Submitted:
10 October 2024
Posted:
10 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Foundations of Wound Healing: Implications for Health and Recovery
1.2. Wound Healing Dynamics, Microbial Biofilms, and Fibrotic Responses in Silicone Mammary Implants
2. From Wound to Peri-SMI Capsular Fibrosis
2.1. Immediate Inflammatory Response and Early Fibrosis
Acute Inflammatory Response
Early Fibrotic Changes
Molecular Signaling Pathways
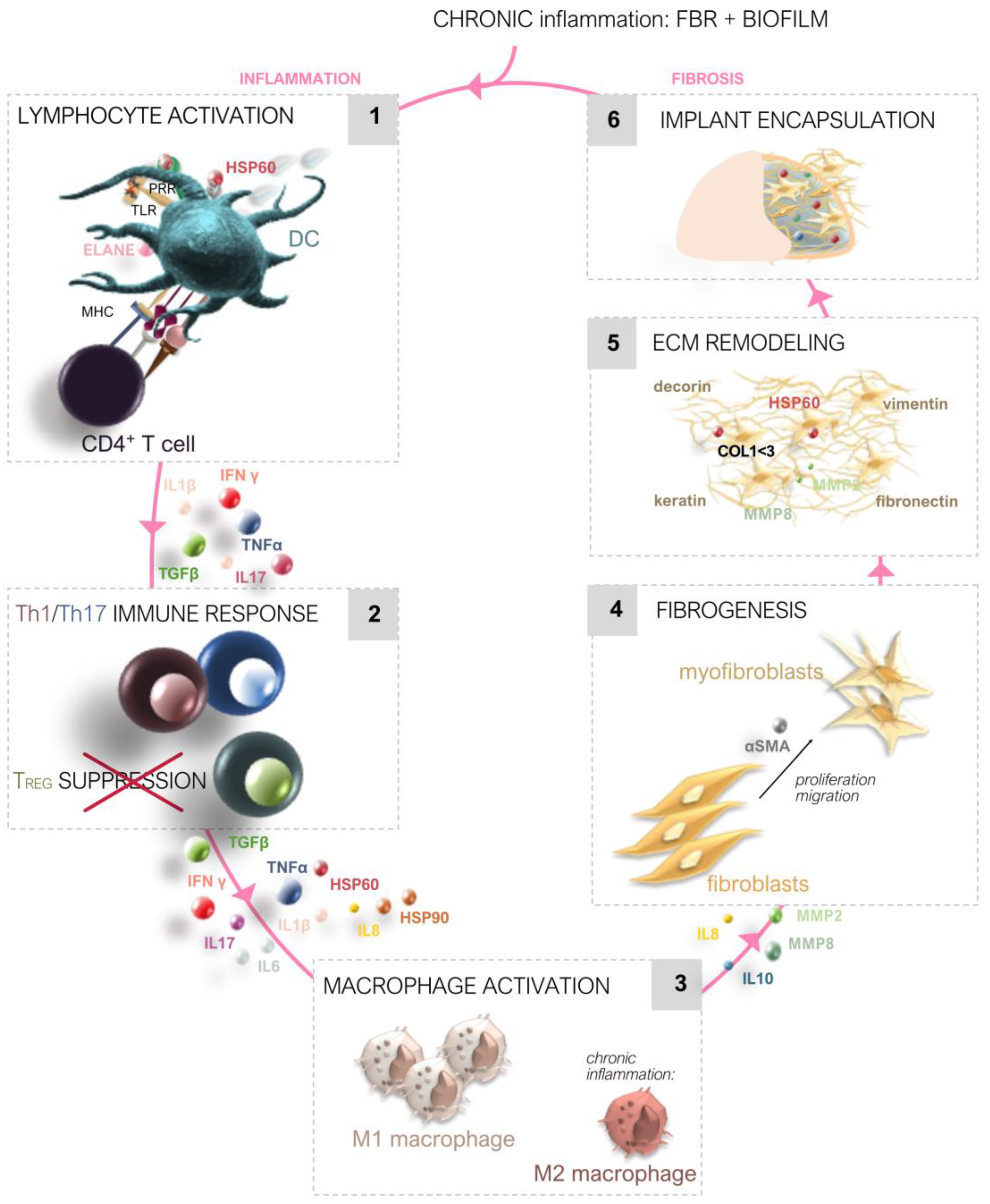
2.2. Chronic Inflammation and Capsular Contracture
Chronic Inflammatory Response
Capsular Contracture Formation
Molecular Signaling Pathways
Immune Cell Interactions
S100 Proteins on Inflammation and Fibrosis
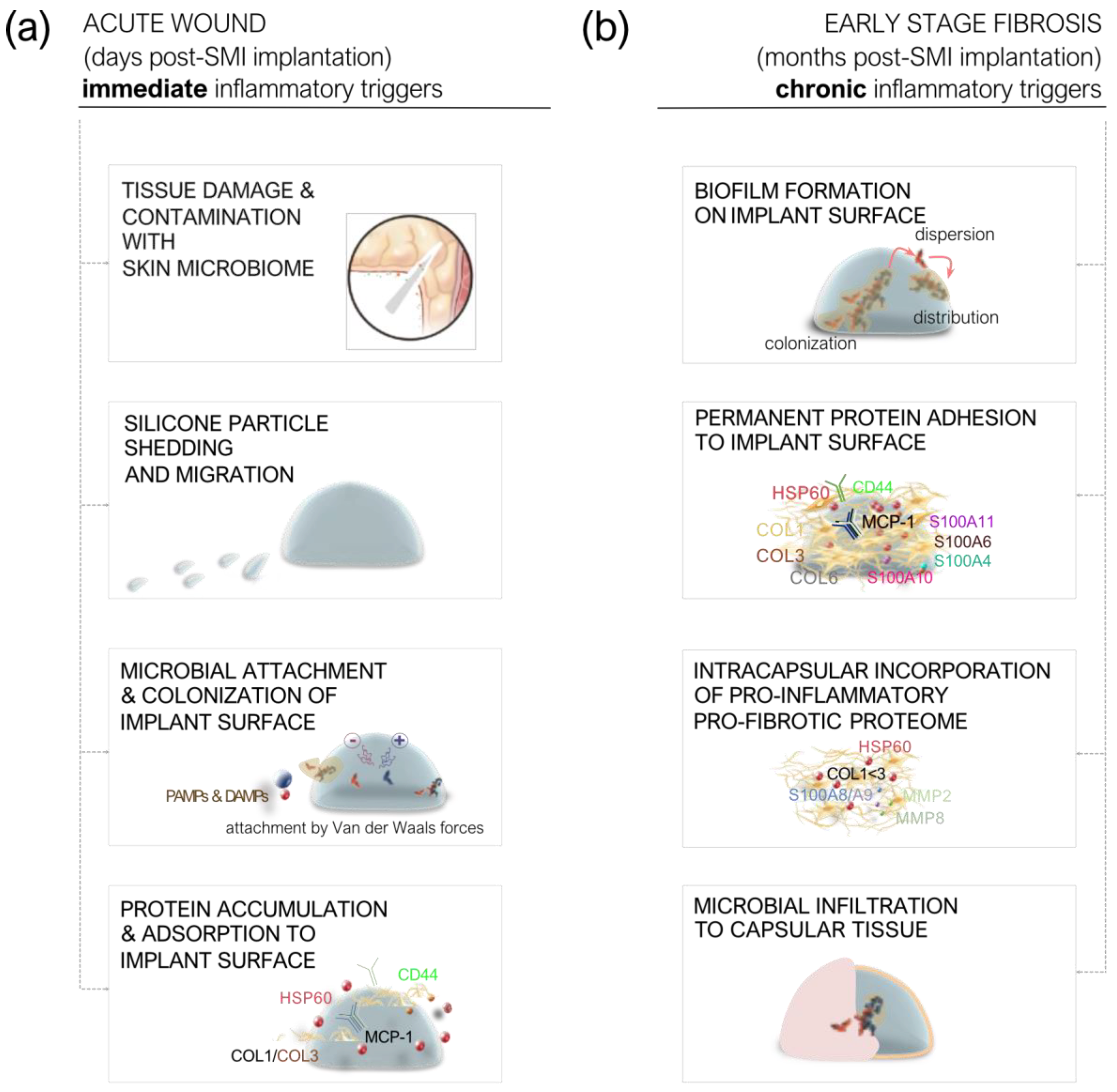
2.3. Immediate and Chronic Inflammatory Triggers Following SMI Insertion
Immediate Post-Implantation Response
Early-Stage Fibrosis (Months Post-Implantation)
2.4. Influence of Surface Characteristics on Inflammation and Fibrotic Pathways
Surface Texture and Inflammation
Surface Characteristics and Cellular Responses
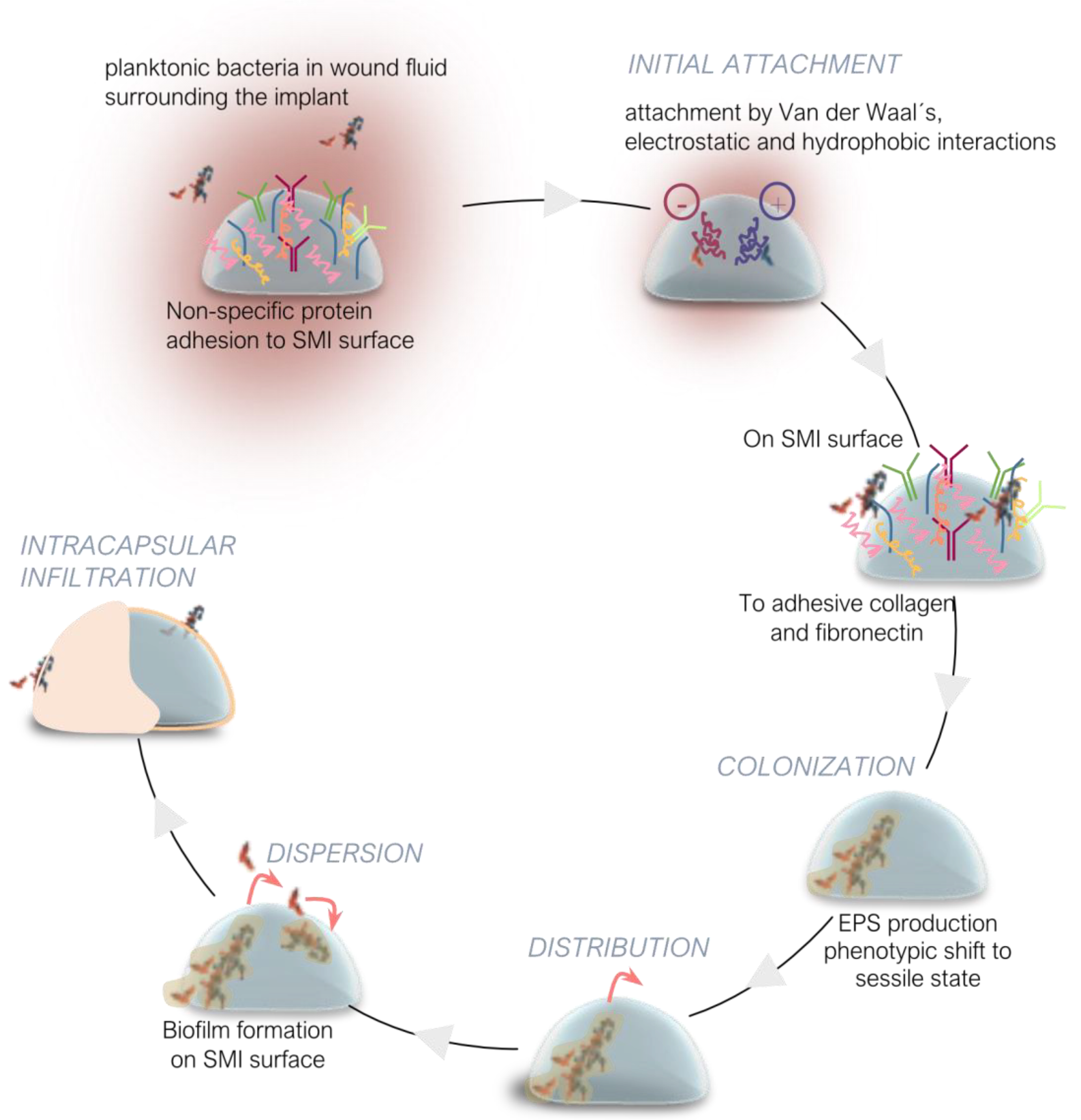
3. Microbial Adhesion, Colonization, and Biofilm Formation on SMI
3.1. Microbial Transmission and Surface Adhesion
3.2. Colonization and Biofilm Formation
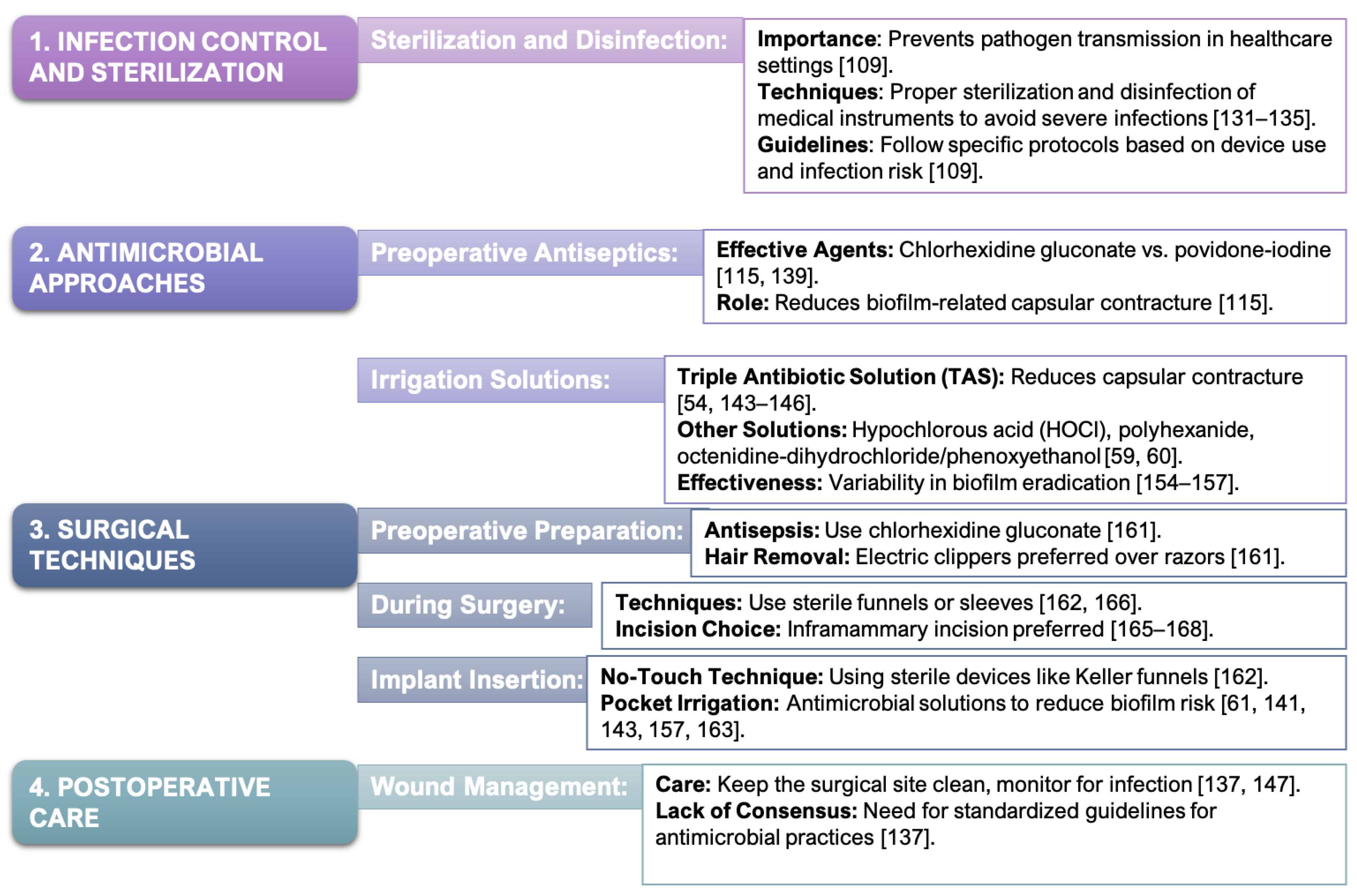
4. Reducing Capsular Contracture: Antimicrobial Strategies in Breast Implant Surgery
4.1. Antimicrobial Approaches to Mitigate Postoperative Infections and Biofilm Formation
4.2. Surgical Techniques for Minimizing Bacterial Contamination
5. Advances in Implant-Shell-Material and Non-Pharmacological Strategies to Prevent Biofilm-Associated Fibrosis
5.1. Physical Modifications with Antimicrobial Properties
Polyurethane Foam Coatings
Influence of Surface Topography on Biofilm Dynamics
