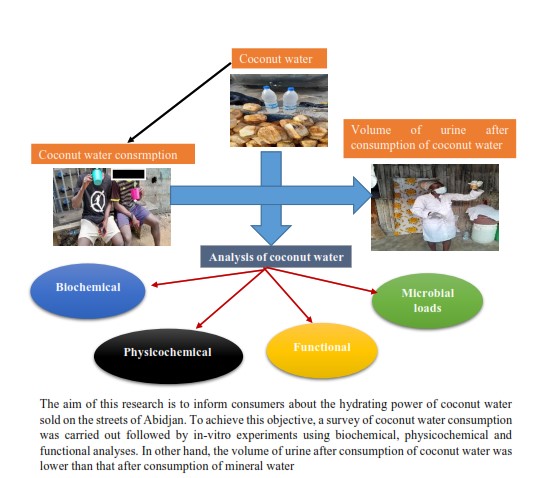
1. Introduction
Coconuts are widely grown in the tropics. The coconut palm is a tropical plant native to Southeast Asia. Over 80% of the area planted to coconut is located in this part of the world [
1]. The remainder is divided between Africa, Latin America, Oceania and the Caribbean. According to the FAO database consulted in 2010, Asia remains the main producer of coconuts, with 83.5% of the global share. Coconuts have spread throughout the intertropical zone by floating on ocean currents, during migrations and human journeys [
1]. In Africa, the coconut palm was introduced by the Portuguese in the 16th century [
2] and is home to 664,000 hectares of coconut groves, including 50,000 hectares in Côte d’Ivoire. With 80% located on the coast, it represents the main cash crop for local populations [
3]. Côte d’Ivoire is the world’s eleventh largest exporter of coconut oil [
4]. In other hand, it is the leading African exporter of coconut products. In terms of yield per hectare, Côte d’Ivoire is among the top performers. All these achievements were made possible by the creation of a research station in 1949 to support the development of the coconut industry [
4].
Fruit species such as coconuts play a significant role in food security and in improving living conditions for the population. On the one hand, they help to improve nutritional balance and health, and on the other, they are a source of income, especially in rural areas. When ripe, the nut contains a few deciliters of liquid, depending on its size, and a thin layer of white gelatinous pulp. Later, the liquid gradually gives way to a larger quantity of firm, fatty pulp, which is exploited under the name of Coprah [
5,
6]. Coconut water, the transparent liquid from coconuts, is a refreshing tropical beverage with a growing international market. This particular fruit juice has original properties due to its mineral salt composition, low soluble sugar content and aroma [
7]. As a result of growing consumer demand, the product, previously consumed locally straight from the fruit, had to be extracted, processed and stabilized to reach international markets. Coca Cola and PepsiCo have recently invested in industrial coconut water production units in Brazil. Nestlé offers a health product based on coconut water in its Nesfluid range [
7].
However, today’s industrial coconut water production units face a number of technological hurdles. Variability in raw material composition can be high, often requiring costly formulation or process adjustments. The current stabilization process destroys most of the flavors and can lead to the appearance of unwanted tastes. However, the hydrating and rehydrating power of coconut water remains a controversial subject. According to [
8], coconut water does not improve hydration as much as mineral water; on the contrary, coconut water could be used as an aid to oral rehydration. The aim of this research is to provide consumers with scientific data on the hydrating power of coconut water sold on the street.
2. Materials and Methods
The material used consisted mainly of fresh coconut water (Coco Nucifera L) (Figure 1). A survey sheet was drawn up to collect information on people’s knowledge and consumption of coconut water.
2.1. Surveys
The surveys took place in the city of Abidjan, more specifically in the communes of Adjamé, Yopougon and Abobo. These three (3) communes were chosen because of their social differences and high population density. The exclusion criteria applied were: children under 10 years of age and people with a medical diagnosis of mental disorder, which would make it difficult to understand the questions that make up the instruments used for data collection. The sampling method adopted for this work is random sampling. The survey was carried out from March 1 to March 31, 2023. The questionnaire was submitted to 385 people. In order to make the questionnaire easier to understand for the respondents and to facilitate their answers, a person with a primary, secondary or higher level of education and who spoke the same ethnic group as the respondents was asked to act as a focal point and interpreter. The questions were double-choice and multiple-choice, with the possibility of 2 or more answers.
2.2. Study Population and Data Collection
For this study, 385 people were interviewed, 147 in the commune of Abobo, 100 in Yopougon and 139 in Adjamé. Population size was determined using formula for an exhaustive independent sample.
N: sample size ; e’‘: margin of error ; t’‘: margin coefficient ; p’‘: population of the study area.
