Submitted:
11 October 2024
Posted:
11 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Phosphorus and Silicon Cycles in Soils
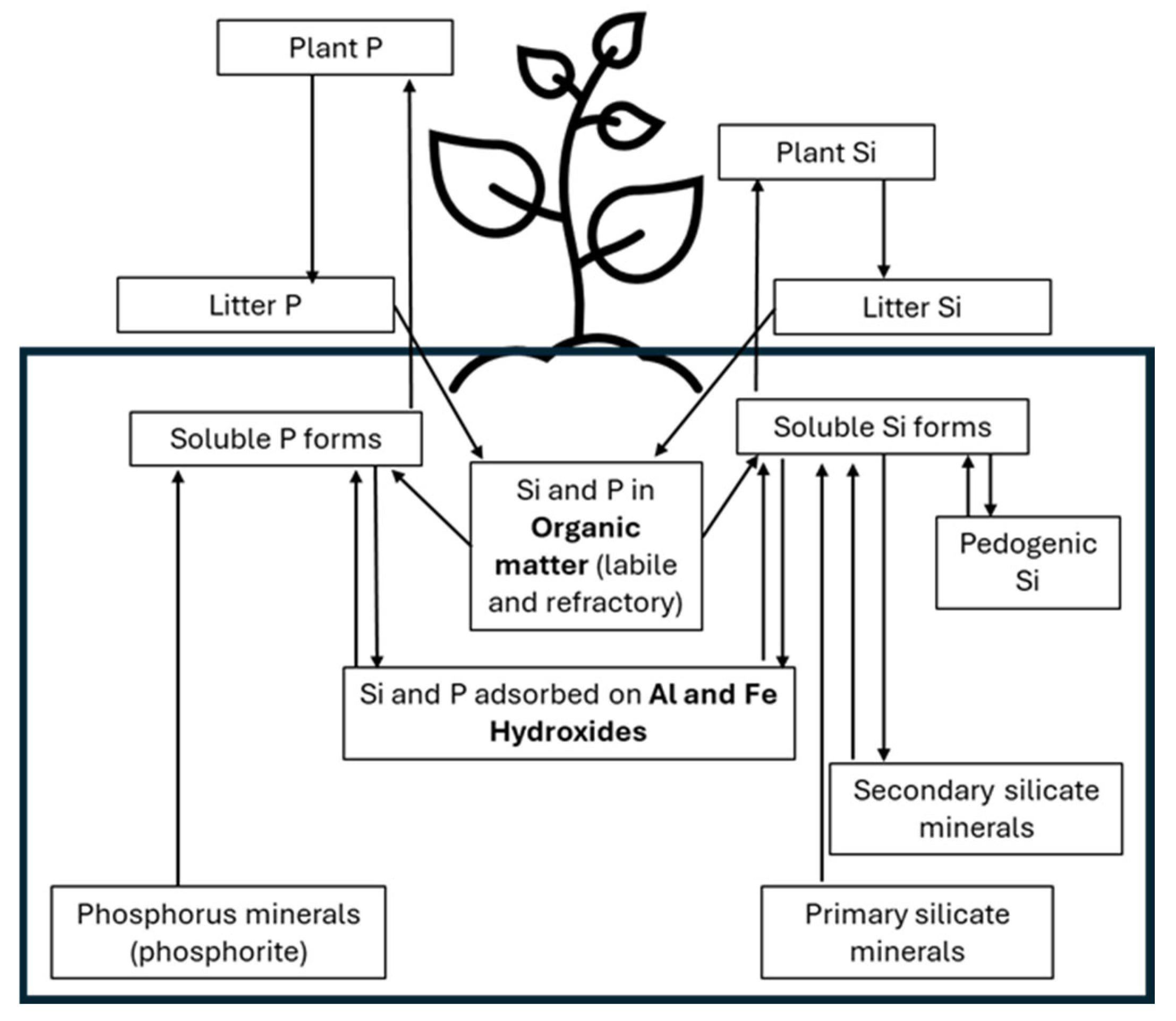
3. The Link between Si and P
The pH Effect
The Si-Uptake Effect
The Si-Soil Effect
4. Moving towards the Future
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shepherd, J.G.; Kleemann, R.; Bahri-Esfahani, J.; Hudek, L.; Suriyagoda, L.; Vandamme, E.; van Dijk, K.C. The Future of Phosphorus in Our Hands. Nutr Cycl Agroecosyst 2016, 104, 281–287. [Google Scholar] [CrossRef]
- Lambers, H.; Plaxton, W.C. Phosphorus: Back to The Roots. In Phosphorus Metabolism in Plants; Wiley Blackwell, 2015; Vol. 48, pp. 3–22 ISBN 9781118958841.