5.2. Biological Matrices with Antimicrobial Properties
Antibiotic-Impregnated Meshes
Spider Silk-Based Meshes
Zwitterionic Polymers
6. Pharmacological Strategies to Prevent Biofilm-Associated Fibrosis in Implants
6.1. Antibacterial Drugs
Systemic Antibacterials
Topical Antibacterials
Drug Incorporation into Implants
6.2. Antifibrotic and Anti-Inflammatory Drugs
Pirfenidone
Halofuginone
Dexamethasone
6.3. Integration of Antimicrobial Strategies
7. Clinical Implications and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noskovicova, N.; Hinz, B.; Pakshir, P. Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction. Cells 2021, 10, 1794. [Google Scholar] [CrossRef]
- Wick, G.; Grundtman, C.; Mayerl, C.; Wimpissinger, T.-F.; Feichtinger, J.; Zelger, B.; Sgonc, R.; Wolfram, D. The Immunology of Fibrosis. Annu. Rev. Immunol. 2013, 31, 107–135. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Safran, T.; Nepon, H.; Chu, C.K.; Winocour, S.; Murphy, A.M.; Davison, P.G.; Dionisopolos, T.; Vorstenbosch, J. Current Concepts in Capsular Contracture: Pathophysiology, Prevention, and Management. Semin. Plast. Surg. 2021, 35, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Kadirvelu, L.; Sivaramalingam, S.S.; Jothivel, D.; Chithiraiselvan, D.D.; Govindarajan, D.K.; Kandaswamy, K. A review on antimicrobial strategies in mitigating biofilm-associated infections on medical implants. Curr. Res. Microb. Sci. 2024, 6, 100231. [Google Scholar] [CrossRef] [PubMed]
- Pool, J. G. 1977. Normal hemostatic mechanisms: a review. The American journal of medical technology 43.
- Furie, Bruce, and Barbara C Furie. 2008. Mechanisms of thrombus formation. Mechanisms of Disease. The New England journal of medicine 359.
