Submitted:
10 October 2024
Posted:
10 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Chemical Materials
2.2. Synthesis of the CuOx/TiO2
2.3. Characterization
2.4. Photocatalytic Reduction Reaction of CO2
3. Results and Discussion
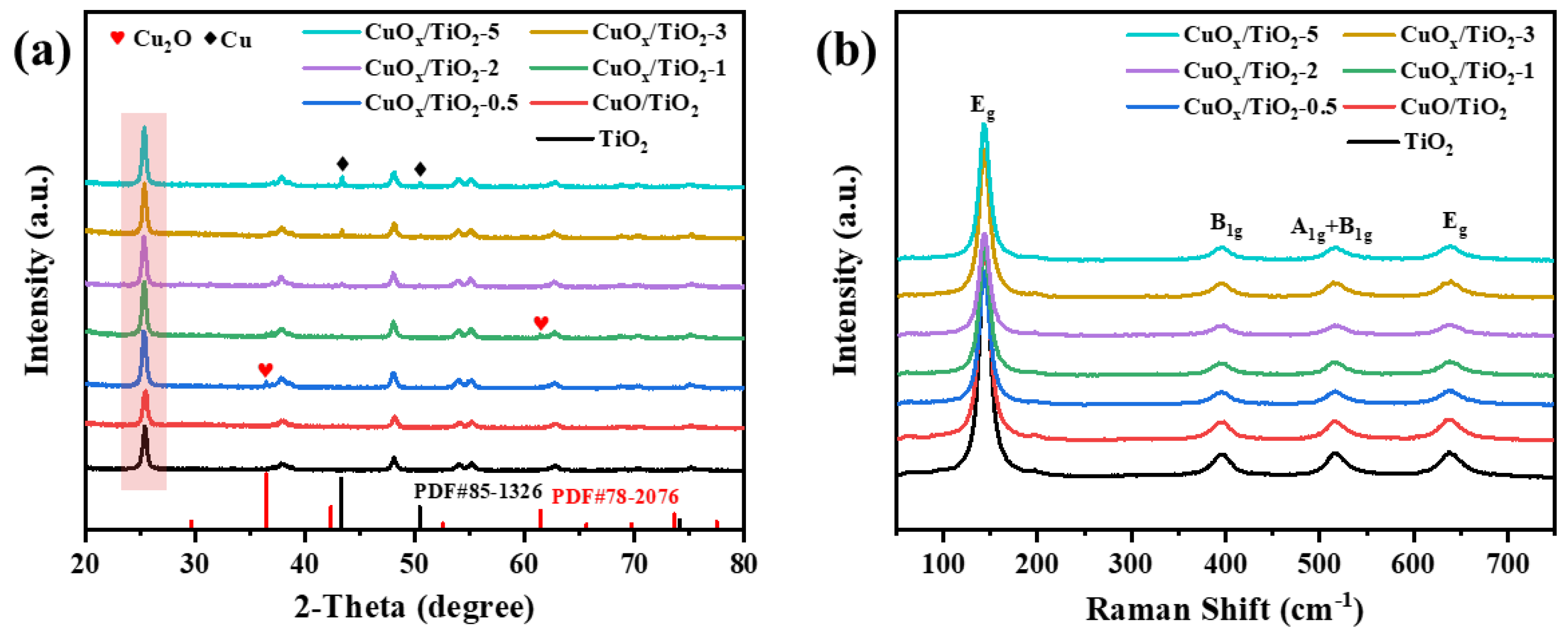
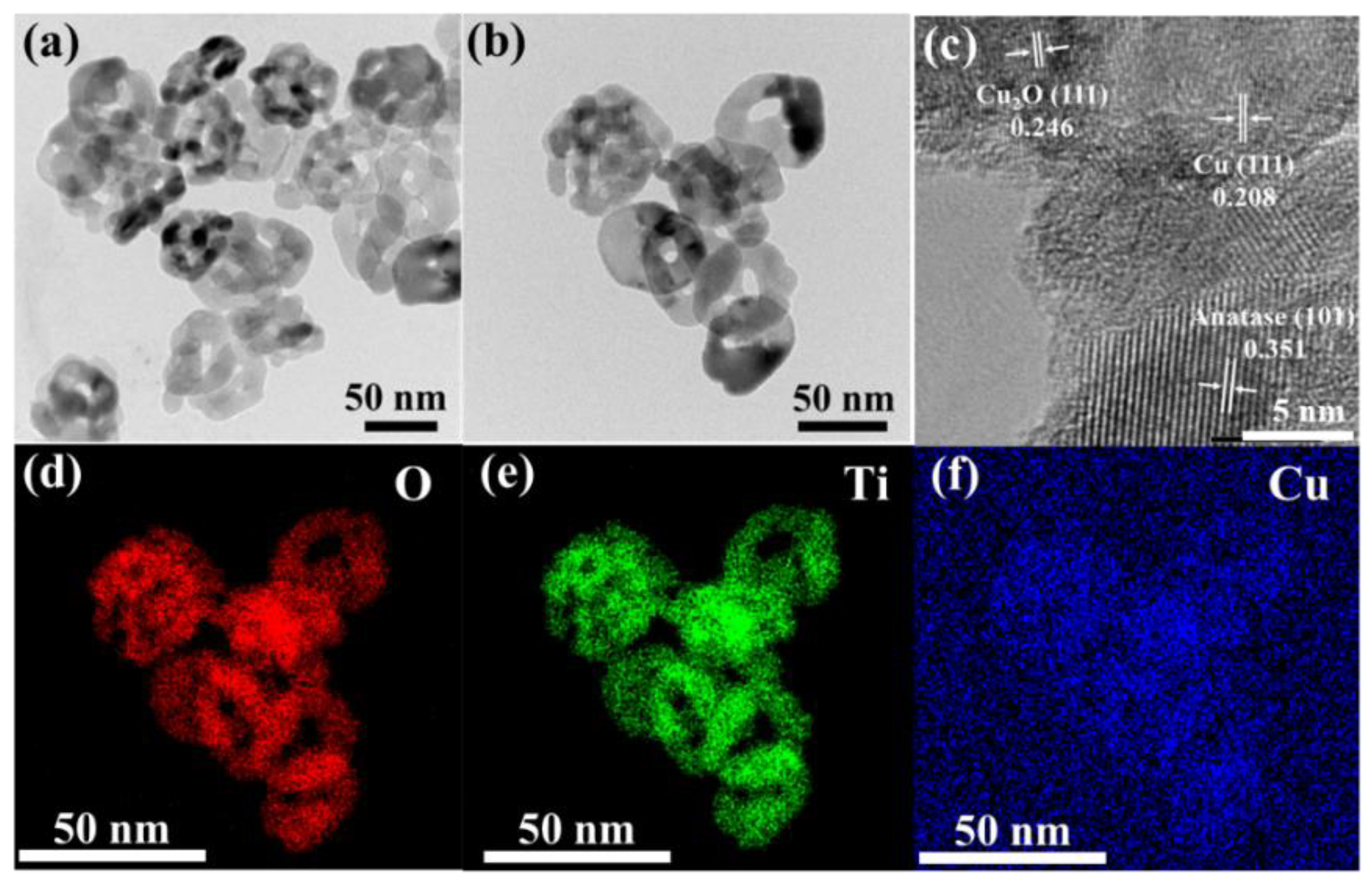
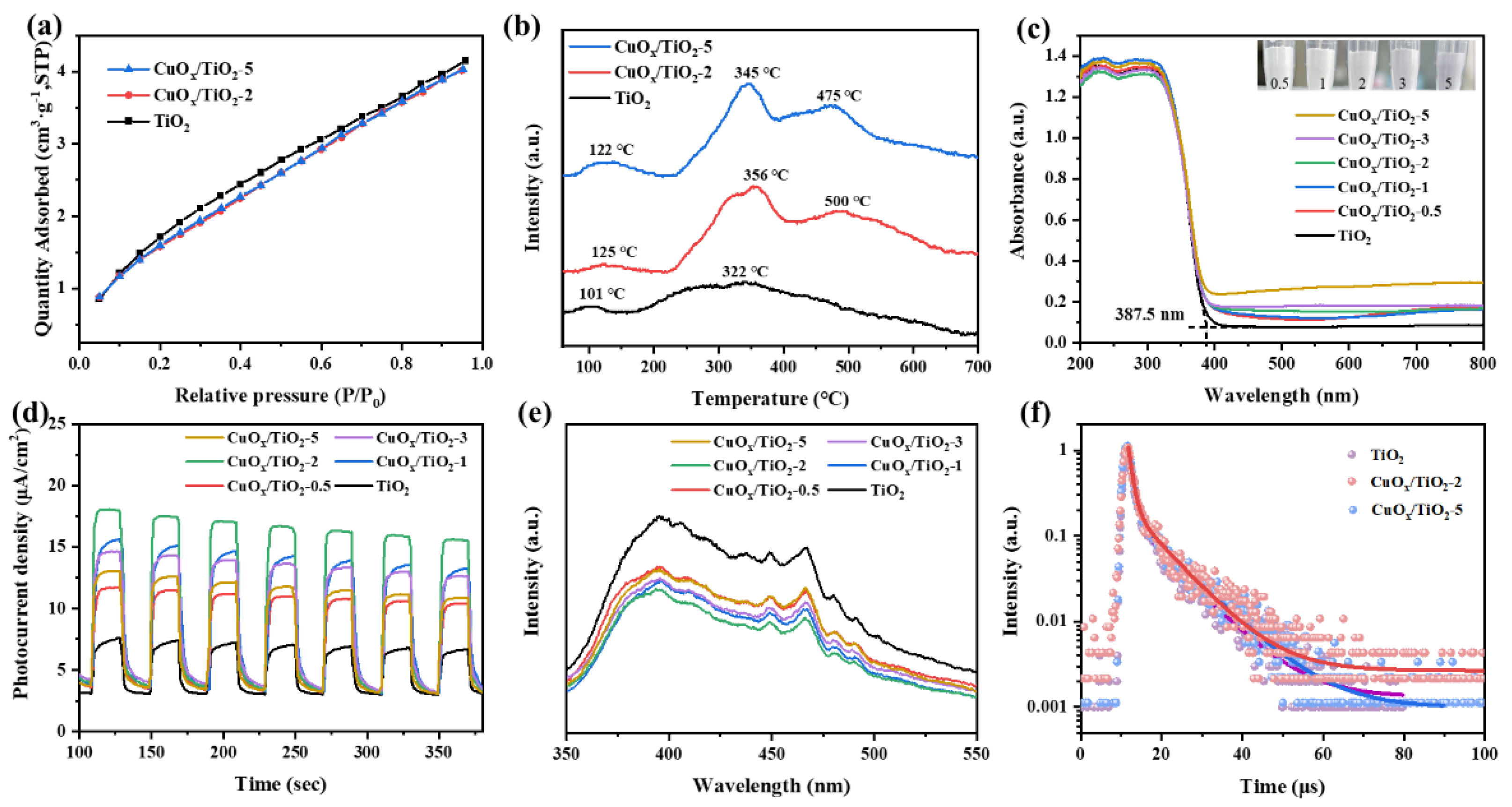
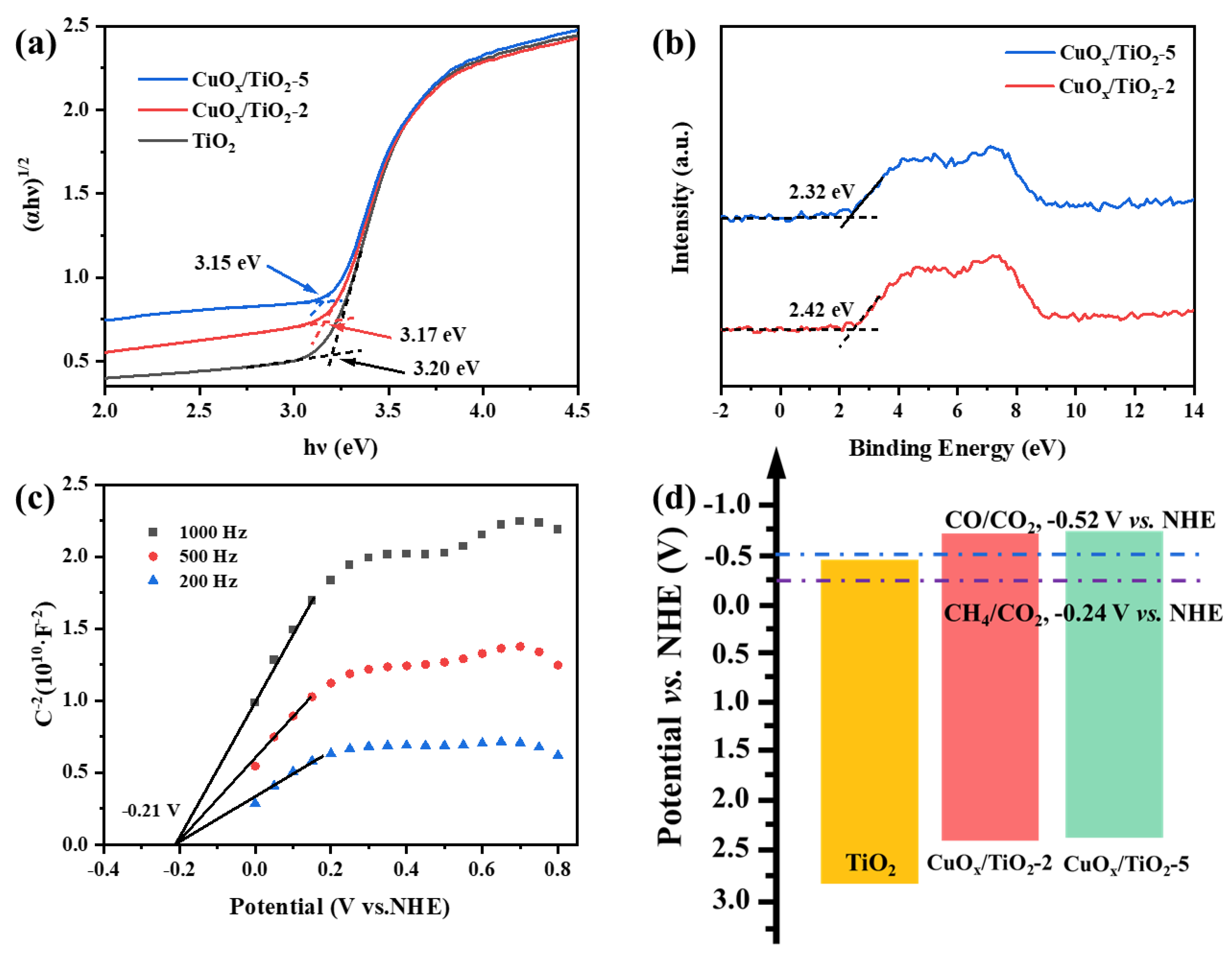
3.1. The Structure and Morphology
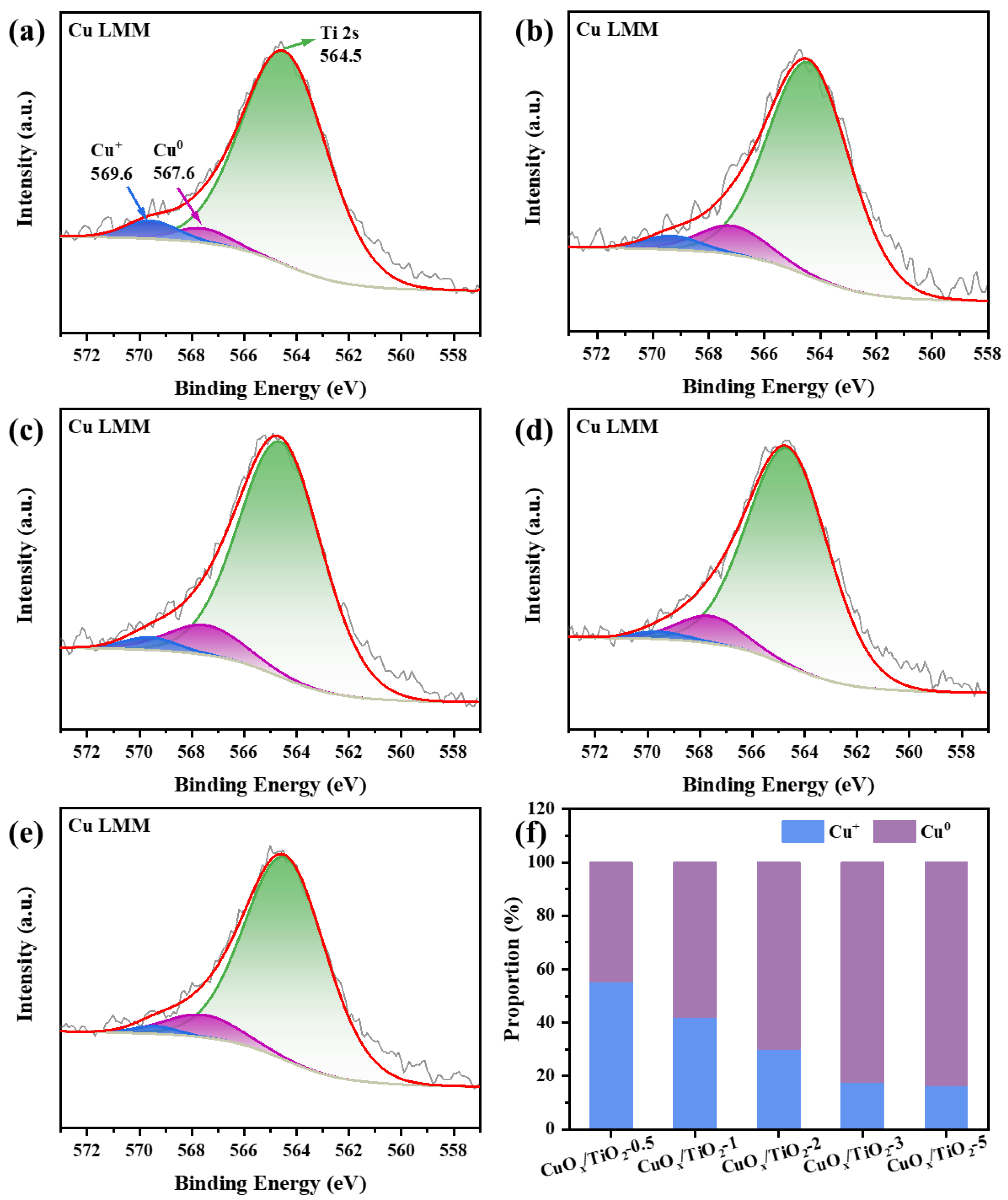
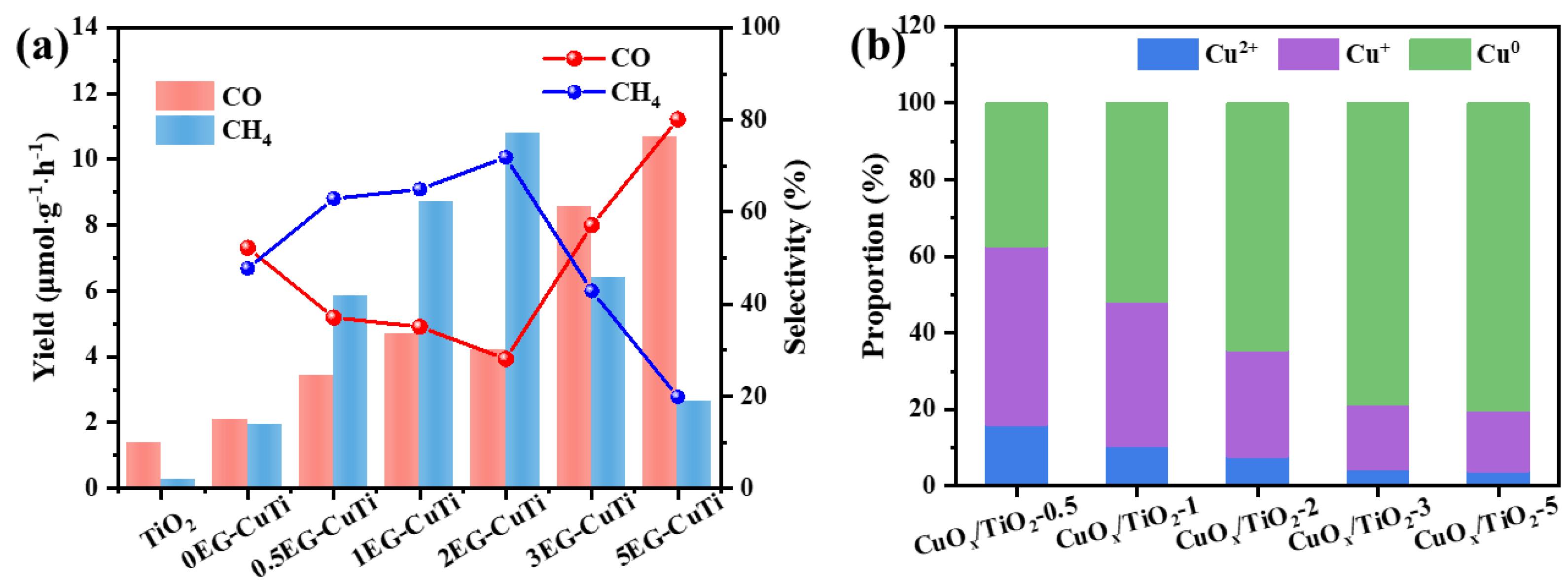
3.2. Copper Valence State on the Surface of CuOx/TiO2
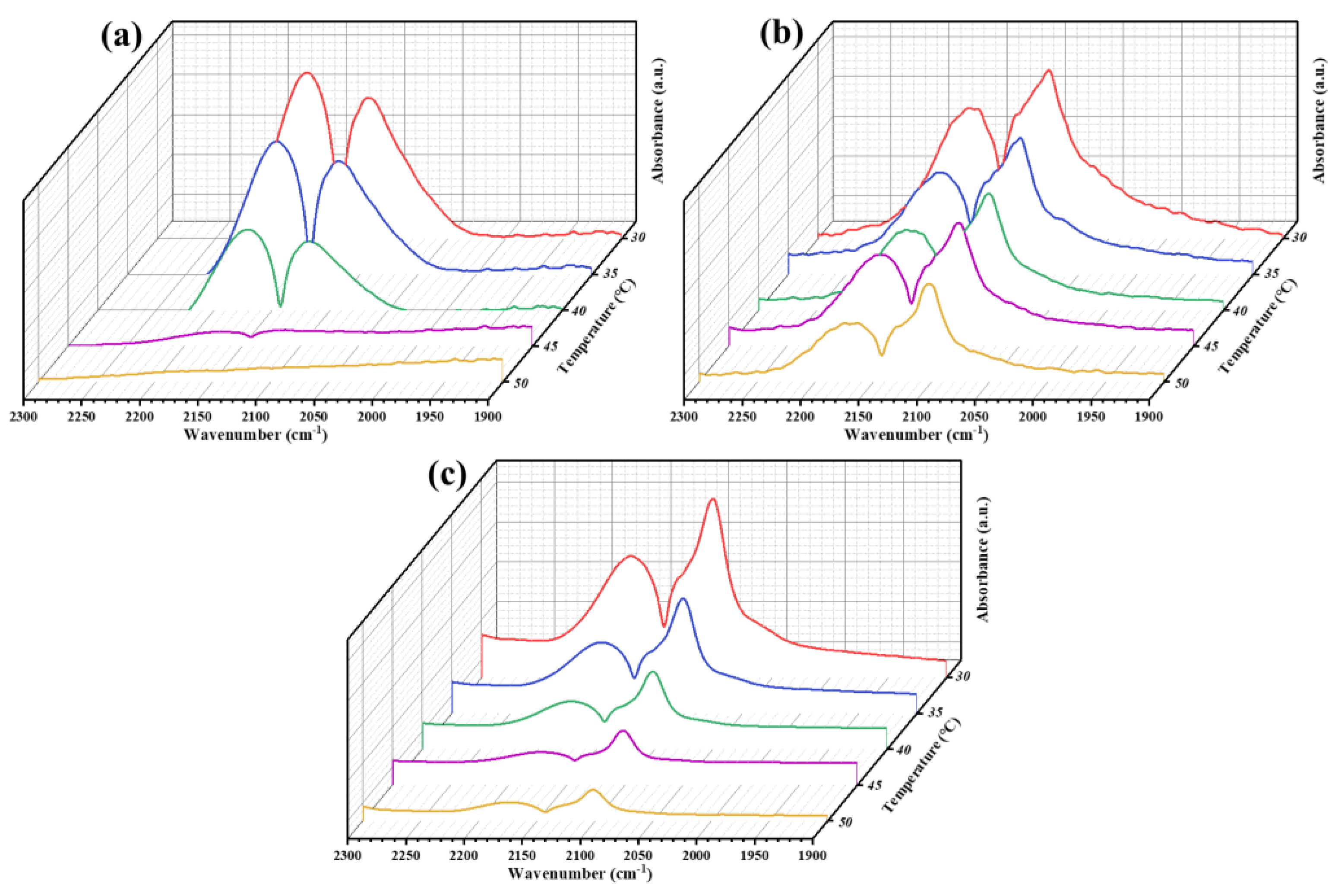
3.3. Performance of Photocatalytic CO2 Reduction
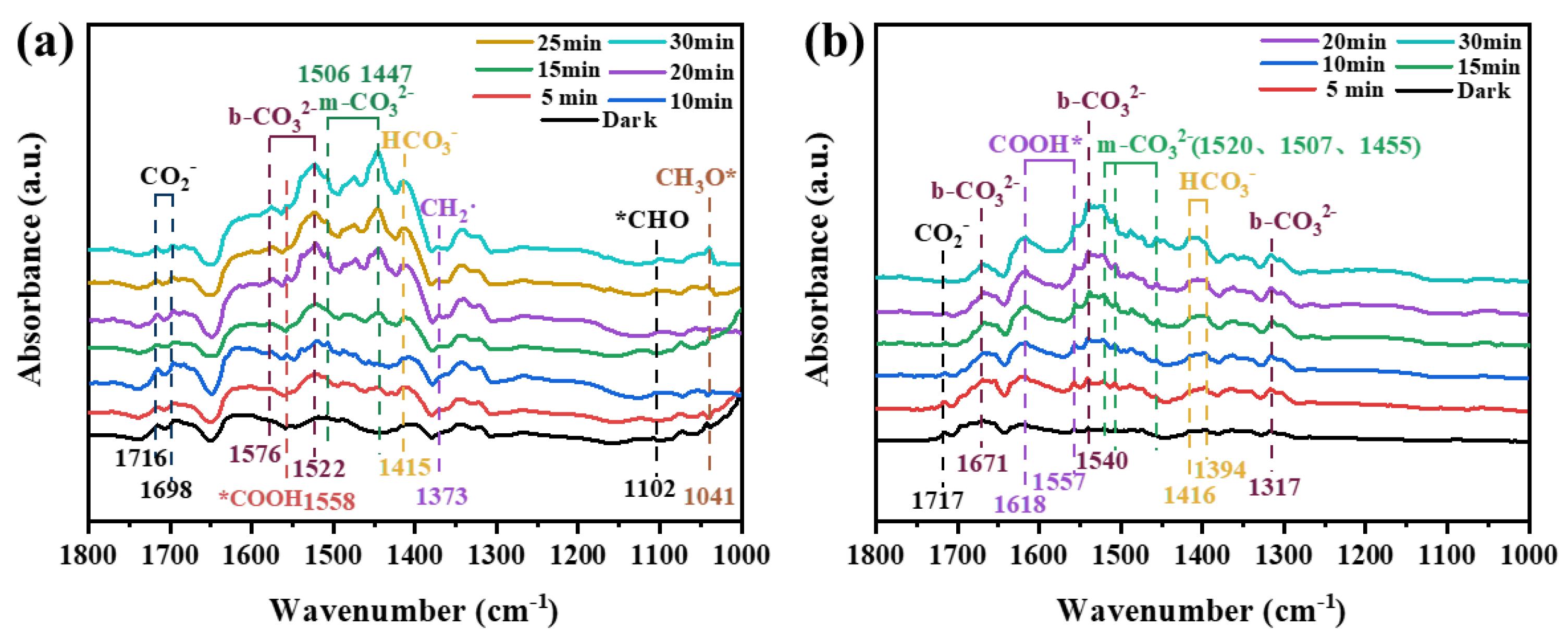
3.4. The Selectivity of Products in Photocatalytic Reduction of CO2
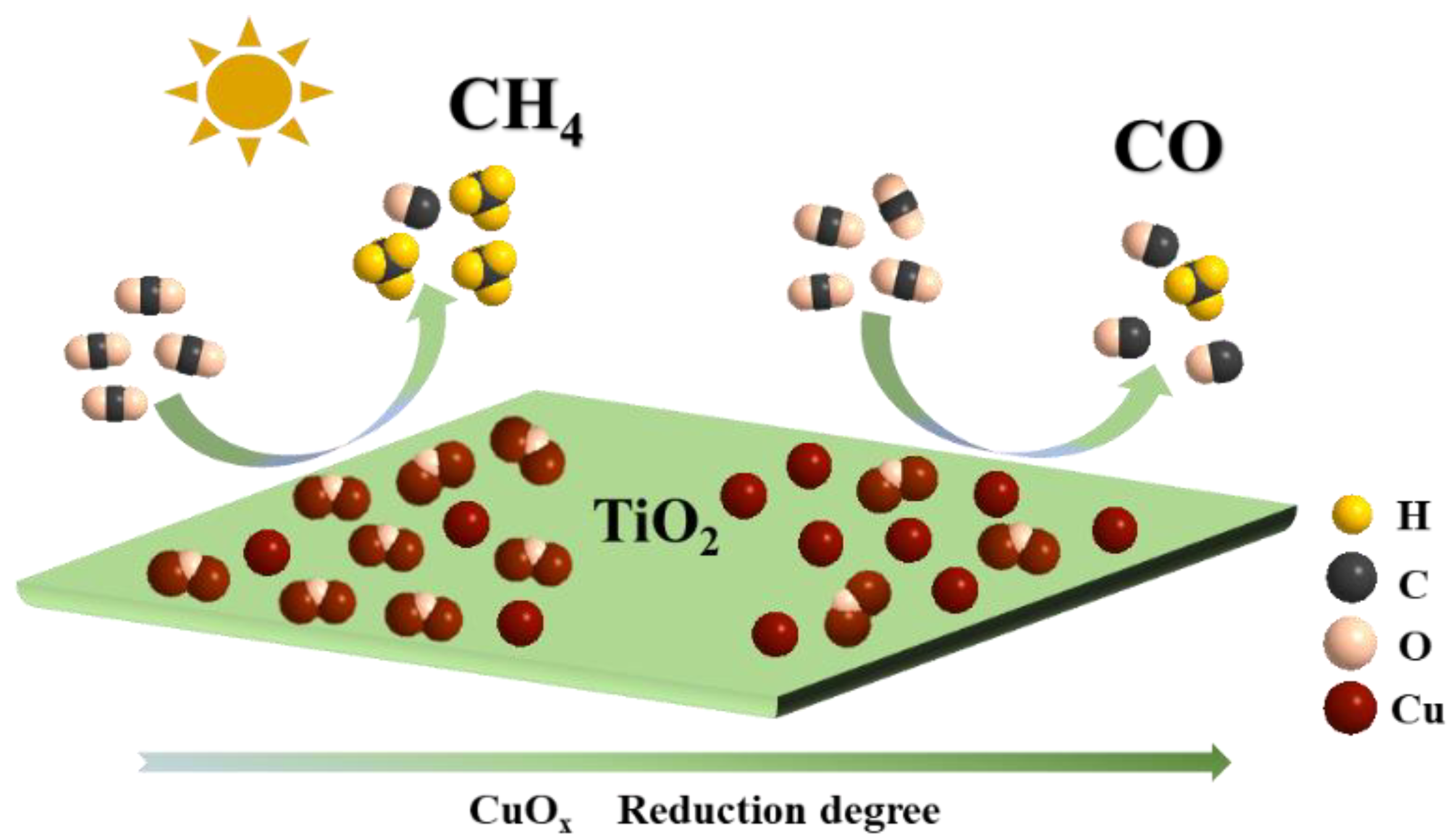
4. Conclusion
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Wang J, Azam W. Natural resource scarcity, fossil fuel energy consumption, and total greenhouse gas emissions in top emitting countries. Geoscience Frontiers 2024, 15, 101757. [Google Scholar] [CrossRef]
- Yao, L. New energy utilization in environmental design and realization. Energy Reports 2022, 8, 9211–9220. [Google Scholar] [CrossRef]
