Submitted:
10 October 2024
Posted:
14 October 2024
You are already at the latest version
Abstract
Global warming has recently intensified research interest in renewable polymer chemistry, with significant attention directed towards lignin nanoparticle (LNPs) synthesis. Despite progress, LNPs industrial application faces challenges: (1) reliance on kraft lignin from declining raw biomass processes, (2) sulfur-rich, and condensed lignin use, (3) complex lignin macroparticles to LNPs conversion, using harmful and toxic solvents, and above all (4) lack of control over the LNPs production process (i.e., anti-solvent precipitation parameters), resulting in excessive variability in properties. In this work, eco-friendly LNPs with tailored properties were produced from a semi-industrial organosolv process by studying anti-solvent precipitation variables. Using first a parametric and then a Fractional Factorial Design, predictions of LNPs size and size distribution as well as zeta-potential were derived from a model over beech by-products organosolv lignin, depending on initial lignin concentration (x1, g/L), solvent flow rate (x2, ml/min), antisolvent composition (x3, H2O/EtOH v/v), antisolvent ratio (x4, solvent/antisolvent v/v) and antisolvent stirring speed (x5, rpm). This novel chemical engineering approach holds promise for overcoming the challenges inherent in industrial lignin nanoparticle production, thereby accelerating the valorization of lignin biopolymers for high value-added applications such as cosmetics (sunscreen or emulsion) and medicine (encapsulation, nanocarriers), a process currently constrained by significant limitations.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents
2.2. Lignin Isolation with Organosolv Process
2.3. Lignin Macroparticles (LMPs) Characterization Properties
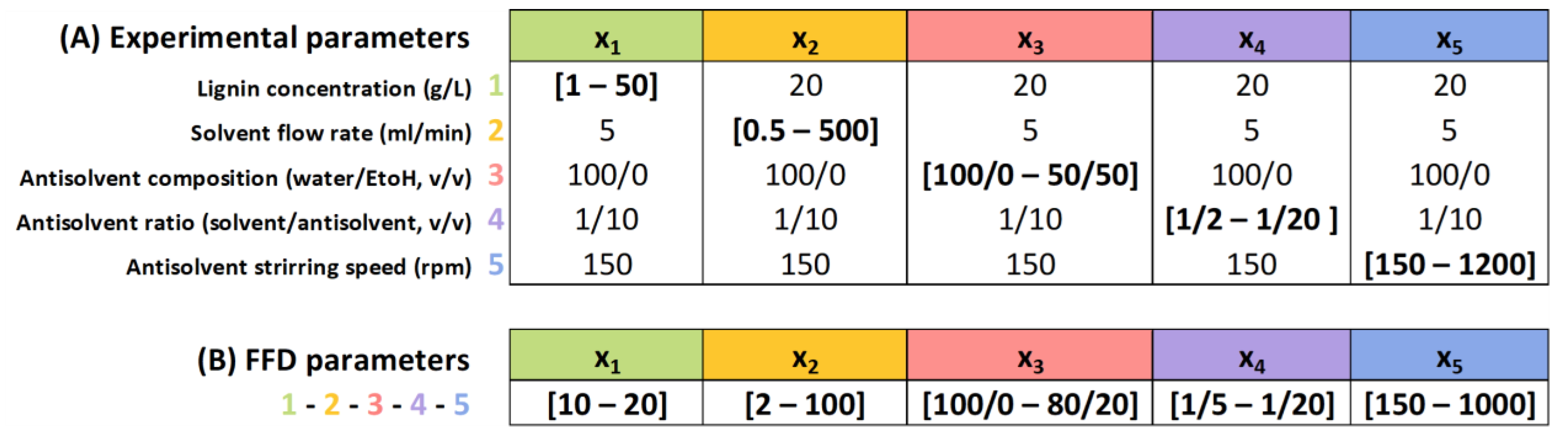
2.4. Lignin Nanoparticles (LNPs) Synthesis and Experimental Design
2.5. LNPs Characterizations
3. Results
3.1. LMPs Isolation from Semi-Industrial Organosolv Reactor