- Locatelli, L.; Colciago, A.; Castiglioni, S.; Maier, J.A. Platelets in Wound Healing: What Happens in Space? Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Su, Y.; Richmond, A. Chemokine Regulation of Neutrophil Infiltration of Skin Wounds. Adv. Wound Care 2015, 4, 631–640. [Google Scholar] [CrossRef]
- He, L.; Marneros, A.G. Macrophages Are Essential for the Early Wound Healing Response and the Formation of a Fibrovascular Scar. Am. J. Pathol. 2013, 182, 2407–2417. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: the role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Namas, R.A.; Mi, Q.; Namas, R.; Almahmoud, K.; Zaaqoq, A.M.; Abdul-Malak, O.; Azhar, N.; Day, J.; Abboud, A.; Zamora, R.; et al. Insights into the Role of Chemokines, Damage-Associated Molecular Patterns, and Lymphocyte-Derived Mediators from Computational Models of Trauma-Induced Inflammation. Antioxidants Redox Signal. 2015, 23, 1370–1387. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat. Immunol. 2011, 12, 1035–1044. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Eilken, H.M.; Adams, R.H. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr. Opin. Cell Biol. 2010, 22, 617–625. [Google Scholar] [CrossRef]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in Wound Healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Kirsner, R.S. Angiogenesis in wound repair: Angiogenic growth factors and the extracellular matrix. Microsc. Res. Tech. 2002, 60, 107–114. [Google Scholar] [CrossRef]
- Mascharak, S.; Desjardins-Park, H.E.; Longaker, M.T. Fibroblast Heterogeneity in Wound Healing: Hurdles to Clinical Translation. Trends Mol. Med. 2020, 26, 1101–1106. [Google Scholar] [CrossRef]
- Kohan, M.; Muro, A.F.; White, E.S.; Berkman, N. EDA-containing cellular fibronectin induces fibroblast differentiation through binding to α4β7integrin receptor and MAPK/Erk 1/2-dependent signaling. FASEB J. 2010, 24, 4503–4512. [Google Scholar] [CrossRef] [PubMed]
- Serini, G.; Bochaton-Piallat, M.-L.; Ropraz, P.; Geinoz, A.; Borsi, L.; Zardi, L.; Gabbiani, G. The Fibronectin Domain ED-A Is Crucial for Myofibroblastic Phenotype Induction by Transforming Growth Factor-β1. J. Cell Biol. 1998, 142, 873–881. [Google Scholar] [CrossRef]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2016, 1863, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmouliere, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Thulabandu, V.; Chen, D.; Atit, R.P. Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip. Rev. Dev. Biol. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- P. , B. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Pakshir, P.; Noskovicova, N.; Lodyga, M.; Son, D.O.; Schuster, R.; Goodwin, A.; Karvonen, H.; Hinz, B. The myofibroblast at a glance. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef]
- Hinz, B. The role of myofibroblasts in wound healing. Curr. Res. Transl. Med. 2016, 64, 171–177. [Google Scholar] [CrossRef]
- Hinz, B.; McCulloch, C.A.; Coelho, N.M. Mechanical regulation of myofibroblast phenoconversion and collagen contraction. Exp. Cell Res. 2019, 379, 119–128. [Google Scholar] [CrossRef]
- Klingberg, F.; Hinz, B.; White, E.S. The myofibroblast matrix: implications for tissue repair and fibrosis. J. Pathol. 2012, 229, 298–309. [Google Scholar] [CrossRef]
- Lindley, L.E.; Stojadinovic, O.; Pastar, I.; Tomic-Canic, M. Biology and Biomarkers for Wound Healing. Plast. Reconstr. Surg. 2016, 138, 18S–28S. [Google Scholar] [CrossRef]
- Hartman, E.; Wallblom, K.; van der Plas, M.J.A.; Petrlova, J.; Cai, J.; Saleh, K.; Kjellström, S.; Schmidtchen, A. Bioinformatic Analysis of the Wound Peptidome Reveals Potential Biomarkers and Antimicrobial Peptides. Front. Immunol. 2021, 11. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.D.O.; Costa, T.F.; de Araújo Andrade, Z.; Medrado, A.R.A.P. Wound Healing—A Literature Review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.F. The role of mast cells in wound healing. Int. Wound J. 2010, 7, 55–61. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Khomtchouk, K.; Santa Maria, P.L. A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms. Clin. Rev. Allergy Immunol. 2020, 58, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Rinkevich, Y. Scars or Regeneration?—Dermal Fibroblasts as Drivers of Diverse Skin Wound Responses. Int. J. Mol. Sci. 2020, 21, 617. [Google Scholar] [CrossRef]
- Cohen, I.K. Lessons from the history of wound healing. Clin. Dermatol. 2007, 25, 3–8. [Google Scholar] [CrossRef]
- Li, H.; Cai, E.; Cheng, H.; Ye, X.; Ma, R.; Zhu, H.; Chang, X. FGA Controls VEGFA Secretion to Promote Angiogenesis by Activating the VEGFR2-FAK Signalling Pathway. Front. Endocrinol. 2022, 13, 791860. [Google Scholar] [CrossRef]
- Margadant, C.; Sonnenberg, A. Integrin–TGF-β crosstalk in fibrosis, cancer and wound healing. Embo Rep. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Jayaraman, P.; Sada-Ovalle, I.; Nishimura, T.; Anderson, A.C.; Kuchroo, V.K.; Remold, H.G.; Behar, S.M. IL-1β Promotes Antimicrobial Immunity in Macrophages by Regulating TNFR Signaling and Caspase-3 Activation. J. Immunol. 2013, 190, 4196–4204. [Google Scholar] [CrossRef]
- Veves, A. ; Veves; Aristidis; Dsc Discussion: Biology and Biomarkers for Wound Healing. Plast. Reconstr. Surg. 2016, 138, 29S–30S. [Google Scholar] [CrossRef]
- Perry, D.; Frame, J. The history and development of breast implants. Ind. Mark. Manag. 2020, 102, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, I.D.; Eleweke, C.; Aladesanwa, F.O.; Onabajo, B.O. Post-mastectomy breast reconstruction: an overview of the state of the art, challenges, and prospects. Int. Surg. J. 2023, 10, 348–354. [Google Scholar] [CrossRef]
- Wick, G.; Backovic, A.; Rabensteiner, E.; Plank, N.; Schwentner, C.; Sgonc, R. The immunology of fibrosis: innate and adaptive responses. Trends Immunol. 2010, 31, 110–119. [Google Scholar] [CrossRef]
- Schoberleitner, I.; Faserl, K.; Sarg, B.; Egle, D.; Brunner, C.; Wolfram, D. Quantitative Proteomic Characterization of Foreign Body Response towards Silicone Breast Implants Identifies Chronological Disease-Relevant Biomarker Dynamics. Biomolecules 2023, 13, 305. [Google Scholar] [CrossRef]
- Schoberleitner, I.; Faserl, K.; Tripp, C.H.; Pechriggl, E.J.; Sigl, S.; Brunner, A.; Zelger, B.; Hermann-Kleiter, N.; Baier, L.; Steinkellner, T.; et al. Silicone implant surface microtopography modulates inflammation and tissue repair in capsular fibrosis. Front. Immunol. 2024, 15, 1342895. [Google Scholar] [CrossRef]
- Mempin, M.; Hu, H.; Chowdhury, D.; Deva, A.; Vickery, K. The A, B and C’s of Silicone Breast Implants: Anaplastic Large Cell Lymphoma, Biofilm and Capsular Contracture. Materials 2018, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Haddouti, E.-M.; Welle, K.; Burger, C.; Wirtz, D.C.; Schildberg, F.A.; Kabir, K. The Effects of Biomaterial Implant Wear Debris on Osteoblasts. Front. Cell Dev. Biol. 2020, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Schoberleitner, I.; Baier, L.; Lackner, M.; Zenz, L.-M.; Coraça-Huber, D.C.; Ullmer, W.; Damerum, A.; Faserl, K.; Sigl, S.; Steinkellner, T.; et al. Surface Topography, Microbial Adhesion, and Immune Responses in Silicone Mammary Implant-Associated Capsular Fibrosis. Int. J. Mol. Sci. 2024, 25, 3163. [Google Scholar] [CrossRef]
