1. Introduction
Intracranial abscesses in children present significant clinical challenges due to their low incidence and nonspecific symptoms, with reported rates of approximately 0.3–1.8 per 100,000 inhabitants annually [
1,
2,
3]. These life-threatening infections can arise from various risk factors, including specific infections, compromised immune systems, and head trauma [
3,
4]. Due to the diverse presentation of symptoms—ranging from neurological deficits and cephalalgia to altered mental status, seizures, and gastrointestinal disturbances such as nausea and vomiting [
1,
2,
5]—the timely diagnosis and management of intracranial abscesses in children remains difficult.
Diagnostic protocols typically involve a combination of laboratory investigations and neuroimaging techniques, particularly computed tomography (CT) and magnetic resonance imaging (MRI) [
4,
6]. The correct identification of the underlying pathogens—commonly streptococci, staphylococci, and anaerobic bacteria—can further complicate the diagnostic process [
4,
6]. Optimal management of these abscesses requires a comprehensive approach that includes anti-infective therapy, neurosurgical intervention, and supportive care. Conservative treatment options may be applicable for smaller or multiple abscesses, with a standard antibiotic regimen often including third-generation cephalosporins and metronidazole [
5,
7,
8]. Furthermore, incorporating agents such as flucloxacillin or vancomycin may be essential for addressing potential methicillin-sensitive Staphylococcus aureus (MSSA) or methicillin-resistant Staphylococcus aureus (MRSA) [
7]. The overall prognosis is contingent upon the severity of the infection, the presence of comorbid conditions, and the swiftness of treatment initiation [
6].
Despite existing literature, the current understanding of intracranial abscesses in pediatric populations remains inadequate, primarily due to the rarity of cases and the absence of robust clinical trials [
9,
10]. This study seeks to bridge this gap by conducting a retrospective analysis of nine pediatric cases of intracranial abscesses treated at a single center in Hamburg, Germany. The primary aim is to statistically evaluate potential associations among clinical features within this cohort, thereby identifying specific disease patterns that may enhance clinical decision-making and facilitate expedited management strategies for this serious condition. This study provides meaningful significant associations between clinical features, delineating specific disease patterns for children with intracranial abscesses.
2. Materials and Methods
2.1. Patient Selection
Through a digital data query from December 2022 to May 2023, all potential pediatric patients under the age of 18 years were identified using the complete set of codes from the International Classification of Diseases Version 10 (ICD-10) that pertain to (potential) intracranial infections and abscesses, with the specific exclusion of cases involving meningitis and meningoencephalitis. The relevant ICD-10 codes are presented in
Supplementary Table S1. Furthermore, to ensure the inclusion of any potentially misclassified patients, all individuals under the age of 18 who underwent neurosurgical procedure were also included in the study cohort. A total of nine cases of intracranial infections were analyzed from eight patients. Comprehensive epidemiological and clinical information was extracted from the digital patient’s chart, focusing on factors such as age, sex, clinical presentation, pathogenesis, pathogens, pathogen isolation, treatment regimen, and anatomical region for further investigation.
2.2. MRI Imaging
All cMRI scans were performed by a 3 Tesla magnetic resonance tomogram (
Figure 2).
2.3. Statistics
Descriptive statistics and data visualization were performed in GraphPad Prism 10. For statistical feature correlation analysis, RStudio 2022.07.2+576 was used and afterwards visualized by GraphPad Prism 10 (
Figure 1). The data was collected in a table and converted to a matrix nominal-binary coded as 0 (not present) and 1 (present) and all combinations of two features were iterated. For each combination, a contingency table was created. First, the Cramer’s V effect size was calculated for all combinations of two features (
Supplementary Table S2). Then, the Fisher’s exact test was performed on the contingency table (
Supplementary Table S3). The p-value was checked for significancy (
Figure 3a). Feature combinations with a Cramer’s V value equal or higher than 0.7 are considered as very strong correlation [
11,
12]. After that, a correlation plot based on the Cramer’s V value of every possible feature combination was created (white = 0, blue = 1). Significant feature combinations of Fisher’s exact test were highlighted with a red rectangle (
Figure 3b).
Figure 1.
