1. Introduction
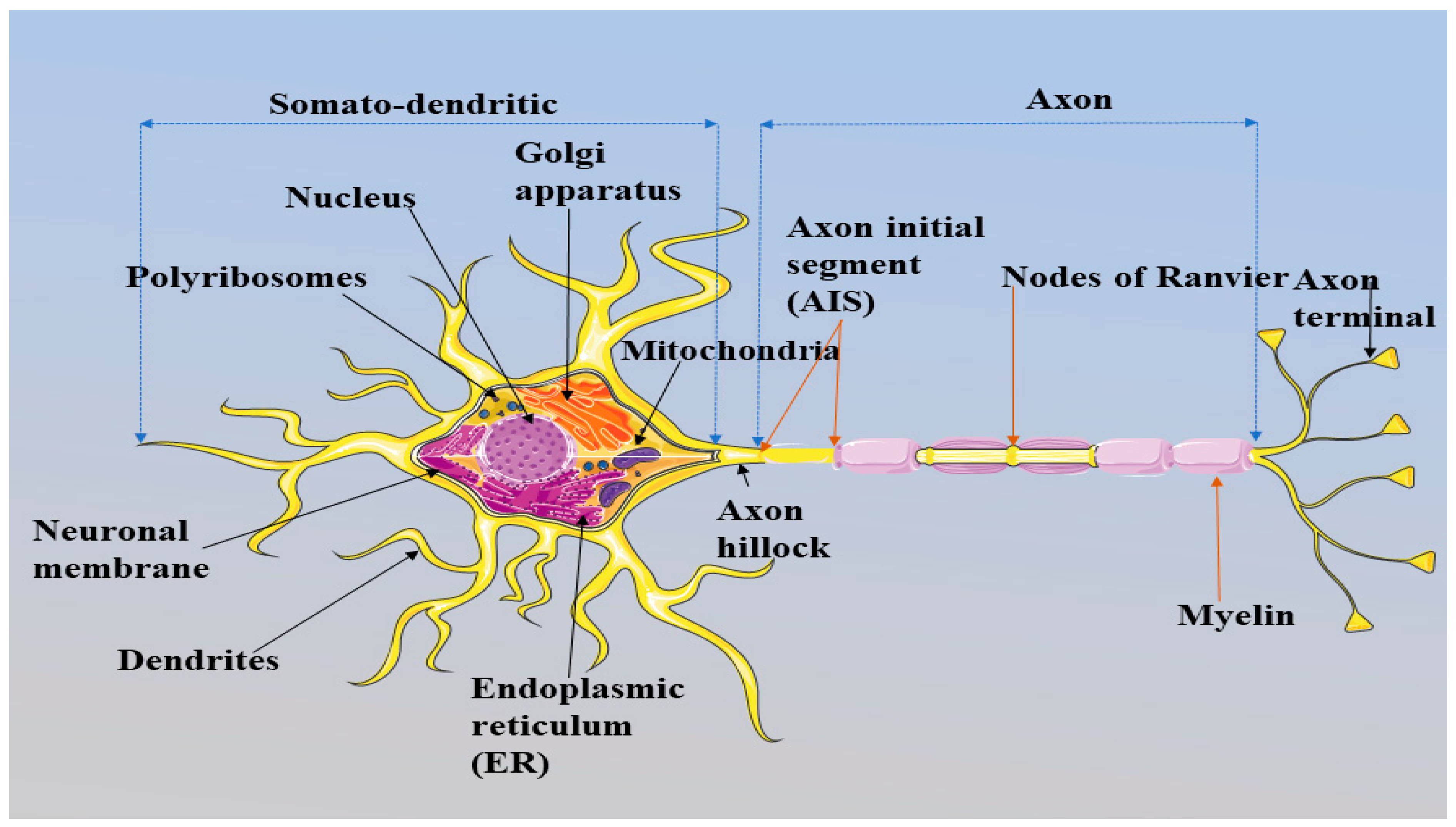
The human brain consists of approximately 100 billion neurons (Fan and Markram, 2019) and a single neuron can be divided into two different compartments: the soma-dendritic and the axonal (Kole et al., 2008, Kole and Stuart, 2012, Leterrier et al., 2015). Soma-dendritic region comprises of nucleus, mitochondria, polyribosomes, dendrites, Golgi apparatus and endoplasmic reticulum (ER) followed by long, narrow, cylindrical structure called axon (Bear et al., 2016) (Fig 1). Excluding the beginning part (axon initial segment -AIS) the axon is covered by myelin sheath (Bear et al., 2016). Gaps between myelin sheath is known as Nodes of Ranvier (NOR) with axon terminal or synaptic buttons at the end. NOR has almost similar molecular organization as AIS (Nelson and Jenkins, 2017, Buffington and Rasband, 2011). Together, all these components contribute in neuronal transmission (Ackerman, 1992). Neuronal transmission is the integration of synaptic inputs into outputs and essential for the communication among neurons (Kole and Stuart, 2012) (Fig 2). As the brain is connected to the rest of the body through neurons, the communication among neurons provide assistance to human body in order to perform basic functions in normal and stress conditions.
Figure 1.
General structure of a neuron. A neuron containing nucleus and all the essential components. Axonal region starts from the axon hillock followed by a non-myelinated region known as the axon initial segment (AIS). Axon region after AIS is covered by myelin sheath and the gaps between myelin sheath are called nodes of Ranvier (NOR), which have similar molecular characteristics to those of the AIS. All together all these components initiate and propagate the AP crucial for neurotransmission between neurons. This figure is created using Servier Medical Art (
http://smart.servier.com).
Figure 1.
General structure of a neuron. A neuron containing nucleus and all the essential components. Axonal region starts from the axon hillock followed by a non-myelinated region known as the axon initial segment (AIS). Axon region after AIS is covered by myelin sheath and the gaps between myelin sheath are called nodes of Ranvier (NOR), which have similar molecular characteristics to those of the AIS. All together all these components initiate and propagate the AP crucial for neurotransmission between neurons. This figure is created using Servier Medical Art (
http://smart.servier.com).
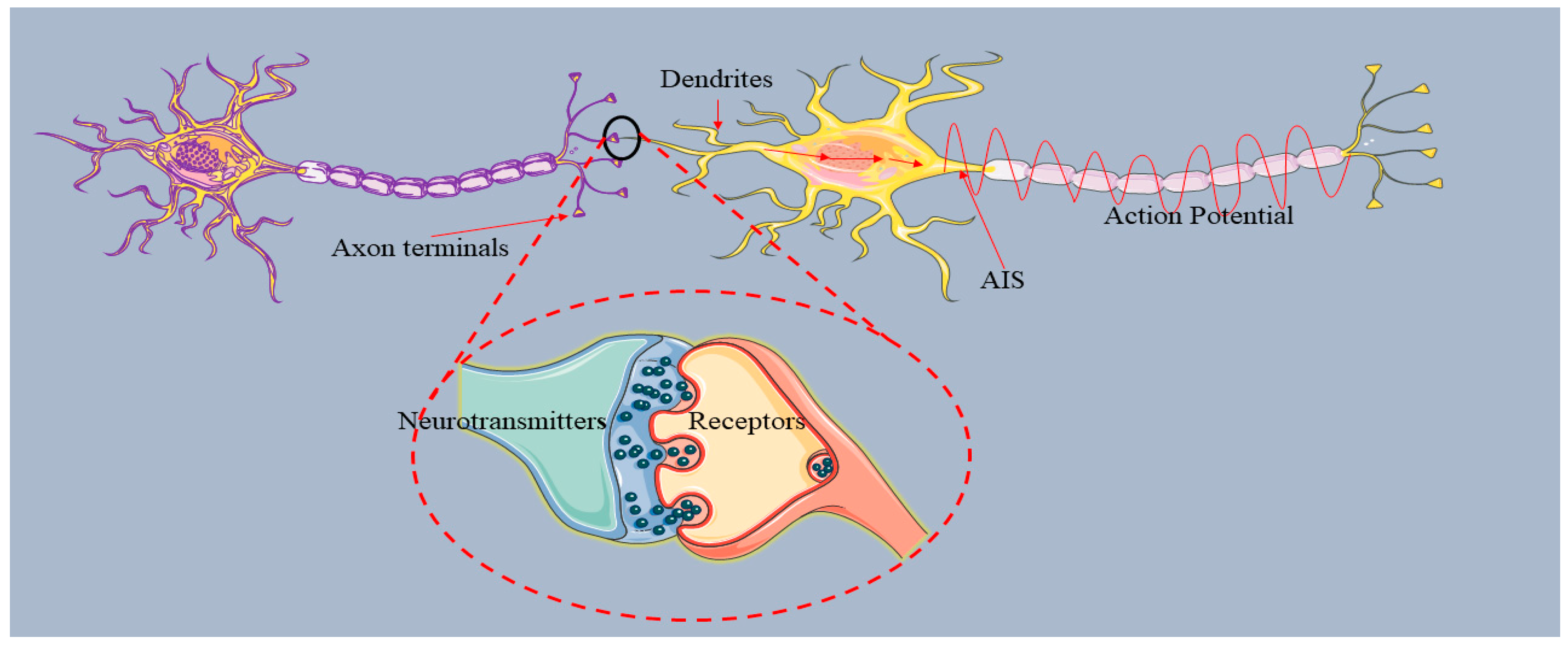
Figure 2.
Schematic representation of information transmission between two neurons.The dendrites of a neuron receive synaptic inputs in the form of chemical signals known as the neurotransmitters (dopamine, acetylcholine, serotonin, glutamate and aspartate) through the axon terminals of other neurons (Koch, 1999). The receptors at dendrites receive the neurotransmitters, which open ligand- gated ion channels, especially calcium channels, in order to convert the chemical signals into electric signals; and the signals are transmitted to the axon for further processing. Axon terminals process final transmission, and the signals again release to the other neurons in the form of neurotransmitters (Bear et al., 2016). The transmission of information among neurons is accomplished through action potentials (AP) (Leterrier et al., 2015). The AP is initiated at the initial part of the axon called AIS (Kole and Stuart, 2012). This figure is created using Servier Medical Art (
http://smart.servier.com).
Figure 2.
Schematic representation of information transmission between two neurons.The dendrites of a neuron receive synaptic inputs in the form of chemical signals known as the neurotransmitters (dopamine, acetylcholine, serotonin, glutamate and aspartate) through the axon terminals of other neurons (Koch, 1999). The receptors at dendrites receive the neurotransmitters, which open ligand- gated ion channels, especially calcium channels, in order to convert the chemical signals into electric signals; and the signals are transmitted to the axon for further processing. Axon terminals process final transmission, and the signals again release to the other neurons in the form of neurotransmitters (Bear et al., 2016). The transmission of information among neurons is accomplished through action potentials (AP) (Leterrier et al., 2015). The AP is initiated at the initial part of the axon called AIS (Kole and Stuart, 2012). This figure is created using Servier Medical Art (
http://smart.servier.com).
The flow of information from the dendrites to the axon terminal through the axon involves various neurological processes and these processes are facilitated through neuronal polarity (Leterrier et al., 2015). Neuronal polarity refers to the compartmentalization of the neurons, which is based on the morphology and functions of the neuron. Proper molecular arrangements in neurons are responsible for the neuronal polarity and the directional flow of information. Transmission of information between neurons, is accomplished through action potentials (AP). An AP is defined as the change in membrane potential due to the efflux and influx of cations (Sodium and Potassium ions) (Fig 3). The AP is initiated at the initial part of the axon, AIS (Kole and Stuart, 2012). AIS, having a complex structure containing various proteins with different functions (Jones and Svitkina, 2016, Li et al., 2005), acts to distinguish between the somato-dendritic and axon compartments (Fig.1). The main proteins in AIS are voltage-gated sodium channels (Nav), voltage-gated potassium channels (Kv), microtubules (MTs), casein kinase 2 (CK2) and ankyrin-G (AnkG). The maintenance of neuronal polarity requires the correct assembly of the proteins present in the AIS. Alterations in neuronal polarity due to mutation or injury to AIS-related proteins can cause various neuro-generative diseases, including Alzheimer’s disease (AD), epilepsy, bipolar disorder (William and Laura, 2013).
The laboratory studies show the evidences for involvement of AIS dysfunction in various neurological diseases. Study conducted using AD transgenic mouse showed low levels of AnkG as compared to wild type mouse (Sun et al., 2014). Additionally, in AD transgenic mouse alteration in protein filtering capability of AIS was noticed (Sun et al., 2014). Filtering mechanism of AIS is essential for the proper localization of protein passing AIS region (Song et al., 2009). In another study using mice with seizures, difference in AIS position was found in comparison with control mice, and the authors concluded that dislocation of AIS could increase the AP initiation threshold (Harty et al., 2013). Strong association of AnkG with bipolar disorder has also been found according to the genome wide association studies (Rueckert et al., 2012). Detailed association of AIS with other neurological diseases are mentioned in section 4 especially, role of AIS in AD pathogenesis.
Given the importance of AIS region in the neuron for proper functionality of the brain, there is a lack of pertinent review of mechanisms behind AP associated with AIS structure. To understand the complex behaviour of this often-overlooked aspect of the brain, mathematical models may play a crucial role. Our object of this paper is to explain in some detail the generation of AP within the context of the players (proteins) in this nano-scale segment in the neuron. Understanding of the plausible interactions and mechanisms that are at play in AIS may shed light to help understand the onset of some of the diseases, especially AD, as previously discussed. In this paper, we focus on the mechanism behind AP and structure of the AP initiation site; AIS. Discussion on AIS architecture contains the structure and functions of key proteins and their role in the AP initiation and AIS assembly. We also discuss the neurological diseases related to the mutation in AIS proteins and particularly the role of AIS proteins in AD pathogenesis based on the experimental evidences. Addition to that in this review we discuss the importance of mathematical modelling with existing models explaining the AP process.
2. History and Mechanism Behind AP
The history of AP is fascinating. Luigi Galvani (1786) became the first researcher to introduce the relationship between electricity and movement in living organisms (DuBois, 2010). He noticed movements in frog legs after using an electric scalpel. His associate, Alessandro Volta, continued his work and invented the galvanic cell. Another scientist, Carlo Matteucci, also conducted the experiments on frog muscles by considering them to be electric conductors and, on the basis of his work, German physician Emil du Bois-Reymond discovered AP (DuBois, 2010). Moreover, the results from AP studies in frogs influenced the scientists to conduct experiments in plants and, in 1873, Sir John Sanderson, encouraged by Charles Darwin, recorded the first AP in insectivorous plants (DuBois, 2010). The voltage clamp techniques developed by Cole and Marmont (1949) helped in understanding APs. The results collected from voltage clamp techniques were used by Hodgkin and Huxley (1952) for the development of a mathematical model explaining AP generation in the squid giant axon. Moreover, the upgrades in patch clamping techniques developed by Neher and Sackman, in 1976, assisted in the proper understanding of currents passing through single ion channels (DuBois, 2010). The AIS region acts as an AP initiator zone due to its molecular organization and, especially, the presence voltage gated ion channels (Na v and Kv channels) with high density (Coombs et al., 1957).
