1. Introduction
Cancer is one of the most common threats to health and life. It constitutes a global problem and is one of the leading causes of human mortality in the world. The most common cancer in women is breast cancer. The increased risk of this cancer is mainly related to factors that are collectively referred to as civilizational. In spite of intensive prevention programs the disease rates are still raising mainly due to increased exposition to environmental risk factors, life style, stress and aging. The main problem in achieving success in the field of effective oncological therapy is the high resistance of cancer cells to the chemotherapy drugs used and, consequently, their resistance to pro-apoptotic signals [
1]. Many studies to date indicate that telomerase may be one of the key factors modulating both processes [
2]. As it has been shown, telomerase (mainly its catalytic subunit hTERT – human telomerase reverse transcriptase) is involved in migration and adhesion, which are the driving mechanisms of the metastasis process [
3]. During metastasis, cell adhesion is decreased to separate cells from the parent tumor and then increased to attach these cells to distant tissues [
4]. Therefore, attempts are being made to identify targets for precision therapy (potentially hTERT is one of them), that would result in attenuated development of the disease or even its elimination.
Before anticancer drugs are implemented, they are tested in vitro and in vivo (including animal experiments) to check the preliminary efficacy, toxicity and pharmacokinetics, and safety. In monolayer cultures, cells have easy access to nutrients and oxygen, resulting in a uniform (to some extent) population of cells in terms of genotype and phenotype. It should be emphasized that cancer cells cultured in such conditions lack the complexity of the structure of a tumor growing in vivo, including vascularization and the presence of inflammatory cells. The monolayer culture is also devoid of extracellular matrix. Therefore, it is necessary to search for alternative experimental models that will facilitate assessment of the response of cancer cells to administered anticancer substances in conditions that resemble in vivo environment [
5]. It seems that the approach based on 3D models could meet these expectations. It is recently gaining more attention since more adequately recapitulates the in vivo conditions – at least when compared to classic 2D research models. Aggregates of cancer cells that are cultured in vitro show the characteristics of a tumor growing in vivo in the early phase of its growth and are therefore considered to be an intermediate form between cells from a single-layer culture and a tumor growing spontaneously. Cell cultures in 3D, especially spheroids, in terms of morphology, consist of cells with a diverse phenotype, in the resting phase of the cell cycle, proliferating cells and cells with necrotic changes located in the center of the spheroid. 3D cultures show a morphological similarity to tumors collected intraoperatively. This similarity is also reflected in the cell-cell interactions, intracellular signaling pathways and gene expression. Consequently, the response of spheroids and tumors growing in vivo to the administered anticancer substances is comparable. Noteworthy, the IC50 (inhibitory concentration) values in a single layer in classic 2D cultures, is usually lower when compared to 3D systems [
5]. Thus we decided to perform a study of the contribution of telomerase/hTERT to breast cancer cells metabolism in a spheroid 3D model of breast cancer in the context of functional assessment of cell-cell interactions.
Telomeres and Telomerase Targeting in Cancer
Telomerase, an enzyme with reverse transcriptase activity that maintains the ends of chromosomes (telomeres), is induced and activated in over 90% of cancer cases. However, it does not occur in most normal cells. Telomerase is therefore an attractive target for both diagnostics and therapy due to its expression profile [
6]. As previously reported hTERT expression levels are observed in MCF7 and MDA-MB-231 cells - Luminal A and Normal-like/Tripple negative models, respectively [
7]; the most common and most difficult to treat [
8]. Due to its activity, telomerase restores telomeric ends leading to unlimited proliferative potential of breast cancer cells [
9]. In vertebrates, telomeres are built of tandem repeats of the TTAGGG sequence, which are bound by a specialized group of protective proteins, collectively called the shelterin complex [
10]. Further telomere protection structures are T-loops and G-quadruplexes, two tertiary DNA structures that protect the ends of telomeres and regulate their length. Identification of the therapeutic agents targeting these regulatory factors may be a good approach to fighting cancer because telomere length is significantly correlated with cancer initiation and progression [
11]. A significant correlation was found between enzyme activity and tumor size, lymph node status, and stage, and with tumor progression [
12].
However, telomerase is also postulated to play some telomere-independent functions that are called noncanonical functions of telomerase. As a result of numerous studies, it has been proven that cells expressing hTERT show increased adhesion to the extracellular matrix [
13,
14]. The adhesion process is particularly important in tumor progression and metastasis formation. During metastasis formation, cell adhesion is downregulated to separate cells from the primary tumor and then induced to communicate the same cells to distant tissues [
3].
Therefore, the aim of this project was to evaluate the effect of telomerase inhibitors TMPyP4, BIBR 1532 or imetelstat on the ability of MCF7 and MDA-MB-231 breast cancer cells to form spheroids in 3D culture. Such approach will reveal the potential of the tested compounds to modulate the metastatic potential of breast cancer cells in vitro.
2. Materials and Methods
Cell Culture
Two cell lines representing different molecular subtypes of breast cancer were enrolled in the study, i.e., (i) MCF7, luminal A (ER/PR+, HER2low, TP53WT), and (ii) MDA MB-231—basal-like subtype, also called triple-negative breast cancer (TNBC; ER/PR-, HER2-, TP53mut). MCF7 cell line, in comparison to the MDA-MB-231 cell line, is a poorly aggressive and non-invasive cell line. Overall, it is being considered to show low metastatic potential. The MCF7 (HTB-22) and MDA-MB-231 (HTB-26) cells were maintained in RPMI-1640 medium (Biowest, Nuaillé, France), supplemented with 10% fetal bovine serum (FBS) (Biowest, Nuaillé, France) at 37 °C in an atmosphere of 5% CO2 and saturated humidity. Both cell lines were obtained from the American Type Culture Collection (ATCC). As previously demonstrated, both cell lines express catalytic telomerase subunit while MCF7 shows higher hTERT protein accumulation than MDA-MB-231. [
7]
Inhibitors
TMPyP4. A potent inhibitor of human telomerase binds strongly to DNA quadruplexes by stacking on the G-tetrads at the core of the quadruplex, resulting in telomerase inhibition [
15,
16,
17,
18]. However, it is postulated that TMPyP4 might not be limited to the influence on telomerase by G-quadruplex stabilization. As demonstrated, photodynamic therapy with TMPyP4 led to the formation of reactive oxygen species and changes in expression of genes involved in oxidative stress response in human hepatoma cell line HepG2 and human glioma cell line U251 [
19]. Moreover, some reports suggest the complexity of TMPyP4 action in cancer cells including not only G4 stabilization but also a contribution to the regulation of expression of some genes engaged in cell metabolism, proliferation, and survival [
20,
21]
BIBR 1532. The non-competitive inhibitor of telomerase activity [
22] exerts antiproliferative effect on numerous leukemia cells while no effect on the proliferative capacity of normal hematopoietic progenitor cell is observed [
23]. BIBR 1532 reduced colony-forming ability, induced telomere length shortening and caused chemotherapeutic sensitization via inhibiting telomerase activity in MCF-7/WT and melphalan-resistant MCF-7/MlnR cell lines [
24]. BIBR 1532 showed cytotoxic properties in a dose-dependent manner in T-cell prolymphocytic leukemia (T-PLL) [
25], and in combination with carboplatin (a chemotherapeutic agent) eliminated ovarian cancer spheroid-forming cells in ES2, SKOV3, and TOV112D cell lines [
26].
