Submitted:
09 October 2024
Posted:
10 October 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Results and Discussion
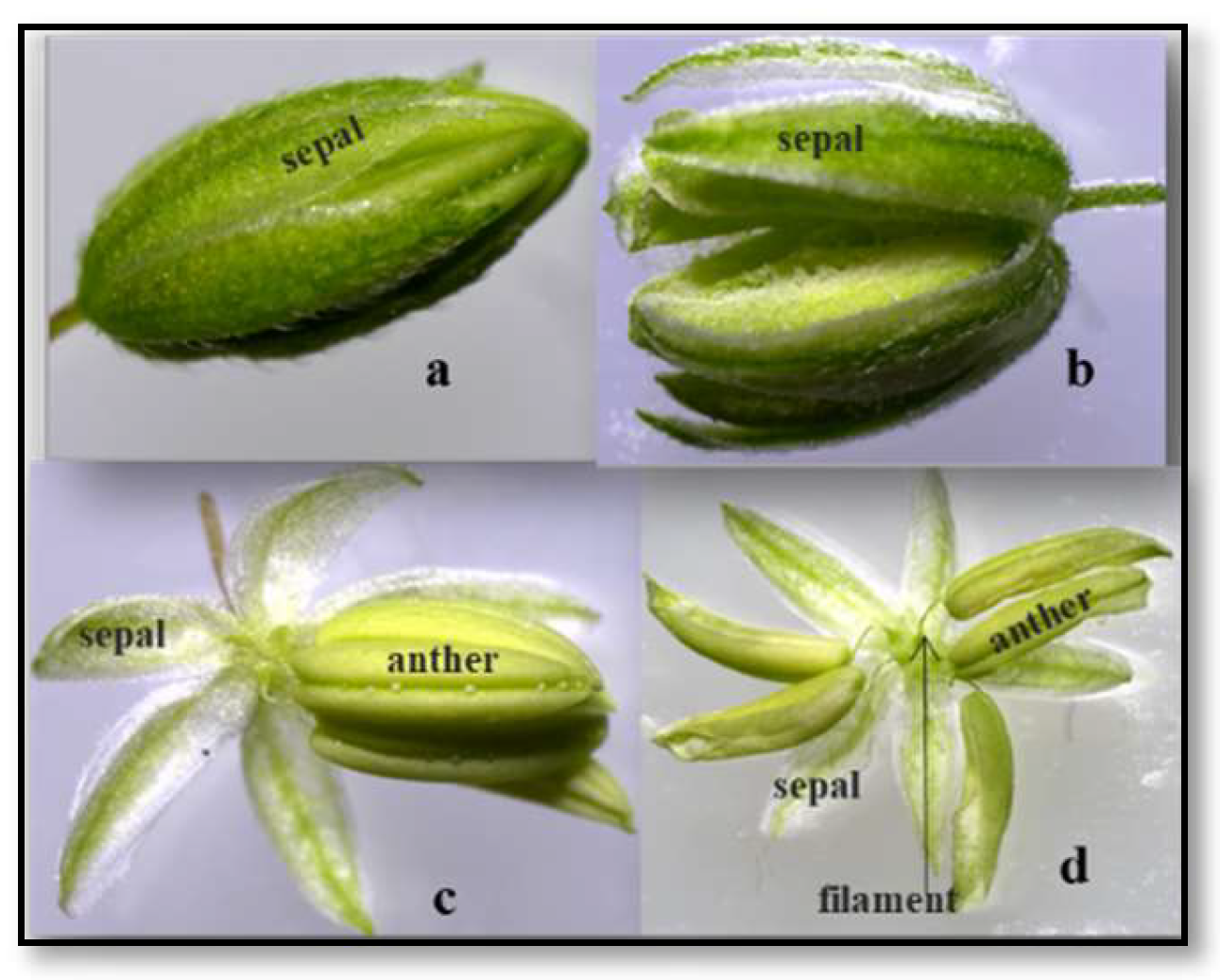
2.1. Floral Characteristics of the Hemp Plant
