Submitted:
11 October 2024
Posted:
12 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. ODAP Content and Macro and Micro-Nutrients Concentrations
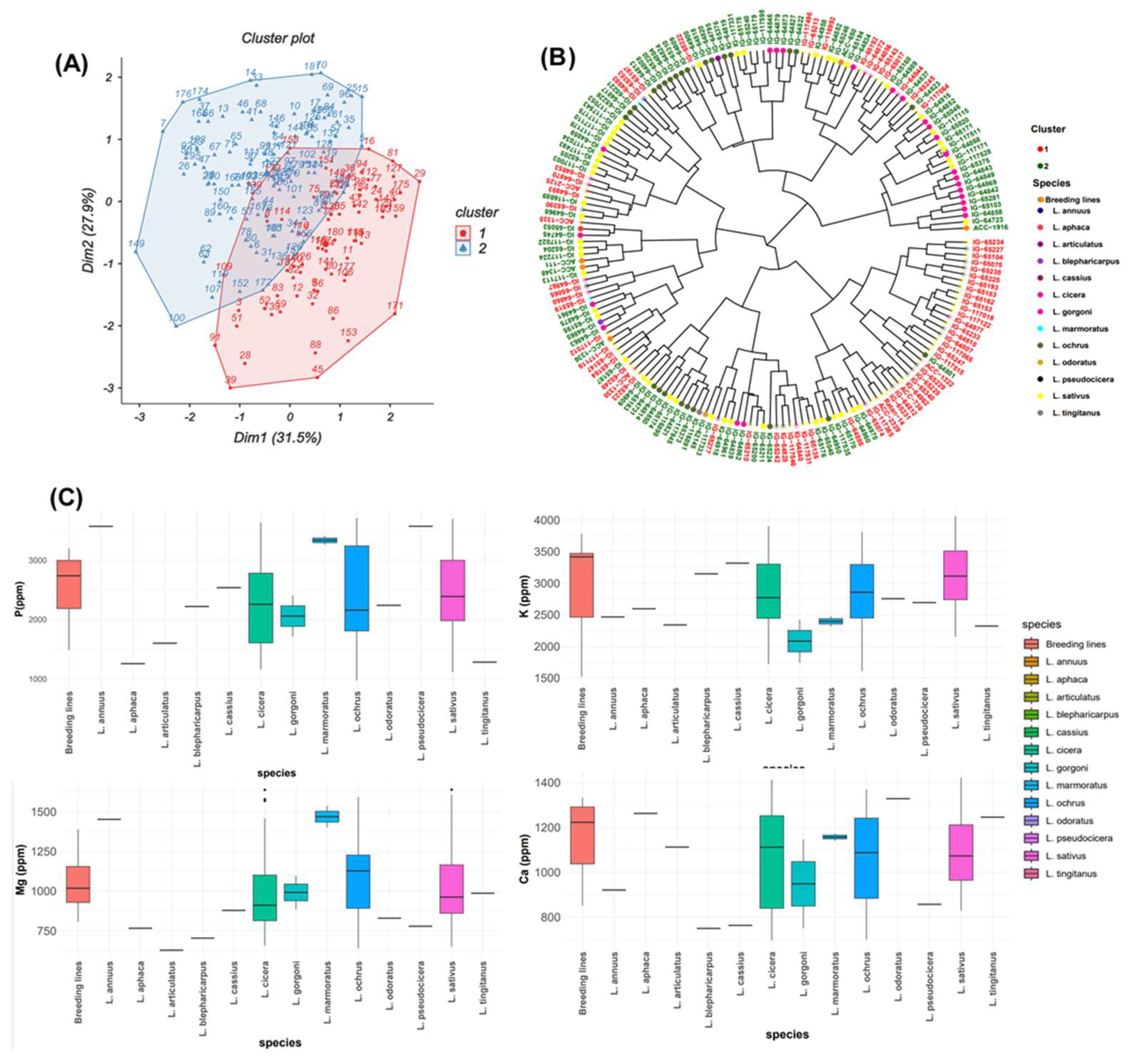
2.2. PCA of Macro-Nutrients
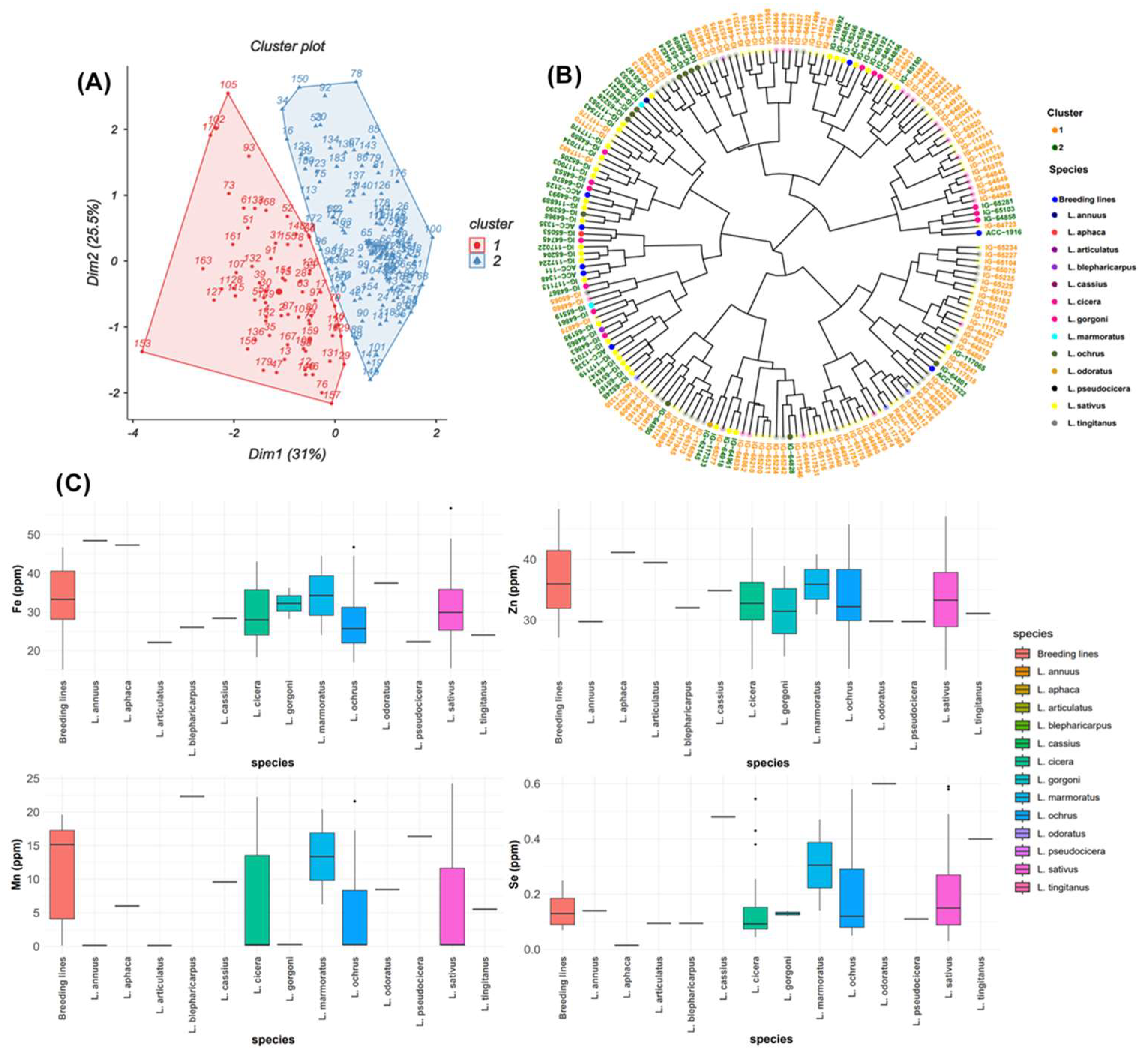
2.3. PCA of Micronutrients
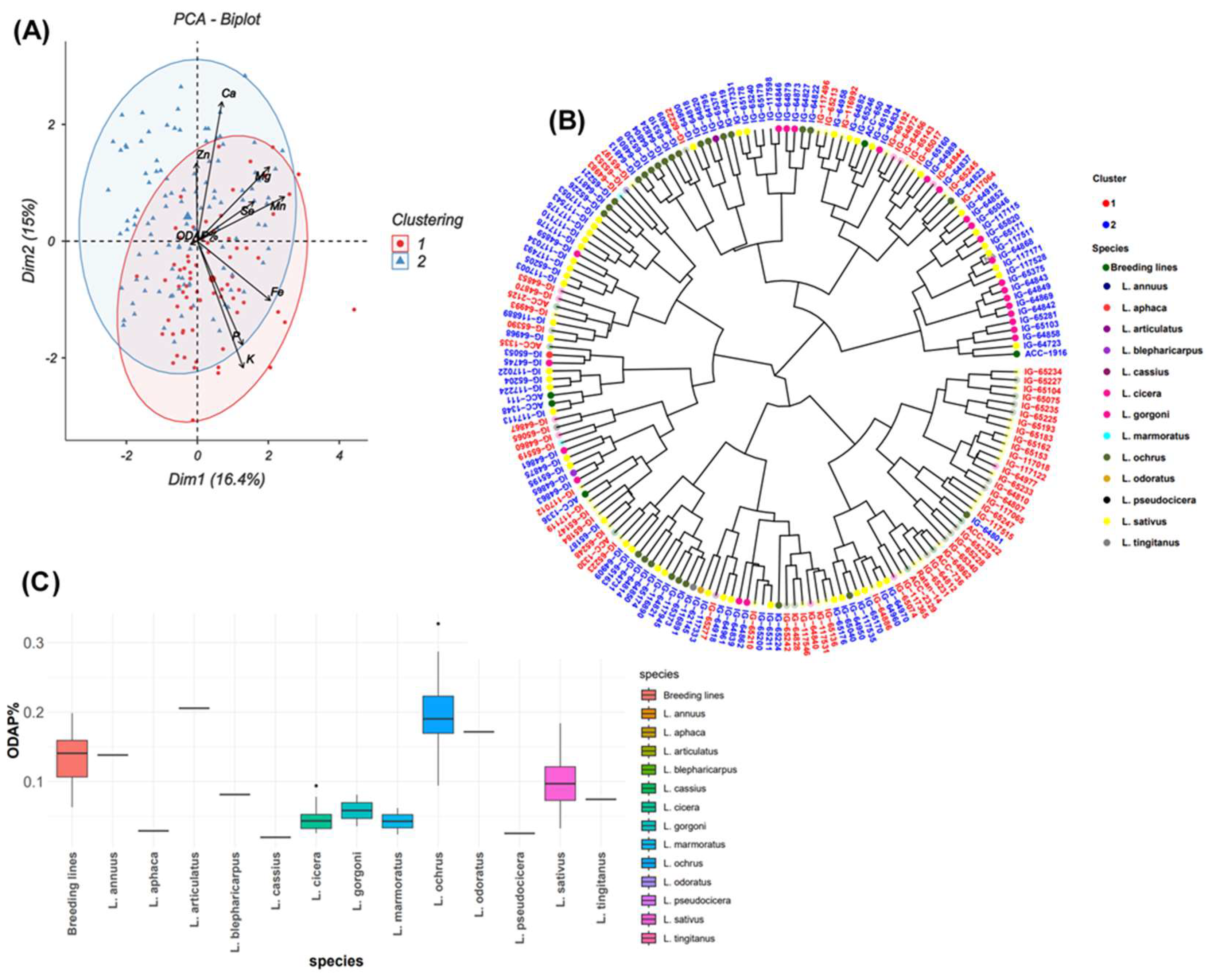
2.4. PCA of ODAP Content
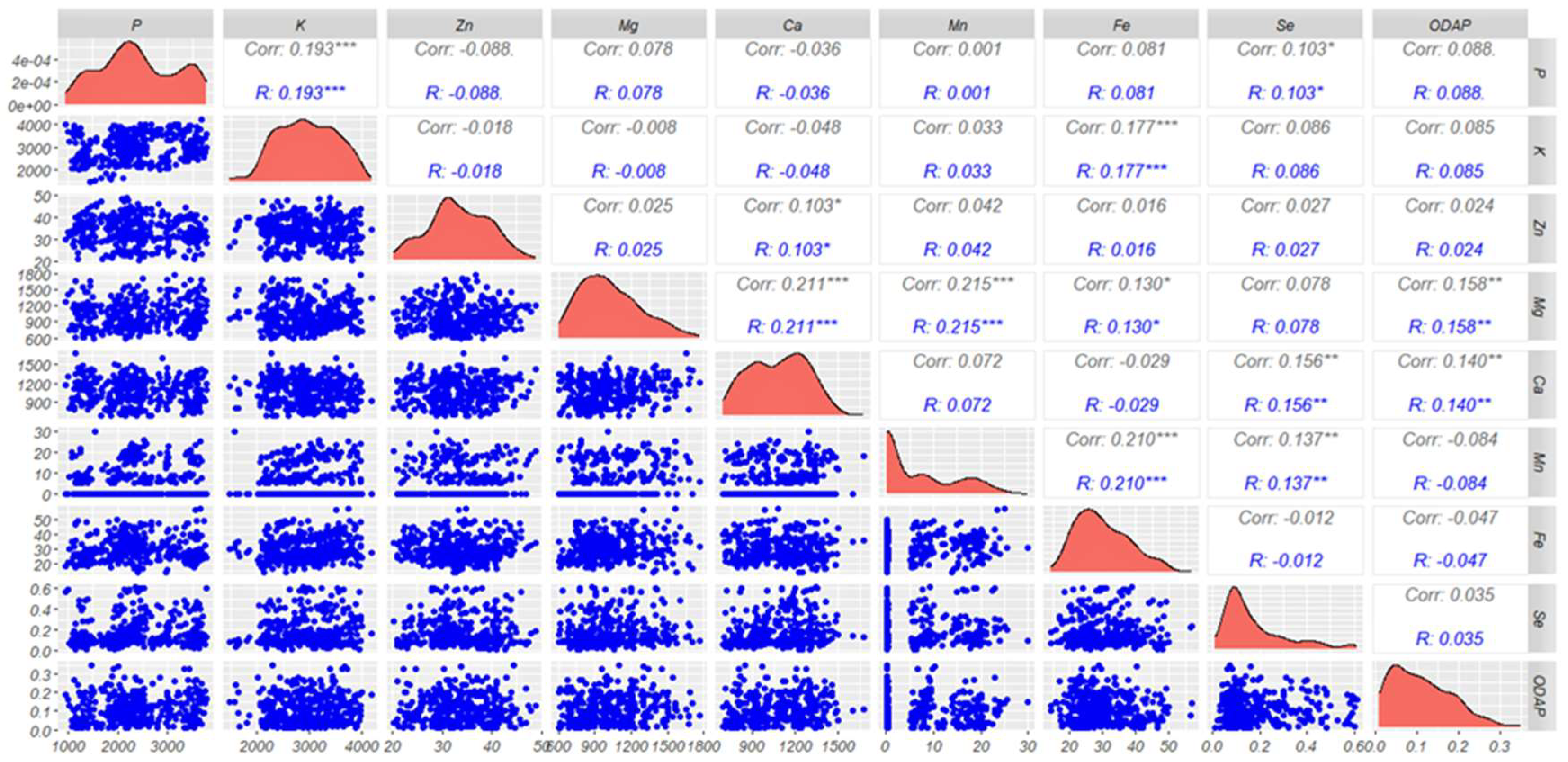
2.5. Correlations among Nutrients and ODAP Contents
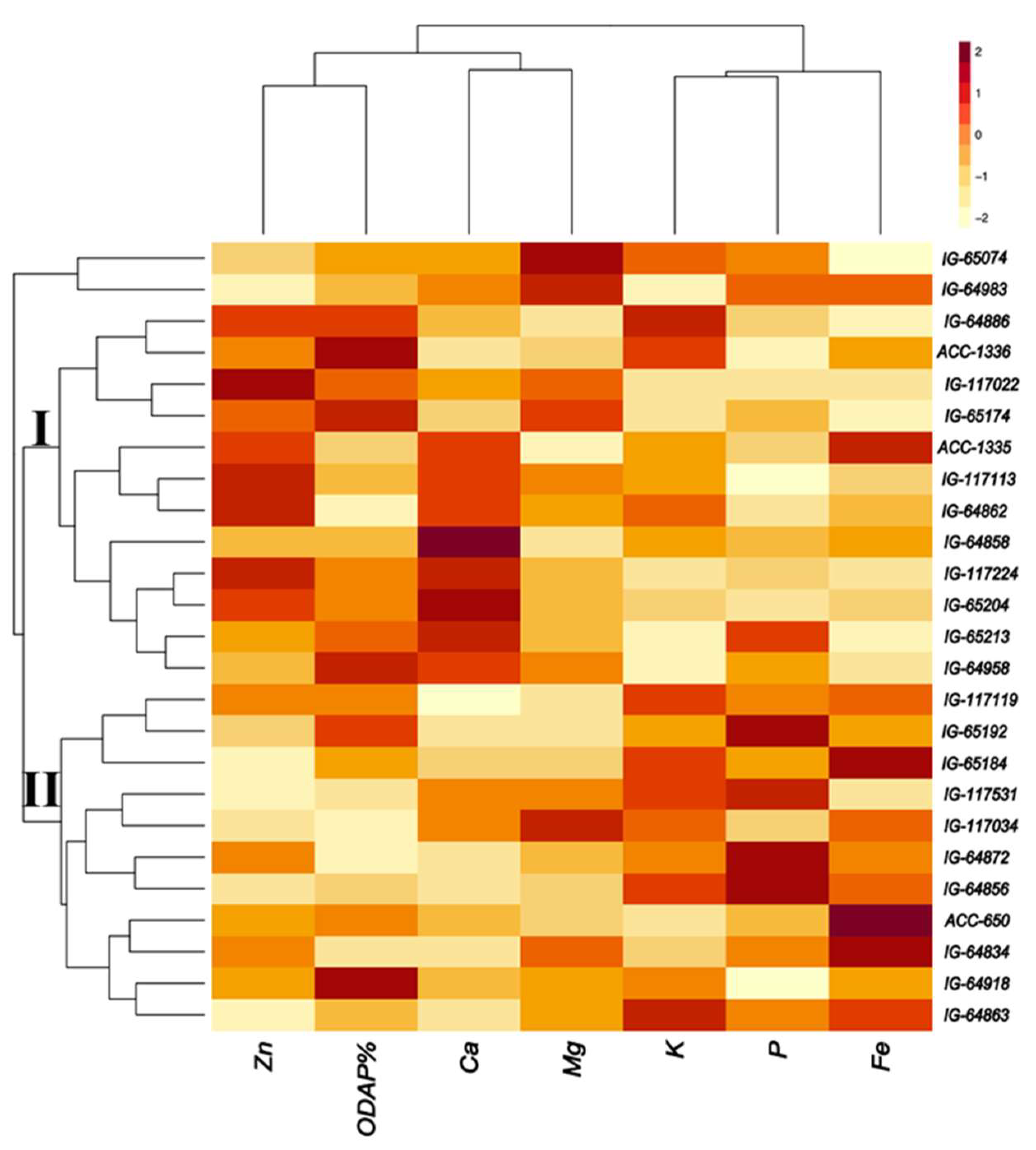
2.6. Best Performing Accessions
3. Discussion
