1. Introduction
The hepatitis A virus (HAV) is an important cause of acute viral hepatitis globally, accounting for 159 million cases of acute hepatitis A, 39 300 deaths, and 2.35 million disability-adjusted life years in 2019 alone [
1,
2,
3]. The virus can be transmitted via the faecal-oral route through ingestion of contaminated food or water, or through close physical contact with an infectious person. Acute hepatitis A typically presents as a self-limiting illness characterized by fever, abdominal pain, nausea, vomiting, or diarrhoea. The disease can progress into inflammatory liver disease and fulminant liver failure in rare (0.015% – 0.5%) severe cases which can be fatal [
1,
4,
5]. Clinical outcomes are strongly correlated with age at infection; while infection in paediatric populations, particularly children under 6 years of age, are often (>90%) asymptomatic, the severity of disease and risk of fatality is increased (>70%) in older age groups [
1,
4,
5]. Improved hygiene and sanitation, food and water safety, and vaccination, are the mainstay of hepatitis A prevention and control [
1].
Low- and middle-income countries with poor sanitary conditions and access to safe drinking water are disproportionately impacted by HAV infection, experiencing 66% and 97% of the global total of cases and deaths, respectively [
1]. In endemic regions like parts of South Asia and sub-Saharan Africa, most children would have been exposed to the virus and developed lifelong immunity after recovery, characterized by ≥90% seroprevalence of immunoglobulin G (IgG) antibodies to the virus (anti-HAV) among the population by 10 years of age [
4,
6]. Emerging evidence suggests that South Africa is in transition from high to intermediate endemicity with anti-HAV seroprevalence reported to only reach >90% among adults ≥25 years of age [
6,
7,
8]. This is concerning, given that populations with intermediate endemicity experience higher incidence of severe clinical outcomes given the greater proportion of susceptible individuals who are at increased risk of symptomatic disease later in adulthood. While the World Health Organization recommends routine vaccination for children ≥12 months of age in countries with intermediate endemicity, the hepatitis A vaccine is currently not part of the South African Expanded Programme on Immunization schedule [
1,
9]. The decision to introduce the vaccine will have to be informed by cost considerations, prioritization of other non-pharmaceutical interventions (improving sanitary conditions, and food and water safety) versus vaccine introduction, and the risk and burden of disease, among other contextual factors. For this purpose, National Immunization Technical Advisory Groups will require robust research evidence including local epidemiological data among key risk groups in order to make expert vaccine policy recommendations.
The disease epidemiology in South Africa is characterized by localized and widespread community and institutional outbreaks with variations across provinces and socio- demographic and economic groups [
9,
10]. Passive laboratory-based surveillance data collected between 2017 and 2020 shows a national hepatitis A incidence rate (based on IgM anti-HAV positivity) of 6 – 10 cases / 100,000 per year among 1 – 9-year-olds, with the highest incidence rates recorded in the Western Cape province (7 – 10 /100,000) [
10]. In a previous nationwide seroprevalence study, it was reported that children 1 – 4 years of age comprised the highest proportion (33.5%) of acute HAV infections, with the highest positivity rates also recorded in the Western Cape [
7]. Further cross-sectional and hospital-based studies have documented the seroprevalence (44.1% anti-HAV positivity in 1 – 7-year-olds), and the occurrence and clinical outcomes (including acute liver failure and death) of symptomatic hepatitis A among children in the Western Cape, often compounded by underlying HIV infection [
8,
11,
12]. Evidence on the seroprevalence of hepatitis A among children living with HIV is scarce. Only one recent hospital-based study assessed the seroprevalence of hepatitis A among children and adolescents (1 – 15 years) living with or without HIV in the Gauteng province and found that while paediatric HIV infection and maternal HIV status were associated with a higher likelihood of testing positive for anti-HAV, these were not significant predictors of hepatitis A seropositivity [
13].
