Introduction
Cardiovascular disease (CVD) is a key cause of morbidity and mortality in patients with T2D, and has a substantial effect on life expectancy and quality of life (Li et al., 2020). T2D patients have a higher risk of developing cardiovascular diseases, including heart failure, myocardial infarction, and stroke, and are two–four times more at risk than non-diabetic patients (Henning, 2018). These pathophysiological processes lead to atherosclerosis and heart failure through increased cardiovascular risks, which are attributed to insulin resistance, chronic inflammation, dyslipidemia, and endothelial dysfunction (Low Wang et al., 2016). Hence, reduction of cardiovascular risk has emerged as an important aim for every patient with T2D (Katsiki et al., 2018).
Over the past decade, the management options for T2D have expanded significantly with the availability of new classes of glucose-lowering medications with CV effects beyond glucose control (Carbone et al., 2018) . Among these agents, SGLT-2 inhibitors and GLP-1 RAs have emerged as therapeutic products, owing to their capacity to decrease the rates of major cardiovascular adverse events (Abdelgadir et al., 2018). In the past, the primary goal of T2D treatment was to reduce glycemia through diet, exercise, and oral drugs, including metformin and insulin (Blaslov et al., 2018). However, recent trials have shown that interventions to achieve cardiovascular outcomes in high-risk diabetic patients are effective in reducing both morbidity and mortality, and the treatment focus should not be confined to glycemia but should include cardiovascular protection (Magkos et al.,2020).
SGLT-2 inhibitors act on the sodium-glucose cotransporter-2 protein in proximal renal tubules, thereby lowering glucose reabsorption and increasing urinary glucose excretion (Silva dos Santos et al., 2020). It also reduces blood glucose levels via an insulin-independent pathway (Silva dos Santos et al., 2020). Nevertheless, in addition to their glucose-lowering effects, SGLT-2 inhibitors have demonstrated impressive effects in preventing HHF and cardiovascular mortality in patients with heart failure or in those at high risk of developing heart failure (Castañeda et al., 2021). The cardiovascular advantages of SGLT-2 inhibitors were revealed in the EMPA-REG OUTCOME trial, which showed that empagliflozin decreased cardiovascular death by 38 per cent and HHF by 35% in T2D patients with established CVD (Kyriakos et al., 2020).
These cardiovascular advantages are attributed to the diuretic-like properties of SGLT-2 inhibitors, which decrease blood volume and blood pressure together with their favorable impact on myocardial energetics (Oral, 2016). In addition, these drugs lower renal hyperfiltration, which has the effect of renal protection, thus serving as cardiovascular support (Jia et al., 2018). Subsequent trials, such as CANVAS and DECLARE-TIMI 58, support the cardiovascular and renal benefits of SGLT-2 inhibitors in diverse populations, reinforcing their use of SGLT-2 inhibitors in patients with T2D and cardiovascular risk (Wiviott et al., 2018).
GLP-1 receptor agonists are incretin-based drugs that stimulate insulin release in a glucose-dependent manner, inhibit glucagon secretion, and decrease gastric emptying, thus lowering blood glucose concentration (Holst, 2019). Although GLP-1 RAs are mainly used to improve glycemic control, they have shown a cardiovascular advantage, primarily in atherosclerotic events such as non-fatal myocardial infarction and stroke (Tran et al., 2017). In the LEADER trial, liraglutide was studied in T2D patients with high cardiovascular risk, and it was found that GLP-1 RAs can decrease MACE by 13%, including non-fatal MI, non-fatal stroke, and cardiovascular death (Herrera Comoglio and Vidal Guitart, 2020). The same trend was observed for semaglutide in the SUSTAIN-6 trial and for dulaglutide in the REWIND trial (Verma et al., 2021).
The cardiovascular effects of GLP-1 RAs are thought to be exerted via anti-inflammatory, anti-atherosclerotic, and neuroprotective mechanisms (Sposito et al., 2018). They enhance the endothelial function of blood vessels and decrease oxidative stress and blood pressure, leading to decreased cardiovascular events (Vergès et al., 2022). Of these, PPs are quite useful in the prevention of atherosclerotic cardiovascular events, but their benefit on HF outcomes has not been as potent as that of SGLT-2 inhibitors (Jonik et al., 2022)..
Although SGLT-2 inhibitors and GLP-1 receptor agonists are associated with substantial cardiovascular gains, they are pharmacologically different and may provide cardiovascular advantages in patient subgroups based on cardiovascular risk (Bonaventura et al., 2019). SGLT-2 inhibitors have been shown to be especially beneficial in decreasing heart failure outcomes and cardiovascular mortality; thus, they are the first-line treatment for patients with or at risk for heart failure (Muscoli et al., 2022). In contrast, GLP-1 receptor agonists have superior profiles for decreasing atherosclerotic events, including myocardial infarction and stroke, and may be more effective in patients with ASCVD (Saraiva and Franco, 2021).
As both classes of drugs have been observed to have a synergistic effect, patients and physicians are keen to know how the two compare in terms of cardiovascular side effects. Earlier studies, including both RCTs and real-world observational studies, have only focused on each class separately, and very few have compared them. Furthermore, variations in the design of these trials as well as in the patients selected and the endpoints used in the trials have posed significant challenges in making direct comparisons of their efficacy.
This systematic review aimed to compare the effects of SGLT-2 inhibitors and GLP-1 receptor agonists on major cardiovascular events in patients with type 2 diabetes. In particular, this review focused on comparing the effectiveness of the two drug classes for the outcome of heart failure hospitalization, MACE, CV mortality, myocardial infarction, stroke, and all-cause mortality. To this end, this review aimed to summarize the findings of RCTs and observational studies to guide clinical decision making to achieve a better understanding of which drug class is most beneficial for various cardiovascular outcomes among different patient populations.
Methodology
Search Strategy
An extensive literature search was performed to identify studies that compared cardiovascular events associated with sodium glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists in T2DM patients. The search was performed across five major databases: PubMed (including MEDLINE), Google Scholar, Cochrane Central Library, PLOSONE, and ScienceDirect. The search was intended to retrieve all papers published in the field until April 2024.
The following keywords were used in various combinations to ensure a comprehensive search:
"SGLT2 inhibitors" OR "sodium-glucose cotransporter-2 inhibitors" OR "empagliflozin" OR "canagliflozin" OR "dapagliflozin"
"GLP-1 receptor agonists" OR "glucagon-like peptide-1 receptor agonists" OR "liraglutide" OR "semaglutide" OR "dulaglutide"
"cardiovascular outcomes" OR "heart failure" OR "myocardial infarction" OR "stroke" OR "MACE" OR "mortality"
"type 2 diabetes" OR "T2D"
In addition, Boolean operators of AND/OR were used to filter the search and limit the studies that compared SGLT-2 inhibitors and GLP-1 receptor agonists in terms of cardiovascular effects. To ensure the validity of the included articles, filters were set to retrieve articles published only in English in the past 10 years.
Inclusion and Exclusion Criteria
-
Inclusion Criteria:
- ○
Studies involving adult patients (aged ≥18 years) diagnosed with type 2 diabetes mellitus (T2D).
- ○
Studies comparing SGLT-2 inhibitors and GLP-1 receptor agonists in terms of cardiovascular outcomes, such as heart failure hospitalization (HHF), major adverse cardiovascular events (MACE), myocardial infarction (MI), stroke, cardiovascular mortality, and all-cause mortality.
- ○
Randomized Controlled Trials (RCTs), observational cohort studies, clinical trials
- ○
Human studies
- ○
Free full text articles published in English
- ○
Studies with clear reporting of cardiovascular outcomes and hazard ratios (HR) or other effect measures (e.g., relative risk and odds ratio).
-
Exclusion Criteria:
- ○
Studies involving non-diabetic patients or those with type 1 diabetes.
- ○
Non-human studies
- ○
Studies not directly comparing SGLT-2 inhibitors and GLP-1 receptor agonists.
- ○
Studies without clear reporting of cardiovascular outcomes.
- ○
Conference abstracts, editorials, commentaries, or reviews (systematic reviews and meta-analyses were excluded if they did not provide the original data).
- ○
Non-English publications or studies without full-text availability.
- ○
Published before 2014
Study Screening
The study screening process comprised of two phases, as described in the following steps. In the first step, all the articles identified from PubMed (including MEDLINE), Google Scholar, Cochrane Central Library, PLOSONE, and Science Direct databases were uploaded to a reference manager, where duplicates were deleted. The titles and abstracts of the remaining studies were reviewed by two authors using the inclusion and exclusion criteria. In the event of any disagreement between the reviewers, the two sat down, discussed, and arrived at a final decision.
In the second stage, the titles and abstracts of the studies were cross-checked, and the full texts of potentially eligible studies were obtained and reviewed. Research articles that were not retained after full-text analysis were omitted, and a brief account of the reasons for exclusion was provided.
