Submitted:
11 October 2024
Posted:
11 October 2024
You are already at the latest version
Abstract
Keywords:
1. General Background
2. Interfacial Charge Transfer Complexes
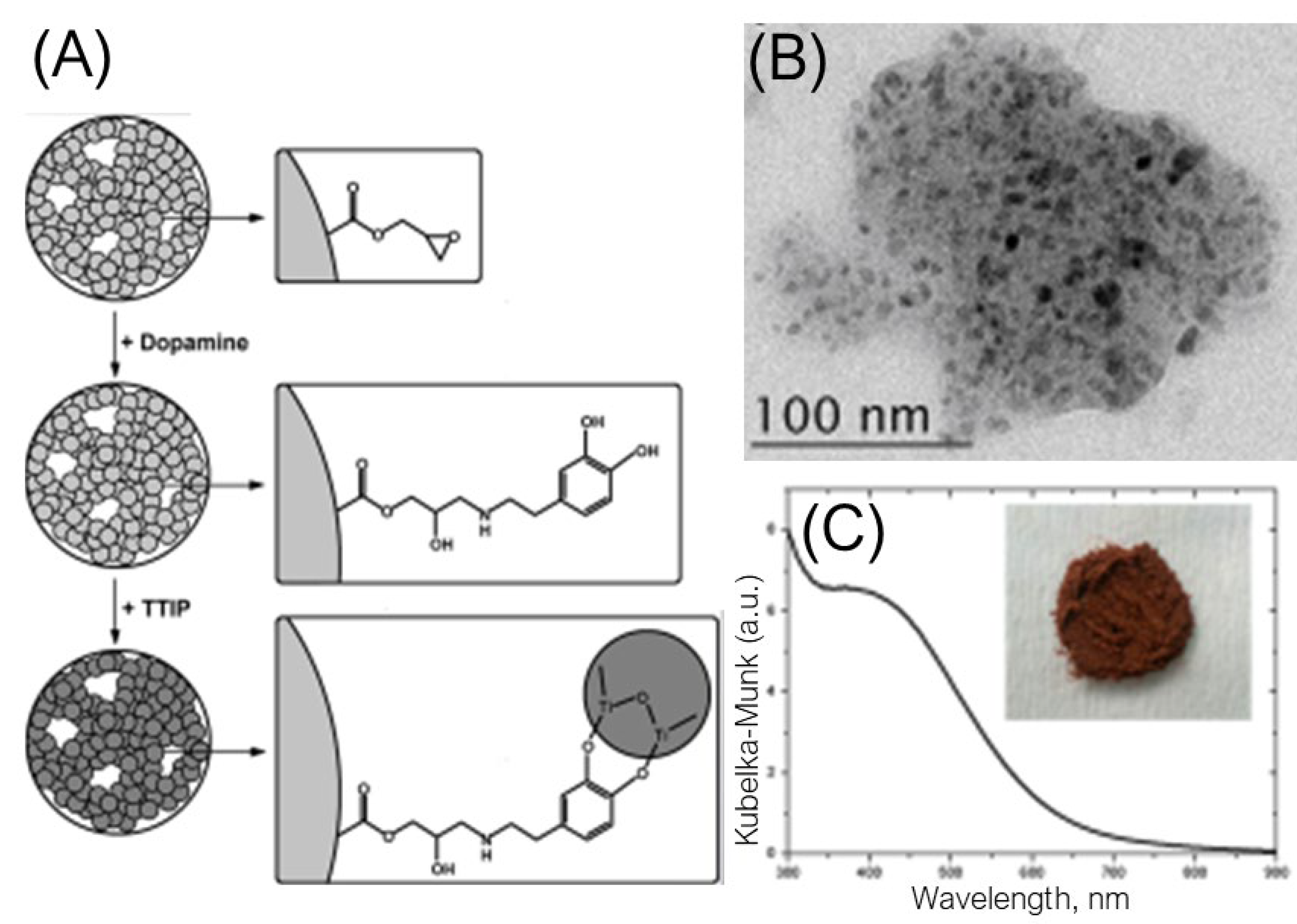
2.1. Synthesis
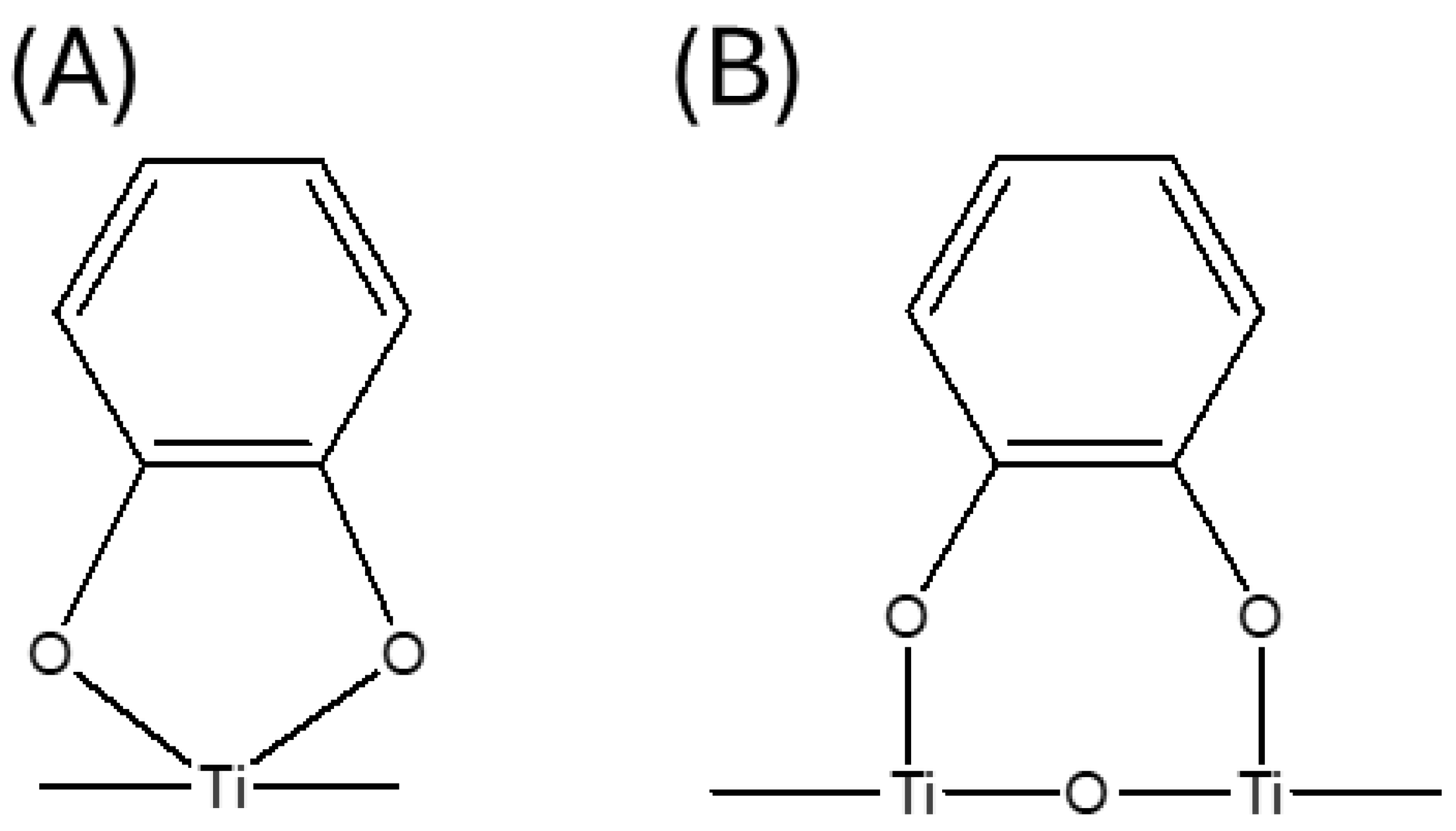
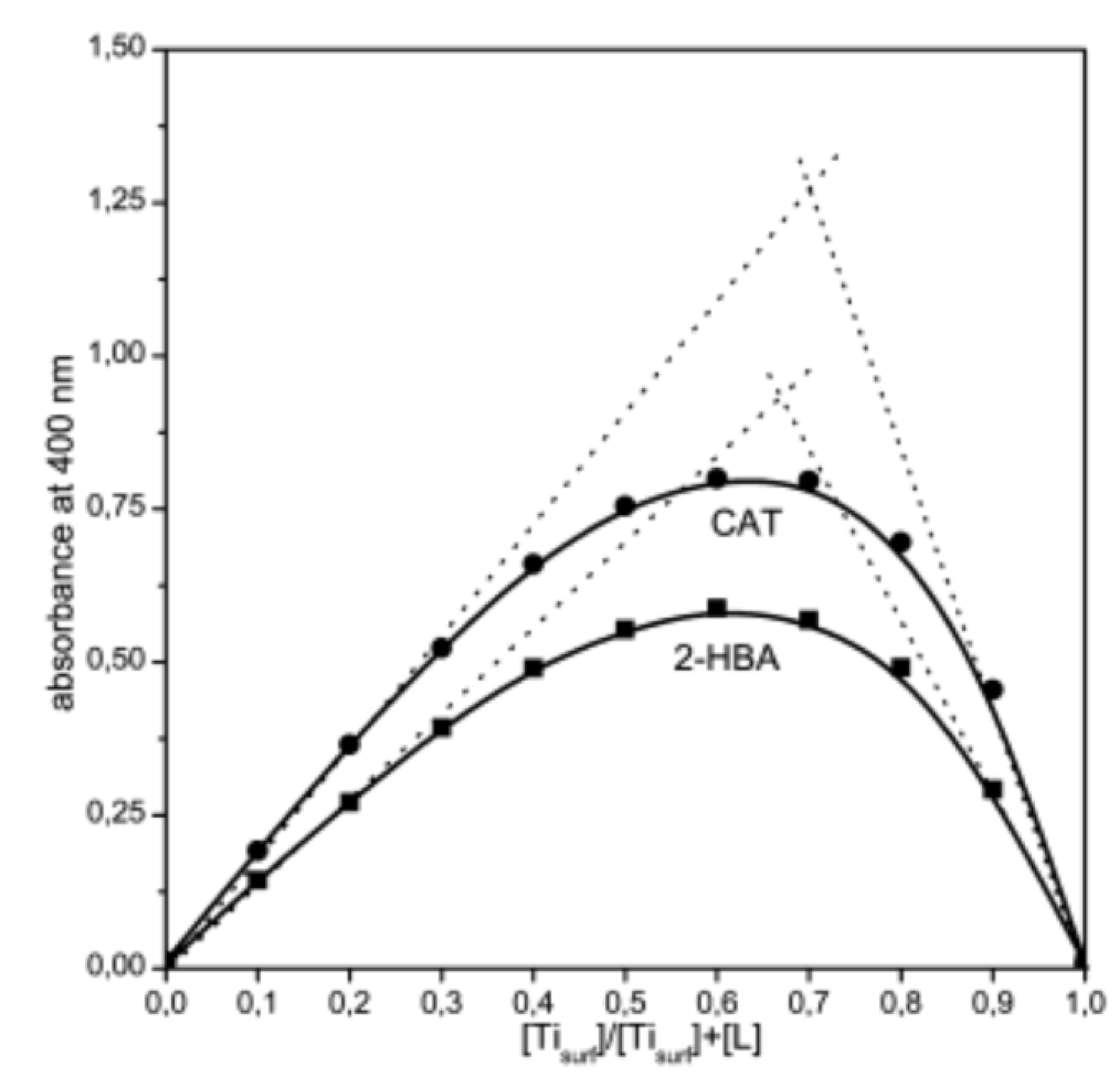
2.2. Composition and Surface Structure
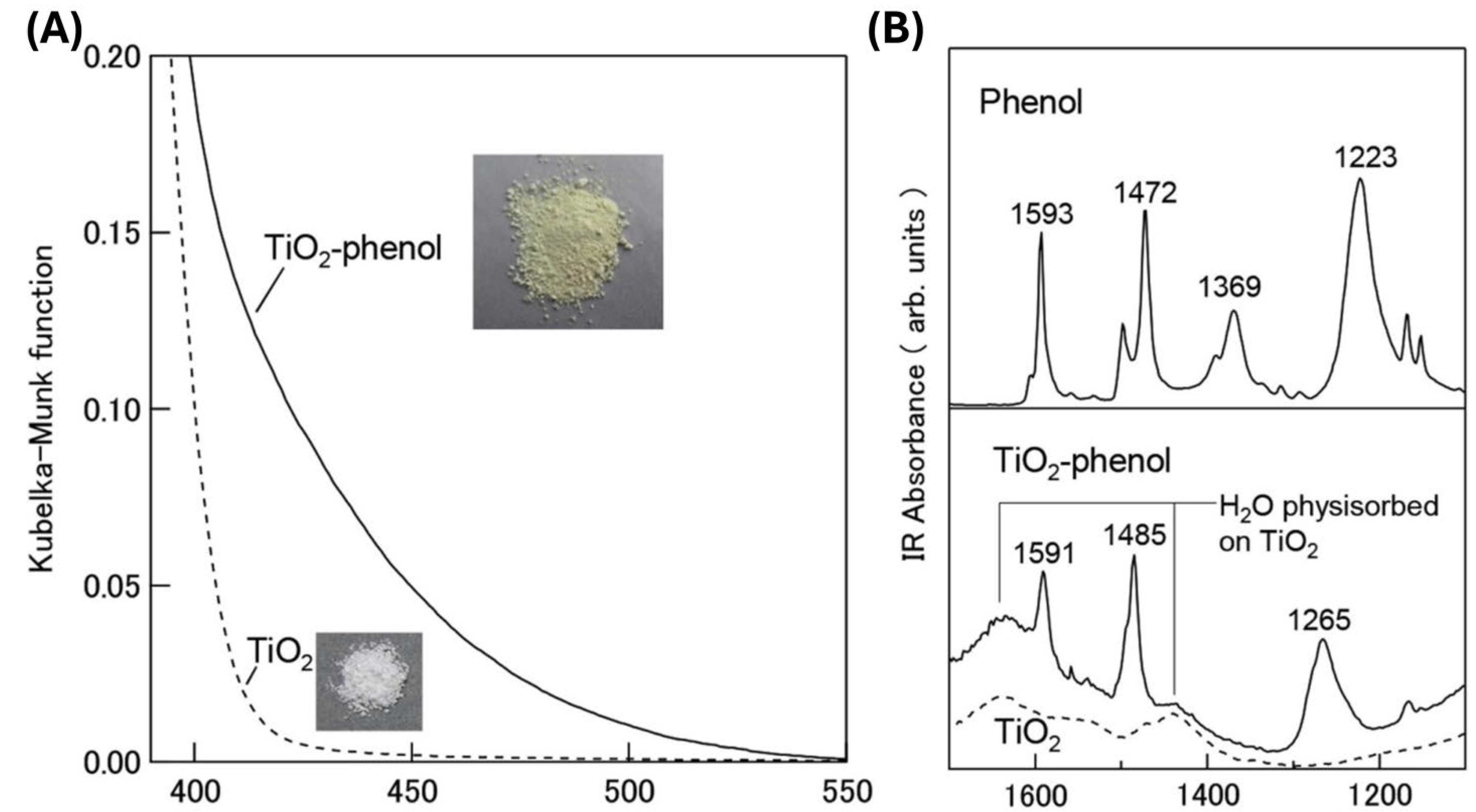
2.3. Fine-Tuning of Optical Properties – The Role of Free Functional Group
