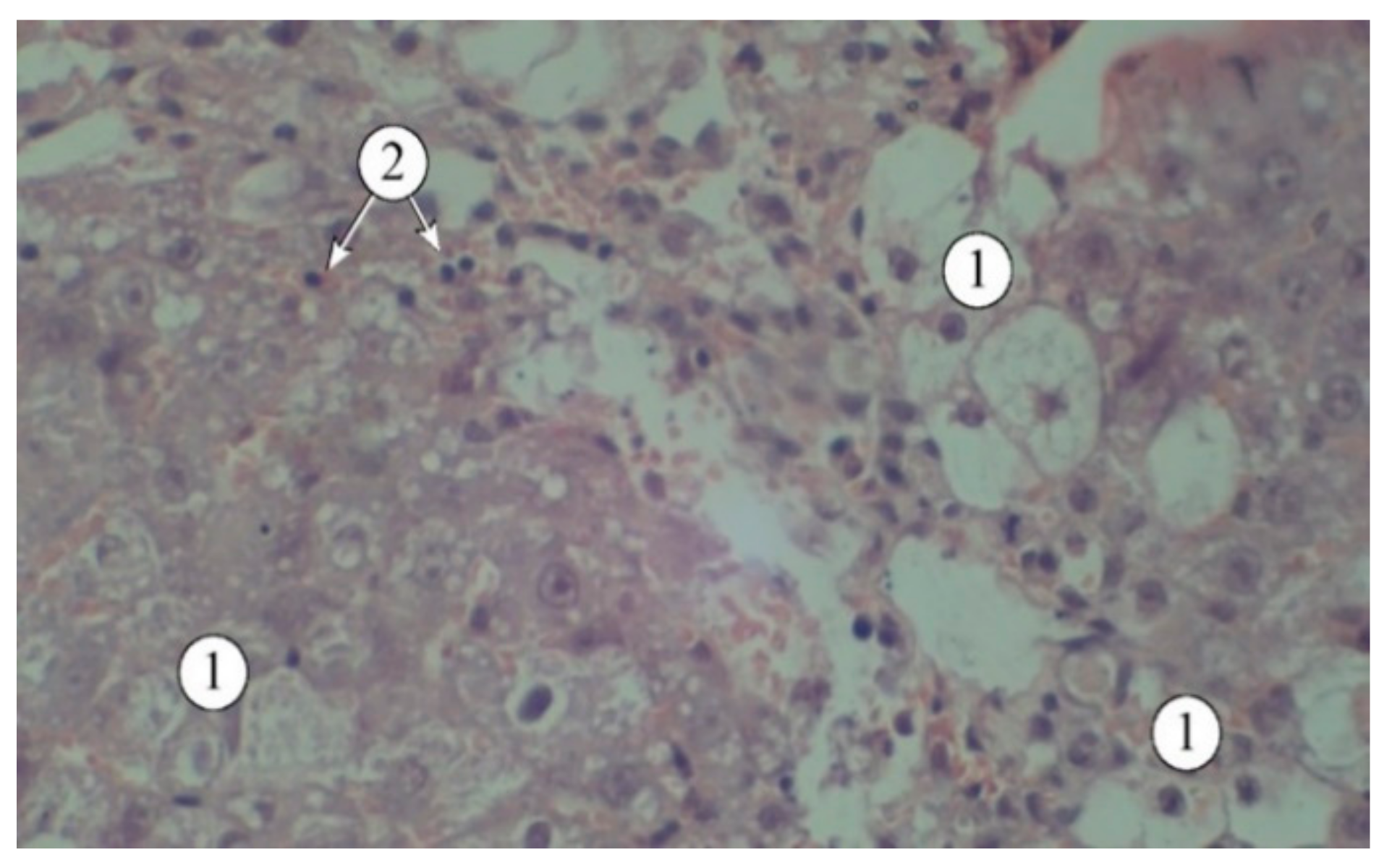
Figure 4) indicate that a single administration of carbon tetrachloride was accompanied by the development of acute toxic liver damage, with a loss of structural integrity, disorganized hepatic plates, and the portal triad not being visualized. The interlobular spaces were expanded and deformed, with pronounced lymphocytic infiltration. The borders of hepatocytes were blurred, and in many fields of view, the nuclei were not visible. There was a pronounced macro- and microvesicular component in the cytoplasm of hepatocytes in the peripheral zone.
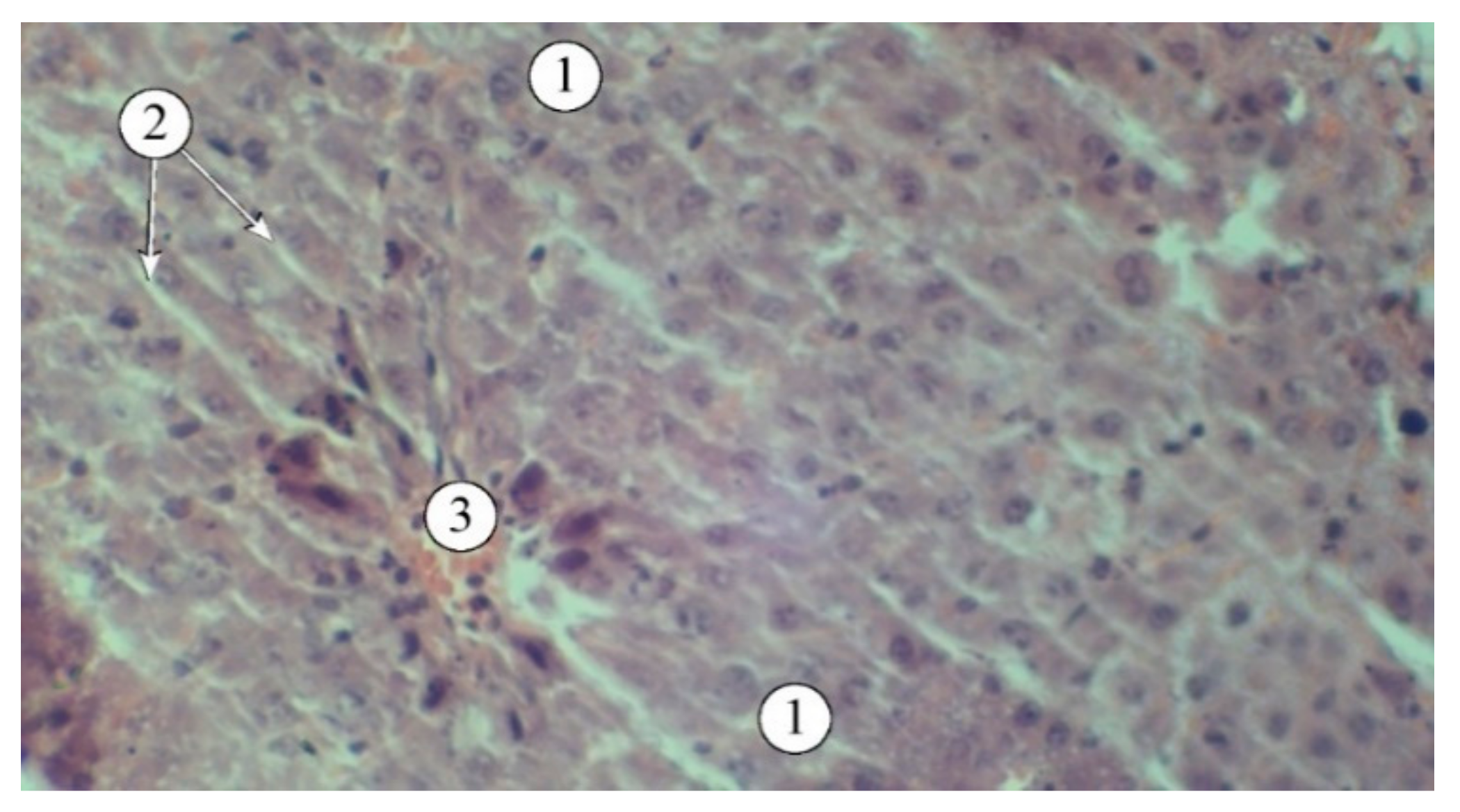
The use of extracts AR1, AR2, AR3, and the comparison drug "Silibor" in the context of experimental hepatitis was associated with a noticeable reduction in pathological manifestations (
Figure 5). The best results were observed in animals that received AR2; the histostructure of the liver was characterized by the organization of hepatic plates and visualization of the components of the portal triad. Hepatocytes in the peripheral zone had a polygonal shape, with their cytoplasm filled with fine-dispersed content and uniformly filled with small vesicles. The intermediate and central zones of the liver lobule demonstrated structured hepatocytes and sinusoids.