- Trojanek, J.B.; Michałkiewicz, J.; Grzywa-Czuba, R.; Jańczyk, W.; Gackowska, L.; Kubiszewska, I.; Helmin-Basa, A.; Wierzbicka-Rucińska, A.; Szalecki, M.; Socha, P. Expression of Matrix Metalloproteinases and Their Tissue Inhibitors in Peripheral Blood Leukocytes and Plasma of Children with Nonalcoholic Fatty Liver Disease. Mediat. Inflamm. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Singh, D.; Rai, V.; Agrawal, D.K. Regulation of Collagen I and Collagen III in Tissue Injury and Regeneration. Cardiol. Cardiovasc. Med. 2023, 07, 5–16. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Strus, M.; Pasich, E. Antibiotic resistance: a “dark side” of biofilm-associated chronic infections. Pol. Arch. Intern. Med. 2013, 123, 309–313. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Deva, Anand K., and Lionel C. Chang. 1999. Bacterial biofilms: A cause for accelerated capsular contracture? Aesthetic Surgery Journal 19. Mosby Inc.: 130–133. [CrossRef]
- Deva, A.K.; Adams, W.P., Jr.; Vickery, K. The Role of Bacterial Biofilms in Device-Associated Infection. Plast. Reconstr. Surg. 2013, 132, 1319–1328. [Google Scholar] [CrossRef]
- del Pozo, Jose L., and Cristina Auba. 2015. Role of biofilms in breast implant associated infections and capsular contracture. Advances in Experimental Medicine and Biology 831. Springer New York LLC: 53–67. [CrossRef]
- Ajdic, D.; Zoghbi, Y.; Gerth, D.; Panthaki, Z.J.; Thaller, S. The Relationship of Bacterial Biofilms and Capsular Contracture in Breast Implants. Aesthetic Surg. J. 2016, 36, 297–309. [Google Scholar] [CrossRef]
- Rieger, U.M.; Mesina, J.; Kalbermatten, D.F.; Haug, M.; Frey, H.P.; Pico, R.; Frei, R.; Pierer, G.; Lüscher, N.J.; Trampuz, A. Bacterial biofilms and capsular contracture in patients with breast implants. Br. J. Surg. 2013, 100, 768–774. [Google Scholar] [CrossRef]
- Ji, L.; Wang, T.; Tian, L.; Song, H.; Gao, M. Roxatidine inhibits fibrosis by inhibiting NF-κB and MAPK signaling in macrophages sensing breast implant surface materials. Mol. Med. Rep. 2020, 21, 161–172. [Google Scholar] [CrossRef]
- Rembe, J.-D.; Huelsboemer, L.; Plattfaut, I.; Besser, M.; Stuermer, E.K. Antimicrobial Hypochlorous Wound Irrigation Solutions Demonstrate Lower Anti-biofilm Efficacy Against Bacterial Biofilm in a Complex in-vitro Human Plasma Biofilm Model (hpBIOM) Than Common Wound Antimicrobials. Front. Microbiol. 2020, 11, 564513. [Google Scholar] [CrossRef] [PubMed]
- Epps, M.T.; Langsdon, S.B.; Pels, T.K.B.; Lee, T.M.; Thurston, T.; Brzezienski, M.A. Antimicrobial Irrigation and Technique during Breast Augmentation: Survey of Current Practice. Plast. Reconstr. Surg. - Glob. Open 2019, 7, e2310. [Google Scholar] [CrossRef]
- Gofstein-Hayuth, D.; Fliss, E.; Barnea, Y.; Legarda, C.; Bracha, G.; Lerner, A.; Lellouche, J.; Carmeli, Y.; Shani, N.; Arad, E. Comparing the efficacy of antimicrobial pocket-irrigation protocols in an in vivo breast implant infection model. J. Plast. Reconstr. Aesthetic Surg. 2023, 85, 165–173. [Google Scholar] [CrossRef]
- Federica, G.; Tommaso, F.; Alessia, C.; Agostino, C.; Florian, B.; Antonio, G.; Nicola, M.D.; Abdallah, R.; Carmela, S.; Lorenzo, S.; et al. Use of Antimicrobial Irrigation and Incidence of Capsular Contracture in Breast Augmentation and Immediate Implant-Based Breast Reconstruction. Aesthetic Plast. Surg. 2023, 47, 2345–2350. [Google Scholar] [CrossRef] [PubMed]
- Pakshir, P.; Hinz, B. The big five in fibrosis: Macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 2018, 68-69, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Mack, M. Inflammation and fibrosis. Matrix Biol. 2018, 68-69, 106–121. [Google Scholar] [CrossRef]
- Moore, Laura Beth, and Themis R. Kyriakides. 2015. Molecular characterization of macrophage-biomaterial interactions. Advances in Experimental Medicine and Biology 865. Springer New York LLC: 109–122. [CrossRef]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [PubMed]
- Sakar, M.S.; Eyckmans, J.; Pieters, R.; Eberli, D.; Nelson, B.J.; Chen, C.S. Cellular forces and matrix assembly coordinate fibrous tissue repair. Nat. Commun. 2016, 7, 11036. [Google Scholar] [CrossRef]
- Schuster, R.; Rockel, J.S.; Kapoor, M.; Hinz, B. The inflammatory speech of fibroblasts. Immunol. Rev. 2021, 302, 126–146. [Google Scholar] [CrossRef]
- Witherel, C.E.; Abebayehu, D.; Barker, T.H.; Spiller, K.L. Macrophage and Fibroblast Interactions in Biomaterial-Mediated Fibrosis. Adv. Heal. Mater. 2019, 8, e1801451. [Google Scholar] [CrossRef]
- Hinz, B.; Celetta, G.; Tomasek, J.J.; Gabbiani, G.; Chaponnier, C. Alpha-Smooth Muscle Actin Expression Upregulates Fibroblast Contractile Activity. Mol. Biol. Cell 2001, 12, 2730–2741. [Google Scholar] [CrossRef]
- Moyer, K.E.M.; Ehrlich, H.P. Capsular Contracture after Breast Reconstruction. Plast. Reconstr. Surg. 2013, 131, 680–685. [Google Scholar] [CrossRef]
- Cheung, D.T.; Benya, P.D.; Perelman, N.; Dicesare, P.E.; Nimni, M.E. A Highly Specific and Quantitative Method for Determining Type III/I Collagen Ratios in Tissues. Matrix 1990, 10, 164–171. [Google Scholar] [CrossRef]
- Akilbekova, D.; Bratlie, K.M. Quantitative Characterization of Collagen in the Fibrotic Capsule Surrounding Implanted Polymeric Microparticles through Second Harmonic Generation Imaging. PLOS ONE 2015, 10, e0130386. [Google Scholar] [CrossRef] [PubMed]
- Dolores, W.; Christian, R.; Harald, N.; Hildegunde, P.; Georg, W. Cellular and molecular composition of fibrous capsules formed around silicone breast implants with special focus on local immune reactions. J. Autoimmun. 2004, 23, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-β signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-H.; Chen, D.-Q.; Wang, Y.-N.; Feng, Y.-L.; Cao, G.; Vaziri, N.D.; Zhao, Y.-Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Interactions 2018, 292, 76–83. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Dondossola, E.; Holzapfel, B.M.; Alexander, S.; Filippini, S.; Hutmacher, D.W.; Friedl, P. Examination of the foreign body response to biomaterials by nonlinear intravital microscopy. Nat. Biomed. Eng. 2016, 1, 1–10. [Google Scholar] [CrossRef]
- Miron, R.J.; Bosshardt, D.D. Multinucleated Giant Cells: Good Guys or Bad Guys? Tissue Eng. Part B Rev. 2018, 24, 53–65. [Google Scholar] [CrossRef]
- McNally, A.K.; Anderson, J.M. Phenotypic expression in human monocyte-derived interleukin-4-induced foreign body giant cells and macrophagesin vitro: Dependence on material surface properties. J. Biomed. Mater. Res. Part A 2014, 103, 1380–1390. [Google Scholar] [CrossRef]
- Volk, S.W.; Wang, Y.; Mauldin, E.A.; Liechty, K.W.; Adams, S.L. Diminished Type III Collagen Promotes Myofibroblast Differentiation and Increases Scar Deposition in Cutaneous Wound Healing. Cells Tissues Organs 2011, 194, 25–37. [Google Scholar] [CrossRef]
- Desmoulière, A.; Chaponnier, C.; Gabbiani, G. Perspective Article: Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005, 13, 7–12. [Google Scholar] [CrossRef] [PubMed]
- de Bakker, E.; Rots, M.; Buncamper, M.E.; Niessen, F.B.; Smit, J.M.; Winters, H.A.H.; Özer, M.; de Vet, H.C.W.; Mullender, M.G. The Baker Classification for Capsular Contracture in Breast Implant Surgery Is Unreliable as a Diagnostic Tool. Plast. Reconstr. Surg. 2020, 146, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Spear, S.L.; Baker, J.L. Classification of capsular contracture after prosthetic breast reconstruction. . 1995, 96, 1119. [Google Scholar] [PubMed]
- Hall-Findlay, E.J.; Findlay, H.; J. , E. Discussion: The Baker Classification for Capsular Contracture in Breast Implant Surgery Is Unreliable as a Diagnostic Tool. Plast. Reconstr. Surg. 2020, 146, 963–963. [Google Scholar] [CrossRef]
- Bachour, Y.; Verweij, S.P.; Gibbs, S.; Ket, J.C.; Ritt, M.J.; Niessen, F.B.; Mullender, M.G. The aetiopathogenesis of capsular contracture: A systematic review of the literature. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 307–317. [Google Scholar] [CrossRef]
- Lan, H.Y. Diverse Roles of TGF-β/Smads in Renal Fibrosis and Inflammation. Int. J. Biol. Sci. 2011, 7, 1056–1067. [Google Scholar] [CrossRef]
