Submitted:
11 October 2024
Posted:
11 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Normal Placental Development, Morphology and Function
2.1. Placental Organogenesis
2.2. Phases of Implantation
2.2.1. Apposition and Adhesion
2.2.2. Invasion
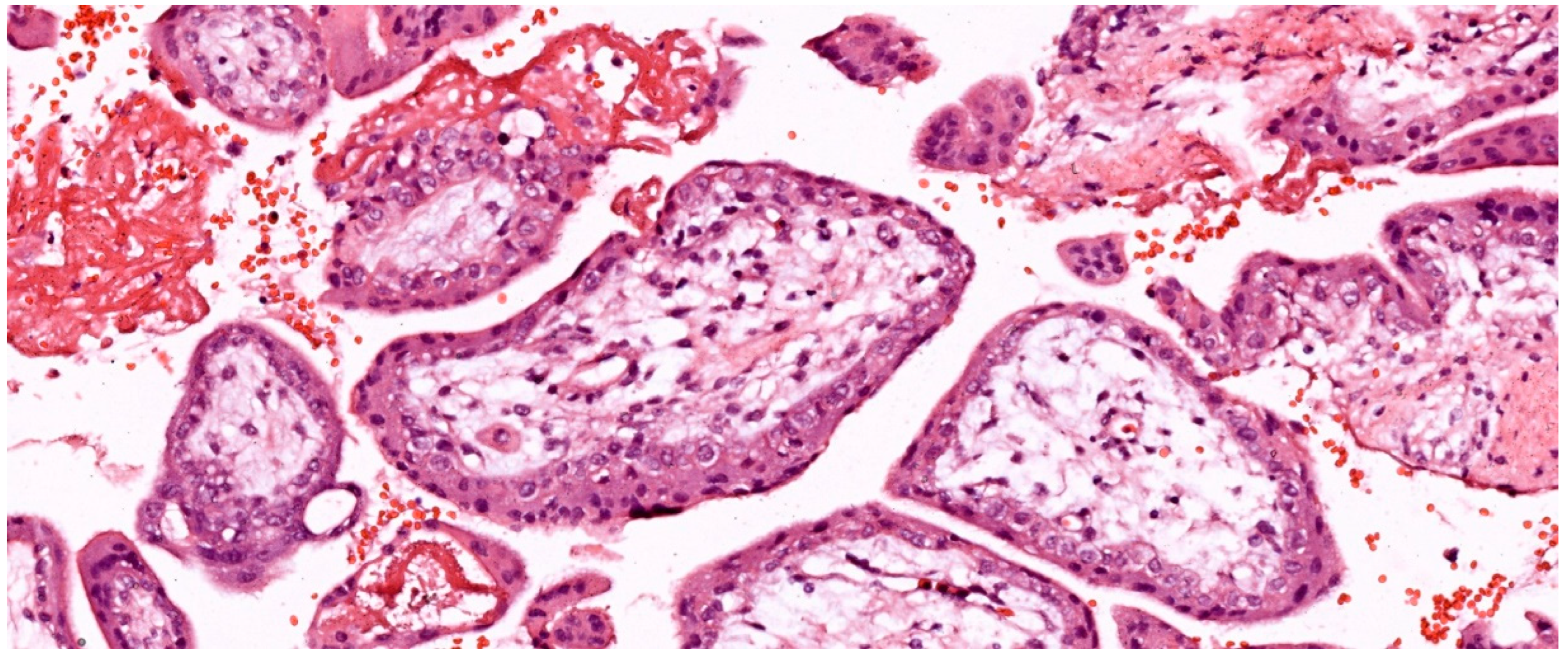
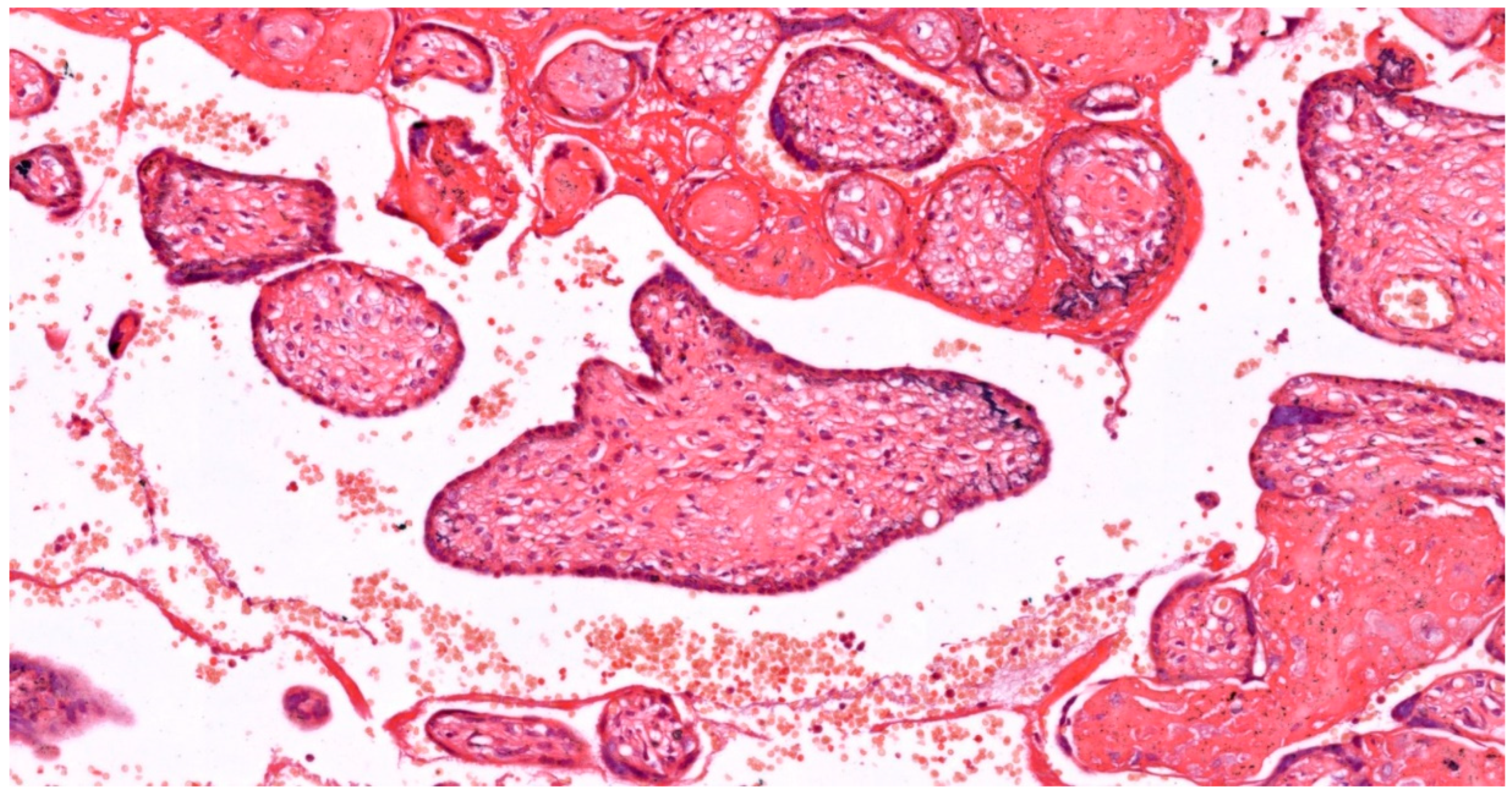
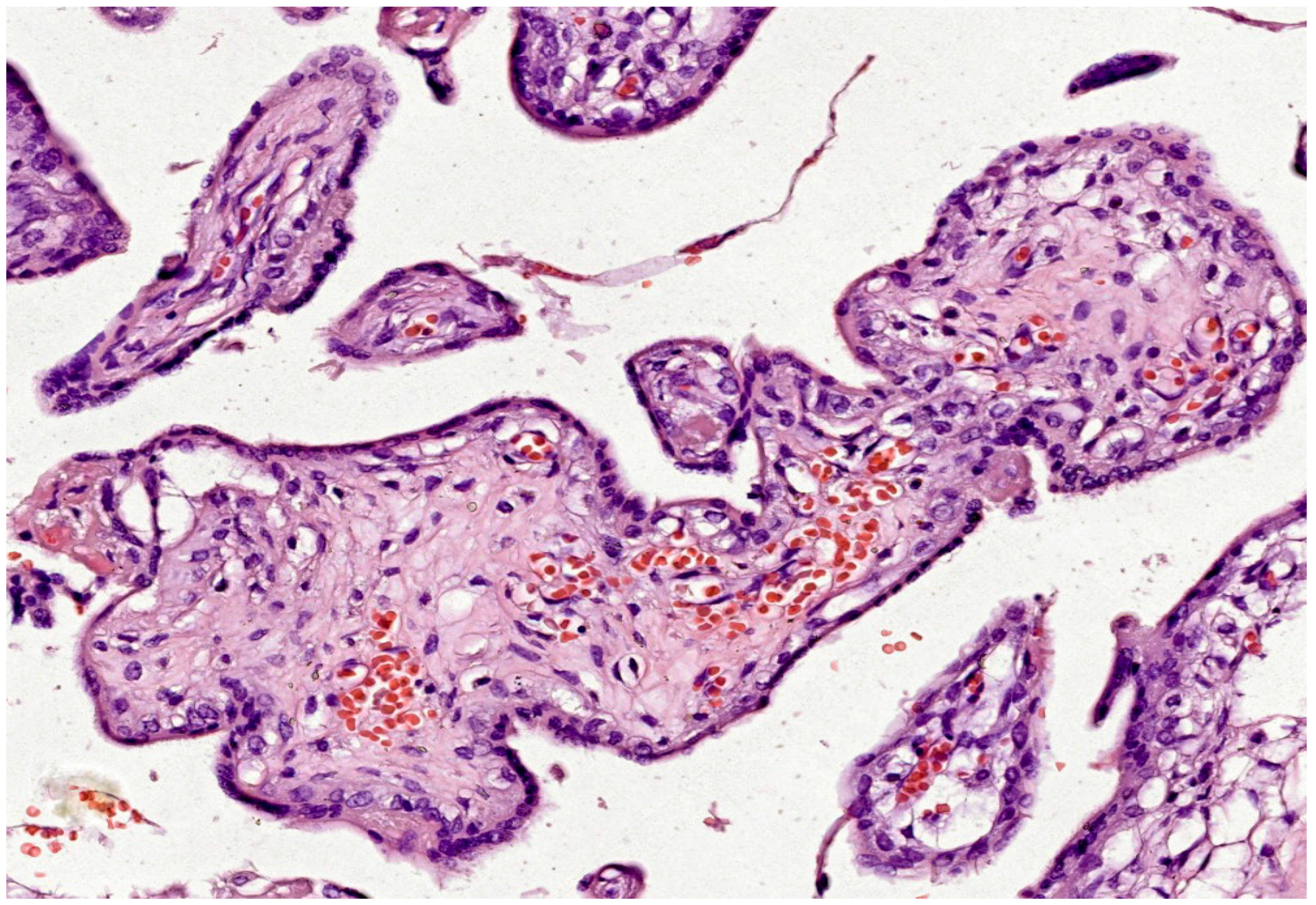
2.3. General Histologic Organization of the Placenta
- Primary Villi: The smallest type, without extensive branching, composed of a central cytotrophoblast core surrounded by peripheral syncytiotrophoblast. Primary villi start to form between day 11 and day 13 post-conception.