Imetelstat (GRN163L, obtained from Johnson & Johnson). This potent and specific telomerase inhibitor is so far the only drug of its class in clinical trials. On June 6, 2024, the Food and Drug Administration approved imetelstat (Rytelo, Geron Corporation), an oligonucleotide telomerase inhibitor, for adults with low- to intermediate-1 risk myelodysplastic syndromes (MDS) with transfusion-dependent anemia requiring four or more red blood cell units over 8 weeks who have not responded to or have lost response to or are ineligible for erythropoiesis-stimulating agents (ESAs). [
27] Imetelstat binds to the telomerase RNA component with high affinity, inhibiting telomerase activity directly, rather than through antisense inhibition of protein translation [
28]. Imetelstat has shown promising results in treating patients with hematological malignancies and solid tumors. Some studies have even reported synergistic effects when Imetelstat was used in combination with other anti-cancer drugs or radiation therapy [
29]. Although, when applied in solid tumors, it usually requires a longer treatment time [
30].
Cell Treatment
Cell were treated for 72 hours with the tested telomerase inhibitors. For each cell line, Petri dishes (6 cm) containing 130,000 cells each were prepared for both cell lines. Then, specific inhibitor compounds were added at specific target concentrations (concentrations were adjusted referring to data obtained in our previous work [
7] and other literature data [
31] as subcytotoxic i.e.,:
TMPyP4 (5µM, 10µM or 20µM)
BIBR 1532 (1µM, 5µM or 10µM)
Imetelstat (1µM,5µM or 10µM)
Mismatch negative control for Imetestat (1µM, 5µM or 10µM).
After 72h treatment, MCF7 and MDA-MB-231 cells were trypsinized from each plate and, after adding telomerase inhibitors at the target concentrations as specified above, transferred to 96-well Ultra-Low Attachment Multiple Well Plates (Corning ULA plates, Merck, Germany) at a density of 5x102 cells/well in six repetitions for a given compound and concentration. The cells were observed and photos were taken every 24h up to three days.
Data Assessment
Results were expressed as representative pictures. All experiments were performed in six repeats.
4. Discussion
Literature data indicate the involvement of hTERT in the process of adhesion and migration of cancer cells [
3]. These processes, in turn, constitute an important element in the development and spread of cancer cells. Modern research aims to identify selective methods of eliminating cancer cells with minimal side effects. In this challenge, one of the most important goals is to limit the metastatic capacity of cancer cells. The regulation of cancer cell adhesion and migration is complex and involves both interactions between proteins (e.g., cell-cell adhesion molecules, cadherins, integrins, MAPKs - mitogen-activated kinases, receptor tyrosine kinases, cytoskeletal proteins) and the modulation of various signaling pathways, which are known to control cell proliferation, survival and differentiation. Telomerase catalytic subunit hTERT is perceived as one of the factors that can promote cell adhesion and migration by influencing the expression of cell adhesion-related genes, but the exact pathway mediating these mechanisms is still unknown [
7].
TMPyP4 Potential to Alter Cancer Cells Adhesion Properties
Numerous studies aim to evaluate the effects of targeting cancer cells for migration and/or adhesion. They include, among others: cationic porphyrin TMPyP4 to inhibit telomerase in human breast cancer cells [
32]. The putative action of TMPyP4 is not limited to its effect on G-quadruplex stabilization. It was shown that TMPyP4 photodynamic therapy led to the formation of reactive oxygen species and changes in the expression of genes involved in the response to oxidative stress. Additionally, TMPyP4 affects the regulation of the expression of some genes involved in cancer cell metabolism, proliferation and survival. These premises initiated studies aimed at revealing how compounds with such biological potential can be used in cancer therapy. Particularly important is the fact that they can cause biological effects in a telomere-independent manner [
7]. As shown TMPyP4 (at a concentration of ≤0.5µM) was capable of inhibiting hTERT expression and telomerase activity in cells of non-small cell lung cancer A549, cervical cancer HeLa, and osteosarcoma cells (U2-OS and SAOS-2) leading to increased cell migration and adhesion of the cells to the matrix. In contrast, higher concentrations (≥2µM) slowed down cancer cell proliferation and increased induction of cell death [
33]. In turn, treatment of MCF7 and MDA-MB-231 cells with the cationic porphyrin TMPyP4 significantly inhibited cancer cell migration and adhesion. It was shown that this inhibitor reduced the metabolic potential of the tested cells in a concentration- and incubation-time-dependent manner [
7]. Similarly, research conducted by Liu et al. showed that the induced expression of hTERT increased cancer cells’ adhesion and migration potential. The experiment also showed that hTERT expression promoted cell adhesion and migration independently of telomerase activity [
3].
BIBR 1532 Potential to Alter Cancer Cells Adhesion Properties
Another telomerase targeting approach is based on using inhibitors that bind non-competitively to telomerase. One well-documented synthetic agent, BIBR 1532, belongs to the non-nucleoside reverse transcriptase inhibitors (NNRTIs) and can effectively bind to the enzyme and block its activity. The specific mechanism for NNRTI inhibitors is not fully understood, although there are two main theories that have been generally accepted. The first states that BIBR 1532 binds to a binding site sufficiently close to the origin of replication to block the replication binding site, thereby inactivating telomerase. Second, BIBR 1532 causes a conformational change in the telomerase structure upon binding with it, which then blocks the activity of the replication binding site.
Scientists from Shahid Beheshti University showed that treatment of MCF7 and MDA-MB-231 cells with a combination of arsenic trioxide (ATO) and BIBR 1532 inhibited the proliferation and colony-forming ability of breast cancer cells. The synergistic effect of ATO and BIBR 1532 resulted in decreased hTERT expression [
34]. In combination dosing studies of the widely used breast cancer chemotherapeutic agent paclitaxel (limited success due to drug resistance) and BIBR 1532, it was shown that the drug combination inhibited MCF7 cell colony formation in a dose-dependent manner [
35]. Interestingly, combination of BIBR 1532 and DOX did not show any significant effect in hTERT protein accumulation in MCF7 cells while in MDA-MB-231 cells the catalytic telomerase subunit was repressed after treatment with BIBR 1532 (48 and 72 h) as well as DOX (48 h) [
7].
Imetelstat Potential to Alter Cancer Cells Adhesion Properties
Another known and tested inhibitor that strongly inhibits telomerase is imetelstat (GRN163L). It was found to affect not only cancer cells, but presumably also cancer stem cells (CSCs). When breast cancer cell lines were treated with imetelstat in vitro, telomerase activity was inhibited in most tumor cells and CSCs. Additionally, imetelstat treatment reduced the fractions of CSCs present in breast cancer cell lines. Differences between expression levels of telomerase activity or telomere length in these cell lines did not correlate with increased CSC sensitivity to imetelstat. This suggests that the mechanism of action on the CSC subpopulation is independent of telomere shortening [
36]. Imetelstat, was shown to bind the hTR RNA messenger component of telomerase, and consequently to stop telomere elongation. Studies conducted in 63 non-small cell lung cancer (NSCLC) cell lines showed that imetelstat inhibited the ability of cells to form colonies [
37].
