Submitted:
12 October 2024
Posted:
14 October 2024
You are already at the latest version
Abstract
Accumulated data indicate that epigenetic regulations, including histone modifications and DNA methylation, are important means for adjusting genes’ expression in response to various stimuli. In contrast to the success in studying the role of DNA methylation in laboratory rodents, the role of DNA methylation in terrestrial snail Helix lucorum has been studied only in behavioral experiments. This prompted us to further investigate the role of DNA methylation and the interaction between DNA methylation and histone acetylation in the mechanisms of neuroplasticity in terrestrial snails using in vitro experiments. Dysregulation of DNA methylation by DNMT inhibitor RG108 suppressed significantly the long-term potentiation (LTP) of synaptic inputs in identified neurons. We then tested whether the RG108-induced weakening of potentiation will be rescued under co-application of histone deacetylase inhibitors sodium butyrate or trichostatin A. RG108-induced LTP deficiency was significantly compensated by increased histone acetylation. These data bring important insight to the functional role of DNA methylation as an important regulatory mechanism and a necessary condition for the development and maintenance of long-term synaptic changes in withdrawal interneurons of terrestrial snails. Moreover, these results support the idea of the interaction of DNA methylation and histone acetylation in the epigenetic regulation of synaptic plasticity.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals
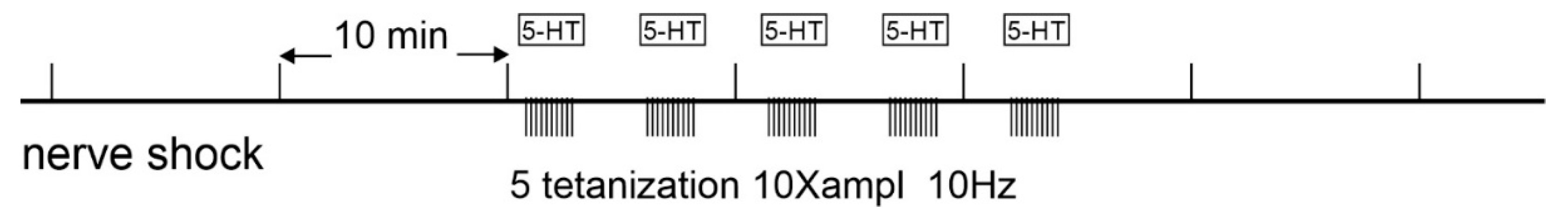
2.2. Electrophysiological Experiments
2.3. Drugs
2.4. Data Analysis
3. Results
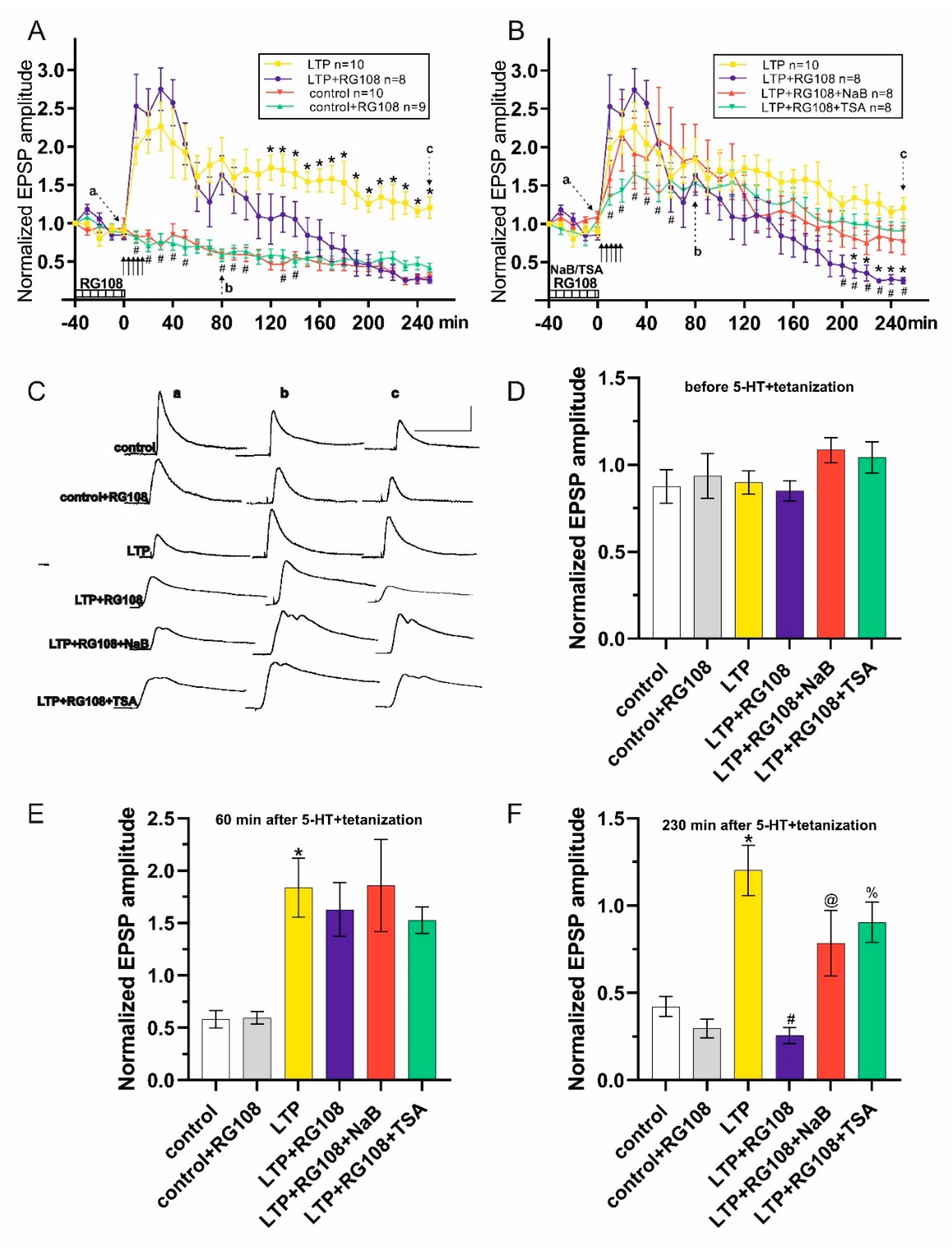
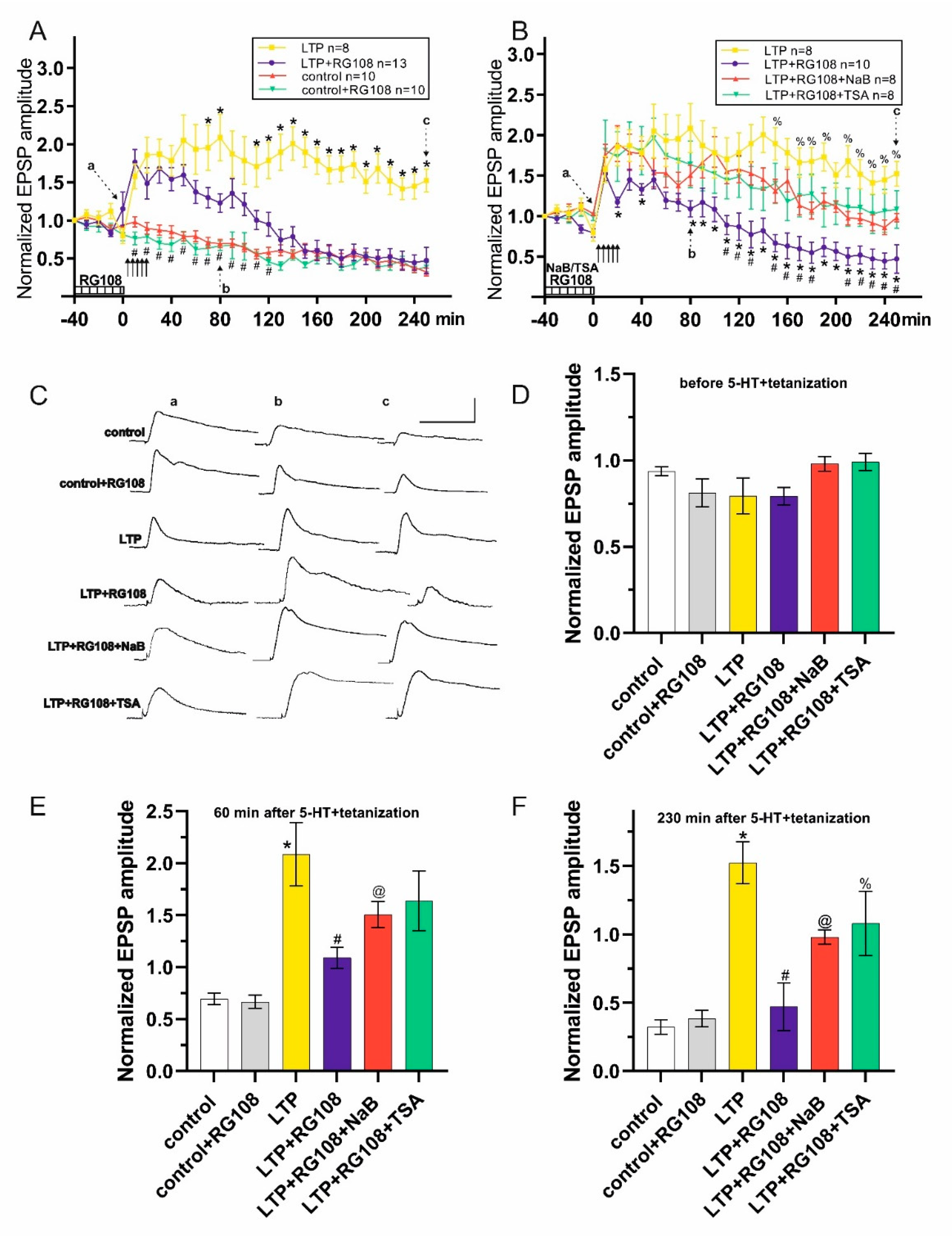
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, R.R.; Wood, M.A. How the epigenome integrates information and reshapes the synapse. Nat. Rev. Neurosci. 2019, 20, 133–147. [CrossRef]
- Oliveira, A.M.M. DNA methylation: a permissive mark in memory formation and maintenance. Learn. Mem. 2016, 23, 587–593. [CrossRef]
- Levenson, J.M.; Roth, T.L.; Lubin, F.D.; Miller, C.A.; Huang, I.-C.; Desai, P.; Malone, L.M.; Sweatt, J.D. Evidence That DNA (Cytosine-5) Methyltransferase Regulates Synaptic Plasticity in the Hippocampus. J. Biol. Chem. 2006, 281, 15763–15773. [CrossRef]