- Secondary Villi: Branching structures with loose connective tissue in the central axis, forming later in the first trimester.
- Tertiary Villi: Begin to form at the end of the third week of gestation, are extensively branched, elongated, and contain well-developed blood vessels within the central axis.
2.3.1. Structure and Development of Chorionic Villi
- 4.
- Stem villi: This type of villi attaches to the chorionic plate and is characterized by a dense fibrous stroma containing both large and small vessels. Vascular structures with smooth muscle develop in the stem chorionic villi. The trophoblast cell layer of stem chorionic villi is partially replaced as pregnancy progresses. The function of stem chorionic villi is to support the structure of the villous "tree". Endocrine activity and maternal-fetal exchange at the level of stem villi are usually negligible [58].
- 5.
- Immature intermediate villi: These are peripheral, immature, bulb-shaped continuations of stem villi. They have a looser or reticular stroma. Hofbauer cells, more prominent blood vessels and a discontinuous layer of cytotrophoblast cells are noted in these villi. The outer layer, the syncytiotrophoblast, remains continuous throughout development. Immature intermediate villi form the basis for growth of the villous "tree". It is considered that maternal-fetal exchange occurs mainly in these villi during the first and second trimesters, until terminal villi differentiate [58].
- 6.
- Mature intermediate villi: These are long, thin, peripheral branches. This type of villi does not have fetal vessels in the stroma. Terminal villi will arise from mature intermediate villi. The increased number of fetal vessels, providing a large exchange surface, makes them important for feto-maternal exchange [6].
- 7.
- Terminal villi: These are connected to stem villi. Terminal villi have a grape-like appearance, characterized by a high degree of capillarization and the presence of highly dilated sinusoids. In the term placenta, terminal villi are smaller, with less stroma, a discontinuous cytotrophoblast cell layer and 4-6 fetal capillaries in cross-section. In terminal villi, fetal capillary vessels and syncytiotrophoblast are separated only by a thin basement membrane, making these villi the most suitable site for maternal-fetal exchange. In the mature placenta, terminal villi represent 40% of the total villous volume of the placenta. Due to their small diameters, the sum of their surfaces represents 50% of the total villous surface area [58]. Terminal villi are considered the functional unit of the placenta. The transfer of electrolytes, O2, CO2 and nutrients between mother and fetus occurs at this level [6].
- 8.
- Mesenchymal villi: represent the most primitive type of villi, from the early stages of pregnancy. The stroma is loose, capillaries are discrete, two layers of surrounding trophoblast cells, a layer of cytotrophoblast cells surrounding the center of the villus and syncytiotrophoblast arranged on the outer villous surface. Fetal capillaries are poorly developed and never show sinusoidal dilatations. The non-vascularized extremities of mesenchymal villi are called villous buds. The function of mesenchymal villi is very primordial at the beginning of pregnancy. These are the site of villous proliferation and fulfill almost all endocrine activities. As pregnancy progresses, their main function is to support villous growth. In the mature placenta, mesenchymal villi represent less than 1% of the total villous volume [58].
2.3.2. Placental Barrier and Materno-Fetal Exchange
- Syncytiotrophoblast Layer: This outermost layer is in direct contact with maternal blood. It plays a role in hormone synthesis and transport.
- Cytotrophoblast Layer: A layer of individual cuboidal cells that provide structural integrity and secrete enzymes that aid in remodeling the maternal vasculature.
- Trophoblast Basement Membrane: The extracellular matrix providing support to trophoblastic cells.
- Villous Core Mesenchyme: Contains fibroblasts, Hofbauer cells, and fetal capillaries that transport nutrients and oxygen.
- Endothelial Basement Membrane: A thin extracellular matrix layer that provides a barrier between fetal blood and the surrounding villous core.
- Fetal Capillary Endothelium: The inner layer that lines fetal blood vessels, allowing for nutrient uptake into fetal circulation.
2.4. Circulatory Changes and Placental Growth Throughout Pregnancy
2.5. Functions of the Placenta
3. Definitions and Mechanisms of Oxidative Stress
3.1. Metabolism of Reactive Oxygen Species
3.1.1. Physiological Roles of Free Radicals
3.1.2. Pathological Effects of Free Radicals
3.2. Antioxidants and Antioxidant Defense Mechanisms
3.3. The Pro-Oxidant-Antioxidant Balance Concept
4. Sources of Oxidative Stress during Pregnancy
5. Oxidative Stress and Placental Development
6. Oxidative Stress and Placental Pathology
6.1. Placental Adaptations to Oxidative Stress
6.2. Associations with Specific Placental Pathologies
6.3. Long-Term Materno-Fetal Consequences
7. Clinical Complications and Oxidative Stress Biomarkers
8. Emerging Therapeutics Targeting Oxidative Stress in Placental Disorders
9. Conclusion and Future Perspectives
- The placenta exists in a delicate redox balance throughout gestation, with physiological levels of ROS playing important signaling roles in placental development and function.
- Disruption of this balance, leading to OS, is implicated in a wide range of placental pathologies and pregnancy complications, including PE, IUGR, gestational diabetes, and preterm birth.
- OS induces structural and functional changes in the placenta, affecting trophoblast differentiation, vascular development, nutrient transport, and hormone production.
- The placenta has evolved various adaptive mechanisms to cope with oxidative challenges, but these can be overwhelmed in pathological conditions.
- The effects of placental OS extend beyond pregnancy, potentially influencing long-term health outcomes for both mother and offspring through epigenetic modifications and other programming mechanisms.
- While antioxidant therapies have shown promise in preclinical studies, translation to effective clinical interventions has been challenging, highlighting the complexity of redox biology in pregnancy.
- Improved biomarkers: Development of more specific and sensitive biomarkers for placental OS could enable earlier detection of at-risk pregnancies and more targeted interventions.
- Personalized approaches: Given the heterogeneity of placental disorders, personalized medicine approaches that consider individual genetic, environmental, and clinical factors may be necessary to effectively manage OS.
- Targeted therapies: Novel drug delivery systems that can specifically target the placenta could improve the efficacy of antioxidant therapies while minimizing systemic effects.
- Timing of interventions: Better understanding of the critical windows of susceptibility to OS during placental development could inform the optimal timing of preventive or therapeutic interventions.
- Long-term follow-up: Extended follow-up studies of both mothers and offspring are needed to fully elucidate the long-term consequences of placental OS and evaluate the efficacy of interventions.
- Integration of multi-omics data: Combining genomic, epigenomic, transcriptomic, proteomic, and metabolomic data could provide a more comprehensive understanding of the complex interplay between OS and placental function.
- Advanced imaging techniques: Development of non-invasive imaging methods to assess placental redox status in vivo could revolutionize the monitoring and management of placental health.
- Microbiome interactions: Exploration of the potential role of the placental and maternal microbiome in modulating OS and placental function.
- Environmental influences: Further investigation of how environmental factors (e.g., air pollution, endocrine disruptors) influence placental OS and development of strategies to mitigate these effects.
- Therapeutic potential of gasotransmitters: Deeper exploration of the therapeutic potential of gasotransmitters (NO, CO, H2S) in managing placental OS and vascular function.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gude, N. M.; Roberts, C. T.; Kalionis, B.; King, R. G. Growth and Function of the Normal Human Placenta. Thromb Res 2004, 114, (5–6). [Google Scholar] [CrossRef] [PubMed]
- Knofler M, Pollheimer J. Human placental trophoblast invasion and differentiation: a particular focus on Wnt signaling. Frontiers in Genetics.
- Myatt, L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta.
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B. J.; Shaman, A.; Gupta, S. The Effects of Oxidative Stress on Female Reproduction: A Review. Reprod Biol Endocrinol 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology.
- Wang Y, Zhao S. Vascular Biology of the Placenta, /: & Claypool Life Sciences; 2010. https, 2010.
- Jauniaux E, Burton GJ. Pathophysiology of histological changes in early pregnancy loss. Placenta.
- Pringle KG, Kind KL, Thompson JG, Roberts CT. Complex interactions between hypoxia inducible factors, oxygen, and progesterone regulate trophoblast giant cell differentiation and migration in mice. Biology of Reproduction.
- Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science, 5332.
- Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. Journal of the Society for Gynecologic Investigation.