3.2. Exploration of 5 Different Experimental Parameters for the Antisolvent Precipitation Method (A)
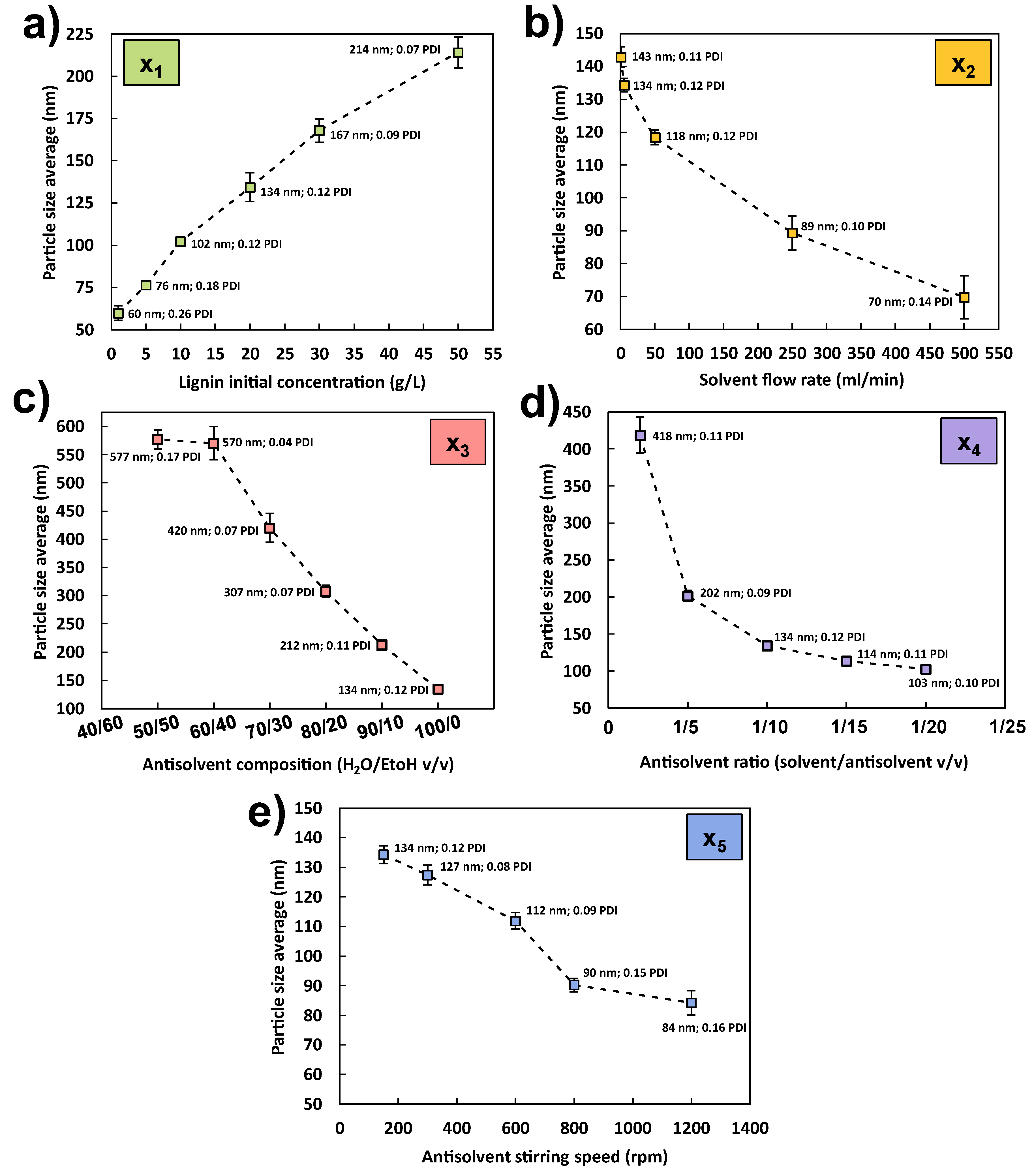
3.2.1. Effect of Lignin Initial Concentration (x1)
3.2.2. Effect of Solvent Flow Rate (x2)
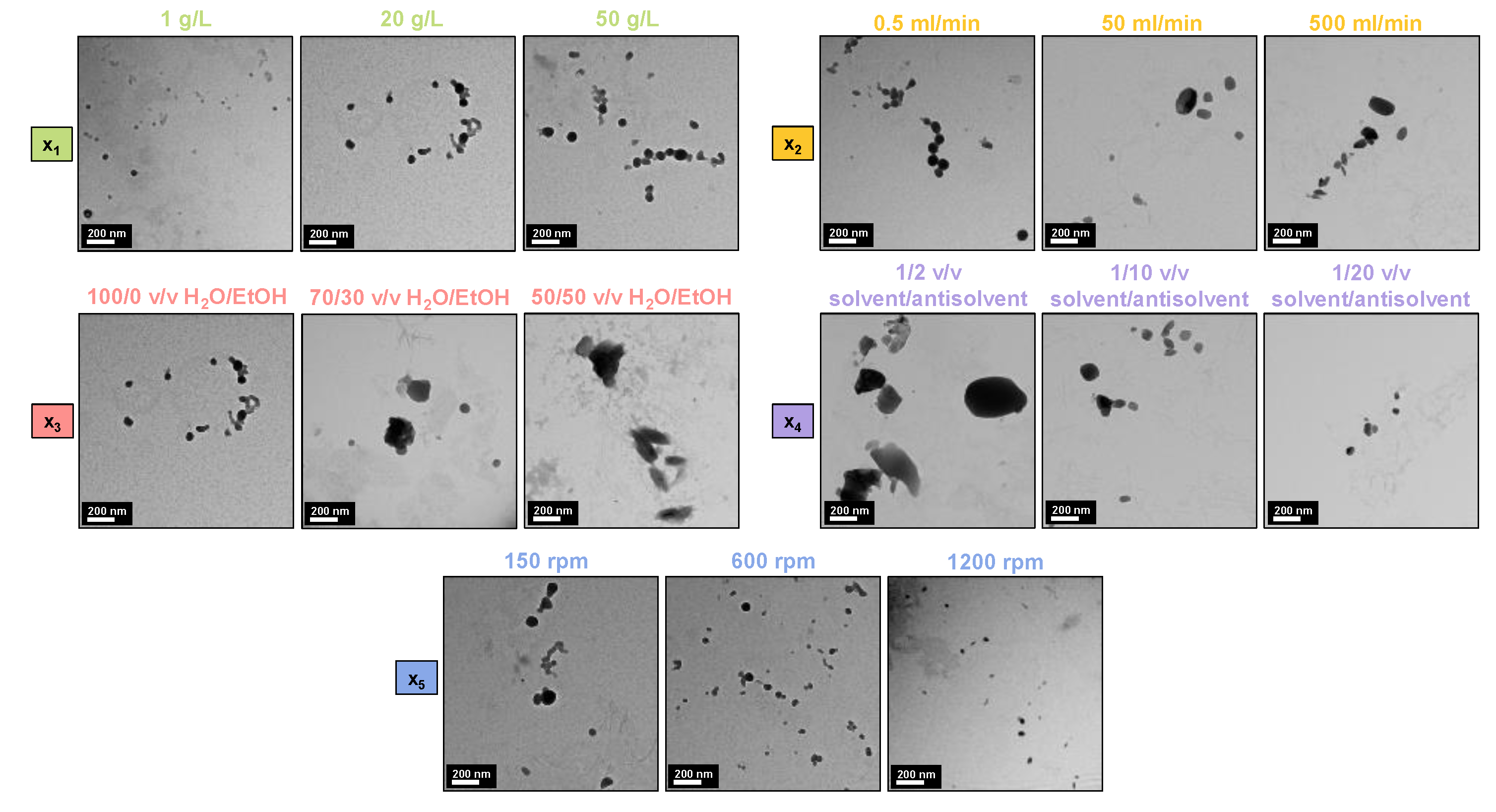
3.2.3. Effect of the Antisolvent Composition (x3)
3.2.4. Effect of the Antisolvent Volume (x4)
3.2.5. Effect of the Antisolvent Stirring Speed (x5)
3.3. Lignin Nanoparticle Prediction Model by Experimental Design (B)
- x1 (initial lignin concentration, g/L): 20 g/L, to prevent aggregation and particle fusion during the nucleation process.
- x2 (solvent flow rate, ml/min): 5 ml/min, to ensure interesting LNPs properties while having a low energy consumption.
- x3 (antisolvent composition, H2O/EtOH, v/v): 100 % H2O, to ensure the correct nucleation process and produce authentic LNPs suspensions while reducing EtOH consumption.
- x4 (antisolvent ratio, solvent/antisolvent, v/v): 1/10, to fine-tune lignin concentration and nucleation while reducing H2O use.