- Mirastschijski, U.; Schnabel, R.; Claes, J.; Schneider, W.; Ågren, M.S.; Haaksma, C.; Tomasek, J.J. Matrix metalloproteinase inhibition delays wound healing and blocks the latent transforming growth factor-β1-promoted myofibroblast formation and function. Wound Repair Regen. 2010, 18, 223–234. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S. T Cells in Fibrosis and Fibrotic Diseases. Front. Immunol. 2020, 11, 1142. [Google Scholar] [CrossRef]
- Iwanaga, N.; Kolls, J.K. Updates on T helper type 17 immunity in respiratory disease. Immunology 2018, 156, 3–8. [Google Scholar] [CrossRef]
- Zhang, S. The role of transforming growth factor β in T helper 17 differentiation. Immunology 2018, 155, 24–35. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Mikami, N.; Wing, J.B.; Tanaka, A.; Ichiyama, K.; Ohkura, N. Regulatory T Cells and Human Disease. Annu. Rev. Immunol. 2020, 38, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, D.; Rabensteiner, E.; Grundtman, C.; Böck, G.; Mayerl, C.; Parson, W.; Almanzar, G.; Hasenöhrl, C.; Piza-Katzer, H.; Wick, G. T Regulatory Cells and TH17 Cells in Peri–Silicone Implant Capsular Fibrosis. Plast. Reconstr. Surg. 2012, 129, 327e–337e. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Ali, S.A. Multifunctional Role of S100 Protein Family in the Immune System: An Update. Cells 2022, 11, 2274. [Google Scholar] [CrossRef]
- Gonzalez, L.L.; Garrie, K.; Turner, M.D. Role of S100 proteins in health and disease. Biochim. et Biophys. Acta (BBA) - Mol. Cell Res. 2020, 1867, 118677. [Google Scholar] [CrossRef]
- Sreejit, Gopalkrishna, Michelle C. Flynn, Mallikarjun Patil, Prasanna Krishnamurthy, Andrew J. Murphy, and Prabhakara R. Nagareddy. 2020. S100 family proteins in inflammation and beyond. Advances in Clinical Chemistry 98. Elsevier: 173–231. [CrossRef]
- Araki, K.; Kinoshita, R.; Tomonobu, N.; Gohara, Y.; Tomida, S.; Takahashi, Y.; Senoo, S.; Taniguchi, A.; Itano, J.; Yamamoto, K.-I.; et al. The heterodimer S100A8/A9 is a potent therapeutic target for idiopathic pulmonary fibrosis. J. Mol. Med. 2020, 99, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Darby, Ian A., and Tim D. Hewitson. 2007. Fibroblast Differentiation in Wound Healing and Fibrosis. International Review of Cytology 257: 143–179. [CrossRef]
- Cappellano, G.; Ploner, C.; Lobenwein, S.; Sopper, S.; Hoertnagl, P.; Mayerl, C.; Wick, N.; Pierer, G.; Wick, G.; Wolfram, D. Immunophenotypic characterization of human T cells after in vitro exposure to different silicone breast implant surfaces. PLOS ONE 2018, 13, e0192108. [Google Scholar] [CrossRef]
- Doloff, J.C.; Veiseh, O.; de Mezerville, R.; Sforza, M.; Perry, T.A.; Haupt, J.; Jamiel, M.; Chambers, C.; Nash, A.; Aghlara-Fotovat, S.; et al. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nat. Biomed. Eng. 2021, 5, 1115–1130. [Google Scholar] [CrossRef]
- Bizjak, M.; Selmi, C.; Praprotnik, S.; Bruck, O.; Perricone, C.; Ehrenfeld, M.; Shoenfeld, Y. Silicone implants and lymphoma: The role of inflammation. J. Autoimmun. 2015, 65, 64–73. [Google Scholar] [CrossRef]
- Brauman, D. Rough Textured Silicone Implants, Bacterial Biofilms, and Capsular Contracture. 2019, 144, 322E–323E. [CrossRef]
- Schoberleitner, I.; Augustin, A.; Egle, D.; Brunner, C.; Amort, B.; Zelger, B.; Brunner, A.; Wolfram, D. Is It All about Surface Topography? An Intra-Individual Clinical Outcome Analysis of Two Different Implant Surfaces in Breast Reconstruction. J. Clin. Med. 2023, 12, 1315. [Google Scholar] [CrossRef]
- Abaricia, J.O.; Farzad, N.; Heath, T.J.; Simmons, J.; Morandini, L.; Olivares-Navarrete, R. Control of innate immune response by biomaterial surface topography, energy, and stiffness. Acta Biomater. 2021, 133, 58–73. [Google Scholar] [CrossRef]
- Love, R.J.; Jones, K.S. The recognition of biomaterials: Pattern recognition of medical polymers and their adsorbed biomolecules. J. Biomed. Mater. Res. Part A 2013, 101A, 2740–2752. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S. 2017. Sterilization and Disinfection. Essentials of Neuroanesthesia. Elsevier: 929. [CrossRef]
- Belay, E.D.; Schonberger, L.B.; Brown, P.; Priola, S.A.; Chesebro, B.; Will, R.G.; Asher, D.M. Disinfection and Sterilization of Prion-Contaminated Medical Instruments. Infect. Control. Hosp. Epidemiology 2010, 31, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W. New Disinfection and Sterilization Methods. Emerg. Infect. Dis. 2001, 7, 348–353. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Guideline for Disinfection and Sterilization of Prion-Contaminated Medical Instruments. Infect. Control. Hosp. Epidemiol. 2010, 31, 107–117. [Google Scholar] [CrossRef]
- Lam, M.; Migonney, V.; Falentin-Daudre, C. Review of silicone surface modification techniques and coatings for antibacterial/antimicrobial applications to improve breast implant surfaces. Acta Biomater. 2020, 121, 68–88. [Google Scholar] [CrossRef]
- Dapunt, U.; Prior, B.; Kretzer, J.P.; Giese, T.; Zhao, Y. Bacterial Biofilm Components Induce an Enhanced Inflammatory Response Against Metal Wear Particles. Ther. Clin. Risk Manag. 2020, ume 16, 1203–1212. [Google Scholar] [CrossRef]
- Carvajal, J.; Carvajal, M.; Chavez, D.; Hernandez, G. Back to Basics: Could the Preoperative Skin Antiseptic Agent Help Prevent Biofilm-Related Capsular Contracture? Aesthetic Surg. J. 2018, 39, 848–859. [Google Scholar] [CrossRef]
- Rezende-Pereira, G.; Albuquerque, J.P.; Souza, M.C.; A Nogueira, B.; Silva, M.G.; Hirata, R.; Mattos-Guaraldi, A.L.; Duarte, R.S.; Neves, F.P.G. Biofilm Formation on Breast Implant Surfaces by Major Gram-Positive Bacterial Pathogens. Aesthetic Surg. J. 2020, 41, 1144–1151. [Google Scholar] [CrossRef]
- Mu, Minchen, Shuhao Liu, William DeFlorio, Li Hao, Xunhao Wang, Karla Solis Salazar, Matthew Taylor, et al. 2023. Influence of Surface Roughness, Nanostructure, and Wetting on Bacterial Adhesion. Langmuir 39. American Chemical Society: 5426–5439. [CrossRef]
- James, G.A.; Boegli, L.; Hancock, J.; Bowersock, L.; Parker, A.; Kinney, B.M. Bacterial Adhesion and Biofilm Formation on Textured Breast Implant Shell Materials. Aesthetic Plast. Surg. 2018, 43, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Josse, J.; Laurent, F.; Diot, A. Staphylococcal Adhesion and Host Cell Invasion: Fibronectin-Binding and Other Mechanisms. Front. Microbiol. 2017, 8, 2433. [Google Scholar] [CrossRef]
- Yoda, I.; Koseki, H.; Tomita, M.; Shida, T.; Horiuchi, H.; Sakoda, H.; Osaki, M. Effect of surface roughness of biomaterials on Staphylococcus epidermidis adhesion. BMC Microbiol. 2014, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Baldassarri, L.; Montanaro, L. In catheter infections by Staphylococcus epidermidis the intercellular adhesion (ica) locus is a molecular marker of the virulent slime-producing strains. J. Biomed. Mater. Res. 2001, 59, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Berne, C.; Ellison, C.K.; Ducret, A.; Brun, Y.V. Bacterial adhesion at the single-cell level. Nat. Rev. Microbiol. 2018, 16, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Boks, N.P.; Norde, W.; van der Mei, H.C.; Busscher, H.J. Forces involved in bacterial adhesion to hydrophilic and hydrophobic surfaces. Microbiology 2008, 154, 3122–3133. [Google Scholar] [CrossRef]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.-J.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Ravaioli, S.; Montanaro, L. Polysaccharide intercellular adhesin in biofilm: structural and regulatory aspects. Front. Cell. Infect. Microbiol. 2015, 5, 7–7. [Google Scholar] [CrossRef]
- Rumbaugh, K.P. , and K. Sauer. 2020. Biofilm dispersion. Nat Rev Microbiol 18: 571–586.