Our studies showed significant differences in the structure of spheroids formed by cells of individual cell lines, with those formed by MCF7 cells being more compact than MDA-MB-231. This may be because MDA-MB-231 cells are characterized as more invasive than MCF7 suggesting different basal adhesion and migration properties. The experiment showed that the cationic porphyrin TMPyP4 affected the ability to form spheroids in both analyzed breast cancer cell lines from the lowest concentration used, i.e., 5µM, and the effect was directly proportional to the concentration (10 and 20 µM, respectively). Breast cancer cells of both lines formed dispersed three-dimensional structures at the highest inhibitor concentration, suggesting a weakening of adhesive properties. The study showed that in both cell lines, the effect of cell dispersion was dependent on the concentration of the inhibitor used, and the ability to form spheroids in MDA-MB-231 cells was also dependent on the incubation time. Alternatively, the non-nucleoside telomerase inhibitor BIBR 1532 used to assess the adhesion of MCF7 and MDA-MB-231 breast cancer cells at concentrations of 1, 5, and 10 µM did not affect the cell-cell adhesion potential of MCF7 line cells. Despite incubation with the compound, the cells’ morphology did not change compared to control cells. However, studies carried out on MDA-MB-231 cells showed the formation of scattered, irregular cell aggregates and the effect of the telomerase inhibitor BIBR 1532 in an incubation time-dependent manner. Further studies with the use of imetelstat at a concentration of 5 and 10µM in MCF7 cells led to a limited ability of the cells to form spheroids. The studies carried out on the MDA-MB-231 cell line demonstrated the inhibitory effect of imetelstat on cell-cell adhesion even at the lowest concentration used, i.e., 1 µM, and this effect was dependent on the incubation time. This effect may indicate the possibility of longer treatment of cells using a lower concentration of the compound, which may be more beneficial for potential patients due to the lower toxicity of BIBR 1532. Noteworthy, the effect of the different telomerase inhibitors was not the same which may reflect their different mechanisms of action. The cationic porphyrin TMPyP4 stabilizes DNA guanine quadruplexes, inhibiting telomerase activity and decreasing gene expression (e.g., c-MYC). It also reduces the level of hTERT transcripts, suggesting two mechanisms of action. Some studies demonstrated that TMPyP4 could reduce the tumor growth rate in xenograft models and prolong the survival of laboratory animals [
20]. Another compound used in the research was the synthetic and selective non-nucleoside inhibitor BIBR 1532, which inhibits recombinant and native human telomerase by interfering with the enzyme’s activity through non-competitive inhibition [
22]. Like the cationic porphyrin, BIBR 1532 acts in a concentration-dependent manner and inhibits telomerase action by decreasing the expression of c-MYC and hTERT [
38]. The third compound tested was the oligonucleotide imetelstat, whose mechanism of action involves binding the messenger RNA component of telomerase, preventing telomere elongation and potent competitive inhibition of telomerase activity [
39]. Interestingly, studies on the effect of imetelstat on CSC subpopulations indicate that the mechanism of action of imetelstat can be independent of telomere shortening [
36].
5. Conclusions
Our observations, together with other reports, demonstrate that telomerase inhibitors reduce the cell-cell adhesion potential of breast cancer cells, which may contribute to the modulation of their metastatic potential. Experiments showing the effect of telomerase/hTERT/telomeres targeting or application of telomerase inhibitors that act also in a telomere-independent manner, suggest their anticancer potential. Experiments performed in 3D culture demonstrate that the induced dispersion of cancer cells after the use of telomerase inhibitors may increase the area of action of the cytotoxic chemotherapy agent, that may affect the observed cytotoxicity effects of adjuvant therapy agents. However, since the results depend on the used agent and treated cell line, these studies require further analysis, taking into account the mechanisms associated with the ability of cancer cells to be released from the tumor into the bloodstream and the possibility of initiating new sites for cancer cell development.
Author Contributions
PK: Investigation, Data curation; KR: Methodology, Visualization; BR: Conceptualization, Funding acquisition, Supervision, Writing—original draft, review and editing.
Funding
This research was funded by the National Science Center, grant 2023/49/B/NZ7/00744.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
Imetelstat and MM were kindly provided by Johnsos & Johnson.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Lipinska, N.; Romaniuk, A.; Paszel-Jaworska, A.; Toton, E.; Kopczynski, P.; Rubis, B. Telomerase and drug resistance in cancer. Cell. Mol. Life Sci. 2017, 74, 4121–4132. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Q.; Ge, Y.; Zhao, Q.; Zheng, X.; Zhao, Y. hTERT promotes cell adhesion and migration independent of telomerase activity. Sci. Rep. 2016, 6, 22886. [Google Scholar] [CrossRef]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. J. Biol. Chem. 2020, 295, 2495–2505. [Google Scholar] [CrossRef]
