Submitted:
11 October 2024
Posted:
11 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Theory
2.1. Basic Principle of DEP
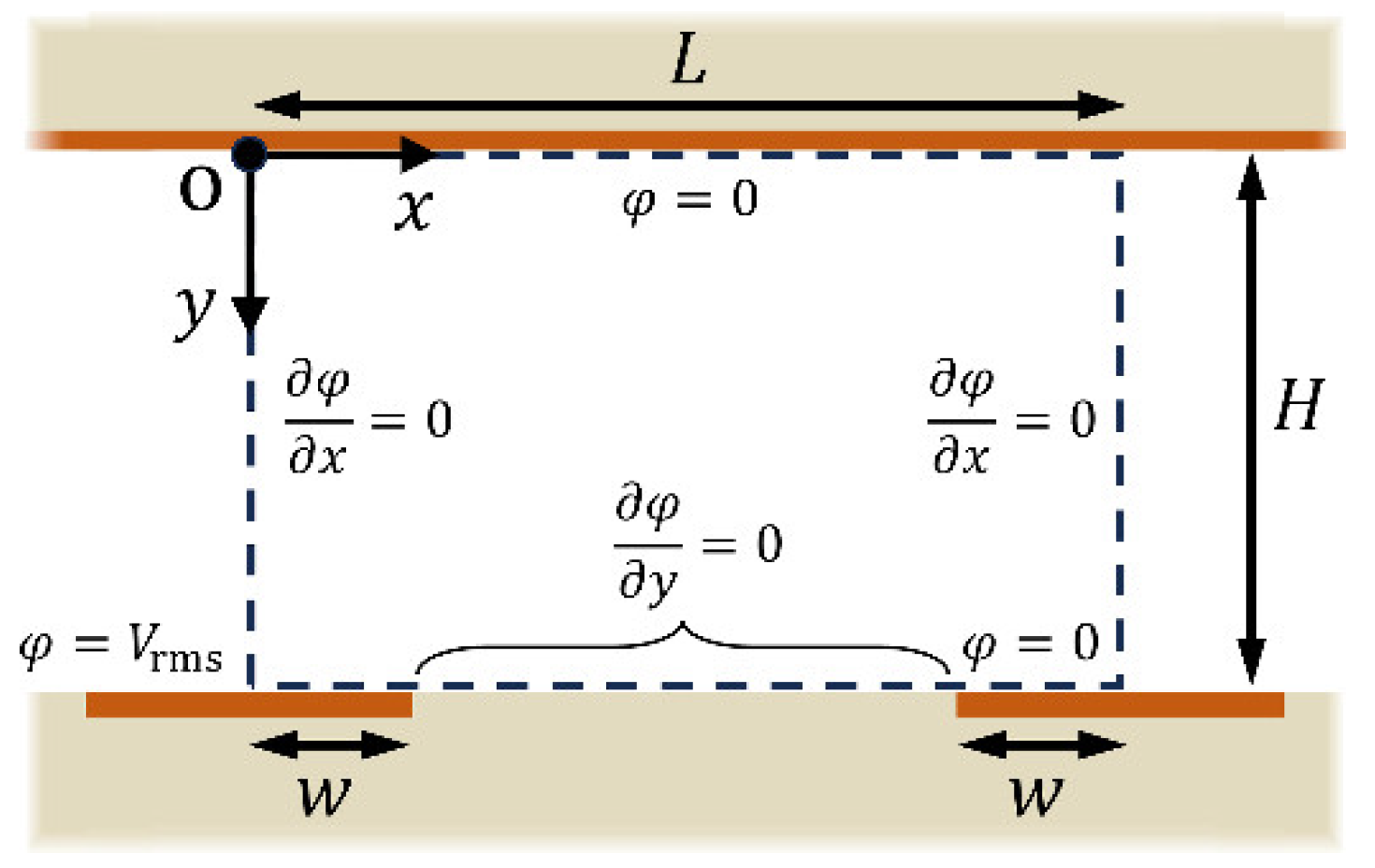
2.2. Electric Field Analysis
2.3. Analytical Solution by Fourier Series Method
2.4. Analytical Form of DEP Force
3. Experimental
3.1. Cell Sample Preparation
3.2. Preparation of Cell Sample Solution
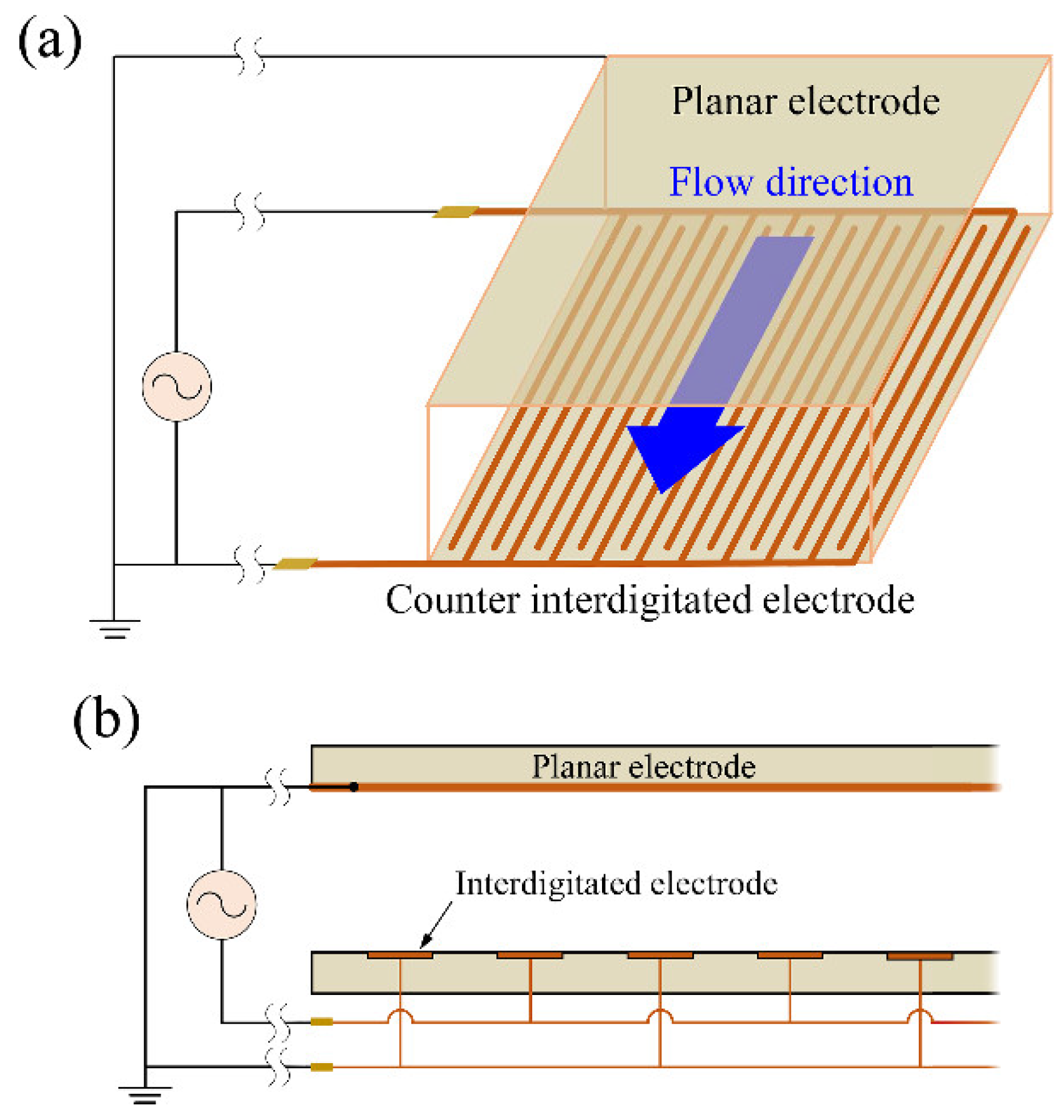
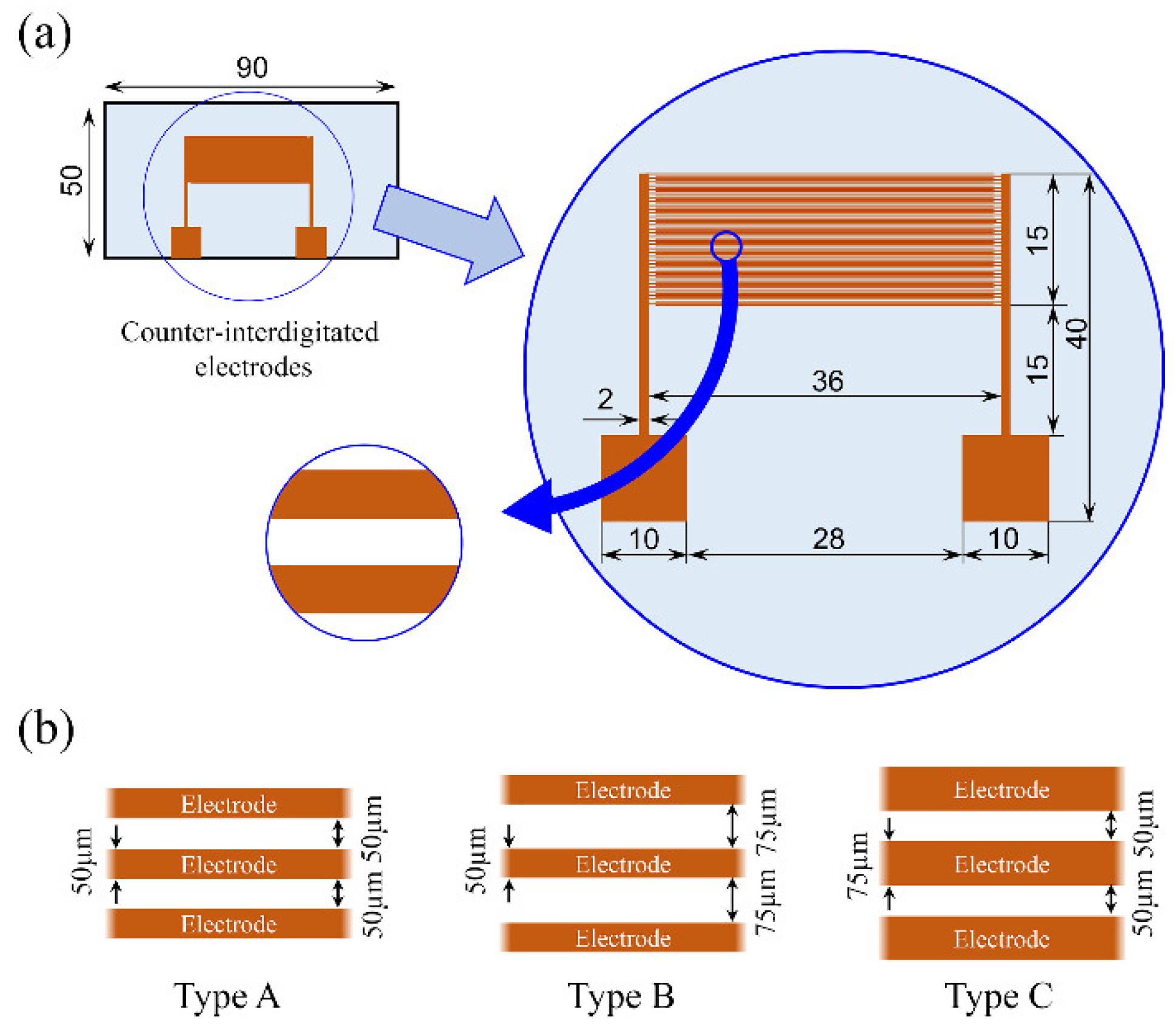
3.3. Fabrication of Cell-Separation Device
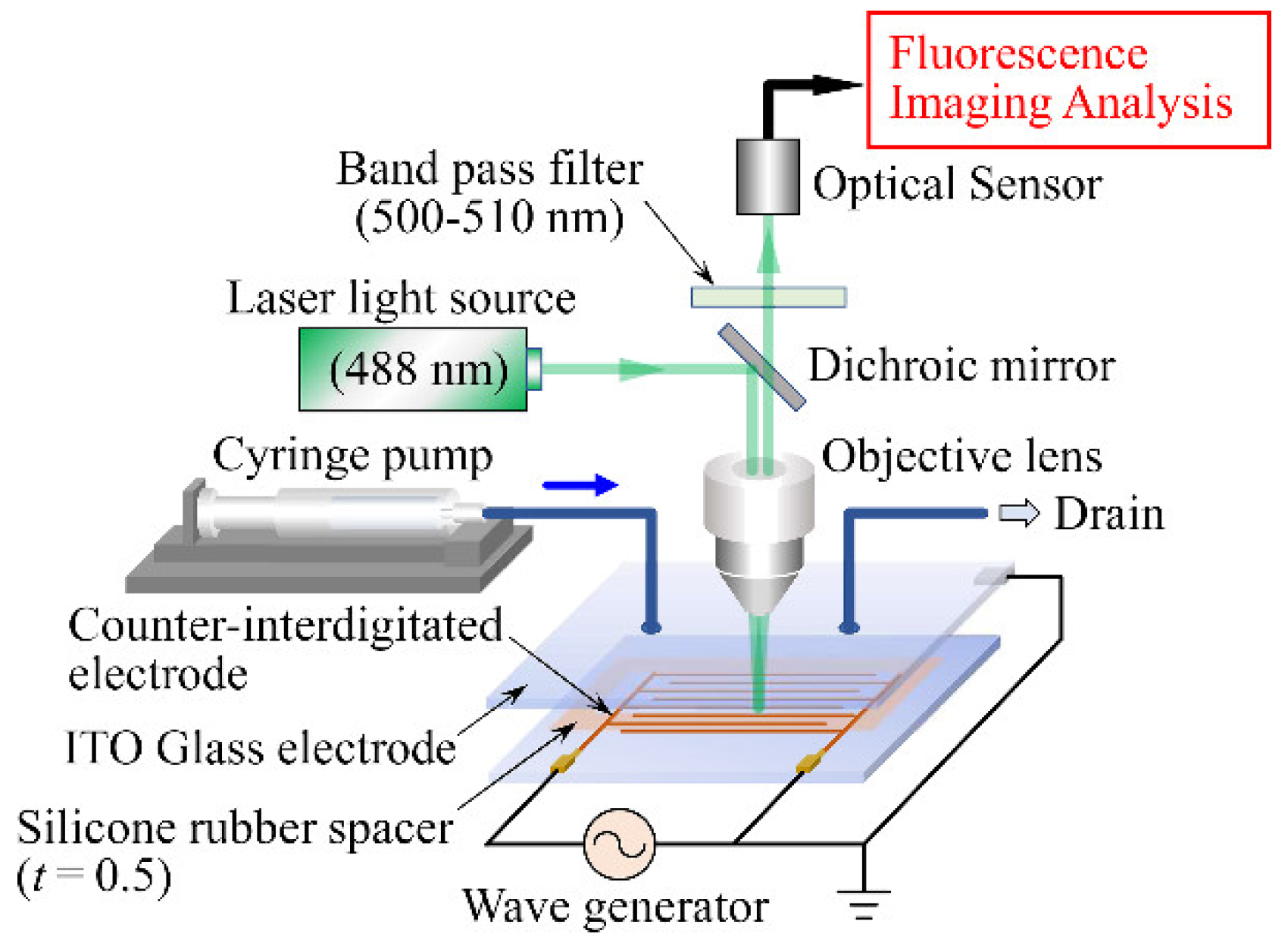
3.4. Experimental Apparatus and Method
4. Results and Discussion
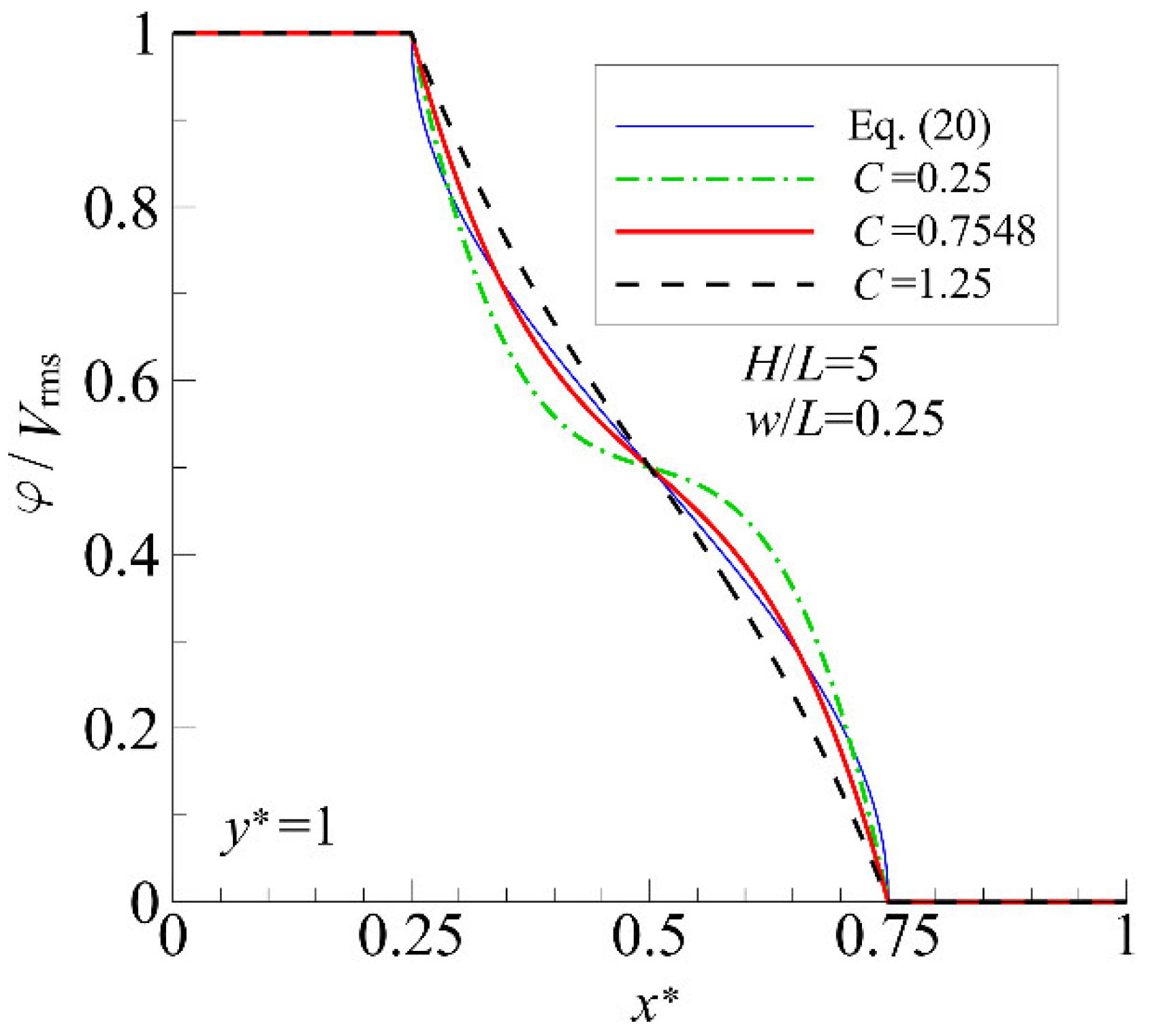
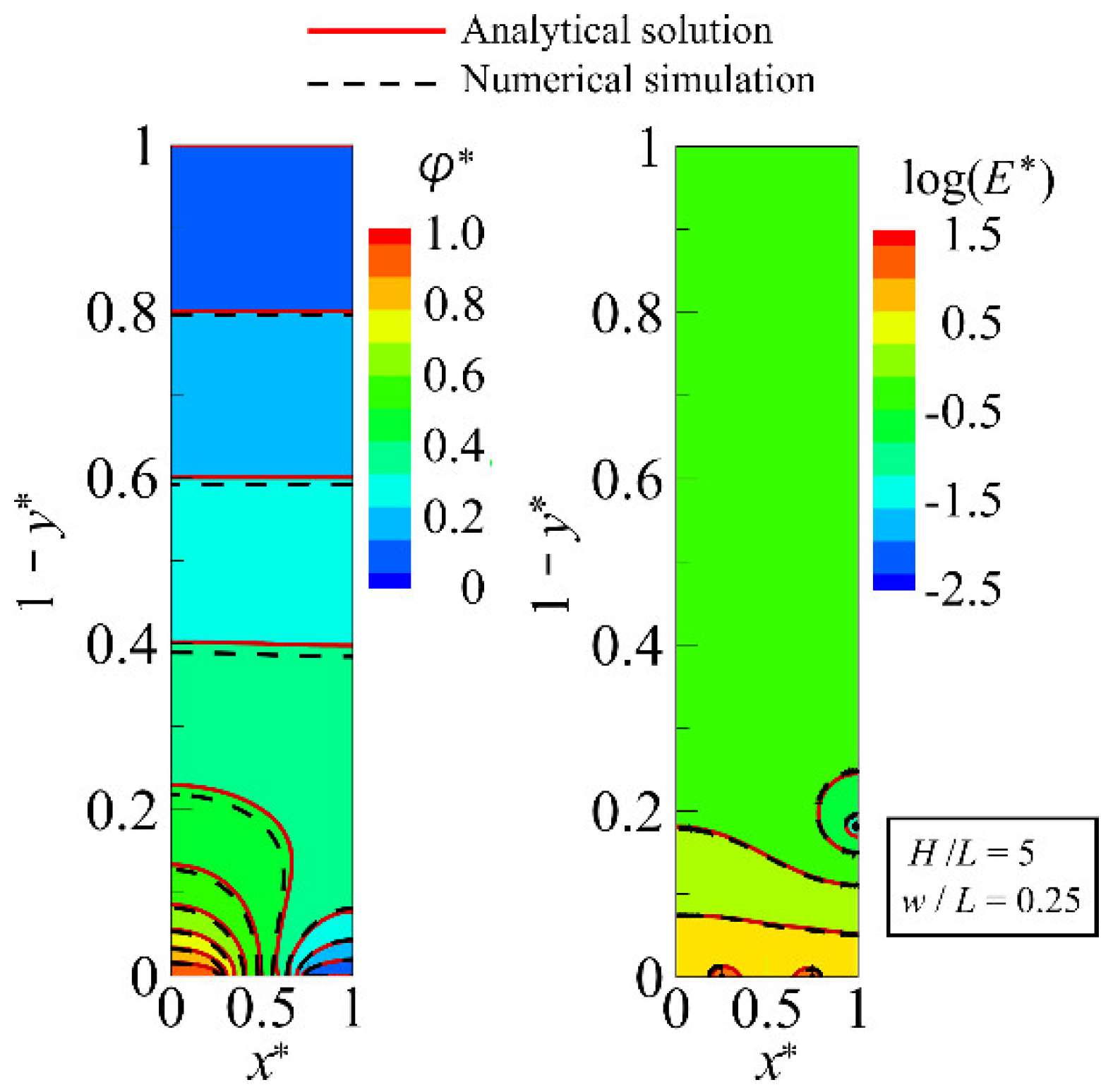
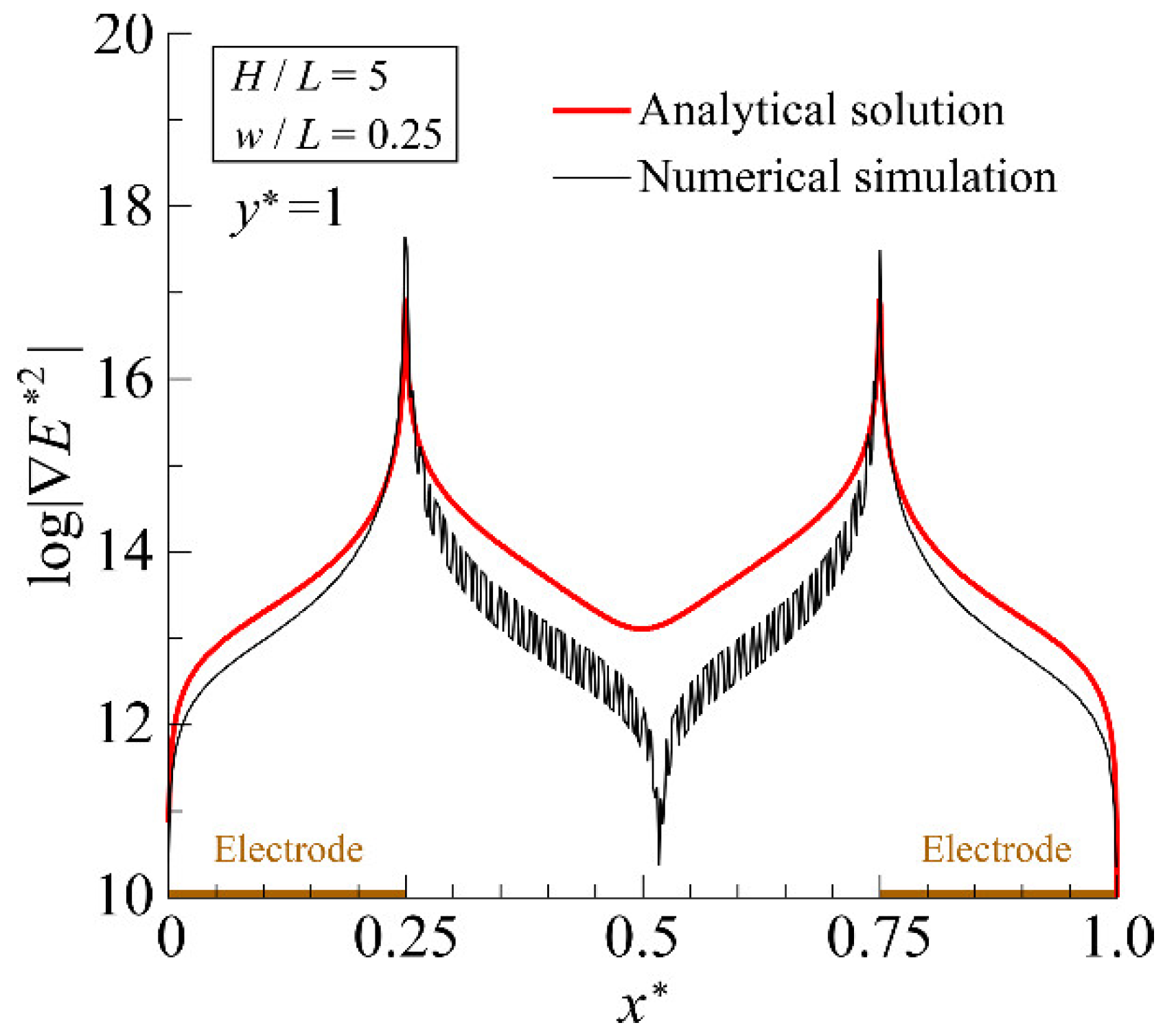
4.1. Accuracy Verification of the Analytical Solution
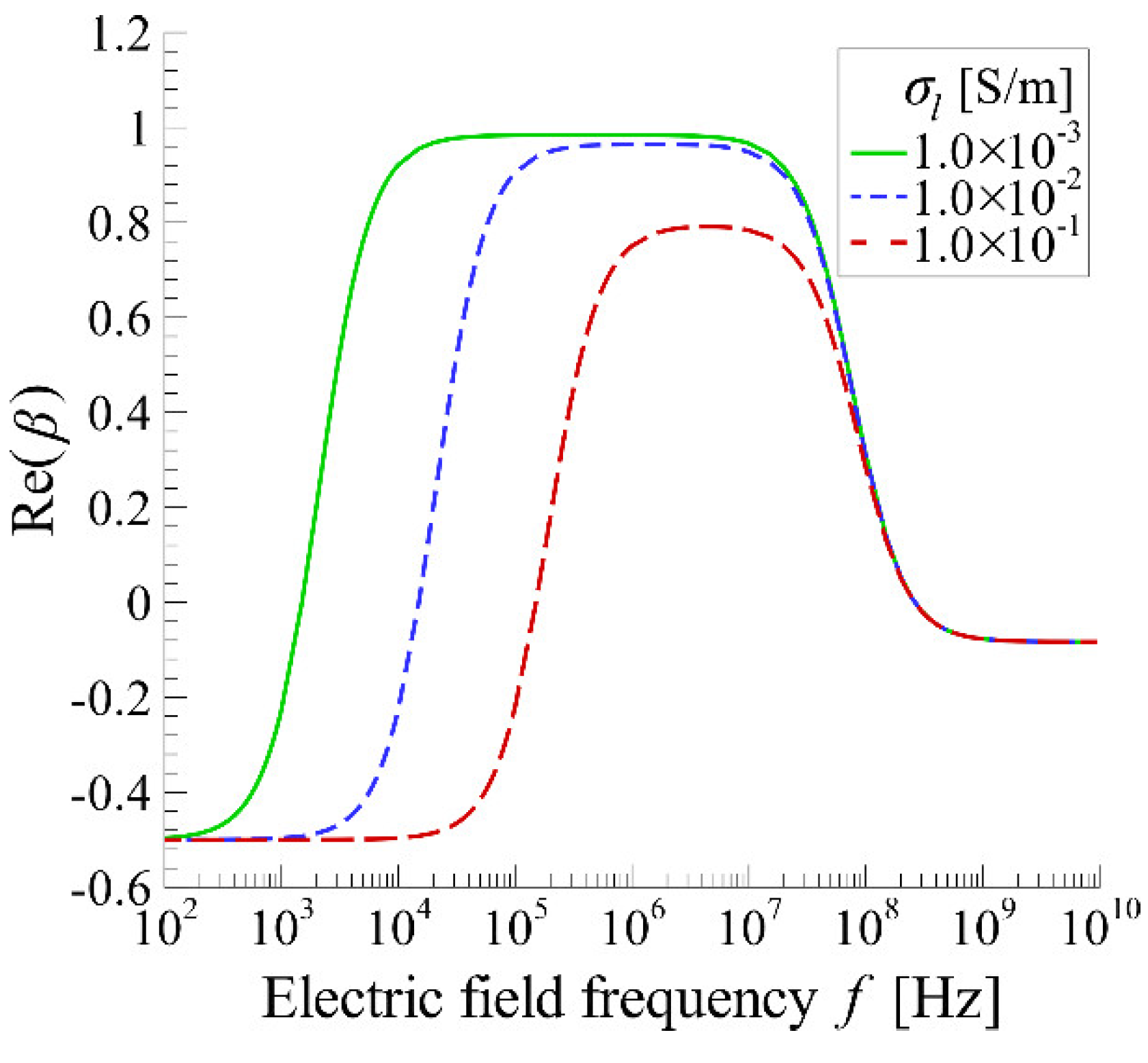
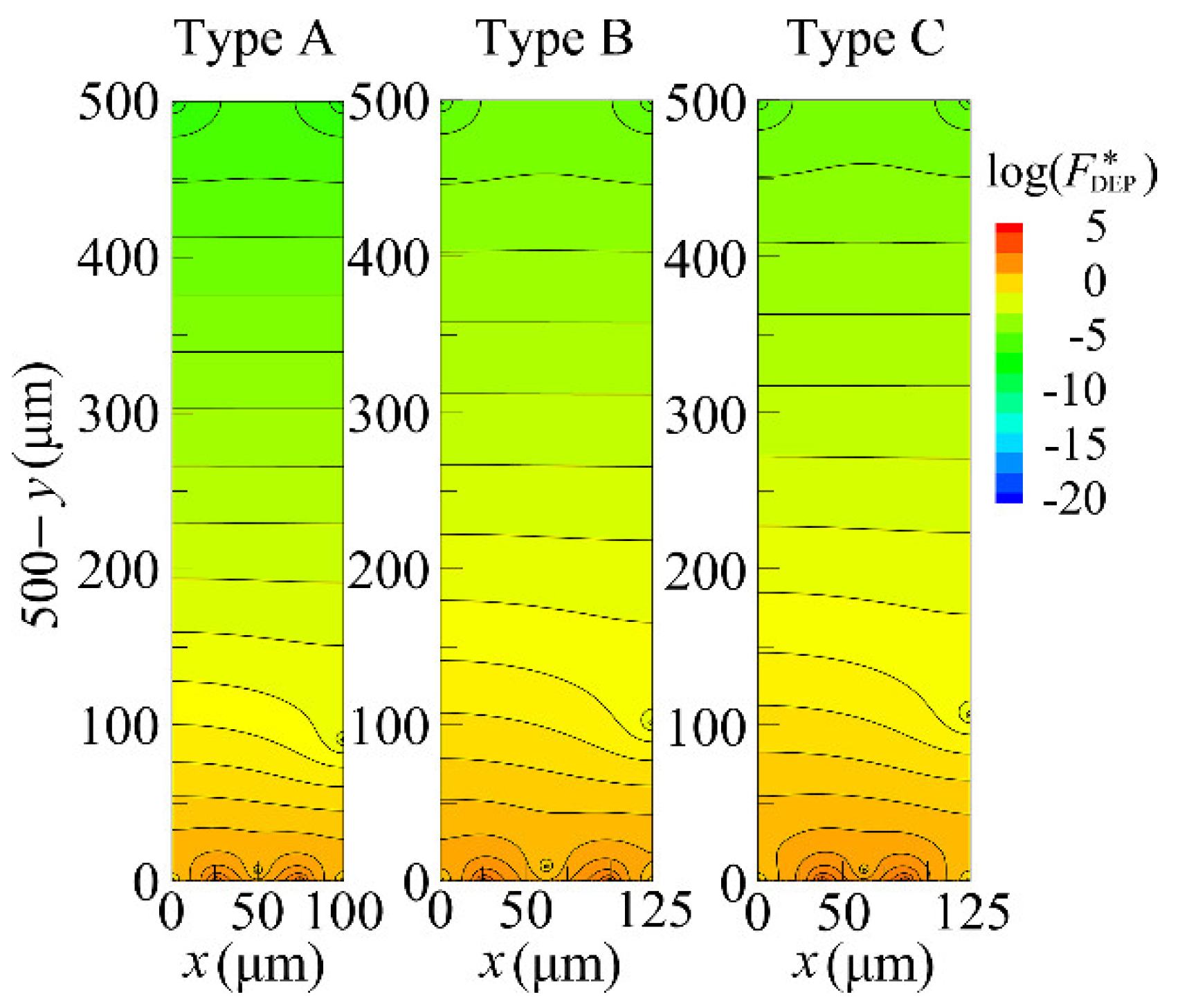
4.2. Distribution of DEP Force
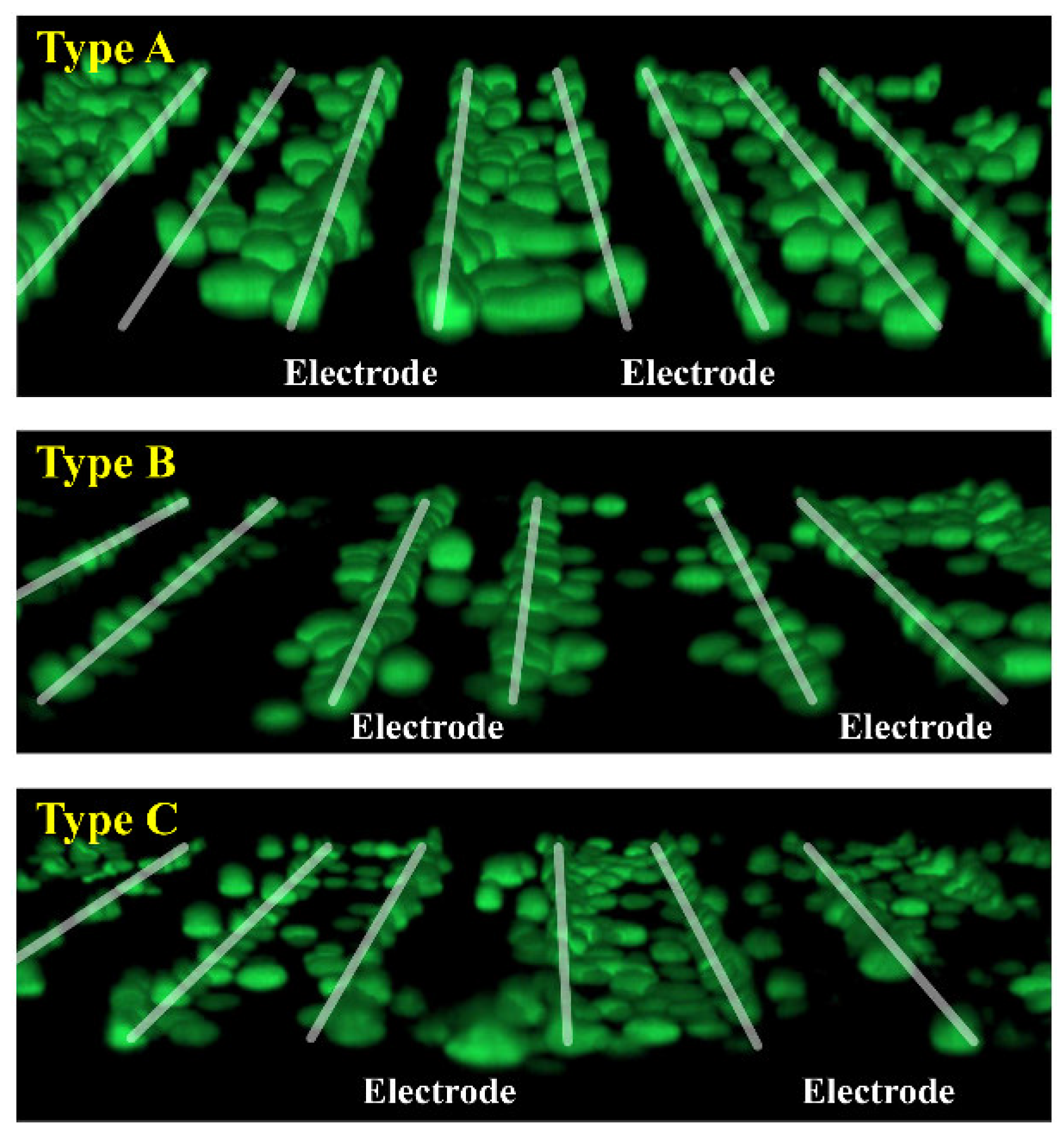
4.3. Experimental Results
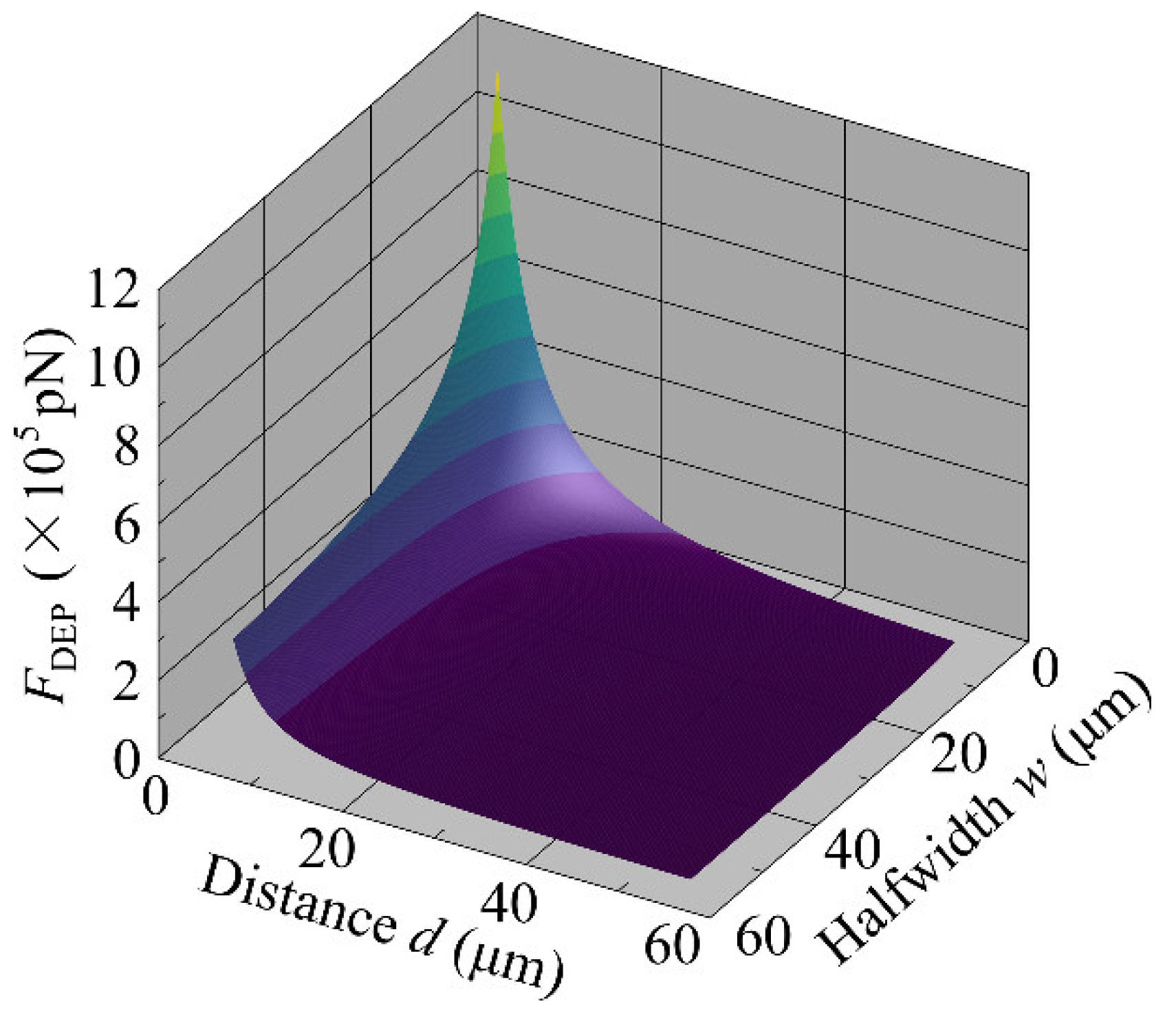
4.4. Relationship between DEP Force and Electrode Width and Spacing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
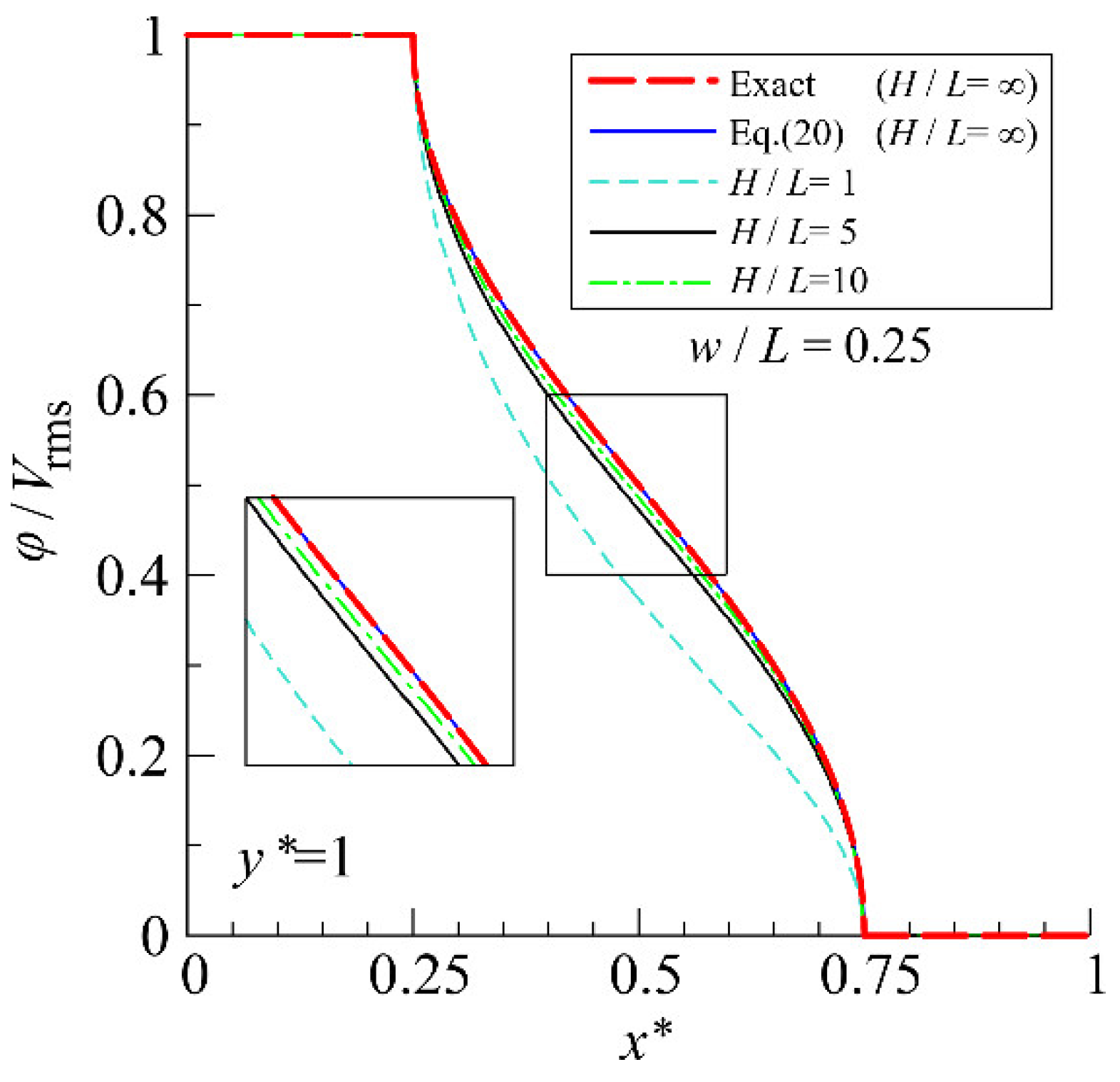
Appendix B
References
- Farasat, M.; Chavoshi, S.M.; Bakhshi, A.; Valipour, A.; Badieirostami, M. A dielectrophoresis-based microfluidic chip for trapping circulating tumor cells using a porous membrane. J Micromech Microeng 2021, 32, 015008. [Google Scholar] [CrossRef]
