Submitted:
09 October 2024
Posted:
10 October 2024
You are already at the latest version
Abstract
Keywords:
Introduction
CBCT: Development, Principles and Clinical Implications
CBCT in Pre-Surgical Evaluation in Implantology
Nasopalatine Canal
Nasal Fossa
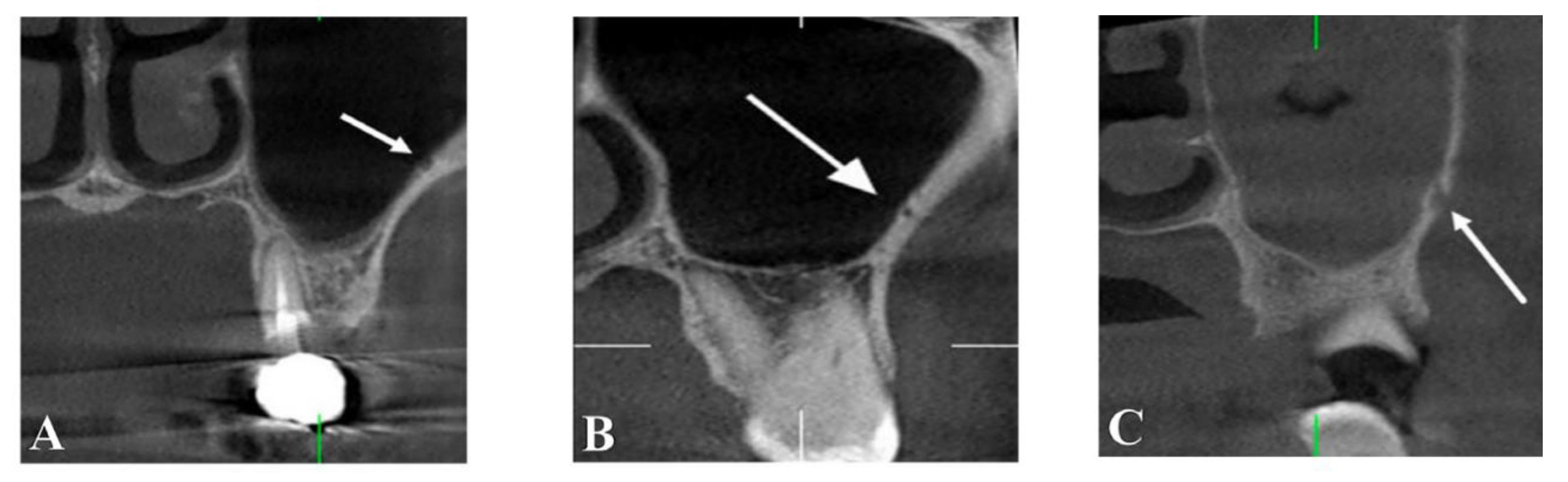
Canalis Sinuosos
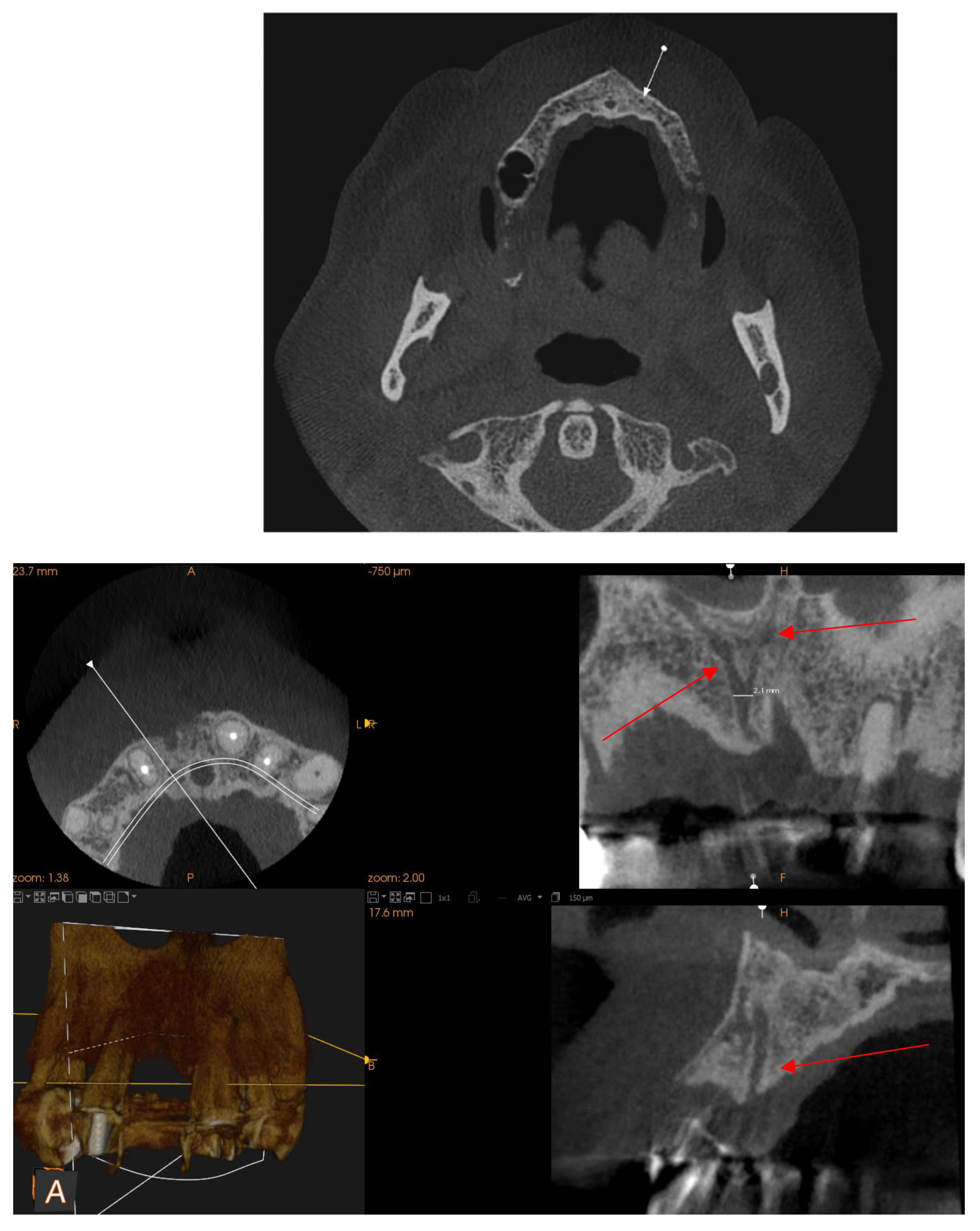
Maxillary Sinus
Septa
Posterior superior alveolar artery
Mentonian Hole
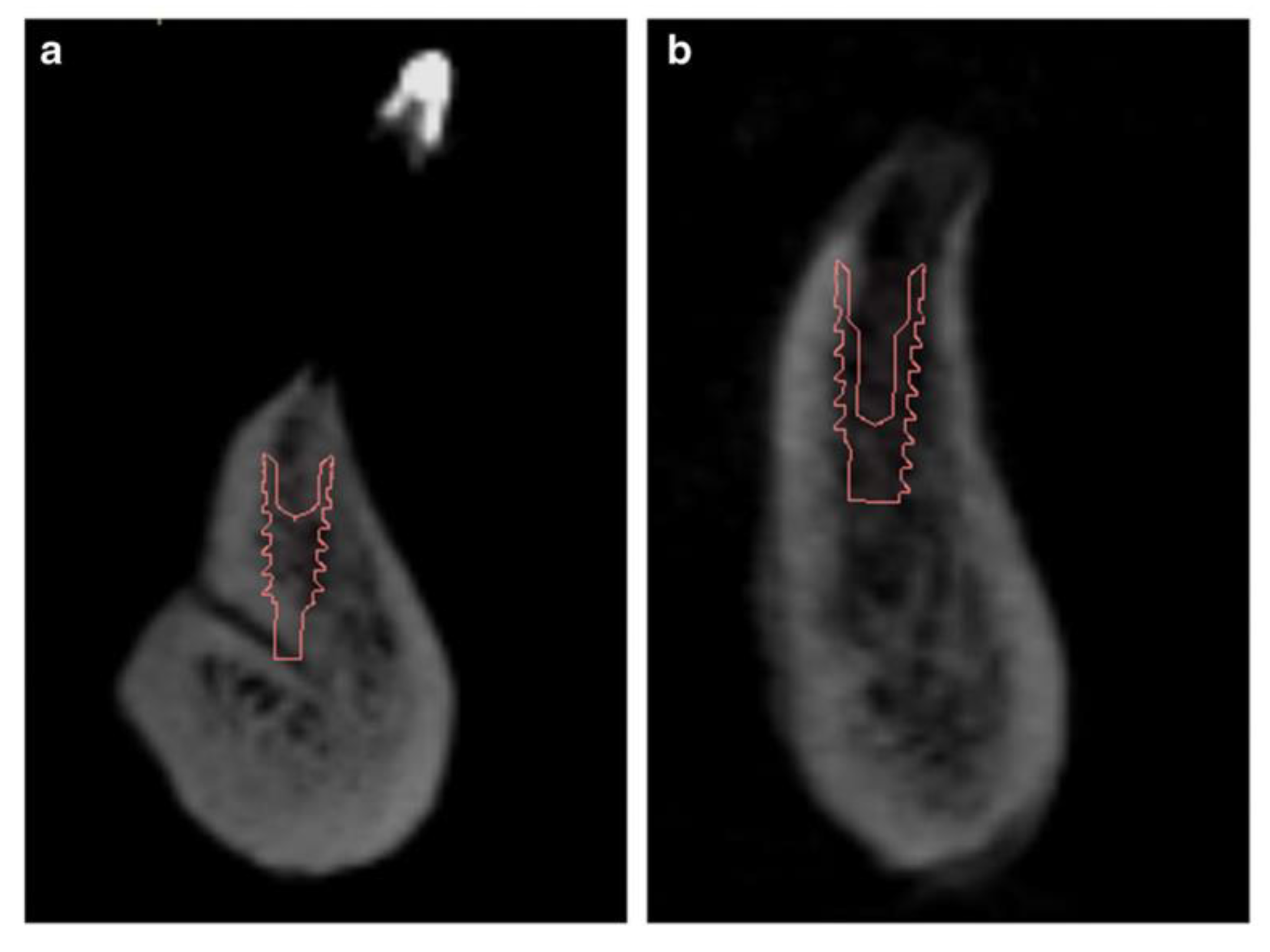
Sublingual Hole and Canal
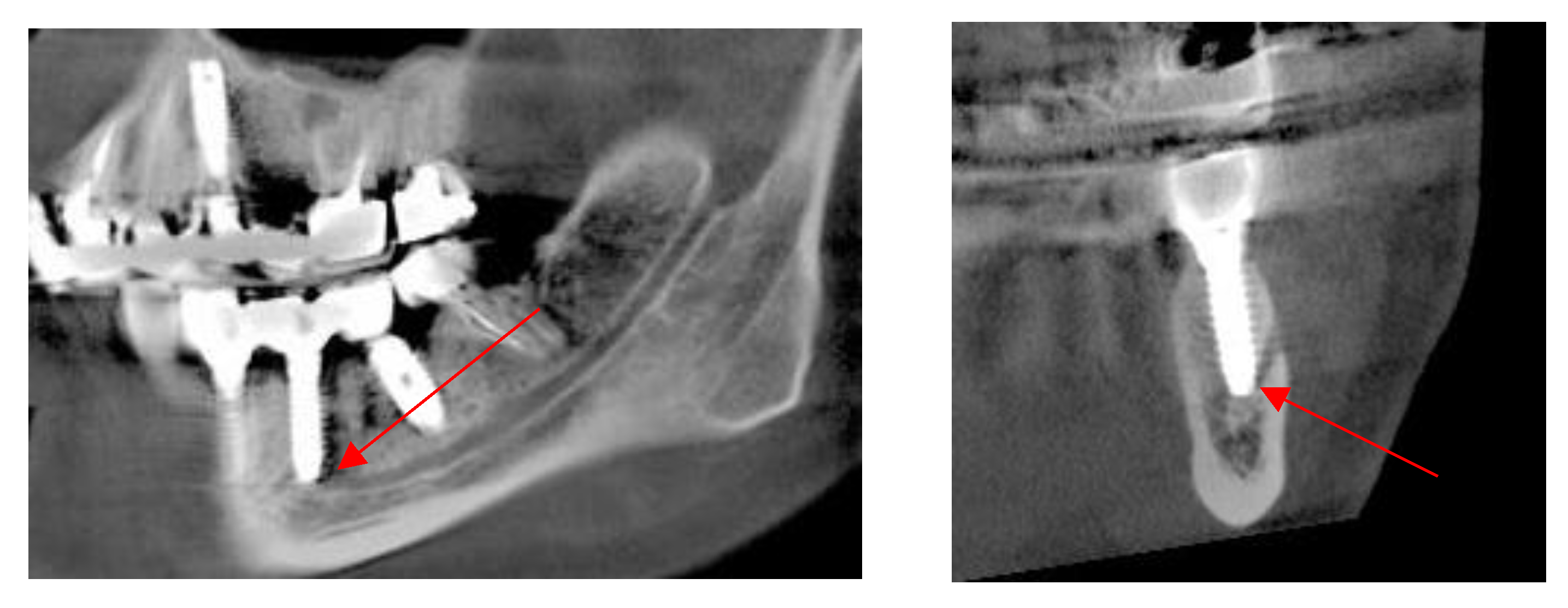
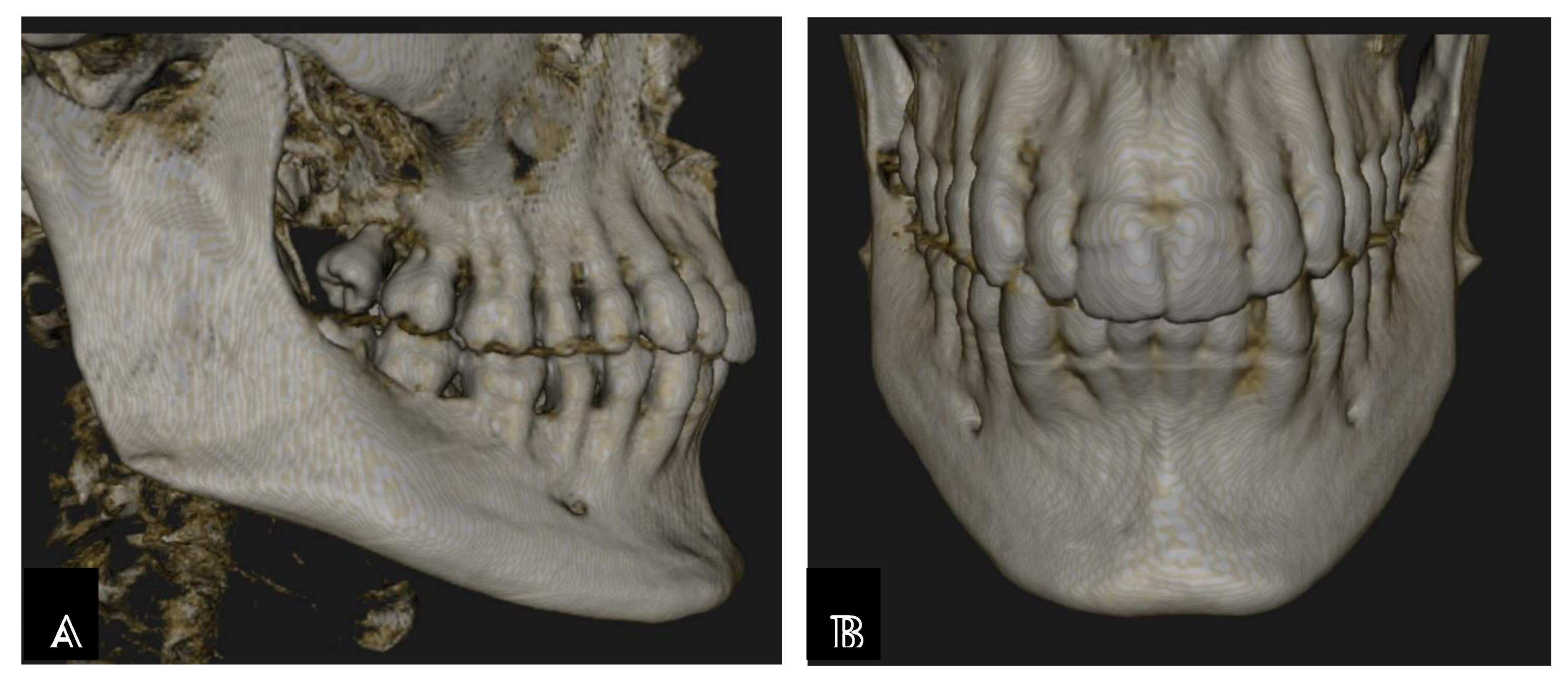
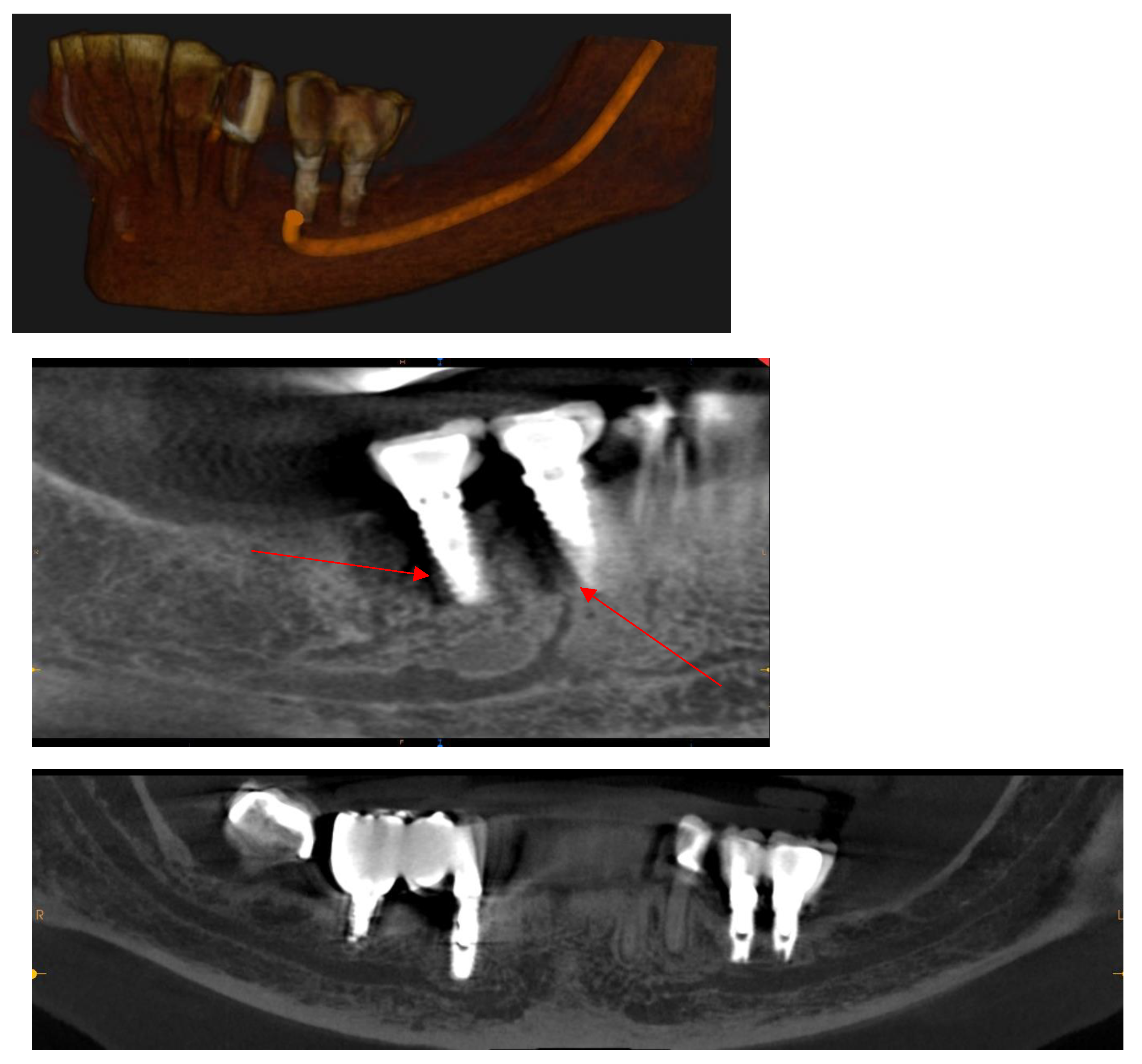
Mandibular Canal
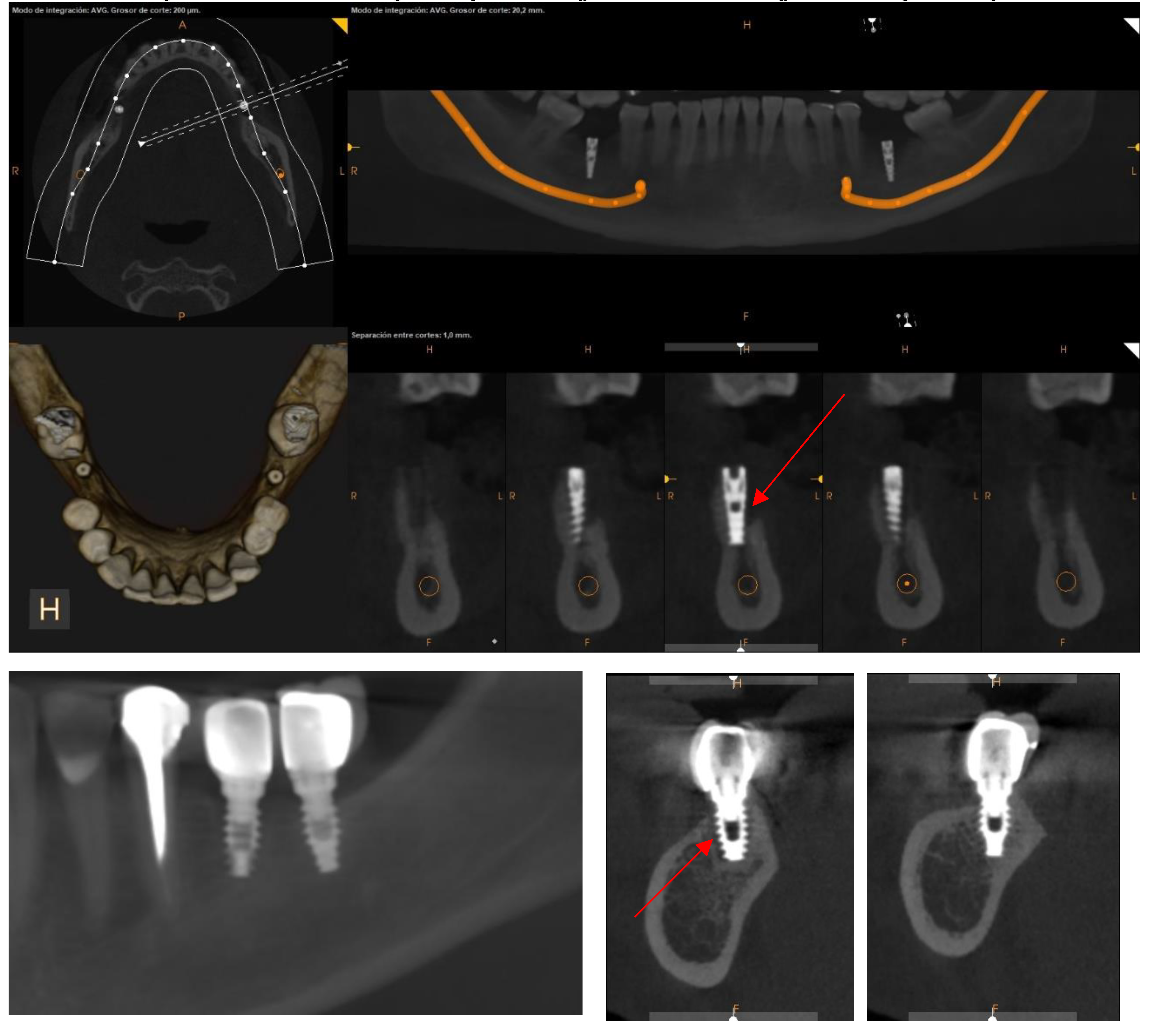
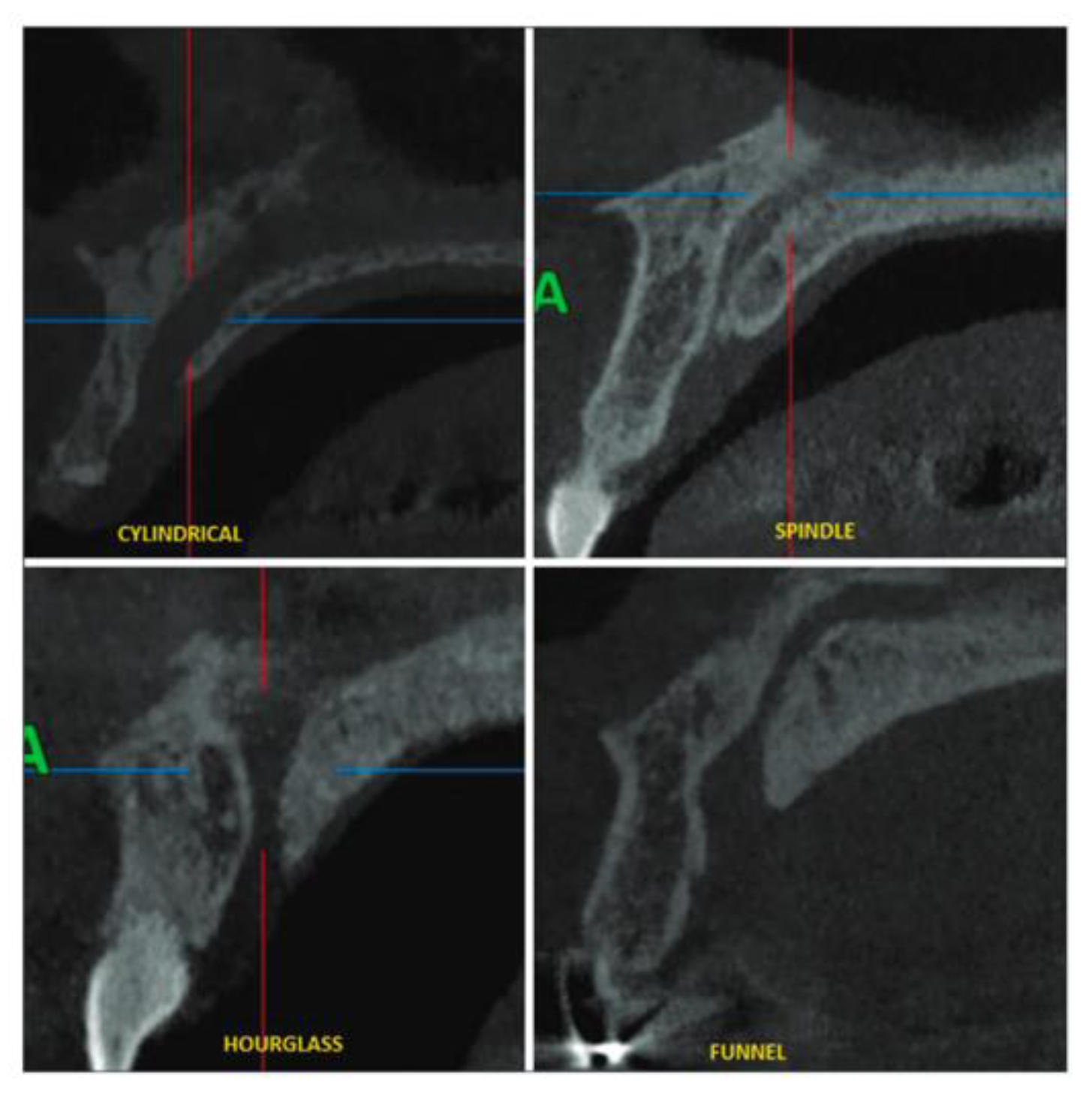
Assessment, Planning and Prevention in the Surgical Context
CBCT in the Post-Surgical Phase: Assessment of Bone Loss and Post-Operative Surgical Complications
Conclusion
References
