Submitted:
10 October 2024
Posted:
11 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
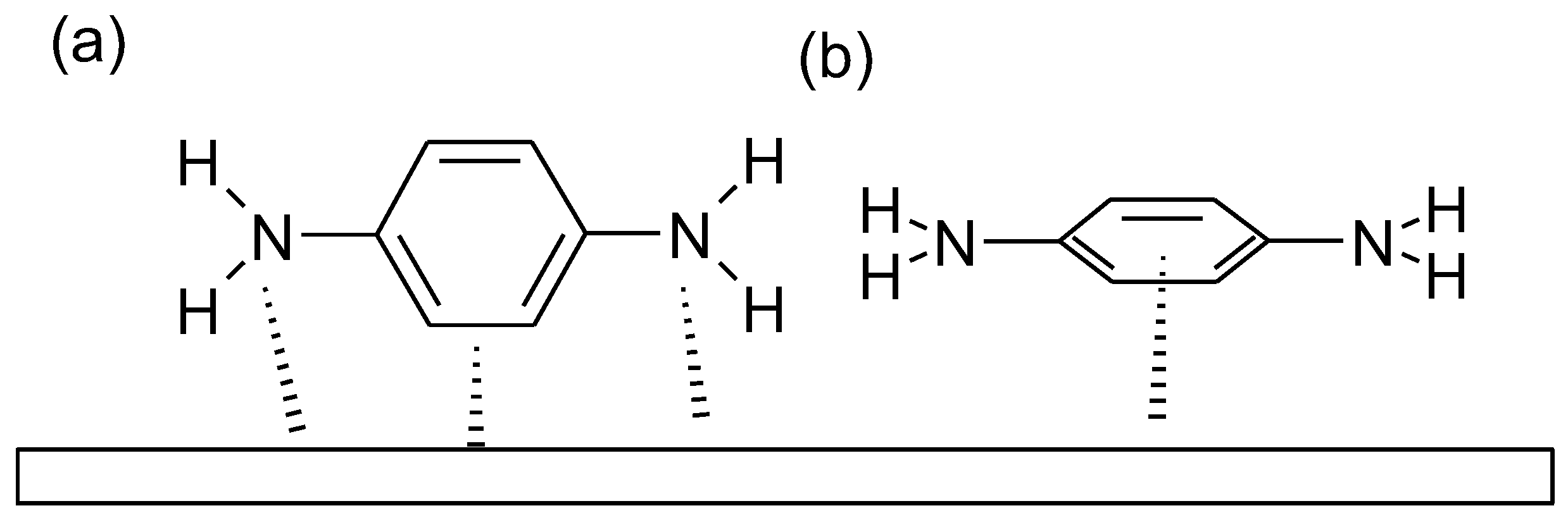
- The attraction between charged as well as neutral molecules and metal through electrostatic forces
- The interaction of the metal with lone electron pairs in the molecule
- Interactions of π-electrons with metal
-
A combination of the first three possibilitiesFurther considerations apply to corrosion inhibitor in more general terms:
- Sufficiently soluble
- Environmentally compatible
- nontoxic
- cheap and sustainable
- chemically stable in the particular environment
1.1. Substituted Benzenes
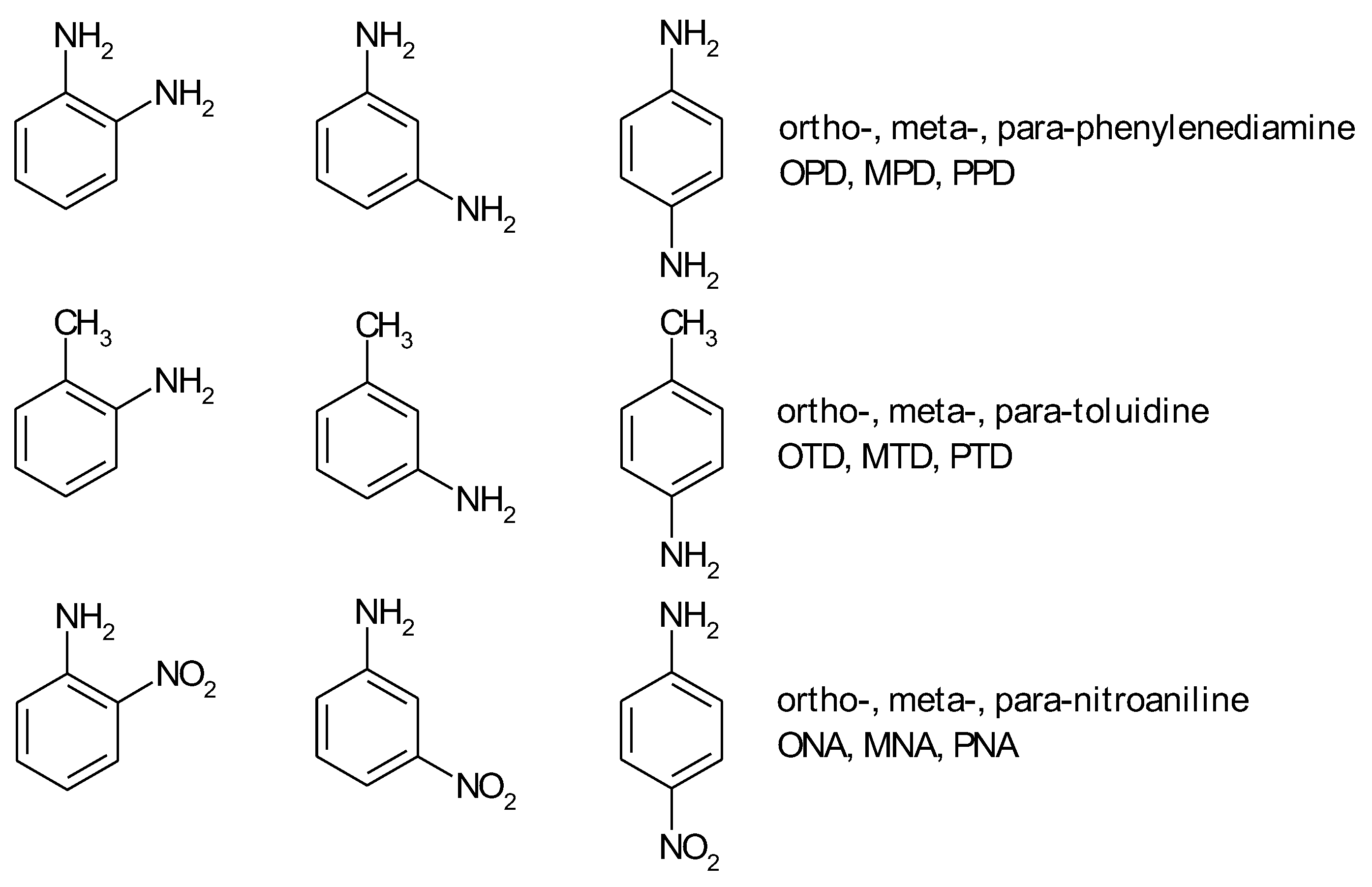

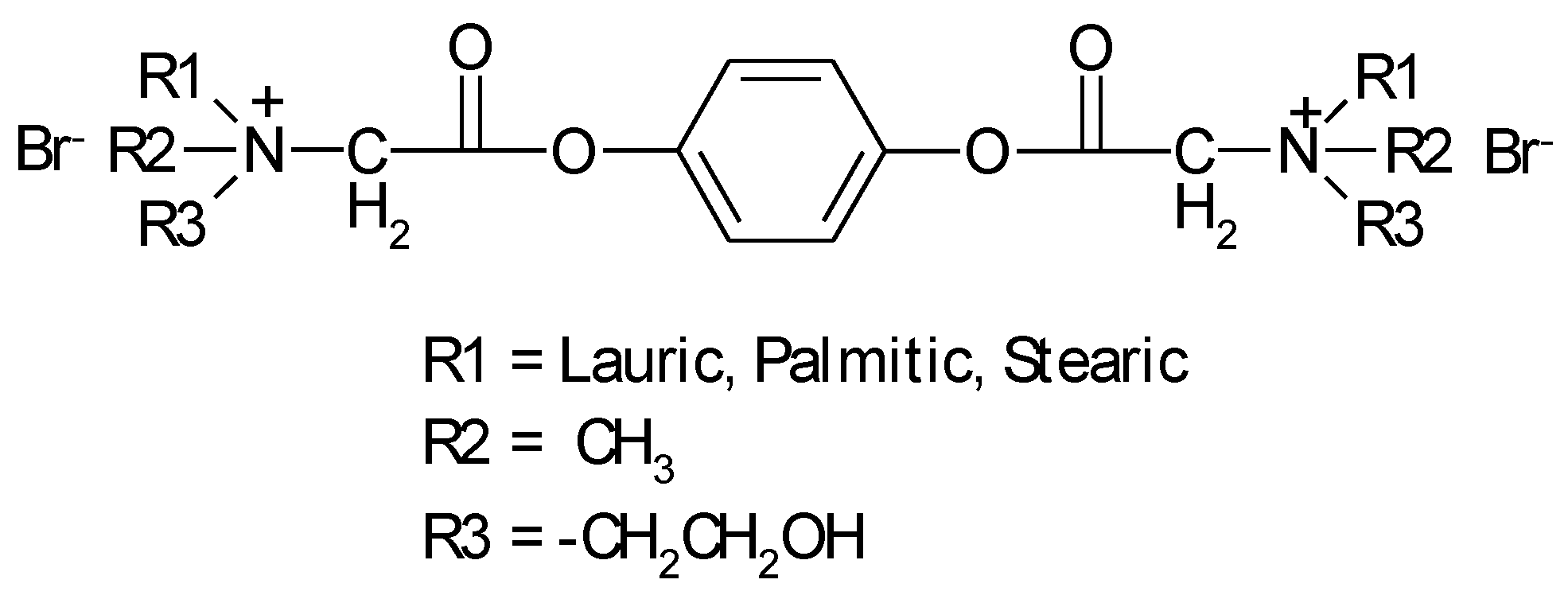
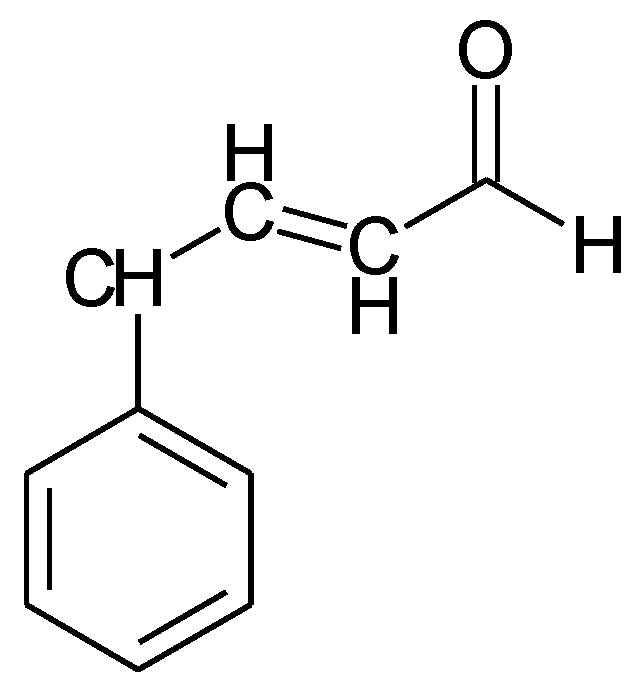
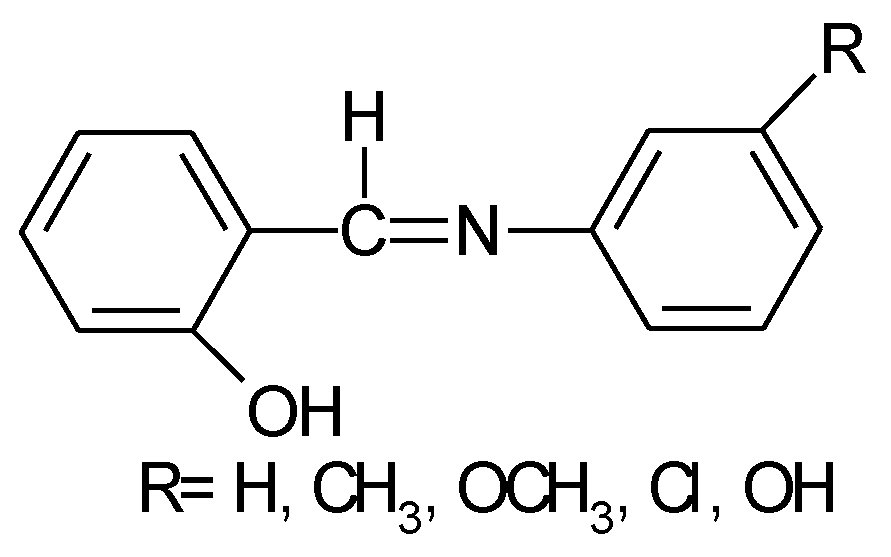

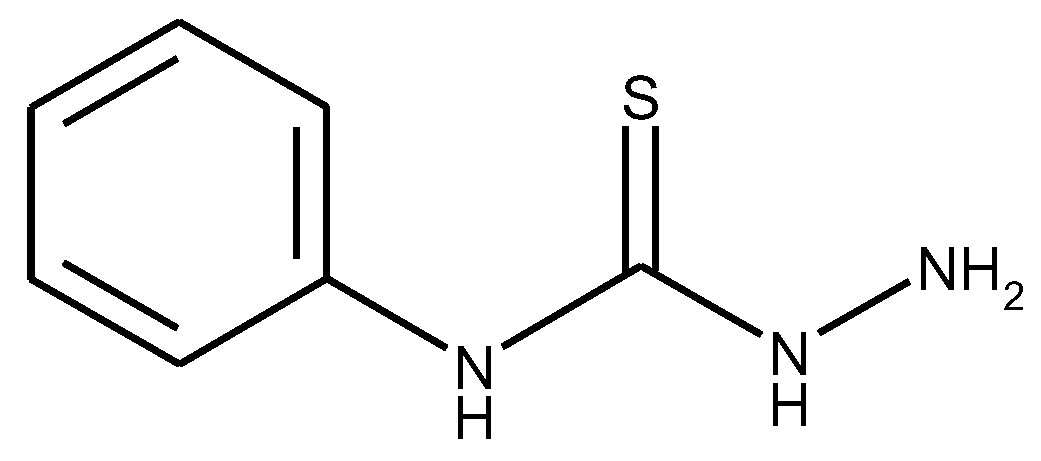
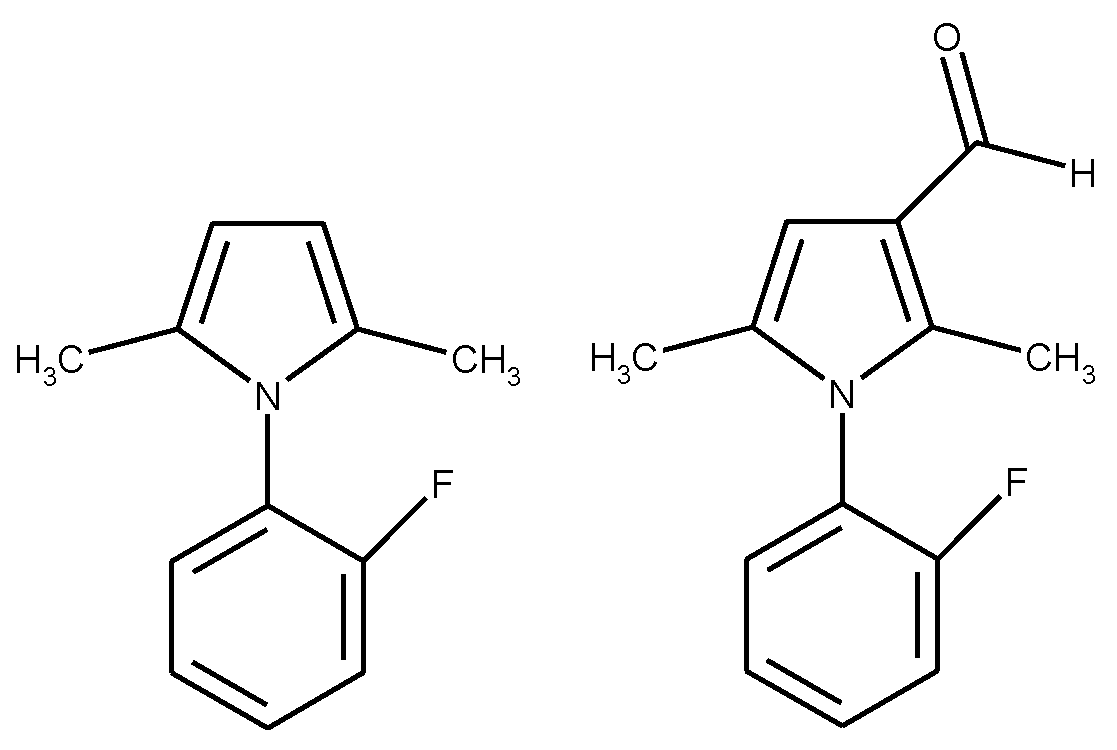
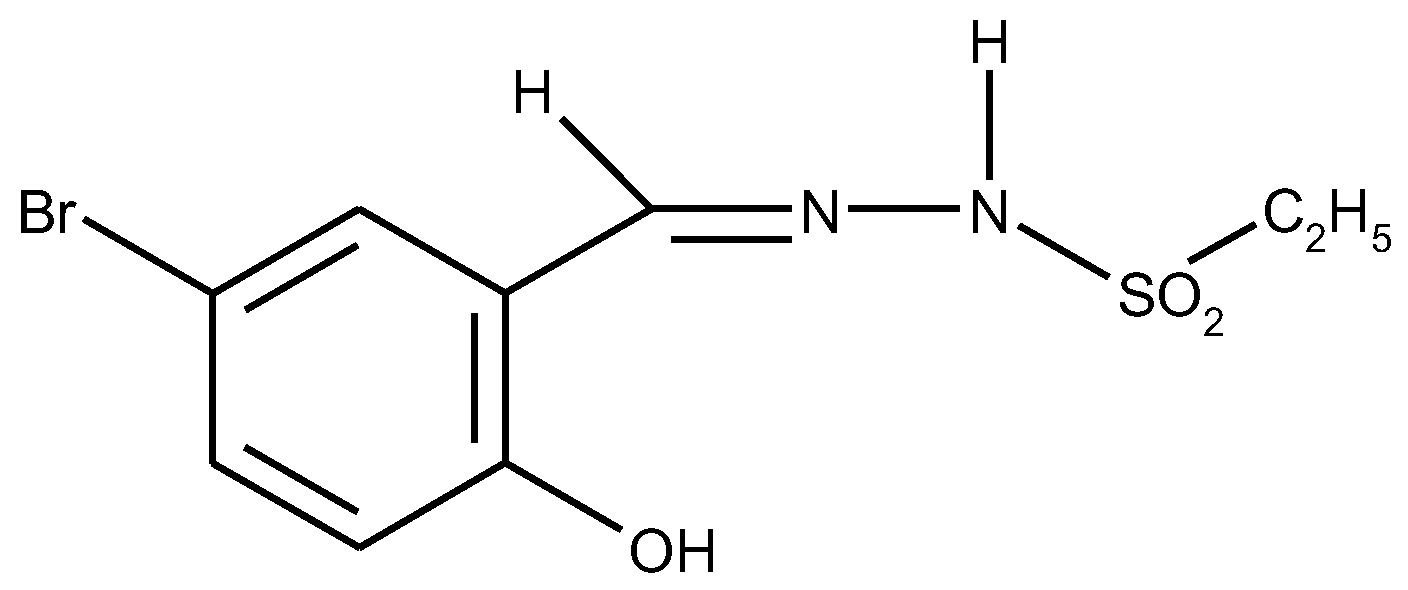
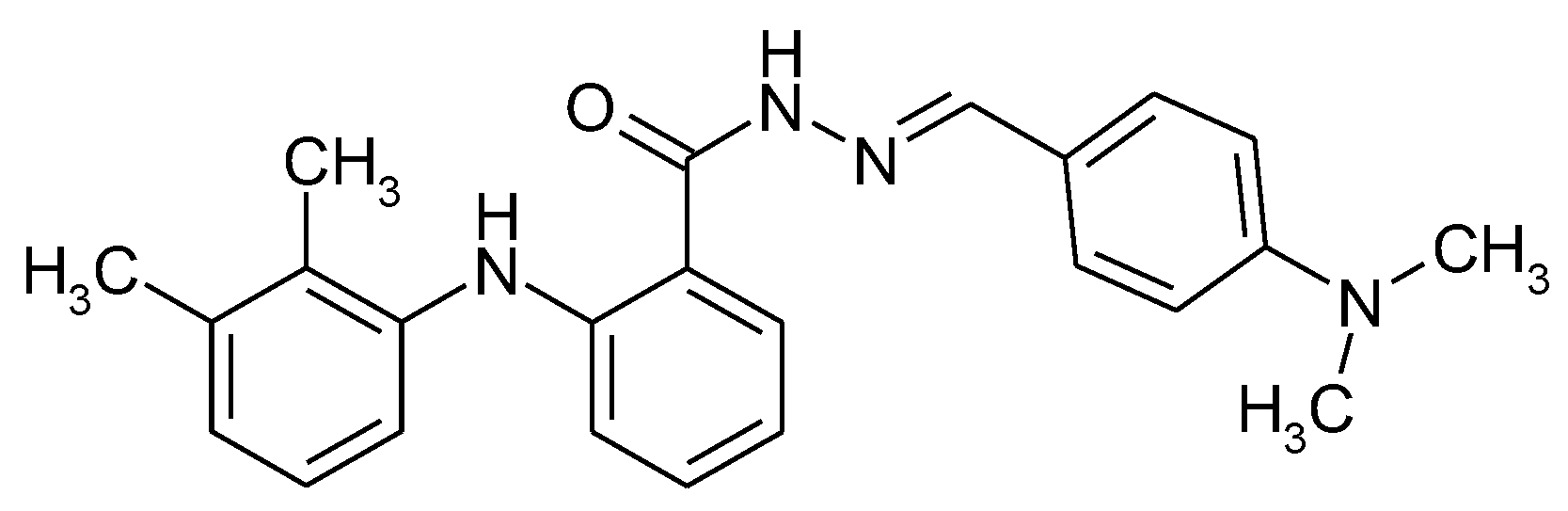
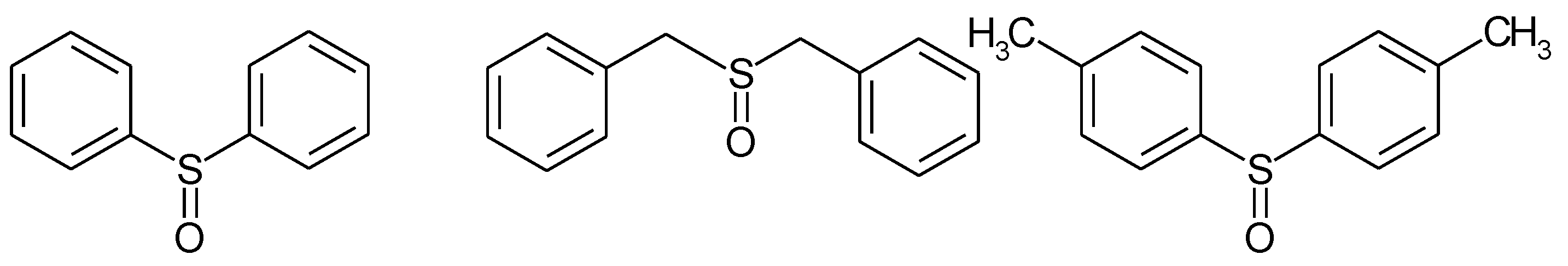