- Lv, B.; Cai, J. Simulation and analysis of geometric parameters based on Taguchi method in YY microfluidic device for circulating tumor cell separation by alternating current dielectrophoresis. J Chromatogr A 2023, 1693, 463894. [Google Scholar] [CrossRef] [PubMed]
- Valijam, S.; Salehi, A.; Andersson, M. Design of a low-voltage dielectrophoresis lab-on-the chip to separate tumor and blood cells. Microfluid Nanofluidics 2023, 27, 22. [Google Scholar] [CrossRef]
- Varmazyari, V.; Habibiyan, H.; Ghafoorifard, H.; Ebrahimi, M.; Ghafouri-Fard, S. A dielectrophoresis-based microfluidic system having double-sided optimized 3D electrodes for label-free cancer cell separation with preserving cell viability. Sci Rep 2022, 12, 12100. [Google Scholar] [CrossRef]
- Lin, S.H.; Su, T.C.; Huang, S.J.; Jen, C.P. Enhancing the efficiency of lung cancer cell capture using microfluidic dielectrophoresis and aptamer-based surface modification. Electrophoresis 2024, 45, 1088–1098. [Google Scholar] [CrossRef]
- Sarno, B.; Heineck, D.; Heller, M. J.; Ibsen, S.D. Dielectrophoresis: Developments and applications from 2010 to 2020. Electrophoresis 2021, 42, 539–564. [Google Scholar] [CrossRef]
- Shokouhmand, H.; Abdollahi, A. Detection of cell-free DNA nanoparticles in insulator based dielectrophoresis systems. J Chromatogr A 2020, 1626, 461262. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.B.; Becker, F.F.; Gascoyne, P.R.C. Introducing dielectrophoresis as a new force field for field-flow fractionation. Biophys J 1997, 73, 1118–1129. [Google Scholar] [CrossRef]