The sample for each study area was obtained using the probabilistic method proportional to the population size in each locality, based on data from the general population census of Côte d’Ivoire.
2.3. Sampling Plan
Sampling took place in Abidjan (Côte d’Ivoire) from March 1 to 15, 2023. The sample was collected in the commune of Adjamé, then transported to the University Laboratory Nangui Abrogoua for microbiological and biochemical analyses. The choice of this site was justified by its availability.
2.4. Collection of Urine from Subjects
In this part of the study, 15 healthy, physically active subjects (7 women and 8 men) were selected for urine collection. The subjects were informed of the purpose of the study and the experimental procedure before signing a consent form. Their mean age, body weight, height and body mass index were determined. Participants were also asked to refrain from strenuous exercise and to refrain from consuming alcoholic beverages in the 24 hours preceding all trials.
2.5. Urine Sampling Procedure
This part of the study was carried out over two (2) days, firstly urine sampling after consumption of mineral water and then urine sampling after ingestion of coconut water. Work began at 8 a.m. and ended at 12 p.m. Participants fasted throughout the experiment. After 20 minutes prior to the volunteers’ arrival, they were invited to consume 500 mL of still mineral water. Their body mass was determined during this study. Participants were then asked to urinate after four (4) hours of the experiment. The same procedure was used for coconut water. Each quantity of urine collected during the study was measured using a graduated plastic jar. The volume of each urine was determined by weighing its mass using a digital balance.
2.6. Determination of Dry Matter and Ash Content
Dry matter and ash content were determined using the [
9] method. Dry matter is determined after taking a ten-gram (10 g) sample mass and oven-drying it (Memmert) at 105 ± 2°C for 24 hours. As for ash content, five grams (5 g) of sample were taken and placed in a muffle furnace (Nobertherm) at 550°C for 24 hours.
2.7. Main Nutrient Composition of Coconut Water
Determination of fat content was carried out using the liquid-liquid extraction method. Liquid-liquid extraction is a purification method based on the difference in solubility of a solute in two immiscible phases. Extraction involves transferring a product from a solvent from which it is difficult to separate (water) to another solvent from which it can be easily isolated (organic solvent). Three consecutive extractions were carried out. For the first extraction, 20 mL of hexane were added to 20 mL of coconut water, the mixture was then vigorously shaken and left to settle. A volume of 10 mL of hexane was added to 20 mL of the decantate from the first extraction, for the second extraction. The same operation was repeated for the third extraction. After lipid extraction, the hexane was evaporated on a rotary evaporator. The tared extraction flask was dried in an oven at 103°C for 20 min. At the end of this operation, the lipid flask was weighed. The experiment was repeated three (3) times, and the total lipid content of the cocoa mucilage juice was determined using the following expression:
M0: mass (g) of empty flask. ; Me: mass (g) of sample. ; M1: mass (g) of the whole (flask + lipids) after incineration.
As regards, the determination of total protein was carried out using the [
9] Kjeldahl method. To carry out mineralization, one gram (1 g) of each sample (me) was added to (20 mL) concentrated sulfuric acid (96%) in the presence of a pinch of mineralization catalyst (selenite). Mineralization was carried out for 2 hours in a Kjeldahl matron at 400°C. The mineralizate was transferred to a 100 mL flask and topped up with distilled water. A 10 mL volume of the mixture was withdrawn and 50 mL of NaOH (40%) added. The mixture was then distilled for 10 minutes, trapping the distillate in a flask containing 20 mL boric acid with mixed indicator (methyl red + bromocresol green). The distillate obtained was titrated with a 0.01 N sulfuric acid solution until it turned from green to pink (V1). A blank test was carried out (V0). The experiment was repeated three (3) times and the percentage of total protein was determined according to the following mathematical relationship:
V0: volume (mL) of sulfuric acid solution poured for blank test. ; V1: volume (mL) of sulfuric acid solution poured for the test (sample). ; N: normality of sulfuric acid solution: 0.01N. ; M0: mass (g) of sample ; 14: atomic mass of nitrogen. ; 6.25: coefficient corresponding to the rate of conversion of nitrogen into protein.
As to, Total carbohydrates (TC) were determined by the calculation method recommended by [
10] and energy value (EV) by [
10]. These methods take into account, on the one hand, moisture, fat, protein, ash and fiber contents and, on the other, energy coefficients relative to the samples.
P: Percentage of crude protein. L: Percentage of total lipids. C: Ash percentage. F: Fiber percentage. G: Percentage of total carbohydrates. H: Moisture content. EV: Energy value in Kcal/ 100 g dry matter (DM).