- Gaêta-Araujo, H.; Alzoubi, T.; Vasconcelos, K.D.F.; Orhan, K.; Pauwels, R.; Casselman, J.W.; Jacobs, R. Cone beam computed tomography in dentomaxillofacial radiology: A two-decade overview. Dentomaxillofacial Radiology 2020, 49, 20200145. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, A.; Kardachi, B.; Bartold, P. Is there a role for the use of volumetric cone beam computed tomography in periodontics? Australian Dental Journal 2012, 57, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Davidopoulou, S.; Karakostas, P.; Batas, L.; Barmpalexis, P.; Assimopoulou, A.; Angelopoulos, C.; Tsalikis, L. Multidimensional 3D-Printed Scaffolds and Regeneration of Intrabony Periodontal Defects: A Systematic Review. Journal of Functional Biomaterials 2024, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Shah, N. Recent advances in imaging technologies in dentistry. World Journal of Radiology 2014, 6, 794. [Google Scholar] [CrossRef] [PubMed]
- Mandelaris, G.A.; Scheyer, E.T.; Evans, M.; Kim, D.; McAllister, B.; Nevins, M.L.; Rios, H.F.; Sarment, D. American Academy of Periodontology Best Evidence Consensus Statement on Selected Oral Applications for Cone-Beam Computed Tomography. Journal of Periodontology 2017, 88, 939–945. [Google Scholar] [CrossRef]