- Sui, L.; Wang, Y.; Ju, L.-H.; Chen, M. Epigenetic regulation of reelin and brain-derived neurotrophic factor genes in long-term potentiation in rat medial prefrontal cortex. Neurobiol. Learn. Mem. 2012, 97, 425–440. [CrossRef]
- Geyer, K.K.; Niazi, U.H.; Duval, D.; Cosseau, C.; Tomlinson, C.; Chalmers, I.W.; Swain, M.T.; Cutress, D.J.; Bickham-Wright, U.; Munshi, S.E.; et al. The Biomphalaria glabrata DNA methylation machinery displays spatial tissue expression, is differentially active in distinct snail populations and is modulated by interactions with Schistosoma mansoni. PLoS Negl. Trop. Dis. 2017, 11, e0005246. [CrossRef]
- Moroz, L.L.; Edwards, J.R.; Puthanveettil, S.V.; Kohn, A.B.; Ha, T.; Heyland, A.; Knudsen, B.; Sahni, A.; Yu, F.; Liu, L.; et al. Neuronal Transcriptome of Aplysia: Neuronal Compartments and Circuitry. Cell 2006, 127, 1453–1467. [CrossRef]
- Cocci, P.; Mosconi, G.; Palermo, F.A. Effects of tributyltin on retinoid X receptor gene expression and global DNA methylation during intracapsular development of the gastropod Tritia mutabilis (Linnaeus, 1758). Environ. Toxicol. Pharmacol. 2021, 88, 103753. [CrossRef]
- Georgescu, M.; Drăghici, G.A.; Oancea, E.-F.; Dehelean, C.A.; Şoica, C.; Vlăduţ, N.-V.; Nica, D.V. Effects of Cadmium Sulfate on the Brown Garden Snail Cornu aspersum: Implications for DNA Methylation. Toxics 2021, 9, 306. [CrossRef]
- Regev, A.; Lamb, M.; Jablonka, E. The Role of DNA Methylation in Invertebrates: Developmental Regulation or Genome Defense? Mol. Biol. Evol. 1998, 15, 880. [CrossRef]
- Rahn, E.J.; Guzman-Karlsson, M.C.; David Sweatt, J. Cellular, molecular, and epigenetic mechanisms in non-associative conditioning: Implications for pain and memory. Neurobiol. Learn. Mem. 2013, 105, 133–150. [CrossRef]
- Jones, P.A. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [CrossRef]
- Law, J.A.; Jacobsen, S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010, 11, 204–220. [CrossRef]
- Lv, H.; Dao, F.-Y.; Zhang, D.; Yang, H.; Lin, H. Advances in mapping the epigenetic modifications of 5-methylcytosine (5mC), N6-methyladenine (6mA), and N4-methylcytosine (4mC). Biotechnol. Bioeng. 2021, 118, 4204–4216. [CrossRef]
