1. Introduction
Chronic venous disease (CVD) is a globally prevalent condition, with risk factors including genetic predisposition, incompetent valves, obesity, and prolonged standing. The condition's progression involves venous reflux in the lower extremities, leading to a range of clinical manifestations [
1,
2]. CVD is a highly prevalent, globally widespread disease, affecting 60-70% of the population, with higher prevalence in Western countries [
3]. The exact pathophysiology is debated, but it involves genetic predisposition, incompetent valves, weakened vascular walls, and increased intravenous pressure [
4]. Risk factors include a family history of venous disease, female sex, older age, body mass index (BMI) >30 kg/m², chronically increased intra-abdominal pressure due to obesity, pregnancy, chronic constipation, or a tumor, hypothyroidism, and other hormonal factors [
5,
6], as well as a history of deep vein thrombosis (DVT) and prolonged standing [
7]. Depending on the topography of the venous reflux and the stage of the disease, clinical manifestations of CVD can range from mild to severe, including telangiectasia, varicose veins, thrombosis, dull aching, cramping or heavy feeling in the legs, tingling sensation in the legs, edema, and pain, which worsens in an orthostatic posture and improves in a clinostatic position, especially when the legs are slightly elevated [
8]. Without proper treatment, CVD progresses to skin changes such as lipodermatosclerosis, with subsequent venous leg ulceration invariably occurring, leading to a significant negative impact on patients' quality of life [
9]. Venous aneurysms, although less common than arterial aneurysms, represent a significant clinical concern due to their potential to cause thromboembolic events. The prevalence of venous aneurysms in patients with chronic venous insufficiency varies, with studies reporting rates between 0.1% to 0.5%. Their impact on patient outcomes, including increased risk of thrombosis and more severe clinical presentations, highlights the importance of early identification and management [
4,
10].
Valvular insufficiency leads to venous reflux in the lower extremities, which is a manifestation of a degenerative process in the venous wall and supporting fascial structures that progressively dilate over time after exposure to high physiological pressures [
10,
11]. As venous reflux progresses, varicose dilatations of the veins occur. The diameter of a healthy great saphenous vein (GSV) usually ranges from 2.5 to 6 mm [
12], depending on factors such as gender, age, height, weight, daily activities, and work. When the venous valves become insufficient, the diameter of the vein slowly increases. An aneurysm can be defined as a focal dilation that is at least two or three times larger than the diameter of the venous trunk. Primary venous aneurysms are focal dilations that are not contained within a varicose vein, making them a relatively rare abnormality [
13]. On the other hand, fusiform dilatations that can appear along the trajectory of a varicose vein are more frequent [
14]. In clinical practice, these morphological peculiarities resulting from GSV degeneration are also considered superficial venous system aneurysms.
Treatment of venous reflux in the lower extremities ranges from conservative methods to interventional procedures, with ablation therapies being central to curative treatment. Conservative treatment options include external compression, lifestyle modifications such as avoiding prolonged standing and straining, exercising, wearing non-restrictive clothing, modifying cardiovascular risk factors, and interventions to reduce peripheral edema; elevating the affected leg; weight loss; and medication, with diosmin being the most commonly used phlebotonic worldwide [
4,
15]. However, curative treatment necessarily involves venous reflux ablation [
16]. Interventional treatments include external laser thermal ablation, endovenous thermal ablation (endovenous laser or radiofrequency ablation), microwave or mechano-chemical ablation, VenaSeal (cyanoacrylate adhesive closure), foam sclerotherapy, and surgery [
17,
18,
19,
20]. Although surgery was once the standard of care, it has largely been replaced by endo-venous thermal ablation, which can be performed under local anesthesia and may offer better outcomes and fewer complications than other treatments. However, a large diameter of the GSV and/or the presence of aneurysms may be difficult to completely close through endovenous procedures [
21], especially considering the degenerative changes that occur in the venous wall in such cases. A targeted therapeutic approach based on the diameter of the insufficient vein is recommended [
22].
This article aims to compare the morphological changes associated with aneurysmal degeneration of the GSV and to correlate these changes with the clinical manifestations. By identifying key differences in the morphoanatomical characteristics between patients with and without venous aneurysms, this study seeks to enhance our understanding of how these changes influence disease progression and treatment outcomes, ultimately guiding more effective clinical management strategies.
2. Materials and Methods
2.1. Study Design and Patients
This study is a retrospective observational analysis focused on the morphological changes occurring in the incompetent great saphenous vein (GSV) and their comparison with clinical features, both with and without aneurysmal degeneration. The study was conducted at the Timis County Emergency Clinical Hospital, Romania, between January 2020 and July 2024. Patients diagnosed with chronic venous disease (CVD) during this period were considered for inclusion. The study cohort was divided into two groups:
Group A: This group consisted of 17 patients with GSV aneurysms, defined as a focal GSV dilation with a diameter ≥ 1.2 cm [
13]. No diffuse vein enlargement specimens were included. The threshold for defining venous aneurysms was selected based on clinical guidelines and existing literature, where a GSV localized dilation > 1.2 cm or greater is commonly considered aneurysmal degeneration [
2,
12,
13,
23]. Despite the fact that insufficient veins diameter can be larger than even 1.5cm, a diffuse dilation contained in a varicose vein is not considered to be an aneurysm.