- Sajjad, H.; Imtiaz, S.; Noor, T.; Siddiqui, Y.H.; Sajjad, A.; Zia, M. Cancer models in preclinical research: A chronicle review of advancement in effective cancer research. Anim. Model. Exp. Med. 2021, 4, 87–103. [Google Scholar] [CrossRef]
- Lipińska, N.; Rubiś, B. Telomerase as a target in diagnosis and treatment of cancer in postmenopausal women. Menopausal Rev. 2013, 6, 478–483. [Google Scholar] [CrossRef]
- Konieczna, N.; Romaniuk-Drapała, A.; Lisiak, N.; Totoń, E.; Paszel-Jaworska, A.; Kaczmarek, M.; Rubiś, B. Telomerase Inhibitor TMPyP4 Alters Adhesion and Migration of Breast-Cancer Cells MCF7 and MDA-MB-231. Int. J. Mol. Sci. 2019, 20, 2670. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S.-C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 1–13. [Google Scholar] [CrossRef]
- Herbert, B.-S.; E Wright, W.; Shay, J.W. Telomerase and breast cancer. Breast Cancer Res. 2001, 3, 1–4. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Hayflick, his limit, and cellular ageing. Nat. Rev. Mol. Cell Biol. 2000, 1, 72–76. [Google Scholar] [CrossRef]
- Palm, W.; de Lange, T. How Shelterin Protects Mammalian Telomeres. Annu. Rev. Genet. 2008, 42, 301–334. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.M.; Osborne, C.K.; Levitt, D.; Wu, F.; Kim, N.W. Telomerase Activity and Survival of Patients With Node-Positive Breast Cancer. JNCI J. Natl. Cancer Inst. 1997, 89, 1874–1881. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Q.; Ge, Y.; Zhao, Q.; Zheng, X.; Zhao, Y. hTERT promotes cell adhesion and migration independent of telomerase activity. Sci. Rep. 2016, 6, 22886. [Google Scholar] [CrossRef]
- Altamura, G.; Martano, M.; Licenziato, L.; Maiolino, P.; Borzacchiello, G. Telomerase Reverse Transcriptase (TERT) Expression, Telomerase Activity, and Expression of Matrix Metalloproteinases (MMP)-1/-2/-9 in Feline Oral Squamous Cell Carcinoma Cell Lines Associated With Felis catus Papillomavirus Type-2 Infection. Front. Veter- Sci. 2020, 7, 148. [Google Scholar] [CrossRef]
- Yamashita, T.; Uno, T.; Ishikawa, Y. Stabilization of guanine quadruplex DNA by the binding of porphyrins with cationic side arms. Bioorganic Med. Chem. 2005, 13, 2423–2430. [Google Scholar] [CrossRef]
- Izbicka, E.; Wheelhouse, R.T.; Raymond, E.; Davidson, K.K.; A Lawrence, R.; Sun, D.; E Windle, B.; Hurley, L.H.; Von Hoff, D.D. Effects of cationic porphyrins as G-quadruplex interactive agents in human tumor cells. Cancer Res. 1999, 59, 639–44. [Google Scholar]
- Anantha, N.V.; Azam, M.; Sheardy, R.D. Porphyrin Binding to Quadruplexed T4G4. Biochemistry 1998, 37, 2709–2714. [Google Scholar] [CrossRef]
- Arthanari, H.; Basu, S.; Kawano, T.L.; Bolton, P.H. Fluorescent dyes specific for quadruplex DNA. Nucleic Acids Res. 1998, 26, 3724–3728. [Google Scholar] [CrossRef]
- Zhuang, X.-Y.; Yao, Y.-G. Mitochondrial dysfunction and nuclear-mitochondrial shuttling of TERT are involved in cell proliferation arrest induced by G-quadruplex ligands. FEBS Lett. 2013, 587, 1656–1662. [Google Scholar] [CrossRef]
- Grand, C.L.; Han, H.; Muñoz, R.M.; Weitman, S.; Von Hoff, D.D.; Hurley, L.H.; Bearss, D.J. The cationic porphyrin TMPyP4 down-regulates c-MYC and human telomerase reverse transcriptase expression and inhibits tumor growth in vivo. Mol Cancer Ther. 2002, 1, 565–73. [Google Scholar]
- Cheng, M.-J.; Cao, Y.-G. TMPYP4 exerted antitumor effects in human cervical cancer cells through activation of p38 mitogen-activated protein kinase. Biol. Res. 2017, 50, 24–24. [Google Scholar] [CrossRef] [PubMed]
- Pascolo, E.; Wenz, C.; Lingner, J.; Hauel, N.; Priepke, H.; Kauffmann, I.; Garin-Chesa, P.; Rettig, W.J.; Damm, K.; Schnapp, A. Mechanism of Human Telomerase Inhibition by BIBR1532, a Synthetic, Non-nucleosidic Drug Candidate. J. Biol. Chem. 2002, 277, 15566–15572. [Google Scholar] [CrossRef] [PubMed]
- El-Daly, H.; Kull, M.; Zimmermann, S.; Pantic, M.; Waller, C.F.; Martens, U.M. Selective cytotoxicity and telomere damage in leukemia cells using the telomerase inhibitor BIBR1532. Blood 2005, 105, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.; Autexier, C. Pharmacological Telomerase Inhibition Can Sensitize Drug-Resistant and Drug-Sensitive Cells to Chemotherapeutic Treatment. Mol Pharmacol. 2005, 68, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Röth, A.; Dürig, J.; Himmelreich, H.; Bug, S.; Siebert, R.; Dührsen, U.; Lansdorp, P.M.; Baerlocher, G.M. Short telomeres and high telomerase activity in T-cell prolymphocytic leukemia. Leukemia 2007, 21, 2456–2462. [Google Scholar] [CrossRef]
- Meng, E.; Taylor, B.; Ray, A.; Shevde, L.A.; Rocconi, R.P. Targeted inhibition of telomerase activity combined with chemotherapy demonstrates synergy in eliminating ovarian cancer spheroid-forming cells. Gynecol. Oncol. 2012, 124, 598–605. [Google Scholar] [CrossRef]
- FDA.gov. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-imetelstat-low-intermediate-1-risk-myelodysplastic-syndromes-transfusion-dependent. (accessed 25 September 2024).