Nevertheless, mineral determination was carried out according to the method of [
11]. A quantity of a white ash. After cooling in a desiccator, the ash was dissolved in 5 mL chloridric acid (20%) and 1 mL concentrated nitric acid. The mixture was placed in a water bath for one hour, and brought up to the mark with distilled water in a 50 mL flask. The elements in the solution were then determined by Atomic Absorption Spectrophotometer (AAS). To avoid interference from the elements Ca and K, lantane chloride was added (5 mL lantane). Minerals were determined using an air-acetylene flame atomic absorption spectrophotometer (AAS 20 type VARIAN). The wavelengths of the minerals K, Zn, Fe, Mg, Mn, Ca and P are 767.6 nm; 214.6 nm; 249 nm; 286 nm; 280.6 nm; 422.71 nm and 885 nm respectively. The assay is repeated three (3) times for each mineral.
2.8. Microbiological Analysis
Ten (10) mL of each sample is weighed under sterile conditions against the flame of a Bunsen burner, and placed in 90 mL of diluent (buffered peptone water) contained in a 100 mL bottle. This mixture was homogenized and a 10
-1 suspension obtained. One (1) mL of the 10
-1 suspension was then homogenized in 9 mL of diluent in a test tube to obtain the 10
-2 dilution. Using the same technique, subsequent dilutions were made up to dilution 10
-x [
12] (Djéni et al., 2011). Mesophilic aerobic bacteria (MAP) were counted on PCA agar (Plate Count Agar; Oxoïd LTD, Basingstore, Hamsphire, England). Inoculation was carried out by incorporation into the agar mass, and involved introducing 1 mL of decimal dilutions into Petri dishes. Then 12 to 15 mL of the previously prepared medium, supercooled at 45°C, were poured into the Petri dish containing the inoculum. The mixture was homogenized by shaking and then left to cool on the bench at room temperature. The seeded media were then incubated at 30°C for 72 hours. (Norme NF V08-051., 1999). Neutral red crystal violet bile lactose agar (VRBL agar) was used for coliform enumeration. Inoculation was carried out by incorporation into the agar mass. Incubation was for 24 hours at 37°C for total coliforms and 44°C for fecal coliforms (NF ISO 4832 July 1991). Colonies appeared purplish-red, round and 0.5 mm in diameter. RAPID’E.coli agar was used for the detection and enumeration of
Escherichia coli (NF ISO 16140, 2003). Inoculation was carried out by spreading and incubation at 37°C for 24 hours. Presumptive Escherichia coli colonies were purple to pink.
Staphylococcus aureus colonies were plated on Baird Parker agar. Inoculation was by spreading and incubation was at 35°C for 24 hours. Presumptive colonies of Staphylococcus aureus were either shiny black, whole, convex, surrounded by clear zones extending into the opaque medium, or shiny black, whole, convex, with no well-defined clear zone, or dark grey [
13].
2.9. Statistical Analysis
One-factor analysis of variance (ANOVA) followed by Tukey’s test were performed with XLSTAT software (Adinosoft Inc., version 2016) to compare the variables analyzed on coconut juice. Differences are considered significant for values of P < 0.05. Pearson correlation analysis to reveal the nature of the correlation between the parameters studied is performed with XLSTAT Version 2016 software.
3. Results and Discussion
3.1. Virtues of Coconut Water According to Age
Coconut water is a refreshing tropical beverage with a booming local market. Thus, this study focused on coconut water with the aim of enlightening consumers on the hydrating power of coconut water sold on the streets through scientific data. The results of the survey revealed that coconut water is very well known to the people questioned (385), and consumption was linked to the virtues attributed by consumers. These beneficial effects vary according to age. The effect most felt by the 20-30 age group was refreshing, with a headcount of 90. However, the observed Chi-square (χ2obs) of 144.08 is higher than the theoretical Chi-square of 36.41. The virtues of coconut water are positively correlated with the age of the people surveyed (Table 1). The use of coconut water as a refreshing drink was reported by [
8] Douthe (2007). These authors claimed that coconut water could be used as an oral rehydration aid to replace fluid loss.
3.2. Timing and Frequency of Consumption of Coconut Juice According to Age
According to the survey data, the timing of coconut juice consumption according to age (20-30 years) was most often taken at snack time, with a headcount of 83 (Table 2). For the same age group, the frequency of consumption was 168. Statistical analysis using the Chi-square (χ2) test showed that the observed Chi-square (χ2obs) value of 138.41 is higher than the theoretical Chi-square of 28.86. There is therefore a link between the frequency of coconut juice consumption and age (Table 3).