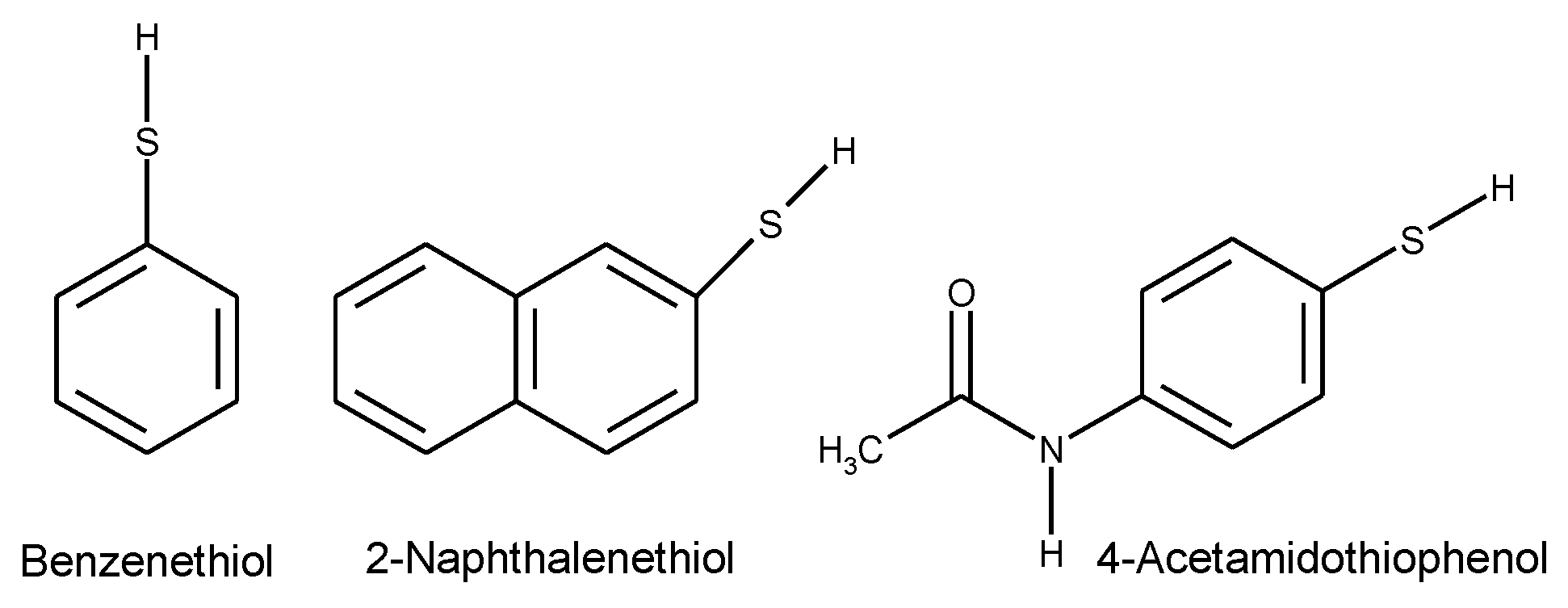
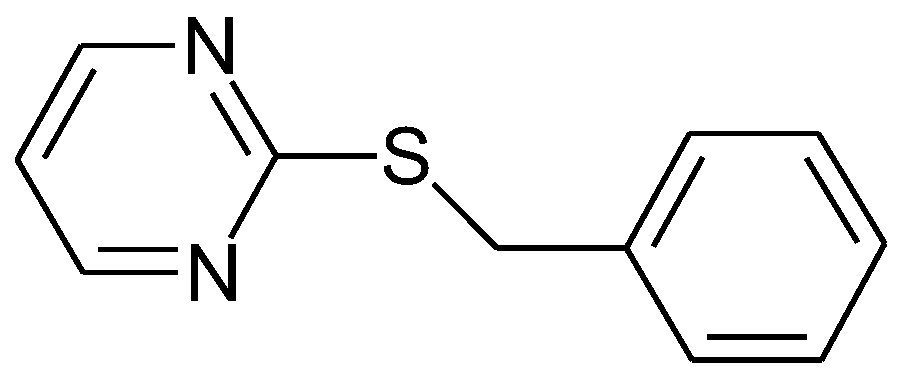
1.2. Substituted Heteroatom-Containing Six-Membered Rings
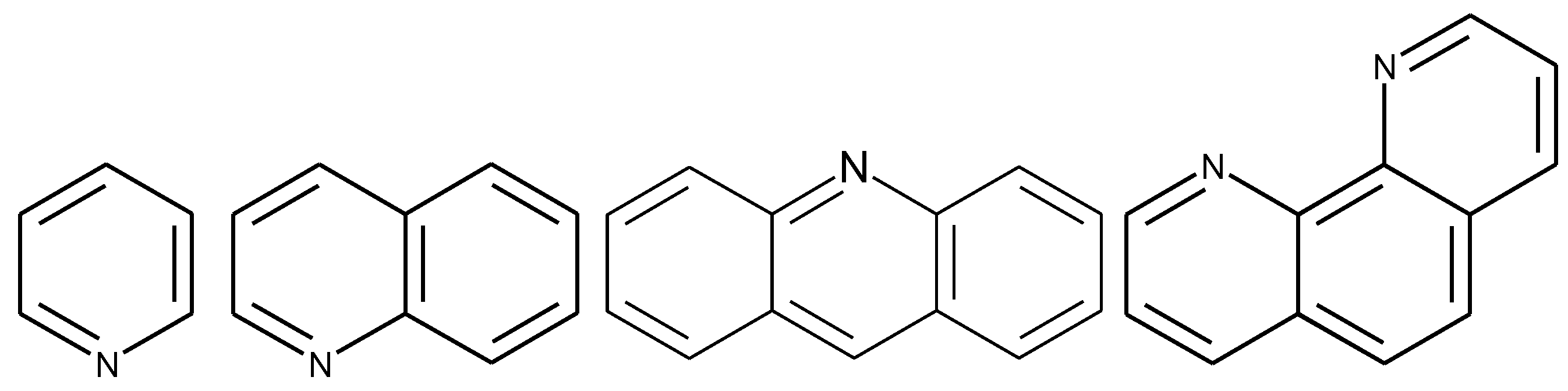
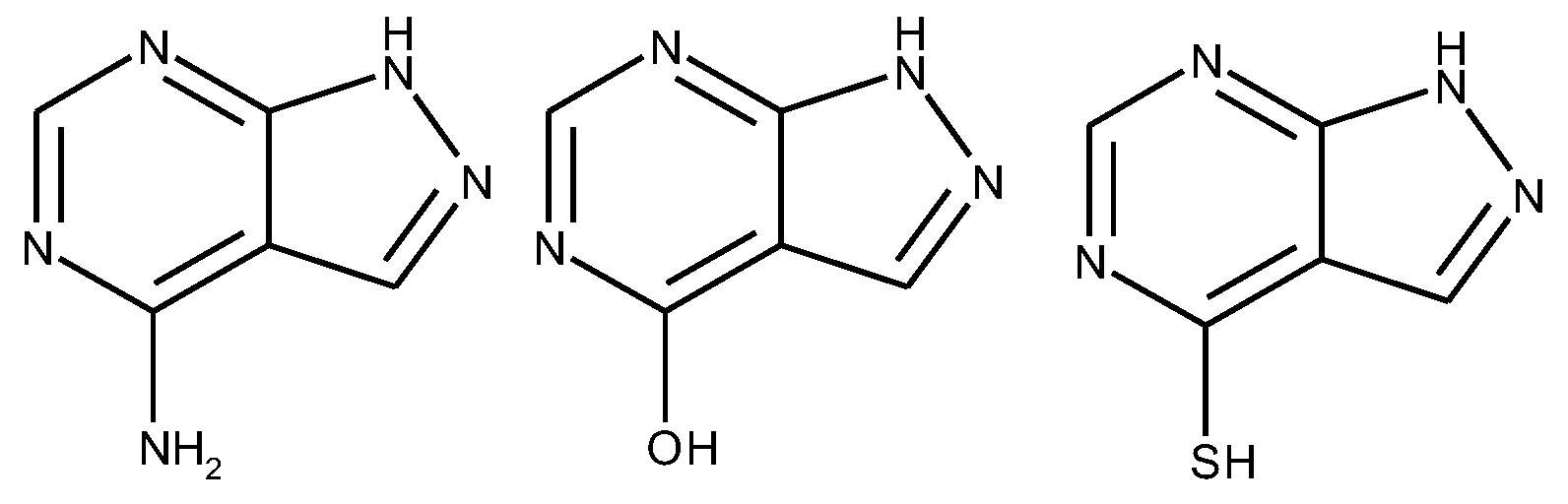

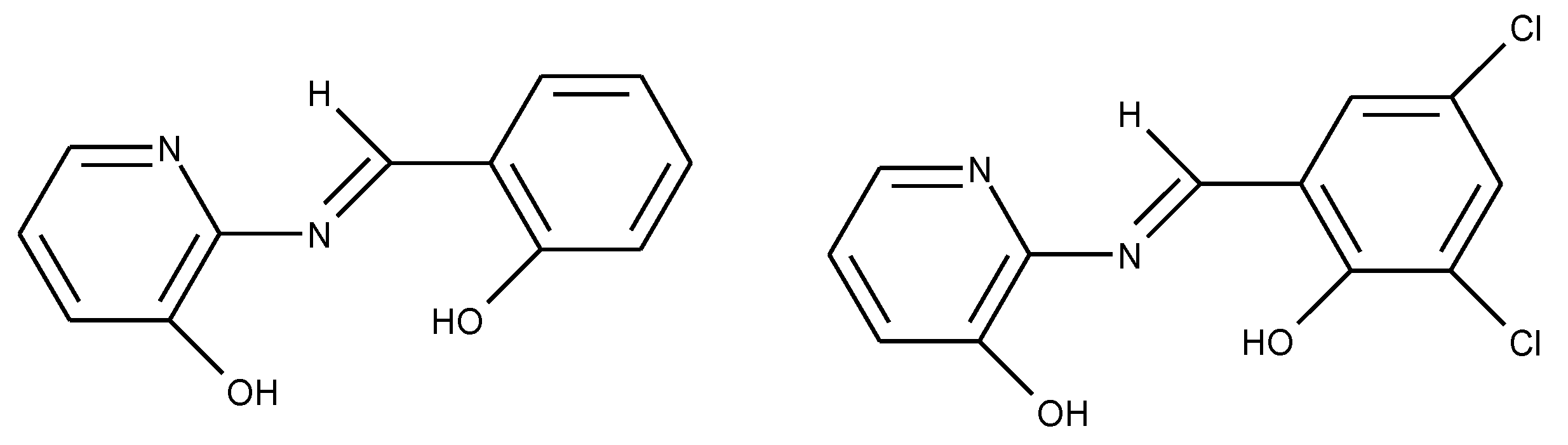
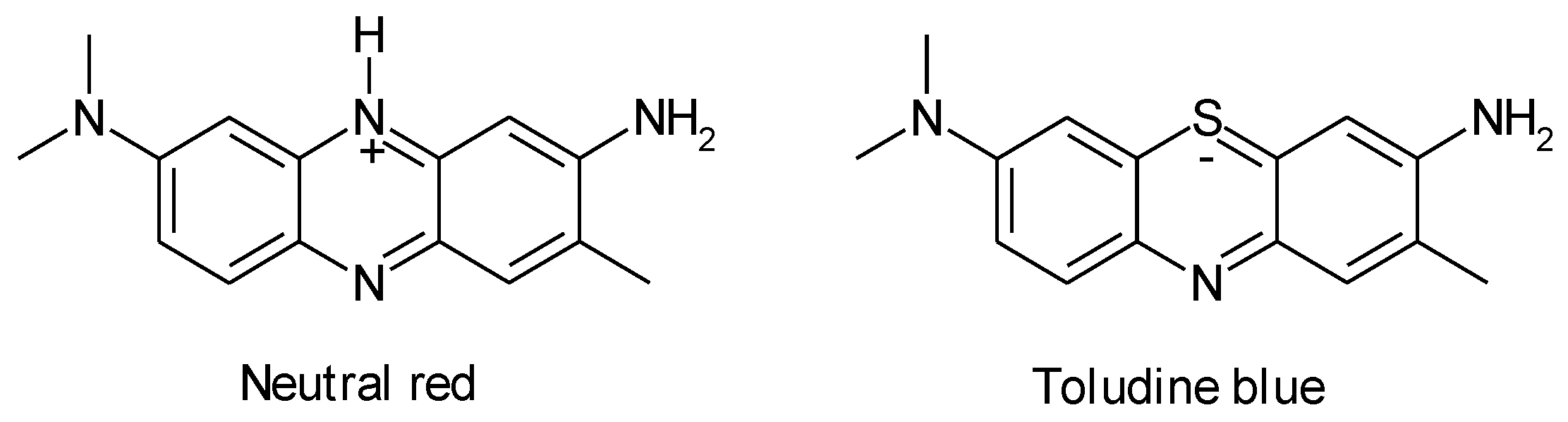
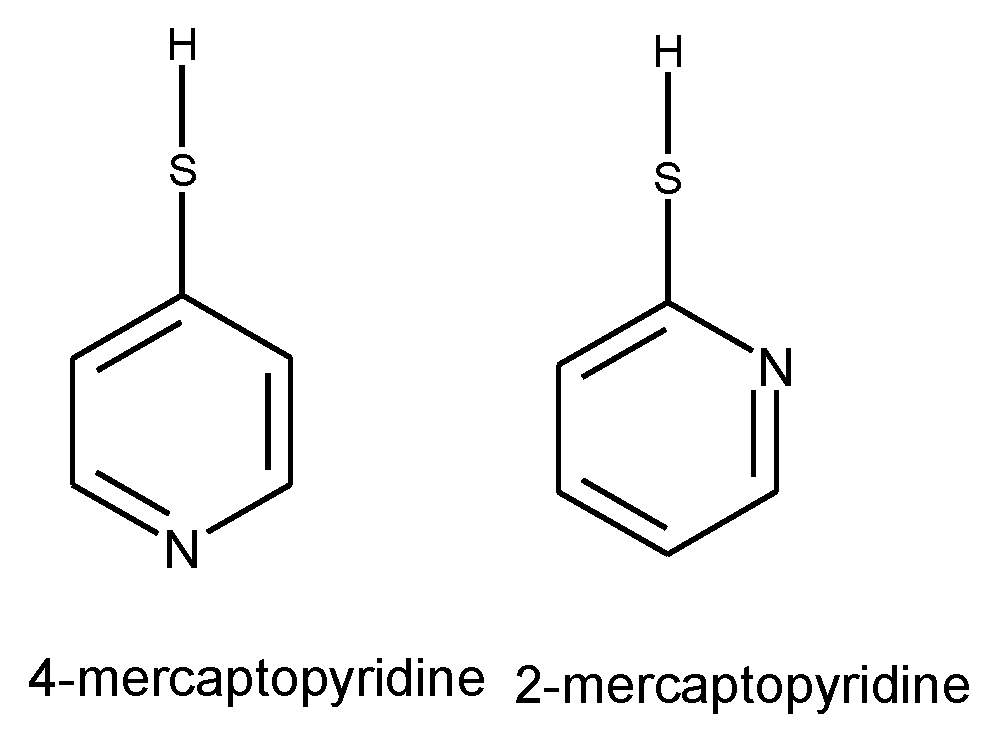
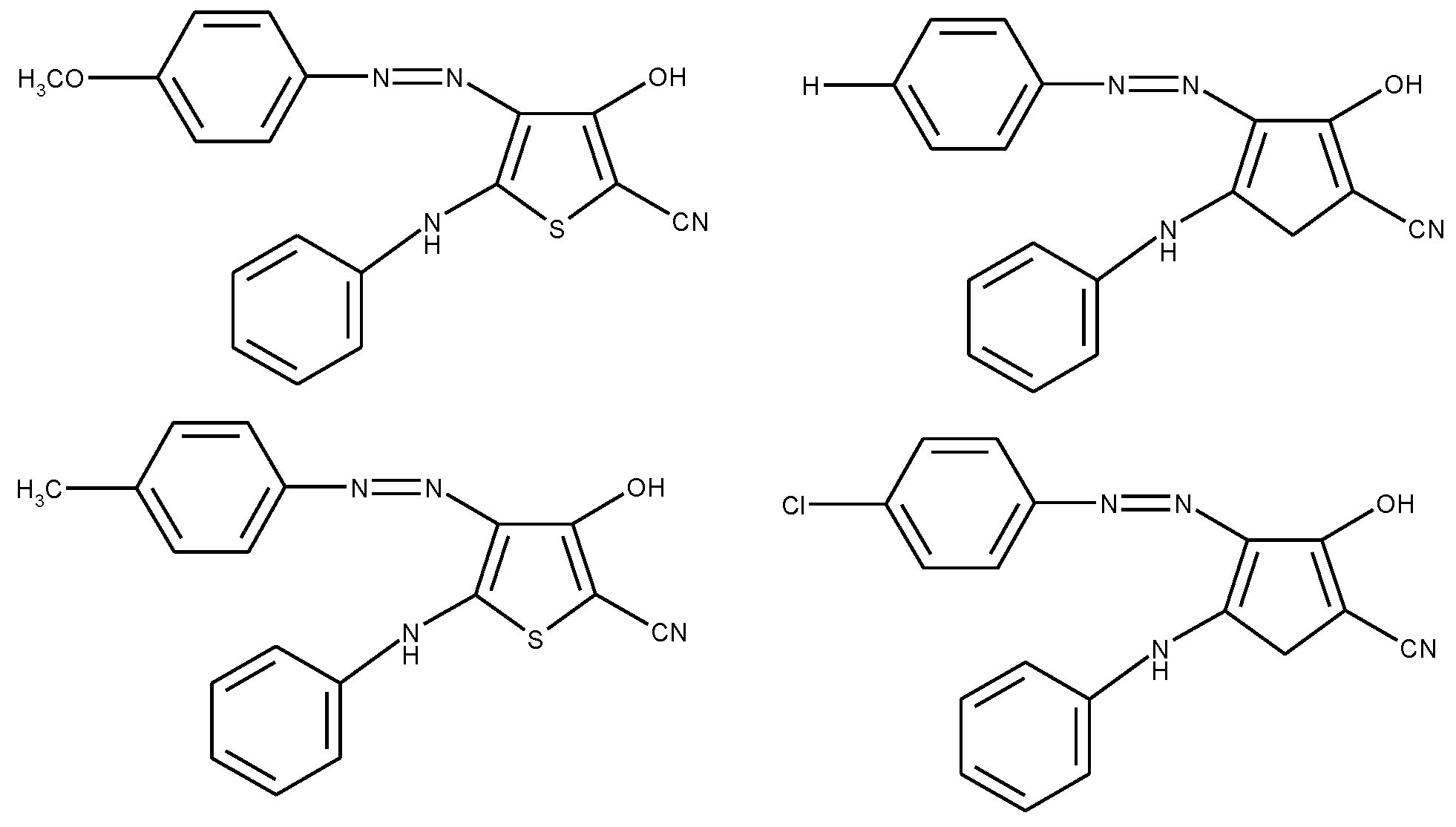
1.3. Substituted Five-Membered Hetero-Atom Containing Rings
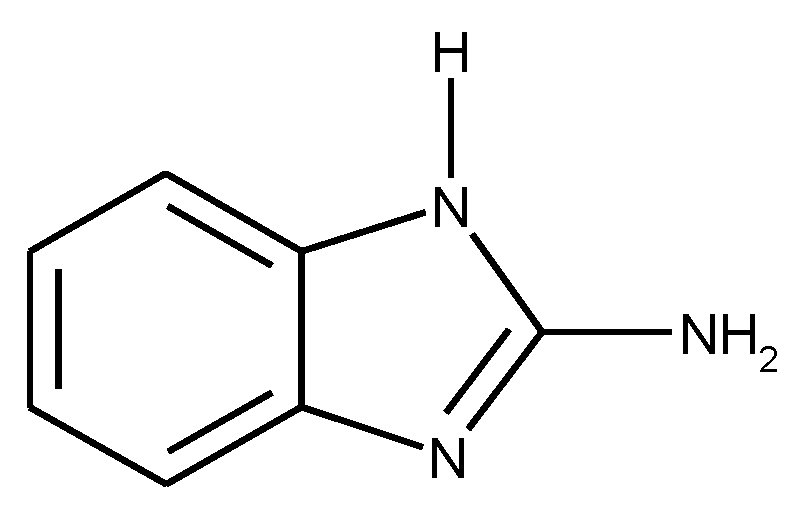
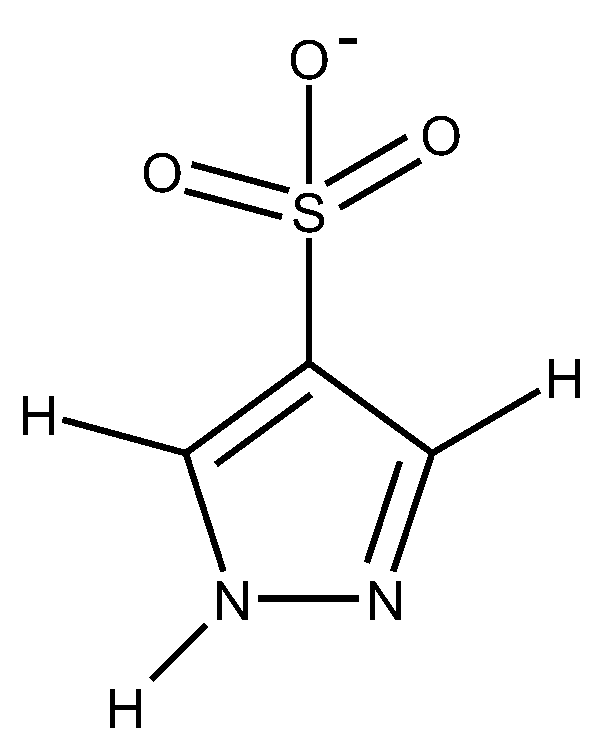
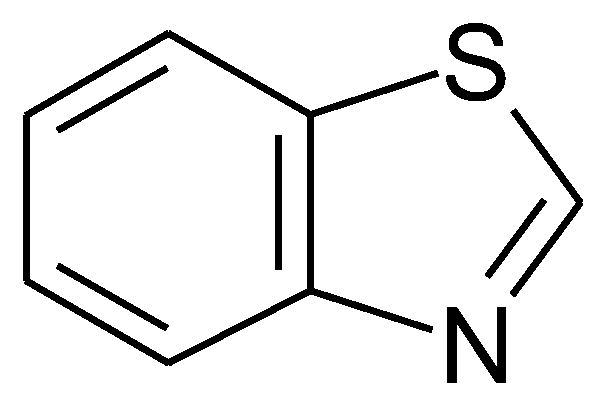
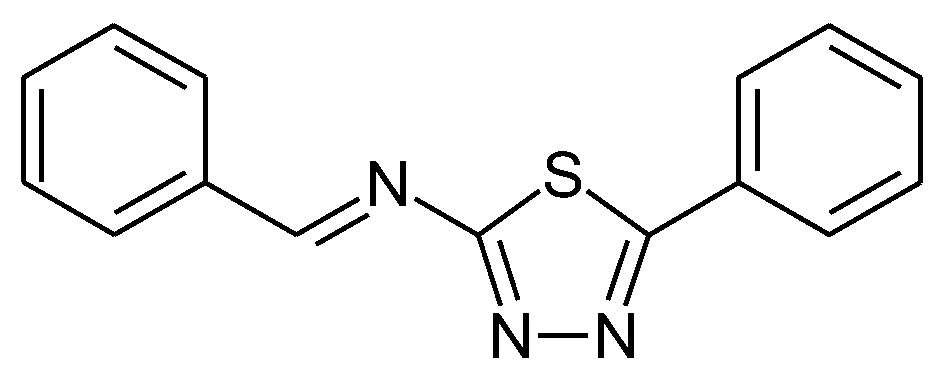
1.4. Natural Compounds, Pharmaceuticals, Drugs, Dyestuffs, and Mixtures
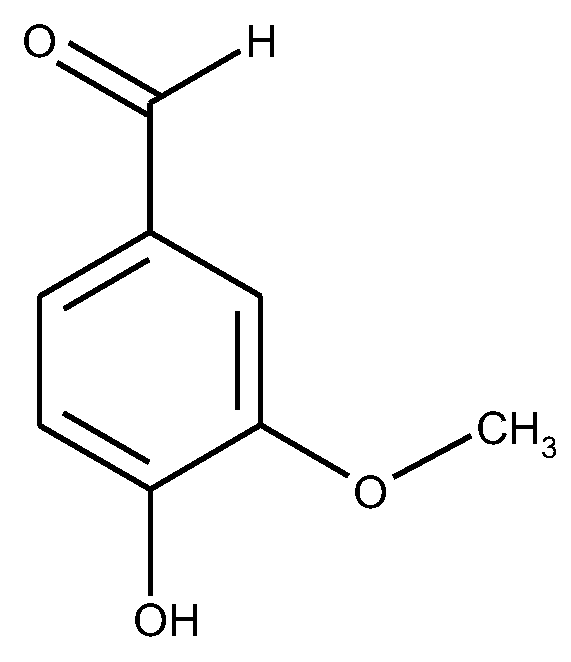

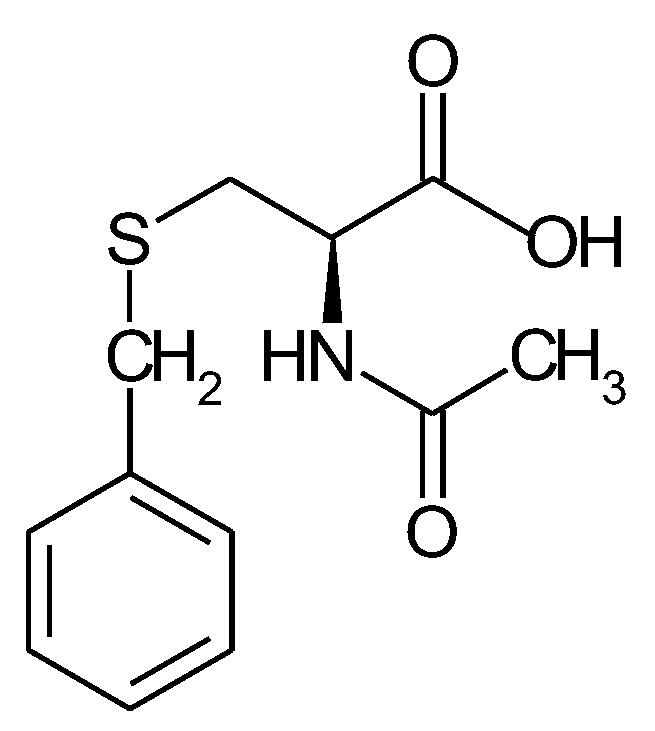
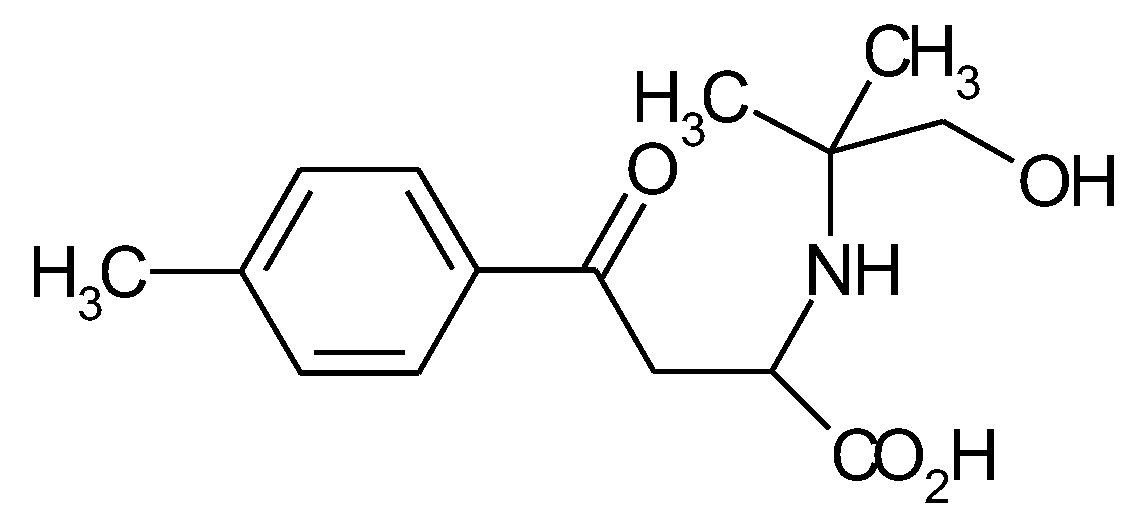
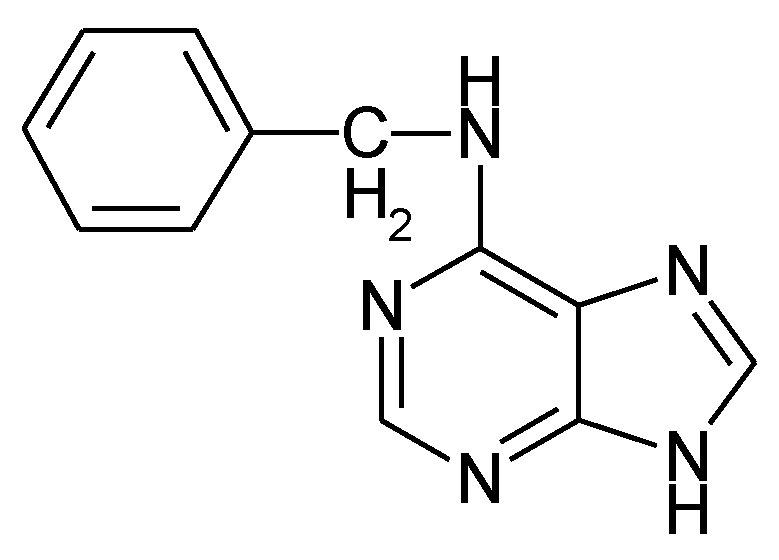
1.5. The State of Things
2. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaesche, H. Die Korrosion der Metalle, 3rd ed.; Springer-Verlag, Berlin, Germany, 1990.