The value of >1.2cm has been used in multiple studies as a marker for clinically significant venous dilation, distinguishing it from simple varicose veins. Although variations exist in the literature, ranging from 1.0 cm to 1.5 cm depending on the study population and methodology [
24], the chosen threshold of 1.2 cm reflects a balance between sensitivity and specificity for detecting aneurysmal changes in the GSV.
Group B: This control group included 58 patients with saphenous vein degeneration but without aneurysms, characterized by a GSV diameter between 0.6 cm and 1.2 cm. This range was chosen to represent significant venous insufficiency without aneurysmal change, as these values are typical in cases of saphenous vein reflux without aneurysm formation.
A total of 75 patients were enrolled in the study after applying the inclusion and exclusion criteria. Inclusion Criteria:
Clinical Diagnosis: They had a clinical diagnosis of chronic venous insufficiency (CVI) confirmed by duplex ultrasound examination, which demonstrated significant venous reflux or saphenous vein dilation.
Surgical Treatment: They underwent open surgical treatment, such as conventional stripping or cryostripping, for saphenous vein insufficiency due to failure of conservative treatments like compression therapy or venotonics. This selection ensured that the study included patients with more advanced stages of CVI, warranting surgical intervention.
Informed Consent: Patients provided informed consent for the use of their clinical and pathological data, ensuring ethical compliance and patient willingness to participate in the study.
Exclusion Criteria: Patients were excluded if they presented any of the following conditions:
Previous Venous Surgery: A history of prior venous surgery on the affected leg, which could alter the anatomical and pathological findings and potentially confound the study results.
Non-compliance: Demonstrated non-compliance with follow-up visits, which could lead to incomplete data collection and impact the reliability of the findings.
Severe Systemic Diseases: Concurrent severe systemic diseases that could independently influence venous pathology: Advanced Heart Failure (NYHA Class III-IV); Chronic Kidney Disease (Stage 4-5), Active Malignancies (especially those in the pelvic or abdominal regions, can compress or invade venous structures, leading to secondary venous insufficiency or obstruction), Severe Peripheral Arterial Disease (PAD); Diabetes Mellitus with poor glycemic control; Severe Liver Disease (Cirrhosis with Portal Hypertension); Chronic Inflammatory Diseases (e.g., Rheumatoid Arthritis, Systemic Lupus Erythematosus); Severe Obesity (BMI > 40 kg/m²).
Alternative Treatments: Patients who were treated exclusively with less invasive methods, such as phlebectomies, endovenous laser therapy, or other conservative approaches, as these treatments might not provide comparable histopathological samples or data.
The small sample size of Group A (n=17) limits the statistical power of the study and may affect the reliability of the results. Larger sample sizes are needed to confirm these findings in future studies. The sample size was constrained by the strict inclusion criteria and the relatively low incidence of aneurysmal degeneration in the studied population. Future studies with larger cohorts are required to validate these results and to provide more robust conclusions regarding the clinical implications of aneurysmal degeneration in saphenous vein insufficiency.
2.2. Clinical and Ultrasound Evaluation
All patients underwent a comprehensive clinical evaluation, including the assessment of venous reflux using Doppler ultrasonography. The examinations were performed using a GE Healthcare Venue40 Doppler ultrasound with a 12L-SC transducer. Reflux was confirmed at the saphenofemoral junction using the Valsalva maneuver in an orthostatic position. The ultrasound evaluations, conducted by the same experienced physician, documented the saphenous vein diameter, wall thickness, and the presence of intraluminal thrombus.
2.3. Histopathological Examination
A total of 75 great saphenous veins (GSV) were analyzed in the Pathology Department. The specimens were fixed in a 10% buffered formalin solution and processed in the Pathology Laboratory. For each specimen, 2-3 cross-sections were taken from areas showing maximum wall thickness, including any existing thrombus. The specimens were manually processed through dehydration in alcoholic solutions, clearing, paraffin embedding, and sectioning at a thickness of 4 micrometers. Two consecutive sections were obtained for each case. These sections were stained using standard protocols with Hematoxylin-Eosin and Masson’s trichrome stains [
25]. Histopathological analysis of the specimens was performed using standardized grading systems to assess intimal hyperplasia, medial fibrosis, disorganization of muscle bundles, and elastic fiber fragmentation [
26]. Each parameter was evaluated with a score from 0 to 3, where 0 indicated no pathologic changes, 1 represented mild changes, 2 moderate changes, and 3 severe changes.
Evaluation of Elastic Fiber Fragmentation:
Elastic fiber fragmentation was quantified as the percentage of discontinuity in the tunica media observed in at least three high-power fields. A semiquantitative scoring system was used, where:
0: No fragmentation or minimal fragmentation (< 10% of the fiber length).
1: Mild fragmentation (10-25% of the fiber length).
2: Moderate fragmentation (25-50% of the fiber length).