Descriptive Analysis. (a) Sex distribution: 55,56% were male and 44,44% female. (b) Age distribution in years: between the age of 2 years to 15 years with a mean age of 10,22 years. (c) Clinical symptoms distribution: headache (15.79%), seizures (10.53%), dizziness (5.26%), meningism (5.26%), ataxia (5.26%) and vomiting (5.26%) which can be summarized as neurological symptoms. Further symptoms were local pain (10.53%) and local mastoiditis (10.53%) which can be summarized as local symptoms. Frontal swelling (10.53%) and orbital swelling (5.26%) which can be described as swelling symptoms, and fever (15.79%). Neurological symptoms make up to 47.36% of all symptoms, while local symptoms contribute 21.06%. Swelling symptoms and fever both make up to 15.79% of all symptoms. (d) Distribution of pathogenesis: 77.78% sinusitis, 11.11% mastoiditis and 11.11% otitis. (e) Outcome: 66.67% of all cases showed an outcome ad integrum, 22.22% a clinical residuum such as mild flaccid paralysis of the left side and small scotoma superior et inferior on the right side and 11.11% of all cases had a relapse. (f) Anatomical region: 55.56% were located frontal and frontobasal, 33.33% temporal and 11.11% cerebellar (11.11%). (g) Isolation of pathogen material: 84.62% of the abscess material could be isolated from the brain and in 15.38% by PCR. (h) Isolated pathogens: Streptococcus anginosus (30.77%), Streptocuccus pyogenes (15.38%), Coagulase-negative staphylococci (CoNS) (15.38%), Methicillin-sensitive Staphylococcus aureus (MSSA) (7.69%), Prevotella nigrescens (7.69%), Eikenella corrodens (7.69%), Acinetobacter baumanii (7.69%) and Aggregatibacter aphrophilus (7.69%). Hence, streptococci were the most common pathogens (46.15%), followed by anaerobic bacteria (30.76%) and staphylococci (23.07%). There was no case in which MRSA was isolated. (i) Isolated pathogens and antibiotic treatment: regarding at all isolated pathogen results (none, mono- and polybacterial results), 88.88% of pathogens were treated with a 3rd generation cephalosporin, 77.77% with metronidazole, 55.55% with vancomycin, 22.22% with both meropenem and flucloxacillin and 11.11% with both amoxicillin/clavulanic acid as well as clindamycin.
Figure 1.
Descriptive Analysis. (a) Sex distribution: 55,56% were male and 44,44% female. (b) Age distribution in years: between the age of 2 years to 15 years with a mean age of 10,22 years. (c) Clinical symptoms distribution: headache (15.79%), seizures (10.53%), dizziness (5.26%), meningism (5.26%), ataxia (5.26%) and vomiting (5.26%) which can be summarized as neurological symptoms. Further symptoms were local pain (10.53%) and local mastoiditis (10.53%) which can be summarized as local symptoms. Frontal swelling (10.53%) and orbital swelling (5.26%) which can be described as swelling symptoms, and fever (15.79%). Neurological symptoms make up to 47.36% of all symptoms, while local symptoms contribute 21.06%. Swelling symptoms and fever both make up to 15.79% of all symptoms. (d) Distribution of pathogenesis: 77.78% sinusitis, 11.11% mastoiditis and 11.11% otitis. (e) Outcome: 66.67% of all cases showed an outcome ad integrum, 22.22% a clinical residuum such as mild flaccid paralysis of the left side and small scotoma superior et inferior on the right side and 11.11% of all cases had a relapse. (f) Anatomical region: 55.56% were located frontal and frontobasal, 33.33% temporal and 11.11% cerebellar (11.11%). (g) Isolation of pathogen material: 84.62% of the abscess material could be isolated from the brain and in 15.38% by PCR. (h) Isolated pathogens: Streptococcus anginosus (30.77%), Streptocuccus pyogenes (15.38%), Coagulase-negative staphylococci (CoNS) (15.38%), Methicillin-sensitive Staphylococcus aureus (MSSA) (7.69%), Prevotella nigrescens (7.69%), Eikenella corrodens (7.69%), Acinetobacter baumanii (7.69%) and Aggregatibacter aphrophilus (7.69%). Hence, streptococci were the most common pathogens (46.15%), followed by anaerobic bacteria (30.76%) and staphylococci (23.07%). There was no case in which MRSA was isolated. (i) Isolated pathogens and antibiotic treatment: regarding at all isolated pathogen results (none, mono- and polybacterial results), 88.88% of pathogens were treated with a 3rd generation cephalosporin, 77.77% with metronidazole, 55.55% with vancomycin, 22.22% with both meropenem and flucloxacillin and 11.11% with both amoxicillin/clavulanic acid as well as clindamycin.