- Herbert, B.-S.; Gellert, G.C.; Hochreiter, A.; Pongracz, K.; E Wright, W.; Zielinska, D.; Chin, A.C.; Harley, C.B.; Shay, J.W.; Gryaznov, S.M. Lipid modification of GRN163, an N3′ → P5′ thio-phosphoramidate oligonucleotide, enhances the potency of telomerase inhibition. Oncogene 2005, 24, 5262–5268. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, J.; Yang, S.; Kuang, Z.; Tan, G.; Yang, G.; Wei, Q.; Guo, Z. Telomerase antagonist imetelstat increases radiation sensitivity in esophageal squamous cell carcinoma. Oncotarget 2017, 8, 13600–13610. [Google Scholar] [CrossRef]
- Goldblatt, E.M.; Gentry, E.R.; Fox, M.J.; Gryaznov, S.M.; Shen, C.; Herbert, B.-S. The telomerase template antagonist GRN163L alters MDA-MB-231 breast cancer cell morphology, inhibits growth, and augments the effects of paclitaxel. Mol. Cancer Ther. 2009, 8, 2027–2035. [Google Scholar] [CrossRef]
- Koziel, J.E.; Herbert, B.-S. The telomerase inhibitor imetelstat alone, and in combination with trastuzumab, decreases the cancer stem cell population and self-renewal of HER2+ breast cancer cells. Breast Cancer Res. Treat. 2015, 149, 607–618. [Google Scholar] [CrossRef]
- Garnique, A.; Rezende-Teixeira, P.; Machado-Santelli, G. Telomerase inhibitors TMPyP4 and thymoquinone decreased cell proliferation and induced cell death in the non-small cell lung cancer cell line LC-HK2, modifying the pattern of focal adhesion. Braz. J. Med Biol. Res. 2023, 56, e12897. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-H.; Nie, X.; Liu, H.-Y.; Fang, Y.-M.; Zhao, Y.; Xia, L.-X. TMPyP4 promotes cancer cell migration at low doses, but induces cell death at high doses. Sci. Rep. 2016, 6, 26592. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, A.; Bashash, D.; Kabuli, M.; Zandi, Z.; Kashani, B.; Zaghal, A.; Mousavi, S.A.; Ghaffari, S.H. Arsenic trioxide and BIBR1532 synergistically inhibit breast cancer cell proliferation through attenuation of NF-κB signaling pathway. Life Sci. 2020, 257, 118060. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sun, L.; Chen, G.; Zheng, D.; Li, L.; Wei, W. A combination of the telomerase inhibitor, BIBR1532, and paclitaxel synergistically inhibit cell proliferation in breast cancer cell lines. Target. Oncol. 2015, 10, 565–573. [Google Scholar] [CrossRef]
- Joseph, I.; Tressler, R.; Bassett, E.; Harley, C.; Buseman, C.M.; Pattamatta, P.; Wright, W.E.; Shay, J.W.; Go, N.F. The Telomerase Inhibitor Imetelstat Depletes Cancer Stem Cells in Breast and Pancreatic Cancer Cell Lines. Cancer Res. 2010, 70, 9494–9504. [Google Scholar] [CrossRef]
- Frink, R.E.; Peyton, M.; Schiller, J.H.; Gazdar, A.F.; Shay, J.W.; Minna, J.D. Telomerase inhibitor imetelstat has preclinical activity across the spectrum of non-small cell lung cancer oncogenotypes in a telomere length dependent manner. Oncotarget 2016, 7, 31639–31651. [Google Scholar] [CrossRef]
- Bashash, D.; Ghaffari, S.H.; Zaker, F.; Hezave, K.; Kazerani, M.; Ghavamzadeh, A.; Alimoghaddam, K.; Mosavi, S.A.; Gharehbaghian, A.; Vossough, P. Direct Short-Term Cytotoxic Effects of BIBR 1532 on Acute Promyelocytic Leukemia Cells Through Induction of p21 Coupled with Downregulation of c-Myc and hTERT Transcription. Cancer Investig. 2012, 30, 57–64. [Google Scholar] [CrossRef]
- Baerlocher, G.M.; Haubitz, M.; Braschler, T.R.; Brunold, C.; Burington, B.; Leibundgut, E.O.; Go, N. Imetelstat inhibits growth of megakaryocyte colony-forming units from patients with essential thrombocythemia. Blood Adv. 2019, 3, 3724–3728. [Google Scholar] [CrossRef]
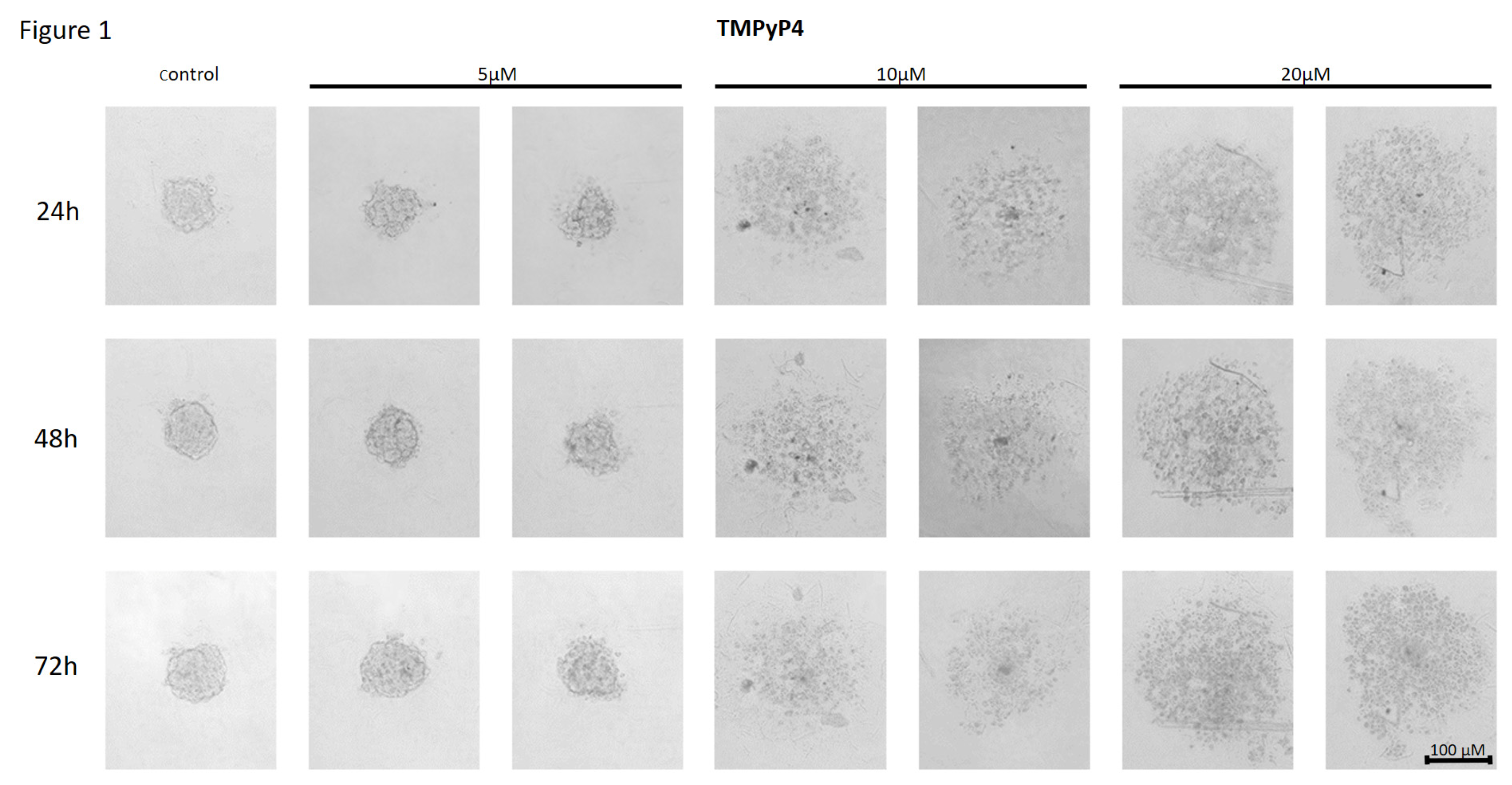
Figure 1.
The effect of telomerase inhibitor, TMPyP4, on the ability of MCF7 to form spheroids. The experiment was performed in 6 replicates for every concentration and time interval. Incubation of the cells with the tested compound lasted 72h, followed by 3D spheroid formation test for up to another 72h, again in the presence of the inhibitor. Pictures were taken using CarlZeiss microscope, 10X. The scale bar shows 100µm. Representative pictures are demonstrated.
Figure 1.
The effect of telomerase inhibitor, TMPyP4, on the ability of MCF7 to form spheroids. The experiment was performed in 6 replicates for every concentration and time interval. Incubation of the cells with the tested compound lasted 72h, followed by 3D spheroid formation test for up to another 72h, again in the presence of the inhibitor. Pictures were taken using CarlZeiss microscope, 10X. The scale bar shows 100µm. Representative pictures are demonstrated.
Figure 2.
Effect of TMPyP4 inhibitor on the ability to form spheroids in MDA-MB-231 cells. The experiment was performed in 6 replicates for every concentration and time interval. Incubation of the cells with the tested compound lasted 72h, followed by 3D spheroid formation test for up to another 72h, again in the presence of the inhibitor. Pictures were taken using CarlZeiss microscope, 10X. The scale bar shows 100µm. Representative pictures are demonstrated.
Figure 2.
Effect of TMPyP4 inhibitor on the ability to form spheroids in MDA-MB-231 cells. The experiment was performed in 6 replicates for every concentration and time interval. Incubation of the cells with the tested compound lasted 72h, followed by 3D spheroid formation test for up to another 72h, again in the presence of the inhibitor. Pictures were taken using CarlZeiss microscope, 10X. The scale bar shows 100µm. Representative pictures are demonstrated.
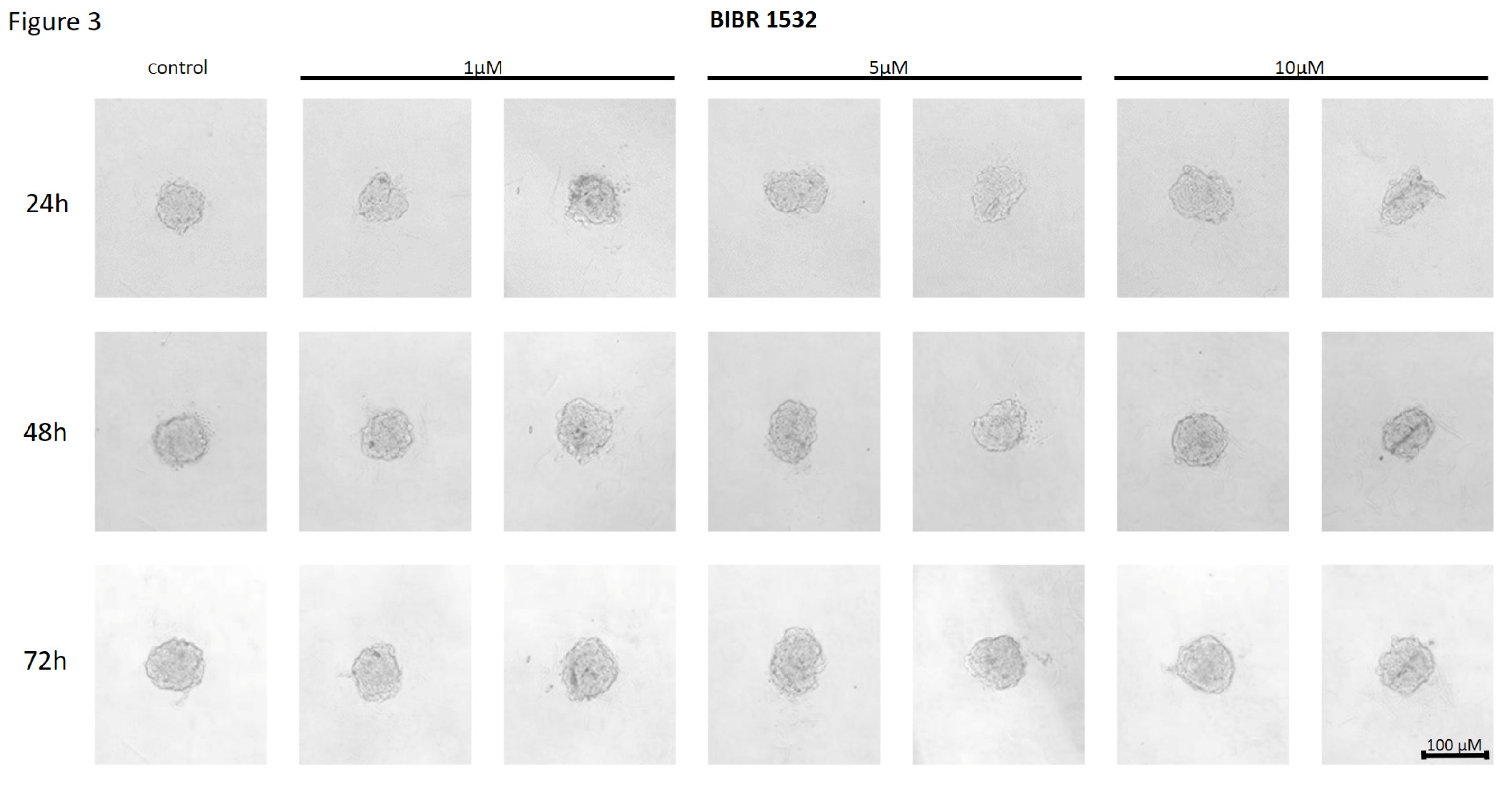
Figure 3.