There is a need for further epidemiological data to improve our limited understanding of the seroprevalence of hepatitis A in the context of paediatric HIV infection. Owing to the success of one of the world’s largest HIV programs aimed at prevention of mother-to-child transmission (PMTCT), there is a growing population of HIV-exposed uninfected (HEU) children in South Africa. Of the 14.8 million HEU children in the world, 90% are from sub-Saharan Africa and 23.8% live in South Africa alone [
14]. Due to potential immune and metabolic alterations following prolonged exposure to maternal HIV infection and antiretroviral prophylaxis in the intrauterine environment, HEU children are reported to experience greater susceptibility and severity of common infectious causes of childhood morbidity and mortality compared to their HIV-unexposed uninfected (HUU) counterparts [
15,
16]. There is also evidence to suggest that HEU children may be at increased risk of other viral hepatitis infections [
17,
18,
19]. Despite this, no other studies have been conducted to better understand whether the seroprevalence of hepatitis A is comparable among HEU children and those living with or without HIV in South Africa. Such evidence gaps compromise rational vaccine decision-making and prioritization of targeted public health interventions. The aim of this study, therefore, is to determine the seroprevalence and risk factors of hepatitis A among children living with HIV, and HEU children, compared to their HUU counterparts.
2. Materials and Methods
2.1. Study Design and Population
We conducted a retrospective cross-sectional study to determine the seroprevalence of hepatitis A among a conveniently sampled population of sera obtained from children receiving in- or out- patient care between 2012 and 2016 from health facilities within the Western Cape province of South Africa. These participants were part of an overarching study which investigated the incidence of pertussis among children presenting with lower respiratory tract infections [
20]. Based on an expected hepatitis A seroprevalence of 44.1% among children in the Western Cape, a sample size of 162 gave a precision of 5% around the point estimate [
8,
21,
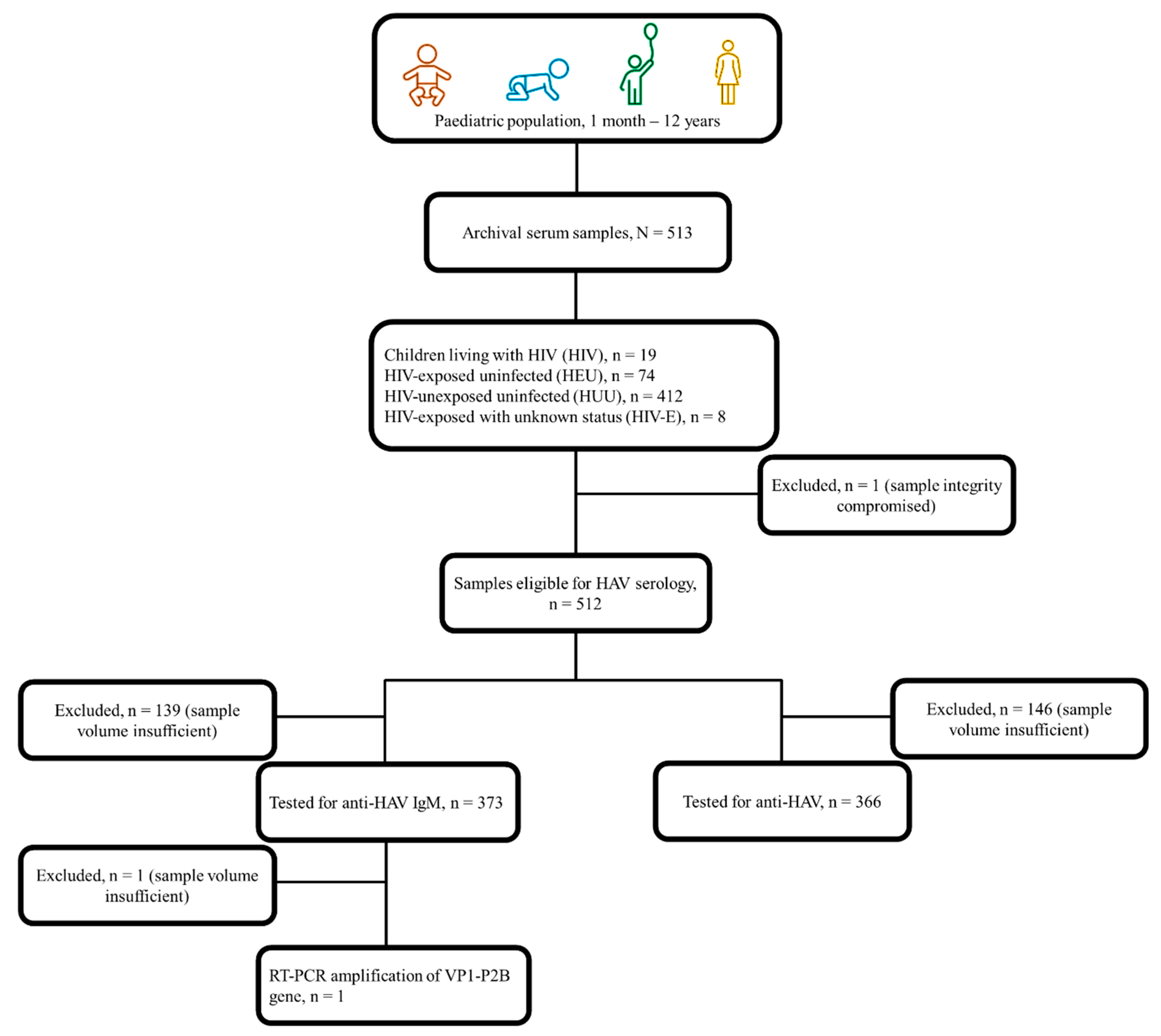
22]. A total of 513 archival sera was available for this study. In addition, participants’ demographic (age, sex, ethnicity), clinical (maternal and child HIV status, vaccination history), and socio-economic (maternal level of education, type of dwelling, access to safe drinking water and other basic amenities) records were assessed. Based on perinatal HIV exposure and infection status, participants were stratified into HIV, HEU, and HUU subgroups. A fourth group of HIV-exposed participants whose infection status was unknown (HIV-E), was also assessed (
