Submitted:
09 October 2024
Posted:
10 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
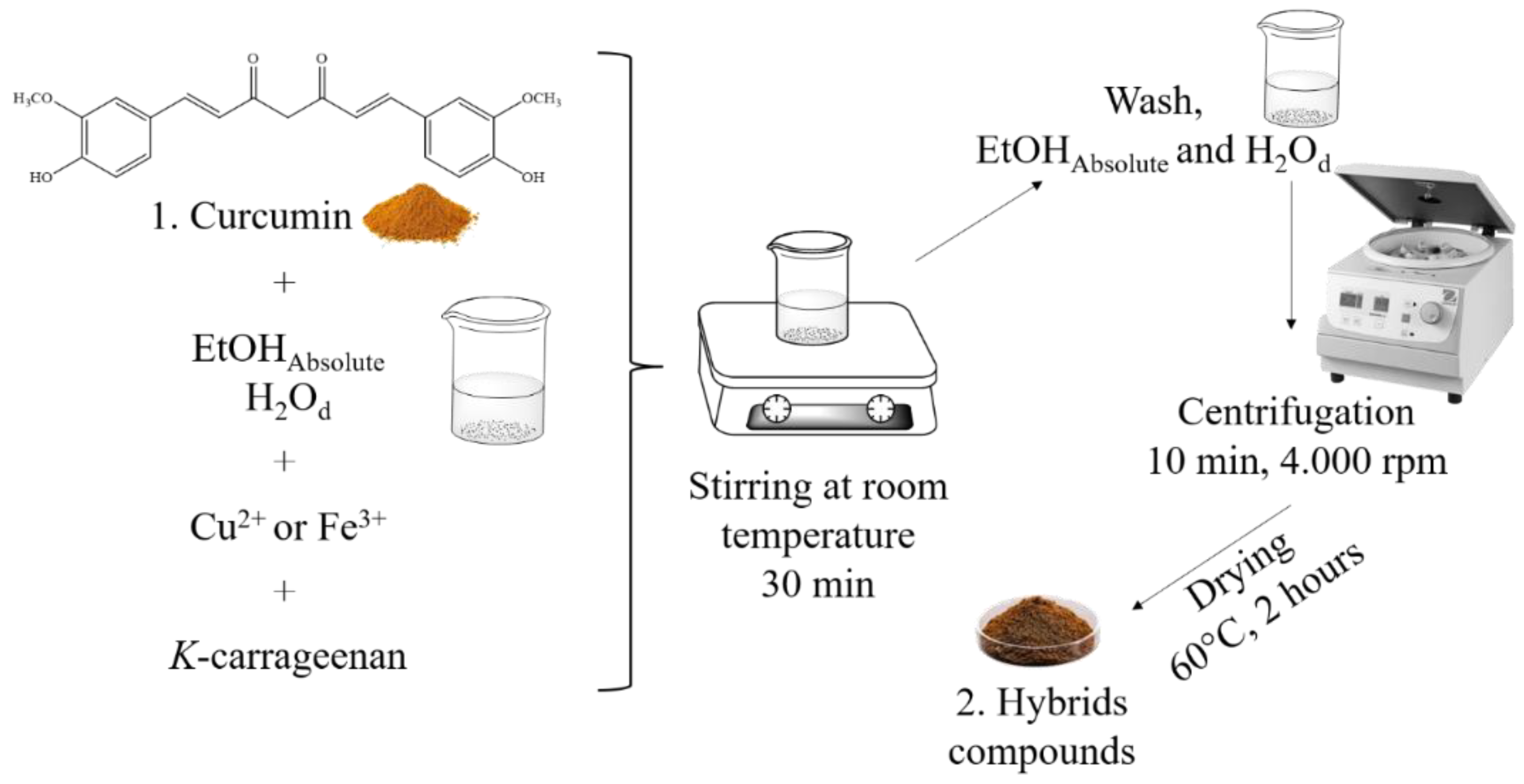
Synthesis of the hybrid materials
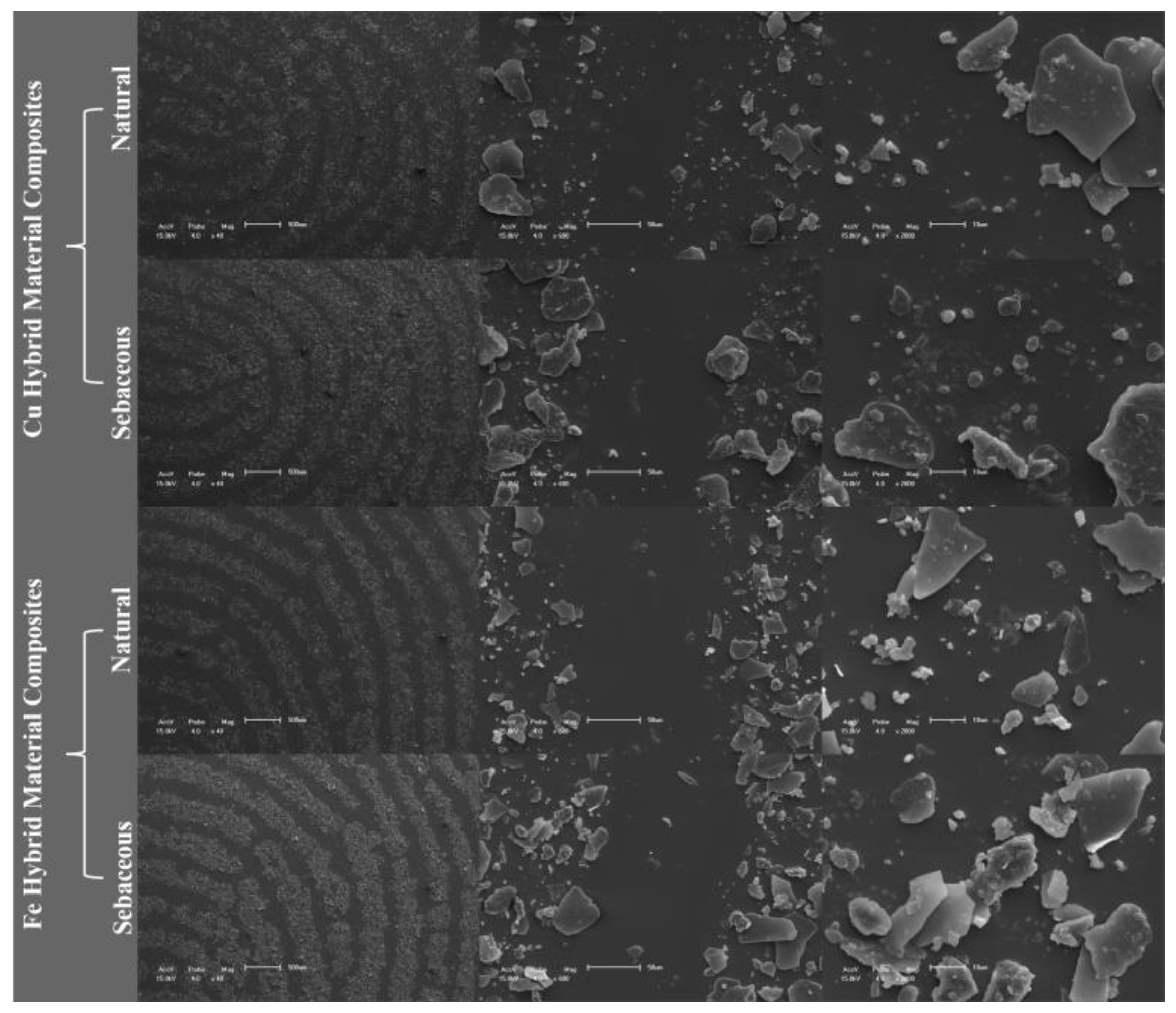
Characterization
High-Performance Liquid Chromatography with UV-Vis Detector (HPLC-UV-Vis)
Preparation of Hybrid Composites
Determination of the Concentration of Cu and Fe in Hybrids
Papilloscopy
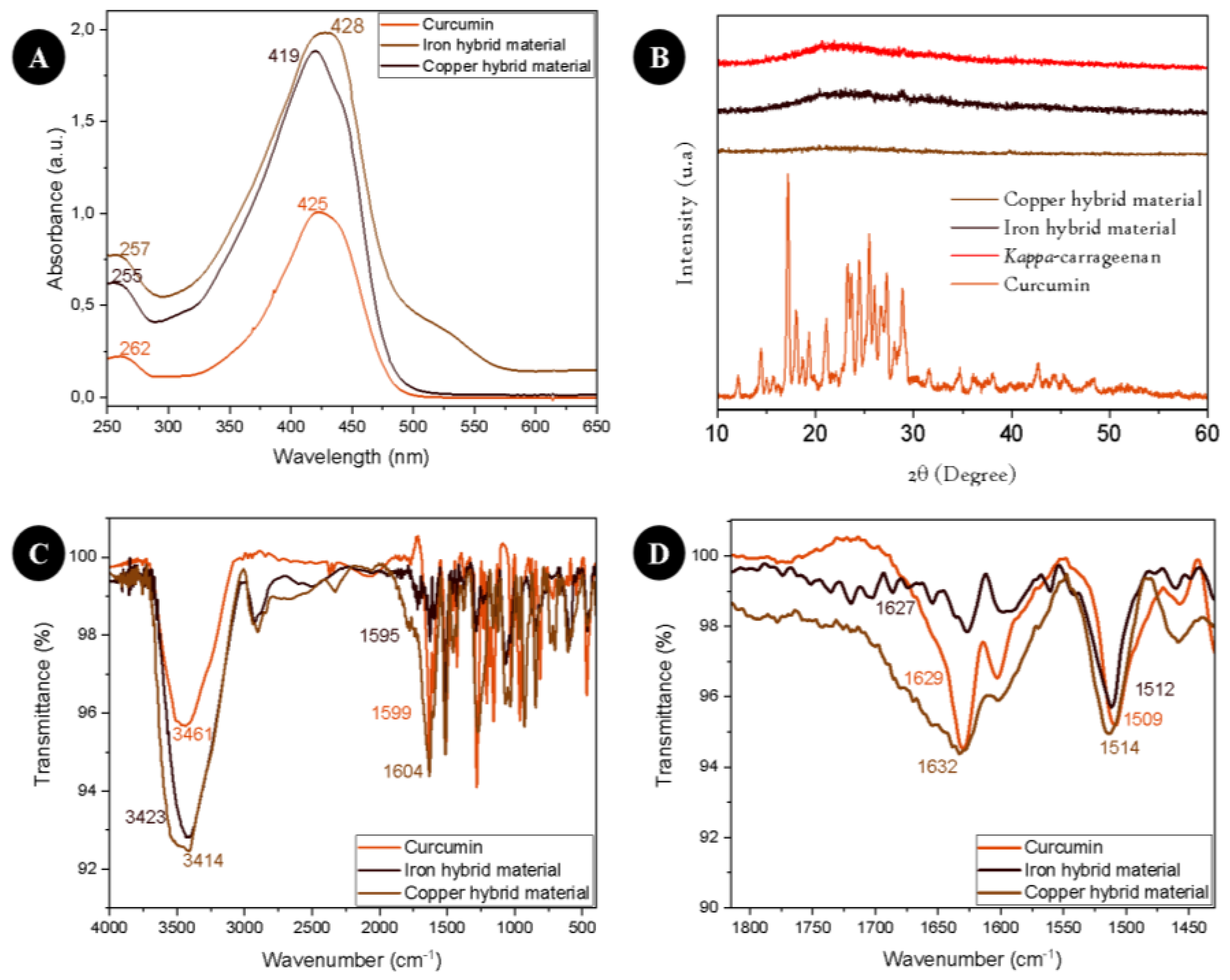
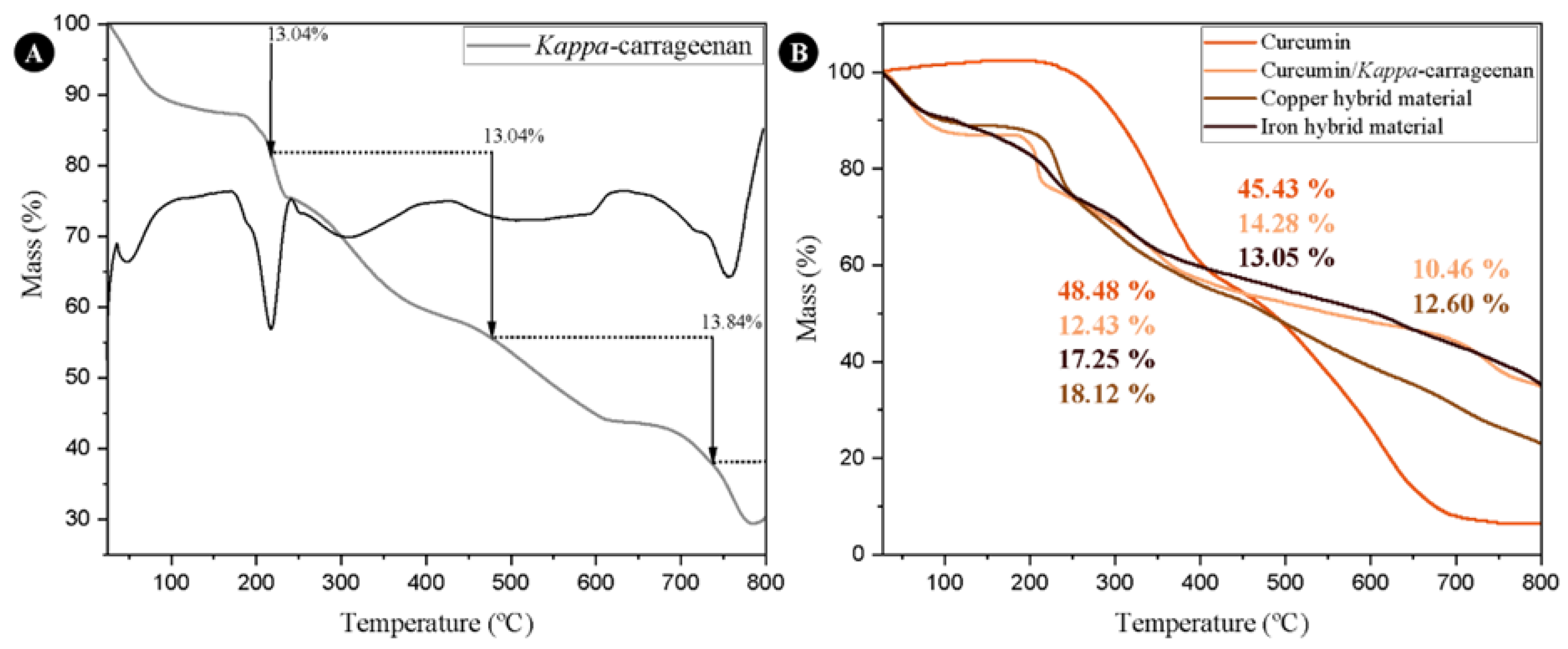
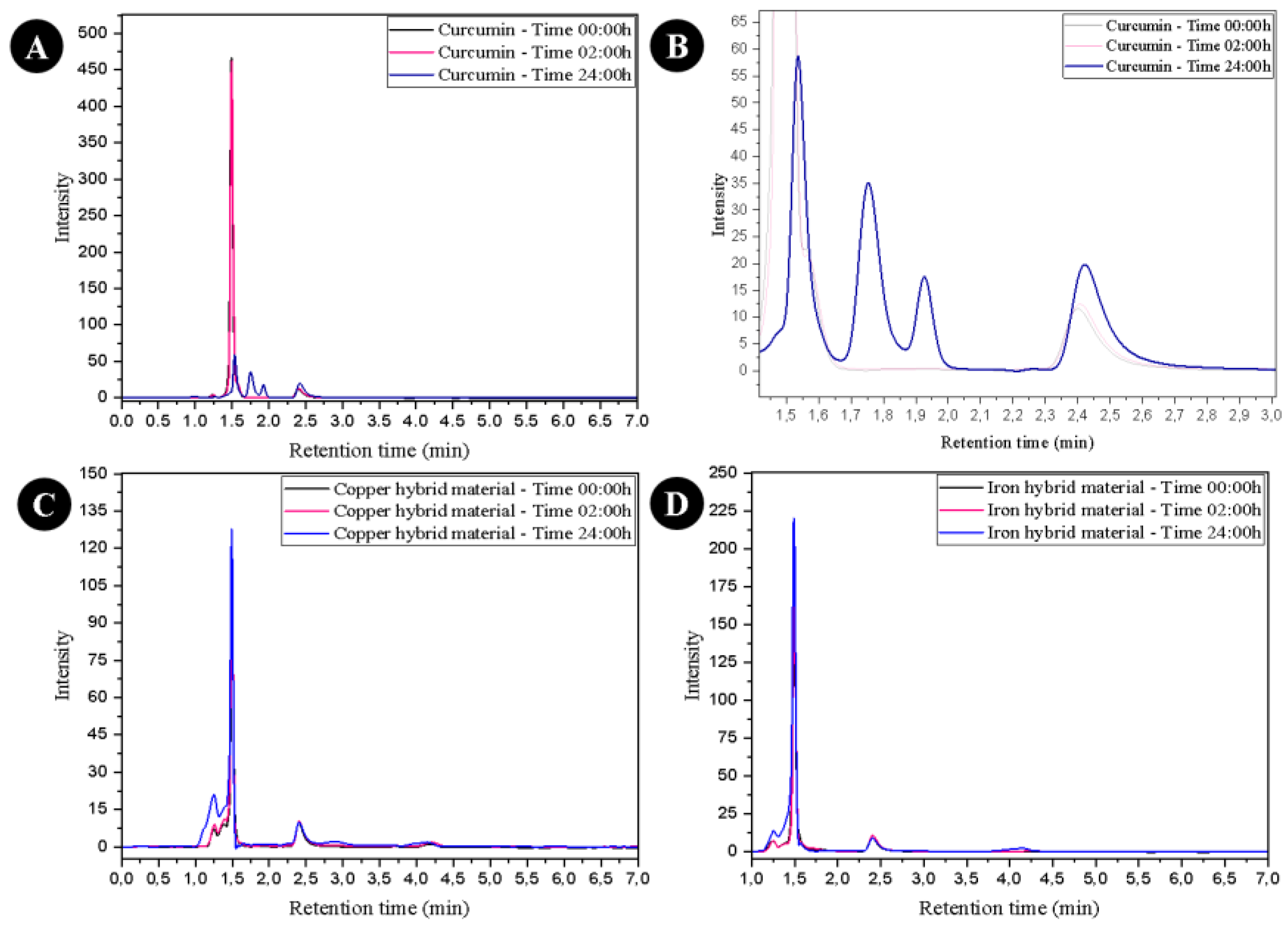
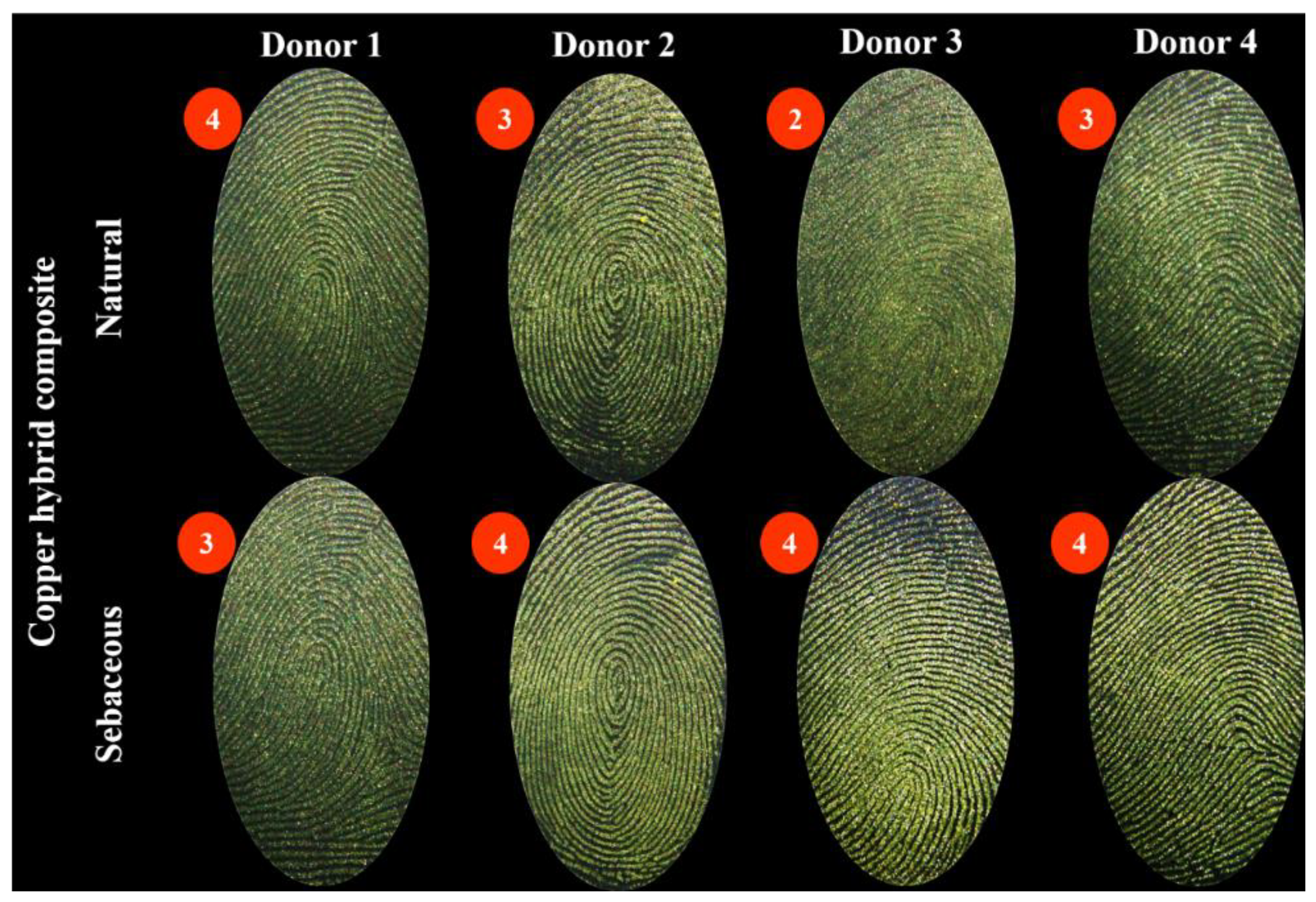
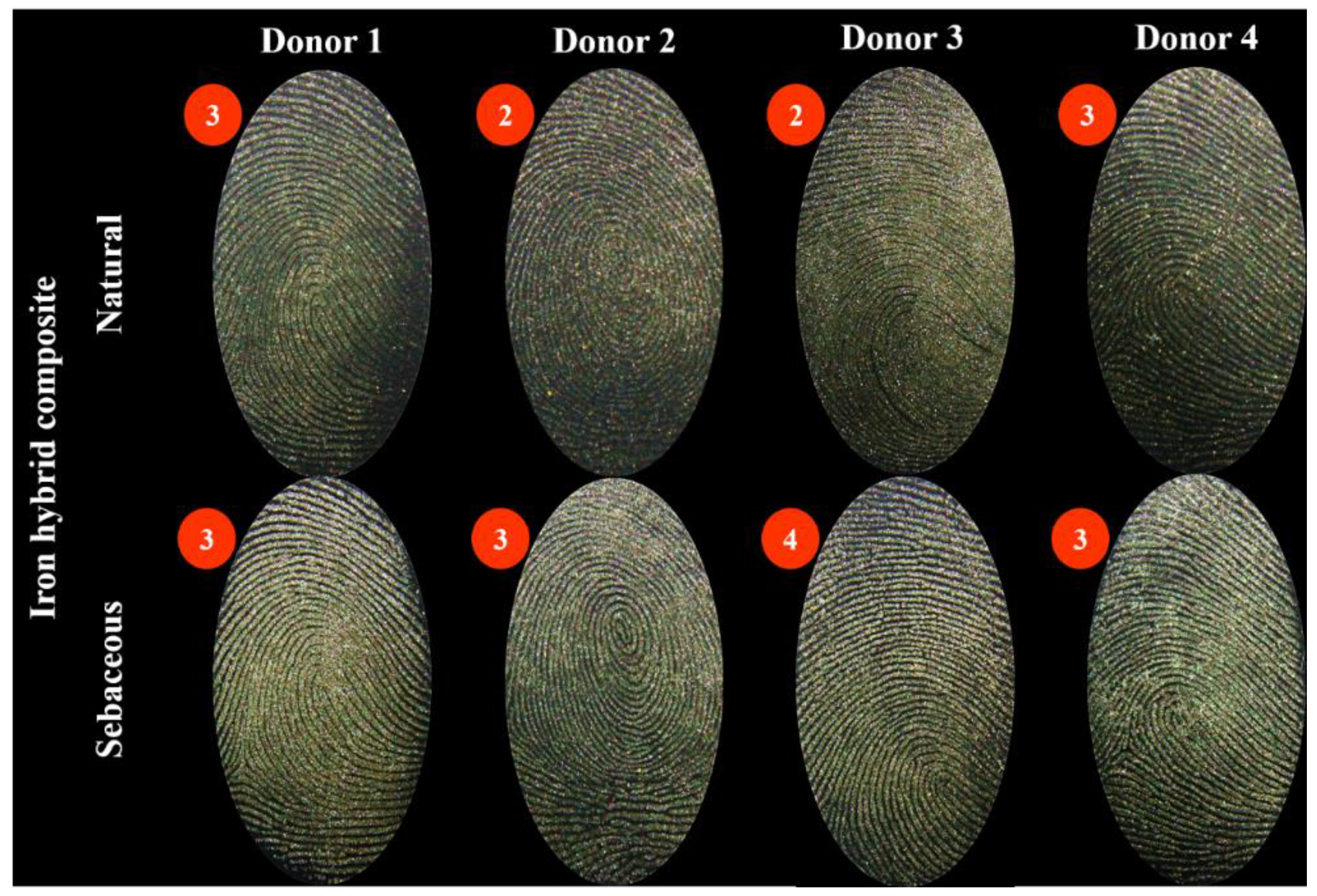
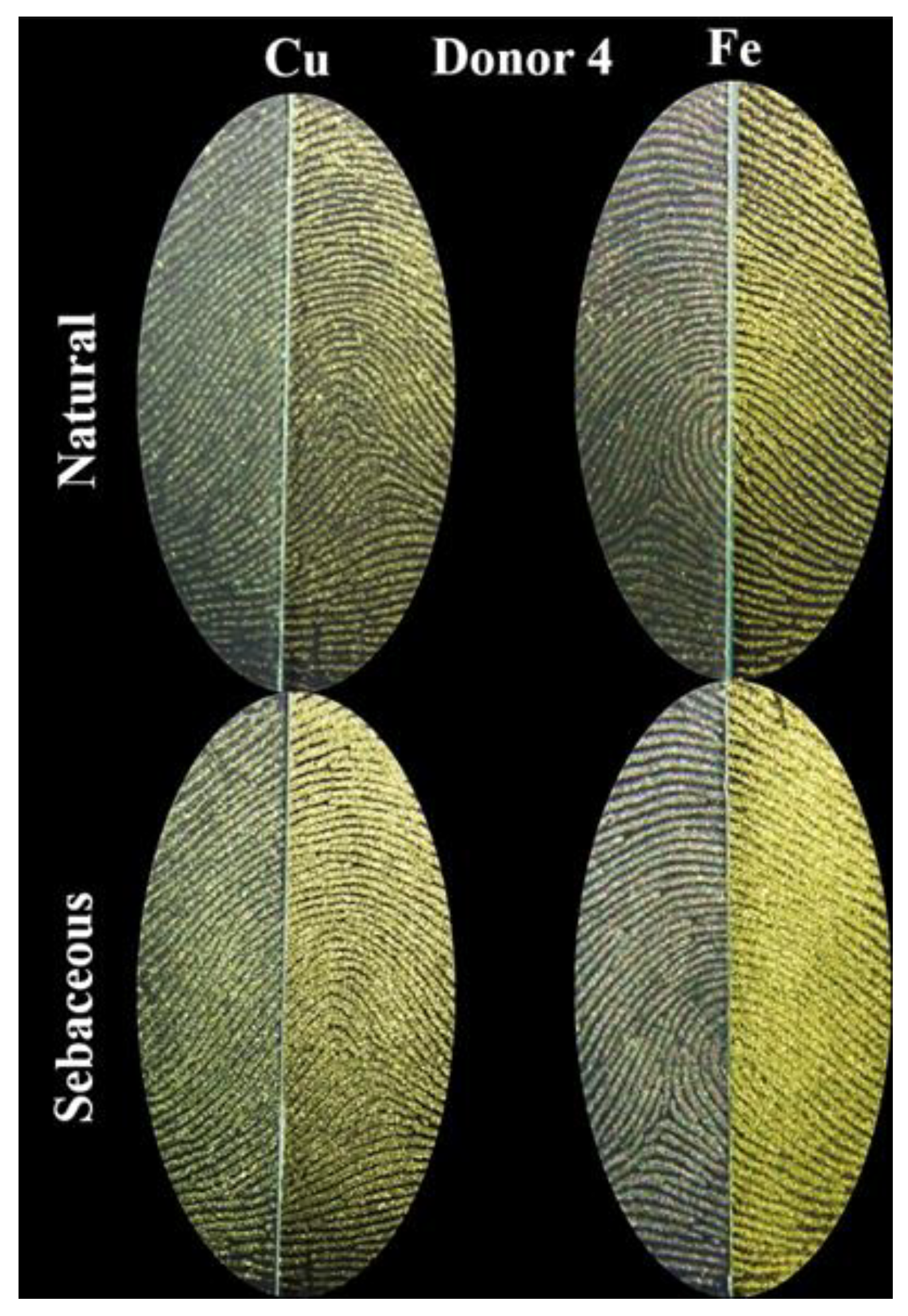
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barros, H.L.; Tavares, L.; Stefani, V. Dye-doped starch microparticles as a novel fluorescent agent for the visualization of latent fingermarks on porous and non-porous substrates. Forensic Chem. 2020, 20, 100264. [Google Scholar] [CrossRef]
