1. Introduction
Hepatitis A virus (HAV) is one of the major causes of acute viral hepatitis and is easily acquired via faecal-oral route [
1,
2,
3]. The clinical presentation and course of the disease vary according to the patient’s age. HAV infection is usually asymptomatic at younger ages. As the age advances, jaundice, fatigue, nausea and other symptoms are more commonly observed. In very rare cases, HAV can cause liver failure and death. The incidence of the disease varies in different regions of the world. It varies with some of the fundamental socioeconomic indicators such as national income per capita, hygiene conditions, access to safe water and sewage infrastructure [
2,
3,
4]. The HAV infection seroprevalence is decreasing worldwide except for underdeveloped and some developing countries; however, HAV infection is still a significant public health issue [
5].
World Health Organization (WHO) divides the geographical regions into high, intermediate and low endemic areas regarding HAV infection [
6,
7]. In high endemic regions with low hygiene conditions, children contact the HAV before ten years of age and develop life-long immunity. In such countries, symptomatic HAV infections and HAV epidemics are rarely encountered [
3]. In intermediate endemic regions, contact with the virus usually occurs later in life, and acute hepatitis with HAV commonly affects adolescents and young adults. As countries and sanitation conditions develop, the age of acquiring HAV infection increases as the incidence rate decreases [
3]. HAV epidemics may occur in low endemic regions, and the course of the disease is more serious and complicated with the advancing age [
2,
4,
5]. Turkey is stated as intermediate endemic country in the worldwide reports [
6,
7,
8].
Hepatitis A virus has only one serotype, so once the disease is survived, life-long immunity with IgG type antibodies occurs [
9]. Infection rates in a population can be tract with IgG anti-HAV, and comments about HAV seroprevalence can be proposed [
3]. Age-specific categorization of HAV seroprevalence enables indirect measurement of age-specific incidence rates of HAV infections, and it is accepted as the best way to explain the status of HAV infection in a country [
10]. Age-specific HAV seroprevalence is also an essential parameter in determining the appropriate age for vaccination and routine immunization [
5,
11].
Many papers study the HAV seroprevalence rates in different provinces in our country. However, there needs to be a paper that systematically evaluates age-specific seroprevalence rates in Turkey. With a systematic approach, we perform a meta-analysis of age-specific seroprevalence rates for the last 23 years.
2. Materials and Methods
2.2. Systematic Literature Search
A systematic search was performed in PubMed, Google Scholar and the online database of the Library of Sakarya University (which searches Web of Science, EBSCOhost, SCOPUS, ULAKBIM Medical database, Turkmedline, etc.) both in Turkish and English languages to identify published articles, conference abstracts, and other online resources. We used a systematic search algorithm similar to the previously proposed ones (
Figure A1) [
1,
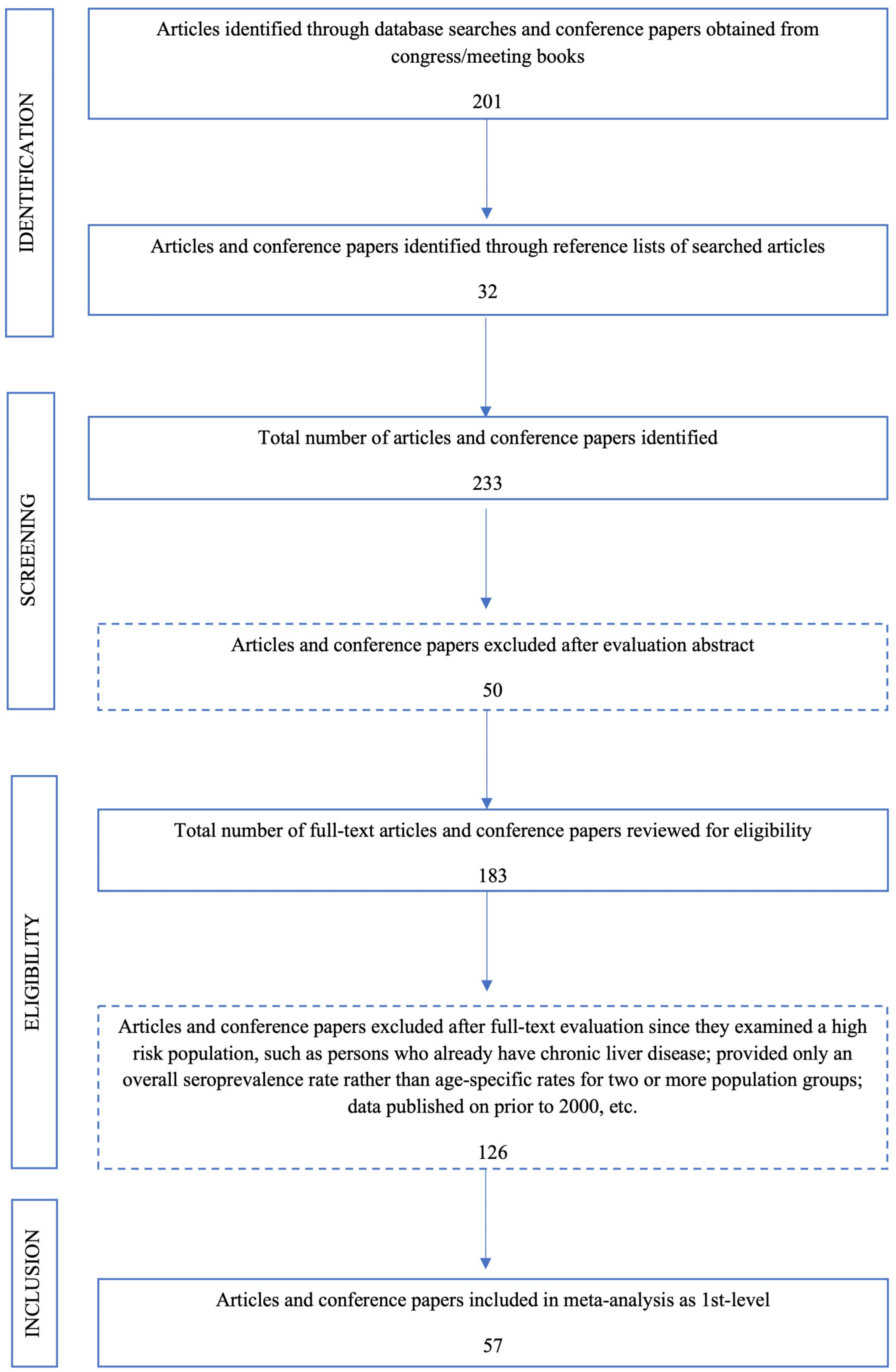
12]. The search was performed using the keywords “hepatitis A”, “HAV”, “hepatitis A seroprevalence in Turkey”, “HAV seroprevalence in Turkey”, “hepatitis A prevalence in Turkey”, “HAV prevalence in Turkey”. We examined all the papers’ reference lists and collected all the published data between 2000 and 2023. Two authors evaluated all the scientific data to determine the eligibility for meta-analysis. Studies from different provinces of Turkey were divided into western, central and eastern regions.
2.2. Categorical Scoring and Extraction of the Data
All the eligible studies were given a quality score according to previously determined criteria [
1,
13,
14]. The basic criteria for categorical scoring are the sampling method, age group ranges, study population, and diagnostic laboratory method.
1- Sampling method: Equally and equiponderant selected from urban and rural areas 10, and randomly chosen from specific group 8, simply and randomly 5, non-randomized 1, not identified 0.
2- Age group ranges: All the ages (0 to over 80) were included 10, 80-100% of all ages 8, 60-80% 6, 40-60% 4, 20-40% 2, less than 20% 1.
