Introduction
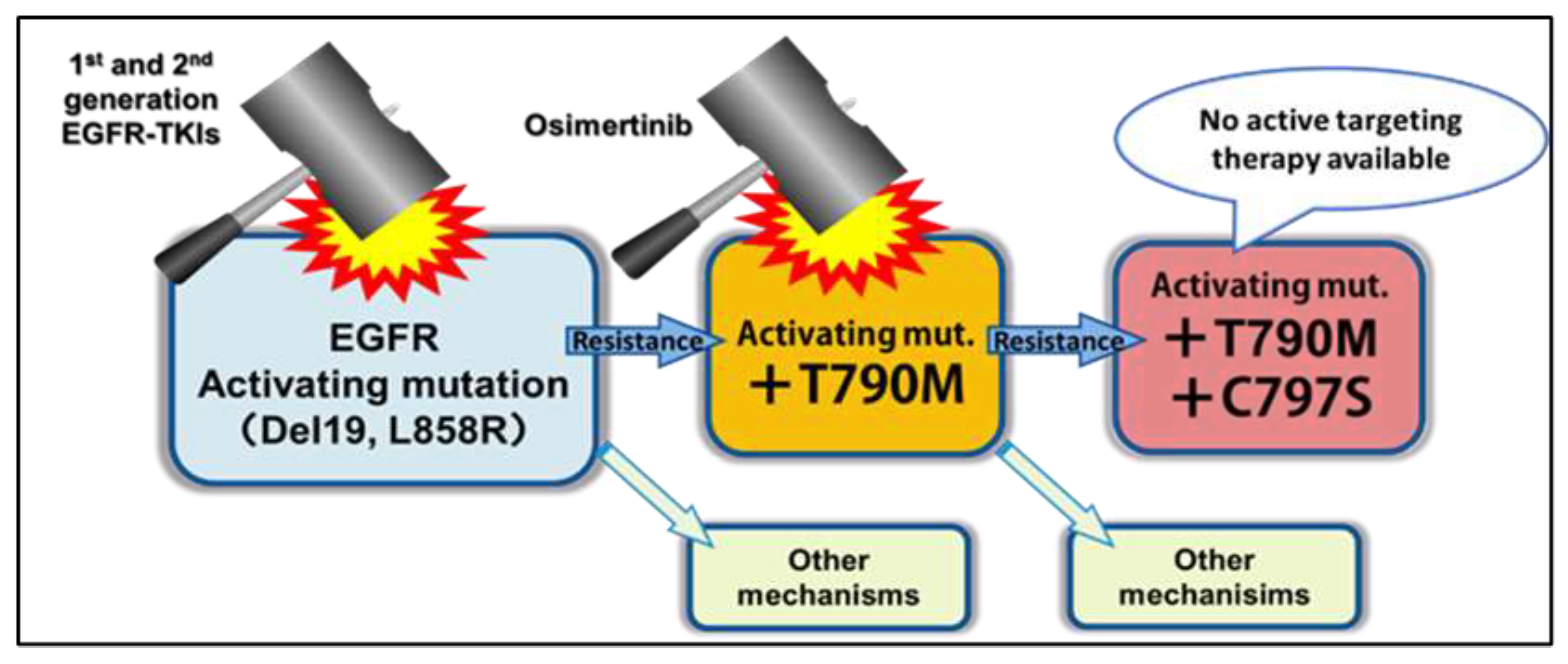
Figure 1.
Diagram of EGFR Mutations, TKIs Used to Treat Them, and Resistance Mechanisms [
3].
Figure 1.
Diagram of EGFR Mutations, TKIs Used to Treat Them, and Resistance Mechanisms [
3].
Lung cancer has been the leading cause of cancer deaths worldwide, primarily among smokers. Lung adenocarcinoma is the most common type of NSCLC. Drug resistance is a major issue in cancer treatment and therefore a main focus of cancer research; it re-mains a large problem in the treatment of cancer, as many patients acquire resistance to a treatment after a median of 9.9 to 20.9 months [
4,
5]. EGFR (also known as human epidermal growth factor receptor [HER1] or ErbB1) is a cell surface protein that regu-lates epithelial tissue development and homeostasis in wild-type organisms and is part of the ErbB family of tyrosine kinases. In wild-type cells, EGFR regulates tissue devel-opment and homeostasis. However, point mutations and amplifications to EGFR may lead to increased cell proliferation and be a driver of tumorigenesis, mostly in breast, lung cancers and glioblastoma [
6]. Exon 19 deletions (del-E746-A750) and an exon 21 point mutation (L858R) make up about 85%-90% of all EGFR mutations in NSCLC and are often called EGFR sensitizing mutations, as they confer sensitivity to EGFR TKIs [
7]. Furthermore, these EGFR mutations are found in about 10% of white, 19% of Black, and 50% of Asian patients with NSCLC [
8]. The group with the highest frequency of EGFR sensitizing mutations are never-smoking Asian women, of which about 63% have these mutations [
9]. NSCLC patients with these mutations are usually treated with EGFR TKIs.
