1. Introduction
Central venous catheter (CVC) insertion is a critical procedure in modern medicine, especially in critical care and emergency settings, providing essential vascular access for blood draws, medication administration and parenteral nutrition [
1,
2,
3]. It is particularly valuable during high-risk surgeries involving significant fluid shifts or blood loss and is commonly used for central venous pressure monitoring [
4]. As populations age, disease severity increases and comorbidity rates rise, the use of CVCs has surged in recent years [
2,
5]. However, contraindications such as severe coagulopathy, patient non-compliance, or local trauma at the insertion site must be carefully considered.
But even under careful consideration of these contraindications, CVCs carry a notable risk of complications, with overall rates ranging from 1.1% to 19% [
1,
2,
6,
7]. Mechanical complications, particularly during insertion, pose significant risks to patient safety, with pneumothorax, hemothorax, and arterial puncture representing the most serious threats [
1,
2,
7,
8]. These mechanical issues occur either during the procedure or as a result of the catheter itself, requiring immediate attention to prevent life-threatening outcomes [
9].
CVCs can be inserted through various anatomical sites, with the internal jugular vein (IJV), subclavian vein (SCV), and femoral vein being the most common. Each site offers distinct advantages and disadvantages that impact clinical decision-making. This study focuses on the IJV and SCV due to their relevance in evaluating the incidence of pneumothorax following CVC procedures.
The IJV is often preferred for its straightforward access and lower risk of pneumothorax compared to the SCV, particularly when larger gauge needles are used or multiple insertion attempts are made [
10]. However, the IJV may have a higher incidence of catheter-related infections, which is why the SCV is preferred in many clinical scenarios due to its lower risk of infection and thrombosis, making it more suitable for long-term access [
1,
11]. Understanding the risk profiles associated with these different insertion sites is crucial for optimizing patient outcomes and minimizing complications related to CVC placement [
1,
10,
11].
The incidence of pneumothorax during CVC insertion varies widely between the two sites, with reported rates ranging from 0.2% to 6.6%. This variability depends on several other factors as well, including patient-related factors (e.g., COPD, abnormal anatomy, prior trauma), catheter type, and clinical factors (e.g., physician experience, prior catheterizations, emergency settings) [
5,
7,
12,
13].
Given the risk of serious complications, particularly pneumothorax, routine chest X-rays have traditionally been performed following CVC insertion to identify post-procedural issues. However, this practice is increasingly being questioned due to concerns over unnecessary costs, radiation exposure, and potential delays in care [
13,
14]. Moreover, post-procedural X-rays can provide a false sense of security, as radiographs, particularly in supine patients, may miss early signs of pneumothorax [
14,
15].
In contrast, routine chest X-rays after CVC removal are rarely performed, as the risk of mechanical complications, such as pneumothorax, is considered negligible. However, CVC removal can still result in rare but life-threatening complications, which may be overlooked due to their rarity. Among these, massive hemothorax and air embolism underscore the importance of continuous monitoring after CVC removal, as early detection and management are crucial in preventing fatal outcomes [
16]. Proper procedural measures - such as keeping the patient supine and applying firm pressure at the insertion site - are essential to minimize these risks [
17].
Despite the rarity of these complications, some hospitals still perform post-procedural imaging to rule out such events, even though international guidelines advise against routine imaging due to its limited clinical benefit [
18]. This inconsistency emphasizes the importance of evidence-based practices in guiding CVC removal protocols.
At the hospital where this study was conducted, the routine use of chest X-rays after both CVC insertion and removal persists, despite guidelines advising against imaging in asymptomatic patients. This practice, driven by habit or institutional inertia, deviates from best practices and has resulted in the accumulation of a comprehensive database of electronic health records (EHR) and radiology reports over many years. Rather than discarding this data, it presents an opportunity to assess real-world complication rates and the cost-effectiveness of these practices, offering valuable insights for improving healthcare protocols and minimizing unnecessary interventions.
These EHRs are ubiquitous in medicine and critical to healthcare delivery, operations, and research. Despite their potential, EHRs are notoriously difficult to process, as over half of the information they contain is in the form of unstructured text (e.g., radiology reports, clinician requests, provider notes, operation reports) and remains largely untapped for secondary use [
19]. Recently, however, newer neural network and deep learning approaches to Natural Language Processing (NLP) have made considerable advances, enabling the systematic analysis of such large datasets [
20].
NLP, a branch of AI focused on interpreting human language, allows for efficient analysis of thousands of radiology reports, identifying key diagnoses like pneumothorax [
21]. These AI-driven tools sift through extensive data, filtering out irrelevant information and streamlining processes with greater accuracy [
22]. This approach transforms previously overlooked or underutilized data into actionable insights, revealing patterns that can inform clinical decision-making and optimize resource use in line with evidence-based guidelines.
However, commercial NLP models often struggle with the complexity of radiology reports, which frequently include lengthy narratives, institution-specific abbreviations, and a mix of normal and abnormal findings [
23]. These models typically emphasize commonly reported normal results, making them less effective at detecting rare but critical complications like pneumothorax.