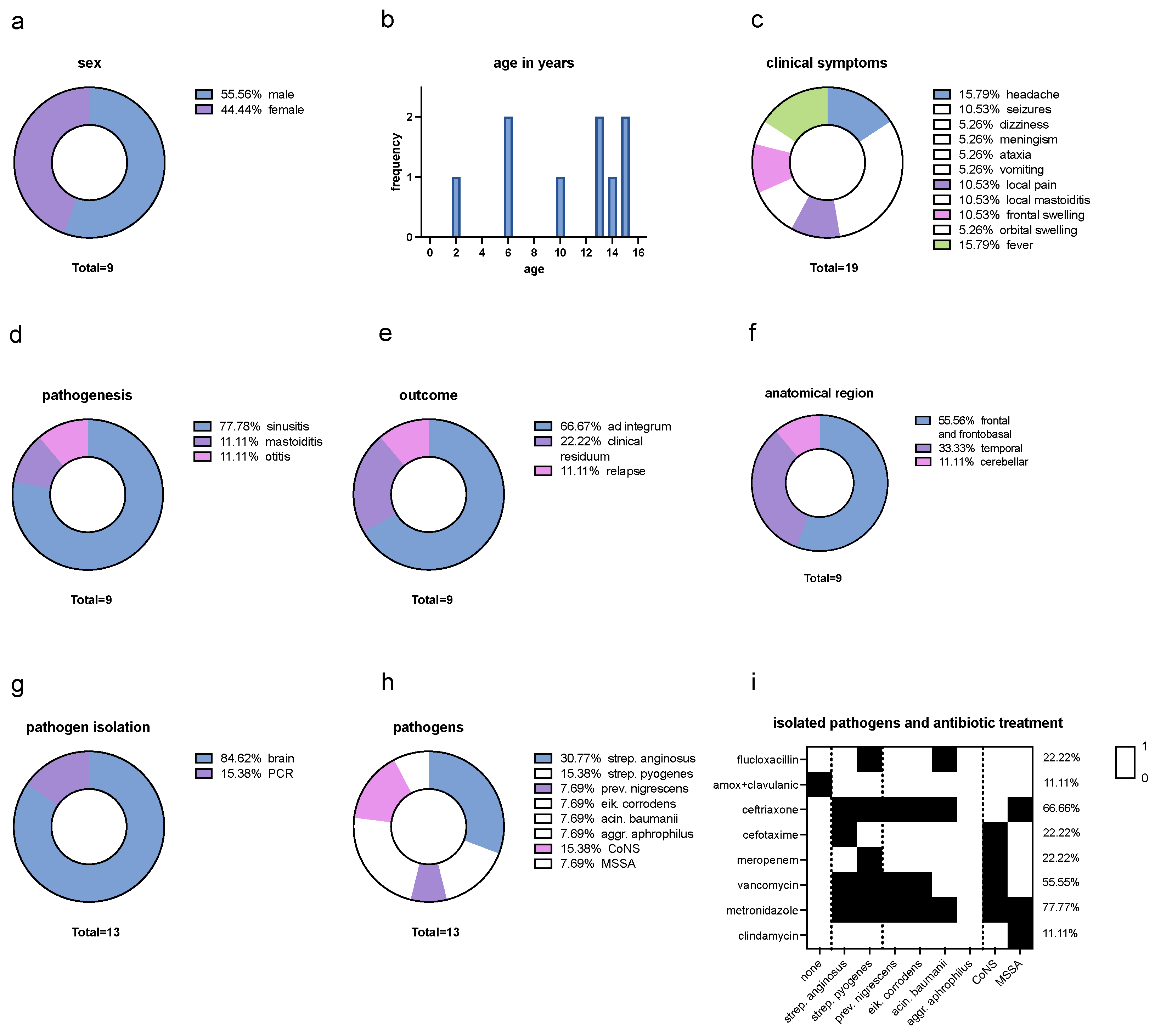
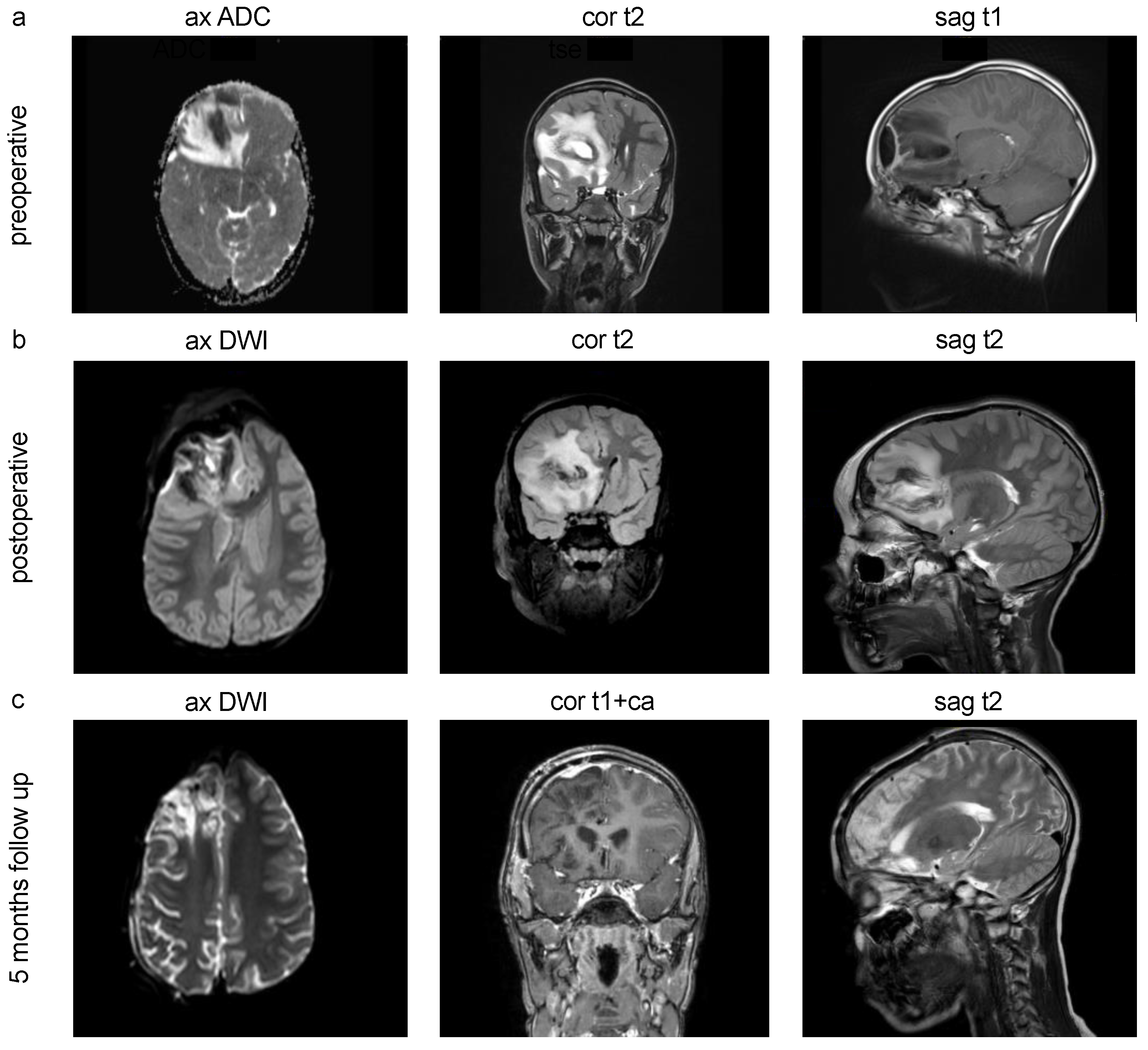
Figure 2.
