Submitted:
11 October 2024
Posted:
12 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ultrasonic-Assisted Ethanol Extraction
2.3. Compositional Analysis of USHE
2.4. High-Performance Liquid Chromatography (HPLC) and Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.5. Antioxidant Capacity of USHE
2.6. Cell Culture
2.7. Cell Viability Analysis
2.8. Analysis of Intracellular ROS Production
2.9. Nuclear Double Staining
2.10. Cell Cycle Analysis
2.11. Annexin V and JC-1 Assays
2.12. Western Blot Analysis
2.13. Statistical Analysis
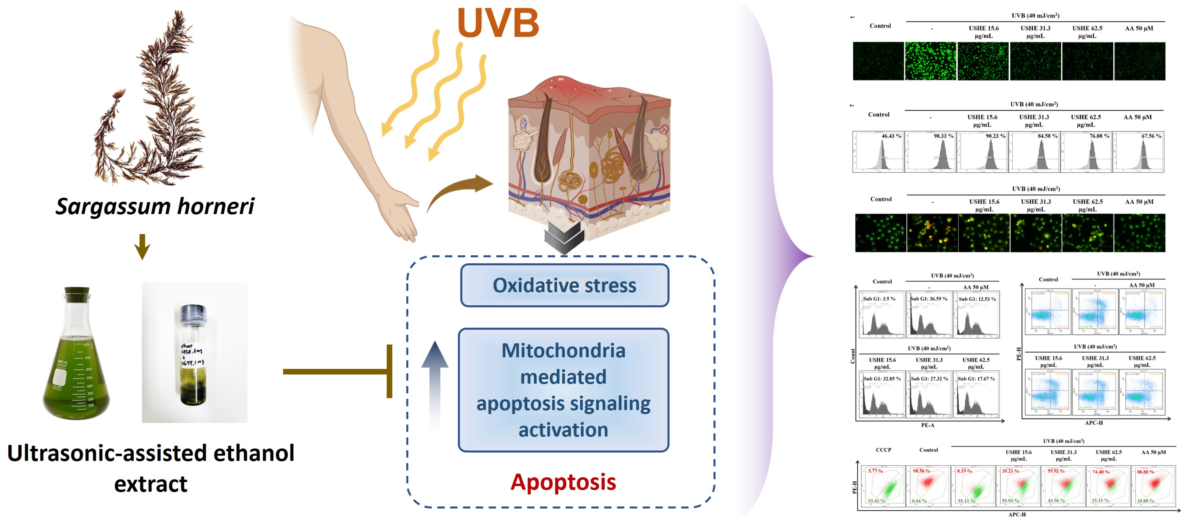
3. Results
3.1. Extraction Yield and Proximate Composition of USHE, and Identification of Active Compounds
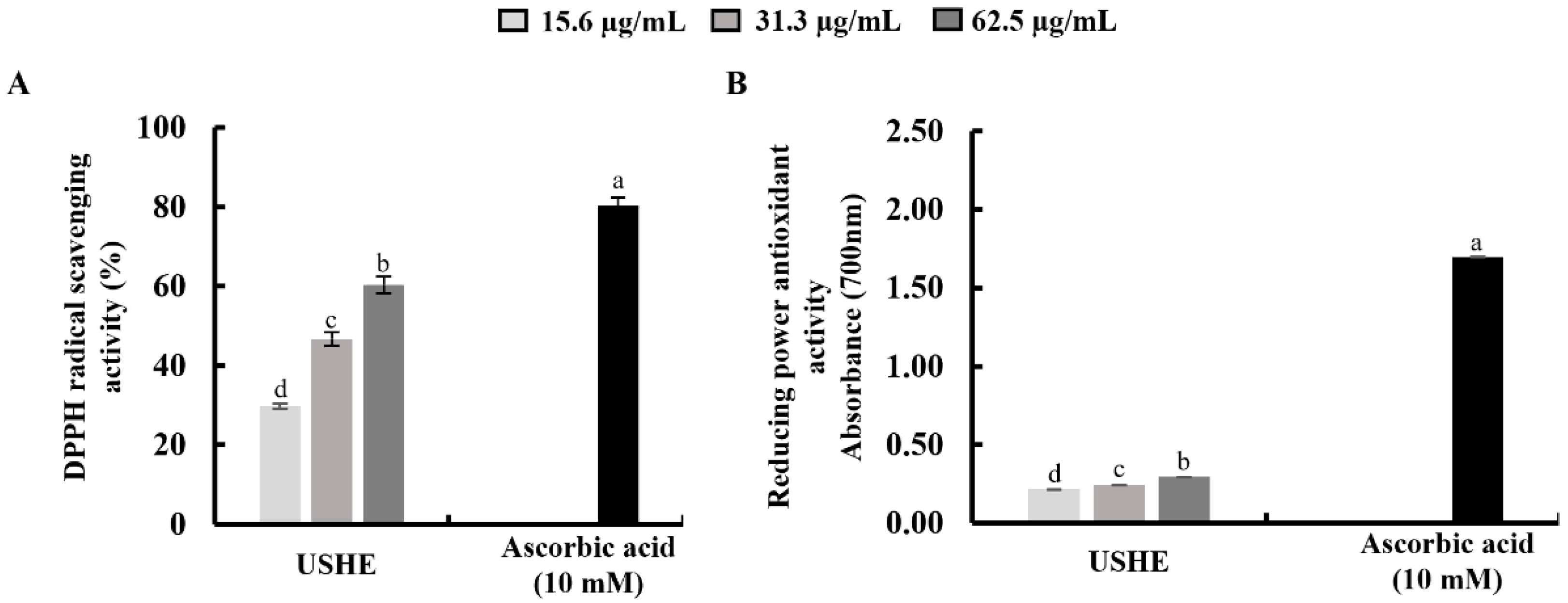
3.2. Antioxidant Capacity of USHE
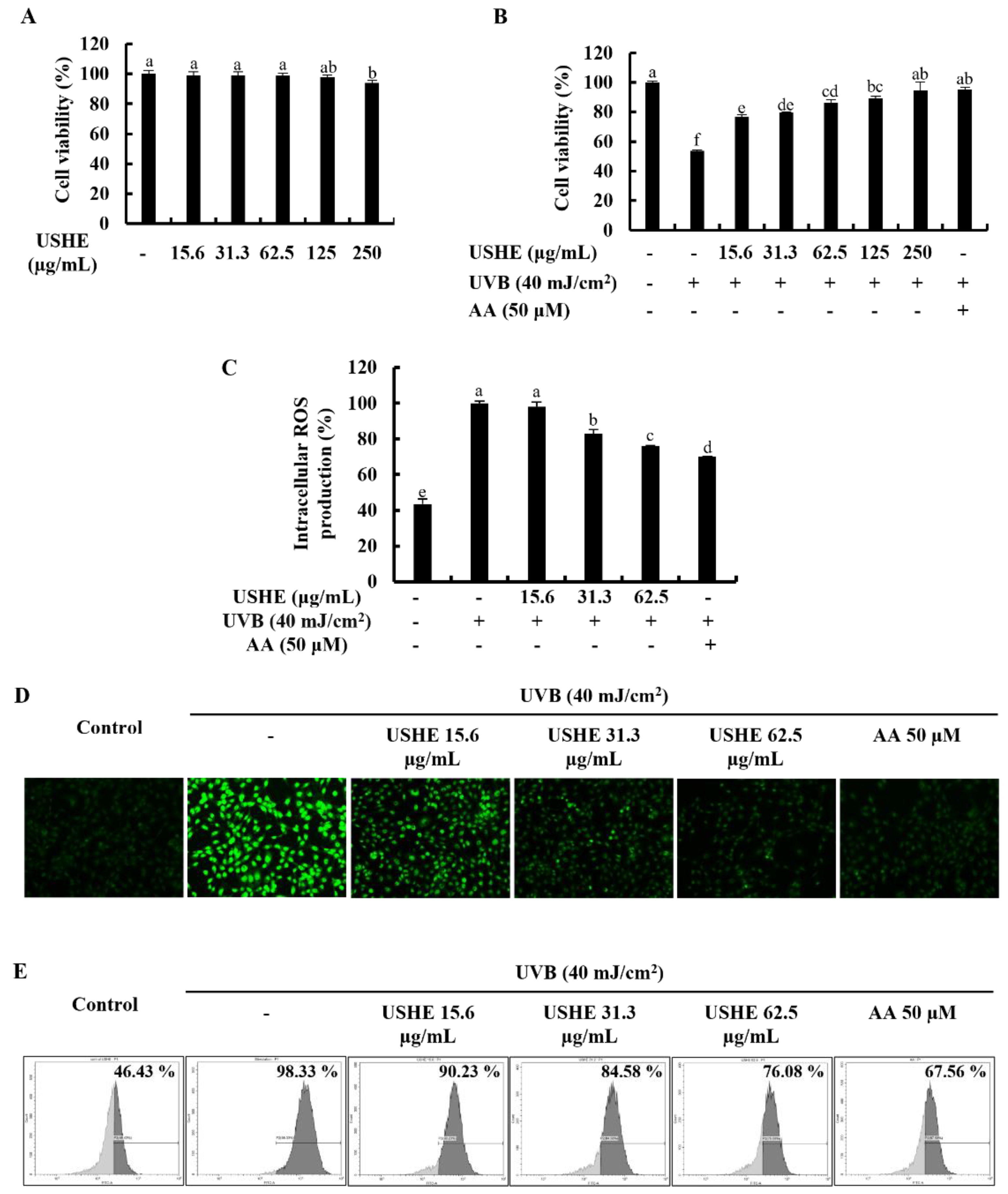
3.3. Effect of USHE on Cell Viability and Intracellular ROS Production in UVB-Exposed HaCaT Keratinocytes
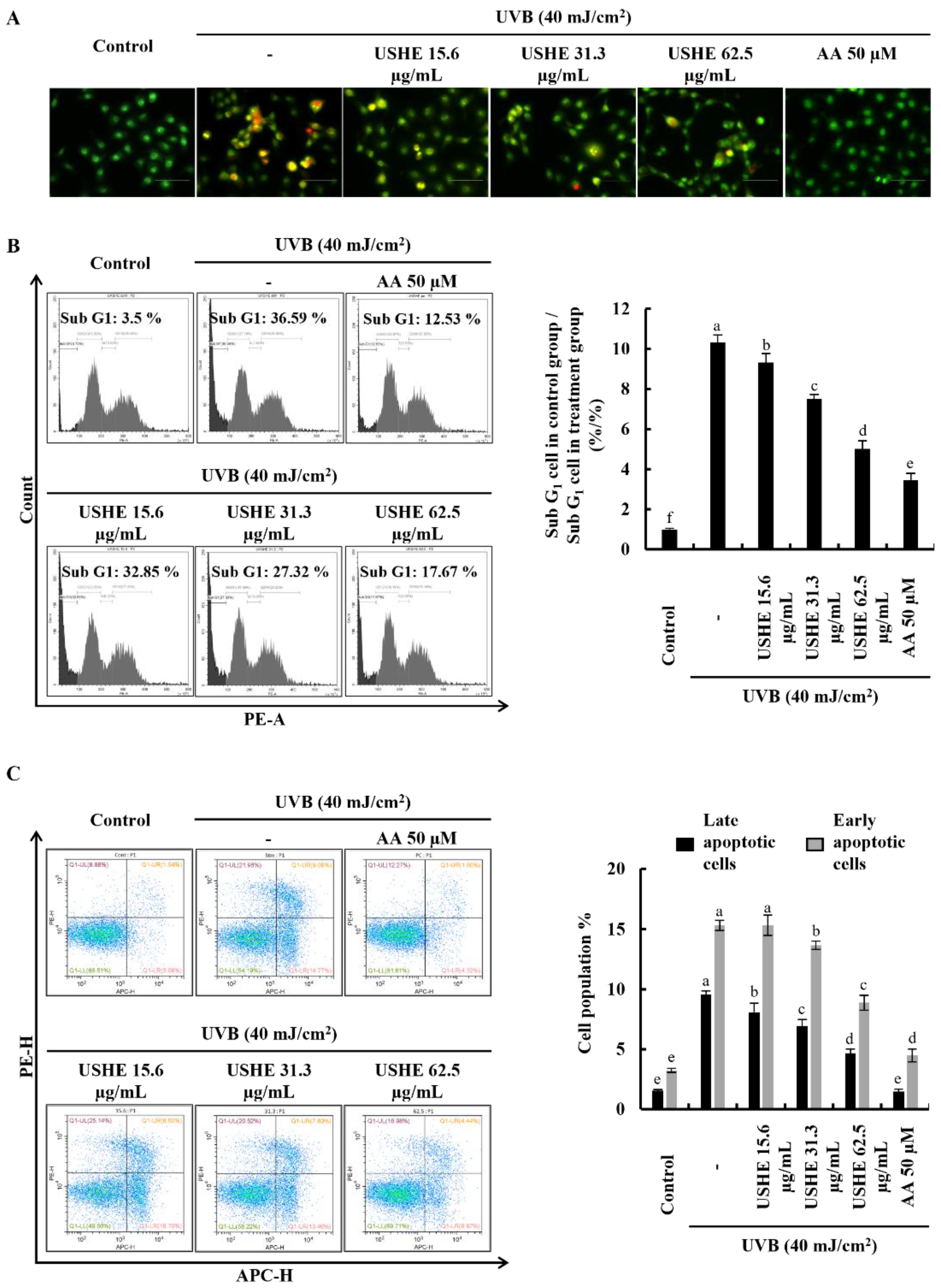
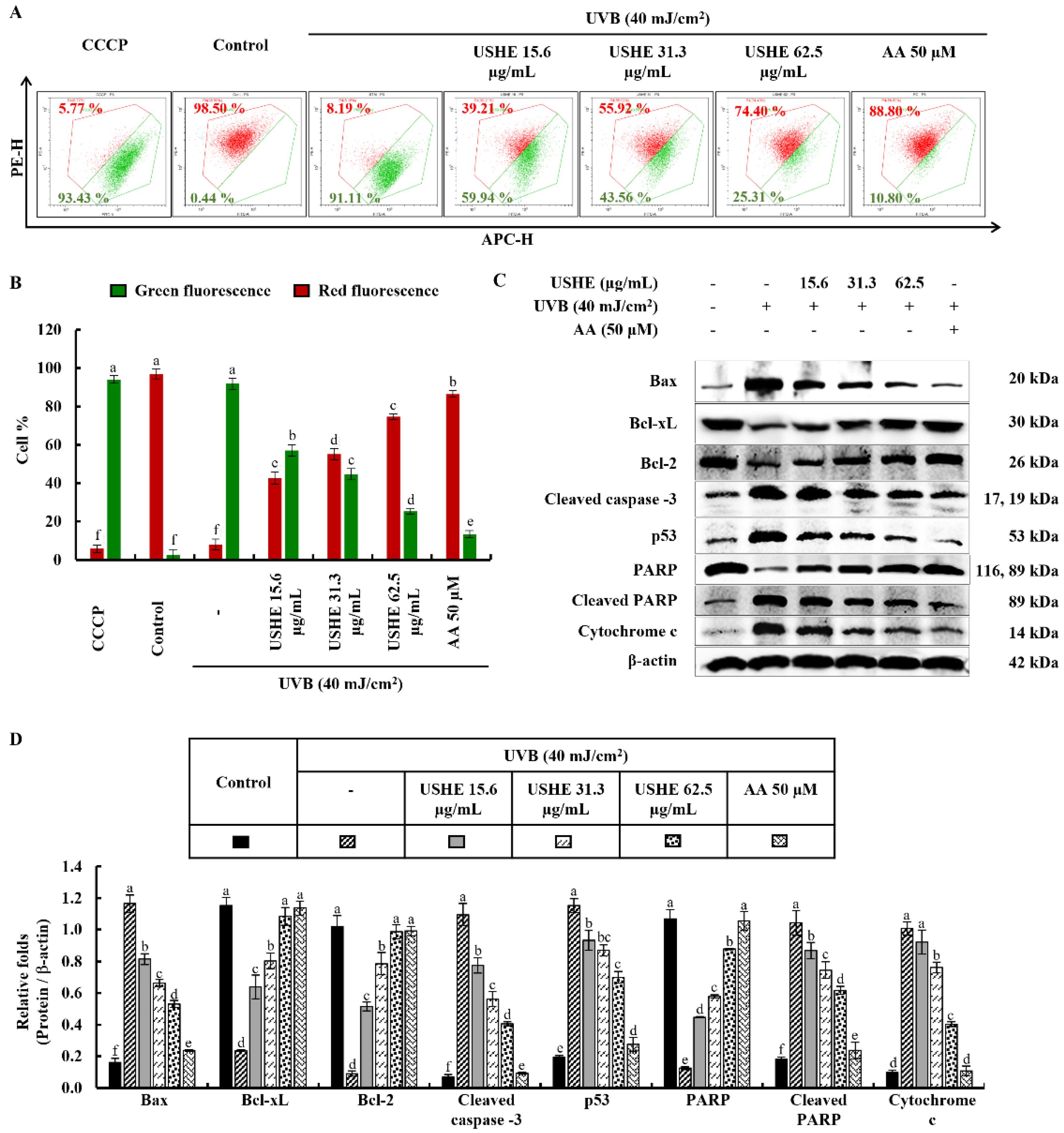
3.4. Effect of USHE on Proportion of Apoptotic Cells in UVB-Exposed HaCaT Keratinocytes
3.5. Effect of USHE against UVB-Exposed Oxidative Stress by Inhibiting Mitochondria-Mediated Apoptotic Signaling Pathway in HaCaT Keratinocytes
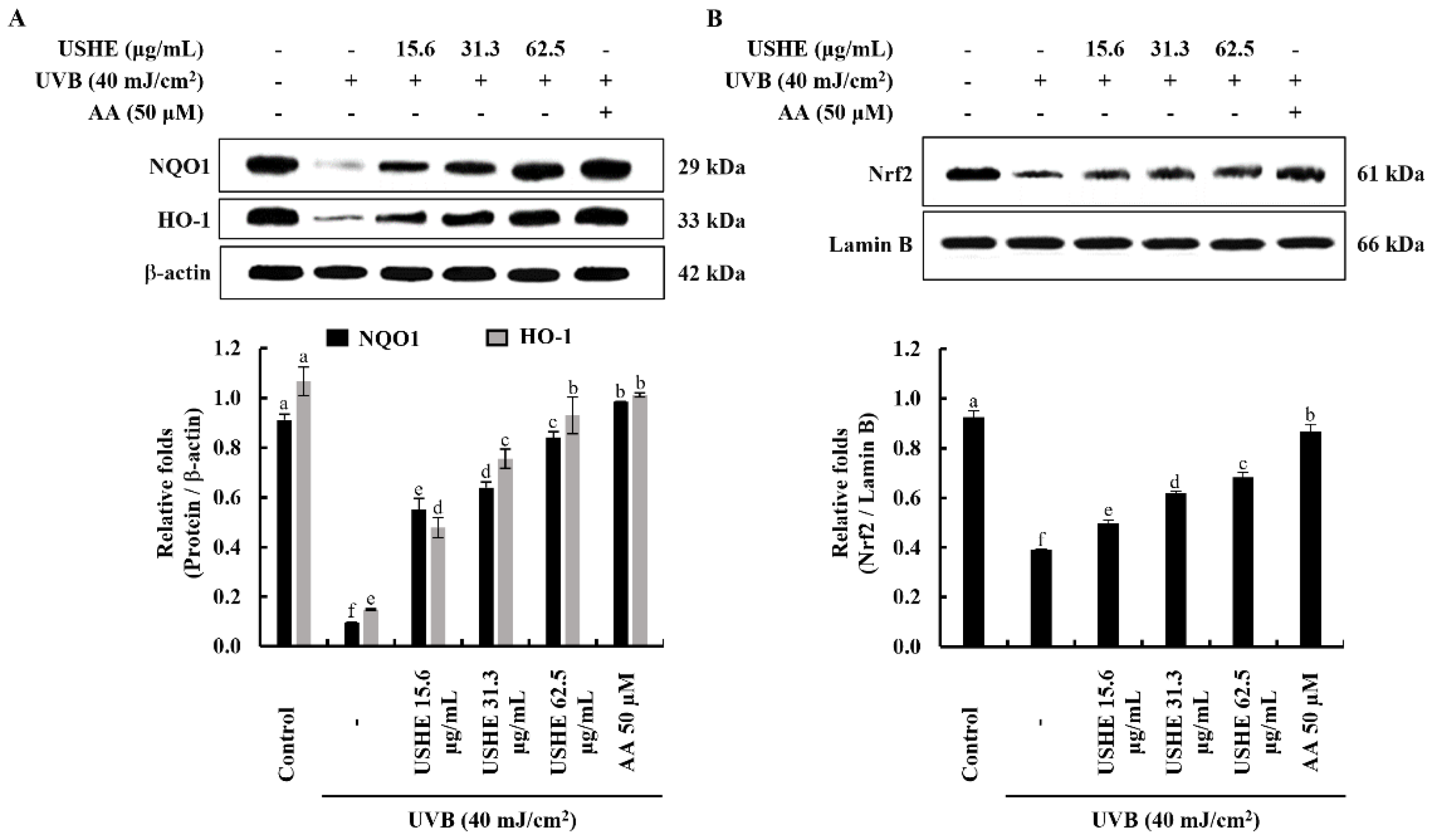
3.6. Effect of USHE on Nrf2/HO-1 Activation in UVB-Exposed HaCaT Keratinocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Taylor, S.C.; Alexis, A.F.; Armstrong, A.W.; Fuxench, Z.C.C.; Lim, H.W. Misconceptions of photoprotection in skin of color. J. Am. Acad. Dermatol. 