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The Uterine Spiral Arteries in Human Pregnancy: Facts and Controversies. Placenta 2006, 27, (9–10). [Google Scholar] [CrossRef]
- Raijmakers, M. T.; Dechend, R.; Poston, L. Oxidative Stress and Preeclampsia: Rationale for Antioxidant Clinical Trials. Hypertension 2004, 44, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.-H.; Burton, G. J. Hypoxia and Reoxygenation: A Possible Mechanism for Placental Oxidative Stress in Preeclampsia. Taiwanese Journal of Obstetrics and Gynecology 2006, 45, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Chappell, L. C.; Morgan, L. Searching for the Causes of Preeclampsia. The Lancet 2006, 367, 56–57. [Google Scholar]
- Roberts, J. M.; Gammill, H. S. Preeclampsia: Recent Insights. Hypertension 2005, 46, 1243–1249. [Google Scholar] [CrossRef]
- Maynard, S. E.; Min, J.-Y.; Merchan, J.; Lim, K.-H.; Li, J.; Mondal, S.; Libermann, T. A.; Morgan, J. P.; Sellke, F. W.; Stillman, I. E.; others. Excess Placental Soluble Fms-like Tyrosine Kinase 1 (sFlt1) May Contribute to Endothelial Dysfunction, Hypertension, and Proteinuria in Preeclampsia. The Journal of Clinical Investigation 2003, 111, 649–658. [Google Scholar] [CrossRef]
- Levine, R. J.; Maynard, S. E.; Qian, C.; Lim, K.-H.; England, L. J.; Yu, K. F.; Schisterman, E. F.; Thadhani, R.; Sachs, B. P.; Epstein, F. H.; others. Circulating Angiogenic Factors and the Risk of Preeclampsia. New England Journal of Medicine 2004, 350, 672–683. [Google Scholar] [CrossRef]
- Baschat, A. A. Pathophysiology of Fetal Growth Restriction: Implications for Diagnosis and Surveillance. Obstetrical & Gynecological Survey 2004, 59, 617–627. [Google Scholar]
- Chen, B.; Longtine, M. S.; Nelson, D. M. Hypoxia Induces Autophagy in Primary Human Trophoblasts. Endocrinology 2012, 153, 4946–4954. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of Cells and Tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Autophagy in the Pathogenesis of Disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Yamaguchi, A. Involvement of Mitochondrial Membrane Potential Loss and Cytochrome c Release in Hydrogen Peroxide-Induced Apoptosis in Osteoblastic Cells. Calcified Tissue International 2003, 72, 541–548. [Google Scholar]
- Ishihara, N.; Matsuo, H.; Murakoshi, H.; Laoag-Fernandez, J. B.; Samoto, T.; Maruo, T. Increased Apoptosis in the Syncytiotrophoblast in Human Term Placentas Complicated by Either Preeclampsia or Intrauterine Growth Retardation. American Journal of Obstetrics and Gynecology 2002, 186, 158–166. [Google Scholar] [CrossRef]
- Myatt, L. Placental Adaptive Responses and Fetal Programming. Journal of Physiology 2006, 572, 25–30. [Google Scholar] [CrossRef]
- Rumbold, A.; Duley, L.; Crowther, C. A.; Haslam, R. R. Antioxidants for Preventing Pre-Eclampsia. Cochrane Database of Systematic Reviews.
- Poston, L.; Igosheva, N.; Mistry, H. D.; Seed, P. T.; Shennan, A. H.; Rana, S.; Karumanchi, S. A.; Chappell, L. C. Role of Oxidative Stress and Antioxidant Supplementation in Pregnancy Disorders. The American Journal of Clinical Nutrition 2011, 94, 1980S–1985S. [Google Scholar] [CrossRef]
- Wang, K.; Ahmad, S.; Cai, M.; Rennie, J.; Fujisawa, T.; Crispi, F.; Baily, J.; Miller, M. R.; Cudmore, M.; Hadoke, P. W.; others. Dysregulation of Hydrogen Sulfide Producing Enzyme Cystathionine γ-Lyase Contributes to Maternal Hypertension and Placental Abnormalities in Preeclampsia. Circulation 2013, 127, 2514–2522. [Google Scholar] [CrossRef]
- Carr, D. J.; Wallace, J. M.; Aitken, R. P.; Milne, J. S.; Mehta, V.; Martin, J. F.; Zachary, I. C.; Peebles, D. M.; David, A. L. Uteroplacental Adenovirus Vascular Endothelial Growth Factor Gene Therapy Increases Fetal Growth Velocity in Growth-Restricted Sheep Pregnancies. Human Gene Therapy 2014, 25, 375–384. [Google Scholar] [CrossRef]
- Gupta, S.; Agarwal, A. The Role of Free Radicals and Antioxidants in Reproduction. Current Opinion in Obstetrics and Gynecology 2005, 17, 263–266. [Google Scholar]
- Wakatsuki, A.; Ikenoue, N. Antioxidant Therapy in Preeclampsia: Lessons from Basic Research Studies. Current Hypertension Reports 2009, 11, 11–16. [Google Scholar]
- Burton, G. J.; Jauniaux, E. Oxidative Stress. Best Practice & Research Clinical Obstetrics & Gynaecology 2011, 25, 287–299. [Google Scholar]
- Roberts, J. M.; Hubel, C. A. The Two Stage Model of Preeclampsia: Variations on the Theme. Placenta 2009, 30, S32–S37. [Google Scholar] [CrossRef] [PubMed]
- Herrick, E. J.; Bordoni, B. Embryology, Placenta. StatPearls, T: Publishing, 2024. [Google Scholar]
- Cunningham, F. G.; Leveno, K. J.; Bloom, S. L. ; McGraw-Hill Education: New York, NY, 2013.Development. In Williams Obstetrics; McGraw-Hill Education: New York, NY, 2013. [Google Scholar]
- Predoi, C. G.; Grigoriu, C.; Vladescu, R.; Mihart, A. E. Placental Damages in Preeclampsia–from Ultrasound Images to Histopathological Findings. Journal of medicine and life 2015, 8, 62–65. [Google Scholar] [PubMed]
- Zia, S. Placental Location and Pregnancy Outcome. Journal of the Turkish German Gynecological Association 2013, 14, 190–193. [Google Scholar] [CrossRef]
- Gibson, D. A.; Simitsidellis, I.; Cousins, F. L.; Critchley, H. O.; Saunders, P. T. Intracrine Androgens Enhance Decidualization and Modulate Expression of Human Endometrial Receptivity Genes. Scientific reports 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Curtis Hewitt, S.; Goulding, E. H.; Eddy, E. M.; Korach, K. S. Studies Using the Estrogen Receptor Alpha Knockout Uterus Demonstrate That Implantation but Not Decidualization-Associated Signaling Is Estrogen Dependent. Biology of reproduction 2002, 67, 1268–1277. [Google Scholar] [CrossRef]
- Guzeloglu-Kayisli, O.; Kayisli, U. A.; Taylor, H. S. The Role of Growth Factors and Cytokines during Implantation: Endocrine and Paracrine Interactions. Seminars in reproductive medicine 2009, 27, 62–79. [Google Scholar] [CrossRef]
- Jinno, M.; Ozaki, T.; Iwashita, M.; Nakamura, Y.; Kudo, A.; Hirano, H. Measurement of Endometrial Tissue Blood Flow: A Novel Way to Assess Uterine Receptivity for Implantation. Fertility and sterility 2001, 76, 1168–1174. [Google Scholar] [CrossRef]
- Saravelos, S. H.; Wong, A. W.; Chan, C. P.; Kong, G. W.; Li, T.-C. Assessment of the Embryo Flash Position and Migration with 3D Ultrasound within 60 Min of Embryo Transfer. Human reproduction 2016, 31, 591–596. [Google Scholar] [CrossRef]
- Vahanian, S. A.; Lavery, J. A.; Ananth, C. V.; Vintzileos, A. Placental Implantation Abnormalities and Risk of Preterm Delivery: A Systematic Review and Metaanalysis. American journal of obstetrics and gynecology 2015, 213, S78–S90. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.-D.; Weng, Y.; Wang, C.-F.; Huang, L.-L.; Zhu, X.-M. Research on the Expression of Integrin Β3 and Leukaemia Inhibitory Factor in the Decidua of Women with Cesarean Scar Pregnancy. BMC pregnancy and childbirth 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rosen, T. Placenta Accreta and Cesarean Scar Pregnancy: Overlooked Costs of the Rising Cesarean Section Rate. Clinics in perinatology 2008, 35, 519–529. [Google Scholar] [CrossRef]
- Macklin, P. S.; McAuliffe, J.; Pugh, C. W.; Yamamoto, A. Hypoxia and HIF Pathway in Cancer and the Placenta. Placenta 2017, 56, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, F.; Galan, A.; Martin, J. J.; Remohi, J.; Pellicer, A.; Simon, C. Hormonal and Embryonic Regulation of Chemokine Receptors CXCR1, CXCR4, CCR5 and CCR2B in the Human Endometrium and the Human Blastocyst. Molecular human reproduction 2003, 9, 189–198. [Google Scholar] [CrossRef]
- Tarrade, A.; Lai Kuen, R.; Malassiné, A.; Tricottet, V.; Blain, P.; Vidaud, M.; Evain-Brion, D. Characterization of Human Villous and Extravillous Trophoblasts Isolated from First Trimester Placenta. Laboratory investigation 2001, 81, 1199–1211. [Google Scholar] [CrossRef]
- Cahill, A. G.; Beigi, R.; Heine, R. P.; Silver, R. M.; Wax, J. R. Placenta Accreta Spectrum. American journal of obstetrics and gynecology 2018, 219, B2–B16. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Godbole, G.; Modi, D. Decidual Control of Trophoblast Invasion. American Journal of Reproductive Immunology 2016, 75, 341–350. [Google Scholar] [CrossRef]
- Plaks, V.; Rinkenberger, J.; Dai, J.; Flannery, M.; Sund, M.; Kanasaki, K.; Ni, W.; Kalluri, R.; Werb, Z. Matrix Metalloproteinase-9 Deficiency Phenocopies Features of Preeclampsia and Intrauterine Growth Restriction. Proceedings of the National Academy of Sciences 2013, 110, 11109–11114. [Google Scholar] [CrossRef]
- Carlino, C.; Rippo, M. R.; Lazzarini, R.; Monsurrò, V.; Morrone, S.; Angelini, S.; Trotta, E.; Stabile, H.; Valentini, S.; Santoni, A.; others. Differential microRNA Expression between Decidual and Peripheral Blood Natural Killer Cells in Early Pregnancy. Human reproduction 2018, 33, 2184–2195. [Google Scholar] [CrossRef]
- Laban, M.; Ibrahim, E. A.; Elsafty, M. S.; Hassanin, A. S. Placenta Accreta Is Associated with Decreased Decidual Natural Killer (dNK) Cells Population: A Comparative Pilot Study. European Journal of Obstetrics & Gynecology and Reproductive Biology 2014, 181, 284–288. [Google Scholar] [CrossRef]
- Bulmer, J. N.; Lash, G. E. Uterine Natural Killer Cells: Time for a Re-Appraisal? F1000Research 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Power, I.; Kam, P. Maternal and Neonatal Physiology. Principles of Physiology for the Anaesthetist, 2011. [Google Scholar]
- Huppertz, B. The Anatomy of the Normal Placenta. Journal of clinical pathology 2008, 61, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Benirschke, K.; Burton, G. J.; Baergen, R. N. Pathology of the Human Placenta; Springer: Berlin, Heidelberg, 2012. [Google Scholar] [CrossRef]
- Kaufmann, P. Basic Morphology of the Fetal and Maternal Circuits in the Human Placenta. Contributions to gynecology and obstetrics 1985, 13, 5–17. [Google Scholar]
- Castellucci, M.; Kaufmann, P. Architecture of Normal Villous Trees. In Pathology of the human placenta; Benirschke, K., Kaufmann, P., Baergen, R., Eds.; Springer, 2006; pp 121–173.