- Gao W, Liang S, Wang R; et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chemical Society Reviews 2020, 49, 8584–8686. [Google Scholar] [CrossRef] [PubMed]
- Herndon, J. Evidence of variable earth-heat production, global non-anthropogenic climate change, and geoengineered global warming and polar melting. Journal of Geography, Environment and Earth Science International 2017, 10, 1–16. [Google Scholar] [CrossRef]
- Lowery C M, Bown P R, Fraass A J; et al. Ecological response of plankton to environmental change: Thresholds for extinction. Annual Review of Earth and Planetary Sciences 2020, 48, 403–429. [Google Scholar] [CrossRef]
- Bhanja P, Modak A, Bhaumik A. Porous organic polymers for CO2 storage and conversion reactions. ChemCatChem 2019, 11, 244–257. [Google Scholar] [CrossRef]
- Liu Y, An Y, Zhu J; et al. Integrated energy storage and CO2 conversion using an aqueous battery with tamed asymmetric reactions. Nature Communications 2024, 15, 977. [Google Scholar] [CrossRef]
- Su Y, Cheng Y, Li Z; et al. Exploring the impact of Nafion modifier on electrocatalytic CO2 reduction over Cu catalyst. Journal of Energy Chemistry 2024, 88, 543–551. [Google Scholar] [CrossRef]
- Wu Z, Gao F, Gao M. Regulating the oxidation state of nanomaterials for electrocatalytic CO2 reduction. Energy & Environmental Science 2021, 14, 1121–1139. [Google Scholar]
- Komarala E P, Alkhoori A A, Zhang X; et al. Design and synthesis of thermally stable single atom catalysts for thermochemical CO2 reduction. Journal of Energy Chemistry 2023, 86, 246–262. [Google Scholar] [CrossRef]
- Liu Y, Shang J, Zhu T. Enhanced thermal-assisted photocatalytic CO2 reduction by RGO/H-CN two-dimensional heterojunction. Journal of Materials Science & Technology 2024, 176, 36–47. [Google Scholar]
- Fu J, Jiang K, Qiu X; et al. Product selectivity of photocatalytic CO2 reduction reactions. Materials Today 2020, 32, 222–243. [Google Scholar] [CrossRef]
- Qu T, Wei S, Xiong Z; et al. Progress and prospect of CO2 photocatalytic reduction to methanol. Fuel Processing Technology 2023, 251, 107933. [Google Scholar] [CrossRef]
- Bushuyev O S, De Luna P, Dinh C T; et al. What should we make with CO2 and how can we make it? Joule 2018, 2, 825–832. [Google Scholar] [CrossRef]