- El-Meligi, A.A. Corrosion preventive strategies as a crucial need for decreasing environmental pollution and saving economics. Rec. Pat. Corros. Sci. 2010, 2, 22-33. [CrossRef]
- Foroulis, Z.A. Corrosion and corrosion inhibition in the petroleum industry. Mater. Corros. 1982, 33, 121-131. [CrossRef]
- Kaesche, H. The corrosion of metals, Springer-Verlag, Berlin, Germany, 2003.
- Kelly, R.G.; Scully, J.R.; Shoesmith, D.W.; Buchheit, R.G. Electrochemical Techniques in Corrosion Science and Engineering, Marcel Dekker, New York, USA 2003.
- Groysman, A. Corrosion for Everybody, Springer, Dordrecht, The Netherlands, 2010.
- Roberg, P.R. Corrosion Engineering Principles and Practice, McGraw Hill, New York, USA, 2008.
- Uhlig's corrosion handbook; Revie, R.W., Uhlig, H.H., Eds.; Wiley, New York, USA 2000.
- Perez, N. Electrochemistry and corrosion science, Kluwer Academic Publisher, New York, USA, 2004.
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control, John Wiley&Sons, Hoboken, USA 2008.
- Sastri, V.S. Green Corrosion Inhibitors, WILEY, Hoboken, USA, 2011.
- Roberge P.R. Corrosion Basics: An Introduction, 2rd ed.; NACE International, Houston, USA 2006.
- APV-Corrosion Handbook, APV, Getzville, USA, 2008.
- Jones, D.A. Principles and Prevention of Corrosion, Prentice Hall, Upper Saddle River, USA 1996.
- Roberge, P.R. Corrosion Inspection and Monitoring, John Wiley&Sons, Hoboken, USA 2007.
- Talbot, D.; Talbot, J. Corrosion science and technology, CRC Press, Boca Raton; USA, 1998.
- Weir, T.W.; Van De Ven, P. Review of organic acids as inhibitors in engine coolants. SAE Techn.Pap. 1996, 960641.
- Edwards W.C. Toxicology of oil field wastes. Hazards to livestock associated with the petroleum industry. The Veterinary clinics of North America. Food animal practice 1989, 5, 363-374. [CrossRef]
- Casanova, L.; Ceriani, F.; Messinese, E.; Paterlini, L.; Beretta, S.; Bolzoni, F.M.; Brenna, A.; Diamanti, M.V.; Ormellese, M.; Pedeferri, M. Recent Advances in the Use of Green Corrosion Inhibitors to Prevent Chloride-Induced Corrosion in Reinforced Concrete, Materials 2023, 16, 7462.
- Shkoor, M.; Jalab, R.; Khaled, M.; Shawkat, T.S.; Korashy, H.M.; Saad, M.; Su, H.L.; Bani-Yaseen, A.D. Experimental and theoretical investigations of the effect of bis-phenylurea-based aliphatic amine derivative as an efficient green corrosion inhibitor for carbon steel in HCl solution, Heliyon 2023, 9, e20254.
- Chafiq, M.; Chaouiki, A.; Lgaz, H.; Salghi, R.; Bhaskar, K.V.; Marzouki, R.; Bhat, K.S.; Ali, I.H.; Khan, M.I.; Chung, L.M. Inhibition performances of spirocyclopropane derivatives for mild steel protection in HCl, Mater. Chem. Phys. 2020, 243, 122582.
- Anitha, R.; Chitra, S.; Hemapriya, V.; Chung, I.M.; Kim, S.H.; Prabakaran, M. Implications of eco-addition inhibitor to mitigate corrosion in reinforced steel embedded in concrete. Constr. Build. Mater. 2019, 213, 246-256. [CrossRef]
- Saurbier, K.; Schultze, J.W.; Geke, J. Temporary inhibitors of corrosion in wet atmosphere: electrochemical investigations of the mechanism and efficiency, Electrochim. Acta 1994, 39, 1171-1178. [CrossRef]
- Rosenfeld, I.L.; Persiantseva, B.P.; Terentiev, P.B. Mechanism of Metal Protection by Volatile Inhibitors. Corrosion 1964, 20, 222-234. [CrossRef]
- Subramanian, A.; Natesan, M.; Muralidharan, V.S.; Balakrishnan, K.; Vasudevan, T. An overview: Vapor phase corrosion inhibitors. Corrosion 2000, 56, 144-155. [CrossRef]
- Quraishi, M.A.; Jamal, D.; Bhardwaj, V. Protection of metals by new vapour phase corrosion inhibitors. Bull. Electrochem. 2004, 20, 459-463.
- Xin, Z.L.; Li, J.; Zhang, D.Q. Development of a novel volatile corrosion inhibitor paper without nitroso-group. Corr. Prot. 2009, 30, 43-45.
- Shkol'nikov, V.M.; Shekhter, Yu.N.; Sidorova, N.N.; Fuks, I.G.; Antipova, K.M.; Koroleva, N.D. Chemical composition of lubricating oils in relation to protective properties. Chem. Technol. Fuels Oils 1973, 9, 264-267. [CrossRef]
- Shekhter, Yu.N. Mechanism of action of oil-soluble corrosion inhibitors. Chem. Technol. Fuels Oils 1966, 2, 185-189. [CrossRef]
- Burant, A.; Selbig, W.; Furlong, E.T.; Higgins, C.P. Trace organic contaminants in urban runoff: Associations with urban land-use. Environm. Pollut. 2018, 242, 2068-2077. [CrossRef] [PubMed]
- Pritchard, J.C.; Mills Hawkins, K.; Cho, Y.M.; Spahr, S.; Struck, S.D.; Higgins, C.P.; Luthy, R.G. Black Carbon-Amended Engineered Media Filters for Improved Treatment of Stormwater Runoff. ACS Environ. Au 2023, 3, 34-46. [CrossRef]
- H.E. Fuchte, N. Beck, E. Bieg, V.J. Bayer, C. Achten, M. Krauss, A. Schäffer, K.E.C. Smith A look down the drain: Identification of dissolved and particle bound organic pollutants in urban runoff waters and sediments. Environm. Poll. 2022, 302, 119047. [CrossRef]
- Knudsen, B.L.; Hjelsvold, M.; Frost, T.K.; Eiken, M.B.; Grini, P.G.; Willumsen, C.F.; Torvik, H. Toward Zero Environmental Impact of the Produced Water. Offshore Europe Conference - Proceedings 2003, 320-325. [CrossRef]
- Ferraz, E.R.A.; De Oliveira, G.A.R.; De Oliveira, D.P. The impact of aromatic amines on the environment: Risks and damages. Front. Biosci. 2012, 4 E, 914-923. [CrossRef]
- Makrides, A.C.; Hackerman, N. Inhibition of acid dissolution of metals. I. Some general observations. J. Phys. Chem. 1955, 59, 707-710. [CrossRef]
- Harvey, T.J.; Walsh, F.C.; Nahlé‚ A.H. A review of inhibitors for the corrosion of transition metals in aqueous acids. J. Mol. Liq. 2018, 266, 160-175. [CrossRef]
- Kuznetsov, Yu.I. Adsorptive passivation of iron by organic acid anions. R. J. Electrochem. 2004, 40, 1287-1291. [CrossRef]
- McCafferty, E. Mechanisms of corrosion control by inhibitors. Society of the Plastics Industry, Reinforced Plastics/Composites Institute, Annual Conference - Proceedings 1979, 279-317.
- Society of the Plastics Industry, Reinforced Plastics/Composites Institute, Annual Conference - Proceedings (H. Leidheiser Ed.) Science Press, Princeton 1979.
- Dehghani, A.; Berdimurodov, E.; Verma, C.; Verma, D.K.; Berdimuradov, K.; Quraishi, M.A.; Aliev, N. Constructing efficacy: a novel perspective on organic corrosion inhibitors and interfacial interactions. Chem. Pap. 2023, 78, 1367-1397. [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Ebenso, E.E.; Quraishi, M.A. Substituents effect on corrosion inhibition performance of organic compounds in aggressive ionic solutions: A review. J. Mol. Liq. 2018, 251, 100-118. [CrossRef]
- Verma, C.; Quraishi, M.A.; Ebenso, E.E. A review on ammonia derivatives as corrosion inhibitors for metals and alloys. in: Green Energy and Technology: Sustainable Ammonia Production (Inamuddin, R. Boddula, A.M. Asiri Ed.) Springer, Cham 2020, p. 49-67. [CrossRef]
- Verma, C.; Alfantazi, A.; Quraishi, M.A.; Rhee, K.Y. Significance of Hammett and Taft substituent constants on bonding potential of organic corrosion inhibitors: Tailoring of reactivity and performance. Coord. Chem. Rev. 2023, 495, 215385. [CrossRef]
- Schleyer, P.V.R.; Jiao, H. What is aromaticity? Pure Appl. Chem. 1996, 68, 209-218. [CrossRef]
- Chauhan, D.S.; Singh, P.; Quraishi, M.A. Quinoxaline derivatives as efficient corrosion inhibitors: Current status, challenges and future perspectives. J. Mol. Liq. 2020, 320, 114387. [CrossRef]
- Carranza, M.S.; Reyes, Y.I.A.; Gonzales, E.C.; Arcon, D.P.; Franco Jr., F.C. Electrochemical and quantum mechanical investigation of various small molecule organic compounds as corrosion inhibitors in mild steel. Heliyon 2021, 7, e07952. [CrossRef]
- Al-Taq, A.A.; Ali, S.A.; Nasr-El-Din, H.A. Inhibition performance of a new series of mono-/diamine-based corrosion inhibitors for HCl solutions. SPE J. 2009, 14, 627-633. [CrossRef]
- Halambek, J.; Grassino, A.N.; Cindrić, I. Inhibition performance of eugenol and linalool on aluminium corrosion: A comparative study. Int. J. Electrochem. Sci. 2020, 15, 857-867. [CrossRef]
- Banerjee, G.; Malhotra, S.N. Contribution to adsorption of aromatic amines on mild steel surface from HCl solutions by impedance, UV, and Raman spectroscopy. Corrosion 1992, 48, 10-15. [CrossRef]
- Holze, R. Surface and Interface Analysis - An Electrochemists Toolbox, Springer, Heidelberg, Germany, 2009. [CrossRef]
- Holze, R. Competition of anchoring groups in adsorption on gold electrodes - A comparative spectroelectrochemical study of 4-mercaptobenzonitrile and aromatic nitriles. J. Solid State Electr. 2013, 17, 1869-1879. [CrossRef]
- Uehara, J.; Aramaki, K. A Surface-Enhanced Raman Spectroscopy Study on Adsorption of Some Sulfur-Containing Corrosion Inhibitors on Iron in Hydrochloric Acid Solutions. J. Electrochem. Soc. 1991, 138, 3245-3251. [CrossRef]
- Durnie, W.H.; De Marco, R.; Jefferson, A.; Kinsella, B.J. In situ SERS study of the adsorption of inhibitors of carbon dioxide corrosion. Surf. Interface Anal. 2003, 35, 536-543. [CrossRef]
- Fockaert, L.I.; Würger, T.; Unbehau, R.; Boelen, B.; Meißner, R.H.; Lamaka, S.V.; Zheludkevich, M.L.; Terryn, H.; Mol, J.M.C. ATR-FTIR in Kretschmann configuration integrated with electrochemical cell as in situ interfacial sensitive tool to study corrosion inhibitors for magnesium substrates, Electrochim. Acta 2020, 345, 136166. [CrossRef]
- Telegdi, J.; Shaban, A.; Kálmán, E. EQCM study of copper and iron corrosion inhibition in presence of organic inhibitors and biocides. Electrochim. Acta 2000, 45, 3639-3647. [CrossRef]
- Kern, P.; Landolt, D. Adsorption of a bromine labeled carboxylic acid corrosion inhibitor on iron measured with EQCM, EIS and XPS. Corros. Sci. 2002, 44, 1809-1824. [CrossRef]
- Murmu, M.; Murmu, N.C.; Ghosh, M.; Banerjee, P. Density functional theory, Monte Carlo simulation and non-covalent interaction study for exploring the adsorption and corrosion inhibiting property of double azomethine functionalised organic molecules. J. Adhes. Sci. Technol. 2022, 36, 2732-2760. [CrossRef]
- Chauhan, D.S.; Verma, C.; Quraishi, M.A. Molecular structural aspects of organic corrosion inhibitors: Experimental and computational insights. J. Molec. Struct. 2021, 1227, 129374. [CrossRef]
- Verma, C.; Alrefaee, S.H.; Rhee, K.Y.; Quraishi, M.A.; Ebenso, E.E. Thiol (-SH) substituent as functional motif for effective corrosion protection: A review on current advancements and future directions. J. Mol. Liq. 2021, 324, 115111. [CrossRef]
- Mercer, W.C. Investigation of carboxylic acids as corrosion inhibitors in engine coolant. ASTM Spec. Tech. Publ. 1993, 3, 44-62. [CrossRef]
- Riggs Jr., O.L.; Every, R.L. Study of Organic Inhibitors For Hydrochloric Acid Attack on Iron. Corrosion 1962, 18, 262-269. [CrossRef]
- Every, R.L.; Riggs Jr., O.L. Organic inhibitors for carbon steel in hydrochloric acid. Mater. Prot. 1964, 3, 46-47.
- Cox, P.F.; Every, R.L.; Riggs, O.L. Study of Aromatic Amine Inhibitors By Nuclear Magnetic Resonance. Corrosion 1964, 20, 299-302. [CrossRef]
- Sutter, E.M.M.; Ammeloot, F.; Pouet, M.J.; Fiaud, C.; Couffignal, R. Heterocyclic compounds used as corrosion inhibitors: Correlation between 13C and 1H NMR spectroscopy and inhibition efficiency. Corros. Sci. 1999, 41, 105-115. [CrossRef]
- Behzadi, H.; Manzetti, S.; Dargahi, M.; Roonasi, P.; Khalilnia, Z. Application of calculated NMR parameters, aromaticity indices and wavefunction properties for evaluation of corrosion inhibition efficiency of pyrazine inhibitors. J. Molec. Struct. 2018, 1151, 34-40. [CrossRef]
- Gopalakrishnan, V.; Balasubramanian, A.; Subramanian, L.; Muhammed, R.I.; Garg, R.; Eddy, N.O. Experimental and Theoretical Analysis on Mild Steel Corrosion Inhibition by Two Novel Compounds (FD and ACP) in Acidic Media. Port. Electrochim. Acta 2022, 41, 223-246. [CrossRef]
- Nandi, M.M.; Banerjee, R. Organic corrosion inhibitors for copper and brass - A review. J. Ind. Chem. Soc. 2014, 91, 977-989.
- Morad, M.S. An electrochemical study on the inhibiting action of some organic phosphonium compounds on the corrosion of mild steel in aerated acid solutions. Corros. Sci. 2000, 42, 1307-1326. [CrossRef]
- Zucchi, F.; Trabanelli, G.; Frignani, A.; Zucchini, M. The inhibition of stress corrosion cracking of stainless steels in chloride solutions. Corros. Sci. 1978, 18, 87-95. [CrossRef]
- Yang, Y.; Wang, Y.; Li, M.X.; Wang, T.; Wang, D.; Wang, C.; Zha, M.; Wang, H.Y. Recent progress in self-repairing coatings for corrosion protection on magnesium alloys and perspective of porous solids as novel carrier and barrier. J. Magnesium Alloys 2023, 11, 3585-3608. [CrossRef]
- Podest, J.J.; Piatti, R.C.V.; Arvia, A.J. The influence of corrosion inhibitors on the periodic oscillations of the current under controlled potential for mild steel in 0.75 M H2SO4, International Congress on Metallic Corrosion - Proceedings Vol. 1, Toronto, June 3-7, 1984 359-362.
- Archer, W.L. Comparison of Chlorinated Solvent-Aluminum Reaction Inhibitors. Ind. Eng. Chem. Prod. Res. Dev. 1979, 18, 131-135. [CrossRef]
- Huang, D.; Hu, J.; Song, G.L.; Guo, X. Inhibition effect of inorganic and organic inhibitors on the corrosion of Mg-10Gd-3Y-0.5Zr alloy in an ethylene glycol solution at ambient and elevated temperatures. Electrochim. Acta 2011, 56, 10166-10178. [CrossRef]
- Michelhaugh, S.L.; Bhardwaj, C.; Cali, G.J.; Bravo, B.G.; Bothwell, M.E.; Berry, G.M.; Soriaga, M.P. The influence of chemisorbed organic monolayers on electrode surface oxidation. Corrosion 1991, 47, 322-328. [CrossRef]
- Bentiss, F.; Lagrenée, M. Heterocyclic compounds as corrosion inhibitors for mild steel in hydrochloric: Acid medium-correlation between electronic structure and inhibition efficiency. J. Mater. Environm. Sci. 2011, 2, 13-17.
- Lukovits, I.; Kosztolányi, T.; Kálmán, E.; Pálinkás, G. Corrosion inhibitors: Correlation between chemical structure and efficiency. NACE - Int.Corros.Conf.Ser. 1999, 1999-April.
- Lukovits, I.; Kálmán, E.; Zucchi, F. Corrosion inhibitors - Correlation between electronic structure and efficiency. Corrosion 2001, 57, 3-8. [CrossRef]
- Kokalj, A.; Lozinsek, M.; Kapun, B.; Taheri, P.; Neupane, S.; Losada-Pérez, P.; Xie, C.; Stavber, S.; Crespo, D.; Renner, F.U.; Mol, A.; Milosev, I. Simplistic correlations between molecular electronic properties and inhibition efficiencies: Do they really exist?, Corros. Sci. 2021, 179, 108856. [CrossRef]
- Lukovits, I.; Shaban, A.; Kálmán E. Corrosion Inhibitors: Quantitative Structure-Activity Relationships. Russ. J. Electrochem. 2003, 39, 177-181. [CrossRef]
- Horner, L.; Meisel, K. Corrosion Inhibitors 23 (1) - Does There Exist a Structure-Efficiency Relation in the Organic Inhibitors of Aluminium Corrosion? Werkst. Korros. 1978, 29, 654-664. [CrossRef]
- Abd El Rehim, S.S.; Hassan, H.H.; Amin, M.A. The corrosion inhibition study of sodium dodecyl benzene sulphonate to aluminium and its alloys in 1.0 M HCl solution. Mater. Chem. Phys. 2003, 78, 337-348. [CrossRef]
- Öztürk, S.; Yildirim, A. Synthesis of aromatic structured di-cationic surfactants used as inhibitors against corrosion of carbon steel in acidic medium. J. Turk. Chem. Soc. A 2018, 5, 819-828. [CrossRef]
- Pianka, H.; Falah, S.; Zanna, S.; Bezborodov, V.; Mikhalyonok, S.; Kuz'menok, N.; Chernik, A.; Xue, Y.; Taleb, A. Anticorrosion Efficiency of Inhibitor Coatings Based on Ammonium Cation with Different Substituents: The Influence of Wettability and Molecular Structure. Coatings 2021, 11, 1512. [CrossRef]
- Chernyad'ev, I.N.; Shein, A.B.; Nedugov, A.N. Study of organic derivatives of the VIa group elements as steel acid corrosion inhibitors. Prot. Met. 2005, 41, 437-442. [CrossRef]
- Chernyad'ev, I.N.; Shein, A.B.; Nedugov, A.N. Effects of arylthio-and arylseleno(methoxy)methanes on acid corrosion of CT3 steel. Prot. Met. 2007, 43, 264-268. [CrossRef]
- Madram, A.R.; Shokri, F.; Sovizi, M.R.; Kalhor, H. Aromatic carboxylic acids as corrosion inhibitors for aluminium in alkaline solution. Portug. Electrochim. Acta 2016, 34, 395-405. [CrossRef]
- Thirumalaikumar, M.; Jegannathan, S. Inhibition effects of nitrones on the corrosion of mild steel in organic acid media. Portug. Electrochim. Acta 2011, 29, 1-8. [CrossRef]
- Katharotiya, P.; Das, S.P. SYNTHESIS, CHARACTERIZATION and INVESTIGATION of CHALCONE AS CORROSION INHIBITORS for MILD STEEL in HYDROCHLORIC ACID. Eur. Chem. Bull. 2021, 10, 199-204.