- Khare, V.; Singla, A. A review on the advancements in chemical examination of composition of latent fingerprint residues. Egypt. J. Forensic Sci. 2022, 12, 1. [Google Scholar] [CrossRef]
- Robson, R.; Ginige, T.; Mansour, S.; Khan, I.; Assi, S. Analysis of fingermark constituents: a systematic review of quantitative studies. Chem. Pap. 2022, 76, 4645–4667. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Chu, J.; Xi, P.; Wang, C.; Liu, R.; Wang, X.; Cheng, B. Dual-mode luminescent multilayer core-shell UCNPs@ SiO2@ TEuTbB nanospheres for high-level anti-counterfeiting and recognition of latent fingerprints. Appl. Surf. Sci. 2022, 581, 152395. [Google Scholar] [CrossRef]
- Ansari, A.A.; Aldajani, K.M.; AlHazaa, A.N.; Albrithen, H.A. Recent progress of fluorescent materials for fingermarks detection in forensic science and anti-counterfeiting. Co-ord. Chem. Rev. 2022, 462, 214523. [Google Scholar] [CrossRef]
- Poletti, T. Avalição química e aplicação de curcuminas derivadas de cinamaldeído como potenciais reveladores de impressões digitais. Dissertation, Master’s degree, Federal University of Pelotas, Pelotas – Brazil, 2021.
- Passos, L.F.; Berneira, L.M.; Poletti, T.; Mariotti, K.d.C.; Carreño, N.L.V.; Hartwig, C.A.; Pereira, C.M.P. Evaluation and characterization of algal biomass applied to the development of fingermarks on glass surfaces. Aust. J. Forensic Sci. 2021, 53, 337–346. [Google Scholar] [CrossRef]
- Pereira, C. M.; Pacheco, B. S.; da Silva, C. C. Curcumin and analogues: chemical and biological aspects, 2017, LAP LAMBERT Academic Publishing.