3- Study population: Whole country 5, whole region or province 4, districts or neighborhoods 3, only one district/neighborhood 1.
4- Diagnostic laboratory method: Chemiluminescence, Enzyme-Linked Immunosorbent Assay (ELISA), Enzyme-Linked Fluorescent Assay (ELFA), etc. methods 2, immunochromatic tests (card tests) 1, not identified 0.
After scoring with the given chart, the mean value and standard deviation (SD) were calculated. Studies with SD+1 and higher were assigned high quality, whereas SD-1 and lower were assigned low quality. Papers lying in between SD ± 1 were accepted as medium quality.
2.3. Inclusion Criteria
Studies published in or after 2000,
Studies containing age-specific seroprevalence data in at least one age group,
Only original articles that do not focus on high-risk groups such as healthcare workers, military personnel, sewer workers, etc., are included.
2.4. Exclusion Criteria
Review articles, epidemic research, animal studies, environmental research,
Studies involving individuals vaccinated with HAV or investigating the efficacy of vaccination, genetics and other laboratory-based studies were excluded.
Studies evaluating only IgM anti-HAV,
Studies examining particular patient groups such as dialysis patients, those with acute or chronic liver disease, liver transplant patients, and other chronic infection patients,
Reports on overall seroprevalence rates and studies on HAV infection incidence rates,
Studies with general seroprevalence rates instead of age-specific values, and
Multicenter studies and studies with inconsistent data were not included in this study.
Duplicate studies that appeared in more than one database were removed. The systematic review and meta-analysis were conducted by the PRISMA guidelines (
Figure A1) [
15,
16].
2.5. Age Groups
We figured out that researchers used various age groupings in different studies, which meant no standard for determining the age groups. We divided ages into four different age groups to analyze and compare the age-specific data more appropriately and accurately. Age groups were classified as 0-<5 years, 5-<15, 15-35 and >35 years. We also preferred not to use overall rates of HAV seroprevalence rates. WHO reports were taken into consideration in determining age groups [
17].
2.6. Statistical Analysis
We calculated pooled estimates with 95% confidence intervals (CI) both within age groups and overall, across all studies, using both fixed and random effects models, by Comprehensive Meta-analysis (CMA) ver. 3.3 software (Biostat, Englewood, USA). Homogeneity across studies was assessed using I square statistic. In the presence of a significant heterogeneity (I2 >50%), Cochrane’s Q test was applied. A level of <0.01 was considered as significant.
Descriptive statistical analyses (mean, SD, etc.), normality distribution, and analysis of differences between groups (One-Way Anova Test) were calculated using SPSS software (IBM SPSS Statistics, Version 25.0; IBM Corp., Armonk, NY, USA). Parametric tests were used for data conforming to normal distribution. A p value of less than 0.05 was considered significant.
3. Results
After a systematic literature search described above, we examined 177 articles and six conference papers with age-specific HAV seroprevalence data. As a result of the database search with keywords, 201 studies were found. In addition, 32 publications were found as a result of the search made from the bibliography of these articles. After examining the abstracts, 50 irrelevant studies and 126 additional studies that did not meet the inclusion criteria were eliminated. A total of 57 original articles were included in this meta-analysis (
Figure A1) [
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61,
62,
63,
64,
65,
66,
67,
68,
69,
70,
71,
72,
73,
74].
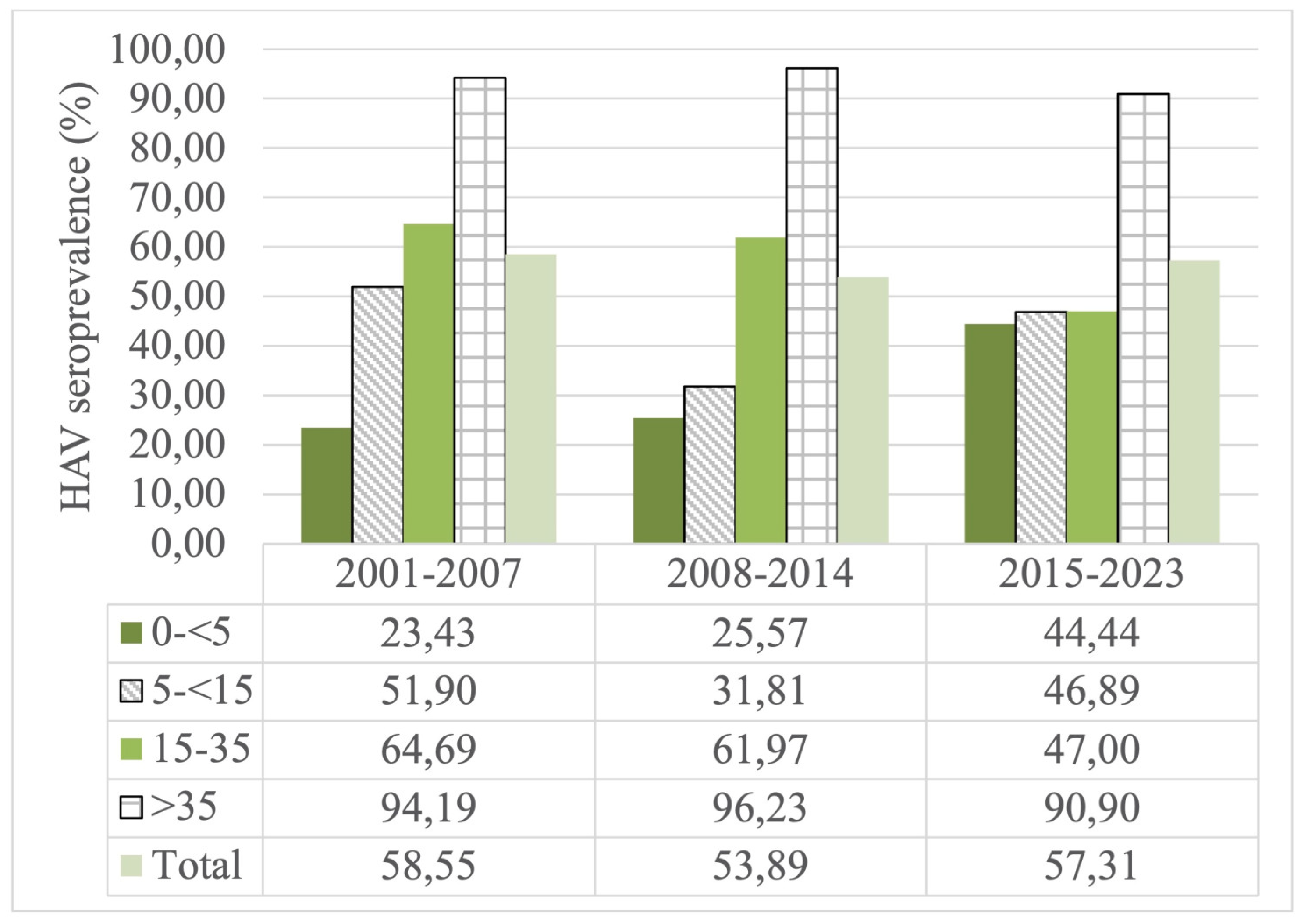
In the 0-<5 age group, the highest HAV prevalence rate was calculated as 44.44%, 25.57% and 23.43% between 2015-2023, 2008-2014 and 2001-2007, respectively. In the 5-<15 age group, the highest HAV prevalence rate was calculated as 51.90%, 46.89% and 31.81% between 2001-2007, 2015-2023, and 2008-2014, respectively. In the 15-35 age group, the highest prevalence rate was calculated as 64.69%, 61.97% and 47% between 2001-2007, 2008-2014, 2015-2023 and, respectively. The highest prevalence rates in the age group of >35 years and above were found to be 96.23%, 94.19% and 90.90 % between 2008-2014, 2001-2007 and 2015-2023, respectively.