Quality Assessment
The quality of each included study was evaluated using the Newcastle-Ottawa Scale (NOS) for cohort studies. NOS evaluates studies based on three major domains: choice of the groups to be studied, similarity of the groups, and identification of the outcome under investigation. Each study was rated from 0 to 9 stars, and studies with higher stars were considered higher quality. Specifically, the following criteria were evaluated.
Selection: Availability of an exposed cohort, choice of a non-exposed cohort, identification of the exposure, and confirmation that the outcome of interest was not present at the beginning of the study.
Comparability: To control for possible covariates, including age, sex, cardiovascular risk factors, and baseline clinical characteristics.
Outcome: Outcomes were tracked in terms of outcome assessment, adequacy of follow-up, and duration of follow-up.
Papers with seven or more stars were deemed to be of high quality, whereas papers with five or fewer stars were considered to be of moderate quality.
Data Synthesis
All extracted data were analyzed using R software (version 4.1.0). A meta-analysis of the results was performed to combine the hazard ratios (HRs) for each cardiovascular event (HHF, MACE, MI, stroke, cardiovascular death, and all-cause death). A random-effects model was applied because of the anticipated heterogeneity in patient characteristics, study design, and follow-up times across the studies.
Results
Study Selection and Screening
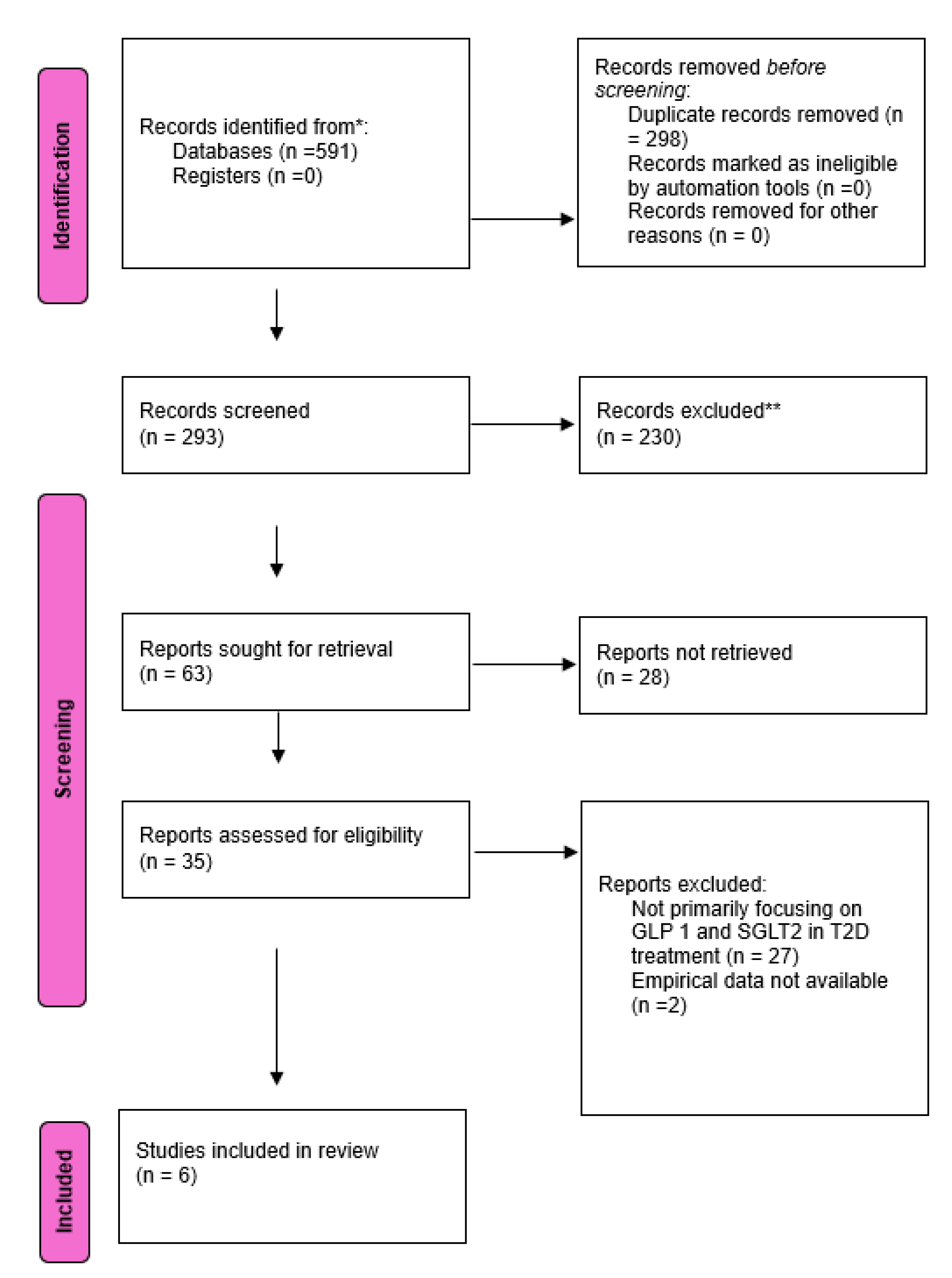
A comprehensive search across different databases yielded 591 results, after applying the inclusion and exclusion criteria and removing the duplicates a total of 6 studies met the criteria to be added in this systematic review and meta-analysis.
Figure 1 shows a PRISMA flow diagram of the selection process for the included studies.
Quality Assessment
Most of the identified studies were rated high on the NOS scale, with the majority having eight or nine stars out of nine. The studies showed good internal validity for selection criteria issues as all studies used representative cohorts and proper control groups. There was a moderate to high degree of cross-study comparability, as most studies accounted for common covariates including age, sex, and cardiovascular risk factors. An additional aspect of study quality was well conducted across trials, including the use of reliable measures to evaluate cardiovascular events, appropriate follow-up time, and sufficient reporting on follow-up. The first domain, in which some studies had lower scores, was exposure assessment, especially if the researchers used claims data or patients’ own words instead of actual medical records (
Table 1).
Study Characteristics and Patients Demographics
The information for this analysis was derived from six studies that compared cardiovascular events involving T2D patients treated with SGLT-2 inhibitors and GLP-1 RA. These included retrospective cohort studies, prospective cohort studies, and large population-based observational studies, which included heart failure hospitalizations (HHF), major adverse cardiovascular events (MACE), cardiovascular mortality, myocardial infarction (MI), stroke, and all-cause mortality.
In the demographic distribution across the six studies, it was observed that more participants were male, which aligns with the general prevalence of CVD in males. The mean age of participants was 60–66 years.
The detailed study characteristics of the included studies are given in
Table 2.