Figure 1). Given the under-representation of HEU paediatric populations in research studies with this specific focus, this purposively sampled population presents a unique opportunity to better understand the burden of hepatitis A and need for tailored interventions in a relatively under-served but growing population in our setting.
2.2. Serological and Molecular Testing for Hepatitis A
To detect the presence of acute and past HAV infections, serum samples were tested in 2023 for anti-HAV IgM and total anti-HAV (IgM/IgG), respectively, using Elecsys® test kits (Roche Diagnostics, Germany). Serological assays were performed on the Cobas® 6000 Analyzer (Roche Diagnostics, Germany) which is a fully automated system used for heterogeneous immunoassays based on the electrochemiluminescence principle for qualitative and quantitative
in vitro determinations. One sample could not be tested for either marker as the Cobas® 6000 Analyzer detected an integrity issue (
Figure 1). In line with the manufacturer’s instructions, samples with a cut-off index (COI) ≥1.0 were considered reactive for anti-HAV IgM while the COI for a positive anti-HAV test was ≤1.0. As an exploratory objective, samples testing positive for IgM anti-HAV were further subjected to a reverse transcription-polymerase chain reaction (RT-PCR) to amplify the viral VP1-P2B gene fragment (393bp) using a set of external (2870P and 3381N) and internal (2896P and 3289N) primers as published previously [
23,
24]. Amplified products were confirmed against positive (supplied by the National Institute for Communicable Diseases of South Africa) and negative controls (PCR-grade H
2O, Roche Diagnostics, Mannheim, Germany) by gel electrophoresis run on a 1% ethidium bromide-stained agarose gel.
2.3. Data Analysis
All statistical analyses were conducted using R version 4.3.1 and R studio version 2023.09.1+494 [
25]. Data visualisations were conducted using Tableau software 2024 [
26]. Maternal and child ages are described using medians with interquartile ranges (IQR). Proportions are presented as percentages with 95% confidence intervals (CI). Subgroup comparisons or associations between dichotomous variables were determined using Chi-square or Fisher’s exact tests depending on the expected cell count. Kruskal-Wallis test was used to determine the differences between the medians of three or more independent groups as appropriate. Probable independent risk factors for past or existing HAV infections were determined by univariable logistic regression analysis. Associations are expressed as odds ratios (OR) with 95% CI. For missing data, we assumed that this occurred completely at random, as missingness did not depend on anything related to the research question and thus unlikely to bias our analysis. We therefore conducted a complete record analysis [
27].