3. Photocatalytic Degradation of Organic Pollutants
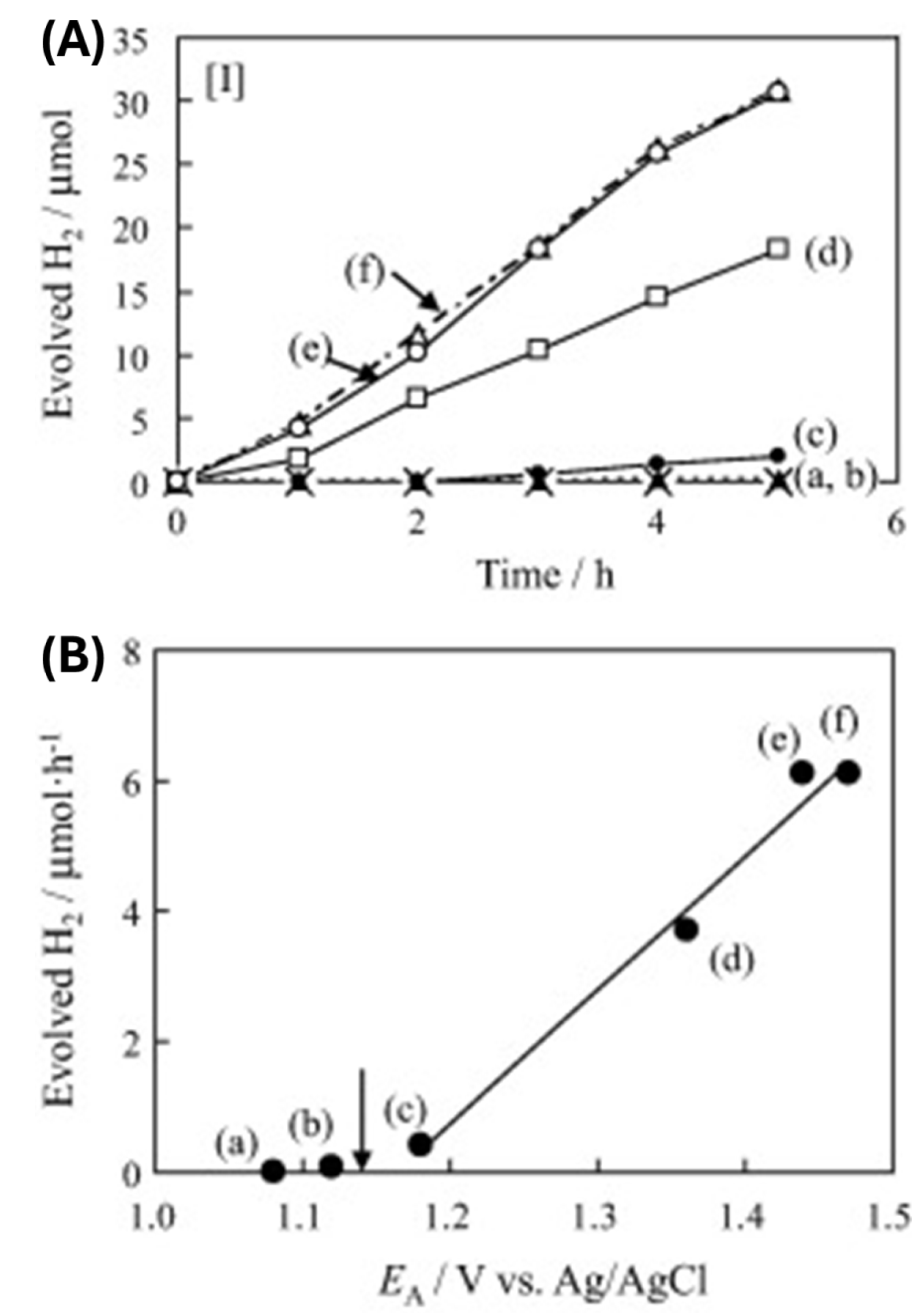
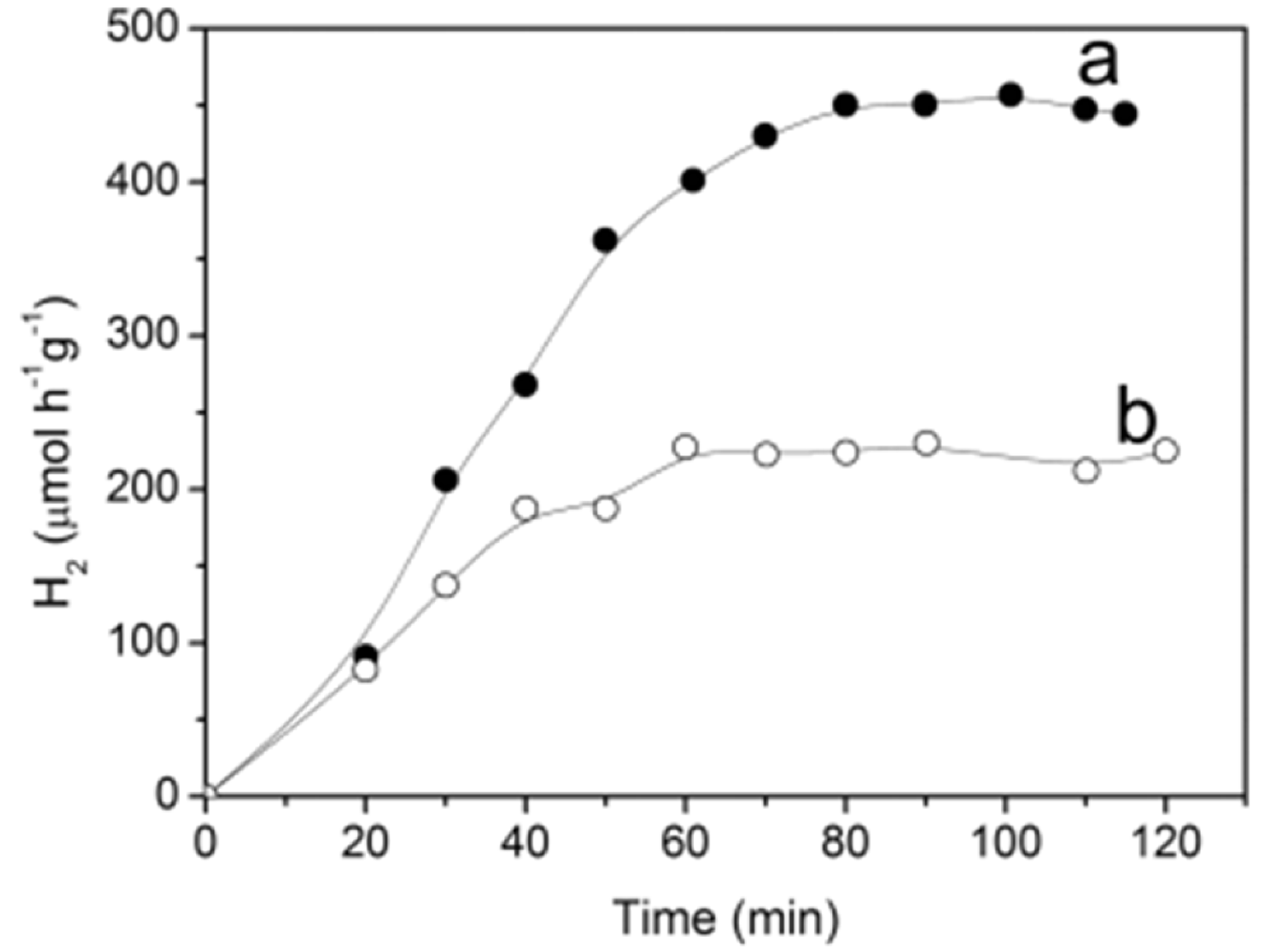
4. Photocatalytic Hydrogen Generation
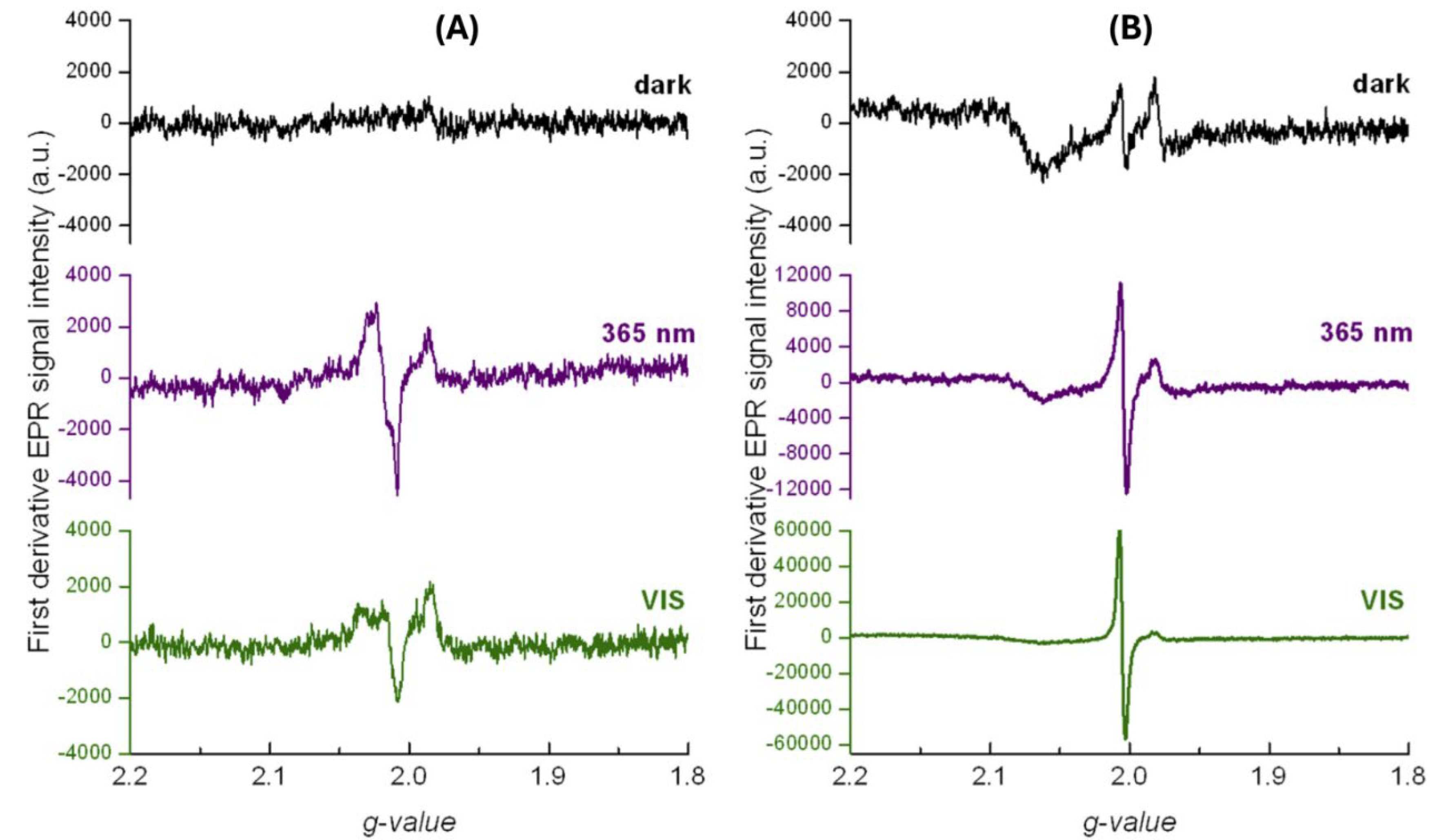
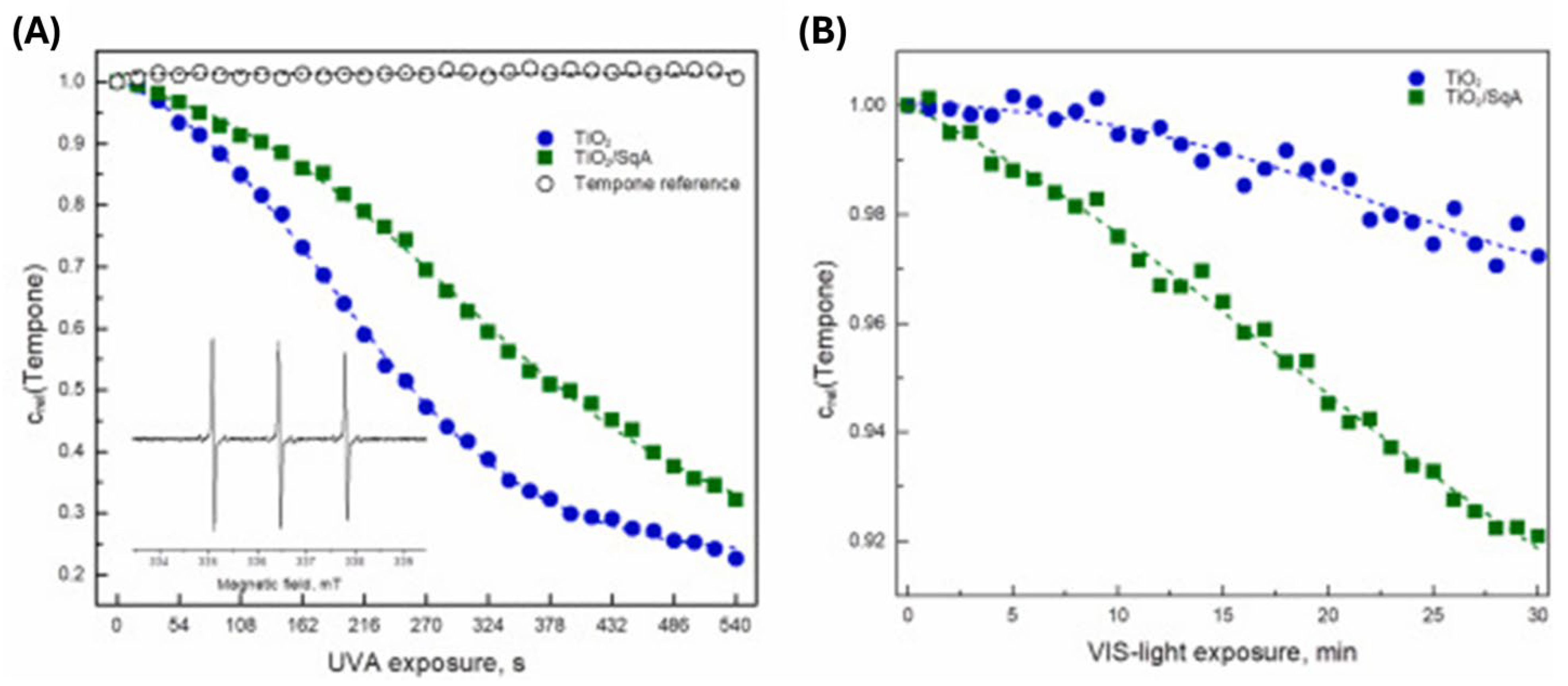
5. Identification of Reactive Species
6. Perspectives for Future Studies
Acknowledgments
References
- A. Fujishima, K. Honda, Electrochemical Photolysis of Water at a Semiconductor Electrode, Nature, 238, 37-38 (1972).