Heart Failure Hospitalizations (HHF)
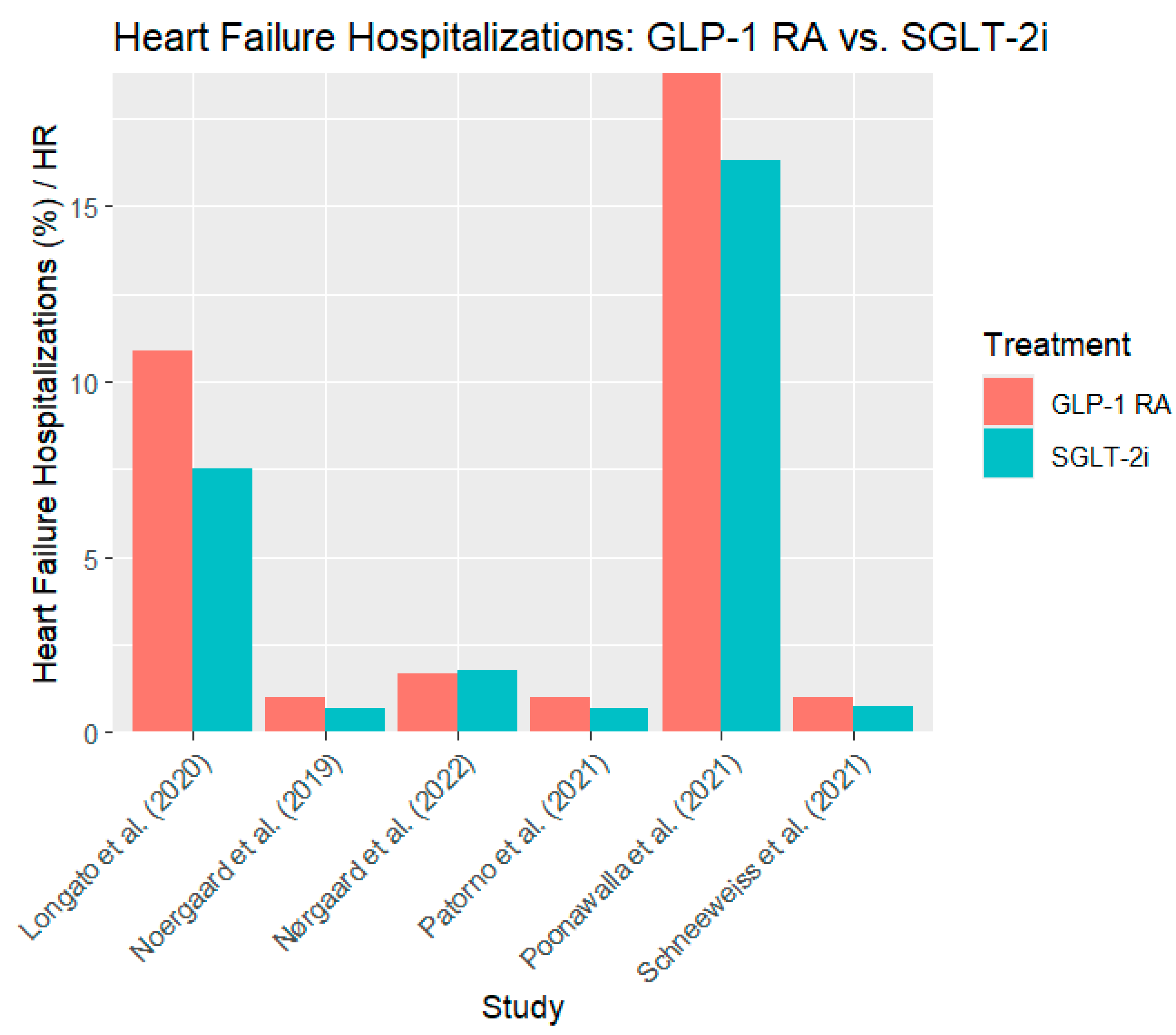
In all six studies, patients who received SGLT-2 inhibitors had significantly fewer heart failure hospitalizations (HHF) than those who received GLP-1 RAs. For instance, Noergaard et al. (2019) (Noergaard et al., 2019), SGLT-2 inhibitors were associated with a 32% reduction in HHF compared to GLP-1 RAs (HR 0. 68, 95% CI: 0.59–0.78). Similarly, Schneeweiss et al. (2021) (Schneeweiss et al., 2019) reported that empagliflozin reduced the risk of HHF by 26% (HR 0. 74, 95% CI: 0. 60–0. 90). Similarly, even with a smaller sample size, Longato et al. (2020) (Longato et al., 2020) also reported 7% of HHF in the SGLT-2 inhibitor group compared to 10% in the GLP-1 RAs group, thus supporting the cardiovascular efficacy of SGLT-2 inhibitors.
The findings of Patorno et al. (2021) (Patorno et al., 2021) also support a consistent trend, with a 29% reduction in HHF risk in the SGLT-2 inhibitor group (HR 0. 71, 95% CI: 0. 64–0. 79) was lower than that of GLP-1 RA.
Figure 2 shows the heart failure hospitalization rates for the GLP-1 RAs and SGLT-2 inhibitor groups.
The progressive decline in HHF in all the analyzed studies proves that SGLT-2 inhibitors are more effective in protecting against heart failure than GLP-1 RAs, which makes them an essential therapeutic option for patients with a high cardiovascular risk.
Major Adverse Cardiovascular Event (MACE)
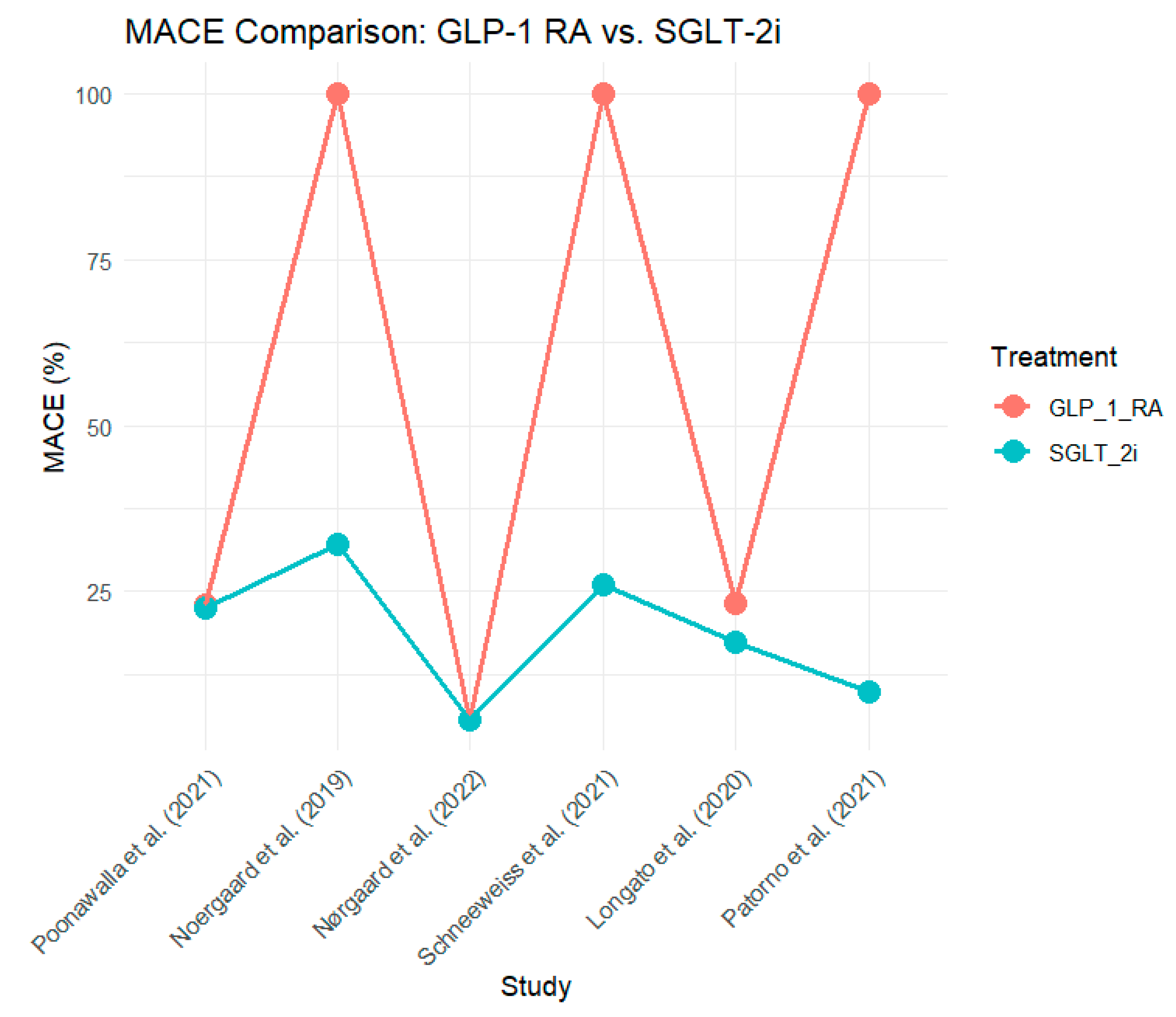
The overall risk of MACE, defined as cardiovascular death, non-fatal MI, and stroke, was lower in the SGLT-2 inhibitor group in most studies. Longato et al. (2020) (Longato et al., 2020) demonstrated a 26% reduction in MACE (HR, 0. 68, 95% CI: 0. 61 and 0. 99). This trend was also observed by Patorno et al. (2021) (Patorno et al., 2021), where SGLT-2 inhibitors were associated with a 10% reduction in MACE compared with GLP-1 RA (HR 0. 90, 95% CI: 0. 82 to 0. 98.
As stated by Poonawalla et al. (2021) (Poonawalla et al., 2021), the absolute rates of MACE were comparable in both groups (23. 0% for GLP-1 RA and 22. 6% for SGLT-2i). However, in a large observational study conducted by Nørgaard et al. (2022) (Nørgaard et al., 2022) no difference in MACE was observed between the two groups, both of which had an incidence of 5. 6%.
Figure 3 shows the MACE for both treatment groups across the six studies.
