1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a tumor with a rising incidence that constitutes 90% of all pancreatic malignancies and is characterized by intricate interactions between genetic, epigenetic, and microenvironmental factors that contribute to an aggressive nature and late-stage diagnosis, resistance to chemotherapy, and early metastasis (1, 2). It develops from the exocrine part of the gland and is one of the deadliest solid tumors in humans. Despite the development of oncology and diagnostic methods, the prognosis for this malignancy has not changed significantly for years, and not more than 10% of patients survive 5 years from diagnosis(3). The poor prognosis of patients with PDAC is largely attributable to the complex interplay of factors within the tumor microenvironment (TME), which not only support tumor growth and progression but also contribute to immune evasion and resistance to conventional treatments(4).
The TME in PDAC is characterized by a dense desmoplastic stroma of activated fibroblasts, extracellular matrix components, and many immune cells that collectively create a hostile environment for therapeutic intervention. This stromal barrier is not only a passive system but also an active contributor to tumor biology, as it secretes growth factors, cytokines, and extracellular matrix proteins that promote tumor survival, invasion, and metastasis(5). The stroma also serves as a physical barrier to the delivery of chemotherapeutic agents and increases the difficulty of treatment(6). Heterogeneity within the tumor microenvironment and differential gene expression have been observed in the tumors of PDAC patients(7-9).
In contrast, pancreatic endocrine tumors (PNETs) are less common and often present different clinical characteristics. PNETs can range from benign, well-differentiated tumors to highly aggressive, poorly differentiated forms, which complicates their management due to variability in clinical behavior and the absence of reliable early biomarkers(10, 11). Functional PNETs that secrete hormones can lead to symptomatic presentations associated with hormone hypersecretion, whereas nonfunctional PNETs are often discovered incidentally during imaging for unrelated conditions(12, 13). Peripheral blood mononuclear cells (PBMNCs) have emerged as promising noninvasive biomarkers because of their accessibility and role in reflecting systemic changes associated with cancer. PBMNCs, which include lymphocytes and monocytes, are isolated from blood via density gradient centrifugation. This technique allows a diverse array of cells to be obtained from a simple blood draw, thus providing a minimally invasive method for studying gene expression associated with various diseases, including cancer(14). In pancreatic cancer, PBMNCs provide insights into the systemic effects of tumors, particularly in terms of gene expression associated with stem cells and progenitor cells. Cancer stem cells (CSCs) are a type of tumor cell characterized by their ability to self-renew and differentiate into various cell types and play crucial roles in tumor initiation, progression, and resistance to therapy(15, 16). At the cellular level, there has been significant interest in identifying biomarkers that reflect the presence and activity of these cells. Among the most promising candidates are genes related to stemness and progenitor cell functions, including NANOG, OCT4, CK19, HES1, PDX1, and INS. These genes are critical not only for maintaining the pluripotency of stem cells but also for their involvement in the pathological processes of tumorigenesis, metastasis, and resistance to therapy(17-19).
The development of pancreatic malignancies is related to metabolic alterations(20). Moreover, genetic alterations in both progenitor genes and stem cell genes and markers have been described in patients with pancreatic neoplastic and inflammatory disorders(21, 22). The challenge of early detection is underscored by the lack of specific and reliable biomarkers that can differentiate PDAC from other pancreatic conditions, including benign neoplasms and chronic pancreatitis. Currently, the only widely accepted biomarker for pancreatic cancer is carbohydrate antigen 19-9 (CA19-9), which is commonly used in clinical practice to monitor disease progression and response to therapy(23). CA19-9 lacks sensitivity and specificity, particularly in the early stages of the disease (24). As a result, novel biomarkers that can facilitate early diagnosis, predict prognosis, and monitor therapeutic responses in PDAC patients are needed.
NANOG and OCT4 are well-known transcription factors that play critical roles in maintaining the pluripotency of embryonic stem cells, and their expression has been associated with poor prognosis in various cancers, including PDAC(25). NANOG has been shown to enhance the self-renewal capabilities of CSCs and promote resistance to chemotherapy by activating survival pathways such as the PI3K/Akt and Wnt/β-catenin signalling cascades(26, 27). OCT4, on the other hand, has been implicated in epithelial‒mesenchymal transition (EMT), a process that endows cancer cells with enhanced migratory and invasive properties, facilitating metastasis(28). CK19, a cytokeratin typically expressed in epithelial cells, is another marker of interest in PDAC. CK19 is widely used as a diagnostic marker for PDAC, distinguishing it from other types of pancreatic neoplasms, such as acinar cell carcinoma and neuroendocrine tumors(29). High CK19 expression has been correlated with a greater incidence of lymph node metastasis and poorer overall survival, indicating its potential role as a prognostic marker(30). Moreover, recent studies suggest that CK19 may play a direct role in the metastatic process, possibly through interactions with the extracellular matrix and modulation of epithelial‒mesenchymal transition (EMT) pathways(31).
In addition to these well-established markers, other genes involved in pancreatic development and function, such as HES1, PDX1, and INS, have also been implicated in pancreatic tumorigenesis. HES1, which is transcriptionally repressed or regulated by the Notch signalling pathway, plays a crucial role in maintaining the undifferentiated state of pancreatic progenitor cells and has been associated with the suppression of differentiation and promotion of tumorigenesis in PDAC(32). PDX1, another key transcription factor, is essential for the development of the pancreas and the differentiation of pancreatic progenitor cells into insulin-producing beta cells(33). Dysregulation of PDX1 has been linked to the development of PDAC, particularly in the context of hyperinsulinaemia and insulin resistance, which are recognized risk factors for pancreatic cancer(33). INS, the gene encoding insulin, has also been implicated in the pathogenesis of PDAC, where aberrant insulin signalling and hyperinsulinaemia are thought to contribute to the metabolic alterations that support tumour growth and survival(34).
While significant progress has been made in understanding the molecular mechanisms underlying PDAC, the identification of reliable biomarkers for early detection and prognosis remains a critical unmet need.
In our study, we comprehensively assessed the mRNA expression profiles of stem cell- and progenitor-cell-associated genes in peripheral blood mononuclear cells from patients with different clinical stages of pancreatic malignancies (PDAC and PNET). By analysing these profiles, we aimed to identify potential biomarkers or biomarker combinations that could facilitate noninvasive diagnosis, prognosis, and monitoring of pancreatic cancer.