- Inoue T F A, Konishi S; et al. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Huang H, Shi R, Li Z; et al. Triphase photocatalytic CO2 reduction over silver-decorated titanium oxide at a gas-water boundary. Angewandte Chemie International Edition 2022, 61, e202200802. [Google Scholar] [CrossRef]
- Liu M, Bao X, Ma F; et al. Enhanced stability and activity towards photocatalytic CO2 reduction via supercycle ALD of Cu and TiO2. Chemical Engineering Journal 2022, 429, 132022. [Google Scholar] [CrossRef]
- Yuan S, Bao X, Chen M; et al. Unravelling the pathway determining the CO2 selectivity in photocatalytic toluene oxidation on TiO2 with different particle size. Chemical Engineering Journal 2023, 470, 144138. [Google Scholar] [CrossRef]
- Ma X, Li D, Xie J; et al. Confined space and heterojunction dual modulation of ZnO/ZnS for boosting photocatalytic CO2 reduction. Solar RRL 2023, 7, 2201093. [Google Scholar] [CrossRef]
- Wang X, Liang F, Gu H; et al. In situ synthesized α-Fe2O3/BCN heterojunction for promoting photocatalytic CO2 reduction performance. Journal of Colloid and Interface Science 2022, 621, 311–320. [Google Scholar] [CrossRef]