- Yang, Q.; Antonov, I.; Castillejos, D.; Nagaraj, A.; Bostwick, C.; Kohn, A.; Moroz, L.L.; Hawkins, R.D. Intermediate-term memory in Aplysia involves neurotrophin signaling, transcription, and DNA methylation. Learn. Mem. Cold Spring Harb. N 2018, 25, 620–628. [CrossRef]
- Rajasethupathy, P.; Antonov, I.; Sheridan, R.; Frey, S.; Sander, C.; Tuschl, T.; Kandel, E.R. A Role for Neuronal piRNAs in the Epigenetic Control of Memory-Related Synaptic Plasticity. Cell 2012, 149, 693–707. [CrossRef]
- Park, J.; Lee, K.; Kim, K.; Yi, S.-J. The role of histone modifications: from neurodevelopment to neurodiseases. Signal Transduct. Target. Ther. 2022, 7, 217. [CrossRef]
- Turner, B.M. Cellular Memory and the Histone Code. Cell 2002, 111, 285–291. [CrossRef]
- Penney, J.; Tsai, L.-H. Histone deacetylases in memory and cognition. Sci. Signal. 2014, 7, re12. [CrossRef]
- Yoshida, M.; Kijima, M.; Akita, M.; Beppu, T. Potent and Specific Inhibition of Mammalian Histone Deacetylase Both in Vivo and in Vitro by Trichostatin A. J. Biol. Chem. 1990, 265, 17174–17179. [CrossRef]
- Kilgore, M.; Miller, C.A.; Fass, D.M.; Hennig, K.M.; Haggarty, S.J.; Sweatt, J.D.; Rumbaugh, G. Inhibitors of Class 1 Histone Deacetylases Reverse Contextual Memory Deficits in a Mouse Model of Alzheimer’s Disease. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2010, 35, 870–880. [CrossRef]
- Alarcón, J.M.; Malleret, G.; Touzani, K.; Vronskaya, S.; Ishii, S.; Kandel, E.R.; Barco, A. Chromatin Acetylation, Memory, and LTP Are Impaired in CBP+/- Mice: A Model for the Cognitive Deficit in Rubinstein-Taybi Syndrome and Its Amelioration. Neuron 2004, 42, 947–959. [CrossRef]
- Zuzina, A.B.; Balaban, P.M. Contribution of histone acetylation to the serotonin-mediated long-term synaptic plasticity in terrestrial snails. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2022, 208, 521–535. [CrossRef]
- Miller, C.A.; Campbell, S.L.; Sweatt, J.D. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol. Learn. Mem. 2008, 89, 599–603. [CrossRef]
- Monsey, M.S.; Ota, K.T.; Akingbade, I.F.; Hong, E.S.; Schafe, G.E. Epigenetic Alterations Are Critical for Fear Memory Consolidation and Synaptic Plasticity in the Lateral Amygdala. PloS One 2011, 6, e19958. [CrossRef]