By developing an NLP algorithm specifically tailored to the language and abbreviations used at this institution, the study harnesses a large dataset of electronic health records to generate evidence-based recommendations for optimizing CVC-related practices. This approach holds the potential to reduce unnecessary healthcare costs and patient exposure to radiation by reevaluating the necessity of routine post-procedural imaging.
2. Materials and Methods
This retrospective cohort study was conducted at the Salzburger Landeskliniken, a network of four university hospitals in Salzburg, Austria. The study focused on analyzing chest X-rays performed between January 1, 2012, and December 31, 2021, following central venous catheter (CVC) placement or removal. Data were collected from electronic health records (EHRs), and the primary aim was to evaluate the incidence of pneumothorax post-procedure.
The initial dataset, retrieved from the EHR system using specific keywords related to CVC procedures, consisted of 26,109 records. Each entry included patient demographic information (age, gender), X-ray date, department of admission, radiologist’s report, and clinician’s request for the X-ray. Sensitive patient information was de-identified, and the dataset was securely stored following institutional ethical guidelines approved by the Salzburger Ethics Committee.
Inclusion criteria were:
Exclusion criteria were:
Patients under 18 years old
Incorrect CVC placement
Follow-up X-rays unrelated to the study objectives
Missing or incomplete data
After applying these criteria, the final dataset included 17,175 patient records, with ages ranging from 18 to 101 years.
A natural language processing (NLP) algorithm was developed to detect newly discovered pneumothorax in radiology reports and clinician requests, while also identifying contraindications such as chest injuries, recent surgeries, or previously known pneumothorax. Clinicians manually identified common phrases and keywords related to pneumothorax, and a list of regular expressions was created to capture these terms, including institutional abbreviations and common misspellings.
Text data preprocessing included converting the reports into lowercase, removing punctuation, and applying custom stopwords. A document-term matrix (DTM) was created with bi-grams (n=2) using the tm and RWeka packages in R, capturing common two-word combinations. Terms that appeared in fewer than 1% of cases were removed to focus on relevant patterns.
To address class imbalance in pneumothorax cases, synthetic minority oversampling (SMOTE) was applied using a recipe from the themis package. The DTM was split into training and test sets (80/20 split), and the random forest model was trained on the balanced dataset with 200 trees and 2 predictors per split (mtry = 2).
The algorithm underwent iterative refinement through manual reviews by a team of three clinicians, who assessed both true positive and false positive findings. Feedback from these reviews led to the incorporation of additional expressions and adjustments, improving detection accuracy.
The final model achieved an accuracy of 93%, with a sensitivity of 97.9% and a specificity of 87.9%. A final manual review by clinicians verified the results and ensured the reliability of the data for further analysis.
The finalized NLP algorithm was applied to the 17,175 records to identify cases of newly discovered pneumothorax within 12 hours of CVC procedures, taking into account the absence of contraindications. The primary outcome was the identification of pneumothorax in the radiology report or clinician’s request.
Descriptive statistics were generated to summarize patient demographics and outcomes. Chi-square tests were conducted to compare pneumothorax rates between CVC insertion and removal procedures. Multivariate logistic regression was used to identify risk factors for pneumothorax, focusing on variables such as age and gender. All statistical analyses were performed using RStudio (2024.04.2) and Microsoft Excel 2016.
Ethical approval for this study was granted by the Salzburger Ethics Committee, ensuring compliance with ethical standards and patient confidentiality on 30. March 2022 (ethical approval code 1032/2022).
3. Results
3.1. Patient Demographics
A total of 17,175 central venous catheter (CVC) procedures were analyzed, conducted between January 1, 2012, and December 31, 2021. The patients' ages ranged from 18 to 101 years (mean: 66.64 years, median: 69 years), with 9,364 male patients (54.5%) and 7,811 female patients (45.5%). Chest X-rays (CXR) were ordered from various clinical departments, with 9,027 cases coming from internal medicine, and smaller numbers from other specialties (
Table 1).
Out of the procedures, 16,380 (95.4%) were CVC insertions, while 795 (4.6%) were CVC removals. CVC insertion durations ranged from 1 to 63 days, with a mean of 11.46 days and a median of 9 days.