4. Materials and Methods
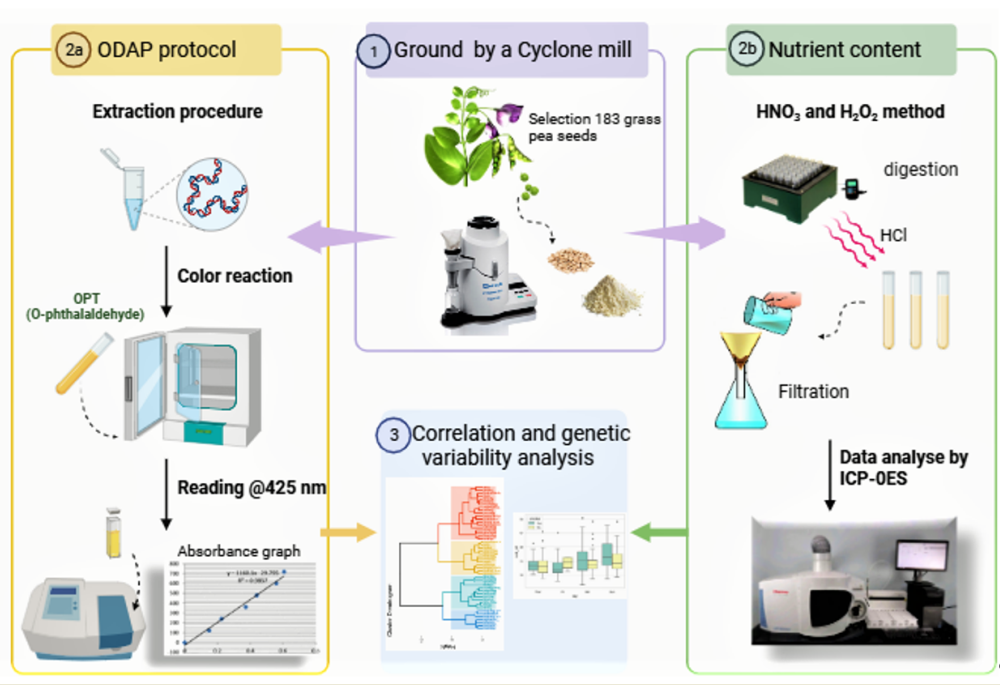
4.1. Plant Material
4.2. ODAP Protocol
4.3. Mineral Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Gupta, P.; Barpete, S.; Sarker, A.; Amri, A.; Mathur, P.N.; Baum, M. Grass pea. In Genetic and Genomic Resources of Grain Legumes Improvement; Singh, M., Upadhyaya, H.D., Bisht, I.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 269–292. [Google Scholar] [CrossRef]
- Kumar, S.; Bejiga, G.; Ahmed, S.; Nakkoul, H.; Sarker, A. Genetic improvement of grass