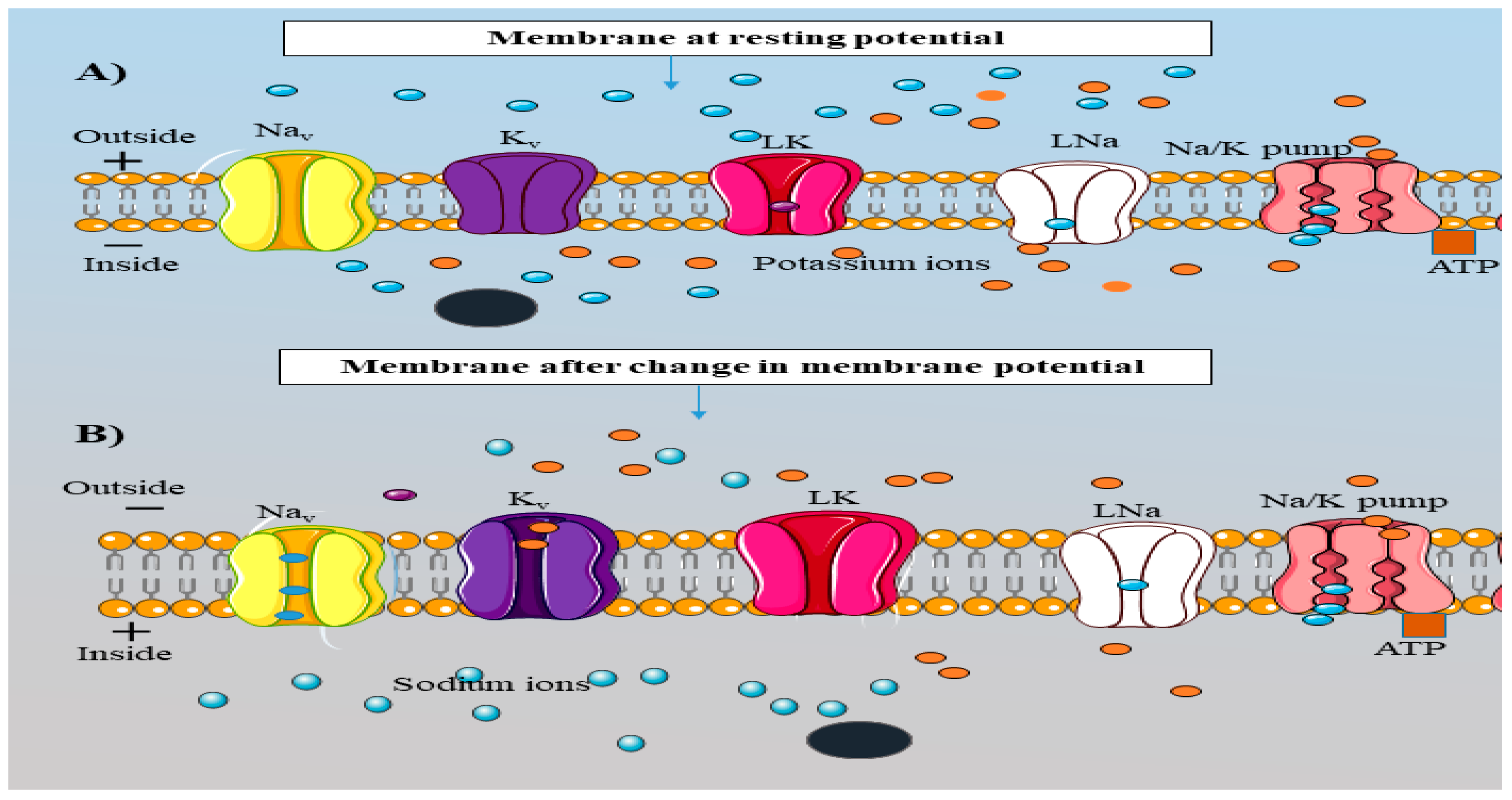
Figure 3.
Schematic representation of change neuronal membrane potential due to AP.The AIS membrane comprises of voltage gated ion channels (Nav, Kv), Na+/K+ pumps and leak channels (LK and LNa). (A) represents the membrane in the resting state where the influx and efflux of cations (Na+ and K+ ions) are almost zero. At the resting state, the Nav and K+ channels remain shut. The membrane voltage in the resting state in most neurons is approximately -70 mV. During the resting state, the net charge inside the membrane is negative, and positive outside the membrane. The inside membrane has a higher K+ ion concentration than Na+ ions. However, the outside membrane contains higher Na+ ions concentration than K+ ions. (B) demonstrates the disturbance in the movement of ions due to stimuli (electric current, light, pressure (mechanical and osmotic)). Stimuli introduce conformational changes into the ion channels resulting in the opening of the Nav and Kv channels. As the Nav channel opens, an influx of Na+ ions occurs and the concentration of Na+ ions inside the membrane increases. This change in the ionic concentration inside the membrane moves the inside voltage towards a positive value (-70 mV to +40 mV) and the outside voltage becomes negative; this is known as depolarization phase. When the Kv channel opens an efflux of K+ ions takes place with the voltage inside the membrane moving again towards its resting value (+40 mV to -70 mV); this is called repolarization phase. Due to the slower movement of Kv, ions membrane potential reaches beyond -70 mV. To generate another AP, the membrane potential should reach a resting value (-70 mV) and this step is accomplished by a Na+/K+ pump. The Na+/K+ pump takes three Na+ ions from inside to outside of the membrane with two K+ ions from outside to inside of the membrane. The black sphere in the figure represents the other proteins present inside the membrane. They cannot pass through due to the absence of a structure similar to ion channels and their larger size as compared to the ions. This figure is created using Servier Medical Art (
http://smart.servier.com).
Figure 3.
Schematic representation of change neuronal membrane potential due to AP.The AIS membrane comprises of voltage gated ion channels (Nav, Kv), Na+/K+ pumps and leak channels (LK and LNa). (A) represents the membrane in the resting state where the influx and efflux of cations (Na+ and K+ ions) are almost zero. At the resting state, the Nav and K+ channels remain shut. The membrane voltage in the resting state in most neurons is approximately -70 mV. During the resting state, the net charge inside the membrane is negative, and positive outside the membrane. The inside membrane has a higher K+ ion concentration than Na+ ions. However, the outside membrane contains higher Na+ ions concentration than K+ ions. (B) demonstrates the disturbance in the movement of ions due to stimuli (electric current, light, pressure (mechanical and osmotic)). Stimuli introduce conformational changes into the ion channels resulting in the opening of the Nav and Kv channels. As the Nav channel opens, an influx of Na+ ions occurs and the concentration of Na+ ions inside the membrane increases. This change in the ionic concentration inside the membrane moves the inside voltage towards a positive value (-70 mV to +40 mV) and the outside voltage becomes negative; this is known as depolarization phase. When the Kv channel opens an efflux of K+ ions takes place with the voltage inside the membrane moving again towards its resting value (+40 mV to -70 mV); this is called repolarization phase. Due to the slower movement of Kv, ions membrane potential reaches beyond -70 mV. To generate another AP, the membrane potential should reach a resting value (-70 mV) and this step is accomplished by a Na+/K+ pump. The Na+/K+ pump takes three Na+ ions from inside to outside of the membrane with two K+ ions from outside to inside of the membrane. The black sphere in the figure represents the other proteins present inside the membrane. They cannot pass through due to the absence of a structure similar to ion channels and their larger size as compared to the ions. This figure is created using Servier Medical Art (
http://smart.servier.com).
The AIS region is heavily populated with all the key players associated with AP, such as Nav, Kv, Na+/K+ pumps and leak channels (Leterrier et al., 2015). Proper functioning of all these proteins is necessary for the independent steps involved in AP (Fig.3). AP is a series of events occurring within the neurons such as depolarization, repolarization and hyper-polarization. All the events are highly depends on the movement of cations (Sodium and Potassium ions) between the external and internal membrane. Depolarization is defined as the change in the inside membrane voltage from negative to positive due to the entry of Na+ ions from outside (Fig.3) and repolarization is the change of the membrane voltage from positive to negative after the efflux of the K+ ions from internal membrane (Fig 3). Hyperpolarization is the phase where the membrane is highly negatively charged (beyond its resting value) due to slow kinetics of Kv channels (Platkiewicz and Brette, 2010, Schmidt-hieber et al., 2008). Nav channels contribute to the depolarization phase, and the repolarization phase depends on the Kv channels. Moreover, the hyperpolarization phase is regulated by Na+/K+ pumps where three Na+ ions are taken out and two K+ ions are brought inside of the membrane (Fig.3) (Platkiewicz and Brette, 2010). Nav and Kv channels work as a passive transporters because the movement of ions takes place from a higher to a lower gradient. However, the Na+/K+ pump relies on active transport and uses adenosine tri-phosphate (ATP) as an energy source (Fig.3).
3. Structural Organization of AIS
The AIS is a non-myelinated region found at the beginning of the axon of a neuron and the size of this region varies between 10 to 60 µm in length (Jones and Svitkina, 2016). Every individual protein within the structure of AIS are necessary for the correct organization and maintenance of the AIS structural assembly. This region is important for maintaining the neuronal polarity (structure, shape and functions) by acting as a diffusion barrier and cytoplasmic selective filter (Jones and Svitkina, 2016, Leterrier et al., 2015, Rasband, 2010). The barrier and filtering capability of the AIS region restricts the entry of dendritic proteins into this region and prevents the transformation of AIS into dendrites. The AIS structure comprises three membranes: plasma, spectrin-actin and microtubule-related (Jones and Svitkina, 2016) (Fig.4). Together, these membranes form the AIS structure, which contains different types of proteins that are essential for the proper functioning of this region. The detailed architecture of the AIS region is explained in Fig (4).
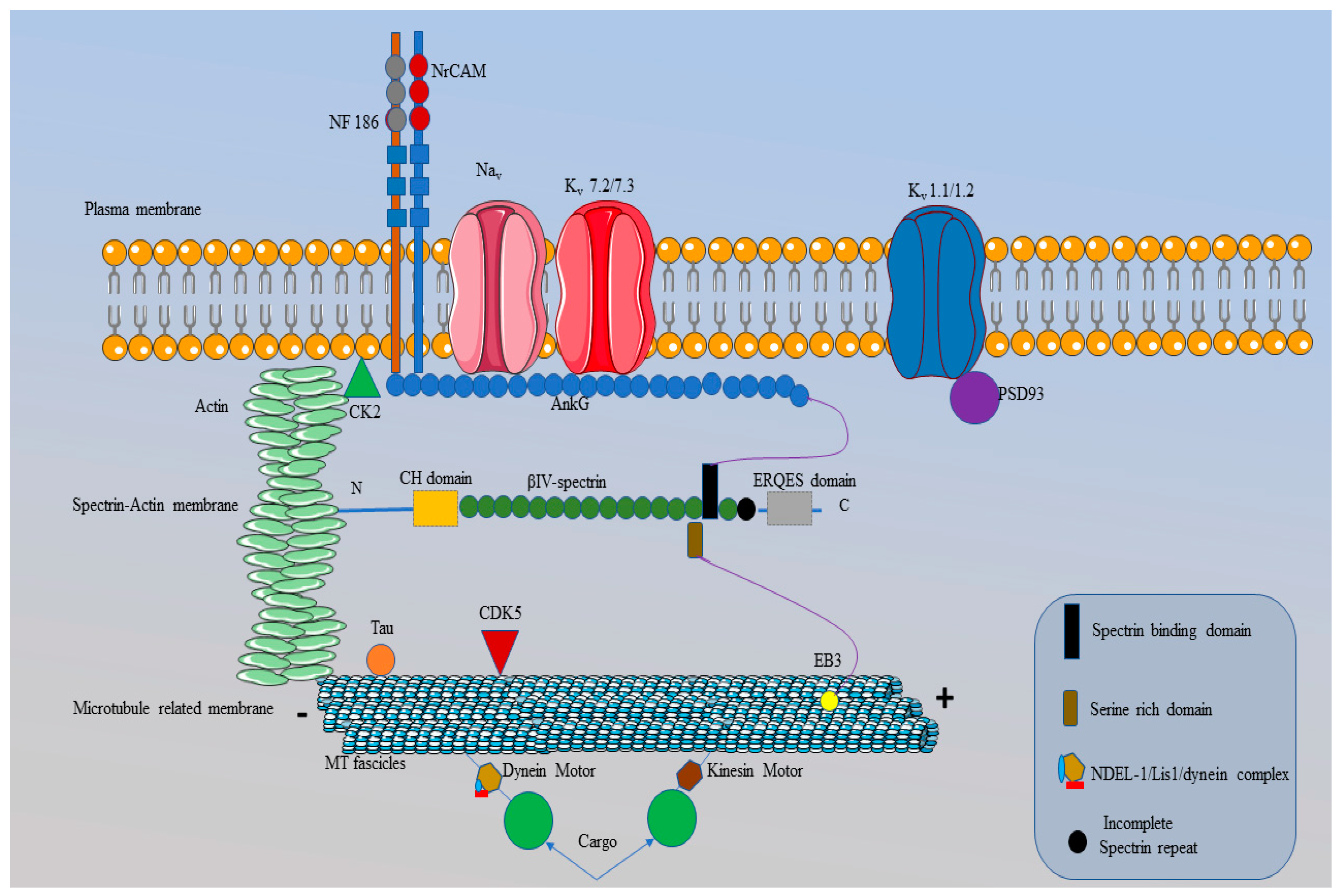
Figure 4.
Intracellular structure of the AIS region. .The plasma membrane of the AIS comprises ions channels (Nav and K¬v channels) and cell adhesion molecules (CAMs), such as NF186 and NrCAM that are restricted by AnkG. The initial part of AnkG (the membrane binding domain (MBD)) is specific for anchoring the Nav, and K¬v channels (Kv 7.2/7.3) and CAMs at the plasma membrane. Proteins, binding with AnkG have a specific amino acid sequence called AnkG binding motifs (ABM), which are absent from Kv 1.1/1.2 and the reason for their binding with PSD 93 (purple circle). CK2 also presents at the plasma membrane and this enhances the interaction between AnkG and the Nav channels. The spectrin-actin membrane present beneath the plasma membrane contains actin and β-IV spectrin and they also restrict entry into the AIS structure through interaction with AnkG. After the MBD, the presence of a spectrin binding domain (black rectangle) in AnkG is responsible for the binding between β-IV spectrin and AnkG. Following the second membrane, AIS contains a microtubule-related membrane that comprises bundles of microtubules known as microtubule fascicles. AnkG also restricts microtubule fascicles due to interactions with an EB3 protein (yellow circle). Moreover, microtubule fascicles contain tau proteins (orange circle) and dynein and kinesin motors. The kinesin and dynein motors are responsible for the transport of proteins (big green circles) from one end of the AIS region to the other while the microtubule fascicles act as the road for these molecular motors. Moreover, CDK5 (red triangle) in the microtubule membrane is responsible for the phosphorylation of NDEL-1, a protein essential for binding with the dynein motor. This figure is created using Servier Medical Art (
https://smart.servier.com).
Figure 4.
Intracellular structure of the AIS region. .The plasma membrane of the AIS comprises ions channels (Nav and K¬v channels) and cell adhesion molecules (CAMs), such as NF186 and NrCAM that are restricted by AnkG. The initial part of AnkG (the membrane binding domain (MBD)) is specific for anchoring the Nav, and K¬v channels (Kv 7.2/7.3) and CAMs at the plasma membrane. Proteins, binding with AnkG have a specific amino acid sequence called AnkG binding motifs (ABM), which are absent from Kv 1.1/1.2 and the reason for their binding with PSD 93 (purple circle). CK2 also presents at the plasma membrane and this enhances the interaction between AnkG and the Nav channels. The spectrin-actin membrane present beneath the plasma membrane contains actin and β-IV spectrin and they also restrict entry into the AIS structure through interaction with AnkG. After the MBD, the presence of a spectrin binding domain (black rectangle) in AnkG is responsible for the binding between β-IV spectrin and AnkG. Following the second membrane, AIS contains a microtubule-related membrane that comprises bundles of microtubules known as microtubule fascicles. AnkG also restricts microtubule fascicles due to interactions with an EB3 protein (yellow circle). Moreover, microtubule fascicles contain tau proteins (orange circle) and dynein and kinesin motors. The kinesin and dynein motors are responsible for the transport of proteins (big green circles) from one end of the AIS region to the other while the microtubule fascicles act as the road for these molecular motors. Moreover, CDK5 (red triangle) in the microtubule membrane is responsible for the phosphorylation of NDEL-1, a protein essential for binding with the dynein motor. This figure is created using Servier Medical Art (
https://smart.servier.com).
3.1. Plasma Membrane
The plasma membrane is the top-most layer of the AIS structure and contains voltage gated ion channels, such as Na v and Kv channels and also cell adhesion molecules (CAMs).
3.1.1. Nav Channels
Nav channels are highly enriched in the plasma membrane of the AIS region (Baranauskas et al., 2013, Katsuki et al., 2009) (Fig 4). Major isoforms of Nav channels are Nav 1.1, 1.2, 1.3 and 1.6 (Akin et al., 2016, Jones and Svitkina, 2016). Out of the four isoforms, all are found in the structure of AIS, except for Nav 1.3. In the early developmental stage, Nav 1.2 is present in the AIS but is later replaced by Nav 1.6 in adult central nervous system (Ogawa and Rasband, 2008). The mechanism involved in this replacement is still unknown. Nav 1.1 and Nav 1.6 are present in the proximal (beginning) and distal (end) parts of the AIS region, respectively (Ogawa and Rasband, 2008, Yoshimura and Rasband, 2014). The proper functioning of the Nav channel depends on its internal structural organization.