In the liver of animals that received the "Silibor" preparation, light-optical examination revealed hepatic plates that were radially oriented towards the central vein and composed of two rows of hepatocytes. The sinusoids appeared as optically translucent slits, without infiltration. The cytoplasm of the hepatocytes was finely granular, with rounded nuclei and visible nucleoli. Individual dystrophically altered hepatocytes were observed in certain fields of view, displaying moderately hypochromatic cytoplasm and nuclei (
Figure 6).
The results obtained from the conducted studies indicate that A. reptans herb extracts exhibit a pronounced hepatoprotective activity in cases of acute toxic liver damage by suppressing peroxide destructive processes and reducing the development of cytolysis syndrome, and they are practically not inferior to the hepatoprotective action of the comparison drug "Silibor." The hepatoprotective activity of A. reptans herb extracts has been studied for the first time.
The study results (
Table 5) show that the anti-exudative activity of AR1, AR3 extracts, and quercetin gradually manifested and reached its maximum within 4 hours from the start of the experiment, indicating a moderate effect of these substances on the suppression of all inflammation mediators, especially prostaglandins. The anti-inflammatory effect is minor and has no pharmacological significance, as a level of pharmacological activity of at least 20% is considered significant for the experimental study of anti-inflammatory agents [
36]. The activity of the AR2 extract increased within 2 hours during the release period of early inflammation mediators (kinin and histamine), confirming the presence of identified polyphenolic compounds with antihistaminic and polyoxygenase activity. The results indicate that, after 2 hours, the AR2 extract exceeded the anti-exudative effect of the reference drug quercetin by more than 1.2 times and maintained this effect for 2 hours. The effect of diclofenac sodium manifested immediately, increased significantly within 2 hours, and reached its maximum 4 hours after the start of the experiment, indicating significant inhibition of all groups of inflammation mediators. In the control group of untreated animals, oedema increased within 4 hours. The study of the anti-inflammatory activity of the tested extracts established that the AR2 extract at a dose of 50 mg/kg exceeded the anti-exudative effect of the reference drug quercetin (anti-exudative effect – 20.52%) after 2 hours. Previously in vivo and in vitro studies have shown that the representatives of
Ajuga L. genus inhibit inflammatory responses by suppressing inflammatory factors (cyclooxygenase-1 (COX-1), cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), nitric oxide (NO), interleukin 8 (IL-8), interleukin 6 (IL-6), and tumour necrosis factor-α (TNF-α) [
5,
37]. Such data is available for some
Ajuga species but lacks them for
A. reptans. The best anti-oxidative and anti-inflammatory activity was observed for
the A. reptans 100 mg dw/mL extract when compared with diclofenac [
7], but in our research the effective therapeutic dose was 50 mg/kg.
The obtained results (
Table 6) indicate that the local application of
A. reptans extracts (using a gauze pad soaked in the tested extracts directly on the wound surface immediately after the wound was made), significantly decreased bleeding time compared to the control group of animals. The most significant reduction in bleeding was caused by applying the AR1 extract, reducing bleeding time by 40.59%, while the hemostatic activity of the "Rotokan" preparation reduced it by 52.61% compared to the control group. Our conducted studies indicate that
A. reptans extracts have a local hemostatic effect. The study's results on the dynamics of the wound healing process with the application of
A. reptans extracts (
Table 7) show that from the 9th day of the local application of the AR2 extract and the comparison drug "Rotokan," complete wound healing occurred. There was no scientific conformation of the hemostatic and wound healing activity of
A. reptans extracts, although, in folk medicine, it is commonly recommended in these cases [
5,
14,
38].
The conducted studies established that A. reptans herb extracts can inhibit the growth of microorganisms to varying degrees, depending on the ethanol concentration used as an extraction agent.
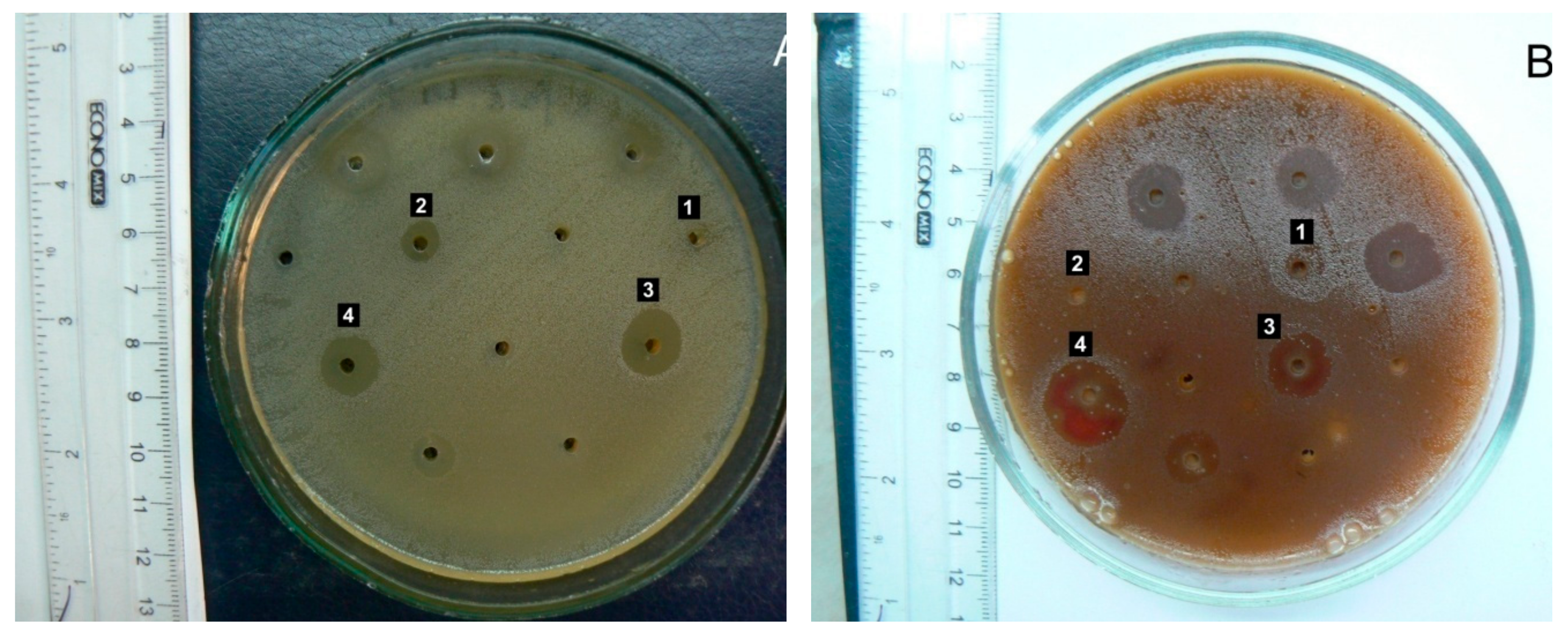
A. reptans extracts demonstrated higher activity against gram-positive microorganisms, with streptococci being particularly sensitive. The AR3 herb extract (extraction agent: 70% ethanol) caused significant inhibition of the growth of pathogenic β-hemolytic streptococci of groups A and G – causative agents of tonsillitis and angina (
Figure 3), as well as β-hemolytic streptococcus group B – a causative agent of inflammatory processes in the female external genital organs. Conditionally pathogenic α-hemolytic streptococci of the oral microflora,
St. oralis,
St. sanguinis, and
St. gordonii (which can cause purulent-inflammatory processes of the oral mucosa and periodontal tissues in dental practice), also showed high sensitivity to the AR3 extract. The growth of
S. sanguinis and
St. oralis was also notably inhibited by the AR2 extract. Regarding the primary causative agent of bacterial respiratory infections (bronchitis, otitis, sinusitis), pneumococcus
St. pneumonia, the AR2 and AR3 extracts exhibited a bacteriostatic effect. Therefore, it can be suggested that
A. reptans herb extracts have potential use in treating streptococcal infections in dental, pediatric, and ENT practices.