pea for low neurotoxin (β-ODAP) content. Food Chem. Toxicol. J. 2011, 49, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Maxted, N.; Goyder, D.J. A new species of Lathyrus sect. Lathyrus (Leguminosae - Papilionoideae) from Turkey. Kew Bull. 1988, 43, 711–714. [Google Scholar] [CrossRef]
- Jackson, M.T.; Yunus, A.G. Variation in the grass pea (L. sativus L.) and wild species. Euphytica 1984, 33, 549–559. [Google Scholar] [CrossRef]
- Hanbury, C.D.; Siddique, K.H.M.; Galwey, N.W.; Cocks, P.S. Genotype-environment interaction for seed yield and ODAP concentration of Lathyrus sativus L. and L. cicera L. in Mediterranean type environments. Euphytica 1999, 110, 45–60. [Google Scholar] [CrossRef]
- Saxena, M.C.; Abd El Moneim, A.M.; Raninam, M. Vetches (Vicia spp.) and chicklings (Lathyrus spp.) in the farming systems in West Asia and North Africa and improvement of these crops at ICARDA. In Potential for Vicia and Lathyrus species as New Grain and Fodder Legumes for Southern Australia; Garlinge, J.R.; Perry, M.W., Eds.; CLIMA, Perth, 1993; pp. 2 - 9.