- Arab, C.; El Kurdi, R.; Patra, D. Effect of pH on the removal of anionic and cationic dyes using zinc curcumin oxide nanoparticles as adsorbent. Mater. Chem. Phys. 2021, 277, 125504. [Google Scholar] [CrossRef]
- Verma, R. K.; Kumari, P.; Maurya, R. K.; Kumar, V.; Verma, R. B.; Singh, R. K. Medicinal properties of turmeric (Curcuma longa L. ): A review. International Journal of Chemical Studies, 2018, 6, 4–1354. [Google Scholar]
- Yang, M.-Y.; Chang, K.-C.; Chen, L.-Y.; Hu, A. Low-dose blue light irradiation enhances the antimicrobial activities of curcumin against Propionibacterium acnes. J. Photochem. Photobiol. B: Biol. 2018, 189, 21–28. [Google Scholar] [CrossRef]
- Maghsoudi, A.; Yazdian, F.; Shahmoradi, S.; Ghaderi, L.; Hemati, M.; Amoabediny, G. Curcumin-loaded polysaccharide nanoparticles: Optimization and anticariogenic activity against Streptococcus mutans. Mater. Sci. Eng. C 2017, 75, 1259–1267. [Google Scholar] [CrossRef]
- Lin, L.; Li, C.; Zhang, D.; Yuan, M.; Chen, C.-H.; Li, M. Synergic Effects of Berberine and Curcumin on Improving Cognitive Function in an Alzheimer’s Disease Mouse Model. Neurochem. Res. 2020, 45, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Buliga, D.-I.; Diacon, A.; Calinescu, I.; Popa, I.; Rusen, E.; Ghebaur, A.; Tutunaru, O.; Boscornea, C.A. Enhancing the light fastness of natural dyes by encapsulation in silica matrix. J. Photochem. Photobiol. A: Chem. 2022, 432, 114085. [Google Scholar] [CrossRef]
- Souza, C. R. A.; Osme, S. F.; Glória, M. B. A. Stability of curcuminoid pigments in model systems. Journal of Food Processing and Preservation, 1997, 21, 353–363. [Google Scholar] [CrossRef]
- Kumar, P.; Saha, T.; Behera, S.; Gupta, S.; Das, S.; Mukhopadhyay, K. Enhanced efficacy of a Cu2+ complex of curcumin against Gram-positive and Gram-negative bacteria: Attributes of complex formation. J. Inorg. Biochem. 2021, 222, 111494. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Felipe, C.; Guzmán-Vargas, A.; Rivera, J.L.; Lima, E. Highly Stable Hybrid Pigments Prepared from Organic Chromophores and Fluorinated Hydrotalcites. Colorants 2024, 3, 125–135. [Google Scholar] [CrossRef]
- Prasad, S.; Lall, R. Zinc-curcumin based complexes in health and diseases: An approach in chemopreventive and therapeutic improvement. J. Trace Elements Med. Biol. 2022, 73, 127023. [Google Scholar] [CrossRef]
- Silvério, F. Preparação e Caracterização de Materiais Híbridos Formados Pela Interação Entre Hidróxidos Duplos Lamelares e Siliconas Aniônicas. Thesis, Doctorate’s degree, Federal University of São Paulo, São Paulo – Brazil, 2009.
