1. Introduction
Hypertrophic cardiomyopathy (HCM) primarily affects diastolic function in both human and cats [
1]. Therefore, the evaluation of diastolic function is important for diagnosis and treatment in patients with HCM. In recent years, it has become possible to measure IVPD using Color M-mode (CMM) in echocardiography. Traditionally, catheterization has been the gold standard for assessing diastolic function. However, its invasive nature presents significant limitations, particularly in veterinary medicine. Therefore, the advent of CMM echocardiography to measure IVPD provides, a non-invasive, simple, and rapid method of assessment has become possible, offering promising clinical applications. It has been demonstrated that the evaluation of intraventricular pressure differences (IVPD) using this method correlates with catheterization, and it is characterized by its non-invasive nature, making it convenient for bedside or routine clinical use [
2,
3,
4]. Diastolic function can be broadly divided into two main categories: "active relaxation," which occurs during early diastole, and "passive relaxation," which occurs during late diastole. Among these, active relaxation, considered crucial for cardiac function, involves the left ventricle actively pulling blood from the left atrium to the left ventricular apex using energy. The pressure differences within the ventricle during this process is referred to as IVPD.
We divided the left ventricle into three segments and defined them as Basal IVPD, Mid IVPD, and Apical IVPD, based on their proximity to the basal region of the heart. Our previous animal studies have shown that Basal IVPD increases with volume overload, while Mid-Apical IVPD decreases when diastolic function declines [
5]. IVPD measured by CMM is in influenced by the size of the heart. Therefore, we used an index called intraventricular pressure gradient (IVPG), which is calculated by dividing IVPD by the length of the left ventricle.
In recent years, advancements in echocardiography technology in small animal clinical practice have increased the detection rate of cardiomyopathies in cats [
6]. Many cases of feline cardiomyopathy progress asymptomatically, and among them, HCM has a particularly high prevalence [
7,
8], making it one of the causes of sudden death in cats. HCM is primarily characterized by diastolic dysfunction, and in clinical practice, it is diagnosed as the HCM phenotype when the left ventricular end-diastolic myocardial wall thickness exceeds 6 mm on echocardiography [
9]. Feline HCM is broadly classified into hypertrophic obstructive cardiomyopathy (HOCM) and hypertrophic non-obstructive cardiomyopathy (HNCM), based on the presence or absence of left ventricular outflow tract obstruction, and left ventricular outflow tract (LVOT) obstruction caused by myocardial hypertrophy or systolic anterior motion (SAM) of the mitral valve leads to an increase in intraventricular pressure [
10].
While conventional echocardiography can assess congestion and diastolic function to some extent, there are several issues that can affect diagnostic accuracy and consistency. One of the notable characteristics in cats is the fusion of left ventricular inflow waveforms, even in cases without heart disease, due to their high heart rate.
For the evaluation of left ventricular diastolic function, indirect indicators such as the E/A ratio (mitral inflow velocity), tissue Doppler imaging (TDI), left atrial diameter and function are typically used [
11,
12,
13,
14]. However, in the case of fusion waves, accurate assessment of mitral inflow and TDI can be challenging.
In this study, we hypothesized that the introduction of IVPD measurement using CMM would enable the evaluation of congestion and diastolic function in feline HCM. Since CMM has high temporal resolution, it allows for analysis even in cats with rapid heart rates. It is expected that new insights into the usefulness of IVPD in the pathophysiology of HOCM and HNCM will be obtained.
2. Materials and Methods
2.1. Animals and Study Protocol
Between October 2021 and April 2023, 18 cats diagnosed with HCM and 10 control cats without heart disease were included in the study, which was conducted at the Dog and cat pediatric hospital. Cats with a left ventricular end-diastolic wall thickness exceeding 6 mm as measured by M-mode echocardiography were classified as the HCM group. Cats with systemic hypertension (systolic blood pressure ≥ 180 mmHg), hyperthyroidism, or secondary HCM due to significant dehydration were excluded. In this study, HOCM was defined by the presence of systolic anterior motion (SAM) of the mitral valve or a left ventricular outflow tract velocity of 250 cm/s or more. Cats with HCM but without LVOT obstruction were classified as the HNCM group.
2.2. Conventional Echocardiography
Echocardiographic examinations were performed using an ultrasound machine (LISENDO880, Fujifilm). Echocardiographic images were stored offline and analyzed using commercial software (described later). The cats were positioned in lateral recumbency, and standard left and right cardiac windows were used to record conventional echocardiographic measurements. From the right parasternal short-axis view at the level of the papillary muscles, the following measurements were reported: left ventricular internal diameter at end-diastole and end-systole (LVIDd, LVIDs), interventricular septal thickness at end-diastole and end-systole (IVSd, IVSs), left ventricular free wall thickness at end-diastole and end-systole (LVPWd, LVPWs), and fractional shortening (FS). From the right parasternal short-axis view at the base of the heart, the left atrial diameter (LAD), aortic diameter (Ao), and left atrium-to-aorta ratio (LA/Ao) were measured in two-dimensional echocardiography. At the level of the left ventricular apex, left ventricular outflow tract velocity (LVOTv) and mitral inflow velocity (Ev) were measured using pulsed-wave Doppler echocardiography. Using tissue Doppler imaging (TDI), early diastolic tissue velocity (e') was measured from the left ventricular free wall (FW). The ratio of Ev to e' (E/e') was then calculated for the left ventricular septum and free wall. All echocardiographic measurements were obtained over three cardiac cycles and averaged. LVPW, IVS, LVID, and FS were measured from the right parasternal short-axis view at the papillary muscle level using M-mode, with the leading-edge to leading-edge method. LA/Ao was measured in B-mode at the base of the heart in early diastole, just after the closure of the aortic valve. Mitral inflow velocity was measured using pulsed-wave Doppler from the left apical four-chamber view, with the sample volume placed at the tips of the mitral valve leaflets. When the E wave and late peak wave (Av) could not be clearly distinguished, the waveform was classified as fused.