2.2. Anther Shape and Size
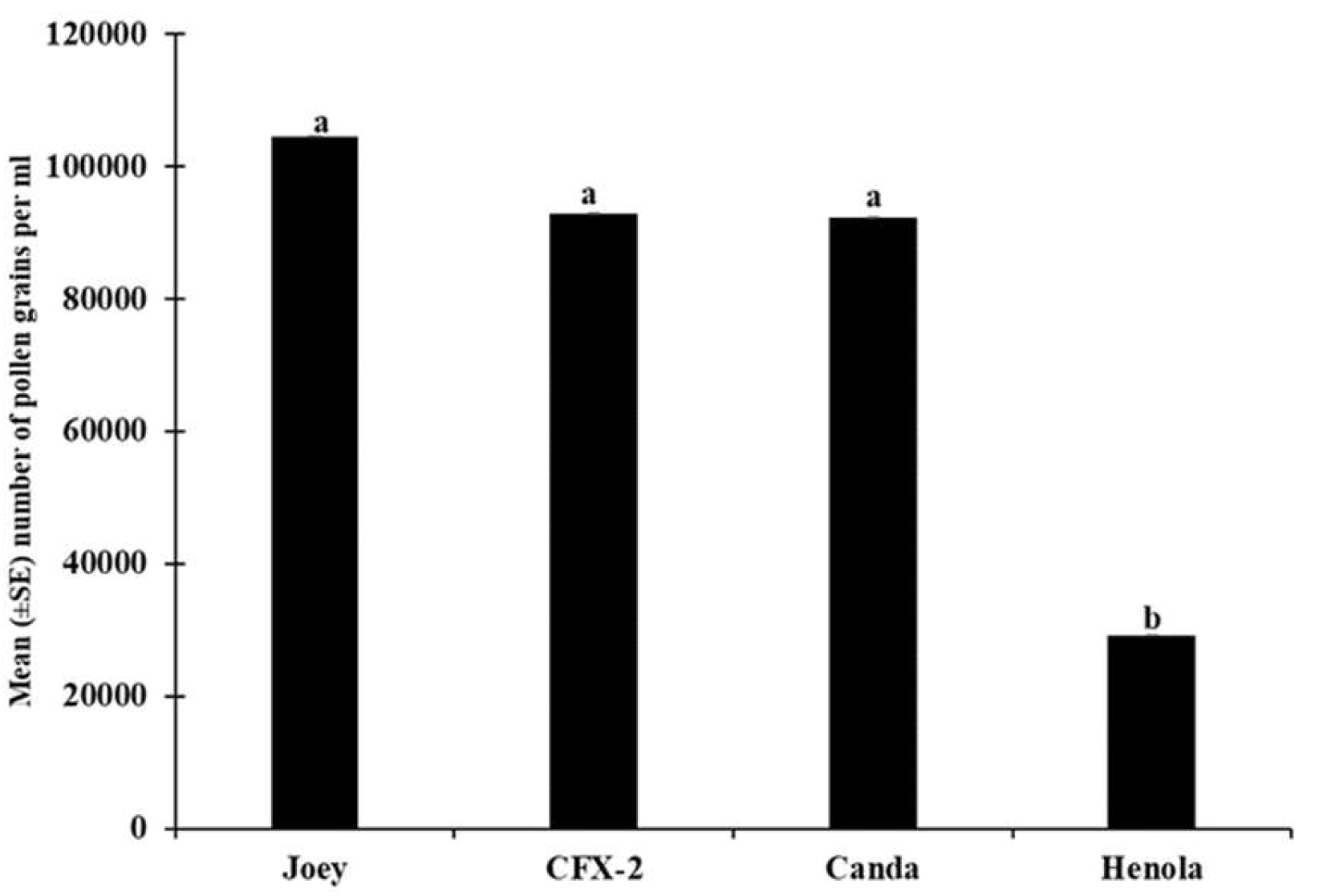
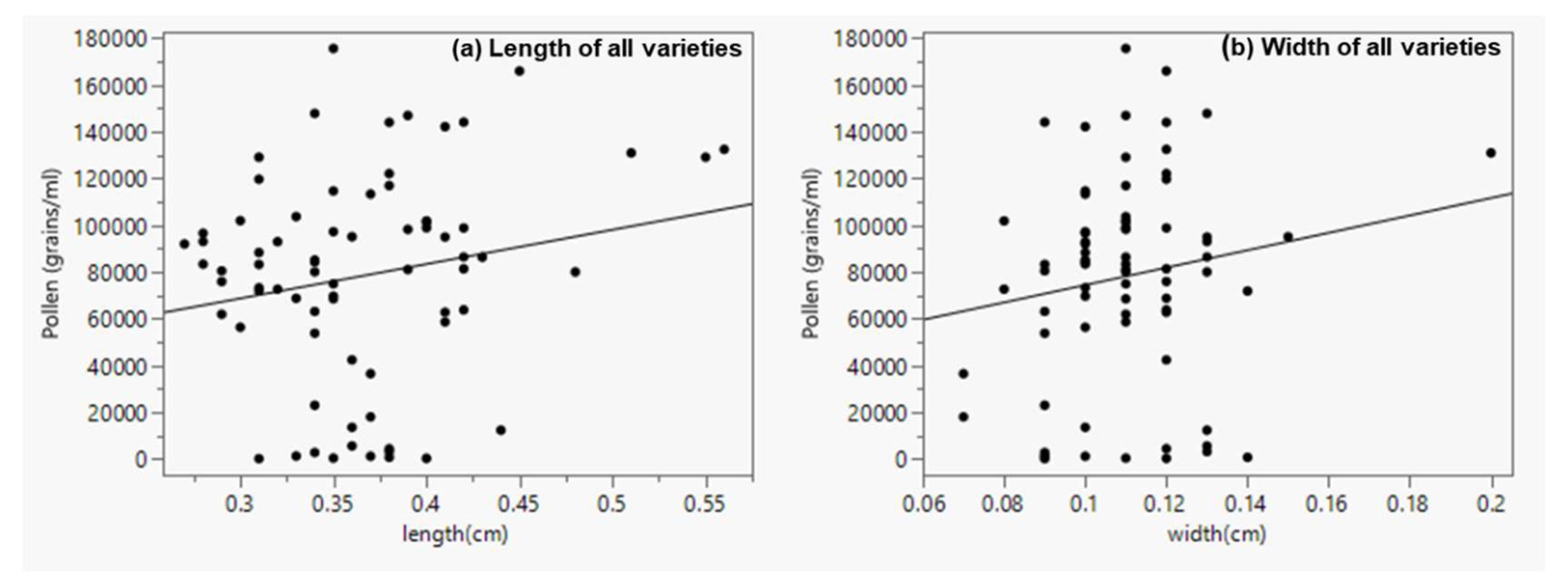
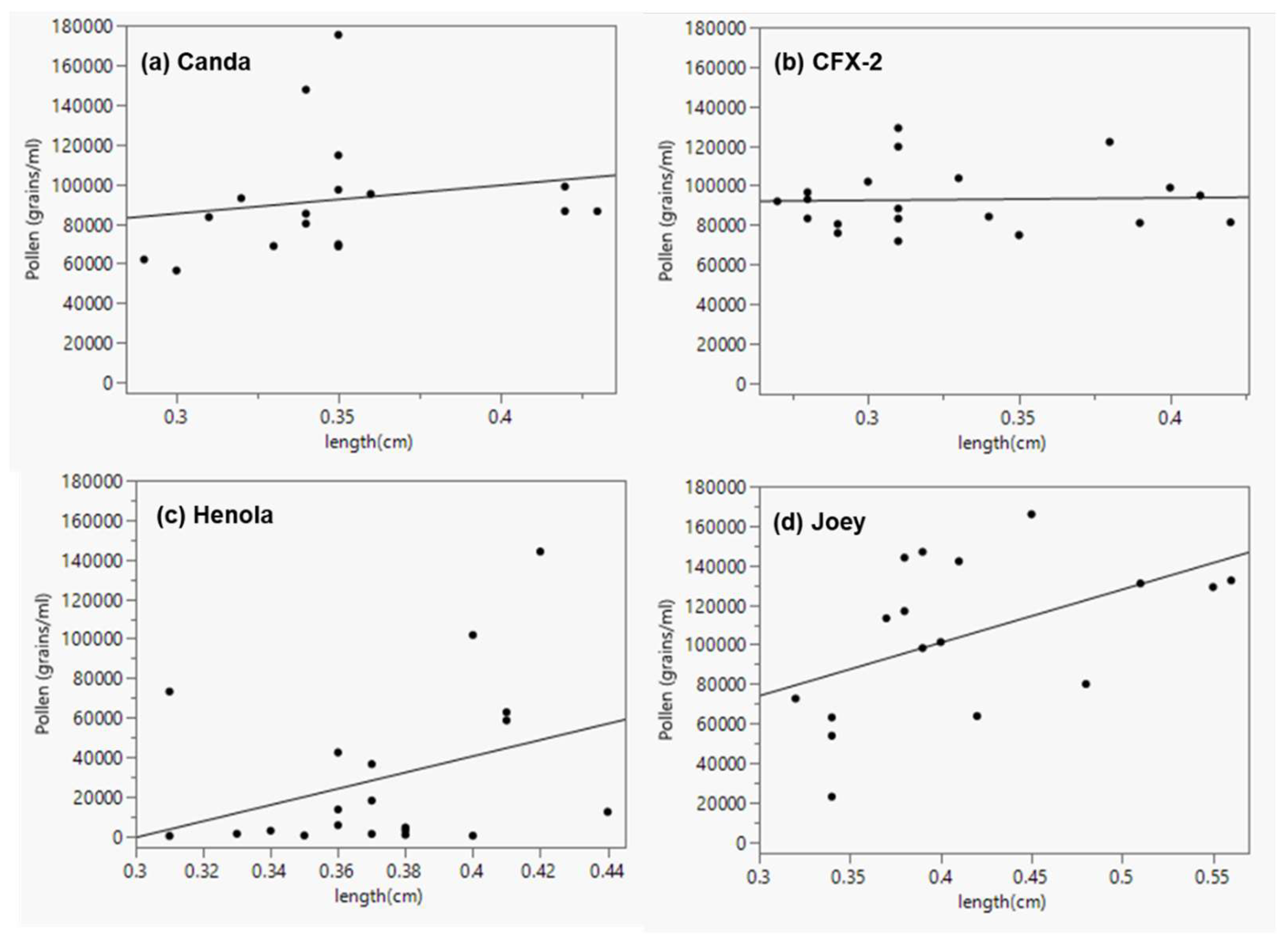
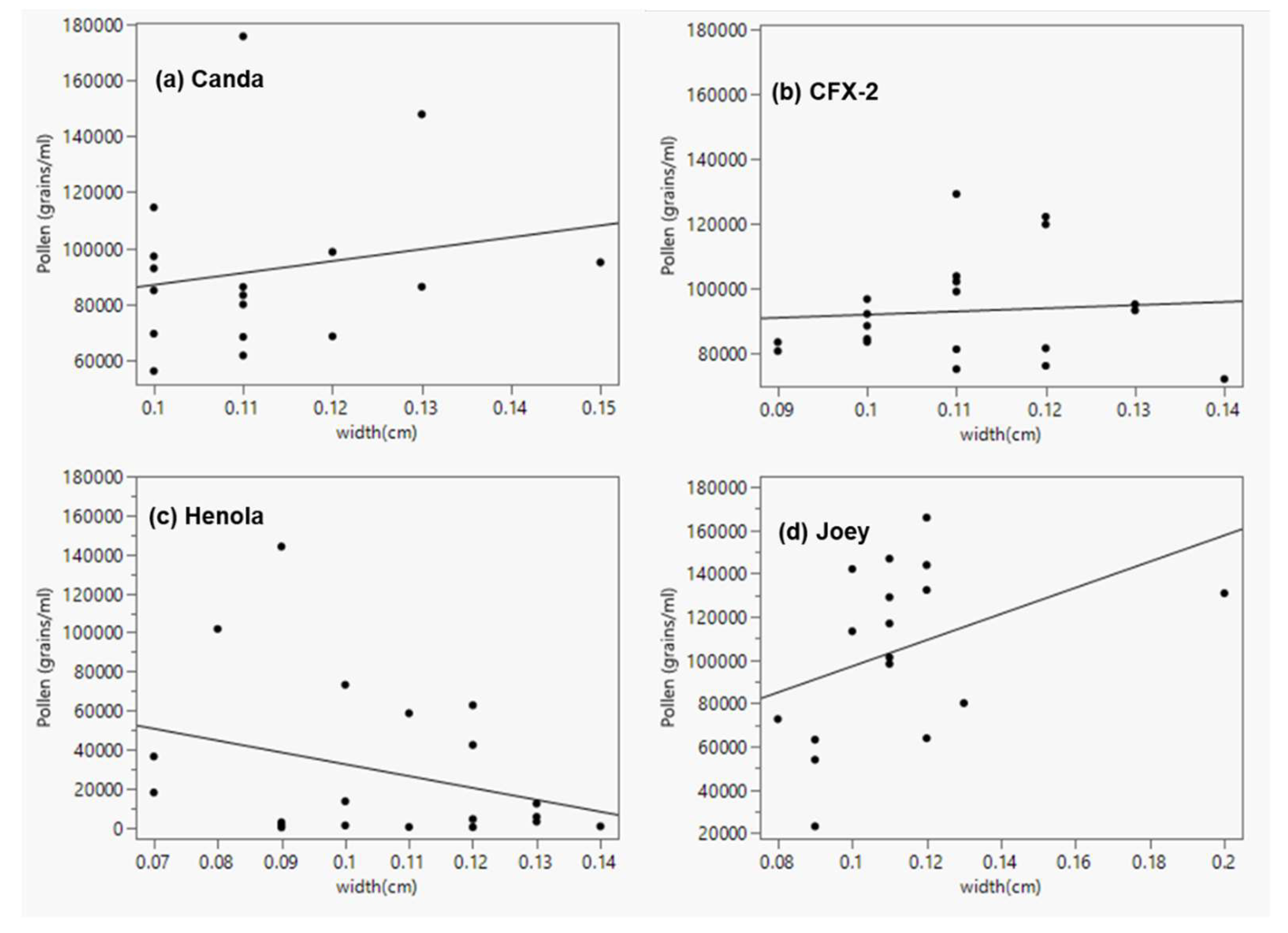
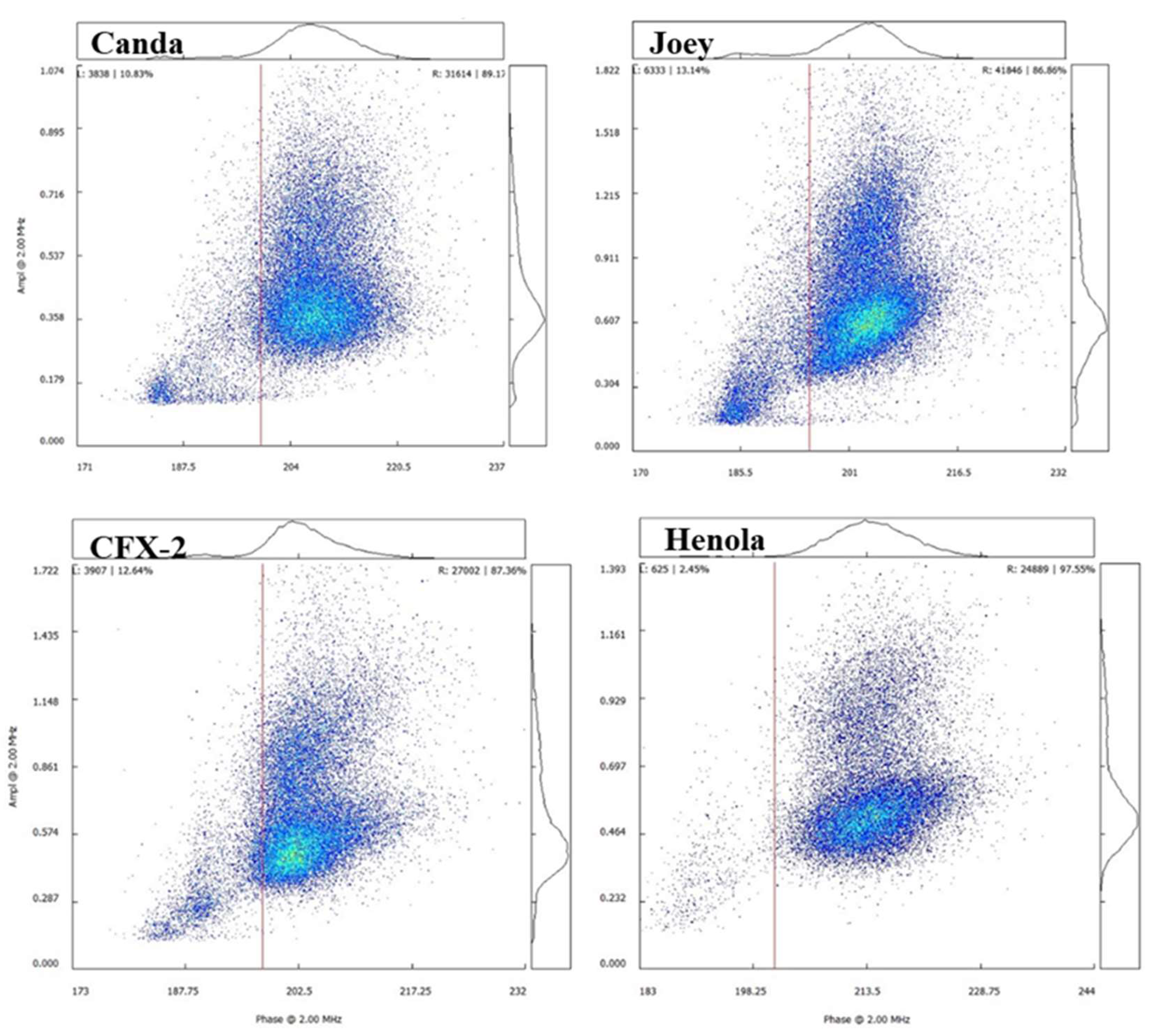