- Cao H, Xue J, Wang Z; et al. Construction of atomically dispersed Cu sites and S vacancies on CdS for enhanced photocatalytic CO2 reduction. Journal of Materials Chemistry A 2021, 9, 16339–16344. [Google Scholar] [CrossRef]
- Li Z, Wang S, Wu J, Zhou W. Recent progress in defective TiO2 photocatalysts for energy and environmental applications. Renewable and Sustainable Energy Reviews 2022, 156, 111980. [Google Scholar] [CrossRef]
- Collado L, Reñones P, Fermoso J; et al. The role of the surface acidic/basic centers and redox sites on TiO2 in the photocatalytic CO2 reduction. Applied Catalysis B: Environmental 2022, 303, 120931. [Google Scholar] [CrossRef]
- Rehman Z U, Bilal M, Hou J; et al. Photocatalytic CO2 reduction using TiO2-based photocatalysts and TiO2 Z-Scheme heterojunction composites: A review. Molecules 2022, 27, 2069. [Google Scholar] [CrossRef]
- Shtyka O, Shatsila V, Ciesielski R; et al. Adsorption and photocatalytic reduction of carbon dioxide on TiO2. Catalysts 2021, 11, 47. [Google Scholar]
- Reñones P, Fresno F, Oropeza F E; et al. Structural and electronic insight into the effect of indium doping on the photocatalytic performance of TiO2 for CO2 conversion. Journal of Materials Chemistry A 2022, 10, 6054–6064. [Google Scholar] [CrossRef]
- Sun X, Li F, Wang Z; et al. Efficient formic acid dehydrogenation on AuPd/N-TiO2, The role of N dopant and the effect of TiO2 crystalline phase. Chemical Engineering Journal 2023, 475, 146143. [Google Scholar] [CrossRef]
- Zhang B, Wang D, Jiao S; et al. TiO2-x mesoporous nanospheres/BiOI nanosheets S-scheme heterostructure for high efficiency, stable and unbiased photocatalytic hydrogen production. Chemical Engineering Journal 2022, 446, 137138. [Google Scholar] [CrossRef]
- Zhang Y, Li Y, Yu H; et al. Interfacial defective Ti3+ on Ti/TiO2 as visible-light responsive sites with promoted charge transfer and photocatalytic performance. Journal of Materials Science & Technology 2022, 106, 139–146. [Google Scholar]
- Lan K, Wang R, Wei Q; et al. Stable Ti3+ defects in oriented mesoporous titania frameworks for efficient photocatalysis. Angewandte Chemie International Edition 2020, 59, 17676–17683. [Google Scholar] [CrossRef]