2. Materials and Methods
2.1. Patients and Study Design
Blood was collected from the forearm veins of patients hospitalized at the Department of Gastroenterology, Pomeranian Medical University (Szczecin, Poland), with pancreatic neoplasms (37 with cancer and 12 with neuroendocrine tumors) in different clinical stages (14 with locally advanced cancer, 23 with metastatic cancer, and 12 with operative neuroendocrine tumors) and from 34 healthy controls. The diagnosis of pancreatic neoplastic disease was confirmed with EUS-guided biopsy, and in every patient, clinical staging was assessed with abdominal computed tomography and chest X-ray. The study was approved by the Bioethics Committee of Pomeranian Medical University kB-0012/43/12. Written informed consent was obtained from all patients.
2.2. Assessment of mRNA in Peripheral Blood
Peripheral blood samples (~20 ml) were collected from forearm veins. Red blood cells were removed from the PB samples via 1X hypotonic lysis solution (BD Pharm Lyse Buffer, BD Bioscience Pharmingen). Total nucleated cells were washed with Dulbecco’s phosphate-buffered saline (DPBS, w/o Ca2+ and Mg2+; HyClone, GE Healthcare Life Sciences) and immediately stored in RNAlater® solution (Invitrogen) at –80°C until genetic analysis. Total RNA was isolated from lysed peripheral blood with an RNeasy Kit (Qiagen). The RNA was reverse-transcribed with a first-strand cDNA synthesis kit and oligo-dT primers (Thermo Fisher). The mRNA expression of 6 stem cell and pancreatic progenitor genes, OCT4 (POU class 5 homeobox 1), NANOG, CK19 (keratin 19), HES1 (hes family bHLH transcription factor 1), INS (insulin), and PDX1 (pancreatic and duodenal homeobox 1), was assessed via