- Fiedler, S.; Shirley, S.G.; Schnelle, T.; Fuhr, G. Dielectrophoretic sorting of particles and cells in a microsystem. Anal Chem 1998, 70, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.; Izquierdo, A.G.; Bakewell, D.; Green, N.G.; Ramos, A. The dielectrophoretic and travelling wave forces generated by interdigitated electrode arrays: Analytical solution using Fourier series. J Phys D Appl Phys 2001, 34, 1553. [Google Scholar] [CrossRef]
- Choi, S.; Park, J.K. Microfluidic system for dielectrophoretic separation based on a trapezoidal electrode array. Lab Chip 2005, 5, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Imasato, H.; Yamakawa, T. Measurement of dielectrophoretic force by employing controllable gravitational force. J Electrophoresis 2008, 52, 1–8. [Google Scholar] [CrossRef]
- Lewpiriyawong, N.; Yang, C.; Lam, Y.C. Continuous sorting and separation of microparticles by size using AC dielectrophoresis in a PDMS microfluidic device with 3-D conducting PDMS composite electrodes. Electrophoresis 2010, 31, 2622–2631. [Google Scholar] [CrossRef]
- So, J.H.; Dickey, M.D. Inherently aligned microfluidic electrodes composed of liquid metal. Lab Chip 2011, 11, 905–911. [Google Scholar] [CrossRef]
- Fathy, J.; Pourmand, A.; Ghavifekr, H. B. Design and simulation of a MEMS based cell separator utilizing 3D travelling-wave dielectrophoresis. Microsyst Technol 2017, 23, 1351–1360. [Google Scholar] [CrossRef]