The effect of telomerase inhibitor, BIBR 1532, on the ability of MCF7 to form spheroids. The experiment was performed in 6 replicates for every concentration and time interval. Incubation of the cells with the tested compound lasted 72h, followed by 3D spheroid formation test for up to another 72h, again in the presence of the inhibitor. Pictures were taken using CarlZeiss microscope, 10X. The scale bar shows 100µm. Representative pictures are demonstrated.
Figure 3.
The effect of telomerase inhibitor, BIBR 1532, on the ability of MCF7 to form spheroids. The experiment was performed in 6 replicates for every concentration and time interval. Incubation of the cells with the tested compound lasted 72h, followed by 3D spheroid formation test for up to another 72h, again in the presence of the inhibitor. Pictures were taken using CarlZeiss microscope, 10X. The scale bar shows 100µm. Representative pictures are demonstrated.
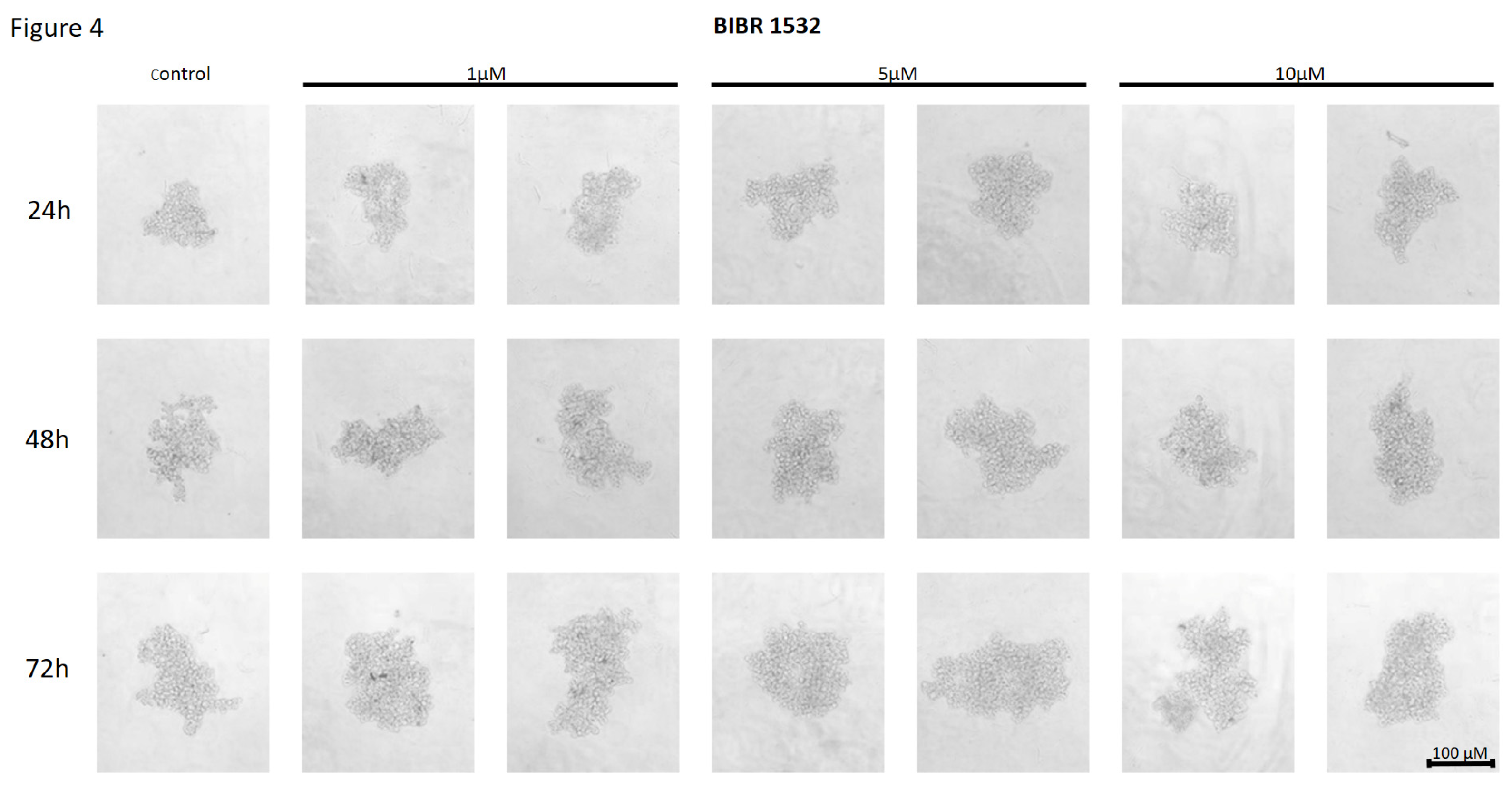
Figure 4.
The effect of telomerase inhibitor, BIBR 1532, on the ability of MDA-MB-231 to form spheroids. The experiment was performed in 6 replicates for every concentration and time interval. Incubation of the cells with the tested compound lasted 72h, followed by 3D spheroid formation test for up to another 72h, again in the presence of the inhibitor. Pictures were taken using CarlZeiss microscope, 10X. The scale bar shows 100µm. Representative pictures are demonstrated.
Figure 4.
The effect of telomerase inhibitor, BIBR 1532, on the ability of MDA-MB-231 to form spheroids. The experiment was performed in 6 replicates for every concentration and time interval. Incubation of the cells with the tested compound lasted 72h, followed by 3D spheroid formation test for up to another 72h, again in the presence of the inhibitor. Pictures were taken using CarlZeiss microscope, 10X. The scale bar shows 100µm. Representative pictures are demonstrated.