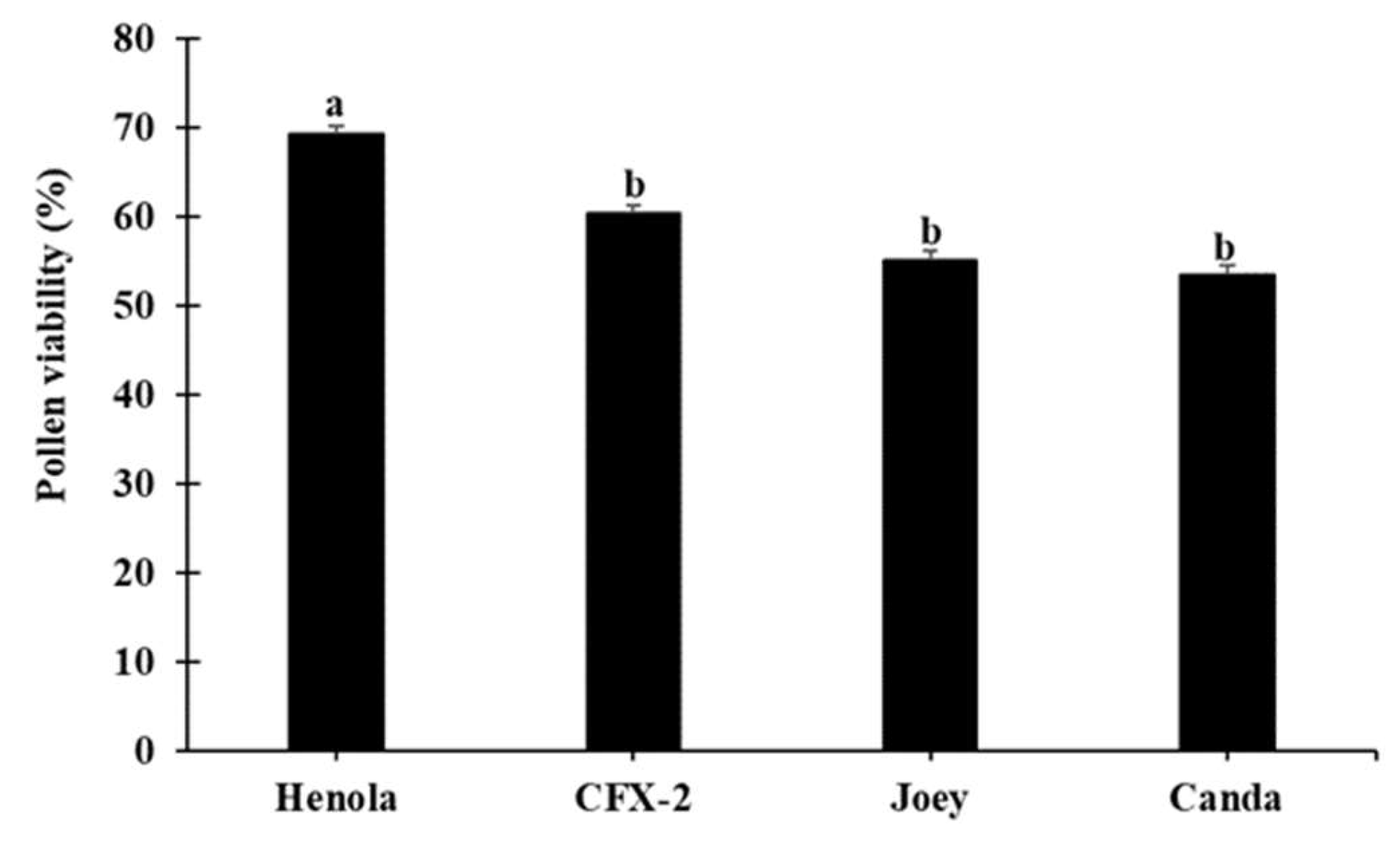
2.3. Pollen Shape, Size, Number, and Viability
3. Materials and Methods
3.1. Study Site and Plant Varieties
3.2. Pollen Viability
3.3. Pollen Staining and Measurement of Pollen Size