- Rosemarin, A.; Ekane, N. The Governance Gap Surrounding Phosphorus. Nutr Cycl Agroecosyst 2016, 104, 265–279. [Google Scholar] [CrossRef]
- Schaetzl, R.; Andeson, S. Soils: Genesis and Geomorphology; 2005; ISBN 9780521812016.
- Horst, W.; Jibrin, J.; Chude, V.; Kamh, M. Agronomic Measures for Increasing P Aviability to Crops. Plant Soil 2001, 237, 211–223. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus Dynamics: From Soil to Plant. Plant Physiol 2011, 156, 997–1005. [Google Scholar] [CrossRef]
- Scholz, R.W.; Ulrich, A.E.; Eilittä, M.; Roy, A. Sustainable Use of Phosphorus: A Finite Resource. Science of the Total Environment 2013, 461–462, 799–803. [Google Scholar] [CrossRef]
- Herrera-Estrella, L.; López-Arredondo, D. Phosphorus: The Underrated Element for Feeding the World. Trends Plant Sci 2016, 21, 461–463. [Google Scholar] [CrossRef]
- Obersteiner, M.; Peñuelas, J.; Ciais, P.; Van Der Velde, M.; Janssens, I.A. The Phosphorus Trilemma. Nat Geosci 2013, 6, 897–898. [Google Scholar] [CrossRef]
- Hart, M.; Quin, B.F. Phosphorus Runoff from Agricultural Land and Direct Fertilizer Effects Phosphorus Runoff from Agricultural Land and Direct Fertilizer Effects : A Review. 2004. [CrossRef]
- Roy, E.D. Phosphorus Recovery and Recycling with Ecological Engineering: A Review. Ecol Eng 2017, 98, 213–227. [Google Scholar] [CrossRef]
- Liang, C.; Liu, X.; Feng, L.; Jin, N.; Lv, J.; Yu, Q. Optimizing Phosphorus Fertilizer Use on the Loess Plateau: Impact on Soil Properties and Crop Production Efficiency. Soil Syst 2024, 8. [Google Scholar] [CrossRef]
- Sattari, S.Z.; Bouwman, A.F.; Giller, K.E.; Van Ittersum, M.K. Residual Soil Phosphorus as the Missing Piece in the Global Phosphorus Crisis Puzzle. Proc Natl Acad Sci U S A 2012, 109, 6348–6353. [Google Scholar] [CrossRef]
- van Dijk, K.C.; Lesschen, J.P.; Oenema, O. Phosphorus Flows and Balances of the European Union Member States. Science of the Total Environment 2016, 542, 1078–1093. [Google Scholar] [CrossRef] [PubMed]
- Rowe, H.; Withers, P.J.A.; Baas, P.; Chan, N.I.; Doody, D.; Holiman, J.; Jacobs, B.; Li, H.; MacDonald, G.K.; McDowell, R.; et al. Integrating Legacy Soil Phosphorus into Sustainable Nutrient Management Strategies for Future Food, Bioenergy and Water Security. Nutr Cycl Agroecosyst 2016, 104, 393–412. [Google Scholar] [CrossRef]
- Keeping, M.G.; Reynolds, O.L. Silicon in Agriculture: New Insights, New Significance and Growing Application. Annals of Applied Biology 2009, 155, 153–154. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.-D. Benefits of Plant Silicon for Crops: A Review. Agron Sustain Dev 2011, 32, 201–213. [Google Scholar] [CrossRef]
- Limmer, M.A.; Mann, J.; Amaral, D.C.; Vargas, R.; Seyfferth, A.L. Silicon-Rich Amendments in Rice Paddies: Effects on Arsenic Uptake and Biogeochemistry. Science of the Total Environment 2018, 624, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E.A.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological Functions of Beneficial Elements. Curr Opin Plant Biol 2009, 12, 267–274. [Google Scholar] [CrossRef]