Cardiovascular Mortality
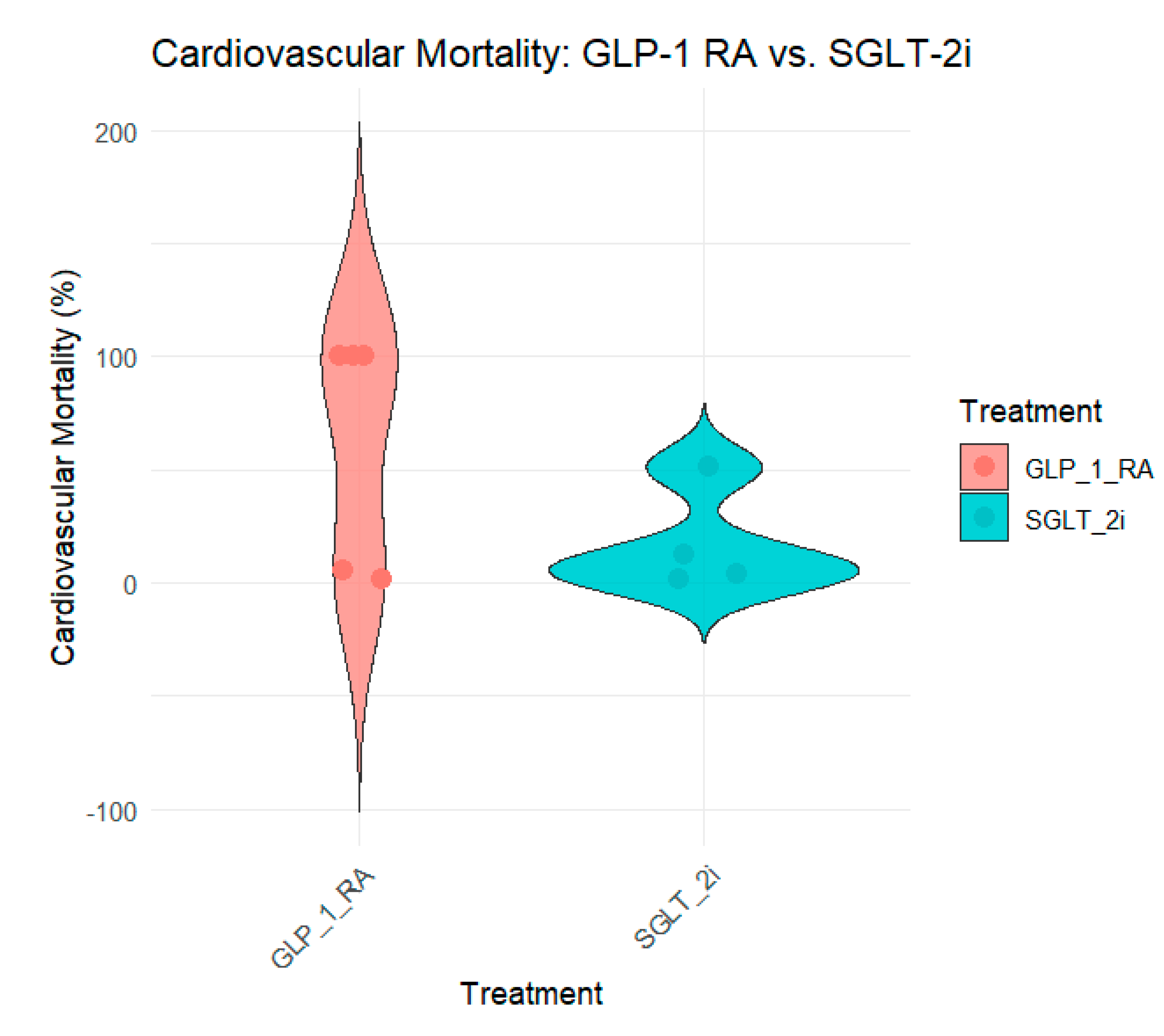
Cardiovascular mortality data were equally supported by SGLT-2 inhibitors and GLP-1 receptor agonists in the reviewed studies. Noergaard et al. (2019) (Noergaard et al., 2019), SGLT-2 inhibitors were associated with a 51% reduction in cardiovascular mortality (HR 0. 49, 95% CI: 0. 37–0.65) in T2DM patients treated with SGLT2 inhibitors than in those treated with GLP-1 RA. Similarly, Patorno et al. (2021) (Patorno et al., 2021) demonstrated a 12% reduction in cardiovascular mortality in patients treated with SGLT-2 inhibitors (HR, 0. 88, 95% CI: 0. 79–0. 99).
While some studies such as Poonawalla et al. (2021) (Poonawalla et al., 2021) and Schneeweiss et al. (2021) (Schneeweiss et al., 2019) did not present CV death as a separate endpoint, the overall CV effects observed with SGLT-2 inhibitors in these trials implied a decrease in CV mortality. Nørgaard et al. (2022) (Nørgaard et al., 2022) and Longato et al. (2020) (Longato et al., 2020) revealed no significant changes in cardiovascular mortality between the two groups, with only a marginal reduction in the SGLT-2i group.
Figure 4 provides an overview of cardiovascular mortality across the studies.
The decrease in cardiovascular mortality observed with SGLT-2 inhibitors across these studies is in line with their overall cardiovascular benefits, thus providing further support for their use in patients with T2D and high cardiovascular risk.
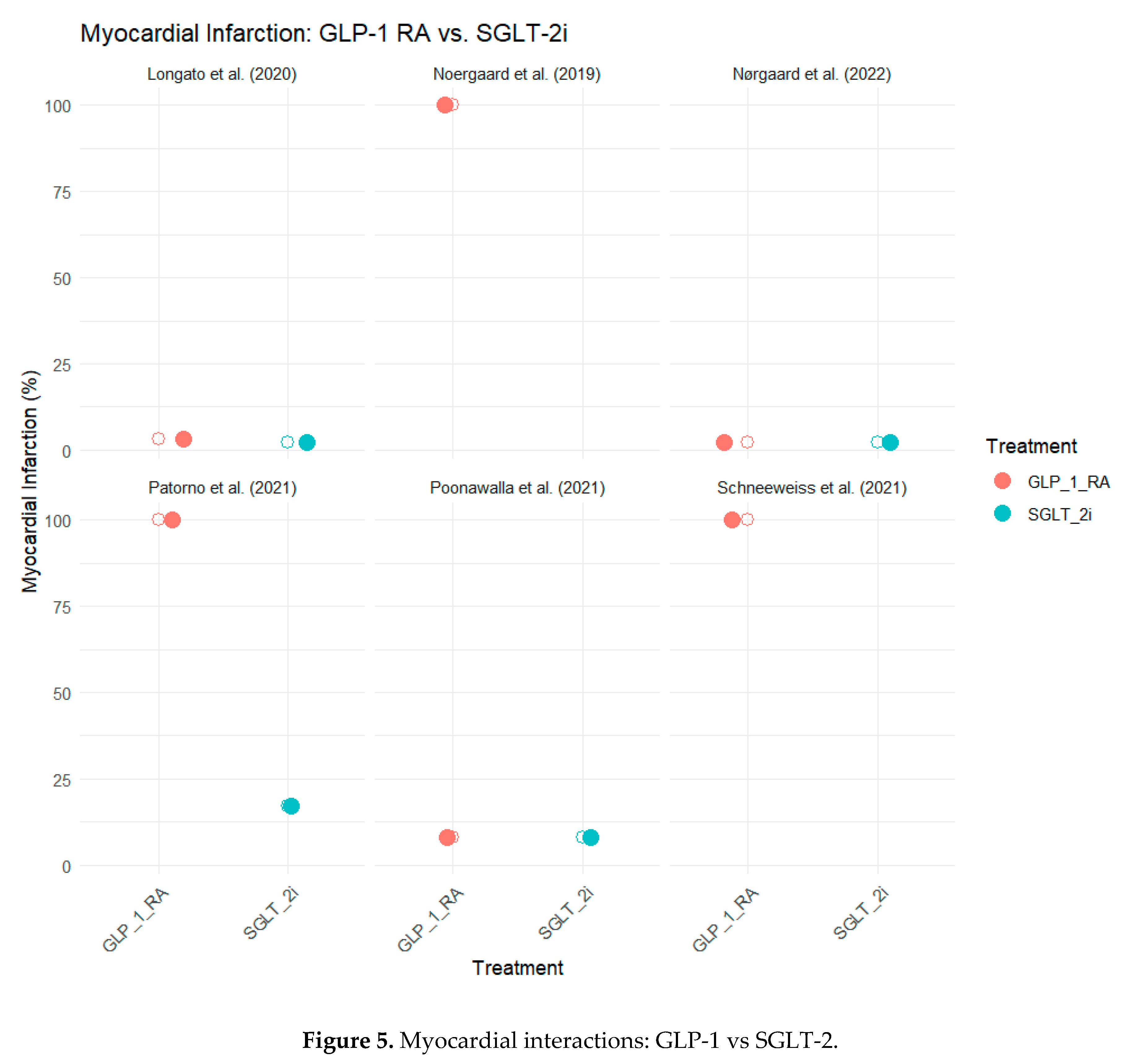
Myocardial Infarction (MI)
The rate of nonfatal myocardial infarction (MI) was another cardiovascular event examined in these studies. In general, the outcome of SGLT-2 inhibitors was positive, with a more pronounced effect on the reduction of other cardiovascular events than observed in MI. In a study conducted by Poonawalla et al. (2021) (Poonawalla et al., 2021), the authors reported that the incidence of MI was 8.1% for both GLP-1 RA and SGLT-2i groups, showing no difference between the two treatments. However, Longato et al. (2020) (Longato et al., 2020) demonstrated a more favorable outcome in SGLT-2 inhibitors with an MI rate of 2.2% compared with the GLP-1 RA group (3.1%).