2022, 86, S9–S17. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Yazar, S.; Young, A.; Norval, M.; De Gruijl, F.; Takizawa, Y.; Rhodes, L.; Sinclair, C.; Neale, R. Human health in relation to exposure to solar ultraviolet radiation under changing stratospheric ozone and climate. Photochemical & Photobiological Sciences 2019, 18, 641–680. [Google Scholar]
- Mapoung, S.; Arjsri, P.; Thippraphan, P.; Semmarath, W.; Yodkeeree, S.; Chiewchanvit, S.; Piyamongkol, W.; Limtrakul, P. Photochemoprotective effects of Spirulina platensis extract against UVB irradiated human skin fibroblasts. S. Afr. J. Bot. 2020, 130, 198–207. [Google Scholar] [CrossRef]
- Leal, A.C.; Corrêa, M.P.; Holick, M.F.; Melo, E.V.; Lazaretti-Castro, M. Sun-induced production of vitamin D3 throughout 1 year in tropical and subtropical regions: Relationship with latitude, cloudiness, UV-B exposure and solar zenith angle. Photochemical & Photobiological Sciences 2021, 20, 265–274. [Google Scholar]
- Xiao, Z.; Yang, S.; Liu, Y.; Zhou, C.; Hong, P.; Sun, S.; Qian, Z.-J. A novel glyceroglycolipid from brown algae Ishige okamurae improve photoaging and counteract inflammation in UVB-induced HaCaT cells. Chemico-Biological Interactions 2022, 351, 109737. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Molecular Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. European journal of medicinal chemistry 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.; Stuppner, H.; Jansen-Dürr, P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef]
