Submitted:
10 October 2024
Posted:
11 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of 22:6PC Lipid Vesicles (Liposomes)
2.3. Oxidation of 22:6PC
2.4. Monitoring of 22:6PC Oxidation
2.4.1. Extraction of Lipids
2.4.2. UV-Visible Absorption Spectroscopy
2.4.3. HPLC
2.3. Preparation of Lipid Samples Containing Lipophilic Antioxidants
2.4. Cell Culture
2.5. Exposure of Cells to Partly Oxidized 22:6PC and/or Light
2.6. Cytotoxicity Assays
2.7. Apoptosis Assays
2.8. Isolation of Bovine Photoreceptor Outer Segments (POS)
2.9. Supplementation of Cells with POS with and Without Partly Oxidized 22:6PC
2.10. Flow Cytometry Analysis of Cell Fluorescence
2.11. Transmission Electron Microscopy (TEM)
2.12. Statistical Analysis
3. Results
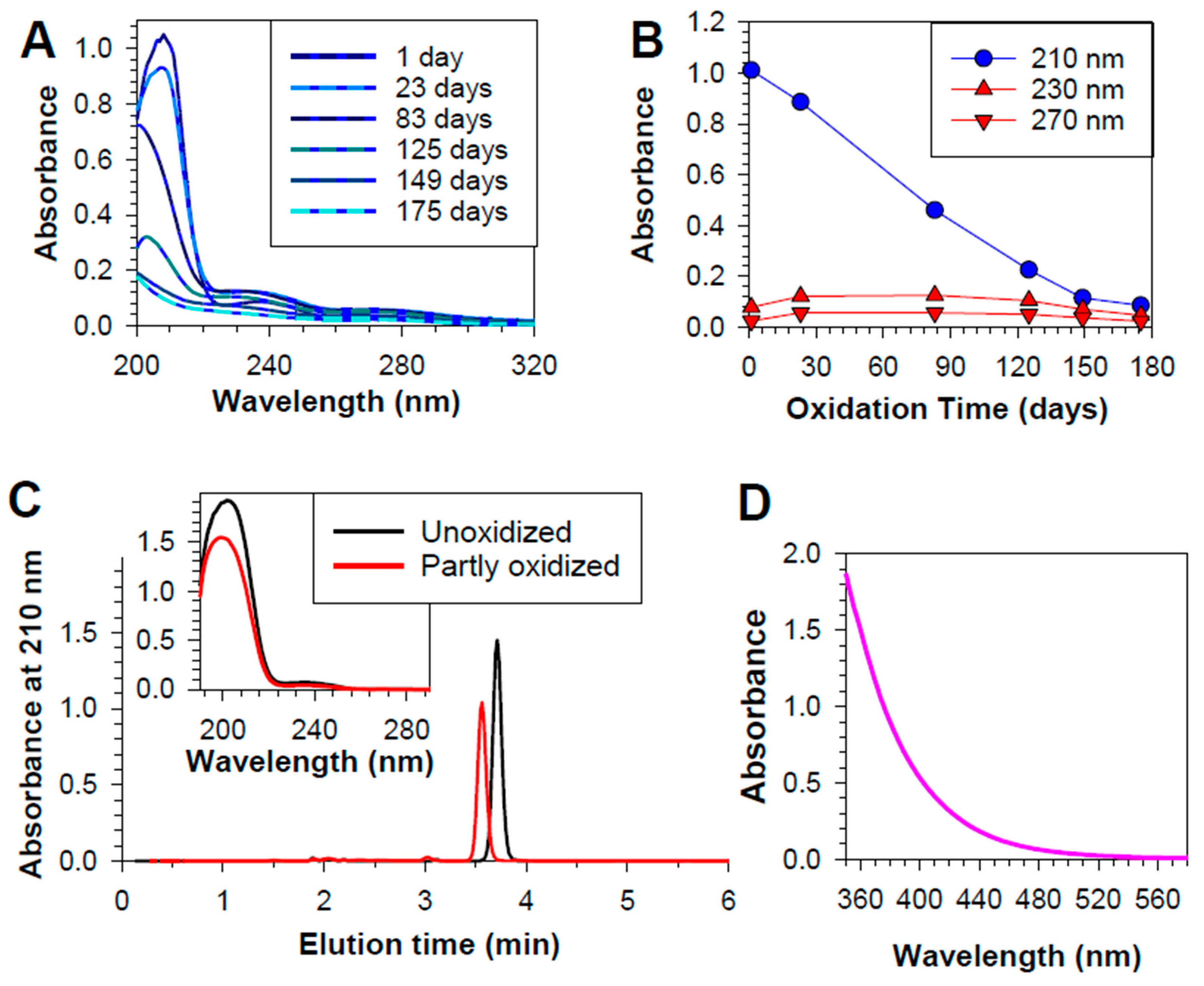
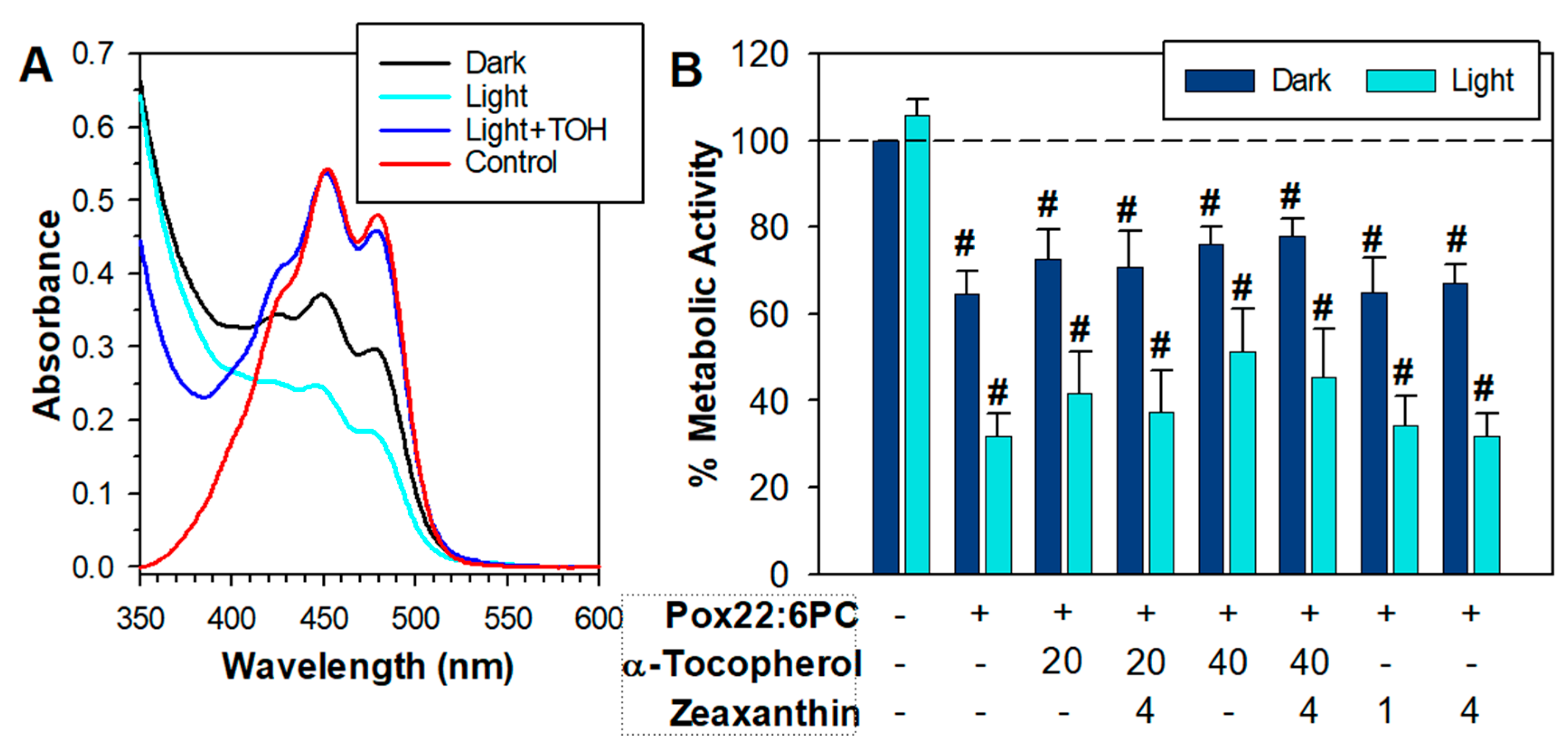
3.1. Partial Oxidation of 22:6PC
3.1. Effects of Partly Oxidized 22:6PC on RPE Cell Viability
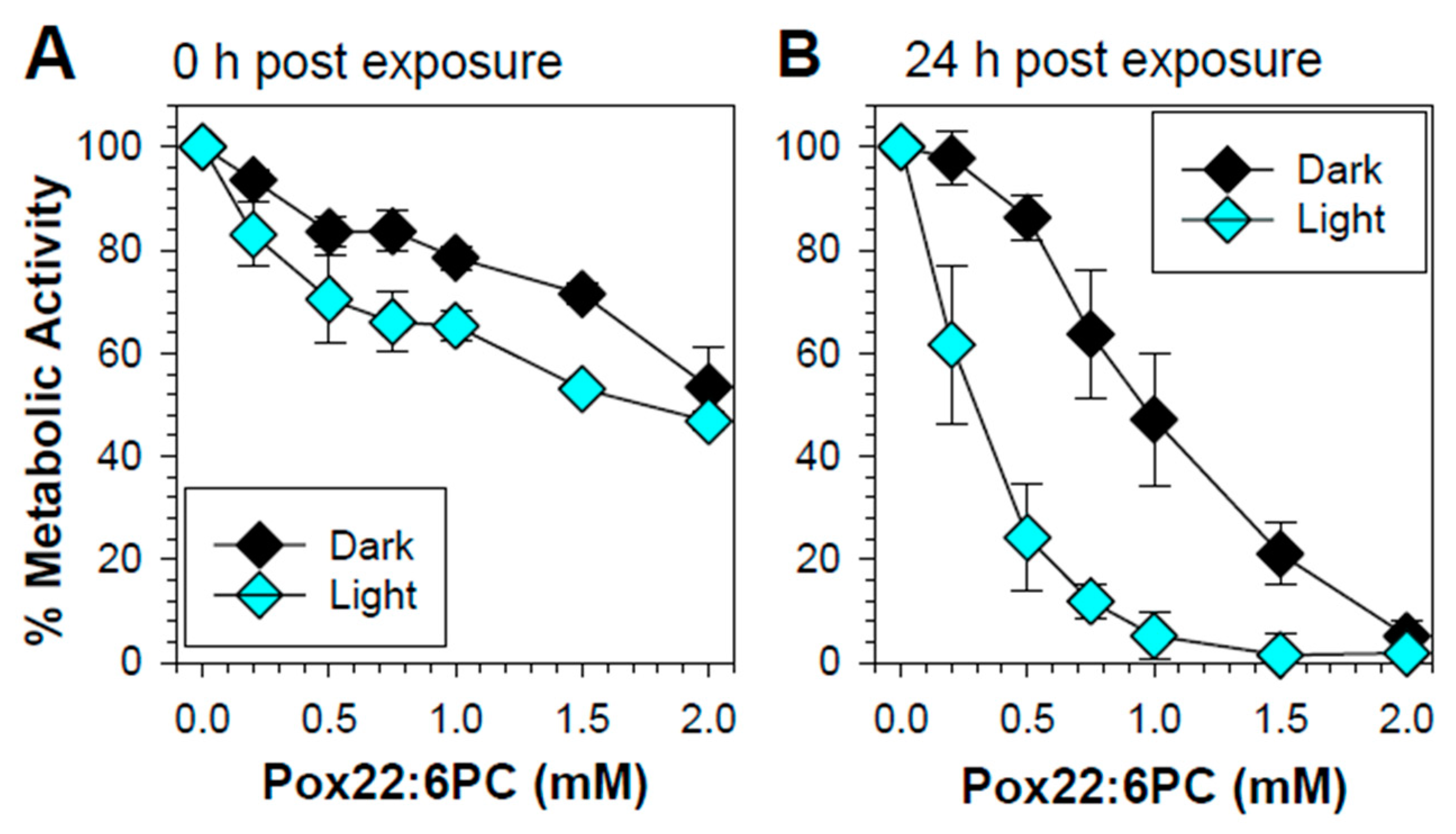
3.1.1. Effect of Partly Oxidized 22:6PC on RPE Cell Metabolic Activity
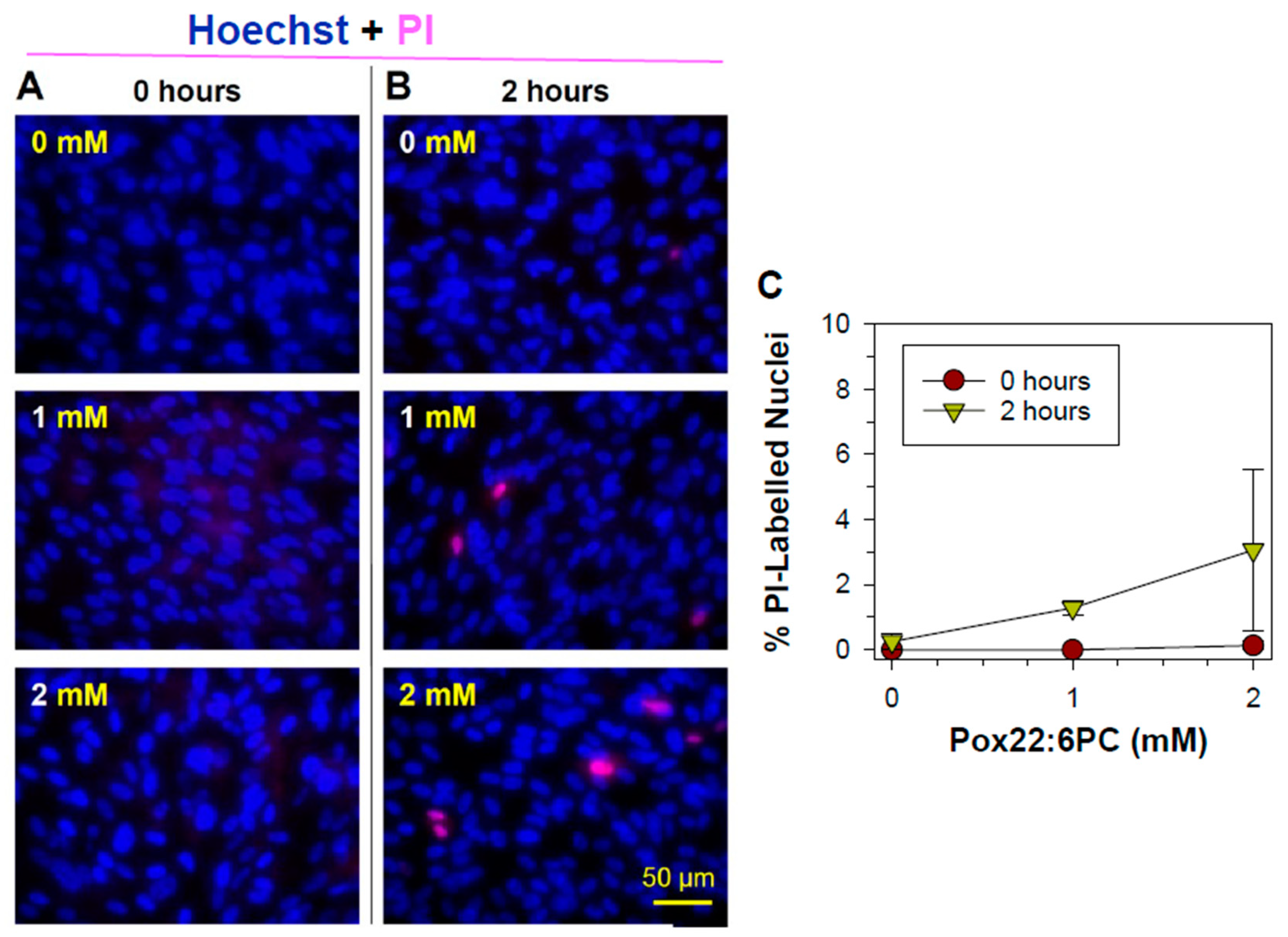
3.1.1. Effects of Oxidized Docosahexaenoate on RPE Plasma Membrane Integrity
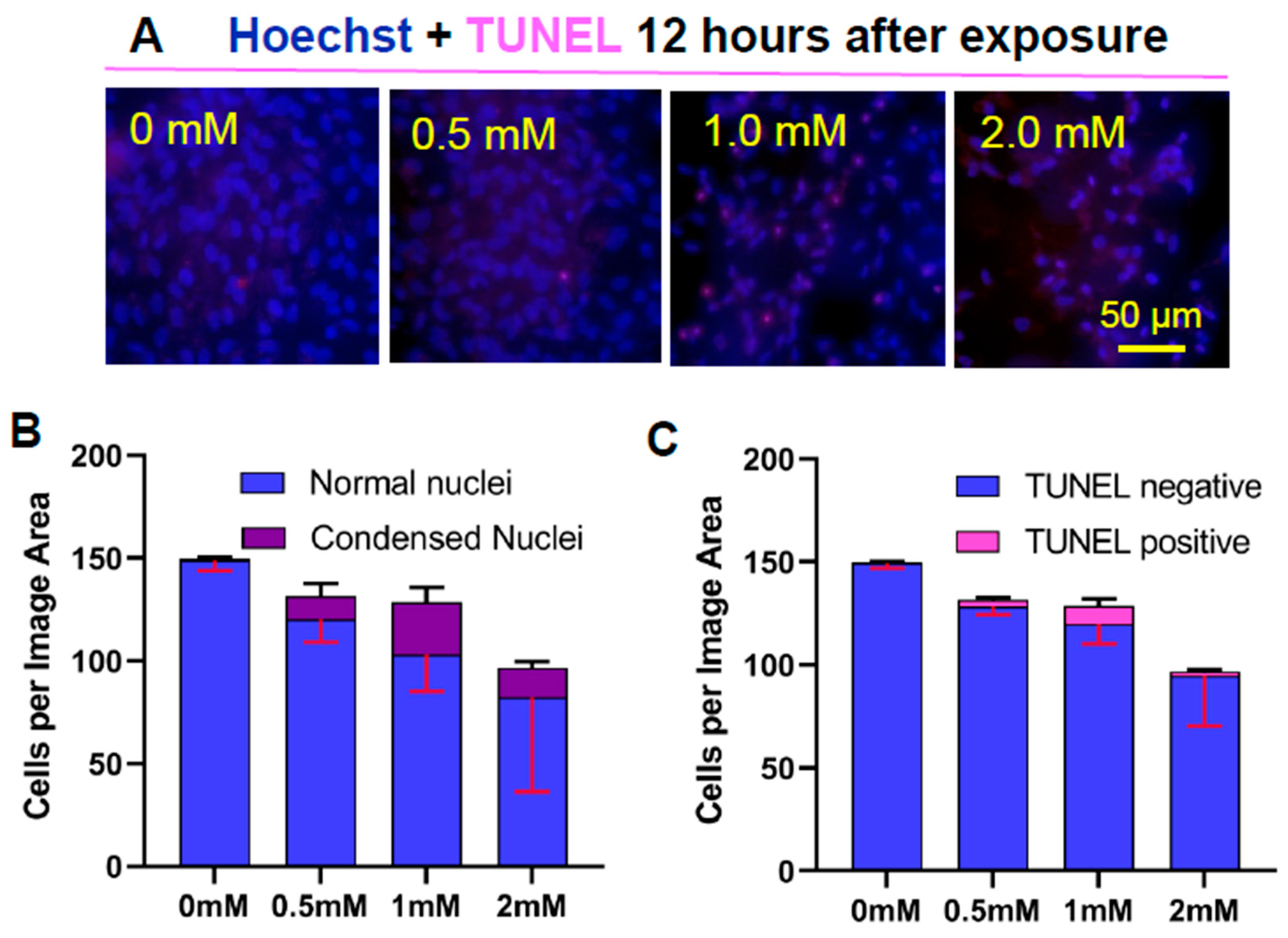
3.1.2. Apoptotic Changes: Nuclear Condensation and DNA Cleavage
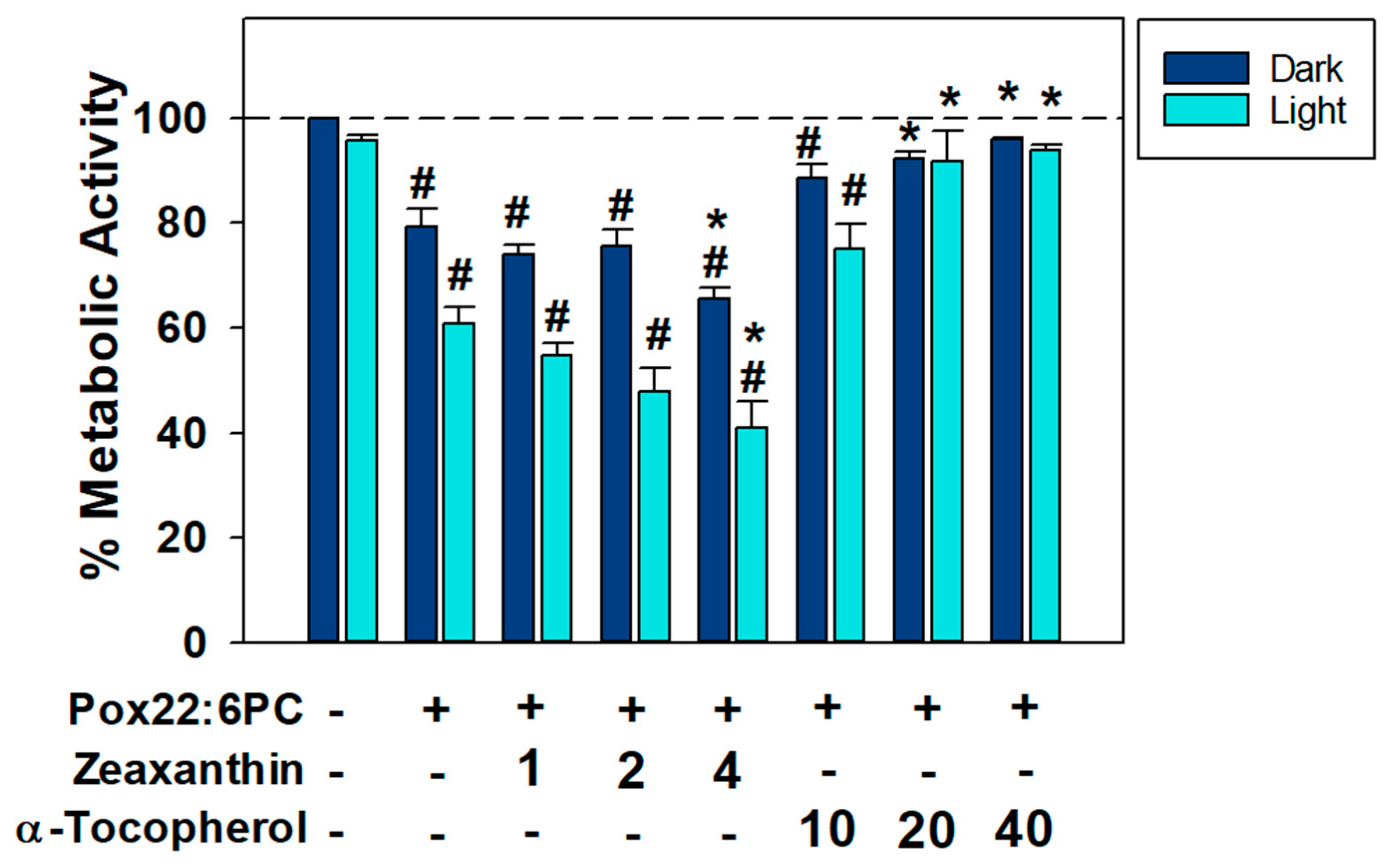
3.2. Effects of Antioxidants on the Toxicity of Partly Oxidized 22:6PC
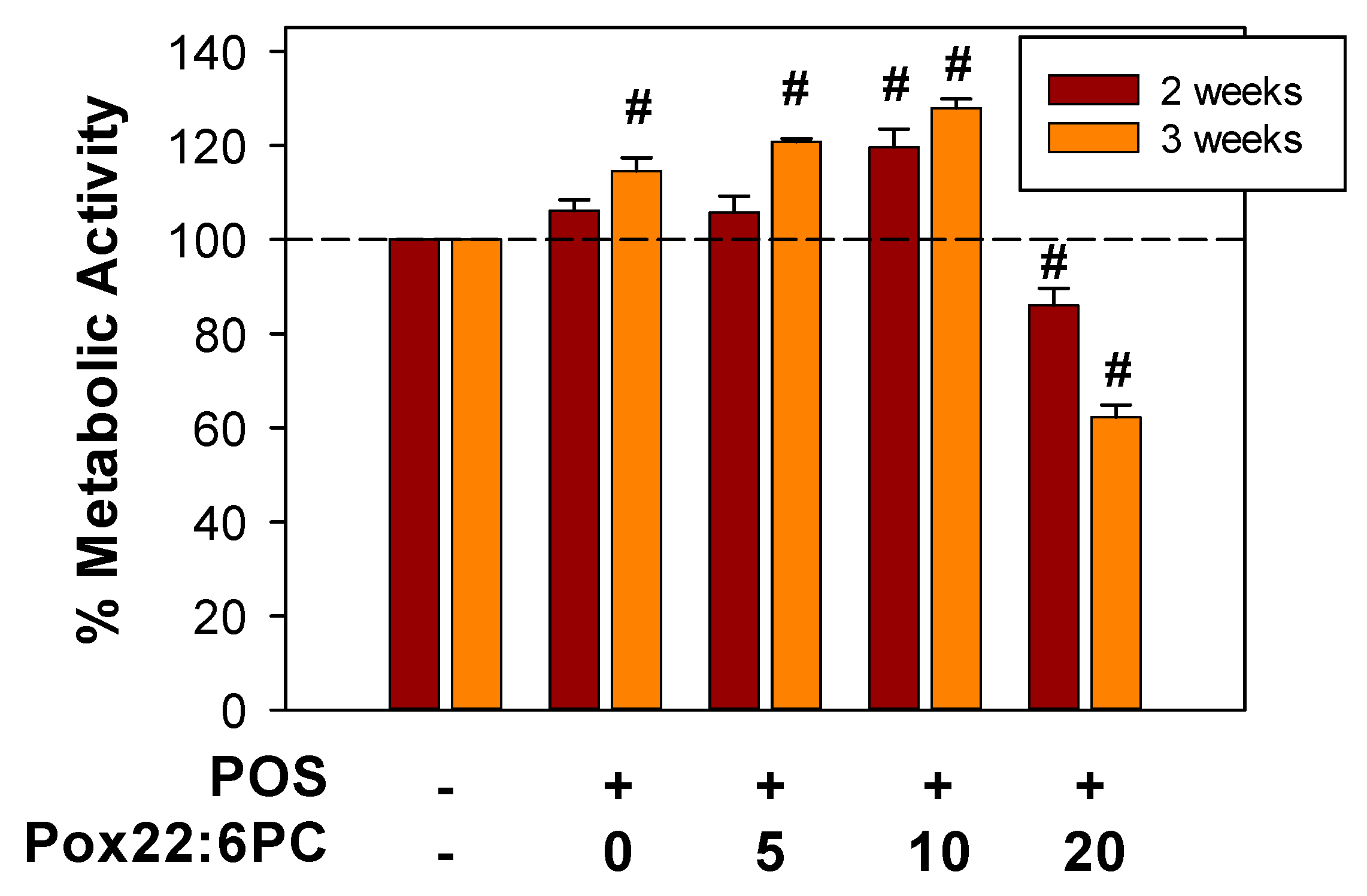
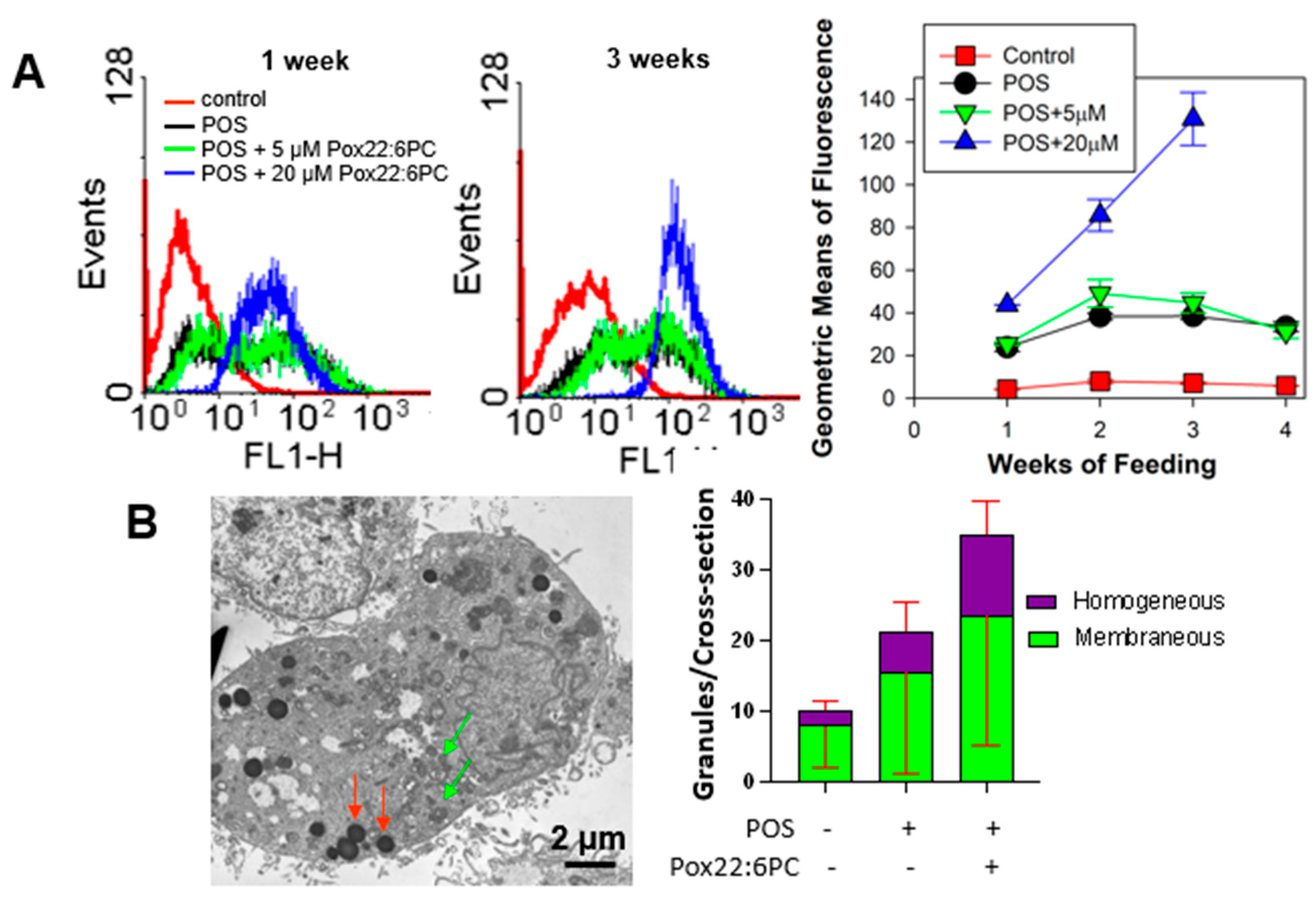
3.3. Effect of Supplementation of Cells with POS Enriched in Partly Oxidized 22:6PC on Metabolic Activity and Formation of Lipofuscin-like Granules
3.3.1. Effect of Supplementation of Cells with POS Enriched in Partly Oxidized 22:6PC on Metabolic Activity
3.3.2. Effect of Supplementation of Cells with POS Enriched in Partly Oxidized 22:6PC on the Formation of Lipofuscin-like Granules
4. Discussion
4.1(. Photo)toxicity of Partly Oxidized 22:6PC
4.2. Effects of Antioxidants on (Photo)toxicity of Partly Oxidized 22:6PC
4.3. Effects of Partly Oxidized 22:6PC on the Formation of Lipofuscin-like Deposits from POS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bazan, H.E.; Bazan, N.G.; Feeney-Burns, L.; Berman, E.R. Lipids in human lipofuscin-enriched subcellular fractions of two age populations. Comparison with rod outer segments and neural retina. Invest Ophthalmol Vis Sci 1990, 31, 1433–1443. [Google Scholar] [PubMed]
- Swinkels, D.; Baes, M. The essential role of docosahexaenoic acid and its derivatives for retinal integrity. Pharmacol Ther 2023, 247, 108440. [Google Scholar] [CrossRef] [PubMed]
- Longoni, B.; Demontis, G.C. Polyunsaturated Lipids in the Light-Exposed and Prooxidant Retinal Environment. Antioxidants 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, D.; Sander, C.L.; Tworak, A.; Gao, F.; Xu, Q.; Skowronska-Krawczyk, D. Dynamic lipid turnover in photoreceptors and retinal pigment epithelium throughout life. Prog Retin Eye Res 2022, 89, 101037. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Gragg, M.; Samuels, I.S.; Parmar, V.M.; Maeda, A.; Park, P.S. Effect of dietary docosahexaenoic acid on rhodopsin content and packing in photoreceptor cell membranes. Biochim Biophys Acta Biomembr 2018, 1860, 1403–1413. [Google Scholar] [CrossRef]
- Rice, D.S.; Calandria, J.M.; Gordon, W.C.; Jun, B.; Zhou, Y.; Gelfman, C.M.; Li, S.; Jin, M.; Knott, E.J.; Chang, B.; et al. Adiponectin receptor 1 conserves docosahexaenoic acid and promotes photoreceptor cell survival. Nat Commun 2015, 6, 6228. [Google Scholar] [CrossRef]