cMRI Imaging of pediatric intracranial abscesses. (a) Preoperative MRI scans of a 10-year-old male patient with pansinusitis and streptococcus anginosus infection, frontal and frontobasal intracranial brain abscess, midline shift subfalcine to the left, compressed right lateral ventricular anterior horn and narrow sulcus drawing with stable spatial conditions. (b) Postoperative control MRI scans after abscess evacuation. (c) 5 months follow up showing no delimitable hygromas or CSF leakage in the condition after neurosurgical hemicraniectomy as well as a known right hemispheric contrast enhancement. ax=axial, cor=coronal, sag=saggital.
Figure 2.
cMRI Imaging of pediatric intracranial abscesses. (a) Preoperative MRI scans of a 10-year-old male patient with pansinusitis and streptococcus anginosus infection, frontal and frontobasal intracranial brain abscess, midline shift subfalcine to the left, compressed right lateral ventricular anterior horn and narrow sulcus drawing with stable spatial conditions. (b) Postoperative control MRI scans after abscess evacuation. (c) 5 months follow up showing no delimitable hygromas or CSF leakage in the condition after neurosurgical hemicraniectomy as well as a known right hemispheric contrast enhancement. ax=axial, cor=coronal, sag=saggital.
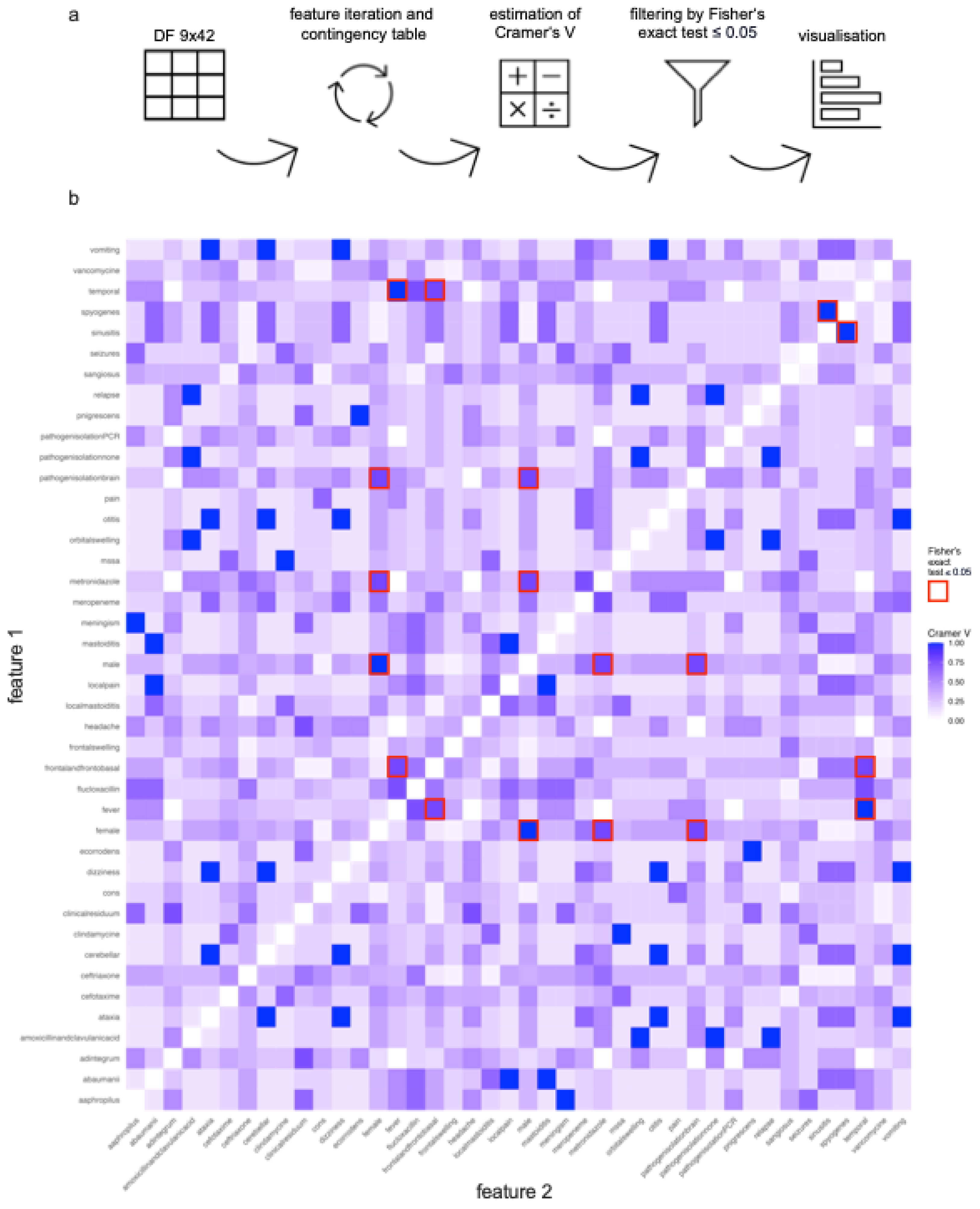
Figure 3.