- Ludlow, J.B.; Timothy, R.; Walker, C.; Hunter, R.; Benavides, E.; Samuelson, D.B.; Scheske, M.J. Effective dose of dental CBCT—a meta analysis of published data and additional data for nine CBCT units. Dentomaxillofacial Radiology 2015, 44, 20140197. [Google Scholar] [CrossRef]
- Lindfors, N.; Ekestubbe, A.; Frisk, F.; Lund, H. Is cone-beam computed tomography (CBCT) an alternative to plain radiography in assessments of dental disease? A study of method agreement in a medically compromised patient population. Clinical Oral Investigations 2024, 28, 127. [Google Scholar] [CrossRef] [PubMed]
- De Faria Vasconcelos, K.; Evangelista, K.; Rodrigues, C.; Estrela, C.; De Sousa, T.; Silva, M. Detection of periodontal bone loss using cone beam CT and intraoral radiography. Dentomaxillofacial Radiology 2012, 41, 64–69. [Google Scholar] [CrossRef]
- Jain, S.; Choudhary, K.; Nagi, R.; Shukla, S.; Kaur, N.; Grover, D. New evolution of cone-beam computed tomography in dentistry: Combining digital technologies. Imaging Science in Dentistry 2019, 49, 179. [Google Scholar] [CrossRef]
- Weiss, R.; Read-Fuller, A. Cone Beam Computed Tomography in Oral and Maxillofacial Surgery: An Evidence-Based Review. Dentistry Journal 2019, 7, 52. [Google Scholar] [CrossRef]
- Kamburoğlu, K. Use of dentomaxillofacial cone beam computed tomography in dentistry. World Journal of Radiology 2015, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, E.; Venkatesh Elluru, S. (2017). CONE BEAM COMPUTED TOMOGRAPHY: BASICS AND APPLICATIONS IN DENTISTRY. Journal of Istanbul University Faculty of Dentistry, 51. [CrossRef]
- Shukla, S.; Chug, A.; Afrashtehfar, K. Role of cone beam computed tomography in diagnosis and treatment planning in dentistry: An update. Journal of International Society of Preventive and Community Dentistry 2017, 7, 125. [Google Scholar] [CrossRef]
- Kumar, M.; Shanavas, M.; Sidappa, A.; Kiran, M. (2015). Cone Beam Computed Tomography—Know its Secrets. Cone Beam Computed Tomography.