- Lee, J.Y.; Lee, S.; Choi, J.H.; Na, K. ι-Carrageenan nanocomposites for enhanced stability and oral bioavailability of curcumin. Biomater. Res. 2021, 25, 32. [Google Scholar] [CrossRef]
- Tan, K.-X.; Ng, L.-L.E.; Loo, S.C.J. Formulation Development of a Food-Graded Curcumin-Loaded Medium Chain Triglycerides-Encapsulated Kappa Carrageenan (CUR-MCT-KC) Gel Bead Based Oral Delivery Formulation. Materials 2021, 14, 2783. [Google Scholar] [CrossRef]
- Wan, H.; Li, C.; Mahmud, S.; Liu, H. Kappa carrageenan reduced-stabilized colloidal silver nanoparticles for the degradation of toxic azo compounds. Colloids Surfaces A: Physicochem. Eng. Asp. 2021, 616, 126325. [Google Scholar] [CrossRef]
- Leitzke, A.; Berneira, L.; Rosa, B.; Moreira, B.; Mariotti, K.; Venzke, D.; Pereira, C. A QUIMICA DE PRODUTOS NATURAIS APLICADOS A REVELADORES DE IMPRESSOES DIGITAIS LATENTES. Quimica Nova 2022, 45, 424–434. [Google Scholar] [CrossRef]
- Bueno, D.T.; Leitzke, A.F.; Crizel, R.L.; Jansen-Alves, C.; Bertizzolo, E.G.; da Silva, J.P.; Sejanes, G.Q.; Mariotti, K.d.C.; de Pereira, C.M.P. Characterization of Bixin by UV-Visible Spectroscopy and HPLC, and Its Application as Latent Fingermark Developer. Analytica 2024, 5, 107–118. [Google Scholar] [CrossRef]
- Balsan, J. D.; Rosa, B. N.; Pereira, C. M.; Santos, C. M. Desenvolvimento de metodologia de revelação de impressão digital latente com chalconas. Química Nova, 2019, 42, 8–845. [Google Scholar] [CrossRef]
- da Rosa, B.N.; da Rosa, M.P.; Poletti, T.; de Lima, N.P.K.; Maron, G.K.; Lopes, B.V.; Mariotti, K.d.C.; Beck, P.H.; Carreno, N.L.V.; de Pereira, C.M.P. Green Composites from Thiophene Chalcones and Rice Husk Lignin: An Alternative of Powder for Latent Fingermark. Surfaces 2022, 5, 481–488. [Google Scholar] [CrossRef]
- da Rosa, B.N.; Maron, G.K.; Lopes, B.V.; Rocha, A.C.S.; Gatti, F.d.M.; Machado, J.O.A.; Barichello, J.M.; Mariotti, K.d.C.; Trossini, G.H.G.; Carreno, N.L.V.; et al. Dimethylaminochalcones with silicon dioxide and zinc oxide as latent fingermark developer powder. Mater. Chem. Phys. 2022, 295, 127033. [Google Scholar] [CrossRef]
- Pacheco, B.S.; Da Silva, C.C.; Da Rosa, B.N.; Mariotti, K.C.; Nicolodi, C.; Poletti, T.; Segatto, N.V.; Collares, T.; Seixas, F.K.; Paniz, O.; et al. Monofunctional curcumin analogues: evaluation of green and safe developers of latent fingerprints. Chem. Pap. 2021, 75, 3119–3129. [Google Scholar] [CrossRef]
- Poletti, T.; Berneira, L.M.; Passos, L.F.; da Rosa, B.N.; de Pereira, C.M.; Mariotti, K.d.C. Preliminary efficiency evaluation of development methods applied to aged sebaceous latent fingermarks. Sci. Justice 2021, 61, 378–383. [Google Scholar] [CrossRef]
- Venzke, D.; Poletti, T.; Rosa, B.N.; Berneira, L.M.; de Lima, N.P.; de Oliveira, T.F.; Carreño, N.L.; Mariotti, K.d.C.; Duarte, L.S.; Nobre, S.M.; et al. Preparation of fluorescent bisamides: A new class of fingermarks developers. Chem. Data Collect. 2021, 33, 100680. [Google Scholar] [CrossRef]
- Lima, N. P.; Rosa, B. N.; Poletti, T.; Moreira, B. C.; Leitzke, A. F.; Mariotti, K. C. , Carreno, N. L. V.; Pereira, C. M. As clássicas hidrazonas como reveladores de impressões digitais: uma proposta de química orgânica experimental. Química Nova, 2023, 46, 215–221. [Google Scholar]
- Leitzke, A.F.; Bueno, D.T.; Poletti, T.; Maron, G.K.; Lopes, B.V.; Morais, E.V.; Inacio, A.P.d.O.L.; Silveira, C.I.; da Silva, J.P.; Dias, D.; et al. The effectiveness of natural indigo/kaolinite composite powder in the development of latent fingermarks. Egypt. J. Forensic Sci. 2024, 14, 19. [Google Scholar] [CrossRef]
- Li, S.; Mu, B.; Yan, P.; Kang, Y.; Wang, Q.; Wang, A. Incorporation of Different Metal Ion for Tuning Color and Enhancing Antioxidant Activity of Curcumin/Palygorskite Hybrid Materials. Front. Chem. 2021, 9, 760941. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Utomo, E.; Domínguez-Robles, J.; Detamornrat, U.; Donnelly, R.F.; Larrañeta, E. A New and Sensitive HPLC-UV Method for Rapid and Simultaneous Quantification of Curcumin and D-Panthenol: Application to In Vitro Release Studies of Wound Dressings. Molecules 2022, 27, 1759. [Google Scholar] [CrossRef] [PubMed]
- Modwi, A.; Ali, M.K.M.; Taha, K.K.; Ibrahem, M.A.; El-Khair, H.M.; Eisa, M.H.; Elamin, M.R.; Aldaghri, O.; Alhathlool, R.; Ibnaouf, K.H. Structural and optical characteristic of chalcone doped ZnO nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 29, 2791–2796. [Google Scholar] [CrossRef]