Statistical Feature Correlation Analysis. (a) Workflow of feature correlation analysis: The data was collected in a table and converted to a matrix nominal-binary coded as 0 (not present) and 1 (present). A code was created iterating over all combinations of two features. For each combination, a contingency table is created. Firstly, the Cramer’s V effect size was calculated for all combinations of two features. Secondly, the Fisher’s exact test was performed on every contingency table. The p-value was checked for significance. After that, a heatmap correlation plot based on the Cramer’s V value of every possible feature combination was created. (b) Statistical feature correlation analysis: 861 different contingency tables were created. 36 feature combinations could be identified with a Cramer’s V value equal or higher than 0.7 which indicates a very strong association. Testing on Fisher’s exact test afterwards, all in all nine feature combinations could be identified as significant with a very strong association: female vs. male (p=0.007) sinusitis vs. streptococcus pyogenes (p=0.028), fever vs. temporal (p=0.012), frontal and frontobasal vs. temporal (p=0.047), fever vs. frontal and frontobasal (p=0.047), female vs. metronidazole (p=0.047), female vs. pathogen isolation brain (p=0.047), male vs. metronidazole (p=0.047) and male vs. pathogen isolation brain (p=0.047).
Figure 3.
Statistical Feature Correlation Analysis. (a) Workflow of feature correlation analysis: The data was collected in a table and converted to a matrix nominal-binary coded as 0 (not present) and 1 (present). A code was created iterating over all combinations of two features. For each combination, a contingency table is created. Firstly, the Cramer’s V effect size was calculated for all combinations of two features. Secondly, the Fisher’s exact test was performed on every contingency table. The p-value was checked for significance. After that, a heatmap correlation plot based on the Cramer’s V value of every possible feature combination was created. (b) Statistical feature correlation analysis: 861 different contingency tables were created. 36 feature combinations could be identified with a Cramer’s V value equal or higher than 0.7 which indicates a very strong association. Testing on Fisher’s exact test afterwards, all in all nine feature combinations could be identified as significant with a very strong association: female vs. male (p=0.007) sinusitis vs. streptococcus pyogenes (p=0.028), fever vs. temporal (p=0.012), frontal and frontobasal vs. temporal (p=0.047), fever vs. frontal and frontobasal (p=0.047), female vs. metronidazole (p=0.047), female vs. pathogen isolation brain (p=0.047), male vs. metronidazole (p=0.047) and male vs. pathogen isolation brain (p=0.047).
3. Results
3.1. Epidemiology
In this monocentric, retrospective study from December 2022 to May 2023, nine cases of intracranial infections were recorded in eight pediatric patients at the University Medical Centre Hamburg-Eppendorf (UKE) in Hamburg, Germany. All patients were treated at the department of pediatrics and underwent neurosurgical treatment for abscess evacuation and sample collection. Investigating all cases, 55,56% were male and 44,44% female (
Figure 1a) between the age of two to 15 years with a mean age of 10,22 years (
Figure 1b).
3.2. Clinical Course
At the initial clinical presentation, a range of diverse symptoms was observed, including headache (15.79%), seizures (10.53%), dizziness (5.26%), meningism (5.26%), ataxia (5.26%) and vomiting (5.26%) which can be summarized as neurological symptoms. Further symptoms were local pain (10.53%) and local mastoiditis (10.53%) which can be summarized as local symptoms, as well as frontal swelling (10.53%) and orbital swelling (5.26%) and fever (15.79%). Neurological symptoms make up to 47.36% of all symptoms, while local symptoms contribute 21.06%. Swelling symptoms and fever both make up to 15.79% of all symptoms (
Figure 1c). In terms of pathogenesis, continuous spread from a preceding infection occurred from 77.78% sinusitis, 11.11% mastoiditis and 11.11% otitis (
Figure 1d). Regarding the outcome, 66.67% of all cases showed an outcome ad integrum, 22.22% a clinical residuum such as mild flaccid paralysis of the left side and small scotoma superior et inferior on the right side and 11.11% of all cases had a relapse (
Figure 1e).