3: Severe fragmentation (> 50% of the fiber length).
Evaluation of Disorganized Muscle Bundles:
Disorganization of muscle bundles was evaluated based on the percentage of disorganized areas relative to the total visual field in at least three high-power fields:
0: No disorganization (< 10% of visual fields examined).
1: Mild disorganization (10-25% of visual fields examined).
2: Moderate disorganization (25-50% of visual fields examined).
3: Severe disorganization (> 50% of visual fields examined).
Two independent pathologists, blinded to the clinical data and group allocation, reviewed all histopathological slides. In cases of disagreement, a consensus was reached through joint re-evaluation. This approach ensured objective and unbiased interpretation of the histological findings. The grading of intimal hyperplasia and medial fibrosis was performed based on established criteria [
13], while elastic fiber fragmentation and disorganization of muscle bundles were quantified as described above.
For the purpose of this study, only scores of 2 and 3 were considered significant and included in the final analysis to highlight moderate to severe pathologic alterations. This approach was chosen to emphasize clinically relevant alterations that are more likely to affect the structural integrity and function of the great saphenous vein. By focusing on these higher scores, the study aims to provide a clearer distinction between normal venous structures and those with more pronounced degenerative changes, which may have greater clinical implications.
Data Collection. The collected data included demographic and clinical characteristics (age, gender, BMI, CVD stage based on the CEAP classification [
27]), as well as morphological details (lumen status, maximum venous diameter, wall thickness, inti-mal and medial thickening, muscle bundle disorganization, and elastic fiber fragmentation).
2.4. Statistical Analysis
Statistical analysis was conducted using MedCalc® Statistical Software version 20.118 (MedCalc Software Ltd, Ostend, Belgium;
https://www.medcalc.org; 2022). For continuous variables, independent t-tests were applied with adjustments for unequal variances when necessary. If assumptions for the t-test were not met, the Mann-Whitney U test was used as a non-parametric alternative. For categorical variables, chi-square tests were used when cell frequencies allowed; otherwise, Fisher's ex-act test was employed. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Patient Demographics and Clinical Characteristics
The study included a total of 75 patients diagnosed with chronic venous disease (CVD), subdivided into two groups: 17 patients with saphenous vein degeneration and venous aneurysms (Group A) and 58 patients with saphenous vein degeneration but without aneurysms (Group B). The mean age of the patients was 53.8 ± 8.1 years in Group A and 55.3 ± 8.4 years in Group B, with no significant difference between the groups (p = 0.526). Gender distribution was similar in both groups, with a slight predominance of females in both (Group A: 59% female, Group B: 61% female, p = 0.280). The mean body mass index (BMI) was significantly higher in Group A (31.4 ± 4.5 kg/m²) compared to Group B (27.4 ± 4.0 kg/m²), with a statistically significant difference between the groups (p = 0.007). The t-test was used to compare continuous variables (age and BMI), while the chi-square test was used for categorical variables (gender).
3.2. Ultrasound Findings
Ultrasound examination revealed significant differences between the two groups in terms of saphenous vein diameter, wall thickness, and the presence of intraluminal thrombus. The mean saphenous vein diameter in Group A was 1.4 ± 0.2 cm, significantly larger than in Group B, where the mean diameter was 0.7 ± 0.1 cm (p < 0.001). Similarly, the wall thickness was greater in Group A (0.4 ± 0.1 cm) compared to Group B (0.2 ± 0.1 cm), with a significant difference (p < 0.001). The presence of intraluminal thrombus was observed in 65% of patients in Group A compared to 22% in Group B. However, with a p-value of 0.808, this difference is not statistically significant, indicating that it may be due to random variability. The presence of venous reflux, confirmed by Doppler ultrasound, was observed in 88% of Group A and 84% of Group B, with no significant difference between the groups (p > 0.05). While venous reflux is a common feature in both groups, its presence without significant diameter increase suggests that reflux alone is not sufficient for aneurysm formation, but rather a combination of factors, including venous wall degeneration and structural changes, is required.