- Jiang, T.; Ren, Y.; Liu, W.; Tang, D.; Tao, Y.; Xue, R.; Jiang, H. Dielectrophoretic separation with a floating-electrode array embedded in microfabricated fluidic networks. Phys Fluids 2018, 30, 112003. [Google Scholar] [CrossRef]
- Beech, J.P.; Keim, K.; Ho, B.D.; Guiducci, C.; Tegenfeldt, J.O. Active posts in deterministic lateral displacement devices. Adv Mater Technol 2019, 4, 1900339. [Google Scholar] [CrossRef]
- Alnaimat, F.; Mathew, B.; Hilal-Alnaqbi, A. Modeling a dielectrophoretic microfluidic device with vertical interdigitated transducer electrodes for separation of microparticles based on size. Micromachines (Basel) 2020, 11, 563. [Google Scholar] [CrossRef]
- Huang, X.; Torres-Castro, K.; Varhue, W.; Salahi, A.; Rasin, A.; Honrado, C.; Brown, A.; Guler, J.; Swami, N. S. Self-aligned sequential lateral field non-uniformities over channel depth for high throughput dielectrophoretic cell deflection. Lab Chip 2021, 21, 835–843. [Google Scholar] [CrossRef]
- Tada, S.; Seki, Y. Analysis of temperature field in the dielectrophoresis-based microfluidic cell separation device. Fluids 2022, 7, 263. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Z.; Liu, Q.; Song, Y. Recent advances in dielectrophoresis-based cell viability assessment. Electrophoresis 2020, 41, 917–932. [Google Scholar] [CrossRef]