real-time quantitative PCR on an ABI 7500 Fast instrument with Power SYBRGreen PCR Master Mix reagent (Thermo Fisher). The real-time conditions were as follows: 95°C (15 s), 40 cycles at 95°C (15 s), and 60°C (1 min). According to melting point analysis, only one PCR product was amplified under these conditions. The mRNA expression of all the genes investigated was normalized to that of the nonregulated reference housekeeping gene b-2 microglobulin (B2M). The data are presented as absolute expression values via the 2ˆdCt formula. The primers (IBBs) used were as follows: Oct-4 (forward) 5-CCCCTGGTGCCGTGAA-3, (reverse) 5-GCAAATTGCTCGAGTTCTTTCTG-3; Nanog (forward) 5-GCAGAAGGCCTCAGCACCTA-3, (reverse) 5-AGGTTCCCAGTCGGGTTCA-3; CK19 (forward), (reverse); HES1 (forward) 5-GCCGCGAGCTATCTTTCTTCA-3, (reverse) 5-ACACGACACCGGATAAACCAA-3; INS (forward) 5-GCAGCCTTTGTGAACCAACA-3, (reverse) 5-TTCCCCGCACACTAGGTAGAGA-3; and PDX1 (forward) 5-AACCGCGTCCAGCTGCCTTTC-3, (reverse) 5-CCGCTTGTTCTCCTCCGGCTC-3.
2.3. Statistical Analysis
Since most quantitative variables, including mRNA expression, showed distributions significantly different from a normal distribution, they are presented as medians with interquartile ranges (Q1--Q3), and nonparametric tests were used: the Mann‒Whitney U test for comparisons between groups and the Spearman rank correlation rank coefficient (Rs) for correlations within groups. Associations with p<0.05 were considered statistically significant.
3. Results
3.1. Patient Characteristics