- Abramovitch, K.; Rice, D.D. Basic Principles of Cone Beam Computed Tomography. Dental Clinics of North America 2014, 58, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Scarfe, W.C.; Farman, A.G. What is Cone-Beam CT and How Does it Work? Dental Clinics of North America 2008, 52, 707–730. [Google Scholar] [CrossRef]
- Howerton, W.B.; Mora, M.A. Advancements in Digital Imaging. The Journal of the American Dental Association 2008, 139, S20–S24. [Google Scholar] [CrossRef]
- Heller, Z.A.; Hogge, M.; Ragan, M.R.; Portnof, J.E. Applications of Cone Beam Computed Tomography Scans in Dental Medicine and Potential Medicolegal Issues. Dental Clinics of North America 2024, 68, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Hussaini, S.; Glogauer, M.; Sheikh, Z.; Al-Waeli, H. CBCT in Dental Implantology: A Key Tool for Preventing Peri-Implantitis and Enhancing Patient Outcomes. Dentistry Journal 2024, 12, 196. [Google Scholar] [CrossRef]
- Jha, S.; Pathi, J.; Sangamesh, N.C.; Singh, D.K.; Sikdar, A.; Dash, M. (2022). Marginal Bone Level and Bone Quality Evaluation Using CBCT after Functional Loading around Dental Implant in the Population of Bhubaneswar, Odisha: A Longitudinal Study. Journal of Pharmaceutical Research International, 6–13. [CrossRef]
- Meer Rownaq Ali, A.B.; Khanam, K.G.K.K.; Mathew, A. CBCT- A Boon for Implant Dentistry. Saudi Journal of Oral and Dental Research 2019, 04, 691–699. [Google Scholar] [CrossRef]
- Zewail, A.M.; Abdullah, A.B.; Aboelhasan, M.F.; El-Ashmawy, M.M.; Ibrahim, A.G. Qualitative Analysis of Maxillary Sinus after Guided Lateral Sinus Lift Procedure with Simultaneous Implant Placement. Al-Azhar Assiut Dental Journal 2024, 7, 103–111. [Google Scholar] [CrossRef]
- Fernández Bodereau, E.; Flores, V.Y.; Naldini, P.; Torassa, D.; Tortolini, P. Clinical Evaluation of the Nasopalatine Canal in Implant-Prosthetic Treatment: A Pilot Study. Dentistry Journal 2020, 8, 30. [Google Scholar] [CrossRef]
- Rai, S.; Misra, D.; Misra, A.; Khatri, M.; Kidwai, S.; Bisla, S.; Jain, P. Significance of morphometric and anatomic variations of nasopalatine canal on cone-beam computed tomography in anterior functional zone—A retrospective study. Annals of Maxillofacial Surgery 2021, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Fokas, G.; Vaughn, V.M.; Scarfe, W.C.; Bornstein, M.M. Accuracy of linear measurements on CBCT images related to presurgical implant treatment planning: A systematic review. Clinical Oral Implants Research 2018, 29, 393–415. [Google Scholar] [CrossRef]
- Guerrero, M.E.; Noriega, J.; Jacobs, R. Preoperative implant planning considering alveolar bone grafting needs and complication prediction using panoramic versus CBCT images. Imaging Science in Dentistry 2014, 44, 213. [Google Scholar] [CrossRef]
- Singhal, M.; Dandriyal, R.; Aggarwal, A.; Agarwal, A.; Yadav, S.; Baranwal, P. Implant placement into the nasopalatine foramen: Considerations from anatomical and surgical point of view. Annals of Maxillofacial Surgery 2018, 8, 347. [Google Scholar] [CrossRef]
- Battaglia, P.; Turri–Zanoni, M.; Lepera, D.; Sica, E.; Karligkiotis, A.; Dallan, I.; Castelnuovo, P. (2016). Endoscopic transnasal approaches to pterygopalatine fossa tumors. Head & Neck, 38. [CrossRef]
- Omura, K.; Nomura, K.; Mori, R.; Ishii, Y.; Tanaka, Y.; Otori, N.; Kojima, H. Advanced Endoscopic Endonasal Approach to the Pterygopalatine Fossa and Orbit: The Endoscopic Tri-port Approach. Journal of Neurological Surgery Part B: Skull Base 2021, 82, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Ranslow, A.N.; Richter, J.P.; Neuberger, T.; Van Valkenburgh, B.; Rumple, C.R.; Quigley, A.P.; Pang, B.; Krane, M.H.; Craven, B.A. Reconstruction and Morphometric Analysis of the Nasal Airway of the White-Tailed Deer ( Odocoileus virginianus ) and Implications Regarding Respiratory and Olfactory Airflow. The Anatomical Record 2014, 297, 2138–2147. [Google Scholar] [CrossRef]
- Oh, S.; Zelig, D.; Aalam, A.A.; Kurtzman, G.M. (2023). Case report: Utilization of Z-Point fixture “Trans-nasal” implants. Annals of Medicine & Surgery. [CrossRef]
- Sanchis, J.; Díaz, J. (2021). Accidental migration of dental implant into the nasal cavity: Spontaneous expulsion through the nose. Journal of Clinical and Experimental Dentistry, 1060; e1057–e1060. [Google Scholar] [CrossRef]
- Yeom, H.-G.; Huh, K.-H.; Yi, W.-J.; Heo, M.-S.; Lee, S.-S.; Choi, S.-C.; Kim, J.-E. Nasal cavity perforation by implant fixtures: Case series with emphasis on panoramic imaging of nasal cavity extending posteriorly. Head & Face Medicine 2023, 19, 37. [Google Scholar] [CrossRef]
- Biafora, M.; Bertazzoni, G.; Trimarchi, M. Maxillary Sinusitis Caused by Dental Implants Extending into the Maxillary Sinus and the Nasal Cavities. Journal of Prosthodontics 2014, 23, 227–231. [Google Scholar] [CrossRef]
- Arruda, J.A.; Silva, P.; Silva, L.; Álvares, P.; Silva, L.; Zavanelli, R.; Rodrigues, C.; Gerbi, M.; Sobral, A.P.; Silveira, M. Dental Implant in the Canalis Sinuosus: A Case Report and Review of the Literature. Case Reports in Dentistry 2017, 2017, 1–5. [Google Scholar] [CrossRef]
- Von Arx, T.; Lozanoff, S.; Sendi, P.; Bornstein, M.M. Assessment of bone channels other than the nasopalatine canal in the anterior maxilla using limited cone beam computed tomography. Surgical and Radiologic Anatomy 2013, 35, 783–790. [Google Scholar] [CrossRef]
- Manhães Júnior, L.R.C.; Villaça-Carvalho, M.F.L.; Moraes, M.E.L.; Lopes, S.L.P.D.C.; Silva, M.B.F.; Junqueira, J.L.C. (2016). Location and classification of Canalis sinuosus for cone beam computed tomography: Avoiding misdiagnosis. Brazilian Oral Research, 30. [CrossRef]
- Shelley, A.; Rushton, V.; Horner, K. Canalis sinuosus mimicking a periapical inflammatory lesion. British Dental Journal 1999, 186, 378–379. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, F.O.; Kassis, E.N.; Manzini, R. (2022). Molecular, cellular and surgical processes of osseointegration for dental implants: A systematic review. 3.
- Hamdy, R.M. (2013). Three-dimensional linear and volumetric analysis of maxillary sinus pneumatization.