- Nerguizian, V.; Alazzam, A.; Roman, D.; Stiharu, I.; Burnier Jr, M. Analytical solutions and validation of electric field and dielectrophoretic force in a bio-microfluidic channel. Electrophoresis 2012, 33, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Alazzam, A.; Roman, D.; Nerguizian, V.; Stiharu, I.; Bhat, R. Analytical formulation of electric field and dielectrophoretic force for moving dielectrophoresis using Fourier series. Microfluid Nanofluid 2010, 9, 1115–1124. [Google Scholar] [CrossRef]
- Chen, R.; Liu, R.; Shen, H. Modeling and analysis of electric field and electrostatic adhesion force generated by interdigital electrodes for wall climbing robots. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems, Tokyo, Japan, 3.11.2013. [Google Scholar]
- Alhammadi, F.; Waheed, W.; El-Khasawneh, B.; Alazzam, A. Mathematical model and verification of electric field and dielectrophoresis in a microfluidic device. In Proceedings of the 2018 Advances in Science and Engineering Technology International Conferences (ASET), Dubai, UAE, 4.4.2020. [Google Scholar]
- Clague, D.; Wheeler, E. Dielectrophoretic manipulation of macromolecules: The electric field. Phys Rev E 2001, 64, 026605. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, M.; Hengster-Movric, K.; Hurák, Z. Green’s function-based control-oriented modeling of electric field for dielectrophoresis. J Appl Phys 2017, 122, 054903. [Google Scholar] [CrossRef]