- Sanchez-Martin, M.J.; Ramon, E.; Torrent-Burgues, J.; Garriga, P. Improved conformational stability of the visual G protein-coupled receptor rhodopsin by specific interaction with docosahexaenoic acid phospholipid. Chembiochem 2013, 14, 639–644. [Google Scholar] [CrossRef]
- Mitchell, D.C.; Niu, S.L.; Litman, B.J. Quantifying the differential effects of DHA and DPA on the early events in visual signal transduction. Chem Phys Lipids 2012, 165, 393–400. [Google Scholar] [CrossRef]
- Dornstauder, B.; Suh, M.; Kuny, S.; Gaillard, F.; Macdonald, I.M.; Clandinin, M.T.; Sauve, Y. Dietary docosahexaenoic acid supplementation prevents age-related functional losses and A2E accumulation in the retina. Invest Ophthalmol Vis Sci 2012, 53, 2256–2265. [Google Scholar] [CrossRef]
- Calder, P.C. Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: Concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie 2020, 178, 105–123. [Google Scholar] [CrossRef]
- Christie, W.W.; Harwood, J.L. Oxidation of polyunsaturated fatty acids to produce lipid mediators. Essays Biochem 2020, 64, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G.; Molina, M.F.; Gordon, W.C. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu Rev Nutr 2011, 31, 321–351. [Google Scholar] [CrossRef] [PubMed]
- Marcheselli, V.L.; Mukherjee, P.K.; Arita, M.; Hong, S.; Antony, R.; Sheets, K.; Winkler, J.W.; Petasis, N.A.; Serhan, C.N.; Bazan, N.G. Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils. Prostaglandins Leukot Essent Fatty Acids 2010, 82, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Marcheselli, V.L.; de Rivero Vaccari, J.C.; Gordon, W.C.; Jackson, F.E.; Bazan, N.G. Photoreceptor outer segment phagocytosis attenuates oxidative stress-induced apoptosis with concomitant neuroprotectin D1 synthesis. Proc Natl Acad Sci U S A 2007, 104, 13158–13163. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Marcheselli, V.L.; Barreiro, S.; Hu, J.; Bok, D.; Bazan, N.G. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci U S A 2007, 104, 13152–13157. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Chawla, A.; Loayza, M.S.; Bazan, N.G. Docosanoids are multifunctional regulators of neural cell integrity and fate: significance in aging and disease. Prostaglandins Leukot Essent Fatty Acids 2007, 77, 233–238. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Marcheselli, V.L.; Serhan, C.N.; Bazan, N.G. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A 2004, 101, 8491–8496. [Google Scholar] [CrossRef]
- Schebb, N.H.; Kuhn, H.; Kahnt, A.S.; Rund, K.M.; O’Donnell, V.B.; Flamand, N.; Peters-Golden, M.; Jakobsson, P.J.; Weylandt, K.H.; Rohwer, N.; et al. Formation, Signaling and Occurrence of Specialized Pro-Resolving Lipid Mediators-What is the Evidence so far? Front Pharmacol 2022, 13, 838782. [Google Scholar] [CrossRef]
- Wu, J.; Cho, E.; Giovannucci, E.L.; Rosner, B.A.; Sastry, S.M.; Willett, W.C.; Schaumberg, D.A. Dietary Intakes of Eicosapentaenoic Acid and Docosahexaenoic Acid and Risk of Age-Related Macular Degeneration. Ophthalmology 2017, 124, 634–643. [Google Scholar] [CrossRef]
- Feher, J.; Kovacs, B.; Kovacs, I.; Schveoller, M.; Papale, A.; Balacco Gabrieli, C. Improvement of visual functions and fundus alterations in early age-related macular degeneration treated with a combination of acetyl-L-carnitine, n-3 fatty acids, and coenzyme Q10. Ophthalmologica 2005, 219, 154–166. [Google Scholar] [CrossRef]