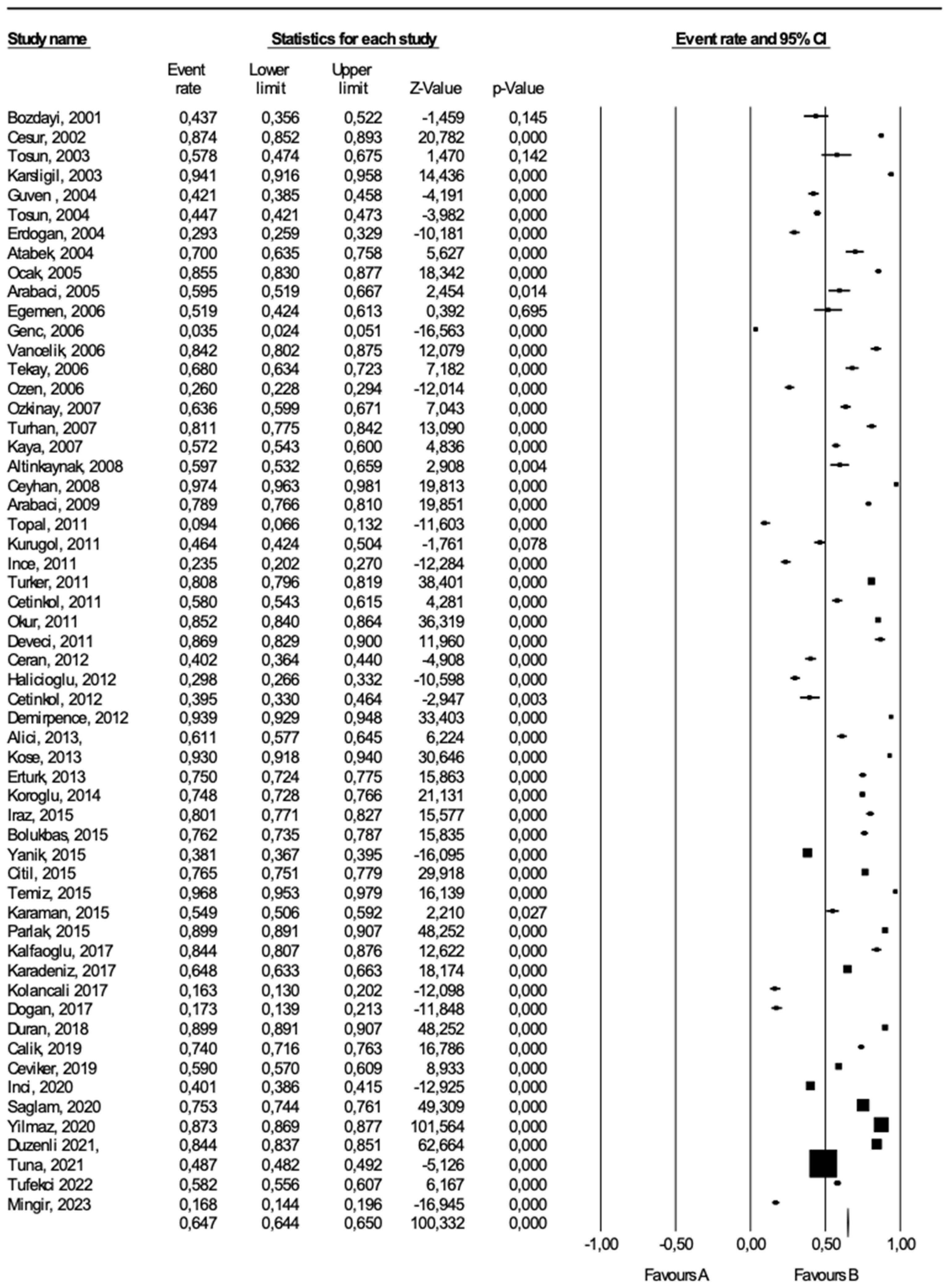
We applied “forest plot analysis” for eligible HAV seroprevalence studies (
Figure A2). We presented the results of both fixed and random effect models. However, setting comments from the random effect model [18–74 in this analysis may appropriate since age-specific HAV seroprevalence studies were heterogeneous.
In this meta-analysis, we evaluated 57 published articles on age-specific HAV seroprevalence, including a total of 157,836 patients. Pooling of the results extracted from all included reports, independent of study design, yielded an event estimation of 64.5% for the random effect model (95% CI: 58.3-70) and 63.7% (95% CI: 63.5-64) for fixed effects model (Q
2 = 25085.65, df = 57, p<0.0001, I
2 = 99.77). In the comprehensive meta-analysis, event rates calculated with fixed and random effect models gave close rates and consequently showed high sensitivity. (
Table A1)
According to categorical scoring, the studies ranged between 7 and 22. The distribution of the articles examined in the meta-analysis according to high, medium and low category scores were 9, 42 and 6, respectively. After removing low quality articles, the point estimate of HAV prevalence in the random effect model shifted from 61,8% (95% CI: 60,5-63,2). The negative effect of articles with low quality scores on seropositivity rates was limited.
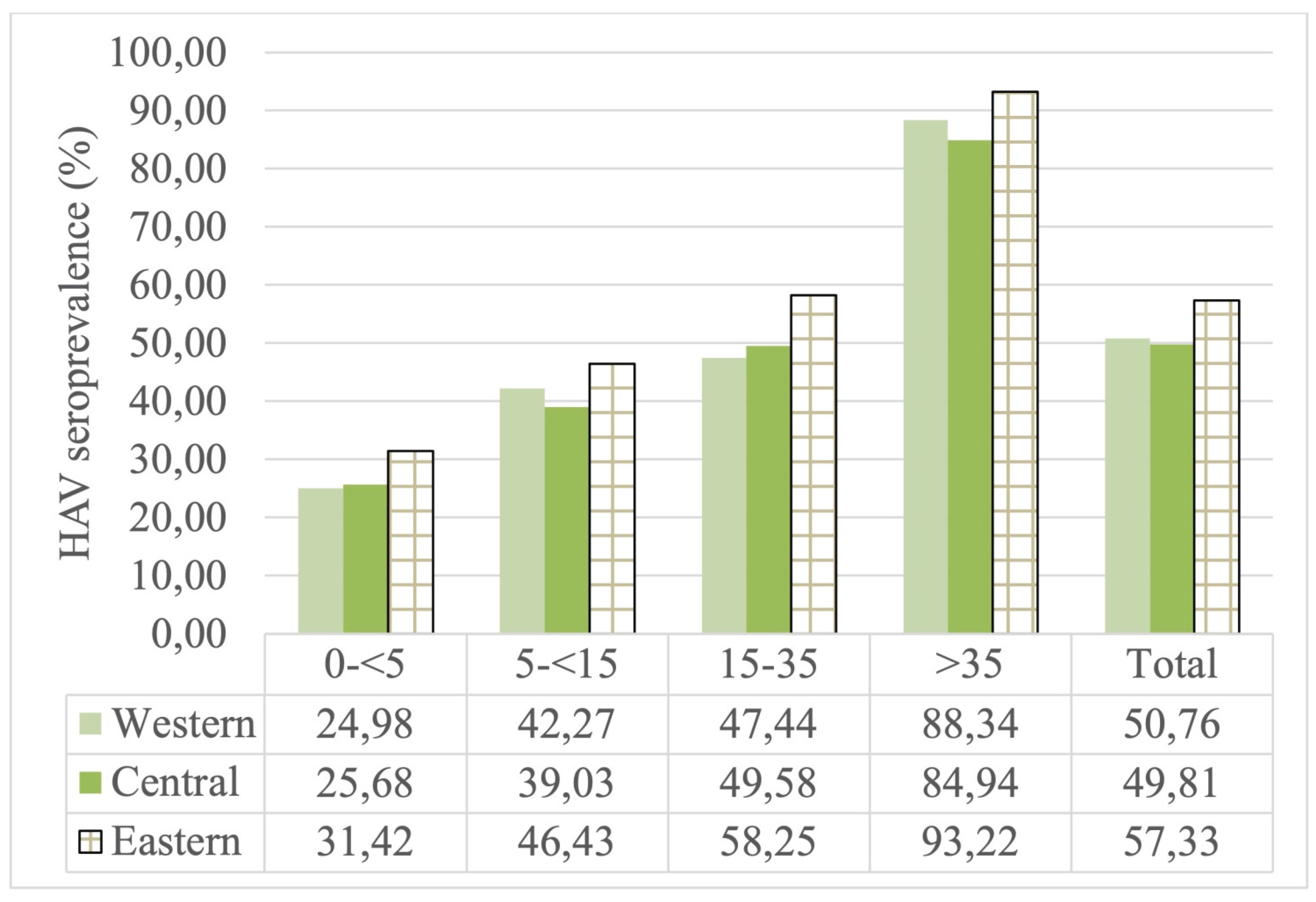
The seroprevalence rate was found to be lower in the central (49.81%) and western (50.76%) regions than in the eastern region (57.33%) in all age groups. A statistically significant difference was found between the seroprevalence rates of the 0-<5 and 15-35 age groups and geographical regions (p<0.05). Additionally, the articles included in the study in the eastern region contain high and medium endemicity notifications. It may be approaching the intermediate endemicity rates of the eastern region, but the lack of data for subsequent years in 2020 does not provide an accurate assessment. As a result, the data that presenting appropriate information of age groups in evaluated studies were indicate that the incidence rate remains higher in eastern region of the country than it is in Turkey’s other regions. (
Figure A3)
The seroprevalence rate was calculated for the 2001-2007 (58,55%), 2008-2014 (53,89%) and 2015-2023 (57.31%) in year groups. A statistically significant increase was detected in the 0-<5 age group over the years (p<0.05). Compare to 2001-2007, a statistically insignificant decrease for HAV seroprevalence was observed both 2008-2014 and 2015-2023 in 5-<15 and 15-35 age groups. A minimal decrease in HAV seroprevalence was observed for the >35 year age group over the 23 years examined in the study. As a result, the studies that presenting appropriate data in age groups shows that HAV seroprevalence has decreased over the years in age groups except for the 0-<5 age group (
Figure A4)
HAV IgG analyses were performed using Enzyme-Linked ImmunoSorbent Assay (ELISA) in 36 of the included studies, Chemiluminescence/Electro-Chemiluminescence Immunoassay (CLIA/ECLIA) in 8, Chemiluminescent Microparticle Immunoassay (CMIA) in 8, Microparticular Enzyme Immunoassay (MPEIA) in 2, Enzyme Linked Fluorescent Assays (ELFA) in 1, and the study method was not reported in 2. No statistically significant difference was found between the method used and HAV IgG seroprevalence (p>0.05).
4. Discussion
From 1990 to 2021, an increase of approximately 13.9% in the incidence of HAV has been reported worldwide [
75]. A meta-analysis conducted in 2024 found a 32% cumulative hospitalization rate among HAV-infected patients despite increased vaccination rates [
76]. This increases the potential burden of HAV outbreaks on the existing healthcare system. Therefore, widespread screening of adults in risk groups for HAV and reporting of these results are important.
Although it is known that the most effective ways to prevent the spread of HAV infections are improved sanitation, food safety and vaccination, the necessary actions have not yet been taken worldwide. This meta-analysis study was performed to promote a better understanding of HAV epidemiology by bringing together individual studies examining HAV seroprevalence rates and diagnostic methods in Turkey; to emphasize the importance of screening tests in high-prevalence subgroups and the need for preventive measures.