- Lambein, F.; Travella, S.; Kuo, Y.H.; Montagu, M.V.; Heijde, M. Grass pea (Lathyrus sativus L.): orphan crop, nutraceutical or just plain food? Planta 2019, 250, 821–838. [Google Scholar] [CrossRef]
- Schulz, S.; Keatinge, J.D.H.; Wells, G.J. Productivity and residual effects of legumes in rice-based cropping systems in a warm-temperate environment: II. Residual effects on rice. Field Crops Res. 1999, 61, 37–49. [Google Scholar] [CrossRef]
- Campbell, C.G.; Mehra, R.B.; Agrawal, S.K.; Chen, Y.Z.; Abd-El-Moneim, A.M.; Khawaja, H.I.T.; Yadav, C.R.; Tay, J.U.; Araya, W.A. Current status and future strategy in breeding grass pea (Lathyrus sativus). Euphytica. 1994, 73, 167–175. [Google Scholar] [CrossRef]
- Kumar, J. Utilization of Lathyrus. In Lathyrus Genetic International Resources Network, proceedings of IPGRI-ICARDA-ICAR Regional Working Group Meeting; Mathur, P.N.; RAmanatha Rao, V.; Arora, R.K., Eds.; New Delhi, India. 1997, p. 57. Available online: https://hdl.handle.net/10568/105246 (accessed on 7 October 2024).
- Tiwari, K.R.; Campbell, C.G. Inheritance of neurotoxin (ODAP) content, flower and seed coat colour in grass pea (Lathyrus sativus L). Euphytica. 1996, 91, 195–203. Available online: http://link.springer.com/article/10.1007/BF00021070 (accessed on 4 October 2024). [CrossRef]
- Campbell, C.G. Grass pea. Lathyrus sativus L.; Institute of Plant Genetics and Crop Plant Research (IPK): Gatersleben, Germany; International Plant Genetic Resources Institute (IPGRI): Rome, Italy, 1997; p. 91. Available online: https://www.researchgate.net/publication/245231747 (accessed on 7 October 2024).
- Abdallah, F.; Kumar, S.; Amri, A.; Mentag, R.; Kehel, Z.; Mejri, R.K.; Triqui, Z.E.A.; Hejjaoui, K.; Baum, M.; Amri, M. Wild Lathyrus species as a great source of resistance for introgression into cultivated grass pea (Lathyrus sativus L.) against broomrape weeds (Orobanche crenata Forsk. and Orobanche foetida Poir.). Crop Sci. 2020, 61, 263–276. [Google Scholar] [CrossRef]
- Tarade, K.M.; Singhal, R.S.; Jayram, R.V.; Pandit, A.B. Kinetics of degradation of ODAP in Lathyrus sativus L. flour during food processing. Food Chem. 2007, 104, 643–649. [Google Scholar] [CrossRef]
- Tamburino, R.; Guida, V.; Pacifico, S.; Rocco, M.; Zarelli, A.; Parente, A.; Maro, A.D. Nutritional values and radical scavenging capacities of grass pea (Lathyrus sativus L.) seeds in Valle Agricola district, Italy. Aust. J. Crop Sci. 2012, 6, 149–156. Available online: https://www.semanticscholar.org/paper/Nutritional-values-and-radical-scavenging-of-grass-Tamburino-Guida/d7823f5c000e7da938d6369f6ae7ccb03681c08f (accessed on 7 October 2024).
- Sen Gupta, D.; Barpete, S.; Kumar, J.; Kumar, S. Breeding for better grain quality in Lathyrus. In Breeding for enhanced nutrition and bio-active compounds in food legumes. D. S., Gupta; S, Gupta; J, Kumar; Eds.; Springer: Kanpur, Uttar Pradesh, India, 2021; pp. 131–156. [CrossRef]
- Rao, S.L.N.; Adiga, P.R.; Sarma, P.S. The isolation and characterization of β-N-oxalyl-l-α, β-diaminopropionic acid: a neurotoxin from the seeds of Lathyrus sativus. Biochemistry. 1964, 3, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Eyzaguirre, P.B.; Paludosi, S.; Hodgkin, T. IPGRI’s strategy for neglected and underutilized species and the human dimension of agrobiodiversity. In Priority-setting for underutilized and neglected plant species of the Mediterranean region. Report of the IPGRI Conference, ICARDA, Aleppo, Syria. IPGRI, Rome, Italy, 9-11 February 1998; pp. 1–20.
- Hanbury, C.D.; Siddique, K.H.M.; Seymour, M.; Jones, R.; MacLeod, B. Growing Ceora grass pea (Lathyrus sativus) in Western Australia. (Ed. SP Department of Agriculture Western Australia and Centre for Legumes in Mediterranean Agriculture. (CLIMA)). Farmnote No. 58, Government of Western Australia. 2005. Available online: https://www.web.uwa.edu.au/__data/assets/pdf_file/0004/920569/ceorafarmnote.pdf (accessed on 3 October 2024).
- Yan, Z.Y.; Spencer, P.S.; Li, Z.X.; Liang, Y.M.; Wang, Y.F.; Wang, C.Y.; Li, F.M. Lathyrus sativus (grass pea) and its neurotoxin ODAP. Phytochemistry. 2006, 67, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Urga, K.; Fufa, H.; Biratu, E.; Husain, A. Evaluation of Lathyrus sativus cultivated in Ethiopia for proximate composition, minerals, β-ODAP and anti-nutritional components. Afr. J. Food Agric. Nutr. Dev. 2005, 5, 1–15. [Google Scholar] [CrossRef]
- Vedna, K. Field evaluation of grass pea (Lathyrus sativus L.) germ plasm for its toxicity in the northwestern hills of India. Lathyrus Lathyrism Newsl. 2001, 2, 82–84. Available online: http://scholar.google.com/scholar_lookup?&title=Field%20evaluation%20of%20grass%20pea%20%28Lathyrus%20sativus%20L.%29%20germ%20plasm%20for%20its%20toxicity%20in%20the%20northwestern%20hills%20of%20India&journal=Lathyrus%20Lathyrism%20Newsl&volume=2&pages=82-84&publication_year=2001&author=Vedna%2CK (accessed on 8 October 2024).