3.2. Incidence of Pneumothorax
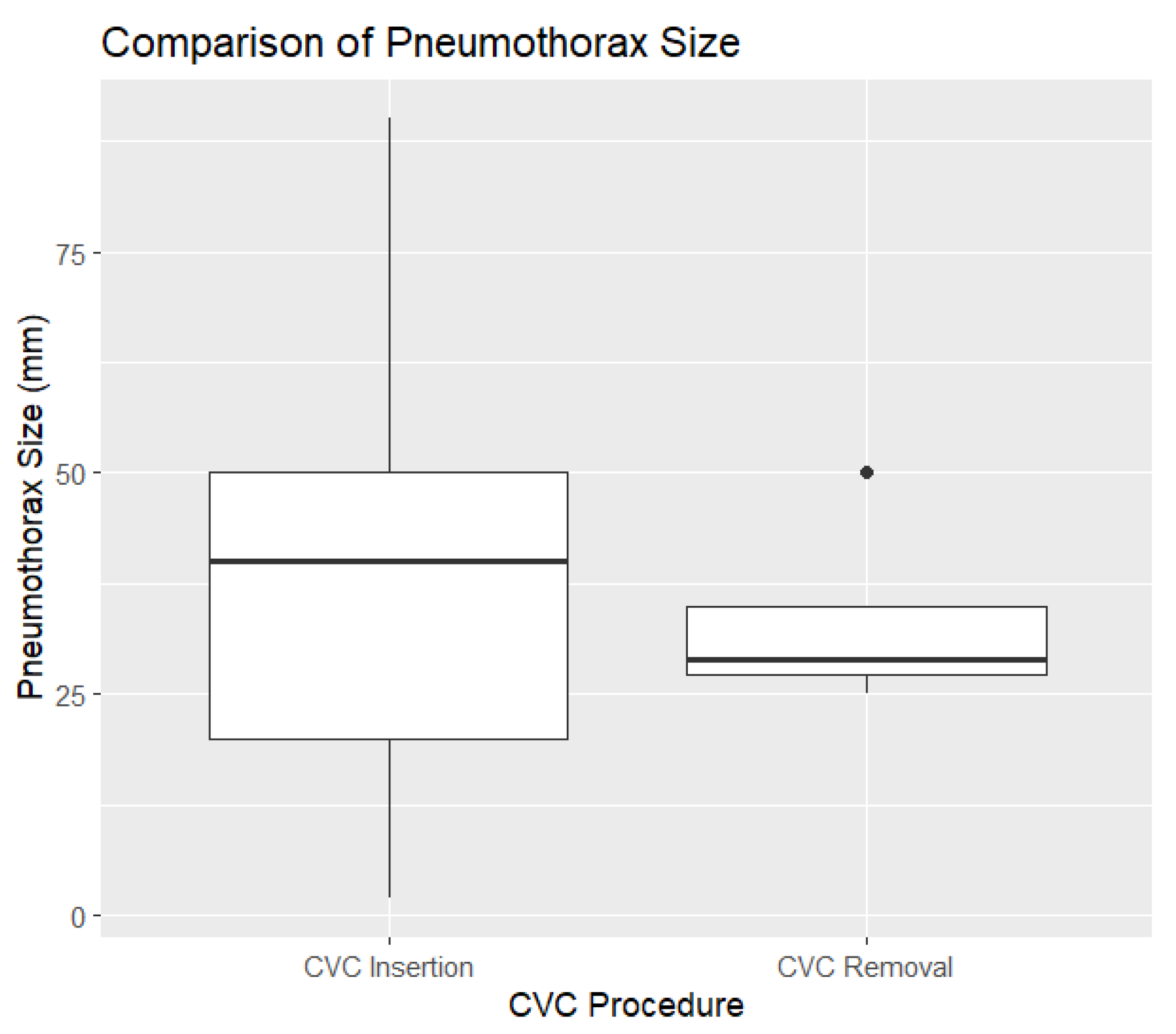
Pneumothorax was observed in 109 cases (0.6%), with 106 cases (1.3%) associated with CVC insertion and only 3 cases (0.02%) following CVC removal. The size of the pneumothoraces varied between 2 mm and 140 mm, with a mean size of 36.93 mm and a median size of 40 mm (
Figure 1).
To assess the effect of central venous catheter (CVC) removal on pneumothorax size, a linear regression model was used. The logistic model examined the relationship between pneumothorax size and whether the central venous catheter (CVC) was removed. The intercept (37.139) suggests an average pneumothorax size of 37 mm when CVC removal is not considered. CVC removal had a non-significant effect, with a coefficient of -3.889, indicating a slight reduction in size (p = 0.713).
3.3. Statistical Analysis
3.3.1. Chi-Square Test
A chi-square test was conducted to compare the incidence of pneumothorax between CVC insertion and removal. The results are presented in the contingency table below.
Table 2.
Contingency Table of Pneumothorax Incidence.
Table 2.
Contingency Table of Pneumothorax Incidence.
| |
No Pneumothorax |
Pneumothorax |
| CVC Insertion |
16,274 |
106 |
| CVC Removal |
792 |
3 |
The test yielded a chi-square value of 0.44965, with 1 degree of freedom and a p-value of 0.5025. These results suggest no statistically significant difference in pneumothorax incidence between CVC insertion and removal.
3.3.2. Logistic Regression for Age and Gender
To evaluate the impact of age and gender on pneumothorax risk, a logistic regression model was used. The model fit statistics included a null deviance of 1300.1 and a residual deviance of 1295.7 with an AIC of 1301.7.
Table 3.
Logistic Regression Analysis for Pneumothorax Risk.
Table 3.