- Querques, G.; Benlian, P.; Chanu, B.; Leveziel, N.; Coscas, G.; Soubrane, G.; Souied, E.H. DHA supplementation for late onset Stargardt disease: NAT-3 study. Clin Ophthalmol 2010, 4, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Group, A.R.; Chew, E.Y.; Clemons, T.; SanGiovanni, J.P.; Danis, R.; Domalpally, A.; McBee, W.; Sperduto, R.; Ferris, F.L. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012, 119, 2282–2289. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study 2 Research, G. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef]
- Souied, E.H.; Delcourt, C.; Querques, G.; Bassols, A.; Merle, B.; Zourdani, A.; Smith, T.; Benlian, P.; Nutritional, A.M.D.T.S.G. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: the Nutritional AMD Treatment 2 study. Ophthalmology 2013, 120, 1619–1631. [Google Scholar] [CrossRef]
- Merle, B.M.; Richard, F.; Benlian, P.; Puche, N.; Delcourt, C.; Souied, E.H. CFH Y402H and ARMS2 A69S Polymorphisms and Oral Supplementation with Docosahexaenoic Acid in Neovascular Age-Related Macular Degeneration Patients: The NAT2 Study. PLoS One 2015, 10, e0130816. [Google Scholar] [CrossRef]
- Querques, G.; Merle, B.M.; Pumariega, N.M.; Benlian, P.; Delcourt, C.; Zourdani, A.; Leisy, H.B.; Lee, M.D.; Smith, R.T.; Souied, E.H. Dynamic Drusen Remodelling in Participants of the Nutritional AMD Treatment-2 (NAT-2) Randomized Trial. PLoS One 2016, 11, e0149219. [Google Scholar] [CrossRef]
- Semeraro, F.; Gambicordi, E.; Cancarini, A.; Morescalchi, F.; Costagliola, C.; Russo, A. Treatment of exudative age-related macular degeneration with aflibercept combined with pranoprofen eye drops or nutraceutical support with omega-3: A randomized trial. Br J Clin Pharmacol 2019, 85, 908–913. [Google Scholar] [CrossRef]
- Piatti, A.; Croce, A.; Mazzacane, D.; Traina, G.; Ambrosino, L.; Boni, L.; Lisi, L.; Cascella, M.C.; Grunberger, A. Effect of 2-year nutritional supplementation on progression of age-related macular degeneration. Eur J Ophthalmol 2020, 30, 376–381. [Google Scholar] [CrossRef]
- Christen, W.G.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Chasman, D.I.; Lee, I.M.; Bubes, V.; Li, C.; Haubourg, M.; Schaumberg, D.A.; et al. Effect of Vitamin D and omega-3 Fatty Acid Supplementation on Risk of Age-Related Macular Degeneration: An Ancillary Study of the VITAL Randomized Clinical Trial. JAMA Ophthalmol 2020, 138, 1280–1289. [Google Scholar] [CrossRef]
- Schwartz, S.G.; Wang, X.; Chavis, P.; Kuriyan, A.E.; Abariga, S.A. Vitamin A and fish oils for preventing the progression of retinitis pigmentosa. Cochrane Database Syst Rev 2020, 6, CD008428. [Google Scholar] [CrossRef]
- Garcia-Layana, A.; Recalde, S.; Hernandez, M.; Abraldes, M.J.; Nascimento, J.; Hernandez-Galilea, E.; Olmedilla-Alonso, B.; Escobar-Barranco, J.J.; Zapata, M.A.; Silva, R.; et al. A Randomized Study of Nutritional Supplementation in Patients with Unilateral Wet Age-Related Macular Degeneration. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.D.L.; Agron, E.; Keane, P.A.; Domalpally, A.; Chew, E.Y.; Age-Related Eye Disease Study Research, G.; Age-Related Eye Disease Study 2 Research, G. Oral Antioxidant and Lutein/Zeaxanthin Supplements Slow Geographic Atrophy Progression to the Fovea in Age-Related Macular Degeneration. Ophthalmology 2024. [Google Scholar] [CrossRef] [PubMed]