2.3. Analysis of Intraventricular Pressure Gradient (IVPG)
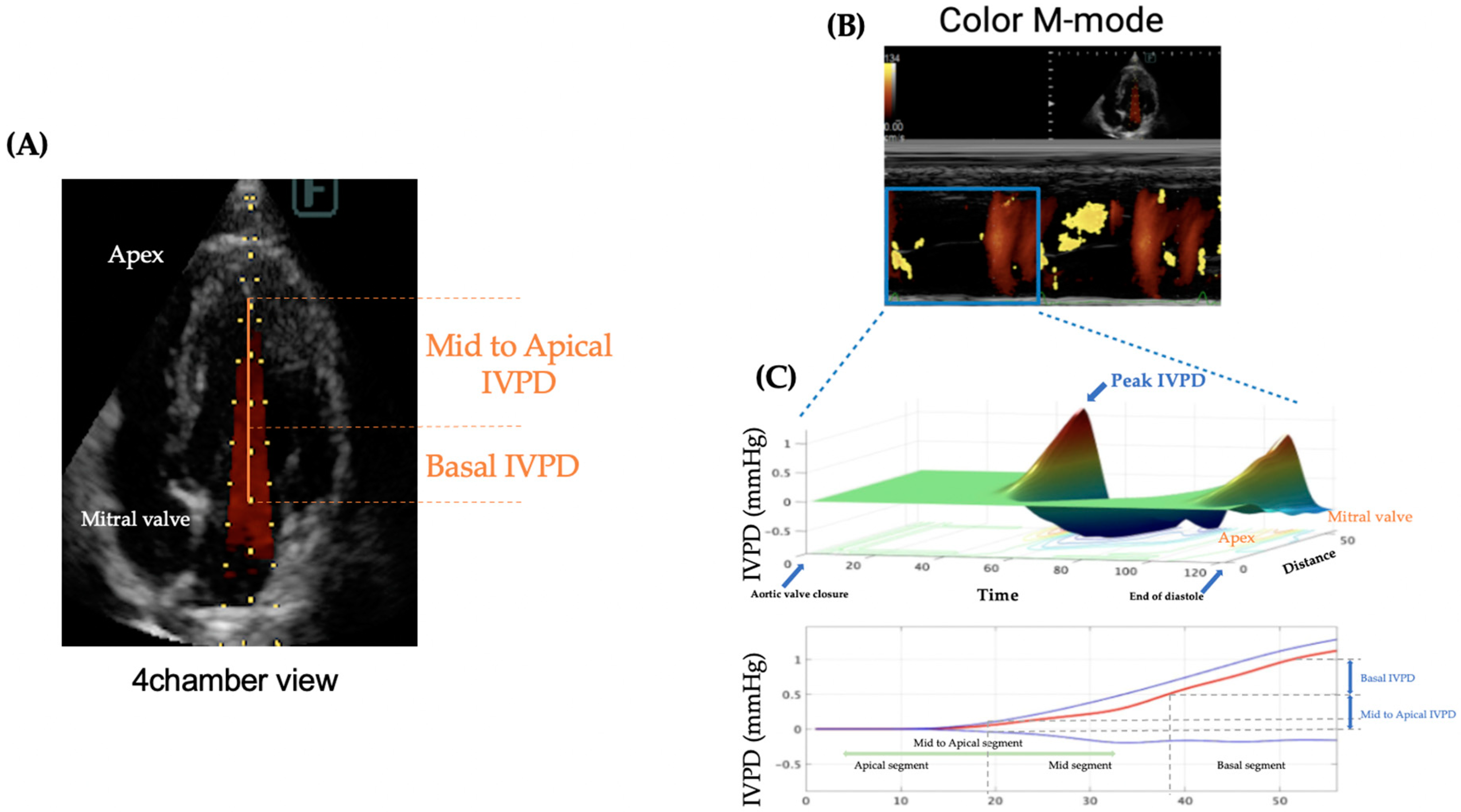
Figure 1 illustrates the process of IVPD measurement. All examinations were performed without sedation, and tests were temporarily halted in cases of rapid increases in heart rate or respiratory rate to avoid stress-induced artifacts. Since cats are particularly susceptible to stress, which can elevate their heart rate merely from visiting the hospital, the examination room was kept dim and quiet to minimize stress. The entire echocardiographic protocol, including CMM, was completed within 10 minutes. Ultrasound settings were adjusted, especially the gain, to appropriately visualize the entire mitral inflow tract and the LV apex from the left apical four-chamber view to trace the IVPD. Pre-settings included a sweep speed of 300 mm/s, color baseline shift of -64, and velocity range set to approximately 1.5 times the recorded mitral inflow velocity at the valve tips to raise the Nyquist limit for color Doppler.
For the image processing of IVPD and IVPG using MATLAB, the time from aortic valve opening to closure, the time from the onset of the Q wave to the start of Ev, and the time from the onset of the Q wave to the peak of the E wave were collected from conventional echocardiography and manually input into a MATLAB dialog box as per the program instructions. Three high-quality images from consecutive heartbeats were selected for each case, and the average values for each patient were used in the analysis.
IVPD and IVPG from CMM were calculated based on a previously validated IVPD measurement method [
2]. IVPD was computed from the images obtained by CMM and processed in MATLAB (The MathWorks, Natick, MA, USA) using the following Euler equation:
Where ∂ represents changes in the followed element, P is pressure, ρ is the constant blood density (1,060 kg/m³), v is velocity, s is the position along the color M-mode line, and t is time. IVPG values were derived from IVPD using the following formula [
15]:
Both IVPG and IVPD were automatically divided into the basal, mid, and apical segments of the left ventricle (LV) by the program [
3]. In this study, only cases where the CMM image of the left ventricular apex could be clearly extracted were included in the analysis, and cases with severe congestion or LV outflow tract obstruction were excluded from the image analysis.
2.4. Statistical Analysis
The statistical methods used included the Mann-Whitney test and the Kruskal-Wallis test. Simple linear regression was performed between traditional Ev and IVPG measurements. The interpretation of effect size was based on the rc value, with rc ≥ 0.5 considered effective. Statistical software used for the analysis was GraphPad Prism Version 8 (GraphPad Software Inc, San Diego, CA, USA), and comparisons were made across all echocardiographic data for the control, HNCM, and HOCM groups. For the Control group, we selected healthy subjects who had no cardiovascular diseases, as confirmed by echocardiography and electrocardiography, and who did not have conditions that could affect the cardiovascular system, such as hypertension, renal dysfunction, or endocrine disorders. In this study, a p-value of <0.05 was considered statistically significant for the analyzed variables.