Enterococcus faecalis, a common causative agent of urological and wound infections, demonstrated quite high sensitivity to AR3. This result deserves special attention due to enterococcus's high natural resistance to most antibiotics groups.
The most common causative agents of purulent-inflammatory processes, staphylococci, were significantly less sensitive to A. reptans herb extracts than streptococci. S. aureus and S. haemolyticus exhibited weak sensitivity to all tested preparations.
Gram-negative bacteria generally showed significantly lower sensitivity to the biologically active compounds of A. reptans extracts. Normal E. coli was found to be completely insensitive to them. However, there was a tendency for AR3 to inhibit the growth of E. fergusonii, which has reduced enzymatic activity, as well as Citrobacter and representatives of the putrefactive intestinal microflora Providencia and Morganella. Therefore, the inclusion of A. reptans herb as an auxiliary component can be considered when developing herbal mixtures and complex herbal remedies for treating mild forms of intestinal dysbiosis.
The antibacterial effects of the essential oil isolated from the aerial part of
A. pseudoiva have been tested before against Gram-positive and Gram-negative bacteria in vitro, including
B. subtilis,
B. cereus,
S. aureus,
E. coli,
E. faecalis,
K. pneumonia,
P. aeruginosa,
S. typhimurium,
L. monocytogenes, and
E. faecium [
5,
39]. The ethanol and methanol extracts from the aerial parts of
A. laxmannii have been shown to exhibit antibacterial activity against
P. aeruginosa,
L. monocytogenes,
E. coli, and
S. typhimurium [
7]. Thus, the results of the antibacterial effects of
A. reptans herb extracts provide new knowledges.
The
A. reptans herb extracts showed weak fungistatic activity against most types of
Candida yeast-like fungi. Previously, it was also shown that
A. reptans extracts are active against Canada spp. and
E. coli [
34,
35].
During the conducted studies, an important pattern was established: the antimicrobial activity of A. reptans herb extracts increases proportionally with the increase in ethanol concentration in the extracting solution. This may indicate the predominantly hydrophobic properties of biologically active compounds that provide the antimicrobial activity of the extracts.
4. Materials and Methods
Plant Raw Materials
The herb of
A. reptans L. was collected during the flowering period in 2021 in the vicinity of the village of Huta, Bohorodchany District, Ivano-Frankivsk Region (48° 39′ 4″ N, 24° 12′ 55″ E 48.651111°, 24.215278°). The herb was collected in the phase of mass flowering and consists of pieces of stems, leaves and flowers. The harvesting of raw materials was carried out in accordance with the collection guidelines, taking into account the specifics of harvesting and ensuring careful handling of the flora. Drying of the medicinal raw material was performed in the shade outdoors, avoiding direct sunlight, and it was stored in paper bags. A total of 2 kg of the dry raw material was obtained. According to the botanical catalogue, the plant's identity was confirmed with the consulting assistance of Professor A.R. Grytsyk from Ivano-Frankivsk National Medical University (IFNMU) [
40]. Voucher specimens No. 551–553 were deposited at the Department of Pharmaceutical Management, Drug Technology, and Pharmacognosy at Ivano-Frankivsk National Medical University.
Phytochemical Research
Flavonoids were studied using high-performance liquid chromatography (HPLC) on an Agilent Technologies 1200 liquid chromatograph. Acetonitrile (A) and 0.1% formic acid solution in water (B) were used as the mobile phase. Elution was carried out in gradient mode: 0 min – A (30%): B (70%); 20 min – A (70%): B (30%); 22 min – A (100%): B (0%); 30 min – A (100%): B (0%). Separation was performed on a Zorbax SB-C18 chromatographic column (3.5 µm, 150 × 4.6 mm) (Agilent Technologies, USA) with a flow rate of 0.25 ml/min. The sample injection volume was 100 µl, and the column temperature was maintained at 25°C. Detection was conducted using a diode-array detector with signal registration at 280 and 365 nm, and spectra recorded in the range of 210–270 nm [
41,
42].
The study of hydroxycinnamic acids was carried out using HPLC on an Agilent Technologies 1200 liquid chromatograph. Methanol (A) and 0.1% formic acid solution in water (B) were used as the mobile phase. Elution was conducted in a gradient mode: 0 min – A (25%): B (75%); 25 min – A (75%): B (25%); 27 min – A (100%): B (0%); 35 min – A (100%): B (0%). Separation was performed on a Zorbax SB-Aq chromatographic column (4.6 mm × 150 mm, 3.5 µm) (Agilent Technologies, USA) with a flow rate of 0.5 ml/min, a column temperature of 30°C, and an injection volume of 4 µl. Detection was conducted using a diode-array detector with signal registration at 250 and 275 nm and absorption spectra fixed in the range of 210–270 nm [
43,
44].
The study of tannin metabolites was carried out on an Agilent Technologies 1200 liquid chromatograph. Methanol (A) and 0.1% formic acid solution in water (B) were used as the mobile phase. Elution was conducted in a gradient mode: 0 min – A (20%): B (80%); 25 min – A (75%): B (25%); 27 min – A (100%): B (0%); 35 min – A (100%): B (0%). Separation was performed on a Zorbax SB-C18 chromatographic column (3.5 µm, 150 × 4.6 mm) (Agilent Technologies, USA) with a flow rate of 0.25 ml/min, a thermostat temperature of 35°C, and an injection volume of 4 µl. Detection was conducted using a diode-array detector with signal registration at 250 and 275 nm and absorption spectra fixed in the range of 210–270 nm [
43,
45].