For the last 20-30 years, countries have been classified as high, moderate, low and very low endemic according to the age of exposure and anti-HAV IgG seropositivity. High endemic region, 90% and above immunity up to the age of 10; moderate endemic region, 50% and above immunity up to the age of 15, less than 90% immunity up to the age of 10; low endemic region is defined as more than 50% immunity up to the age of 30 and less than 50% immunity up to the age of 15; very low endemic region is defined as 50% and above immunity up to the age of 30 [
2]. In studies conducted before 2010, Turkey was described as moderately endemic [
24]. When the endemicity levels of the studies included in this meta-analysis were examined, it was seen that 17 were high, 6 were very high, 28 were moderate and 6 were low endemic. The endemicity level in Turkey was detected at a low level in all periods covering the years 2001-2007, 2008-2014 and 2015-2023. However, it was determined that HAV IgG seropositivity rate 0-<5 years of age increased in the period 2015-2023. Additionally, It interpret thought that the low HAV seroprevalence rates in the 0-<5, 5-<15 and 15-35 age groups between 2008-2014 may be due to the studies mostly conducted in the western and central Anatolian regions.
The HAV vaccination program was included in the Childhood Vaccination Program (CVP) at the end of 2012 and is applied to children born after March 1, 2011 in 2 doses at 18 and 24 months in Turkey [
77]. Unfortunately, current scientific publications are insufficient to demonstrate the effects of CVP for HAV immunity. Only two studies reporting seropositivity and vaccination information for the 0-<5 age group reported anti-HAV positivity above 90%. The insufficient sample size in these two studies that relative weights are 0,49 and 1,95 does not allow making an evaluation for future perspective of CVP.
When we excluded low-quality articles, we found minimal decrease in the prevalence rates in the meta-analysis. In order to prevent misinterpretations in the future, studies should be carefully planned with a more comprehensive and equal sample population reflecting the entire country, covering all age groups and taking into account the CVP.
Regional differences have been reported in Brazil, Mexico, Iran and Italy [
1,
78,
79,
80]. Heterogeneous seroprevalence rates were observed in different regions of Turkey (
Figure A3). The western part of our country shows a lower seroprevalence rate due to its more developed socioeconomic status. Except the 0-<5 age group, seroprevalence rates gradually decreased in the central and western regions. The seroprevalence rate in the eastern region was higher than in other regions. Additionally, since there was insufficient data after 2020, we have not been able to comment on eastern region
s’ seropositivity rates latest statue. Standardization of screening and confirmatory tests may be recommended to minimize regional differences. However, no significant difference was found between the method used in our study and seroprevalence. Therefore, screening tests and immunization should be conducted in regions with low socioeconomic status and should be given the same attention as in the least developed regions.
It is known that more than 90% of children living in low socioeconomic level regions and more than 90% of young adults in developing countries have HAV seropositivity [
81]. In our study, regardless of regional differences, from 2001 to 2023, HAV seropositivity in the 5-<15, 15-35 and >35 age groups decreased gradually by 5.0%, 17.7% and 3.29%, respectively. When CVP is ignored, this situation can be evaluated as an improvement in drinking water systems and sanitation conditions in Turkey. But any break in hygiene may expose the population to an epidemic in regions with high immigration. Therefore, basic lifestyle information such as socioeconomic status, access to clean water resources and living area (rural, urban) must be presented in new planned studies.