- Priyan Shanura Fernando, I.; Kim, K.-N.; Kim, D.; Jeon, Y.-J. Algal polysaccharides: Potential bioactive substances for cosmeceutical applications. Crit. Rev. Biotechnol. 2019, 39, 99–113. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Ahn, G. Fucoidan refined by Sargassum confusum indicate protective effects suppressing photo-oxidative stress and skin barrier perturbation in UVB-induced human keratinocytes. International Journal of Biological Macromolecules 2020, 164, 149–161. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Heo, S.-J.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.-J.; Kim, M.-J.; Sanjeewa, K.K.A.; Lee, K.; Ahn, G. (−)-loliolide isolated from sargassum horneri abate uvb-induced oxidative damage in human dermal fibroblasts and subside ecm degradation. Marine Drugs 2021, 19, 435. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Ko, S.-C.; Cha, S.-H.; Kang, D.-H.; Park, H.-S.; Choi, Y.-U.; Kim, D.; Jung, W.-K.; Jeon, Y.-J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicology in vitro 2009, 23, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Kirindage, K.G.I.S.; Jayasinghe, A.M.K.; Han, E.-J.; Jee, Y.; Kim, H.-J.; Do, S.G.; Fernando, I.P.S.; Ahn, G. Fucosterol isolated from dietary brown alga Sargassum horneri protects TNF-α/IFN-γ-stimulated human dermal fibroblasts via regulating Nrf2/HO-1 and NF-κB/MAPK pathways. Antioxidants 2022, 11, 1429. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, E.