- M.R. Hoffmann, S.T. Martin, W. Choi, D.W. Bahnemann, Environmental applications of semiconductor photocatalysis, Chem. Rev., 95, 69-96 (1995).
- A. Kudo, Y. Miseki, Heterogeneous photocatalyst materials for water splitting, Chem. Soc. Rev., 38, 253-278 (2009).
- T. Bak, J. Nowotny, M. Rekas, C.C. Sorrell, Photo-electrochemical hydrogen generation from water using solar energy. Materials-related aspects, Int. J. Hydrogen Energ., 27, 991-1022 (2002).
- M. Kosmulski, The significance of the difference in the point of zero charge between rutile and anatase, Adv. Colloid Interface Sci., 99, 255-264 (2002).
- A. Kraeutler, A.J. Bard, Photoelectrosynthesis of Ethane from Acetate Ion at an n-Type TiO2 Electrode. The Photo-Kolbe Reaction, J. Am. Chem. Soc., 99, 7729-7731 (1977).
- A. Kraeutler, A.J. Bard, Heterogeneous Photocatalytic Decomposition of Saturated Carboxylic Acids on TiO2 Powder. Decarboxylative Route to Alkanes, J. Am. Chem. Soc., 100, 5985-5992 (1978).
- S.T. Martin, H. Herrmann, W. Choi, M.R. Hoffmann, Time-resolved microwave conductivity. Part 1.‒TiO2 photoreactivity and size quantization. Trans. Faraday Soc., 90, 3315-3323 (1994).
- S.T. Martin, H. Herrmann, M.R. Hoffmann, Time-resolved microwave conductivity. Part 2.‒Quantum-sized TiO2 and the effect of adsorbates and light intensity on charge-carrier dynamics, Faraday Soc., 90, 3323-3330 (1994).
- C.G. Silva, R. Juárez, T. Marino, R. Molinari, H. García, Influence of Excitation Wavelength (UV or Visible Light) on the Photocatalytic Activity of Titania Containing Gold Nanoparticles for the Generation of Hydrogen or Oxygen from Water, J. Am. Chem. Soc., 133, 595-602 (2011).
- A. Meng, L. Zhang, B. Cheng, J. Yu, Dual Cocatalysts in TiO2 Photocatalysis, Adv. Mater., 31, 1807660 (2019).
- R. Asahi, T. Morikawa, K. Aoki, Y. Taga, Visible-light photocatalysis in nitrogen doped titanium oxides, Science, 293, 269-271 (2001).
- S. Sakthivel, H. Kisch, Angew. Daylight photocatalysis by carbon-modified titanium dioxide, Chem. Int. Ed., 42, 4908-4911 (2003).
- X. Chen, C. Burda, The Electronic Origin of the Visible-Light Absorption Properties of C-, N- and S-Doped TiO2 Nanomaterials, J. Am. Chem. Soc., 130, 5018-5019 (2008).
- J. Kiwi, E. Pelizzetti, M. Visca, M. Grätzel, Sustained Water Cleavage by Visible Light, J. Am. Chem. Soc., 103, 6324-6329 (1981).
- B. O’Regan, M. Grätzel, A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films, Nature, 353, 737-740 (1991).
- S. Shen, L. Zhao, Z. Zhou, L. Guo, Enhanced Photocatalytic Hydrogen Evolution over Cu-Doped ZnIn2S4 under Visible Light Irradiation, J. Phys. Chem. C, 112, 16148-16155 (2008).
- J. Low, J. Yu, M. Jaroniec, S. Wageh, A.A. Al-Ghamdi, Heterojunction Photocatalysts, Adv. Mater., 29, 1601694 (2017).
- Q. Xu, L. Zhang, J. Yu, S. Wageh, A.A. Al-Ghamdi, M. Jaroniec, Direct Z-scheme photocatalysts: Principles, synthesis, and applications, Mater. Today, 21, 1042-1063 (2018).
- T. Rajh, J.M. Nedeljković, L.X. Chen, O. Poluektov, M.C. Thurnauer, Improving optical and charge separation properties of nanocrystalline TiO2 by surface modification with vitamin C, J. Phys. Chem. B, 103, 3515-3519 (1999).
- S. Higashimoto, T. Nishi, M. Yasukawa, M. Azuma, Y. Sakata, H. Kobayashi, Photocatalysis of titanium dioxide modified by catechol-type interfacial surface complexes (ISC) with different substituted groups, J. Catal., 329, 286-291 (2015).
- B. Milićević, V. Ðorđević, D. Lončarević, S.P. Ahrenkiel, M.D. Dramićanin, J.M. Nedeljković, Visible light absorption of surface modified TiO2 powders with bidentate benzene derivatives, Micropor. Mesopor. Mat., 217, 184-189 (2015).
- Yao, C. Peng, P. Lu, Y. He, W. Zhang, Q. Zhang, Fabrication of Tiron-TiO2 charge-transfer complex with excellent visible-light photocatalytic performance, Mat. Chem. Phys., 184, 298-305 (2016).
- Vukoje, T. Kovač, J. Džunuzović, E. Džunuzović, D. Lončarević, S.P. Ahrenkiel, J.M. Nedeljković, Photocatalytic ability of visible-light-responsive TiO2 nanoparticles, J. Phys. Chem. C, 120, 18560-18569 (2016).
- S. Günes, N. Marjanović, J.M. Nedeljković, N.S. Sariciftci, Photovoltaic characterization of hybrid solar cells using surface modified TiO2 nanoparticles and poly(3-hexyl)thiophene, Nanotechnology, 19, 424009 (2008).
- J. Fujisawa, M. Nagata, Efficient light-to-current conversion by organic-inorganic interfacial charge-transfer transitions in TiO2 chemically adsorbed with 2-anthroic acid, Chem. Phys. Lett., 619, 180-184 (2015).
- S.-B. Ko, T.I. Ryu, A.-N. Cho, J. Fujisawa, H. Segawa, N.-G. Park, Visible light absorption and photoelectrochemical activity of colorless molecular 1,3-bis (dicyanomethylidene)indane (BDMI) by surface complexation on TiO2, Phys. Chem. Chem. Phys., 17, 18541-18546 (2015).
- M. Shahriari-Khalaji, F. Zabihi, A. Bahi, D. Sredojević, J.M. Nedeljković, D.K. Macharia, M. Ciprian, S. Yang, F. Ko, Photon-driven bactericidal performance of surface-modified TiO2 nanofibers, J. Mater. Chem. C, 11, 5796-5805 (2023).
- I.A. Janković, Z.V. Šaponjić, M.I. Čomor, J.M. Nedeljković, Surface modification of colloidal TiO2 nanoparticles with bidentate benzene derivatives, J. Phys. Chem. C, 113, 12645-12652 (2009).
- P. Persson, R. Bergström, S. Lunell, Quantum Chemical Study of Photoinjection Processes in Dye-Sensitized TiO2 Nanoparticles. J. Phys. Chem. B, 104, 10348-10351 (2000).
- J. Fujisawa, M. Hanaya, Light harvesting and direct electron injection by interfacial charge-transfer transitions between TiO2 and carboxy-anchor dye LEG4 in dye-sensitized solar cells, J. Phys. Chem. C, 122, 8-15 (2018).
- T. Rajh, Z. Šaponjić, J. Liu, N.M. Dimitrijević, N.F. Scherer, M. Vega-Arroyo, P. Zapol, L.A. Curtiss, M.C. Thurnauer, Charge transfer across the nanocrystalline-DNA interface: probing DNA recognition, Nano Lett., 4, 1017-1023 (2004).
- L.X. Chen, T. Rajh, W. Jäger, J. Nedeljković, M.C. Thurnauer, X-ray absorption reveals surface structure of titanium dioxide nanoparticles, J. Synchrotron Rad., 6, 445-447 (1999).
- I.M. Dugandžić, D.J. Jovanović, L.T. Mančić, N. Zheng, S.P. Ahrenkiel, O.B. Milošević, Z.V. Šaponjić, J.M. Nedeljković, Surface modification of submicronic TiO2 particles prepared by ultrasonic spray pyrolysis for visible light absorption, J. Nanopart. Res., 14, 1157-1167 (2012).
- I.M. Dugandžić, D.J. Jovanović, L.T. Mančić, O.B. Milošević, S.P. Ahrenkiel, Z.V. Šaponjić, J.M. Nedeljković, Ultrasonic spray pyrolysis of surface modified TiO2 nanoparticles with dopamine, Mater. Chem. Phys., 143, 233-239 (2013).
- J. Fujisawa, R. Muroga, M. Hanaya, Interfacial charge-transfer transitions in a TiO2-benzenedithiol complex with Ti–S–C linkages, Phys. Chem. Chem. Phys., 17, 29867-29873 (2015).
- J. Fujisawa, S. Matsumura, M. Hanaya, A single Ti‒O‒C linkage induces interfacial charge-transfer transitions between TiO2 and a π-conjugated molecule, Chem. Phys. Lett., 657, 172-176 (2016).
- J. Fujisawa, T. Eda, G. Giorgi, M. Hanaya, Visible-to-Near-IR Wide-Range Light Harvesting by Interfacial Charge-Transfer Transitions between TiO2 and p-Aminophenol and Evidence of Direct Electron Injection to the Conduction Band of TiO2, J. Phys. Chem. C, 121, 18710-18716 (2017).
- D.N. Sredojević, T. Kovač, E. Džunuzović, V. Ðorđević, B.N. Grgur, J.M. Nedeljković, Surface-modified TiO2 powders with phenol derivatives: A comparative DFT and experimental study, Chem. Phys. Lett., 686, 167-172 (2017).
- Z. Barbieriková, D. Dvoranová, V. Brezová, E. Džunuzović, D.N. Sredojević, V. Lazić, J.M. Nedeljković, Visible-light-responsive surface-modified TiO2 powder with 4-chlorophenol: A combined experimental and DFT study, Opt. Mater., 89, 237-242 (2019).
- V. Lazić, D. Sredojević, A. Ćirić, J.M. Nedeljković, G. Zelenková, M. Férová, T. Zelenka, M.P. Chavhan, V. Slovák, The photocatalytic ability of visible-light-responsive interfacial charge transfer complex between TiO2 and Tiron, J. Photoch. Photobio. A, 449, 115394 (2024).
- I.A. Janković, Z.V. Šaponjić, E.S. Džunuzović, J.M. Nedeljković, New hybrid properties of TiO2 nanoparticles surface modified with catecholate type ligands, Nanoscale Res. Lett., 5, 81-88 (2010).
- T.D. Savić, I.A. Janković, Z.V. Šaponjić, M.I. Čomor, D.Ž. Veljković, S.D. Zarić, J.M. Nedeljković, Surface modification of anatase nanoparticles with fused catecholate type ligands: a combined DFT and experimental study of optical properties, Nanoscale, 4, 1612-1619 (2012).
- T.D. Savić, Z.V. Šaponjić, M.I. Čomor, J.M. Nedeljković, M.D. Dramićanin, M.G. Nikolić, D.Ž. Veljković, S.D. Zarić, I.A. Janković, Surface modification of anatase nanoparticles with fused ring salicylate-type ligands (3-hydroxy-2-naphtoic acids): a combined DFT and experimental study of optical properties, Nanoscale, 5, 7601-7612 (2013).
- T.D. Savić, M.I. Čomor, J.M. Nedeljković, D.Ž. Veljković, S.D. Zarić, V.M. Rakić, I.A. Janković, The effect of substituents on the surface modification of anatase nanoparticles with catecholate-type ligands: a combined DFT and experimental study, Phys. Chem. Chem. Phys., 16, 20796-20805 (2014).
- T.D. Savić, M.I. Čomor, N.D. Abazović, Z.V. Šaponjić, M.T. Marinović-Cincović, D.Ž. Veljković, S.D. Zarić, I.A. Janković, Anatase nanoparticles surface modified with fused ring salicylate-type ligands (1-hydroxy-2-naphthoic acids): a combined DFT and experimental study, J. Alloy Compd., 630, 226-235 (2015).
- V. Bajić, B. Spremo-Potparević, L. Živković, A. Čabarkapa, J. Kotur-Stevuljević, E. Isenović, D. Sredojević, I. Vukoje, V. Lazić, S.P. Ahrenkiel, J.M. Nedeljković, Surface-modified TiO2 nanoparticles with ascorbic acid: Antioxidant properties and efficiency against DNA damage in vitro, Colloid Surface B, 155, 323-331 (2017).
- D. Dekanski, B. Spremo-Potparević, V. Bajić, L. Živković, D. Topalović, D.N. Sredojević, V. Lazić, J.M. Nedeljković, Acute toxicity study in mice of orally administrated TiO2 nanoparticles functionalized with caffeic acid, Food Chem. Toxicol., 115, 42-48 (2018).
- D.K. Božanić, G.A. Garcia, L. Nahon, D. Sredojević, V. Lazić, I. Vukoje, S.P. Ahrenkiel, V. Djoković, Ž. Šljivančanin, J.M. Nedeljković, Interfacial Charge Transfer Transitions in Colloidal TiO2 Nanoparticles Functionalized with Salicylic acid and 5-Aminosalicylic acid: A Comparative Photoelectron Spectroscopy and DFT Study, J. Phys. Chem. C, 123, 29057-29066 (2019).