Logistic Regression Analysis for Pneumothorax Risk.
| Predictor |
Estimate |
Std. Error |
Z Value |
P-value |
| Intercept |
-4.396298 |
0.398671 |
-11.027 |
< 2e-16 |
| Age |
-0.011809 |
0.006003 |
-1.967 |
0.0492 * |
| Gender |
0.200964 |
0.194861 |
1.031 |
0.3024 |
The results indicate a slight but statistically significant decrease in pneumothorax risk with increasing age (p = 0.0492), while gender was not a significant predictor (p = 0.3024).
3.4. NLP Algorithm Performance
The natural language processing (NLP) algorithm developed to detect pneumothorax in radiology reports demonstrated high accuracy, with a final accuracy rate of 93%. Sensitivity was 97.9%, and specificity was 87.9%, as confirmed by manual review.
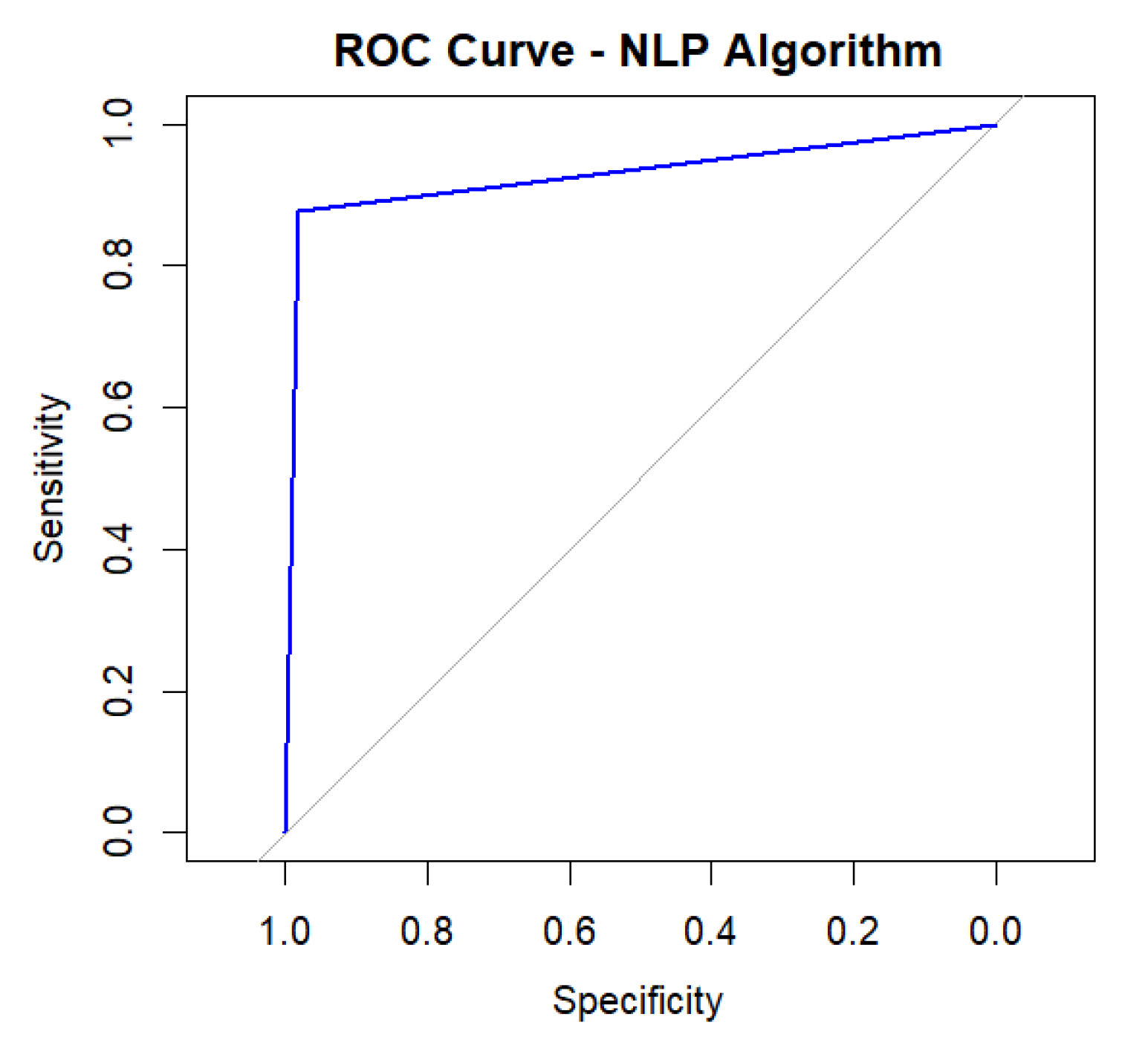
The performance of the natural language processing (NLP) algorithm was further evaluated using a Receiver Operating Characteristic (ROC) curve. The area under the curve (AUC) was calculated to be 0.9283, reflecting the algorithm's strong ability to distinguish between pneumothorax-positive and pneumothorax-negative cases (
Figure 2).
This demonstrates the model's high discriminatory power, with an optimal balance between detecting true positives while minimizing false positives.