- x5 (antisolvent stirring speed, rpm): 150 rpm, combined with the solvent flow rate, enhances mixing and supersaturation, thereby improving nucleation. This value also optimizes the LNPs properties while maintaining low energy consumption.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gabrielli, P.; Rosa, L.; Gazzani, M.; Meys, R.; Bardow, A.; Mazzotti, M.; Sansavini, G. Net-Zero Emissions Chemical Industry in a World of Limited Resources. One Earth 2023, 6, 682–704. [Google Scholar] [CrossRef]
- McMichael, P.S.; Hoque, M.; Dos Santos, F.B.; French, V.; Foster, E.J. Binary Mixture of Subcritical Water and Acetone: A Hybrid Solvent System towards the Production of Lignin Nanoparticles. React. Chem. Eng. 2024, 9, 226–234. [Google Scholar] [CrossRef]
- Michelin, M.; Ruiz, H.A.; Silva, D.P.; Ruzene, D.S.; Teixeira, J.A.; Polizeli, M.L.T.M. Cellulose from Lignocellulosic Waste. In Polysaccharides; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, 2015; pp. 475–511. ISBN 978-3-319-16297-3. [Google Scholar]
- Sharma, V.; Tsai, M.-L.; Nargotra, P.; Chen, C.-W.; Sun, P.-P.; Singhania, R.R.; Patel, A.K.; Dong, C.-D. Journey of Lignin from a Roadblock to Bridge for Lignocellulose Biorefineries: A Comprehensive Review. Science of The Total Environment 2023, 861, 160560. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Gokhale, D.V. Lignocellulosic Biomass: Hurdles and Challenges in Its Valorization. Appl Microbiol Biotechnol 2019, 103, 9305–9320. [Google Scholar] [CrossRef]
- Brosse, N.; Mohamad Ibrahim, M.N.; Abdul Rahim, A. Biomass to Bioethanol: Initiatives of the Future for Lignin. ISRN Materials Science 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Nader, S.; Guzman, F.; Becar, R.; Segovia, C.; Fuentealba, C.; Peirera, M.; Mauret, E.; Brosse, N. Lignocellulosic Micro and Nanofibrillated Cellulose Produced by Steam Explosion for Wood Adhesive Formulations. Journal of Renewable Materials 2022, 10, 263–271. [Google Scholar] [CrossRef]
- Bugg, T.D.H. The Chemical Logic of Enzymatic Lignin Degradation. Chem. Commun. 2024, 60, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Boarino, A.; Klok, H.-A. Opportunities and Challenges for Lignin Valorization in Food Packaging, Antimicrobial, and Agricultural Applications. Biomacromolecules 2023, 24, 1065–1077. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as Renewable Raw Material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef]
- Chakar, F.S.; Ragauskas, A.J. Review of Current and Future Softwood Kraft Lignin Process Chemistry. Industrial Crops and Products 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Kataya, G.; Cornu, D.; Bechelany, M.; Hijazi, A.; Issa, M. Biomass Waste Conversion Technologies and Its Application for Sustainable Environmental Development—A Review. Agronomy 2023, 13, 2833. [Google Scholar] [CrossRef]
- Kumar, A.; Anushree; Kumar, J.; Bhaskar, T. Utilization of Lignin: A Sustainable and Eco-Friendly Approach. Journal of the Energy Institute 2020, 93, 235–271. [CrossRef]
- Beisl, S.; Loidolt, P.; Miltner, A.; Harasek, M.; Friedl, A. Production of Micro- and Nanoscale Lignin from Wheat Straw Using Different Precipitation Setups. Molecules 2018, 23, 633. [Google Scholar] [CrossRef] [PubMed]
- Ab Rasid, N.S.; Shamjuddin, A.; Abdul Rahman, A.Z.; Amin, N.A.S. Recent Advances in Green Pre-Treatment Methods of Lignocellulosic Biomass for Enhanced Biofuel Production. Journal of Cleaner Production 2021, 321, 129038. [Google Scholar] [CrossRef]