- Wang J, Wang K, He Z; et al. Solvent-induced synthesis of hierarchical TiO2 nanoflowers with tunable morphology by monolayer self-assembly for probing the photocatalytic performance. Journal of Nanostructure in Chemistry 2022, 12, 1075–1087. [Google Scholar] [CrossRef]
- Xu Y, Wang F, Lei S; et al. In situ grown two-dimensional TiO2/Ti3CN MXene heterojunction rich in Ti3+ species for highly efficient photoelectrocatalytic CO2 reduction. Chemical Engineering Journal 2023, 452, 139392. [Google Scholar] [CrossRef]
- Yuan R, Wang M, Liao L; et al. 100% N2O inhibition in photocatalytic NOx reduction by carbon particles over Bi2WO6/TiO2 Z-scheme heterojunctions. Chemical Engineering Journal 2023, 453, 139892. [Google Scholar] [CrossRef]
- Xiao J, Chen C, Chen S; et al. Insight into the significantly enhanced photocatalytic CO2 reduction performance of Pt/MnOx dual cocatalysts on sea-urchin-like anatase TiO2 microspheres. Chemical Engineering Journal 2021, 425, 131627. [Google Scholar] [CrossRef]
- Zhang L, Hussain S, Li Q, Yang J. PdCu alloy anchored defective titania for photocatalytic conversion of carbon dioxide into methane with 100% selectivity. Journal of Energy Chemistry 2024, 91, 254–265.