- Castellucci, M.; Kosanke, G.; Verdenelli, F.; Huppertz, B.; Kaufmann, P. Villous Sprouting: Fundamental Mechanisms of Human Placental Development. Human reproduction update 2000, 6, 485–494. [Google Scholar] [CrossRef]
- Enache, A.; Ciocan, V.; Muresan, C. O.; Cut, T. G.; Novacescu, D.; Paul, C.; Andreescu, N.; Mihailescu, A.; Raica, M.; Dumache, R. Postmortem Documentation of SARS-CoV-2 in Utero and Postpartum Transmission, through Amniotic Fluid, Placental, and Pulmonary Tissue RT-PCR. Applied Sciences 2021, 11. [Google Scholar] [CrossRef]
- Burton, G. J.; Jauniaux, E. Obstetrics: Normal and Problem Pregnancies. https://shop.elsevier.com/books/obstetrics-normal-and-problem-pregnancies/gabbe/978-0-323-32108-2 (accessed 2024-10-09).
- Pringle, K. G.; Kind, K. L.; Sferruzzi-Perri, A. N.; Thompson, J. G.; Roberts, C. T. Beyond Oxygen: Complex Regulation and Activity of Hypoxia Inducible Factors in Pregnancy. Hum Reprod Update 2010, 16, 415–431. [Google Scholar] [CrossRef]
- Myatt, L.; Cui, X. Oxidative Stress in the Placenta. Histochemistry and cell biology 2004, 122. [Google Scholar] [CrossRef]
- Kingdom, J.; Huppertz, B.; Seaward, G.; Kaufmann, P. Development of the Placental Villous Tree and Its Consequences for Fetal Growth. European journal of obstetrics, gynecology, and reproductive biology 2000, 92. [Google Scholar] [CrossRef]
- Moffett, A.; Loke, C. Immunology of Placentation in Eutherian Mammals. Nat Rev Immunol 2006, 6, 584–594. [Google Scholar] [CrossRef]
- Malassiné, A.; Frendo, J.; Evain-Brion, D. A Comparison of Placental Development and Endocrine Functions between the Human and Mouse Model. Human reproduction update 2003, 9. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, S. K.; Campbell, J. P. Placental Structure, Function and Drug Transfer. Continuing Education in Anaesthesia Critical Care & Pain 2015, 15, 84–89. [Google Scholar] [CrossRef]
- Mushambi, M.; Pinnock, C.; Lin, T.; Smith, T. Physiology of Pregnancy. In Fundamentals of Anaesthesia; Greenwich Medical Media Ltd: London, 2002. [Google Scholar]
- Desforges, M.; Sibley, C. Placental Nutrient Supply and Fetal Growth. International Journal of Developmental Biology 2010, 54, (2–3). [Google Scholar] [CrossRef] [PubMed]
- Knipp, G.; Audus, K.; Soares, M. Nutrient Transport across the Placenta. Advanced Drug Delivery Reviews 1999, 38, 41–58. [Google Scholar] [CrossRef]
- Malek, A. Role of IgG Antibodies in Association with Placental Function and Immunologic Diseases in Human Pregnancy. Expert Review of Clinical Immunology 2013, 9, 235–249. [Google Scholar] [CrossRef]
- Pacifici, G. M.; Nottoli, R. Placental Transfer of Drugs Administered to the Mother. Clinical pharmacokinetics 1995, 28, 235–269. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative medicine and cellular longevity 2017, 2017. [Google Scholar] [CrossRef]
- Sato, H.; Shibata, H.; Shimizu, T.; Shibata, S.; Toriumi, H.; Ebine, T.; Kuroi, T.; Funakubo, M.; Abe, M.; Suzuki, N. Differential Cellular Localization of Antioxidant Enzymes in the Trigeminal Ganglion. Neuroscience 2013, 248, 345–358. [Google Scholar] [CrossRef]
- Navarro-Yepes, J.; Zavala-Flores, L.; Anandhan, A.; Wang, F.; Skotak, M.; Chandra, N.; Li, M.; Pappa, A.; Martinez-Fong, D.; Del Razo, L. M.; others. Antioxidant Gene Therapy against Neuronal Cell Death. Pharmacology & therapeutics 2014, 142, 206–230. [Google Scholar] [CrossRef]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E. N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and Human Diseases. Clinica chimica acta 2014, 436, 332–347. [Google Scholar] [CrossRef]
- Al-Gubory, K. H.; Garrel, C.; Faure, P.; Sugino, N. Roles of Antioxidant Enzymes in Corpus Luteum Rescue from Reactive Oxygen Species-Induced Oxidative Stress. Reproductive biomedicine online 2012, 25, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A. K. Free Radicals: Health Implications and Their Mitigation by Herbals. British Journal of Medicine and Medical Research 2015, 7, 438–457. [Google Scholar] [CrossRef]
- Droge, W. Free Radicals in the Physiological Control of Cell Function. Physiological reviews 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Genestra, M. Oxyl Radicals, Redox-Sensitive Signalling Cascades and Antioxidants. Cellular signalling 2007, 19, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Raijmakers, M. T. M.; Burton, G. J.; Jauniaux, E.; Seed, P. T.; Peters, W. H. M.; Steegers, E. A. P.; Poston, L. Placental NAD (P) H Oxidase Mediated Superoxide Generation in Early Pregnancy. Placenta 2006, 27, (2–3). [Google Scholar] [CrossRef]
- Taniyama, Y.; Griendling, K. K. Reactive Oxygen Species in the Vasculature: Molecular and Cellular Mechanisms. Hypertension 2003, 42, 1075–1081. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M. Metals, Toxicity and Oxidative Stress. Current medicinal chemistry 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.; Moncol, J.; Izakovic, M.; Mazur, M. Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chemico-biological interactions 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Nishida, N.; Arizumi, T.; Takita, M.; Kitai, S.; Yada, N.; Hagiwara, S.; Inoue, T.; Minami, Y.; Ueshima, K.; Sakurai, T.; others. Reactive Oxygen Species Induce Epigenetic Instability through the Formation of 8-Hydroxydeoxyguanosine in Human Hepatocarcinogenesis. Digestive diseases 2013, 31, (5–6). [Google Scholar] [CrossRef]
- Deponte, M. Glutathione Catalysis and the Reaction Mechanisms of Glutathione-Dependent Enzymes. Biochimica et Biophysica Acta (BBA)-General Subjects 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Nicol, C. J.; Zielenski, J.; Tsui, L.-C.; Wells, P. G. An Embryoprotective Role for Glucose-6-Phosphate Dehydrogenase in Developmental Oxidative Stress and Chemical Teratogenesis. The FASEB Journal 2000, 14, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Tempfer, C.; Unfried, G.; Zeillinger, R.; Hefler, L.; Nagele, F.; Huber, J. C. Endothelial Nitric Oxide Synthase Gene Polymorphism in Women with Idiopathic Recurrent Miscarriage. Human reproduction 2001, 16, 1644–1647. [Google Scholar] [CrossRef] [PubMed]
- Al-Kunani, A. S.; Knight, R.; Haswell, S. J.; Thompson, J. W.; Lindow, S. W. The Selenium Status of Women with a History of Recurrent Miscarriage. BJOG: An International Journal of Obstetrics & Gynaecology 2001, 108, 1094–1097. [Google Scholar] [CrossRef]
- Zhang, K. Integration of ER Stress, Oxidative Stress and the Inflammatory Response in Health and Disease. International journal of clinical and experimental medicine 2010, 3. [Google Scholar]
- Zhang, K.; Kaufman, R. J. From Endoplasmic-Reticulum Stress to the Inflammatory Response. Nature 2008, 454, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Walter, P. Signal Integration in the Endoplasmic Reticulum Unfolded Protein Response. Nature reviews Molecular cell biology 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Jauniaux, E.; Watson, A.; Hempstock, J.; Bao, Y.-P.; Skepper, J.; Burton, G. Onset of Maternal Arterial Blood Flow and Placental Oxidative Stress: A Possible Factor in Human Early Pregnancy Failure. The American journal of pathology 2000, 157, 2111–2122. [Google Scholar] [CrossRef]
- Burton, G. J. Oxygen, the Janus Gas; Its Effects on Human Placental Development and Function. Journal of anatomy 2009, 215, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L.; Cui, X. Role of Placental Oxidative Stress in Abnormal Placental Function and Fetal Programming of Cardiovascular Disease. Nutrition reviews 2004, 62 (suppl_2), S28–S32. [Google Scholar]
- Holland, O. J.; Cuffe, J. S.; Dekker Nitert, M.; Callaway, L.; Kwan Cheung, K. A.; Radenkovic, F.; Perkins, A. V. Placental Mitochondrial Function and Structure in Gestational Disorders. Placenta 2017, 54, 2–9. [Google Scholar] [CrossRef]
- Pereira, R. D.; De Long, N. E.; Wang, R. C.; Yazdi, F. T.; Holloway, A. C.; Raha, S. Angiogenesis in the Placenta: The Role of Reactive Oxygen Species Signaling. BioMed research international 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Liu, G.-S.; Dusting, G. J.; Chan, E. C. NADPH Oxidase-Dependent Redox Signaling in TGF-β-Mediated Fibrotic Responses. Redox biology 2014, 2, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Many, A.; Westerhausen-Larson, A.; Kanbour-Shakir, A.; Roberts, J. M. Xanthine Oxidase and Xanthine Dehydrogenase Activity and Gene Expression in Normal Placenta. Placenta 2000, 21, 788–793. [Google Scholar]
- Hung, T.; Skepper, J.; Burton, G. Hypoxia-Reoxygenation: A Potent Inducer of Apoptotic Changes in the Human Placenta and Possible Etiological Factor in Preeclampsia. Circulation research 2002, 90, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Kukor, Z.; Valent, S.; Toth, M. Excessive Activation of Poly (ADP-Ribose) Synthetase by Oxidative Stress in the Placenta of Women with Intrauterine Growth Retardation. Placenta 2000, 21, (2–3). [Google Scholar]
- Krause, B. J.; Hanson, M. A.; Casanello, P. Characterization of Placental Nitric Oxide Synthase Activity and Nitric Oxide Production in Labor and Preeclampsia. Placenta 2011, 32, 671–676. [Google Scholar]