- Adamcyk, J.; Beisl, S.; Amini, S.; Jung, T.; Zikeli, F.; Labidi, J.; Friedl, A. Production and Properties of Lignin Nanoparticles from Ethanol Organosolv Liquors—Influence of Origin and Pretreatment Conditions. Polymers 2021, 13, 384. [Google Scholar] [CrossRef]
- Gao, W.; Fatehi, P. Lignin for Polymer and Nanoparticle Production: Current Status and Challenges. Can J Chem Eng 2019, 97, 2827–2842. [Google Scholar] [CrossRef]
- Ibrahim, H.H.; Bilsborrow, P.E.; Phan, A.N. Intensification of Pre-Treatment and Fractionation of Agricultural Residues. Chemical Engineering and Processing - Process Intensification 2021, 159, 108231. [Google Scholar] [CrossRef]
- Rambaran, T.; Schirhagl, R. Nanotechnology from Lab to Industry – a Look at Current Trends. Nanoscale Adv. 2022, 4, 3664–3675. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Lange, H.; Crestini, C.; Henn, A.; Österberg, M. Lignin for Nano- and Microscaled Carrier Systems: Applications, Trends, and Challenges. ChemSusChem 2019, 12, 2039–2054. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X.; Shi, Y. A Review on Lignin Antioxidants: Their Sources, Isolations, Antioxidant Activities and Various Applications. International Journal of Biological Macromolecules 2022, 210, 716–741. [Google Scholar] [CrossRef]
- Almeida, F.; Margarida, A.; Seixas, N.; Pinto, R.J.B.; Silvestre, A.J.D.; Da Costa Lopes, A.M. Sustainable Tailoring of Lignin Nanoparticles Assisted by Green Solvents. ChemNanoMat 2024, 10, e202400010. [Google Scholar] [CrossRef]
- Qian, Y.; Zhong, X.; Li, Y.; Qiu, X. Fabrication of Uniform Lignin Colloidal Spheres for Developing Natural Broad-Spectrum Sunscreens with High Sun Protection Factor. Industrial Crops and Products 2017, 101, 54–60. [Google Scholar] [CrossRef]
- Hussin, M.H.; Appaturi, J.N.; Poh, N.E.; Latif, N.H.A.; Brosse, N.; Ziegler-Devin, I.; Vahabi, H.; Syamani, F.A.; Fatriasari, W.; Solihat, N.N.; et al. A Recent Advancement on Preparation, Characterization and Application of Nanolignin. International Journal of Biological Macromolecules 2022, 200, 303–326. [Google Scholar] [CrossRef] [PubMed]
- Girard, V.; Fragnières, L.; Chapuis, H.; Brosse, N.; Marchal-Heussler, L.; Canilho, N.; Parant, S.; Ziegler-Devin, I. The Impact of Lignin Biopolymer Sources, Isolation, and Size Reduction from the Macro- to Nanoscale on the Performances of Next-Generation Sunscreen. Polymers 2024, 16. [Google Scholar] [CrossRef]
- Zhang, Z.; Terrasson, V.; Guénin, E. Lignin Nanoparticles and Their Nanocomposites. Nanomaterials 2021, 11, 1336. [Google Scholar] [CrossRef]
- Tang, Q.; Qian, Y.; Yang, D.; Qiu, X.; Qin, Y.; Zhou, M. Lignin-Based Nanoparticles: A Review on Their Preparations and Applications. Polymers 2020, 12, 2471. [Google Scholar] [CrossRef]
- Freitas, F.M.C.; Cerqueira, M.A.; Gonçalves, C.; Azinheiro, S.; Garrido-Maestu, A.; Vicente, A.A.; Pastrana, L.M.; Teixeira, J.A.; Michelin, M. Green Synthesis of Lignin Nano- and Micro-Particles: Physicochemical Characterization, Bioactive Properties and Cytotoxicity Assessment. International Journal of Biological Macromolecules 2020, 163, 1798–1809. [Google Scholar] [CrossRef]
- Myint, A.A.; Lee, H.W.; Seo, B.; Son, W.-S.; Yoon, J.; Yoon, T.J.; Park, H.J.; Yu, J.; Yoon, J.; Lee, Y.-W. One Pot Synthesis of Environmentally Friendly Lignin Nanoparticles with Compressed Liquid Carbon Dioxide as an Antisolvent. Green Chem. 2016, 18, 2129–2146. [Google Scholar] [CrossRef]
- Nair, S.S.; Sharma, S.; Pu, Y.; Sun, Q.; Pan, S.; Zhu, J.Y.; Deng, Y.; Ragauskas, A.J. High Shear Homogenization of Lignin to Nanolignin and Thermal Stability of Nanolignin-Polyvinyl Alcohol Blends. ChemSusChem 2014, 7, 3513–3520. [Google Scholar] [CrossRef]