- Balaban, P.M. Cellular mechanisms of behavioral plasticity in terrestrial snail. Neurosci. Biobehav. Rev. 2002, 26, 597–630. [CrossRef]
- Zyuzina, A.B.; Balaban, P.M. Extinction and Reconsolidation of Memory. Neurosci. Behav. Physiol. 2017, 47, 74–82. [CrossRef]
- Zuzina, A.B.; Balaban, P.M. Contribution of Transglutaminase to the Induction and Maintenance of Long-Term Synaptic Potentiation in Neurons in the Common Snail. Neurosci. Behav. Physiol. 2023, 53, 590–596. [CrossRef]
- Zuzina, A.B.; Balaban, P.M. Contribution of Epigenetic Mechanisms to the Formation, Maintenance, and Reconsolidation of a Long-Term Food-Related Aversive Memory in Terrestrial Snails. Neurosci. Behav. Physiol. 2024, 54, 138–148. [CrossRef]
- Zuzina, A.B.; Vinarskaya, A.K.; Balaban, P.M. Histone deacetylase inhibitors rescue the impaired memory in terrestrial snails. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2020, 206, 639–649. [CrossRef]
- Zuzina, A.B.; Vinarskaya, A.Kh. Increased histone acetylation levels or a serotonin precursor reinstate the context memory impaired by the serotonin receptor blocker methiothepin. Neurosci. Behav. Physiol. 2023, 53, 460–472. [CrossRef]
- Zuzina, A.B.; Vinarskaya, A.K.; Balaban, P.M. DNA Methylation Inhibition Reversibly Impairs the Long-Term Context Memory Maintenance in Helix. Int. J. Mol. Sci. 2023, 24, 14068. [CrossRef]
- Kolotova, D.E.; Malyshev, A.Yu.; Balaban, P.M. Histone Deacytylase Inhibitor Enhances Long-Term Synaptic Potentiation in Neurons of a Grape Snail. J. Evol. Biochem. Physiol. 2021, 57, 704–708. [CrossRef]
- Bravarenko, N.I.; Korshunova, T.A.; Malyshev, A.Y.; Balaban, P.M. Synaptic contact between mechanosensory neuron and withdrawal interneuron in terrestrial snail is mediated by l-glutamate-like transmitter. Neurosci. Lett. 2003, 341, 237–240. [CrossRef]
- Ter-Markaryan, A.G.; Palikhova, T.A.; Sokolov, E.N. Effect of atropine and d-tubocurarine on the monosynaptic connections between identified neurons in the central nervous system of the edible snail. Neurosci. Behav. Physiol. 1991, 21, 37–38. [CrossRef]
- Zuzina, A.B.; Vinarskaya, A.K.; Balaban, P.M. Increase in serotonin precursor levels reinstates the context memory during reconsolidation. Invertebr. Neurosci. IN 2019, 19, 8. [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2013, 38, 23–38. [CrossRef]