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Fey, P.D.; Olson, M.E. Current concepts in biofilm formation of Staphylococcus epidermidis. Futur. Microbiol. 2010, 5, 917–933. [Google Scholar] [CrossRef] [PubMed]
- Pajkos, A.; Deva, A.K.; Vickery, K.; Cope, C.; Chang, L.; Cossart, Y.E. Detection of Subclinical Infection in Significant Breast Implant Capsules. Plast. Reconstr. Surg. 2003, 111, 1605–1611. [Google Scholar] [CrossRef]
- Sopwith, W.; Hart, T.; Garner, P. Preventing infection from reusable medical equipment: a systematic review. BMC Infect. Dis. 2002, 2, 4–4. [Google Scholar] [CrossRef] [PubMed]
- Biron, F.; Verrier, B.; Peyramond, D. Transmission of the Human Immunodeficiency Virus and the Hepatitis C Virus. New Engl. J. Med. 1997, 337, 348–349. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, T.; Hogg, L.; Budge, E.; Duncan, A.; Coia, J. Mycobacterium chelonae isolated from rinse water within an endoscope washer–disinfector. J. Hosp. Infect. 2000, 45, 332–334. [Google Scholar] [CrossRef]
- Schelenz, S.; French, G. An outbreak of multidrug-resistant Pseudomonas aeruginosa infection associated with contamination of bronchoscopes and an endoscope washer-disinfector. J. Hosp. Infect. 2000, 46, 23–30. [Google Scholar] [CrossRef]
- Srinivasan, A.; Wolfenden, L.L.; Song, X.; Mackie, K.; Hartsell, T.L.; Jones, H.D.; Diette, G.B.; Orens, J.B.; Yung, R.C.; Ross, T.L.; et al. An Outbreak ofPseudomonas aeruginosaInfections Associated with Flexible Bronchoscopes. New Engl. J. Med. 2003, 348, 221–227. [Google Scholar] [CrossRef]
- Cohen, J.B.; Carroll, C.; Tenenbaum, M.M.; Myckatyn, T.M. Breast Implant–Associated Infections. Plast. Reconstr. Surg. 2015, 136, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Palubicka, A.; Jaworski, R.; Wekwejt, M.; Swieczko-Zurek, B.; Pikula, M.; Jaskiewicz, J.; Zielinski, J. Surgical Site Infection after Breast Surgery: A Retrospective Analysis of 5-Year Postoperative Data from a Single Center in Poland. Medicina 2019, 55, 512. [Google Scholar] [CrossRef]
- Prantl, L.; Momeni, A.; Brebant, V.; Kuehlmann, B.; Heine, N.; Biermann, N.; Brix, E. Recommendations for the Use of Antibiotics in Primary and Secondary Esthetic Breast Surgery. Plast. Reconstr. Surg. - Glob. Open 2020, 8, e2590. [Google Scholar] [CrossRef]
- Yalanis, G.C.; Liu, E.-W.; Cheng, H.-T. Efficacy and Safety of Povidone-Iodine Irrigation in Reducing the Risk of Capsular Contracture in Aesthetic Breast Augmentation. Plast. Reconstr. Surg. 2015, 136, 687–698. [Google Scholar] [CrossRef]
- Burkhardt, B.R.; Dempsey, P.D.; Schnur, P.L.; Tofield, J.J. Capsular Contracture. Plast. Reconstr. Surg. 1986, 77, 919–930. [Google Scholar] [CrossRef]
- Brandon, H.; Brandon, D.H.J.; Young, V.L.; Jerina, D.K.L.; Wolf, C.J.; Adams, J.W.P.; Watson, M.E. Mechanical analysis of explanted saline-filled breast implants exposed to betadine pocket irrigation. Aesthetic Surg. J. 2002, 22, 438–445. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (2017). 2023. Premarket Approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?ID=402786. Accessed October 30.
- Adams, W.P.; Conner, W.C.H.; Barton, F.E.; Rohrich, R.J. Optimizing Breast-Pocket Irrigation: The Post-Betadine Era. Plast. Reconstr. Surg. 2001, 107, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Jewell, M.L.; Adams, W.P. Betadine and Breast Implants. Aesthetic Surg. J. 2018, 38, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.P. Commentary on: Surgical Site Irrigation in Plastic Surgery: What is Essential? Aesthetic Surg. J. 2017, 38, 276–278. [Google Scholar] [CrossRef]
- Viola, G.M.; Rolston, K.V.; Butler, C.; Selber, J.; Reece, G.; Clemens, M.; Villa, M.; Raad, I.I.; Baumann, D. Evaluation of Current Perioperative Antimicrobial Regimens for the Prevention of Surgical Site Infections in Breast Implant-based Reconstructive Surgeries. Plast. Reconstr. Surg. - Glob. Open 2019, 7, e2342. [Google Scholar] [CrossRef]
- Zhadan, O.; Becker, H. Surgical Site Irrigation in Plastic Surgery. Aesthetic Surg. J. 2017, 38, 265–273. [Google Scholar] [CrossRef]
- McKinnon, P.S.; Davis, S.L. Pharmacokinetic and Pharmacodynamic Issues in the Treatment of Bacterial Infectious Diseases. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, N.; Mazzocchi, M.; Fioramonti, P.; Bistoni, G. The Effects of Zafirlukast on Capsular Contracture: Preliminary Report. Aesthetic Plast. Surg. 2006, 30, 513–520. [Google Scholar] [CrossRef]
- Rubino, C.; Brongo, S.; Pagliara, D.; Cuomo, R.; Abbinante, G.; Campitiello, N.; Santanelli, F.; Chessa, D. Infections in breast implants: a review with a focus on developing countries. J. Infect. Dev. Ctries. 2014, 8, 1089–1095. [Google Scholar] [CrossRef]
- Jianu, D.M.; Săndulescu, O.; Berciu, I.; Blidaru, A.; Filipescu, M.; Vartic, M.; Cobani, O.; Jianu. A.; Tălăpan, D.; Dorobăț, O.; et al. Microbiologic Safety of the Transareolar Approach in Breast Augmentation. Aesthetic Surg. J. 2015, 36, 51–57. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Yang, S.; Zhang, Y. Peri-Operative Antibiotic Prophylaxis Does Not Reduce Surgical Site Infection in Breast Cancer. Surg. Infect. 2020, 21, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Monroig, K.B.; Ghosh, K.B.; Marquez, J.E.M.; Medrano, C.B.; Marmor, W.A.B.; McAuliffe, P.B.; Ferrier, A.B.; Kapadia, K.; Rogoff, H.B.; Huston, T.M.; et al. Do Postoperative Prophylactic Antibiotics Reduce Highly Virulent Infections? Ann. Plast. Surg. 2020, 85, S50–S53. [Google Scholar] [CrossRef] [PubMed]
- Gil Conesa, M.; Martínez, N.C.; Luque, J.D.M.; Poveda, M.D.; Villar, D.R.; Caravaca, G.R. Evaluation of compliance with the antibiotic prophylaxis protocol in breast surgery and its effect on the incidence of surgical infection. An. del Sist. Sanit. de Navar. 2019, 42, 139–146. [Google Scholar] [CrossRef]
- Doherty, C.; McClure, J.A.; Baxter, N.N.; Brackstone, M. Complications From Postmastectomy Radiation Therapy in Patients Undergoing Immediate Breast Reconstruction: A Population-Based Study. Adv. Radiat. Oncol. 2022, 8, 101104. [Google Scholar] [CrossRef]
- Adams, W.P.; Culbertson, E.J.; Deva, A.K.; Magnusson, M.R.; Layt, C.; Jewell, M.L.; Mallucci, P.; Hedén, P. Macrotextured Breast Implants with Defined Steps to Minimize Bacterial Contamination around the Device: Experience in 42,000 Implants. Plast. Reconstr. Surg. 2017, 140, 427–431. [Google Scholar] [CrossRef]
- Hu, H.; Sleiman, J.; Johani, K.; Vickery, K. Hypochlorous Acid Versus Povidone-Iodine Containing Irrigants: Which Antiseptic is More Effective for Breast Implant Pocket Irrigation? Aesthetic Surg. J. 2018, 38, 723–727. [Google Scholar] [CrossRef]
- Brody, G.S. On the Safety of Breast Implants. Plast. Reconstr. Surg. 1997, 100, 1314–1321. [Google Scholar] [CrossRef]
- Steiert, A.; Sorg, H.; Boyce, M. Capsular contracture by silicone breast implants: possible causes, biocompatibility, and prophylactic strategies. Med Devices: Évid. Res. 2013, 6, 211–8. [Google Scholar] [CrossRef]
- Risks and Complications of Breast Implants | FDA. 2022. https://www.fda.gov/medical-devices/breast-implants/risks-and-complications-breast-implants. Accessed November 21.
- Jewell, M.L.; Bengtson, B.P.; Smither, K.; Nuti, G.; Perry, T. Physical Properties of Silicone Gel Breast Implants. Aesthetic Surg. J. 2018, 39, 264–275. [Google Scholar] [CrossRef]
- Henriksen, T.F.; Fryzek, J.P.; Hölmich, L.R.; McLaughlin, J.K.; Kjøller, K.; Høyer, A.P.; Olsen, J.H.; Friis, S. Surgical Intervention and Capsular Contracture After Breast Augmentation. Ann. Plast. Surg. 2005, 54, 343–351. [Google Scholar] [CrossRef]
- Namnoum, J.D.; Largent, J.; Kaplan, H.M.; Oefelein, M.G.; Brown, M.H. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Moyer, HR, B Ghazi, A Losken - Plastic and Reconstructive Surgery, and undefined 2011. 2024. Sterility in breast implant placement: the Keller funnel and the “no touch” technique. journals.lww.comHR Moyer, B Ghazi, A LoskenPlastic and Reconstructive Surgery, 2011•journals.lww.com. https://journals.lww.com/plasreconsurg/fulltext/2011/10001/Capsular_Contracture_Rate_in_Low_Risk_Population.14.aspx. Accessed August 27.