4. Discussion
The findings of this study align with current literature, questioning the necessity of routine chest X-rays after CVC procedures [
15]. While complications such as arterial puncture, hematoma and infection remain concerns—with incidence rates of 2% for arterial puncture, 4% for hematoma, and 9% for sepsis—the rates of pneumothorax and catheter malposition are low enough to suggest that routine post-procedural imaging may be unnecessary for asymptomatic patients [
24]. Recent studies report pneumothorax rates ranging from 0.2% to 6.6% after CVC insertion, reinforcing the notion that routine chest radiographs may not be justified given the low complication incidence [
5,
7,
12,
13].
Although chest X-rays have traditionally been the standard for detecting pneumothorax post-CVC, recent studies indicate that ultrasound offers a more accurate, immediate, and cost-effective alternative [
15]. Alrajhi et al. demonstrated that ultrasound has a sensitivity of 90.9% and specificity of 98.2% for pneumothorax detection, far surpassing chest radiographs, which have a sensitivity of just 50.2% [
9]. This significant disparity reveals that post-procedural X-rays may provide a false sense of security, as early signs of pneumothorax can easily be missed, particularly in supine patients during chest radiographs [
14,
15].
Despite these findings, the routine use of chest X-rays after CVC procedures continues in many clinical settings, often driven by institutional habits rather than evidence supporting a transition to ultrasound for pneumothorax detection [
15]. The reluctance to adopt newer practices like ultrasound stems from several barriers, including the upfront costs of ultrasound machines, the resources required to train clinicians, and legal concerns [
25]. Many institutions remain hesitant to adopt ultrasound, largely due to the perception that it requires extensive training compared to traditional imaging methods. However, data show that with proper instruction and supervision, ultrasound yields similar success rates in CVC placement and complication prevention as conventional methods [
26,
27].
Financial analyses have also indicated modest labor cost savings when ultrasound is used in emergency departments and intensive care units to confirm catheter positioning and exclude pneumothorax [
27,
28,
29]. Institutional inertia also plays a significant role, as many clinicians rely on established practices, even in the absence of updated evidence [
30]. Legal concerns exacerbate this inertia, with providers fearing potential repercussions for deviating from established protocols, even when newer, proven methods are available [
15,
25].
While physicians are generally well aware of the risks of catheter insertion, complications from CVC removal often receive less attention, despite the procedure carrying its own set of hazards. Inadequate training, particularly in removal techniques, has been linked to increased risks, including air embolism. Ensuring that only trained personnel handle CVC removal is key to reducing these potentially life-threatening complications [Ingram Paul]. Risks such as bleeding, catheter fracture, thrombus dislodgement, and infection, though rare, can lead to severe consequences if not properly managed [
16]. Proper education and adherence to protocols are essential to prevent these events in this routine procedure.
To address these challenges within the framework of value-based healthcare, developing standardized protocols ensures imaging is performed only when clinically necessary. This approach enhances patient care while reducing unnecessary radiation exposure and healthcare costs, aligning with value-based principles by prioritizing patient-centered, evidence-driven practices. By implementing clear guidelines that favor ultrasound over chest X-rays, hospitals can streamline patient management, promote more efficient care delivery, and reduce unwarranted interventions, thus contributing to both improved outcomes and resource optimization [
31].
As electronic health records (EHR) are increasingly integrated with Artificial Intelligence (AI) tools, hospitals can now mine vast datasets, uncovering trends and insights that would be challenging to identify manually. Recent advances in natural language processing (NLP), an interdisciplinary research field that aims to develop algorithms for the computational understanding of written and spoken languages, have facilitated this automatic annotation of a large volume of medical reports [
23].
Given the fast growth of digital data and the growing need for automated language processing, NLP has become an indispensable technology in healthcare with its most prominent applications in text classification, question answering, speech recognition, language translation, chat bots, and the generation or summarization of texts [
19]. Over the past decade, the progress of NLP has been accelerated by deep learning techniques, in conjunction with increasing hardware capabilities and the availability of massive text corpora [
20].
NLP's role is crucial for transforming unstructured EHR data into actionable insights, especially in radiology, where it helps detect critical conditions like pneumothorax. These integrations of EHR also reflect a broader trend of AI being used to optimize clinical workflows and improve patient outcomes. By processing the massive amount of unstructured text found in EHRs, NLP algorithms unlock valuable insights that would otherwise be challenging to extract manually. This capability aligns with the increasing reliance on evidence-based guidelines, allowing healthcare providers to make better-informed decisions with greater speed and accuracy [
32].
However, commercial NLP models often struggle with the complex, institution-specific language found in radiology reports [
33]. This complexity, which includes detailed narratives and specialized abbreviations, can reduce the accuracy of identifying specific conditions like pneumothorax [
34].
These challenges have prompted the adoption of more advanced NLP techniques, which have shown promise in overcoming these language complexities [
35,
36]. Techniques such as word embedding, deep learning, and transformers, such as the bidirectional encoder representations from transformers (BERT) model, have significantly improved the accuracy and efficiency of medical report analysis in recent years by enhancing a NLP’s capability to understand semantic context [
33,
37,
38].