3. Results
3.1. Patient Data
The most common breed in the Control group was American Shorthair and Maine Coon (n=2), followed by Scottish Fold (n=1), British Shorthair (n=1), Siberian (n=1), Abyssinian (n=1), Selkirk Rex (n=1), and Minuet (n=1).The most common breed in the HCM group was Scottish Fold and mixed breed(n=5), followed by American Shorthair (n=3), Munchkin (n=2), Russian Blue (n=1), American Curl (n=1), Persian (n=1). The median age of the Control group was 1 years (range: 0.25–9 years), and the median ag e of the HCM group was 4 years (range: 0.5–15 years), and the median body weight of the Control group was 2.95 kg (range: 1.65–5.1 kg), and the median body weight of the HCM group was 4.25 kg (range: 2.36–6.3 kg). The control group consisted of 6 males and 4 females, and the HCM group consisted of 12 males and 6 females. Of the 18 cats diagnosed with HCM, 11 were classified into the HOCM group, while 7 were classified into the HNCM group. In the HOCM group, 8 cats were classified as ACVIM stage B1, 1 cat as stage B2, and 2 cats as stage C. In the HNCM group, 5 cats were classified as ACVIM stage B1, and 2 as stage C. Of the 18 cats diagnosed with HCM, 13 were treated with beta-blockers (10 in stage B1, 1 in B2, and 1 in C), 4 were treated with ACEi (3 in stage B1 and 1 in C), 3 were treated with antiplatelet agents, and 1 each in stage C were treated with anticoagulants and diuretics.
3.2. Conventional Echocardiography
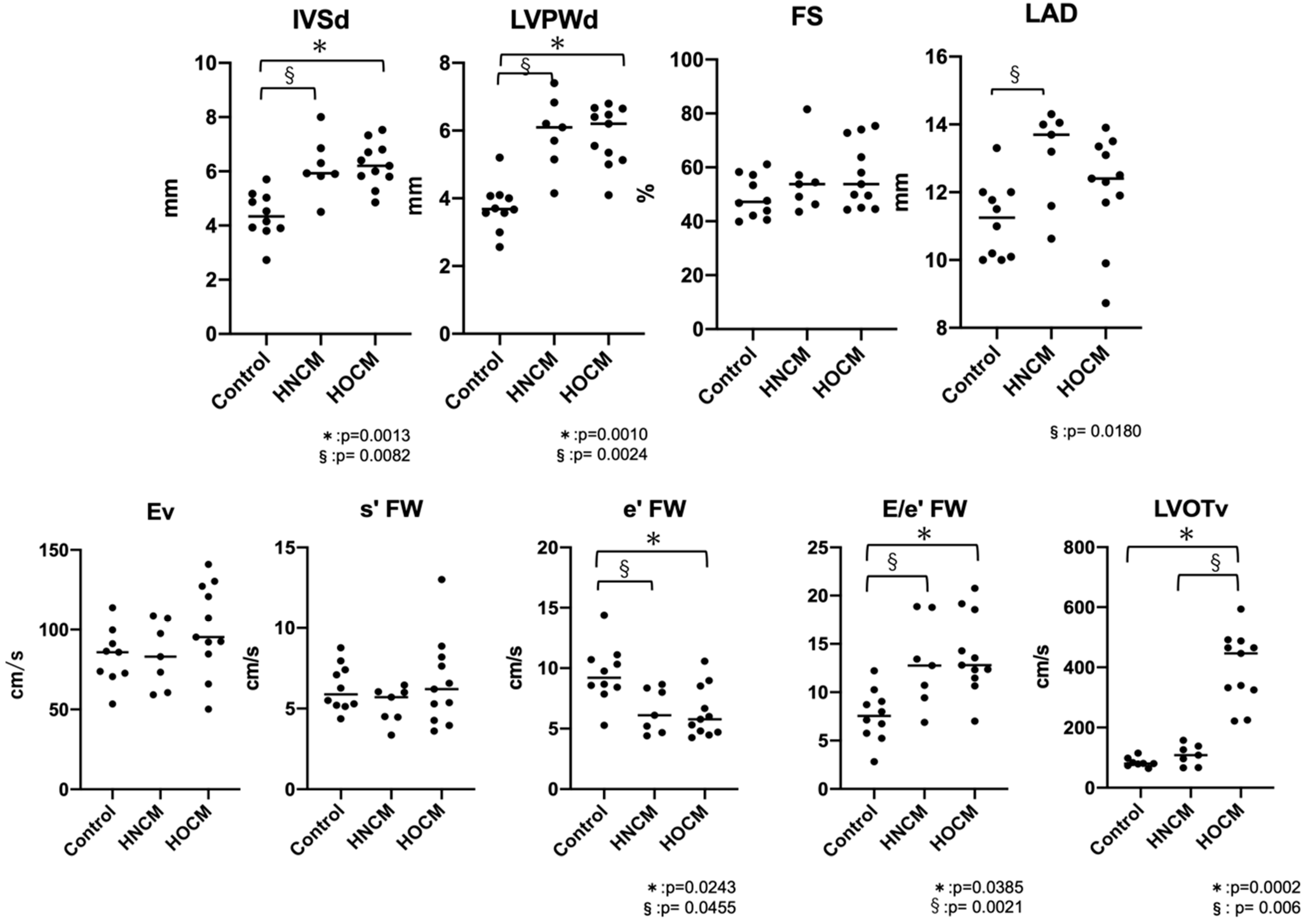
The results of the conventional echocardiography are shown in
Table 1 and
Figure 2. IVSd and LVPWd were significantly increased in both the HOCM and HNCM groups compared to the control group (IVSd: Control vs HNCM p=0.0082, Control vs HOCM p=0.0013, LVPWd: Control vs HNCM p=0.0024, Control vs HOCM p=0.001). For LAD the HNCM group showed a significant increase compared to the control group (p=0.0270), but there was no marked enlargement of the left atrial diameter across the entire HCM group (LAD < 16 mm). In cases with fusion waves, there were 6 cats in Control group, 2 cats in HNCM group and 6 cats in HOCM group. There were no significant differences in Ev between the three groups, but e' FW was significantly reduced in both the HOCM and HNCM groups compared to the control group (p=0.0243, 0.0455), and E/e' FW was significantly increased (p=0.0385, 0.0021). No significant differences were observed between the HOCM and HNCM groups, except for LVOTv (p=0.0076).