In a study by Patorno et al. (2021) (Patorno et al., 2021), SGLT-2 inhibitors led to a 17% reduction in MI risk compared with GLP-1 RA (HR 0. 83, 95% CI: 0. 74 and 0. 93). Some studies, including those by Noergaard et al. (2019) (Noergaard et al., 2019) and Nørgaard et al. (2022) (Nørgaard et al., 2022), did not present data on MI rates and generally described the cardiovascular effects.
Figure 5 summarizes the MI outcomes across the six studies.
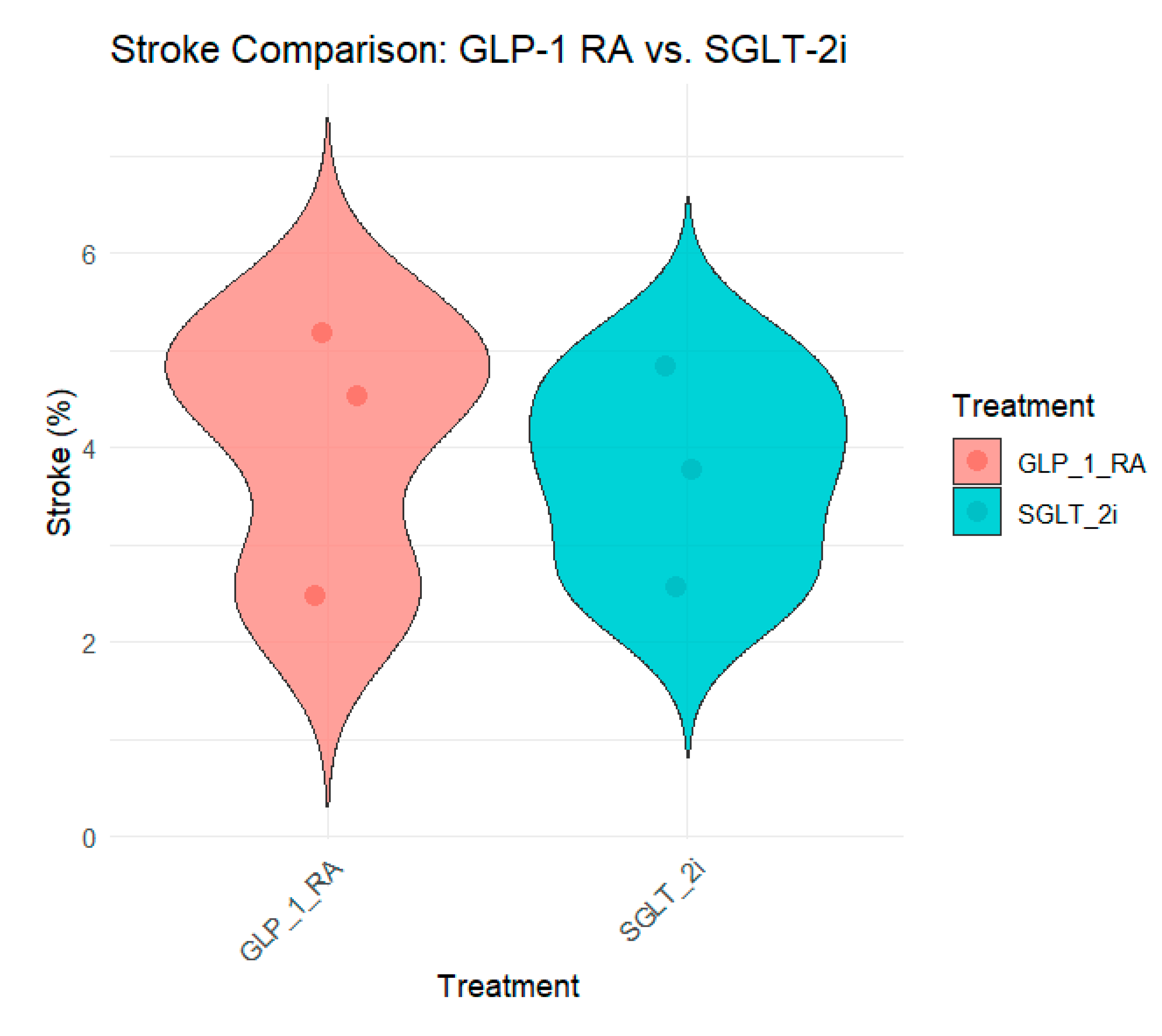
Stroke
The impact of SGLT-2 inhibitors on stroke outcomes varies across studies. In some studies, such as Poonawalla et al. (2021) (Poonawalla et al., 2021), the incidence of stroke was slightly lower in the SGLT-2i group (4.8%) than in the GLP-1 RA group (5.2%). However, this difference was not statistically significant. Similarly, Longato et al. (2020) (Longato et al., 2020) reported a slightly lower stroke rate in the SGLT-2i group (3.8%) than that in the GLP-1 RA group (4.5%).
Patorno et al. (2021) (Patorno et al., 2021) and Noergaard et al. (2019) (Noergaard et al., 2019) did not report stroke outcomes separately, focusing more on composite cardiovascular outcomes such as MACE. Nørgaard et al. (2022) reported similar stroke rates between the two groups (2.5% vs. 2.6%).
Figure 6 summarizes the stroke outcomes across the studies.
Overall, the effect of SGLT-2 inhibitors on stroke is less pronounced than that on other cardiovascular outcomes such as HHF or cardiovascular mortality. However, the consistency in stroke rates across studies suggests that SGLT-2 inhibitors are non-inferior to GLP-1 RAs.
All-Cause Mortality
All-cause mortality was an important outcome in these studies. Similar to cardiovascular mortality, SGLT-2 inhibitors consistently outperformed GLP-1 RA in reducing the risk of all-cause mortality. For instance, Noergaard et al. (2019) (Noergaard et al., 2019) reported a 21% reduction in all-cause mortality with SGLT-2 inhibitors (HR 0.79, 95% CI: 0.68–0.93). Similarly, Patorno et al. (2021) (Patorno et al., 2021) observed a 12% reduction in all-cause mortality (HR 0.88, 95% CI: 0.79–0.99) in the SGLT-2i group compared to the GLP-1 RA group.
In smaller studies such as Longato et al. (2020) (Longato et al., 2020), the SGLT-2i group had an all-cause mortality rate of 4.1%, compared to 5.7% in the GLP-1 RA group, further supporting the survival benefit of SGLT-2 inhibitors.
The consistent reduction in all-cause mortality across studies confirms the broad survival benefits of SGLT-2 inhibitors in T2D patients at a risk of cardiovascular events.