3. Results
3.1. Characteristics of the Study Population
The demographic characteristics of the study population are presented in
Table 1. Among the 513 participants in this study, there was an overrepresentation of males (54.0%, n=277), children under the age of 1-year (53.8%, n=276), and children of Black African ethnicity (65.7%, n=337). Where crèche or pre-school attendance was concerned, 25.1% (n=129) of the study population, ranging in age from 2 months – 12 years had ever attended or were attending crèche at the time of participating in the overarching study. Most children (76.8%, n=394) had received mixed feeding (breast and formula fed) within the first 4 months of life. All mothers were screened for HIV infection in line with national antenatal guidelines and based on maternal clinical records, 19.7% (101/513) had been diagnosed with HIV, 50.5% (51/101) of whom reported being on antiretroviral therapy. When stratified by perinatal HIV exposure, most participants fell in the HUU (80.3%, n=412) subgroup (
Table 1). The distribution of demographic characteristics among the HIV-exposed strata (HIV, n=19; HEU, n=74, and HIV-E, n= 8) is shown in Supplementary
Table 1.
The median (IQR) maternal age was 29 (25 – 34) years with most mothers (87.3% [448/513]) being ≥22 years at the time of enrolment in the overarching study (
Table 2). The overwhelming majority of mothers (92.6% [475/513]) had received a secondary school education (grade 9 – 12) or higher (tertiary education including university) and this was comparable across the subgroups (
Table 2). Based on available self-reported asset ownership (including type of dwelling, access to basic amenities), employment, and education, the study population (N=513) could be categorized into low (20.3%, n=104), lower-middle (6.6%, n=34), upper-middle (48.1%, n=247), and high (12.5%, n=64) socio-economic quartiles. The HIV-exposed subgroup had a relatively higher proportion (25.7%, n=26) of mothers in the low socio-economic quartile. In terms of access to basic amenities and sanitation, 64.1% (264/412) of the HUU subgroup lived in brick housing while 66.4% (67/101) of their HIV-exposed counterparts, lived in informal dwellings made of wood or shacks, tin, zinc, or iron sheeting (Supplementary
Table 2). The highest proportion of those with access to indoor taps was found in the HUU subgroup (51.9% [214/412]) while majority of the overall study population (84.8% [435/513]) had access to flush toilets with limited use of pit latrines (13.1% [67/513]) or the bucket system (1.6% [8/513]) (
Table 2).
3.2. Prevalence of Hepatitis A
Of the 513 archival sera, 71.3% (n=366) and 72.7% (n=373) had sufficient volumes and could be tested for anti-HAV and anti-HAV IgM, respectively (
Figure 1). Overall, 63.5% (326/513) could be tested for both anti-HAV and anti-HAV IgM, while 12.6% (47/373) of those tested for anti-HAV IgM could not be tested for anti-HAV and 10.9% (40/366) of those tested for anti-HAV could not be tested or anti-HAV IgM. Of those tested, 56.6% (207/366 [95% CI 51.4-61.8]) were positive for anti-HAV while 0.5% (2/373 [95% CI 0.09-2.13]) had evidence of an acute HAV infection. Peak anti-HAV seropositivity (95.9%, 117/122 [95% CI 90.2-98.4]) was observed among those ≤6 months of age, suggestive of the rate of transplacental transfer of anti-HAV. Excluding <1-year-olds with possible persistence of passively transferred anti-HAV, seropositivity of anti-HAV and anti-HAV IgM in those 1 – 12 years of age was 19.3% (37/192 [95% CI 14.1-25.7]) and 1.1% (2/188 [95% CI 1.29-3.79]), respectively. Seroprevalence of hepatitis A declined with increasing age, reaching 40.0% (2/5 [95% CI 5.27-85.3]) by age 10 years and older (
Figure 2).
The two cases of acute HAV infection were detected in a 2- and 3-year-old who were in the HIV and HEU subgroups, respectively. Of these two cases, only one participant had sufficient serum for detection of viral RNA using RT-PCR. This participant was in the convalescent phase of infection (tested positive for both anti-HAV IgM and anti-HAV) characterized by low or undetectable viremia. Consequently, the RT-PCR was negative and the viral VP1-P2B gene fragment could not be amplified.