3.3. Color M-Mode Echocardiography for IVPG
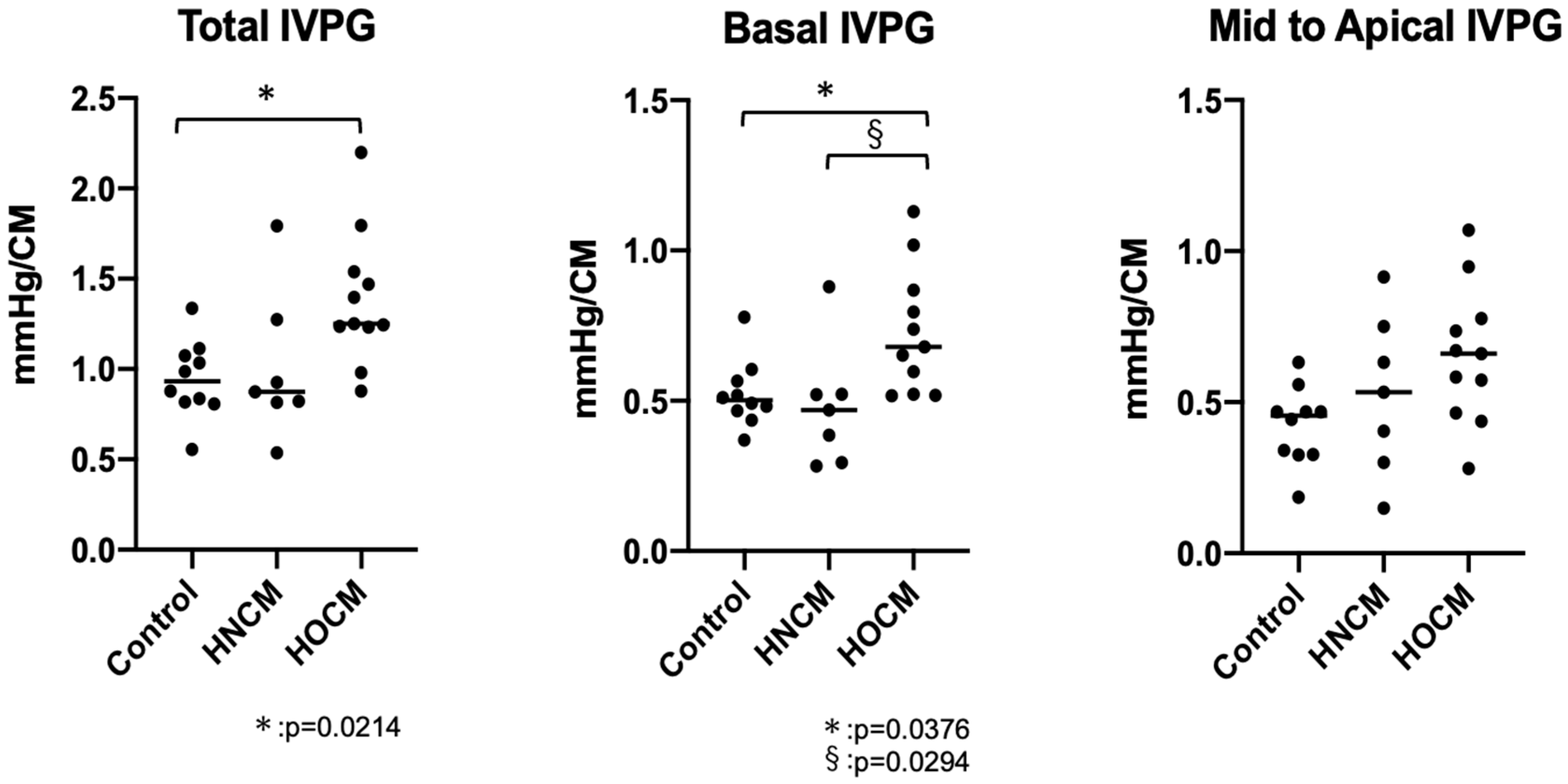
In this study, IVPG analysis was not possible in three cats with HOCM due to the presence of severe left ventricular outflow tract (LVOT) obstruction. In total, IVPG analysis was successful in 25 of 28 cases, with an analysis rate of approximately 89.3%. However, data from the three cats that could not be analyzed were able to be analyzed after treatment and were included in this study. Previous reports on healthy cats showed a correlation between Ev and IVPG [
12] ; however, in this study, no significant correlation was found between Ev and Basal IVPG (p=0.0072, rc=0.496). Additionally, there was no correzation between Total IVPG and Mid to Apical IVPG with Ev (rc=0.418, 0.310). The HOCM group showed a significant increase in Total IVPG (p=0.0214) and Basal IVPG (p=0.0376) compared to the control group (
Figure 3, Tabel 2). Basal IVPG was significantly higher in the HOCM group compared to the HNCM group (P=0.0294).
4. Discussion
4.1. Previous Achievements in IVPG
The evaluation of IVPG using CMM has already begun clinical research in human medicine. Among childhood cancer survivors who received cardiotoxic chemotherapy, both Mid to Apical IVPD and Total IVPD decreased [
16]. This finding suggest that the assessment of diastolic function will become increasingly important for determining treatment decisions and timing of interventions in the future. Additionally, from infancy to adolescence, Mid to Apical IVPD increases as left ventricular relaxation progresses [
2]. It has also been elucidated in human medicine that IVPD or IVPG changes with growth. These studies suggest that IVPG can also function as a new marker for assessing small heart, such as those of infants. Regarding segmental IVPD and diastolic function, Iwano et al. concluded that in patients with diastolic heart failure, the increase in left atrial pressure maintains Basal IVPD, which sustains the peak E velocity despite a decrease in left ventricular suction force [
17]. One significant advantage of segmental IVPD and IVPG is that they allow identification of which part of the left ventricle generates active suction, a crucial factor [
18]. Steine et al. reported, using an ischemic experimental model, that the peak of early diastolic IVPD decreased only in the apical region. Ohara et al. found that patients with diastolic dysfunction, despite having preserved ejection fraction, showed reduced adrenaline-induced enhancement of IVPD from the mid-ventricle to the apex, which was consistent with a decrease in apical suction ability [
19].By dividing the ventricle into segments in this way, it μay be possible to determine whether blood is being “sucked” into the apex “pushed” out of it.