- Vazquez, B.; Given, K.S.; Houston, G.C. Breast augmentation: A review of subglandular and submuscular implantation. Aesthetic Plast. Surg. 1987, 11, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-H.; Samuel, M.; Tan, B.-K.; Song, C. Capsular Contracture in Subglandular Breast Augmentation with Textured versus Smooth Breast Implants: A Systematic Review. Plast. Reconstr. Surg. 2006, 118, 1224–1236. [Google Scholar] [CrossRef]
- Khandwekar, A.; Rho, C.K. Modulation of cellular responses on engineered polyurethane implants. J. Biomed. Mater. Res. Part A 2012, 100A, 2211–2222. [Google Scholar] [CrossRef]
- Manav, S.; Ayhan, M.S.; Deniz, E.; Özkoçer, E.; Elmas. ; Yalinay, M.; Şahin, E. Capsular contracture around silicone miniimplants following bacterial contamination: an in vivo comparative experimental study between textured and polyurethane implants. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 1747–1757. [Google Scholar] [CrossRef]
- Duxbury, P.J.; Harvey, J.R. Systematic review of the effectiveness of polyurethane-coated compared with textured silicone implants in breast surgery. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 452–460. [Google Scholar] [CrossRef]
- Daka, J.N.; Chawl, A.S. Release of Chemicals from Polyurethane Foam in the Meme Breast Implant. Biomater. Artif. Cells Immobil. Biotechnol. 1993, 21, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Harmeling, J.X.; Cinca, K.P.; Andrinopoulou, E.-R.; Corten, E.M.L.; A Mureau, M. Long-term outcomes of two-stage, immediate and delayed breast reconstruction with polyurethane-covered versus textured implants: protocol of a prospective, multicentre randomised controlled trial (TIPI trial). BMJ Open 2021, 11, e044219. [Google Scholar] [CrossRef]
- Encinas, N.; Yang, C.-Y.; Geyer, F.; Kaltbeitzel, A.; Baumli, P.; Reinholz, J.; Mailänder, V.; Butt, H.-J.; Vollmer, D. Submicrometer-Sized Roughness Suppresses Bacteria Adhesion. ACS Appl. Mater. Interfaces 2020, 12, 21192–21200. [Google Scholar] [CrossRef]
- Alessandri-Bonetti, M.; Jeong, T.; Vaienti, L.; De La Cruz, C.; Gimbel, M.L.; Nguyen, V.T.; Egro, F.M. The Role of Microorganisms in the Development of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Pathogens 2023, 12, 313. [Google Scholar] [CrossRef]
- Rolph, R.; Farhadi, J. The use of meshes and matrices in breast reconstruction. Br. J. Hosp. Med. 2018, 79, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Jacombs, A.; Allan, J.; Hu, H.; Valente, P.M.; Wessels, W.L.F.; Deva, A.K.; Vickery, K. Prevention of Biofilm-Induced Capsular Contracture With Antibiotic-Impregnated Mesh in a Porcine Model. Aesthetic Surg. J. 2012, 32, 886–891. [Google Scholar] [CrossRef]
- Ruff, E.S.; Hirase, T.; Rude, M.J. Evaluation of Antibiotic-Impregnated Mesh in Preventing the Recurrence of Capsular Contracture. Aesthetic Surg. J. 2018, 39, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Borkner, C.B.; Wohlrab, S.; Möller, E.; Lang, G.; Scheibel, T. Surface Modification of Polymeric Biomaterials Using Recombinant Spider Silk Proteins. ACS Biomater. Sci. Eng. 2016, 3, 767–775. [Google Scholar] [CrossRef]
- Weiss, A.C.G.; Herold, H.M.; Lentz, S.; Faria, M.; Besford, Q.A.; Ang, C.-S.; Caruso, F.; Scheibel, T. Surface Modification of Spider Silk Particles to Direct Biomolecular Corona Formation. ACS Appl. Mater. Interfaces 2020, 12, 24635–24643. [Google Scholar] [CrossRef]
- Salehi, S.; Koeck, K.; Scheibel, T. Spider Silk for Tissue Engineering Applications. Molecules 2020, 25, 737. [Google Scholar] [CrossRef] [PubMed]
- Scheibel, T. Spider silks: recombinant synthesis, assembly, spinning, and engineering of synthetic proteins. Microb. Cell Factories 2004, 3, 14–14. [Google Scholar] [CrossRef]
- Kumari, S.; Lang, G.; DeSimone, E.; Spengler, C.; Trossmann, V.T.; Lücker, S.; Hudel, M.; Jacobs, K.; Krämer, N.; Scheibel, T. Engineered spider silk-based 2D and 3D materials prevent microbial infestation. 2020, 41, 21–33. [CrossRef]
- Kumari, S.; Bargel, H.; Scheibel, T. Recombinant Spider Silk–Silica Hybrid Scaffolds with Drug-Releasing Properties for Tissue Engineering Applications. Macromol. Rapid Commun. 2019, 41, e1900426. [Google Scholar] [CrossRef]
- Zeplin, P.H.; Maksimovikj, N.C.; Jordan, M.C.; Nickel, J.; Lang, G.; Leimer, A.H.; Römer, L.; Scheibel, T. Spider Silk Coatings as a Bioshield to Reduce Periprosthetic Fibrous Capsule Formation. Adv. Funct. Mater. 2014, 24, 2658–2666. [Google Scholar] [CrossRef]
- Erathodiyil, N.; Chan, H.-M.; Wu, H.; Ying, J.Y. Zwitterionic polymers and hydrogels for antibiofouling applications in implantable devices. Mater. Today 2020, 38, 84–98. [Google Scholar] [CrossRef]
- Mi, L.; Jiang, S. Integrated Antimicrobial and Nonfouling Zwitterionic Polymers. Angew. Chem. Int. Ed. 2014, 53, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yuan, Z.; Jain, P.; Hung, H.-C.; He, Y.; Lin, X.; McMullen, P.; Jiang, S. De novo design of functional zwitterionic biomimetic material for immunomodulation. Sci. Adv. 2020, 6, eaba0754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Z.; Bai, T.; Carr, L.; Ella-Menye, J.-R.; Irvin, C.; Ratner, B.D.; Jiang, S. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 2013, 31, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jain, P.; Ma, J.; Smith, J.K.; Yuan, Z.; Hung, H.-C.; He, Y.; Lin, X.; Wu, K.; Pfaendtner, J.; et al. Trimethylamine N -oxide–derived zwitterionic polymers: A new class of ultralow fouling bioinspired materials. Sci. Adv. 2019, 5, eaaw9562. [Google Scholar] [CrossRef] [PubMed]
- Jansen, L.E.; Amer, L.D.; Chen, E.Y.-T.; Nguyen, T.V.; Saleh, L.S.; Emrick, T.; Liu, W.F.; Bryant, S.J.; Peyton, S.R. Zwitterionic PEG-PC Hydrogels Modulate the Foreign Body Response in a Modulus-Dependent Manner. Biomacromolecules 2018, 19, 2880–2888. [Google Scholar] [CrossRef]
- Adams, W.P., Jr.; Rios, J.L.; Smith, S.J. Enhancing Patient Outcomes in Aesthetic and Reconstructive Breast Surgery Using Triple Antibiotic Breast Irrigation: Six-Year Prospective Clinical Study. Plast. Reconstr. Surg. 2006, 118, 46S–52S. [Google Scholar] [CrossRef]
- Giordano, S.; Peltoniemi, H.; Lilius, P.; Salmi, A. Povidone-Iodine Combined With Antibiotic Topical Irrigation to Reduce Capsular Contracture in Cosmetic Breast Augmentation. Aesthetic Surg. J. 2013, 33, 675–680. [Google Scholar] [CrossRef]
- Drinane, J.J.; Bergman, R.S.; Folkers, B.L.; Kortes, M.J. Revisiting Triple Antibiotic Irrigation of Breast Implant Pockets. Plast. Reconstr. Surg. - Glob. Open 2013, 1, e55–e55. [Google Scholar] [CrossRef]
- Jewell, M.L.; Hariri, S.; E Lantz, E.; Jewell, H.L.; Strickland, A.D.; Leung, B.K. In Vitro Evaluation of Common Antimicrobial Solutions Used for Breast Implant Soaking and Breast Pocket Irrigation—Part 1: Efficacy Against Planktonic Bacteria. Aesthetic Surg. J. 2020, 41, 1242–1251. [Google Scholar] [CrossRef]
- Baker, J.E.; Seitz, A.P.; Boudreau, R.M.; Skinner, M.J.B.; Beydoun, A.B.; Kaval, N.; Caldwell, C.C.; Gulbins, E.M.; Edwards, M.J.; Gobble, R.M. Doxycycline-Coated Silicone Breast Implants Reduce Acute Surgical-Site Infection and Inflammation. Plast. Reconstr. Surg. 2020, 146, 1029–1041. [Google Scholar] [CrossRef]
- Nguyen, L.; Afshari, A.; Green, J.; Joseph, J.; Yao, J.; Perdikis, G.; Higdon, K.K. Post-Mastectomy Surgical Pocket Irrigation With Triple Antibiotic Solution vs Chlorhexidine Gluconate: A Randomized Controlled Trial Assessing Surgical Site Infections in Immediate Tissue Expander Breast Reconstruction. Aesthetic Surg. J. 2021, 41, NP1521–NP1528. [Google Scholar] [CrossRef] [PubMed]
- Unlu, R.E.; Yilmaz, A.D.; Orbay, H.; Can, B.; Tekdemir, I.; Sensoz, O. Influence of Rifampin on Capsule Formation Around Silicone Implants in a Rat Model. Aesthetic Plast. Surg. 2007, 31, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Gancedo, M.; Ruiz-Corro, L.; Salazar-Montes, A.; Rincón, A.R.; Armendáriz-Borunda, J. Pirfenidone Prevents Capsular Contracture After Mammary Implantation. Aesthetic Plast. Surg. 2007, 32, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, C.J.; Ruhrmund, D.W.; Pan, L.; Seiwert, S.D.; Kossen, K. Antifibrotic activities of pirfenidone in animal models. Eur. Respir. Rev. 2011, 20, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Fayzullin, A.; Churbanov, S.; Ignatieva, N.; Zakharkina, O.; Tokarev, M.; Mudryak, D.; Khristidis, Y.; Balyasin, M.; Kurkov, A.; Golubeva, E.N.; et al. Local Delivery of Pirfenidone by PLA Implants Modifies Foreign Body Reaction and Prevents Fibrosis. Biomedicines 2021, 9, 853. [Google Scholar] [CrossRef] [PubMed]
- Grattendick, K.; Nakashima, J.; Feng, L.; Giri, S.; Margolin, S. Effects of three anti-TNF-α drugs: Etanercept, infliximab and pirfenidone on release of TNF-α in medium and TNF-α associated with the cell in vitro. Int. Immunopharmacol. 2008, 8, 679–687. [Google Scholar] [CrossRef]
