Submitted:
10 October 2024
Posted:
11 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Phosphors Preparation
2.2. Characterizations
3. Results and Discussion
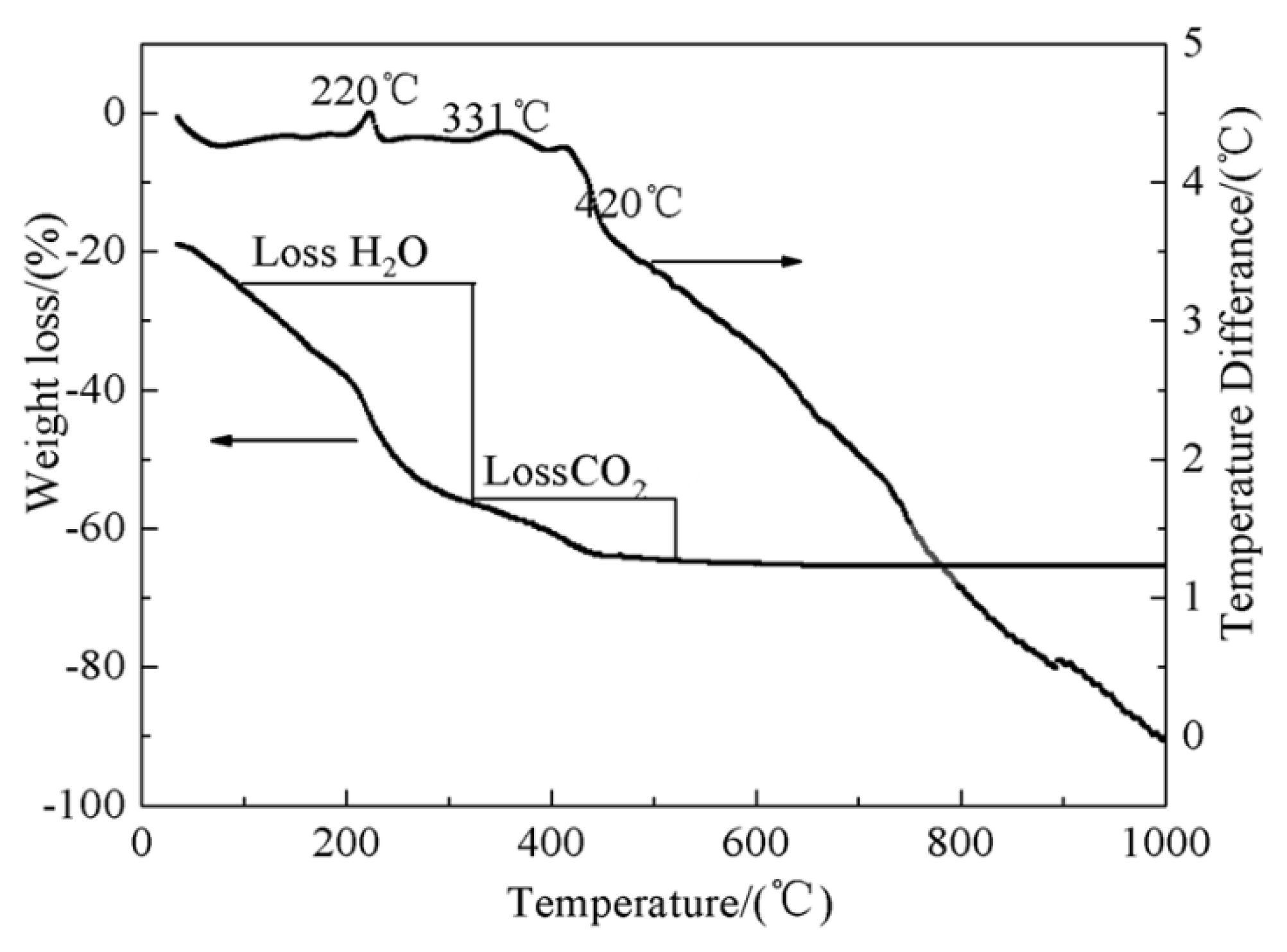
3.1. TG-DTA Analysis of Na5Zn2Gd1-x(MoO4)6: x Eu3+