- Kanti, V.; Mohan, V.; Haritha, K.; Md, Z.; Praveen, K. Fetal Hemoglobin in Umbilical Cord Blood as a Marker of Neonatal Hypoxia in Nigerian Newborns. International Journal of Gynecology & Obstetrics 2014, 126, 176–179. [Google Scholar]
- Işık, H.; Aynıoğlu, Ö.; Tımbıl, A.; Sahbaz, A.; Harma, M.; Yılmaz, N.; Demircan, B.; Demir, M.; Alakuş, Ü.; Harma, M.; others. Oxidative Stress Markers in Severe Preeclampsia and Preeclampsia-Related Perinatal Morbidity—Preliminary Report. Ginekologia polska 2015, 86, 292–297. [Google Scholar]
- Redman, C. W.; Sargent, I. L. Reduced Placental Antioxidant Defenses in Preeclampsia Are Associated with Increased Placental Oxidative Stress. The American journal of pathology 2009, 175, 1058–1069. [Google Scholar]
- Germain, S. J.; Sacks, G. P.; Sooranna, S. R.; Sargent, I. L.; Redman, C. W. Preeclampsia: An Excessive Maternal Inflammatory Response to Pregnancy. American journal of obstetrics and gynecology 2007, 196, 416–e1. [Google Scholar]
- Aycicek, A.; Ipek, A. Maternal Active or Passive Smoking Causes Oxidative Stress in Placental Tissue. European journal of pediatrics 2008, 167, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, J. C.; Herring, A. H.; Thorp, J. M. Air Pollution and Stillbirth Risk: Exposure to Airborne Particulate Matter during Pregnancy Is Associated with Fetal Death. PloS one 2014, 9. [Google Scholar]
- Myatt, L.; Maloyan, A. Placental Oxidative Stress: From Miscarriage to Preeclampsia. Journal of the Society for Gynecologic Investigation 2016, 23, 69–79. [Google Scholar]
- Saben, J.; Lindsey, F.; Zhong, Y.; Thakali, K.; Badger, T. M.; Andres, A.; Gomez-Acevedo, H.; Shankar, K. Maternal Obesity Is Associated with a Lipotoxic Placental Environment. Placenta 2014, 35, 171–177. [Google Scholar] [CrossRef]
- Burton, G. J.; Watson, A. L.; Hempstock, J.; Skepper, J. N.; Jauniaux, E. Uterine Glands Provide Histiotrophic Nutrition for the Human Fetus during the First Trimester of Pregnancy. The Journal of Clinical Endocrinology & Metabolism 2002, 87, 2954–2959. [Google Scholar] [CrossRef]
- Burton, G. J.; Hempstock, J.; Jauniaux, E. Oxygen, Early Embryonic Metabolism and Free Radical-Mediated Embryopathies. Reprod BioMed Online 2003, 6, 84–96. [Google Scholar] [CrossRef]
- Hustin, J.; Schaaps, J. P. Echographic and Anatomic Studies of the Maternotrophoblastic Border during the First Trimester of Pregnancy. Am J Obstet Gynecol 1987, 157, 162–168. [Google Scholar] [CrossRef]
- Burton, G. J.; Jauniaux, E.; Watson, A. L. Maternal Arterial Connections to the Placental Intervillous Space during the First Trimester of Human Pregnancy; the Boyd Collection Revisited. Am J Obstet Gynecol 1999, 181, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Hempstock, J.; Greenwold, N. Trophoblastic Oxidative Stress in Relation to Temporal and Regional Differences in Maternal Placental Blood Flow in Normal and Abnormal Early Pregnancies. Am J Pathol 2003, 162, 115–125. [Google Scholar] [CrossRef]
- Watson, A. L.; Palmer, M. E.; Jauniaux, E. Variations in Expression of Copper/Zinc Superoxide Dismutase in Villous Trophoblast of the Human Placenta with Gestational Age. Placenta 1997, 18, 295–299. [Google Scholar] [CrossRef]
- Watson, A. L.; Skepper, J. N.; Jauniaux, E. Changes in the Concentration, Localisation and Activity of Catalase within the Human Placenta during Early Gestation. Placenta 1998, 19, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Schoots, M.; Gordijn, S.; Scherjon, S.; van Goor, H.; Hillebrands, J. Oxidative Stress in Placental Pathology. Placenta 2018, 69. [Google Scholar] [CrossRef] [PubMed]
- Burton, G. J.; Jauniaux, E. Oxidative Stress and Placental Dysfunction. Oxidative Medicine and Cellular Longevity 2019, 2019, 1–12. [Google Scholar]
- Cohen, M. C.; Machado, N.; Moros-Torrico, Y. Fetal Vascular Malperfusion. Archives of Pathology & Laboratory Medicine 2020, 144, 216–222. [Google Scholar]
- Stanek, J. Maternal Vascular Malperfusion of the Placental Bed: A Review. Journal of Pathology and Translational Medicine 2021, 55, 1–13. [Google Scholar]
- Kim, C. J.; Romero, R.; Chaemsaithong, P.; Kim, J.-S. Chronic Placental Inflammation in Twin Pregnancies. Journal of Pathology 2015, 237, 484–497. [Google Scholar]
- Redline, R. W. Placental Pathology: A Systematic Approach with Clinical Correlations. Placenta 2012, 29, S86–S91. [Google Scholar] [CrossRef] [PubMed]
- Redline, R. W. Fetal Thrombotic Vasculopathy: A Review. Surgical and Experimental Pathology 2015, 2, 1–13. [Google Scholar]
- Tandu-Umba, B.; Mbangama, M. A. Chorioamnionitis and Pregnancy Outcomes: A Meta-Analysis. Public Health 2016, 138, 13–21. [Google Scholar]
- Ananth, C. V.; Lavery, J. A.; Vintzileos, A. M.; Skupski, D. W.; Varner, M.; Saade, G.; Biggio, J.; Williams, M. A.; Wapner, R. J.; Wright, J. D. Placental Abruption: Clinical Features and Diagnosis. UpToDate 2015. [Google Scholar]
- Nguyen, N. M. P.; Slim, R. Gestational Trophoblastic Disease: Molecular Genetics and Early Diagnosis. Current Opinion in Obstetrics and Gynecology 2018, 30, 95–100. [Google Scholar]
- Bellamy, L.; Casas, J.-P.; Hingorani, A. D.; Williams, D. J. Pre-Eclampsia and Risk of Cardiovascular Disease and Cancer in Later Life: Systematic Review and Meta-Analysis. BMJ 2007, 335. [Google Scholar] [CrossRef] [PubMed]
- Basit, S.; Wohlfahrt, J.; Boyd, H. A. Pre-Eclampsia and Risk of Dementia Later in Life: Nationwide Cohort Study. BMJ 2018, 363, k4109. [Google Scholar] [CrossRef] [PubMed]
- Behrens, I.; Basit, S.; Melbye, M.; Lykke, J. A.; Wohlfahrt, J.; Bundgaard, H.; Thilaganathan, B.; Boyd, H. A. Risk of Post-Pregnancy Hypertension in Women with a History of Hypertensive Disorders of Pregnancy: Nationwide Cohort Study. BMJ 2017, 358, j3078. [Google Scholar] [CrossRef]
- Davis, E. F.; Lazdam, M.; Lewandowski, A. J.; Worton, S. A.; Kelly, B.; Kenworthy, Y.; Adwani, S.; Wilkinson, A. R.; McCormick, K.; Sargent, I.; others. Cardiovascular Risk Factors in Children and Young Adults Born to Preeclamptic Pregnancies: A Systematic Review. Pediatrics 2012, 129, e1552–e1561. [Google Scholar] [CrossRef]
- Godfrey, K. M.; Reynolds, R. M.; Prescott, S. L.; Nyirenda, M.; Jaddoe, V. W.; Eriksson, J. G.; Broekman, B. F. Influence of Maternal Obesity on the Long-Term Health of Offspring. The lancet Diabetes & endocrinology 2017, 5, 53–64. [Google Scholar]
- Noubiap, J. J.; Bigna, J. J.; Nansseu, J. R.; Nyaga, U. F.; Balti, E. V.; Echouffo-Tcheugui, J. B.; Kengne, A. P. Prevalence of Dyslipidaemia among Adults in Africa: A Systematic Review and Meta-Analysis. The Lancet Global Health 2018, 6, e998–e1007. [Google Scholar] [CrossRef]
- Reynolds, R. M.; Allan, K. M.; Raja, E. A.; Bhattacharya, S.; McNeill, G.; Hannaford, P. C.; Sarwar, N.; Lee, A. J.; Bhattacharya, S.; Norman, J. E. Maternal Obesity during Pregnancy and Premature Mortality from Cardiovascular Event in Adult Offspring: Follow-up of 1 323 275 Person Years. BMJ 2013, 347, f4539. [Google Scholar] [CrossRef]
- Vikse, B. E.; Irgens, L. M.; Leivestad, T.; Skjaerven, R.; Iversen, B. M. Preeclampsia and the Risk of End-Stage Renal Disease. New England Journal of Medicine 2008, 359, 800–809. [Google Scholar] [CrossRef]
- Vounzoulaki, E.; Khunti, K.; Abner, S. C.; Tan, B. K.; Davies, M. J.; Gillies, C. L. Progression to Type 2 Diabetes in Women with a Known History of Gestational Diabetes: Systematic Review and Meta-Analysis. BMJ 2020, 369, m1361. [Google Scholar] [CrossRef]
- Wu, P.; Haththotuwa, R.; Kwok, C. S.; Babu, A.; Kotronias, R. A.; Rushton, C.; Zaman, A.; Fryer, A. A.; Kadam, U.; Chew-Graham, C. A.; others. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circulation: Cardiovascular Quality and Outcomes 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Ching, T.; Ha, J.; Song, M.-A.; Tiirikainen, M.; Molnar, J.; Berry, M. J.; Towner, D.; Garmire, L. X. Genome-Scale Hypomethylation in the Cord Blood DNAs Associated with Early Onset Preeclampsia. Clinical epigenetics 2015, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T. D.; Mathiesen, E. R.; Hansen, T.; Pedersen, O.; Jensen, D. M.; Lauenborg, J.; Damm, P. High Prevalence of Type 2 Diabetes and Pre-Diabetes in Adult Offspring of Women with Gestational Diabetes Mellitus or Type 1 Diabetes: The Role of Intrauterine Hyperglycemia. Diabetes care 2008, 31, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Dachew, B. A.; Scott, J. G.; Mamun, A.; Alati, R. Pre-Eclampsia and the Risk of Attention-Deficit/Hyperactivity Disorder in Offspring: Findings from the ALSPAC Birth Cohort Study. Psychiatry research 2019, 272, 392–397. [Google Scholar] [CrossRef]
- Forno, E.; Young, O. M.; Kumar, R.; Simhan, H.; Celed’on, J. C. Maternal Obesity in Pregnancy, Gestational Weight Gain, and Risk of Childhood Asthma. Pediatrics 2014, 134, e535–e546. [Google Scholar] [CrossRef]