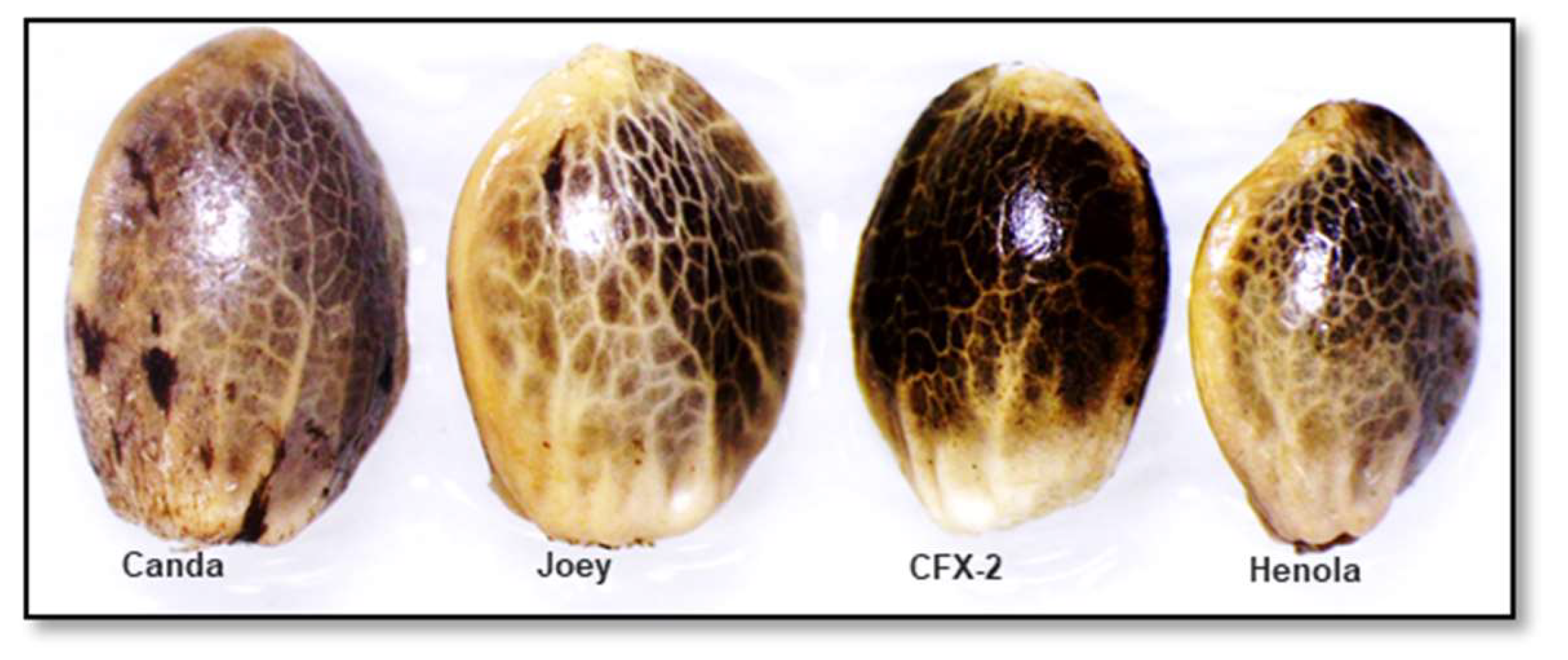
3.4. Flower and Seed Size
3.5. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amaducci, S.; Gusovious, H.J. Hemp - Cultivation, Extraction and Processing. In Industrial Applications of Natural Fibres: Structure, Properties and Technical Applications, Müssig, J., Ed.; John Wiley and Sons Ltd.: The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, United Kingdom, 2010, pp. 109- 134.