In seroprevalence studies, it is a matter of concern whether the selected sample represents the general prevalence. Therefore, the exclusion criteria we used should be taken into account when interpreting the results. Our study excluded groups with a high prevalence of HAV infection (such as healthcare workers, chronic hepatitis patients, students living in dormitories, prisoners, military personnel), known vaccinated samples, and studies that included data from immunosuppressed patients. Despite these exclusion criteria and the total sample size, a potential selection bias cannot be completely excluded.
Despite the 12 years that have passed, information on HAV immunity obtained by vaccination cannot be presented due to the insufficient or incomplete data of the publications that meet the inclusion criteria in this study. The vaccination should be taken into account in new studies to reveal the effects of CVP. Otherwise, it will not be possible to reveal or interpret the future perspective of HAV vaccination.
It has been suggested that antibodies against viral hepatitis viruses may have protective roles against coronavirus infection [
82,
83]. In related studies, the protective role of HAV antibodies and immune system stimulation in patients has been pointed out [
82]. During the COVID-19 pandemic, a milder increase in the number of cases and a lower mortality rate compared to developed countries have been reported in underdeveloped countries [
84,
85]. This situation can be explained by the cross-protection of existing immunity against viruses such as HAV, which are endemic in underdeveloped geographies. However, there are also studies reporting that there is no significant relationship between the two [
86]. The ongoing anti-vaccine sentiment during the COVID-19 pandemic may have affected the HAV prevalence, which decreased between 2015 and 2023, especially after the age of 15.
4.1. Strengths and Limitations
This study is one of the few analyses that bring together HAV prevalence and serological diagnostic methods worldwide and was conducted for the first time in Turkey. There was high heterogeneity among the reports from different regions. Heterogeneity was investigated with subgroup analysis. Medical characteristics of the patients such as chronic diseases and vaccination status may have contributed to this heterogeneity. Although subgroup analyses were conducted, the aforementioned factors could not be taken into account due to the lack of data in the studies. In addition, the age ranges in the studies reporting seroprevalence for age groups were quite diverse. Therefore, data that did not fit the age groups we determined were excluded when performing statistical analyses.
5. Conclusions
A systematic meta-analysis of age-specific seroprevalence rates of HAV in Turkey over the last 23 years provides important data into the changing epidemiology of the virus. HAV seroprevalence has increased significantly in the 0-<5 ages, associated with the launch of the HAV vaccination program in 2012. While overall prevalence remains concerning, especially in certain age groups and regions, trends suggest a gradual shift towards lower endemicity.
The heterogeneity observed across region highlights the impact of socioeconomic factors and access to sanitation on HAV transmission dynamics. Further HAV seroprevalence studies should be planned by including sociodemographic data such as patient gender, age group, access to clean water, vaccination status, ethnicity, occupation, income level, living area, region of residence, as well as the diagnostic method used and sensitivity and specificity values calculated when the relevant method is compared with the reference method. It is vital that studies adopt standardized methodologies and include a comprehensive demographic representation to better assess the effectiveness of public health interventions. Ensuring the homogeneity of the data will contribute to epidemiological research.
Continuous monitoring and evaluation of the impact of the vaccination program on seroprevalence will be important in guiding public health strategies and achieving long-term control of HAV in Turkey. As we move forward, prioritizing high-quality research and addressing regional disparities will be vital in our efforts to combat HAV and protect public health.
Author Contributions
Conceptualization, I.H.C., M.K., T.D., H.A.T.; formal analysis I.H.C., M.K., T.D., H.A.T.; investigation, I.H.C., M.K., T.D., H.A.T., E.P.K.K.; methodology, I.H.C.; project administration, I.H.C.; visualization, I.H.C., E.P.K.K; resources, I.H.C., M.K., T.D., H.A.T.; writing—original draft preparation, I.H.C., M.K., T.D., H.A.T., E.P.K.K.; writing—review and editing, I.H.C., M.K., T.D., H.A.T., E.P.K.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Since this study is a meta-analysis study and does not carry any risks, patient consent was waived.
Data Availability Statement
The authors declare that all related data are available from the corresponding author upon reasonable request.
Conflicts of Interest
All authors declare that they have no conflicts of interest.
Appendix A
Forest plot analyses of HAV seroprevalence in the age groups 0-<5, 5-<15, 15-35 and >35.
Appendix B
Figure A1.
Flowchart scheme for systematic literature search and selection of studies.
Figure A1.
Flowchart scheme for systematic literature search and selection of studies.
Figure A2.
Forest plot analysis of the age-specific HAV seroprevalence studies in Turkey.
Figure A2.
Forest plot analysis of the age-specific HAV seroprevalence studies in Turkey.
Table A1.
Event rate values according to age-specific HAV seroprevalence studies in Turkey.
Table A1.
Event rate values according to age-specific HAV seroprevalence studies in Turkey.
Age Groups
|
Event rates for fixed and random effect models (95% CI) |
Fixed (%)
(95% CI) |
Random (%)
(95% CI) |
I2
|
Q2
|
| 0-<5 |
49.1
(47.3-50.9) |
35.4
(23.8-48.9) |
97.88 |
849.22 |
| 5-<15 |
54.6
(53.4-55.7) |
47.3
(37.5-57.3) |
98.39 |
1620.64 |
| 15-35 |
66.1
(65.6-66.6) |
70.7
(62.9-77.5) |
99.48 |
5784.07 |
| >35 |
69.2
(68.5-69.8) |
95.8
(93-97.5) |
99.50 |
5241.76 |
| Total |
63.7
(63.5-64) |
64.5
(58.3-70.2) |
99.77 |
25085.65 |
Figure A3.
The HAV seroprevalence rates according to regions in Turkey.
Figure A3.
The HAV seroprevalence rates according to regions in Turkey.
Figure A4.
The age groups HAV seroprevalence rates over the years in Turkey.
Figure A4.
The age groups HAV seroprevalence rates over the years in Turkey.
References
- Farajzadegan, Z.; Mirmoghtadaee, P.; Mostafavi, S.N.; Hoseini, S.G.; Jamshidi, F.; Kelishadi, R. Systematic Review and Meta-Analysis on The Age-Specific Seroprevalence of Hepatitis A in Iran. J. Res. Med. Sci. 2014, 19, S56–S63. [Google Scholar] [PubMed]
- Jacobsen, K.H.; Wiersma, S.T. Hepatitis A Virus Seroprevalence by Age and World Region, 1990 and 2005. Vaccine 2010, 28, 6653–6657. [Google Scholar] [CrossRef] [PubMed]
- Melhem, N.M.; Talhouk, R.; Rachidi, H.; Ramia, S. Hepatitis A Virus in The Middle East and North Africa Region: A New Challenge. Viral Hepatitis J. 2014, 21, 605. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.; Zaratti, L.; Meleleo, C.; Serino, L.; Sorbara, D. Hepatitis A: Epidemiology and Prevention in Developing Countries. World J. Hepatol. 2012, 4(3), 68–73. [Google Scholar] [CrossRef]
- Mistik, R. Epidemiology of the Hepatitis A Virus Infections. In Viral Hepatit, 1st ed.; Tabak, F., Tosun, S., Eds.; Istanbul Tip Kitabevi: Istanbul, Turkey, 2013. [Google Scholar]
- WHO Position Paper on Hepatitis A Vaccines: June 2012-Recommendations. Vaccine 2013, 31(2), 285–286. [CrossRef]
- CDC. The Centers for Disease Control and Prevention Advisory Committee on Immunisation Practices; Vaccines and Immunizations; 2014. Available online: http://www.cdc.gov/vaccines/vpd-vac/hepa/in-short-adult.htm. (accessed on 30.01.2015).