- Tekle Haimanot, R.; Abegaz, B.M.; Wuhib, E.; Kassina, A.; Kidane, Y.; Kebede, N.; Alemu, T.; Spencer, P.S. Pattern of Lathyrus sativus (grass pea) consumption and Beta-N-oxalyl-a, β -diaminopropionic acid (β -ODAP) content of food samples in the lathyrism endemic region of northwest Ethiopia. Nutri. Res. 1993, 13, 1113–1126. [Google Scholar] [CrossRef]
- Barpete, S.; Gupta, P.; Khawar, K.M.; Kumar, S. Effect of cooking methods on protein content and neurotoxin (β-ODAP) concentration in grass pea (Lathyrus sativus L.) grains. CyTA – J. of Food. 2021, 19, 448–456. [Google Scholar] [CrossRef]
- Hailu, D.; Abera, S.; Teka, T.A. Effects of processing on nutritional composition and anti-nutritional factors of grass pea (Lathyrus sativus L): A review. Food Sci. Qual. Manag. 2015, 36, 61–70. Available online: https://www.researchgate.net/publication/303700648 (accessed on 8 October 2024).
- Abd-El-Moneim, A.M.; van Dorrestein, B.; Baum, M.; Mulugeta, W. Improving the nutritional quality and yield potential of grass pea (Lathyrus sativus L.). Food Nutr. Bull. 2000, 21, 493–496. [Google Scholar] [CrossRef]
- Spencer, P.S.; Ludolph, A.; Dwivedi, M.P.; Roy, D.N.; Hugon, J.; Schaumburg, H.H. Lathyrism: evidence for role of the neuroexcitary amino acid BOAA. Lancet ii. 1986, 1066–1067. [Google Scholar] [CrossRef]
- White, C.L.; Hanbury, C.D.; Young, P.; Phillips, N.; Wiese, S.C.; Milton, B.; Avidson, R.H.; Siddique, K.H.M.; Harris, D. The nutritional value of Lathyrus cicera and Lupinus angustifolius grain for sheep. Anim. Feed Sci. Technol. 2002, 99, 45–64. [Google Scholar] [CrossRef]
- Özyazıcı, M.A.; Açıkbaş, S. Forage quality and mineral composition of common grass pea (Lathyrus sativus L.) genotypes. Int. J. Elem. 2023, 28, 405–421. [Google Scholar] [CrossRef]
- Hanbury, C.D.; White, C.L.; Mullan, B.P.; Siddique, K.H.M. The potential of Lathyrus sativus L. and L. cicera L. grain for use as animal feed: A review. Anim. Feed Sci. Tech. 2000, 87, 1–27. [Google Scholar] [CrossRef]
- El Haddad, N.; Choukri, H.; Ghanem, M.E.; Smouni, A.; Mentag, R.; Rajendran, K.; Hejjaoui, K.; Maalouf, F.; Kumar, S. High temperature and drought stress effects on growth, yield and nutritional quality with transpiration response to vapor pressure deficit in lentil. Plants. 2021, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Choukri, H.; El Haddad, N.; Aloui, K.; Hejjaoui, K.; El-Baouchi, A.; Smouni, A.; Thavarajah, D.; Maalouf, F.; Kumar, S. Effect of high temperature stress during the reproductive stage on grain yield and nutritional quality of lentil (Lens culinaris medicus). Front. Food. Sci. Technol. 2022, 9, 857469. [Google Scholar] [CrossRef]
- Farida Traoré, F.; El-Baouchi, A.; En-nahli, Y.; Hejjaoui, K.; Metougui, M.L.; Hamwieh, A.; Sohail, Q.; Istanbuli, T.; Boughribil, S.; Amri, M. Exploring the genetic variability and potential correlations between nutritional quality and agro-physiological traits in Kabuli chickpea germplasm collection (Cicer arietinum L.). Front. Plant Sci. 2022, 13, 905320. [Google Scholar] [CrossRef]
- Lisiewska, Z.; Korus, A.; Kmiecik, W.; Gebczynski, P. Effect of maturity stage on the content of ash components in raw and preserved grass pea (Lathyrus sativus L.) seeds. Int. Food Sci. Nutr. 2006, 57, 39–45. [Google Scholar] [CrossRef]
- Chavan, U.D.; Shahidi, F.; Bal, A.K.; McKenzie, D.B. Physico-chemical properties and nutrient composition of beach pea (Lathyrus maritimus L.). Food Chem. 1999, 66, 43–50. [Google Scholar] [CrossRef]
- Grela, E.R.; Rybiński, W.; Klebaniuk, R.; Matras, J. Morphological characteristics of some accessions of grass pea (Lathyrus sativus L.) grown in Europe and nutritional traits of their seeds. Genet. Resour. Crop. Evol. 2010, 57, 693–701. [Google Scholar] [CrossRef]
- Grela, E.R.; Rybiński, W.; Matras, J.; Sobolewska, S. Variability of phenotypic and morphological characteristics of some Lathyrus sativus L.and Lathyrus cicera L. accessions and nutritional traits of their seeds. Genet. Resour. Crop. Evol. 2012, 59, 1687–1703. [Google Scholar] [CrossRef]
- Hanbury, C.D.; Siddique, K.H.M. Registration of 'Chalus' Lathyrus cicera L. Crop. Sci. 2010, 40, 1199. [Google Scholar] [CrossRef]
- Aletor, V.A.; Abd El-Moneim, A.; Goodchild, A.V. Evaluation of the seeds of selected lines of three Lathyrus spp for p-N-Oxalylamino-L-Alanine (BOAA), Tannins, Trypsin Inhibitor Activity and Certain In-vitro Characteristic. J. Sci. Food Agric. 1994, 65, 143–151. [Google Scholar] [CrossRef]
- Urga, K.; Fite, A.; Kebede, B. Nutritional and anti-nutritional factors of grass pea germ plasm. Bull. Chem. Soc. Ethiop. 1995, 9, 9–16. Available online: https://www.ajol.info/index.php/bcse/article/view/74085/64747 (accessed on 8 October 2024).