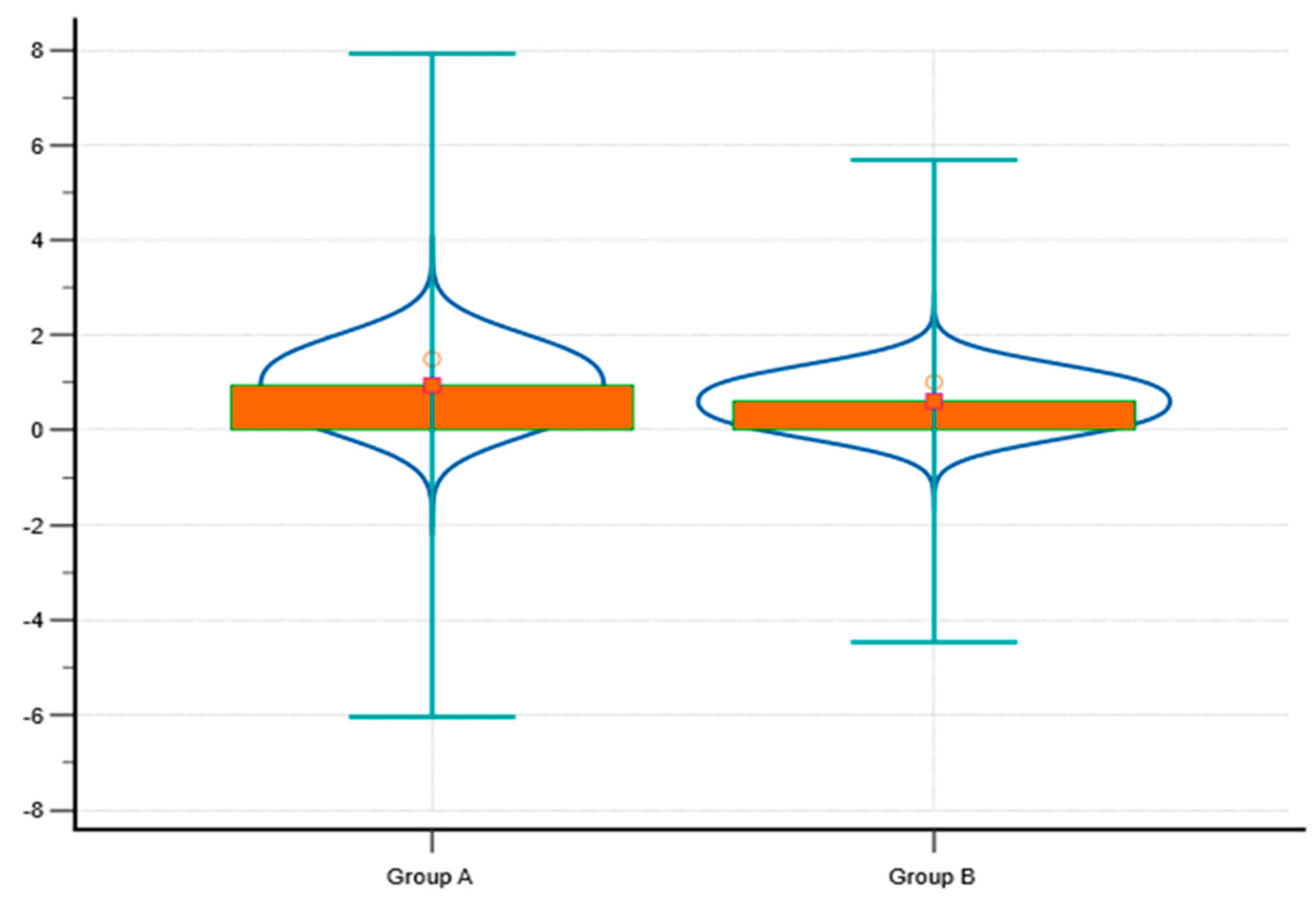
To further illustrate the differences between Group A and Group B in terms of saphenous vein diameter and wall thickness, a violin plot combined with a box plot has been generated (
Figure 1). This graph visually represents the distribution of values within each group, allowing for a detailed comparison of central tendencies and variability. Group A shows a higher median and broader distribution for saphenous vein diameter compared to Group B, reflecting the larger average vein diameter observed in this group (1.4 ± 0.2 cm vs. 0.7 ± 0.1 cm). The increased wall thickness in Group A is also reflected in the graph, with a higher median and greater variability compared to Group B, aligning with the association between larger vein diameters, greater wall thickness, and more advanced venous disease.
3.3. Histopathological Findings
Histopathological analysis revealed significant structural differences between Group A and Group B (
Table 1). The histopathological analysis showed that patients with larger saphenous vein diameters (Group A) exhibited significantly more severe morpho-anatomical disruptions than those with smaller diameters (Group B). Notably, 55% of Group A presented with severe elastic fiber fragmentation compared to 29% in Group B (p < 0.01). Disorganized muscle bundles were also more prominent in Group A (55%) compared to Group B (42%, p < 0.0001). These findings indicate that while it might be expected for more dilated veins to show some degree of structural disruption, the degree and nature of these disruptions, such as pronounced elastic fiber fragmentation and severe disorganization of muscle bundles, suggest a distinct and more advanced pathophysiological process in aneurysmal veins. This indicates a higher degree of structural disruption in the venous wall in Group A, suggesting a more advanced degenerative process in patients with venous aneurysms. Fragmentation of elastic fibers in the tunica media was markedly more severe in Group A, with 55% of cases showing this feature, compared to 29% in Group B (p < 0.01). This fragmentation highlights the loss of elasticity and structural support in the venous wall, contributing to aneurysm formation in Group A.
3.4. Clinical Outcomes
Patients in Group A exhibited more severe clinical outcomes as reflected by a longer duration of hospitalization and higher complication rates. The mean duration of hospitalization was significantly longer in Group A, averaging 2.1 ± 0.6 days, com-pared to 1.5 ± 0.6 days in Group B (p = 0.0023). This suggests that the presence of venous aneurysms is associated with a more complex and severe disease course, necessitating extended medical care and monitoring. Complications such as hematoma and ecchymosis were more frequently observed in Group A, with hematoma occurring in 18% of patients and ecchymosis in 24%, compared to 8% and 10%, respectively, in Group B. Although these differences in complication rates were not statistically significant (p > 0.05), the trend suggests a greater clinical burden in patients with venous aneurysms.