When comparing those 1 – 12 years of age with (HIV, HEU, and HIV-E subgroups) and without (HUU subgroup) perinatal HIV exposure, the seropositivity of anti-HAV was 51.6% (16/30) and 29.2% (47/161), respectively (p = 0.015). That for anti-HAV IgM was 6.7% (2/30) in the HIV-exposed subgroup with no cases of acute infection detected among HUU children. The seropositivity of anti-HAV across subgroups and age strata is presented in
Figure 3 and
Figure 4.
Anti-HAV seropositivity followed similar trends regardless of perinatal HIV exposure or infection status, declining after the first 6 months of life and peaking among those ≥5 years, but never reaching higher than 50% among those 10 years and older (
Figure 4). Among HIV-exposed children, anti-HAV seropositivity was comparably higher in the HIV (60%, 6/10 [95% CI 27.4-86.3]) than the HEU subgroups (45%, 9/20 [95% CI 23.8-68]).
3.3. Risk factors of Hepatitis A Seropositivity
We assessed potential independent risk factors of hepatitis A seropositivity among study participants who tested positive for at least one marker (anti-HAV and/or anti-HAV IgM). Overall, n=206 participants tested positive for anti-HAV alone, n=1 for both anti-HAV and anti-HAV IgM, and n=1 for anti-HAV IgM only. Thus n=208 participants were included in the logistic regression analysis (
Supplementary Table S3). Compared to <1 year-olds, children between the ages of 1 – 4 years had a lower likelihood of being seropositive with an OR of 0.23 [95% CI 0.14-0.36]. Further regression analysis did not identify additional significant associations with other socio- demographic or economic variables including maternal HIV exposure.
4. Discussion
This study reports evidence on the seroprevalence of hepatitis A among a paediatric population of HIV-exposed infected and uninfected children, compared to their HIV-unexposed uninfected counterparts in South Africa. Overall, age-specific trends in anti-HAV seropositivity appear to be comparable among HIV-exposed and HIV-unexposed children. Perinatal HIV exposure was not found to be an independent risk factor for hepatitis A seropositivity in early childhood. Our findings are consistent with others suggesting an intermediate hepatitis A endemicity (<90% anti-HAV positivity by age 10 years) in South Africa [
6,
7,
8]. The implication of this is that the level of natural immunity within the population remains insufficient to protect against symptomatic HAV infection and severe clinical outcomes later in adulthood without timely and appropriate public health intervention.
We found that among ≤6-month-olds, anti-HAV seropositivity was near universal at 95.1% and could serve as a proxy for the seroprevalence of hepatitis A among pregnant women and mothers in our study setting. The detection of anti-HAV among newborns is associated with transplacental transfer of antibodies which provide temporary protection against HAV infection [
26]. Concentrations of passively transferred maternal antibodies remain high during the first 6 months of life but wane significantly between months 7 – 12 [
26,
27,28,29]. This was also observed in our study, demonstrated by a decline in anti-HAV seropositivity among 7 – 11-month-old participants across all subgroups. Cases of acute HAV infection were only observed after the first year of life – which coincides with the decline in protective maternal anti-HAV – and only among children living with perinatal HIV exposure. In a hospital-based hepatitis A seroprevalence study conducted between 2018 and 2019, du Plessis et al. report an anti-HAV IgM seroprevalence of 2.62% among 1 – 15-year-olds [
13]. Although they do not disaggregate this seroprevalence rate between those living with and without HIV infection, it is lower than the 6.7% anti-HAV IgM seroprevalence identified in our HIV-exposed subgroup of 1 – 12-year-olds. With cases detected in 2- and 3-year-olds, our findings are also consistent with the peak age range (1 – 4 years) for acute HAV infection among children in South Africa [
7]. The public health significance of HAV-HIV co-infection lies in its association with a longer duration of HAV viremia and shedding in stool, increasing the likelihood of onward spread in settings with poor sanitation and hygiene practices [
4,30].