We compared basic clinical data, including age, height, weight, BMI, and biochemical features, such as white blood count and platelets (PLT), erythrocytes (ERY), CRP and Ca19.9 counts, between patients with pancreatic cancer and neuroendocrine tumors and healthy controls. Our analysis revealed significantly greater leukocyte counts, CRP and Ca 19.9 levels in PDAC patients and lower weights, BMIs, and erythrocyte counts than in healthy controls. The PNETS patients were significantly younger than the healthy controls were.
Table 1 presents the baseline characteristics of all included patients.
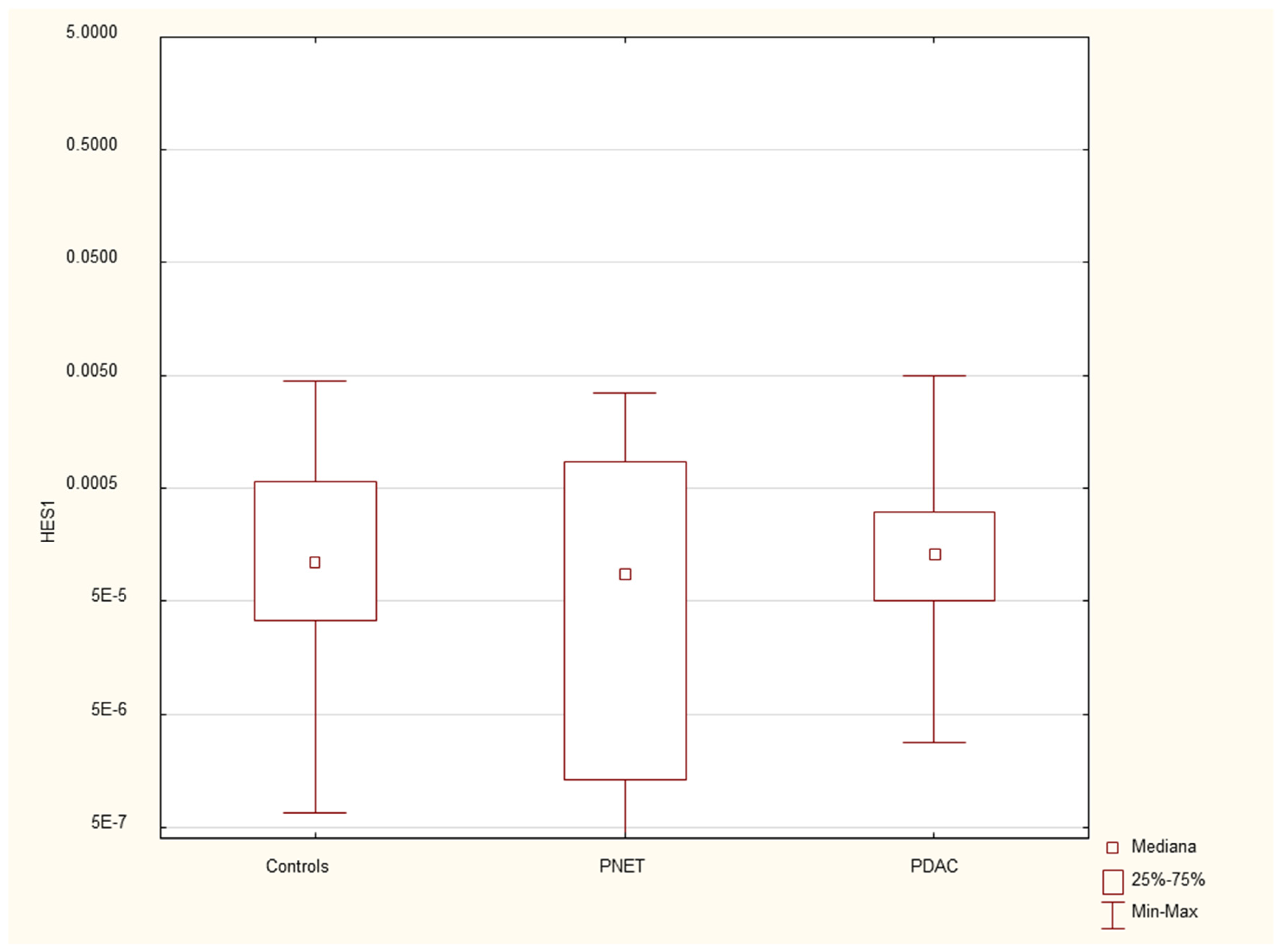
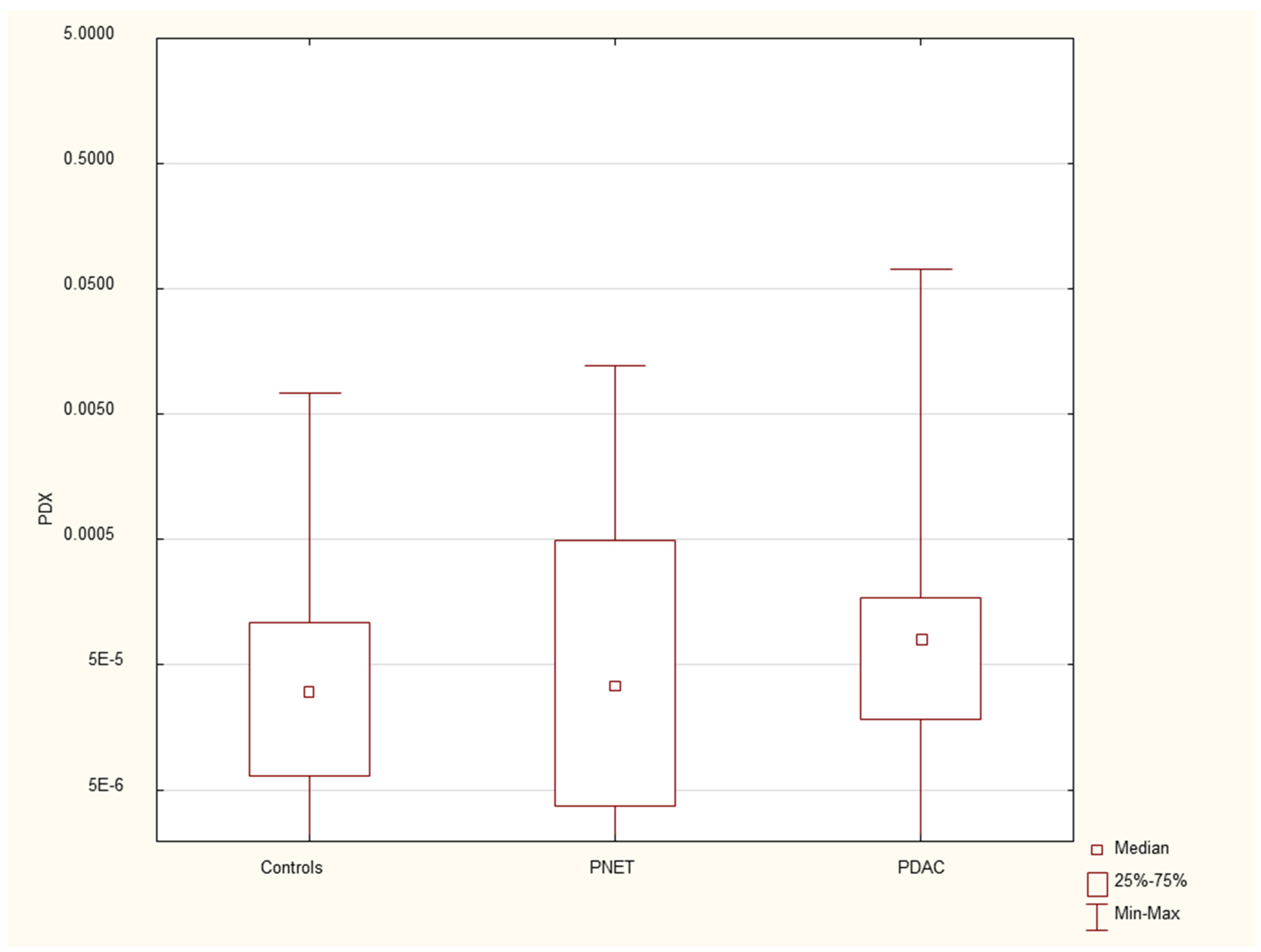
3.2. Expression of mRNAs Encoding Stem Cell and Progenitor Pancreatic Genes in PB Patients with Pancreatic Neoplastic Disorders
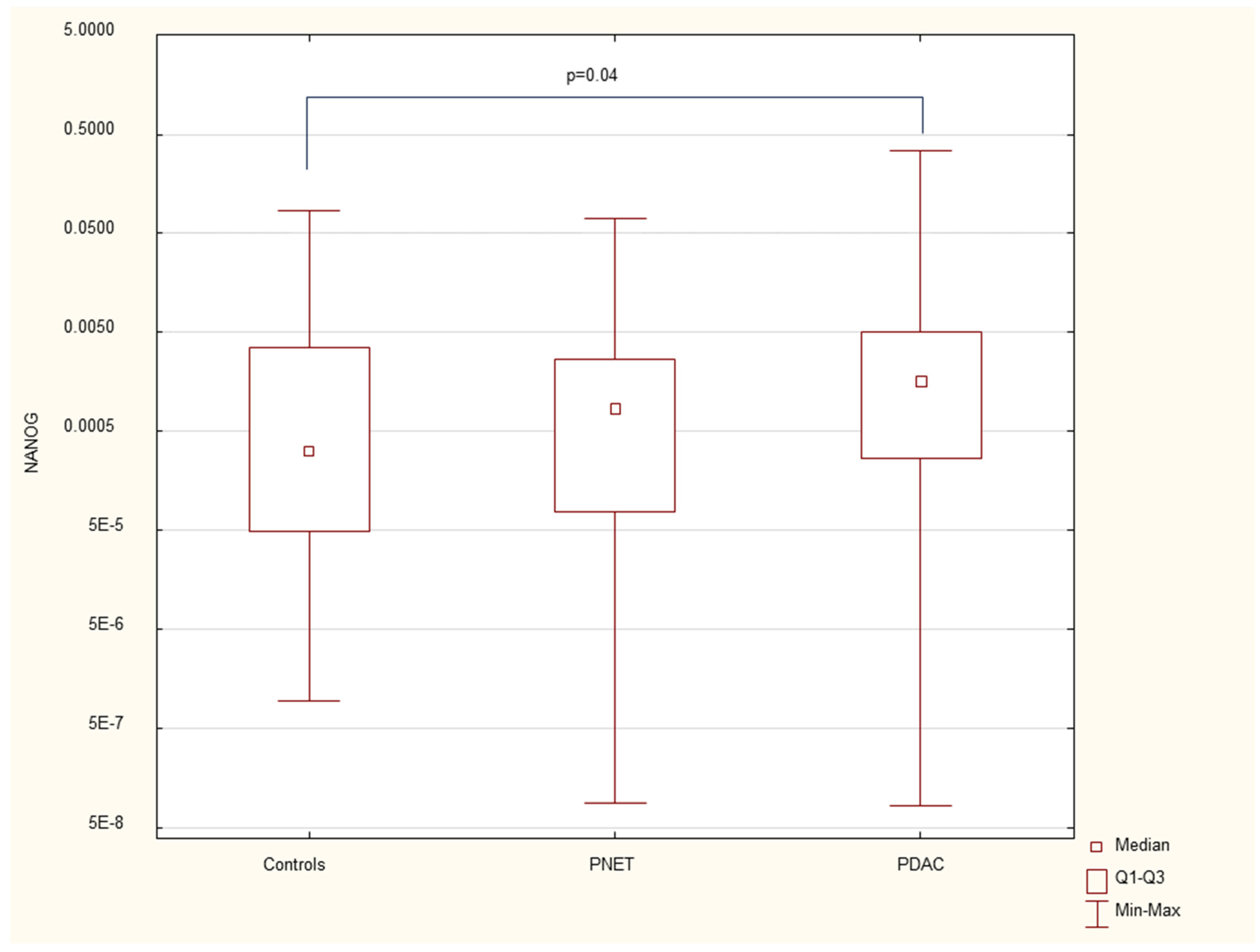
We found that the expression of genes characteristic of early stem cells,
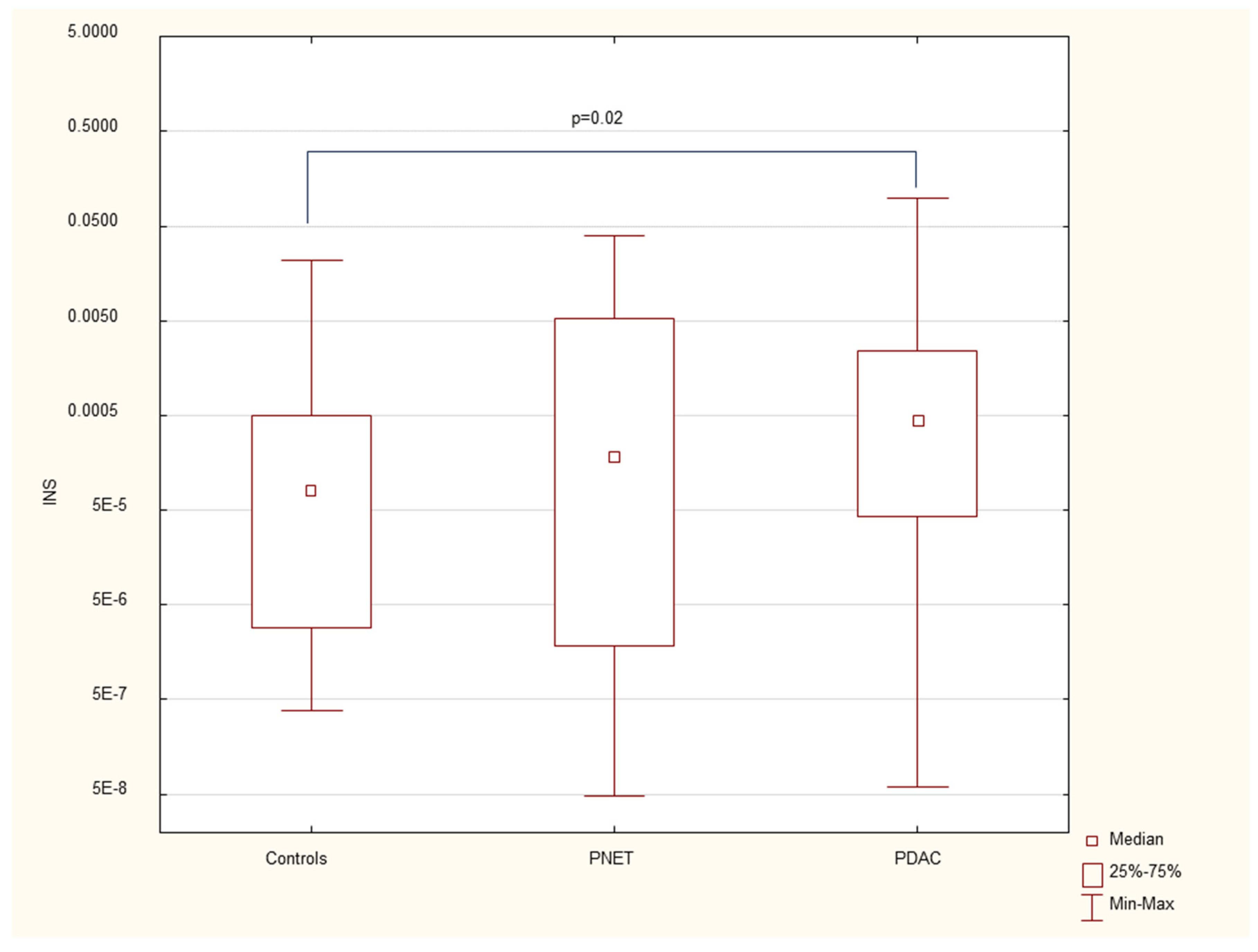
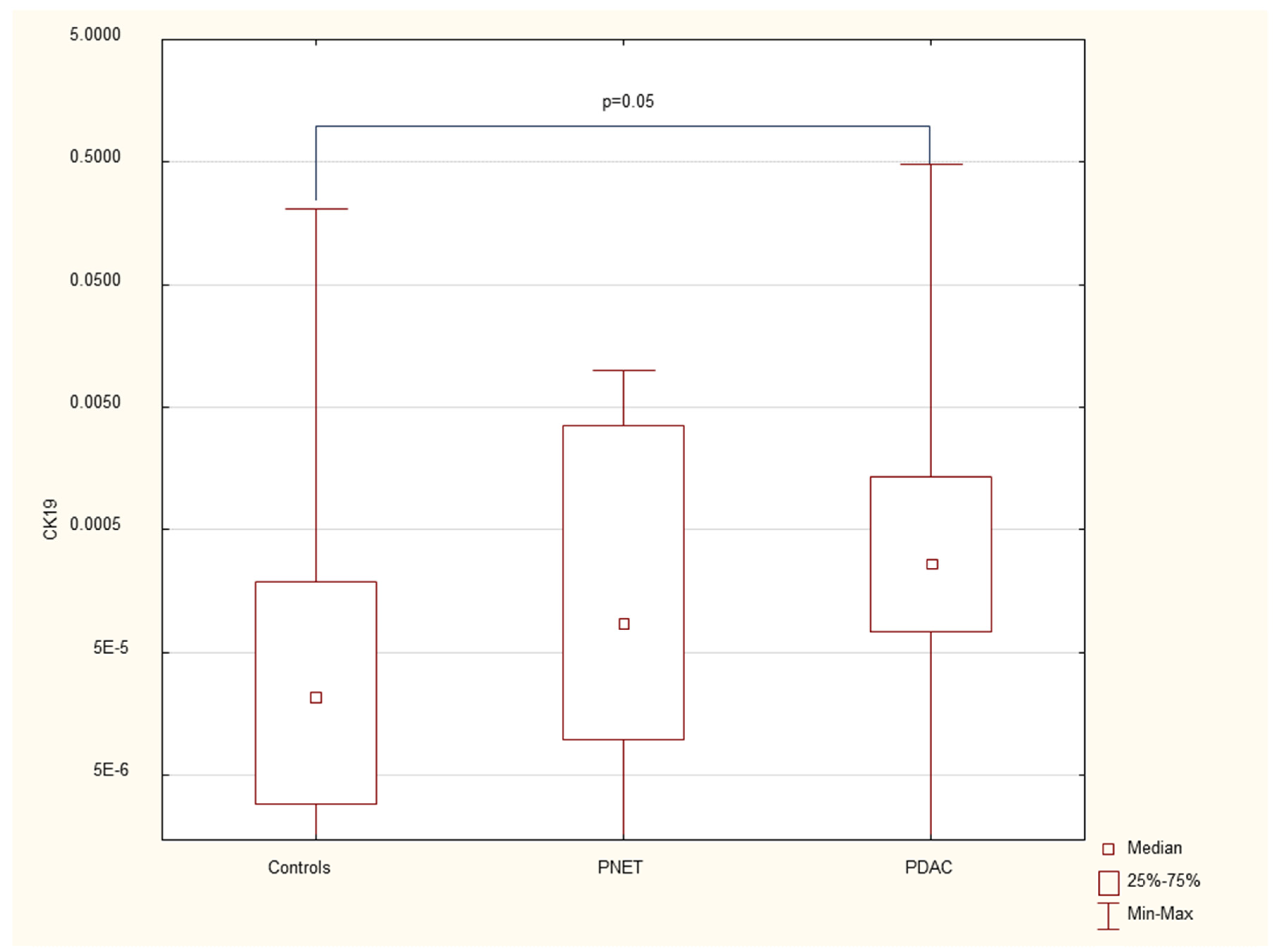
NANOG (p=0.03), and the expression of genes encoding insulin and cytokeratin 19 (
INS p=0.02,
CK19 p=0.005) were increased in pancreatic cancer patients. However, it was not significantly different under other neoplastic (neuroendocrine) conditions. There were no significant differences in genetic expression between patients with different stages of pancreatic cancer (locally advanced and metastatic).
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6 and
Table 2 present the expression of genes associated with neoplastic disorders. The gene expression data and basic clinical characteristics of patients with locally advanced and metastatic pancreatic cancer are presented in
Table 3.
3.3. Correlations
In pancreatic cancer patients; statistical analysis revealed no significant correlations between the expression of genes and clinical (height; weight; BMI) or biochemical data (WBC; ERY; PLT; CRP; and Ca 19.9); apart from CK19 overexpression; which was correlated with inflammatory markers (both CRP (Rs=+0.34; p=0.04) and WBC (Rs=+0.51; p=0.001)) in patients with pancreatic cancer.
In neuroendocrine tumor patients, statistical analysis revealed no significant correlations between the expression of genes and clinical or biochemical data, with the exception of a positive correlation between HES1 gene expression and WBCs (Rs=+0.77, p=0.003). The correlations between mRNA gene expression and clinical and biochemical patient characteristics are presented in supplementary Tables S1 and S2.
4. Discussion
Pancreatic cancer continues to be one of the most significant challenges in oncology. The majority of patients with PDAC are diagnosed at advanced stages, and the absence of reliable markers for early detection, combined with resistance to chemotherapy, contributes to its poor prognosis. The five-year survival rate is less than 5%, making PDAC one of the deadliest solid tumors in humans(35).
In our study, we comprehensively analysed the mRNA expression of progenitor and stem cell genes in the peripheral blood of patients with pancreatic neoplastic disorders, including cancer and neuroendocrine tumors. The results revealed that the mRNA expression of certain stem cell and progenitor genes in the peripheral blood was significantly greater in cancer patients than in controls, regardless of the disease stage. However, this pattern was not observed in patients with neuroendocrine tumors, where lower mRNA expression of some progenitor and stem cell genes was observed. Among the most notable findings, in our view, are the overexpression of NANOG and CK19 (and their association with inflammation) in PDAC.