Although the comparison of CEAP categories using Fisher’s exact test did not yield statistically significant results (p-values > 0.24), a trend toward higher CEAP values was observed in patients with larger saphenous veins, particularly with a higher proportion of C3 cases in Group A (53%) and fewer C4 cases compared to C2. The relatively lower number of C4 cases likely contributed to the lack of statistical significance. Nonetheless, this trend aligns with the hypothesis that patients with larger saphenous veins tend to present with more advanced stages of chronic venous disease. This underscores the need for further studies with larger sample sizes to confirm this potential relationship and its clinical implications.
4. Discussion
This study highlights significant differences in morpho-anatomic features and clinical outcomes between patients with saphenous vein degeneration with and with-out venous aneurysms. The findings suggest that the presence of venous aneurysms is associated with more severe structural degeneration and poorer clinical outcomes, re-quiring a more tailored therapeutic approach [
28].
Although it could be assumed that more dilated veins would naturally exhibit disrupted morphology, our study provides histopathological evidence that highlights the advanced stage of venous wall degeneration in patients with larger saphenous vein diameters. Specifically, the fragmentation of elastic fibers and disorganization of muscle bundles seen in these patients is not merely an extension of venous insufficiency but represents a distinct progression toward aneurysmal degeneration. This finding underscores the importance of recognizing these advanced structural changes, as they may require more aggressive therapeutic approaches. The detailed nature of these morpho-anatomical disruptions—such as the degree of elastic fiber fragmentation and the severity of muscle disorganization—adds a new layer of understanding to the management of patients with venous aneurysms, emphasizing the need for targeted treatment strategies [
29].
The morphoanatomical changes observed in the group with venous aneurysms are likely driven by a combination of hemodynamic stress and structural remodeling of the venous wall. Several studies have highlighted the role of increased wall tension and mechanical stress in the progression of venous dilation and aneurysm formation. For instance, it has been well-documented that the degradation of extracellular matrix components, particularly elastin and collagen, plays an important role in weakening the venous wall structure. This degradation is largely mediated by the overexpression of matrix metalloproteinases (MMPs), particularly MMP-2 and MMP-9, which are known to degrade elastin and other structural proteins within the venous wall [
30].
The study has shown that venous aneurysms, especially in the great saphenous vein, are characterized by severe degeneration of the venous wall, which is an essential factor in their formation. For example, Aggarwal V. emphasizes the role of reduced elastin and increased fibrosis in the progression of venous aneurysms [
31]. This study emphasizes the molecular basis of venous wall degeneration, highlighting in particular the disruption of the balance between MMPs and their inhibitors, which contribute to the weakening of the venous structure [
32,
33]. In addition, another publication discusses how venous aneurysms are not simply passive vein widenings but are active pathologic entities involving complex cellular and molecular changes that lead to significant structural damage, making these veins prone to further complications and requiring more aggressive clinical interventions [
34].These structural changes not only underscore the need for personalized treatment strategies, but also suggest that patients with such advanced morphological changes are at higher risk of poor clinical outcomes. Consequently, these findings advocate for a more aggressive therapeutic approach in the management of patients with venous aneurysms, which may involve earlier surgical intervention and closer postoperative monitoring to prevent complications associated with these severe changes in the venous wall. Detailed understanding of these mechanisms may provide better treatment protocols and improve the prognosis of patients with saphenous vein aneurysms [
35].
The significant differences in saphenous vein diameter and wall thickness between the two groups, as detected by ultrasound, further emphasize the utility of imaging in identifying patients at higher risk of complications. The correlation between these imaging findings and histopathological changes reinforces the importance of a multi-modal assessment in the management of chronic venous disease (CVD). The presence of intraluminal thrombus in a higher percentage of patients with aneurysms also underscores the thrombogenic potential of aneurysmal veins. This finding is consistent with existing studies that suggest an increased risk of thromboembolic events in patients with venous aneurysms due to altered blood flow dynamics and stasis within the dilated segments of the vein [
36].
The more severe clinical outcomes observed in patients with venous aneurysms (Group A), including prolonged hospitalization and increased complication rates, underscore the substantial clinical burden these patients bear. Although the differences in complication rates between Group A and Group B were not statistically significant, the trend suggests that venous aneurysms contribute to a more complex and severe clinical course, necessitating more intensive postoperative care and closer monitoring to pre-vent complications. Although the CEAP classification comparison did not reach statistical significance (p-values > 0.24), a noticeable trend was observed, with Group A (patients with larger saphenous veins) showing more advanced CEAP stages, particularly C3. The lower number of C4 cases in both groups likely contributed to the lack of statistical significance. Nevertheless, the observed correlation between increased saphenous vein diameter and more advanced disease stages is clinically relevant. This trend, consistent with prior research, emphasizes the need for further investigation with larger cohorts to validate these findings and explore their potential clinical implications [
37]. The presence of venous aneurysms, as an indicator of severe structural degeneration, suggests that these patients may experience faster disease progression and a greater risk of complications if not managed appropriately [
38].