- de Souza, T.M.; Cordeiro, R.F.B.; Viana, G.M.; Aguiar, L.C.S.; de Senna, L.F.; Malta, L.F.B.; D'Elia, E. Inclusion compounds of dibenzylthiourea with hydroxypropylated-cyclodextrins for corrosion protection of carbon steel in acidic medium. J. Molec. Struct. 2016, 1125, 331-339. [CrossRef]
- Torres, V.V.; Rayol, V.A.; Magalhaes, M.; Viana, G.M.; Aguiar, L.C.S.; Machado, S.P.; Orofino, H.; D'Elia, E. Study of thioureas derivatives synthesized from a green route as corrosion inhibitors for mild steel in HCl solution. Corr. Sci. 2014, 79, 108-118. [CrossRef]
- Gopiraman, M.; Selvakumaran, N.; Kesavan, D.; Kim, I.S.; Karvembu, R. Chemical and physical interactions of 1-benzo yl-3,3 disubstituted thiourea derivatives on mild steel surface: Corrosion inhibition in acidic media. Ind. Eng. Chem. Res. 2012, 51, 7910-7922. [CrossRef]
- Hamitouche, H.; Khelifa, A.; Kouache, A.; Moulay, S. Study of the inhibiting effect of a quaternary ammoniumsurfactants mixture synthesized from petroleum fraction (reformate) against the carbon steel corrosion in HCl 1 M, Res. Chem. Intermed. 2014, 40, 2859-2872. [CrossRef]
- Trofimov, V.A.; Spurkin, V.G.; Ablyazova, T.O.; Bocharov, A.A. New inhibitors of hydrogen sulfide corrosion for turbine oils. Khim. Tekhnol. Topl. Mas. 1997, 2, 14-15. [CrossRef]
- Babu, B.R.; Holze, R. Corrosion and hydrogen permeation inhibition for mild steel in HCl by isomers of organic compounds. Brit. Corr. J. 2000, 35, 204-209. [CrossRef]
- Hackermann , N.; Makrides, A.C. Action of polar organic inhibitors in acid dissolution of metals. Ind. Eng. Chem. 1954, 46, 523-527. [CrossRef]
- Holze, R. Potential- and pH-dependent adsorption of aniline on silver as evidenced with surface enhanced Raman spectroscopy. Electrochim. Acta 1987, 32, 1527-1532. [CrossRef]
- Holze, R. The adsorption of aniline on gold: a SERS study. J. Electroanal. Chem. 1988, 250, 143-157. [CrossRef]
- Mann, C.A.; Lauer, B.E.; Hultin, C.T. Organic Inhibitors of Corrosion: Aromatic Amines. Ind. Eng. Chem. 1936, 28, 1048-1051. [CrossRef]
- Blomgren, E.; Bockris J.O’. The adsorption of aromatic amines at the interface: Mercury-aqueous acid solution. J. Phys. Chem. 1959, 63, 1475-1484. [CrossRef]
- Xie, X.; Holze; R. Experimental methods in corrosion research. ChemTexts 2018,4, 5. [CrossRef]
- Xie, X.; Holze, R. Experimentelle Methoden in der Korrosionsforschung. Bunsen-Magazin 2018, 20, 100-117.
- Weber, E.; Richter, E.; Holze, R. o-Toluidine in electrochemistry – an overview. J. Solid State Electr. 2022, 26, 1097-1114. [CrossRef]
- Roscher, J.; Liu, D.; Holze, R. Concentration-dependent corrosion inhibition with electrochemical energy conversion systems by a disubstituted aromatic: A comparison of methods. Electrochem. Energy Technol. 2021, 7, 38-43.
- Roscher, J.; Liu, D.; Holze, R. A comparison of methods for corrosion inhibitor assessment: Mild steel protected by disubstituted aromatics. Mater. Corros. 2022, 73, 254-258. [CrossRef]
- Khaled, K.F.; Hackerman, N. Ortho-substituted anilines to inhibit copper corrosion in aerated 0.5 M hydrochloric acid. Electrochim. Acta 2004, 49, 485-495. [CrossRef]
- Khaled, K.F.; Hackerman, N. Investigation of the inhibitive effect of ortho-substituted anilines on corrosion of iron in 0.5 M H2SO4 solutions. Mater. Chem. Phys. 2003, 82, 949-960. [CrossRef]
- Talati, J.D.; Patel, G.A. Effect of substituted anilines on the corrosion of aluminium-manganese alloy in phosphoric acid. Br. Corros. J. 1976, 11, 47-51. [CrossRef]
- Talati, J.D.; Patel, G.A. Toluidines as corrosion inhibitors for 3S aluminium in trichloracetic acid. J. Electrochem. Soc. India 1978, 27, 87-90.
- Desai, M.N.; Shah, G.J. Aromatic Amines Inhibitors of the Corrosion of Aluminium-57 S in Hydrochloric Acid. Corros. Trait. Prot. Finition 1972, 20, 391-396.
- 1 Choudhary, R.S.; Singh, D.D.N.; Agarwal, V.V. Inhibitive action of some para-substituted aromatic amines towards corrosion of 1060 aluminium in nitric acid solution. J. Electrochem. Soc. India 1978, 27, 91-94. [CrossRef]
- Desai, M.N.; Desai, Y.B.; Gandhi, M.H. Molecular structure of amines and their inhibitive action for the corrosion of Al-2S in HCl. Corros. Sci. 1971, 11, 397-402. [CrossRef]
- Desai, M.N.; Patel, R.P. Aromatic amines as corrosion inhibitors for aluminium 3S in hydrochloric acid. Anti-Corros. Methods Mater. 1972, 19, 12-14. [CrossRef]
- Desai, M.N. Corrosion Inhibitors for Aluminium Alloys. Mater. Corros. 1972, 23, 475-482. [CrossRef]
- Stupnišek-Lisac, E.; Brnada, A.; Mance, A.D. Secondary amines as copper corrosion inhibitors in acid media. Corros. Sci. 2000, 42, 243-257. [CrossRef]
- Zhao, J.; Zhang, N.; Qu, C.; Zhang, J.; Zhang, X. Comparison of the corrosion inhibitive effect of anaerobic and aerobic cigarette butts water extracts on N80 steel at 90 øc in hydrochloric acid solution. Ind. Eng. Chem. Res. 2010, 49, 12452-12460. [CrossRef]
- Hosseini, S.M.A.; Salari, M.; Jamalizadeh, E.; Jafari, A.H. Electrochemical and quantum chemical studies of aromatic amines on the steel corrosion in acid solution. Corrosion 2012, 68, 600-609. [CrossRef]
- Badran, B.M.; Abdel Fattah, A.A.; Abdul Azim, A.A. New corrosion inhibitors based on fatty materials-II. Epoxidized fatty materials modified with aromatic amines. Corros. Sci. 1982, 22, 525-536. [CrossRef]
- Mousaa, I.M. Gamma irradiation processed (epoxidized soybean fatty acids/p-substituted aromatic amines) adducts as corrosion inhibitors for UV-curable steel coatings. Prog. Organ. Coat. 2017, 111, 220-230. [CrossRef]
- El-Haddad, M.N.; Fouda, A.E.A.S. Corrosion inhibition effect and adsorption of aniline derivatives on QD36 steel surface in acidic solution. Prot. Met. Phys. Chem. Surf. 2013, 49, 753-762. [CrossRef]
- Rana, S.S.; Desai, M.N. Inhibition of corrosion of copper in nitric acid. Ind. J. Technol. 1967, 5, 393-395.
- Anejjar, A.; Salghi, R.; Id El Mouden, O.; Ebenso, Eno.E.; Zougagh, M.; Hammouti, B. Corrosion Inhibition of Carbon Steel in 1M HCl Solution by 2-amino-1-methylbenzene (2-methylaniline). Int. J. Electrochem. Sci. 2014, 9, 8380-8391. [CrossRef]
- Desai, M.N.; Desai, M.B. Aromatic amines as inhibitors of the corrosion of mild steel in hydrochloric acid solution. Trans. SAEST 1981, 16, 77-87.
- Quraishi, M.A.; Jamal, D. Dianils: New and effective corrosion inhibitors for oil-well steel (N-80) and mild steel in boiling hydrochloric acid. Corrosion 2000, 56, 156-160. [CrossRef]
- Quraishi, M.A.; Jamal, D. The influence of some condensation products on corrosion inhibition of mild steel in acidic solutions. Anti-Corros. Methods Mater. 2000, 47, 233-240. [CrossRef]
- Dagdag, O.; Safi, Z.; Erramli, H.; Cherkaoui, O.; Wazzan, N.; Guo, L.; Verma, C.; Ebenso, E.E.; El Harfi, A. Adsorption and anticorrosive behavior of aromatic epoxy monomers on carbon steel corrosion in acidic solution: Computational studies and sustained experimental studies. RSC Adv. 2019, 9, 14782-14796. [CrossRef] [PubMed]
- Dagdag, O.; Safi, Qiang, Y.; Z.; Erramli, H.; Guo, L.; Verma, C.; Ebenso, E.E.; Kabir; A.; Wazzan, N.; El Harfi, A. Synthesis of Macromolecular Aromatic Epoxy Resins as Anticorrosive Materials: Computational Modeling Reinforced Experimental Studies ACS Omega 2020, 5, 3151-3164. [CrossRef]
- Dagdag, O.; Safi, Z.; Hsissou, R.; Erramli, H.; El Bouchti, M.; Wazzan, N.; Guo, L.; Verma, C.; Ebenso, E.E.; El Harfi, A. Epoxy pre-polymers as new and effective materials for corrosion inhibition of carbon steel in acidic medium: Computational and experimental studies. Sci. Rep. 2019, 9, 11715. [CrossRef] [PubMed]
- Hsissou, R.; Dagdag, O.; Abbout, S.; Benhiba, F.; Berradi, M.; El Bouchti, M.; Berisha, A.; Hajjaji, N.; Elharfi, A. Novel derivative epoxy resin TGETET as a corrosion inhibition of E24 carbon steel in 1.0 M HCl solution. Experimental and computational (DFT and MD simulations) methods. J. Mol. Liq. 2019, 284, 182-192. [CrossRef]
- Dagdag, O.; Safi, Z.; Wazzan, N.; Erramli, H.; Guo, L.; Mkadmh, A.M.; Verma, C.; Ebenso, E.E.; El Gana, L.; El Harfi, A. Highly functionalized epoxy macromolecule as an anti-corrosive material for carbon steel: Computational (DFT, MDS), surface (SEM-EDS) and electrochemical (OCP, PDP, EIS)studies. J. Mol. Liq. 2020, 302, 112535. [CrossRef]
- Dagdag, O.; Safi, Z.; Erramli, H.; Wazzan, N.; Guo, L.; Verma, C.; Ebenso, E.E.; Kaya, S.; El Harfi, A. Epoxy prepolymer as a novel anti-corrosive material for carbon steel in acidic solution: Electrochemical, surface and computational studies. Mater. Today Commun. 2020, 22, 100800. [CrossRef]
- Dagdag, O.; Haldhar, R.; Kim, S.C.; Safi, Z.S.; Wazzan, N.; Mkadmh, A.M.; Berisha, A.; Berdimurodov, E.; Jodeh, S.; Nwanna, E.E.; Akpan, E.D.; Ebenso, E.E. Synthesis, physicochemical properties, theoretical and electrochemical studies of tetraglycidyl methylenedianiline. J. Molec. Struct. 2022, 1265, 133508. [CrossRef]
- Hsissou, R.; Abbout, S.; Berisha, A.; Berradi, M.; Assouag, M.; Hajjaji, N.; Elharfi, A. Experimental, DFT and molecular dynamics simulation on the inhibition performance of the DGDCBA epoxy polymer against the corrosion of the E24 carbon steel in 1.0 M HCl solution. J. Molec. Struct. 2019, 1182, 340-351. [CrossRef]
- Boughoues, Y.; Benamira, Messaadia, L.; Bouider, N.; Abdelaziz, S. Experimental and theoretical investigations of four amine derivatives as effective corrosion inhibitors for mild steel in HCl medium. RSC Adv. 2020, 10, 24145-24158. [CrossRef]
- Donya, A.P.; Pakter, M.K.; Shalimova, M.A.; Lambin, V.N. The effect of polar substituents in aniline and pyridine derivatives on the inhibition of steel corrosion in acids. Prot.Met. 2002, 38, 216-219. [CrossRef]
- Khusnitdinov, R.N.; Gataullin, R.R. Preparation of Metal Corrosion Inhibitors from Arylamines and Butadiene. Russ. J. Appl. Chem. 2022, 95, 575-581. [CrossRef]
- Mahida, M.B.; Chaudhari, H.G. Aromatic amines as corrosion inhibitors for zinc in hydrochloric acid. J. Chem. Pharm. Res. 2012, 4, 5195-5201.
- Desai, M.N.; Thakar, B.C.; Shah, D.K.; Gandhi, M.H. Cathodic protection of 70/30 brass in 2.0 M nitric acid in the presence. of organic corrosion inhibitors. Br. Corros. J. 1975, 10, 39-40. [CrossRef]
- Desai, M.N.; Shah, Y.C.; Gandhi, M.H. The structure of amines and their inhibitive action on the corrosion of 63Cu-37Zn in HNO3. Corros. Sci. 1969, 9, 65-70. [CrossRef]
- Desai, M.N.; Shah, V.K. Aromatic amines as corrosion inhibitors for 70/30 brass in nitric acid. Corros. Sci. 1972, 12, 725-726, 727-730. [CrossRef]
- Desai, M.N.; Shah, Y.C.; Punjani, B.K. Inhibition of the corrosion of 63/37 brass in nitric acid. Br. Corros. J. 1969, 4, 309-314. [CrossRef]
- Brüschke, H.; Waller, F.; Ebert, K.H. Partial Current Density/Potential Curves of Nickel Electrodes in Air-Saturated Hydrochloric Acid in the Presence of Various Amines. Werkst. Korros. 1976, 27, 227-231. [CrossRef]
- Tandon, H.C.; Kumar, S.; Singh, L. AM1 and M3 Studies in Corrosion of Mild Steel in Presence of Ani(li)ne and its Derivatives. Asian J. Chem. 2003, 15, 1190-1192.
- Obot, I.B.; Madhankumar, A. Synergistic effect of iodide ion addition on the inhibition of mild steel corrosion in 1 M HCl by 3-amino-2-methylbenzylalcohol. Mater. Chem. Phys. 2016, 177, 266-275. [CrossRef]
- Al-Sabagh, A.M.; Kandil, N.G.; Ramadan, O.; Amer, N.M.; Mansour, R.; Khamis, E.A. Novel cationic surfactants from fatty acids and their corrosion inhibition efficiency for carbon steel pipelines in 1 M HCl. Egypt. J. Petroleum 2011, 20, 47-57. [CrossRef]
- Al-Sabagh, A.M.; Kandile, N.G.; Amer, N.; Ramadan, O. Khamis, E.A. Quaternary Ammonium Salts from Hydrolyzed Fatty Oil Based on Novel Tertiary Amines Used as Corrosion Inhibitors for Pipelines Carbon Steel at Acid Job in Petroleum Industry. J. Dispers. Sci. Technol. 2012, 33, 1307-1320. [CrossRef]
- De Almeida Rangel, H.; Merҫon, F. Study of ultraviolet fluorescence emission in the quantification of corrosioninhibitor of the quaternary ammonium salt type in water. Quim. Nova 2012, 35, 1287-1293. [CrossRef]
- Bacskai, R.; Schroeder, A.H.; Young, D.C. Hydrocarbon-soluble alkylaniline/formaldehyde oligomers as corrosion inhibitors. J. Appl. Polym. Sci. 1991, 42, 2435-2441. [CrossRef]
- Annand, R.R.; Hurd, R.M.; Hackerman, N. Adsorption of Monomeric and Polymeric Amino Corrosion Inhibitors on Steel. J. Electrochem. Soc. 1965, 112, 138-144. [CrossRef]
- Manivel , P.; Venkatachari, G. The inhibitive effect of poly(p-toluidine) on corrosion of iron in 1M HCl solutions. J. Appl. Polym. Sci. 2007, 104, 2595-2601. [CrossRef]
- Beck, F.; Schrötz, M. Thin polyheteroaromatic interlayers on commodity metals for corrosion protection. Mater. Sci. Forum 1998, 289-292, 1217-1228.
- Madhusudhana, G.; Santhi, R.J. Synthesis, Characterization and Corrosion Behavior of Isomers of Conducting Poly-Toluidine on Mild Steel in Acid Medium. Int. J. Sci. Res. 2015, 4, 1645-1650.
- Ashraf Abdelfadeel, M.; Hussain, A.I.; El-Ziaty, A.K.; Morsi, S.M.M.; Khorshed, L.A.; Shaban, S.S. Effect of poly(Aniline-CoO-Toluidine) loaded on emulsion paints with different pigments on corrosion inhibition of mild steel. Egypt. J. Chem. 2021, 64, 2711-2721.
- Farahati, R.; Ghaffarinejad, A.; Rezania, H.J.; Mousavi-Khoshdel, S.M.; Behzadi, H. Sulfonated aromatic polyamide as water-soluble polymeric corrosion inhibitor of copper in HCl. Coll. Surf. A 2019, 578, 123626. [CrossRef]
- Unnisa, C.B.N.; Nirmala Devi, G.; Hemapriya, V.; Chitra, S.; Chung, I.M.; Kim, S.H.; Prabakaran, M. Linear polyesters as effective corrosion inhibitors for steel rebars in chloride induced alkaline medium – An electrochemical approach. Constr. Build. Mater. 2018, 165, 866-876. [CrossRef]
- Unnisa, C.N.; Chitra, S. Comparative study of adsorption of linear aliphatic/aromatic polyesters at metal/0.5 M H2SO4 interface. J. Environm. Chem. Eng. 2018, 6, 6714-6722. [CrossRef]
- Farag, A.A.; Ismail, A.S.; Migahed, M.A. Inhibition of carbon steel corrosion in acidic solution using some newly polyester derivatives. J. Mol. Liq. 2015, 211, 915-923. [CrossRef]
- Aly, K.I.; Abd El-Lateef, H.M.; Yehia, N.; Khodairy, A.; Sayed, M.M.; El-Remaily, A.E.A.A.A. Novel polyesters based on indazole moiety: Synthesis, characterization and applicability as efficient inhibitors for acidic X-65-steel corrosion, React. Funct. Polym. 2021, 166, 105001. [CrossRef]
- Odewunmi, N.A.; Mazumder, M.A.J.; Ali, S.A.; Alharbi, B.G. Hydroquinone Decorated with Alkyne, Quaternary Ammonium, and Hydrophobic Motifs to Mitigate Corrosion of X-60 Mild Steel in 15 wt.% HCl. Chem. Asian J. 2021, 16, 801-821. [CrossRef] [PubMed]
- Negm, N.A.; Mohamed, A.S. Surface and thermodynamic properties of diquaternary bola-form amphiphiles containing an aromatic spacer. J. Surfact. Deterg. 2004, 7, 23-30. [CrossRef]
- Sherif, E.M.; Park, S.M. Effects of 1,4-naphthoquinone on aluminum corrosion in 0.50 M sodium chloride solutions. Electrochim. Acta 2006, 51, 1313-1321. [CrossRef]
- Salman, M.; Ansari, K.R.; Srivastava, V.; Chauhan, D.S.; Haque, J.; Quraishi, M.A. Chromeno naphthyridines based heterocyclic compounds as novel acidizing corrosion inhibitors: Experimental, surface and computational study. J. Mol. Liq. 2021, 322, 114825. [CrossRef]
- Desai, M.N.; Shah, G.J.; Gandhi, M.H. Corrosion inhibitors for aluminium in hydrochloric acid. Anti-Corros. Meth. Mater. 1972, 19, 12-15. [CrossRef]
- Krishnan, M.; Subramanyan, N. The influence of some aldehydes on the corrosion and anodic behaviour of aluminium in sodium hydroxide solution. Corros. Sci. 1977, 17, 893-900. [CrossRef]
- Talati, J.D.; Joshi, N.H. Aldehydes as corrosion inhibitors for aluminium-manganese alloys in potassium hydroxide. Mater. Corros. 1978, 29, 461-468. [CrossRef]
- Desai, M.N.; Thakar, B.C.; Gandhi, M.H. Aromatic aldehyde as corrosion inhibitors for 70/30 brass in potassium persulphate. Ind. J. Technol. 1974, 12, 85-86.
- Badea, T.; Badea, G.E.; Cojocaru, A. The inhibition action of some aliphatic and aromatic aldehydes on mild steel corrosion in 1 M Hd solution. Effect of the inhibitor concentration and medium temperature. Rev. Roum. Chim. 2004, 49, 37-47.
- Salman, H.E.; Balakit, A.A.; Albo Hay Allah, M.A. Study of the corrosion inhibitive effect and adsorption process of two azo-aldehydes on carbon steel in 1 M H2SO4. IOP Conf. Ser. Mater. Sci. Engin. 2019, 571, 012078. [CrossRef]
- Desai, M.N.; Shah, G.V.; Pandya, M.M. Azomethines derived from substituted aromatic aldehydes as corrosion inhibitors for aluminium - 56S in hydrochloric acid. Trans. SAEST 1981, 16, 221-231.
- Growcock, F..; Lopp, V.R. Film formation on steel in cinnamaldehyde-inhibited hydrochloric acid. Corrosion 1988, 44, 248-254. [CrossRef]
- Agarwal, P.; Landolt, D. Protection of steel by aromatic carboxylic acid corrosion inhibitors. Mater. Sci. Forum 1998, 289-292, 1229-1236. [CrossRef]
- Agarwal, P.; Landolt, D. Effect of anions on the efficiency of aromatic carboxylic acid corrosion inhibitors in near neutral media: experimental investigation and theoretical modeling. Corros. Sci. 1998, 40, 673-691. [CrossRef]
- Aquino-Torres, E.; Camacho-Mendoza, R.L.; Gutierrez, E.; Rodriguez, J.A.; Feria, L.; Thangarasu, P.; Cruz-Borbolla, J. The influence of iodide in corrosion inhibition by organic compounds on carbon steel: Theoretical and experimental studies. Appl. Surf. Sci. 2020, 514, 145928. [CrossRef]
- Moussa, M.N.; El-Tagoury, M.M.; Radi, A.A.; Hassan, S.M. Carboxylic acids as corrosion inhibitors for aluminium in acidic and alkaline solutions. Anti-Corros. Methods Mater. 1990, 37, 4-8. [CrossRef]
- Hassan, S.M.; El-Tagoury, M.M.; Radi, A.A. Aromatic acid derivatives as corrosion inhibitors for aluminium in acidic and alkaline solutions. Anti-Corros. Methods Mater. 1990, 37, 8-11. [CrossRef]
- Talati, J.D.; Pandya, J.M. Anilines as corrosion inhibitors for an aluminium-copper alloy in phosphoric acid. Corros. Sci. 1976, 16, 603-612. [CrossRef]
- Patel, R.B.; Pandya, J.M.; Lal, K. Toluidines as corrosion inhibitors for aluminium-copper alloy in hydrochloric acid. Trans. SAEST 1982, 17, 321-324.