- Barão, L. The Use of Si-Based Fertilization to Improve Agricultural Performance. J Soil Sci Plant Nutr 2022. [Google Scholar] [CrossRef]
- Katz, O.; Puppe, D.; Kaczorek, D.; Prakash, N.B.; Schaller, J. Silicon in the Soil–Plant Continuum: Intricate Feedback Mechanisms within Ecosystems. Plants 2021, 10. [Google Scholar] [CrossRef]
- Barão, L.; Alaoui, A.; Hessel, R. Identifying and Comparing Easily Accessible Frameworks for Assessing Soil Organic Matter Functioning. Agronomy 2023, 13. [Google Scholar] [CrossRef]
- Rovira, P.; Ramón Vallejo, V. Labile and Recalcitrant Pools of Carbon and Nitrogen in Organic Matter Decomposing at Different Depths in Soil: An Acid Hydrolysis Approach; 2002; Vol. 107;
- Robertson2000.
- Cabrera, M.L.; Kissel, D.E.; Vigil, M.F. Nitrogen Mineralization from Organic Residues: Research Opportunities.
- Zou, X.; Binkley, D.; Doxtader, K.G. A New Method for Estimating Gross Phosphorus Mineralization and Immobilization Rates in Soils. Plant Soil 1992, 147, 243–250. [Google Scholar] [CrossRef]
- Piperno, D. Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists; Press, A., Ed. 2006. [Google Scholar]
- Rashid, I.; Mir, S.H.; Zurro, D.; Dar, R.A.; Reshi, Z.A. Phytoliths as Proxies of the Past. Earth Sci Rev 2019, 194, 234–250. [Google Scholar] [CrossRef]
- Fraysse, F.; Pokrovsky, O.S.; Schott, J.; Meunier, J.D. Surface Chemistry and Reactivity of Plant Phytoliths in Aqueous Solutions. Chem Geol 2009, 258, 197–206. [Google Scholar] [CrossRef]
- Struyf, E.; Smis, A.; Damme, S.; Meire, P.; Conley, D.J. The Global Biogeochemical Silicon Cycle. Silicon 2009, 1, 207–213. [Google Scholar] [CrossRef]
- Ruttenberg, K.C. The Global Phosphorus Cycle. In Encyclopedia of Ocean Sciences; 2008; pp. 585–643.
- Barão, L.; Clymans, W.; Vandevenne, F.; Meire, P.; Conley, D.J.; Struyf, E. Pedogenic and Biogenic Alkaline-Extracted Silicon Distributions along a Temperate Land-Use Gradient. Eur J Soil Sci 2014, 65. [Google Scholar] [CrossRef]
- Schaller, J.; Puppe, D.; Kaczorek, D.; Ellerbrock, R.; Sommer, M. Silicon Cycling in Soils Revisited. Plants 2021, 10, 1–36. [Google Scholar] [CrossRef]
- Barão, L.; Teixeira, R.; Vandevenne, F.; Ronchi, B.; Unzué-Belmonte, D.; Struyf, E. Silicon Mobilization in Soils: The Broader Impact of Land Use. Silicon 2020, 12, 1529–1538. [Google Scholar] [CrossRef]
- Haynes, R.J. A Contemporary Overview of Silicon Availability in Agricultural Soils. Journal of Plant Nutrition and Soil Science 2014, 177, 831–844. [Google Scholar] [CrossRef]
- Sommer, M.; Kaczorek, D.; Kuzyakov, Y.; Breuer, J. Silicon Pools and Fluxes in Soils and Landscapes - A Review. Journal of Plant Nutrition and Soil Science 2006, 169, 310–329. [Google Scholar] [CrossRef]
- Wilson, M.J. Weathering of the Primary Rock-Forming Minerals: Processes, Products and Rates. Clay Miner 2004, 39, 233–266. [Google Scholar] [CrossRef]
- Dahanayake, K.; Subasinghe, S.M.N.D. 1989.