In a hospital-based study conducted in São Paulo, Brazil, assessing the prevalence of hepatitis A antibodies in children born to mothers living with HIV, Gouvêa et al. found an overall hepatitis A seroprevalence of 26%, with a higher seroprevalence in children living with HIV (35.5%) than in HEU children (16.7%) aged 2 – 10 years [31]. However, this study did not include an HUU subgroup. Among 1 – 12-year-olds in our study, the seroprevalence of hepatitis A was 19.3%, with a relatively higher prevalence rate in the HIV (60%) than the HEU (45%) subgroups, although the HUU subgroup (29.2%) had the lowest proportion of participants testing positive for anti-HAV overall. The hepatitis A seroprevalence rate in our study (19.3%) is considerably lower than the 44.1% reported among 1 – 7-year-olds (HIV status unknown) in 2015 in the Western Cape province [
8]. A recent systematic review by Patterson et al. estimates that the seroprevalence of hepatitis A among children and adolescents (1 – 18 years) in Africa is 57%, consistent with an intermediate endemicity [
6]. While no other studies have assessed the seroprevalence of hepatitis A among HEU children in South Africa, one other study conducted in children (1 – 15 years) living with and without HIV found rates ranging between 24.0 – 85.7% and 18.6% – 100%, respectively [
13]. Differences in hepatitis A seroprevalence rates may be associated with variable socio-economic status among study populations. In the du Plessis et al. study, increased age and living in informal dwellings, rather than HIV status, were statistically associated with HAV seropositivity [
13]. In our study, the level of maternal education was high, and most participants were in the upper-middle socio-economic quartile, lived in formal housing, and had good access to safe drinking water (in-door taps) and sewage disposal systems (flush toilets). The socio-economic characteristics of our study population is consistent with findings from the national household survey [32,33]. Previous studies have shown that common risk factors for HAV infection in other low- and middle-income countries, include low level of knowledge about hepatitis, low family income, over-crowding, poor hygiene practices or sanitary conditions, and poor access to safe drinking water [
4,34,35].
The findings of this study should be considered in light of some limitations including the retrospective study design, the modest sample size across HIV and age subgroups, and the use of a hospital-based population who may have risk profiles and health seeking behaviours that are not representative of the general population. Despite this, we contribute crucial evidence to the limited body of knowledge on the seroprevalence and risk factors of hepatitis A among HIV-exposed infected and uninfected children. Future research directions should include prospective studies in community-representative populations in order to enhance the generalizability of findings and guide public health policy and practice.
5. Conclusions
The findings of this study underscore the intermediate endemicity of hepatitis A in South Africa even in the context of perinatal HIV exposure. We show a high level of maternal transfer of anti-HAV among children under 6 months of age regardless of perinatal HIV exposure, with acute HAV infections only observed among HIV-exposed 1 – 4-year-olds. Overall, the seroprevalence of hepatitis A among children between 1 – 12 years of age was 19.3%, and trends in anti-HAV seropositivity were comparable in HIV-exposed infected and uninfected, as well as unexposed uninfected children. There is a need to scale-up public health interventions given the high susceptibility rate within the population and the risk of localized and widespread outbreaks in South Africa. Such interventions should include intensive risk communication to the public including pregnant women, and parents and caregivers. In this regard, PMTCT services could also serve as useful entry points for raising awareness about hepatitis A and improving adherence to good hygiene practices and prevention and control strategies. Relevant government institutions should increase the provision of safe water, optimize wastewater treatment and management, and improve the socioeconomic conditions within all communities as a matter of national priority in order to reduce the risk of hepatitis A outbreaks. Further to this, when making evidence-based decisions on whether to introduce the hepatitis A vaccine, African-based National Immunization Technical Advisory Groups should include epidemiological data on the seroprevalence of hepatitis A among the growing population of HEU children.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Figure S1: title; Table S1: title; Video S1: title.
Author Contributions
Conceptualization, E.A-D; methodology, E.A-D., L.R., L.M.M., and O.E.S.; software, N.B.; validation, G.S., B.M.K. and R.M.; formal analysis, E.A-D. and N.B.; investigation, E.A-D., L.R., L.M.M., and O.E.S. ; resources, E.A-D.; data curation, E.A-D., L.R., L.M.M., O.E.S., N.B. and R.M.; writing—original draft preparation, E.A-D.; writing—review and editing, L.R., L.M.M., O.E.S., N.B., G.S., B.M.K., and R.M.; visualization, N.B.; funding acquisition, E.A-D. All authors have read and agreed to the published version of the manuscript.