- Jacobsen, K.H.; Koopman, J.S. Declining Hepatitis A Seroprevalence: A Global Review and Analysis. Epidemiol. Infect. 2004, 132(6), 1005–1022. [Google Scholar] [CrossRef]
- Kurkela, S.; Pebody, R.; Kafatos, G.; Andrews, N.; Hesketh, L.M.; Nardone, A. Comparative Hepatitis A Seroepidemiology in 10 European Countries. Epidemiol. Infect. 2012, 140(12), 2172–2181. [Google Scholar] [CrossRef]
- Mohd Hanafiah, K.; Wiersma, S.T.; Jacobsen, K.H. Challenges to Mapping the Health Risk of Hepatitis A Virus Infection. Int. J. Health Geographics 2011, 10. [Google Scholar] [CrossRef]
- Tosun, S. The Changing Viral Hepatitis Epidemiology in our Country. ANKEM Dergisi 2013, 27 (Suppl 2), 128–134. [Google Scholar]
- Itani, T.; Jacobsen, K.H.; Nguyen, T.; Wiktor, S.Z. A New Method for Imputing Country-Level Estimates of Hepatitis A Virus Endemicity Levels in the Eastern Mediterranean Region. Vaccine 2014, 32(46), 6067–6074. [Google Scholar] [CrossRef]
- Dean, A.S.; Crump, L.; Greter, H.; Schelling, E.; Zinsstag, J. Global Burden of Human Brucellosis: A Systematic Review of Disease Frequency. PLoS Neglected Trop. Dis. 2012, 6(10), 1–9. [Google Scholar] [CrossRef] [PubMed]
- Loney, P.L.; Chambers, L.W.; Bennett, K.J.; Roberts, J.G.; Stratford, P.W. Critical Appraisal of the Health Research Literature: Prevalence or Incidence of a Health Problem. Chronic Dis. Can. 1998, 19(4), 170–176. [Google Scholar] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009, 6, 332. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Thombs, B.D.; Welch, V.; Altman, D.G.; Pottenger, A.; McKenzie, J.E.; Brennan, S.E.; Cogo, E.; Li, T.; Tsertsvadze, A.; Moher, D.; Liberati, A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4(1), 1. [Google Scholar] [CrossRef]
- WHO. The Global Prevalence of Hepatitis A Virus Infection and Susceptibility: A Systematic Review, 2010. Available online: https://iris.who.int/bitstream/handle/10665/70180/WHO_IVB_10.01_eng.pdf. (accessed on 01.06.2024).
- Bozdayi, G.; Ozden, A.; Donderici, O.; Cetinkaya, H. Variation of Seropositivity Rates for Hepatitis A Virus in Primary School Students in Ankara for the Last Decade. Hepatology 2001, 35(2), 285. [Google Scholar]
- Cesur, S.; Akin, K.; Dogaroglu, I.; Birengel, S.; Balik, I. Hepatitis A and Hepatitis E Seroprevalence in the Adults of Ankara Region. Hepatology 2002, 36(1), 79. [Google Scholar]
- Tosun, S.; Kasirga, E.; Ertan, P.; Aksu, S. Evidence Against the Fecal-oral Route of Transmission for Helicobacter pylori Infection in Childhood. Med. Sci. Monit. 2003, 9(11), 489–492. [Google Scholar]
- Karsligil, T.; Eksi, F.; Balci, I.; Belgin, R. The Seroprevalence of Hepatitis A and E in Our Region. Viral Hepatitis J. 2003, 8(3), 155–159. [Google Scholar]
- Guven, F.; Erkum, A.Y.; Erkum, T.; Say, A. Seroprevalence of Hepatitis A in Children Between 0-15 Years Old. Medical Bulletin of Zeynep Kamil 2004, 35(1), 41–44. [Google Scholar]
- Tosun, S.; Ertan, P.; Kasirga, E.; Umit, A. Changes in Seroprevalence of Hepatitis A in Children and Adolescents in Manisa, Turkey. Pediatrics Int. 2004, 46, 669–672. [Google Scholar] [CrossRef]
- Erdogan, M.S.; Otkun, M.; Tatman-Otkun, M.; Akata, F.; Ture, M. The Epidemiology of Hepatitis A Virus Infection in Children, in Edirne, Turkey. Eur. J. Epidemiol. 2004, 19(3), 267–273. [Google Scholar] [CrossRef] [PubMed]
- Atabek, M.E.; Findik, D.; Gulyuz, A.; Erkul, I. Prevalence of Anti-HAV and Anti-HEV Antibodies in Konya, Turkey. Health Policy 2004, 67, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Ocak, S.; Kaya, H.; Cetin, M.; Inandi, T. Seropositivity of Hepatitis A and B According to Age and Sex in Preoperative Patients in Antakya. Viral Hepatitis J. 2005, 10(3), 169–175. [Google Scholar]
- Arabaci, F.; Demirli, H. The Seroprevalence of Hepatitis A and B in Children 6-10 Years of Age. Turkish Journal of Infection 2005, 19(4), 457–460. [Google Scholar]
- Egemen, A.; Yilmaz, O.; Akil, I.; Altuglu, I. Evaluation of association between hepatitis A and Helicobacter pylori infections and routes of transmission. Turkish J. Pediatr. 2006, 48(2), 135–139. [Google Scholar]
- Genc, G.; Yilmaz, G.; Karacan, C.; Atay, N.; Yoney, A. Hepatitis A Seroprevalence Among 2-6 Year-Old Children of Low Socioeconomic Class Families. Ege Journal of Medicine 2006, 28(4), 188. [Google Scholar] [CrossRef]
- Vancelik, S.; Guraksin, A.; Alp, H. Hepatitis A seroepidemiology in Eastern Turkey. East Afr. Med. J. 2006, 83(2), 86–90. [Google Scholar] [CrossRef]
- Tekay, F. Hakkâri Devlet Hastanesine basvuran 0-14 yas grubu cocuklarda hepatit A sikligi. Hepatitis A Frequency in Children of Between 0-14 Age Group Who Had Consulted at Hakkari Province Hospital. Hepatology 2006, 33(4), 245.