- Garcia, M.; Clague, D. The 2D electric field above a planar sequence of independent strip electrodes. J Phys D Appl Phys 2000, 33, 1747. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.B.; Becker, F.; Gascoyne, P.R.C. A theoretical method of electrical field analysis for dielectrophoretic electrode arrays using Green’s theorem. J Phys D Appl Phys 1996, 29, 1649–1660. [Google Scholar] [CrossRef]
- Bai, S.; Tang, Y.; Ruan, L.; Song, R.; Chen, H.; Du, Y.; Lin, H.; Tang, Y.; Shan, Y. Investigation into the influence of interdigital parameters on electrochemical performance for in-plane supercapacitors via mathematical modeling and conformal mapping techniques. J Energy Storage 2023, 65, 107287. [Google Scholar] [CrossRef]
- Blume, S. O.; Ben-Mrad, R.; Sullivan, P.E. Modelling the capacitance of multi-layer conductor-facing interdigitated electrode structures. Sens Actuators B Chem 2015, 213, 423–433. [Google Scholar] [CrossRef]
- Pampin, R.S.; Raskin, J.; Huynen, I.; Flandre, D. Electrodes-oxide-semiconductor device for biosensing: Renewed conformal analysis and multilayer algorithm. J Electroanal Chem 2020, 856, 113651. [Google Scholar] [CrossRef]
- Sun, T.; Green, N.G.; Gawad, S.; Morgan, H. Analytical electric field and sensitivity analysis for two microfluidic impedance cytometer designs. IET Nanobiotechnol 2007, 1, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Morgan, H.; Green, N.G. Analytical solutions of ac electrokinetics in interdigitated electrode arrays: Electric field, dielectrophoretic and traveling-wave dielectrophoretic forces. Phys Rev E 2007, 76, 046610. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Crupi, G.; Ocket, I.; Bao, J.; Ceyssens, F.; Kraft, M.; Nauwelaers, B.; Schreurs, D. Numerical modeling of two microwave sensors for biomedical applications. Int J Numer Model El 2021, 34, e2810. [Google Scholar] [CrossRef]
- Ghomian, T.; Hihath, J. Review of dielectrophoretic manipulation of micro and nanomaterials: Fundamentals, recent developments, and challenges. IEEE Trans Biomed Eng 2022, 70, 27–41. [Google Scholar] [CrossRef]
- Miura, T.; Uno, S. Computer simulation for electrochemical impedance of a living cell adhered on the inter-digitated electrode sensors. Jpn J Appl Phys 2019, 58, SBBG15. [Google Scholar]
- Santos-Neto, I.S.d.; Carvalho, C.D.; Filho, G.B.A.; Andrade, C.D.S.S.; Santos, G.C. d.O.; Barros, A.K.; Neto, J.V.d.F.; Casas, V.L.P.; Alencar, L.M.R.; Lopes, A.J.O. Interdigitated electrode for electrical characterization of commercial pseudo-binary biodiesel–diesel blends. Sensors 2021, 21(21), 7288. [Google Scholar] [CrossRef]
- Zaman, M.A.; Padhy, P.; Ren, W.; Wu, M.; Hesselink, L. Microparticle transport along a planar electrode array using moving dielectrophoresis. J Appl Phys 2021, 130(3), 034902. [Google Scholar] [CrossRef]
- Tada, S.; Hayashi, M.; Eguchi, M.; Tsukamoto, A. High-throughput separation of cells by dielectrophoresis enhanced with 3D gradient AC electric field. Biomicrofluidics 2017, 11, 064110. [Google Scholar] [CrossRef]
- Duffy, D.G. Mixed Boundary Value Problems, 1st ed.; Chapman and Hall/CRC: New York, USA, 2008; pp. 1–14. [Google Scholar]