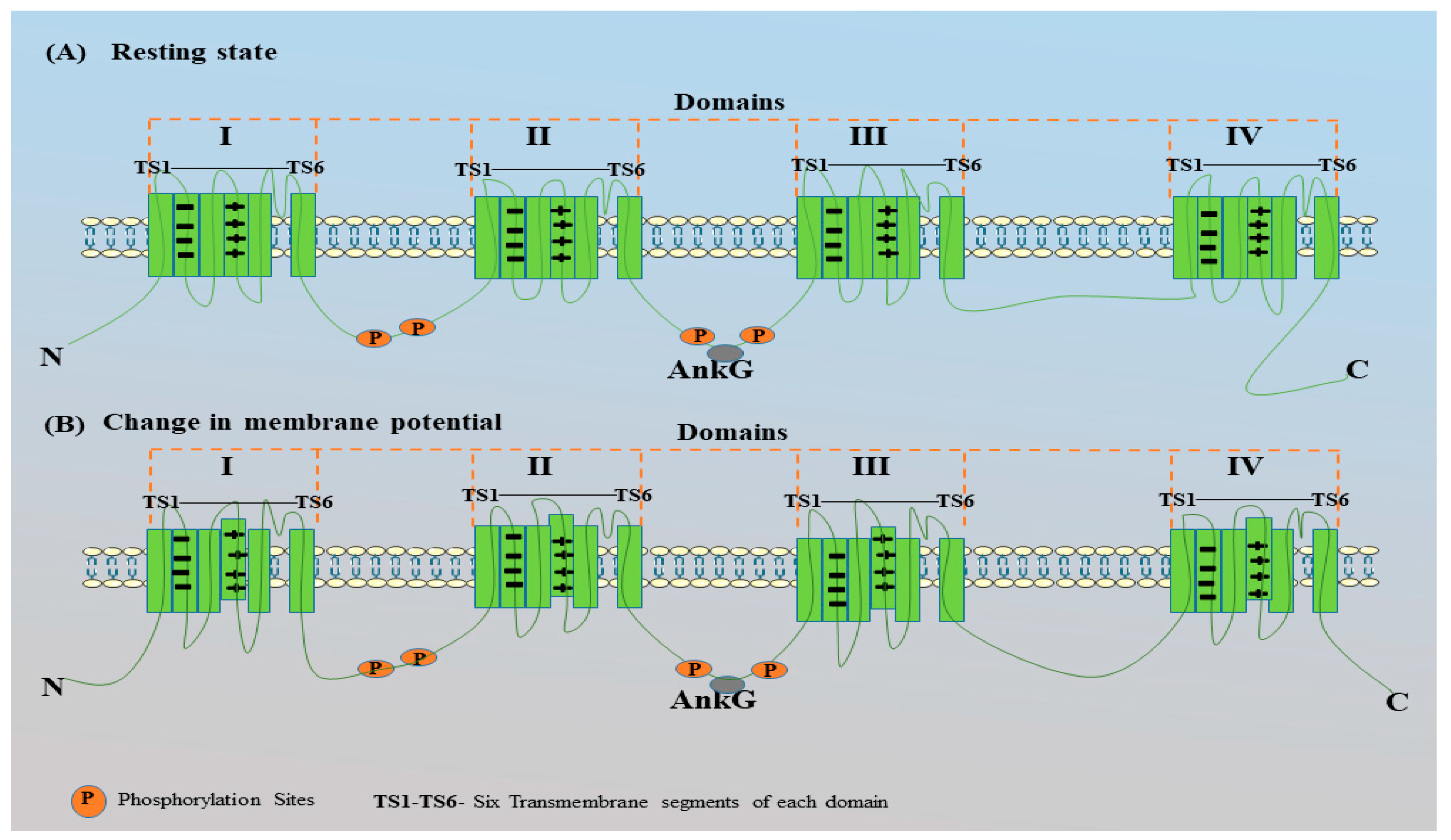
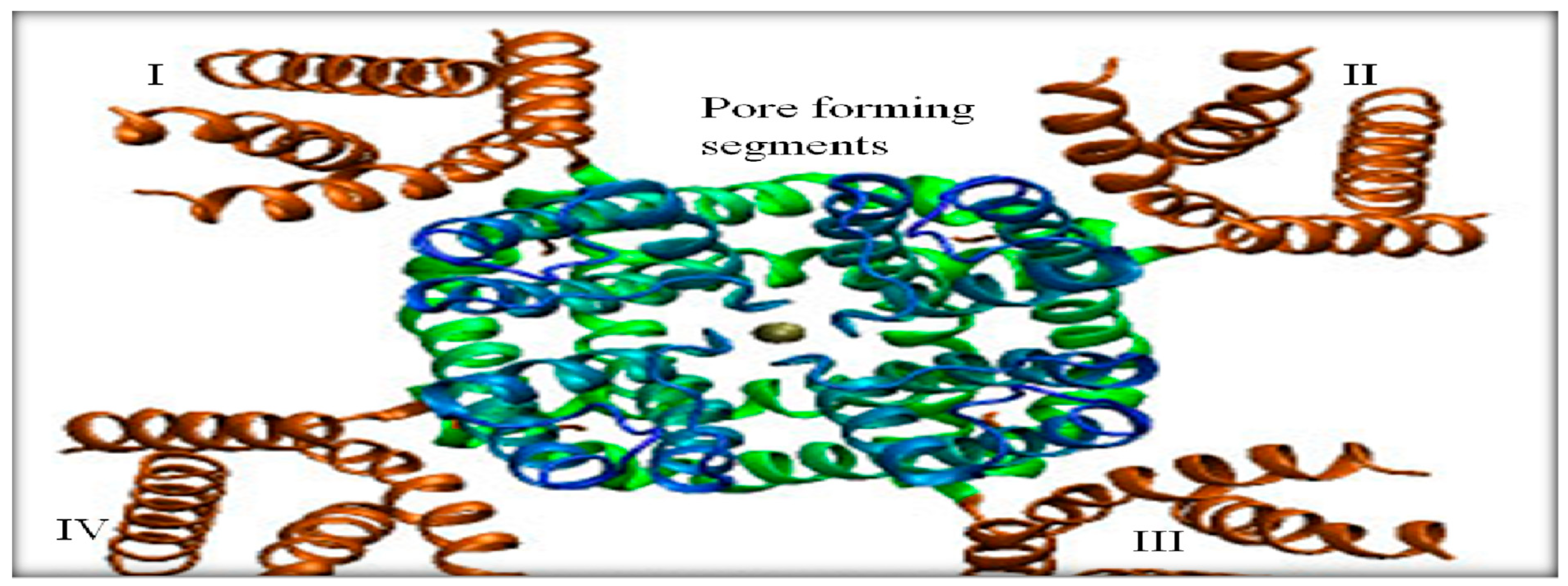
The Nav channel has four domains (I-IV) and every domain has six transmembrane segments (TS1-TS6) (Jones and Svitkina, 2016) (Fig 5, 6). TS1- TS4 of all the four domains are sensitive towards voltage and are known as voltage sensing segments. However, the TS5-TS6 segments of all domains are known as pore forming segments and are responsible for the entry of Na+ ions. The presence of the Nav channel in the AIS region is highly dependents on AnkG and CK2. Clustering of the Nav channels in the AIS is carried out by binding with AnkG. The Nav channel has an ankyrin-binding motif (ABM) present between domains II and III that binds to the membrane-binding domain (MBD) of AnkG (Boiko et al., 2003, Leterrier and Dargent 2014, Xu and Cooper, 2015, Zhou et al., 1998) (Fig.6).
CK2 facilitates the phosphorylation of Nav channels to increase their affinity towards AnkG before binding (Rasband, 2008, Yamada and Kuba, 2016). Phosphorylation of various sites present in the AIS motif of Nav channels modulates the channel and its subtypes (Schafer et al., 2009). Experimental records have shown the presence of 61 phosphorylation sites in Nav 1.2, 28 in Nav 1.1, and 13 in Nav 1.6 (Baek et al., 2014, Fache et al., 2004). Nav channels have four potential phosphorylation sites within its AIS motif, named as serine at positions 1112, 1123, 1124 and 1126. Mutational studies on these four serine sites in Nav channels showed a massive reduction in Nav channel concentrations in AIS. However, a single mutation by replacing each serine with alanine one by one did not alter Nav channel trafficking (Bréchet et al., 2008, Fache et al., 2004). In contrast, double and triple serine mutations have shown a large reduction in Nav channel concentrations in the structure of AIS. Out of four serine sites, any single serine mutation, together with a glutamate (E1111) mutation, impairs the segregation of Nav channels in the AIS structure (Bréchet et al., 2008, Fache et al., 2004). Moreover, the E1111 residue is also critical for Nav channels trafficking towards AIS (Bréchet et al., 2008). E1111 in the AIS motif is not a phosphorylation site for CK2 but still plays a significant part in Nav channel trafficking. This may be because of the acidic nature of E1111, which enhances phosphorylation near serine or threonine (Bréchet et al., 2008, Fache et al., 2004).
Figure 5.
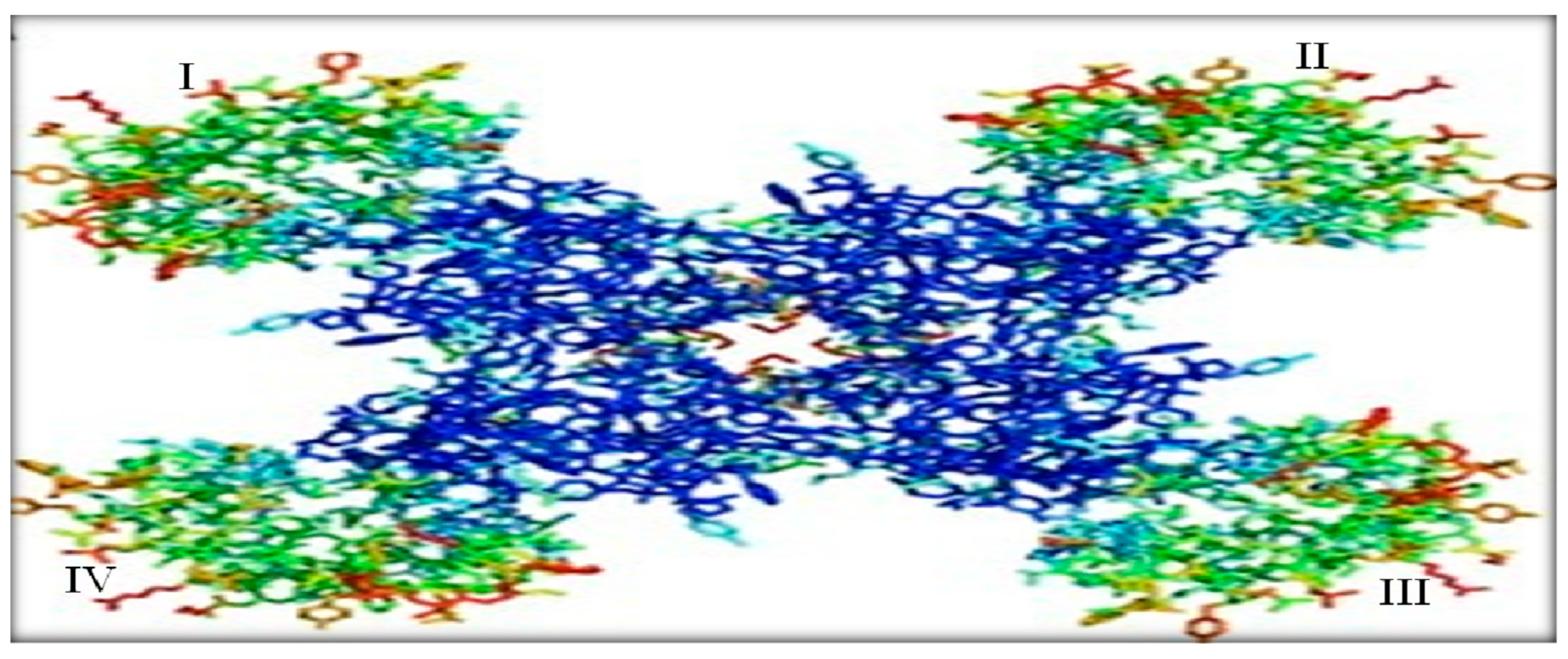
Crystallographic structure of a Nav channel. The green region at the corners represents the transmembrane segments (TS1-TS4) of the four domains (I-IV) and the blue region refers to the pore-forming segments formed by TS5-TS6 of each domain. In the middle, the gap shows the path for the influx and efflux of Na+ ions during opening and closing of Nav channels due to changes in the membrane potential. Image retrieved from Catterall ,(2010).
Figure 5.
Crystallographic structure of a Nav channel. The green region at the corners represents the transmembrane segments (TS1-TS4) of the four domains (I-IV) and the blue region refers to the pore-forming segments formed by TS5-TS6 of each domain. In the middle, the gap shows the path for the influx and efflux of Na+ ions during opening and closing of Nav channels due to changes in the membrane potential. Image retrieved from Catterall ,(2010).
To understand the accumulation of Nav channels into the AIS region, Fache et al. (2004) conducted an experiment using chimeric CD4 containing a II-III linker of Nav 1.2. In this experiment chimeric CD4 is supposed to mimic the function of Nav 1.2 channels due to the presence of the II-III linker of Nav 1.2 in chimeric CD4. When chimeric CD4 was then transfected into laboratory-extracted neurons the results clearly showed that chimeric CD4 was restricted to the AIS region. This restriction was due to the interaction of chimeric CD4 with the MBD of AnkG (Fache et al., 2004).
Similar to the AIS region, Nav channels are also present in the soma region of neurons. However, the density of Nav channels in this region are different and more extensive than those in the soma region (Rasband, 2010). The high number of Nav channels in the AIS region is supported by Kole et al. (2008), and their experimental results suggest that the density of Nav channels in this region is higher by 50-fold than that in the soma. They also conclude that AIS requires a minimum Nav channel density of 2500 Psµm-2 for AP initiation and backpropagation (Kole et al., 2008). The high density of Nav channels in the structure of the AIS supports the AIS skeleton to overcome the load induced during AP initiation. Moreover, due to their high density, Nav channels in the AIS activate and deactivate much faster than the Na¬v ¬channels present in the soma. Due to the high density of Nav channels in the AIS region, the membrane potential changes at a lower voltage as compared to the soma. The density of Nav channels in the AIS region can be influenced by other proteins present, such as the fibroblast growth factor 14 (FGF14) and subunits of Nav channels (Ogawa and Rasband 2008, Yoshimura and Rasband ,2014) .
Mutations of FGF14 can result in the loss of Nav channel density in the AIS. According to in vitro studies, Nav channel mutations result in a reduction in the frequency of Nav channels and the Na+ current in the AIS region (Xu and Shrager 2005). Therefore, reductions in Nav channels can be responsible for the high voltage requirement to initiate an AP. Overall, Nav channels in the AIS region are the key players and any alteration can disturb the initiation of AP there due to the large voltage requirement. Mutations in the Nav channel can develop medical conditions in humans, such as cognitive impairment and poor coordination of hand, speech and eye movements, known as the spinocerebellar ataxia (poor coordination between hands, speech and eye movements (Ogawa and Rasband, 2008). Moreover, alteration in Nav channels may be responsible for the breakdown of AnkG when activated by Ca2+-dependent cysteine calpain and this process is known as the proteolysis (Kole and Stuart 2012). Proteolysis of AnkG can result in the disruption of the AIS structure due to the weak binding of proteins with AnkG.
Figure 6.
Schematic representation of Nav channels in resting state and during change in membrane potential.
Figure 6.
Schematic representation of Nav channels in resting state and during change in membrane potential.