- Herzog, E. M.; Eggink, A. J.; Reijnierse, A.; Kerkhof, M. A.; de Krijger, R. R.; Roks, A. J.; Reiss, I. K.; Nigg, A. L.; Eilers, P. H.; Steegers, E. A.; others. Impact of Early-and Late-Onset Preeclampsia on Features of Placental and Newborn Vascular Health. Placenta 2017, 49, 72–79. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Ondrackova, M.; Pirkova, P.; Kestlerova, A.; Novotna, V.; Hympanova, L.; Krofta, L. Expression Profile of C19MC microRNAs in Placental Tissue in Pregnancy-Related Complications. DNA and cell biology 2015, 34, 437–457. [Google Scholar] [CrossRef]
- Luyckx, V. A.; Bertram, J. F.; Brenner, B. M.; Fall, C.; Hoy, W. E.; Ozanne, S. E.; Vikse, B. E. Effect of Fetal and Child Health on Kidney Development and Long-Term Risk of Hypertension and Kidney Disease. The Lancet 2013, 382, 273–283. [Google Scholar] [CrossRef]
- Maher, G. M.; O’Keeffe, G. W.; Kearney, P. M.; Kenny, L. C.; Dinan, T. G.; Mattsson, M.; Khashan, A. S. Association of Hypertensive Disorders of Pregnancy with Risk of Neurodevelopmental Disorders in Offspring: A Systematic Review and Meta-Analysis. JAMA psychiatry 2018, 75, 809–819. [Google Scholar] [CrossRef]
- Mando, C.; De Palma, C.; Stampalija, T.; Anelli, G. M.; Figus, M.; Novielli, C.; Parisi, F.; Clementi, E.; Ferrazzi, E.; Cetin, I. Placental Mitochondrial Content and Function in Intrauterine Growth Restriction and Preeclampsia. American Journal of Physiology-Endocrinology and Metabolism 2014, 306, E404–E413. [Google Scholar] [CrossRef]
- Tuovinen, S.; Eriksson, J. G.; Kajantie, E.; R"aikk"onen, K. Maternal Hypertensive Pregnancy Disorders and Cognitive Functioning of the Offspring: A Systematic Review. Journal of the American Society of Hypertension 2014, 8, 832–847. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Delahanty, L. M.; Buka, S. L.; Rothman, K. J.; Hivert, M.-F.; James-Todd, T. Maternal Hypertensive Disorders of Pregnancy and Offspring Risk of Hypertension: A Population-Based Cohort Study. Pediatrics 2020, 145. [Google Scholar]
- Beauchamp, B.; Thrush, A. B.; Quizi, J.; Antoun, G.; McIntosh, N.; Al-Dirbashi, O. Y.; Patti, M.-E.; Harper, M.-E. Undernutrition during Pregnancy in Mice Leads to Dysfunctional Cardiac Muscle Respiration in Adult Offspring. Bioscience reports 2015, 35. [Google Scholar] [CrossRef]
- Sipos, P. I.; Ramma, W.; Brosens, J. J.; Pijnenborg, R.; Ahmed, A. Oxidative Stress and the Induction of Cyclooxygenase Enzymes and Apoptosis in the Murine Placenta. Placenta 2013, 34, 150–158. [Google Scholar]
- Biron-Shental, T.; Sukenik-Halevy, R.; Sharon, Y.; Goldberg-Bittman, L.; Kidron, D.; Fejgin, M. D.; Amiel, A. Short Telomeres May Play a Role in Placental Dysfunction in Preeclampsia and Intrauterine Growth Restriction. American journal of obstetrics and gynecology 2010, 202, 381–e1. [Google Scholar] [CrossRef]
- Cox, L. S.; Redman, C. The Role of Cellular Senescence in Ageing of the Placenta. Placenta 2017, 52, 139–145. [Google Scholar] [CrossRef]
- Hausvater, A.; Giannone, T.; Sandoval, Y.-H. G.; Doonan, R. J.; Antonopoulos, C. N.; Matsoukis, I. L.; Petridou, E. T.; Daskalopoulou, S. S. The Association between Preeclampsia and Arterial Stiffness. Journal of hypertension 2012, 30, 17–33. [Google Scholar] [CrossRef]
- Niki, E. Lipid Peroxidation: Physiological Levels and Dual Biological Effects. Free radical biology and medicine 2009, 47, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Spickett, C. M.; Pitt, A. R. Protein Oxidation: Role in Signalling and Detection by Mass Spectrometry. Amino acids 2010, 39, 1055–1067. [Google Scholar] [CrossRef]
- Cai, Z.; Yan, L.-J. Protein Oxidation and Peroxidation. Current opinion in food science 2013, 2, 69–75. [Google Scholar]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein Carbonyl Groups as Biomarkers of Oxidative Stress. Clinica chimica acta 2006, 329, (1–2). [Google Scholar] [CrossRef] [PubMed]
- Mert, I.; Oruc, A. S.; Yuksel, S.; Cakar, E. S.; Buyukkagnici, U.; Karaer, A.; Danisman, N. Oxidative Stress and Maternal Obesity: Feto-Placental Unit Interaction. Placenta 2012, 33, 789–794. [Google Scholar]
- Birben, E.; Sahiner, U. M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organization Journal 2012, 5, 1–11. [Google Scholar] [CrossRef]
- Cooke, M. S.; Evans, M. D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA Damage: Mechanisms, Mutation, and Disease. The FASEB journal 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox biology 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Wu, C.; Yosef, N.; Thalhamer, T.; Zhu, C.; Xiao, S.; Kishi, Y.; Regev, A.; Kuchroo, V. K. Oxidative Stress and Vascular Disease. Arteriosclerosis, thrombosis, and vascular biology 2004, 24, 816–823. [Google Scholar]
- Hybertson, B. M.; Gao, B.; Bose, S. K.; McCord, J. M. Oxidative Stress in Health and Disease: The Therapeutic Potential of Nrf2 Activation. Molecular aspects of medicine 2011, 32, (4–6). [Google Scholar] [CrossRef]
- Morgan, M. J.; Liu, Z. Crosstalk between NFkB and the PI3-Kinase/AKT Pathway Can Be Targeted in Primary Effusion Lymphoma (PEL) Cell Lines for Efficient Apoptosis. PloS one 2011, 6. [Google Scholar]
- Picard, M.; Zhang, J.; Hancock, S.; Derbeneva, O.; Golhar, R.; Golik, P.; O’Hearn, S.; Levy, S.; Potluri, P.; Lvova, M.; others. Mitochondrial DNA Copy Number Threshold in mtDNA Depletion Myopathy. Neurology 2016, 86, 424–433. [Google Scholar]
- Yakes, F. M.; Van Houten, B. Mitochondrial DNA Damage Is More Extensive and Persists Longer than Nuclear DNA Damage in Human Cells Following Oxidative Stress. Proceedings of the National Academy of Sciences 1997, 94, 514–519. [Google Scholar] [CrossRef]
- Sá, C. P. N. de; Jiménez, M. F.; Rosa, M. W.; Arlindo, E. M.; Ayub, A. C. K.; Cardoso, R. B.; Kreitchmann, R.; El Beitune, P. Evaluation of Angiogenic Factors (PlGF and sFlt-1) in Pre-Eclampsia Diagnosis. Rev Bras Ginecol Obstet 2020, 42, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lin, Y.; Yang, J.; Wang, S.; Gao, L.; Bi, Y.; Wang, Y. Mitochondrial Dysfunction and Oxidative Stress in Selective Fetal Growth Restriction. Placenta 2024, 156, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Saucedo, R.; Ortega-Camarillo, C.; Ferreira-Hermosillo, A.; Díaz-Velázquez, M. F.; Meixueiro-Calderón, C.; Valencia-Ortega, J. Role of Oxidative Stress and Inflammation in Gestational Diabetes Mellitus. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Menon, R. Oxidative Stress Damage as a Detrimental Factor in Preterm Birth Pathology. Front Immunol 2014, 5, 567. [Google Scholar] [CrossRef] [PubMed]
- Joó, J. G.; Sulyok, E.; Bódis, J.; Kornya, L. Disrupted Balance of the Oxidant–Antioxidant System in the Pathophysiology of Female Reproduction: Oxidative Stress and Adverse Pregnancy Outcomes. Curr Issues Mol Biol 2023, 45, 8091–8111. [Google Scholar] [CrossRef] [PubMed]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef]
- Poston, L.; Briley, A. L.; Seed, P. T.; Kelly, F. J.; Shennan, A. H. Vitamin C and Vitamin E in Pregnant Women at Risk for Pre-Eclampsia (VIP Trial): Randomised Placebo-Controlled Trial. The Lancet 2006, 367, 1145–1154. [Google Scholar] [CrossRef]
- Ahmed, A.; Williams, D. J.; Cheed, V.; Middleton, L. J.; Ahmad, S.; Wang, K.; Vince, A. T.; Hewett, P.; Spencer, K.; Khan, K. S.; others. Pravastatin for Early-Onset Preeclampsia: A Randomized, Blinded, Placebo-Controlled Trial. BJOG: An International Journal of Obstetrics & Gynaecology 2020, 127, 478–488. [Google Scholar]
- Aljunaidy, M. M.; Morton, J. S.; Kirschenman, R.; Phillips, T.; Case, C. P.; Cooke, C.-L. M.; Davidge, S. T. Targeting Mitochondrial Function to Treat Quiescent Tumor Cells in Solid Tumors. International journal of molecular sciences 2018, 19. [Google Scholar]
- Groom, K. M.; McCowan, L. M.; Stone, P. R.; Chamley, L. C.; McLintock, C. Sildenafil Citrate for Fetal Growth Restriction. Obstetrics & Gynecology 2019, 133, 1241–1248. [Google Scholar]
- Kaandorp, J. J.; van Bel, F.; Veen, S.; Derks, J. B.; Groenendaal, F.; Rijken, M.; Roze, E.; Venema, M. M.; Rademaker, C. M.; Bos, A. F.; others. Allopurinol as a Therapeutic Option in the Treatment of Cardiac Ischaemia/Reperfusion Injury: A Systematic Review of Animal Studies. Cardiovascular Research 2015, 105, 334–341. [Google Scholar]
- Li, J.; Stouffs, M.; Serrander, L.; Banfi, B.; Bettiol, E.; Charnay, Y.; Steger, K.; Krause, K.-H.; Jaconi, M. E. Nox2 Contributes to the Arterial Endothelial Specification of Mouse Induced Pluripotent Stem Cells by Upregulating Notch Signaling. Scientific reports 2018, 8, 1–13. [Google Scholar]
- Mao, L.; Wang, H.; Wang, X.; Tian, L.; Yao, J. Nrf2 Activators: A Novel Strategy to Promote Oligodendrocyte Survival in Multiple Sclerosis? Multiple Sclerosis Journal 2018, 24, 1761–1774. [Google Scholar]
- Miller, S. L.; Yawno, T.; Alers, N. O.; Castillo-Melendez, M.; Supramaniam, V. G.; VanZyl, N.; Sabaretnam, T.; Loose, J. M.; Drummond, G. R.; Walker, D. W.; others. Antenatal Antioxidant Treatment with Melatonin to Decrease Newborn Neurodevelopmental Deficits and Brain Injury Caused by Fetal Growth Restriction. Journal of pineal research 2014, 56, 283–294. [Google Scholar] [CrossRef]
- Rayman, M. P. Selenium and Human Health. The Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J. M.; Myatt, L.; Spong, C. Y.; Thom, E. A.; Hauth, J. C.; Leveno, K. J.; Pearson, G. D.; Wapner, R. J.; Varner, M. W.; Thorp Jr, J. M.; others. Vitamins C and E to Prevent Complications of Pregnancy-Associated Hypertension. New England Journal of Medicine 2010, 362, 1282–1291. [Google Scholar] [CrossRef]