Identification and quantitative analysis of substances were conducted by comparison with standard reference materials (Sigma-Aldrich Products, Burlington, MA, USA)
Volatile compounds research by gas chromatography-mass spectrometry (GC-MS). The qualitative composition and content (mg/kg) of volatile compounds were determined using gas chromatography-mass spectrometry (GC-MS) on an Agilent Technologies 6890 chromatograph with a mass spectrometric detector 5973. A capillary HP-5ms chromatographic column with an internal diameter of 0.25 mm and a length of 30 m was used. The carrier gas, helium, had a flow rate of 1.0 ml/min, and the sample introduction heater was maintained at a temperature of 250°C. The column’s thermostat temperature was programmed to increase from 60°C to 320°C at a rate of 7°C/min. Components were identified based on the general patterns of molecular fragmentation of organic compounds under electron impact, and by comparing the obtained spectra with the data from the NIST05 and WILEY 2007 mass spectra libraries, which contain over 470,000 spectra. The AMDIS and NIST identification software programs were used for data analysis [
31,
46].
Study of steroid compounds (phytosterols) using gas chromatography/mass spectrometry (GC/MS) was conducted on an Agilent Technologies 6890 chromatograph with a mass spectrometric detector 5973, equipped with a capillary column HP-5ms (0.25 mm in diameter, 30 m in length). Helium was used as the carrier gas, with a flow rate of 1.0 ml/min, and the sample introduction heater was set to 350°C. The thermostat temperature was programmed to increase from 150°C to 300°C at a rate of 7°C/min. Components were identified using the mass spectra library combined with the AMDIS and NIST identification software programs [
47]. The quantitative content of steroids (X mg/kg) was determined using the internal standard method, applying the formula:
where P1 is the area of the peak of the studied substance; 50 is the mass of the internal standard introduced into the sample (μg); P2 is the area of the standard peak; and m is the weight of the extract (g).
The quantitative determination of vitamin K content in the studied samples of
A. reptans herb extracts was carried out using the spectrophotometric method [
48]. A sample of the extract (500 mg) was placed in a 50 ml conical flask and extracted three times with 70% ethanol, using 25 ml each time, by heating in a boiling water bath for 15 minutes. The extracts were filtered while hot into a 100 ml flask and then washed with 10 ml of 70% ethanol. 4 ml of a 10% lead acetate solution was added to the hot extract solutions and heated in a water bath for 3 minutes to coagulate the precipitate. The solution was then cooled to room temperature, filtered into a 100 ml volumetric flask, and brought to volume with 70% ethanol. A 5 ml portion of the resulting solution was transferred to a volumetric flask and diluted with the same solvent to 50 ml. The optical density of the extract was measured at a wavelength of 230 nm in a 10 mm cell using a spectrophotometer, with 70% ethanol serving as the reference solution. The optical density of a 10% Vikasol solution was measured in parallel. The vitamin K content (X) recalculated to Vikasol was determined using the formula:
where A is the optical density of the test solution; m is the weight of the medicinal plant raw material (g); 420 is the specific absorption coefficient of Vikasol in 70% ethanol; and W is the mass loss on drying (%).
The content of free organic acids, recalculated as malic acid, in the extracts was determined by titration according to the pharmacopeial method [
25,
49].
The quantitative determination of ascorbic acid was carried out using the titrimetric method, following the pharmacopeial procedure [
50,
51].
The identification and determination of the content of amino acids in
A. reptans herb extracts was carried out based on the SE “Ivano-Frankivsk Scientific and Production Center for Standardization, Metrology and Certification” (accreditation certificate No. 2H098 dated June 20, 2014) [
20,
21]. Analysis was conducted using an amino acid analyzer AAA T-339 M. (Mikrotekhnika, Prague, CRSR) compared with the concentration of standard amino acid hydrolysates according to DSTU ISO 13903:2005. Quantification was performed using standard solutions of amino acids (TU 6-09-3147-83) [
52].
The quantitative determination of tannins, expressed as pyrogallol, was performed using the spectrophotometric method in accordance with the pharmacopeial method DFU 2.0 [
25,
26].
The quantitative determination of the total flavonoid content, expressed as rutin, was performed using the spectrophotometric method according to the pharmacopeial method DFU 2.0 [
25,
53].
The quantitative determination of hydroxycinnamic acids, expressed as chlorogenic acid, in the extracts was conducted using the spectrophotometric method at a wavelength of 325 nm [
54,
55].
Pharmacological Research
The pharmacological and toxicological properties of
A. reptans herb extracts were studied according to the "General Ethical Principles of Experiments on Animals" (Ukraine, 2001), which comply with the provisions of the "European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes" (Strasbourg, 1986) [
56,
57,
58]. Laboratory animals were kept according to the current "Sanitary Rules for the Arrangement, Equipment, and Maintenance of Experimental Biological Clinics (Vivaria)" at a temperature of 18–20°C and relative humidity of 50–55%. Feeding was conducted with a complete diet according to a standard scheme. Before the experiment began, the animals underwent a 7-day adaptation period during which their clinical condition was carefully observed daily. Throughout the study, observations were made regarding the clinical condition and behaviour of the experimental animals. After the end of the experiment, the animals were euthanized via decapitation under ether anaesthesia. The research was approved by the Bioethics Commission of the Ivano-Frankivsk National Medical University (protocol №121/21 from 13.05.2021).
Acute Toxicity Research. The acute toxicity of A. reptans herb extracts was studied with the consulting assistance of Professor A.O. Klymenko from the Department of Biological and Medical Chemistry named after Academician H.O. Babenko, Professor O.G. Popadynets from the Department of Human Anatomy, and Professor Ya.S. Hudyvok from the Department of Pharmacology at IFNMU.
Biochemical parameters were studied at the Bioelementology Center of the Ivano-Frankivsk National Medical University (accreditation certificate No. 037/14 dated April 29, 2014). Laboratory mice weighing 19–23 g were used in the experiment. To determine the parameters of acute toxicity of A. reptans herb extracts, the animals were divided into six groups (6 animals in each group):
Group I – intact animals (purified water);
Group II – AR1 extract (extraction agent – purified water);
Group III – AR2 extract (extraction agent – 50% ethanol);
Group IV – AR3 extract (extraction agent – 70% ethanol);
Group V – 50% ethanol.
The solutions of the substances were administered intragastrically using a probe, ensuring that the solution volume did not exceed 0.5 ml. The dose was calculated in mg of active substance per 1 kg of body weight. Observations were conducted over 14 days. After the experiment, the animals were euthanized via decapitation under ether anaesthesia. Internal organs were examined, and blood samples were collected for biochemical analysis [
32].
Hepatoprotective Activity Research. The study of the hepatoprotective activity of Ajuga reptans L. herb extracts – AR1 (extraction agent: purified water), AR2 (extraction agent: 50% ethanol), and AR3 (extraction agent: 70% ethanol) – was conducted using a model of acute carbon tetrachloride (CCl₄) hepatitis [
33,
59]. The domestically produced hepatoprotective agent "Silibor" was used as a comparison drug.