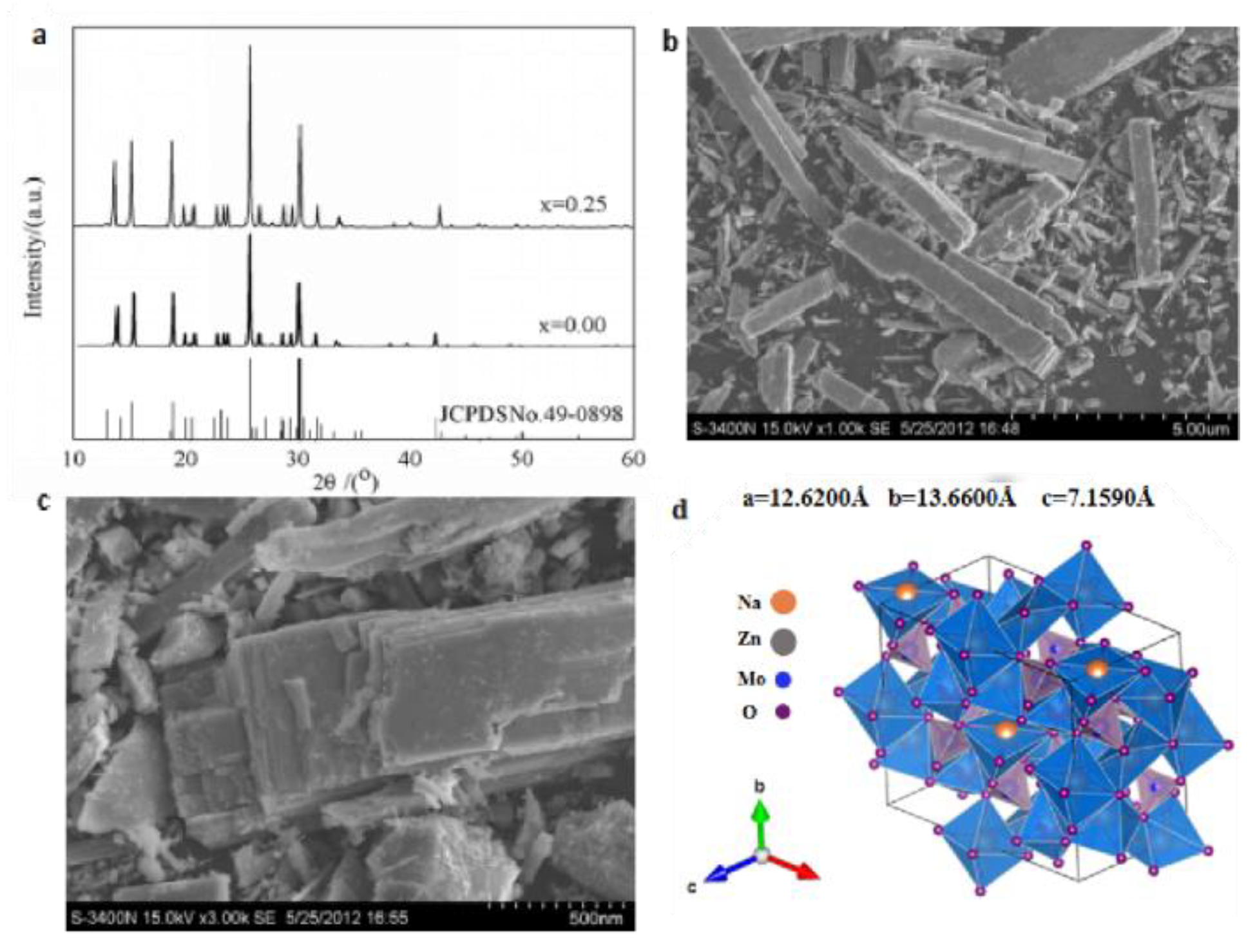
3.2. Structure and Morphology of Na5Zn2Gd1-x(MoO4)6: x Eu3+
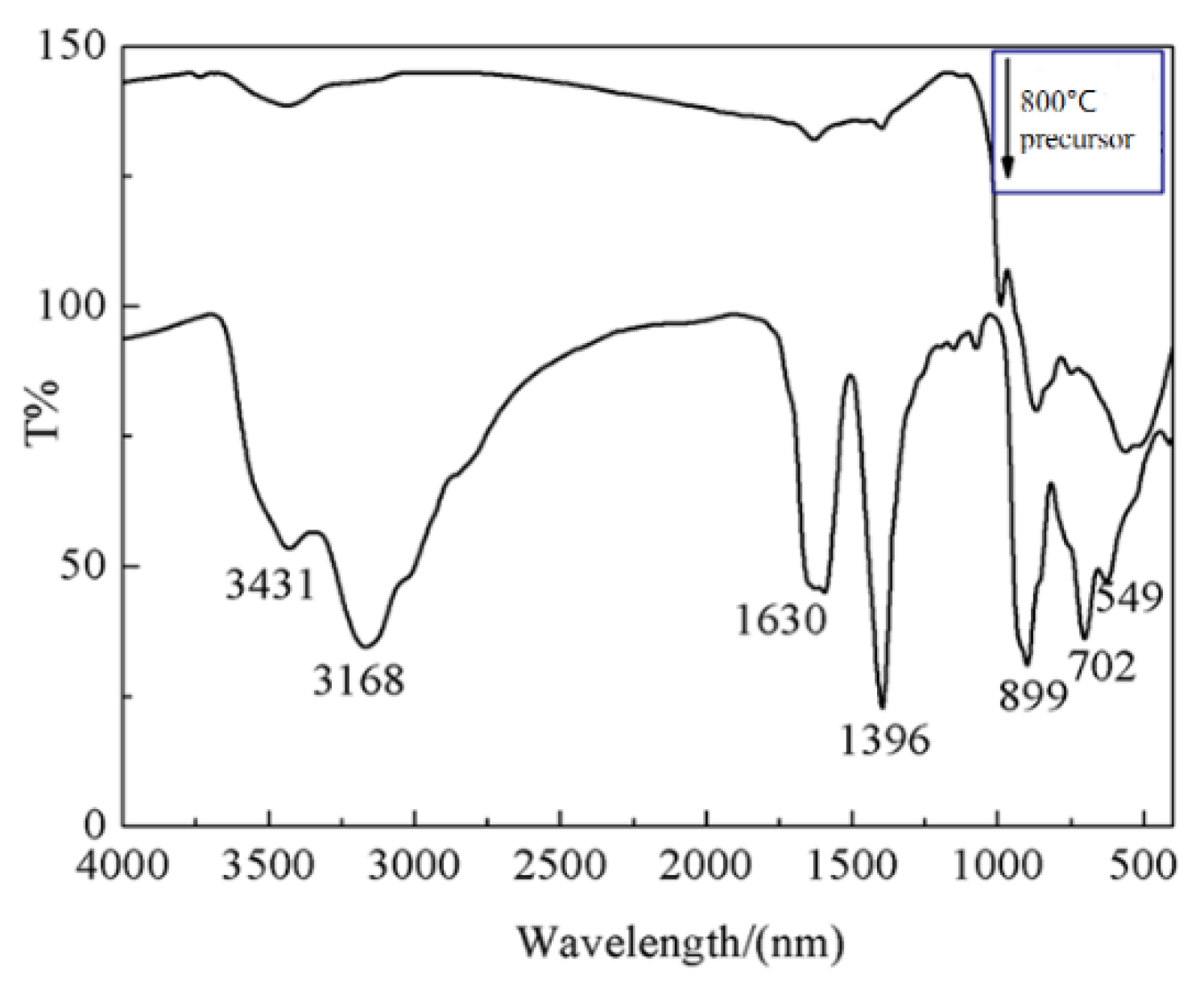
3.3. FTIR spectra of Na5Zn2Gd0.75(MoO4)6: 0.25 Eu3+
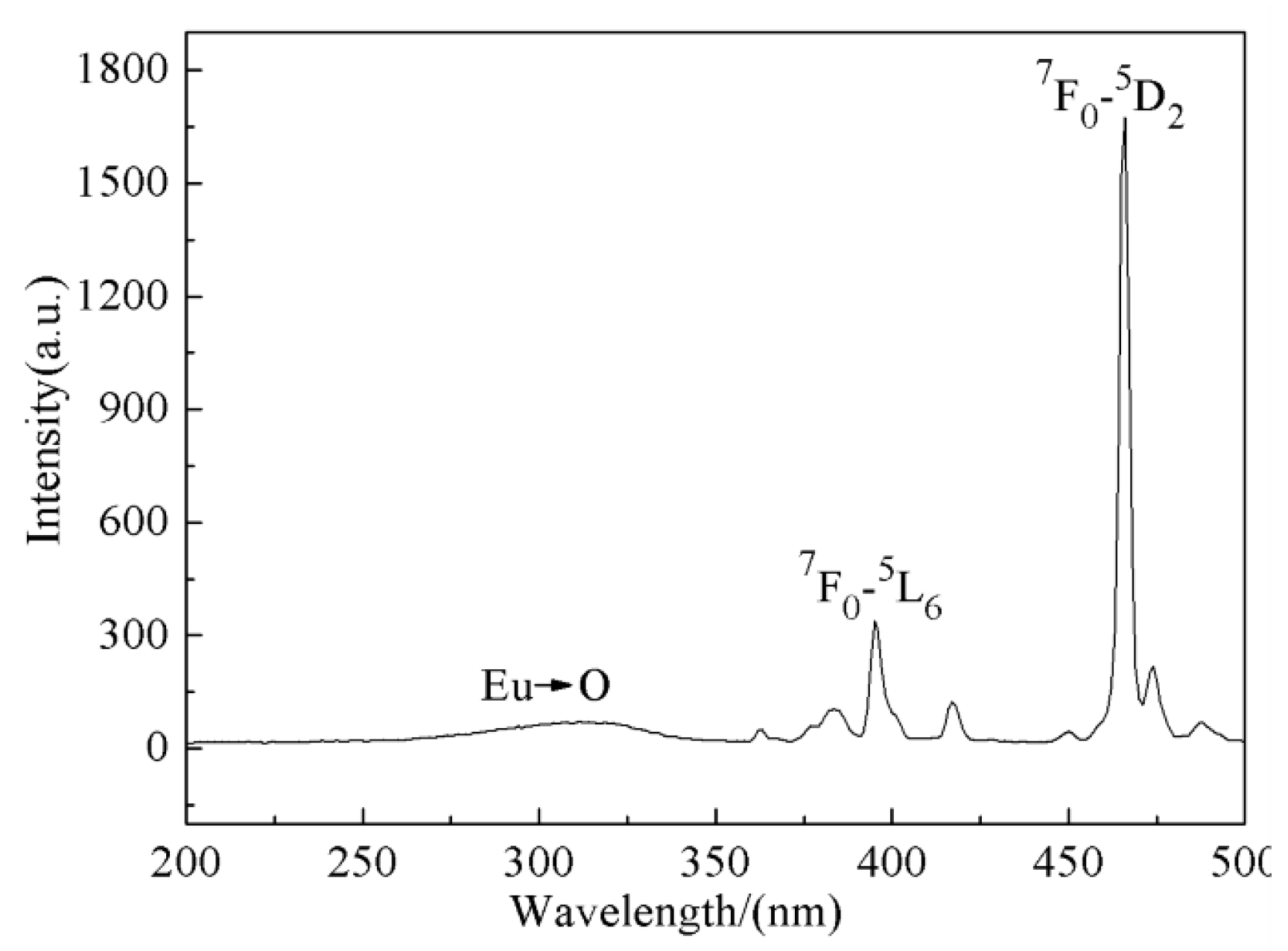
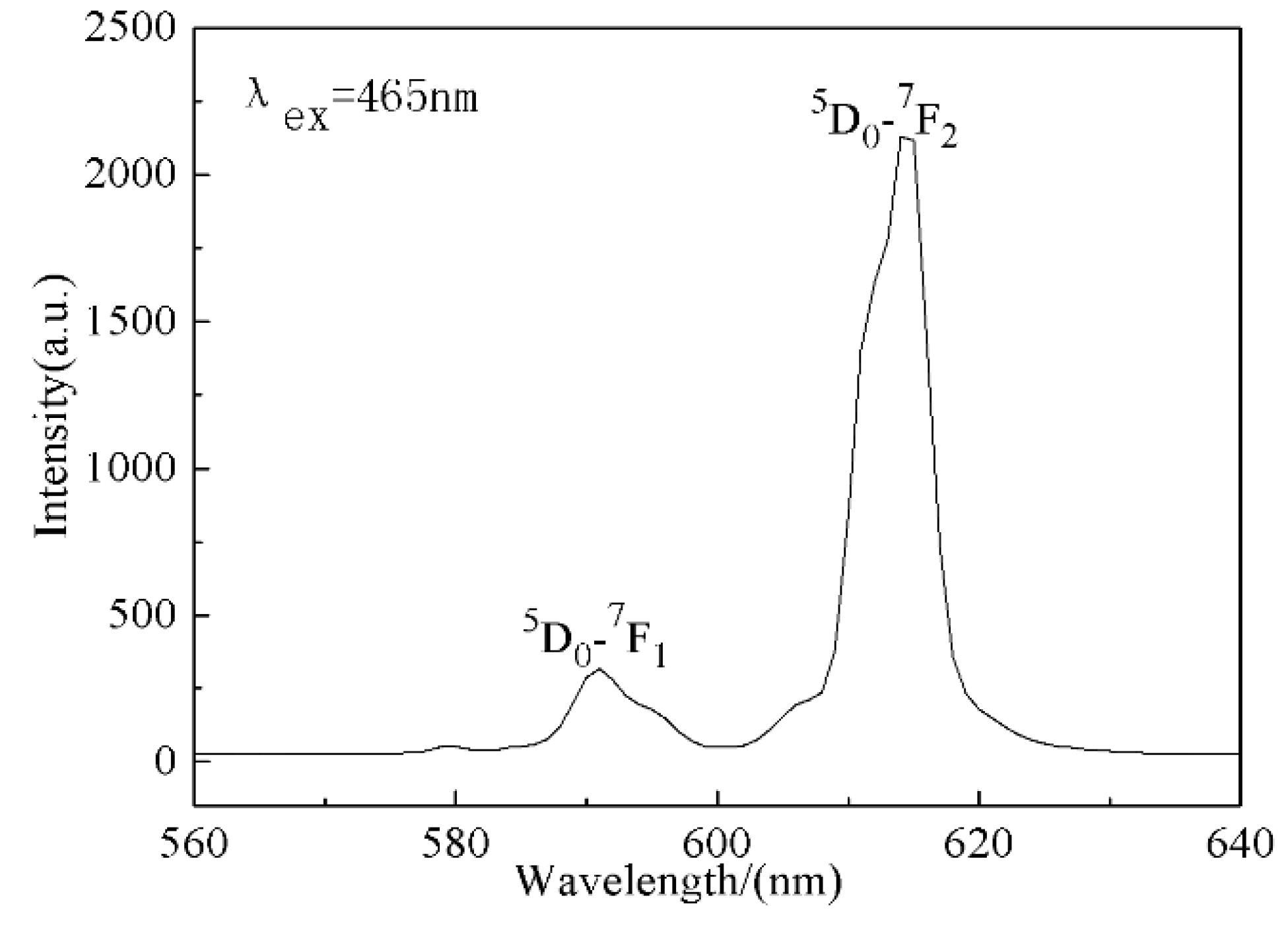