- Rózanowska, M.B. Lipofuscin, Its Origin, Properties, and Contribution to Retinal Fluorescence as a Potential Biomarker of Oxidative Damage to the Retina. Antioxidants 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press Inc.: New York, NY, USA, 2007. [Google Scholar]
- He, X.; Hahn, P.; Iacovelli, J.; Wong, R.; King, C.; Bhisitkul, R.; Massaro-Giordano, M.; Dunaief, J.L. Iron homeostasis and toxicity in retinal degeneration. Prog Retin Eye Res 2007, 26, 649–673. [Google Scholar] [CrossRef] [PubMed]
- Hahn, P.; Milam, A.H.; Dunaief, J.L. Maculas affected by age-related macular degeneration contain increased chelatable iron in the retinal pigment epithelium and Bruch’s membrane. Arch Ophthalmol 2003, 121, 1099–1105. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem Biophys Res Commun 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Tanito, M.; Elliott, M.H.; Kotake, Y.; Anderson, R.E. Protein modifications by 4-hydroxynonenal and 4-hydroxyhexenal in light-exposed rat retina. Invest. Ophthalmol. Vis. Sci. 2005, 46, 3859–3868. [Google Scholar] [CrossRef]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A 2002, 99, 14682–14687. [Google Scholar] [CrossRef]
- Gu, X.; Meer, S.G.; Miyagi, M.; Rayborn, M.E.; Hollyfield, J.G.; Crabb, J.W.; Salomon, R.G. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem 2003, 278, 42027–42035. [Google Scholar] [CrossRef]
- Ng, K.P.; Gugiu, B.; Renganathan, K.; Davies, M.W.; Gu, X.; Crabb, J.S.; Kim, S.R.; Rozanowska, M.B.; Bonilha, V.L.; Rayborn, M.E.; et al. Retinal pigment epithelium lipofuscin proteomics. Mol Cell Proteomics 2008, 7, 1397–1405. [Google Scholar] [CrossRef]
- Handa, J.T.; Cano, M.; Wang, L.; Datta, S.; Liu, T. Lipids, oxidized lipids, oxidation-specific epitopes, and Age-related Macular Degeneration. Biochim Biophys Acta Mol Cell Biol Lipids 2017, 1862, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res 2017, 60, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Linetsky, M.; Guo, J.; Udeigwe, E.; Ma, D.; Chamberlain, A.S.; Yu, A.O.; Solovyova, K.; Edgar, E.; Salomon, R.G. 4-Hydroxy-7-oxo-5-heptenoic acid (HOHA) lactone induces apoptosis in retinal pigment epithelial cells. Free Radical Biology and Medicine 2020, 152, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.S.; Linetsky, M.; Li, H.; Ayyash, N.; Gardella, A.; Salomon, R.G. 4-Hydroxy-7-oxo-5-heptenoic acid lactone can induce mitochondrial dysfunction in retinal pigmented epithelial cells. Free Radic Biol Med 2020, 160, 719–733. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Linetsky, M.; Gu, X.; Ayyash, N.; Gardella, A.; Salomon, R.G. Light-induced generation and toxicity of docosahexaenoate-derived oxidation products in retinal pigmented epithelial cells. Exp Eye Res 2019, 181, 325–345. [Google Scholar] [CrossRef]
- Linetsky, M.; Bondelid, K.S.; Losovskiy, S.; Gabyak, V.; Rullo, M.J.; Stiadle, T.I.; Munjapara, V.; Saxena, P.; Ma, D.M.; Cheng, Y.S.; et al. 4-Hydroxy-7-oxo-5-heptenoic Acid Lactone Is a Potent Inducer of the Complement Pathway in Human Retinal Pigmented Epithelial Cells. Chem. Res. Toxicol. 2018, 31, 666–679. [Google Scholar] [CrossRef]
- Wang, H.; Linetsky, M.; Guo, J.H.; Yu, A.O.; Salomon, R.G. Metabolism of 4-Hydroxy-7-oxo-5-heptenoic Acid (HOHA) Lactone by Retinal Pigmented, Epithelial Cells. Chem. Res. Toxicol. 2016, 29, 1198–1210. [Google Scholar] [CrossRef]