- Ozen, M.; Yologlu, S.; Isik, Y.; Tekerekoglu, M.S. Anti-HAV IgG Seropositivity in Children Aged Between 2-16 Years Who Were Admitted to Turgut Ozal Medical Center. Turkish Archives of Pediatrics 2006, 41(1), 36–40. [Google Scholar]
- Ozkinay, F.; Kurugol, Z.; Koturoglu, G.; Ozacar, T.; Altuglu, I.; Vardar, F.; Ozer, A.; Geyik, M.; Yilmaz, N. The Epidemiology of Hepatitis A İnfection in the Population of Bornova, Izmir, Turkey. Ege Journal of Medicine 2007, 46(1), 1. [Google Scholar]
- Turhan, E.; Cetin, M. The Seroprevalence of Viral Hepatitis A in Patients Who Had Consulted at Mustafa Kemal University of Medicine Faculty. Viral Hepatitis J. 2007, 12(1), 30–34. [Google Scholar] [CrossRef]
- Kaya, D.; Guler, E.; Ekerbicer, H.C.; Dilber, C.; Karabiber, H.; Guler, S.; Davutoglu, M.; Ciragil, P. Hepatitis A Seroprevalence and its Relationship with Environmental Factors in Children of Different Age Groups in Kahramanmaras, Eastern Mediterranean region of Turkey. Viral Hepatitis J. 2007, 14(12), 830–834. [Google Scholar] [CrossRef] [PubMed]
- Altinkaynak, S.; Selimoglu, M.A.; Ertekin, V.; Kilicaslan, B. Epidemiological Factors Affecting Hepatitis A Seroprevalence in Childhood in a Developing Country. Eurasian J. Med. 2008, 40(1), 25–28. [Google Scholar] [PubMed]
- Ceyhan, M.; Yildirim, I.; Kurt, N.; Uysal, G.; Dikici, B.; Ecevit, C.; Koyuncu, A.; Akman, S. Differences in Hepatitis A Seroprevalence Among Geographical Regions in Turkey: a Need for Regional Vaccination Recommendations. Viral Hepatitis J. 2008, 15 (Suppl 2), 69–72. [Google Scholar] [CrossRef] [PubMed]
- Arabaci, F.; Oldacay, M. The Seroprevalence of Hepatitis A in Different Age Groups and Hepatitis A İncidence in Acute Hepatitis Cases in the Canakkale Province. J. Pediatric Infect. 2009, 3, 58–61. [Google Scholar]
- Topal, E.; Hatipoglu, N.; Turel, O.; Aydogmus, C.; Hatipoglu, H.; Erkal, S.; Erkal, S.; Ozkaya, H.; Demirtas, A. Seroprevalence of Hepatitis A and Hepatitis A Vaccination Rate in Preschool Age in Istanbul. J. Pediatric Infect. 2011, 1, 12. [Google Scholar] [CrossRef]
- Kurugol, Z.; Aslan, A.; Turkoglu, E.; Koturoglu, G. Changing epidemiology of hepatitis A infection in Izmir, Turkey. Vaccine 2011, 29(37), 6259–6261. [Google Scholar] [CrossRef]
- Ince, O.T.; Yalcin, S.S.; Yurdakok, K.; Ozmert, E.N. Hepatitis A Seroprevalence Among Infants Aged 12 Months in Ankara. Turkish J. Pediatr. 2011, 53(1), 114–116. [Google Scholar] [CrossRef]
- Turker, K.; Balci, E.; Bati, S.; Hascuhadar, M.; Savas, E. Ulkemizde Hepatit A Enfeksiyonunun Degisen Epidemiyolojisi. Turk Mikrobiyol. Cem. Derg. 2011, 41(4), 143–148. [Google Scholar] [CrossRef]
- Cetinkol, Y.; Yildirim, A.A. The Evaluation of the HBsAg, anti-HBs, anti-HCV and anti-HAV IgG Results in Medical Career College Students. Viral Hepatitis J. 2012, 18(1), 23–25. [Google Scholar] [CrossRef]
- Okur, M.; Erbey, F.; Acar, M.N.; Kaya, A.; Guven, A. The Seropositivity of Hepatitis A in Children Between 0-18 Years in the Van Province and Around. Duzce Med. J. 2011, 13(2), 6–9. [Google Scholar]
- Deveci, U.; Ustun, C.; Hamanca, O. Seroprevalence of hepatitis A virus among children aged 1-16 years in Eastern Anatolia, Turkey. Afr. J. Microbiol. Res. 2011, 5(32), 5969–5971. [Google Scholar] [CrossRef]
- Ceran, N.; Yuksel Kocdogan, F.; Mert, D.; Erdem, I.; Dede, B.; Adaleti, R.; Bostan, O.; Arslan, H. Hepatitis A Seroprevalence in Children and Young Adults in Istanbul, Turkey: Seroprevalence Change and Associated Factors. Viral Hepatitis J. 2012, 19(1), 72–76. [Google Scholar] [CrossRef] [PubMed]
- Halicioglu, O.; Akman, S.A.; Tatar, B.; Atesli, R.; Kose, S. Hepatitis A Seroprevalence in Children And Adolescents Aged 1–18 Years Among a Low Socioeconomic Population in Izmir, Turkey. Travel Med. Infect. Dis. 2012, 10, 43–47. [Google Scholar] [CrossRef]
- Cetinkol, Y.; Altuncekic Yildirim, A. The Seroprevalence of Viral Hepatitis A in Patients Who Had Been Consulted at Unye State Hospital. Med. J. Kocatepe 2011, 12(1), 18–22. [Google Scholar]
- Demirpence, O.; Tezcan, S.I.; Degirmen, E.; Mert, D.; Gumus, A.; Celen, M.K. Seroprevalence of HAV, HBV, HCV and HIV in People Admitted to Batman State Hospital. Viral Hepatitis J. 2012, 18(1), 6–10. [Google Scholar] [CrossRef]
- Alici, O.; Agalar, C.; Yazicilar, H.A. Hepatitis A Seroprevalence in Patients who Admitted to Training and Research Hospital in Istanbul. Viral Hepatitis J. 2013, 19(3), 100–114. [Google Scholar] [CrossRef]
- Kose, S.; Mandiracioglu, A.; Cavdar, G.; Ulu, Y.; Nohutcu, N.; Gurbuz, I.; Erdem, S. The Seroprevalence of Hepatitis A in Adults in Izmir: Prior to Introducing Vaccine into Routine Vaccination Program. Nobel Medicus 2013, 9(3), 49–53. [Google Scholar]
- Erturk, A.; Copur Cicek, A.; Cure, E.; Akdogan, R.A. Seroprevalence of Hepatitis A in Rize Province and Different Adult Age Groups. Viral Hepatitis J. 2013, 19(2), 85–88. [Google Scholar] [CrossRef]
- Koroglu, M.; Demiray, T.; Terzi, H.A.; Altindis, M. Seroprevalence of Hepatitis A among Different Age Groups in Sakarya and Review of The Literature. Viral Hepatitis J. 2014, 20(3), 110–114. [Google Scholar] [CrossRef]
- Iraz, M.; Gultepe, B.; Doymaz, M.Z. Seroprevalence of Hepatitis A in The Adult Age Groups. Abant Med. J. 2015, 4(1), 54–58. [Google Scholar] [CrossRef]
- Bolukbas, B.; Mengeloglu, Z.; Tas, T. Seroprevalence Rates of Hepatitis A Virus in Different Age Groups in the Province of Bolu. Abant Med. J. 2015, 4(4), 331–333. [Google Scholar] [CrossRef]
- Yanik, K.; Akbal, A.U.; Erdil, M.; Karadag, A.; Eroglu, C.; Gunaydin, M. Evaluation of the Prevalence of Hepatitis A in Samsun Vicinity. Viral Hepat J. 2015, 21(1), 23–27. [Google Scholar] [CrossRef]
- Citil, B.; Sayiner, H.; Akgun, S.; Aksoz, S. The Seroprevalence of Hepatitis A in Adiyaman. J. Contemp. Med. 2015, 157–162. [Google Scholar] [CrossRef]
- Temiz, H.; Ozbek, E.; Toprak, S.F.; Onur, A.; Ertugrul, S. Hepatitis A seroprevalence in patients who admitted to a training and research hospital in Southeast Anatolia. Dicle Med. J. 2015, 42(4), 485–489. [Google Scholar] [CrossRef]
- Karaman, S.; Karaman, K.; Kizilyildiz, B.S.; Ceylan, N.; Kaba, S.; Parlak, M.; Beger, B.; Ceylan, A. Seroprevalence of Hepatitis A and Associated Factors Among 1-15 Year Old Children in Eastern Turkey. Int. J. Clin. Exp. Med. 2015, 8(10), 19394–199. [Google Scholar]
- Parlak, M.; Guven, A.; Erdin, B.N.; Bayram, Y. Seroprevalence of Hepatitis A Virus Among Child and Adult Age Groups Admitted to a Training and Research Hospital. Viral Hepat J. 2015, 21(1), 20–22. [Google Scholar] [CrossRef]
- Kalfaoglu, H.; Zeytinoglu, A.; Ocek, Z.A. Izmir ilinde hepatit A virusu ve hepatit E virusu seroprevalansi. FLORA 2017, 22(1), 17–28. [Google Scholar] [CrossRef]
- Karadeniz, A.; Akduman Alasehir, E.; Yesilbag, Z.; Balikci, A.; Yaman, G. The seroprevalence of hepatitis A in Istanbul, Turkey. Marmara Med. J. 2017, 30(1), 14–17. [Google Scholar] [CrossRef]
- Kolancali, N.; Onal, Z.S.; Aksaray, S.; Nuhoglu, C. Evaluation of the Seroprevalence of Hepatitis A and Vaccination Status in Children Aged Two and Sixteen Years. Viral Hepat J. 2017, 23(2), 46–49. [Google Scholar] [CrossRef]
- Dogan, E.; Sevinc, E.; Kuru, C. Seroprevalence of HAV, HBV, and HCV in Pediatric Patients in Karabuk Province. The Turkish Journal of Academic Gastroenterology 2017, 16(3), 97–100. [Google Scholar] [CrossRef]
- Duran, I.; Nazik, S. Seroprevalence of Hepatitis A in Pediatric Age Groups in Bingol Province. JAREM 2018, 8(1), 15–18. [Google Scholar] [CrossRef]
- Calik, S.; Tosun, S.; Ari, A.; Ertan, P.; Umit, A.; Çetin, M.; Yildirim, A. Hepatitis A Seroprevalence in Different Age Groups in a Region With Low and Moderate Socioeconomic Level in Izmir Province: Results of a Fieldwork. Klimik Derg. 2019, 32(3), 310–314. [Google Scholar] [CrossRef]
- Alkan Ceviker, S.; Gunal, O.; Kilic, S.S.; Koksal, E.; Tahmaz, A. Seroprevalence of Hepatıtıs A Virus Among Different Age Groups in The Provınce of Samsun. Balikesir Health Sciences Journal, 2019, 8(2), 81–86.