First to Third-Generation EGFR-TKIs and Their Uses and Limitations
First-generation EGFR TKIs (such as gefitinib and erlotinib) and second-generation EGFR TKIs (such as afatinib and dacomitinib) are used in clinical practice as first-line treatment on patients with EGFR-sensitizing mutations (although osimertinib, a third-generation TKI, is recommended by the National Comprehensive Cancer Net-work® [NCCN®] as a treatment option when the mutation is discovered prior to first line therapy) [
8]. First-generation TKIs inhibit the receptors of EGFR by reversibly binding to the intracellular kinase domain of EGFR, inhibiting autophosphorylation and therefore reducing intracellular signaling cascades that predispose cells to en-hanced proliferation. However, most patients acquire resistance to these agents after a median of 10.9 to 14.7 months [
10,
11,
12]. The most common secondary resistance mechanism is the EGFR T790M mutation, which is the cause of about 50-60% of ac-quired resistance to first-generation and second-generation TKIs [
13,
14]. The T790M mutation causes resistance by increasing EGFR’s affinity for ATP back to wild-type levels, causing EGFR-TKIs to be outcompeted by ATP and their efficacy decreased [
15]. Second-generation TKIs were designed in response to acquired resistance to first gen-eration TKIs due to alternate signaling pathways through other members of the ErbB family. These second-generation TKIs irreversibly inhibit EGFR, HER2, HER3, and HER4 (all members of the ErbB family), overcoming a factor of resistance that was present in their first-generation counterparts; however, T790M still renders sec-ond-generation TKIs ineffective [
16].
Osimertinib is a third-generation EGFR TKI used as first-line or adjuvant treatment in patients with locally advanced or metastatic NSCLC with EGFR sensitizing mutations and T790M; however, it has also been approved for first-line use for patents with just EGFR-sensitizing mutations [
17]. It has proven to be superior in terms of progression free survival (PFS) over first and second generation TKIs in most populations [
18,
19,
20]. However, as with its predecessors, resistance eventually develops. Mech-anisms of resistance (in order of frequency) include EGFR exon 20 C797S mutations (more frequent in second-line setting) or due to the development of MET amplification (more common in first line setting. C797S prevents osimertinib from forming covalent bonds in the ATP-binding domain of EGFR kinase, resulting in EGFR-TKI dysfunction [
21].
CRISPR/Cas9 Based Treatment
One compelling solution to the problem of acquired drug resistance in cancer treat-ment— particularly since resistance is acquired through mutations— is to correct the mutation with a genome editing approach. The CRISPR/Cas9 system has allowed the ability to specifically target and edit mammalian genome with precision. Furthermore, recent FDA approval of CRISPR-based therapies targeting germline-caused sickle cell diseases has underscored the clinical relevance of this approach. Casgevy (ex-agamglogene autotemcel) is the first FDA approved CRISPR based treatment and has had positive outcomes with Sickle Cell Disease (SCD) in patients with recurrent vaso-occlusive crises and are twelve years of age and older. Patients’ hematopoietic stem cells are extracted and edited using a CRISPR/Cas9 system and a lentiviral vector. The edited stem cells are reintroduced to the patient and increase the production of fe-tal hemoglobin, which replaces sickled blood cells [
22].
In the past decade, there have been various studies on the use of CRISPR-based gene editing for treatment of a multitude of diseases and disorders. Heart-1 is a Phase 1b clinical trial utilizing VERVE-101, a CRISPR-based gene editing therapy that is deliv-ered in vivo to hepatocytes via a lipid nanoparticle vehicle to inactivate PCSK9, a gene that causes low density lipoprotein-cholesterol (LDL-C) to raise to dangerously high levels, resulting in plaque buildup in the arteries. This treatment was administered by a single intravenous infusion in patients with Heterozygous familial hypercholester-olemia. There was durable 39%-55% reduction of LDL-C and no evidence of off-target editing in liver cells. This demonstrated the first proof-of-concept for in vivo DNA base editing in humans [
23].