Given these findings, there is a clear need for personalized treatment strategies in patients with significant venous wall degeneration. Less invasive procedures, such as endovenous laser ablation (EVLA) and radiofrequency ablation (RFA), may be less effective in patients with large aneurysmal veins due to the difficulty in achieving complete closure. In such cases, open surgical approaches may be more effective in reducing the risk of complications and preventing disease recurrence. The success of these treatments is closely related to the diameter and condition of the vein, with larger and more degenerative veins presenting a higher risk of recanalization and treatment failure [
39,
40].
The clinical implications of these findings are significant. Patients with venous aneurysms not only exhibit greater structural damage in the venous wall but also pre-sent with more severe clinical outcomes, such as increased body mass index (BMI) and longer hospital stays. This suggests that the presence of venous aneurysms could be an indicator of more advanced disease, requiring closer monitoring and potentially more aggressive therapeutic interventions. The association between venous aneurysms and higher rates of complications, such as thrombosis, highlights the need for early detection and tailored treatment strategies to prevent further progression of the disease and improve patient outcomes.
Finally, while this study provides important insights, several limitations should be acknowledged. The retrospective design limits the ability to establish causality, and the relatively small sample size may restrict the generalizability of the findings. Additionally, the lack of long-term follow-up data precludes the assessment of the durability of treatment outcomes. Future research should focus on prospective studies with larger cohorts and longer follow-up periods to validate these findings and refine the management strategies for patients with venous aneurysms. Further investigation into the molecular mechanisms driving elastic fiber fragmentation and other degenerative changes in the venous wall could also lead to the development of targeted therapies aimed at preventing or slowing the progression of venous aneurysms in CVD patients [
41].
In conclusion, the results of this study underscore the need for a nuanced approach to the management of CVD, particularly in patients with advanced structural degeneration of the saphenous vein. By better understanding the morphological and clinical differences between patients with and without venous aneurysms, clinicians can improve patient outcomes through more tailored and effective treatment strategies.
5. Conclusions
The findings from this study suggest that saphenous vein degeneration with associated venous aneurysms may lead to more pronounced morphoanatomical changes and more severe clinical outcomes. These observations indicate that patients with such conditions could benefit from closer monitoring and potentially more aggressive treatment approaches. However, given the small sample size and the retrospective nature of this study, these conclusions should be viewed as preliminary. Further research with larger, prospective studies is necessary to validate these findings and to develop more definitive clinical management guidelines for patients with saphenous vein degeneration and venous aneurysms.
Author Contributions
Conceptualization, D.G.S. and R.P.; methodology, D.G.S.; software, R.P.; validation, D.G.S. and R.P.; formal analysis, D.G.S. and R.P.; investigation, D.G.S.; resources, D.G.S.; data curation, R.P.; writing—original draft preparation, D.G.S. and R.P.; writing—review and editing, D.G.S. and R.P.; visualization, D.G.S.; supervision, D.G.S and R.P.; project administration, R.P. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to acknowledge VICTOR BABES UNIVERSITY OF MEDICINE AND PHARMACY TIMISOARA for their support in covering the costs of publication for this research paper.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Timis County Emergency Clinical Hospital 390/20.04.2023.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Matei, S.-C.; Dumitru, C.Ș.; Oprițoiu, A.-I.; Marian, L.; Murariu, M.-S.; Olariu, S. Female Gonadal Venous Insufficiency in a Clinical Presentation Which Suggested an Acute Abdomen—A Case Report and Literature Review. Medicina 2023, 59, 884. [CrossRef]
- Munteanu, K.; Ghirlea, O.; Breban-Schwarzkopf, D.; Dănilă, A.-I.; Iacob, R.-G.; Petrache, I.A.; Cozma, G.V.; Bordianu, A.; Bolintineanu, S.L. The Great Saphenous Vein Proximal Part: Branches, Anatomical Variations, and Their Implications for Clinical Practice and Venous Reflux Surgery. J. Cardiovasc. Dev. Dis. 2024, 11, 242. [CrossRef]
- Matei, S.C.; Matei, M.; Anghel, F.M.; Carabenciov, E.; Murariu, M.S.; Olariu, S. Utility of routine laboratory tests in the as-sessmentof chronic venous disease progression in female patients. Exp. Ther. Med. 2022, 24, 571. [CrossRef]
- Raetz, J.; Wilson, M.; Collins, K. Varicose Veins: Diagnosis and Treatment. Am Fam Physician. 2019, 99, 682-688.