- Dahiya, B.S. Seed morphology as an indicator for low neurotoxin in Lathyrus sativus. Qualitas Plantarium. Plant Foods Hum. Nutr. 1976, 25, 391–394. [Google Scholar] [CrossRef]
- Pandey, R.L.; Kashyap, O.P.; Sharma, R.N.; Nanda, H.C.; Geda, A.K.; Nair, S. Catalogue on grass pea (Lathyrus sativus L.) germplasm. Indira Gandhi Krishi Vishwavidyalaya, Raipur, India. 2008, p. 213. Available online: http://krishikosh.egranth.ac.in/handle/1/5810141369 (accessed on 8 October 2024).
- Leakey, C. Khesari dhal: The poisonous pea. J. Appropr. Technol. 1979, 6, 15–16. Available online: https://scholar.google.com/scholar_lookup?title=Khesari%20dalthe%20poisonous%20pea&publication_year=1979&author=C.%20Leakey (accessed on 8 October 2024).
- Robertson, L.D.; Abd-El-Moneim, A. Status of Lathyrus germplasm held at ICARDA and its use in breeding programs. In Lathyrus Genetic Resources Network, Proceedings of a IPGRI-ICARDA-ICAR Regional Working Group Meeting; Mathur, P.N., Rao, V.R., Arora, R.K., Eds.; New Delhi, India, 8-10 December 1997, pp. 30–41. Available online: https://cgspace.cgiar.org/server/api/core/bitstreams/d259bae7-ccf4-4a10-897c-a90af077062d/content (accessed on 8 October 2024).
- Tadesse, W.; Bekele, E. Variation and association of morphological and biochemical characters of grass pea (Lathyrus sativus L.). Euphytica. 2003, 130, 315–324. [Google Scholar] [CrossRef]
- Eichinger, P.C.H.; Rothnie, N.E.; Delaere, I.; Tate, M.E. New technologies for toxin analyses in food legumes. In Linking research and marketing opportunities for pulses in the 21st century; Knight, R., Ed.; Kluwer Academic Publishers: Dordrecht, 2000; pp. 685–692. [Google Scholar] [CrossRef]
- Siddique, K.H.M.; Loss Herwig, S.P.; Wilson, J.M. Growth, yield and neurotoxin (ODAP) concentration of three Lathyrus species in Mediterranean type environments of Western Australia. Aus. J. Exp. Agric. 1996, 36, 209–218. [Google Scholar] [CrossRef]
- Sammour, R.H.; Abd El-Zahar, M.; Salwa, B.; Walla, B. Genetic variability of some quality traits in Lathyrus spp. germplasm. Acta Agric. Slov. 2007, 90, 33–43. [Google Scholar] [CrossRef]
- Sammour, R.H.; Abd El-Zahar, M.; Salwa, B.; Walla, T. Genetic variations in accessions of Lathyrus sativus L. Acta Bot. Croat. 2007, 66, 1–13. Available online: https://hrcak.srce.hr/11767 (accessed on 8 October 2024).
- Basaran, U.; Acar, Z.; Karacan, M.; Onar, A.N. Variation and correlation of morpho-agronomic traits and biochemical contents (protein and ß-ODAP) in Turkish grass pea (Lathyrus sativus L.) landraces. Turk. J. Field. Crops. 2013, 18, 166–173. Available online: https://www.researchgate.net/publication/286365155 (accessed on 7 October 2024).
- Bandana, C.; Khanin, P.; Priyanka, D.; Ranjan, B.A.; Hemen, K.; Samindra, B. Nutritional evaluation of few grass pea (Lathyrus sativus L.) genotypes of Assam. Ind. J. Agric. Biochem. 2022, 35, 155–158. [Google Scholar] [CrossRef]
- Hanbury, C.D.; Siddique, K.H.M.; Galwcy, N.W.; Cocks, P.S. Genotype-environment interaction for seed yield and ODAP concentration of Lathyrus sativus L. and L. cicera L. in Mediterranean type environments. Euphytica. 2000, 110, 45–60. [Google Scholar] [CrossRef]
- Wuletaw, T. Stability of grass pea (Lathyrus sativus L.) varieties for β-ODAP content and grain yield in Ethiopia. Lathyrus Lathyrism Newsl. 2003, 3, 32–34. [Google Scholar] [CrossRef]
- Arslan, M. Variation of some seed trace element contents in grass pea (Lathyrus sativus L.) genotypes from Turkey. Fresenius Environ. Bull. 2017, 26, 3676–3684. Available online: https://www.researchgate.net/publication/348805447 (accessed on 6 October 2024).
- Basaran, U.; Gulumser, E.; Dogrusoz, M.; Mut, H. Variation and association in Lathyrus species based on seed biochemical constituents. Range Manag. Agrofor. 2019, 40, 124–130. Available online: https://hdl.handle.net/11552/1346 (accessed on 7 October 2024).