The strategies used in these treatments can be applied to the reversal of T790M and C797S. To reverse a point mutation, a homology-directed repair (HDR) pathway ap-pears to be the desired mechanism to achieve precise editing. In comparison to the nonhomologous end joining (NHEJ) pathway, HDR tends to be less efficient due to competition with the NHEJ pathway. However, there have been numerous strategies found to increase the efficiency of the HDR pathway such as inhibiting the NHEJ pathway, regulating HDR-related factors, cell cycle synchronization, optimally de-signing the donor DNA template, and optimizing the proximity of the CRISPR and donor DNA components [
24].
The proposed process to implement this would consist of an initial biopsy and Next Generation Sequencing (NGS), the designation of a CRISPR/Cas9 to directly target a specific somatic mutation, the delivery to the cancer, and another biopsy to determine if the treatment was successful. This proposed treatment is specifically for patients with lung adenocarcinoma who have acquired resistance to the TKIs used for treat-ment due to T790M or C797S mutations. This should be determined through NGS of EGFR exon 20 (where both T790M and C797S occur), which would likely be tested in tandem with other commonly mutated regions of EGFR, exons 18 to 21 [
3]. Knowing exactly which mutations exist in the cancer will allow for the proper usage of CRISPR/Cas9 and the genome editing would be applied in vivo. In order to design a single guide RNA (sgRNA) for a CRISPR/Cas9 system, you must identify a protospac-er-adjacent motif (PAM) adjacent to the target site (NGG for Cas9). The sample used for NGS would be acquired through a biopsy of the tumor via bronchoscopy or in the case of metastasis to pleura, via thoracentesis.
Delivery of the CRISPR/Cas9 System
The CRISPR/Cas9 system may be delivered in the following ways: 1) directly to the tumor via intra-tumoral injection delivery utilizing robotic bronchoscopy, 2) to the pulmonary vasculature via a pulmonary artery catheter and/or selective bronchial embolization, 3) intrapleural administration via chest tube in the case of pleural me-tastasis. Itra-tumoral injection delivery via bronchoscopy is performed under general anesthesia and requires endotracheal intubation. The bronchoscope is directed manu-ally or by robotic platform into the edge of the lung and a needle would be advanced into the tumor to deliver the CRISPR/Cas9 system. Complications may be anesthesia related (central nervous system toxicity, hemodynamic instability, methemoglo-binemia, malignant hyperthermia), bleeding, pneumothorax (1% to 3%), cardiac ar-rhythmias, vocal cord injury, pneumomediastinum, hypoxia and rarely death [
25].
Because there is dual blood supply to the lung— arterial blood via the bronchial arter-ies from the aorta and the pulmonary artery from the right ventricle— depending on where the blood comes from to the tumor, delivery to the pulmonary vasculature via a pulmonary artery (PA) catheter and/or selective bronchial embolization is possible. A PA catheter is inserted into a large vein (eg. femoral or brachial) and advanced to the right atrium, right ventricle and floated to the pulmonary artery and the CRISPR/Cas9 system would be delivered to the branch leading to the tumor. For bronchial emboliza-tion, a catheter is inserted into the femoral artery and advanced to the bronchial ar-tery. Potential complications may include pneumothorax, hemothorax, arrhythmias, valve rupture, cardiac perforation, pulmonary infarction [
26].
In the case of pleural metastasis, intrapleural administration via chest tube may be ap-plicable. Chest tube placement (chest tube thoracostomy) is placed with local anesthe-sia and can be used to deliver the CRISPR/Cas9 system into the pleural cavity. Alterna-tively, an indwelling pleural catheter can be inserted in a similar fashion and can also be used for persistent pleural effusions and also deliver CRISPR to the pleural cavity. Both can be performed as an outpatient however, pain at the insertion site is common [
27]. Potential complications may include bleeding, infection, injury to spleen, liver, heart or aorta [
28].
To assess the efficacy of the treatment a re-biopsy should be performed with NGS and if successful, TKI therapy should be continued.