- Guo, J.H.; Linetsky, M.; Yu, A.O.; Zhang, L.; Howell, S.J.; Folkwein, H.J.; Wang, H.; Salomon, R.G. 4-Hydroxy-7-oxo-5-heptenoic Acid Lactone Induces Angiogenesis through Several Different Molecular Pathways. Chem. Res. Toxicol. 2016, 29, 2125–2135. [Google Scholar] [CrossRef]
- Wang, H.; Linetsky, M.; Guo, J.H.; Choi, J.; Hong, L.; Chamberlain, A.S.; Howell, S.J.; Howes, A.M.; Salomon, R.G. 4-Hydroxy-7-oxo-5-heptenoic Acid (HOHA) Lactone is a Biologically Active Precursor for the Generation of 2-(ω-Carboxyethyl)pyrrole (CEP) Derivatives of Proteins and Ethanolamine Phospholipids. Chem. Res. Toxicol. 2015, 28, 967–977. [Google Scholar] [CrossRef]
- Wang, H.; Guo, J.H.; West, X.X.Z.; Bid, H.K.; Lu, L.; Hong, L.; Jang, G.F.; Zhang, L.; Crabb, J.W.; Linetsky, M.; et al. Detection and Biological Activities of Carboxyethylpyrrole Ethanolamine Phospholipids (CEP-EPs). Chem. Res. Toxicol. 2014, 27, 2015–2022. [Google Scholar] [CrossRef]
- Rózanowska, M.B.; Pawlak, A.; Rózanowski, B. Products of ˙docosahexaenoate oxidation as contributors to photosensitising properties of retinal lipofuscin. Int. J. Mol. Sci. 2021, 22, 3525. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Rozanowska, M.; Pawlak, A.; Rozanowski, B.; Skumatz, C.; Zareba, M.; Boulton, M.E.; Burke, J.M.; Sarna, T.; Simon, J.D. Age-related changes in the photoreactivity of retinal lipofuscin granules: role of chloroform-insoluble components. Invest Ophthalmol Vis Sci 2004, 45, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- DeMar, J.C., Jr.; Wensel, T.G.; Anderson, R.E. Biosynthesis of the unsaturated 14-carbon fatty acids found on the N termini of photoreceptor-specific proteins. J. Biol. Chem. 1996, 271, 5007–5016. [Google Scholar] [CrossRef]
- Kim, R.S.; LaBella, F.S. Comparison of analytical methods for monitoring autoxidation profiles of authentic lipids. J. Lipid Res. 1987, 28, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Wrona, M.; Rozanowska, M.; Sarna, T. Zeaxanthin in combination with ascorbic acid or alpha-tocopherol protects ARPE-19 cells against photosensitized peroxidation of lipids. Free Radic. Biol. Med. 2004, 36, 1094–1101. [Google Scholar] [CrossRef]
- Dunn, K.C.; Aotaki-Keen, A.E.; Putkey, F.R.; Hjelmeland, L.M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res 1996, 62, 155–169. [Google Scholar] [CrossRef]
- Rozanowska, M.B.; Rozanowski, B. Photodegradation of Lipofuscin in Suspension and in ARPE-19 Cells and the Similarity of Fluorescence of the Photodegradation Product with Oxidized Docosahexaenoate. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Rozanowska, M.B.; Czuba-Pelech, B.; Rozanowski, B. Is There an Optimal Combination of AREDS2 Antioxidants Zeaxanthin, Vitamin E and Vitamin C on Light-Induced Toxicity of Vitamin A Aldehyde to the Retina? Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Rozanowska, M.; Handzel, K.; Boulton, M.E.; Rozanowski, B. Cytotoxicity of all-trans-retinal increases upon photodegradation. Photochem Photobiol 2012, 88, 1362–1372. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival - application to proliferation and cytotoxicity assays. Journal of Immunological Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Gavrieli, Y.; Sherman, Y.; Ben-Sasson, S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992, 119, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Papermaster, D.S. Preparation of retinal rod outer segments. Methods in Enzymology 1982, 81, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.; Marshall, J. Effects of increasing numbers of phagocytic inclusions on human retinal pigment epithelial cells in culture: a model for aging. Br J Ophthalmol 1986, 70, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Recknagel, R.O.; Glende, E.A. Spectrophotometric detection of lipid conjugated dienes. Methods in Enzymology 1984, 105, 331–337. [Google Scholar] [CrossRef]