- A. Todorović, K. Bobić, F. Veljković, S. Pejić, S. Glumac, S. Stanković, T. Milovanović, I. Vukoje, J.M. Nedeljković, S. Radojević Škodrić, S.B. Pajović, D. Drakulić, Comparable Toxicity of Surface-Modified TiO2 Nanoparticles: An In Vivo Experimental Study on Reproductive Toxicity in Rats, Antioxidants, 13, 231 (2024).
- T.S. Kovač, E.S. Džunuzović, J.V. Džunuzović, B. Milićević, D.N. Sredojević, E.N. Brothers, J.M. Nedeljković, Visible light absorption of TiO2 nanoparticles surface-modified with vitamin B6: A comparative experimental and DFT study, J. Serb. Chem. Soc., 83, 899-909 (2018).
- J. Fujisawa, S. Kato, M. Hanaya, Detailed study of a TiO2-phenol complex using deuterated phenol, Chem. Phys. Lett., 803, 139833 (2022).
- J. Fujisawa, S. Kato, M. Hanaya, Substituent effects on interfacial charge-transfer transitions in a TiO2-phenol complex, Chem. Phys. Lett., 827, 140688 (2023).
- T. Rajh, D.M. Tiede, M.C. Thurnauer, Surface modification of TiO2 nanoparticles with bidentate ligands studied by EPR spectroscopy J. Non-Cryst. Solids, 205-207, 815-820 (1996).
- M.C. Thurnauer, T. Rajh, D.M. Tiede, Surface Modification of TiO2: Correlation between Structure, Charge Separation and Reduction Properties, Acta Chem. Scand., 51, 610-618 (1997).
- J. Fujisawa, R. Muroga, M. Hanaya, Interfacial charge-transfer transitions and reorganization energies in sulfur-bridged TiO2-x-benzenedithiol complexes (x: o, m, p), Phys. Chem. Chem. Phys., 18, 22286‒22292 (2016).
- B. Milićević, V. Ðorđević, D. Lončarević, J.M. Dostanić, S.P. Ahrenkiel, M.D. Dramićanin, D. Sredojević, N.M. Švrakić, J.M. Nedeljković, Charge-transfer complex formation between TiO2 nanoparticles and thiosalicylic acid: A comprehensive experimental and DFT study, Opt. Mater., 73, 163-171 (2017).
- J. Fujisawa, N. Kaneko, M. Hanaya, Interfacial Charge-Transfer Transitions in TiO2 Nanoparticles Adsorbed with 4-Mercaptobenzenoic Acid: Carboxy versus Thiol Anchor and Adsorbate-to-TiO2 versus TiO2-to-Adsorbate Charge Transfer, J. Phys. Chem. C, 125, 13534-13541 (2021).
- J. Fujisawa, S. Kato, M. Hanaya, Interfacial Charge-Transfer Transitions in Weakly Coupled TiO2 Complexes with Aromatic Monothiols for Visible-Light Photocatalytic Reactions, J. Phys. Chem. C, 126, 11602-11610 (2022).
- S. Manzhos, R. Jono, K. Yamashita, J. Fujisawa, M. Nagata, H. Segawa, Study of Interfacial Charge Transfer Bands and Electron Recombination in the Surface Complexes of TCNE, TCNQ, and TCNAQ with TiO2, J. Phys. Chem. C, 115, 21487-21493 (2011).
- J. Fujisawa, M. Nagata, M. Hanaya, Charge-transfer complex versus σ-complex formed between TiO2 and bis(dicyanomethylene) electron acceptors, Phys. Chem. Chem. Phys., 17, 27343‒27356 (2015).
- B. Moongraksathum, P.T. Hsu, Y.W. Chen, Photocatalytic activity of ascorbic acidmodified.
- TiO2 sol prepared by the peroxo sol‒gel method. J. Sol-Gel Sci. Technol., 78, 647-659 (2016).
- J. Fujisawa, N. Kikuchi, M. Hanaya, Coloration of tyrosine by organic-semiconductor interfacial charge-transfer transitions, Chem. Phys. Lett., 664, 178-183 (2016).
- L. Tian, J. Xu, A. Alnafisah, R. Wang, X. Tan, N.A. Oyler, L. Liu, X. Chen, A novel green TiO2 photocatalyst with surface charge-transfer complex of Ti and hydrazine groups, Chem. Eur. J., 23, 5345-5351 (2017).
- Y. Sun, J.B. Mwandeje, L.M. Wangatia, F. Zabihi, J. Nedeljkovic, S. Yang, Enhanced Photocatalytic Performance of Surface-Modified TiO2 Nanofibers with Rhodizonic Acid, Adv. Fiber Mater., 2, 118-122 (2020).
- J. Fujisawa, Interfacial charge-transfer transitions between TiO2 and indole, Chem. Phys. Lett., 739, 136974 (2020).
- Z. Barbieriková, M. Šimunková, V. Brezová, D. Sredojević, V. Lazić, D. Lončarević, J.M. Nedeljković, Interfacial charge transfer complex between TiO2 and non-aromatic ligand squaric acid, Opt. Mater., 123, 111918 (2022).
- J. Fujisawa, S. Kato, M. Hanaya, Interfacial Charge-Transfer Transitions between TiO2 Nanoparticles and Benzoic Acid Derivatives J. Phys. Chem. C, 125, 25075-25086 (2021).
- J. Fujisawa, S. Kato, M. Hanaya, Aromatic-ring dependence of interfacial charge-transfer transitions between TiO2 nanoparticles and aromatic carboxylic acids, Chem. Phys. Lett., 788, 139274 (2022).
- J. Fujisawa, S. Kato, M. Hanaya, Adsorption, Electronic, and Optical Properties of TiO2–Pyridine Complexes: General Principles for Interfacial Charge-Transfer Transitions, J. Phys. Chem. C, 127, 17888-17895 (2023).
- D. Jaušovec, M. Božić, J. Kovač, J. Štrancar, V. Kokol, Synergies of phenolic-acids’ surface-modified titanate nanotubes (TiNT) for enhanced photo-catalytic activities, J. Colloid Interf. Sci., 38, 277-290 (2015).
- M.M. Medić, M. Vasić, A.R. Zarubica, L.V. Trandafilović, G. Dražić, M.D. Dramićanin, J.M. Nedeljković, Enhanced photoredox chemistry in surface-modified Mg2TiO4 nano-powders with bidentate benzene derivatives, RSC Adv., 6, 94780-94786 (2016).
- J. Fujisawa, T. Eda, M. Hanaya, Interfacial Charge-Transfer Transitions in BaTiO3 Nanoparticles Adsorbed with Catechol, J. Phys. Chem. C, 120, 21162-21168 (2016).
- J. Fujisawa, T. Eda, M. Hanaya, Comparative study of conduction-band and valence-band edges of TiO2, SrTiO3, and BaTiO3 by ionization potential measurements, Chem. Phys. Lett., 685, 23-26 (2017).
- T. Eda, J. Fujisawa, M. Hanaya, Inorganic Control of Interfacial Charge-Transfer Transitions in Catechol-Functionalized Titanium Oxides Using SrTiO3, BaTiO3, and TiO2, J. Phys. Chem. C, 122, 16216-16220 (2018).
- Z. Barbieriková, D. Lončarević, J. Papan, I.D. Vukoje, M. Stoiljković, S.P. Ahrenkiel, J.M. Nedeljković, Photocatalytic hydrogen evolution over surface-modified titanate nanotubes by 5-aminosalicylic acid decorated with silver nanoparticles, Adv. Powder Technol., 31, 4683-4690 (2020).
- M. Prekajski-Ðordević, I. Vukoje, V. Lazić, V. Ðordević, D. Sredojević, J. Dostanić, D. Lončarević, S.P. Ahrenkiel, M.R. Belić, J.M. Nedeljković, Electronic structure of surface complexes between CeO2 and benzene derivatives: A comparative experimental and DFT study, Mater. Chem. Phys., 236, 121816 (2019).
- V. Lazić, L.S. Živković, D. Sredojević, M.M. Fernandes, S. Lanceros-Mendez, S.P. Ahrenkiel, J.M. Nedeljković, Tuning Properties of Cerium Dioxide Nanoparticles by Surface Modification with Catecholate-type of Ligands, Langmuir, 36, 9738-9746 (2020).
- V. Lazić, A. Pirković, D. Sredojević, J. Marković, J. Papan, S.P. Ahrenkiel, I. Janković-Častvan, D. Dekanski, M. Jovanović-Krivokuća, J.M. Nedeljković, Surface-modified ZrO2 nanoparticles with caffeic acid: Characterization and in vitro evaluation of biosafety for placental cells, Chem.-Biol. Interact., 347, 109618 (2021).
- A. Zarubica, D. Sredojević, R. Ljupković, M. Randjelović, N. Murafa, M. Stoiljković, V. Lazić, J.M. Nedeljković, Photocatalytic ability of visible-light-responsive hybrid ZrO2 particles, Sustain. Energ. Fuels, 7, 2279-2287 (2023).
- M. Dukić, D. Sredojević, M. Férová, V. Slovak, D. Lončarević, J. Dostanić, H. Šalipur, V. Lazić, J.M. Nedeljković, Interfacial charge transfer complexes between ZnO and benzene derivatives: Characterization and photocatalytic hydrogen production, Int. J. Hydrogen Energ., 62, 628-636 (2024).
- J. Fujisawa, N. Kaneko, M. Hanaya, Interfacial charge-transfer transitions in ZnO induced exclusively by adsorption of aromatic thiols, Chem. Comm., 56, 4090-4093 (2020).
- J. Fujisawa, M. Hanaya, Linkage Dependence of Interfacial Charge-Transfer Transitions in ZnO: Carboxylate versus Sulfur Linker, J. Phys. Chem. A, 125, 5903-5910 (2021).
- J. Fujisawa, Definitive assignment and mechanistic study of interfacial charge-transfer transitions between ZnO and benzenethiol, Chem. Phys. Lett., 778, 138774 (2021).
- J. Fujisawa, S. Kato, M. Hanaya, Interfacial Charge-Transfer Transitions between ZnO Nanoparticles and Aliphatic Thiols, J. Phys. Chem. C, 127, 15300-15306 (2023).
- V. Ðordević, J. Dostanić, D. Lončarević, S.P. Ahrenkiel, D.N. Sredojević, N. Švrakić, M. Belić, J.M. Nedeljković, Hybrid visible-light responsive Al2O3 particles, Chem. Phys. Lett., 685, 416-421 (2017).
- V. Ðorđević, D.N. Sredojević, J. Dostanić, D. Lončarević, S.P. Ahrenkiel, N. Švrakić, E. Brothers, M. Belić, J.M. Nedeljković, Visible light absorption of surface-modified Al2O3 powders: A comparative DFT and experimental study, Micropor. Mesopor. Mat., 273, 41-49 (2019).
- A. Zarubica, R. Ljupković, J. Papan, I. Vukoje, S. Porobic, S.P. Ahrenkiel, J.M. Nedeljković, Visible-light-responsive Al2O3 powder: Photocatalytic study, Opt. Mater., 106, 110013 (2020).
- V. Lazić, I. Smičiklas, J. Marković, D. Lončarević, J. Dostanić, S.P. Ahrenkiel, J.M. Nedeljković, Antibacterial ability of supported silver nanoparticles by functionalized hydroxyapatite with 5-aminosalicylic acid, Vacuum, 148, 62-68 (2018).
- I.D. Smičiklas, V.M. Lazić, L.S. Živković, S.J. Porobić, S.P. Ahrenkiel, J.M. Nedeljković, Sorption of divalent heavy metal ions onto functionalized biogenic hydroxyapatite with caffeic acid and 3,4-dihydroxybenzoic acid, J. Environ. Sci. Heal. A, 54, 899-905 (2019).
- D. Sredojević, S. Stavrić, V. Lazić, S.P. Ahrenkiel, J.M. Nedeljković, Interfacial charge transfer complex formation between silver nanoparticles and aromatic amino acids, Phys. Chem. Chem. Phys., 24, 16493-16500 (2022).
- D. Finkelstein-Shapiro, S.K. Davidowski, P.B. Lee, C. Guo, G.P. Holland, T. Rajh, K.A. Gray, J.L. Yarger, M. Calatayud, Direct evidence of chelated geometry of catechol on TiO2 by a combined solid-state NMR and DFT study, J. Phys. Chem. C, 120, 23625-23630 (2016).
- P. Job, Formation and Stability of Inorganic Complexes in Solution, Ann. Chim., 9, 113-203 (1928).
- L.X. Chen, T. Rajh, Z. Wang, M.C. Thurnauer, XAFS studies of surface structures of TiO2 nanoparticles and photocatalytic reduction of metal ions, J. Phys. Chem. B, 101, 10688-10697 (1997).
- H.A. Benesi, J.H. Hildebrand, A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons, J. Am. Chem. Soc., 71, 2703-2707 (1949).
- J. Moser, S. Punchihewa, P.P. Infelta, M. Grätzel, Surface Complexation of Colloidal Semiconductors Strongly Enhances Interfacial Electron-Transfer Rates, Langmuir, 7, 3012-3018 (1991).
- S.T. Martin, J.M. Kesselman, D.S. Park, N.S. Lewis, M.R. Hoffman, Surface Structures of 4-Chlorocatechol Adsorbed on Titanium Dioxide, Environ. Sci. Technol., 30, 2535-2542 (1996).
- P. Rodriguez, M.A. Blesa, A.E. Regazzoni, Surface Complexation at the TiO2 (anatase)/AqueousSolution Interface: Chemisorption of Catechol, J. Colloid Interface Sci., 177, 122-131 (1996).
- A.E. Regazzoni, P. Mandelbaum, M. Matsuyoshi, S. Schiller, S.A. Bilmes, M.A. Blesa, Adsorption and Photooxidation of Salicylic Acid on Titanium Dioxide: A Surface Complexation Description, Langmuir, 14, 868-874 (1998).
- D.V. Heyd, B. Au, Fluorescence development during 514 nm irradiation of catechol adsorbed on nanocyrstalline titanium dioxide, J. Photochem. Photobiol. A: Chem., 174, 62-70 (2005).
- A.D. Weisz, A.E. Regazzoni, M.A. Blesa, ATR–FTIR study of the stability trends of carboxylate complexes formed on the surface of titanium dioxide particles immersed in water, Solid State Ionics, 143, 125-130 (2001).