- Salentijn, E.M..; Zhang, Q.; Amaducci, S.; Yang, M.; Trindade, L.M. New developments in fiber hemp (Cannabis sativa L.) breeding. Ind. Crop. Prod. 2015, 68, 32-41.
- Schluttenhofer, C.; Yuan, L. (2017). Challenges towards revitalizing hemp: A multifaceted crop. Trends Plant Sci. 2017, 22, 917-929.
- Market Data Forecast. Global Industrial Hemp Market Size, Share, Trends, COVID-19 Impact & Growth Analysis Report—Segmented by Type, Application and Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa)—Industry Forecast (2022 to 2027). Available online: https://www.marketdataforecast.com/market-reports/industrial-hemp-market (accessed on 13 August 2024).
- Johnson, R. Defining Hemp: A Fact Sheet; Congressional Research Service: Washington, DC, USA, 2019.
- Malone, T.; Gomez, K. Hemp in the United States: A Case Study of Regulatory Path Dependence. Appl. Econ. Perspect. Policy 2019, 1–16.
- USDA-NASS. Agricultural Statistic 2023; National Hemp Report; USDA-NASS: Washington, DC, USA, 2023.
- Dingha, B.; Sandler, L.; Bhowmik, A.; Akotsen-Mensah, C.; Jackai, L.; Gibson, K.; Turco, R. Industrial Hemp Knowledge and Interest among North Carolina Organic Farmers in the United States. Sustainability 2019, 11, 2691.
- Kaur, G.; Kander, R. The Sustainability of Industrial Hemp: A Literature Review of Its Economic, Environmental, and Social Sustainability. Sustainability 2023, 15, 6457.
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The Seed of Industrial Hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935.
- Fiani, B.; Sarhadi, K.J.; Soula, M.; Zafar, A.; Quadri, S.A. Current application of cannabidiol (CBD) in the management and treatment of neurological disorders. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2020, 41, 3085–3098.
- Britch, S.C.; Babalonis, S.; Walsh, S.L. Cannabidiol: Pharmacology and therapeutic targets. Psychopharmacology 2021, 238, 9–28.
- Castillo-Arellano, J.; Canseco-Alba, A.; Cutler, S.J.; León, F. The Polypharmacological Effects of Cannabidiol. Molecules 2023, 28, 3271.
- Wang, X.; Zhang, H.; Liu, Y.; Xu, Y.; Yang, B.; Li, H.; Chen, L. An overview on synthetic and biological activities of cannabidiol (CBD) and its derivatives. Bioorg. Chem. 2023, 140, 106810.
- Chundawat, S.P.S.; Beckham, G.T.; Himmel, M.E.; Dale, B.E. Deconstruction of lignocellulosic biomass to fuels and chemicals. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 121-145.
- Hu, R.; Lim, J.K. Fabrication and mechanical properties of completely biodegradable hemp fiber reinforced polylactic acid composites. J. Compos. Mater. 2016, 41, 1655-1669.
- Angelini, L.G.; Tavarini S.; Candilo, M.D. Performance of new and traditional fiber hemp (Cannabis sativa L.) cultivars for novel application: Stem bark, and core yield and chemical composition. J. Nat. Fibers 2016,13, 238-252.
- Cherney, J.H.; Small, E. Industrial Hemp in North America: Production, Politics and Potential. Agronomy 2016, 6, 58.
- Zhao, J.; Xu, Y.; Wang, W.; Griffin, J.; Roozeboom, K.; Wang, D. Bioconversion of industrial hemp biomass for bioethanol production: A review. Fuel 2020, 281, 118725.
- Placido, D.F.; Lee, C.C. Potential of Industrial Hemp for Phytoremediation of Heavy Metals. Plants 2022, 11, 595.
- Pollastro, F.; Minassi, A.; Fresu, L.G. (2018). Cannabis phenolics and their bioactivities. Curr. Med. Chem. 2018, 25, 1160-1185.
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hempseed in food industry: Nutritional value, health benefits, and industrial applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 282-308.
- Chen, T.; He, J.; Zhang, J.; Zhang, H.; Qian, P.; Hao, J.; Li, L. Analytical Characterization of Hempseed (Seed of Cannabis sativa L.) Oil from Eight Regions in China. J. Diet. Suppl. 2010, 7, 117–129.
- House, J.D.; Neufeld, J.; Leson, G. Evaluating the Quality of Protein from Hemp Seed (Cannabis sativa L.) Products Through the Use of the Protein Digestibility-Corrected Amino Acid Score Method. J. Agric. Food Chem. 2010, 58, 11801–11807.