- Ulloa, Dominguez, J.; Lopez, P.F.; Ulloa, J.H.; Salazar, J.; Penagos, A. Prevalence of hypothyroidism in adults with chronic venous insufficiency. Acta Phlebol 2018, 19, 112-4. [CrossRef]
- Matei, M.; Vlad, M.M.; Golu, I.; Dumitru, C.Ș.; De Scisciolo, G.; Matei, S.C. Can Routine Laboratory Tests Be Suggestive in Determining Suspicions of Malignancy in the Case of Thyroid Nodules?. Medicina (Kaunas) 2023, 59, 1488. [CrossRef]
- Taengsakul, N. Risk Factors for and Treatment of Chronic Venous Disease in Thai Patients. Vasc Health Risk Manag 2022, 18, 667-676. [CrossRef]
- Matei, S.-C.; Dumitru, C.Ș.; Radu, D. Measuring the Quality of Life in Patients with Chronic Venous Disease before and Short Term after Surgical Treatment—A Comparison between Different Open Surgical Procedures. J. Clin. Med. 2022, 11, 7171. [CrossRef]
- Matei, S.-C.; Dumitru, C.S.; Fakhry, A.M.; Ilijevski, N.; Pešić, S.; Petrović, J.; Crăiniceanu, Z.P.; Murariu, M.-S.; Olariu, S. Bacterial Species Involved in Venous Leg Ulcer Infections and Their Sensitivity to Antibiotherapy—An Alarm Signal Regarding the Seriousness of Chronic Venous Insufficiency C6 Stage and Its Need for Prompt Treatment. Microorganisms 2024, 12, 472. [CrossRef]
- Baliyan, V.; Tajmir, S.; Hedgire, S.S.; Ganguli, S.; Prabhakar, A.M. Lower extremity venous reflux. Cardiovasc Diagn Ther. 2016, 6, 533-543. [CrossRef]
- Matei, S.C.; Matei, M.; Anghel, F.M.; Derban, M.D.; Olariu, A.; Olariu, S. Impact of statin treatment on patients diagnosed with chronic venous disease. Morphological analysis of the venous wall and clinical implications. Phlebology. 2022, 37, 188-195. [CrossRef]
- Bendelic, A.; Catereniuc, I. Vena saphena magna – peculiarities of origin, trajectory and drainage. Mold Med J. 2020, 63, 26-31. [CrossRef]
- Matei, S.C.; Matei, M.; Anghel, F.M.; Olariu, A.; Olariu, S. Great saphenous vein giant aneurysm. Acta Phlebol. 2022, 23, 87–92. [CrossRef]
- Pascarella, L.; Al-Tuwaijri, M.; Bergan, J.J.; Mekenas, L.M. Lower extremity superficial venous aneurysms. Ann Vasc Surg. 2005, 19, 69-73. [CrossRef]
- Cazaubon, M.; Benigni, J.P.; Steinbruch, M.; Jabbour, V.; Gouhier-Kodas, C. Is There a Difference in the Clinical Efficacy of Diosmin and Micronized Purified Flavonoid Fraction for the Treatment of Chronic Venous Disorders? Review of Available Evidence. Vasc Health Risk Manag. 2021, 17, 591-600. [CrossRef]
- Matei, S.C.; Radu-Teodorescu, D.; Murariu, M.S.; Dumitru, C.Ș.; Olariu, S. Cryostripping Versus Conventional Safenectomy in Chronic Venous Disease Treatment: A Single Center Retrospective Cohort Study. Chirurgia (Bucur). 2024, 119, 56-64. [CrossRef]
- Matei, S.C.; Radu-Teodorescu, D. 12th Balkan Venous Forum: New insights in venous disease treatment. Acta Phlebol. 2024, 25, 49. [CrossRef]
- Zhang, J.; Lin, Y.; Zhang, L.; Geng, C.; Huang, W.; Yang, Q.; Zeng, W.; He, C. Comparison of one-year outcomes and quality of life between radiofrequency ablation and microwave ablation in the treatment of lower extremity varicose veins: A retrospec-tive cohort study. Phlebology. 2024. [CrossRef]
- Gibson, K.; Ferris, B. Cyanoacrylate closure of incompetent great, small and accessory saphenous veins without the use of post-procedure compression: Initial outcomes of a post-market evaluation of the VenaSeal System (the WAVES Study). Vascular, 2017, 25, 149–156. [CrossRef]
- Matei, S.C.; Matei, M.; Anghel, F.M.; Murariu, M.S.; Olariu, S. Cryostripping-A Safe and Efficient Alternative Procedure in Chronic Venous Disease Treatment. J Clin Med. 2022, 11, 5028. [CrossRef]
- Starodubtsev, V. Lukyanenko M, Karpenko A, Ignatenko P. Endovenous laser ablation in patients with wide diameter of the proximal segment of the great saphenous vein: Comparison of methods. Phlebology. 2015, 30, 700-705. [CrossRef]
- Chernukha, L.; Voloshyn, O.; Suzdalenko, O.; Gubka, V.; Machuskui, S.; Pavlychenko, V. Comparison of staged versus one shot varicose veins treatment: Depending on tributaries diameter. Phlebology, 2024. [CrossRef]
- De Maeseneer, Marianne G. ESVS Guidelines Committee, Document Reviewers, et al. European Journal of Vascular and Endovascular Surgery. European Society for Vascular Surgery (ESVS) 2022 Clinical Practice Guidelines on the Management of Chronic Venous Disease of the Lower Limbs., Volume 63, Issue 2, 184 – 267.