- A.D. Weisz, L. García Rodenas, P.J. Morando, A.E. Regazzoni, M.A. Blesa, FTIR study of the adsorption of single pollutants and mixtures of pollutants onto titanium dioxide in water: oxalic and salicylic acids, Catal. Today, 76, 103-112 (2002).
- P.Z. Araujo, C.B. Mendive, L.A. García Rodenas, P.J. Morando, A.E. Regazzoni, M.A. Blesa, D. Bahnemann, FT-IR–ATR as a tool to probe photocatalytic interfaces, Colloid Surface A, 265, 73-80 (2005).
- P.Z. Araujo, P.J. Morando, M.A. Blesa, Interaction of Catechol and Gallic Acid with Titanium Dioxide in Aqueous Suspensions. 1. Equilibrium Studies, Langmuir, 21, 3470-3474 (2005).
- T. Rajh, L.X. Chen, K. Lukas, T. Liu, M.C. Thurnauer, D.M. Tiede, Surface Restructuring of Nanoparticles: An Efficient Route for Ligand-Metal Oxide Crosstalk, J. Phys. Chem. B, 106, 10543-10552 (2002).
- M. Chen, Y.G. Feng, X. Wang, T.C. Li, J.Y. Zhang, D.J. Qian, Silver nanoparticles capped by oleylamine: formation, growth, and self-organization, Langmuir, 23, 5296-5304 (2007).
- S. Mourdikoudis, L.M. Liz-Marzan, Oleylamine in nanoparticle synthesis, Chem. Mater., 25, 1465-1476 (2013).
- V. Lazić, K. Mihajlovski, A. Mraković, E. Illés, M. Stoiljković, S.P. Ahrenkiel, J.M. Nedeljković, Antimicrobial activity of silver nanoparticles supported by magnetite, ChemistrySelect, 4, 4018-4024 (2019).
- J. Fujisawa, S. Matsumura, M. Hanaya, Facile and rapid visualization of colorless endocrine disruptor bisphenol A by interfacial charge-transfer transitions with TiO2 nanoparticles, ChemistrySelect, 2, 6097-6099 (2017).
- J. Fujisawa, T. Eda, M. Hanaya, Facile, quick and selective visible-light sensing of phenol-containing drug molecules acetaminophen and biosol by use of interfacial charge transfer transitions with TiO2 nanoparticles, Chem. Phys. Lett., 684, 328-332 (2017).
- U. Stafford, K.A. Gray, P.V. Kamat, Photocatalytic Degradation of 4-Chlorophenol: The Effects of Varying TiO2 Concentration and Light Wavelength, J. Catal., 167, 25-32 (1997).
- A. Mills, R.H. Davies, D. Worsley, Water purification by semiconductor photocatalysis, Chem. Soc. Rev., 22, 417-425 (1993).
- A. Mills, S. Morris, Photomineralization of 4-chlorophenol sensitized by titanium dioxide: a study of the initial kinetics of carbon dioxide photogeneration, J. Photochem. Photobiol. A: Chem., 71, 75-83 (1993).
- A.G. Agrios, K.A. Gray, E. Weitz, Photocatalytic Transformation of 2,4,5-Trichlorophenol on TiO2 under Sub-Band-Gap Illumination, Langmuir, 19, 1402-1409 (2003).
- A.G. Agrios, K.A. Gray, E. Weitz, Narrow-Band Irradiation of a Homologous Series of Chlorophenols on TiO2: Charge-Transfer Complex Formation and Reactivity, Langmuir, 20, 5911-5917 (2004).
- S. Kim, W. Choi, Visible-Light-Induced Photocatalytic Degradation of 4-Chlorophenol and Phenolic Compounds in Aqueous Suspension of Pure Titania: Demonstrating the Existence of a Surface-Complex-Mediated Path, J. Phys. Chem. B, 109, 5143-5149 (2005).
- T. Paul, P. Miller, T.J. Strathmann, Visible-Light-Mediated TiO2 Photocatalysis of Fluoroquinolone Antibacterial Agents, Environ. Sci. Technol., 41, 4720-4727 (2007).
- M. Li, P. Tang, Z. Hong, M. Wang, High efficient surface-complex-assisted photodegradation of phenolic compounds in single anatase titania under visible-light, Colloid Surface A, 318, 285-290 (2008).
- N. Wang, L. Zhu, Y. Huang, Y. She, Y. Yu, H. Tanga, Drastically enhanced visible-light photocatalytic degradation of colorless aromatic pollutants over TiO2 via a charge-transfer complex path: A correlation between chemical structure and degradation rate of the pollutants, J. Catal., 266, 199-206 (2009).
- S. Higashimoto, N. Kitao, N. Yoshida, T. Sakura, M. Azuma, H. Ohue, Y. Sakata, Selective photocatalytic oxidation of benzyl alcohol and its derivatives into corresponding aldehydes by molecular oxygen on titanium dioxide under visible light irradiation, J. Catal., 266, 279-285 (2009).
- S. Higashimoto, N. Suetsugu, M. Azuma, H. Ohue, Y. Sakata, Efficient and selective oxidation of benzylic alcohol by O2 into corresponding aldehydes on a TiO2 photocatalyst under visible light irradiation: Effect of phenyl-ring substitution on the photocatalytic activity, J. Catal., 274, 76-83 (2010).
- S. Higashimoto, K. Okada, T. Morisugi. M. Azuma, H. Ohue, T.-H. Kim, M. Matsuoka, M. Anpo, Effect of Surface Treatment on the Selective Photocatalytic Oxidation of Benzyl Alcohol into Benzaldehyde by O2 on TiO2 Under Visible Light, Top. Catal., 53, 578-583 (2010).
- S. Girish Kumar, L. Gomathi Devi, Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected results and Related Mechanism on Interfacial Charge Transfer Carrier Transfer Dynamics, J. Phys. Chem. A, 115, 13211-13241 (2011).
- S. Higashimoto, R. Shirai, Y. Osano, M. Azuma, H. Ohue, Y. Sakata, H. Kobayashi, Influence of metal ions on the photocatalytic activity: Selective oxidation of benzyl alcohol on iron (III) ion-modified TiO2 using visible light, J. Catal., 311, 137-143 (2014).
- G. Kim, S.-H. Lee, W. Choi, Glucose–TiO2 charge transfer complex-mediated photocatalysis under visible light, Appl. Catal. B: Environ., 162, 463-469 (2015).
- A. Houas, H. Lachheb, M. Ksibi, E. Elaloui, C. Guillard, J.-M. Herrmann, Photocatalytic degradation pathway of methylene blue in water, Appl. Catal. B: Environ., 31, 145-157 (2001).
- R. Djellabi, M. Ghorab, G. Cerrato, S. Morandi, S. Gatto, V. Oldani, A. Di Michele, C. Bianchi, Photoactive TiO2−Montmorillonite Composite for Degradation of Organic Dyes in Water, J. Photochem. Photobio. A, 295, 57-63 (2014).
- B. Ealet, M.H. Elyakhloufi, E. Gillet, M. Ricci, Electronic and crystallographic structure of γ-alumina thin films, Thin Solid Films, 250, 92-100 (1994).
- A. Panáček, L. Kvítek, R. Prucek, M. Kolář, R. Večeřová, N. Pizúrová,V.K. Sharma, T. Nevĕčná, R. Zbořil, Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity, J. Phys. Chem. B, 110, 16248-16253 (2006).
- T. Yuranova, A.G. Rincon, A. Bozzi, S. Parra, C. Pulgarin, P. Albers, J. Kiwi, Antibacterial textiles prepared by RF-plasma and vacuum-UV mediated deposition of silver, J. Photochem. Photobiol. A, 161, 27-34 (2003).
- V. Ilić, Z. Šaponjić, V. Vodnik, B. Potkonjak, P. Jovančić, J. Nedeljković, M. Radetić, The influence of silver content on antimicrobial activity and color of cotton fabrics functionalized with Ag nanoparticles, Carbohyd. Polym., 78, 564-569 (2009).
- V.A. Oyanedel-Craver, J.A. Smith, Sustainable colloidal-silver-impregnated ceramic filter for point-of-use water treatment, Environ. Sci. Technol., 42, 927-933 (2008).
- S. Davidović, V. Lazić, M. Miljković, M. Gordić, M. Sekulić, M. Marinović-Cincović, I.S. Ratnayake, S.P. Ahrenkiel, J.M. Nedeljković, Antibacterial ability of immobilized silver nanoparticles in agar-agar films co-doped with magnesium ions, Carbohyd. Polym., 224, 115187 (2019).
- V. Lazić, V. Vivod, Z. Peršin, M. Stoiljković, I.S. Ratnayake, P.S. Ahrenkiel, J.M. Nedeljković, V. Kokol, Dextran-coated silver nanoparticles for improved barrier and controlled antimicrobial properties of nanocellulose films used in food packaging, Food Packaging Shelf, 26, 100575 (2020).
- W.A. Daoud, J.H. Xin, Low temperature sol-gel processed photocatalytic titania coating, J. Sol-Gel Sci. Technol., 29, 25-29 (2004).
- C. Pulgarin, J. Kiwi, V. Nadtochenko, Mechanism of photocatalytic bacterial inactivation on TiO2 films involving cell-wall damage and lysis, Appl. Catal. B-Environ., 128, 179-183 (2012).
- W.A. Daoud, J.H. Xin, Y.H. Zhang, Surface functionalization of cellulose fibers with titanium dioxide nanoparticles and their combined bactericidal activities, Surf. Sci., 599: 69-75 (2005).
- D. Mihailović, Z. Šaponjić, M. Radoičić, T. Radetić, P. Jovančić, J Nedeljković, M. Radetić, Functionalization of polyester fabrics with alginates and TiO2 nanoparticles, Carbohyd. Polym., 79, 526-532 (2010).
- D. Mihailović, Z. Šaponjić, R. Molina, N. Pauč, P. Jovančić, J. Nedeljković, M. Radetić, Improved properties of oxygen and argon RF plasma-activated polyester fabrics loaded with TiO2 nanoparticles, ACS Appl. Mater. Inter., 2, 1700-1706 (2010).
- J.A. Rengifo-Herrera, E. Mielczarski, J. Mielczarski, N.C. Castillo, J. Kiwi, C. Pulgarin, Escherichia coli inactivation by N, S co-doped commercial TiO2 powders under UV and visible light, Appl. Catal. B-Environ., 84, 448-456 (2008).
- D. Meng, X. Liu, Y. Xie, Y. Du, Y. Yang, C. Xiao, Antibacterial Activity of Visible Light-Activated TiO2 Thin Films with Low Level of Fe Doping, Adv. Mater. Sci. Eng., 5819805 (2019).
- Y.V. Pleskov, Y.Y. Gurevich, in Semiconductor Photoelectrochemistry, ed. P.N. Bartlett, Plenum, New York, 1986.
- H. Schwarz, R. Dodson, Reduction Potentials of CO2-and the Alcohol Radicals, J. Phys. Chem., 93, 409-414 (1989).
- S. Ikeda, C. Abe, T. Torimoto, B. Ohtani, Photochemical hydrogen evolution from aqueous triethanolamine solutions sensitized by binaphthol-modified titanium(IV) oxide under visible-light irradiation, J. Photoch. Photobio. A, 160, 61-67 (2003).
- Z.H.N. Al-Azri, W.-T. Chen, A. Chan, V. Jovic, T. Ina, H. Idriss, G.I.N. Waterhouse, The roles of metal co-catalysts and reaction media in photocatalytic hydrogen production: Performance evaluation of M/TiO2 photocatalysts (M = Pd, Pt, Au) in different alcohol–water mixtures, J. Catal., 329, 355-367 (2015).
- E.J. Hart, J.W. Boag, Absorption Spectrum of the Hydrated Electron in Water and in Aqueous Solutions, J. Am Chem. Soc., 84, 4090-4095 (1962).
- J.K. Thomas, J. Rabani, M.S. Matheson, E.J. Hart, S. Gordon, Absorption Spectrum of the Hydroxyl Radical, J. Phys. Chem., 70, 2409-2410 (1966).
- D. Duonghong, J. Ramsden, Michael Grätzel, Dynamics of Interfacial Electron-Transfer Processes in Colloidal Semiconductor Systems, J. Am. Chem. Soc., 104, 2977-2985 (1982).
- R.F. Howe, M. Grӓtzel, EPR Observation of Trapped Electrons in Colloidal TiO2, J. Phys. Chem., 89, 4495-4499 (1985).
- O.I. Micic, Y. Zhang, K.R. Cromack, A.D. Trifunac, M.C. Thurnauer, Trapped Holes on TiO2 Colloids Studied by Electron Paramagnetic Resonance, J. Phys. Chem., 97, 7277-7283 (1993).
- D. Dvoranová, V. Brezová, M. Mazúr, M.A. Malati, Investigations of metal-doped titanium dioxide photocatalysts, Appl. Catal. B Env., 37, 91-105 (2002).
- M. Chiesa, M.C. Paganini, S. Livraghi, E. Giamello, Charge trapping in TiO2 polymorphs as seen by Electron Paramagnetic Resonance spectroscopy, Phys. Chem. Chem. Phys., 15, 9435-9447 (2013).
- E.G. Panarelli, S. Livraghi, S. Maurelli, V. Polliotto, M. Chiesa, E. Giamello, Role of surface water molecules in stabilizing trapped hole centres in titanium dioxide (anatase) as monitored by electron paramagnetic resonance, J. Photoch. Photobio. A, 322-323, 27-34 (2016).
- B. Ohtani, O. Prieto-Mahaney, D. Li, R. Abe, What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test, J. Photoch. Photobio. A, 216, 179-182 (2010).
- M.C. Patterson, N.D. Keibart, L.W. Kiruri, C.A. Thibodeaux, S. Lomnicki, R.L. Kurtz, E.D. Poliakoff, B. Dellinger, P.T. Sprunger, EPFR formation from phenol adsorption on Al2O3 and TiO2: EPR and EELS studies, Chem. Phys., 422, 277-282 (2013).
- M.C. Patterson, C.A. Thibodeaux, O. Kizilkaya, R.L. Kurtz, E.D. Poliakoff, P.T. Sprunger, Electronic signatures of a model pollutant-particle system: chemisorbed phenol on TiO2 (110), Langmuir, 31, 3869-3875 (2015).
- Landolt-Börnstein, Numerical Data and Functional Relationships in Science and Technology. Vol. 17 Magnetic Properties of Free radicals. Subvolume e, Radicals centered on heteroatoms with Z>7 and selected anion radicals. pp. 35-249.
- G. Zhang, G. Kim, W. Choi, Visible light driven photocatalysis mediated via ligand-to- metal charge transfer (LMCT): an alternative approach to solar activation of titania, Energy Environ. Sci., 7, 954-966 (2014).