- Brenchley, W.E.; Maskell, E.J.; Warington, K. The Inter-Relation Between Silicon and Other Elements in Plant Nutrition. Ann Appl Biol 1927, XIV, 45–82. [Google Scholar] [CrossRef]
- Fisher, R.A. A Preliminary Note on the Effect of Sodium Silicate in Increasing the Yield of Barley. 1929. [CrossRef]
- Ma, J.; Takahashi, E. Effect of Silicate on Phosphate Availability for Rice in a P‐Deficient Soil. 1991. [Google Scholar]
- Ma, J.; Takahashi, E. Effect of Silicon on the Growth and Phosphorus Uptake of Rice; 1990; Vol. 126;
- Snyder, G.H.; Jones, D.B.; Gascho, G.J.; Jones, D.S. Silicon Fertiliz-Ation Ofrice on Everglades Histosols; 1986; Vol. 50;
- Agostinho, F.B.; Tubana, B.S.; Martins, M.S.; Datnoff, L.E. Effect of Different Silicon Sources on Yield and Silicon Uptake of Rice Grown under Varying Phosphorus Rates. Plants 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Deren, C.W. Changes in Nitrogen and Phosphorus Concentrations of Silicon-Fertilized Rice Grown on Organic Soil. J Plant Nutr 1997, 20, 765–771. [Google Scholar] [CrossRef]
- Kostic, L.; Nikolic, N.; Bosnic, D. Silicon Increases Phosphorus ( P ) Uptake by Wheat under Low P Acid Soil Conditions. Plant Soil 2017, 447–455. [Google Scholar] [CrossRef]
- Rezakhani, L.; Motesharezadeh, B.; Tehrani, M.M.; Etesami, H.; Mirseyed Hosseini, H. Phosphate–Solubilizing Bacteria and Silicon Synergistically Augment Phosphorus (P) Uptake by Wheat (Triticum Aestivum L.) Plant Fertilized with Soluble or Insoluble P Source. Ecotoxicol Environ Saf 2019, 173, 504–513. [Google Scholar] [CrossRef]
- Rezakhani, L.; Motesharezadeh, B.; Tehrani, M.M.; Etesami, H.; Mirseyed Hosseini, H. Effect of Silicon and Phosphate-Solubilizing Bacteria on Improved Phosphorus (P) Uptake Is Not Specific to Insoluble P-Fertilized Sorghum (Sorghum Bicolor L.) Plants. J Plant Growth Regul 2020, 39, 239–253. [Google Scholar] [CrossRef]
- Frank Stephano, M.; Geng, Y.; Cao, G.; Wang, L.; Meng, W.; Meiling, Z. Effect of Silicon Fertilizer and Straw Return on the Maize Yield and Phosphorus Efficiency in Northeast China. Commun Soil Sci Plant Anal 2021, 52, 116–127. [Google Scholar] [CrossRef]
- Owino-Gerroh, C.; Gascho, G.J.; Phatak, S.C. Pigeonpea Response to Silicon, Phosphorus, and Rhizobium Inoculation in an Acid Coastal Plain Soil. J Plant Nutr 2005, 28, 797–804. [Google Scholar] [CrossRef]
- Neu, S.; Schaller, J.; Dudel, E.G. Silicon Availability Modifies Nutrient Use Efficiency and Content, C:N:P Stoichiometry, and Productivity of Winter Wheat (Triticum Aestivum L.). Sci Rep 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Guntzer, F.; Keller, C.; Meunier, J.D. Benefits of Plant Silicon for Crops: A Review. Agron Sustain Dev 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Besharat, S.; Barão, L.; Cruz, C. New Strategies to Overcome Water Limitation in Cultivated Maize: Results from Sub-Surface Irrigation and Silicon Fertilization. J Environ Manage 2020, 263. [Google Scholar] [CrossRef]