-A.; Ahn, G.; Jee, Y.; Jeon, Y.-J. Anti-inflammatory activity of a sulfated polysaccharide isolated from an enzymatic digest of brown seaweed Sargassum horneri in RAW 264.7 cells. Nutr. Res. Pract. 2017, 11, 3–10. [Google Scholar] [CrossRef]
- Han, E.J.; Kim, H.-S.; Sanjeewa, K.K.A.; Jung, K.; Jee, Y.; Jeon, Y.-J.; Fernando, I.P.S.; Ahn, G. Sargassum horneri as a functional food ameliorated IgE/BSA-induced mast cell activation and passive cutaneous anaphylaxis in mice. Marine drugs 2020, 18, 594. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.J.; Jee, Y.; Jeon, Y.-J.; Kim, H.J. Antioxidant potential of Sargassum horneri extract against urban particulate matter-induced oxidation. Food Sci. Biotechnol. 2020, 29, 855–865. [Google Scholar] [CrossRef]
- Herath, K.H.I.N.M.; Cho, J.; Kim, A.; Kim, H.-S.; Han, E.J.; Kim, H.J.; Kim, M.S.; Ahn, G.; Jeon, Y.-J.; Jee, Y. Differential modulation of immune response and cytokine profiles of Sargassum horneri ethanol extract in murine spleen with or without Concanavalin A stimulation. Biomed. Pharmacother. 2019, 110, 930–942. [Google Scholar] [CrossRef]
- Zhao, D.; Zheng, L.; Qi, L.; Wang, S.; Guan, L.; Xia, Y.; Cai, J. Structural features and potent antidepressant effects of total sterols and β-sitosterol extracted from Sargassum horneri. Marine drugs 2016, 14, 123. [Google Scholar] [CrossRef]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, H.-S.; Jeon, Y.-J.; Jee, Y.; Kim, K.-N.; Lee, K.; Fernando, I.P.S.; Ahn, G. Sargassum horneri (Turner) C. Agardh ethanol extract attenuates fine dust-induced inflammatory responses and impaired skin barrier functions in HaCaT keratinocytes. J. Ethnopharmacol. 2021, 273, 114003. [Google Scholar] [CrossRef]