- Pryor, W.A.; Castle, L. Chemical methods for the detection of lipid hydroperoxides. Methods in Enzymology 1984, 105, 293–299. [Google Scholar] [CrossRef]
- Corongiu, F.P.; Banni, S.; Dess, M.A. Conjugated dienes detected in tissue lipid extracts by second derivative spectrophotometry. Free Radical Biology and Medicine 1989, 7, 183–186. [Google Scholar] [CrossRef]
- Butovich, I.A.; Lukyanova, S.M.; Bachmann, C. Dihydroxydocosahexaenoic acids of the neuroprotectin D family: synthesis, structure, and inhibition of human 5-lipoxygenase. J. Lipid Res. 2006, 47, 2462–2474. [Google Scholar] [CrossRef]
- Butovich, I.A. On the structure and synthesis of neuroprotectin D1, a novel anti-inflammatory compound of the docosahexaenoic acid family. J. Lipid Res. 2005, 46, 2311–2314. [Google Scholar] [CrossRef]
- Knight, J.; Taylor, G.W.; Wright, P.; Clare, A.S.; Rowley, A.F. Eicosanoid biosynthesis in an advanced deuterostomate invertebrate, the sea squirt (Ciona intestinalis). Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids 1999, 1436, 467–478. [Google Scholar] [CrossRef]
- Kuklev, D.V.; Smith, W.L. Synthesis of long chain n-3 and n-6 fatty acids having a photoactive conjugated tetraene group. Chemistry and Physics of Lipids 2004, 130, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Artigas, J.M.; Felipe, A.; Navea, A.; Fandino, A.; Artigas, C. Spectral transmission of the human crystalline lens in adult and elderly persons: color and total transmission of visible light. Invest Ophthalmol Vis Sci 2012, 53, 4076–4084. [Google Scholar] [CrossRef] [PubMed]
- Rozanowska, M.; Edge, R.; Land, E.J.; Navaratnam, S.; Sarna, T.; Truscott, T.G. Scavenging of Cation Radicals of the Visual Cycle Retinoids by Lutein, Zeaxanthin, Taurine, and Melanin. Int J Mol Sci 2023, 25. [Google Scholar] [CrossRef] [PubMed]
- Rozanowska, M.B.; Czuba-Pelech, B.; Landrum, J.T.; Rozanowski, B. Comparison of Antioxidant Properties of Dehydrolutein with Lutein and Zeaxanthin, and their Effects on Cultured Retinal Pigment Epithelial Cells. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Wrona, M.; Korytowski, W.; Rozanowska, M.; Sarna, T.; Truscott, T.G. Cooperation of antioxidants in protection against photosensitized oxidation. Free Radic Biol Med 2003, 35, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Rozanowska, M.; Bakker, L.; Boulton, M.E.; Rozanowski, B. Concentration dependence of vitamin C in combinations with vitamin E and zeaxanthin on light-induced toxicity to retinal pigment epithelial cells. Photochem Photobiol 2012, 88, 1408–1417. [Google Scholar] [CrossRef]
- Tsung, Y.C.; Chung, C.Y.; Wan, H.C.; Chang, Y.Y.; Shih, P.C.; Hsu, H.S.; Kao, M.C.; Huang, C.J. Dimethyl Sulfoxide Attenuates Acute Lung Injury Induced by Hemorrhagic Shock/Resuscitation in Rats. Inflammation 2017, 40, 555–565. [Google Scholar] [CrossRef]
- Różanowska, M.; Różanowski, B. Uptake and photoprotection in cultured RPE cells. In Carotenoids: Physical, Chemical, and Biological Functions and Properties, Landrum, J.T., Ed.; CRC Press: 2010; pp. 309–364.