- Iwanaga, J.; Wilson, C.; Lachkar, S.; Tomaszewski, K.A.; Walocha, J.A.; Tubbs, R.S. (2018). Clinical anatomy of the maxillary sinus: Application to sinus floor augmentation.
- Whyte, A.; Boeddinghaus, R. (2019). The maxillary sinus: Physiology, development and imaging anatomy. The Maxillary Sinus.
- Li, L.; Fu, Y.; Huang, S.; Lai, Z.; Ge, J. (2020). An Analysis of The Relationship between The Maxillary Molars and The maxillary sinus oor in Adult Patient Using Cone-beam Computed Tomography.
- Nogami, S.; Yamauchi, K.; Tanuma, Y.; Odashima, K.; Matsui, A.; Tanaka, K.; Takahashi, T. (2016). Removal of dental implant displaced into maxillary sinus by combination of endoscopically assisted and bone repositioning techniques: A case report.
- Çam, K.; Zengi̇N, A.Z. (2024). Evaluation of the Localization of Posterior Superi̇or Alveolar Artery and Infraorbital Foramen Originating From the Same Source by Using Conic Beam Computed Tomography. [CrossRef]
- Khalighi Sigaroudi, A.; Dalili Kajan, Z.; Rastgar, S.; Neshandar Asli, H. Frequency of different maxillary sinus septal patterns found on cone-beam computed tomography and predicting the associated risk of sinus membrane perforation during sinus lifting. Imaging Science in Dentistry 2017, 47, 261. [Google Scholar] [CrossRef] [PubMed]
- Raghav, M.; Karjodkar, F.; Sontakke, S.; Sansare, K. Prevalence of incidental maxillary sinus pathologies in dental patients on cone-beam computed tomographic images. Contemporary Clinical Dentistry 2014, 5, 361. [Google Scholar] [CrossRef]
- Assari, A.; Alotaibi, N.; Alajaji, M.A.; Alqarni, A.; Ali Alarishi, M. Characteristics of Maxillary Sinus Septa: A Cone-Beam Computed Tomography Evaluation. International Journal of Dentistry 2022, 2022, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hungerbühler, A.; Rostetter, C.; Lübbers, H.-T.; Rücker, M.; Stadlinger, B. Anatomical characteristics of maxillary sinus septa visualized by cone beam computed tomography. International Journal of Oral and Maxillofacial Surgery 2019, 48, 382–387. [Google Scholar] [CrossRef]
- Dworski, K.; Mazurek, M.; Domański, J. Posterior superior alveolar artery – an anatomical and clinical case report. Medical Journal of Cell Biology 2023, 11, 93–97. [Google Scholar] [CrossRef]
- Tofangchiha, M.; Hematzadeh, S.; Vali, M.E.; Ghonche, M.R.A.; Mirzadeh, M.; Reda, R.; Testarelli, L. Anatomical localization of posterior superior alveolar artery: A retrospective study by cone-beam computed tomography. Dental and Medical Problems 2022, 59, 407–412. [Google Scholar] [CrossRef]
- McDaniel, C.R.; Johnson, T.M.; Stancoven, B.W.; Lincicum, A.R. Distribution of the intraosseous branch of the posterior superior alveolar artery relative to the posterior maxillary teeth. Imaging Science in Dentistry 2024, 54, 121. [Google Scholar] [CrossRef]
- Radmand, F.; Razi, T.; Baseri, M.; Gavgani, L.F.; Salehnia, F.; Faramarzi, M. Anatomic evaluation of the posterior superior alveolar artery using cone-beam computed tomography: A systematic review and meta-analysis. Imaging Science in Dentistry 2023, 53, 177. [Google Scholar] [CrossRef]
- Tehranchi, M.; Taleghani, F.; Shahab, S.; Nouri, A. Prevalence and location of the posterior superior alveolar artery using cone-beam computed tomography. Imaging Science in Dentistry 2017, 47, 39. [Google Scholar] [CrossRef]
- Mardinger, O.; Abba, M.; Hirshberg, A.; Schwartz-Arad, D. Prevalence, diameter and course of the maxillary intraosseous vascular canal with relation to sinus augmentation procedure: A radiographic study. International Journal of Oral and Maxillofacial Surgery 2007, 36, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Hakiem Tawfieq, A.; Ali Hasan, H.; Abd Alrazaq Hassan, M.; Salah Khlfi, M. Localization of the position of vital anatomical structures in the lateral wall of maxillary sinus during different surgical intervention using cone beam computed tomography. Diyala Journal of Medicine 2023, 25, 61–71. [Google Scholar] [CrossRef]
- Al-Mahalawy, H.; Al-Aithan, H.; Al-Kari, B.; Al-Jandan, B.; Shujaat, S. Determination of the position of mental foramen and frequency of anterior loop in Saudi population. A retrospective CBCT study. The Saudi Dental Journal 2017, 29, 29–35. [Google Scholar] [CrossRef]
- Fishel, D.; Buchner, A.; Hershkowith, A.; Kaffe, I. Roentgenologic study of the mental foramen. Oral Surgery, Oral Medicine, Oral Pathology 1976, 41, 682–686. [Google Scholar] [CrossRef]
- Juodzbalys, G.; Wang, H.-L.; Sabalys, G. (2010). Anatomy of Mandibular Vital Structures. Part I: Mandibular Canal and Inferior Alveolar Neurovascular Bundle in Relation with Dental Implantology. Journal of Oral and Maxillofacial Research, 1. [CrossRef]
- Parnia, F.; Moslehifard, E.; Hafezeqoran, A.; Mahboub, F.; Mojaver-Kahnamoui, H. (2012). Characteristics of anatomical landmarks in the mandibular interforaminal region: A cone-beam computed tomography study. Medicina Oral Patología Oral y Cirugia Bucal, e420–e425. [CrossRef]
- Vásquez, C.A.P. (2019). Tesis para optar el título de Especialista en Radiología Bucal y Maxilofacial.
- Hidalgo, R.; Alejandro; San Pedro, V. ; Jaime. Relación del Foramen Lingual con Espinas Mentonianas y Evaluación del Conducto Lingual Mediante Radiografías Periapicales y Extraorales. International journal of odontostomatology 2010, 4, 295–302. [Google Scholar] [CrossRef]
- Pérez-Vásquez, A.; Vilma, R. CARACTERIZACIÓN DEL FORAMEN LINGUAL MANDIBULAR MEDIANTE TOMOGRAFÍA COMPUTARIZADA DE HAZ CÓNICO. Odontología Activa Revista Científica 2019, 4, 1–8. [Google Scholar] [CrossRef]
- Okumuş, Ö.; Dumlu, A. Prevalence of bifid mandibular canal according to gender, type and side. Journal of Dental Sciences 2019, 14, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, F.S.; Alegre, P. (2013). VARIAÇÕES NA ANATOMIA DA MANDÍBULA: BIFURCAÇÃO DO CANAL MANDIBULAR E DO DEFEITO ÓSSEO DE STAFNE.