- Alam, M.; Bandeali, S.J.; Virani, S.S.; Jneid, H.M.; Shahzad, S.A.; Ramanathan, K.B.; Kar, B.; Kleiman, N.S.; Lakkis, N. Clinical outcomes of percutaneous interventions in saphenous vein grafts using drug-eluting stents compared to bare-metal stents: a comprehensive meta-analysisof all randomized clinical trials. Clinical cardiology, 2012, 35, 291–296. [CrossRef]
- Dumitru, C.S.; Ceausu, A.R.; Gaje, N.P.; Suciu, C.S.; Raica, M. Proliferating Lymphatic Endothelial Cells as a Prognostic Marker in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 9793. [CrossRef]
- Déglise, S.; Bechelli, C.; Allagnat, F. Vascular smooth muscle cells in intimal hyperplasia, an update. Frontiers in physiology, 2023, 13, 1081881. [CrossRef]
- Zegarra, T.I.; Tadi, P. CEAP classification of venous disorders. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Gensicke, N.; Nicholson, R.; Sharp, W. Lower extremity aneurysmal degeneration of great saphenous venous allograft bypass in an adolescent boy. J Vasc Surg Cases Innov Tech. 2021, 8, 5-8. [CrossRef]
- Choi, J.Y., Lee, J.H., Kwon, O.J. Association between the saphenous vein diameter and venous reflux on computed tomography venography in patients with varicose veins. PloS one, 2022, 17, e0263513. [CrossRef]
- Katoh, K. Effects of Mechanical Stress on Endothelial Cells In Situ and In Vitro. Int J Mol Sci. 2023, 24, 16518. [CrossRef]
- Aggarwal, V. Pathogenesis and management of superficial venous aneurysms through a case of thrombosed large great sa-phenous vein aneurysm. Vascular, 2021, 29, 297–300. [CrossRef]
- Dumitru, C.S.; Raica, M. Vascular Endothelial Growth Factor Family and Head and Neck Squamous Cell Carcinoma. Anti-cancer research, 2023, 43, 4315–4326. [CrossRef]
- Dumitru, C.S.; Raica, M. A Splice Form of VEGF, a Potential Anti-Angiogenetic Form of Head and Neck Squamous Cell Cancer Inhibition. Int. J. Mol. Sci. 2024, 25, 8855. [CrossRef]
- Teter, K.A.; Maldonado, T.M., Adelman, M.A. A systematic review of venous aneurysms by anatomic location. Journal of vascular surgery. Venous and lymphatic disorders, 2018, 6, 408–413. [CrossRef]
- Ghosh, B.; Sibi, Krishna, T.; Boini, A.; Castillo, Miranda, J.C.D.; Sinha, M.; Bansal, R.; Visconti-Lopez, F.J.; Mesfin, Girma, S.; Aliye, Asfaw, Y. Risk factors associated with saphenous vein graft aneurysm after coronary artery bypass graft. Annals of medicine and surgery, 2012, 85, 5604–5610. [CrossRef]
- Bontekoe, J.; Matsumura, J.; Liu, B. Thrombosis in the pathogenesis of abdominal aortic aneurysm. JVS Vasc Sci. 2023, 4:100106. [CrossRef]
- Lessiani, G.; Gazzabin, L.; Cocco, G.; Corvino, A.; D'Ardes, D.; Boccatonda, A. Understanding CEAP Classification: Insights from an Italian Survey on Corona Phlebectatica and Recurrent Active Venous Ulcers by Vascular Specialists. Medicina (Kaunas). 2024, 60, 618. [CrossRef]
- Racman, M.; Kafol, J.; Jug, B.; Stankovic, M.; Piljic, D.; Ksela, J. Rapidly Growing and Ruptured Great Saphenous Vein An-eurysm in a Liver Transplant Patient. Medicina 2024, 60, 290. [CrossRef]
- Hamann, S.A.S.; Timmer-de Mik, L.; Fritschy, W.M.; Kuiters, G.R.R.; Nijsten, T.E.C.; van den Bos, R.R. Randomized clinical trial of endovenous laser ablation versus direct and indirect radiofrequency ablation for the treatment of great saphenous varicose veins. The British journal of surgery, 2019, 106, 998–1004. [CrossRef]
- Ivan, V.; Teodorescu, M.; Tilincă, M.; Radu, D.; Olariu, S.; Pop, S.; Icma, I.; Ivan, C.; Mogoşanu, M.; Puşcaşiu, T.; Banciu, D. O tehnică nouă de tratament a varicelor [ A new technique for the treatment of varicose veins]. Chirurgia (Bucur) 2001, 527-532.
- Stougiannou, T.M.; Christodoulou, K.C.; Georgakarakos, E.; Mikroulis, D.; Karangelis, D. Promising Novel Therapies in the Treatment of Aortic and Visceral Aneurysms. J Clin Med. 2023, 12, 5878. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).