The experiments were conducted on male white rats weighing 0.18–0.22 kg, divided into six groups (6 animals in each group). Animals in Groups I–III were subcutaneously administered Ajuga reptans L. herb extracts (extraction agents: purified water, 50% ethanol, and 70% ethanol, respectively). The animals in Group IV received the comparison drug "Silibor" at a dose of 25 mg/kg. Group V was the control group that received no treatment, and Group VI consisted of intact animals. Liver pathology was induced by a single subcutaneous injection of a 50% oily carbon tetrachloride solution at a dose of 0.8 ml per 0.1 kg of body weight to the animals in the first to fifth groups over two days with a 24-hour interval. The studied extracts and the comparison drug were administered one hour before and two hours after the introduction of the hepatotropic poison.
The choice of carbon tetrachloride as the intoxicant was based on its high selective hepatotoxicity. Poisoning of experimental animals with this xenobiotic leads to morphological and biochemical changes that closely resemble acute liver damage of various etiologies in humans [
60,
61].
The animals were euthanized by decapitation on the third day after the first injection of carbon tetrachloride, after which blood samples were collected, and the liver was isolated. The effectiveness of the investigated extracts was assessed based on biochemical and functional parameters of the liver and blood serum, which were determined 24 hours after the last injection of carbon tetrachloride.
One of the criteria for evaluating the hepatoprotective effect of the studied extracts was the survival rate of the animals. The mortality rate in the control group was 16.7%, while all animals in the other groups survived until the end of the experiment. The intensity of oxidative destructive changes in the animals' bodies was assessed by measuring the content of thiobarbituric acid (TBA) reactive substances in blood serum and liver homogenate. The activity of alanine aminotransferase (ALT), which is a hepatospecific marker of cytolysis, was also determined in blood serum. The ALT activity was measured using the unified dinitrophenylhydrazine method of Reitman and Frankel with a standard set of reagents from the "SIMKO Ltd" company [
33].
The level of lipid peroxidation products, specifically TBA-reactive substances, was assessed by their reaction with 2-thiobarbituric acid using a spectrophotometric method according to the technique developed by E.N. Korobeynikova, using biochemical kits from a domestic manufacturer ("Felicit-Diagnostics," Ukraine) [
62].
The study of the anti-inflammatory activity of A. reptans extracts was conducted with the consultative assistance of Dr. H.M. Ersteniuk, Doctor of Biological Sciences, Professor of the Department of Biological and Medical Chemistry named after Academician H.O. Babenko.
The experiment was conducted on non-linear white rats of both sexes weighing 180–220 g (n=48), which were standardized according to physiological and biochemical parameters. The animals were kept under vivarium conditions in accordance with sanitary and hygienic norms.
To assess the impact of
A. reptans extracts on the progression of the exudative phase of the inflammatory process, a model of acute aseptic inflammation — carrageenan-induced rat paw oedema — was employed. This model is characterized by the cyclooxygenase pathway of inflammation as described in the methodology by Trynus F.P. et al. [
63]. In this model, 0.1 ml of a 1% carrageenan solution was injected subplantarly into the paw pad of a rat under aseptic conditions to induce inflammation. Carrageenan-induced oedema inhibits the synthesis of serotonin, kinins, histamine, and prostaglandins. Serotonin and histamine are inhibited within 0.5–1.5 hours; kinins between 1.5 and 2.5 hours; and prostaglandins from 2.5 to 5.5 hours.
Using a mechanical oncometer developed by A.S. Zakharevskyi, changes in the rat's paw volume were measured at the start of the study and dynamically 1, 2, and 4 hours after introducing the phlogogenic agent. The changes in animals receiving the test preparations were compared with those in the control group. Results were calculated and expressed as percentages [
33].
For comparison, under similar conditions, the anti-exudative effect of quercetin, a plant-derived preparation with proven anti-exudative activity, and diclofenac sodium, a standard nonsteroidal anti-inflammatory drug, was studied [
64].
The experimental animals were divided into seven groups, with six animals in each group. The animals in the first to third groups were administered extracts AR1 (extraction agent: purified water), AR2 (extraction agent: 50% ethanol), and AR3 (extraction agent: 70% ethanol), respectively. The fourth group received 50% ethanol; the fifth group received quercetin orally at a dose of 50 mg/kg of body weight, and the sixth group received diclofenac sodium intraperitoneally at a dose of 8 mg/kg of body weight. The seventh group served as the control group. Forty minutes before the injection of the phlogogenic agent, the animals were administered the test extracts and comparison drugs according to the group assignment. Control animals received an equivalent volume of physiological saline.
The anti-exudative activity of the substances tested was determined as a percentage of the degree of reduction in oedema in the animals that received the test preparations compared to the control group.
Research on Hemostatic and Wound-Healing Effects. The study of the wound-healing and hemostatic properties of A. reptans herb extracts was conducted with the consultative assistance of Professor O.G. Popadynets from the Department of Human Anatomy and Professor Ya.S. Hudyvok from the Department of Pharmacology at IFNMU.
The wound-healing activity of
A. reptans herb extracts was investigated on clinically healthy guinea pigs over a 16-day period. The tested extracts were applied twice a day, starting from the first day until complete healing. Dynamic observation was conducted on the 3rd, 5th, 7th, 9th, 14th, and 16th days to evaluate the inflammatory response, conduct planimetry, and determine the rate of wound healing [
65]. The preparation "Rotokan," a combined herbal medicine containing tinctures of chamomile flowers, calendula flowers, and yarrow herb, was used as a comparison.
A linear incision wound (wound model) was made by cutting through all skin layers and the muscle layer on the lateral surface of the abdomen using a scalpel. The operational area was pre-shaved, treated with a 5% iodine solution, and anaesthetized using barbamil anaesthesia (0.8 ml per 100 mg of a 1% aqueous barbamil solution). The size of the model wound was 1.5 × 0.3 cm. The duration of bleeding was determined by measuring the time (using Duke's method) from the moment of tissue incision until spontaneous hemostasis.
Research on Antimicrobial Activity and Microbiological Purity. The study of the potential antimicrobial activity of A. reptans herb extracts against the growth of pure cultures of gram-positive bacteria and yeast was conducted at the Department of Microbiology, Virology, and Immunology at IFNMU, with the consultative assistance of the head of the department, Doctor of Medical Sciences, Professor R.V. Kutsyk.
The objective of the study was to assess the antimicrobial activity of the aqueous extract AR1 and aqueous -ethanol extracts AR2 and AR3. The antimicrobial activity of the extracts was evaluated using clinical isolates of antibiotic-sensitive and antibiotic-resistant microorganisms. Bacterial cultures were identified based on the biochemical microtests "STAPHYtest 16," "ENTEROtest 24," and "NEFERMENTtest 24" (Lachema, Czech Republic), as well as by considering a set of morphological and cultural characteristics according to the recommendations of the 9th edition of "Bergey's Manual of Determinative Bacteriology" [
66]. Cultures of yeast-like fungi were identified based on 40 biochemical tests using the VITEK 2 system with VITEK 2 YST ID cards (bioMérieux, France).
The antimicrobial effect of the plant extracts was examined using the agar diffusion micromethod. This method is highly sensitive and allows reliable differentiation between active and inactive extracts [
67,
68]. Petri dishes placed on a horizontal, flat surface were filled with 30 ml of agar. After the medium solidified, wells with a diameter of 4.0 mm were created using a special punch with equal edges. The agar was uniformly seeded with a suspension of the test culture (concentration 1×10⁷ CFU/ml). In the experimental wells, 20 μl of the plant extracts were placed, while 20 μl of extractants (50% and 70% ethanol) were added to the control wells. Pure solvents in concentrations corresponding to their content in the preparations were used as controls.