- Hsu, J.-T.; Huang, H.-L.; Fuh, L.-J.; Li, R.-W.; Wu, J.; Tsai, M.-T.; Shen, Y.-W.; Tu, M.-G. Location of the Mandibular Canal and Thickness of the Occlusal Cortical Bone at Dental Implant Sites in the Lower Second Premolar and First Molar. Computational and Mathematical Methods in Medicine 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Chavarry, N.G.M. Dental implants placed buccally to the mandibular canal in molar regions with severe vertical bone loss: Case reports. Rio de Janeiro Dental Journal (Revista Científica Do CRO-RJ) 2019, 4, 107–113. [Google Scholar] [CrossRef]
- Angelopoulos, C.; Aghaloo, T. Imaging Technology in Implant Diagnosis. Dental Clinics of North America 2011, 55, 141–158. [Google Scholar] [CrossRef]
- Silva, I.M.D.C.C.; Freitas, D.Q.D.; Ambrosano, G.M.B.; Bóscolo, F.N.; Almeida, S.M. Bone density: Comparative evaluation of Hounsfield units in multislice and cone-beam computed tomography. Brazilian Oral Research 2012, 26, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Chugh, T.; Jain, A.K.; Jaiswal, R.K.; Mehrotra, P.; Mehrotra, R. Bone density and its importance in orthodontics. Journal of Oral Biology and Craniofacial Research 2013, 3, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, M.; Stefanelli, L.V.; Pacifici, A.; Pacifici, L.; Barbato, E. How Accurate Is CBCT in Measuring Bone Density? A Comparative CBCT-CT In Vitro Study. Clinical Implant Dentistry and Related Research 2014, 16, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Köseoğlu Seçgin, C.; Karslıoğlu, H. ; Özemre, Mö.; Orhan, K. (2021). Gray value measurement for the evaluation of local alveolar bone density around impacted maxillary canine teeth using cone beam computed tomography. Medicina Oral Patología Oral y Cirugia Bucal, e669–e675. [CrossRef]
- Genç, T.; Duruel, O.; Kutlu, H.; Dursun, E.; Karabulut, E.; Tözüm, T. Evaluation of anatomical structures and variations in the maxilla and the mandible before dental implant treatment. Dental and Medical Problems 2018, 55, 233–240. [Google Scholar] [CrossRef]
- Javed, F.; Romanos, G.E. Impact of Diabetes Mellitus and Glycemic Control on the Osseointegration of Dental Implants: A Systematic Literature Review. Journal of Periodontology 2009, 80, 1719–1730. [Google Scholar] [CrossRef]
- Madrid, C.; Sanz, M. (2009). What impact do systemically administrated bisphosphonates have on oral implant therapy? A systematic review. Clinical Oral Implants Research, 20, 87–95. [CrossRef]
- Ismail, A.; Shaddox, L.; Santamaria, M.; Sabbagh, M. (2022). Peri-implantitis: A Review to Simplify a Mystifying Disease. Medical Research Archives, 10. [CrossRef]