3.3. Treatment and Etiology
As soon as an intracranial abscess was suspected, a cMRI imaging was performed. After an interdisciplinary case discussion between pediatricians and neurosurgeons, a prompt neurosurgical treatment was performed within 24 hours. The abscess formation was mircosurgically obtained and sent for microbiological and histopathological diagnosis. Abscess drainage was not used in any case. Extubation was performed promptly in the pediatric intensive care unit, followed by starting the empirical antibiotic treatment until the pathogen-specific antibiogram was available.
In nine cases of eight patients, a total of 13 different pathogens could be isolated. Regarding the intracranial anatomical abscess region, 55.56% were located frontal and frontobasal (
Figure 2), 33.33% temporal and 11.11% cerebellar (11.11%) (
Figure 1f). Pathogen material was isolated in 84.62% of all cases using abscess material from the brain and in 15.38% by PCR (
Figure 1g). In one case, no pathogens could be isolated. Blood cultures were sterile in all cases. The isolated pathogens are Streptococcus anginosus (30.77%), Streptocuccus pyogenes (15.38%), Coagulase-negative staphylococci (CoNS) (15.38%), Methicillin-sensitive Staphylococcus aureus (MSSA) (7.69%), Prevotella nigrescens (7.69%), Eikenella corrodens (7.69%), Acinetobacter baumanii (7.69%) and Aggregatibacter aphrophilus (7.69%) (
Figure 1h). Hence, streptococci were the most common pathogens (46.15%), followed by anaerobic bacteria (30.76%) and staphylococci (23.07%). There was no case in which MRSA was isolated.
In 66.66% of all cases, a 3rd generation cephalosporin was administered along with metronidazole. In 44.44% of all cases, flucloxacillin or vancomycin were added to the regimen. One patient received clindamycin additionally. The patient who was readmitted with a relapsed infection was treated with amoxicillin/clavulanic acid in the first episode. Meropenem in combination with vancomycin were initiated in 22.22% of all cases. The empirical anti-infective therapy demonstrated an efficacy of 77.77% against the identified pathogens. In the initial episode of the patient who had a reinfection detection of a causing pathogen was not possible. Retrospectively, with the isolation of Strep. anginosus, an insufficient antibacterial activity of amoxicillin/clavulanic acid may be considered. Looking at all isolated pathogen results (none, mono- and polybacterial results), 88.88% of pathogens were treated with a 3rd generation cephalosporin, 77.77% with metronidazole, 55.55% with vancomycin, 22.22% with both meropenem and flucloxacillin and 11.11% with both amoxicillin/clavulanic acid as well as clindamycin (
Figure 1i).
3.4. Statistical Feature Correlation Analysis
To analyze the various features of all cases and identify, if there is a significant association between two feature, Cramer’s V test and Fisher’s exact test was performed. With the Cramer’s V test, one can measure the strength of the relationship between two categorical variables. While V=0 displays a weak association, V=1 indicates a strong association without giving a statement on the direction. The Fisher’s exact test is then used to examine the significancy of measured associations between two categorical variables, especially with a small number of cases.
Figure 3a shows the workflow. With 42 different features, the number of feature combinations excluding same feature comparison is 861 leading to an individual contingency table for each feature combination (
Supplementary Table S2). 36 feature combinations could be identified with a V value equal or higher than 0.7 which indicates a very strong association [
11,
12]. Testing on Fisher’s exact test, all in all nine feature combinations could be identified as significant. All of them display a Cramer’s V value higher than 0.7 speaking for a very strong association: female vs. male (p=0.007), sinusitis vs. streptococcus pyogenes (p=0.028), fever vs. temporal (p=0.012), frontal and frontobasal vs. temporal (p=0.047), fever vs. frontal and frontobasal (p=0.047), female vs. metronidazole (p=0.047), female vs. pathogen isolation brain (p=0.047), male vs. metronidazole (p=0.047) and male vs. pathogen isolation brain (p=0.047) (
Figure 3b,
Supplementary Table S3).
4. Discussion
4.1. Recognition of a Specific Disease Pattern of Children with Intracranial Abscesses
Here we compare the treatment regime used in our study with the current literature. Intracranial abscesses in children are a rare and potentially fatal diseases. Knowledge about clinical, diagnostic, and therapeutic parameters is important for optimal treatment. In our study we could not find a difference in sex. The mean age with 10.2 years aligns with previous research [
13]. Regarding the distribution of clinical symptoms, we found that half of the clinical symptoms were neurological, while the remaining half were split between swelling, fever, and local symptoms, suggesting that the occurrence of these symptom complexes should be present in the differential diagnosis of intracranial abscess [
14,
15]. The prevalence of streptococci and staphylococci mirrors the distribution observed in previous studies. Our study highlights that anaerobes constitute a larger proportion than what is commonly reported in the existing literature [
16].