The Nav channel comprises four domains (I-IV) (green) and each domain contains six transmembrane segments (TS1-TS6). Out of the six trans-membrane segments TS1 – TS4 are voltage sensing segments while TS5 and TS6 are pore forming segments. Na+ ions are transported through the TS5 and TS6 segments when the Nav channels are open. Various phosphorylation sites are present in Nav channels and they enhance the interaction of these channels with AnkG after phosphorylation by CK2. Binding the Nav channels with AnkG depends on the AIS motif between the II and III domains of the Nav channels. (A) represents the Nav channel at its resting state (no change in voltage across the membrane), where the TS4 of all four domains is stabilized inside this structure due to the ionic pair interactions between the positively charged residues (lysine or arginine) of TS4 and the negatively charged residues of TS2. (B) shows the outward movement of the TS4 segments in all domains. The movement of TS4 segments is due to changes in the membrane voltage. As voltage sensing segments (TS1-TS4), recognize the strong voltage input, the ionic pair between the TS4 and TS2 segments of all domains break and the TS4 segment moves outwards, which leads to conformational changes in the Nav channels. Conformational changes result in the movement of Na+ ions from outside the membrane to inside the membrane. The influx of Na+ ions results in a change in the membrane voltage from negative to positive; this is called the depolarization of the membrane. This figure is created using Servier Medical Art (
https://smart.servier.com)
3.1.2. Kv Channels
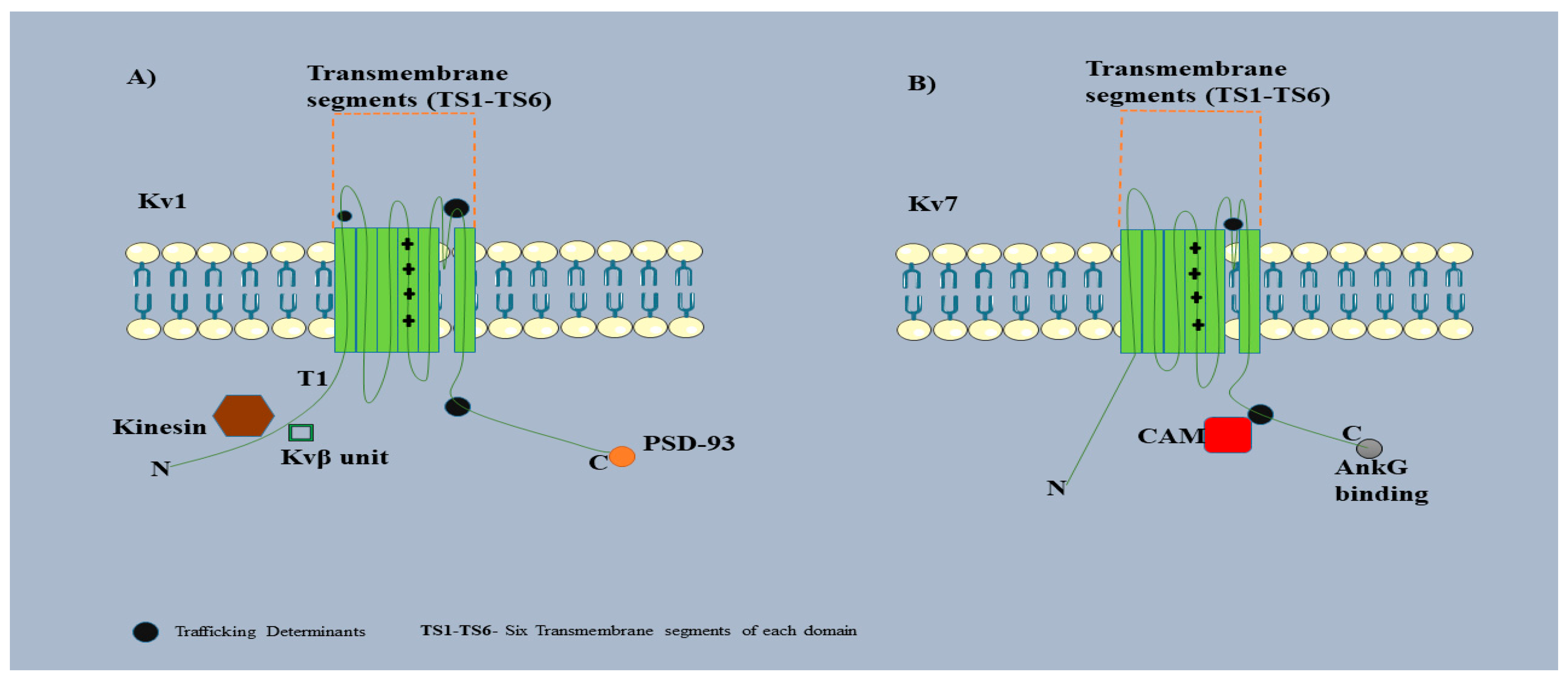
The Kv channel is another type of voltage gated ion channel present in the plasma membrane of AIS region (Fig.4). Kv channels are different in structure and formation as well as function compared to Nav channels. Kv channels contain only one domain with six transmembrane segments (TS1-TS6) (Fig 7, 8) (Papandréou et al., 2015). Out of the six segments, TS1 to TS4 are known as voltage sensory modules and the remaining two segments (TS5 and TS6) are pore-forming modules. Kv channels consist a tetramerization domain (T1) at the N-terminal and this is responsible for the binding of Kv β subunits and other proteins, such as kinesin motors. Kv β subunits increase the binding affinity of Kv channels towards the AIS. There are several isoforms of Kv channels, such as KCNQ2/3, Kv 1.1/1.2/1.4, and Kv 2.2, all of which are completely different from each other (Papandréou et al., 2015). Kv 1.1 and Kv 1.2 can be found at the end segment of the AIS region and share space with Nav 1.6 (Rasband, 2010).
The accumulation of different Kv channel subunits into the AIS region is different from each other. Kv 2/3 or Kv 7.2/7.3 is restricted in the AIS by AnkG because of the presence of ABM, similar to the Nav channels (Jones and Svitkina, 2016, Ogawa and Rasband, 2008). However, Kv 1.1 and 1.2 do not bind with AnkG due to the absence of ABM and are anchored into structure of AIS through postsynaptic density protein 93 (PSD93) or chapsin 11 (Leterrier and Dargent, 2014, Yoshimura and Rasband, 2014). PSD93 is a part of the PDZ domain family that contains the membrane-associated guanylated kinase family of scaffolding proteins (MAGUK) (Ogawa and Rasband, 2008). Kv channel trafficking in AIS is enhanced phosphorylation by cyclin dependent kinase 5 (CDK5).
Figure 7.
Crystallographic structure of the Kv channel.
Figure 7.
Crystallographic structure of the Kv channel.
The brown extensions at the corners represent the transmembrane segments (TS1-TS4) serving as voltage sensing modules. TS5-TS6 are known as pore forming segments (blue and green region). Due to the change in membrane potential, conformational changes occur within the Kv channel and this results in the opening of the channel (centre blue and green region). Opening of Kv channel helps in changing the membrane voltage from positive to negative due the efflux of K+ ions. Image retrieved from Schulten et al., (2006).
Figure 8.
Schematic representation of the Kv channel.
Figure 8.
Schematic representation of the Kv channel.
Both channels, Kv 1 (A) and Kv 7 (B), have almost similar structures of a single domain divided into six transmembrane segments (TS1-TS6). The TS4 segment comprises positive residues that act as a voltage sensing module and, together with TS1-TS3, T5 and T6 comprise the pore forming segments. Several amino acids in both types of Kv channels act as the trafficking determinants (TDs) (black circle) and are responsible for the maintenance, expression and trafficking of the Kv channels (Papandréou et al., 2015). The TDs at Kv channels also regulate the interaction of Kv channels with other proteins. The N-terminal of Kv 1 channels consists of a tetramerization domain (T1) and this serves as a binding site for the kinesin motors and β subunits of the Kv 1 channel. The C-terminal of the Kv 1 channel has a binding motif that is responsible for the restriction of the Kv 1 channel into the AIS region through PSD 93 (orange circle). Kv 7 channels have TDs (black circle) near the C-terminal and are responsible for the binding of CAMs. Tethering the Kv 7 channels into the AIS region depends on ABM, which is present at the end of the C-terminal. This figure is created using Servier Medical Art (
http://smart.servier.com)
In the AIS region, the Kv channels modulate AP firing as well as the shape, initiation and spike patterning by restricting neuronal excitability and repolarizing the membrane by allowing an efflux of K+ ions from the membrane. (Rowan et al., 2014, Sánchez-Ponce and DeFelipe ,2012). Mutation in any of the Kv channels can lead to alterations in these functions. Moreover, mutation in particular Kv channel subunits (KCNQ 2/3 or Kv 7.2/7.3 ) can cause epilepsy in new-born human babies (Jensen et al., 2017) due to a defect in Kv channel trafficking into the AIS region, which can reduce the size of the K+ current.
3.1.3. Cell Adhesion Molecules (CAMs)
CAMs of the L1 family are also enriched in the AIS region. Neurofascin 186 (NF186) and neuronal cell adhesion molecules (NrCAM) are examples of CAMs available in the plasma membrane of the AIS. Both NF186 and NrCAM have a AnkG binding motif and this motif helps in the interaction of CAMs with AnkG (Fig. 4)(Jones and Svitkina, 2016). Mutation of NF186 results in the inhibition of gephyrin, which weakens the GABAergic synapses by inhibiting the clustering of GABAA receptors (GABAAR). Overall, CAMs maintain the GABAergic synapse between the axon terminal and the AIS region, which is crucial for AP initiation. In-vivo studies reveal that NF-186 is important for stabilizing the AIS region, and the recruitment of NrCAM into this region (Kriebel et al., 2011, Zonta et al., 2011).
3.2. Spectrin-Actin Membrane
The second membrane of the AIS structure lies beneath the plasma membrane and is composed of βIV spectrin and actin (Fig 4). The main function of this membrane is to provide mechanical support to the AIS region (Zhang and Rasband, 2016).
3.2.1. Spectrin
The spectrin family of cytoskeleton proteins was first noticed in erythrocytes as a rod shape, and it is a tetramer with two units each of α and β subunits (Tse et al., 2001). It is antiparallel heterodimer made up of α and β subunits with a length of ~100 nm that interact in a head to head way resulting in the formation of a tetramer ~200 nm long (Goodman et al., 1995, Viel and Branton, 1996). In the erythrocyte membrane, the subunits of spectrin have 106 residues and they form a helix bundle (Berghs et al., 2000). The α subunit of spectrin has 20 repeats of spectrin and an SH3 domain at the ninth segment (Saka et al., 2014) . Two EF-hand motifs are present between the 20th spectrin repeat and the C-terminal (Berghs et al., 2000, Jones and Svitkina, 2016). The β subunit of spectrin has 17 spectrin repeats, actin-binding domain with protein 4.1, adducin and phosphatidyl-inositol 4, 5-biphosphate (PIP2) at the N-terminal and a pleckstrin homology domain (PH) towards the C-terminal (Saka et al., 2014). All β-spectrin binds with AnkG on spectrin repeat 15. In mammals, the five genes of β-spectrin are present but βIV-spectrin is found in the AIS region, which has been localized in the mouse at chromosome 19q13.13 ( Jones and Svitkina, 2016, Tse et al., 2001). After alternative splicing, the βIV-spectrin generates six isoforms, from βIV-spectrin Σ1 to Σ6. βIV-spectrin Σ1 is the longest isoform and has 36 exons and 2559 amino acids. In the brain, the size of βIV-spectrin Σ1 is ~9.0 kb and this has been detected on day 19 during development; the concentration of the βIV-spectrin Σ1 continues to increase until adulthood (Berghs et al., 2000). The βIV-spectrin Σ6 isoform does not have any actin-binding domain at the N-terminal, and a total of nine repeats of spectrin are present in the full size of 4.7 kb (Komada and Soriano, 2002). The recruitment of βIV spectrin is highly dependent on AnkG (Yang et al., 2007). The ERQES domain (a specific domain) with positively and negatively charged residues, is present after the 17th repeat and before the PH domain. This proline-rich domain is not present in other β spectrin isoforms and is highly charged because of the presence of glutamate, glutamine and arginine at 23.3%, 8.8%, and 26.6%, respectively (Komada and Soriano, 2002). The βIV-spectrin and AnkG play a crucial role in the correct localization of membrane proteins in the AIS region. According to various studies, the βIV-spectrin participates partly in the localization of AnkG and, together, they help the AIS region act as a barrier and decrease the concentration of somato-dendritic proteins, such as GluR1 and telecephalin, before they enter the AIS region (Nishimura et al., 2007). An optical tweezer experiment by Winckler et al.( 1999) showed that βIV-spectrin regulates the lateral mobility of L1 CAMs, and this function of βIV-spectrin indirectly depends on the interaction of L1 CAMs with AnkG . According to genetic studies on C. elegans, β-spectrin maintains the function of nerve axons to overcome the stress that occurs during movement, so a deficiency of β-spectrin can lead to paralysis (Baines, 2010).
3.2.2. Actin
This actin-based structure provides passage to axonal proteins and restricts the entry of dendritic proteins into the axon (Balasanyan et al., 2017) (Fig.4). In cultured cortical neurons, the existence of actin can be noticed at three days-in-vitro (DIV 3) by 58% and 100% by DIV 7 (Watanabe et al., 2012). The actin patches available at the axons are higher than in the dendrites and, in the AIS region, they are present 13 µm away from the cell body (Watanabe et al., 2012). SEM imaging reveals the structure of actin by showing the presence of actin filaments and synaptopodin. Synaptopodin is a protein, known as the actin-interacting regulatory protein, that has multiple binding sites for α-actinin (Sánchez-Ponce and Blázquez-Llorca, 2012). α-actinin comes from the family of actin-binding proteins that crosslinks and bundles actin filaments. α-actinin creates short branched packages of actin filaments (Sánchez-Ponce and Blázquez-Llorca 2012). All actin filaments are present towards the positive end of the cell body (Watanabe et al., 2012) and they result in the formation of a diffusion barrier and this prevents the entry of unnecessary protein into the axon.
Clustering of transmembrane proteins with the spectrin-actin based cytoskeleton via AnkG is essential for the creation of the diffusion barrier in AIS. The diffusion barrier develops when all the proteins are available in this region. According to the picket fence model, the formation of the diffusion barrier reduces the mobility of membrane proteins in the structure of AIS because of the crowding of the proteins. In contrast, Albrecht et al. (2016) conducted a study to propose a new model called the ‘actin fence model’. To test this model, single glycosylphosphatidylinositol-anchored GFP (GPI-GFP) trajectories were measured within the AIS of primary rat hippocampal neurons using high-density single-particle tracking (SPT). SPT analysis was undertaken at different time points. Reduction in the mobility of GPI-GFP in the AIS region was noticed after DIV 3. The outcomes of this study revealed that GPI-GFP was restricted in this region because of the actin cytoskeleton and there was also a reduction observed in the mobility of membrane proteins between adjacent actin rings (Albrecht et al., 2016). In contrast, Nakada et al. stated that the formation of barrier takes place at DIV 10; however, treatment with jasplakinolide (an actin promoting agent) enhanced the polymerization of f-actin resulting in the creation of a barrier at DIV 5 (Nakada et al., 2003).
3.3. Microtubule-Related Membrane
The microtubule-related membrane is essential for the transportation of various proteins that act as a pathway for the molecular transporters or molecular motors. Moreover, various post-translation modifications (PTMs), such as phosphorylation acetylation, also contribute to the correct functioning of the microtubules.
3.3.1. Microtubules (MTs)
MTs are hollow in shape with a 25 nm diameter (Hoogenraad and Bradke, 2009, Kapitein and Hoogenraad, 2011) (Fig 4,9). In the AIS region, MTs are present in bundles known as fascicles. Fascicle formation may occur because of the interaction of MTs with AnkG through an end binding protein (EB) (Jones and Svitkina, 2016). MT fascicles are collections of MTs that align parallel to each other and then cross-link into a bundle. There are three to seven fascicles in total, and the number of MTs present in fascicles range from 2-25 (Chung et al., 2016, Jones and Svitkina, 2016).
MTs consist of αβ-tubulin, tau, an end binding protein, spastin and motor proteins. αβ-tubulin, a heterodimer made up of straight protofilaments (Chung et al., 2016, Tapia et al., 2010). Tubulin dimers maintain the MTs dynamics. MTs requires the histone deacetylase 6 (HDAC6) enzyme to deacetylase tubulin and tau (Tsushima et al., 2015). HDAC6 is involved in axon development, and the inhibition of HDAC6 can cause the loss of AIS assembly (Tapia et al., 2010, Tsushima et al. 2015). Microtubule-associated proteins, such as tau, end binding protein (EB), spastin and motor proteins, maintain the dynamics of the MTs (Kapitein et al., 2010, Chung et al., 2016) . The EB protein is found in three forms EBs (1-3), and are known as plus-end tracking proteins. EB1 and EB3 have unique roles and play a part in axonal transport. EB3 acts as a link between AnkG and MT through the EB homology domain (Leterrier et al., 2011, Leterrier and Dargent, 2014). The properties of MTs are modified with the help of CK2. A number of experimental analyses have provided evidence showing that inhibition of CK2 increases the acetylation of tubulin by three times the rate and impairs the function of the MTs (Sanchez-Ponce et al., 2011). Post-translational modifications (PTMs) of tyrosination, detyrosination, polyglutamylation and polyglycylation, are examples of modifications that take place in MTs (Leterrier and Dargent, 2014). MAP dysfunction leads to various diseases, such as AD, amyotrophic lateral sclerosis, multiple sclerosis, and chronic traumatic encephalopathy, in athletes suffering concussion (Chung et al., 2016, Kapitein and Hoogenraad, 2011).The presence of MTs in the form of fascicles and their involvement in cargo transport make MTs a noteworthy member of the AIS region (Jones and Svitkina, 2016, Nakata and Hirokawa, 2003). MT also plays a vital role in structure of the AIS by maintaining its functions and assembly.
3.3.2. MTs Based Protein Transport
MTs participate in protein trafficking with the help the kinesin and the dynein superfamily (Gumy and Hoogenraad, 2018, Leterrier and Dargent, 2014, Xiao and Jan, 2009). An alteration in any of these motors results in damage to the assembly of AIS and forces the axons to acquire the molecular characteristics of dendrites (Sanchez-Ponce et al., 2011). The motor protein binds with the motors and moves along with MTs. Adenosine triphosphate (ATPs) is the energy source for the mobility of these motors (Encalada and Goldstein, 2014, Jenkins et al., 2012).
Figure 9.
Schematic representation of a microtubule in the AIS.
Figure 9.
Schematic representation of a microtubule in the AIS.