After 24 hours of cultivation, the diameters of the inhibition zones for bacterial test cultures were determined. Fungistatic activity was registered after 2 days, and fungicidal activity was registered after 4 days of cultivation. Digital images of the cultures on the plates were obtained and processed using the computer program Image Tool 2.0 (UTHSCSA ImageTool 2.0, The University of Texas Health Science Center in San Antonio, 1995–1996).
Staphylococci, α- and β-hemolytic streptococci, enterococci, bacilli, enterobacteria, pseudomonads, and yeast-like fungi were used as controls [
66]. Clinical isolates of antibiotic-sensitive and antibiotic-resistant microorganisms were used to study the antimicrobial activity of the extracts.
The microbiological purity of the extracts was determined according to the requirements of the State Pharmacopoeia of Ukraine 5.1.4 N 2.6.13 N. Category 3 B [
25].
Figure 1.
The scheme of the results of the chromatographic analysis of organic acids in Ajuga reptans L. herb: 1 – tartaric acid; 2 – oxalic acid; 3 – succinic acid; 4 – lactic acid; 5 – citric acid; 6 – ascorbic acid; 7 – benzoic acid; 8 – malic acid; 9 – solution of AR2 extract.
Figure 1.
The scheme of the results of the chromatographic analysis of organic acids in Ajuga reptans L. herb: 1 – tartaric acid; 2 – oxalic acid; 3 – succinic acid; 4 – lactic acid; 5 – citric acid; 6 – ascorbic acid; 7 – benzoic acid; 8 – malic acid; 9 – solution of AR2 extract.
Figure 2.
Chromatogram of phenolic compounds in Ajuga reptans L. herb extracts: 1 - 3 – extracts of A. reptans; 4 – A. reptans extract (extraction agent: 50% ethanol); 5 – A. reptans extract (extraction agent: 70% ethanol); 6 – comparison solution (rutin, caffeic acid, quercetin, apigenin-7-glucoside, isoquercitrin, hyperoside, chlorogenic acid, luteolin, apigenin); 7 – comparison solution (apigenin, ferulic acid).
Figure 2.
Chromatogram of phenolic compounds in Ajuga reptans L. herb extracts: 1 - 3 – extracts of A. reptans; 4 – A. reptans extract (extraction agent: 50% ethanol); 5 – A. reptans extract (extraction agent: 70% ethanol); 6 – comparison solution (rutin, caffeic acid, quercetin, apigenin-7-glucoside, isoquercitrin, hyperoside, chlorogenic acid, luteolin, apigenin); 7 – comparison solution (apigenin, ferulic acid).
Figure 3.
Antimicrobial activity of Ajuga reptans L. herb extracts prepared using AR1 (extraction agent: purified water), AR2 (extraction agent: 50% ethanol), and AR3 (extraction agent: 70% ethanol) against cultures of Enterococcus faecalis (A) and β-hemolytic Streptococcus group G (B).
Figure 3.
Antimicrobial activity of Ajuga reptans L. herb extracts prepared using AR1 (extraction agent: purified water), AR2 (extraction agent: 50% ethanol), and AR3 (extraction agent: 70% ethanol) against cultures of Enterococcus faecalis (A) and β-hemolytic Streptococcus group G (B).
Figure 4.
Structural features of liver lobules under the influence of the toxicant. 1 – destructured hepatocytes, 2 – lymphocytes. Staining: hematoxylin and eosin. Magnification: ×200.
Figure 4.
Structural features of liver lobules under the influence of the toxicant. 1 – destructured hepatocytes, 2 – lymphocytes. Staining: hematoxylin and eosin. Magnification: ×200.
Figure 5.
Structural features of liver lobules in animals administered AR2: 1 – hepatic plates, 2 – sinusoids, 3 – blood vessels. Staining: hematoxylin and eosin. Magnification: ×200.
Figure 5.
Structural features of liver lobules in animals administered AR2: 1 – hepatic plates, 2 – sinusoids, 3 – blood vessels. Staining: hematoxylin and eosin. Magnification: ×200.
Figure 6.
Structural features of liver lobules in animals that received the "Silibor" preparation. 1 – hepatic plates, 2 – sinusoid, 3 – central vein. Staining: hematoxylin and eosin. Magnification: ×200.
Figure 6.
Structural features of liver lobules in animals that received the "Silibor" preparation. 1 – hepatic plates, 2 – sinusoid, 3 – central vein. Staining: hematoxylin and eosin. Magnification: ×200.
Table 1.
The content of biologically active substances in the extracts of Ajuga reptans L. herb.
Table 1.
The content of biologically active substances in the extracts of Ajuga reptans L. herb.
| № |
Name of Identified Compound |
Quantitative Content, mg/100 g |
| AR1 |
AR2 |
AR3 |
| Carboxylic Acid |
| 1 |
Quinic acid |
146.31± 4.12 |
158.87 ± 9.43 |
149.14 ± 11.76 |
| Phenolic Acids |
| 2 |
Gallic acid |
48.03 ± 2.33 |
68.44 ± 4.54 |
83,23 ± 6.52 |
| 3 |
Benzoic acid |
102.22 ± 7.09 |
168.59 ± 11.29 |
184.82 ± 9.07 |
| 4 |
Syringic acid |
27.28 ± 1.62 |
33.22 ± 1.58 |
41.96 ± 2.01 |
| Hydroxycinnamic Acids |
| 5 |
Caffeic acid |
326.48 ± 12.17 |
343.60 ± 31.99 |
311.62 ± 23.07 |
| 6 |
p- Coumaric acid |
420.62 ± 23.22 |
432.63 ± 28.32 |
376.96 ± 31.44 |
| 7 |
trans-Ferulic acid |
79.96 ± 5.13 |
87.22 ± 5.65 |
64.74 ± 2.12 |
| 8 |
Sinapic acid |
261.56 ± 21.12 |
284.43 ± 21.45 |
398.23 ± 27.87 |
| 9 |
trans- Cinnamic acid |
113.87 ± 5.08 |
126.27 ± 7.67 |
107.34 ± 12.61 |
| Flavonoids |
| 10 |
Rutin |
2384.71 ± 207.87 |
3465.43 ± 167.34 |
3256.74 ± 267.21 |
| 11 |
Quercetin-3-glucoside |
166.52 ± 6.14 |
173.25 ± 23.45 |
208.45 ± 26.32 |
| 12 |
Naringin |
146.97± 5.18 |
156.43 ± 9.23 |
187.97 ± 20.02 |
| 13 |
Neohesperidin |
330.67 ± 12.16 |
374.28 ± 27.65 |
401.27 ± 31.89 |
| 14 |
Quercetin |
1325.53 ± 115.54 |
1453.22 ± 117.39 |
1543.64 ± 107.86 |
| 15 |
Luteolin |
312.32 ± 21.62 |
341.57 ± 25.02 |
376.54 ± 28.51 |
| 16 |
Apigenin |
117.85 ± 7.02 |
134.67 ± 8.48 |
153.75 ± 18.84 |
| 17 |
Naringenin |
100.51 ± 4.89 |
112.92 ± 10.12 |
132.83 ± 10.08 |
| Tannin Metabolites |
| 18 |
Pyrocatechin |
53.44 ± 2.45 |
65.21 ± 8.55 |
77.15 ± 4.45 |
| 19 |
Epicatechin |
395.93 ± 30.34 |
356.78 ± 12.68 |
401.48 ± 36.33 |
| 20 |
Epicatechin gallate |
1295.58 ± 108.32 |
1129.42 ± 159.56 |
1209.21 ± 143.67 |
| 21 |
Gallocatechin |
1079.72 ± 76.28 |
985.46 ± 76.09 |
1067.53 ± 109.78 |
| Group content, % |
| Vitamin K1 (spectrophotometry) |
0.73 ± 0.02 |
0.81 ± 0.04 |
0.95 ± 0.07 |
| Organic acids (titrimetry) |
2.29 ± 0.05 |
2.03 ± 0.04 |
1.89 ± 0.04 |
| Ascorbic acid (titrimetry) |
0.13 ± 0.01 |
0.09 ± 0.02 |
0.08 ± 0.01 |
| Total polyphenols (spectrophotometry) |
14.25 ± 0.74 |
15.11 ± 0.57 |
15.41 ± 0.82 |
| Total tannins (spectrophotometry) |
3.72 ± 0.30 |
3.08 ± 0.31 |
2.81 ± 0.21 |
| Total flavonoids (spectrophotometry) |
5.37 ± 0.76 |
6.93 ± 0.48 |
7.07 ± 0.71 |
| Total hydroxycinnamic acids (spectrophotometry) |
5.58 ± 0.07 |
5.91 ± 0.09 |
5.80 ± 0.03 |
Table 2.