- Kirindage, K.G.I.S.; Fernando, I.P.S.; Jayasinghe, A.M.K.; Han, E.-J.; Dias, M.K.H.M.; Kang, K.-P.; Moon, S.-I.; Shin, T.-S.; Ma, A.; Ahn, G. Moringa oleifera hot water extract protects Vero cells from hydrogen peroxide-induced oxidative stress by regulating mitochondria-mediated apoptotic pathway and Nrf2/HO-1 signaling. Foods 2022, 11, 420. [Google Scholar] [CrossRef]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochimica et Biophysica Acta (BBA)-General Subjects 2005, 1721, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Kirindage, K.G.I.S.; Jayasinghe, A.M.K.; Han, E.-J.; Han, H.-J.; Kim, K.-N.; Wang, L.; Heo, S.-J.; Jung, K.-S.; Ahn, G. Phlorofucofuroeckol-A refined by edible brown algae Ecklonia cava indicates anti-inflammatory effects on TNF-α/IFN-γ-stimulated HaCaT keratinocytes and 12-O-tetradecanoylphorbol 13-acetate-induced ear edema in BALB/c mice. J. Funct. Foods 2023, 109, 105786. [Google Scholar] [CrossRef]
- Kirindage, K.G.I.S.; Jayasinghe, A.M.K.; Cho, N.; Cho, S.H.; Yoo, H.M.; Fernando, I.P.S.; Ahn, G. Fine-dust-induced skin inflammation: Low-molecular-weight fucoidan protects keratinocytes and underlying fibroblasts in an integrated culture model. Marine Drugs 2022, 21, 12. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Kirindage, K.G.I.S.; Jayasinghe, A.M.K.; Han, E.J.; Dias, M.K.H.M.; Kang, K.P.; Moon, S.I.; Shin, T.S.; Ma, A.; Jung, K. Hot Water Extract of Sasa borealis (Hack.) Makino & Shibata Abate Hydrogen Peroxide-Induced Oxidative Stress and Apoptosis in Kidney Epithelial Cells. Antioxidants 2022, 11, 1013. [Google Scholar] [CrossRef]
- Balicki, S.; Pawlaczyk-Graja, I.; Gancarz, R.; Capek, P.; Wilk, K.A. Optimization of Ultrasound-Assisted Extraction of Functional Food Fiber from Canadian Horseweed (Erigeron canadensis L.). ACS omega 2020, 5, 20854–20862. [Google Scholar] [CrossRef]
- Kirindage, K.G.I.S.; Jayasinghe, A.M.K.; Ko, C.-I.; Ahn, Y.-S.; Heo, S.-J.; Oh, J.-Y.; Kim, E.-A.; Cha, S.-H.; Ahn, G. Unveiling the Potential of Ultrasonic-Assisted Ethanol Extract from Sargassum horneri in Inhibiting Tyrosinase Activity and Melanin Production in B16F10 Murine Melanocytes. Frontiers in Bioscience-Landmark 2024, 29, 194. [Google Scholar] [CrossRef]
- Chi, H.K.; Van, N.T.H.; Hang, T.T.N.; Duc, T.M.; Ha, T.T.H. Intracellular reactive oxygen species scavenging effect of fucosterol isolated from the brown alga Sargassum crassifolium in Vietnam. Vietnam Journal of Marine Science and Technology 2020, 20, 308–316. [Google Scholar] [CrossRef]
- Pawlowska, E.; Szczepanska, J.; Koskela, A.; Kaarniranta, K.; Blasiak, J. Dietary polyphenols in age-related macular degeneration: protection against oxidative stress and beyond. Oxidative medicine and cellular longevity 2019, 2019. [Google Scholar] [CrossRef]