- Riggs Jr., O.L.; Morrison, K.L.; Brunsell, D.A. Inhibitor development for titanium corrosion. Corrosion 1979, 35, 356-360. [CrossRef]
- Deyab, M.A. Corrosion inhibition of heat exchanger tubing material (titanium) in MSF desalination plants in acid cleaning solution using aromatic nitro compounds. Desalination 2018, 439, 73-79. [CrossRef]
- Orlov, S.N.; Bogachev, N.A.; Glukhoedov, N.A.; Mereshchenko, A.S.; Mikailova, R.A.; Skripkin, M.Yu.Formation of oxide films on titanium alloys under the conditions of the primary circuit of light-water nuclear reactors (a review). Int. J. Corros. Scale Inhib. 2022, 11, 1026-1040.
- Abdallah, M.; Asghar, B.H.; Zaafarany, I.; Sobhi, M. Synthesis of some aromatic nitro compounds and its applications as inhibitors for corrosion of carbon steel in hydrochloric acid solution. Prot. Met. Phys. Chem. Surf. 2013, 49, 485-491. [CrossRef]
- Saha, S.K.; Banerjee, P. Introduction of newly synthesized Schiff base molecules as efficient corrosion inhibitors for mild steel in 1 M HCl medium: An experimental, density functional theory and molecular dynamics simulation study. Mater. Chem. Front. 2018, 2, 1674-1691. [CrossRef]
- Li, S.; Chen, S.; Lei, S.; Ma, H.; Yu, R.; Liu, D. Investigation on some Schiff bases as HCl corrosion inhibitors for copper. Corros. Sci. 1999, 41, 1273-1287. [CrossRef]
- Quraishi, M.A.; Ajmal, M.; Shere, S. Investigation on some aromatic Schiffs bases as acid corrosion inhibitors for mild steel. Bull. Electrochem. 1996, 12, 523-525.
- Dutta, A.; Saha, S.K.; Banerjee, P.; Patra, A.K.; Sukul, D. Evaluating corrosion inhibition property of some Schiff bases for mild steel in 1 M HCl: Competitive effect of the heteroatom and stereochemical conformation of the molecule. RSC Adv. 2016, 6, 74833-74844. [CrossRef]
- Saha, S.Kr.; Dutta, A.; Ghosh, P.; Sukul, D.; Banerjee, P. Adsorption and corrosion inhibition effect of schiff base molecules on the mild steel surface in 1 M HCL medium: A combined experimental and theoretical approach. Phys. Chem. Chem. Phys. 2015, 17, 5679-5690. [CrossRef]
- Saha, S.K.; Dutta, A.; Ghosh, P.; Sukul, D.; Banerjee, P. Novel Schiff-base molecules as efficient corrosion inhibitors for mild steel surface in 1 M HCl medium: Experimental and theoretical approach. Phys.Chem.Chem.Phys. 2016, 18, 17898-17911. [CrossRef] [PubMed]
- Popov, Yu.V.; Korchagina, .K.; Chicherina, G.V. Synthesis and reactions of Schiff bases containing an m-phenoxyphenyl group: I. N-aryl-m-phenoxybenzylidene-amines and N-aryl-N’-(m-phenoxybenzylidene)hydrazines. Russ. J. Org. Chem. 2001, 37, 677-679. [CrossRef]
- Shokry, H.; Yuasa, M.; Sekine, I.; Issa, R.M.; El-Baradie, H.Y.; Gomma, G.K. Corrosion inhibition of mild steel by Schiff base compounds in various aqueous solutions: Part 1. Corros. Sci. 1998, 40, 2173-2186. [CrossRef]
- Allah, M.A.A.H.; Balakit, A.A.; Salman, H.I.; Abdulridha, A.A.; Sert, Y. New Heterocyclic Compound as Carbon Steel Corrosion Inhibitor in 1 M H2SO4, High Efficiency at Low Concentration: Experimental and Theoretical Studies. J. Adhes. Sci. Technol. 2023, 37, 525-547. [CrossRef]
- Upadhyay, R.K.; Mathur, S.P. Effect of Schiff's Bases as Corrosion Inhibitors on Mild Steel in Sulphuric Acid. E-J. Chem. 2007, 4, 408-414. [CrossRef]
- Oyeneyin, O.E.; Ojo, N.D.; Ipinloju, N.; James, A.C.; Agbaffa, E.B. Investigation of Corrosion Inhibition Potentials of Some Aminopyridine Schiff Bases Using Density Functional Theory and Monte Carlo Simulation. Chemistry Africa 2022, 5, 319-332. [CrossRef]
- Madkour, L.H.; Zinhome, U.A. Inhibition effect of Schiff base compounds on the corrosion of iron in nitric acid and sodium hydroxide solutions. J. Corros. Sci. Eng. 2010, 13, 34.
- Madkour, L.H.; Elroby, S.K. Aminic nitrogen-bearing polydentate Schiff base compounds as corrosion inhibitors for iron in acidic and alkaline media: A combined experimental and DFT studies. J. Corros. Sci. Eng. 2014, 17, 4.
- Leila, B.; Djahida, H.; Djamila, A.; Saida, M.; Salah, C. Inhibitive properties and Quantum chemical calculations of a new synthesized schiff base 1-[(3hydroxyphenylamino) methylene]-naphtalen-2-one for XC48 in hydrochloric acid solution. Int. J. Electrochem. Sci. 2018, 13, 6734-6755. [CrossRef]
- Murmu, M.; Saha, S.K.; Murmu, N.C.; Banerjee, P. Amine cured double Schiff base epoxy as efficient anticorrosive coating materials for protection of mild steel in 3.5% NaCl medium. J. Mol. Liq. 2019, 278, 521-535. [CrossRef]
- Salman, H.E.; Balakit, A.A.; Abdulridha, A.A.; Makki, S.Q. Synthesis of new aromatic azo-schiff compound as carbon steel corrosion inhibitor in 1 M H2SO4; High efficiency at low concentration. IOP Conf. Ser. Mater. Sci. Engin. 2019, 571, 012077. [CrossRef]
- Li, X.; Chen, L.; Xie, B., Lai, C.; He, J.; Feng, J.; Yang, Y.; Ji, R.; Liu, M. Two semi flexible nonplanar double Schiff bases as corrosion inhibitors for mild steel in HCl solution: Experimental and theoretical investigations. J. Env. Chem. Eng. 2023, 11, 110077. [CrossRef]
- Elaraby, A.; Qasim, K.F.; Mohamed, S.K.; El-Sharkawy, E.A.; Abdelhamed, S. Di-imine Schiff base inhibitor for carbon steel corrosion in 1 M HCl: Electrochemical, surface and theoretical investigations. J. Env. Chem. Eng. 2024, 12, 111861. [CrossRef]
- Shanmughan, S.K.; Krishnakutty, B.T.; Raphael, V.P.; Kakkasery, J.T. Anti-metal Deteriorating and Antiviral Potency of a Novel Polynuclear Schiff base, Derived from Anthrone. Orient. J. Chem. 2022, 38, 44-55. [CrossRef]
- Furtado, L.B.; Leoni, G.B.; Nascimento, R.C.; Santos, P.H.C.; Henrique, F.J.F.S.; Guimaraes, M.J.O.C.; Brasil, S.L.D.C. Experimental and Theoretical Studies of Tailor-made Schiff Bases as Corrosion Inhibitors for Carbon Steel in HCl. Mater. Res. 2023, 26, e20220398. [CrossRef]
- Satpati, S.; Suhasaria, A.; Ghosal, S.; Dey, S.; Sukul, D. Interaction of newly synthesized dipeptide Schiff bases with mild steel surface in aqueous HCl: Experimental and theoretical study on thermodynamics, adsorption and anti-corrosion characteristics. Mater. Chem. Phys. 2023, 296, 127200. [CrossRef]
- Enad, M.A.; Kadhim, M.Y.; Abdulnabi, A.S. Corrosion inhibition efficiency and adsorption of Azo schiff base chelate surfactant at carbon steel in hydrochloric acid interface. J. Global Pharma Technol. 2020, 12, 651-656.
- Salman, H.E.; Balakit, A.A.; Abdulridha, A.A. New aromatic azo-schiff bases as carbon steel corrosion inhibitor in 1 M H2SO4. Orient. J. Chem. 2018, 34, 2471-2476. [CrossRef]
- Upadhyay, R.K.; Anthony, S.; Mathur, S.P. Schiff's Bases as Inhibitors of Mild Steel Corrosion in Hydrochloric Acid. Russ. J. Electrochem.(Elektrokhimiya) 2007(2007), 43(43), 238-241(252-256). [CrossRef]
- Omar, I.H.; Zucchi, F.; Trabanelli, G. Schiff bases as corrosion inhibitors of copper and its alloys in acid media. Surf. Coat. Technol. 1986, 29, 141-151. [CrossRef]
- Hovey, R.J.; O'Connell, J.J.; Martell, A.E. Inner complex chelates. II.Analogs and polar substituted analogs of bisacetylacetoneethylenediimine and its metal chelates. J. Am. Chem. Soc. 1959, 81, 3189-3192. [CrossRef]
- Aljibori, H.S.; Abdulzahra, O.H.; Al Adily, A.J.; Al-Azzawi, W.K.; Al-Amiery, A.A.; Kadhum, A.A.H. Corrosion inhibition effects of concentration of 2-oxo-3-hydrazonoindoline in acidic solution, exposure period, and temperature. Int. J. Corros. Scale Inhib. 2023, 12, 438-457. [CrossRef]
- Mary, R.N.; Nazareth, R.; Suchetan, P.A.; Potla, K. Schiff Bases Derived from Triazoles as Corrosion Inhibitors for Maraging Steel in Acid Mixtures: Experimental and Theoretical Studies. Polyc. Arom. Compds. 2023, 43, 2788-2809. [CrossRef]
- Sudha, P.; Menaka, S.; Elango, K.P. Effect of structure, solvent and temperature on the kinetics of corrosion of mild steel in acid medium. Trans. SAEST 2004, 39, 17-21.
- Elemike, E.E.; Onwudiwe, D.C.; Nwankwo, H.U.; Hosten, E.C. Synthesis, crystal structure, electrochemical and anti-corrosion studies of Schiff base derived from o-toluidine and o-chlorobenzaldehyde. J. Mol. Struct. 2017, 1136, 253-262. [CrossRef]
- Talati, J.D.; Desai, M.N., Shah, N.K. Meta-Substituted aniline-N-salicylidenes as corrosion inhibitors of zinc in sulphuric acid. Mater. Chem. Phys. 2005, 93, 54-64. [CrossRef]
- Babashkina, M.G.; Panova, E.V.; Alkhimova, L.E.; Safin, D.A. Salen: Insight into the Crystal Structure, Hirshfeld Surface Analysis, Optical Properties, DFT, and Molecular Docking Studies. Polyc. Arom. Compds. 2023, 43, 5116-5138. [CrossRef]
- Gupta, N.K.; Verma, C.; Quraishi, M..; Mukherjee, A.K. Schiff's bases derived from l-lysine and aromatic aldehydes as green corrosion inhibitors for mild steel: Experimental and theoretical studies. J. Mol. Liq. 2016, 215, 47-57. [CrossRef]
- Alharthi, N.H.; El-Hashemy, M.A.; Derafa, W.M.; Althobaiti, I.O.; Altaleb, H.A. Corrosion inhibition of mild steel by highly stable polydentate schiff base derived from 1, 3-propanediamine in aqueous acidic solution. J. Saudi Chem. Soc. 2022, 26, 101501. [CrossRef]
- Saha, S.Kr.; Murmu, M.; Murmu, N.C.; Banerjee, P. Synthesis, characterization and theoretical exploration of pyrene based Schiff base molecules as corrosion inhibitor. J. Molec. Struct. 2021, 1245, 131098. [CrossRef]
- Xhanari, K.; Finsgar, M. Organic corrosion inhibitors for aluminium and its alloys in acid solutions: A review. RSC Adv. 2016, 6, 62833-62857. [CrossRef]
- Lashgari, M.; Malek, A.M. Fundamental studies of aluminum corrosion in acidic and basic environments: Theoretical predictions and experimental observations. Electrochim. Acta 2010, 55, 5253-5257. [CrossRef]
- Patel, N.K.; Makwana, S.C.; Patel, K.C. Action of phenols on the corrosion of 3S aluminium in hydrochloric acid solutions. Mater. Corros. 1973, 24, 964-966. [CrossRef]
- Müller, B.; Förster, I. Corrosion Inhibition of Zinc Pigments in Aqueous Alkaline Media by Aromatic Hydroxy Compounds. Corrosion 1996, 52, 786-789. [CrossRef]
- Müller, B. Corrosion inhibition of aluminium pigment by disubstituted benzene derivatives. Werkst. Korr. 1999, 50, 213-218. [CrossRef]
- Müller, B.; Kubitzki, G.; Kinet, G. Aromatic 2-hydroxy-oximes as corrosion inhibitors for aluminium and zinc pigments. Corros. Sci. 1998, 40, 1469-1477. [CrossRef]
- Zohreh, S.; Wan, B.J.; Zain, S.M. Aluminium corrosion inhibition using benzene-1,2,4,5-tetracarboxylic dianhydride (PMDH). Anti-Corr. Methods Mater. 2010, 57, 21-27. [CrossRef]
- Saleh, R.M.; Shams El Din, A.M. Efficiency of organic acids and their anions in retarding the dissolution of aluminium. Corros. Sci. 1972, 12, 689-697. [CrossRef]
- Zor, S.; Özkazanҫ, H.; Arslan, T.; Kandemirli, F. Inhibition effects of 4-phenyl-3-thiosemicarbazide on the corrosion of aluminum in 0.1 M HCl: Theoretical and experimental studies. Corrosion 2010, 66, 0450061-0450067. [CrossRef]
- El-Deeb, M.M.; Mohamed, S.M. Corrosion inhibition of aluminum with a 3-(10-sodium sulfonate decyloxy) aniline monomeric surfactant and its analog polymer in a 0.5M hydrochloric acid solution. J. Appl. Polym. Sci. 2011, 122, 3030-3037. [CrossRef]
- Elsharif, A.M.; Abubshait, S.A.; Abdulazeez, I.; Abubshait, H.A. Synthesis of a new class of corrosion inhibitors derived from natural fatty acid: 13-Docosenoic acid amide derivatives for oil and gas industry. Arab. J. Chem. 2020, 13, 5363-5376. [CrossRef]
- Grubač, Z.; Babić, R.; Metikoš-Huković, M. Application of substituted N-arylpyrroles in the corrosion protection of aluminium in hydrochloric acid. J. Appl. Electrochem. 2002, 32, 431-438. [CrossRef]
- Metikoš-Huković, M.; Babić, R.; Grubač, Z. The study of aluminium corrosion in acidic solution with nontoxic inhibitors. J. Appl. Electrochem. 2002, 32, 35-41. [CrossRef]
- Ayta, A.; Özmen, Ü.; Kabasakaloğlu, M. Investigation of some Schiff bases as acidic corrosion of alloy AA3102. Mater. Chem. Phys. 2005, 89, 176-181. [CrossRef]
- Fouda, A.E.A.S.; Al-Sarawy, A.A.; Radwan, M.S. Some aromatic hydrazone derivatives as inhibitors for the corrosion of C-steel in phosphoric acid solution. Annal. Chim. 2006, 96, 85-96. [CrossRef]
- Chafai, N.; Chafaa, S.; Benbouguerra, K.; Hellal, A.; Mehri, M. Synthesis, spectral analysis, anti-corrosive activity and theoretical study of an aromatic hydrazone derivative. J. Molec. Struct. 2019, 1181, 83-92. [CrossRef]
- Chaitra, T.K.; Mohana, K.N.; Tandon, H.C. Evaluation of newly synthesized hydrazones as mild steel corrosion inhibitors by adsorption, electrochemical, quantum chemical and morphological studies. Arab J. Basic Appl. Sci. 2018, 25, 45-55. [CrossRef]
- Lgaz, H.; Chung, I.M.; Albayati, M.R.; Chaouiki, A.; Salghi, R.; Mohamed, S.K. Improved corrosion resistance of mild steel in acidic solution by hydrazone derivatives: An experimental and computational study. Arab. J. Chem. 2020, 13, 2934-2954. [CrossRef]
- Kumari, P.P.; Shetty, P.; Rao, S.A. Electrochemical measurements for the corrosion inhibition of mild steel in 1ÿM hydrochloric acid by using an aromatic hydrazide derivative. Arab. J. Chem. 2017) 10, 653-663. [CrossRef]
- Quraishi, M.A.; Sardar, R.; Jamal, D. Corrosion inhibition of mild steel in hydrochloric acid by some aromatic hydrazides. Mater.Chem.Phys. 2001, 71, 309-313. [CrossRef]
- Singh, A.K.; Thakur, S.; Pani, B.; Ebenso, E.E.; Quraishi, M.A.; Pandey, A.K. 2-Hydroxy-N-((Thiophene-2-yl)methylene)benzohydrazide: Ultrasound-Assisted Synthesis and Corrosion Inhibition Study. ACS Omega 2018, 3, 4695-4705. [CrossRef] [PubMed]
- Mohan, P.; Usha, R.; Kalaignan, G.P.; Muralidharan, V.S. Inhibition effect of benzohydrazide derivatives on corrosion behaviour of mild steel in 1 M HCl. J. Chem. 2013, 2013, 541691. [CrossRef]
- Poojary, N.G.; Kumari, P.; Rao, S.A. 4-Hydroxyl-N’-[(3-Hydroxy-4-Methoxyphenyl) Methylidene] Benzohydrazide] as Corrosion Inhibitor for Carbon Steel in Dilute H2SO4. J. Fail. Anal. Preven. 2021, 21, 1264-1273. [CrossRef]
- Fouda, A.S.; Mohamed, M.T.; Soltan, M.R. Role of Some Benzohydrazide Derivatives as Corrosion Inhibitors for Carbon Steel in HCl Solution. J. Electrochem. Sci. Technol. 2013, 4, 61-70. [CrossRef]
- Ichchou, I.; Larabi, L.; Rouabhi, H.; Harek, Y.; Fellah, A. Electrochemical evaluation and DFT calculations of aromatic sulfonohydrazides as corrosion inhibitors for XC38 carbon steel in acidic media. J. Molec. Struct. 2019, 1198, 126898. [CrossRef]
- El Ashry, E.S.H.; El Nemra, A.; Essawy, S.A.; Ragab, S. Corrosion inhibitors part 31: Quantum chemical studies on the efficiencies of some aromatic hydrazides and Schiff bases as corrosion inhibitors of steel in acidic medium. Arkivoc 2006, 2006, 205-220. [CrossRef]
- Hassan, A.; Numin, M.S.; Jumbri, K.; Kee, K.E.; Borhan, N.; Daud, N.M.R.N.M.; Nor, A.M.; Suhor, M.F.; Wahab, R.A. Density Functional Theory Studies on New Possible Biobased Gemini Corrosion Inhibitors Derived from Fatty Hydrazide Derivatives. ACS Omega 2023, 8, 23945-23952. [CrossRef]
- Tansuğ, G.; Kicir, N.; Demirkol, O.; Giray, E.S.; Tuken, T. Synthesis and application of phenylcarbamodithioate compound for steel protection. J. Adhes. Sci. Technol. 2016, 30, 1984-2000. [CrossRef]
- Trabanelli, G.; Zucchi, F.; Gullini, G.; Carassiti, V. Correlation of the structure and the inhibitive action of some sulphoxides. Br. Corros. J. 1969, 4, 212-215. [CrossRef]
- Li, Y.; Wang, Y.; Zhang, S.; Miao, L.; Wei, M.; Wang, K. Corrosion inhibition of aromatic acids on Al-7075 anode for Al-air batteries with alkaline electrolyte. J. Power Sources 2022, 523, 231042. [CrossRef]
- Trabanelli, G. Inhibitors. An old remedy for a new challenge. Corrosion 1991, 47, 410-419. [CrossRef]
- Caprioli, F.; Martinelli, A.; Di Castro, V.; Decker, F. Effect of various terminal groups on long-term protective properties of aromatic SAMs on copper in acidic environment. J. Electroanal. Chem. 2013, 693, 86-94. [CrossRef]
- Blobner, F.; Abufager, P.N.; Han, R.; Bauer, J.; Duncan, D.A.; Maurer, R.J.; Reuter, K.; Feulner, P.; Allegretti, F. Thiolate-Bonded Self-Assembled Monolayers on Ni(111): Bonding Strength, Structure, and Stability. J. Phys. Chem. C 2015, 119, 15455-15468. [CrossRef]
- Maege, I.; Jaehne, E.; Henke, A.; Adler, H.J.P.; Bram, C.; Jung, C.; Stratmann, M. Ultrathin organic layers for corrosion protection. Macromol. Symp. 1998, 126, 7-24. [CrossRef]
- Felhösi, I.; Kálmán, E.; Póczik, P. Corrosion protection by self-assembly. Russ.J.Electrochem. 2002, 38, 230-237. [CrossRef]
- Samide, A.; Bibicu, I.; Rogalski, M.; Preda, M. A study of the corrosion inhibition of carbon-steel in diluted ammonia media using 2-mercapto-benzothiazol (MBT). Acta Chim. Slov. 2004, 51, 127-136.
- Wu, S.; Chen, Z.; Qiu, Y.; Guo, X. Corrosion Protection of Copper by Self-Assembled Monolayers Modified in Aqueous Micellar Solution. J. Electrochem. Soc. 2012, 159, C277-C282. [CrossRef]
- Antonijevic, M.M.; Petrovic, M.B. Copper corrosion inhibitors. A review. Int. J. Electrochem. Sci. 2008, 3, 1-28. [CrossRef]
- Caprioli, F.; Decker, F.; Marrani, A.G.; Beccari, M.; Di Castro, V. Copper protection by self-assembled monolayers of aromatic thiols in alkaline solutions. Phys. Chem. Chem. Phys. 2010, 12, 9230-9238. [CrossRef]
- Caprioli, F.; Martinelli, A.; Gazzoli, D.; Di Castro, V.; Decker, F. Enhanced protective properties and structural order of self-assembled monolayers of aromatic thiols on copper in contact with acidic aqueous solution. J. Phys. Chem. C 2012, 116, 4628-4636. [CrossRef]
- Caprioli, F.; Decker, F.; Di Castro, V. Durable Cu corrosion inhibition in acidic solution by SAMs of Benzenethiol, J. Electroanal. Chem. 2011, 657, 192-195. [CrossRef]
- Lai, Y.; Gao, Y.; Yao, X.; Zhang, C.; Wen, L.; Jin, Y. Inhibition and adsorption behavior of thiophenol derivatives on copper corrosion in saline medium. J. Adh. Sci. Technol. 2022, 36, 875-894. [CrossRef]
- Ruan, L.; Zhang, Z.; Huang, X.; Lyu, Y.; Wen, Y.; Shang, W.; Wu, L. Evaluation of Corrosion Inhibition of Two Schiff Bases Self-Assembled Films on Carbon Steel in 0.5 M HCl. Int. J. Electrochem. Sci. 2017, 12, 103-115. [CrossRef]
- Behpour, M.; Mohammadi, N. Investigation of inhibition properties of aromatic thiol self-assembled monolayer for corrosion protection. Corros. Sci. 2012, 65, 331-339. [CrossRef]
- Chen, Y.; Tang, Z.; Tong, R.; Wang, Q. Effect of Schiff bases structure on corrosion inhibition efficiency of copper. J. Chin. Soc. Corr. Prot. 2007, 27, 156-161.