Based on our study, the high prevalence of sinusitis with the above-mentioned pathogens as the underlying cause of the development of intracranial infections goes in line with the current literature [
13,
17]. When examining the timing of these intracranial abscess cases, it is noteworthy that they increased following the relaxation of COVID-19 pandemic restrictions. This may suggest that the reduced transmission of respiratory pathogens during the pandemic, including bacteria and viruses causing sinusitis, may have influenced the rise in intracranial infections observed in our study period. This could represent a rebound effect, which might have implications for the targeted treatment and prevention of sinusitis and its complications. In terms of the affected anatomical areas, our findings show that half of the infections occurred in the frontal region and a third in the temporal region. This distribution likely reflects sinusitis as a predominant pathway for infection. [
13,
17]. Hereby, the ability to isolate pathogens in a highly significant proportion of cases using material from surgery suggests that access to proper diagnostic procedures appears a reliable method comparing our studies (84%) to the known literature (60-89%) [
18,
19]. Moreover, the use of 3rd generation cephalosporin and metronidazole as empiric antibiotic treatments in most cases appears to be effective [
4,
20].
In our cases, abscess drainage was not employed, which is an alternative surgical treatment option used in other neurosurgical centers. The continuation of antibiotic treatment was tailored based on the specific recommendations of the in-house infectious disease guidelines and varied accordingly. Following the mentioned treatment regime, most cases in our analysis showed a favorable outcome (resitutio ad integrum) without a death event and a significant reduction in the follow-up cMRI (
Figure 2c).
4.2. Feature Analysis Reveals Clinical Feature Combinations to Be Considered for Early Diagnostics and Treatment
Our study showed a statistically significant strong association between both male and female sexes, suggesting that the occurrence of intracranial abscesses in children is independent of the gender. Additionally, a strong association was observed between the clinical symptoms of sinusitis and the pathogen Streptococcus pyogenes, indicating that in such cases, early consideration of an intracranial abscess is crucial and may be diagnosed by early imaging, preferably with cMRI [
21]. Additionally, a statistically significant strong correlation was found between the clinical symptoms of fever and the anatomical presence of the abscess in the temporal and frontal/frontobasal regions. Consequently, fever serves as a crucial clinical marker for abscesses formation in the frontal and temporal areas [
22]. Successful pathogen isolation from surgery was also found to be statistically significantly associated with both male and female pediatric patients, implying that this is independent of gender. Moreover, the use of metronidazole was identified as an important component of the antibiotic therapy, showing a statistically significant strong association in both male and female patients.
4.3. Decreased Overall Transmission of Respiratory Pathogens during COVID-19 Pandemic Maybe Led to a Shift of Incidence and a Seasonal Increase of Intracerebral Abscesses in Children due to a Catch-Up Effect
During the pandemic, the implementation of strict rules and guidelines, such as social distancing, wearing masks, and increased emphasis on hand hygiene, aimed to reduce the transmission of not only the COVID-19 virus but also other respiratory pathogens [
23]. Most of the Covid-19 measures in Hamburg and Schleswig-Holstein ended with the expiry of the hotspot regulation in April 2022. These preventive measures could have resulted in a decreased overall transmission of respiratory pathogens, including those commonly associated with sinusitis [
24]. Nevertheless, physiologically, infections build up immunity, which protects against re-infection by the same pathogen, at least for a certain period, which is called adaptive immune response. This protection wears off again after a few years [
25]. The easing of hygiene measures may result in a resurgence of infections, as many children have grown more vulnerable to respiratory pathogens after a prolonged period without exposure. Cohen et al. published a highly discussed hypothesis in 2021 that the lack of immune stimulation due to the reduced circulation of microbial agents may induced an “immunity debt” which could have negative consequences when the pandemic was under control and non-pharmaceutical intervention (NPIs) are lifted [
26,
27]. Nevertheless, the occurrence of intracranial infections in children necessitates additional research and analysis. Factors such as changes in healthcare-seeking behavior, delayed diagnoses, or differences in the presentation of symptoms during the pandemic may also contribute to the observed patterns. Therefore, additional research and data collection are needed to fully understand whether reduced exposure to pathogens has an impact on the increased development of intracranial abscesses in children.
4.4. Methodological Classification and Clinical Recommendation for the Management of Children with Intracranial Abscesses
In conclusion, our study provides valuable insights into the associations and clinical characteristics of pediatric patients with intracranial abscesses. However, it is important to acknowledge the limitations of our study, including its retrospective study design, single-center setting, and sample size. To overcome these limitations, further research is needed, particularly in larger, multicenter studies. Additionally, investigating potential confounding factors and incorporating longitudinal follow-up would strengthen the validity of these findings. However, with this study, we have made a statistical contribution to the treatment regimen for intracranial abscesses in children, an area where there is only limited data available.
5. Conclusions
Taken together, we had no death events and approximately 22% of patients with clinical residuals in our study. Given the severity of this condition, immediate imaging, ideally with cMRI, is essential in cases where there is clinical suspicion based on symptoms like sinusitis, fever, and neurological issues. Prompt surgical drainage of the abscess should be aimed for, postoperative calculated antibiotic therapy should be initiated immediately and covered following the antibiogram. Here, we show significant associations of clinical features of children with intracranial abscesses revealing a specific disease pattern that could be useful in clinical practice for decision making and fast disease management. Nevertheless, further research is needed to expand upon these findings.