- Besharat, S.; Pinto, J.C.; Fernandes, M.; Miguel, A.; Cruz, C.; Barão, L. Biofertilizers and Silicon Fertilization as a Sustainable Option for Maize Production. Silicon 2024, 16, 877–889. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Duan, Y.; Feng, R.; Gong, H. Silicon Reduces Long-Term Cadmium Toxicities in Potted Garlic Plants. Acta Physiol Plant 2016, 38. [Google Scholar] [CrossRef]
- Wang, H.; Wen, S.; Chen, P.; Zhang, L.; Cen, K. Mitigation of Cadmium and Arsenic in Rice Grain by Applying Different Silicon Fertilizers in Contaminated Fields. Environ. Sci. Pollut. Res. 2016, 3781–3788. [Google Scholar] [CrossRef] [PubMed]
- J., Koski-Vähälä; Hartikainen, H.; Tallberg, P.; Si, H. Phosphorus Mobilization from Various Sediment Pools in Response to Increased PH and Silicate Concentration The Contribution of Different Inorganic P Pools in The; 2001. [Google Scholar]
- Koski-Vähälä, J.; Hartikainen, H. Surface Water Quality Assessment of the Risk of Phosphorus Loading Due to Resuspended Sediment; 2001. [Google Scholar]
- Eneji, A.E.; Inanaga, S.; Muranaka, S.; Li, J.; Hattori, T.; An, P.; Tsuji, W. Growth and Nutrient Use in Four Grasses under Drought Stress as Mediated by Silicon Fertilizers. J Plant Nutr 2008, 31, 355–365. [Google Scholar] [CrossRef]
- Cornelis, J.T.; Delvaux, B.; Georg, R.B.; Lucas, Y.; Ranger, J.; Opfergelt, S. Tracing the Origin of Dissolved Silicon Transferred from Various Soil-Plant Systems towards Rivers: A Review. Biogeosciences 2011, 8, 89–112. [Google Scholar] [CrossRef]
- Tallberg, P.; Tréguer, P.; Beucher, C.; Corvaisier, R. Potentially Mobile Pools of Phosphorus and Silicon in Sediment from the Bay of Brest: Interactions and Implications for Phosphorus Dynamics. Estuar Coast Shelf Sci 2008, 76, 85–94. [Google Scholar] [CrossRef]
- Cornelis, J.T.; Delvaux, B. Soil Processes Drive the Biological Silicon Feedback Loop. Funct Ecol 2016, 30, 1298–1310. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of Soil Inorganic P in the Rhizosphere as Affected by Root-Induced Chemical Changes: A Review; 2001; Vol. 237;
- Datnoff, L.E.; Snyder, G.H.; Korndörfer, G.H. Silicon in Agriculture; 2001; ISBN 9780874216561.
- Ma, J.F.; Takahashi, E. Soil, Fertilizer, and Plant Silicon Research in Japan. 2002. [Google Scholar]
- Bent, E. Silicon Solutions - Part III Horticulture. In Silicon Solutions - Helping plants to help themselves; 2014; pp. 102–126.
- Matichenkov, V.; Bocharnikova, E. Si in Horticultural Industry. In Plant Mineral Nutrition and Pesticide Management; 2004.
- IPCC Part A: Global and Sectoral Aspects. (Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change); 2014.
- Guntzer, F.; Keller, C.; Poulton, P.R.; McGrath, S.P.; Meunier, J.D. Long-Term Removal of Wheat Straw Decreases Soil Amorphous Silica at Broadbalk, Rothamsted. Plant Soil 2012, 352, 173–184. [Google Scholar] [CrossRef]
- Vandevenne, F.; Struyf, E.; Clymans, W.; Meire, P. Agricultural Silica Harvest: Have Humans Created a New Loop in the Global Silica Cycle? Front Ecol Environ 2012, 10, 243–248. [Google Scholar] [CrossRef]
- Barão, L.; Teixeira, R. The Environmental Impacts of Silicon as an Alternative to Phosphorus Fertilizers. Proceeding of LCA Food 2016 Conference 2016. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