The content of volatile substances and phytosterols in the extracts of Ajuga reptans L. herb.
Table 2.
The content of volatile substances and phytosterols in the extracts of Ajuga reptans L. herb.
| Substance Name |
Class |
Content, mg/kg |
| AR2 |
AR3 |
| 2-Heptanone |
Ketones |
11.56 |
13.43 |
| 3- Heptanol |
Alcohols |
4.49 |
5.78 |
| α- Linalool |
Terpenoids |
13.30 |
17.83 |
| α- Terpineol |
Monoterpenic alcohols |
9.36 |
10.12 |
| Farnesene |
Aliphatic sesquiterpenes |
7.90 |
9.33 |
| Hexahydrofarnesyl acetone |
Sesquiterpene derivatives |
83.55 |
96.32 |
| α- Bergamotene |
Sesquiterpenes |
6.83 |
9.71 |
| Linoleic acid |
Esters |
77.19 |
68.23 |
| Methyl linoleate |
Esters |
138.97 |
141.52 |
| Squalene |
Acyclic triterpene |
15.38 |
21.83 |
| Ergosterol |
Sterol |
281.69 |
298.43 |
| Isocholesterol |
Sterol |
74.10 |
76.32 |
| Pregna-5,17-dien-3-ol |
Sterol |
843.91 |
903.08 |
| Stigmasterol |
Sterol |
1546.20 |
1789.34 |
| β- Sitosterol |
Sterol |
144.07 |
153.59 |
| Olean-12-en |
Sterol |
133.07 |
139.12 |
| 3β-stearyloxy-ers-12-en |
Sterol |
272.86 |
307.32 |
| Stigmasta-5,22-dien-3-ol acetate |
Sterol |
196.06 |
203.45 |
Table 3.
Amino acid content in the extracts of Ajuga reptans L. herb.
Table 3.
Amino acid content in the extracts of Ajuga reptans L. herb.
| Amino Acid |
Chemical Formula |
Content of Free Amino Acids, мг/100 г |
| AR1 |
AR2 |
AR3 |
| Monoaminomonocarboxylic |
| Alanine |
C3H7O2N |
14.03± 0.07 |
11.83± 0.09 |
10,98± 0,08 |
| Valine |
C5H11O2N |
12.67± 0.07 |
14.67± 0.07 |
16,82± 0,04 |
| Glycine |
C2H5O2N |
15.45± 0.15 |
13.81±0.06 |
12,89± 0,11 |
| Isoleucine |
C6H13O2N |
11.93± 0.06 |
11.64±0.03 |
10,81± 0,10 |
| Leucine |
C6H13O2N |
19.73± 0.17 |
18.21±0.02 |
17,88± 0,07 |
| Methionine |
C5H11O2NS |
9.07± 0.06 |
7.23±0.03 |
7,82± 0,12 |
| Serine |
C3H7O3N |
19.03± 0.14 |
17.13±0.11 |
17,86± 0,16 |
| Threonine |
C4H9O3N |
11.04± 0.11 |
10.33±0.08 |
10,72± 0,09 |
| Phenylalanine |
C9H11O2N |
10.06± 0.09 |
11.81±0.06 |
13,58± 0,13 |
| Cysteine |
C6H12N2O4S2 |
3.82± 0.07 |
4.11±0.07 |
4,67± 0,07 |
| Total |
126.83 |
120.77 |
124.03 |
| Monoaminodicarboxylic |
| Aspartic acid |
C4H7O4N |
29.81± 0.21 |
28.93±0.13 |
26,09± 0,19 |
| Glutamic acid |
C5H9O4N |
49.87± 0.18 |
54.91±0.22 |
55,83± 0,15 |
| Total |
79.87 |
83.84 |
81.92 |
| Diaminomonocarboxylic |
| Arginine |
C6H14O2N |
33.76± 0.17 |
35.16±0.09 |
41,82± 0,13 |
| Lysine |
C6H14O2N |
9.53± 0.07 |
8.71±0.07 |
9,07± 0,08 |
| Total |
43.29 |
43.87 |
50.89 |
| Heterocyclic |
| Histidine |
C6H9O2N |
8.83± 0.08 |
9.64±0.06 |
9,87± 0,04 |
| Tryptophan |
C11H12N2O2 |
0.71± 0.02 |
0.93±0.02 |
0,89± 0,04 |
| Total |
9.54 |
10.57 |
10.76 |
| Overall total content |
259.53 |
259.05 |
267.60 |
Table 4.
The effect of Ajuga reptans L. extracts on the biochemical parameters of blood serum and liver condition in acute carbon tetrachloride-induced hepatitis.
Table 4.
The effect of Ajuga reptans L. extracts on the biochemical parameters of blood serum and liver condition in acute carbon tetrachloride-induced hepatitis.
| Biochemical and hematological indicators |
Research Objects |
| Silibor |
AR1 |
AR2 |
AR3 |
50% Oil Solution CCl₄ |
Intact Animals |
| Number of animals |
6 |
6 |
6 |
6 |
6 |
6 |
| Dose, mg/0.1 kg |
2.5 |
2.5 |
2.5 |
2.5 |
0.8 mL |
- |
| ALT, µmol/hour.ml |
3.70 ± 0.08 |
4.01 ± 0.09 |
4.61 ± 0.12** |
4.89 ± 0.09 |
6.12 ± 0.03** |
1.07 ± 0.04** |
| AST, µmol/hour.ml |
3.25 ± 0.09 |
2.30 ± 0.05 |
2.93 ± 0.06 |
3.83 ± 0.02 |
4.03 ± 0.05 |
1.13 ± 0.04 |
| TBA-reactive substances, nmol/ml |
3.45 ± 0.02 |
3.42 ± 0.02** |
3.52 ±0.07* |
3.50 ± 0.02 |
5.47 ± 0.07** |
3.27 ± 0.01 |
| Arginase, µmol/0.1 ml |
0.85 ± 0.02 |
0.61 ± 0.01 |
0.70 ± 0.02 |
0.68 ± 0.01 |
1.21 ± 0.11 |
0.58 ± 0.02 |
| Ceruloplasmin, u.o. |
25.5 ± 0.05 |
25.41 ± 0.05 |
24.89 ± 0.05 |
25.01 ± 0.47 |
19.01 ± 0.08 |
27.00 ± 0.04 |
| Liver Homogenate |
| TBA-reactive substances, µmol/g |
34.15 ± 0.03* |
30.35 ± 0.04** |
34.06 ± 0.12** |
34.23 ± 0.06 |
64.31 ± 0.02** |
27.23 ± 0.05 |
Table 5.