Funding
This independent research was supported by the Gilead Sciences Research Scholars Program in Global Public Health.
Institutional Review Board Statement
Ethics approval to conduct this study was obtained from the University of Cape Town Faculty of Health Sciences Human Research Ethics Committee (reference number: 371/2011).
Informed Consent Statement
Written informed consent and ascent (<18-year-olds) was obtained from parents or caregivers prior to being enrolled into the overarching study. As part of the consenting process, participants were asked to indicate their approval for blood samples and records to be used in future related studies. We only included those samples for which consent for future use was obtained. Where parents’ or caregivers’ consent could not be confirmed or where they explicitly declined consent for samples to be tested in future studies, these were excluded from the current study.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank the National Institute for Communicable Diseases of South Africa for providing the hepatitis A positive control used in the reverse transcription-polymerase chain reaction conducted in this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- World Health Organization WHO Position Paper on Hepatitis A Vaccines – October 2022 Available online:. Available online: https://www.who.int/publications-detail-redirect/who-wer9740-493-512 (accessed on 24 October 2023).
- World Health Organization Global Hepatitis Report, 2017 Available online:. Available online: https://www.who.int/publications-detail-redirect/9789241565455 (accessed on 7 January 2024).
- The Institute for Health Metrics and Evaluation Acute Hepatitis A — Level 4 Cause Available online:. Available online: https://www.healthdata.org/results/gbd_summaries/2019/acute-hepatitis-level-4-cause (accessed on 7 January 2024).
- Migueres, M.; Lhomme, S.; Izopet, J. Hepatitis A: Epidemiology, High-Risk Groups, Prevention and Research on Antiviral Treatment. Viruses 2021, 13, 1900. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.-C.; Jeong, S.-H. Natural History, Clinical Manifestations, and Pathogenesis of Hepatitis A. Cold Spring Harb Perspect Med 2018, 8, a031708. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.; Abdullahi, L.; Hussey, G.D.; Muloiwa, R.; Kagina, B.M. A Systematic Review of the Epidemiology of Hepatitis A in Africa. BMC Infect Dis 2019, 19, 651. [Google Scholar] [CrossRef] [PubMed]
- Haeri Mazanderani, A.; Motaze, N.V.; McCarthy, K.; Suchard, M.; du Plessis, N.M. Hepatitis A Virus Seroprevalence in South Africa - Estimates Using Routine Laboratory Data, 2005-2015. PLoS One 2019, 14, e0216033. [Google Scholar] [CrossRef] [PubMed]
- Enoch, A.; Hardie, D.R.; Hussey, G.D.; Kagina, B.M. Hepatitis A Seroprevalence in Western Cape Province, South Africa: Are We in Epidemiological Transition? S Afr Med J 2019, 109, 314–318. [Google Scholar] [CrossRef] [PubMed]
- National Department of Health National Guidelines for the Management of Viral Hepatitis Available online:. Available online: https://knowledgehub.health.gov.za/elibrary/national-guidelines-management-viral-hepatitis (accessed on 24 October 2023).