- V. Nikšić, M. Malček Šimunková, Z. Dyrčíková, D. Dvoranová, V. Brezová, D. Sredojević, J.M. Nedeljković, V. Lazić, Photoinduced reactive species in interfacial charge transfer complex between TiO2 and taxifolin: DFT and EPR study, Opt. Mater., 152, 115454 (2024).
- V. Brezová, D. Dvoranová, A. Staško, Characterization of titanium dioxide photoactivity following the formation of radicals by EPR spectroscopy, Res. Chem. Intermed., 33, 251-268 (2007).
- K. Jomová, L. Hudecova, P. Lauro, M. Simunkova, S.H. Alwasel, I.M. Alhazza, M. Valko, A switch between antioxidant and prooxidant properties of the phenolic compounds myricetin, morin, 3′,4′-dihydroxyflavone, taxifolin and 4-hydroxy-coumarin in the presence of copper(II) ions: a spectroscopic, absorption titration and dna damage study, Molecules, 24, 4335 (2019).
- A. Staško, V. Brezová, S. Biskupič, V. Misík, The potential pitfalls of using 1,1- dipehnylpicrylhydrazyl to characterize antioxidant in mixed water solvent, Free Radic. Res., 41, 379-390 (2007).
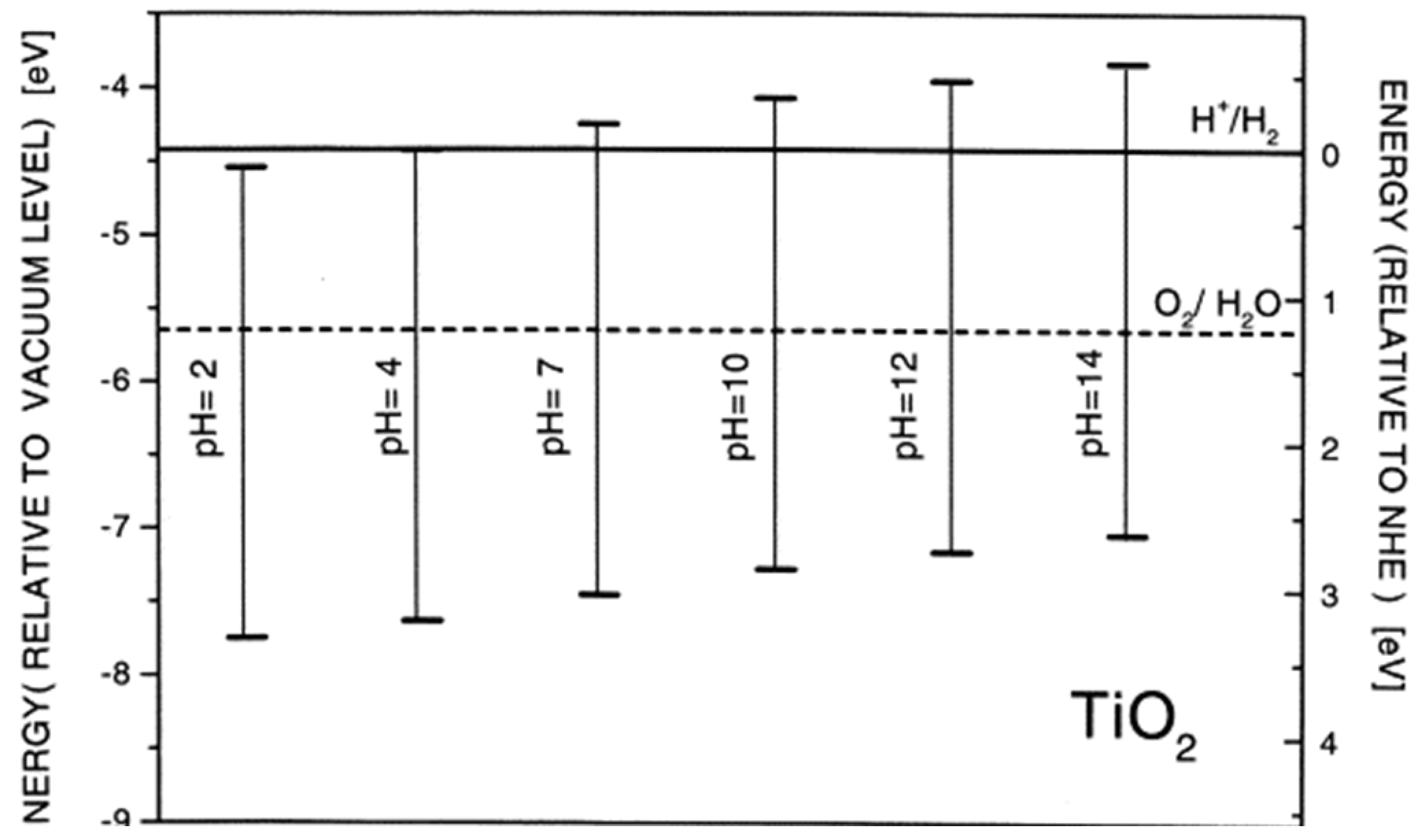
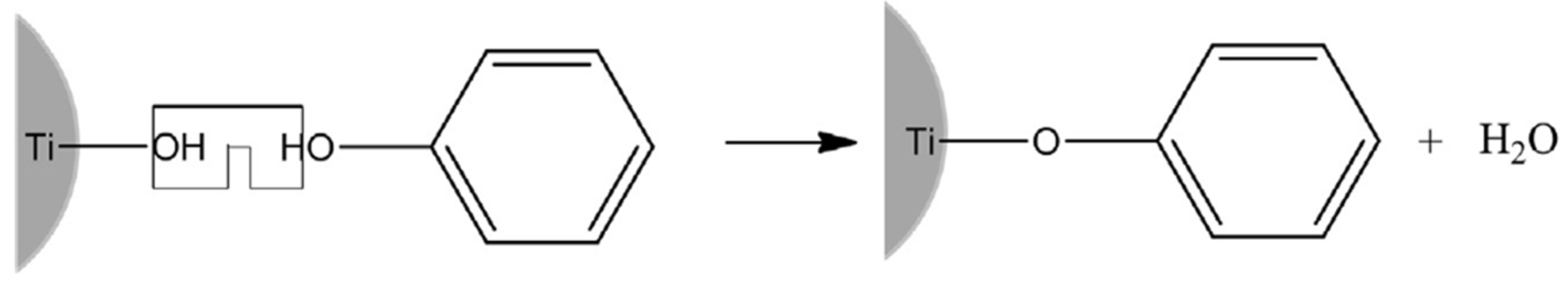
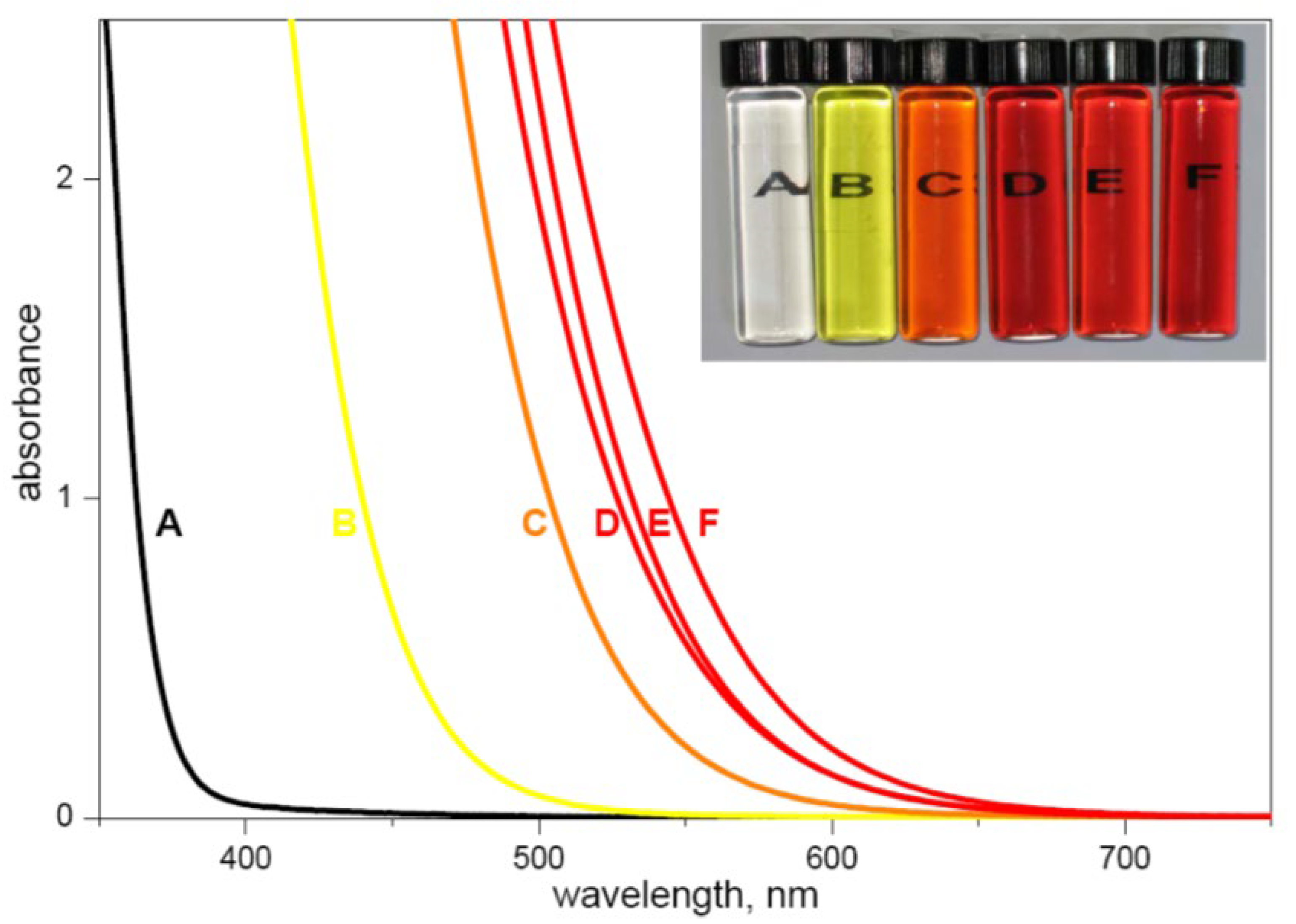
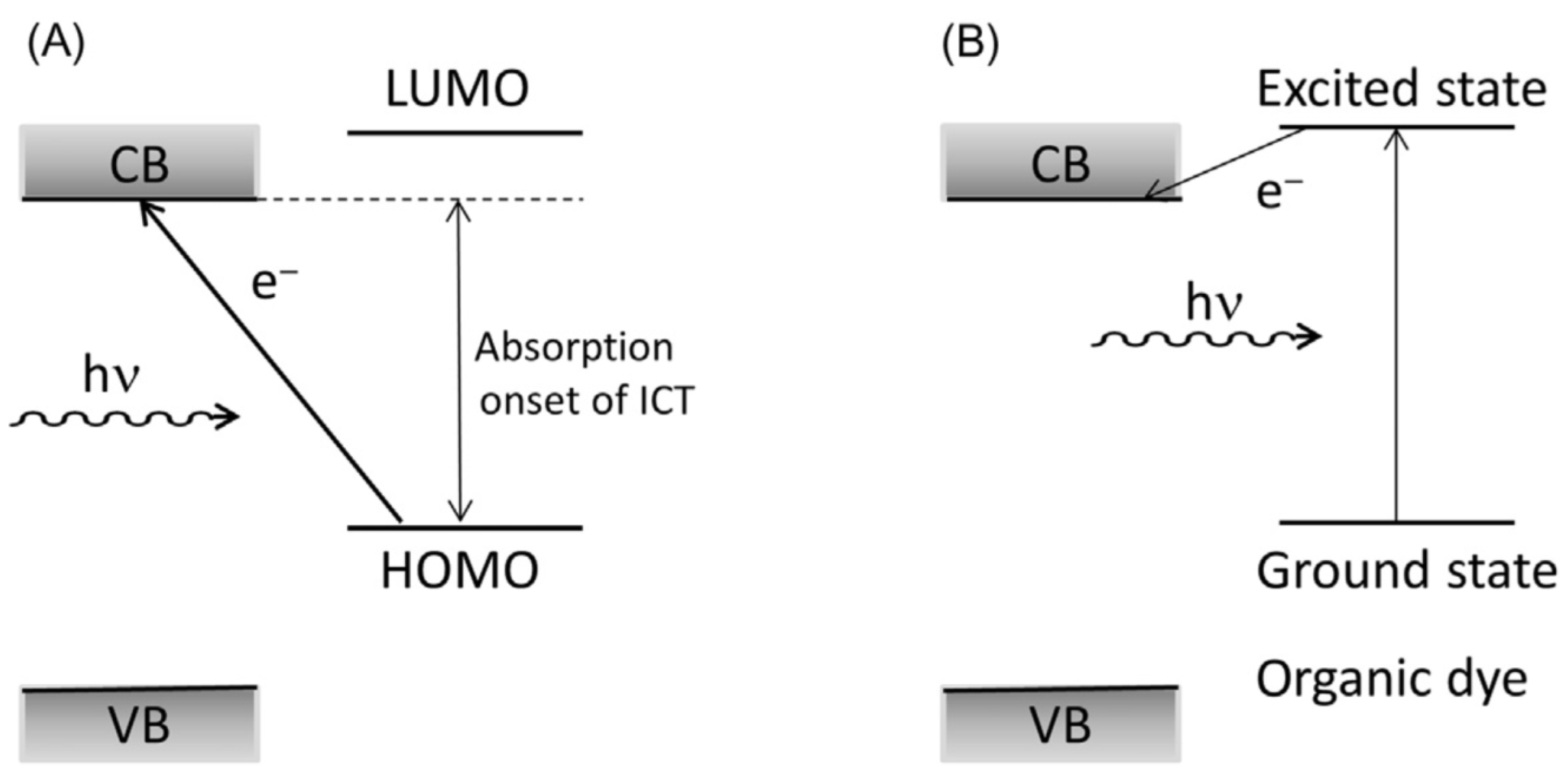
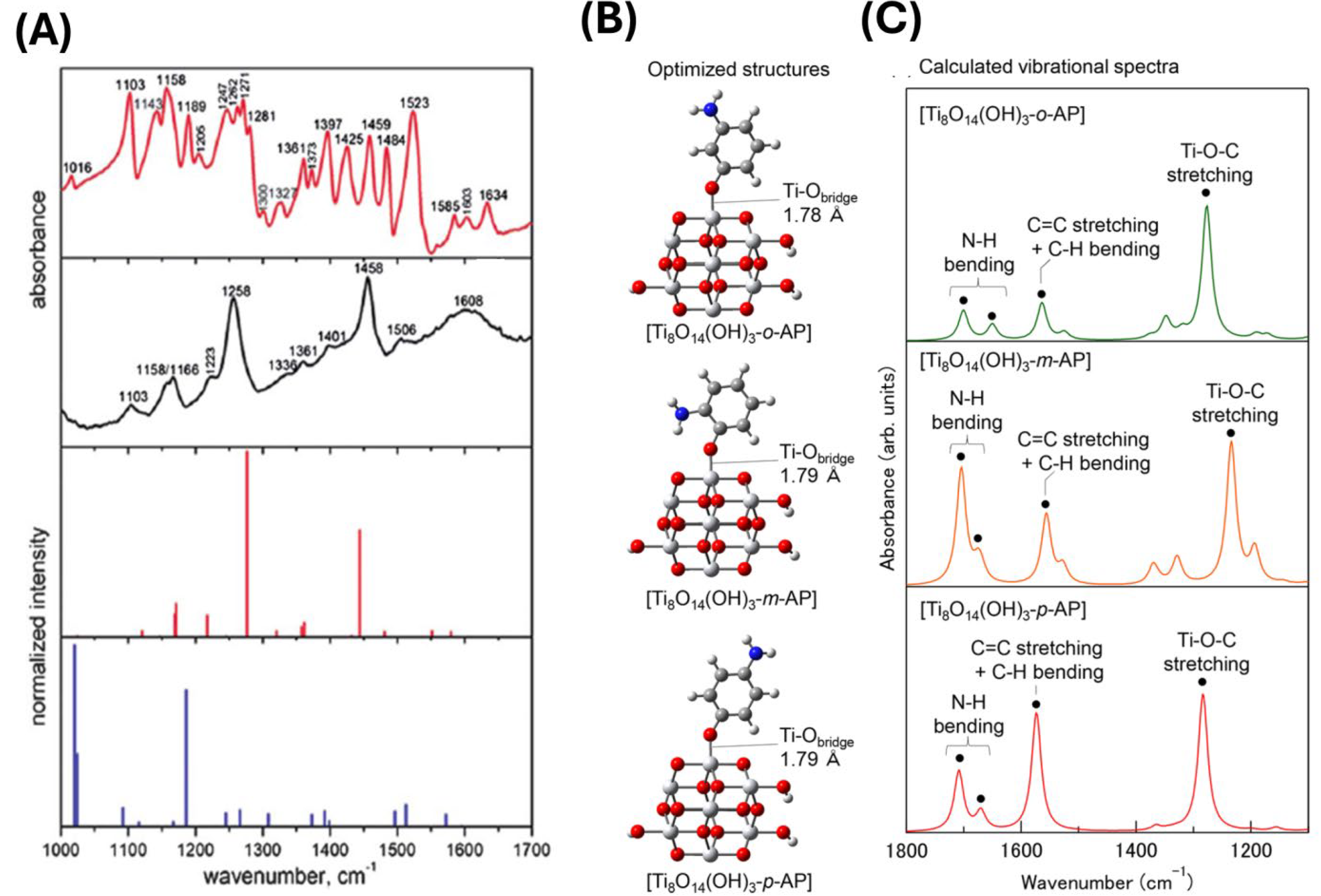
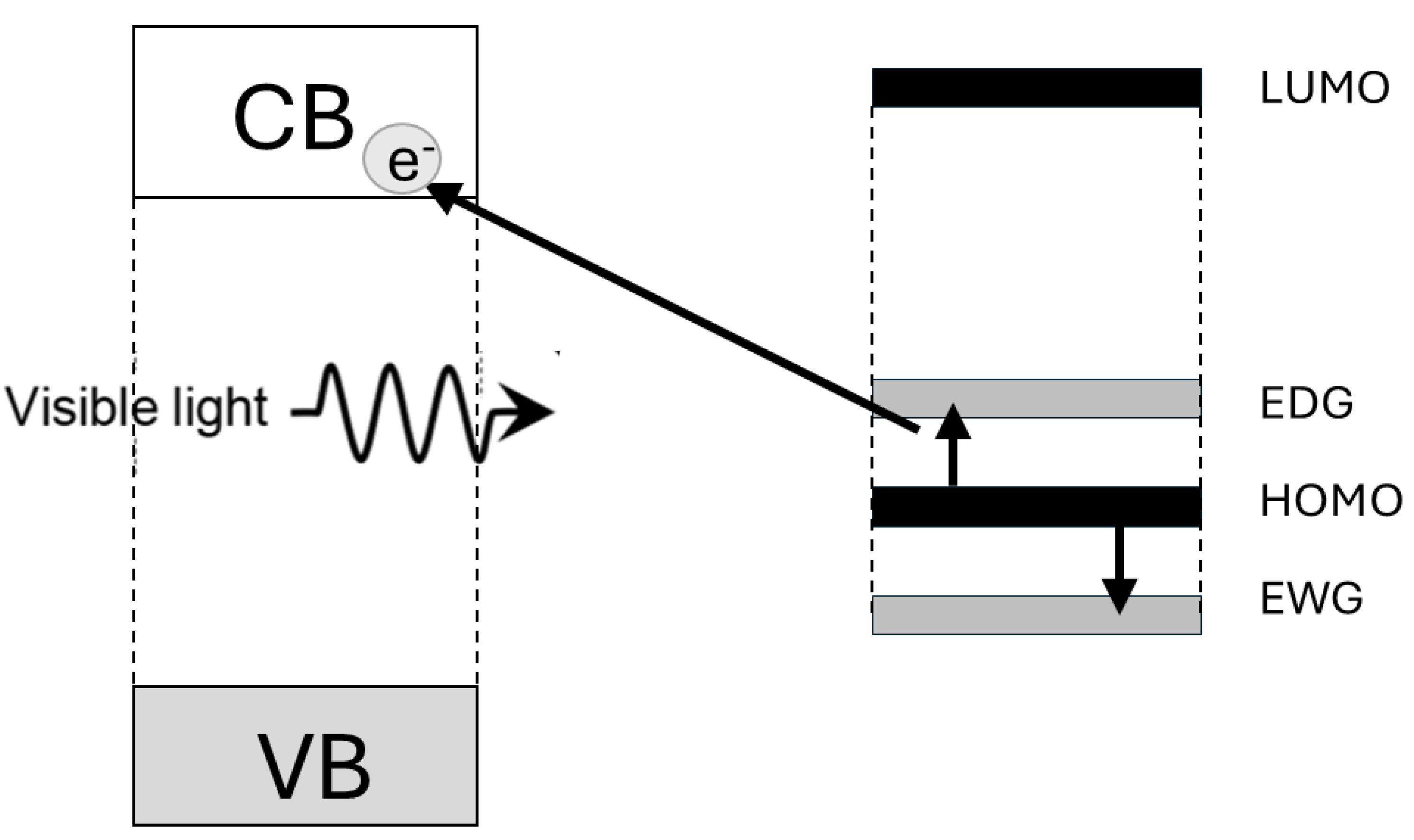
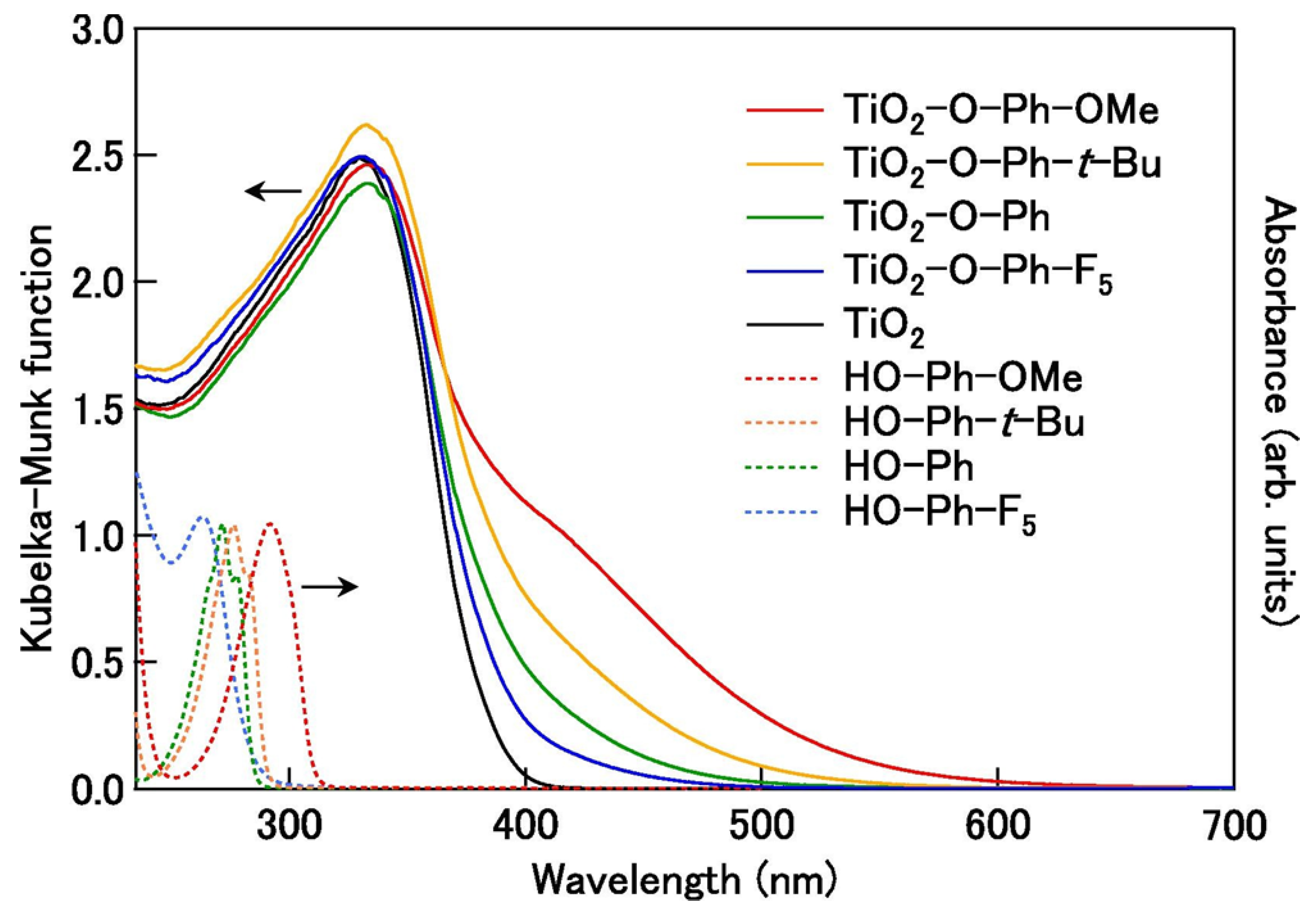