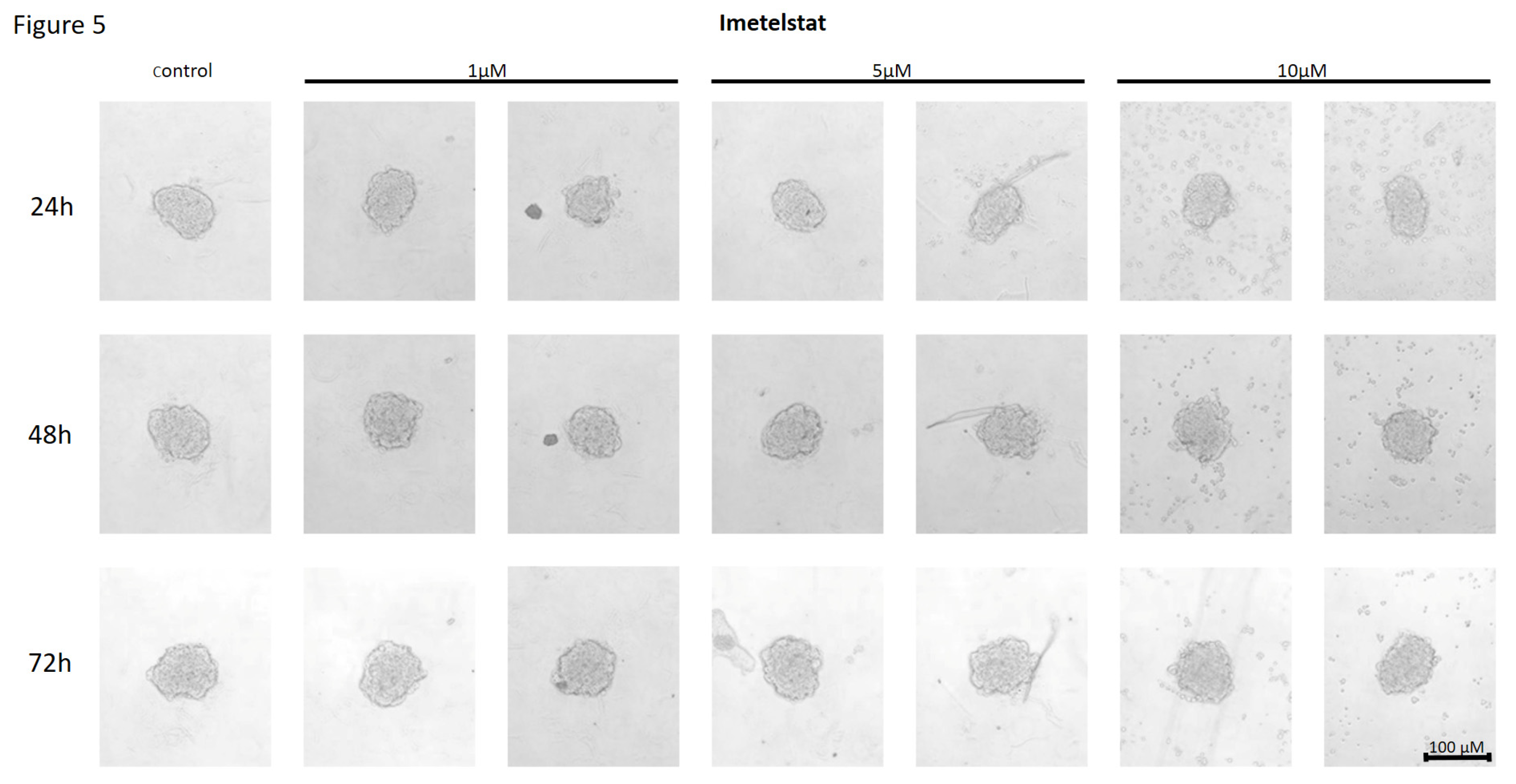
Figure 5.
The effect of telomerase inhibitor, imetelstat, on the ability of MCF7 to form spheroids. The experiment was performed in 6 replicates for every concentration and time interval. Incubation of the cells with the tested compound lasted 72h, followed by 3D spheroid formation test for up to another 72h, again in the presence of the inhibitor. Pictures were taken using CarlZeiss microscope, 10X. The scale bar shows 100µm. Representative pictures are demonstrated.
Figure 5.
The effect of telomerase inhibitor, imetelstat, on the ability of MCF7 to form spheroids. The experiment was performed in 6 replicates for every concentration and time interval. Incubation of the cells with the tested compound lasted 72h, followed by 3D spheroid formation test for up to another 72h, again in the presence of the inhibitor. Pictures were taken using CarlZeiss microscope, 10X. The scale bar shows 100µm. Representative pictures are demonstrated.
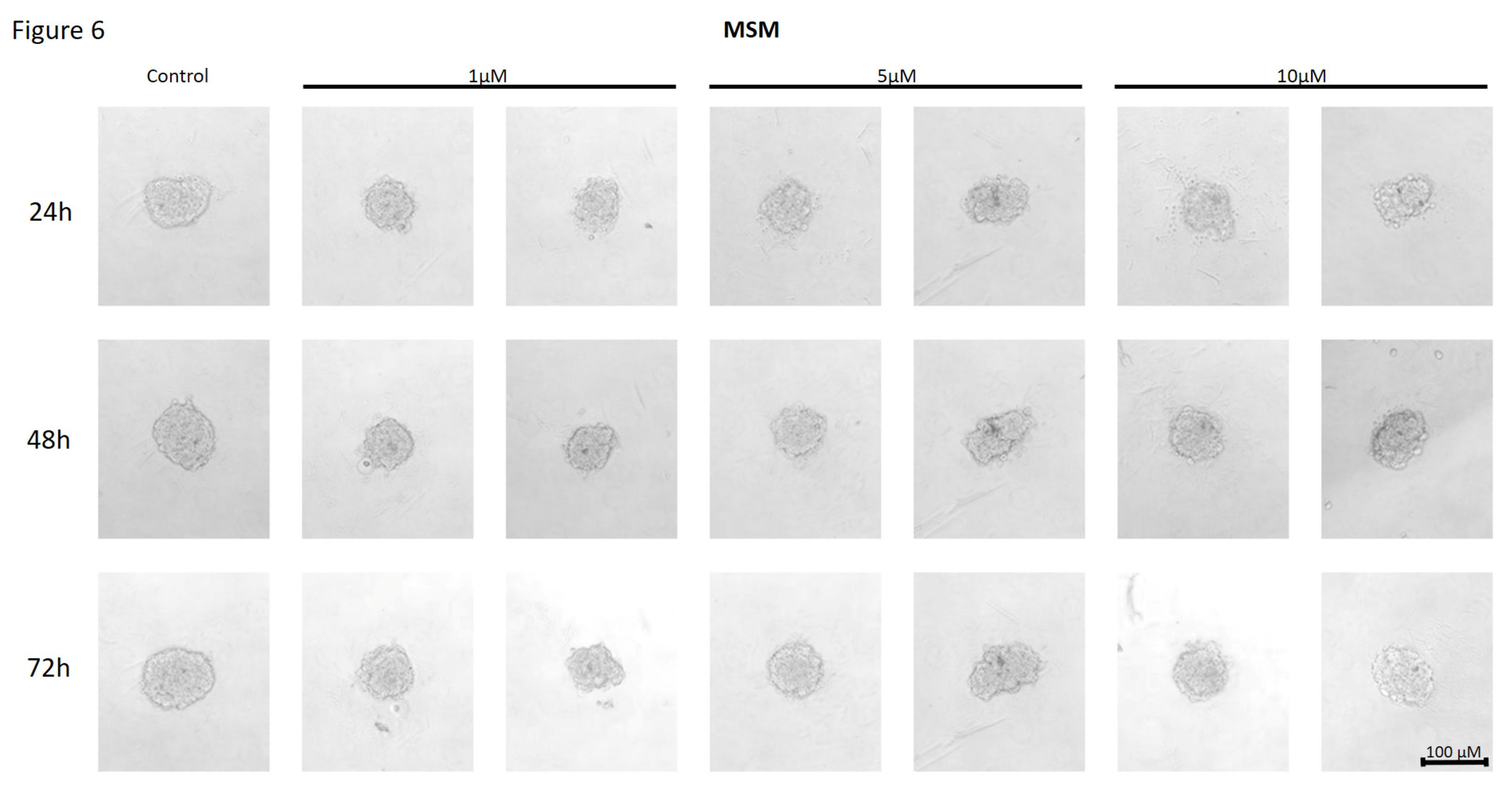
Figure 6.