This overexpression of stem cell markers, including NANOG, is in line with our previous finding of increased numbers of stem cells in the PB of patients with PDAC(22). NANOG is a gene that controls stem cell stemness, and its expression is correlated with stage and prognosis in PDAC and is related to the development of metastases (36).
However, in our study. We did not find a significant difference in the expression of NANOG in patients with locally advanced and metastatic cancer. One possible explanation could be that in both metastatic and inoperable cancer, there is infiltration of vessels and the presence of cancer cells in circulation. Our previous observations, along with those of previous murine studies, allowed us to draw conclusions about the role of mobilized bone marrow stem cells in supporting the growth and development of cancer metastases(21, 37). Notably, NANOG is a marker of pluripotency and is expressed in some populations of stem cells, and NANOG expression(38), in the present study, was not detected in the serum of patients with neuroendocrine tumors. This finding highlights the potential diagnostic value of this marker for discriminating pancreatic cancer from other tumors. These findings also support our previous observations related to the role of pluripotent stem cells in the pathogenesis of pancreatic cancer(39, 40).
CK-19 is a type of filament protein called a keratin expressed in the epithelium of the gastrointestinal tract. CK19 is a biomarker commonly associated with tumors of epithelial origin and circulating tumor cells in the bloodstream, serving as an indicator for epithelial-derived tumors. (16). Its expression has been observed in various types of cancers, including breast, lung, and pancreas cancers, and in patients with pancreatic neuroendocrine tumors (17-19). CK19 has been consistently associated with poor prognosis, tumor progression, and metastatic development in these cancers(16). In our study, we also observed that CK19 mRNA expression was elevated exclusively in the serum of pancreatic cancer patients and was positively correlated with systemic inflammation, as indicated by increased WBC and CRP levels, two commonly used clinical inflammatory markers.
The correlation between inflammation and stem cell expression was previously demonstrated for stem cells in PDAC(39, 41). In this group of patients, complement cascade elements play a role in stem cell trafficking to the peripherial blood of patients with PDAC(17). In particular, components such as C3a and C5a play crucial roles in modulating the inflammatory environment in PDAC. They enhance immune cell recruitment and stimulate cytokine release, contributing to the distribution of CK-19-positive tumor cells as circulating tumor cells(42). This interaction highlights CK19’s dual role as both a marker of epithelial tumors and a component of Inflammation-driven tumor progression in PDAC. Moreover, the number of some interleukins is associated with the number of circulating stem cells(43). There is increasing data showing that inflammatory elements, including cytokines such as IL-6, IL-1β, and TNF-α, play important roles in cancer initiation, growth, and metastasis development(44, 45). IL-6, for instance, activates the JAK/SSTAT3 signalling pathway, which is critical for promoting CK19 transcription and sustaining tumor cell proliferation and invasiveness(46). IL-6 is also known to increase CRP levels, directly linking CK19 expression with systemic inflammatory markers such as the WBC count and CRP level(47). The desmoplastic PDAC microenvironment is complex, and the interplay between its components, including inflammatory elements, determines cancer progression (10). In addition, TNF-α and IL-1β activate the NF-κB pathway, a master regulator of inflammation and oncogenesis in PDAC, further increasing CK19 expression. The persistent activation of NF-κB in the PDAC microenvironment results in a feedback loop that amplifies cytokine secretion, driving both tumor progression and the inflammatory response(48). Moreover, carcinogenesis shares some similarities with the process of inflammation, and inflammation may trigger cancer development(40, 49). This is also observed clinically, and examples of preneoplastic inflammatory states that lead to cancer development include chronic atrophic gastritis and gastric cancer, ulcerative colitis and colon cancer and chronic pancreatitis and pancreatic cancer(49, 50).
In contrast to previous studies in which CK-19 expression has been identified as a marker of poor prognosis in PNETs, particularly in advanced or metastatic cases, our study did not observe significant CK-19 expression in patients with PNETs(46). This may be explained by the characteristics of the patient population included in our study, which consisted primarily of individuals with early-stage, nonmetastatic PNETs. It is possible that CK-19 expression becomes more prominent in more aggressive or advanced disease stages and thus may not be relevant in early tumor presentation. Furthermore, when PNETs are compared with PDAC, a more aggressive form of pancreatic cancer, CK-19 expression patterns may differ, as PDAC often presents at more advanced stages where poor prognostic markers such as CK-19 are more prevalent. These findings suggest that CK-19 may be more useful as a prognostic biomarker in late-stage PNETs or PDAC patients than in early, localized PNET patients.
INS is a gene encoding insulin, and in our study, elevated INS expression was associated with PDAC. Previous studies have shown that hyperinsulinaemia and insulin resistance are factors related to increased incidence and mortality in patients with PDAC, with insulin potentially promoting the development of preneoplastic pancreatic conditions(26, 27, 34). This could not be confirmed in our study because of the lack of survival data. Similar to the CK19 and NANOG genes, its expression was observed in PB only in cancer patients. Notably, insulin resistance is frequently observed in PDAC patients and serves as both a predisposing factor and an early symptom of the disease. This resistance is one of the mechanisms leading to diabetes in these patients.
The tissue degeneration process, Involving the replacement of the pancreatic parenchyma with fibrosis or the development of edema or necrosis, followed by the onset of endocrine and exocrine gland insufficiency, forms the core of pancreatitis. Unsurprisingly, disturbances in the expression of genes involved in pancreatic development, such as HES1 and PDX1, were observed in our study. These genes belong to the family of transcriptional activators. PDX1 plays crucial roles in maintaining B-cell mass, function, and identity, which are essential for the expression of insulin, somatostatin, glucokinase, islet amyloid polypeptide and glucose transporter type 2(51). Its defects are responsible for pancreatic agenesis and the development of diabetes (maturity-onset diabetes of the young type 4-MODY4)(52, 53). The exact role of the HES1 protein in the adult pancreas has yet to be determined. It is expressed in the duct and centroacinar cells in the gland and is involved in the differentiation of ductal and acinar cells under normal and inflammatory conditions. Additionally, it is involved in the formation of preneoplastic states, such as acinar to ductal neoplasia and PANiN, and may contribute to Kras-driven pancreatic tumorigenesis(32).
The limitation of our study is the lack of heterogeneity among cancer patients, as only those with advanced-stage disease (metastatic or locally advanced disease) were included. Evaluating mRNA genes in early-stage pancreatic cancer seems to be an interesting area of future research. Another limitation is the lack of long-term survival data, which could be correlated with gene expression and the diverse clinical characteristics of patients, such as age and sex, across various pancreatic disorders.