The effect of Ajuga reptans L. extracts on the increase in paw volume and suppression of the inflammatory response in rat limbs.
Table 5.
The effect of Ajuga reptans L. extracts on the increase in paw volume and suppression of the inflammatory response in rat limbs.
| Group№ |
Tested substance |
Dose, mg/kg |
, n=8 /
Inflammatory Response Inhibition, % |
| 1 hour |
2 hours |
4 hours |
| I |
AR1 |
100 |
43.80±0.07* |
58.43±0.04 |
82.34±0.08** |
| 2.82 |
5.8 |
9.84 |
| II |
AR2 |
100 |
42.88±0.04* |
56.45±0.02** |
45.52±0.03 |
| 6.8 |
15.7 |
20.52 |
| III |
AR3 |
100 |
49.03±0.03 |
62.46±0.03 |
65.35±0.04 |
| 4.7 |
6.3 |
11.8 |
| IV |
Ethanol 50% |
|
48.82±0.02* |
62. 46±0.03 |
82.3±0.03** |
| 2.0 |
4.4 |
6.2 |
| V |
Quercetin |
50 |
46.57±0.19 |
63.2±0.15 |
61.09±0.15 |
| 5.8 |
13.4 |
17.2 |
| VI |
Diclofenac sodium |
8 |
39.17±0.93** |
33.60±0.05* |
35.52±0.03* |
| 17.97 |
31.54 |
45.14 |
| VII |
Control |
- |
44.88±0.04 |
71.67±0.02 |
132.29±0.04 |
Table 6.
The effect of Ajuga reptans L. herb extracts and the reference preparation "Rotokan" on the duration of bleeding.
Table 6.
The effect of Ajuga reptans L. herb extracts and the reference preparation "Rotokan" on the duration of bleeding.
| № |
Tested Substance |
, c |
Reduction in Bleeding Time, % |
| 1 |
AR1 |
90.45 ±1.88 |
40.59 |
| 2 |
AR2 |
110.35 ±1.12 |
27.52 |
| 3 |
AR3 |
125.10 ±1.44 |
17.83 |
| 4 |
«Rotokan» |
72.15 ±1.11 |
52.61 |
| 5 |
Intact animals |
152.25 ±1.65 |
- |
Table 7.
Dynamics of the wound healing process applyingon of Ajuga reptans L. herb extracts.
Table 7.
Dynamics of the wound healing process applyingon of Ajuga reptans L. herb extracts.
| Experimental Animal Groups |
Determination of the wound healing area as a percentage of the total wound surface area, % |
| 3 day |
5 day |
7 day |
9 day |
14 day |
16 day |
| AR1 |
4.30 ±0.12 |
10.45 ±0.10 |
46.90 ±0.13 |
74.25 ±0.21 |
92.80 ±0.13 |
100 |
| AR2 |
7.00 ±0.16 |
25.83 ±0.13 |
62.92 ±0.14 |
100 |
100 |
100 |
| AR3 |
6.75 ±0.13 |
24.75 ±0.12 |
60.44 ±0.12 |
83.63 ±0.12 |
100 |
100 |
| «Rotokan» |
7.50 ±0.12 |
28.35 ±0.12 |
69.45 ±0.17 |
100 |
100 |
100 |
| Intact animals |
4.20 ±0.16 |
19.15 ±0.11 |
55.25 ±0.14 |
70.14 ±0.14 |
92.24±0.11 |
100 |
Table 8.
Antimicrobial activity of Ajuga reptans L. herb extracts.
Table 8.
Antimicrobial activity of Ajuga reptans L. herb extracts.
| Microorganism |
Ajuga reptans L. herb extracts |
| AR1 |
AR2 |
AR3 |
| Staphylococci |
| Staphylococcus (S.) aureus |
0 |
0 |
6.36 ± 0.28 |
| S. haemolyticus |
6.58 ± 0.12 |
6.95 ± 0.46 |
5.84 ± 0.27 |
| S. epidermidis |
0 |
5.01 ± 0.64 |
4.48 ± 0.30 |
| Enterococci |
| Enterococcus faecalis |
3.83 ± 0.20 |
7.29 ± 0.34* |
11.49 ± 0.61* |
| β-Hemolytic streptococci |
| Streptococcus (St.) pyogenes (GroupA) |
0 |
11.96 ± 0.67* |
13.99 ± 0.67* |
| St. agalacticae (Group B) |
0 |
0 |
9.31 ± 0.49* |
| St. group G |
4.39 ± 0.09 |
4.65 ± 0.26 |
12.27 ± 0.10* |
| α-Hemolytic streptococci |
| St. gordonii |
4.63 ± 0.12 |
4.51 ± 0.30 |
13.24 ± 0.60* |
| St. sanguinis |
0 |
11.82 ± 0.40* |
0 |
| St. oralis |
[8.96 ± 0.61]* |
12.14 ± 0.34* |
12.13 ± 0.13 |
| St. pneumoniae |
[6.37 ± 0.24]* |
[10.86 ±0.30]* |
[11.01 ±1.57]* |
| Bacilli |
| Bacillus subtilis |
4.07 ± 0.29 |
8.29 ± 0.48* |
4.48 ± 0.69 |
| Enterobacteria |
| Escherichia coli |
0 |
0 |
5.80 ± 0.42 |
| Escherichia fergusonii |
0 |
0 |
3.86 ± 0.19 |
| Citrobacter freundii |
0 |
3.89 ± 0.08 |
12.45 ± 0.50* |
| Morganella morganii |
4.07 ± 0.14 |
4.44 ± 0.35 |
5.66 ± 0.10* |
| Providencia rettgeri |
0 |
0 |
9.09 ± 0.21* |
| Providencia stuartii |
0 |
5.10 ± 0.44 |
8.61 ± 0.46* |
| Klebsiella pneumoniae |
0 |
0 |
5.68 ± 0.40 |
| Pseudomonads |
| Pseudomonas aeruginosa |
0 |
0 |
0 |
| Yeast-like Fungi of the Genus Candida |
| Candida albicans |
0 |
0 |
[10.36 ± 0.48] |
| C. tropicalis |
0 |
0 |
[5.35 ± 0.63] |
| C. lusitaniae |
3.92±0.16 |
5.25±0.72 |
[4.98 ± 0.55] |
| C. lipolytica |
0 |
4.65±0.55 |
[5.83 ± 0.28] |
| C. kefyr |
3.10 ± 0.16 |
4.38 ± 0.29 |
[9.55 ± 0.35] |