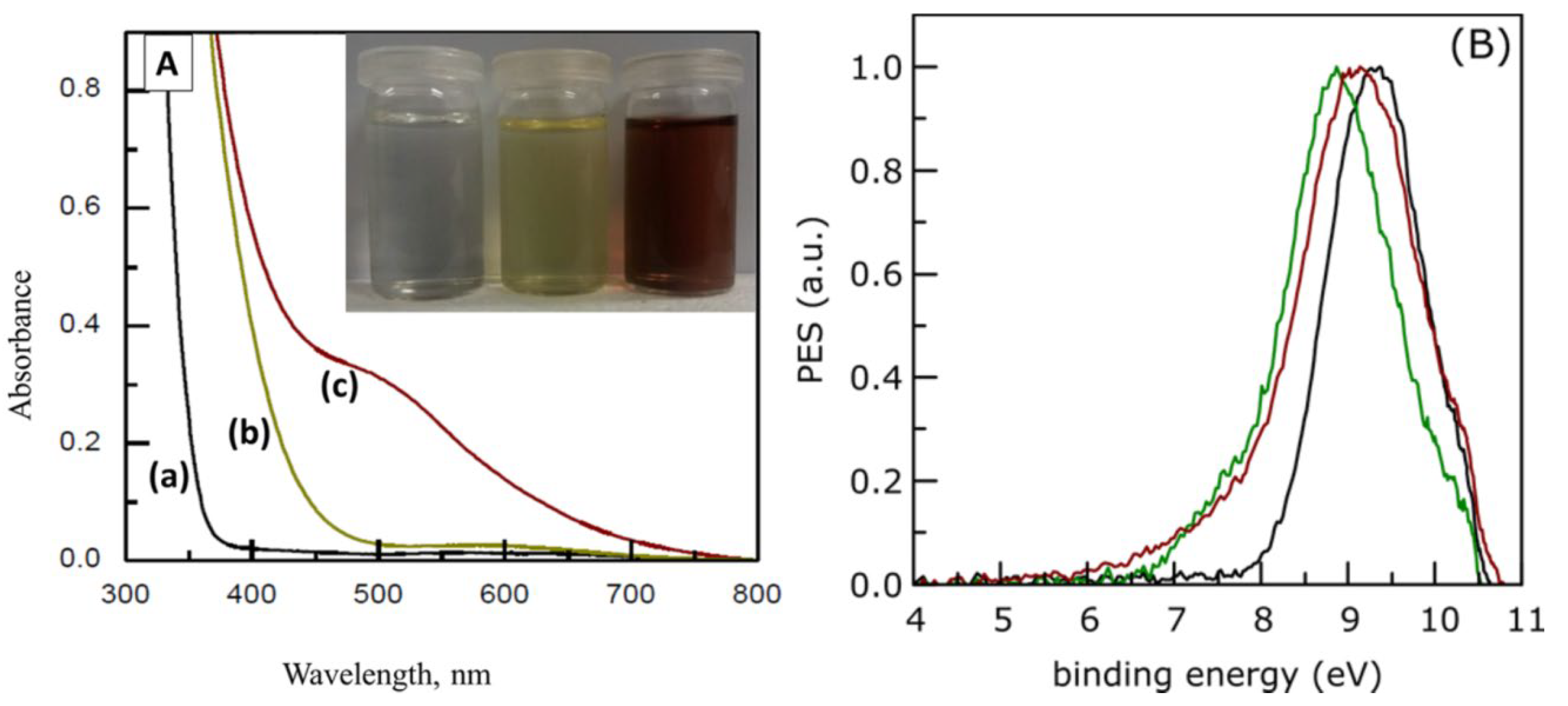
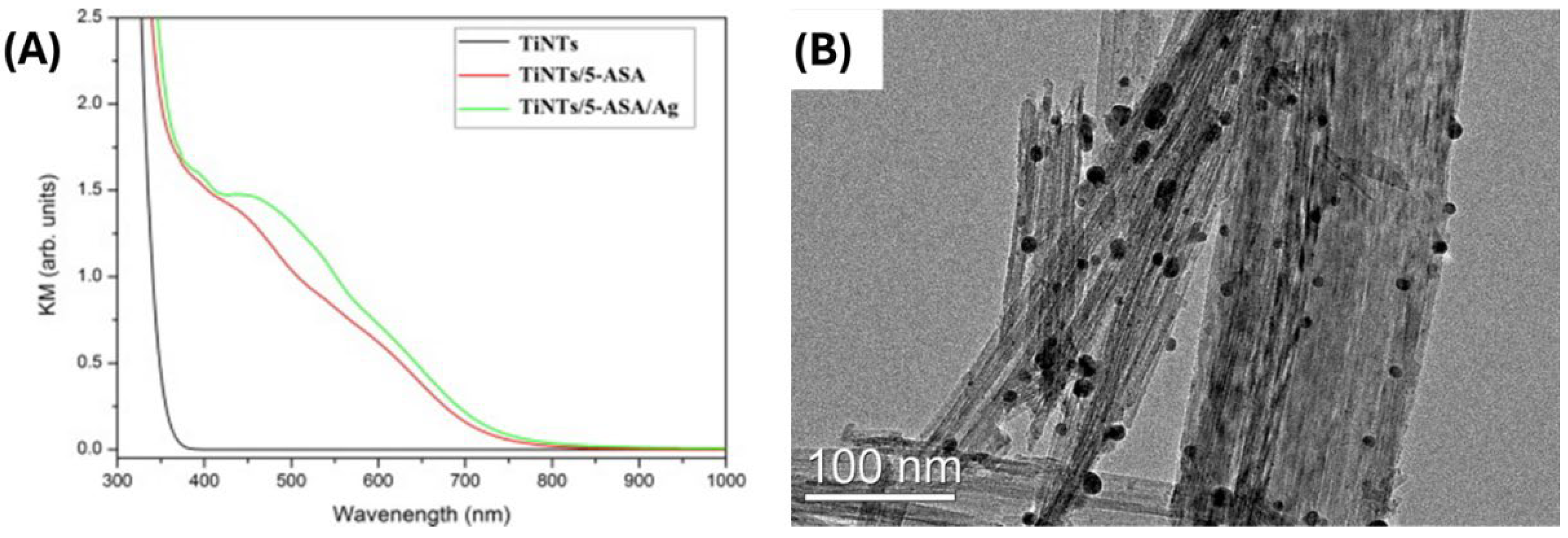
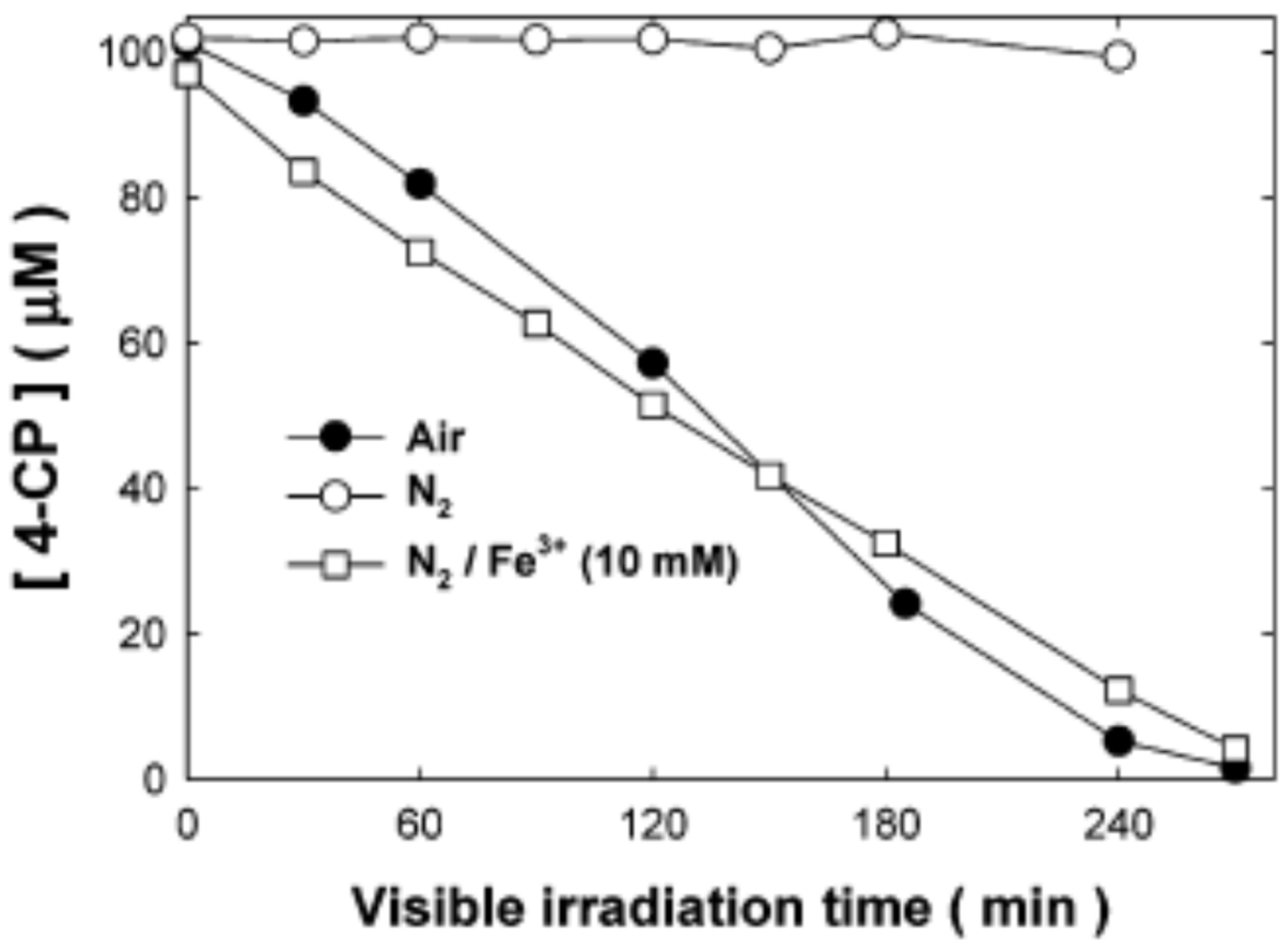
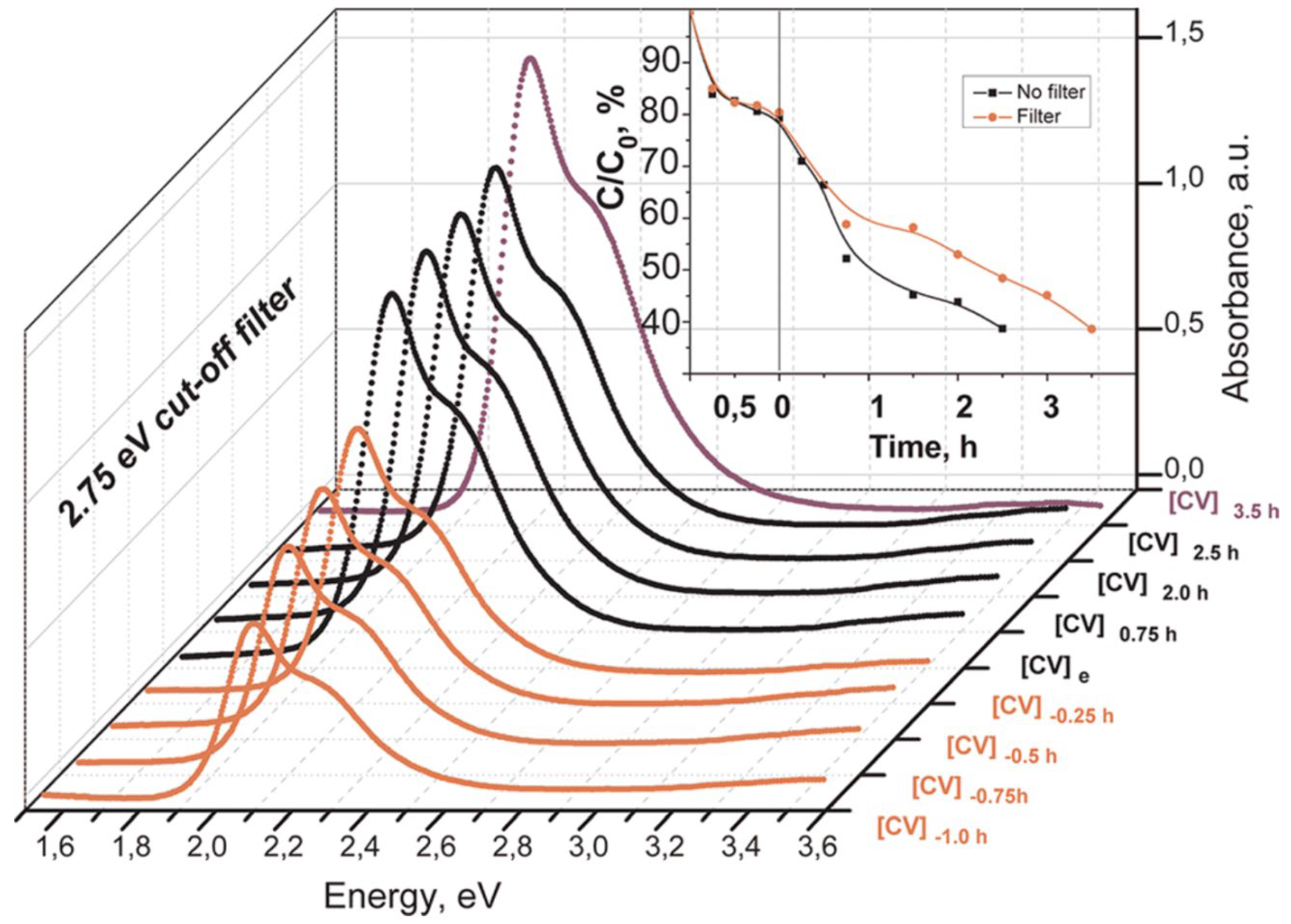
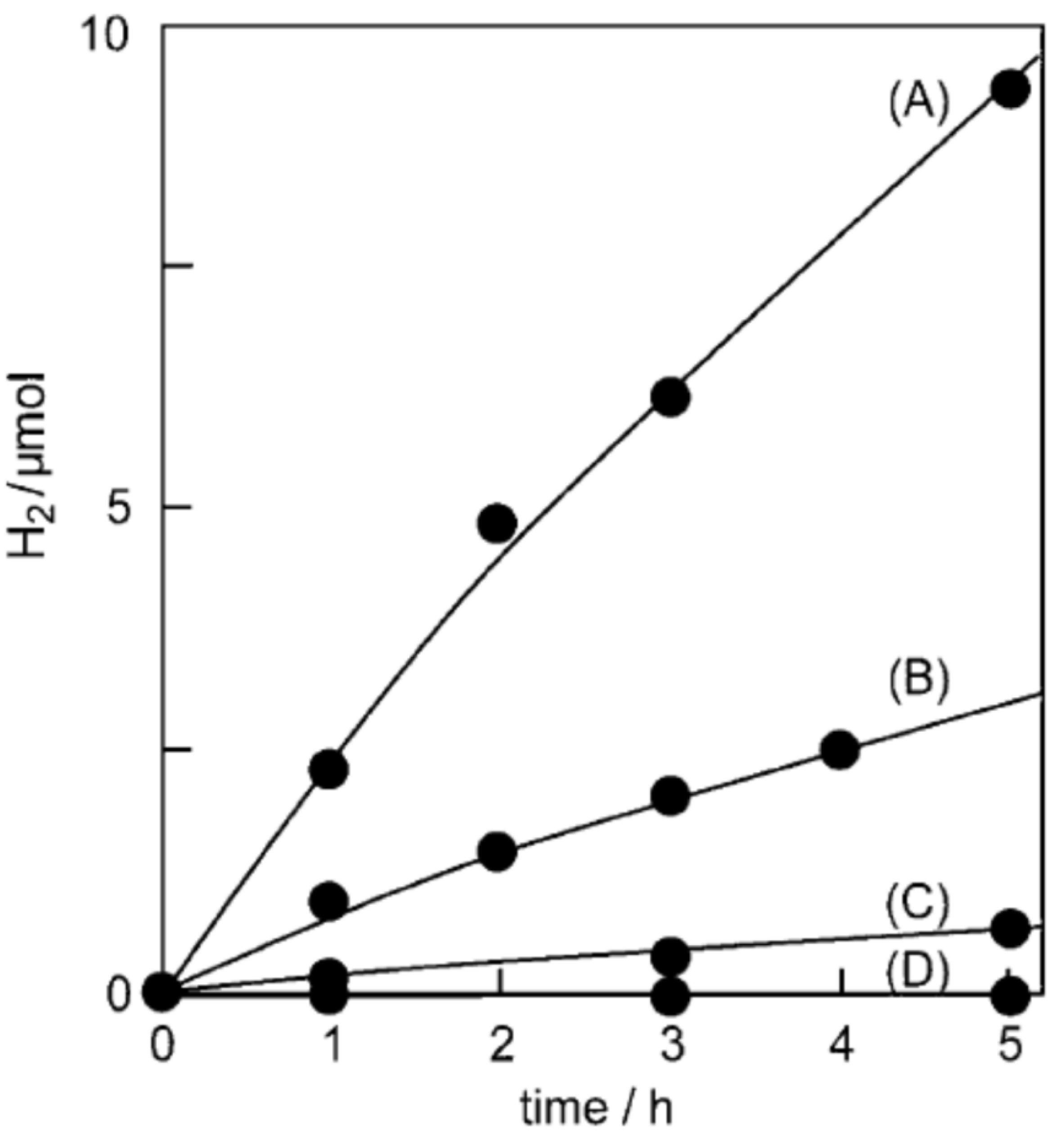
| Me‒Ox | Type of ligand | |||
|---|---|---|---|---|
| Two Me‒O‒C linkages | Single Me‒O‒C linkage | Me‒S‒C linkage or linkages | Miscellaneous | |
| TiO2 | 21-25, 29, 33-35, 41-50 | 26, 37-40, 51-53 | 36, 54-59 | 20, 27, 28, 31, 60-70 |
| Titanates | 72-76 | |||
| CeO2 | 77, 78 | |||
| ZrO2 | 79-80 | |||
| ZnO | 81 | 82-85 | ||
| Al2O3 | 86-88 | |||
| HAP* | 89, 90 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