3.4. Luminescent Properties of Na5Zn2Gd(MoO4)6: Eu3+
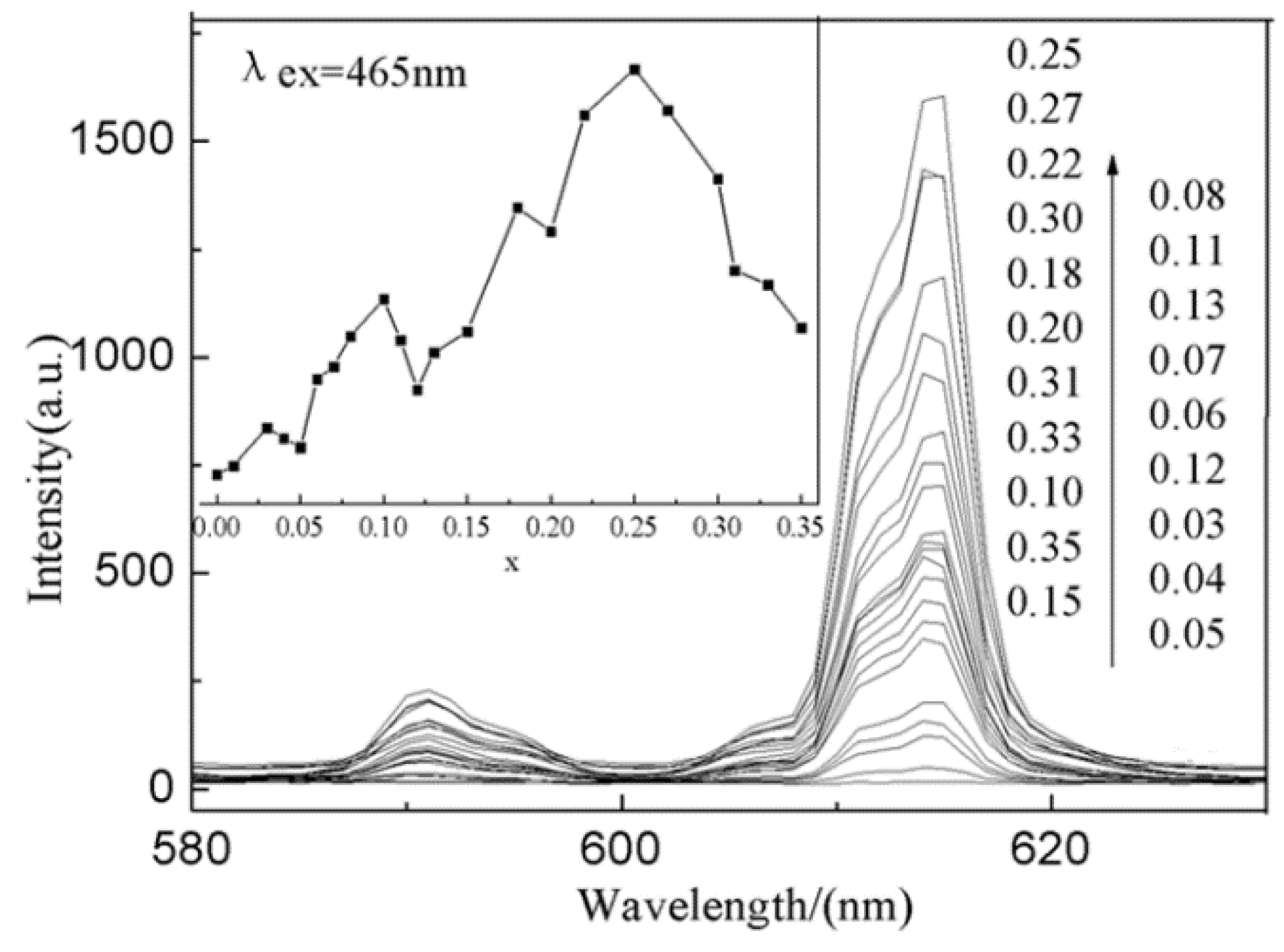
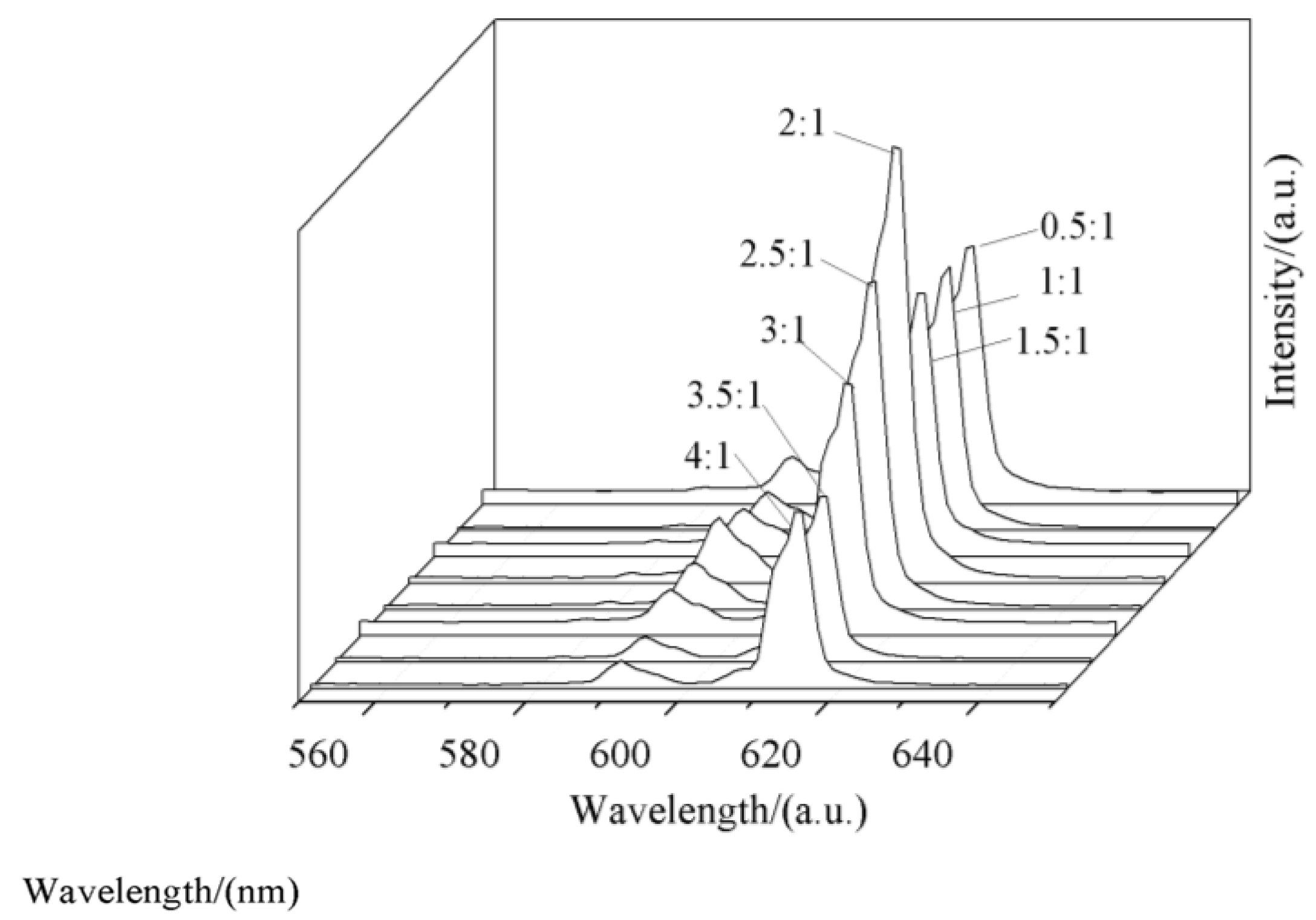
3.5. Effect of Doped-Eu3+ Concentration
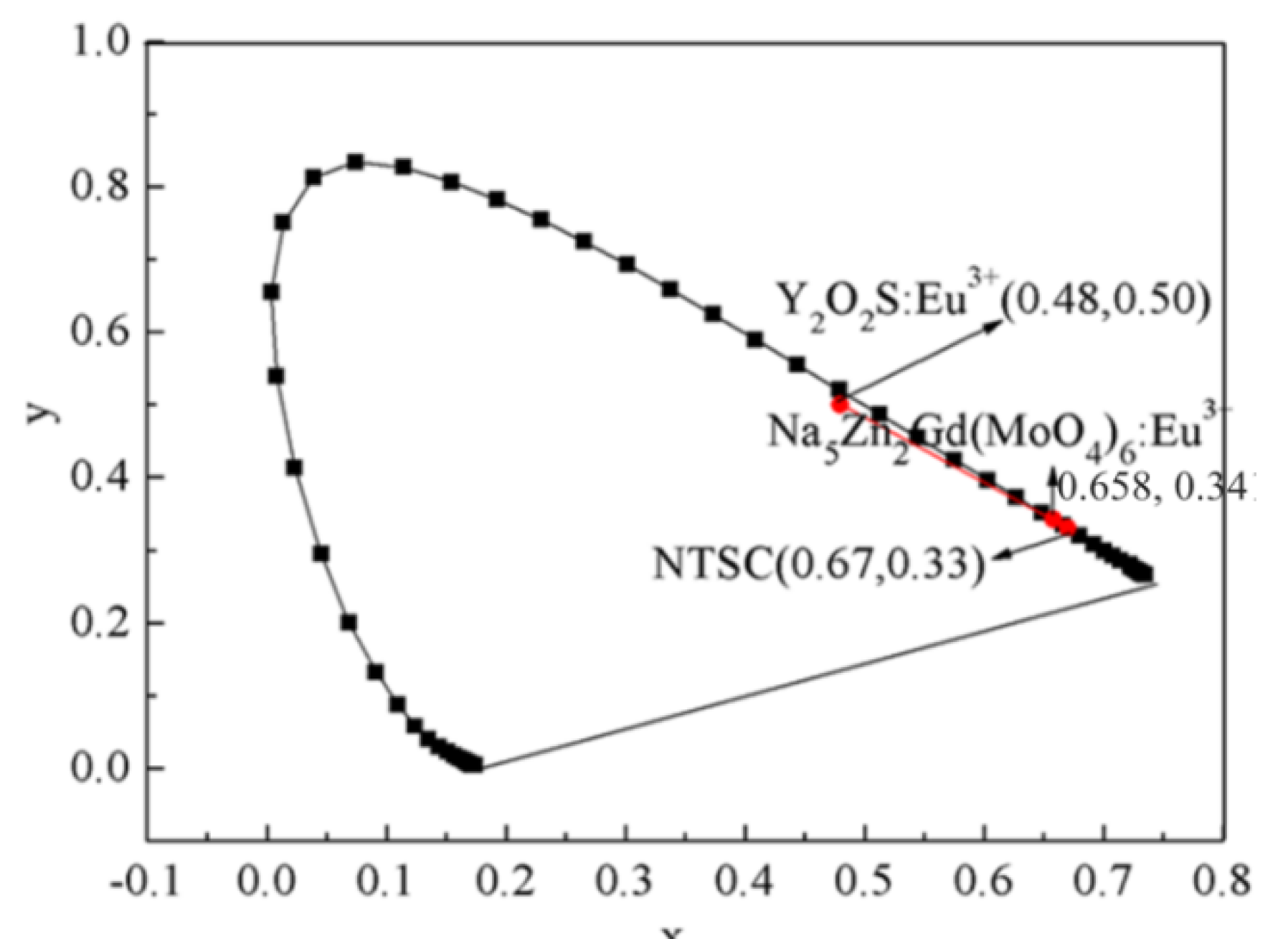
3.6. CIE Color Coordinates of Na5Zn2Gd0.75(MoO4)6: 0.25 Eu3+