- Maity, S.; Jarome, T.J.; Blair, J.; Lubin, F.D.; Nguyen, P.V. Noradrenaline goes nuclear: epigenetic modifications during long-lasting synaptic potentiation triggered by activation of β-adrenergic receptors. J. Physiol. 2016, 594, 863–881. [CrossRef]
- Pearce, K.; Cai, D.; Roberts, A.C.; Glanzman, D.L. Role of protein synthesis and DNA methylation in the consolidation and maintenance of long-term memory in Aplysia. eLife 2017, 6, e18299. [CrossRef]
- Sunada, H.; Riaz, H.; de Freitas, E.; Lukowiak, K.; Swinton, C.; Swinton, E.; Protheroe, A.; Shymansky, T.; Komatsuzaki, Y.; Lukowiak, K. Heat stress enhances LTM formation in Lymnaea: role of HSPs and DNA methylation. J. Exp. Biol. 2016, 219, 1337–1345. [CrossRef]
- Rothwell, C.M.; Lukowiak, K.D. Impairing DNA methylation obstructs memory enhancement for at least 24 hours in Lymnaea. Commun. Integr. Biol. 2017, 10, e1306616. [CrossRef]
- Lukowiak, K.; Heckler, B.; Bennett, T.E.; Schriner, E.K.; Wyrick, K.; Jewett, C.; Todd, R.P.; Sorg, B.A. Enhanced memory persistence is blocked by a DNA methyltransferase inhibitor in the snail Lymnaea stagnalis. J. Exp. Biol. 2014, 217, 2920–2929. [CrossRef]
- Wu, C.-F.; Zhang, D.-F.; Zhang, S.; Sun, L.; Liu, Y.; Dai, J.-J. Optimizing treatment of DNA methyltransferase inhibitor RG108 on porcine fibroblasts for somatic cell nuclear transfer. Reprod. Domest. Anim. Zuchthyg. 2019, 54, 1604–1611. [CrossRef]
- Stresemann, C.; Brueckner, B.; Musch, T.; Stopper, H.; Lyko, F. Functional Diversity of DNA Methyltransferase Inhibitors in Human Cancer Cell Lines. Cancer Res. 2006, 66, 2794–2800. [CrossRef]
- Brueckner, B.; Garcia Boy, R.; Siedlecki, P.; Musch, T.; Kliem, H.C.; Zielenkiewicz, P.; Suhai, S.; Wiessler, M.; Lyko, F. Epigenetic Reactivation of Tumor Suppressor Genes by a Novel Small-Molecule Inhibitor of Human DNA Methyltransferases. Cancer Res. 2005, 65, 6305–6311. [CrossRef]
- Carraway, H.E.; Malkaram, S.A.; Cen, Y.; Shatnawi, A.; Fan, J.; Ali, H.E.A.; Abd Elmageed, Z.Y.; Buttolph, T.; Denvir, J.; Primerano, D.A.; et al. Activation of SIRT6 by DNA hypomethylating agents and clinical consequences on combination therapy in leukemia. Sci. Rep. 2020, 10, 10325. [CrossRef]