By fine-tuning our NLP model to the hospital’s database and terminology, we achieved higher sensitivity and specificity in analyzing radiology reports related to CVC procedures. This approach is in line with the latest research, which highlights the importance of machine learning in improving NLP performance [
39].
The algorithm demonstrated robust performance, with an accuracy of 93%, a sensitivity of 97.9%, and a specificity of 87.9%, alongside an AUC of 0.9283. Navarro et al. reviewed 94 studies, with 30 published in the last three years, highlighting that machine learning methods were employed in 68 studies, while 22 used a combination of machine learning and rule-based approaches. The highest F1 scores among these studies reached 93.25%, which compares favorably to the performance of our model [
40].
Notably, while many studies rely on public datasets like i2b2, the proprietary dataset and manual review used in this study emphasize the importance of customization for specific clinical tasks, such as pneumothorax detection. By implementing NLP pipelines that are both precise and practical, hospitals can enhance clinical decision-making, minimize unnecessary interventions, and ultimately improve patient care. However, even though NLP applications in radiology have become more accurate, there is still a need for better clinical integration to fit seamlessly into real-world medical workflows [
41]. This underscores the growing role of NLP in medical diagnostics and reinforces, that adapting algorithms to specialized clinical contexts can lead to improved outcomes, rather than solely relying on generalized public datasets.
By eliminating unnecessary post-CVC imaging and leveraging AI-driven tools like NLP, hospitals can improve the efficiency and cost-effectiveness of healthcare delivery. This approach aligns with the principles of value-based medicine, which prioritize evidence-based, patient-centered care by reducing unnecessary interventions without compromising patient safety [
42]. As healthcare shifts towards this model, technologies like NLP play a crucial role in optimizing both clinical outcomes and resource management, ensuring that interventions are guided by actual clinical benefit and not by habitual practices.
The limitations of this study include several factors that could affect the generalizability and validity of the findings. First, the dataset is restricted to chest X-rays containing specific search terms in the physician’s request, meaning some X-rays for CVC insertion or removal may have been overlooked. Additionally, while the NLP algorithm was customized to the institution's unique language and abbreviations, this limits its broader applicability. Lastly, the study focuses primarily on post-procedural pneumothorax, excluding other complications like infections or catheter misplacement, potentially overlooking broader CVC-related risks. Further research is needed to address these gaps and assess long-term outcomes related to reduced post-procedural imaging.
5. Conclusions
This study highlights the effectiveness of tailored NLP algorithms in detecting pneumothorax in radiology reports, thereby minimizing the need for unnecessary imaging after CVC procedures. Given the lack of a significant difference in pneumothorax incidence between CVC insertion and removal, and existing guidelines suggesting chest X-rays are unnecessary after CVC removal, these findings further suggest that routine post-insertion imaging may also be unnecessary for asymptomatic patients. This supports a shift towards more efficient, evidence-based practices in line with value-based healthcare principles.
Author Contributions
Conceptualization, M.B., C.D. and F.W.; methodology, M.B.; software, M.B.; validation, M.B., V.M., and T.W.; formal analysis, M.B.; investigation, M.B.; resources, C.D. and F.W.; data curation, M.B.; writing—original draft preparation, M.B.; writing—review and editing, M.B. and C.D.; visualization, M.B.; supervision, C.D.; project administration, C.D. and F.W.; funding acquisition, F.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of LAND SALZBURG (protocol code 1032/2022, 30. March 2022).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study, which involved the evaluation of anonymized data. No identifiable patient information was used, and the study adhered to institutional guidelines for data protection and ethical research practices.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author due to ethical and privacy restrictions regarding patient information.