3.3. Characterization of the Physico-Chemical and Biochemical Parameters of Coconut Water
Evaluation of the physico-chemical and biochemical parameters of the coconut water sample revealed that these parameters were nature-dependent. Coconut water is rich in natural sugars and contains many important nutrients and minerals not found in sufficient quantities anywhere else. However, when coconut water is analyzed, nutrients can be found in varying degrees. Apart from total carbohydrate content, coconut water contains small amounts of lipids (0.18±0.0282%) and proteins (0.075±0.0007%). Coconut water is therefore not a significant source of protein and fat. The main component of the coconut water sample was a carbohydrate of 4.5±0.0%, this carbohydrate content could give coconut water the sports drink appearance (Table 4). Several authors recommend coconut water as a sports drink [
14]. Foods high in phosphorus and low in calcium make the body acidic, depleting it of calcium and other minerals and increasing inflammation. High levels of phosphorus (276.1±10.1823 mg) versus calcium (35.05±0.6363 mg) in the diet have been shown to cause the body to draw calcium from its own reserves (the bones) to compensate for the differences (Table 4). Over a long period, this can dramatically affect bones in a negative way [
15]. On the other hand, the low protein and fat content of coconut water as in this study has been reported by [
14,
16,
17,
18] Probably the virtues attributed to coconut water could be due to phenolic compounds and antioxidant activity [
19] (Rahmanian et al.
, 2015).
3.4. Microbiological Analysis of Coconut Water
Microbiological analysis of coconut water samples focused on microbial enumerations. Of all the microbes enumerated, only aerobic mesophilic germs were found with significant loads. None of the coconut water samples in this study met global microbiological criteria or standards. According to [
20], the microbial load in coconut water should be less than 5.10
3 CFU/mL for GAM, less than 10 total coliforms and no faecal coliforms. According to the samples, high loads of GAM (7.10
3 CFU/mL) were found in street coconut water (Table 5). These high microbial loads could be due to contamination caused by poor hygiene in the sales area [
21], as well as frequent unsanitary handling of coconut water and cross-contamination [
22]. Despite its natural character, coconut water presents a potential hazard for consumers due to its high concentration of micro-organisms.
3.5. Evolution of Urine Volume after Ingestion of Coconut Water and Mineral Water
With regard to the quantity of urine after ingestion of mineral water and coconut water, the results showed that throughout the duration of the experiment, urine was more abundant when subjects consumed only mineral water than when they consumed coconut water. The average volume of urine collected after mineral water consumption ranged from 138.5 ml at T1 (1 hour after ingestion) to 66.6 ml at T4 (4 hours after ingestion). The quantity of urine collected after ingestion of coconut water decreased from T1 (41.67 ml) to T3 (26 ml), then increased slightly from T3 (26 ml) to T4 (36.33 ml). (Figure 2.). This may be explained by the high sodium content of coconut water (220.45±0.4949 mg). Indeed, a substance rich in sodium stimulates water retention when it is in excess [
14,
22]. Coconut water simplifies the evacuation of excess water and toxic substances from the body. Several studies and research point out that it may be more beneficial than mineral water for rehydration, especially after vigorous exercise. Coconut water appears to be more hydrating than mineral water.
4. Conclusions
At the end of this study, coconut water was mainly consumed for its fortifying, digestive and refreshing properties. In other hand, coconut water is more hydrating than mineral water. It is therefore advisable to raise public awareness of coconut water as a hydrating drink. Despite its functional properties, coconut water sold on the street has not always been of good microbiological quality. To avoid contamination of coconut water sold on the street, it is advisable to make vendors aware of good hygiene practices.
Author Contributions
This work was carried out in collaboration between all authors. Conceptualization and designing of the research work, F.C and A.S-P.N ; Execution of field/lab experiments and data collection, E.R.K ; K.L.K and Y.M.G.T; Analysis of data and interpretation, W.H.C and S.J.A ; Preparation of manuscript, K.M.J-P.B
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data Availability Statement
The data files associated with this study have been submitted along with this manuscript and are available upon request. Please contact the Corresponding Author with any questions or concerns.
Acknowledgements
The authors would like to thank all those who agreed to take part in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gunn, B.F. , Baudouin, L., Olsen, K.M. Independent Origins of Cultivated Coconut (Cocos nucifera L.) in the Old World Tropics. PLoS ONE 2011, 6, e21143. [Google Scholar] [CrossRef] [PubMed]
- De Nuce de, L. & Wuidart, W.,. Les cocotiers Grands à Port-Bouët (Côte d’Ivoire) 1-Grand Ouest Africain, Grand de Mozambique, Grand de Polynésie, Grand de Malaisie. Oléagineux.1979, 34 (7), 339-349.