- Feng, J.J.; Krishnamoorthy, S.; Chen, Z.J.; Makhijani, V.B. Numerical and analytical studies of AC electric field in dielectrophoretic electrode arrays. In Proceedings of the Technical Proceedings of the 2002 International Conference on Modeling and Simulation of Microsystems, Puerto Rico, USA, 22.4.2002. [Google Scholar]
- Jones, T.B. Electromechanics of Particles, 2nd ed.; Cambridge University Press: Cambridge, UK, 1995; pp. 34–48. [Google Scholar]
- Henslee, E.A.; Sano, M.B.; Rojas, A.D.; Schmelz, E.M.; Davalos, R.V. Selective concentration of human cancer cells using contactless dielectrophoresis. Electrophoresis 2011, 32, 2523–2529. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, V.; Bolopion, A.; Gauthier, M. Analytical formulation of the electric field induced by electrode arrays: Towards automated dielectrophoretic cell sorting. Micromachines (Basel) 2017, 8, 253. [Google Scholar] [CrossRef]
| Symbol | Values | |
|---|---|---|
| RMS voltage | V | |
| electric permittivity of the solution | F/m | |
| electric conductivity of the solution | S/m | |
| cell diameter | 15 m | |
| height of the microchannel | 500 m | |
| real part of the CM factor | 0.98 | |
| number of terms of the series expansion | 100,000 | |
| electrode width and spacing (:d) | Type A | (50 μm:50 μm) |
| Type B | (50 μm:75 μm) | |
| Type C | (75 μm:50 μm) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