- Huang, H.; Fu, Y.; Li, F.; Wang, Z.; Zhang, S.; Wang, X.; Wang, Z.; Li, H.; Gao, F. Orderly self-assembly of new ionic copolymers for efficiently protecting copper in aggressive sulfuric acid solution. Chem. Eng. J. 2020, 384, 123293. [CrossRef]
- Hou, B.S.; Zhang, Q..; Li, Y.Y.; Zhu, G.Y.; Lei, Y.; Wang, X.; Liu, H.F.; Zhang, G.A. In-depth insight into the inhibition mechanism of pyrimidine derivatives on the corrosion of carbon steel in CO2-containing environment based on experiments and theoretical calculations. Corros. Sci. 2021, 181, 109236. [CrossRef]
- Troquet, M.; Labbe, J.P.; Pagetti, J. The mechanism of the inhibition of zinc corrosion in 1N HCl solution by tetraphenylphosphonium bromide. Corros. Sci. 1981, 21, 101-117. [CrossRef]
- He, Z.; Li, J. Inhibitive effect of aromatic phosphonium salts on corrosion of aluminium in aluminium perchlorate solution. J. Chin. Soc. Corr. Prot. 1996, 16, 239-240.
- Szklarska-Smialowska, Z.; Dus, B. Effect of some organic phosphorus compounds on the corrosion of low carbon steel in hydrochloric acid solutions. Corrosion 1967, 23, 130-141. [CrossRef]
- Dus, B.; Szklarska-Smialowska, Z. Effect of some phosphoroorganic compounds on te corrosion rate of varios metals in acid solutions. Corrosion 1972, 28, 105-113. [CrossRef]
- Zhao, W.; Xia, M.; Lei, W.; Wang, F. Quantum chemistry studies of organophosphorus corrosion inhibitors. J. Chin. Soc. Corros. Prot. 2002, 22, 217-220.
- Shein, A.B.; Nedugov, A.N. Examination of trialkyl-substituted sulfonium, selenonium, and telluronium salts as inhibitors of acid corrosion of iron and steel. Prot. Met. 2000, 36, 240-243. [CrossRef]
- Verma, C.; Abdellattif, M.H.; Alfantazi, A.; Quraishi, M.A. N-heterocycle compounds as aqueous phase corrosion inhibitors: A robust, effective and economic substitute. J. Mol. Liq. 2021, 340, 117211. [CrossRef]
- Abd El-Maksoud, S.A.; Fouda, A.S. Some pyridine derivatives as corrosion inhibitors for carbon steel in acidic medium. Mater. Chem. Phys. 2005, 93, 84-90. [CrossRef]
- Lashkari, M.; Arshadi, M.R. DFT studies of pyridine corrosion inhibitors in electrical double layer: Solvent, substrate, and electric field effects. Chem.Phys. 2004, 299, 131-137. [CrossRef]
- Hau, N.N.; Huong, D.Q. Effect of aromatic rings on mild steel corrosion inhibition ability of nitrogen heteroatom-containing compounds: Experimental and theoretical investigation. J. Mol. Struct. 2023, 1277, 134884. [CrossRef]
- Selvaraj, K.; Sponton, M.E.; Arumugam, H.; Kannaiyan, S.K.; Ayyavu, C.; Casarino, A.F.; Estenoz, D.A.; Al-Lohedan, H.;Kumar, M.; Balu, R.; Kannaiyan, D. Synthesis of new quinoline derivatives based on mono-functional polybenzoxazines for oil-water separation, anti-corrosion and antibacterial applications. Compos. Interfaces 2024, 31, 665-682. [CrossRef]
- Granese, S.L.; Rosales, B.M.; Oviedo, C.; Zerbino, J.O. The inhibition action of heterocyclic nitrogen organic compounds on Fe and steel in HCl media. Corros. Sci. 1992, 33, 1439-1453. [CrossRef]
- Frignani, A.; Zucchit, F.; Monticelli, C. Inhibition of iron corrosion in different acid media by N-Decyl-Pyridinium derivatives. Brit. Corros. J. 1983, 18, 19-24. [CrossRef]
- Schmitt, G.; Bedbur, K. Investigations on structural and electronic effects in acid inhibitors by AC impedance. Mater. Corros. 1985, 36, 273-278. [CrossRef]
- Özcan, M.; Toffoli, D.; Üstünel, H.; Dehri, İ. Insights into surface-adsorbate interactions in corrosion inhibition processes at the molecular level. Corros. Sci. 2014, 80, 482-486. [CrossRef]
- Mahmoud, N.F.H.; El-Sewedy, A. Multicomponent Reactions, Solvent-Free Synthesis of 2-Amino-4-aryl-6-substituted Pyridine-3,5-dicarbonitrile Derivatives, and Corrosion Inhibitors Evaluation. J. Chem. 2018, 2018, 7958739. [CrossRef]
- Anwer, K.E.; Farag, A.A.; Mohamed, E.A.; Azmy, E.M.; Sayed, G.H. Corrosion inhibition performance and computational studies of pyridine and pyran derivatives for API X-65 steel in 6 M H2SO4. J. Ind. Eng. Chem. 2021, 97, 523-538. [CrossRef]
- Mendoza-Huizar, L.H.; Rios-Reyes, C.H.; Palomar-Pardavé‚ M.E. A semiempirical PM6 study of some aminopyrimidine derivatives and their interaction with an iron surface. ECS Trans. 2009, 20, 507-517. [CrossRef]
- Onyeachu, I.; Quraishi, M.A.; Obot, I.B.; Haque, J. Newly synthesized pyrimidine compound as CO2 corrosion inhibitor for steel in highly aggressive simulated oilfield brine. J. Adhes. Sci. Technol. 2019, 33, 1226-1247. [CrossRef]
- Hmamou, D.B.; Zarrouk, A.; Salghi, R.; Zarrok, H.; Ebenso, E.E.; Hammouti, B.; Kabanda, M.M.; Benchat, N.; Benali, O. Experimental and theoretical studies of the adsorption and corrosion inhibition of 6-phenylpyridazine-3(2H)-thione on Carbon Steel in 2.0 M H3PO4 solution. Int. J. Electrochem. Sci. 2014, 9, 120-138. [CrossRef]
- Iravani, D.; Esmaeili, N.; Guo, L.; Akbarinezhad, E. Experimental and computational study of aromatic ring effects on corrosion inhibition in the H2S media. Mater. Today Commun. 2023, 35, 105559. [CrossRef]
- Pruthviraj, R.D.; Prakash, C.H. Aromatic quinoxaline as corrosion inhibitor for Zn-Al alloy in 3% NaCl solution. Int. J. Chem. Sci. 2012, 10, 1096-1100.
- Saoudi, N.; Bellaouchou, A.; Guenbour, A.; Ben Bachir, A.; Essassi, E.M.; El Achouri, M. Aromatic quinoxaline as corrosion inhibitor for bronze in aqueous chloride solution. Bull. Mater. Sci. 2010, 33, 313-318. [CrossRef]
- Skrypnik, Yu.G.; Doroshenko, T.F.; Lyashchuk, S.N. Electronic and steric effects in inhibitive action of pyridine on acid corrosion. Zash. Metal. 1991, 27, 243-247.
- Eddy, N.O.; Ita, B.I. Theoretical and experimental studies on the inhibition potentials of aromatic oxaldehydes for the corrosion of mild steel in 0.1 M HCl. J. Molec. Model. 2011, 17, 633-647. [CrossRef]
- El-Mekabaty, A.; Habib, O.M.O. Synthesis and evaluation of some novel additives as antioxidants and corrosion inhibitors for petroleum fractions. Petrol. Sci. 2014, 11, 161-173. [CrossRef]
- Mehdiyeva, G.M. 8-Allyl-1,3-benzoxazines as Hydrogen Sulfide Corrosion Inhibitors and Biocides in Crude Oil Extraction. Petroleum Chem. 2023, 63, 394-402. [CrossRef]
- Babić-Samardžija, K.; Khaled, K.F.; Hackerman, N. N-heterocyclic amines and derivatives as corrosion inhibitors for iron in perchloric acid. Anti-Corros. Methods Mater. 2005, 52, 11-21. [CrossRef]
- Singaravelu, P.; Bhadusha, N.; Dharmalingam, V. Inhibitive Effect of Organic Inhibitors on the Corrosion of Mild Steel in Acidic Medium. Int. J. Life Sci. Pharma Res. 2022, 12, L40-L50. [CrossRef]
- Xu, Y.; Zhang, S.; Li, W.; Guo, L.; Xu, S.; Feng, L.; Madkour, L.H. Experimental and theoretical investigations of some pyrazolo-pyrimidine derivatives as corrosion inhibitors on copper in sulfuric acid solution. Appl. Surf. Sci. 2018, 459, 612-620. [CrossRef]
- Salehzadeh, J.; Nasiri, F. A versatile solvent-free synthesis of novel pyrazolone-1,3-dithiolan and pyrazolone-1,3-dithiole hybrids. Res. Chem. Interm. 2023, 49, 4621-4637. [CrossRef]
- Ashassi-Sorkhabi, H.; Shaabani, B.; Seifzadeh, D. Corrosion inhibition of mild steel by some schiff base compounds in hydrochloric acid. Appl. Surf. Sci. 2005, 239, 154-164. [CrossRef]
- Ayta, A.; Bilgiҫ, S.; Gece, G.; Ancin, N.; Öztas, S.G. Experimental and theoretical study of the inhibition effects of some Schiff bases as corrosion inhibitors of aluminium in HCl. Mater. Corros. 2012, 63, 729-734. [CrossRef]
- Şafak, S.; Duran, B.; Yurt, A.; Türkoğlu, G. Schiff bases as corrosion inhibitor for aluminium in HCl solution. Corros. Sci. 2012, 54, 251-259. [CrossRef]
- Al-Labban, H.M.Y.; Sejjad, F.A.A.; Sahap, E.H. Corrosion inhibitory influences of synthetic schiff base on aluminum in hydrochloric acid. Int. J. Pharm. Res. 2020, 12, 1304-1309. [CrossRef]
- Gomma, G.K.; Wahdan, M.H. Schiff bases as corrosion inhibitors for aluminium in hydrochloric acid solution. Mater. Chem. Phys. 1995, 39, 209-213.
- Fakrudeen, S.P.; Raju V, B. Electrochemical Behaviour of AA6061 Alloy in 1M Hydrochloric Acid using Schiff Base Compounds as Corrosion Inhibitors. J. Mater. Environ. Sci. 2013, 4, 326-337.
- Muniandy, M.T.; Abdul Rahim, A.; Osman, H.; Mohd Shah, A.; Yahya, S.; Bothi Raja, P. INVESTIGATION OF SOME SCHIFF BASES AS CORROSION INHIBITORS FOR ALUMINIUM ALLOY IN 0.5 M HYDROCHLORIC ACID SOLUTIONS. Surf. Rev. Lett. 2011, 18, 127-133. [CrossRef]
- Bansiwal, A.; Anthony, P.; Mathur, S.P. Inhibitive effect of some Schiff bases on corrosion of aluminium in hydrochloric acid solutions. Br. Corr. J. 2000, 35, 301-303. [CrossRef]
- Ashassi-Sorkhabi, H.; Shabani, B.; Aligholipour, B.; Seifzadeh, D. The effect of some Schiff bases on the corrosion of aluminum in hydrochloric acid solution. Appl. Surf. Sci. 2006, 252, 4039-4047. [CrossRef]
- Yurt, A.; Aykin, Ö Diphenolic Schiff bases as corrosion inhibitors for aluminium in 0.1M HCl: Potentiodynamic polarisation and EQCM investigations. Corros. Sci. 2011, 53, 3725-3732. [CrossRef]
- Yurt,A.; Ulutas, S.; Dal, H. Electrochemical and theoretical investigation on the corrosion of aluminium in acidic solution containing some Schiff bases. Appl. Surf. Sci. 2006, 253, 919-925. [CrossRef]
- Bautista García, J.E.; Imbert Palafox, J.L.; Veloz Rodríguez, M.A.; Vázquez García, R.Á.; Gómez Gómez, J.V. Bacterial biodegradability of inhibitors of chiral imine type corrosion and catabolic prediction. Rev. Int. Contam. Amb. 2015, 31, 415-426.
- Rbaa, M.; Galai, M.; Ouakki, M.; Hsissou, R.; Berisha, A.; Kaya, S.; Berdimurodov, E.; Lakhrissi, B.; Zarrouk, A. Synthesis of new halogenated compounds based on 8-hydroxyquinoline derivatives for the inhibition of acid corrosion: Theoretical and experimental investigations. Mater. Today Commun. 2022, 33, 104654. [CrossRef]
- Al-Abdallah, M.M.; Al-Talib, M.; Tashtoush, H. Inhibiting effect of substituted 1,3,5-oxadiazinium salts on the dissolution of copper in nitric acid. Ind. J. Technol. 1989, 27, 168-170.
- Schmitt, G.; Bedbur, K. INVESTIGATIONS ON STRUCTURAL AND ELECTRONIC EFFECTS IN ACID INHIBITORS BY ac IMPEDANCE.. Proc.9th Int.Congr.Metal.Corros. 1984, 112-119. [CrossRef]
- Kurbanov, F.K.; Nasimov, E.; Kuchkarov, A.B.; Sadykov, K.M. INVESTIGATION OF THE INHIBITOR PROPERTIES OF N-PROPARGYL DERIVATIVES OF AROMATIC AMINES IN HYDROCHLORIC ACID. Prot. Met. 1976, 12, 392-393.
- Ebenso, E.E.; Kabanda, M.M.; Murulana, L.C.; Singh, A.K.; Shukla, S.K. Electrochemical and quantum chemical investigation of some azine and thiazine dyes as potential corrosion inhibitors for mild steel in hydrochloric acid solution. Ind. Engin. Chem. Res. 2012, 51, 12940-12958. [CrossRef]
- Obot, I.B.; Kaya, S.; Kaya, C.; Tüzün, B. Theoretical evaluation of triazine derivatives as steel corrosion inhibitors: DFT and Monte Carlo simulation approaches. Res. Chem. Intermed. 2016, 42, 4963-4983. [CrossRef]
- Shukla, S.K.; Singh, A.K.; Quraishi, M.A. Triazines: Efficient corrosion inhibitors for mild steel in hydrochloric acid solution. Int. J. Electrochem. Sci. 2012, 7, 3371-3389. [CrossRef]
- Obot, I.B.; Onyeachu, I.B.; Umoren, S.A. Pyrazines as Potential Corrosion Inhibitors for Industrial Metals and Alloys: A Review. J. Bio. Tribo. Corr. 2018, 4, 18. [CrossRef]
- Hassan, N.; Holze, R. A comparative electrochemical study of electrosorbed 2- and 4-mercaptopyridines and their application as corrosion inhibitors at C60 steel. J. Chem.Sci. 2009, 121, 693-701. [CrossRef]
- Fouda, A.S.; Alsawy, T.F.; Ahmed, E.S.; Abou-Elmagd, B.S. Performance of some thiophene derivatives as corrosion inhibitors for 304 stainless steel in aqueous solutions. Res. Chem. Intermed. 2013, 39, 2641-2661. [CrossRef]
- Fernine, Y.; Arrousse, N.; Haldhar, R.; Raorane, C.J.; Ech-chihbi, E.; Kim, S.C.; El Hajjaji, F.; Alami, A.; Ebn Touhami, M.; Taleb, M. Novel thiophene derivatives as eco-friendly corrosion inhibitors for mild steel in 1 M HCl solution: Characterization, electrochemical and computational (DFT and MC simulations) methods. J. Environm. Eng. 2022, 10, 108891. [CrossRef]
- Tantawy, A.H.; Soliman, K.A.; Abd El-Lateef, H.M. Experimental and computational approaches of sustainable quaternary bisammonium fluorosurfactants for corrosion inhibition as protective films at mild steel/H2SO4 interface. Coll. Surf. A 2021, 614, 126141. [CrossRef]
- Pandimuthu, G.; Muthukrishnan, P.; Rameshkumar, S.; Paramasivaganesh, K.; Sankar, A. Charge Transfer Resistance and Adsorption performance of a New Pyrrole derivative on Mild steel in Acidic media: Antibacterial studies. Orient. J. Chem. 2021, 37, 779-790. [CrossRef]
- Caldona, E.B., Zhang, M.; Liang, G.; Hollis, T.K.; Webster, C.E.; Jr Smith, D.W.; Wipf, D.O. Corrosion inhibition of mild steel in acidic medium by simple azole-based aromatic compounds. J. Electroanal. Chem. 2021, 880, 114858. [CrossRef]
- Wang, D.; Wang, Z. Experimental studies of structure and inhibition efficiency of imidazoline derivatives. J. Chin. Soc. Corros. Prot. 2001, 21, 116-121. [CrossRef]
- Quraishi, M.A.; Sardar, R. Aromatic triazoles as corrosion inhibitors for mild steel in acidic environments. Corrosion 2002, 58, 748-755. [CrossRef]
- Deng, Q.; Jeschke, S.; Jakeria, M.R.; White, P.; Hirth, S.; Eiden, P.; Gorges, J.N., Chen, X.B.; Keil, P., Cole, I. Synergistically and sustainably performed inhibitors for galvanised steel against aqueous corrosion. Corr. Sci. 2023, 213, 110984. [CrossRef]
- Popova, A.; Christov, M.; Zwetanova, A. Effect of the molecular structure on the inhibitor properties of azoles on mild steel corrosion in 1 M hydrochloric acid. Corros. Sci. 2007, 49, 2131-2143. [CrossRef]
- Nwankwo, H.U.; Olasunkanmi, L.O.; Ebenso, E.E. Electrochemical and Computational Studies of Some Carbazole Derivatives as Inhibitors of Mild Steel Corrosion in Abiotic and Biotic Environments. J. Bio. Tribo. Corros. 2018, 4, 13. [CrossRef]
- Prethaler, A.; Vogl, T.; Mori, G.; Havlik, W.; Zehethofer, G.; Hönig, S.; Rosenberg, E. Efficiency of two gas and gas condensate inhibitors in a laboratory two phase flow. EUROCORR 2013 - European Corrosion Congress 2013.
- Holze R. Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology, New Series, Group IV: Physical Chemistry, Volume 9: Electrochemistry, Subvolume B: Ionic Conductivities of Liquid Systems, Part 1: Molten Salts and Ionic Liquids, W. Martienssen, M.D. Lechner, Eds., Springer-Verlag, Berlin, Germany, 2016.
- Zeng, X.; Zheng, X.; Guo, L.; Xu, Q.; Huang, H.; Tan, B. Three imidazole ionic liquids as green and eco-friendly corrosion inhibitors for mild steel in sulfuric acid medium. J. Mol. Liq. 2021, 324, 115063. [CrossRef]
- Mazumder, M.A.J.; Ali, S.A.; Al-Muallem, H.A. 2-(P-ALKOXYPHENYL)-2-IMIDAZOLINES AND THEIR USE AS CORROSION INHIBITORS, US 20160347988A1, 01.12.2016.
- Hou, Y.; Zhu, L.; He, K.; Yang, Z.; Ma, S.; Lei, J. Synthesis of three imidazole derivatives and corrosion inhibition performance for copper. J. Mol. Liq. 2022, 348, 118432. [CrossRef]
- Daoudi, W.; El Ibrahimi, B.; Dagdag, O.; Berdimurodov, E.; Guo, L.; Ebenso, E.E.; Oussaid, A.; El Aatiaoui, A. New chlorophenyl-imidazole derivative as a novel corrosion inhibitor in the gas and oil industry. J. Phys. Chem. Sol. 2023, 179, 111409. [CrossRef]
- Martin, J.A.; Valone, F.W. The Existence of Imidazoline Corrosion Inhibitors. Corrosion 1985, 41, 281-287. [CrossRef]
- Nahlé, A.; Al-Tuniji, R.; Abu-Abdoun, I.; Abdel-Rahman, I. Corrosion inhibition of 1,4-di (1-vinyl-3-methylimidazolium) benzene dibromide on mild steel in HCL solution. Portug. Electrochim. Acta 2016, 34, 197-211. [CrossRef]
- Xiong, S.; Wu, H.; Liu, Z.; Wang, Y.; Lin, W. Study on corrosion behavior of new corrosion inhibitor imidazole derivatives for mild steel in oil-in-water emulsion. Surf. Interface Anal. 2021, 53, 418-431. [CrossRef]
- Srivastava, V.; Salman, M.; Chauhan, D.S.; Abdel-Azeim, S.; Quraishi, M.A. (E)-2-styryl-1H-benzo[d]imidazole as novel green corrosion inhibitor for carbon steel: Experimental and computational approach. J. Mol. Liq. 2021, 324, 115010. [CrossRef]
- Zuriaga-Monroy, C.; Oviedo-Roa, R.; Montiel-Sánchez, L.E.; Vega-Paz, A.; Marín-Cruz, J.; Martínez-Magadán, J.M. Theoretical Study of the Aliphatic-Chain Length's Electronic Effect on the Corrosion Inhibition Activity of Methylimidazole-Based Ionic Liquids. Ind. Engin. Chem. Res. 2016, 55, 3506-3516. [CrossRef]
- Jovancicevic, V.; Ramachandran, S.; Prince, P. Inhibition of Carbon Dioxide Corrosion of Mild Steel by Imidazolines and Their Precursors. Corrosion 1999, 55, 449-455. [CrossRef]
- Braga, T. G.; Martín, R. L.; McMahon, J. A.; Oude Alink, B. A.; Outlaw, B. T. Combinations of Imidazolines and Wetting Agents as Environmentally Acceptable Corrosion Inhibitors. U.S. Patent US6338819 B1. 16.02.1999.
- Ismail, A.; Irshad, H.M.; Zeino, A.; Toor, I.H. Electrochemical Corrosion Performance of Aromatic Functionalized Imidazole Inhibitor Under Hydrodynamic Conditions on API X65 Carbon Steel in 1ÿM HCl Solution. Arab. J. Sci. Engin. 2019, 44, 5877-5888. [CrossRef]
- Bhargava, G.; Ramanarayanan, T.A.; Gouzman, I.; Bernasek, S.L. Corrosion inhibitor - iron interactions: A study combining surface science and electrochemistry. ECS Trans. 2006, 1, 195-206. [CrossRef]
- Bhargava, G.; Ramanarayanan, T.A.; Gouzman, I.; Abelev, E.; Bemasek, S.L. Inhibition of iron corrosion by imidazole: An electrochemical and surface science study. Corrosion 2009, 65, 308-317. [CrossRef]
- Prashanth, M.K.; Kumar, C.B.P.; Prathibha, B.S.; Raghu, M.S.; Kumar, K.Y.; Jagadeesha, M.B.; Mohana, K.N.; Krishna, H. Effect of OH, NH2 and OCH3 groups on the corrosion inhibition efficacy of three new 2,4,5-trisubstituted imidazole derivatives on mild steel in acidic solutions: Experimental, surface and DFT explorations. J. Mol. Liq. 2021, 329, 115587. [CrossRef]
- Costa, S.N.; Almeida-Neto, F.W.Q.; Campos, O.S.; Fonseca, T.S.; de Mattos, M.C.; Freire, V.N.; Homem-de-Mello, P.; Marinho, E.S.; Monteiro, N.K.V.; Correia, A.N.; de Lima-Neto, P. Carbon steel corrosion inhibition in acid medium by imidazole-based molecules: Experimental and molecular modelling approaches. J. Mol. Liq. 2021, 326, 115330. [CrossRef]
- Cao, J.; Guo, C.; Guo, X.; Chen, Z. Inhibition behavior of synthesized ZIF-8 derivative for copper in sodium chloride solution. J. Mol. Liq. 2020, 311, 113277. [CrossRef]
- Obot, I.B. Theoretical design of new benzimidazole derivatives as steel corrosion inhibitors in CO2 corrosive environment: DFT and Monte Carlo approaches. European Corrosion Congress, EUROCORR 2015, 2015, 1, 390-401.
- Popova, A.K.; Machkova, M.S.; Djambova, A.G.; Zwetanova, A.; Raicheva, S.N. Relationship between chemical structure parameters and inhibitor efficiency of some azoles. Bulg. Chem. Commun. 2008, 40, 300-305.
- Chervinskii, A.Yu.; Shein, A.B.; Vdovichenko, A.N.; Morozova, T.L.; Kapkan, L.M. Inhibition of acid steel corrosion by substituted benzimidazoles. Prot. Met. 1991, 26, 517-519.