Acknowledgments
We would like to acknowledge the support provided by the administrative and IT teams at our hospital and the MEL support institute.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Teja, B.; Bosch, N.A.; Diep, C.; Pereira, T.V.; Mauricio, P.; Sklar, M.C.; Sankar, A.; Wijeysundera, H.C.; Saskin, R.; Walkey, A.; et al. Complication Rates of Central Venous Catheters: A Systematic Review and Meta-Analysis. JAMA Internal Medicine 2024, 184, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Tsotsolis, N.; Tsirgogianni, K.; Kioumis, I.; Pitsiou, G.; Baka, S.; Papaiwannou, A.; Karavergou, A.; Rapti, A.; Trakada, G.; Katsikogiannis, N.; et al. Pneumothorax as a complication of central venous catheter insertion. Ann Transl Med 2015, 3, 40. [Google Scholar] [CrossRef] [PubMed]
- Kehagias, E.; Galanakis, N.; Tsetis, D. Central venous catheters: Which, when and how. British Journal of Radiology 2023, 96. [Google Scholar] [CrossRef]
- Hill, B.; Smith, C. Central venous pressure monitoring in critical care settings. British journal of nursing 2021, 30, 230–236. [Google Scholar] [CrossRef]
- Ablordeppey, E.A.; Huang, W.; Holley, I.; Willman, M.; Griffey, R.; Theodoro, D.L. Clinical practices in central venous catheter mechanical adverse events. Journal of Intensive Care Medicine 2022, 37, 1215–1222. [Google Scholar] [CrossRef]
- Kusminsky, R.E. Complications of Central Venous Catheterization. Journal of the American College of Surgeons 2007, 204, 681–696. [Google Scholar] [CrossRef]
- Björkander, M.; Bentzer, P.; Schött, U.; Broman, M.E.; Kander, T. Mechanical complications of central venous catheter insertions: A retrospective multicenter study of incidence and risks. Acta Anaesthesiologica Scandinavica 2019, 63, 61–68. [Google Scholar] [CrossRef]
- Polderman, K.H.; Girbes, A.R. Central venous catheter use: Part 1: Mechanical complications. Intensive care medicine 2002, 28, 1–17. [Google Scholar] [CrossRef]
- Alrajhi, K.; Woo, M.Y.; Vaillancourt, C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest 2012, 141, 703–708. [Google Scholar] [CrossRef]
- Imai, E.; Watanabe, J.; Okano, H.; Yokozuka, M. Efficacy and safety of supraclavicular versus infraclavicular approach for subclavian vein catheterisation: An updated systematic review and meta-analysis of randomised controlled trials. Indian Journal of Anaesthesia 2023, 67, 486–496. [Google Scholar] [CrossRef]
- Sakuraya, M.; Okano, H.; Yoshihiro, S.; Niida, S.; Kimura, K. Insertion site of central venous catheter among hospitalized adult patients: A systematic review and network meta-analysis. Frontiers in medicine 2022, 9, 960135. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.A.; Narasimhan, M.; Berger, J.S.; Mayo, P.H.; Rosen, M.J.; Schneider, R.F. Mechanical complications of central venous catheters. Journal of intensive care medicine 2006, 21, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Prishchepova, A.; Abramovitz, A.M.; Dudaie, R.S.; Buchanan, P.M. Bedside ultrasound as an alternative to chest radiograph in detecting complications associated with central venous catheter placement: a retrospective cohort study. Journal of Emergency and Critical Care Medicine 2021, 5. [Google Scholar] [CrossRef]
- Molgaard, O.; Nielsen, M.; Handberg, B.; Jensen, J.; Kjaergaard, J. Routine X-ray control of upper central venous lines: Is it necessary? Acta anaesthesiologica scandinavica 2004, 48, 685–689. [Google Scholar] [CrossRef]
- Brindley, P.; Deschamps, J.; Milovanovic, L.; Buchanan, B. Are routine chest radiographs still indicated after central line insertion? A scoping review. Journal of the Intensive Care Society 2024, 25, 190–207. [Google Scholar] [CrossRef]
- Dib, J.; Boutari, R.; Yaacoub, B.; Al Khalil, N.; Al Ayoubi, M.; Itani, O. Hemothorax Occurring After Central Venous Catheter Removal: A Case Report. International Journal of Clinical Research 2023, 3, 37–41. [Google Scholar] [CrossRef]
- Ingram, P.; Sinclair, L.; Edwards, T. The safe removal of central venous catheters. Nursing Standard (through 2013) 2006, 20, 42. [Google Scholar] [CrossRef]
- Drewett, S. Removal of central venous access devices. Central venous catheters. Wiley-Blackwell, Chichester, 2009; 238–248. [Google Scholar]
- Li, I.; Pan, J.; Goldwasser, J.; Verma, N.; Wong, W.P.; Nuzumlalı, M.Y.; Rosand, B.; Li, Y.; Zhang, M.; Chang, D. Neural natural language processing for unstructured data in electronic health records: a review. Computer Science Review 2022, 46, 100511. [Google Scholar] [CrossRef]
- Li, J.; Dada, A.; Puladi, B.; Kleesiek, J.; Egger, J. ChatGPT in healthcare: a taxonomy and systematic review. Computer Methods and Programs in Biomedicine 2024, 108013. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Hu, S.; Shen, Y.; Lan, J.; Jiang, B.; de Bock, G.H.; Vliegenthart, R.; Chen, X.; Xie, X. Development and multicenter validation of chest X-ray radiography interpretations based on natural language processing. Communications medicine 2021, 1, 43. [Google Scholar] [CrossRef]
- Casey, A.; Davidson, E.; Poon, M.; Dong, H.; Duma, D.; Grivas, A.; Grover, C.; Suárez-Paniagua, V.; Tobin, R.; Whiteley, W. A systematic review of natural language processing applied to radiology reports. BMC medical informatics and decision making 2021, 21, 179. [Google Scholar] [CrossRef] [PubMed]