- Mili, M.; Hashmi, S.A.R.; Tilwari, A.; Rathore, S.K.S.; Naik, A.; Srivastava, A.K.; Verma, S. Preparation of Nanolignin Rich Fraction from Bamboo Stem via Green Technology: Assessment of Its Antioxidant, Antibacterial and UV Blocking Properties. Environmental Technology 2023, 44, 416–430. [Google Scholar] [CrossRef]
- Rizal, S.; Alfatah, T.; Abdul Khalil, H.P.S.; Yahya, E.B.; Abdullah, C.K.; Mistar, E.M.; Ikramullah, I.; Kurniawan, R.; Bairwan, R.D. Enhanced Functional Properties of Bioplastic Films Using Lignin Nanoparticles from Oil Palm-Processing Residue. Polymers 2022, 14, 5126. [Google Scholar] [CrossRef] [PubMed]
- Abbati De Assis, C.; Greca, L.G.; Ago, M.; Balakshin, M.Yu.; Jameel, H.; Gonzalez, R.; Rojas, O.J. Techno-Economic Assessment, Scalability, and Applications of Aerosol Lignin Micro- and Nanoparticles. ACS Sustainable Chem. Eng. 2018, 6, 11853–11868. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rosas, R.; Bedia, J.; Lallave, M.; Loscertales, I.G.; Barrero, A.; Rodríguez-Mirasol, J.; Cordero, T. The Production of Submicron Diameter Carbon Fibers by the Electrospinning of Lignin. Carbon 2010, 48, 696–705. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Ferrer, M.L.; Del Monte, F. Ice-Templated Materials: Sophisticated Structures Exhibiting Enhanced Functionalities Obtained after Unidirectional Freezing and Ice-Segregation-Induced Self-Assembly. Chem. Mater. 2008, 20, 634–648. [Google Scholar] [CrossRef]
- Chen, N.; Dempere, L.A.; Tong, Z. Synthesis of pH-Responsive Lignin-Based Nanocapsules for Controlled Release of Hydrophobic Molecules. ACS Sustainable Chem. Eng. 2016, 4, 5204–5211. [Google Scholar] [CrossRef]
- Richter, A.P.; Bharti, B.; Armstrong, H.B.; Brown, J.S.; Plemmons, D.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. Synthesis and Characterization of Biodegradable Lignin Nanoparticles with Tunable Surface Properties. Langmuir 2016, 32, 6468–6477. [Google Scholar] [CrossRef]
- Frangville, C.; Rutkevičius, M.; Richter, A.P.; Velev, O.D.; Stoyanov, S.D.; Paunov, V.N. Fabrication of Environmentally Biodegradable Lignin Nanoparticles. ChemPhysChem 2012, 13, 4235–4243. [Google Scholar] [CrossRef]
- Li, H.; Deng, Y.; Liang, J.; Dai, Y.; Li, B.; Ren, Y.; Qiu, X.; Li, C. Direct Preparation of Hollow Nanospheres with Kraft Lignin: A Facile Strategy for Effective Utilization of Biomass Waste. BioResources 2016, 11, 3073–3083. [Google Scholar] [CrossRef]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.-L.; Hult, E.-L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A Simple Process for Lignin Nanoparticle Preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef]
- Lepeltier, E.; Bourgaux, C.; Couvreur, P. Nanoprecipitation and the “Ouzo Effect”: Application to Drug Delivery Devices. Advanced Drug Delivery Reviews 2014, 71, 86–97. [Google Scholar] [CrossRef]
- Liu, X.; Ni, S.; Chen, X.; Li, Z.; Fu, Y.; Qin, M.; Zhang, F. Green Fabrication of Fabric by Ethanol/Water Solvent Mediated Self-Assembly of Homogeneous Lignin for Oil-Water Separation. Green chemistry : an international journal and green chemistry resource : GC 2024.
- Zwilling, J.D.; Jiang, X.; Zambrano, F.; Venditti, R.A.; Jameel, H.; Velev, O.D.; Rojas, O.J.; Gonzalez, R. Understanding Lignin Micro- and Nanoparticle Nucleation and Growth in Aqueous Suspensions by Solvent Fractionation. Green Chem. 2021, 23, 1001–1012. [Google Scholar] [CrossRef]