- Plouguerné, E.; da Gama, B.A.; Pereira, R.C.; Barreto-Bergter, E. Glycolipids from seaweeds and their potential biotechnological applications. Frontiers in cellular and infection microbiology 2014, 4, 174. [Google Scholar] [CrossRef]
- Wang, T.; Jonsdottir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food chemistry 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical biochemistry 1996, 239, 70–76. [Google Scholar] [CrossRef]
- de Pedro, I.; Alonso-Lecue, P.; Sanz-Gómez, N.; Freije, A.; Gandarillas, A. Sublethal UV irradiation induces squamous differentiation via a p53-independent, DNA damage-mitosis checkpoint. Cell Death Dis. 2018, 9, 1094. [Google Scholar] [CrossRef]
- Garg, C.; Sharma, H.; Garg, M. Skin photo-protection with phytochemicals against photo-oxidative stress, photo-carcinogenesis, signal transduction pathways and extracellular matrix remodeling—An overview. Ageing Research Reviews 2020, 62, 101127. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: a critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- De Jager, T.; Cockrell, A.; Du Plessis, S. Ultraviolet light induced generation of reactive oxygen species. Ultraviolet Light in Human Health, Diseases and Environment 2017, 15-23.
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Heo, S.-J.; Ahn, G. Fucoidan Fractionated from Sargassum coreanum via Step-Gradient Ethanol Precipitation Indicate Promising UVB-Protective Effects in Human Keratinocytes. Antioxidants 2021, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Ahn, G. Step