- Chen, M.; Cheng, Y.; Xu, H.; Wu, J.; Xue, M.; Zhang, X. Molecular structure of imidazoline inhibitor and quantum chemical analysis of corrosion inhibition performance of Zn atom. E3S Web Conf. 2020, 213, 01027. [CrossRef]
- Xhanari, K.; Finsgar, M. The first electrochemical and surface analysis of 2-aminobenzimidazole as a corrosion inhibitor for copper in chloride solution. New J. Chem. 2017, 41, 7151-7161. [CrossRef]
- Neupane, S.; Losada-Pérez, P.; Tiringer, U.; Taheri, P.; Desta, D.; Xie, C.; Crespo, D.; Mol, A.; Milosev, I.; Kokalj, A.;Renner, F.U. Study of Mercaptobenzimidazoles As Inhibitors for Copper Corrosion: Down to the Molecular Scale, J. Electrochem. Soc. 2021, 168, 051504. [CrossRef]
- Babić-Samardžija, K.; Lupu, C.; Hackerman, N.; Barron, A.R.; Luttge, A. Inhibitive properties and surface morphology of a group of heterocyclic diazoles as inhibitors for acidic iron corrosion. Langmuir 2005, 21, 12187-12196. [CrossRef] [PubMed]
- Quraishi, M.A.; Sardar, R. Corrosion inhibition of mild steel in acid solutions by some aromatic oxadiazoles. Mater. Chem. Phys. 2003, 78, 425-431. [CrossRef]
- Xiong, S.; Sun, J.; Xu, Y.; Yan, X. Adsorption behavior of tautomeric forms of 2-aminino-5-mercato-1,3,4-thiadizole as corrosion inhibitor on copper surface. Anti-Corros. Meth. Mater. 2016, 63, 452-460. [CrossRef]
- Zhang, Q.; Hua, Y. Corrosion inhibition of aluminum in hydrochloric acid solution by alkylimidazolium ionic liquids. Mater. Chem. Phys. 2010, 119, 57-64. [CrossRef]
- El-Haddad, M.N.; Fouda, A.S. Electroanalytical, quantum and surface characterization studies on imidazole derivatives as corrosion inhibitors for aluminum in acidic media. J. Mol. Liq. 2015, 209, 480-486. [CrossRef]
- El-Shafei, A.A.; El-Maksoud, S.A.A.; Fouda, A.S. The role of indole and its derivatives in the pitting corrosion of Al in neutral chloride solution. Corros. Sci. 2004, 46, 579-590. [CrossRef]
- Farghaly, T.A.A.; Fawzy, A.; Alsharief, H.H.H.; Alqarni, N.; Bahir, A.A.; Riyadh, S.M.M.; Khalil, K.D.D. Investigation of inhibition efficiencies of Novel bis-oxindole and bis(spiro(triazole-oxindole)) for the corrosion of copper in sulfuric acid medium. Polyc. Arom. Compds. 2024, 44, 1258-1272. [CrossRef]
- Verma, D.K.; Sahu, R.; Berdimurodov, E.; Verma, C.; Quraishi, M.A.; Jain, V.K.; Berdimuradov, K. Isatin as a new core in the development of corrosion inhibitors: A comprehensive review. J. Molec. Struct. 2023, 1294, 136313. [CrossRef]
- Fernando, I.R.; Daskalakis, N.; Demadis, K.D.; Mezei, G. Cation effect on the inorganic-organic layered structure of pyrazole-4-sulfonate networks and inhibitory effects on copper corrosion. New J. Chem. 2010, 34, 221-235. [CrossRef]
- Fernando, I.R.; Jianrattanasawat, S.; Daskalakis, N.; Demadis, K.D.; Mezei, G. Mapping the supramolecular chemistry of pyrazole-4-sulfonate: Layered inorganic-organic networks with Zn2+, Cd2+, Ag+, Na+ and NH4+, and their use in copper anticorrosion protective films. CrystEngComm 2012, 14, 908-919. [CrossRef]
- Khaled, K.F.; Amin, M.A. Electrochemical and molecular dynamics simulation studies on the corrosion inhibition of aluminum in molar hydrochloric acid using some imidazole derivatives. J. Appl. Electrochem. 2009, 39, 2553-2568. [CrossRef]
- Fouda, A.S.; Abdel-Latif, E.; Helal, H.M.; El-Hossiany, A. Synthesis and Characterization of Some Novel Thiazole Derivatives and Their Applications as Corrosion Inhibitors for Zinc in 1 M Hydrochloric Acid Solution. Russ. J. Electrochem. 2021, 57, 159-171. [CrossRef]
- Traisnel, M.; Kadia, L.El.; Bentiss, F.; Lagrenée, M.; Vezin, H. 3,5-Bis(n-Methoxyphenyl)-4-Amino-1,2,4-Triazoles as inhibitors of corrosion of mild steel in acidic media, EUROCORR 2004 - European Corrosion Conference: Long Term Prediction and Modelling of Corrosion 2004.
- Abdennabi, A.M.S.; Abdulhadi, A.I.; Abu-Orabi, S.T.; Saricimen, H. The inhibition action of 1(benzyl)1-H-4,5-dibenzoyl1,2,3-triazole on mild steel in hydrochloric acid media. Corros. Sci. 1996, 38, 1791-1800. [CrossRef]
- Liu, L.; Pan, X.; Zhang, Q.; Qian, J. Corrosion inhibition and olecular structure of thiadiazole derivatives in sulfur-ethanol system. CIESC J. 2014, 65, 4039-4048.
- A.M.S. Abdennabi, A.I. Abdulhadi, S. Abu-Orabi Relationship between the molecular structure and the inhibition performance of triazole compounds using electrochemical methods. Anti-Corros. Methods Mater. 1998, 45, 103-108. [CrossRef]
- Domínguez-Crespo, M.A.; Zepeda-Vallejo, L.G.; Torres-Huerta, A.M.; Brachetti-Sibaja, S.B.; Palma-Ramírez, D.; Rodríguez-Salazar, A.E.; Ontiveros-de la Torre, D.E. New Triazole and Isoxazole Compounds as Corrosion Inhibitors for Cu-Ni (90/10) Alloy and Galvanized Steel Substrates. Metall. Mater. Trans. A 2020, 51, 1822-1845. [CrossRef]
- Rao, N.; Johnson, D.A.; Lu, F.F.; Nghiem, N.P. Elucidation of components of aromatic triazole demand in cooling water systems and development of more environmentally friendly yellow metal corrosion inhibitor. J. Cooling Tower Inst. 1997, 18, 30-34, 36-38, 40-42, 44-45.
- Huntscha, S.; Hofstetter, T.B.; Schymanski, E.L.; Spahr, S.; Hollender, J. Biotransformation of benzotriazoles: Insights from transformation product identification and compound-specific isotope analysis. Environm. Sci. Technol. 2014, 48, 4435-4443. [CrossRef]
- Postigo, C.; Barceló, D. Synthetic organic compounds and their transformation products in groundwater: Occurrence, fate and mitigation. Sci. Tot. Environm. 2015, 503-504, 32-47. [CrossRef]
- Muthukumar, N.; Maruthamuthu, S.; Palaniswamy, N. Water-soluble inhibitor on microbiologically influenced corrosion in diesel pipeline. Coll. Surf. B 2006, 53, 260-270. [CrossRef] [PubMed]
- Rajasekar, A.; Maruthamuthu, S.; Palaniswamy, N.; Rajendran, A. Biodegradation of corrosion inhibitors and their influence on petroleum product pipeline. Microbiol. Res. 2007, 162, 355-368. [CrossRef] [PubMed]
- Muthukumar, N.; Maruthamuthu, S.; Mohanan, .; Palaniswamy, N. Influence of an oil soluble inhibitor on microbiologically influenced corrosion in a diesel transporting pipeline. Biofouling 2007, 23, 395-404. [CrossRef] [PubMed]
- Korpics, C.J. Aromatic triazoles as corrosion inhibitors of copper and copper alloys. Anti-Corros. Meth. Mater. 1974, 21, 11-13. [CrossRef]
- Rawat, N.S.; Udayabhanu, G.; Arora, R.K. Studies on the effect of chloride ions on the inhibition of mild steel corrosion by some nitrogeneous aromatic compounds in sulfuric acid medium. Trans. SAEST 1985, 20, 63-66.
- Fent, K.; Chew, G.; Li, J.; Gomez, E. Benzotriazole UV-stabilizers and benzotriazole: Antiandrogenic activity in vitro and activation of aryl hydrocarbon receptor pathway in zebrafish eleuthero-embryos. Sci.Tot.Environ. 2014, 482-483, 125-136. [CrossRef]
- Wu, L.; Suchana, S.; Flick, R.; Kuemmel, S.; Richnow, H.; Passeport, E. Carbon, hydrogen and nitrogen stable isotope fractionation allow characterizing the reaction mechanisms of 1H-benzotriazole aqueous phototransformation. Water Res 2021, 203, 117519. [CrossRef]
- Wu, J.S.; Nobe, K. Effects of substituted benzotriazoles on the electrochemical behavior of copper in H2SO4. Corrosion 1981, 37, 223-225. [CrossRef]
- Loto, R.T. Anti-corrosion performance of 1,3-Benzothiazole on 410 Martensitic stainless steel in H2SO4. Surf. Rev. Lett. 2017, 24, 1750121. [CrossRef]
- Khaled, K.F.; El-Maghraby, A. Experimental, Monte Carlo and molecular dynamics simulations to investigate corrosion inhibition of mild steel in hydrochloric acid solutions. Arab. J. Chem. 2014, 7, 319-326. [CrossRef]
- 1 Al-Bghdadi, S.B.; Hanoon, M.M.; Odah, J.F.; Shaker, L.M.; Al-Amiery, A.A. Benzylidene as Efficient Corrosion Inhibition of Mild Steel in Acidic Solution. Proceedings 2019, 41, 27. [CrossRef]
- Gece, G.; Bilgiҫ, S. Quantum chemical study of some cyclic nitrogen compounds as corrosion inhibitors of steel in NaCl media. Corros. Sci. 2009, 51, 1876-1878. [CrossRef]
- Şahin, M.; Bilgiç, S.; Yılmaz, H. The inhibition effects of some cyclic nitrogen compounds on the corrosion of the steel in NaCl mediums. Appl. Surf. Sci. 2002, 195, 1-7. [CrossRef]
- Şahin, M.; Bilgiç, S. The inhibition effects of some heterocyclic nitrogenous compounds on the corrosion of the steel in CO2-saturated NaCl solutions. Anti-Corros. Meth. Mater. 2003, 50, 34-39. [CrossRef]
- Pham, T.L.M.; Phung, T.K.; Thang, H.V. DFT insights into the adsorption mechanism of five-membered aromatic heterocycles containing N, O, or S on Fe(110) surface. Appl. Surf. Sci. 2022, 583, 152524. [CrossRef]
- Shen, M.; Furman, A.; Kharshan, R. Investigation of bio-based aromatic acids as corrosion inhibitor. NACE - Int.Corros.Conf.Ser. 2015, 2015-January.
- Billiet, H.A.H. Amines from environmental sources. J. Chromatog. Library 1992, 51, B583-B595.
- Varvara, S.; Bostan, R.; Bobis, O.; Gaina, L.; Popa, F.; Mena, V.; Souto, R.M. Propolis as a green corrosion inhibitor for bronze in weakly acidic solution. Appl. Surf. Sci. 2017, 426, 1100-1112. [CrossRef]
- Ganash, A.A.; Alshelaly, A.H. Extensive electrochemical study and surface examinations combined with DFT theoretical approaches of Zoffa extracts as green corrosion inhibitors for ASTM/A36 steels in acidic solutions. J. Adhes. Sci. Technol. 2022, 36, 1247-1278. [CrossRef]
- Shen, M.; Furman, A.; Kharshan, R. Investigation of bio-based aromatic acids as corrosion inhibitors. Mater.Perf. 2016, 55, 52-57.
- Chandler, C.; Kharshan, M.; Furman, A. SUGAR BEETS AGAINST CORROSION. Corros. Rev. 2002, 20, 379. [CrossRef]
- El-Etre, A.Y. Inhibition of acid corrosion of aluminum using vanillin. Corros. Sci. 2001, 43, 1031-1039. [CrossRef]
- 1 Furtado, L.B.; Nascimento, R.C.; Henrique, F.J.F.S.; Guimaraes, M.J.O.C.; Rocha, J.C.; Ponciano, J.A.C.; Seidl, P.R. Effects of temperature, concentration and synergism on green Schiff bases synthesized from vanillin in applications as corrosion inhibitors for carbon steel in well stimulation. J. Petr. Sci. Eng. 2022, 213, 110401. [CrossRef]
- Hadisaputra, S.; Abhi Purwoko, A.; Hamdiani, S.; Nuryono, N. Which anthocyanin is the best corrosion inhibitor? IOP Conf. Ser. Mater. Sci. Engin. 2019, 509, 012129. [CrossRef]
- Oni, A. Mild-steel corrosion and inhibition by aromatic amino acid in hot H2SO4. Corr. Prev. Contr. 1992, 39, 128-130.
- Zeng, N.; Zhao, H.; Luo, C.; Liu, Y.; Wang, C.; Ma, T. Roles and mechanistic analysis of adenine as a green inhibitor in chemical mechanical polishing. J. Appl. Electrochem. 2021, 51, 1479-1489. [CrossRef]
- Abd-El-Nabey, B.A.; Khalil, N.; Mohamed, A. Inhibition by amino acids of the corrosion of steel in acid. Surf. Technol. 1985, 24, 383-389. [CrossRef]
- Loto, R.T.; Loto, C.A. Data on evaluation of the corrosion inhibition effect of l-alpha-aminoisocaproate on high carbon steel corrosion in Dilute Acid Media. Medziagotyra 2019, 25, 422-426. [CrossRef]
- Purnima, Goyal, S.; Luxami, V. Corrosion inhibition mechanism of aromatic amino acids for steel in alkaline pore solution simulating carbonated concrete environment. Mater. Corros. 2024, 75, 39-60. [CrossRef]
- Fu, J.J.; Li, S.N.; Wang, Y.; Liu, X.D.; Lu, L.D. Computational and electrochemical studies on the inhibition of corrosion of mild steel by l-Cysteine and its derivatives. J. Mater. Sci. 2011, 46, 3550-3559. [CrossRef]
- Eddy, N.O. Experimental and theoretical studies on some amino acids and their potential activity as inhibitors for the corrosion of mild steel, part 2. J. Adv. Res. 2011, 2, 35-47. [CrossRef]
- Shehu, N.U.; Gaya, U.I.; Muhammad, A.A. Influence of side chain on the inhibition of aluminium corrosion in HCl by α-amino acids. Appl. Sci. Engin. Prog. 2019, 12, 186-197. [CrossRef]
- Saurbier, K.; Mendorf, V.; Schultze, J.W.; Geke, J.; Penninger, J.; Roßmaier, H. Toluylalanine as an inhibitor of atmospheric corrosion. Corros. Sci. 1992, 33, 1351-1359. [CrossRef]
- Li, .; Deng, S.; Fu, H.; Li, T. Adsorption and inhibition effect of 6-benzylaminopurine on cold rolled steel in 1.0 M HCl. Electrochim. Acta 2009, 54, 4089-4098. [CrossRef]
- Haruna, K.; Saleh, T.A.Dopamine functionalized graphene oxide (DGO) as a corrosion inhibitor against X60 carbon steel corrosion in a simulated acidizing environ ment; An electrochemical, weight loss, SERS, and computational study. Surf.Interf. 2024, 44, 103688. [CrossRef]
- Al-Mazaideh, G.M.; Abu-Sbeih, K.A.; Khalil, S.M. Computational Calculations of Chitosan Fragments as Corrosion Inhibitors of Metals. J. Chem. Biol. Phys. Sci. 2017, 7, 398-409.
- Umoren, S.U.; Eduok, U.M. Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: A review. Carbohydr. Polym. 2016, 140, 314-341. [CrossRef]
- Alsabagh, A.M.; Elsabee, M.Z.; Moustafa, Y.M.; Elfky, A.; Morsi. R.E. Corrosion inhibition efficiency of some hydrophobically modified chitosan surfactants in relation to their surface active properties. Egypt. J. Petroleum 2014, 23, 349-359. [CrossRef]
- Al-Mazaideh, G.M.; Al-Quran, S.A. Inhibitive action of Chamomile extract on the corrosion of Iron: Density Functional Theory. Mor.J.Chem. 2018, 6, 195-202.
- Saranya, J.; Vagdevi, K.; Jyothirmai, N.; Anusuya, N.; Benhiba, F.; Warad, I.; Zarrouk, A., Application of quercetin as a green inhibitor to prevent mild steel corrosion in the petroleum industry: Experimental and modelling techniques. Chem. Data Coll. 2024, 50, 101125. [CrossRef]
- Baildya, N.; Ghosh, N.N.; Chattopadhyay, A.P. Anti-corrosive properties of quercetin and its derivatives on Fe(111) surface: a quantum chemical approach. SN Appl. Sci. 2019, 1, 735. [CrossRef]
- Sukul, D.; Pal, A.; Saha, S.K.; Satpati, S.; Adhikari, U.; Banerjee, P. Newly synthesized quercetin derivatives as corrosion inhibitors for mild steel in 1 M HCl: Combined experimental and theoretical investigation. Phys. Chem. Chem. Phys. 2018, 20, 6562-6574. [CrossRef] [PubMed]
- Al-Mazeideh, G.M. Carbohydrates as Green Corrosion Inhibitors of Copper: Ab initio Study. Jord. J. Chem. 2017, 12, 189-200.
- C.C. Ong, K.A. Karim Inhibitory effect of red onion skin extract on the corrosion of mild steel in acidic medium. Chem. Engin. Trans. 2017, 56, 913-918.
- Fouda, A.S.; Rashwan, S.M.; Abo-Mosallam, H.A. Fennel seed extract as green corrosion inhibitor for 304 stainless steel in hydrochloric acid solutions. Desalin. Water Treat. 2014, 52, 5175-5186. [CrossRef]
- Fouda, A.E.A.S.; Nazeer, A.A.; El-Khateeb, A.Y.; Fakih; M. Cinnamon plant extract as corrosion inhibitor for steel used in waste water treatment plants and its biological effect on escherichia coli. J. Kor. Chem. Soc. 2014, 58, 359-365. [CrossRef]
- Loto, R.T. Corrosion Inhibition Performance of the Synergistic Effect of Rosmarinus officinalis and 5-Bromovanillin on 1018 Carbon Steel in Dilute Acid Media. J. Fail. Anal. Preven. 2017, 17, 1031-1043. [CrossRef]
- Loto, R.T. Surface coverage and corrosion inhibition effect of Rosmarinus officinalis and zinc oxide on the electrochemical performance of low carbon steel in dilute acid solutions. Res. Phys. 2018, 8, 172-179. [CrossRef]
- Loto, R.T. Inhibition studies of the synergistic effect of Rosemary oil and zinc oxide on S41003 ferritic steel corrosion in dilute sulphuric and hydrochloric acid solutions. Orient. J. Chem. 2018, 34, 240-254. [CrossRef]
- Loto, R.T.; Oghenerukewe, E. Inhibition studies of Rosmarinus officinalis on the pitting corrosion resistance 439LL ferritic stainless steel in dilute sulphuric acid. Orient. J. Chem. 2016, 32, 2813-2832. [CrossRef]
- Santos, A.M.; Aquino, I.P.; Cotting, F.; Aoki, I.V.; de Melo, H.G.; Capelossi, V.R. Evaluation of Palm Kernel Cake Powder (Elaeis guineensis Jacq.) as Corrosion Inhibitor for Carbon Steel in Acidic Media. Met. Mater. Intern. 2020, 27, 1519-1530. [CrossRef]
- Dai, J.; An, X. Corrosion inhibition properties of Camellia chrysantha flower extract for Q235 in 1 M HCl solution. Int. J. Electrochem. Sci. 2023, 18, 100080. [CrossRef]
- Zaher, A.; Aslam, R.; Lee, H.S.; Khafouri, A.; Boufellous, M.; Alrashdi, A..; El Aoufir, Y.; Lgaz, H.; Ouhssine, M. A combined computational & electrochemical exploration of the Ammi visnaga L. extract as a green corrosion inhibitor for carbon steel in HCl solution. Arab. J. Chem. 2022, 15, 103573. [CrossRef]
- Eddy, N.O.; Odoemelam, S.A.; Ogoko, E.C.; Ukpe, R.A.; Garg, R.; Anand, B. Experimental and quantum chemical studies of synergistic enhancement of the corrosion inhibition efficiency of ethanol extract of Carica papaya peel for aluminum in solution of HCl. Res. Chem. 2022, 4, 100290. [CrossRef]
- Royani, A.; Aigbodion, V.S.;. Hanafi, M.; Mubarak, N.M.; Verma, C.; Alfantazi, A.; Manaf, A. Enhancing the corrosion inhibition performance of Tinospora cordifolia extract using different fractions of methanol solvent on carbon steel corrosion in a seawater-simulated solution. App. Surf. Sci. Adv. 2023, 18, 100465. [CrossRef]
- Vaszilcsin, C.G.; Putz, M.V.; Dan, M.L.; Medeleanu, M. MYRICETIN AS CORROSION INHIBITOR FOR METALS IN ALCOHOLIC SOLUTIONS. Studia UBB Chemie 2023, 23-36. [CrossRef]
- Bathily, M.; Cisse, K.; Youssefi, Y.; Ou-ani, O.; Oucheikh, L.; Ngom, B.; Znini, M.; Gassama, D.; Costa, J. Evaluation of the Inhibiting Power of Essential Oil Extracted from Cloves (Syzygium Aromaticum) on Corrosion of Steel C24 In Medium HCl 1M. Anal. Bioanal. Electrochem. 2023, 15, 891-913.
- Abdelaali, B.; El Menyiy, N.; El Omari, N.; Benali, T.; Guaouguaou, F.E.; Salhi, N.; Naceiri Mrabti, H.; Bouyahya, A. Phytochemistry, Toxicology, and Pharmacological Properties of Origanum elongatum. Evidence-based Complementary and Alternative Medicine 2021, 2021, 6658593. [CrossRef]
- Boutaoui, N.; Acila, M.; Boulahbel, H.; Achouri, B.; Zaiter, L.; Benayache, F.; Benayache, S.; D' Agostino, I.; Campestre, C.; Locatelli, M. Assessment of The Green Corrosion Inhibition Performances of Thymus Algeriensis Microwave-Assisted Extracts on Mild Steel in Acid Solution. Anal.Bioanal.Electrochem. 2023, 15, 938-955.
- Coiai, S.; Campanella, B.; Paulert, R.; Cicogna, F.; Bramanti, E.; Lazzeri, A.; Pistelli, L.; Coltelli, M.B. Rosmarinic Acid and Ulvan from Terrestrial and Marine Sources in Anti-Microbial Bionanosystems and Biomaterials. Appl. Sci. 2021, 11, 9249. [CrossRef]
- Hachelef, H.; Mehdaoui, R.; Hachama, K.; Amara, M.; Khelifa, A.; Benmoussat, A.; Hadj Meliani, M.; Suleiman, R.K. Evaluation of Inhibition Efficiency of Thymus Extract as a Corrosion Inhibitor of Aluminum Alloy 5083 in an Ethylene Glycol/NaCl Corrosive Medium. Corros. Sci. Technol. 2023, 22, 314-321.
- Bathily, M.; Ngom, B.; Mbengue, M.; Gassama, D. Evaluation of the inhibitory action of essential oil from Eucalyptus globulus leaves on the corrosion of mild carbon steel in 1M HCl medium. Ovidius Univ. Annal. Chem. 2023, 34, 1-7. [CrossRef]
- El-Housseiny, S.; Abdel-Gaber, A.M.; Rahal, H.T.; Beqai, F.T. Eco-friendly corrosion inhibitor for mild steel in acidic media. Int. J. Corros. Scale Inhib. 2022, 11, 1516-1538.
- Torres Hernandez, J.R.; Del Angel Meraz, E.; Corvo Perez. E.F. Tradescantia spathacea: New green corrosion inhibitor for SAE 1010 steel in acid medium. Int. J. Corros. Scale Inhib. 2023, 12, 61-83.
- Sidine, M.O.; Lahbib, H.; Hadou, A.; Ba, M.; Benmessaoud, M.; Ben Amor, Y.; Ould, B. Elemine Usage of Grewia bicolor Juss leaves extract as green corrosion inhibitor for Steel XC40 in sulfuric acid medium. Int. J. Corros. Scale Inhib. 2023, 12, 961-983.