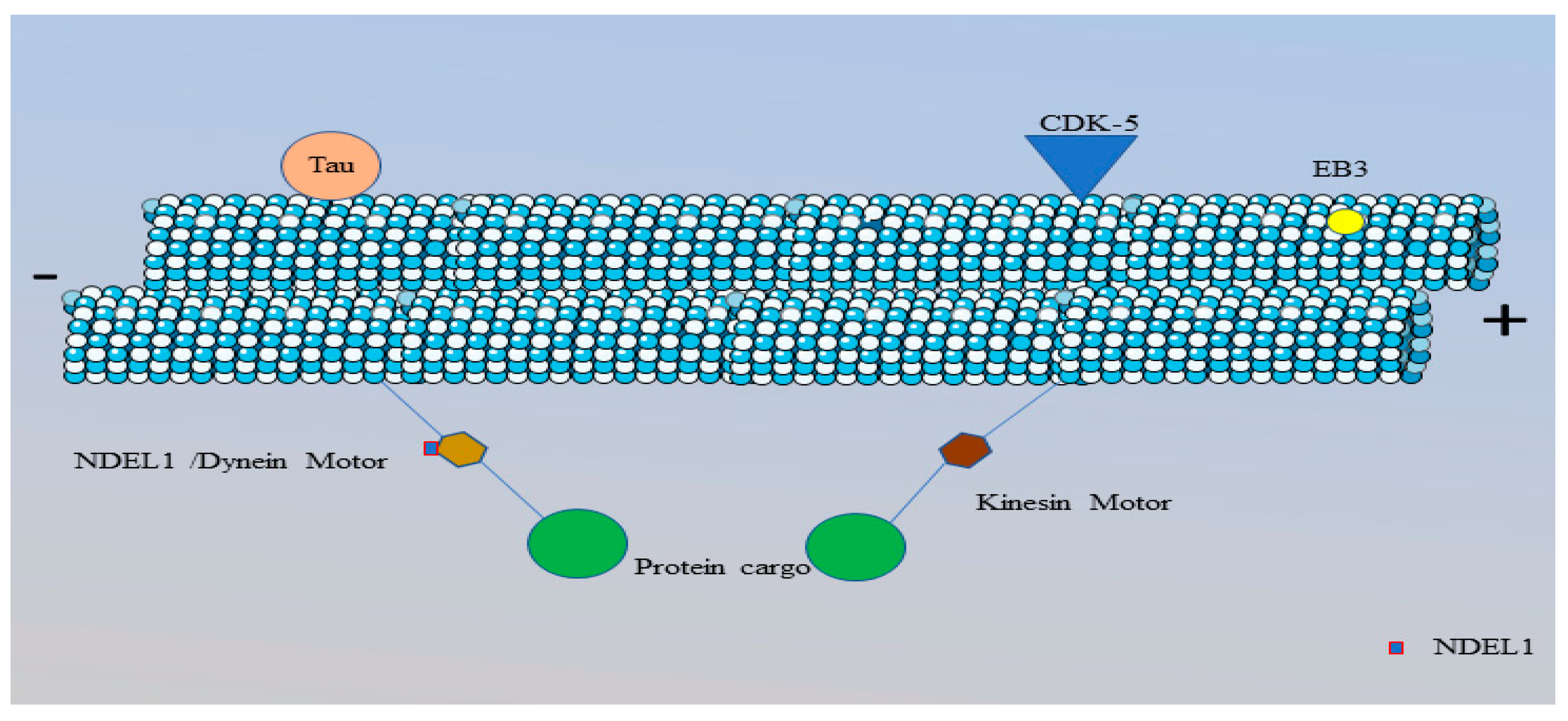
Microtubules are located in the AIS in the form of bundles called fascicles. Each fascicle contains 2-25 microtubules parallel to each other. Microtubules act as pathways for the transportation of proteins through the molecular motors, kinesin (brown hexagon) and dynein (gold hexagon). Kinesin motors move towards the plus end of the microtubule and dynein motors move towards the minus end. Transportation of protein cargo (green circle) towards the axon terminal and soma is accomplished by kinesin and dynein motors, respectively. The dynein motors are accompanied by NDEL1 (red outlined rectangle) that are responsible for the activation of dynein motors. A microtubule consists of tau (orange circle) and the hyper-phosphorylation of tau can disrupt the function of other proteins present in this region. The activity of the microtubules is maintained by CDK-5 kinase (blue triangle). Microtubule binding with AnkG relies on the EB3 protein (yellow circle) and this binding is important for the correct localization of the microtubules within the AIS region. This figure is created using Servier Medical Art (
http://smart.servier.com).
3.3.2.1. Kinesin Motors
Kinesin motors move along MTs towards their minus end (Leterrier and Dargent, 2014). The kinesin family has 45 types of kinesin proteins (Barry et al., 2014). Kinesin-1 is the main motor protein for axonal transport (Fig.9). It contains a heavy chain dimer at the N-terminal and two kinesin light chains at the C-terminal. KIF5A, KIF5B, KIF5C are three isoforms of kinesin-1 (Leterrier and Dargent, 2014). The N-terminal of kinesin has a stalk domain (responsible for dimerization through the coiled-coil region), and the C-terminal is a binding site for protein that needs to be transported (Barry et al., 2014). KIF5 is known for the transport of the vesicle associated membrane protein (VAMP2) and the amyloid precursor protein (APP) to the axon, and N-methyl-d-aspartate (NR2B) transport by, KIF17, to the dendrites (another protein from the kinesin family) (Xiao and Jan, 2009). Song et al (2009) conducted a number of experiments using chimeric KIF17, with a KIF5B tail, and chimeric KIF5B, with a KIF17 tail, to understand the presence cytoplasmic selective filter transport in AIS. Outcomes indicate that chimeric KIF 17 took VAMP2 as cargo, and chimeric KIF5B took NR2B as cargo; hence, the results showed no evidence of VAMP2 in the axons while, on the other hand, NR2B was found in both axons and dendrites. This indicates that KIF5B can transport cargoes in both axons and dendrites and the presence of a cytoplasmic filter in the AIS region has been found between DIV 3 and 5 (Song et al., 2009). Transport through kinesin motors is important for neuronal polarity because kinesin family proteins are responsible for the precise supply of the components in both axons and dendrites (Jenkins et al., 2012).
3.3.2.2. Dynein Motors
Another motor that also co-localizes with kinesin is called dynein, which moves along the MTs but towards the minus end (Kuijpers et al., 2016, Vallee et al., 2004) (Fig.9). The dynein motor is known for the transportation of Golgi bodies from the axons and the rerouting of soma-dendritic protein cargos to the soma. Activation of the dynein motor depends on the nuclear distribution element like 1 (NDEL1) (Egan et al., 2012, Klinman et al., 2017). The presence of CDK-5 was also noticed in the AIS region and this promotes the phosphorylation of NDEL1 (Klinman et al., 2017). The phosphorylated form of NDEL 1 binds with the dynein motor. Alterations in CDK-5 impair the formation NDEL-dynein binding; thus, affecting the assembly of the AIS structure to changes in protein transport (Kapitein et al., 2010, Klinman et al., 2017). NDEL1 enrichment in the AIS region is due to it binding with AnkG, while the subsequent depletion of NDEL1 results in the inactivation of the dynein motor and this allows the entry of dendritic proteins into the proximal axon. Binding of NDEL1 and AnkG occurs at the C-terminal of NDEL1. The activation of dynein forces the cargo containing dendritic proteins to move back to the soma. Strong binding between dynein motors and MTs prevent the exclusion of dynein motors from the AIS structure (Klinman et al., 2017).
3.4. Importance of AnkG in AIS
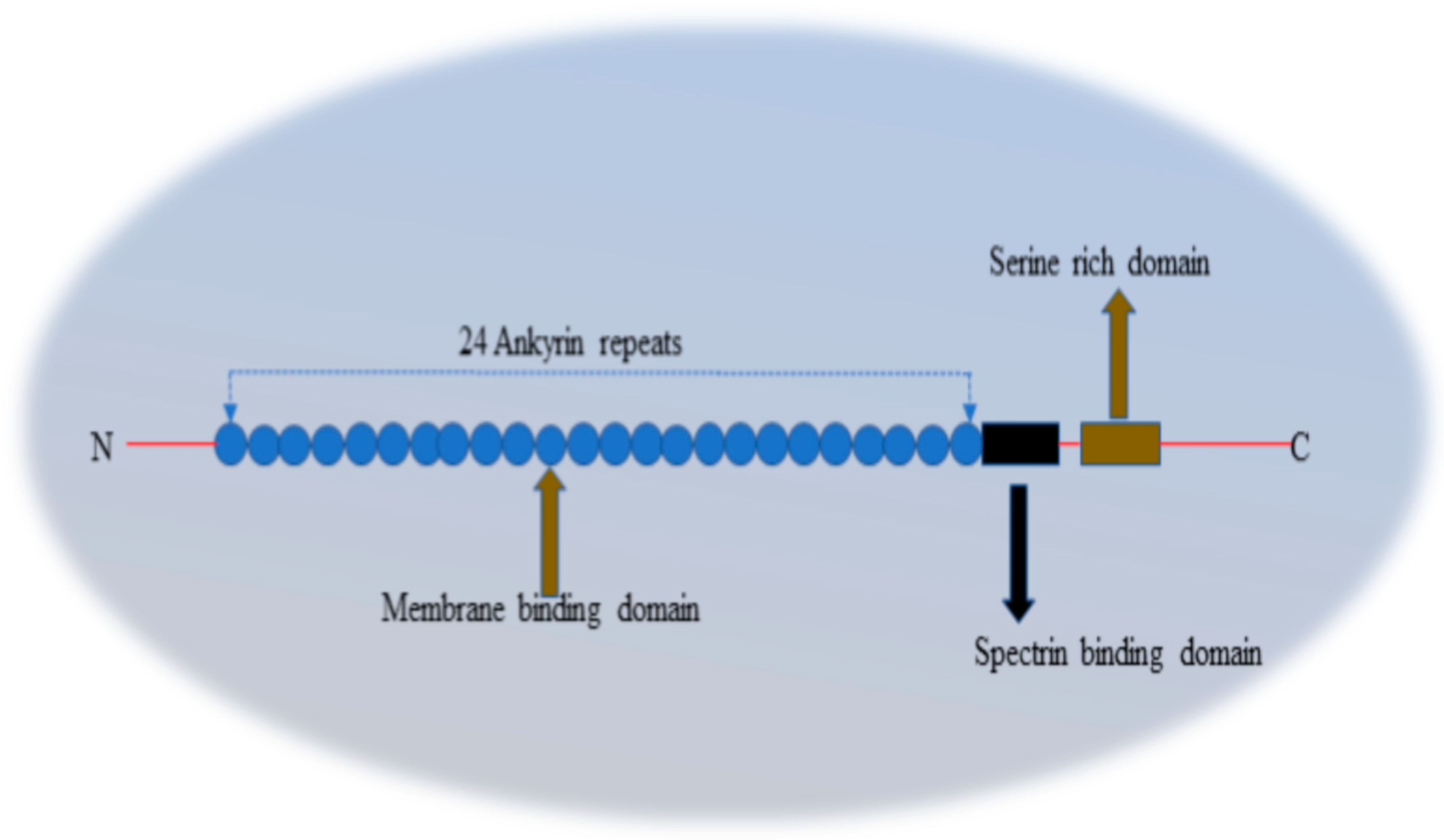
The ankyrins are a group of scaffolding proteins. In vertebrates, ankyrin is present in three isoforms: AnkR, AnkB, and AnkG, that have similar molecular structures (Leterrier and Dargent, 2014, Smith and Penzes, 2018). AnkR is known as the first isoform of ankyrin and is crucial for the maintenance of the shape and functioning of erythrocytes (Smith and Penzes, 2018). AnkB is present in tissues, including the brain, thymus, and heart and skeleton muscles. In the heart, AnkB plays a critical role in the organization of the transport and ion channels, as the correct localization of these components is necessary for correct Ca+ signalling and heart function. The third, and most important, ankyrin, AnkG, can be found in almost all tissues of the brain, epithelium, kidney, and muscles. In the brain, AnkG is available in three different isoform protein sizes, i.e., 190 kDa, 270 kDa and 480 kDa (Smith and Penzes, 2018). AnkG is crucial for AIS assembly and the maintenance of neuronal polarity (Cunha and Mohler, 2009). AnkG is the first protein that clusters at the proximal axon; however, the mechanism behind this is still unknown. The presence of AnkG in the proximal axon maybe due to teamwork between the multiple domains present at AnkG (He et al., 2012). AnkG is the chief organizer of the AIS region because the accumulation and expression of almost all AIS proteins depends on AnkG. AnkG consists of an MBD at the N-terminal made up of 24 ankyrin repeats, a spectrin-binding domain, a serine-rich domain (only in the 270 and) 480 kDa isoforms with a long tail and a C-terminal domain (Fig 10). The MBD of AnkG is responsible for the recruitment of ion channels (Nav, Kv), molecular motors and CAMs (NF186, NrCAM) into the AIS (Carolina, 1998, Cunha and Mohler, 2009, Hedstrom et al., 2008, Nelson and Jenkins, 2017, Zhou et al., 1998). The interaction of AnkG with the second membrane, the spectrin-actin membrane, is accomplished by the spectrin binding domain with the help of a ZU5 motif of 160 amino acids. Other motifs, DAR999 and S2417, are also essential for the interaction of spectrin with the small and large isoforms of AnkG (Smith and Penzes, 2018). A regulatory domain, also known as the death domain, is an unstructured domain with 300 amino acids, which regulates the interactions of all proteins with AnkG binding domains (Nelson and Jenkins, 2017).
One of the most important functions of AnkG is to maintain neuronal polarity. Neuronal polarity in the AIS region is maintained by the correct localization of AIS proteins and depends highly on AnkG. AnkG interacts with the ion channels, spectrin isoforms and actin, and stabilizes them within the structure (Smith and Penzes, 2018). The tight interaction of proteins with AnkG within the AIS region favours AP initiation. Injury or mutations can cause weak binding between AIS-related proteins and AnkG. The weaker binding of proteins with AnkG increases the probability of the removal of protein from the structure (Leterrier et al., 2010) Every membrane protein within this region is directly or indirectly associated with AnkG. AnkG and its binding members are known for targeting the GABAergic inter-neuronal presynaptic inputs that are necessary for controlling the AP firing frequency and the excitability of neurons in the brain region (Ango et al., 2004). GABAergic interneurons are composed of GABAAR clusters, and the organization of GABAergic interneurons is dependent on the binding of GABAAR with a scaffolding protein, called gephyrin, which is indirectly associated with NF186 (Smith and Penzes, 2018).
Any alteration in AnkG results in the disassembly of the AIS structure and disruption in neuronal polarity (Le Bras et al., 2014) (Fig 11). A mutation in AnkG has a direct impact on the expression of membrane proteins and their functions. The absence of AnkG can cause disturbances in the filtering or transport capability of the AIS region where unnecessary proteins or dendritic proteins enter the axon region. The entry of these dendritic proteins forces the axon to adapt dendritic molecular characteristics and this means the axon will perform the same functions as the dendrites. The loss of actin, spectrin, and CAMs by 90%, 90%, and 98%, respectively, has been noticed in AnkG mutated mice (Hedstrom et al., 2008). A study of purkinje cells with an AnkG mutation demonstrated the importance of AnkG in AIS by observing that they contained were no voltage gated ion channels (Nav and Kv channels) due to the absence of AnkG (Zhou et al., 1998). The lack of ion channels suggested the absence of interactions between the voltage gated ion channels and AnkG in AIS. The absence of voltage gated ion channels can also lead to the non-initiation of AP and their generation at the AIS (Zhou et al., 1998).
Figure 10.
Pictorial representation of the structure of AnkG.
Figure 10.
Pictorial representation of the structure of AnkG.
AnkG is responsible for the recruitment of some important proteins in the AIS region, such as ion channels, CAMs and others. AnkG comprises a membrane binding domain (MBD) (blue circles) made up of 24 ankyrin repeats. The MBD is responsible for anchoring both the Nav and Kv channels and CAMs in AIS. Recruitment of ion channels into AIS play a crucial role in AP initiation in this region. Close to the MBD, AnkG has a spectrin binding domain (black rectangle) which binds β-IV spectrin with AnkG and provides strength to the spectrin-actin membrane to perform functions, such as cytoplasmic filters and diffusion barriers. Actin, in the AIS, is connected to AnkG through β-IV spectrin because the N-terminal of β-IV spectrin binds with actin and the C-terminal binds with the spectrin binding domain of AnkG. Alongside the spectrin binding domain, AnkG contains a serine rich domain that is only present in AnkG with size of 270 and480 kDa. The final domain of AnkG is known as the regulatory/death domain and its function is to maintain the interactions between all the proteins and AnkG by regulating all the domains present in AnkG.
Figure 11.
Pictorial representation of the role of AnkG in the AIS region.
Figure 11.
Pictorial representation of the role of AnkG in the AIS region.