- Vonapartis, E.; Aubin, M.P.; Seguin, P.; Mustafa, A.F.; Charron, J.B. Seed composition of ten industrial hemp cultivars approved for production in Canada. J. Food Compos. Anal. 2015, 39, 8–12.
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance-A review. Life Sci. 2018, 203, 255–267.
- Alonso-Esteban, J.I.; González-Fernández, M.J.; Fabrikov, D.; Sánchez-Mata, M.; Torija-Isasa, E.; Guil-Guerrero, J.L. Fatty acids and minor functional compounds of hemp (Cannabis sativa L.) seeds and other Cannabaceae species. J. Food Compos. Anal. 2023, 115, 104962.
- Orlando, G.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Carradori, S.; Di Simone, S.; Ciferri, M.C.; Zengin, G.; Ak, G.; et al. Water Extract from Inflorescences of Industrial Hemp Futura 75 Variety as a Source of Anti-Inflammatory, Anti-Proliferative and Antimycotic Agents: Results from In Silico, In Vitro and Ex Vivo Studies. Antioxidants 2020, 9, 437.
- di Giacomo, V.; Recinella, L.; Chiavaroli, A.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Politi, M.; Antolini, M.D.; Acquaviva, A.; et al. Metabolomic Profile and Antioxidant/Anti-Inflammatory Effects of Industrial Hemp Water Extract in Fibroblasts, Keratinocytes and Isolated Mouse Skin Specimens. Antioxidants 2021, 10, 44.
- Alonso-Esteban, J.I.; Pinela, J.; Ćirić, A.; Calhelha, R.C.; Soković, M.; Ferreira, I.C.F.R.; Barros, L.; Torija-Isasa, E.; Sánchez-Mata, M.d.C. Chemical composition and biological activities of whole and dehulled hemp (Cannabis sativa L.) seeds. Food Chem. 2022, 374, 131754.
- Serventi, L.; Flores, G.A.; Cusumano, G.; Barbaro, D.; Tirillini, B.; Venanzoni, R.; Angelini, P.; Acquaviva, A.; Di Simone, S.C.; Orlando, G.; et al. Comparative Investigation of Antimicrobial and Antioxidant Effects of the Extracts from the Inflorescences and Leaves of the Cannabis sativa L. cv. strawberry. Antioxidants 2023, 12, 219.
- Small, E.; Antle, T. A Preliminary Study of Pollen Dispersal in Cannabis sativa in Relation to Wind Direction. J. Ind. Hemp 2003, 8, 37-50.
- Kurtz, L.E.; Brand, M.H.; Lubell-Brand, J.D. Production of tetraploid and triploid hemp. HortSci. 2020, 55, 1703-1707.
- Gómez-Mena, C.; Honys, D.; Datla, R.; Testillano, P.S. Advances in pollen research: biology, biotechnology, and plant breeding applications. Front. Plant Sci. 2022, 13, 876502.
- Todd, J.; Song, H.; Van Acker, R. Does pollination alter the cannabinoid composition and yield of extracts from hemp (Cannabis sativa L. cv. Finola) flowers?. Ind. Crop. and Prod. 2022, 183, 114989.
- Ushiyama, T.; Du, M.; Inoue, S.; Shibaike, H.; Yonemura, S.; Kawashima, S.; Amano, K. Three-dimensional prediction of maize pollen dispersal and cross-pollination, and the effects of windbreaks. Environ. Biosafety Res. 2009, 8, 183-202.
- Meier, C.; Mediavilla ,V. Factors influencing the yield and the quality of hemp essential oil. J. Int. Hemp Assoc. 1998, 5, 16–20.
- Capital Press. Hemp boom spurs cross-pollination disputes. https://www.capitalpress.com/state/oregon/hemp-boom-spurs-cross-pollination-disputes/article_efd1e99c-c903-11e9-8bdd-73e58f5946b5.html Available online (accessed on 06 July 2024).
- National Cannabis Industry Association (NCIA). Cross-pollination poised to prompt litigation in light of new USDA hemp rules. Available online https://thecannabisindustry.org/member-blog-cross-pollination-poised-to-prompt-litigation-in-light-of-new-usda-hemp-rules/ (accessed on 06 July 2024).
- Chabbert, B.; Kurek, B.; Beherec, O. Physiology and botany of industrial hemp. In Hemp Industrial Production and Uses; Bouloc, P., Ed.; Cabi Publication: Wallingford, UK, 2013; pp. 27–47.
- Amaducci, S.; Scordia, D.; Liu, F.H.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S.L. Key cultivation techniques for hemp in Europe and China. Ind. Crop. Prod. 2015, 68, 2-16.
- Leme, F.M.; Schönenberger, J.; Staedler, Y.M.; Teixeira, S.P. Comparative floral development reveals novel aspects of structure and diversity of flowers in Cannabaceae. Bot. J. Linn. Soc. 2020,193, 64–83.
- Hesami, M.; Pepe, M.; Jones, A.M.P. Morphological Characterization of Cannabis sativa L. Throughout Its Complete Life Cycle. Plants 2023, 12, 3646.
- Renner, S.S.; Ricklefs, R.E. “Dioecy and its correlates in the flowering plants.” Am. J. Bot. 1995, 82, 596-606.
- Frankowski, J.; Wawro, A.; Batog, J.; Burczyk, H. New Polish Oilseed Hemp Cultivar Henola - Cultivation, Properties and Utilization for Bioethanol Production. J. Nat. Fibers. 2021, 19, 7283–7295.
- Fernández-Illescas, F.; Nieva, J.; Márquez-García, B.; Muñoz-Rodríguez, A. Pollen production in halophytic species of the Chenopodiaceae in a Mediterranean marsh. Grana. 2010, 49, 300–307.
- Faegri, K.; Iverson, J.; Kaland, P.E.; Krzywinski, K. Textbook of pollen analysis, 4th ed.; John Wiley and Sons: Chichester, UK, 1989; 328.