- Wan Nik, W.B.; Ravindran, K.; Al-Amiery, A.A.; Izionworu, V.O.; Fayomi, O.S. I.; Berdimurodov, E.; Daoudi, W.; Zulkifli, F.; Dhande, D.Y. Piper betle extract as an eco-friendly corrosion inhibitor for aluminium alloy in hydrochloric acid media, Int. J. Corros. Scale Inhib. 2023, 12, 948-960.
- Rahal, H.T.; Abdel-Gaber, A.M.; El Khatib, L.W.; El-Housseiny, S. Evaluation of Fragaria ananassa and Cucurbita pepo L Leaf Extracts as natural green corrosion inhibitors for copper in 0.5 M hydrochloric acid solution. Int. J. Corros. Scale Inhib. 2023, 12, 1417-1440.
- El Nemr, A.; Elhebshi, A.; Ashour, I.; El-Deab, M.S.; Barghout, N.A.; Eddy, N.O.; Ragab, S. Camphor tree bark extract as green corrosion inhibitor of LCS in 0.5 M H2SO4 with and without salt effect. J. Chin. Chem. Soc. 2024, 71, 174-196. [CrossRef]
- Feng, L.; Zhang, S.; Hao, L.; Du, H.; Pan, R.; Huang, G.; Liu, H. Cucumber (Cucumis sativus L.) Leaf Extract as a Green Corrosion Inhibitor for Carbon Steel in Acidic Solution: Electrochemical, Functional and Molecular Analysis. Molecules 2022, 27, 3826. [CrossRef]
- Temitayo, O.O.; Vawe, H.; Johnson, J.; Ezekiel, A.; Ilesanmi, O. Investigation on Corrosion Inhibition of Hura Crepitans for 2101 Duplex Stainless Steel in an Acidic Environment. Adv. Sci. Technol. Res. 2022, 16, 56-63.
- Hynes, N.R.J.; Vignesh, N.J.; Barile, C.; Velu, P.S.; Baskaran, T.; Jappes, J.T.W.; Al-Khashman, O.Al.; Brykov, M.; Ene, A. Green Corrosion Inhibition on Carbon-Fibre-Reinforced Aluminium Laminate in NaCl Using Aerva Lanata Flower Extract. Polymers 2022, 14, 1700. [CrossRef]
- Beniaich, G.; Beniken, M.; Salim, R.; Arrousse, N.; Ech-chihbi, E.; Rais, Z.; Sadiq, A.; Nafidi, H.A.; Bin Jardan, Y.A.; Bourhia, M. Anticorrosive Effects of Essential Oils Obtained from White Wormwood and Arar Plants. Separations 2023, 10, 396. [CrossRef]
- Zhao, C.; Lin, B.; Zhou, X.; Zhu, J.; Duan, T.; Xu, Y. Corrosion Inhibition and Adsorption Behavior of Musa Basjoo Siebold Leaves Extract on Mild Steel in H3PO4 Solution: Experimental and Theoretical Studies. Prot. Met. Phys. Chem.Surf. 2023, 59, 1010-1027. [CrossRef]
- Mohammed, N.J.; Othman, N.K.; Al-Gburi, A.J.A.; Yusop, R.M. Date Palm Seed Extract for Mild Steel Corrosion Prevention in HCl Medium. Separations 2023, 10, 54. [CrossRef]
- Zhao, C.; Lin, B.; Zhou, X.; Zhu, J.; Duan, T.; Xu, Y. Corrosion Inhibition and Adsorption Behavior of Musa Basjoo Siebold Leaves Extract on Mild Steel in H3PO4 Solution: Experimental and Theoretical Studies. Prot. Met. Phys. Chem. Surf. 2023, 59, 778-795. [CrossRef]
- Enabulele, D.O.; Bamigboye, G.O.; Solomon, M.M.; Durodola, B. Exploration of the Corrosion Inhibition Potential of Cashew Nutshell on Thermo-Mechanically Treated Steel in Seawater. Arab. J. Sci. Eng. 2023, 48, 223-237. [CrossRef]
- A.M. Santos, I.P. Aquino, F. Cotting, I.V. Aoki, H.G. de Melo, V.R. Capelossi Evaluation of Palm Kernel Cake Powder (Elaeis guineensis Jacq.) as Corrosion Inhibitor for Carbon Steel in Acidic Media. Met. Mater. Int. 2021, 27, 1519-1530. [CrossRef]
- Abbasi, S. Ghaffari, N. Safa, M. Ferdosi Sumac Extract for Effective Aluminum Corrosion Inhibition in HCl Solution. J.Mater.Engin.Perf. 2023. [CrossRef]
- Acidi, A.; Sedik, A.; Rizi, A.; Bouasla, R.; Rachedi, K.O.; Berredjem, M.; Delimi, A.; Abdennouri, A.; Ferkous, H.; Yadav, K.K.; Alam, M.; Ernst, B.; Bengureba, Y. Examination of the main chemical components of essential oil of Syzygium aromaticum as a corrosion inhibitor on the mild steel in 0.5 M HCl medium. J. Mol. Liq. 2023, 391, 123423. [CrossRef]
- Lin, B.; Shao, J.; Zhao, C.; Zhou, X.; He, F.; Xu, Y. Passiflora edulis Sims peel extract as a renewable corrosion inhibitor for mild steel in phosphoric acid solution. J. Mol. Liq. 2023, 375, 121296. [CrossRef]
- Abdellattif, M.H.; Alrefaee, S.H.; Dagdag, O.; Verma, C.; Quraishi, M.A. Calotropis procera extract as an environmental friendly corrosion Inhibitor: Computational demonstrations. J. Mol. Liq. 2021, 337, 116954. [CrossRef]
- Golafshani, M.G.; Tavakoli, H.; Hosseini, S.A.; Akbari, M. MD and DFT computational simulations of Caffeoylquinic derivatives as a bio-corrosion inhibitor from quince extract with experimental investigation of corrosion protection on mild steel in 1M H2SO4. J. Molec. Struct. 2023, 1275, 134701. [CrossRef]
- Eddy, N.O.; Awe, F.; Ebenso; E.E. Adsorption and inhibitive properties of ethanol extracts of leaves of Solanum melongena for the corrosion of mild steel in 0.1 M HCl. Int. J. Electrochem. Sci. 2010, 5, 1996-2011. [CrossRef]
- Eddy, N.O.; Odiongenyi, A.O.; Ameh, P.O.; Ebenso, E.E. Corrosion inhibition potential of Daniella oliverri gum exudate for mild steel in acidic medium. Int. J. Electrochem. Sci. 2012, 7, 7425-7439. [CrossRef]
- Znini, M.; Bouklah, M.; Majidi, L.; Kharchouf, S.; Aouniti, A.; Bouyanzer, A.; Hammouti, B.; Costa, J.; Al-Deyab, S.S. Chemical composition and inhibitory effect of Mentha spicata essential oil on the corrosion of steel in molar hydrochloric acid, Int. J. Electrochem. Sci. 2011, 6, 691-704. [CrossRef]
- Barbouchi, M.; Benzidia, B.; Idrissi, M.E.; Choukrad, M. Pistacia lentiscus l. Essential oils as a green corrosion inhibitors for iron in neutral chloride media. Anal. Bioanal. Electrochem. 2019, 11, 333-348.
- Suleiman, I.Y.; Yaro, S.A.; Abdulwahab, M.; Salihu, S.A.; Ogheneme, O.C. Phytochemical and spectroanalytical characterizations of some plants extract as green corrosion inhibitors. J. Mater.Environm. Sci. 2017, 8, 3423-3432.
- 1 Salvador, C.; Carlos, B.; Alvaro, R. Inhibitory effect of extracts of Amella radican on corrosion of carbon steel sheets in HCl solution. J. Eng. Appl. Sci. 2017, 12, 5247-5252.
- Nnaji, N.J.N.; Obi-Egbedi, N.O.; Okoye, C.O.B. Cashew nut testa tannin: Assessing its effects on the corrosion of aluminium in HCL. Portug. Electrochim. Acta 2014, 32, 157-182. [CrossRef]
- Okewale, A.; Omoruwuo, F. Neem leaf extract as a corrosion inhibitor on mild steel in acidic solution. Int. J. Engin. Res. Africa 2018, 35, 208-220. [CrossRef]
- Rosli, N.R.; Yusuf, S.M.; Sauki, A.; Razali, W.M.R.W. Musa sapientum (Banana) peels as green corrosion inhibitor for mild steel. Key Engin. Mater. 2019, 797, 230-239. [CrossRef]
- Fekkar, G.; Yousfi, F.; Elmsellem, H.; Aiboudi, M.; Ramdani, M.; Abdel-Rahman, I.; Hammouti, B.; Bouyazza, L. Eco-friendly chamaerops humilis l. Fruit extract corrosion inhibitor for mild steel in 1 M HCL. Int. J. Corros. Scale Inhib. 2020, 9, 446-459.
- Boumezzourh, A.; Ouknin, M.; Chibane, E.; Costa, J.; Bouyanzer, A.; Hammouti, B.; Majidi, L. Inhibition of tinplate corrosion in 0.5 M H2C2O4 medium by Mentha pulegium essential oil. Int. J. Corros. Scale Inhib. 2020, 9, 152-170.
- Haldhar, R.; Prasad, D.; Saxena, A.; Kaur, A. Corrosion resistance of mild steel in 0.5 M H2SO4 solution by plant extract of Alkana tinctoria: Experimental and theoretical studies. Eur. Phys. J. Plus 2018, 133, 356. [CrossRef]
- Mousaa, I.M. Preparation and comparative study of eco-friendly anticorrosion inhibitors based on aliphatic and aromatic compounds for electron beam curable steel coatings. Anti-Corros. Meth. Mater. 2020, 67, 367-377. [CrossRef]
- Thomas, A.; Prajila, M.; Shainy, K.M.; Joseph, A. A green approach to corrosion inhibition of mild steel in hydrochloric acid using fruit rind extract of Garcinia indica (Binda). J. Mol. Liq. 2020, 312, 113369. [CrossRef]
- Haldhar, R.; Prasad, D.; Saxena, A.; Kumar, R. Experimental and theoretical studies of Ficus religiosa as green corrosion inhibitor for mild steel in 0.5 M H2SO4 solution. Sustain. Chem. Pharm. 2018, 9, 95-105. [CrossRef]
- Vasyliev, G.; Vorobiova, V. Rape grist extract (Brassica napus) as a green corrosion inhibitor for water systems. Mater. Today. Proc. 2019, 6, 178-186. [CrossRef]
- Agi, A.; Junin, R.; Zakariah, M.I.; Bukkapattanam, T.B. Effect of Temperature and Acid Concentration on Rhizophora mucronata Tannin as a Corrosion Inhibitor. J. Bio. Tribo. Corros. 2018, 4, 5. [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Electronic/atomic level fundamental theoretical evaluations combined with electrochemical/surface examinations of Tamarindus indiaca aqueous extract as a new green inhibitor for mild steel in acidic solution (HCl 1 M). J. Taiwan Inst. Chem. Engin. 2019, 102, 349-377. [CrossRef]
- Fang, Y.; Suganthan, B.; Ramasamy, R.P. Electrochemical characterization of aromatic corrosion inhibitors from plant extracts. J. Electroanal. Chem. 2019, 840, 74-83. [CrossRef]
- Furtado, L.B.; Nascimento, R.C.; Seidl, P.R.; Guimarães, M.J.O.C.; Costa, L.M.; Rocha, J.C.; Ponciano, J.A.C.P. Eco-friendly corrosion inhibitors based on Cashew nut shell liquid (CNSL) for acidizing fluids. J. Mol. Liq. 2019, 284, 393-404. [CrossRef]
- Agi, A.; Junin, R.; Rasol, M.; Gbadamosi, A.; Gunaji, R. Treated Rhizophora mucronata tannin as a corrosion inhibitor in chloride solution. PLoS ONE 2018, 13, e0200595. [CrossRef] [PubMed]
- Martinez, S. Inhibitory mechanism of mimosa tannin using molecular modeling and substitutional adsorption isotherms. Mater. Chem. Phys. 2003, 77, 97-102. [CrossRef]
- Fouda, A.S.; El-Etre, A.Y.; Mahmoud, M. Plant doum (Hyphaenethebaica l.) as a safe corrosion inhibitor for carbon steel in a solution of HCl. Int. J. Corros. Scale Inhib. 2021, 10, 1547-1565.
- Fouda, A.E.A.S.; Salem, A.M.; Wahba, A.M.; Abd El-Maksous, S.A.; El-Haddad, M.N. Experimental and theoretical optimization of Chelidonium Majus (Papaveraceae) extract as an environmentally friendly inhibitor for corrosion of α-brass in nitric acid solution. Mater. Prot. 2023, 64, 239-255. [CrossRef]
- Okeniyi, J.O.; Okeniyi, E.T.; Ogunlana, O.O.; Owoeye, T.F.; Ogunlana, O.E. Investigating biochemical constituents of Cymbopogon citratus leaf: Prospects on total corrosion of concrete steel-reinforcement in acidic-sulphate medium, TMS 2017 146th Annual Meeting & Exhibition Supplemental Proceedings Part F6 (2017) 341-351.
- Lin, B.L.; Shao, J.J.; Xu, Y.Y.; Lai, Y.M.; Zhao, Z.N. Adsorption and corrosion of renewable inhibitor of Pomelo peel extract for mild steel in phosphoric acid solution. Arab. J. Chem. 2021, 14, 103114. [CrossRef]
- Hassannejad, H.; Nouri, A. Sunflower seed hull extract as a novel green corrosion inhibitor for mild steel in HCl solution. J. Mol. Liq. 2018, 254, 377-382. [CrossRef]
- Hussin, M.H.; Rahim, A.A.; Mohamad Ibrahim, M.N.; Brosse, N. Improved corrosion inhibition of mild steel by chemically modified lignin polymers from Elaeis guineensis agricultural waste. Mater. Chem. Phys. 2015, 163, 201-212. [CrossRef]
- Hajar, H.M.; Zulkifli, F.; Mohd Sabri, M.G.; Fitriadhy, A.; Wan, W.B. Nik Lawsonia inermis performance as corrosion inhibitor for mild steel in seawater. Int. J. ChemTech Res. 2016, 9, 600-608.
- Luo, X.; Chen, B.; Li, J.; Lan, B.; Zhou, C.; Ren, Z.; Ci, C.; Liu, Y. Synthesis of natural glucomannan derivative as a highly-efficient green inhibitor for mild steel in the simulated seawater. J. Ind. Engin. Chem. 2023, 124, 132-146. [CrossRef]
- Singh, A.; Ebenso, E.E.; Quraishi, M.A. Corrosion Inhibition of Carbon Steel in HCl Solution by Some Plant Extracts. Int. J. Corros. 2012, 2012, 897430. [CrossRef]
- Bilgiç, S. Plant extracts as corrosion inhibitors for mild steel in HCl media – Review I. Int. J. Corros. Scale Inhib. 2021, 10, 145–175.
- Bilgiç, S. Plant extracts as corrosion inhibitors for mild steel in H2SO4 and H3PO4 media - Review II. Int. J. Corros. Scale Inhib. 2022, 11, 1-42.
- Daouda, D.; Douadi, T.; Ghobrini, D.; Lahouel, N.; Hamani, H. Investigation of some phenolic-type antioxidants compounds extracted from biodiesel as green natural corrosion inhibitors; DFT and molecular dynamic simulation, comparative study. AIP Conf. Proc. 2019, 2190, 020098.
- Ghelichkhah, Z.; Dehkharghani, F.K.; Sharifi-Asl, S.; Obot, I.B.; Macdonald, D.D.; Farhadi, K.; Avestan, M.S.; Petrossians, A. The inhibition of type 304LSS general corrosion in hydrochloric acid by the New Fuchsin compound. Corros. Sci. 2021, 178, 109072. [CrossRef]
- Ganjoo, R.; Verma, C.; Kumar, A.; Quraishi, M.A. Colloidal and interface aqueous chemistry of dyes: Past, present and future scenarios in corrosion mitigation. Adv. Coll. Interf. Sci. 2023, 311, 102832. [CrossRef]
- Buier, R.; Szabo, G.S.; Katona, G.; Muntean, N.; Muresan, L.M. Influence of pH on the Inhibiting Characteristics of Cresol Red Incorporated in Chitosan Coatings on Zinc. Metals 2023, 13, 1958. [CrossRef]
- Fouda, A.S.; Mostafa, H.A.; El-Abbasy, H.M. Antibacterial drugs as inhibitors for the corrosion of stainless steel type 304 in HCl solution. J. Appl. Electrochem. 2010, 40, 163-173. [CrossRef]
- Adardour, L.; Lgaz, H.; Salghi, R.; Larouj, M.; Jodeh, S.; Zougagh, M.; Hamed, O.; Taleb, M. Corrosion inhibition of steel in phosphoric acid by Sulfapyridine: Experimental and theoretical studies. Der Pharmacia Lettre 2016, 8, 173-185.
- Tasić, Ž.Z.; Petrović Mihajlović, M.B.; Radovanović, M.B.; Simonović, A.T.; Antonijević; M.M. Experimental and theoretical studies of paracetamol as a copper corrosion inhibitor. J. Mol. Liq. 2021, 327, 114817. [CrossRef]
- Verma, C.; Quraishi, M.A.; Rhee, K.Y. Present and emerging trends in using pharmaceutically active compounds as aqueous phase corrosion inhibitors. J. Mol. Liq. 2021, 328, 115395. [CrossRef]
- Ituen, E.; Akaranta, O.; James, A. Influence of Clomipramine-Based Blends on Corrosion Behaviour of J55 Steel in Simulated Oilfield Acidizing Solution. J. Bio. Tribo. Corros. 2017, 3, 23. [CrossRef]
- Abeng, F.E.; Ikpi, M.E.; Okafor, P.C.; Anadebe, V.C.; Chukwuike, V.I.; Uwakwe, K.J.; Ikeuba, A.I.; Okafor, N.A.; Anaekwe, N.O. Corrosion inhibition of API 5L X-52 pipeline steel in oilfield acidizing solution by gentamicin and sulfamethoxazole: experimental, plane-wave density functional theory (PWDFT) and the generalized-gradient approximation (GGA) simulations. J. Adhes. Sci. Technol. 2022, 36, 2438-2461. [CrossRef]
- Monisha, R.; Ramkumar, S.; Nalini, D. Experimental and density function theory calculations to investigate the adsorption of an anti-inflammatory drug on aluminum surface in acid solution. NACE - Int. Corr. Conf. Ser. 2016, 4, 2703-2717.
- Bedair, M.A.; Abuelela, A.M.; Alshareef, M.; Owda, M.; Eliwa, E.M. Ethyl ester/acyl hydrazide-based aromatic sulfonamides: facile synthesis, structural characterization, electrochemical measurements and theoretical studies as effective corrosion inhibitors for mild steel in 1.0 M HCl. RSC Adv. 2022, 13, 186-211. [CrossRef]
- El-Haddad, M.N.; Fouda, A.E.A.S. Evaluation of Curam drug as an ecofriendly corrosion inhibitor for protection of stainless steel-304 in hydrochloric acid solution: Chemical, electrochemical, and surface morphology studies. J. Chin. Chem. Soc. 2021, 68, 826-836. [CrossRef]
- Fouda, A.S.; El-Abbasy, H.M. Inhibitive action of ampicillin and benzyl penicillin drugs for corrosion of type 304 stainless steel in 1.0 M HCl solution. Corrosion 2012, 68, 015002. [CrossRef]
- Fouda, A.S.; El-Ewady, G.; Ali, A.H. Modazar as promising corrosion inhibitor of carbon steel in hydrochloric acid solution. Green Chem.Lett.Rev. 2017, 10, 88-100. [CrossRef]
- Beltran-Perez, C.; Serrano, A.A.A.; Solis-Rosas, G.; Martinez-Jimenez, A.; Orozco-Cruz, R.; Espinoza-Vazquez, A.; Miralrio, A. A General Use QSAR-ARX Model to Predict the Corrosion Inhibition Efficiency of Drugs in Terms of Quantum Mechanical Descriptors and Experimental Comparison for Lidocaine. Int. J. Molec. Sci. 2022, 23, 5086. [CrossRef]
- Chen, W.; Xiao, W. Corrosion Inhibition Effect of flubendazole for Carbon Steel in 0.5 M H2SO4. Int. J. Electrochem. Sci. 2022, 17, 220427. [CrossRef]
- Arslan, T.; Kandemirli, F.; Ebenso, E.E.; Love, I.; Alemu, H. Quantum chemical studies on the corrosion inhibition of some sulphonamides on mild steel in acidic medium. Corros. Sci. 2009, 51, 35-47. [CrossRef]
- Murulana, L.C.; Kabanda, M.M.; Ebenso, E.E. Investigation of the adsorption characteristics of some selected sulphonamide derivatives as corrosion inhibitors at mild steel/hydrochloric acid interface: Experimental, quantum chemical and QSAR studies. J. Mol. Liq. 2016, 215, 763-779. [CrossRef]
- Vaszilcsin, N.; Kellenberger, A.; Dan, M.L.; Duca, D.A.; Ordodi, V.L. Efficiency of Expired Drugs Used as Corrosion Inhibitors: A Review. Materials 2023, 16, 5555. [CrossRef] [PubMed]
- Peng, M.; He, S.; Wu, C.; Chen, Z.; Cen, H. Corrosion inhibition of extracts from the expired traditional Chinese patent medicines for Q235 carbon steel in hydrochloric acid. Sust. Chem. Pharm. 2023, 36, 101326. [CrossRef]
- El-Haddad, M.N.; Fouda, A.S. Inhibition Effect and Adsorption Behavior of New Azodye Derivatives on Corrosion of Carbon Steel in Acid Medium. J. Dispers. Sci. Technol. 2013, 34, 1471-1480. [CrossRef]
- Alrazzak, N.A. SYNTHESIS OF NEW AZO COMPOUNDS BASED ON 4-AMINOSALICYLIC ACID AND STUDY ANTI-CORROSIVE ACTIVITY. Bull. Chem. Soc. Ethiopia 2024, 38, 473-479. [CrossRef]
- Ramkumar, S.; Nalini, D.; Quraishi, M.A.; Ebenso, E.E.; Verma, C. Anti-corrosive property of bioinspired environmental benign imidazole and isoxazoline heterocyclics: A cumulative studies of experimental and DFT methods. J. Heterocyc. Chem. 2020, 57, 103-119. [CrossRef]
- Song, X.; Qiu, G.; Qu, P.; Yang, H.; He, X. Density function theory studies on the structure of AMT inhibitor. J. Wuhan Univ. Technol. Mater. Sci.Ed. 2006, 21, 61-64.
- Oyeneyin, O.E.; Ojo, N.D.; Ipinloju, N.; Agbaffa, E.B.; Emmanuel, A.V. Investigation of the corrosion inhibition potentials of some 2-(4-(substituted) arylidene)-1H-indene-1,3-dione derivatives: density functional theory and molecular dynamics simulation. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 132. [CrossRef]
- Shafi, A.; Timiri Sathyamurthy, R.D.; Seetharaman, J.; Sambanthan, M.; Murugesan, R.; Sundaram, S.; Bhanumathy Ramarathinam, R. Molecular docking, quantum chemical computational and vibrational studies on bicyclic heterocycle "6-nitro-2,3-dihydro-1,4-benzodioxine": Anti-cancer agent. Comp. Biol. Chem. 2020, 86, 107226. [CrossRef]
- Holze, R. Optical and Electrochemical Band Gaps in Mono-, Oligo-, and Polymeric Systems: A Critical Reassessment. Organometallics 2014, 33, 5033-5042. [CrossRef]
- Sastri, V.S.; Perumareddi, J.R. Selection of Corrosion Inhibitors for Use in Sour Media. Corrosion 1994, 50, 432-437. [CrossRef]
- Sayós, R.; González, M.; Costa, J.M. On the use of quantum chemical methods as an additional tool in studying corrosion inhibitor substances. Corros. Sci. 1986, 26, 927-934. [CrossRef]
| 1 | Designations of alloys are apparently not stated in an internationally accepted format, sometimes they are just called by naming the majority metal. To avoid confusion the term alloy is always inserted when required irrespective whatever in the original report has been stated. |
| 2 | It should be noted that some triazoles are not aromatic. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