4. Conclusions
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mariyam Thoma, Prabhakar Rao, M. DeePa, et al.: Novel Powellit-based red-emitting PhosPhors: CaLa1-xNbMoO8: Eux3+ for white light emtting diodes. J. Solid State Chem.182(l), 203-207 (2008). [CrossRef]
- J.Y. Park, H.K. Yang.: Development of red-emitting La2ZnTiO6: Eu3+ phosphors for WLED and visualization of latent fingerprint applications. Mater. Today Commun. 31, 103391 (2022). [CrossRef]
- Hu Y, ZhuangW, YeH, et al.: A novel red phosphor for white light emitting diodes. J. Alloy. Compd., 390(1-2), 226-229 (2005). [CrossRef]
- Kai, Z. : Progress in the Preparation of Phosphor for White Light Emitting Diode. Materials Review (2005).
- Sun Xiaojuan, Zhang Jiahua, ZhangXia, et al.: A single white phosphor suitable for near ultraviolet excitation applied to new generation white LED lighting. Chin. J. Lumin. 26 (3), 404-406 (2005).
- WuHao, Pan Yuexiao, GuoChangfeng, et al.: Fabrication and properties of rare earth phosphors and their applications in white-light LEDs. Chin. J. Lumin. 27 (2), 201-205 (2006).