- Talukdar, D. Recent progress on genetic analysis of novel mutants and aneuploid research in grass pea (Lathyrus sativus L.). Afr. J. Agric. Res. 2009, 4, 1549–1559. [Google Scholar] [CrossRef]
- Rao, S.L.N. A sensitive and specific colorimetric method for the determination of α, β-diaminopropionic acid and the Lathyrus sativus neurotoxin. Anal. Biochem. 1978, 86, 386–395. [Google Scholar] [CrossRef]
- Sen Gupta, D.; Thavarajah, D.; McGee, R.; Coyne, C.; Kumar, S.; Thavarajah, P. Genetic diversity among cultivated and wild lentils for iron, zinc, copper, calcium and magnesium concentrations. Aust. J. Crop Sci. 2016, 10, 1381–1387. [Google Scholar] [CrossRef]
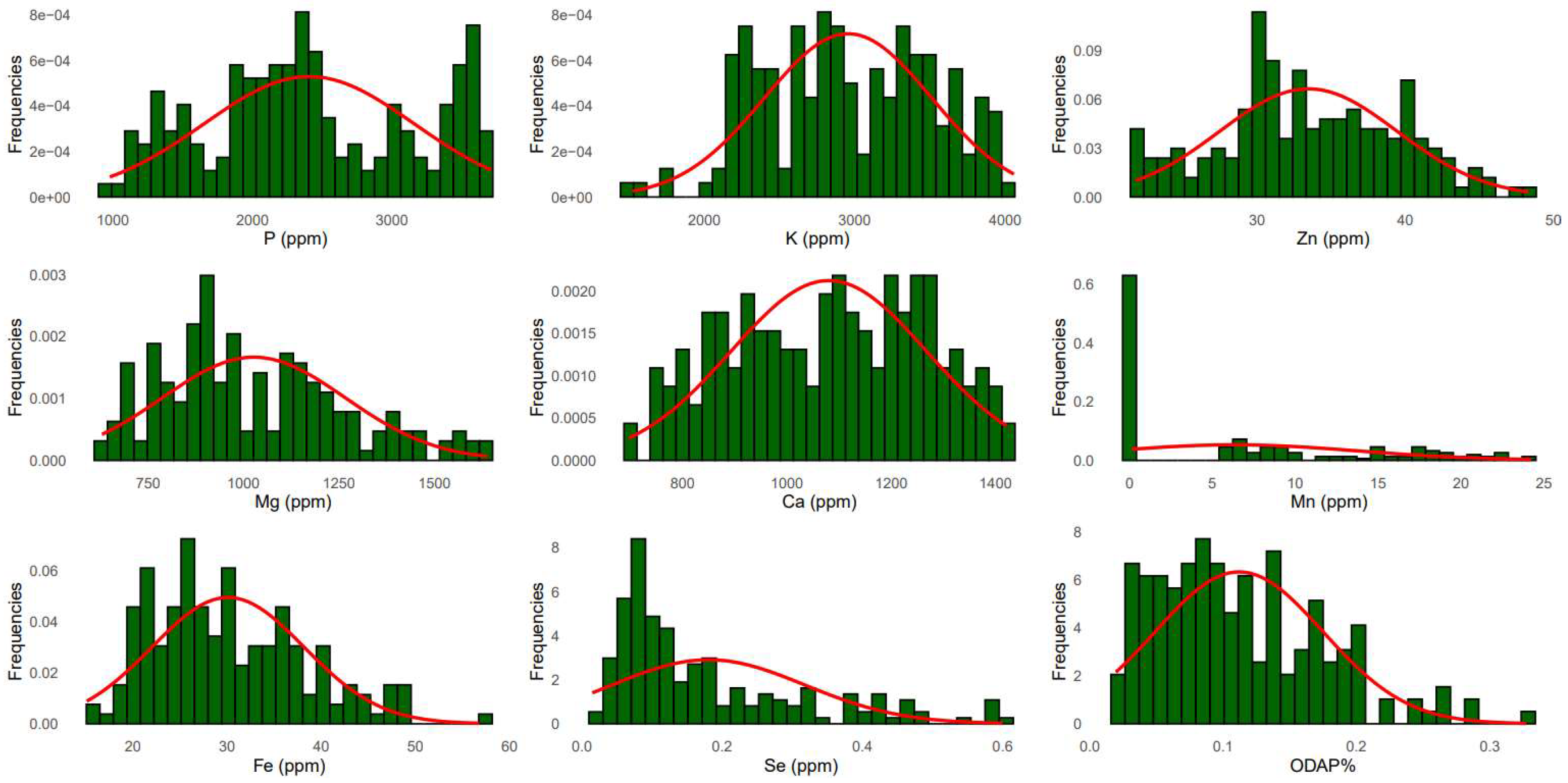
| Min. | Max. | Mean | F | R2 | Heritability (h2) |
CV (%) |
Genotypic Variance | LSD (95%) |
|
|---|---|---|---|---|---|---|---|---|---|
| P | 953.7 (IG65224) |
3788.9 (IG64807) |
2405.8 | 63.9** | 0.9 | 0.5 | 31.6 | 308479.4 | 177.9 |
| K | 1478.9 (ACC1916) |
4189.1 (IG117365) |
2953.8 | 40.9** | 0.9 | 0.4 | 19.0 | 157105.1 | 169.2 |
| Ca | 688.4 (IG64834) |
1678.4 (IG116889) |
1080.1 | 5.7** | 0.8 | 0.5 | 18.9 | 23025.8 | 74.6 |
| Mg | 601.1 (IG64858) |
1765.4 (IG65074) |
1027.5 | 10.4** | 0.9 | 0.5 | 24.3 | 33138.6 | 69.6 |
| Mn | 0.1 (IG117018) |
30.0 (ACC1916) |
6.3 | 21.5** | 0.9 | 0.6 | 122.5 | 37.5 | 9.6 |
| Fe | 14.4 (ACC1330) |
57.1 (IG117012) |
30.1 | 75.3** | 0.9 | 0.6 | 27.0 | 43.6 | 6.0 |
| Zn | 20.3 (IG65147) |
48.7 (ACC1348) |
33.4 | 48.4** | 0.9 | 0.2 | 18.0 | 8.3 | 8.39 |
| Se | 0.01 (IG65053) |
0.6 (IG62145) |
0.1 | 66.3** | 0.9 | 0.7 | 77.2 | 0.02 | 0.1 |
| ODAP% | 0.01 (IG65277) |
0.3 (IG65340) |
0.1 | 2.5** | 0.7 | 0.5 | 66.0 | 38.6 | 5.0 |
| PC1 | PC2 | PC3 | PC4 | |
|---|---|---|---|---|
| P | 0.044 | 0.630 | -0.014 | 0.335 |
| K | 0.052 | 0.753 | -0.033 | -0.068 |
| Ca | 0.055 | -0.250 | 0.678 | 0.177 |
| Mg | 0.599 | -0.191 | 0.187 | 0.524 |
| Mn | 0.734 | 0.003 | 0.178 | -0.141 |
| Fe | 0.646 | 0.252 | -0.246 | -0.134 |
| Zn | -0.019 | -0.156 | 0.373 | -0.416 |
| Se | 0.038 | 0.391 | 0.717 | -0.078 |
| ODAP % | -0.242 | 0.075 | 0.104 | 0.706 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