- Girard, V.; Chapuis, H.; Brosse, N.; Canilho, N.; Marchal-Heussler, L.; Ziegler-Devin, I. Lignin Nanoparticles: Contribution of Biomass Types and Fractionation for an Eco-Friendly Production. ACS Sustainable Chem. Eng. 2024, 12, 7055–7068. [Google Scholar] [CrossRef]
- Ma, M.; Dai, L.; Xu, J.; Liu, Z.; Ni, Y. A Simple and Effective Approach to Fabricate Lignin Nanoparticles with Tunable Sizes Based on Lignin Fractionation. Green chemistry : an international journal and green chemistry resource : GC 2020, 22, 211–217. [Google Scholar] [CrossRef]
- Pylypchuk, I.V.; Riazanova, A.; Lindström, M.E.; Sevastyanova, O. Structural and Molecular-Weight-Dependency in the Formation of Lignin Nanoparticles from Fractionated Soft- and Hardwood Lignins. Green Chem. 2021, 23, 3061–3072. [Google Scholar] [CrossRef]
- Schneider, W.D.H.; Dillon, A.J.P.; Camassola, M. Lignin Nanoparticles Enter the Scene: A Promising Versatile Green Tool for Multiple Applications. Biotechnology Advances 2021, 47, 107685. [Google Scholar] [CrossRef]
- Pylypchuk, I.V.; Karlsson, M.; Lindén, P.A.; Lindström, M.E.; Elder, T.; Sevastyanova, O.; Lawoko, M. Molecular Understanding of the Morphology and Properties of Lignin Nanoparticles: Unravelling the Potential for Tailored Applications. Green Chem. 2023, 25, 4415–4428. [Google Scholar] [CrossRef]
- Ju, T.; Zhang, Z.; Li, Y.; Miao, X.; Ji, J. Continuous Production of Lignin Nanoparticles Using a Microchannel Reactor and Its Application in UV-Shielding Films. RSC Adv. 2019, 9, 24915–24921. [Google Scholar] [CrossRef]
- Li, H.; Deng, Y.; Wu, H.; Ren, Y.; Qiu, X.; Zheng, D.; Li, C. Self-Assembly of Kraft Lignin into Nanospheres in Dioxane-Water Mixtures. Holzforschung 2016, 70, 725–731. [Google Scholar] [CrossRef]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y.; Chu, F. Preparation and Formation Mechanism of Size-Controlled Lignin Nanospheres by Self-Assembly. Industrial Crops and Products 2017, 100, 146–152. [Google Scholar] [CrossRef]
- Manisekaran, A.; Grysan, P.; Duez, B.; Schmidt, D.F.; Lenoble, D.; Thomann, J.-S. Solvents Drive Self-Assembly Mechanisms and Inherent Properties of Kraft Lignin Nanoparticles (<50 Nm). Journal of Colloid and Interface Science 2022, 626, 178–192. [Google Scholar] [CrossRef]
- Li, X.; Shen, J.; Wang, B.; Feng, X.; Mao, Z.; Sui, X. Acetone/Water Cosolvent Approach to Lignin Nanoparticles with Controllable Size and Their Applications for Pickering Emulsions. ACS Sustainable Chem. Eng. 2021, 9, 5470–5480. [Google Scholar] [CrossRef]
- Luo, T.; Wang, C.; Ji, X.; Yang, G.; Chen, J.; Janaswamy, S.; Lyu, G. Preparation and Characterization of Size-Controlled Lignin Nanoparticles with Deep Eutectic Solvents by Nanoprecipitation. Molecules 2021, 26, 218. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liao, Y.; Jiang, Z.; Sun, Q.; Guo, X.; Zhang, W.; Hu, C.; Luque, R.; Shi, B.; Sels, B.F. Solvent Effect on the Production of Spherical Lignin Nanoparticles. Green Chem. 2023, 25, 993–1003. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Lange, H.; Ago, M.; Crestini, C. Understanding Lignin Aggregation Processes. A Case Study: Budesonide Entrapment and Stimuli Controlled Release from Lignin Nanoparticles. ACS Sustainable Chem. Eng. 2018, 6, 9342–9351. [Google Scholar] [CrossRef]
- He, Q.; Ziegler-Devin, I.; Chrusciel, L.; Obame, S.N.; Hong, L.; Lu, X.; Brosse, N. Lignin-First Integrated Steam Explosion Process for Green Wood Adhesive Application. ACS Sustainable Chem. Eng. 2020, 8, 5380–5392. [Google Scholar] [CrossRef]
- Rao, X.; Liu, Y.; Zhang, Q.; Chen, W.; Liu, Y.; Yu, H. Assembly of Organosolv Lignin Residues into Submicron Spheres: The Effects of Granulating in Ethanol/Water Mixtures and Homogenization. ACS Omega 2017, 2, 2858–2865. [Google Scholar] [CrossRef]