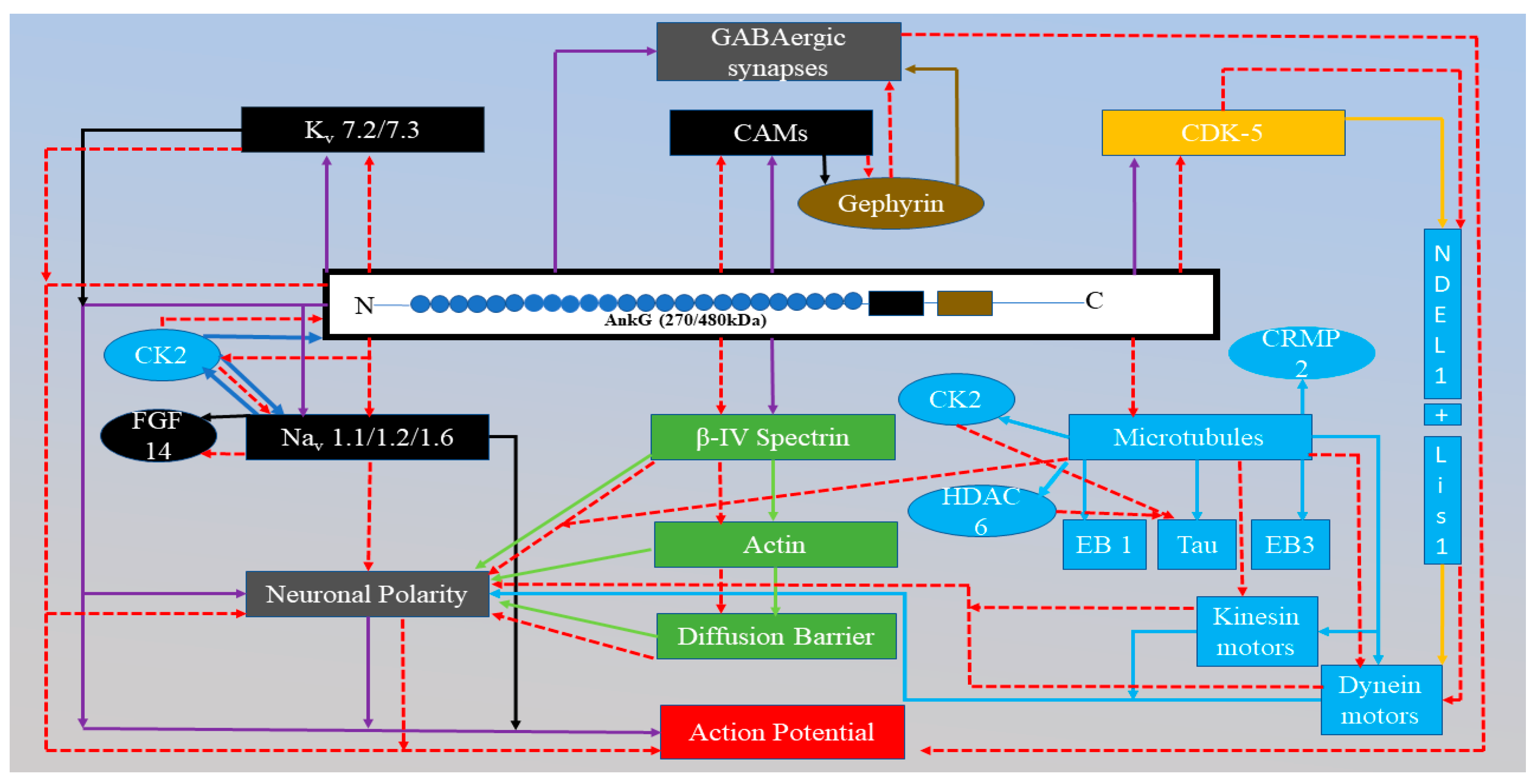
All the purple arrows represent the relationships between AnkG and other proteins and the relationships between AnkG and the specific function of the AIS region (diffusion barrier, action potential or neuronal polarity). In the AIS, AnkG maintains the neuronal polarity (light grey). Neuronal polarity is maintained by diffusion barrier ability of the AIS region. For this region to act as a diffusion barrier is only possible when all the necessary proteins are recruited correctly. Recruitment of proteins in AIS region is dependent on AnkG. Moreover, the correct localization of proteins in the AIS region promotes the initiation of AP (red rectangle). AnkG recruits the Kv and Nav channels and CAMs (black rectangle) and they are responsible for the depolarization and repolarization phase of the AP process, respectively. CAMs bind with the gephyrin protein, which plays a crucial part in the GABAergic synapses (gold sphere). In addition, AnkG also recruits β¬-IV spectrin and this is associated with actin, together, they act as a diffusion barrier and this helps in maintaining neuronal polarity. AnkG also has a relationship with microtubules (blue rectangles) connected through the EB3 protein. Microtubules act as the pathway for the molecular motors (kinesin and dynein) (blue rectangles) and are responsible for the transport of protein through the axons. The transport ability of molecular motors also plays a part in maintaining neuronal polarity by transporting the correct proteins in and out of the axons. The activation of the dynein motors depends on AnkG because NDEL-1 is an activator, and this binds to the dynein motors after interaction with AnkG. The red dotted line indicates the alteration in proteins due to the mutation in AnkG and this has a direct negative impact on AP initiation in the AIS region.
4. Diseases Related to the AIS Dysfunction
Several studies have shown the association of AIS proteins in the diseases such as epilepsy, bipolar disorder, schizophrenia and many more (Fujiwara et al., 2003, Huang et al., 2017, Jr, 2005, Rima et al., 2013, Saeed et al., 2014, Blair, 2012, Guglielmi, 2015, Zuberi et al., 2010). Alteration in the voltage-gated ion channels in this region can disrupt the proper signalling between neurons (Kress and Mennerick , 2010, Leterrier et al., 2010, Yoshimura and Rasband, 2014). The studies indicate the degeneration of axons in patients with multiple sclerosis (MS) and the improper localization of Nav channels may be due to the altered axonal transport that occurs as a result of this disease (
Table 1).
4.1. Possible Role of AIS in AD
AD is the most common neurodegenerative disease in humans thus, it is the most common cause of dementia and as yet there is no known cure for AD. The main characteristics of AD are its progressiveness and unalterable cognitive function and memory loss. The exact pathophysiology and pathogenesis of the disease is not known. The two categories of AD, based on the age of onset, are early-onset or familial AD (FAD) and sporadic AD (SAD). Only 5% of all AD cases are FAD and 90-95% are SAD (William and Laura, 2013). In an AD mice model, the area around Aβ plaques showed synaptic losses, axonal swelling and mutation in the neuronal network (Marin et al., 2016). Aβ plaques could damage the near or around region of AIS by targeting AnkG and βIV spectrin (Marin et al., 2016, Sohn et al., 2016). Moreover, a study on AD transgenic mice showed a decrease in the density and length of this region (Sohn et al., 2016). Alterations in the density and length may be the result of the calpain-mediated proteolysis of AnkG and βIV spectrin (Buffington and Rasband, 2011, Marin et al., 2016). The proteolysis of AnkG reduces its concentration and dismantles the structure of AIS by loosening the proteins anchored by AnkG. Dismantling of this structure results in the disruption of neuronal polarity by abolishing the functions of important proteins present in this region.
MTs are one of the main components of the AIS region and are found in an impaired state after the inhibition of histone deacetylase 6 (HDAC6) (Tapia et al., 2010, Tsushima et al., 2015, Kadavath et al., 2015). A study stated that HDAC6 inhibition by inducing extracellular soluble Aβ or HDAC6 inhibitors (TSA, valproic acid, sodium butyrate) into AIS could lead to the excessive acetylation of tau and tubulin, and this deteriorates MTs function. This may be because of the acetylated tau decreases its binding capability to MTs and increases the mobility of the EB3 protein. However, stabilized MTs has EB3 proteins with low mobility (Sohn et al., 2016, Tsushima et al., 2015). Another possibility is that the acetylated tau may be attached to other AIS proteins impairing their functions in AIS (Sohn et al., 2016).
The hyper-phosphorylation of tau has also been noticed in the AIS region, leading to the impairment of MTs dynamics and this is possibly responsible for the distortion of tau into the soma-dendritic compartment via mutations in the transport motor proteins (Kinesin and Dynein) in MTs ( Li et al., 2011, Sohn et al., 2016). An experimental investigation of the rtg4510 mouse strain revealed that hyper-phosphorylation of tau reduces AP firing frequency via disrupting MTs dynamics by preventing binding of the EB3 protein to MTs. This study also displayed the relocation of the AIS region after abnormal hyper-phosphorylation and this relocation may increase the AP threshold (Gong and Iqbal, 2008, Hatch et al., 2017) Other than tau, hyper-phosphorylation of FGF14 also impacts on the density of AIS by inhibiting the activity of GSK-3. Consequently the inhibition of this kinase family member reduces the expression of the FGF14-Nav complex (Hsu et al., 2017).
Sun et al. (2014) observed the effect of miRNA-342-5p on the hippocampal neurons of AD transgenic and wild type (WT) mice. Western blot analysis showed a significant decrease in AnkG protein levels in APP/PS1 hippocampal neurons at 2, 3, 5 DIV (Sun et al., 2014). Another study by the same research group was undertaken on the cytoplasmic filtering capability of the AIS. They employed dextrans of different sizes, 10 kDa and 70 kDa, in WT and AD mice. The diffusion of small dextrans (10 kDa) into axons was noticed in both types of mice, but the 70 kda dextran did not pass into the axons in WT mice. However, in AD mice 70 kDa dextran was found in the axons. This indicates a defect in the cytoplasmic filtering capability of the AIS region in AD mice. Defects in the filtering mechanism could be due to the low levels of AnkG in AD mice (Sun et al., 2014). On the other hand, SH-SY5Y cells showed no change in AnkG-related mRNA levels but displayed low AnkG levels. These results indicate that the direct targeting of AnkG mRNA 3` UTR by miRNA-342-5p represses the translation process of proteins but not mRNA degradation. The low level of AnkG proteins could affect protein filtering and trafficking in the AIS region and may contribute to AD pathogenesis (Sun et al., 2014).
5. Mathematical Modelling of AP
Because of the enormous amount of data involved in molecular biology it is not easy to provide logical reasoning for every reaction occurring in a biological system. However, the use of mathematical models is beneficial for explaining biological processes using both mathematics and data generated in laboratories together. There are two types of mathematical models: first, models based on mathematics; and secondly, models based on experimental findings and human thinking (Gunawardena, 2014). Mathematical models comprise assumptions developed from a combination of ideas and from the literature. Assumptions in the model dictate the conclusions; and the assumptions are based on logic. If the assumptions are made accurately, there is a greater possibility that the conclusions are also correct. Based on the conclusions, it is possible to predict the behaviour of a system, including the type of interactions taking place within the system (Bailey et al., 2002). Incorrect assumptions can result in conclusions containing errors but can still predict the behaviour of the system based on the percentage accuracy of the assumptions. Complexity in a model does not guarantee correct predictions for any system; however, even a simple model with correct assumptions can draw accurate conclusions and explain the behaviour of complex systems (Bailey et al., 2002). Predictions by mathematical models have the ability to surpass human predictions. Biological processes depend on various factors, such as rate constants and the initial concentration of proteins. Use of mathematical models can help in predicting the parameters influencing protein interactions within a biological system.
In 1952, Hodgkin and Huxley defined the characteristics of AP by using voltage clamp technique on squid giant axon (Hodgkin and Huxley, 1952). They recorded the Na+ and K+ at different membrane potential and characterized their kinetic changes as a function of membrane potential. Additionally, they recorded the overlapping current and named as leak current. Hodgkin and Huxley model (HH model) gave an idea about the presence of ion channels and various gates present in them. In model the inside membrane potential was set to 0 and external membrane potential was recorded. According to the HH model observations, during the change in the membrane potential, Na+ ions enter inside the membrane and depolarize the membrane. However, efflux of K+ ions initiate slower than the influx of the Na+ ions. Hodgkin and Huxley gave set of equations by using electric circuit to define AP at squid giant axon.
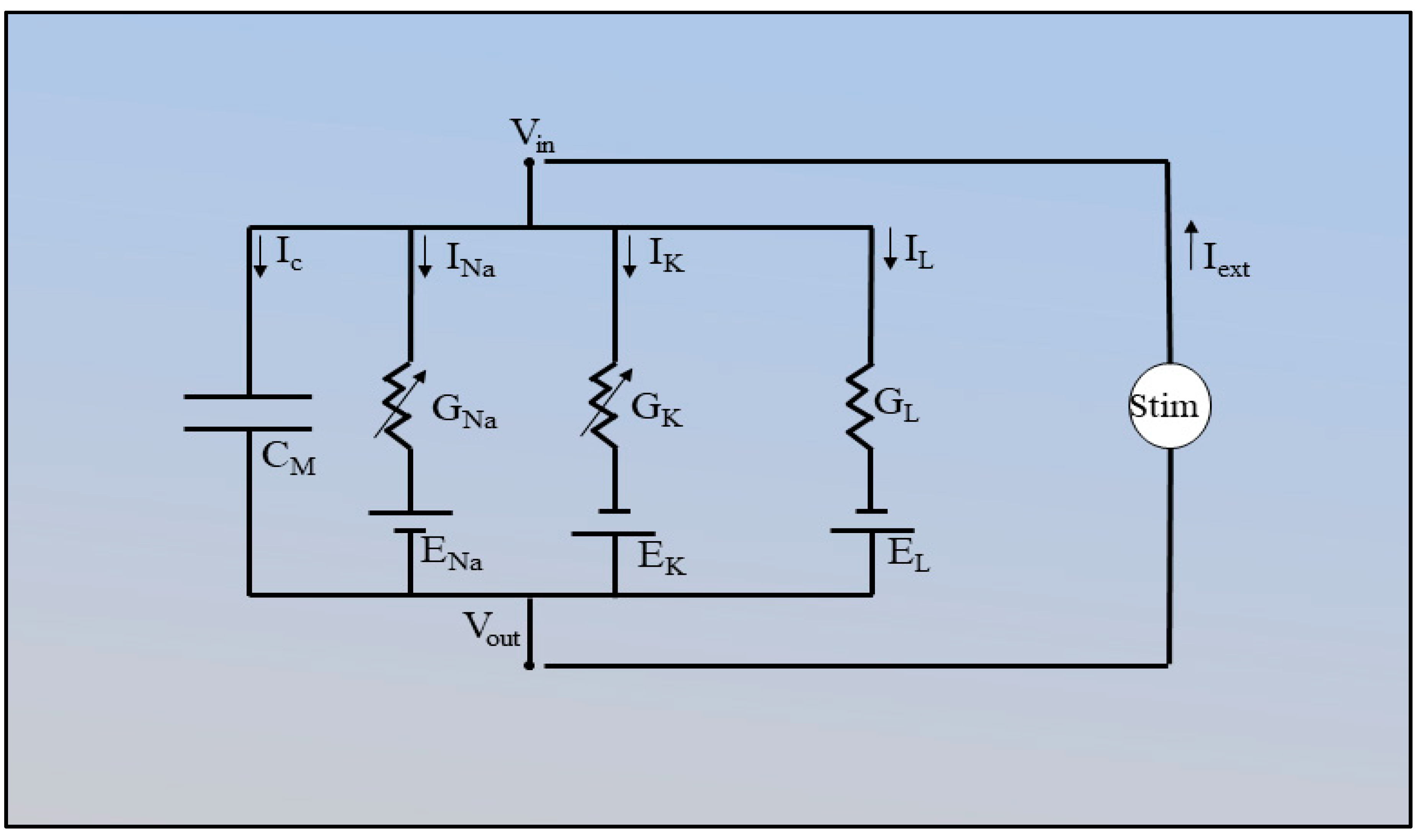
Figure 11.
Circuit diagram used in the HH model.
Figure 11.
Circuit diagram used in the HH model.
I
c, I
Na , I
K and I
L are capacitive currents through the Na
v channel, K
v channel and leak channels, respectively. G
Na, G
K, G
L represent the conductance of Na
+, K
+ and leak ions, respectively. G
Na and G
K are voltage dependent and G
L is a constant in the HH model. E
Na, E
K, E
L are the equilibrium potentials of the Na
+, K
+ and leak ions and C
M represents the membrane capacitance. Stim, in the circuit, is the external current applied to the electrode. V
in and V
out are the voltages coming in out from the circuit.
I
c is the capacitive current and C
m is the membrane capacitance (µF/cm
2) and this is defined as the rate of change in the charge of the membrane voltage (V
m (t)). The behaviour of the electric circuit used in HH model can, therefore, be written as:
Eq. (2) demonstrates the change in membrane potential due to the passing of ions through the membrane. I
ext is the current from the external source (intracellular electrode) applied to the giant squid axon in the model. In the HH model, the total ionic current (I
ionic) is the sum of the three types of current in the membrane: the sodium (I
Na), potassium (I
K), and leak (I
L). The I
ionic current is assumed through Ohm’s law:
where V is the voltage, I, is the current and R is the resistance. Eq. (3) can be written in terms of conductance (G) and G is the inverse of the resistance (G= 1/R). Unit of V is (mV).