- Jo M, Choi S, Jo J H; et al. Utility of squaraine dyes for dye-sensitized photocatalysis on water or carbon dioxide reduction. ACS Omega 2019, 4, 14272–14283. [Google Scholar] [CrossRef]
- Chon B, Choi S, Seo Y; et al. InP-quantum dot surface-modified TiO2 catalysts for sustainable photochemical carbon dioxide reduction. ACS Sustainable Chemistry & Engineering 2022, 10, 6033–6044. [Google Scholar]
- Liu M, Zheng L, Bao X; et al. Substrate-dependent ALD of Cux on TiO2 and its performance in photocatalytic CO2 reduction. Chemical Engineering Journal 2021, 405, 126654. [Google Scholar] [CrossRef]
- Feng X, Pan F, Tran B Z; et al. Photocatalytic CO2 reduction on porous TiO2 synergistically promoted by atomic layer deposited MgO overcoating and photodeposited silver nanoparticles. Catalysis Today 2020, 339, 328–336. [Google Scholar] [CrossRef]
- Zheng Y, Duan Z, Liang R; et al. Shape-dependent performance of Cu/Cu2O for photocatalytic reduction of CO2. ChemSusChem 2022, 15, e202200216. [Google Scholar] [CrossRef]
- Zhu Z, Yang C, Hwang Y; et al. Fuel generation through photoreduction of CO2 on novel Cu/BiVO4. Materials Research Bulletin 2020, 130, 110955. [Google Scholar] [CrossRef]
- Deng Y, Wan C, Li C; et al. Synergy effect between facet and zero-valent copper for selectivity photocatalytic methane formation from CO2. ACS Catalysis 2022, 12, 4526–4533. [Google Scholar] [CrossRef]
- Wang Z, Song H, Pang H; et al. Photo-assisted methanol synthesis via CO2 reduction under ambient pressure over plasmonic Cu/ZnO catalysts. Applied Catalysis B: Environmental 2019, 250, 10–16. [Google Scholar] [CrossRef]
- Wang T, Chen L, Chen C; et al. Engineering catalytic interfaces in Cuδ+/CeO2-TiO2 photocatalysts for synergistically boosting CO2 reduction to ethylene. ACS Nano 2022, 16, 2306–2318. [Google Scholar] [CrossRef]
- Wu Y A, McNulty I, Liu C; et al. Facet-dependent active sites of a single Cu2O particle photocatalyst for CO2 reduction to methanol. Nature Energy 2019, 4, 957–968. [Google Scholar] [CrossRef]
- Kreft S, Schoch R, Schneidewind J; et al. Improving selectivity and activity of CO2 reduction photocatalysts with oxygen. Chem 2019, 5, 1818–1833. [Google Scholar] [CrossRef]
- Yuan L, Hung S, Tang Z; et al. Dynamic evolution of atomically dispersed Cu species for CO2 photoreduction to solar fuels. ACS Catalysis 2019, 9, 4824–4833. [Google Scholar] [CrossRef]
- Jiang Z, Sun W, Miao W; et al. Living atomically dispersed Cu ultrathin TiO2 nanosheet CO2 reduction photocatalyst. Advanced Science 2019, 6, 1900289. [Google Scholar] [CrossRef]
- Lee B H, Park S, Kim M; et al. Reversible and cooperative photoactivation of single-atom Cu/TiO2 photocatalysts. Nature Materials 2019, 18, 620–626. [Google Scholar] [CrossRef]
- Zhang W, Huang C, Xiao Q; et al. Atypical oxygen-bearing copper boosts ethylene selectivity toward electrocatalytic CO2 reduction. Journal of the American Chemical Society 2020, 142, 11417–11427. [Google Scholar] [CrossRef]
- Eren B, Heine C, Bluhm H; et al. Catalyst chemical state during CO Oxidation reaction on Cu(111) studied with ambient-pressure X-ray photoelectron spectroscopy and near edge X-ray adsorption fine structure sSpectroscopy. Journal of the American Chemical Society 2015, 137, 11186–11190. [Google Scholar] [CrossRef] [PubMed]
- Chang X, Wang T, Zhao Z; et al. Tuning Cu/Cu2O interfaces for the reduction of carbon dioxide to methanol in aqueous solutions. Angewandte Chemie International Edition 2018, 57, 15415–15419. [Google Scholar] [CrossRef] [PubMed]
- Xing M, Zhou Y, Dong C; et al. Modulation of the reduction potential of TiO2–x by fluorination for efficient and selective CH4 generation from CO2 photoreduction. Nano Letters 2018, 18, 3384–3390. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