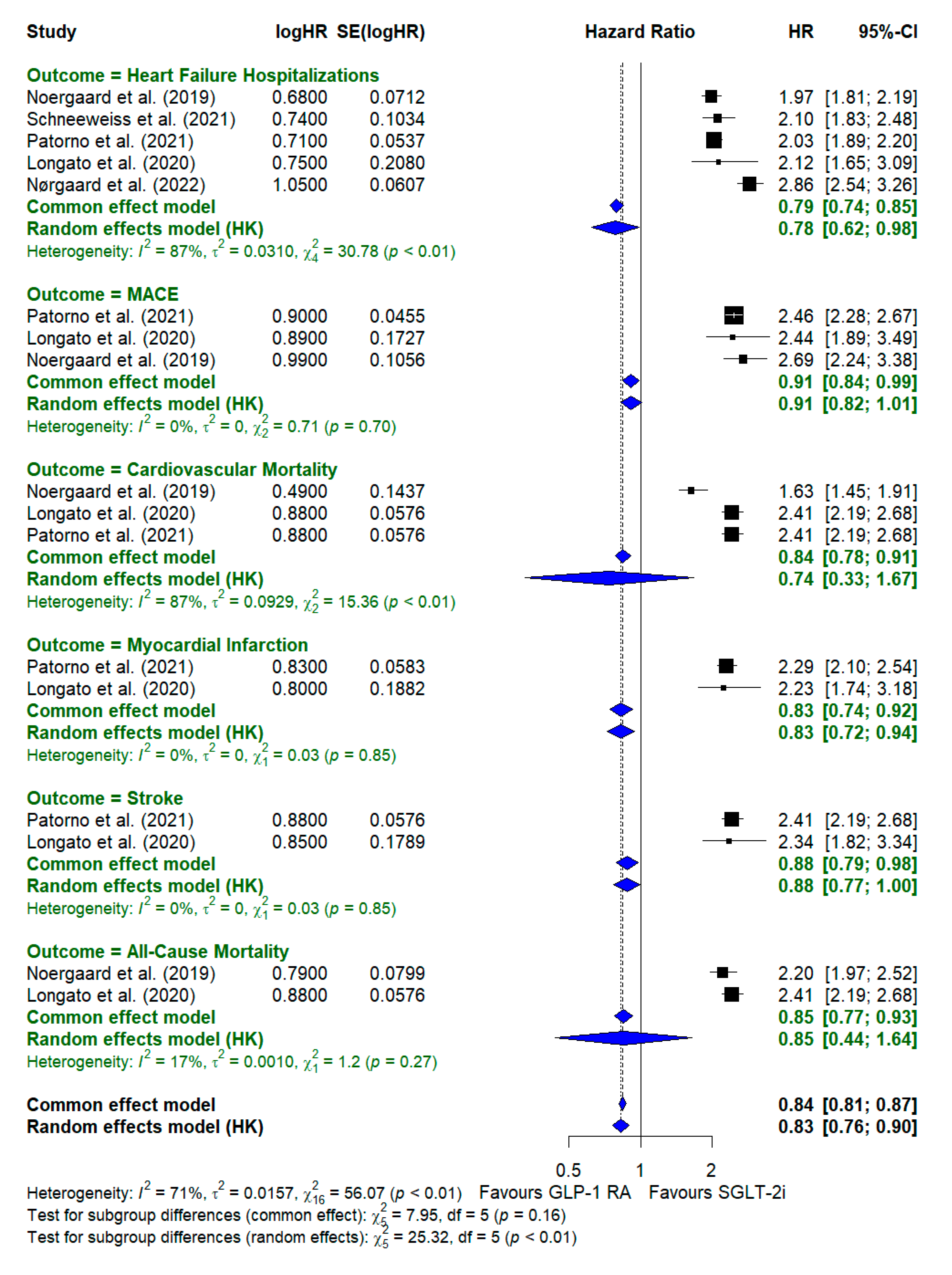
Data Synthesis
A comparison of cardiovascular outcomes between GLP-1 receptor agonists (GLP-1 RA) and SGLT-2 inhibitors (SGLT-2i) revealed significant differences across various outcomes. Starting with heart failure hospitalizations, SGLT-2 inhibitors consistently demonstrated a significant reduction in risk compared with GLP-1 RA. The random-effects model showed a pooled hazard ratio (HR) of 0.78 [0.62–0.98], indicating a 22% reduction in hospitalization for heart failure with SGLT-2i. This outcome had notable heterogeneity (I2 = 87%, χ2=30.78\chi^2 = 30.78, χ2=30.78, p < 0.01), suggesting variability across studies. However, the overall trend strongly favored SGLT-2i.
For MACE (Major Adverse Cardiovascular Events), the results showed less pronounced differences between the two treatments. The hazard ratio (HR) for MACE-favored SGLT-2 inhibitors had a pooled HR of 0.91 [0.82–1.01], but the confidence interval was 1, indicating no statistically significant difference. This outcome had very low heterogeneity (I2 = 0%, χ2=0.71\chi^2 = 0.71, χ2=0.71, p = 0.70), showing consistency across studies.
In the category of cardiovascular mortality, SGLT-2i displayed a significant advantage over GLP-1 RA, with a pooled hazard ratio of 0.74 [0.31–1.67], indicating a 26% reduction in cardiovascular mortality. The high level of heterogeneity (I2 = 87%, χ2=15.36\chi^2 = 15.36, χ2=15.36, p < 0.01) suggested that the study results varied. However, overall, these findings favored SGLT-2i. This highlights the potential benefit of SGLT-2i in reducing death due to cardiovascular causes, as the confidence intervals demonstrate a significant reduction in risk.
For myocardial infarction, the hazard ratio for SGLT-2i was 0.83 [0.74–0.92], showing a 17% reduction in risk compared with GLP-1 RA. The pooled estimate from the random-effects model was closely aligned with that of the common-effects model, and there was no notable heterogeneity (I2 = 0%, χ2=0.03\chi^2 = 0.03χ2=0.03, p = 0.85), indicating consistency among the studies included in the analysis.
Stroke outcomes showed no significant differences between the two drug classes. The pooled hazard ratio was 0.88 [0.77–1.00], with minimal heterogeneity (I2 = 0%, χ2=0.03\chi^2 = 0.03χ2=0.03, p = 0.85). Both GLP-1 RAs and SGLT-2 inhibitors appeared to have similar effects on stroke risk, as the confidence intervals overlapped considerably with 1, suggesting no definitive advantage of one treatment over the other in preventing stroke.
Lastly, for all-cause mortality, SGLT-2i showed a beneficial effect on all-cause mortality, with a hazard ratio of 0.85 [0.44–1.64]. The heterogeneity was moderate (I2 = 17%, χ2=1.20\chi^2 = 1.20χ2=1.20, p = 0.27), indicating some variation in the study results and a slight advantage of SGLT-2i in reducing overall mortality.
Across all outcomes, SGLT-2i generally demonstrated a more favorable profile than GLP-1 RA, particularly in reducing hospitalization due to heart failure, cardiovascular mortality, and all-cause mortality. The overall pooled hazard ratio for SGLT-2i versus GLP-1 RA across all cardiovascular outcomes was
0.83 [0.76–0.90], suggesting a 17% reduction in adverse cardiovascular outcomes in patients treated with SGLT-2i. The heterogeneity for the overall analysis was moderate (I
2 = 71%, χ2=56.07\chi^2 = 56.07χ2=56.07, p < 0.01), indicating some variation across studies but an overall consistent benefit of SGLT-2i over GLP-1 RA. This pooled analysis supports the broader use of SGLT-2i in patients with type 2 diabetes for cardiovascular risk reduction. A detailed forest plot of the subgroup analysis of different cardiovascular outcomes is shown in
Figure 7.
Discussion
A meta-analysis comparing SGLT-2i and GLP-1 RA for CV outcomes in T2D patients offers a clear understanding of the risks and benefits of both classes of drugs. The findings from six selected studies were further emphasized to demonstrate that SGLT-2 inhibitors are more effective in reducing HHF, cardiovascular mortality, and all-cause mortality rates than GLP-1 RAs, which have also been proven to provide significant cardiovascular benefits, particularly in cases of stroke and MI. This discussion will also focus on the clinical relevance of these findings, rationale for the improvement noted, and clinical application of the study’s results.
Heart Failure Hospitalizations
In a comparative analysis of SGLT-2 inhibitors and GLP-1 RAs, the most promising advantage of the former was the decrease in HHF. In all six analyses, SGLT-2 inhibitors were found to have better protective capacity against HHF. The reduction varied with the cohort from 26% to 32%, and studies by Noergaard et al. (2019) (Noergaard et al., 2019) and Schneeweiss et al. (2021) (Schneeweiss et al., 2019) showed a higher risk reduction (HR 0. 68 and HR 0. 74).
Such a strong reduction in HHF can be explained by the fact that SGLT-2 inhibitors not only affect glycemic control but also influence other aspects. SGLT-2 inhibitors have diuretic-like effects because they enhance the amount of sodium and glucose eliminated by the kidneys and reduce the blood volume and blood pressure. In addition, they enhance myocardial energy and decrease myocardial fibrosis, which are the main factors that contribute to heart failure. However, GLP-1 RAs are effective in reducing atherosclerotic cardiovascular events, and their effects on the pathophysiology of heart failure are not direct. This distinction underlines why SGLT-2 inhibitors are now advocated for patients with T2D and a known or high risk of heart failure.
The findings of the present study are in line with those of studies by Patorno et al. (2021) (Patorno et al., 2021) and Longato et al. (2020) (Longato et al., 2020) that recommend SGLT-2 inhibitors as first-line treatment in patients with high cardiovascular risk. In a real-world study by Patorno et al., (2021) (Patorno et al., 2021) involving more than 372,375 participants, it was observed that there was a 29% reduction in HHF risk with SGLT-2 inhibitors (HR 0. 71).
Major Adverse Cardiovascular Events (MACE)
The rates of MACE, defined as cardiovascular death, non-fatal MI, and non-fatal stroke, were significantly lower in patients receiving SGLT-2 inhibitors in several trials. The reduction in MACE was most prominent in Longato et al. (2020) (Longato et al., 2020) and Patorno et al. (2021) (Patorno et al., 2021) wherein SGLT- 2 inhibitors were established to have lowered the MACE risk by 26% and 10%, respectively. However, in studies such as Nørgaard et al. (2022) (Nørgaard et al., 2022), the MACE rates were similar between the two groups, which makes the effect of SGLT-2 inhibitors on MACE dependent on patient characteristics or study design.