- Fioravanti, M.I.A.; Pizano, F.P.; Rebellato, A.P.; Milani, R.F.; Morgano, M.A.; Bragotto, A.P.A. Turmeric products: Evaluation of curcumin and trace elements. Food Res. Int. 2024, 196, 115028. [Google Scholar] [CrossRef]
- Sears, V.; Bleay, S.; Bandey, H.; Bowman, V. A methodology for finger mark research. Sci. Justice 2011, 52, 145–160. [Google Scholar] [CrossRef]
- Subhan, M.A.; Alam, K.; Rahaman, M.S.; Rahman, M.A.; Awal, R. Synthesis and Characterization of Metal Complexes Containing Curcumin (C21H20O6) and Study of their Anti-microbial Activities and DNA-binding Properties. J. Sci. Res. 2013, 6, 97–109. [Google Scholar] [CrossRef]
- Zhou, S.-S.; Xue, X.; Wang, J.-F.; Dong, Y.; Jiang, B.; Wei, D.; Wan, M.-L.; Jia, Y. Synthesis, optical properties and biological imaging of the rare earth complexes with curcumin and pyridine. J. Mater. Chem. 2012, 22, 22774–22780. [Google Scholar] [CrossRef]
- Sayyar, Z.; Malmiri, H.J. Photocatalytic and antibacterial activities study of prepared self-cleaning nanostructure surfaces using synthesized and coated ZnO nanoparticles with Curcumin nanodispersion. Z. Fur Krist. Mater. 2018, 234, 307–328. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Development and characterization of carrageenan/grapefruit seed extract composite films for active packaging. Int. J. Biol. Macromol. 2014, 68, 258–266. [Google Scholar] [CrossRef]
- Chen, X.; Zou, L.-Q.; Niu, J.; Liu, W.; Peng, S.-F.; Liu, C.-M. The Stability, Sustained Release and Cellular Antioxidant Activity of Curcumin Nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef]
- Halevas, E.; Papadopoulos, T.; Swanson, C.; Smith, G.; Hatzidimitriou, A.; Katsipis, G.; Pantazaki, A.; Sanakis, I.; Mitrikas, G.; Ypsilantis, K.; et al. In-depth synthetic, physicochemical and in vitro biological investigation of a new ternary V(IV) antioxidant material based on curcumin. J. Inorg. Biochem. 2018, 191, 94–111. [Google Scholar] [CrossRef]
- Halevas, E.; Pekou, A.; Papi, R.; Mavroidi, B.; Hatzidimitriou, A.G.; Zahariou, G.; Litsardakis, G.; Sagnou, M.; Pelecanou, M.; Pantazaki, A.A. Synthesis, physicochemical characterization and biological properties of two novel Cu(II) complexes based on natural products curcumin and quercetin. J. Inorg. Biochem. 2020, 208, 111083. [Google Scholar] [CrossRef] [PubMed]
- Kulal, P.; Badalamoole, V. Hybrid nanocomposite of kappa-carrageenan and magnetite as adsorbent material for water purification. Int. J. Biol. Macromol. 2020, 165, 542–553. [Google Scholar] [CrossRef] [PubMed]
- de França, B.M.; Oliveira, S.S.; Souza, L.O.; Mello, T.P.; Santos, A.L.; Forero, J.S.B. Synthesis and photophysical properties of metal complexes of curcumin dyes: Solvatochromism, acidochromism, and photoactivity. Dye. Pigment. 2022, 198, 110011. [Google Scholar] [CrossRef]
- Prasad, S.; DuBourdieu, D.; Srivastava, A.; Kumar, P.; Lall, R. Metal–Curcumin Complexes in Therapeutics: An Approach to Enhance Pharmacological Effects of Curcumin. Int. J. Mol. Sci. 2021, 22, 7094. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; El Rayess, Y.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Bonifácio, D.; Martins, C.; David, B.; Lemos, C.; Neves, M.; Almeida, A.; Pinto, D.; Faustino, M.; Cunha. Photodynamic inactivation of Listeria innocua biofilms with food-grade photosensitizers: a curcumin-rich extract of Curcuma longa vs commercial curcumin. J. Appl. Microbiol. 2018, 125, 282–294. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, B.H. Preparation of curcuminoid microemulsions fromCurcuma longaL. to enhance inhibition effects on growth of colon cancer cells HT-29. RSC Adv. 2018, 8, 2323–2337. [Google Scholar] [CrossRef]
- Khurana, Amrik; Ho, C. -T. High Performance Liquid Chromatographic Analysis of Curcuminoids and Their Photo-Oxidative Decomposition Compounds in Curcuma Longal. J. Liq. Chromatogr. 1988, 11, 11–2295. [Google Scholar]
- Chávez, D.; Garcia, C.; Oliva, J.; Diaz-Torres, L. A review of phosphorescent and fluorescent phosphors for fingerprint detection. Ceram. Int. 2020, 47, 10–41. [Google Scholar] [CrossRef]
- Vadivel, R.; Nirmala, M.; Anbukumaran, K. Commonly available, everyday materials as non-conventional powders for the visualization of latent fingerprints. Forensic Chem. 2021, 24, 100339. [Google Scholar] [CrossRef]
- De Alcaraz-Fossoul, J.; Patris, C.M.; Muntaner, A.B.; Feixat, C.B.; Badia, M.G. Determination of latent fingerprint degradation patterns—a real fieldwork study. Int. J. Leg. Med. 2012, 127, 857–870. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