- Inci, H.; Asgin, N.; Inci, F.; Adahan, D. Seroprevalence of Viral Hepatitis According to Age Groups in İndividuals Applying to a University Hospital Family Medicine Clinic. Konuralp Med. J. 2020, 12(1), 34–38. [Google Scholar] [CrossRef]
- Saglam, M.; Celik, C.; Taskin Kafa, A.H.; Hasbek, M. Evaluation of Hepatitis A Seroprevalence and Epidemiologic Data of Patients Applying to A Medical Faculty Hospital. Viral Hepat J. 2020, 26(2), 104–109. [Google Scholar] [CrossRef]
- Yilmaz, A. Hepatitis A Seroprevalence in Erzurum, Turkey. Ann Agric Environ Med. 2020, 27(3), 481–484. [Google Scholar] [CrossRef]
- Duzenli, T.; Koseoglu, H.; Ucer, S.; Akçin, A.; Şen, A.; Tamer, A. Seroprevalence of hepatitis A virus according to age groups in Northern Anatolia of Turkey. Turk J Acad Gastroenterol. 2021, 20, 136–142. [Google Scholar] [CrossRef]
- Tuna, D.K.; Dicle, Y.; Aydin, E. Two-year seroprevalence of hepatitis and HIV in blood samples taken from patients applying to primary healthcare facilities. Van Med J. 2021, 28(3), 404–411. [Google Scholar] [CrossRef]
- Tufekci, E.F.; Calisir, B.; Yasar Duman, M.; Kilinc, C. Evaluation of Hepatitis A Seroprevalence in Kastamonu Province, Turkey. Med Records. 2022, 4(3), 428–432. [Google Scholar] [CrossRef]
- Mingir, S.; Sensoy, N.; Demirturk, N. Evaluation of Hepatitis A and Hepatitis C Serologies and Hepatitis B Vaccine Application Responses In Adolescent Children. TJFMPC. 2023, 17(1), 126–131. [Google Scholar] [CrossRef]
- Cao, G.; Jing, W.; Liu, J.; Liu, M. The global trends and regional differences in incidence and mortality of hepatitis A from 1990 to 2019 and implications for its prevention. Hepatology international, 2021, 15(5), 1068–1082. [CrossRef]
- Gandhi, A.P.; Al-Mohaithef, M.; Aparnavi, P.; Bansal, M.; Satapathy, P.; Kukreti, N.; Rustagi, S.; Khatib, M.N.; Gaidhane, S.; Zahiruddin, Q.S. Global outbreaks of foodborne hepatitis A: Systematic review and meta-analysis. Heliyon, 2024, 10(7), e28810. [CrossRef]
- Turkiye Cumhuriyeti Saglik Bakanligi. Hepatit A asi Uygulamasi Ust Yazisi. Accessed online: https://dosyaism.saglik.gov.tr/Eklenti/12472,20121008-1509-hskdan-hepatit-a-asisinin-uygulanmasi-hakkinda-yazipdf.pdf?0 (accessed on 31.07.2018).
- Ximenes, R.A.; Martelli, C.M.; Amaku, M.; Sartori, A.M.; de Soárez, P.C.; Novaes, H.M.; Pereira, L.M.; Moreira, R.C.; Figueiredo, G.M.; de Azevedo, R.S.; Hepatitis Study Group. Modelling The Force of Infection for Hepatitis A in an Urban Population-Based Survey: A Comparison of Transmission Patterns in Brazilian Macro-Regions. PLoS One. 2014, 9(5), e94622. [CrossRef]
- Lazcano-Ponce, E.; Conde-Gonzalez, C.; Rojas, R.; DeAntonio, R.; Romano-Mazzotti, L.; Cervantes, Y. Ortega-Barria, E. Seroprevalence of Hepatitis A Virus in a Cross-Sectional Study in Mexico: Implications for Hepatitis A Vaccination. Hum Vaccin. Immunother. 2013, 9(2), 375–381. [CrossRef]
- Ansaldi, F.; Bruzzone, B.; Rota, M.C.; Bella, A.; Ciofi degli Atti, M.; Durando, P.; Gasparini, R.; Icardi, G. Serologic Study Group. Hepatitis A Incidence and Hospital-Based Seroprevalence in Italy: A Nationwide Study. Eur. J. Epidemiol. 2008, 23(1), 45–53. [CrossRef]
- Gust, I.D. Epidemiological Patterns of Hepatitis A in Different Parts of the World. Vaccine. 1992, 10(1), 56–58. [Google Scholar] [CrossRef]
- arialioglu, F.; Belen Apak, F.B.; Haberal, M. Can Hepatitis A Vaccine Provide Protection Against COVID-19?. Experimental and clinical transplantation : official journal of the Middle East Society for Organ Transplantation, 2020, 18(2), 141–143. [CrossRef]
- Sarialioğlu, F.; Belen, F.B.; Hayran, K.M. Hepatitis A susceptibility parallels high COVID-19 mortality. Turkish Journal of Medical Sciences, 2021, 51(1), 382–384. [CrossRef]
- COVID-19 CORONAVIRUS PANDEMIC. Accessed online: https://www.worldometers.info/coronavirus (accessed on 8.10.2024).
- Yaqinuddin, A. Cross-immunity between respiratory coronaviruses may limit COVID-19 fatalities. Medical Hypotheses, 2020, 144, 110049. [CrossRef]
- Abdollahi, A.; Salarvand, S.; Mehrtash, V.; Jafarzadeh, B.; Ghalehtaki, R.; Nateghi, S. Is There A Correlation Between COVID-19 and Hepatitis A and Hepatitis E Serum Antibody Level?. Iranian Journal of Pathology, 2022, 17(1), 71–74. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).