- Assa, R. , Konan vemlin, J., Padres, A., Agbo N. and Sie, R. Diagnostic de la cocoteraie paysanne du littoral ivoirien. Science et nature. 2006, 3, 113–120. [Google Scholar]
- Amrizal, I. Coconut Statistical Yearbook. Asian and Pacific Coconut Community (APCC), Jakarta (India). 2003. 233 p.
- TIS. Copra expeller. Transport Information Service from German Marine Insurers, 2013a.
- TIS. Copra extraction meal. Transport Information Service from German Marine Insurers, 2013b.
- Teklehaimanot, Z. Influence of the Origin of Stem Cutting, Season of Collection and Auxin Application on the Vegetative Propagation of African Sandalwood (Osyris lanceolata) in Tanzania. The Southern African Forestry Journal. 2004, 201, 13–24. [Google Scholar] [CrossRef]
- Douthe, M-L.K. Influence de la varieté et du stade de la maturité des noix de coco (cocos nucifera L.) sur le mode de récolte et la qualité de l’eau de coco. Thèse de Master Europeen natura en transformation des produits tropicaux à vocation alimentaire. 2007; 75P.
- AOAC. Official Methods of Analysis, 15th edition. Association of Official, 1990.
- FAO. Directives relatives à la conversion d’unités, de dénominateurs et d’expressions - Version 1.0. 2015, P1-41.
- Kularate, K. and Fretas, C. Epiphytie lichens as biomonitors of airborne heavy metal pollution. Environmental and experimental botany. 2013, 88, 24–32. [Google Scholar] [CrossRef]
- Djéni, N.T. , N’Guessan, K.F., Toka, D.M., Kouame, K.A. and Dje, K.M. Quality of attieke (a fermented cassava product) frorn the three ma.in processing zones in Côte d’Ivoire. Food Control. 2011, 7, 37–41. [Google Scholar]
- Capita, R. , Alonso-Calleja, M.C.B. and Garcia-Fernandez, M.C. Assessment of Baird-Parker agar as screening test for determination of Staphylococcus aureus in poultry meat. Journal Microbiology. 2001, 39, 321–325. [Google Scholar]
- Yong, J.W. , Ge, L., Ng, Y.F., Tan, S.N. The chemical composition and biological properties of coconut (Cocos nucifera L.) water. Molecules. 2009, 14, 5144–5164. [Google Scholar] [CrossRef] [PubMed]
- Appiah, F. , Asibuo, J.Y. and Kumah, P. Physicochemical and functional properties of bean flours of three cowpea (Vigna unguiculata L. Walp) varieties in Ghana. African Journal of Food Science. 2011, 5, 100–104. [Google Scholar]
- Arditti, J. Micropropagation of Orchids, 2nd ed.; Blackwell Publishing: Oxford, UK, 2008; Volume II. [Google Scholar]
- Prades, A. , Dornier, M., Diop, N., Pain, J.P. Coconut water uses, composition and properties: A review. Fruits. 2011, 67, 87-107.
- Navarro-Cruz, A.R. , Obdulia Vera-Lopez, O., Segura-Badilla, O., Lazcano-Hernandez, M., Kammar-García, M., Aguilar-Alonso, P., Ramírez-Calixto, J. Use of coconut water (Cocus nucifera L) for the development of a symbiotic functional drink. Heliyon,. 2020, 6, e03653. [Google Scholar]
- Rahmanian, N., Siti Hajar Bt, A., Homayoonfard, M., Ali, N.J., Rehan, M., Sadef, Y.and Nizami, A.S. Analysis of Physiochemical Parameters to Evaluate the Drinking Water Quality in the State of Perak, Malaysia. Hindawi Publishing Corporation Journal of Chemistry, 2015, Article ID 716125, 10 pages.
- FAO, New sports drink: coconut water, Fruit Process. 2007, 10 (12), 491–496.
- Theodore, J.D. , Knight-Jones, M., Bernard, H., Mwansa M. S., Yona, S. and Delia, G. Microbial Contamination and Hygiene of Fresh Cow’s Milk Produced by Smallholders in Western Zambia Int. J. Environ. Res. 2016, 13, 737. [Google Scholar] [CrossRef]
- Wahauwouélé, H.C. ,, Fatoumata, C., Majoie, G. T., Pierre Alain, K.K., Karnon, C., Edi Guy, A.S.Y., Michael, A.W. Nutritional profile and functional properties of coconut water marketed in the streets of Abidjan (Côte d’Ivoire). Scientific African, 2023, 20, e01616. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).