IVPD and IVPG can help clarify conditions that conventional echocardiography alone cannot explain, proving to be excellent indicators of diastolic function [
20]. It is believed that IVPD is influenced not only by volume load and diastolic function but also by the size of the heart and age. However, IVPG is adjusted by the left ventricular long-axis length, making it less affected by heart size [
2,
21]. In veterinary medicine, IVPG is still in the experimental stage, but our study using dogs with PDA (Patent Ductus Arteriosus) demonstrated that IVPG responds more consistently to preload reduction than to left ventricular relaxation. This result supports the clinical utility of CMM-derived IVPG measurements in assessing diastolic function in dogs with PDA at an early stage [
22] Since the heart morphology among dog breeds is relatively similar, with adequately sized intracardiac cavities, dogs are well-suited for IVPG measurements. In diseases like PDA, where the intracardiac cavity is further enlarged, IVPG measurements become even easier. In contrast, cats have smaller intracardiac cavities, making IVPG measurements more challenging than in dogs. However, we have measured IVPG in 106 healthy cats, demonstrating that IVPG measurement is feasible in cats and revealing that it is more susceptible to increased heart rate and short IVRT (Isovolumetric Relaxation Time) [
23]. In this process, the need for future research to evaluate segmental IVPG changes in cats with HCM became evident, particularly to clarify how CMM-estimated IVPG can be applied clinically in feline cardiology. This study aims to demonstrate the clinical utility of IVPG in HCM cases and to further elucidate the impact of left ventricular outflow tract obstruction on IVPG by classifying HCM into HOCM and HNCM.
4.2. Characteristics of the Study Group and Differences in Echocardiographic Features between HOCM and HNCM
This study included 18 cats (11 with HOCM, 7 with HNCM) and 10 control cats. The relatively small sample size and the inability to match the age and body weight distribution between groups are limitations. The IVPG analysis was conducted by inputting CMM image data into a Matlab analysis program. However, in cases where analysis was not feasible, errors occurred. In some cases, in this study, the analysis could not be performed. All of these unmeasurable cases were HOCM cases, which had severe congestion or significant LVOT obstruction, and turbulence near the apex of the heart. For these cases, data from earlier stages of the disease, when the condition was milder, were used, meaning that IVPG data for more severe conditions were not reflected. The fact that measurement is impossible in severe cases poses a significant problem for the examination method, which must be addressed. In cases where the heart rate increased due to excitement, special care was required to calm the animal during the echocardiographic examination [
23].
Previous studies on healthy cats have also shown that heart rate affects IVPG, underscoring the importance of conducting exams in a more natural state to obtain reliable data, regardless of whether analysis is possible. In this study group, both IVSd and LVPWd were elevated in both the HOCM and HNCM groups, indicating myocardial hypertrophy in both groups. The measurement of e’ using TDI is relatively independent of volume load [
24,
25], and the decrease in e’ observed in both the HOCM group and HNCM group in this study suggests left ventricular relaxation impairment or decreased myocardial flexibility in HCM.
However, there was no difference in FS, and there were no cases of marked left atrial enlargement in terms of LAD. There were no significant differences in the E wave between the groups, suggesting that left atrial pressure elevation was not severe. Although the increase in the E/e’ ratio implies an elevation in left ventricular end-diastolic pressure (LVEDP) [
26,
27,
28], and hence an increase in left atrial pressure, the E/e’ values were not significantly higher than normal, and since no morphological changes like atrial enlargement were observed, it can be concluded that the congestion was mild. From conventional echocardiography findings, both the HOCM and HNCM groups exhibit left ventricular relaxation dysfunction or reduced myocardial flexibility due to myocardial hypertrophy, with mild congestion, and no significant differences were observed between the two groups. While the difference in LVOT velocities allows for easy differentiation between HOCM and HNCM using conventional echocardiography, it is interesting that there were no differences between the groups in terms of disease severity. Kizilbash et al. reported that a single measurement of the left ventricular out flow tract is insufficient to define the severity of dynamic left ventricular outflow tract obstruction [
29]. In report on cats, it has been noted that the use of pimobendan in HOCM dose not cause sudden hemodynamic changes [
30]. However, in cases with SAM, the use of pimobendan has been reported to worsen the condition due to hypotension [
31]. This suggests that useful information for treatment selection may not be obtained. In HNCM, there is no outflow tract obstruction, so the primary pathology is impaired diastolic function. On the other hand, in HOCM, the degree of LVOT obstruction may vary during the cardiac cycle, potentially influencing the internal environment of the left ventricle. Conventional echocardiography may not be useful in assessing this difference.
4.3. Is Basal Congestion?
By dividing IVPG into three segments, it becomes possible to categorize pathological conditions in greater detail. Basal IVPG, in particular, is thought to correlate with left atrial pressure [
17]. In this study, Basal IVPD was elevated only in the HOCM group compared to the control group. This finding suggests that evaluating heart function in feline HOCM may be challenging based solely on left atrial enlargement. At the same time, it highlights the importance of Basal IVPG as a key indicator in assessing congestion in feline HOCM. In HOCM, the hypertrophy of the interventricular septum narrows the left ventricular outflow tract, creating resistance to blood flow from the left ventricle to the aorta. This obstruction increases blood flow velocity near the left ventricular base (around the outflow tract), which likely leads to an elevation in IVPG. Particularly during systole, a significant pressure difference between the left ventricle and aorta is generated, contributing to the rise in Basal IVPG. The dynamic obstruction characteristic of HOCM, where sudden worsening occurs during certain parts of the cardiac cycle, may also result in a marked increase in IVPG at the ventricular base, which is likely reflected in this study. Furthermore, in HOCM, a phenomenon called SAM often occurs, where the anterior mitral valve leaflet is pulled into the left ventricular outflow tract. This changes the direction of blood flow at the ventricular base, causing a sharp increase in flow velocity, which likely leads to a further elevation in Basal IVPG. Additionally, mitral regurgitation caused by SAM can lead to an increase in left atrial pressure and volume overload in the left ventricle. This may raise the pressure on the basal side on the heart, potentially causing an increase in Basal IVPG. Given that the severity of HOCM and HNCM in this study is considered mild, the elevation of Basal IVPG observed only in the HOCM group should be understood as being caused by factors other than congestion or increased left atrial pressure. In studies on canine Patent Ductus Arteriosus (PDA), Basal IVPG was a good parameter for assessing congestion, but in cats, particularly those with HOCM, Basal IVPG cannot be considered a highly sensitive indicator of congestion. It is important to recognize that various effects associated with left ventricular outflow tract obstruction may also cause elevated Basal IVPG in these cases.