- Rolnik, D. L.; Wright, D.; Poon, L. C.; O’Gorman, N.; Syngelaki, A.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; others. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. New England Journal of Medicine 2017, 377, 613–622. [Google Scholar] [CrossRef]
- Shahin, A. Y.; Hassanin, I. M.; Ismail, A. M.; Kruessel, J. S.; Hirchenhain, J. Effect of Oral N-Acetyl Cysteine on Recurrent Preterm Labor Following Treatment for Bacterial Vaginosis. International Journal of Gynecology & Obstetrics 2009, 104, 44–48. [Google Scholar]
- Stanley, J. L.; Sulek, K.; Andersson, I. J.; Davidge, S. T.; Kenny, L. C.; Sibley, C. P.; Mandal, R.; Wishart, D. S.; Broadhurst, D. I.; Baker, P. N. Antioxidant Therapies in Hypertensive Disorders of Pregnancy. Nutrients 2012, 4, 1282–1302. [Google Scholar]
- Wang, R. Cystathionine Gamma-Lyase Deficiency and Overproliferation of Smooth Muscle Cells. Cardiovascular research 2013, 97, 478–486. [Google Scholar]
- Al Wattar, B. H.; Dodds, J.; Placzek, A.; Beresford, L.; Spyreli, E.; Moore, A.; Gonzalez Carreras, F. J.; Austin, F.; Murugesu, N.; Roseboom, T. J.; others. Mediterranean-Style Diet in Pregnant Women with Metabolic Risk Factors (ESTEEM): A Pragmatic Multicentre Randomised Trial. PLoS medicine 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Coluccio, A.; Benedetti, F.; Crudele, F.; Falzarano, M. S.; Neri, M.; Gualandi, F.; Scotton, C.; Gherardi, S.; Ballestri, M.; Spelta, F.; others. Gene Therapy for Inherited Muscle Diseases: Where Genetics Meets Rehabilitation Medicine. Frontiers in genetics 2018, 9, 114. [Google Scholar]
- Gurung, S.; Greening, D. W.; Catt, S.; Salamonsen, L.; Evans, J. Placental Extracellular Vesicles and Feto-Maternal Communication. Placenta 2021, 109, 27–37. [Google Scholar]
- Keelan, J. A.; Baskaran, V.; Kelly, A. J.; Labhasetwar, V. Nanoparticle-Based Therapeutic Approaches for the Treatment of Inflammatory Diseases. Journal of controlled release 2015, 219, 519–535. [Google Scholar]
- Makrides, M.; Gould, J. F.; Gawlik, N. R.; Yelland, L. N.; Smithers, L. G.; Anderson, P. J.; Gibson, R. A. Effect of Omega-3 Long-Chain Polyunsaturated Fatty Acid Supplementation during Pregnancy on Cognitive Outcomes in Children. Jama 2019, 322, 826–833. [Google Scholar]
- Pels, A.; Derks, J.; Elvan-Taşpinar, A.; van Drongelen, J.; de Boer, M.; Duvekot, H.; van Laar, J.; van Eyck, J.; Al-Nasiry, S.; Sueters, M.; others. Sildenafil Citrate Therapy for Severe Early-Onset Intrauterine Growth Restriction. BJOG: An International Journal of Obstetrics & Gynaecology 2015, 122, 384–391. [Google Scholar]
- Sunderji, S.; Sibai, B. The Use of Etanercept in Refractory Preeclampsia. American Journal of Obstetrics & Gynecology MFM 2019, 1. [Google Scholar]
- Syngelaki, A.; Nicolaides, K. H.; Balani, J.; Hyer, S.; Akolekar, R.; Kotecha, R.; Pastides, A.; Shehata, H. Metformin versus Placebo in Obese Pregnant Women without Diabetes Mellitus. New England Journal of Medicine 2016, 374, 434–443. [Google Scholar] [CrossRef]
| Source | Description | Key Features |
|---|---|---|
| Fluctuations in Oxygenation [93,94] | - Dramatic changes in O2 levels during pregnancy - Transition from hypoxic to normoxic/hyperoxic environment |
- First trimester: <20 mmHg SpO₂ - After 12 weeks: >50 mmHg SpO₂ - Syncytiotrophoblast particularly vulnerable |
| Mitochondrial Sources [95,96] | - Electron leakage from respiratory chain - Primary source of superoxide radicals |
- 1-2% of O2 consumed converted to superoxide - Increased in conditions like PE |
| NADPH Oxidases [97,98] | - Dedicated ROS-producing enzymes - Multiple isoforms expressed in placenta |
- NOX1, NOX2, NOX4, NOX5 present - Involved in physiological signaling/pathology |
| Xanthine Oxidase [99,100] | - Activated during ischemia-reperfusion events - Converts xanthine to uric acid, producing ROS |
- Increased activity in PE - Contributes to oxidative damage during blood flow fluctuations |
| Uncoupled NO Synthase [101,102] | - NOS produces O2- instead of NO when uncoupled - Often due to tetrahydrobiopterin deficiency |
- Reduces NO bioavailability - Contributes to endothelial dysfunction |
| Auto-oxidation of Hemoglobin [103,104] | - Hb from maternal blood in intervillous space - Can release free iron, a potent pro-oxidant |
- Increased in conditions with placental damage - Contributes to lipid peroxidation |
| Inflammatory Cells [105,106] | - Maternal immune cells in placental bed - Can produce ROS through respiratory burst |
- Increased in inflammatory conditions - Contribute to oxidative damage in infection/ PE |
| Environmental Factors [107,108] | - External sources increasing placental OS | - Maternal smoking - Air pollution - Radiation exposure - Certain medications - Alcohol consumption |
| Maternal Conditions [109,110] | - Systemic conditions affecting placental oxidative balance | - Diabetes mellitus - Obesity - Advanced maternal age - Hypertensive disorders |
| Pathology | Role of Oxidative Stress | Histological Features |
|---|---|---|
| Maternal Vascular Malperfusion | - Impairs trophoblast invasion - Causes endothelial dysfunction - Increases villous trophoblast apoptosis |
- Decidual arteriopathy - Accelerated villous maturation - Distal villous hypoplasia - Increased syncytial knots - Infarction and retroplacental hemorrhage |
| Fetal Vascular Malperfusion | - Causes endothelial damage leading to thrombosis - Increases inflammation and coagulation |
- Villous stromal-vascular karyorrhexis - Avascular villi - Thrombosis in fetal vessels |
| Chronic Villitis | - Inflammatory cell infiltration increases ROS production - Promotes further inflammation |
- Lymphohistiocytic infiltrate in villi - Destruction of villous architecture - Fibrinoid necrosis |
| Chorioamnionitis | - Activates maternal neutrophils leading to respiratory burst - Bacterial products directly induce ROS production |
- Neutrophil infiltration in chorion and amnion - Funisitis - Potential secondary fetal vascular malperfusion |
| Placental Abruption | - Oxidative damage predisposes to abruption - Hemorrhage and ischemia-reperfusion increase OS |
- Retroplacental hematoma - Compressed villi adjacent to abruption site - Secondary ischemic changes in affected regions |
| Gestational Trophoblastic Diseases | - Implicated in abnormal trophoblast proliferation and differentiation - Alters angiogenesis |
- Hydropic swelling of villi - Trophoblast hyperplasia - Abnormal vasculature |
| Category | Consequence | Mechanism | Summary of Evidence | |
|---|---|---|---|---|
| Maternal | CV Risk | ↑ risk of CV disease | Persistent endothelial dysfunction, OS and inflammation | 2-4 fold ↑ risk of future CV events in women with history of PE |
| Hypertension | Vascular remodeling, altered renin-angiotensin system | ↑ risk of chronic hypertension after hypertensive disorders of pregnancy | ||
| Metabolic Risk | Type 2 diabetes | Persistent β-cell dysfunction, insulin resistance | Up to 7-fold ↑ risk after gestational diabetes | |
| Metabolic syndrome | Persistent OS, inflammation | ↑ prevalence after PE and gestational diabetes | ||
| Renal Risk | Chronic kidney disease | Persistent renal endothelial dysfunction, microvascular damage | 4-5 fold ↑ risk after PE | |
| Cognitive Function | Cognitive decline and dementia | Cerebrovascular effects of OS and inflammation | ↑ risk of vascular dementia after PE | |
| Offspring Programming | CV Risk | Hypertension | Altered vascular development, epigenetic changes in CV regulatory genes | ↑ blood pressure in childhood and young adulthood after exposure to PE |
| ↑ CV disease risk | Early vascular dysfunction, altered lipid metabolism | ↑ CV risk factors in offspring exposed to maternal obesity or diabetes | ||
| Metabolic Risk | Obesity | Altered hypothalamic circuits regulating appetite and metabolism | ↑ risk of childhood obesity after exposure to maternal obesity or gestational diabetes | |
| Type 2 diabetes | Impaired pancreatic β-cell development and function | ↑ risk in offspring exposed to maternal diabetes | ||
| Neurodevelopmental Outcomes | Autism spectrum disorders | Oxidative damage to developing neurons, altered neurotransmitter systems | ↑ risk after exposure to PE and other pregnancy complications | |
| ADHD | Altered neurodevelopment due to OS and inflammation | Association between maternal PE and offspring ADHD | ||
| Cognitive impairment | Oxidative damage to developing brain, altered cerebral blood flow | Lower cognitive scores in children born after preeclamptic pregnancies | ||
| Immune Function | Allergic diseases | Altered immune system development due to OS | ↑ risk of asthma and allergies in offspring exposed to maternal obesity and gestational diabetes | |
| Autoimmune diseases | OS-induced changes in T cell differentiation | Some evidence for ↑ risk of type 1 diabetes after PE | ||
| Renal Function | Reduced nephron population | OS impact on nephrogenesis | Reduced kidney size and function in offspring exposed to PE or IUGR | |