- Esau, T.; Puryer, J.; McNally, L.; O’Sullivan, D. Patient understanding and recall of risks and complications of dental implant treatment following informed consent. Faculty Dental Journal 2016, 7, 16–21. [Google Scholar] [CrossRef]
- Khongkhunthian, P.; Jomjunyong, K.; Reichart, A.P. Accuracy of Cone Beam Computed Tomography for Dental Implant Treatment Planning. Chiang Mai University Journal of Natural Sciences 2017, 16. [Google Scholar] [CrossRef]
- Jacobs, R.; Salmon, B.; Codari, M.; Hassan, B.; Bornstein, M.M. Cone beam computed tomography in implant dentistry: Recommendations for clinical use. BMC Oral Health 2018, 18, 88. [Google Scholar] [CrossRef]
- Oh, T.; Yoon, J.; Misch, C.E.; Wang, H. The Causes of Early Implant Bone Loss: Myth or Science? Journal of Periodontology 2002, 73, 322–333. [Google Scholar] [CrossRef]
- Arai, Y.; Takashima, M.; Matsuzaki, N.; Takada, S. (2024). Marginal bone loss in dental implants: A literature review of risk factors and treatment strategies for prevention. Journal of Prosthodontic Research, JPR_D_23_00223. [CrossRef]
- Song, D.; Shujaat, S.; De Faria Vasconcelos, K.; Huang, Y.; Politis, C.; Lambrichts, I.; Jacobs, R. Diagnostic accuracy of CBCT versus intraoral imaging for assessment of peri-implant bone defects. BMC Medical Imaging 2021, 21, 23. [Google Scholar] [CrossRef]
- Rydholm, H.; Von Corswant, C.; Denison, H.; Jensen, J.M.; Lehmann, A.; Ruth, M.; Söderlind, E.; Aurell-Holmberg, A. Reducing Adverse Effects During Drug Development: The Example of Lesogaberan and Paresthesia. Clinical Therapeutics 2016, 38, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Worthington, H.V. (2013). Interventions for replacing missing teeth: Antibiotics at dental implant placement to prevent complications. Cochrane Database of Systematic Reviews, 2019. [CrossRef]
- Hossein, K.; Dahlin, C.; Bengt, A. Influence of Different Prophylactic Antibiotic Regimens on Implant Survival Rate: A Retrospective Clinical Study. Clinical Implant Dentistry and Related Research 2005, 7, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Goodacre, C.J.; Bernal, G.; Rungcharassaeng, K. ; Kan. (2004). Common complications with implants and implant prostheses: What complications are associated with dental implants? Evidence-Based Dentistry, 5, 70–71. [CrossRef]
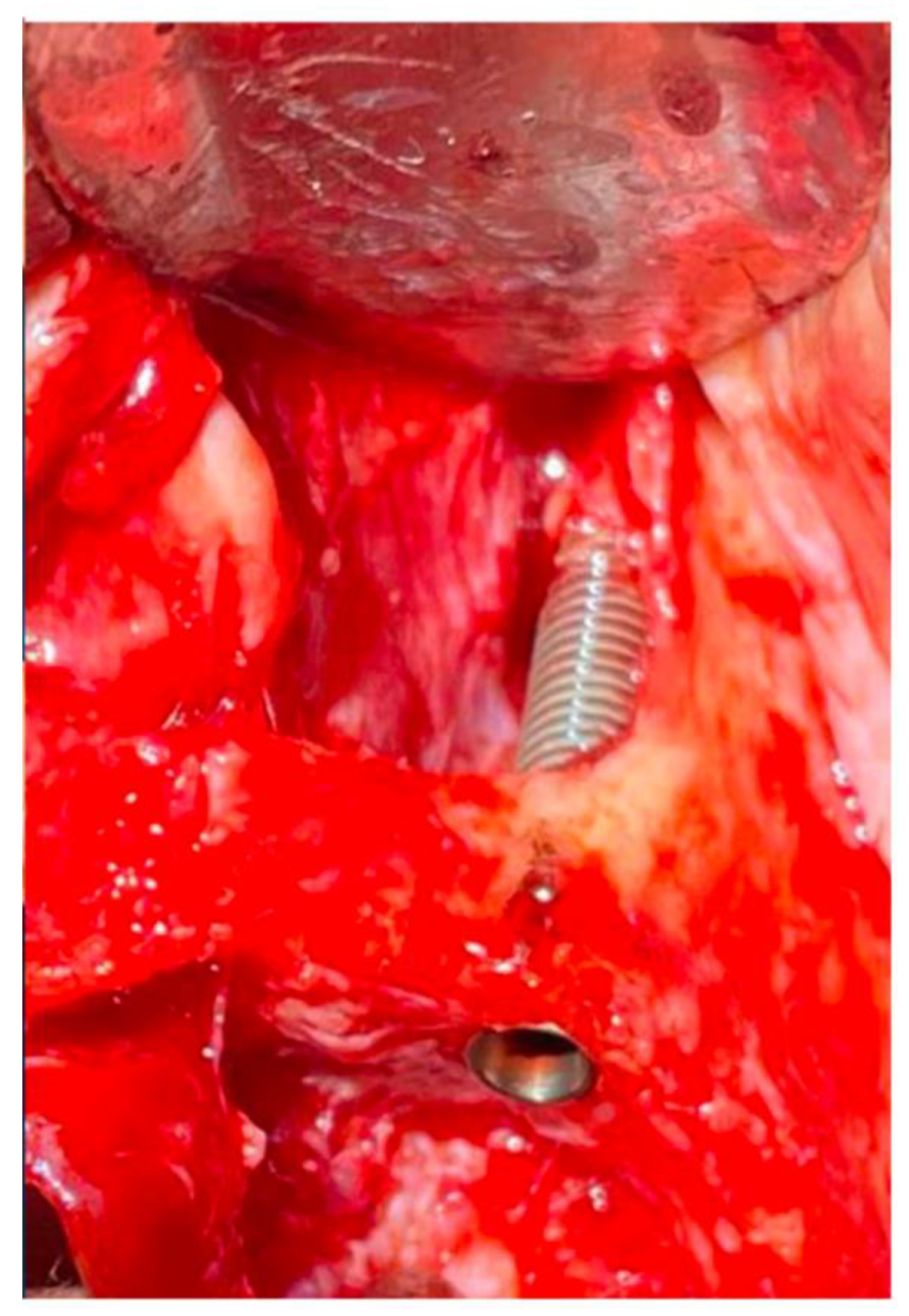
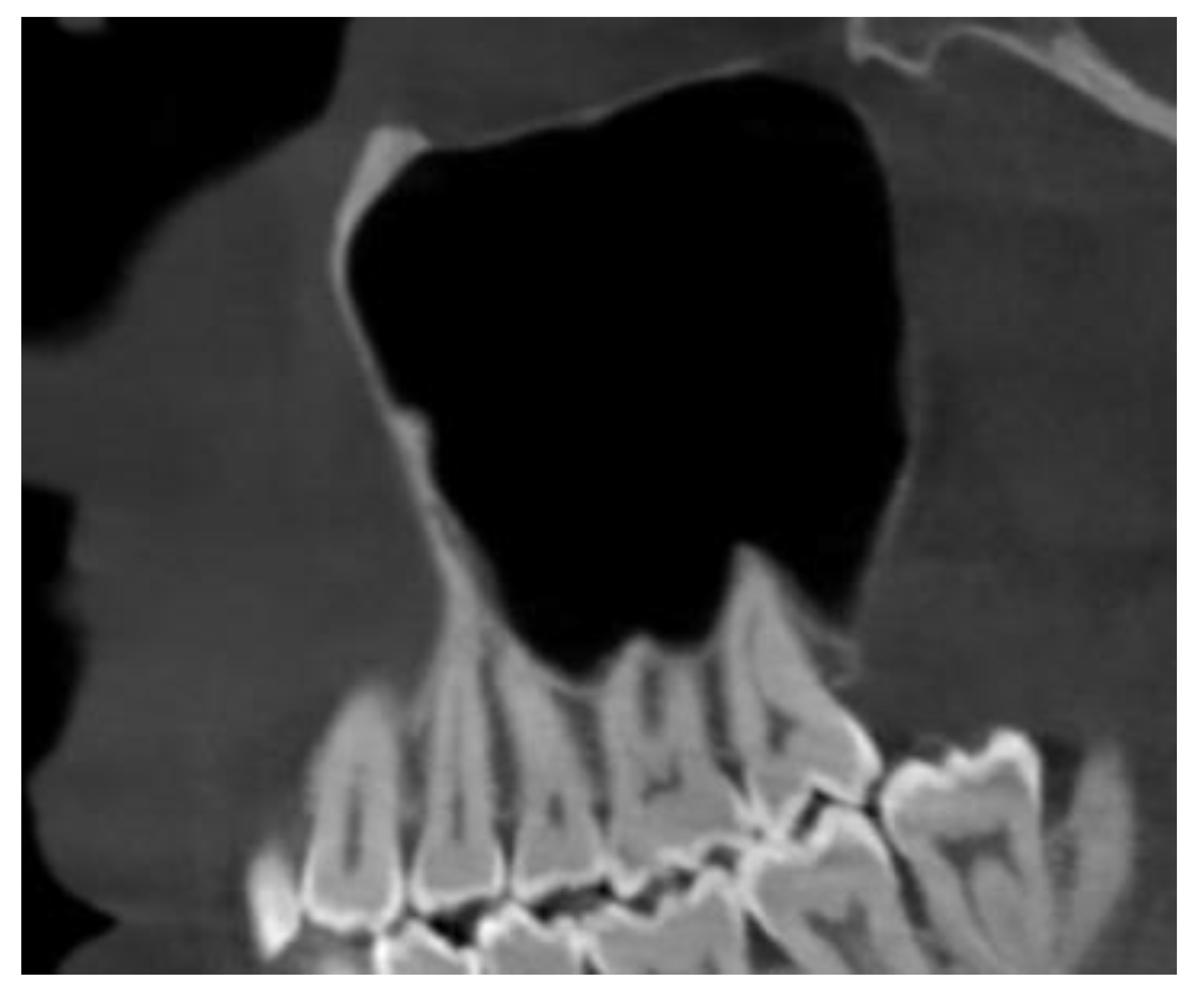
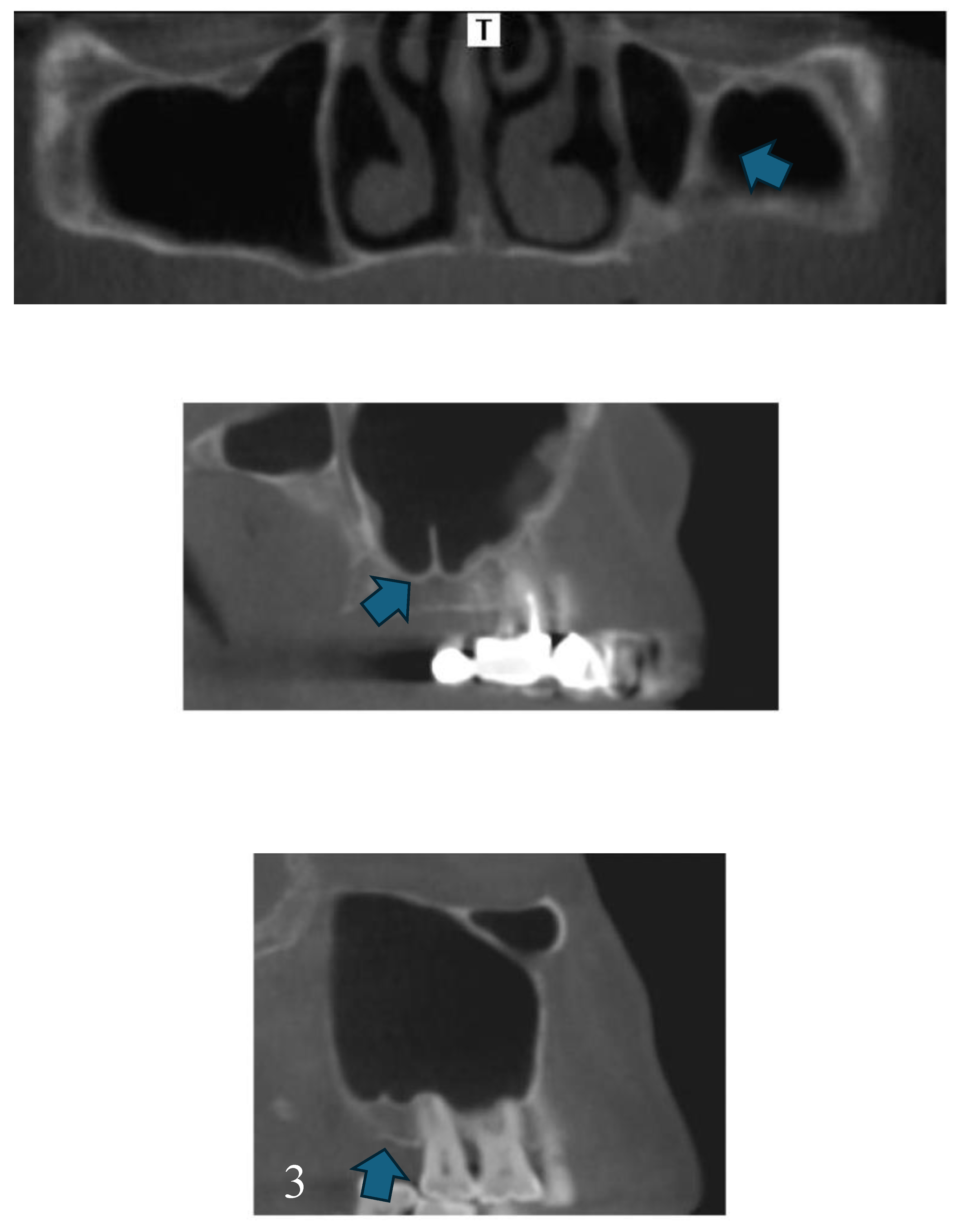
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