The effect of mismatch (MSM) negative control on the ability of MCF7 to form spheroids. The experiment was performed in 6 replicates for every concentration and time interval. Incubation of the cells with the tested compound lasted 72h, followed by 3D spheroid formation test for up to another 72h, again in the presence of the inhibitor. Pictures were taken using CarlZeiss microscope, 10X. The scale bar shows 100µm. Representative pictures are demonstrated.
Figure 6.
The effect of mismatch (MSM) negative control on the ability of MCF7 to form spheroids. The experiment was performed in 6 replicates for every concentration and time interval. Incubation of the cells with the tested compound lasted 72h, followed by 3D spheroid formation test for up to another 72h, again in the presence of the inhibitor. Pictures were taken using CarlZeiss microscope, 10X. The scale bar shows 100µm. Representative pictures are demonstrated.
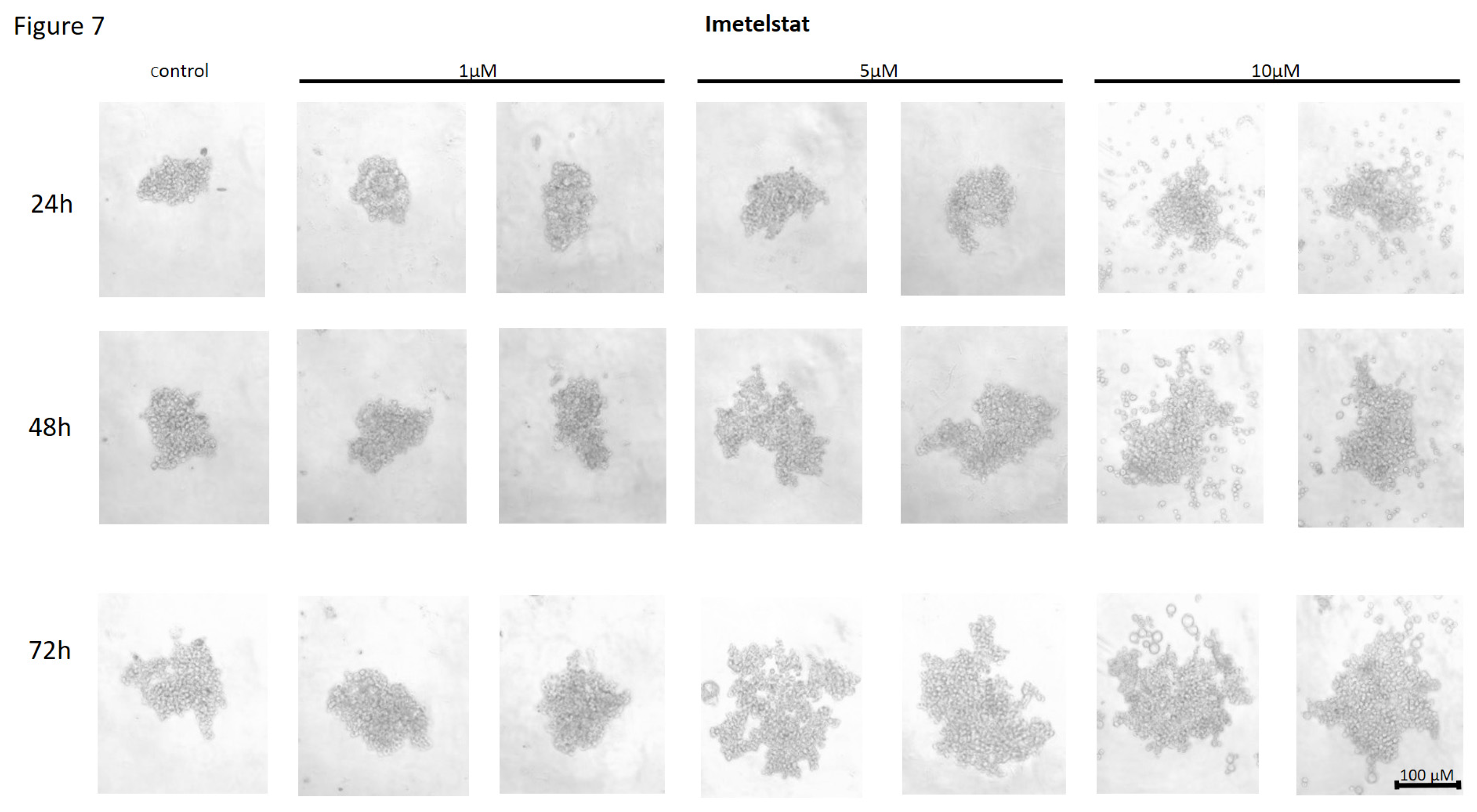
Figure 7.
The effect of telomerase inhibitor, imetelstat, on the ability of MDA-MB-231 to form spheroids. The experiment was performed in 6 replicates for every concentration and time interval. Incubation of the cells with the tested compound lasted 72h, followed by 3D spheroid formation test for up to another 72h, again in the presence of the inhibitor. Pictures were taken using CarlZeiss microscope, 10X. The scale bar shows 100µm. Representative pictures are demonstrated.
Figure 7.
The effect of telomerase inhibitor, imetelstat, on the ability of MDA-MB-231 to form spheroids. The experiment was performed in 6 replicates for every concentration and time interval. Incubation of the cells with the tested compound lasted 72h, followed by 3D spheroid formation test for up to another 72h, again in the presence of the inhibitor. Pictures were taken using CarlZeiss microscope, 10X. The scale bar shows 100µm. Representative pictures are demonstrated.
Figure 8.
The effect of mismatch negative control on the ability of MDA-MB-231 to form spheroids. The experiment was performed in 6 replicates for every concentration and time interval. Incubation of the cells with the tested compound lasted 72h, followed by 3D spheroid formation test for up to another 72h, again in the presence of the inhibitor. Pictures were taken using CarlZeiss microscope, 10X. The scale bar shows 100µm. Representative pictures are demonstrated.
Figure 8.
The effect of mismatch negative control on the ability of MDA-MB-231 to form spheroids. The experiment was performed in 6 replicates for every concentration and time interval. Incubation of the cells with the tested compound lasted 72h, followed by 3D spheroid formation test for up to another 72h, again in the presence of the inhibitor. Pictures were taken using CarlZeiss microscope, 10X. The scale bar shows 100µm. Representative pictures are demonstrated.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).