The CRISPR/Cas9 system that is encoded by plasmid will be encapsulated by a lipid nanoparticle. Lipid nanoparticles are one of the most common non viral nucleic acid delivery methods and can protect the CRISPR/Cas9 system from destruction from nu-clases and enter target cells via endocytosis [
29].
Conclusion
When patients acquire resistance to all of the EGFR-TKIs on the market, often their next best option is chemotherapy. However, osimertinib has shown to be the better treatment for these patients– the median progression-free survival (PFS) of osimertinib is 18.9 months [
19] compared to 5.2 months on platinum-based chemotherapy and the objective response rate is about 70% with osimertinib and 30% with chemotherapy [
30]. Because of this, many researchers are working on a fourth-generation TKI that specifically targets C797S, however, it appears that inevitably, there will be acquired resistance to those new TKIs through various, unpredictable means. Therefore, rather than developing a new TKI when resistance is discovered, it seems reasonable to sug-gest a treatment that re-allows osimertinib to be effective.
Author Contributions
Conceptualization: M.K.A.N.; project administration: Q.D., M.P.L. ; su-pervision: Q.D., M.P.L.; writing- original draft: M.K.A.N.; writing—review and editing: M.K.A.N. , Q.D., M.P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article material, further inquiries can be directed to the corresponding author.
Acknowledgments
Eric A. Crawley, MD, FCCP, Pulmonology, Hawaii Pacific Health Medical Group, is thanked for his insight on the potential delivery methods of CRISPR; Melvin P. Palalay, MD Oncology and Hematology, Hawaii Cancer Care is thanked for his review and critique of the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lim SM, Cho BC, Han JY, et al. Phase 1/2 clinical trial of JIN-A02, a 4th generation EGFR-TKI, in patients with 3rd generation EGFR-TKI resistance in EGFR mutated advanced/metastatic non-small cell lung cancer (NSCLC). Journal of Clinical Oncology. 2024;42(16_suppl):TPS8658-TPS8658. Accessed September 26, 2024. [CrossRef]
- Mansour MA, AboulMagd AM, Abbas SH, Abdel-Rahman HM, Abdel-Aziz M. Insights into fourth generation selective in-hibitors of (C797S) EGFR mutation combating non-small cell lung cancer resistance: a critical review. RSC Advances. 2023;13(27):18825-18853 Accessed September 26, 2024. [CrossRef]
- Uchibori K, Inase N, Araki M, et al. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nature Communications. 2017;8. Accessed September 1, 2024. Available online: https://www.jfcr.or.jp/english/chemotherapy/pickup/backnumber_005.html.
- Myers DJ, Wallen JM. Lung Adenocarcinoma. In: StatPearls [Internet]. StatPearls Publishing; 2020. Accessed August 19, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519578/#:~:text=Lung%20adenocarcinoma%20is%20the%20most,death%20in%20the%20United%20States.
- Vendrell JA, Quantin X, Aussel A, Solassol I, Serre I, Solassol J. EGFR-dependent mechanisms of resistance to osimertinib de-termined by ctDNA NGS analysis identify patients with better outcome. Translational Lung Cancer Research. 2021;10(11):4084-4094. Accessed August 26, 2024. [CrossRef]
- Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Molecular Oncology. 2018;12(1). Accessed August 21, 2024. [CrossRef]
- Lund-Iversen M, Kleinberg L, Fjellbirkeland L, Helland Å, Brustugun OT. Clinicopathological Characteristics of 11 NSCLC Patients with EGFR-Exon 20 Mutations. Journal of Thoracic Oncology. 2012;7(9):1471-1473. Accessed August 16, 2024. [CrossRef]
- Riely GJ, Wood DE, Ettinger DS, et al. Non–Small Cell Lung Cancer, Version 4.2024, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network. 2024;22(4):249-274. Accessed August 20, 2024. [CrossRef]
- Ha SY, Choi SJ, Cho JH, et al. Lung cancer in never-smoker Asian females is driven by oncogenic mutations, most often in-volving EGFR. Oncotarget. 2015;6(7):5465-5474. Accessed August 20, 2024. [CrossRef]
- Sequist L v., Yang JCH, Yamamoto N, et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients With Metastatic Lung Adenocarcinoma With EGFR Mutations. Journal of Clinical Oncology. 2013;31(27):3327-3334. Accessed August 20, 2024. [CrossRef]
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. The Lancet Oncology. 2017;18(11):1454-1466. Accessed August 20, 2024. [CrossRef]
- Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. The Lancet Oncology. 2016;17(5):577-589. Accessed August 22, 2024. [CrossRef]
- Wu SG, Liu YN, Tsai MF, et al. The mechanism of acquired resistance to irreversible EGFR tyrosine kinase inhibitor-afatinib in lung adenocarcinoma patients. Oncotarget. 2016;7(11):12404-12413. Accessed August 20, 2024. Available online: https://pubmed.ncbi.nlm.nih.gov/26862733/.