- Kale, K.; Jadhav, K. Replace and report: NLP assisted radiology report generation. arXiv, 2023; arXiv:2306.17180 2023. [Google Scholar]
- Singh, R.; Patel, N.; Mehta, N.; Singh, G.; Patel, N. Central venous catheterization-related complications in a cohort of 100 hospitalized patients: An observational study. Journal of Acute Disease 2023, 12, 169–172. [Google Scholar] [CrossRef]
- Ablordeppey, E.A.; Keating, S.M.; Brown, K.M.; Theodoro, D.L.; Griffey, R.T.; James, A.S. Implementation of ultrasound after central venous catheter insertion: A qualitative study in early adopters. The Journal of Vascular Access 2023, 24, 879–888. [Google Scholar] [CrossRef] [PubMed]
- De Cassai, A.; Geraldini, F.; Pasin, L.; Boscolo, A.; Zarantonello, F.; Tocco, M.; Pretto, C.; Perona, M.; Carron, M.; Navalesi, P. Safety in training for ultrasound guided internal jugular vein CVC placement: a propensity score analysis. BMC anesthesiology 2021, 21, 1–6. [Google Scholar] [CrossRef]
- Tran, Q.K.; Foster, M.; Bowler, J.; Lancaster, M.; Tchai, J.; Andersen, K.; Matta, A.; Haase, D.J. Emergency and critical care providers’ perception about the use of bedside ultrasound for confirmation of above-diaphragm central venous catheter placement. Heliyon 2020, 6. [Google Scholar] [CrossRef]
- Ablordeppey, E.A.; Koenig, A.M.; Barker, A.R.; Hernandez, E.E.; Simkovich, S.M.; Krings, J.G.; Brown, D.S.; Griffey, R.T. Economic evaluation of ultrasound-guided central venous catheter confirmation vs chest radiography in critically ill patients: a labor cost model. Western Journal of Emergency Medicine 2022, 23, 760. [Google Scholar] [CrossRef]
- Austin, S.E.; Tran, Q.; Pourmand, A.; Matta, A.; Haase, D. Comments on “Economic Evaluation of Ultrasound-guided Central Venous Catheter Confirmation vs Chest Radiography in Critically Ill Patients: A Labor Cost Model”. Western Journal of Emergency Medicine: Integrating Emergency Care with Population Health 2023, 24. [Google Scholar] [CrossRef]
- Alhussein, R.M.; Alyahya, B.A.; Alashaikh, A.S.; Malabarey, M.A.; Alrajhi, K.N.; Al Aseri, Z.A. Barriers to the use of ultrasound guidance in central venous catheter placement by emergency physicians in Saudi Arabia: a cross-sectional study. Signa Vitae 2023, 1, 8. [Google Scholar]
- Keating, S.; James, A.; Griffey, R.; Ablordeppey, E. Barriers and facilitators of de-implementing chest X-rays after central venous catheter insertion. Annals of Emergency Medicine 2020, 76, S102. [Google Scholar] [CrossRef]
- Schopow, N.; Osterhoff, G.; Baur, D. Applications of the natural language processing tool ChatGPT in clinical practice: comparative study and augmented systematic review. JMIR Medical Informatics 2023, 11, e48933. [Google Scholar] [CrossRef]
- Pons, E.; Braun, L.M.; Hunink, M.M.; Kors, J.A. Natural language processing in radiology: a systematic review. Radiology 2016, 279, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; McAuley, J.; Lu, X.; Du, J.; Chang, E.Y.; Gentili, A.; Hsu, C.-N. RadBERT: adapting transformer-based language models to radiology. Radiology: Artificial Intelligence 2022, 4, e210258. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.W.; Chong, J.J. Review of natural language processing in radiology. Neuroimaging Clinics 2020, 30, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Sorin, V.; Barash, Y.; Konen, E.; Klang, E. Deep learning for natural language processing in radiology—fundamentals and a systematic review. Journal of the American College of Radiology 2020, 17, 639–648. [Google Scholar] [CrossRef]
- Linna, N.; Kahn Jr, C.E. Applications of natural language processing in radiology: A systematic review. International Journal of Medical Informatics 2022, 163, 104779. [Google Scholar] [CrossRef]
- Chen, L.; Shah, R.; Link, T.; Bucknor, M.; Majumdar, S.; Pedoia, V. Bert model fine-tuning for text classification in knee OA radiology reports. Osteoarthritis and Cartilage 2020, 28, S315–S316. [Google Scholar] [CrossRef]
- Liang, S.; Kades, K.; Fink, M.; Full, P.; Weber, T.; Kleesiek, J.; Strube, M.; Maier-Hein, K. Fine-tuning BERT models for summarizing German radiology findings. In Proceedings of the Proceedings of the 4th Clinical Natural Language Processing Workshop, 2022; pp. 30–40.
- Navarro, D.F.; Ijaz, K.; Rezazadegan, D.; Rahimi-Ardabili, H.; Dras, M.; Coiera, E.; Berkovsky, S. Clinical named entity recognition and relation extraction using natural language processing of medical free text: A systematic review. International Journal of Medical Informatics 2023, 177, 105122. [Google Scholar] [CrossRef]
- Bobba, P.S.; Sailer, A.; Pruneski, J.A.; Beck, S.; Mozayan, A.; Mozayan, S.; Arango, J.; Cohan, A.; Chheang, S. Natural language processing in radiology: Clinical applications and future directions. Clinical Imaging 2023, 97, 55–61. [Google Scholar] [CrossRef]
- Porter, M.E.; Teisberg, E.O. Redefining health care: creating value-based competition on results; Harvard business press: 2006.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).