4.4. Differences in IVPG between HNCM and HOCM
Caution is also needed when interpreting IVPG other than Basal IVPG. In our previous animal experiments, it was shown that an increase in volume load leads to an increase in Basal IVPG, while a decrease in diastolic function causes a reduction in Mid to Apical IVPG [
3,
5]. A similar analysis was conducted in this study, but significant increases were observed only in the HOCM group. In this group, Total and Basal IVPG showed an increase.
In HNCM, the main pathology was believed to be impaired diastolic function, as there is no outflow tract obstruction. We hoped that using IVPD would allow for a detailed assessment of the pressure gradient within the left ventricle, helping us evaluate relaxation dysfunction and abnormal diastolic dynamics. However, unfortunately, we could not find any significant differences compared to the control group. This suggests that using IVPD alone may not be sufficient to evaluate the pathophysiology of HNCM. On the other hand, the observed increases in Total and Basal IVPG in the HOCM group also require careful consideration. As mentioned earlier, the rise in Basal IVPG is likely due to the factors previously discussed, but since Basal IVPG is part of the Total and Mid to Apical IVPG measurements, the increase in these areas may also be influenced by the elevated Basal IVPG. In studies on humans and dogs, the heart is divided into three segments, and only Basal IVPG near the atrium is considered to be influenced by left atrial pressure. However, given that the feline heart is smaller and more compact, it is possible that left atrial pressure affects not only Basal IVPG but also Mid and Apical IVPG. Therefore, it may be necessary to reconsider the method of segmenting IVPG when applied to cats.
4.5. The Significance of IVPG in Diastolic Function in Cats
An increase in IVPG in HOCM cases may have significant clinical implications. While this study could not fully elucidate all aspects, an elevated IVPG may be closely associated with the pathophysiology, symptoms, prognosis, and treatment strategies of HOCM, indicating the need for further research. Traditionally, LVOT velocity has been used to assess the severity of LVOT obstruction in HOCM. Although this measure is valid as an indicator of increased left ventricular pressure due to obstruction, it is unclear whether it accurately reflects the overall condition of the left ventricle. There are reports in human medicine suggesting that IVPG better reflects the dynamic changes in LVOT obstruction in HOCM.
The classic study by Spirito et al. emphasizes that a high IVPG leads to hemodynamic instability, increasing the risk of syncope, heart failure symptoms, and sudden cardiac death. In particular, it explains that patients with outflow tract obstruction have a worse prognosis compared to those without obstruction, as indicated by the IVPG [
32]. Moreover, the report by Maron et al. compared the clinical outcomes of HCM patients with and without left ventricular outflow tract obstruction. It showed that patients with an IVPG of 50 mmHg or higher at rest or during exercise had a significantly higher risk of worsening symptoms, progression to heart failure, and sudden death. Specifically, a high IVPG at rest is a strong predictor of poor prognosis [
33].
Given these findings, many studies suggest that elevated IVPG is associated with poor prognosis in HOCM patients [
34]. It is also highly likely that IVPG can serve as an excellent indicator for evaluating the impact of LVOT obstruction on the left ventricle in cats. To assess whether managing IVPG and appropriate treatment can improve prognosis, it is essential for IVPG to become more widely utilized in veterinary clinical practice. Once sufficient cases have been gathered, clinical trials focusing on prognosis should be conducted. Schober et al. supported that propagation velocity in CMM correlates with tau when using catheterization and serves as an indicator of diastolic function that is less affected by volume load [
12]. As discussed, while high IVPG is often the focus, it is also necessary to consider cases where IVPG may be low. When left ventricular contractility significantly decreases, the pumping function of the myocardium weakens, reducing the pressure difference inside the heart, which can result in low IVPG. For example, in dilated-phase HCM or end-stage heart failure, the intraventricular pressure gradient may be low. In cats, the heart is small, and the myocardial wall is relatively thick, often resulting in a narrow ventricular chamber. Due to this anatomical feature, diastolic function is extremely important in feline hearts. In particular, generating a high IVPG during fast heart rates is necessary to ensure the left ventricle fills with blood during diastole [
35]. Even in healthy individuals, the presence of fused waves may be advantageous in maintaining high IVPG. By evaluating IVPG, it may be possible to assess whether the overall IVPG is being maintained, which could also lead to a broader assessment of diastolic function.
Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
5. Conclusions
Traditional echocardiographic indicators have primarily focused on morphological perspectives and blood flow velocity. In this study, conventional echocardiography did not reveal differences in pathophysiology between the HOCM and HNCM groups. However, by using IVPG for evaluation, increases in Total and Basal IVPG in the HOCM group were detectable. While HOCM and HNCM can be easily differentiated by LVOT velocity, considering the mechanism of IVPG elevation in HOCM, IVPG may better reflect the condition. The pathophysiology of HOCM is influenced by many factors, making its assessment challenging. Traditional echocardiographic indicators required comprehensive knowledge and experience to evaluate the combination of various metrics. However, IVPG offers a simpler and more valuable method for evaluating the impact of left ventricular outflow tract obstruction on the condition.
Limitations
Although the number of cats included in this study is small, the data provided, and its clinical relevance are solid. In addition, the age of the patients was relatively young, and treatment varied from patient to patient. Finally, we did not perform a comparative evaluation of CMM and catheterized IVPD in the present study, as previous studies have shown that CMM-derived IVPD and IVPG correlate strongly with tau measured by invasive catheterization [
15,
36].