- Wang S, Cang S, Liu D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. Journal of Hematology & Oncology. 2016;9(1):34. Accessed September 15, 2024. [CrossRef]
- Yun CH, Mengwasser KE, Toms A v., et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(6). Accessed September 15, 2024. [CrossRef]
- Shah R, Lester JF. Tyrosine Kinase Inhibitors for the Treatment of EGFR Mutation-Positive Non–Small-Cell Lung Cancer: A Clash of the Generations. Clinical Lung Cancer. 2020;21(3). Accessed September 15, 2024. [CrossRef]
- TAGRISSO- osimertinib tablet, film coated. DailyMed. Published online April 29, 2024. Accessed September 1, 2024. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5e81b4a7-b971-45e1-9c31-29cea8c87ce7.
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR -Mutated Advanced Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2018;378(2):113-125. Accessed September 26, 2024. [CrossRef]
- Yang Y, Liu Q, Cao L, et al. Osimertinib versus afatinib in patients with T790M-positive, non-small-cell lung cancer and multiple central nervous system metastases after failure of initial EGFR-TKI treatment. BMC Pulmonary Medicine. 2021;21(1):172. Ac-cessed September 26, 2024. [CrossRef]
- Ito K, Morise M, Wakuda K, et al. A multicenter cohort study of osimertinib compared with afatinib as first-line treatment for EGFR-mutated non-small-cell lung cancer from practical dataset: CJLSG1903. ESMO Open. 2021;6(3):100115. Accessed Sep-tember 26, 2024. [CrossRef]
- He J, Huang Z, Han L, Gong Y, Xie C. Mechanisms and management of 3rd-generation EGFR-TKI resistance in advanced non-small cell lung cancer (Review). International Journal of Oncology. 2021;59(5):90. Accessed September 15, 2024. [CrossRef]
- FDA Approves First Gene Therapies to Treat Patients with Sickle Cell Disease. Food and Drug Administration. Published online December 8, 2023; Accessed September 15, 2024. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapies-treat-patients-sickle-cell-disease.
- Horie T, Ono K. VERVE-101: a promising CRISPR-based gene editing therapy that reduces LDL-C and PCSK9 levels in HeFH patients. European Heart Journal - Cardiovascular Pharmacotherapy. 2024;10(2):89-90. Accessed September 26, 2024. [CrossRef]
- Feng S, Wang Z, Li A, et al. Strategies for High-Efficiency Mutation Using the CRISPR/Cas System. Frontiers in Cell and De-velopmental Biology. 2022;9 Accessed September 22, 2024. [CrossRef]
- Mahmoud N, Vashisht R, Sanghavi DK, et al. Bronchoscopy Stat Pearls Internet 2023; Accessed September 1, 2024; 1. Available online: https://www.ncbi.nlm.nih.gov/books/NBK448152/.
- Rodriguez Ziccardi M, Khalid N. Pulmonary Artery Catheter, Stat Pearls; 2023; Accessed September 5, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482170/.
- Luketich JD, Kiss M, Hershey J, et al. Chest Tube Insertion: A Prospective Evaluation of Pain Management. The Clinical Journal of Pain. 1998;14(2):152-154. ; Accessed September 15, 2024. [CrossRef]
- Ravi C, McKnight CL. Chest Tube. In: StatPearls [Internet]. StatPearls Publishing; 2024. Accessed September 14, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459199/.
- Huang L, Liao Z, Liu Z, Chen Y, Huang T, Xiao H. Application and Prospect of CRISPR/Cas9 Technology in Reversing Drug Resistance of Non-Small Cell Lung Cancer. Frontiers in pharmacology. 2022;13:900825. Accessed August 7, 2024. [CrossRef]
- He J, Huang Z, Han L, Gong Y, Xie C. Mechanisms and management of 3rd-generation EGFR-TKI resistance in advanced non-small cell lung cancer (Review). International Journal of Oncology. 2021;59(5):90. Accessed August 5, 2024. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).