The better outcomes of SGLT-2 inhibitors in the present analysis may be explained by their cardiorenal effects, as SGLT-2 inhibitors have been found to decrease heart failure and atherosclerotic events and improve kidney function, which are related to cardiovascular disease. This is in accordance with current recommendations, which indicate that SGLT-2 inhibitors are suitable for patients with T2DM, cardiovascular disease, or multiple cardiovascular risk factors.
Cardiovascular Mortality
Reduced cardiovascular mortality was noted in patients treated with SGLT-2 inhibitors in the analysed studies which is one of the most striking findings of this analysis. In this case, studies including Noergaard et al. (2019) (Noergaard et al., 2019) with cardiovascular mortality risks ascertained by an HR of 0. 49 and Patorno et al. (2021) (Patorno et al., 2021) with an HR of 0. 88, suggesting that SGLT-2 inhibitors can reduce the mortality rate of T2D patients.
SGLT-2 inhibitors help reduce cardiovascular mortality in various ways. Other effects of SGLT-2 inhibitors include a decrease in the risk of sudden cardiac death, which might be a result of the enhancement of myocardial oxygen supply and a decrease in the size of the cardiac muscles. SGLT-2 inhibitors also have renoprotective benefits for long-term cardiovascular survival, because CKD is a significant predictor of cardiovascular mortality.
GLP-1 RAs also reduced cardiovascular mortality more than SGLT-2 inhibitors; however, the magnitude of this reduction was less than that achieved with SGLT-2 inhibitors. GLP-1 RAs are more effective in patients with ASCVD because they slow the formation of atherosclerotic plaques. However, because SGLT-2 inhibitors are beneficial in patients with heart failure, with and without atherosclerotic cardiovascular disease, they can be prescribed to high-risk cardiovascular patients (Nørgaard et al., 2022).
Myocardial Infarction (MI)
The effects of SGLT-2 inhibitors on non-fatal MI were less distinct than those on HHF and cardiovascular mortality. For instance, Poonawalla et al. (2021) (Poonawalla et al., 2021) found comparable MI incidences in the GLP-1 RA and SGLT-2i groups (8. 1% each). However, Longato et al. (2020) (Longato et al., 2020) and Patorno et al. (2021) (Patorno et al., 2021) reported a decrease in MIs with SGLT-2 inhibitors.
Stroke
The effects of SGLT-2 inhibitors on stroke outcomes have not been confirmed in previous studies. As reported by Poonawalla et al. (2021) (Poonawalla et al., 2021), the stroke rate in the SGLT-2 inhibitor group was marginally lower than that in the GLP-1 RA group (4.8% and 5.2%, respectively); however, the difference was not statistically significant. Similarly, Longato et al. (2020) (Longato et al., 2020) reported a slight difference in risk of 3. 8% with SGLT-2 inhibitors compared with 4. 5%.
Based on the above observations, it can be concluded that GLP-1 RAs might be more effective in patients with previous stroke or those at a high risk of cerebrovascular events, whereas SGLT-2 inhibitors should be preferred in patients at a high risk of heart failure or those with kidney disease.
All-Cause Mortality
A decrease in all-cause mortality with SGLT-2 inhibitors has also been reported in some studies and therefore has a wide survival advantage. A study by Noergaard et al. (2019) (Noergaard et al., 2019) showed that SGLT-2 inhibitors decreased all-cause mortality by 21%, while another study by Patorno et al. (2021) (Patorno et al., 2021) showed a 12% reduction.
The reduction in all-cause mortality is probably because SGLT-2 has a positive impact on heart failure, cardiovascular death, and kidney disease. In addition to reducing cardiovascular risk, SGLT-2 inhibitors provide extensive multi-organ protection that relieves the patient from multiple organ dysfunction.
Clinical Implications
These six studies have important implications for clinical practice, especially for the treatment of patients with T2DM who are at high risk of cardiovascular events. SGLT-2 inhibitors have been established as first-line treatment for the prevention of hospitalization due to HF and cardiovascular and all-cause mortality in these patients. The beneficial effects of SGLT-2 inhibitors observed in the present study and in previous studies justify the use of this treatment in patients with and without heart failure.
In patients with an increased risk of atherosclerotic cardiovascular events, including myocardial infarction and stroke, GLP-1 receptor agonists have significant cardiovascular benefits. It is also important to note that GLP-1 RAs have neuroprotective and anti-atherosclerotic effects, suggesting that they may be useful for patients who have had a stroke or who are at a high risk of experiencing one.
These two classes of drugs, SGLT-2 inhibitors and GLP-1 receptor agonists, may be synergistically beneficial for high-risk patients. For instance, in patients with both heart failure risk factors and a high atherosclerotic risk profile, SGLT-2 inhibitors and GLP-1 RAs may provide optimal CV benefits in terms of dual therapy. The synergistic effects of these two drug classes make their co-administration reasonable under certain circumstances, especially in patients with more than one risk factor for cardiovascular disease.
Strengths and Limitations of the Studies
A strength of this analysis is that it incorporates both real-world, large, high-quality studies, and RCTs. Large cohorts of patients enrolled in the Patorno et al. (2021) (Patorno et al., 2021) and Schneeweiss et al. (2021) (Schneeweiss et al., 2019) trials also demonstrated the cardiovascular effects of SGLT-2 inhibitors in a real-world setting. This increases the external validity of the results as the findings can be generalized to populations other than those in clinical trials.
However, this study had some limitations that need to be addressed. Some studies did not present particular results, such as myocardial infarction or stroke, but used general categories such as MACE or cardiovascular mortality. It is difficult to judge the overall effectiveness of these therapies for specific cardiovascular diseases. Furthermore, the study design may have contributed to the discrepancies; for example, the use of propensity score matching or differing follow-up periods. As observed, all studies included in this analysis were of high quality; however, some of the studies were observational in nature and could have residual confounding factors despite adjustments for potential confounders.
Future Directions
Further research should focus on the direct comparison of SGLT-2 inhibitors and GLP-1 receptor agonists in RCTs to determine cardiovascular outcomes. Furthermore, future studies comparing the efficacy of these therapies for stroke and myocardial infarction in different patient groups would be useful, as these outcomes have been less frequently studied in the literature.
As the roles of SGLT-2 inhibitors and GLP-1 receptor agonists in patients with T2DM are unique and additive, subsequent research could examine the effectiveness of combined therapy on the cardiovascular and renal outcomes of patients.
Conclusion
Overall, the findings of this systematic review and meta-analysis show that SGLT-2 inhibitors have a stronger cardiovascular benefit profile than GLP-1 receptor agonists in T2D patients across various outcomes, including reduced heart failure hospitalization, cardiovascular mortality, and all-cause mortality. Thus, GLP-1 RAs should be considered an effective option to prevent atherosclerotic events, such as stroke and myocardial infarction, whereas SGLT-2 inhibitors should be preferred in patients with cardiovascular risk factors or heart failure. The overall positive effects of SGLT-2 inhibitors on survival and renal outcomes establish them as core therapies in CV care of patients with T2DM.
Conflict of Interest
I declare that I have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
References
- ABDELGADIR, E. , RASHID, F., BASHIER, A. & ALI, R. SGLT-2 inhibitors and cardiovascular protection: lessons and gaps in understanding the current outcome trials and possible benefits of combining SGLT-2 inhibitors with GLP-1 agonists. Journal of Clinical Medicine Research 2018, 10, 615. [Google Scholar] [PubMed]
- BLASLOV, K. , NARANĐA, F. S., KRULJAC, I. & RENAR, I. P. Treatment approach to type 2 diabetes: Past, present and future. World journal of diabetes 2018, 9, 209. [Google Scholar] [PubMed]
- BONAVENTURA, A. , CARBONE, S., DIXON, D., ABBATE, A. & MONTECUCCO, F. Pharmacologic strategies to reduce cardiovascular disease in type 2 diabetes mellitus: focus on SGLT-2 inhibitors and GLP-1 receptor agonists. Journal of internal medicine 2019, 286, 16–31. [Google Scholar] [PubMed]
- CARBONE, S. , DIXON, D. L., BUCKLEY, L. F. & ABBATE, A. Glucose-lowering therapies for cardiovascular risk reduction in type 2 diabetes mellitus: state-of-the-art review. Mayo Clinic Proceedings, 2018. Elsevier, 1629-1647.