So, considering all the equations above, I
ionic can be written as:
GNa, GK and GL represent the conductance of the potassium, sodium and leak channels. The individual ionic currents can be positive or negative. This depends on whether the membrane voltage is below or above the equilibrium potential. The unit of G is m.mho/cm2. In Eqs. (5) ENa, EK and EL are the equilibrium potentials for each ion (Na+, K+, L+).
The movement of ions from the ion channels is based on the behaviour of gates present in the respective channels and the conductance of particular channels relies on these gates. The Eq. (6, 7) indicates the relationship between conductance and the gates within the channels and currents due to the movement of ions through their respective channels. The K
v channel comprises four identical gates denoted as n, and the Na
v channel also comprises four gates but three are identical (m) and one is different (h). The conductance of the Na
v and K
v channels can be written as:
Eq. (6) n refers to the activation gate and n4 represents the four n gates of the Kv channel. gkmax is the maximum conductance when all the gates are open. Eq. (7) explains that the Nav channel conductance is equal to the product of all the gates (three activation gates (m3) and one inactivation gate (h)), and gNamax is the maximum conductance when all the activation gates are open.
From Eqs. (6) and (7) we can modify Eq. (5):
Eq. 8 explains the total ionic current passing through the membrane during AP.
α (opening of gate) and β (closing of gate) are the voltage dependent transient rate constant. (1-n), (1-h) and (1-m) was the inactivation of the respective gate.
The HH model has been remarkably successful in both unfolding and calculating a large number of neuronal properties. Extensions of this model, integrating a variety of voltage-dependent channel types beyond the original HH pair, have been very extensively used in research throughout the world.
In 1981, Morris and Lecar predicted the behaviour of AP on Branacle muscle cells by using equations similar to the HH model (Koch, 1999). However, in this model Ca
2+ was considered in place of Na
+ in HH model. According to the Morris – Lecar predictions total current in muscle cell is defined as:
where g
L, g
Ca, g
K was equal to the 8,3,40 mS/cm
2 respectively and V
K, V
Ca and V
L was set to -70, 120 and -50 respectively.
In this model, Ca2+ act similar to the Na+ in the HH model but do not inactivate. In the heart, Ca2+ play significant role in producing repetitive pacemaker AP.
Experiments in 1950s gave evidence about the initiation of AP at proximal axon. Electrophysiological recordings and voltage- sensitive dye imaging studies confirmed the initiation of AP at AIS. Study conducted by Kole et al., (2008) revealed the importance of Nav channel density in the AP initiation at AIS. Computational experiment was conducted by using morphological model and simulations was carried out in NEURON simulation software. Simulation results was observed at different Nav channel density until the experimental observations was reproduced. According to the results after three computing three different morphology model, minimum 2500 (pS µm-2) of Nav channel density was required for the AP initiation. Moreover, this study gave ample evidence that Nav channel density at AIS is higher than the soma (at least 50 times) (Kole et al., 2008). Similarly based on the morphology, Hallermann et al (2012) conducted computational study to understand the consumption of ATP during AP. Simulation results indicated that 90% of the energy was consumed by the axon during AP propagation. Total consumption of 400 to 800*106 molecules was noticed during AP propagation depending on the cell morphology. This study introduced a morphology model of cortical layer 5 pyramidal neuron which has been used by various studies to understand the the AP initiation and propagation (Hallermann et al., 2012).
Investigation carried out by Telenczuk et al. (2018) focussing on the consequences of the AP initiation mechanism on the extracellular signatures of AP. In this study also morphology model mentioned in Hallermann et al. (2012) was used with alternate parameters. Results of this study proposed the formation of dipole between soma and the AIS at AP initiation. Soma- AIS dipole contribute to the extracellular field and its consequences on the shape and amplitude of the extracellular AP.
6. Summary and Future Directions
Extensive experimental and computational investigations suggested that AIS region is important for the AP initiation in order to execute the neurotransmission. As AIS is enriched with various important proteins such as AnkG, Nav channel, Kv channels and MTs. All the proteins in this region are associated with AnkG, because recruitment of all the important proteins at AIS is depend on their binding with AnkG. The experimental studies demonstrated the negative impact on the protein trafficking and loss of Nav, Kv channels from the AIS structure after mutations in AnkG. Also, AIS acts as selective filter and diffusion barrier in order to restrict the entry of the unnecessary protein into the axon. Protein trafficking of proteins towards axon and dendrites are accomplished through kinesin and dynein motors respectively. CK2 mediated phosphorylation also plays critical role in the recruitment of Nav channels in AIS. Experimental evidences proved that serine specific mutation in Nav channels could altered the binding with AnkG necessary for the Nav accumulation in AIS. Computational investigations demonstrated the importance of Nav channel and minimum density of 2500 Psµm-2 is required to initiate the AP at AIS.
In future, studies should focus on the protein interactions taking place at AIS including protein binding with AnkG. Moreover, studies should investigate the post translation modifications such as phosphorylation because as mentioned in Hallermann et al. (2012), axon consumed 90% of the total energy during AP propagation. In addition to that, experiments should be conducted to understand the mechanism for the recruitment of AnkG into the AIS. Future studies should also focus on the protein trafficking by molecular motors and their role in various neurological diseases after alteration in their mechanism.
Overall, AIS as a differentiator between axon and dendrites is an important contributor in the proper functioning of a neuron and researchers should conduct more experiments in order to define its involvement in neuronal functions. With modelling it is easy to test the hypothesis and assumptions in order to understand the importance of AIS proteins in AP initiation. Moreover, we can also investigate more deeply on the AIS contribution in various neurological diseases.
Acknowledgement
We would like to thank Dr Yan Zhang from School of Life Sciences (Peeking University), China for her encouragement of this work.
Abbreviations
AIS; Action initial segment; ER; Endoplasmic reticulum; AP; Action potential; Nav; Voltage gated Sodium channels; Kv ; Voltage gated Potassium channels; CK2; Casein Kinase 2; AD; Alzheimer’s disease; AnkG; Ankyrin G; PTM; Post translational modifications; ABM; Ankyrin binding motif; MBD; Membrane binding domain; TS; Transmembrane segments; CAMs; Cell adhesion molecules; FGF; Fibroblast growth factor; PSD93; Post synaptic density 93; TDs; Traffic determinants; NF186; Neurofascin 186; NrCAM; Neuronal cell adhesion molecules; PH; Pleckstrin homology domain; PIP2; phosphatidyl-inositol ; 5 -biphosphate; DIV; Days in-vitro; GPI; glycosyl-phospha-tidyl-nositol; SPT; Single particle tracking; MTs; Microtubules ; HDAC6; Histone deacetylase 6; EB; End binding protein; ATP; Adenosine tri phosphate; CDK-5; Cyclin dependent kinase; VAMP; Vesicle associated membrane protein; APP; Amyloid precursor protein; NDEL 1; Nuclear distribution like 1; FAD; Familial Alzheimer’s disease; SAD; Sporadic Alzheimer’s disease; NFT; Neurofibrillary tangles; AChEIs; Acetocholinesterase inhibitors; PHF, Paired helical filaments
References
- Ackerman. S. (1992)."Discovering the brain". National academy of Sciences.
- Akin, E.J.; Solé, L.; Johnson, B.; Beheiry, M. el, Masson, J.B.; Krapf, D.; Tamkun, M.M. Single-Molecule Imaging of Nav1.6 on the Surface of Hippocampal Neurons Reveals Somatic Nanoclusters. Biophysical Journal 2016, 111, 1235–1247. [Google Scholar] [CrossRef]
- Albrecht, D.; Winterflood, C.M.; Sadeghi, M.; Tschager, T.; Ewers, H. Nanoscopic compartmentalization of membrane protein motion at the axon initial segment. Journal of Cell Biology 2016, 215, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Ango, F.; Di Cristo, G.; Higashiyama, H.; Bennett, V.; Wu, P.; Huang, Z.J. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at Purkinje axon initial segment. Cell 2004, 119, 257–272. [Google Scholar] [CrossRef]
- Baek, J.H.; Rubinstein, M.; Scheuer, T.; Trimmer, J.S. Reciprocal changes in phosphorylation and methylation of mammalian brain sodium channels in response to seizures. Journal of Biological Chemistry 2014, 289, 15363–15373. [Google Scholar] [CrossRef] [PubMed]
- Baines, A.J. Évolution Du Squelette Membranaire Dépendant De La Spectrine. Transfusion Clinique et Biologique 2010, 17, 95–103. [Google Scholar] [CrossRef]
- Balasanyan, V.; Watanabe, K.; Dempsey, W.P.; Lewis, T.L.; Trinh, L.A.; Arnold, D.B. Structure and Function of an Actin-Based Filter in the Proximal Axon. Cell Reports 2017, 21, 2696–2705. [Google Scholar] [CrossRef]
- Baranauskas, G.; David, Y.; Fleidervish, I.A. Spatial mismatch between the Na+ flux and spike initiation in axon initial segment. Proceedings of the National Academy of Sciences 2013, 110, 4051–4056. [Google Scholar] [CrossRef]
- Barry, J.; Gu, Y.; Jukkola, P.; O’Neill, B.; Gu, H.; Mohler, P.J.; … Gu, C. Ankyrin-G Directly Binds to Kinesin-1 to Transport Voltage-Gated Na+Channels into Axons. Developmental Cell 2014, 28, 117–131. [Google Scholar] [CrossRef]
- Berghs, S.; Aggujaro, D.; Dirkx, R.; Maksimova, E.; Stabach, P.; Hermel, J.M.; … Solimena, M. βIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. Journal of Cell Biology 2000, 151, 985–1001. [Google Scholar] [CrossRef]
- Bi, C.; Wu, J.; Jiang, T.; Liu, Q.; Cai, W.; Yu, P.; … Sun, Z.S. Mutations of ANK3 identified by exome sequencing are associated with Autism susceptibility. Human Mutation 2012, 33, 1635–1638. [Google Scholar] [CrossRef]
- Blair, R.D. G. Temporal Lobe Epilepsy Semiology. Epilepsy Research and Treatment 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Boiko, T.; Van Wart, A.; Caldwell, J.H.; SR, L. , Trimmer, J.S.; Matthews, G. Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. TL - 23. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 2003, 23, 2306–2313. [Google Scholar] [CrossRef]
- Bréchet, A.; Fache, M.P.; Brachet, A.; Ferracci, G.; Baude, A.; Irondelle, M.; … Dargent, B. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. Journal of Cell Biology 2008, 183, 1101–1114. [Google Scholar] [CrossRef]
- Buffington, S.A.; Rasband, M.N. The axon initial segment in nervous system disease and injury. European Journal of Neuroscience 2011, 34, 1609–1619. [Google Scholar] [CrossRef]
- Carolina, N. Restriction of 480/270-kD Ankyrin 1998, 142, 1571–1581.
- Chung, P.J.; Song, C.; Deek, J.; Miller, H.P.; Li, Y.; Choi, M.C.; … Safinya, C.R. Tau mediates microtubule bundle architectures mimicking fascicles of microtubules found in the axon initial segment. Nature Communications 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Coombs, B.J. S. Curtis, D.R.; Eccles, J.C. (1957). Spike Potential, 232–249.
- Cunha, S.R.; Mohler, P.J. Ankyrin protein networks in membrane formation and stabilization. Journal of Cellular and Molecular Medicine 2009, 13, 4364–4376. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.J.; Tan, K.; Reck-Peterson, S.L. Lis1 is an initiation factor for dynein-driven organelle transport. Journal of Cell Biology 2012, 197, 971–982. [Google Scholar] [CrossRef]
- Encalada, S.E.; Goldstein, L.S. B. Biophysical Challenges to Axonal Transport: Motor-Cargo Deficiencies and Neurodegeneration. Annual Review of Biophysics 2014, 43, 141–169. [Google Scholar] [CrossRef]
- Evans, M.D.; Dumitrescu, A.S.; Kruijssen, D.L. H. , Taylor, S.E.; Grubb, M.S. Rapid Modulation of Axon Initial Segment Length Influences Repetitive Spike Firing. Cell Reports 2015, 13, 1233–1245. [Google Scholar] [CrossRef]
- Fache, M.P.; Moussif, A.; Fernandes, F.; Giraud, P.; Garrido, J.J.; Dargent, B. Endocytotic elimination and domain-selective tethering constitute a potential mechanism of protein segregation at the axonal initial segment. Journal of Cell Biology 2004, 166, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Sugawara T, Mazaki-Miyazaki E, & Takahashi Y. Current Literature Scn1a In Smei, Icegtc, And Gefs+: Alphabet Soup Or Emerging Genotypic-Phenotypic Clarity? Mutations of Sodium Channel α-Subunit Type 1 (SCN1A) in Intractable Childhood Epilepsies with Frequent Generalized Tonic-clonic Seizures. 2003, 1, 6–7.