Author Contributions
Conceptualization, M.H, M.W, K.M and R.T.; Echocardiography, M.H and M.W.; Formal analysis, M.H.; Methodology, K.T; Software Programming; K.T.; Investigation; A.T, A.Y. and K.T.; Writing—original draft preparation, M.H.; Writing—review and editing, R.T.; Project administration, R.T. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was received.
Institutional Review Board Statement
The animal study was reviewed and approved by the Ethical Committee of the Institute of rescue animal medicine (IRAM2021-01).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to acknowledge Koji Abe and the students of the Department of Veterinary Surgery for their help and support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nishimura, R.A.; Holmes, D.R., Jr. Clinical practice. Hypertrophic obstructive cardiomyopathy. N Engl J Med 2004, 350, 1320-1327. [CrossRef]
- Takahashi, K.; Nii, M.; Takigiku, K.; Toyono, M.; Iwashima, S.; Inoue, N.; Tanaka, N.; Matsui, K.; Shigemitsu, S.; Yamada, M.; et al. Development of suction force during early diastole from the left atrium to the left ventricle in infants, children, and adolescents. Heart Vessels 2019, 34, 296-306. [CrossRef]
- Matsuura, K.; Shiraishi, K.; Sato, K.; Shimada, K.; Goya, S.; Uemura, A.; Ifuku, M.; Iso, T.; Takahashi, K.; Tanaka, R. Left ventricular vortex and intraventricular pressure difference in dogs under various loading conditions. Am J Physiol Heart Circ Physiol 2019, 316, H882-H888. [CrossRef]
- Notomi, Y.; Popovic, Z.B.; Yamada, H.; Wallick, D.W.; Martin, M.G.; Oryszak, S.J.; Shiota, T.; Greenberg, N.L.; Thomas, J.D. Ventricular untwisting: a temporal link between left ventricular relaxation and suction. Am J Physiol-Heart C 2008, 294, H505-H513. [CrossRef]
- Yairo, A.; Mandour, A.S.; Matsuura, K.; Yoshida, T.; Ma, D.; Kitpipatkun, P.; Kato, K.; Cheng, C.J.; El-Husseiny, H.M.; Tanaka, T.; et al. Effect of Loading Changes on the Intraventricular Pressure Measured by Color M-Mode Echocardiography in Rats. Diagnostics (Basel) 2021, 11. [CrossRef]
- Egenvall, A.; Nodtvedt, A.; Haggstrom, J.; Strom Holst, B.; Moller, L.; Bonnett, B.N. Mortality of life-insured Swedish cats during 1999-2006: age, breed, sex, and diagnosis. J Vet Intern Med 2009, 23, 1175-1183. [CrossRef]
- Ferasin, L.; Sturgess, C.P.; Cannon, M.J.; Caney, S.M.; Gruffydd-Jones, T.J.; Wotton, P.R. Feline idiopathic cardiomyopathy: a retrospective study of 106 cats (1994-2001). J Feline Med Surg 2003, 5, 151-159. [CrossRef]
- Payne, J.R.; Brodbelt, D.C.; Luis Fuentes, V. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). J Vet Cardiol 2015, 17 Suppl 1, S244-257. [CrossRef]
- Luis Fuentes, V.; Abbott, J.; Chetboul, V.; Cote, E.; Fox, P.R.; Haggstrom, J.; Kittleson, M.D.; Schober, K.; Stern, J.A. ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. J Vet Intern Med 2020, 34, 1062-1077. [CrossRef]
- Maron, B.J.; Maron, M.S.; Wigle, E.D.; Braunwald, E. The 50-year history, controversy, and clinical implications of left ventricular outflow tract obstruction in hypertrophic cardiomyopathy from idiopathic hypertrophic subaortic stenosis to hypertrophic cardiomyopathy: from idiopathic hypertrophic subaortic stenosis to hypertrophic cardiomyopathy. J Am Coll Cardiol 2009, 54, 191-200. [CrossRef]
- Payne, J.R.; Borgeat, K.; Connolly, D.J.; Boswood, A.; Dennis, S.; Wagner, T.; Menaut, P.; Maerz, I.; Evans, D.; Simons, V.E.; et al. Prognostic indicators in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2013, 27, 1427-1436. [CrossRef]
- Schober, K.E.; Fuentes, V.L.; Bonagura, J.D. Comparison between invasive hemodynamic measurements and noninvasive assessment of left ventricular diastolic function by use of Doppler echocardiography in healthy anesthetized cats. Am J Vet Res 2003, 64, 93-103. [CrossRef]
- Kiatsilapanan, A.; Surachetpong, S.D. Assessment of left atrial function in feline hypertrophic cardiomyopathy by using two- dimensional speckle tracking echocardiography. BMC Vet Res 2020, 16, 344. [CrossRef]
- Khouri, S.J.; Maly, G.T.; Suh, D.D.; Walsh, T.E. A practical approach to the echocardiographic evaluation of diastolic function. J Am Soc Echocardiogr 2004, 17, 290-297. [CrossRef]
- Yotti, R.; Bermejo, J.; Antoranz, J.C.; Desco, M.M.; Cortina, C.; Rojo-Alvarez, J.L.; Allue, C.; Martin, L.; Moreno, M.; Serrano, J.A.; et al. A noninvasive method for assessing impaired diastolic suction in patients with dilated cardiomyopathy. Circulation 2005, 112, 2921-2929. [CrossRef]
- Shigemitsu, S.; Takahashi, K.; Yazaki, K.; Kobayashi, M.; Yamada, M.; Akimoto, K.; Tamaichi, H.; Fujimura, J.; Saito, M.; Nii, M.; et al. New insight into the intraventricular pressure gradient as a sensitive indicator of diastolic cardiac dysfunction in patients with childhood cancer after anthracycline therapy. Heart Vessels 2019, 34, 992-1001. [CrossRef]