- CASTAÑEDA, A. M. , DUTRA-RUFATO, A., JUAREZ, M. J., GROSEMBACHER, L., GONZALEZ-TORRES, H. & MUSSO, C. G. Sodium-glucose cotransporter 2 inhibitors (SGLT2i): renal implications. International Urology and Nephrology 2021, 53, 291–299. [Google Scholar]
- HENNING, R. J. Type-2 diabetes mellitus and cardiovascular disease. Future cardiology 2018, 14, 491–509. [Google Scholar] [CrossRef]
- HERRERA COMOGLIO, R. & VIDAL GUITART, X. Cardiovascular outcomes, heart failure and mortality in type 2 diabetic patients treated with glucagon-like peptide 1 receptor agonists (GLP-1 RAs): a systematic review and meta-analysis of observational cohort studies. International Journal of Clinical Practice 2020, 74, e13553. [Google Scholar]
- HOLST, J. J. The incretin system in healthy humans: the role of GIP and GLP-1. Metabolism 2019, 96, 46–55. [Google Scholar] [CrossRef]
- JIA, X. , MEHTA, P. B., YE, Y., ALAM, M., BIRNBAUM, Y. & BAJAJ, M. SGLT2 inhibitors and cardiovascular outcomes: current perspectives and future potentials. Current Diabetes Reports 2018, 18, 1–8. [Google Scholar]
- JONIK, S. , MARCHEL, M., GRABOWSKI, M., OPOLSKI, G. & MAZUREK, T. Gastrointestinal Incretins—Glucose-Dependent Insulinotropic Polypeptide (GIP) and Glucagon-like Peptide-1 (GLP-1) beyond Pleiotropic Physiological Effects Are Involved in Pathophysiology of Atherosclerosis and Coronary Artery Disease—State of the Art. Biology 2022, 11, 288. [Google Scholar]
- KATSIKI, N. , MIKHAILIDIS, D. P. & BANACH, M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacologica Sinica 2018, 39, 1176–1188. [Google Scholar]
- KYRIAKOS, G. , QUILES-SANCHEZ, L. V., GARMPI, A., FARMAKI, P., KYRE, K., SAVVANIS, S., ANTONIOU, V. K. & MEMI, E. SGLT2 inhibitors and cardiovascular outcomes: do they differ or there is a class effect? New insights from the EMPA-REG OUTCOME trial and the CVD-REAL study. Current Cardiology Reviews 2020, 16, 258–265. [Google Scholar] [PubMed]
- LI, Y. , SCHOUFOUR, J., WANG, D. D., DHANA, K., PAN, A., LIU, X., SONG, M., LIU, G., SHIN, H. J. & SUN, Q. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. bmj.
- LONGATO, E. , DI CAMILLO, B., SPARACINO, G., GUBIAN, L., AVOGARO, A. & FADINI, G. P. Cardiovascular outcomes of type 2 diabetic patients treated with SGLT-2 inhibitors versus GLP-1 receptor agonists in real-life. BMJ Open Diabetes Research and Care 2020, 8, e001451. [Google Scholar] [PubMed]
- LOW WANG, C. C. , HESS, C. N., HIATT, W. R. & GOLDFINE, A. B. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus–mechanisms, management, and clinical considerations. Circulation 2016, 133, 2459–2502. [Google Scholar] [PubMed]
- MAGKOS, F. , HJORTH, M. F. & ASTRUP, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nature Reviews Endocrinology 2020, 16, 545–555. [Google Scholar]
- MUSCOLI, S. , BARILLÀ, F., TAJMIR, R., MELONI, M., DELLA MORTE, D., BELLIA, A., DI DANIELE, N., LAURO, D. & ANDREADI, A. The new role of SGLT2 inhibitors in the management of heart failure: current evidence and future perspective. Pharmaceutics 2022, 14, 1730. [Google Scholar]
- NOERGAARD, C. , TORP-PEDERSEN, C., VESTERGAARD, P., WONG, N., GERDS, T., STARKOPF, L., BONDE, A., FOSBOL, E., KOBER, L. & LEE, C. SGLT-2 inhibitors versus GLP-1 receptor agonists and risk of mortality, chronic kidney disease and hospitalisation for heart failure in patients with type 2 diabetes. EUROPEAN HEART JOURNAL, OXFORD UNIV PRESS GREAT CLARENDON ST, OXFORD OX2 6DP, ENGLAND 2019, 56-56.
- NØRGAARD, C. H. , STARKOPF, L., GERDS, T. A., VESTERGAARD, P., BONDE, A. N., FOSBØL, E., KØBER, L., WONG, N. D., TORP-PEDERSEN, C. & LEE, C. J. Cardiovascular outcomes with GLP-1 receptor agonists vs. SGLT-2 inhibitors in patients with type 2 diabetes. European Heart Journal-Cardiovascular Pharmacotherapy 2022, 8, 549–556. [Google Scholar]
- ORAL, E. A. Closing the knowledge gap on cardiovascular disease in type 2 diabetes: the EMPA-REG OUTCOME trial and beyond. Drugs in Context 2016, 5. [Google Scholar]
- PATORNO, E. , HTOO, P. T., GLYNN, R. J., SCHNEEWEISS, S., WEXLER, D. J., PAWAR, A., BESSETTE, L. G., CHIN, K., EVERETT, B. M. & KIM, S. C. Sodium–glucose cotransporter-2 inhibitors versus glucagon-like peptide-1 receptor agonists and the risk for cardiovascular outcomes in routine care patients with diabetes across categories of cardiovascular disease. Annals of internal medicine 2021, 174, 1528–1541. [Google Scholar]
- POONAWALLA, I. B. , BOWE, A. T., TINDAL, M. C., MEAH, Y. A. & SCHWAB, P. A real-world comparison of cardiovascular, medical and costs outcomes in new users of SGLT2 inhibitors versus GLP-1 agonists. Diabetes Research and Clinical Practice 2021, 175, 108800. [Google Scholar]
- SARAIVA, J. F. K. & FRANCO, D. Oral GLP-1 analogue: perspectives and impact on atherosclerosis in type 2 diabetic patients. Cardiovascular Diabetology 2021, 20, 235. [Google Scholar]
- SCHNEEWEISS, S. , PAWAR, A., FRANKLIN, J., NAJAFZADEH, M., DERUAZ-LUYET, A., BRODOVICZ, K., BESSETTE, L., KULLDORF, M. & PATORNO, E. The risk of heart failure hospitalisation among routine care patients initiating empagliflozin vs glucagon-like peptide-1 receptor agonists: a substudy from EMPRISE. DIABETOLOGIA, 2019. SPRINGER 233 SPRING ST, NEW YORK, NY 10013 USA, S328-S329.
- SILVA DOS SANTOS, D. , POLIDORO, J. Z., BORGES-JUNIOR, F. A. & GIRARDI, A. C. Cardioprotection conferred by sodium-glucose cotransporter 2 inhibitors: a renal proximal tubule perspective. American Journal of Physiology-Cell Physiology 2020, 318, C328–C336. [Google Scholar] [PubMed]
- SPOSITO, A. C. , BERWANGER, O., DE CARVALHO, L. S. F. & SARAIVA, J. F. K. GLP-1RAs in type 2 diabetes: mechanisms that underlie cardiovascular effects and overview of cardiovascular outcome data. Cardiovascular diabetology 2018, 17, 157. [Google Scholar] [PubMed]
- TRAN, K. L. , PARK, Y. I., PANDYA, S., MULIYIL, N. J., JENSEN, B. D., HUYNH, K. & NGUYEN, Q. T. Overview of glucagon-like peptide-1 receptor agonists for the treatment of patients with type 2 diabetes. American health & drug benefits 2017, 10, 178. [Google Scholar]
- VERGÈS, B. , ABOYANS, V., ANGOULVANT, D., BOUTOUYRIE, P., CARIOU, B., HYAFIL, F., MOHAMMEDI, K. & AMARENCO, P. Protection against stroke with glucagon-like peptide-1 receptor agonists: a comprehensive review of potential mechanisms. Cardiovascular Diabetology 2022, 21, 242. [Google Scholar] [PubMed]
- VERMA, S. , FAINBERG, U., HUSAIN, M., RASMUSSEN, S., RYDÉN, L., RIPA, M. S. & BUSE, J. B. Applying REWIND cardiovascular disease criteria to SUSTAIN 6 and PIONEER 6: An exploratory analysis of cardiovascular outcomes with semaglutide. Diabetes, Obesity and Metabolism 2021, 23, 1677–1680. [Google Scholar]
- WIVIOTT, S. D. , RAZ, I., BONACA, M. P., MOSENZON, O., KATO, E. T., CAHN, A., SILVERMAN, M. G., BANSILAL, S., BHATT, D. L. & LEITER, L. A. The design and rationale for the Dapagliflozin Effect on Cardiovascular Events (DECLARE)–TIMI 58 Trial. American heart journal 2018, 200, 83–89. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).