- Gong, C.-X. , & Iqbal, K. Hyperphosphorylation of Microtubule-Associated Protein Tau: A Promising Therapeutic Target for Alzheimer Disease. Current Medicinal Chemistry 2008, 15, 2321–2328. [Google Scholar] [CrossRef]
- Goodman, S.R.; Zimmer, W.E.; Blair Clark, M.; Zagon, I.S.; Barker, J.E.; Bloom, M.L. Brain spectrin: Of mice and men. Brain Research Bulletin 1995, 36, 593–606. [Google Scholar] [CrossRef]
- Guglielmi, L. Update on the implication of potassium channels in autism: K+ channelautism spectrum disorder. Frontiers in Cellular Neuroscience 2015, 9, 1–14. [Google Scholar] [CrossRef]
- Gumy, L.F.; Hoogenraad, C.C. Local mechanisms regulating selective cargo entry and long-range trafficking in axons. Current Opinion in Neurobiology 2018, 51, 23–28. [Google Scholar] [CrossRef]
- Harty, R.C.; Kim, T.H.; Thomas, E.A.; Cardamone, L.; Jones, N.C.; Petrou, S.; Wimmer, V.C. Axon initial segment structural plasticity in animal models of genetic and acquired epilepsy. Epilepsy Research 2013, 105, 272–279. [Google Scholar] [CrossRef]
- Hatch, R.J.; Wei, Y.; Xia, D.; Götz, J. Hyperphosphorylated tau causes reduced hippocampal CA1 excitability by relocating the axon initial segment. Acta Neuropathologica 2017, 133, 717–730. [Google Scholar] [CrossRef]
- He, M.; Jenkins, P.; Bennett, V. Cysteine 70 of ankyrin-G is S-palmitoylated and is required for function of ankyrin-G in membrane domain assembly. Journal of Biological Chemistry 2012, 287, 43995–44005. [Google Scholar] [CrossRef]
- Hedstrom, K.L.; Ogawa, Y.; Rasband, M.N. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. Journal of Cell Biology 2008, 183, 635–640. [Google Scholar] [CrossRef]
- Hoogenraad, C.C.; Bradke, F. Control of neuronal polarity and plasticity - a renaissance for microtubules? Trends in Cell Biology 2009, 19, 669–676. [Google Scholar] [CrossRef]
- Hsu, W.C. J. , Wildburger, N.C.; Haidacher, S.J.; Nenov, M.N.; Folorunso, O.; Singh, A.K.; … Laezza, F. PPARgamma agonists rescue increased phosphorylation of FGF14 at S226 in the Tg2576 mouse model of Alzheimer’s disease. Experimental Neurology 2017, 295, 1–17. [Google Scholar] [CrossRef]
- Huang, W.; Liu, M.; Yan, S.F.; Yan, N. Structure-based assessment of disease-related mutations in human voltage-gated sodium channels. Protein and Cell 2017, 8, 401–438. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. A Quantitative Description of Membrane Current and Application to Conduction and Excitation in Nerve. The Journal of Physiology. 1952, 500–544. [Google Scholar] [CrossRef]
- Jegla, T.; Nguyen, M.M.; Feng, C.; Goetschius, D.J.; Luna, E.; van Rossum, D.B.; … Rolls, M.M. Bilaterian Giant Ankyrins Have a Common Evolutionary Origin and Play a Conserved Role in Patterning the Axon Initial Segment. PLoS Genetics 2016, 12, 1–31. [Google Scholar] [CrossRef]
- Jenkins, B.; Decker, H.; Bentley, M.; Luisi, J.; Banker, G. A novel split kinesin assay identifies motor proteins that interact with distinct vesicle populations. Journal of Cell Biology 2012, 198, 749–761. [Google Scholar] [CrossRef]
- Jensen, C.S.; Watanabe, S.; Stas, J.I.; Klaphaak, J.; Yamane, A.; Schmitt, N.; Misonou, H. Trafficking of Kv2.1 Channels to the Axon Initial Segment by a Novel Non-Conventional Secretory Pathway. The Journal of Neuroscience 2017, 37, 3510–3516. [Google Scholar] [CrossRef]
- Jones, S.L.; Svitkina, T.M. Axon Initial Segment Cytoskeleton: Architecture, Development, and Role in Neuron Polarity. Neural Plasticity 2016, 2016. [Google Scholar] [CrossRef]
- Jr, A.G. Inherited disorders of voltage-gated sodium channels. Journal of Clinical Investigation 2005, 115. [Google Scholar] [CrossRef]
- Kadavath, H.; Hofele, R.V.; Biernat, J.; Kumar, S.; Tepper, K.; Urlaub, H.; … Zweckstetter, M. Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proceedings of the National Academy of Sciences 2015, 112, 7501–7506. [Google Scholar] [CrossRef] [PubMed]
- Kaphzan, H.; Buffington, S.A.; Jung, J.I.; Rasband, M.N.; Klann, E. Alterations in Intrinsic Membrane Properties and the Axon Initial Segment in a Mouse Model of Angelman Syndrome. Journal of Neuroscience 2011, 31, 17637–17648. [Google Scholar] [CrossRef] [PubMed]
- Kapitein, L.C.; Hoogenraad, C.C. Which way to go? Cytoskeletal organization and polarized transport in neurons. Molecular and Cellular Neuroscience 2011, 46, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Kapitein, L.C.; Schlager, M.A.; Kuijpers, M.; Wulf, P.S.; van Spronsen, M.; MacKintosh, F.C.; Hoogenraad, C.C. Mixed Microtubules Steer Dynein-Driven Cargo Transport into Dendrites. Current Biology 2010, 20, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, T.; Ailani, D.; Hiramoto, M.; Hiromi, Y. Intra-axonal Patterning: Intrinsic Compartmentalization of the Axonal Membrane in Drosophila Neurons. Neuron 2009, 64, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Klinman, E.; Tokito, M.; Holzbaur, E.L. F. CDK5-dependent activation of dynein in the axon initial segment regulates polarized cargo transport in neurons. Traffic 2017, 18, 808–824. [Google Scholar] [CrossRef]
- Kole, M.H. P. , Ilschner, S.U.; Kampa, B.M.; Williams, S.R.; Ruben, P.C.; Stuart, G.J. Action potential generation requires a high sodium channel density in the axon initial segment. Nature Neuroscience 2008, 11, 178–186. [Google Scholar] [CrossRef]
- Kole, M.H. P. , & Stuart, G.J. Signal Processing in the Axon Initial Segment. Neuron 2012, 73, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Komada, M.; Soriano, P. ??IV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. Journal of Cell Biology 2002, 156, 337–348. [Google Scholar] [CrossRef]
- Kress, G.J.; Mennerick, S. (2010). NIH Public Access 158, 211–222. [CrossRef]
- Kriebel, M.; Metzger, J.; Trinks, S.; Chugh, D.; Harvey, R.J.; Harvey, K.; Volkmer, H. The cell adhesion molecule neurofascin stabilizes axo-axonic GABAergic terminals at the axon initial segment. Journal of Biological Chemistry 2011, 286, 24385–24393. [Google Scholar] [CrossRef]
- Kuijpers, M.; van de Willige, D.; Freal, A.; Chazeau, A.; Franker, M.A.; Hofenk, J.; … Hoogenraad, C.C. Dynein Regulator NDEL1 Controls Polarized Cargo Transport at the Axon Initial Segment. Neuron 2016, 89, 461–471. [Google Scholar] [CrossRef]
- Le Bras, B.; Fréal, A.; Czarnecki, A.; Legendre, P.; Bullier, E.; Komada, M.; … Couraud, F. In vivo assembly of the axon initial segment in motor neurons. Brain Structure and Function 2014, 219, 1433–1450. [Google Scholar] [CrossRef]
- Leterrier, C.; Vacher, H.; Fache, M.-P. , d’Ortoli, S.A.; Castets, F.; Autillo-Touati, A.; Dargent, B. End-binding proteins EB3 and EB1 link microtubules to ankyrin G in the axon initial segment. Proceedings of the National Academy of Sciences 2011, 108, 8826–8831. [Google Scholar] [CrossRef] [PubMed]
- Leterrier, Christophe, Brachet, A. ; Fache, M.P.; Dargent, B. Voltage-gated sodium channel organization in neurons: Protein interactions and trafficking pathways. Neuroscience Letters 2010, 486, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Leterrier, Christophe, & Dargent, B. No Pasaran! Role of the axon initial segment in the regulation of protein transport and the maintenance of axonal identity. Seminars in Cell and Developmental Biology 2014, 27, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Leterrier, Christophe, Potier, J. ; Caillol, G.; Debarnot, C.; Rueda Boroni, F.; Dargent, B. Nanoscale Architecture of the Axon Initial Segment Reveals an Organized and Robust Scaffold. Cell Reports 2015, 13, 2781–2793. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kumar, Y.; Zempel, H.; Mandelkow, E.M.; Biernat, J.; Mandelkow, E. Novel diffusion barrier for axonal retention of Tau in neurons and its failure in neurodegeneration. EMBO Journal 2011, 30, 4825–4837. [Google Scholar] [CrossRef]
- Li, Y.C.; Cheng, C.X.; Li, Y.N.; Shimada, O.; Atsumi, S. Beyond the initial axon segment of the spinal motor axon: Fasciculated microtubules and polyribosomal clusters. Journal of Anatomy 2005, 206, 535–542. [Google Scholar] [CrossRef]
- Marin, M.A.; Ziburkus, J.; Jankowsky, J.; Rasband, M.N. Amyloid-β plaques disrupt axon initial segments. Experimental Neurology 2016, 281, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Nakada, C.; Ritchie, K.; Oba, Y.; Nakamura, M.; Hotta, Y.; Iino, R.; … Kusumi, A. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nature Cell Biology 2003, 5, 626–632. [Google Scholar] [CrossRef]
- Nakata, T.; Hirokawa, N. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. Journal of Cell Biology 2003, 162, 1045–1055. [Google Scholar] [CrossRef]
- Nelson, A.D.; Jenkins, P.M. Axonal Membranes and Their Domains: Assembly and Function of the Axon Initial Segment and Node of Ranvier. Frontiers in Cellular Neuroscience 2017, 11, 1–17. [Google Scholar] [CrossRef]
- Nishimura, K.; Akiyama, H.; Komada, M.; Kamiguchi, H. βIV-spectrin forms a diffusion barrier against L1CAM at the axon initial segment. Molecular and Cellular Neuroscience 2007, 34, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Rasband, M.N. The functional organization and assembly of the axon initial segment. Current Opinion in Neurobiology 2008, 18, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Peltola, M.A.; Kuja-Panula, J.; Liuhanen, J.; Võikar, V.; Piepponen, P.; Hiekkalinna, T.; … Rauvala, H. AMIGO-Kv2.1 Potassium Channel Complex Is Associated with Schizophrenia-Related Phenotypes. Schizophrenia Bulletin 2016, 42, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Platkiewicz, J.; Brette, R. A Threshold Equation for Action Potential Initiation 2010, 6. [CrossRef]
- Rasband, M.N. Na + channels get anchored...with a little help. Journal of Cell Biology 2008, 183, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Rasband, M.N. The axon initial segment and the maintenance of neuronal polarity. Nature Reviews Neuroscience 2010, 11, 552–562. [Google Scholar] [CrossRef]
- Rima, N.; Nicole, C.; Mathilde, C.; Giulia, B.; Charles, B.; Celia, D.; … Catherine, C. Encephalopathy in children with Dravet syndrome is not a pure consequence of epilepsy. Orphanet Journal of Rare Diseases 2013, 8, 176. [Google Scholar]
- Rowan, M.J. M. , Tranquil, E.; Christie, J.M. Distinct Kv Channel Subtypes Contribute to Differences in Spike Signaling Properties in the Axon Initial Segment and Presynaptic Boutons of Cerebellar Interneurons. Journal of Neuroscience 2014, 34, 6611–6623. [Google Scholar] [CrossRef]
- Rueckert, E.H.; Barker, D.; Ruderfer, D.; Bergen, S.E.; O’dushlaine, C.; Luce, C.J.; … Sklar, P. Cis-acting regulation of brain-specific ANK3 gene expression by a genetic variant associated with bipolar disorder. Molecular Psychiatry 2013, 18, 922–929. [Google Scholar] [CrossRef]
- Saeed, M.; Azam, M.; Shabbir, N.; Qamar, S.A. Is "benign Childhood Epilepsy with Centrotemporal Spikes" Always Benign? Iranian Journal of Child Neurology 2014, 8, 38–43. [Google Scholar]
- Saka, S.K.; Honigmann, A.; Eggeling, C.; Hell, S.W.; Lang, T.; Rizzoli, S.O. Organization of the Plasma Membrane. Nature Communications 2014, 5, 1–14. [Google Scholar] [CrossRef]
- Sánchez-Ponce, D.; Blázquez-Llorca, L.; Defelipe, J.; Garrido, J.J.; Muñoz, A. Colocalization of α-actinin and synaptopodin in the pyramidal cell axon initial segment. Cerebral Cortex 2012, 22, 1648–1661. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ponce, D.; DeFelipe, J.; Garrido, J.J.; Muñoz, A. Developmental Expression of Kv Potassium Channels at the Axon Initial Segment of Cultured Hippocampal Neurons. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ponce, D.; Muñoz, A.; Garrido, J.J. Casein kinase 2 and microtubules control axon initial segment formation. Molecular and Cellular Neuroscience 2011, 46, 222–234. [Google Scholar] [CrossRef]
- Schafer, D.P.; Jha, S.; Liu, F.; Akella, T.; McCullough, L.D.; Rasband, M.N. Disruption of the Axon Initial Segment Cytoskeleton Is a New Mechanism for Neuronal Injury. Journal of Neuroscience 2009, 29, 13242–13254. [Google Scholar] [CrossRef]
- Schmidt-hieber, C.; Jonas, P.; Bischofberger, J. (2008). Action potential initiation and propagation in hippocampal mossy fibre axons, 7, 1849–1857. [CrossRef]
- Smith, K.R.; Penzes, P. Ankyrins: Roles in synaptic biology and pathology. Molecular and Cellular Neuroscience 2018. [Google Scholar] [CrossRef]
- Sohn, P.D.; Tracy, T.E.; Son, H.I.; Zhou, Y.; Leite, R.E. P. , Miller, B.L.; … Gan, L. Acetylated tau destabilizes the cytoskeleton in the axon initial segment and is mislocalized to the somatodendritic compartment. Molecular Neurodegeneration 2016, 11, 1–13. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Y.; Gu, M.; Zhang, Y. MiR-342-5p decreases ankyrin G levels in Alzheimer’s disease transgenic mouse models. Cell Reports 2014, 6, 264–270. [Google Scholar] [CrossRef]
- Tapia, M.; Wandosell, F.; Garrido, J.J. Impaired function of hdac6 slows down axonal growth and interferes with axon initial segment development. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Tse, W.T.; Tang, J.; Jin, O.; Korsgren, C.; John, K.M.; Kung, A.L.; … Lux, S.E. A New Spectrin, βIV, Has a Major Truncated Isoform that Associates with Promyelocytic Leukemia Protein Nuclear Bodies and the Nuclear Matrix. Journal of Biological Chemistry 2001, 276, 23974–23985. [Google Scholar] [CrossRef]
- Tsushima, H.; Emanuele, M.; Polenghi, A.; Esposito, A.; Vassalli, M.; Barberis, A.; … Chieregatti, E. HDAC6 and RhoA are novel players in Abeta-driven disruption of neuronal polarity. Nature Communications 2015, 6. [Google Scholar] [CrossRef]
- Vallee, R.B.; Williams, J.C.; Varma, D.; Barnhart, L.E. Dynein: An Ancient Motor Protein Involved in Multiple Modes of Transport. Journal of Neurobiology 2004, 58, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Viel, A.; Branton, D. Spectrin: On the path from structure to function. Current Opinion in Cell Biology 1996, 8, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Al-Bassam, S.; Miyazaki, Y.; Wandless, T.J.; Webster, P.; Arnold, D.B. Networks of Polarized Actin Filaments in the Axon Initial Segment Provide a Mechanism for Sorting Axonal and Dendritic Proteins. Cell Reports 2012, 2, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- William, T.; Laura, B. 2013 Alzheimer’s disease facts and figures. Alzheimer’s and Dementia 2013, 9, 208–245. [Google Scholar] [CrossRef]
- Winckler, B.; Forscher, P.; Mellman, I. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature 1999, 397, 698–701. [Google Scholar] [CrossRef]
- Xiao, S.; Jan, L.Y. A Gate Keeper for Axonal Transport. Cell 2009, 136, 996–998. [Google Scholar] [CrossRef]
- Xu, X.; Shrager, P. Dependence of axon initial segment formation on Na+ channel expression. Journal of Neuroscience Research 2005, 79, 428–441. [Google Scholar] [CrossRef]
- Yamada, R.; Kuba, H. Structural and Functional Plasticity at the Axon Initial Segment. Frontiers in Cellular Neuroscience 2016, 10, 1–7. [Google Scholar] [CrossRef]
- Yang, Y.; Ogawa, Y.; Hedstrom, K.L.; Rasband, M.N. βIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. Journal of Cell Biology 2007, 176, 509–519. [Google Scholar] [CrossRef]
- Yoshimura, T.; Rasband, M.N. Axon initial segments: Diverse and dynamic neuronal compartments. Current Opinion in Neurobiology 2014, 27, 96–102. [Google Scholar] [CrossRef]
- Zhang, C.; Rasband, M.N. Cytoskeletal control of axon domain assembly and function. Current Opinion in Neurobiology 2016, 39, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Lambert, S.; Malen, P.L.; Carpenter, S.; Boland, L.M.; Bennett, V. Ankyrin(G) is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. Journal of Cell Biology 1998, 143, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Cordner, Z.A.; Xiong, J.; Chiu, C.-T. , Artola, A.; Zuo, Y.; … Ross, C.A. Genetic disruption of ankyrin-G in adult mouse forebrain causes cortical synapse alteration and behavior reminiscent of bipolar disorder. Proceedings of the National Academy of Sciences 2017, 114, 10479–10484. [Google Scholar] [CrossRef] [PubMed]
- Zonta, B.; Desmazieres, A.; Rinaldi, A.; Tait, S.; Sherman, D.L.; Nolan, M.F.; Brophy, P.J. A Critical Role for Neurofascin in Regulating Action Potential Initiation through Maintenance of the Axon Initial Segment. Neuron 2011, 69, 945–956. [Google Scholar] [CrossRef]
- Zuberi, S.M.; Eunson, L.H.; Spauschus, A.; De Silva, R.; Tolmie, J.; Wood, N.W.; … Hanna, M.G. A novel mutation in the human voltage-gated potassium channel gene (Kv1.1) associates with episodic ataxia type 1 and sometimes with partial epilepsy (Brain 122:PART 5 (817-825)). Brain 2010, 133, 1569. [Google Scholar] [CrossRef]
Table 1.
Neurological disorders associated with AIS dysfunction.
Table 1.
Neurological disorders associated with AIS dysfunction.
| AIS Protein |
Disorder |
Symptoms |
Source |
| Nav
|
Generalized epilepsy with febrile seizure plus (GEFS+)
Benign familial neonatal-infantile seizures (BFNS)
Epileptic encephalopathy early infantile 6, 11, and 13 (EIEE (6, 11, 13))
Intractable childhood epilepsy with generalized tonic-clonic seizures (ICEGTC)
Dravet syndrome |
Epilepsy syndrome with febrile convulsions
Recurrent seizures in newborn babies
Tonic spasms occur within three months of life. Can be 100 times per day for up to 10 seconds each
Children in the first year of life with recalcitrant seizures and cognitive decline
Severe clonic seizures activated by fever in the first year of life |
(Fujiwara et al., 2003)
(Jr 2005)
(Huang et al., 2017)
(Huang et al., 2017)
(Rima et al., 2013) |
| Kv
|
Episodic ataxia 1 (EA1)
BFNS
Benign childhood epilepsy with centro-temporal spikes (BECTS)
Temporal lobe epilepsy (TLE)
Autism |
Severe discoordination with or without muscle movement
Recurrent seizures in new-born human babies
Idiopathic epilepsy with seizures generally occurring in sleep in children from 2-13 years of age
Seizures in the temporal lobe; it is a location or focal form of epilepsy
Disorder with difficulty in speech, nonverbal communication and repetitive behaviour |
(Zuberi et al., 2010)
(Saeed et al., 2014)
(Blair, 2012)
(Guglielmi, 2015) |
| AnkG |
Bipolar disorder
Alzheimer’s disease (AD)
Schizophrenia
Angelman syndrome |
Disorder with manic depression
Neurodegenerative disease and the main cause of dementia
Disorder with abnormal behaviour, not understanding reality and unrealistic thinking and difficulty in speech
Genetic disorder with specific facial appearance, seizures and speaking problems |
(Zhu et al., 2017)
(Sun et al., 2014)
(Bi et al., 2012)
(Kaphzan et al., 2011) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).