- Brosse, N.; Hussin, M.H.; Rahim, A.A. Organosolv Processes. In Biorefineries; Wagemann, K., Tippkötter, N., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer International Publishing: Cham, 2017; Volume 166, pp. 153–176. ISBN 978-3-319-97117-9. [Google Scholar]
- Nitsos, C.; Stoklosa, R.; Karnaouri, A.; Vörös, D.; Lange, H.; Hodge, D.; Crestini, C.; Rova, U.; Christakopoulos, P. Isolation and Characterization of Organosolv and Alkaline Lignins from Hardwood and Softwood Biomass. ACS Sustainable Chem. Eng. 2016, 4, 5181–5193. [Google Scholar] [CrossRef]
- Zevallos Torres, L.A.; Lorenci Woiciechowski, A.; De Andrade Tanobe, V.O.; Karp, S.G.; Guimarães Lorenci, L.C.; Faulds, C.; Soccol, C.R. Lignin as a Potential Source of High-Added Value Compounds: A Review. Journal of Cleaner Production 2020, 263, 121499. [Google Scholar] [CrossRef]
- El Hage, R.; Brosse, N.; Chrusciel, L.; Sanchez, C.; Sannigrahi, P.; Ragauskas, A. Characterization of Milled Wood Lignin and Ethanol Organosolv Lignin from Miscanthus. Polymer Degradation and Stability 2009, 94, 1632–1638. [Google Scholar] [CrossRef]
- El Hage, R.; Brosse, N.; Sannigrahi, P.; Ragauskas, A. Effects of Process Severity on the Chemical Structure of Miscanthus Ethanol Organosolv Lignin. Polymer Degradation and Stability 2010, 95, 997–1003. [Google Scholar] [CrossRef]
- Beisl, S.; Adamcyk, J.; Friedl, A. Direct Precipitation of Lignin Nanoparticles from Wheat Straw Organosolv Liquors Using a Static Mixer. Molecules 2020, 25, 1388. [Google Scholar] [CrossRef] [PubMed]
- Vermaas, J.V.; Crowley, M.F.; Beckham, G.T. Molecular Lignin Solubility and Structure in Organic Solvents. ACS Sustainable Chem. Eng. 2020, 8, 17839–17850. [Google Scholar] [CrossRef]
- Conner, C.G.; Veleva, A.N.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. Scalable Formation of Concentrated Monodisperse Lignin Nanoparticles by Recirculation-Enhanced Flash Nanoprecipitation. Part & Part Syst Charact 2020, 37, 2000122. [Google Scholar] [CrossRef]
- Tao, J.; Chow, S.F.; Zheng, Y. Application of Flash Nanoprecipitation to Fabricate Poorly Water-Soluble Drug Nanoparticles. Acta Pharmaceutica Sinica B 2019, 9, 4–18. [Google Scholar] [CrossRef] [PubMed]

| 1. Raw Material (%) | Cellulose | Hemicellulose | Lignin | Extractives | Ashes | Total |
| 47.8 ± 1.5 | 22.5 ± 0.9 | 23.7 ± 0.2 | 2.7 ± 0.3 | 0.7 ± 0 | 97.4 ± 2.9 | |
| 2. Organosolv solid residue (%) | Cellulose | Hemicellulose | Lignin | Mass loss yield (wt %) | LMPs purity (%) | LMPs isolation yields (wtc %) |
| 51.5 ± 1.8 | 13.0 ± 0.7 | 13.0 ± 0.4 | 46.0 ± 0.1 | 93.8 ± 0.3 | 70.8 ± 0.2 |
| Run | Design Factors (25-1) | Design response | ||||
| x1 | x2 | x3 | x4 | x5 | LNPs size average | |
| 1 | 10 | 100 | 80 | 5 | 150 | 323 ± 6 |
| 2 | 20 | 2 | 100 | 5 | 1000 | 112 ± 3 |
| 3 | 10 | 100 | 80 | 20 | 1000 | 109 ± 2 |
| 4 | 20 | 2 | 100 | 20 | 150 | 114 ± 4 |
| 5 | 20 | 100 | 80 | 5 | 1000 | 266 ± 2 |
| 6 | 10 | 2 | 100 | 5 | 150 | 128 ± 3 |
| 7 | 20 | 100 | 80 | 20 | 150 | 173 ± 3 |
| 8 | 10 | 2 | 100 | 20 | 1000 | 50 ± 2 |
| 9 | 20 | 2 | 80 | 5 | 150 | 340 ± 6 |
| 10 | 10 | 100 | 100 | 5 | 1000 | 87 ± 3 |
| 11 | 20 | 2 | 80 | 20 | 1000 | 127 ± 3 |
| 12 | 10 | 100 | 100 | 20 | 150 | 62 ± 2 |
| 13 | 10 | 2 | 80 | 5 | 1000 | 237 ± 5 |
| 14 | 20 | 100 | 100 | 5 | 150 | 134 ± 3 |
| 15 | 10 | 2 | 80 | 20 | 150 | 153 ± 3 |
| 16 | 20 | 100 | 100 | 20 | 1000 | 73 ± 2 |
| 17 | 15 | 51 | 90 | 12.5 | 575 | 132 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