Despite these limitations, our study is the first to comprehensively assess the mRNA expression of both stem cells and progenitor genes across a spectrum of neoplastic pancreatic disorders.
5. Conclusions
The mRNA expression of selected stem and progenitor genes (NANOG, INS, CK19) in peripheral blood mononuclear cells (PBMNCs) has potential as a valuable marker for pancreatic cancer, with a notable correlation with coexisting inflammation. These findings underscore the importance of studying mRNA expression in peripheral blood to enhance our understanding of pancreatic neoplasms. This approach may contribute to improving early detection and enabling more personalized, noninvasive strategies for managing these aggressive diseases.
Author Contributions
Conceptualization, KD.MT.KS.MD. TS; Methodology, MT, KD, KS. ; Software, KS. ; Data Curation, KD.AK.KP. ; Writing–Original Draft Preparation, KD.MD.AK. KP, TS; Writing–Review & Editing, KD.MT.KS.MD.; Visualization, KS.MT. ; Supervision, TS.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022.
- Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47-52. [CrossRef]
- Dąbkowski K, Bogacka B, Tarnowski M, Starzyńska T. [Pancreatic cancer microenvironment]. Polski merkuriusz lekarski: organ Polskiego Towarzystwa Lekarskiego. 2016;41(246):296-302. [CrossRef]
- Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nature reviews Clinical oncology. 2020;17(9):527-40. [CrossRef]
- Mercanti L, Sindaco M, Mazzone M, Di Marcantonio MC, Piscione M, Muraro R, et al. PDAC, the Influencer Cancer: Cross-Talk with Tumor Microenvironment and Connected Potential Therapy Strategies. Cancers (Basel). 2023;15(11). [CrossRef]
- Bulle A, Lim KH. Beyond just a tight fortress: contribution of stroma to epithelial-mesenchymal transition in pancreatic cancer. Signal Transduct Target Ther. 2020;5(1):249. [CrossRef]
- Galindo-Vega A, Maldonado-Lagunas V, Mitre-Aguilar IB, Melendez-Zajgla J. Tumor Microenvironment Role in Pancreatic Cancer Stem Cells. Cells. 2023;12(12). [CrossRef]
- Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris JPt, et al. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer cell. 2012;22(6):737-50. [CrossRef]
- Gidekel Friedlander SY, Chu GC, Snyder EL, Girnius N, Dibelius G, Crowley D, et al. Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer cell. 2009;16(5):379-89. [CrossRef]
- Sun J. Pancreatic neuroendocrine tumors. Intractable Rare Dis Res. 2017;6(1):21-8.
- Ro C, Chai W, Yu VE, Yu R. Pancreatic neuroendocrine tumors: biology, diagnosis,and treatment. Chin J Cancer. 2013;32(6):312-24. [CrossRef]
- Dumlu EG, Karakoç D, Özdemir A. Nonfunctional Pancreatic Neuroendocrine Tumors: Advances in Diagnosis, Management, and Controversies. Int Surg. 2015;100(6):1089-97. [CrossRef]
- Anderson CW, Bennett JJ. Clinical Presentation and Diagnosis of Pancreatic Neuroendocrine Tumors. Surg Oncol Clin N Am. 2016;25(2):363-74. [CrossRef]
- Alexovič M, Uličná C, Sabo J, Davalieva K. Human peripheral blood mononuclear cells as a valuable source of disease-related biomarkers: Evidence from comparative proteomics studies. Proteomics Clin Appl. 2024;18(2):e2300072. [CrossRef]
- Vaz AP, Ponnusamy MP, Seshacharyulu P, Batra SK. A concise review on the current understanding of pancreatic cancer stem cells. J Cancer Stem Cell Res. 2014;2. [CrossRef]
- MacLean MR, Walker OL, Arun RP, Fernando W, Marcato P. Informed by Cancer Stem Cells of Solid Tumors: Advances in Treatments Targeting Tumor-Promoting Factors and Pathways. International journal of molecular sciences. 2024;25(7). [CrossRef]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer research. 2007;67(3):1030-7. [CrossRef]
- La Rosa S, Rigoli E, Uccella S, Novario R, Capella C. Prognostic and biological significance of cytokeratin 19 in pancreatic endocrine tumours. Histopathology. 2007;50(5):597-606. [CrossRef]
- Marui S, Nishikawa Y, Shiokawa M, Yokode M, Matsumoto S, Muramoto Y, et al. Context-Dependent Roles of Hes1 in the Adult Pancreas and Pancreatic Tumor Formation. Gastroenterology. 2022;163(6):1613-29.e12. [CrossRef]
- Skubisz K, Dąbkowski K, Samborowska E, Starzyńska T, Deskur A, Ambrozkiewicz F, et al. Serum Metabolite Biomarkers for Pancreatic Tumors: Neuroendocrine and Pancreatic Ductal Adenocarcinomas-A Preliminary Study. Cancers (Basel). 2023;15(12). [CrossRef]
- Dąbkowski K, Łabędź-Masłowska A, Dołęgowska B, Safranow K, Budkowska M, Zuba-Surma E, et al. Evidence of Stem Cells Mobilization in the Blood of Patients with Pancreatitis: A Potential Link with Disease Severity. Stem cells international. 2022;2022:5395248. [CrossRef]
- Starzynska T, Dabkowski K, Blogowski W, Zuba-Surma E, Budkowska M, Salata D, et al. An intensified systemic trafficking of bone marrow-derived stem/progenitor cells in patients with pancreatic cancer. Journal of cellular and molecular medicine. 2013. [CrossRef]
- Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol. 2012;3(2):105-19. [CrossRef]
- Amaral MJ, Oliveira RC, Donato P, Tralhão JG. Pancreatic Cancer Biomarkers: Oncogenic Mutations, Tissue and Liquid Biopsies, and Radiomics-A Review. Digestive diseases and sciences. 2023;68(7):2811-23. [CrossRef]
- Roy S, Dukic T, Keepers Z, Bhandary B, Lamichhane N, Molitoris J, et al. SOX2 and OCT4 mediate radiation and drug resistance in pancreatic tumor organoids. Cell Death Discov. 2024;10(1):106. [CrossRef]