| Epigenetic Mechanisms | DNA methylation changes | OS stress-induced alterations in methylation patterns | Altered methylation in genes related to metabolism and vascular function in cord blood after PE | |
| Histone modifications | OS influence on histone-modifying enzymes | Changes in histone acetylation in placentas from complicated pregnancies | ||
| microRNA alterations | OS-induced changes in microRNA expression | Altered placental microRNA profiles in PE and IUGR | ||
| Mitochondrial Effects | mtDNA mutations | Oxidative damage to mitochondrial DNA | ↑ mtDNA mutations in placentas from complicated pregnancies | |
| Altered mitochondrial dynamics | Changes in fission/fusion balance | Persistent alterations in offspring tissues after IUGR | ||
| Vascular and Endothelial Effects | Endothelial progenitor cell dysfunction | Reduced number and function of EPCs | Observed in women with history of PE | |
| ↑ arterial stiffness | Vascular remodeling, endothelial dysfunction | Observed in both mothers and offspring years after preeclamptic pregnancies | ||
| Cellular Senescence | Telomere shortening | Oxidative damage to telomeres | Shorter telomeres in placentas from complicated pregnancies | |
| Senescence-associated secretory phenotype | Persistent low-grade inflammation and pro-oxidant state | ↑ senescence markers in preeclamptic placentas | ||
| Biomarker Category | Examples | Significance in Placental Pathology |
|---|---|---|
| Lipid Peroxidation Products | MDA F2-isoprostanes 4-HNE |
Indicate oxidative damage to cellular membranes |
| Protein Oxidation Markers | Protein carbonyls AOPP Nitrotyrosine |
Reflect oxidative damage to placental proteins |
| DNA/RNA Oxidation | 8-OHdG 8-hydroxyguanosine |
Indicate oxidative damage to nucleic acids |
| Antioxidant Status | SOD, CAT, GPx, Vitamins C and E, Glutathione |
Reflect the placenta's ability to counteract OS |
| Redox-Sensitive Transcription Factors | Nrf2 NF-κB |
Indicate changes in gene expression due to OS |
| Mitochondrial Dysfunction Markers | mtDNA copy number Electron transport chain enzyme activities |
Reflect OS-induced mitochondrial damage |
| Gasotransmitters | NO metabolites H₂S levels CO production |
Indicate OS and vascular dysfunction. |
| Complication | Role of OS | Key Findings | Potential Biomarkers |
|---|---|---|---|
| Preeclampsia | - Placental ischemia/reperfusion injury - Endothelial dysfunction - Systemic inflammation |
- Increased placental OS markers - Reduced antioxidant capacity - Increased circulating anti-angiogenic factors |
- sFlt-1/PlGF ratio - Malondialdehyde - 8-isoprostane - Nitrotyrosine |
| Fetal Growth Restriction | - Impaired placental development - Reduced nutrient transport - Mitochondrial dysfunction |
- Increased placental oxidative damage - Altered placental gene expression - Reduced placental antioxidant enzymes |
- F2-isoprostanes - Protein carbonyls - 8-OHdG - Mitochondrial DNA damage |
| Gestational Diabetes Mellitus | - Hyperglycemia-induced ROS production - Mitochondrial dysfunction - Advanced glycation end products |
- Increased lipid peroxidation - Reduced antioxidant defenses - Altered placental insulin signaling |
- 8-isoprostane - Advanced glycation end products - Reduced glutathione/oxidized glutathione ratio |
| Preterm Birth | - Inflammation-induced OS - Premature rupture of membranes - Activation of labor pathways |
- Increased OS markers in amniotic fluid - Reduced antioxidant capacity in maternal circulation - Oxidative damage to fetal membranes |
- F2-isoprostanes in amniotic fluid - Myeloperoxidase - Matrix metalloproteinases |
| Recurrent Pregnancy Loss | - Impaired trophoblast invasion - Endothelial dysfunction - Oxidative damage to oocytes/embryos |
- Increased OS markers in maternal circulation - Reduced antioxidant capacity - Oxidative DNA damage in placental tissues |
- 8-OHdG - Lipid hydroperoxides - Total antioxidant capacity |
| Placental Abruption | - Acute ischemia-reperfusion injury - Activation of inflammatory cascades - Systemic OS in severe cases |
- Increased markers of oxidative damage in placental tissue - Elevated inflammatory mediators - Potential alterations in coagulation factors |
- Malondialdehyde - Protein carbonyls - Inflammatory cytokines (e.g., IL-6, TNF-α) |
| Approach | Examples | Rationale | Evidence/Status |
|---|---|---|---|
| Antioxidant Supplementation | Vitamins C and E | Free radical scavengers, regenerate other antioxidants | Large RCTs (VIP, DAPIT) showed no benefit in preventing PE. Potential issues with high doses interfering with physiological ROS signaling. |
| Selenium | Essential component of glutathione peroxidase and other selenoproteins | Observational studies show lower selenium in PE. Limited intervention studies with mixed results. | |
| N-acetylcysteine (NAC) | Precursor to glutathione, enhances cellular antioxidant capacity | Small studies show potential benefits in recurrent pregnancy loss and preterm labor. Larger trials needed. | |
| Melatonin | Potent antioxidant that can cross the placenta | Shown to reduce OS and improve outcomes in animal models of IUGR and PE. Early-stage clinical trials ongoing. | |
| Targeting Specific ROS Sources | MitoQ, SkQ1 (mitochondria-targeted antioxidants) | Accumulate in mitochondria to reduce mitochondrial OS | Promising results in preclinical models of PE and IUGR. Phase 2 trial of MitoQ in PE completed (results pending). |
| Apocynin, VAS2870 (NADPH oxidase inhibitors) | Reduce ROS production from a major enzymatic source | Mostly in preclinical stages. Apocynin shown to improve endothelial function in animal models of PE. | |
| Enhancing Endogenous Antioxidant Systems | Allopurinol (xanthine oxidase inhibitor) | Reduce ROS production during ischemia-reperfusion events | Some studies in PE and fetal hypoxia. APEX trial ongoing for fetal neuroprotection. |
| Sulforaphane, bardoxolone methyl (Nrf2 activators) | Stimulate endogenous antioxidant gene expression | Promising preclinical data, but concerns about potential teratogenicity. Sulforaphane is being studied in gestational diabetes. | |
| Targeting Placental Vasculature and Angiogenesis | SOD mimetics (e.g., tempol), catalase mimetics | Provide enzymatic antioxidant activity without affecting gene expression | Beneficial effects in animal models of PE and IUGR. Human studies limited. |
| L-arginine, sildenafil citrate (NO donors) | Improve placental blood flow and have antioxidant effects | Some positive results in IUGR. STRIDER trials for sildenafil in severe early-onset IUGR (mixed results, safety concerns). | |
| Anti-inflammatory Approaches | GYY4137, sodium hydrosulfide (H₂S donors) | H₂S has vasodilatory and antioxidant properties | Promising preclinical data in PE and IUGR models. Human studies in early stages. |
| Pravastatin | Pleiotropic effects including improved endothelial function and reduced OS | Encouraging results in animal models and small human studies. StAmP trial ongoing for prevention of PE. | |
| Low-dose aspirin | Anti-inflammatory and antiplatelet effects | Recommended for prevention of PE in high-risk women. Meta-analyses show 10-20% risk reduction. | |
| Modulation of Placental Metabolism | Omega-3 fatty acids | Anti-inflammatory effects and precursors to specialized pro-resolving mediators | Some studies show reduced risk of preterm birth, but effects on PE are inconsistent. ORIP trial showed no benefit for PE prevention. |
| Targeted anti-cytokine therapies (e.g., TNF-α inhibitors) | Reduce inflammatory signaling that can exacerbate OS | Mostly in preclinical stages for pregnancy complications. Case reports of TNF-α inhibitor use in refractory PE. | |
| Metformin | Activates AMPK, potentially improving mitochondrial function and reducing OS | Benefits shown in gestational diabetes. EMPOWaR and MOP trials found no benefit for obese pregnant women. Ongoing research in PE prevention. | |
| Novel and Emerging Approaches | Dietary interventions (e.g., Mediterranean diet) | Reduce metabolic stress and inflammation | Some observational studies show benefits. ESTEEM trial showed reduced gestational diabetes risk with Mediterranean diet. |
| Extracellular vesicle-based therapies | Deliver antioxidants or supportive factors directly to the placenta | Early preclinical research. Potential for targeted delivery of therapeutic cargo. | |
| CRISPR-based approaches | Correct genetic factors predisposing to OS or enhance antioxidant gene expression | Theoretical at this stage for placental disorders. Ethical concerns for human application. | |
| Nanomedicine (e.g., nanoparticle-based antioxidant delivery) | Improve targeting and efficacy of antioxidant therapies | Preclinical studies ongoing. Potential for enhanced placental drug delivery. | |
| Gasotransmitter therapies (e.g., inhaled NO, CO-releasing molecules) | Modulate vascular function and reduce OS | Some clinical trials for inhaled NO in preterm IUGR. CO-RMs in preclinical stages. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