- Bhowmik, S.; Datta, B.K. Pollen production in relation to ecological class of some hydrophytes and marsh plants. Am. J. Plant Sci. 2013, 4, 324-332.
- Milatović, D.; Nikolić, D.; Janković, S.; Janković, D.; Stanković, J. Morphological characteristics of male reproductive organs in some walnut (Juglans regia L.) genotypes. Sci. Hortic. 2020, 272, 109587.
- Pinheiro-Costa, B.K.; Mesquita-Neto, J.N.; Rego, J.O.; Schlindwein, C. Trade off between quantity and size of pollen grains in the heterandrous flowers of Senna pendula (Fabaceae). Acta Bot. Bras. 2018, 32, 446-453.
- Trevizan, R.; Caetano,A.P.S.; Brito, V.L.G.; Oliveira, P.E.; Telles, F.J. Stamen and pollen heteromorphism linked to the division of labour in Melastomataceae species, Flora. 2023, 305, 152315.
- Hao, K.; Tian, Z.X.; Wang, Z.C.; Huang, S.Q. Pollen grain size associated with pollinator feeding strategy. Proc. Biol. Sci. 2020, 287, 20201191.
- Dingha, B.N.; Jackai, L.E. Chemical Composition of Four Industrial Hemp (Cannabis sativa L.) Pollen and Bee Preference. Insects 2023, 14, 668.
- Ackerman, J.D. Abiotic pollen and pollination: ecological, functional, and evolutionary perspectives. Plant Syst. Evol. 2000, 222, 167–185.
- Floraflex. Hemp Farming Regulations and Licensing: Navigating Legal Requirements. Available online: https://floraflex.com/default/blog/post/hemp-farming-regulations-and-licensing-navigating-legal-requirements (accessed August 16 2024).
- Cabezudo, B.; Recio, M.; Sánchez-Laulhé, J.M.; Trigo, M.D.M.; Toro, F.J.; Polvorinos, F. Atmospheric transportation of marihuana pollen from North Africa to the southwest of Europe. Atmos. Environ. 1997, 31, 3323-3328.
- Brunet, Y.; Foueillassar, X.; Audran, A.; Garrigou, D.; Dayau, S.; Tardieu, L. Evidence for long-range transport of viable maize pollen. In 1st European Conference on the Coexistence of Genetically Modified Crops with Conventional and Organic Crops, Boelt, B., Slagelse, Eds.; Danish Institute of Agricultural Sciences: Helsingor, Denmark, 2003; pp. 74–76.
- Hofmann, F.; Epp, R.; Kruse, L. et al. Monitoring of Bt-Maize pollen exposure in the vicinity of the nature reserve Ruhlsdorfer Bruch in northeast Germany 2007 to 2008. Environ. Sci. Eur. 2010, 22, 229–251.
- Sieracka, D.; Zaborowicz, M.; Frankowski, J. Identification of Characteristic Parameters in Seed Yielding of Selected Varieties of Industrial Hemp (Cannabis sativa L.) Using Artificial Intelligence Methods. Agriculture 2023, 13, 1097.
- Impe, D.; Reitz, J.; Köpnick, C.; Rolletschek, H.; Börner, A.; Senula, A.; Nagel, M. Assessment of pollen viability for wheat. Front. Plant Sci. 2020, 10, 1588.
- Teleszko, M.; Zając, A.; Rusak, T. Hemp Seeds of the Polish ‘Bialobrzeskie’ and ‘Henola’ Varieties (Cannabis sativa L. var. sativa) as Prospective Plant Sources for Food Production. Molecules 2022, 27, 1448.
- Stramkale, V.; Morozova, I.; Černova, L.; Stramkalis, A. Industrial Hemp Varieties Productivity Potential in the Latvian Climatic Conditions. In Proceedings of the 14th International Scientific and Practical Conference. 15-16 June 2023.
- Lan, Y.; Zha, F.; Peckrul, A.; Hanson, B.; Johnson, B.; Rao, J.; Chen, B. Genotype x Environmental Effects on Yielding Ability and Seed Chemical Composition of Industrial Hemp (Cannabis sativa L.) Varieties Grown in North Dakota, USA. J. Am. Oil. Chem. Soc. 2019, 96, 1417-1425.
- Bajwa, P.; Singh, S.; Singh, M.; Kafle, A.; Parkash, V.; et al. Assessing the production potential of industrial hemp in the semi-arid west Texas. Technol. Agron. 2023, 3,17.
- Matthews, F.R.; Bramlett, D.L. Pollen Quantity and Viability Affect Seed Yields from Controlled Pollinations of Loblolly Pine. South. J. Appl. For. 1986, 10, 78-80.
- Moon, Y.; Cha, Y.; Lee, J.; Kim, K.; Kwon, D.; Kang, Y. Investigation of Suitable Seed Sizes, Segregation of Ripe Seeds, and Improved Germination Rate for the Commercial Production of Hemp Sprouts (Cannabis sativa L.). J. Sci. Food Agric. 2020, 100, 2819-2827.
- Gimeno-Martínez, D.; Igual, M.; García-Segovia, P.; Martínez-Monzó, J.; Navarro-Rocha, J. Characterisation of the Fat Profile of Different Varieties of Hemp Seeds (Cannabis sativa L.) for Food Use. Biol. Life Sci. Forum 2023, 26, 89.
- Cheung, K.C.; Di Berardino, M.; Schade-Kampmann, G.; Hebeisen, M.; Pierzchalski, A.; Bocsi, J.; Mittag, A.; Tárnok, A. Microfluidic impedance-based flow cytometry. Cytometry A 2010, 77, 648-66.
- Xu Y, Xie X, Duan Y, Wang L, Cheng Z, Cheng J. A review of impedance measurements of whole cells. Biosens. Bioelectron. 2016, 77, 824-36.




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