gradient alcohol precipitation for the purification of low molecular weight fucoidan from Sargassum siliquastrum and its UVB protective effects. International journal of biological macromolecules 2020, 163, 26–35. [Google Scholar] [CrossRef]
- Ziegler, A.; Jonason, A.S.; Leffellt, D.J.; Simon, J.A.; Sharma, H.W.; Kimmelman, J.; Remington, L.; Jacks, T.; Brash, D.E. Sunburn and p53 in the onset of skin cancer. Nature 1994, 372, 773–776. [Google Scholar] [CrossRef]
- Lugli, E.; Troiano, L.; Ferraresi, R.; Roat, E.; Prada, N.; Nasi, M.; Pinti, M.; Cooper, E.L.; Cossarizza, A. Characterization of cells with different mitochondrial membrane potential during apoptosis. Cytometry Part A 2005, 68, 28–35. [Google Scholar] [CrossRef]
| Sample | Yield (%) 1 | Carbohydrate (%)2 | Crude protein (%)2 | Total phenolic compounds (%)2 |
|---|---|---|---|---|
| USHE | 29.50 ± 1.32 | 9.83 ± 0.87 | 15.01 ± 1.06 | 6.97 ± 0.36 |
| RT (min) |
m/z ([M-H]) |
Formula | Δppm | Compound |
|---|---|---|---|---|
| 16.12 | 527.2518 | C23H43O11S | -0.0192 | 1-O-Tetradecanoyl-3-O-(6′-sulfo-a-D-quinovopyranosyl) glycerol |
| 17.17 | 553.3187 | C25H45O11S | -0.0504 | 1-O-(11-Hexadecenoyl)-3-O-(6′-sulfo-a-D-quinovopyranosyl) glycerol |
| 19.02 | 555.3054 | C25H47O11S | -0.0215 | 1-O-Hexadecanoyl-3-O-(6′-sulfo-a-D-quinovopyranosyl) glycerol |
| 20.13 | 581.3199 | C27H49O11S | -0.0007 | 1-O-(9-Octadecenoyl)-3-O-(6′-sulfo-a-D-quinovopyranosyl) glycerol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