- Zeplin, P.H.; Larena-Avellaneda, A.; Schmidt, K. Surface Modification of Silicone Breast Implants by Binding the Antifibrotic Drug Halofuginone Reduces Capsular Fibrosis. Plast. Reconstr. Surg. 2010, 126, 266–274. [Google Scholar] [CrossRef]
- Pines, M.; Nagler, A. Halofuginone: A Novel Antifibrotic Therapy. Gen. Pharmacol. Vasc. Syst. 1998, 30, 445–450. [Google Scholar] [CrossRef]
- Granot, I.; Halevy, O.; Hurwitz, S.; Pines, M. Halofuginone: An inhibitor of collagen type I synthesis. Biochim. et Biophys. Acta (BBA) - Gen. Subj. 1993, 1156, 107–112. [Google Scholar] [CrossRef]
- McGaha, T.L.; Bona, C.; Phelps, R.G.; Spiera, H. Halofuginone, an Inhibitor of Type-I Collagen Synthesis and Skin Sclerosis, Blocks Transforming-Growth-Factor-β-Mediated Smad3 Activation in Fibroblasts. J. Investig. Dermatol. 2002, 118, 461–470. [Google Scholar] [CrossRef]
- Guimier, E.; Carson, L.; David, B.; Lambert, J.M.; Heery, E.; Malcolm, R.K. Pharmacological Approaches for the Prevention of Breast Implant Capsular Contracture. J. Surg. Res. 2022, 280, 129–150. [Google Scholar] [CrossRef] [PubMed]
- Colak, O.; Ozer, K.; Dikmen, A.; Ozakinci, H.; Ozkaya, O. Evaluation of Safe Systemic Immunosuppression Created with Dexamethasone in Prevention of Capsular Contracture: A Glance to Distinct Perspectives with Toll-Like Receptors. Aesthetic Plast. Surg. 2018, 42, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Wilk, M.; Hessler, R.; Mugridge, K.; Jolly, C.; Fehr, M.; Lenarz, T.; Scheper, V. Impedance Changes and Fibrous Tissue Growth after Cochlear Implantation Are Correlated and Can Be Reduced Using a Dexamethasone Eluting Electrode. PLOS ONE 2016, 11, e0147552. [Google Scholar] [CrossRef] [PubMed]
| Biological Mechanisms | Wound Healing Dynamics | Fibrotic Responses in SMI |
| Pathophysiological Process | Restoration of tissue integrity and function through hemostasis, inflammation, proliferation, and remodeling. | Formation of a fibrous capsule around implants due to inflammatory response and biofilm formation. |
| Initial Response | Hemostasis: Constriction of blood vessels, platelet activation, clot formation to prevent blood loss and provide a scaffold for healing. | Immediate Immune Response: Activation of innate immune system, recruitment of immune cells (neutrophils, macrophages) due to microbial contamination and protein adsorption. |
| Molecular Components | Platelets, Fibrinogen, Fibrin: Form a clot that acts as a barrier and temporary scaffold. | Microorganisms, Proteins: Microbial biofilm formation on the implant surface, leading to persistent immune stimulation. |
| Inflammatory Phase | Neutrophils and Macrophages: Clear debris, combat infection, release cytokines and MMPs to modulate the inflammatory response. | Chronic Inflammation: Persistent inflammation due to biofilm, leading to prolonged immune response and ongoing ECM remodeling. |
| Proliferation Phase | Fibroblast Proliferation, ECM Deposition, Angiogenesis, Epithelialization: Formation of new tissue, collagen production, and blood vessel formation. | Fibrous Capsule Formation: Collagen and ECM deposition around the implant, leading to capsule formation. |
| Remodeling Phase | Collagen Realignment, Cross-Linking, Continual ECM Remodeling: Strengthening of new tissue and refinement of the wound architecture. | Capsular Contracture: Excessive collagen deposition and ECM remodeling leading to a contracted and thickened fibrous capsule. |
| Impact on Healing | Prevention of Infection, Scar Formation: Effective healing reduces infection risk, minimizes scar formation, and restores tissue function. | Infection Risk: Biofilms create a persistent infection risk that complicates healing and exacerbates fibrosis. |
| Category | Strategy | Mechanism | Key Points |
|
Physical Modifications with Antimicrobial Properties |
Polyurethane Foam Coatings | Surface modification to disrupt fibrotic tissue formation | Initial reduction in fibrotic capsule formation, concerns over toxicity from degradation products [169,170,171,172]. |
|
Surface Topography |
Rough surfaces increase biofilm formation | Rougher surfaces (Ra 60 µm) enhance bacterial adhesion and biofilm maturity, leading to infections and capsular contracture [45,47,105,175]. | |
|
Biological Matrices with Antimicrobial Properties |
Antibiotic- Impregnated Meshes |
Localized antibiotic delivery to reduce biofilm formation | Effective in reducing bacterial colonization and biofilm formation with sustained antimicrobial activity [176,177,178,179]. |
|
Spider Silk- Based Meshes |
Inhibits bacterial adhesion and fibrotic tissue formation | Biocompatible, reduces fibroblast proliferation, and collagen deposition [180,181,182,183,184,185,186]. | |
|
Zwitterionic Polymers |
Superior hydrophilicity, preventing protein adsorption and microbial adhesion | Resistant to microbial colonization, preventing foreign body response and fibrotic capsules [187,188,189,190,191]. | |
|
Pharmacological Strategies |
Systemic Antibacterials (Cefazolin, Gentamicin) |
Prophylactic antibiotics to prevent infections before and during surgery | Effective against gram-positive and gram-negative bacteria in breast implant surgeries [192,193]. |
|
Topical Antibacterials (Bacitracin, Chlorhexidine) |
Direct application to reduce bacterial load and biofilm formation | Applied during surgery to prevent contamination and infection [192,194,195,196]. | |
|
Drug Incorporation into Implants (Rifampin) |
Localized, sustained antimicrobial effect from drug-coated implant surfaces | Reduces bacterial colonization and biofilm formation directly at the implant site [197]. | |
| Antifibrotic and Anti-inflammatory Drugs | Pirfenidone | Reduces inflammation and fibroblast activity | Shown to reduce capsule thickness in preclinical models, potential use in biofilm-associated fibrosis [198,199,200,201]. |
| Halofuginone | Inhibits collagen synthesis and T helper 17 cell differentiation | Reduces fibrosis and capsule formation around implants, promising antifibrotic properties [202,203,204,205]. | |
| Dexamethasone | Reduces inflammation and collagen production by modulating cytokine activity | Decreases fibrous tissue formation and inflammation, improving implant surgery outcomes [194,207,208]. | |
|
Integration of Antimicrobial and Antifibrotic Strategies |
Combined Approaches |
Use of both antimicrobial and antifibrotic strategies to prevent biofilm formation and fibrosis | Combining antibiotics with local antiseptic irrigation and antifibrotic agents enhances efficacy in preventing biofilm-associated fibrosis. |
| Clinical Aspect | Challenges | Current Strategies | Future Directions |
|
Biofilm Formation on SMIs |
Persistent biofilms protect bacteria from immune responses and antimicrobials, leading to chronic inflammation and fibrosis. | Antimicrobial prophylaxis, antimicrobial coatings, and advanced material surfaces designed to reduce biofilm formation. | Develop targeted antimicrobial strategies that penetrate biofilms. Biomimetic materials that release antimicrobials in response to biofilm formation. |
|
Chronic Inflammation and Fibrosis |
Biofilm matrix limits immune cell infiltration and antimicrobial effectiveness, leading to thick fibrous capsules. | Anti-inflammatory and antifibrotic agents (pirfenidone, halofuginone, dexamethasone) to reduce fibrosis. | Incorporate anti-inflammatory and antifibrotic agents into implant materials. Personalized treatment approaches based on patient immune responses. |
| Antimicrobial Resistance | Resistance to traditional antimicrobial treatments is rising, making it difficult to manage biofilm infections. | Preoperative prophylaxis with systemic antibiotics; antimicrobial materials such as antibiotic-impregnated meshes. | Novel drug delivery systems (e.g., nanoparticles or localized reservoirs) that enhance antimicrobial efficacy and overcome resistance. |
|
Advanced Materials |
Existing materials may not fully prevent microbial adhesion or fibrosis. | Antibiotic-impregnated and spider silk-based meshes, zwitterionic polymers. | Development of smart materials that dynamically respond to microbial threats, change surface properties, or release antimicrobials. |
|
Clinical Guidelines and Practice |
Variability in practices and lack of standardized guidelines lead to inconsistent management of biofilm complications. | General antimicrobial prophylaxis and surface modifications for reducing biofilm risk. | Standardized clinical guidelines for antimicrobial prophylaxis, coatings, and antifibrotic treatments across clinical settings. |
| Personalized Medicine | Generalized treatments may not consider individual patient factors, leading to suboptimal outcomes. | Uniform antibiotic and antifibrotic regimens based on general risk profiles. | Personalized treatments tailored to patient-specific factors (microbial flora, immune responses, genetic predisposition). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