- Kaemmerer, E.; Schutt, F.; Krohne, T.U.; Holz, F.G.; Kopitz, J. Effects of lipid peroxidation-related protein modifications on RPE lysosomal functions and POS phagocytosis. Invest Ophthalmol Vis Sci 2007, 48, 1342–1347. [Google Scholar] [CrossRef]
- Nilsson, S.E.; Sundelin, S.P.; Wihlmark, U.; Brunk, U.T. Aging of cultured retinal pigment epithelial cells: oxidative reactions, lipofuscin formation and blue light damage. Doc Ophthalmol 2003, 106, 13–16. [Google Scholar] [CrossRef]
- Wihlmark, U.; Wrigstad, A.; Roberg, K.; Brunk, U.T.; Nilsson, S.E. Lipofuscin formation in cultured retinal pigment epithelial cells exposed to photoreceptor outer segment material under different oxygen concentrations. APMIS 1996, 104, 265–271. [Google Scholar] [CrossRef]
- Wihlmark, U.; Wrigstad, A.; Roberg, K.; Brunk, U.T.; Nilsson, S.E. Formation of lipofuscin in cultured retinal pigment epithelial cells exposed to pre-oxidized photoreceptor outer segments. APMIS 1996, 104, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, D.; Wu, Y.; Ji, B. Docosahexaenoic acid aggravates photooxidative damage in retinal pigment epithelial cells via lipid peroxidation. J Photochem Photobiol B 2014, 140, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Neiteler, A.; Palakkan, A.A.; Gallagher, K.M.; Ross, J.A. Oxidative stress and docosahexaenoic acid injury lead to increased necroptosis and ferroptosis in retinal pigment epithelium. Sci Rep 2023, 13, 21143. [Google Scholar] [CrossRef] [PubMed]
- Rozanowska, M.; Sarna, T. Light-induced damage to the retina: role of rhodopsin chromophore revisited. Photochem Photobiol 2005, 81, 1305–1330. [Google Scholar] [CrossRef]
- Stubbs, G.W.; Litman, B.J. Effect of alterations in the amphipathic microenvironment on the conformational stability of bovine opsin. 1. Mechanism of solubilization of disk membranes by the nonionic detergent, octyl glucoside. Biochemistry 1978, 17, 215–219. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Ma, X.; Bai, X. Maresin-1 inhibits high glucose induced ferroptosis in ARPE-19 cells by activating the Nrf2/HO-1/GPX4 pathway. BMC Ophthalmol 2023, 23, 368. [Google Scholar] [CrossRef]
- Calandria, J.M.; Do, K.V.; Kala-Bhattacharjee, S.; Obenaus, A.; Belayev, L.; Bazan, N.G. cRel and Wnt5a/Frizzled 5 Receptor-Mediated Inflammatory Regulation Reveal Novel Neuroprotectin D1 Targets for Neuroprotection. Cell Mol Neurobiol 2023, 43, 1077–1096. [Google Scholar] [CrossRef]
- Calandria, J.M.; Do, K.V.; Kala-Bhattacharjee, S.; Obenaus, A.; Belayev, L.; Bazan, N.G. Correction to: cRel and Wnt5a/Frizzled 5 Receptor-Mediated Inflammatory Regulation Reveal Novel Neuroprotectin D1 Targets for Neuroprotection. Cell Mol Neurobiol 2023, 43, 1097–1103. [Google Scholar] [CrossRef]
- Antony, R.; Lukiw, W.J.; Bazan, N.G. Neuroprotectin D1 induces dephosphorylation of Bcl-xL in a PP2A-dependent manner during oxidative stress and promotes retinal pigment epithelial cell survival. J Biol Chem 2010, 285, 18301–18308. [Google Scholar] [CrossRef]
- Calandria, J.M.; Bazan, N.G. Neuroprotectin D1 Modulates the Induction of Pro-Inflammatory Signaling and Promotes Retinal Pigment Epithelial Cell Survival DuringOxidative Stress. Adv Exp Med Biol 2010, 664, 663–670. [Google Scholar] [CrossRef]
- Calandria, J.M.; Marcheselli, V.L.; Mukherjee, P.K.; Uddin, J.; Winkler, J.W.; Petasis, N.A.; Bazan, N.G. Selective survival rescue in 15-lipoxygenase-1-deficient retinal pigment epithelial cells by the novel docosahexaenoic acid-derived mediator, neuroprotectin D1. J Biol Chem 2009, 284, 17877–17882. [Google Scholar] [CrossRef] [PubMed]
- Simón, M.V.; Agnolazza, D.L.; German, O.L.; Garelli, A.; Politi, L.E.; Agbaga, M.P.; Anderson, R.E.; Rotstein, N.P. Synthesis of docosahexaenoic acid from eicosapentaenoic acid in retina neurons protects photoreceptors from oxidative stress. J. Neurochem. 2016, 136, 931–946. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Brush, R.S.; Elliott, M.H.; Wicker, L.D.; Henry, K.R.; Anderson, R.E. High levels of retinal membrane docosahexaenoic acid increase susceptibility to stress-induced degeneration. J Lipid Res 2009, 50, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Finnemann, S.C.; Febbraio, M.; Shan, L.; Annangudi, S.P.; Podrez, E.A.; Hoppe, G.; Darrow, R.; Organisciak, D.T.; Salomon, R.G.; et al. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium: a potential mechanism for modulating outer segment phagocytosis under oxidant stress conditions. J Biol Chem 2006, 281, 4222–4230. [Google Scholar] [CrossRef]
- Organisciak, D.T.; Darrow, R.M.; Jiang, Y.L.; Blanks, J.C. Retinal light damage in rats with altered levels of rod outer segment docosahexaenoate. Invest Ophthalmol Vis Sci 1996, 37, 2243–2257. [Google Scholar]
- Orban, T.; Johnson, W.M.; Dong, Z.; Maeda, T.; Maeda, A.; Sakai, T.; Tsuneoka, H.; Mieyal, J.J.; Palczewski, K. Serum levels of lipid metabolites in age-related macular degeneration. FASEB J 2015, 29, 4579–4588. [Google Scholar] [CrossRef]
- Vidal, E.; Jun, B.; Gordon, W.C.; Maire, M.A.; Martine, L.; Gregoire, S.; Khoury, S.; Cabaret, S.; Berdeaux, O.; Acar, N.; et al. Bioavailability and spatial distribution of fatty acids in the rat retina after dietary omega-3 supplementation. J Lipid Res 2020, 61, 1733–1746. [Google Scholar] [CrossRef]
- Sugasini, D.; Yalagala, P.C.R.; Subbaiah, P.V. Correction: Sugasini et al. Efficient Enrichment of Retinal DHA with Dietary Lysophosphatidylcholine-DHA: Potential Application for Retinopathies. Nutrients 2020, 12, 3114. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Sugasini, D.; Yalagala, P.C.R.; Subbaiah, P.V. Efficient Enrichment of Retinal DHA with Dietary Lysophosphatidylcholine-DHA: Potential Application for Retinopathies. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- Schnebelen-Berthier, C.; Acar, N.; Pouillart, P.; Thabuis, C.; Rodriguez, B.; Depeint, F.; Clerc, E.; Mathiaud, A.; Bourdillon, A.; Baert, B.; et al. Incorporation of lutein and docosahexaenoic acid from dietary microalgae into the retina in quail. Int J Food Sci Nutr 2015, 66, 222–229. [Google Scholar] [CrossRef]
- Ramchani-Ben Othman, K.; Cercy, C.; Amri, M.; Doly, M.; Ranchon-Cole, I. Dietary Supplement Enriched in Antioxidants and Omega-3 Protects from Progressive Light-Induced Retinal Degeneration. Plos One 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Schnebelen, C.; Gregoire, S.; Pasquis, B.; Joffre, C.; Creuzot-Garcher, C.P.; Bron, A.M.; Bretillon, L.; Acar, N. Dietary n-3 and n-6 PUFA enhance DHA incorporation in retinal phospholipids without affecting PGE(1) and PGE (2) levels. Lipids 2009, 44, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Schnebelen, C.; Viau, S.; Gregoire, S.; Joffre, C.; Creuzot-Garcher, C.P.; Bron, A.M.; Bretillon, L.; Acar, N. Nutrition for the eye: different susceptibility of the retina and the lacrimal gland to dietary omega-6 and omega-3 polyunsaturated fatty acid incorporation. Ophthalmic Res 2009, 41, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Sekine, S.; Saito, M. Effect of docosahexaenoic acid and ascorbate on peroxidation of retinal membranes of ODS rats. Free Radic Res 2003, 37, 419–424. [Google Scholar] [CrossRef]
- Prokopiou, E.; Kolovos, P.; Kalogerou, M.; Neokleous, A.; Nicolaou, O.; Sokratous, K.; Kyriacou, K.; Georgiou, T. Omega-3 Fatty Acids Supplementation: Therapeutic Potential in a Mouse Model of Stargardt Disease. Invest Ophthalmol Vis Sci 2018, 59, 2757–2767. [Google Scholar] [CrossRef]
- Prokopiou, E.; Kolovos, P.; Georgiou, C.; Kalogerou, M.; Potamiti, L.; Sokratous, K.; Kyriacou, K.; Georgiou, T. Omega-3 fatty acids supplementation protects the retina from age-associated degeneration in aged C57BL/6J mice. BMJ Open Ophthalmol 2019, 4, e000326. [Google Scholar] [CrossRef]