Supplementary Materials
The following supporting information can be downloaded at:
Preprints.org, Supplementary Table 1: ICD10 Codes, 2: Cramer’s V test, 3: Fisher’s t-test.
Author Contributions
MM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review and editing; MK: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review and editing; FG: Data curation, Investigation, Validation, Writing – review and editing; FL: Formal analysis, Investigation, Resources, Validation, Writing – review and editing; LD: Conceptualization, Project administration, Methodology, Resources, Supervision, Writing – review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank the nurses, laboratory assistants and surgical assistants for their commitment to the children’s recovery process.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alvis Miranda, H., et al., Brain abscess: Current management. J Neurosci Rural Pract, 2013. 4(Suppl 1): p. S67-81. [CrossRef]
- Brouwer, M.C., J.M. Coutinho, and D. van de Beek, Clinical characteristics and outcome of brain abscess: systematic review and meta-analysis. Neurology, 2014. 82(9): p. 806-13. [CrossRef]
- Sahbudak Bal, Z., et al., Brain Abscess in Children: A Rare but Serious Infection. Clin Pediatr (Phila), 2018. 57(5): p. 574-579. [CrossRef]
- Frazier, J.L., E.S. Ahn, and G.I. Jallo, Management of brain abscesses in children. Neurosurg Focus, 2008. 24(6): p. E8. [CrossRef]
- Brouwer, M.C. and D. van de Beek, Epidemiology, diagnosis, and treatment of brain abscesses. Curr Opin Infect Dis, 2017. 30(1): p. 129-134. [CrossRef]
- Bonfield, C.M., J. Sharma, and S. Dobson, Pediatric intracranial abscesses. J Infect, 2015. 71 Suppl 1: p. S42-6. [CrossRef]
- Felsenstein, S., et al., Clinical and microbiologic features guiding treatment recommendations for brain abscesses in children. Pediatr Infect Dis J, 2013. 32(2): p. 129-35. [CrossRef]
- Sonneville, R., et al., An update on bacterial brain abscess in immunocompetent patients. Clin Microbiol Infect, 2017. 23(9): p. 614-620. [CrossRef]
- English, B.K. and A.H. Gaur, The use and abuse of antibiotics and the development of antibiotic resistance. Adv Exp Med Biol, 2010. 659: p. 73-82. [CrossRef]
- Kollef, M.H., Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis, 2000. 31 Suppl 4: p. S131-8. [CrossRef]
- Kim, H.Y., Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restor Dent Endod, 2017. 42(2): p. 152-155. [CrossRef]
- McHugh, M.L., The chi-square test of independence. Biochem Med (Zagreb), 2013. 23(2): p. 143-9. [CrossRef]
- Raffaldi, I., et al., Brain abscesses in children: an Italian multicentre study. Epidemiol Infect, 2017. 145(13): p. 2848-2855. [CrossRef]
- Cole, T.S., et al., Pediatric focal intracranial suppuration: a UK single-center experience. Childs Nerv Syst, 2012. 28(12): p. 2109-14. [CrossRef]
- Ozsurekci, Y., et al., Brain abscess in childhood: a 28-year experience. Turk J Pediatr, 2012. 54(2): p. 144-9.
- Mameli, C., et al., Brain abscess in pediatric age: a review. Childs Nerv Syst, 2019. 35(7): p. 1117-1128. [CrossRef]
- Shachor-Meyouhas, Y., et al., Brain abscess in children - epidemiology, predisposing factors and management in the modern medicine era. Acta Paediatr, 2010. 99(8): p. 1163-7. [CrossRef]
- Canpolat, M., et al., Brain abscesses in children: results of 24 children from a reference center in Central Anatolia, Turkey. J Child Neurol, 2015. 30(4): p. 458-67.
- Lee, C.G., et al., Brain abscess in Korean children: A 15-year single center study. Korean J Pediatr, 2010. 53(5): p. 648-52. [CrossRef]
- Gelabert-Gonzalez, M., et al., Management of brain abscess in children. J Paediatr Child Health, 2008. 44(12): p. 731-5. [CrossRef]
- Lundy, P., et al., Intracranial subdural empyemas and epidural abscesses in children. J Neurosurg Pediatr, 2019. 24(1): p. 14-21. [CrossRef]
- Sheehan, J.P., et al., Brain abscess in children. Neurosurg Focus, 2008. 24(6): p. E6. [CrossRef]
- Kirsch, F., et al., Personal Protective Measures during the COVID-19 Pandemic in Germany. Int J Infect Dis, 2022. 121: p. 177-183. [CrossRef]
- Jung, C.M., et al., Impact of Non-Pharmaceutical Interventions on the Incidence and Treatment of Chronic Rhinosinusitis during the COVID-19 Pandemic: A Nationwide Retrospective Cohort Study. J Clin Med, 2023. 12(20). [CrossRef]
- Marshall, J.S., et al., An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol, 2018. 14(Suppl 2): p. 49. [CrossRef]
- Cohen, R., et al., Immune debt: Recrudescence of disease and confirmation of a contested concept. Infect Dis Now, 2023. 53(2): p. 104638. [CrossRef]
- Cohen, R., et al., Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect Dis Now, 2021. 51(5): p. 418-423. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).