- Prabdial-Sing, N.; Motaze, V.; Manamela, J.; McCarthy, K.; Suchard, M. Establishment of Outbreak Thresholds for Hepatitis A in South Africa Using Laboratory Surveillance, 2017-2020. Viruses 2021, 13, 2470. [Google Scholar] [CrossRef] [PubMed]
- Solomons, R.S.; Rabie, H.; Nel, E.; Cotton, M. An Overview of Hepatitis A at Tygerberg Children’s Hospital. South African Journal of Child Health 2008, 2, 43–45. [Google Scholar]
- Patterson, J.; Cleary, S.; Silal, S.P.; Hussey, G.D.; Enoch, A.; Korsman, S.; Goddard, E.; Setshedi, M.; Spearman, W.C.; Kagina, B.M.; et al. A Retrospective Study Assessing the Clinical Outcomes and Costs of Acute Hepatitis A in Cape Town, South Africa. BMC Infect Dis 2022, 22, 45. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, N.M.; Haeri Mazanderani, A.; Motaze, N.V.; Ngobese, M.; Avenant, T. Hepatitis A Virus Seroprevalence among Children and Adolescents in a High-Burden HIV Setting in Urban South Africa. Sci Rep 2022, 12, 20688. [Google Scholar] [CrossRef] [PubMed]
- Slogrove, A.L.; Powis, K.M.; Johnson, L.F.; Stover, J.; Mahy, M. Global Estimates of Children HIV Exposed and Uninfected in the Evolving HIV Epidemic: 2000 to 2018. Lancet Glob Health 2020, 8, e67–e75. [Google Scholar] [CrossRef] [PubMed]
- Slogrove, A.L.; Goetghebuer, T.; Cotton, M.F.; Singer, J.; Bettinger, J.A. Pattern of Infectious Morbidity in HIV-Exposed Uninfected Infants and Children. Frontiers in Immunology 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- du Toit, L.D.V.; Prinsloo, A.; Steel, H.C.; Feucht, U.; Louw, R.; Rossouw, T.M. Immune and Metabolic Alterations in Children with Perinatal HIV Exposure. Viruses 2023, 15, 279. [Google Scholar] [CrossRef] [PubMed]
- Chotun, N.; Nel, E.; Cotton, M.F.; Preiser, W.; Andersson, M.I. Hepatitis B Virus Infection in HIV-Exposed Infants in the Western Cape, South Africa. Vaccine 2015, 33, 4618–4622. [Google Scholar] [CrossRef] [PubMed]
- Tamandjou Tchuem, C.; Cotton, M.F.; Nel, E.; Tedder, R.; Preiser, W.; Violari, A.; Bobat, R.; Hovind, L.; Aaron, L.; Montepiedra, G.; et al. Viral Hepatitis B and C in HIV-Exposed South African Infants. BMC Pediatrics 2020, 20, 563. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raya, B.; Kollmann, T.R.; Marchant, A.; MacGillivray, D.M. The Immune System of HIV-Exposed Uninfected Infants. Front Immunol 2016, 7, 383. [Google Scholar] [CrossRef] [PubMed]
- Muloiwa, R.; Dube, F.S.; Nicol, M.P.; Zar, H.J.; Hussey, G.D. Incidence and Diagnosis of Pertussis in South African Children Hospitalized With Lower Respiratory Tract Infection. Pediatr Infect Dis J 2016, 35, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Charan, J.; Biswas, T. How to Calculate Sample Size for Different Study Designs in Medical Research? Indian J Psychol Med 2013, 35, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Dhand, N.K.; Khatkar, M.S. Sample Size Calculator for Estimating a Single Proportion Available online:. Available online: https://statulator.com/SampleSize/ss1P.html (accessed on 30 January 2024).
- Hutin, Y.J.; Pool, V.; Cramer, E.H.; Nainan, O.V.; Weth, J.; Williams, I.T.; Goldstein, S.T.; Gensheimer, K.F.; Bell, B.P.; Shapiro, C.N.; et al. A Multistate, Foodborne Outbreak of Hepatitis A. National Hepatitis A Investigation Team. N Engl J Med 1999, 340, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Nainan, O.V.; Armstrong, G.L.; Han, X.-H.; Williams, I.; Bell, B.P.; Margolis, H.S. Hepatitis A Molecular Epidemiology in the United States, 1996–1997: Sources of Infection and Implications of Vaccination Policy. The Journal of Infectious Diseases 2005, 191, 957–963. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R: A Language and Environment for Statistical Computing Available online:. Available online: https://www.r-project.org/ (accessed on 12 January 2023).
- Tableau Software Tableau Desktop 2024.
- Lee, K.J.; Tilling, K.M.; Cornish, R.P.; Little, R.J.A.; Bell, M.L.; Goetghebeur, E.; Hogan, J.W.; Carpenter, J.R. Framework for the Treatment and Reporting of Missing Data in Observational Studies: The Treatment And Reporting of Missing Data in Observational Studies Framework. Journal of Clinical Epidemiology 2021, 134, 79–88. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).