- Zhao Y, Qin C, Zhao B, Wang Y, Li Z, Li T, et al. Pancreatic cancer stemness: dynamic status in malignant progression. J Exp Clin Cancer Res. 2023;42(1):122. [CrossRef]
- Safa AR. Epithelial-mesenchymal transition: a hallmark in pancreatic cancer stem cell migration, metastasis formation, and drug resistance. J Cancer Metastasis Treat. 2020;6. [CrossRef]
- Zhang Q, Han Z, Zhu Y, Chen J, Li W. The Role and Specific Mechanism of OCT4 in Cancer Stem Cells: A Review. Int J Stem Cells. 2020;13(3):312-25. [CrossRef]
- Menz A, Bauer R, Kluth M, Marie von Bargen C, Gorbokon N, Viehweger F, et al. Diagnostic and prognostic impact of cytokeratin 19 expression analysis in human tumors: a tissue microarray study of 13,172 tumors. Human pathology. 2021;115:19-36. [CrossRef]
- Ferrara B, Pignatelli C, Cossutta M, Citro A, Courty J, Piemonti L. The Extracellular Matrix in Pancreatic Cancer: Description of a Complex Network and Promising Therapeutic Options. Cancers (Basel). 2021;13(17). [CrossRef]
- Raskov H, Gaggar S, Tajik A, Orhan A, Gögenur I. The Matrix Reloaded-The Role of the Extracellular Matrix in Cancer. Cancers (Basel). 2023;15(7). [CrossRef]
- Nishikawa Y, Kodama Y, Shiokawa M, Matsumori T, Marui S, Kuriyama K, et al. Hes1 plays an essential role in Kras-driven pancreatic tumorigenesis. Oncogene. 2019;38(22):4283-96. [CrossRef]
- Oliver-Krasinski JM, Stoffers DA. On the origin of the beta cell. Genes & development. 2008;22(15):1998-2021. [CrossRef]
- Zhang AMY, Chu KH, Daly BF, Ruiter T, Dou Y, Yang JCC, et al. Effects of hyperinsulinemia on pancreatic cancer development and the immune microenvironment revealed through single-cell transcriptomics. Cancer Metab. 2022;10(1):5. [CrossRef]
- Dabkowski K, Bogacka B, Tarnowski M, Starzynska T. [Pancreatic cancer microenvironment]. Polski merkuriusz lekarski: organ Polskiego Towarzystwa Lekarskiego. 2016;41(246):296-302.
- Gao S, Pan Y, Song L, Dong L, Weng LI, Wang P, et al. Nanog Predicts Poor Prognosis in Human Pancreatic Cancer and Is Downregulated by QingyihuaJi Formula in Pancreatic Cancer Stem Cells. Evid Based Complement Alternat Med. 2016;2016:7028289. [CrossRef]
- Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, et al. Gastric cancer originating from bone marrow-derived cells. Science (New York, NY). 2004;306(5701):1568-71. [CrossRef]
- Lahlil R, Scrofani M, Barbet R, Tancredi C, Aries A, Hénon P. VSELs Maintain their Pluripotency and Competence to Differentiate after Enhanced Ex Vivo Expansion. Stem Cell Rev Rep. 2018;14(4):510-24. [CrossRef]
- Starzyńska T, Dąbkowski K, Błogowski W, Zuba-Surma E, Budkowska M, Sałata D, et al. An intensified systemic trafficking of bone marrow-derived stem/progenitor cells in patients with pancreatic cancer. Journal of cellular and molecular medicine. 2013;17(6):792-9. [CrossRef]
- Dabkowski K, Labedz-Maslowska A, Zuba-Surma E, Starzynska T. [Role of the bone marrow derived stem cells in pancreatic inflammatory disorders]. Polski merkuriusz lekarski: organ Polskiego Towarzystwa Lekarskiego. 42. Poland2017. p. 137-41.
- Błogowski W, Deskur A, Budkowska M, Sałata D, Madej-Michniewicz A, Dąbkowski K, et al. Selected cytokines in patients with pancreatic cancer: a preliminary report. PloS one. 2014;9(5):e97613. [CrossRef]
- Hart PC, Rajab IM, Alebraheem M, Potempa LA. C-Reactive Protein and Cancer-Diagnostic and Therapeutic Insights. Front Immunol. 2020;11:595835. [CrossRef]
- Blogowski W, Deskur A, Budkowska M, Salata D, Madej-Michniewicz A, Dabkowski K, et al. Selected cytokines in patients with pancreatic cancer: a preliminary report. PloS one. 2014;9(5):e97613. [CrossRef]
- Shadhu K, Xi C. Inflammation and pancreatic cancer: An updated review. Saudi journal of gastroenterology: official journal of the Saudi Gastroenterology Association. 2019;25(1):3-13. [CrossRef]
- Hausmann S, Kong B, Michalski C, Erkan M, Friess H. The role of inflammation in pancreatic cancer. Advances in experimental medicine and biology. 2014;816:129-51. [CrossRef]
- Cen D, Chen J, Li Z, Zhao J, Cai X. Prognostic significance of cytokeratin 19 expression in pancreatic neuroendocrine tumor: A meta-analysis. PloS one. 2017;12(11):e0187588. [CrossRef]
- Han X, Zhao J, Ji Y, Xu X, Lou W. Expression of CK19 and KIT in resectable pancreatic neuroendocrine tumors. Tumour Biol. 2013;34(5):2881-9. [CrossRef]
- Rex J, Lutz A, Faletti LE, Albrecht U, Thomas M, Bode JG, et al. IL-1β and TNFα Differentially Influence NF-κB Activity and FasL-Induced Apoptosis in Primary Murine Hepatocytes During LPS-Induced Inflammation. Frontiers in physiology. 2019;10:117.
- Ling S, Feng T, Jia K, Tian Y, Li Y. Inflammation to cancer: The molecular biology in the pancreas (Review). Oncology letters. 2014;7(6):1747-54. [CrossRef]
- Lüttges J, Klöppel G. Precancerous conditions of pancreatic carcinoma. J Hepatobiliary Pancreat Surg. 2000;7(6):568-74. [CrossRef]
- Tang ZC, Chu Y, Tan YY, Li J, Gao S. Pancreatic and duodenal homeobox-1 in pancreatic ductal adenocarcinoma and diabetes mellitus. Chin Med J (Engl). 2020;133(3):344-50. [CrossRef]
- Zhang Y, Fang X, Wei J, Miao R, Wu H, Ma K, et al. PDX-1: A Promising Therapeutic Target to Reverse Diabetes. Biomolecules. 2022;12(12). [CrossRef]
- Ebrahim N, Shakirova K, Dashinimaev E. PDX1 is the cornerstone of pancreatic β-cell functions and identity. Front Mol Biosci. 2022;9:1091757. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).