- Neeraj S, Kijoma N, Cheetham A K. Novel red phosphors for solid-state lighting: the system NaM(WO4)2-x(MoO4)x: Eu3+ (M= Gd, Y, Bi). Chem. Phys. Lett. 387 (1), 2 (2003). [CrossRef]
- C.F. Guo, T. Chen, L. Luan, et al.: Luminescent properties of R2(MoO4)3:Eu3+ (R=La, Y, Gd) phosphors prepared by sol-gel method. J. Phys. Chem. Solid. 69, 1905–1911 (2008). [CrossRef]
- C.F. Guo, F. Gao, L.F. Liang, et al.: Synthesis, characterization and luminescent properties of novel red emitting phosphor Li3Ba2Ln3(MoO4)8: Eu3+ (Ln=La, Gd and Y) for white light-emitting diodes. J. Alloy. Comp. 479, 607-612 (2009). [CrossRef]
- P. Du, J.S. Yu.: Synthesis and luminescent properties of Eu3+-activated Na0.5Gd0.5MoO4: a strong re-emitting phosphor for LED and FED applications. J. Lumin. 179, 451–456 (2016). [CrossRef]
- Li X, Yang Z P, Guan L, et al.: Synthesis and properties of Eu3+ activated strontium molybdate phosphor. J. Rare Earths, 25, 706 (2007). [CrossRef]
- Yaoyao Li, Huiya Li, Yan Li, et al.: Preparation of a novel red KBaGd(MoO4)3:Eu3+ phosphor by sol-gel method and its luminescent properties. Opt. Mater. 144, 114336 (2023). [CrossRef]
- Wang ZL, Liang HB, Zhou LY, et al.: NaEu0.96Sm0.04(MoO4)2 as a promising red-emitting phosphor for LED solid-state lighting prepared by the Pechini process. J. Lumin. 128(1), 147-154 (2007). [CrossRef]
- Gao Fei, Liang Lifang, Guo Chongfeng.: Preparation and luminescence of red light-emitting phosphors based on Li3Ba2Ln(3-x)Eux(MoO4)8 by sol-gel method. Chin. J. Lumin. 30(5), 610-616 (2009).
- Chen, F, Akram, M.N, Chen, X.Y.: Improved photoluminescence performance of Eu3+-doped Y2(MoO4)3 red-emitting phosphor via orderly arrangement of the crystal lattice. Molecules. 28, 1014 (2023). [CrossRef]
- Xie, H.D, Chen, C, Li, J, et al.: Sol-gel synthesis and luminescent performance of Eu3+, Lu3+ co-doped Ca0.3Sr0.7Mo1−xWxO4 red-emitting phosphor. Inorg. Nano Met. Chem. 51, 1297-1305 (2020). [CrossRef]
- Zhao Li, Ju-long Cheng, Ya-nan Wang, et al.: Rapid synthesis of BaMoO4:Eu3+ red phosphors using microwave irradiation. Luminescence. 38(8), 1414-1421 (2023). [CrossRef]
- Kong L, Sun H, Nie Y, et al.: Luminescent Properties and Charge Compensator Effects of SrMo0.5W0.5O4: Eu3+ for White Light LEDs. Molecules. 28, 2681 (2023). [CrossRef]
- S.F.Solodovnikov, E.G. Khaikina, Z.A. Solodovnikova.: New families of lithium containing triple molybdates and the stabilizing role of lithium in their structure formation. Dokl. Chem. 416(l), 207-212 (2007). [CrossRef]
- O. M. Basovich, E. G. Khaikina.: Formation Laws for Scheelite-like Triple Molybdates LiMLn2 -(MoO4)4. Russ. J. Inorg. Chem. 51 (7), 160-1164 (2006). [CrossRef]
- T. Ji, E. Ha, M. Wu, et al.: Controllable hydrothermal synthesis and photocatalytic performance of Bi2MoO6 nano/microstructures. Catalysts. 10(10), 1161(2020). [CrossRef]
- H. Li, S. Zhang, S. Zhou, et al.: Crystalline size effect on the energy transfer from Mo-O groups to Eu3+ ions in R2MoO6: Eu (R¼La, Gd, and Y) Crystals. J. Phys. Chem. C. 113, 13115-13120 (2009). [CrossRef]
- X. Huang, S. Wang, B. Li, et al.: High-brightness and high-color purity red-emitting Ca3Lu(AlO)3(BO3)4: Eu3+ phosphors with internal quantum efficiency close to unity for near-ultraviolet-based white-light-emitting diodes. Opt. Lett. 43(6),130-1310 (2018). [CrossRef]
- Gupta, S. Singh, S. Bhagwan, et al.: Rare earth (RE) doped phosphors and their emerging applications: a review. Ceram. Int. 47(14), 19282-19303 (2021). [CrossRef]
- B. Han, J. Zhang, P. Li, et al.: Synthesis and luminescence properties of Eu3+ doped high temperature form of Bi2MoO6. J. Electron. Mater. 44(3), 102-103 (2015).
- LI Zhao, WANG Yongfeng, CAO Jing, et al.: Hydrothermal synthesis and luminescent properties of BaMoO4: Sm3+ red phosphor. J. Rare Earths. 34 (2), 143-146 (2016). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