- Iwano, H.; Kamimura, D.; Fox, E.; Hall, M.; Vlachos, P.; Little, W.C. Altered spatial distribution of the diastolic left ventricular pressure difference in heart failure. J Am Soc Echocardiogr 2015, 28, 597-605 e591. [CrossRef]
- Ohara, T.; Niebel, C.L.; Stewart, K.C.; Charonko, J.J.; Pu, M.; Vlachos, P.P.; Little, W.C. Loss of adrenergic augmentation of diastolic intra-LV pressure difference in patients with diastolic dysfunction: evaluation by color M-mode echocardiography. JACC Cardiovasc Imaging 2012, 5, 861-870. [CrossRef]
- Steine, K.; Stugaard, M.; Smiseth, O.A. Mechanisms of retarded apical filling in acute ischemic left ventricular failure. Circulation 1999, 99, 2048-2054. [CrossRef]
- Cho, J.S.; Shrestha, S.; Kagiyama, N.; Hu, L.; Ghaffar, Y.A.; Casaclang-Verzosa, G.; Zeb, I.; Sengupta, P.P. A Network-Based "Phenomics" Approach for Discovering Patient Subtypes From High-Throughput Cardiac Imaging Data. JACC Cardiovasc Imaging 2020, 13, 1655-1670. [CrossRef]
- Matsuura, K.; Sato, K.; Shimada, K.; Goya, S.; Uemura, A.; Iso, T.; Yazaki, K.; Yilmaz, Z.; Takahashi, K.; Tanaka, R. Changes in left ventricular blood flow during diastole due to differences in chamber size in healthy dogs. Sci Rep 2020, 10, 1106. [CrossRef]
- Hirose, M.; Mandour, A.S.; Goya, S.; Hamabe, L.; Matsuura, K.; Yoshida, T.; Watanabe, M.; Shimada, K.; Uemura, A.; Takahashi, K.; et al. Color M-Mode Echocardiography for Non-Invasive Assessment of the Intraventricular Pressure in Dogs Before and After Ductus Arteriosus Occlusion: A Retrospective Study. Front Vet Sci 2022, 9, 908829. [CrossRef]
- Matsuura, K.; Bach, M.B.T.; Takahashi, K.; Willesen, J.L.; Koch, J.; Tanaka, R. Non-invasive assessment of left ventricular relaxation property using color M-mode-derived intraventricular pressure gradients in cats. J Vet Cardiol 2022, 41, 236-248. [CrossRef]
- Garcia, M.J.; Palac, R.T.; Malenka, D.J.; Terrell, P.; Plehn, J.F. Color M-mode Doppler flow propagation velocity is a relatively preload-independent index of left ventricular filling. J Am Soc Echocardiogr 1999, 12, 129-137. [CrossRef]
- Sohn, D.W.; Chai, I.H.; Lee, D.J.; Kim, H.C.; Kim, H.S.; Oh, B.H.; Lee, M.M.; Park, Y.B.; Choi, Y.S.; Seo, J.D.; et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 1997, 30, 474-480. [CrossRef]
- Ommen, S.R.; Nishimura, R.A.; Appleton, C.P.; Miller, F.A.; Oh, J.K.; Redfield, M.M.; Tajik, A.J. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation 2000, 102, 1788-1794. [CrossRef]
- Oyamada, J.; Toyono, M.; Shimada, S.; Aoki-Okazaki, M.; Tamura, M.; Takahashi, T.; Harada, K. Noninvasive estimation of left ventricular end-diastolic pressure using tissue Doppler imaging combined with pulsed-wave Doppler echocardiography in patients with ventricular septal defects: a comparison with the plasma levels of the B-type natriuretic Peptide. Echocardiography 2008, 25, 270-277. [CrossRef]
- Masutani, S.; Saiki, H.; Kurishima, C.; Kuwata, S.; Tamura, M.; Senzaki, H. Assessment of ventricular relaxation and stiffness using early diastolic mitral annular and inflow velocities in pediatric patients with heart disease. Heart Vessels 2014, 29, 825-833. [CrossRef]
- Kizilbash, A.M.; Heinle, S.K.; Grayburn, P.A. Spontaneous variability of left ventricular outflow tract gradient in hypertrophic obstructive cardiomyopathy. Circulation 1998, 97, 461-466. [CrossRef]
- Ward, J.L.; Kussin, E.Z.; Tropf, M.A.; Tou, S.P.; DeFrancesco, T.C.; Keene, B.W. Retrospective evaluation of the safety and tolerability of pimobendan in cats with obstructive vs nonobstructive cardiomyopathy. J Vet Intern Med 2020, 34, 2211-2222. [CrossRef]
- Gordon, S.G.; Saunders, A.B.; Roland, R.M.; Winter, R.L.; Drourr, L.; Achen, S.E.; Hariu, C.D.; Fries, R.C.; Boggess, M.M.; Miller, M.W. Effect of oral administration of pimobendan in cats with heart failure. J Am Vet Med Assoc 2012, 241, 89-94. [CrossRef]
- Spirito, P.; Seidman, C.E.; McKenna, W.J.; Maron, B.J. The management of hypertrophic cardiomyopathy. N Engl J Med 1997, 336, 775-785. [CrossRef]
- Maron, M.S.; Olivotto, I.; Betocchi, S.; Casey, S.A.; Lesser, J.R.; Losi, M.A.; Cecchi, F.; Maron, B.J. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med 2003, 348, 295-303. [CrossRef]
- Yazaki, K.; Suzuki, A.; Shiga, T.; Minami, Y.; Arai, K.; Ashihara, K.; Shoda, M.; Hagiwara, N. Left intraventricular pressure gradient in hypertrophic cardiomyopathy patients receiving implantable cardioverter-defibrillators for primary prevention. BMC Cardiovasc Disord 2021, 21, 106. [CrossRef]
- Popovic, Z.B.; Richards, K.E.; Greenberg, N.L.; Rovner, A.; Drinko, J.; Cheng, Y.; Penn, M.S.; Fukamachi, K.; Mal, N.; Levine, B.D.; et al. Scaling of diastolic intraventricular pressure gradients is related to filling time duration. Am J Physiol Heart Circ Physiol 2006, 291, H762-769. [CrossRef]
- Nagueh, S.F. Heart failure with preserved ejection fraction: insights into diagnosis and pathophysiology. Cardiovasc Res 2021, 117, 999-1014. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).