- Sun, G.Y.; Appenteng, M.K.; Li, R.; Woo, T.; Yang, B.; Qin, C.; Pan, M.; Cieslik, M.; Cui, J.; Fritsche, K.L.; et al. Docosahexaenoic Acid (DHA) Supplementation Alters Phospholipid Species and Lipid Peroxidation Products in Adult Mouse Brain, Heart, and Plasma. Neuromolecular Med 2021, 23, 118–129. [Google Scholar] [CrossRef]
- Miralles-Perez, B.; Mendez, L.; Nogues, M.R.; Sanchez-Martos, V.; Fortuno-Mar, A.; Ramos-Romero, S.; Hereu, M.; Medina, I.; Romeu, M. Effects of a Fish Oil Rich in Docosahexaenoic Acid on Cardiometabolic Risk Factors and Oxidative Stress in Healthy Rats. Mar Drugs 2021, 19. [Google Scholar] [CrossRef]
- Starcevic, K.; Filipovic, N.; Galan, A.; Micek, V.; Gudan Kurilj, A.; Masek, T. Hepatic Lipogenesis and Brain Fatty Acid Profile in Response to Different Dietary n6/n3 Ratios and DHA/EPA Supplementation in Streptozotocin Treated Rats. Mol Nutr Food Res 2018, 62, e1701007. [Google Scholar] [CrossRef]
- Allard, J.P.; Kurian, R.; Aghdassi, E.; Muggli, R.; Royall, D. Lipid peroxidation during n-3 fatty acid and vitamin E supplementation in humans. Lipids 1997, 32, 535–541. [Google Scholar] [CrossRef]
- Zaloga, G.P. Narrative Review of n-3 Polyunsaturated Fatty Acid Supplementation upon Immune Functions, Resolution Molecules and Lipid Peroxidation. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Mane, S.; Kumari, P.; Singh, A.; Taneja, N.K.; Chopra, R. Amelioration for oxidative stability and bioavailability of N-3 PUFA enriched microalgae oil: an overview. Crit Rev Food Sci Nutr 2024, 64, 2579–2600. [Google Scholar] [CrossRef] [PubMed]
- Hands, J.M.; Anderson, M.L.; Cooperman, T.; Frame, L.A. A Multi-Year Rancidity Analysis of 72 Marine and Microalgal Oil Omega-3 Supplements. J Diet Suppl 2024, 21, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Jairoun, A.A.; Shahwan, M.; Zyoud, S.H. Fish oil supplements, oxidative status, and compliance behaviour: Regulatory challenges and opportunities. PLoS One 2020, 15, e0244688. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Bannenberg, G.; Rice, H.B.; Schutt, E.; MacKay, D. Oxidation in EPA- and DHA-rich oils: An overview. Lipid Technol. 2016, 28, 55–59. [Google Scholar] [CrossRef]
- Beltrame, G.; Ahonen, E.; Damerau, A.; Gudmundsson, H.G.; Haraldsson, G.G.; Linderborg, K.M. Lipid Structure Influences the Digestion and Oxidation Behavior of Docosahexaenoic and Eicosapentaenoic Acids in the Simulated Digestion System. J Agric Food Chem 2023, 71, 10087–10096. [Google Scholar] [CrossRef]
- Floros, S.; Toskas, A.; Pasidi, E.; Vareltzis, P. Bioaccessibility and Oxidative Stability of Omega-3 Fatty Acids in Supplements, Sardines and Enriched Eggs Studied Using a Static In Vitro Gastrointestinal Model. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; Agron, E.; Domalpally, A.; Keenan, T.D.L.; Vitale, S.; Weber, C.; Smith, D.C.; Christen, W.; Group, A.R. Long-term Outcomes of Adding Lutein/Zeaxanthin and omega-3 Fatty Acids to the AREDS Supplements on Age-Related Macular Degeneration Progression: AREDS2 Report 28. JAMA Ophthalmol 2022, 140, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Sliney, D.H. Eye protective techniques for bright light. Ophthalmology 1983, 90, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Rozanowska, M.; Rozanowski, B.; Boulton, M. Photobiology of the retina: Light damage to the retina. http://photobiology.info/Rozanowska.html. In Photobiological Sciences Online: http://www.photobiology.info; Smith, K.C., Ed.; American Society for Photobiology: Herndon, VA, USA, 2009. [Google Scholar]
- Ham, W.T., Jr.; Ruffolo, J.J., Jr.; Mueller, H.A.; Clarke, A.M.; Moon, M.E. Histologic analysis of photochemical lesions produced in rhesus retina by short-wave-length light. Invest Ophthalmol Vis Sci 1978, 17, 1029–1035. [Google Scholar]
- Buettner, G.R. The pecking order of free radicals and antioxidants: lipid peroxidation, -tocopherol, and ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, A.; Ishikura, M.; Konishi, K.; Nagaoka, S.; Mukai, K. Kinetic study of the prooxidant effect of alpha-tocopherol. Hydrogen abstraction from lipids by alpha-tocopheroxyl radical. Lipids 2009, 44, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Lamberson, C.R.; Xu, L.; Muchalski, H.; Montenegro-Burke, J.R.; Shmanai, V.V.; Bekish, A.V.; McLean, J.A.; Clarke, C.F.; Shchepinov, M.S.; Porter, N.A. Unusual kinetic isotope effects of deuterium reinforced polyunsaturated fatty acids in tocopherol-mediated free radical chain oxidations. J Am Chem Soc 2014, 136, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Firsov, A.M.; Franco, M.S.F.; Chistyakov, D.V.; Goriainov, S.V.; Sergeeva, M.G.; Kotova, E.A.; Fomich, M.A.; Bekish, A.V.; Sharko, O.L.; Shmanai, V.V.; et al. Deuterated polyunsaturated fatty acids inhibit photoirradiation-induced lipid peroxidation in lipid bilayers. J Photochem Photobiol B 2022, 229, 112425. [Google Scholar] [CrossRef]
- Jerzykiewicz, M.; Cwielag-Piasecka, I.; Witwicki, M.; Jezierski, A. a-Tocopherol impact on oxy-radical induced free radical decomposition of DMSO: Spin trapping EPR and theoretical studies. Chemical Physics 2011, 383, 27–34. [Google Scholar] [CrossRef]
- Edge, R.; Truscott, T.G. Singlet Oxygen and Free Radical Reactions of Retinoids and Carotenoids-A Review. Antioxidants (Basel) 2018, 7. [Google Scholar] [CrossRef]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms-A Comprehensive Review. Antioxidants (Basel) 2020, 9. [Google Scholar] [CrossRef]
- Boehm, F.; Edge, R.; Truscott, T.G. Anti- and pro-oxidative mechanisms comparing the macular carotenoids zeaxanthin and lutein with other dietary carotenoids - a singlet oxygen, free-radical in vitro and ex vivo study. Photochem Photobiol Sci 2020, 19, 1001–1009. [Google Scholar] [CrossRef]
- Kalariya, N.M.; Ramana, K.V.; Srivastava, S.K.; van Kuijk, F.J. Genotoxic effects of carotenoid breakdown products in human retinal pigment epithelial cells. Curr Eye Res 2009, 34, 737–747. [Google Scholar] [CrossRef]
- Kalariya, N.M.; Ramana, K.V.; Srivastava, S.K.; van Kuijk, F.J. Carotenoid derived aldehydes-induced oxidative stress causes apoptotic cell death in human retinal pigment epithelial cells. Exp Eye Res 2008, 86, 70–80. [Google Scholar] [CrossRef]
- Rapp, L.M.; Maple, S.S.; Choi, J.H. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Invest. Ophthalmol. Vis. Sci. 2000, 41, 1200–1209. [Google Scholar] [PubMed]
- Sommerburg, O.G.; Siems, W.G.; Hurst, J.S.; Lewis, J.W.; Kliger, D.S.; van Kuijk, F.J. Lutein and zeaxanthin are associated with photoreceptors in the human retina. Curr Eye Res 1999, 19, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Finnemann, S.C.; Silverstein, R.L. Differential roles of CD36 and alphavbeta5 integrin in photoreceptor phagocytosis by the retinal pigment epithelium. J Exp Med 2001, 194, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Brunk, U.T.; Wihlmark, U.; Wrigstad, A.; Roberg, K.; Nilsson, S.E. Accumulation of lipofuscin within retinal pigment epithelial cells results in enhanced sensitivity to photo-oxidation. Gerontology 1995, 41 Suppl 2, 201–212. [Google Scholar] [CrossRef]
- Krohne, T.U.; Stratmann, N.K.; Kopitz, J.; Holz, F.G. Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells. Exp Eye Res 2010, 90, 465–471. [Google Scholar] [CrossRef]
- Lei, L.; Tzekov, R.; McDowell, J.H.; Smith, W.C.; Tang, S.; Kaushal, S. Formation of lipofuscin-like material in the RPE Cell by different components of rod outer segments. Exp Eye Res 2013, 112, 57–67. [Google Scholar] [CrossRef]
- Chen, C.L.; Chen, Y.H.; Liang, C.M.; Tai, M.C.; Lu, D.W.; Chen, J.T. Glucosamine-Induced Autophagy through AMPK(-)mTOR Pathway Attenuates Lipofuscin-Like Autofluorescence in Human Retinal Pigment Epithelial Cells In Vitro. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