- Cai, D.; Chen, S.; Glanzman, D.L. Postsynaptic Regulation of Long-Term Facilitation in Aplysia. Curr. Biol. CB 2008, 18, 920–925. [CrossRef]
- Sossin, W.S. Isoform specificity of protein kinase Cs in synaptic plasticity. Learn. Mem. Cold Spring Harb. N 2007, 14, 236–246. [CrossRef]
- Zhou, L.; Baxter, D.A.; Byrne, J.H. Contribution of PKC to the maintenance of 5-HT-induced short-term facilitation at sensorimotor synapses of Aplysia. J. Neurophysiol. 2014, 112, 1936–1949. [CrossRef]
- Manseau, F.; Fan, X.; Hueftlein, T.; Sossin, W.S.; Castellucci, V.F. Ca2+-Independent Protein Kinase C Apl II Mediates the Serotonin-Induced Facilitation at Depressed Aplysia Sensorimotor Synapses. J. Neurosci. 2001, 21, 1247–1256. [CrossRef]
- Hu, J.-Y.; Chen, Y.; Schacher, S. Protein Kinase C Regulates Local Synthesis and Secretion of a Neuropeptide Required for Activity-Dependent Long-Term Synaptic Plasticity. J. Neurosci. 2007, 27, 8927–8939. [CrossRef]
- Villareal, G.; Li, Q.; Cai, D.; Fink, A.E.; Lim, T.; Bougie, J.K.; Sossin, W.S.; Glanzman, D.L. Role of Protein Kinase C in the Induction and Maintenance of Serotonin-Dependent Enhancement of the Glutamate Response in Isolated Siphon Motor Neurons of Aplysia californica. J. Neurosci. 2009, 29, 5100–5107. [CrossRef]
- Mellor, H.; Parker, P.J. The extended protein kinase C superfamily. Biochem. J. 1998, 332(Pt 2), 281–292. [CrossRef]
- Cai, D.; Pearce, K.; Chen, S.; Glanzman, D.L. Protein Kinase M Maintains Long-Term Sensitization and Long-Term Facilitation in Aplysia. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 6421–6431. [CrossRef]
- Hastings, M.; Farah, C.A.; Sossin, W.S. Roles of Protein Kinase C and Protein Kinase M in Aplysia Learning. In Handbook of Behavioral Neuroscience; Menzel, R., Benjamin, P.R., Eds.; Invertebrate Learning and Memory; Elsevier: Amsterdam, The Netherlands, 2013; Volume 22, pp. 221–235. [CrossRef]
- Newton, A.C. Protein Kinase C: Structural and Spatial Regulation by Phosphorylation, Cofactors, and Macromolecular Interactions. Chem. Rev. 2001, 101, 2353–2364. [CrossRef]
- Sossin, W.S.; Abrams, T.W. Evolutionary Conservation of the Signaling Proteins Upstream of Cyclic AMP-Dependent Kinase and Protein Kinase C in Gastropod Mollusks. Brain. Behav. Evol. 2009, 74, 191–205. [CrossRef]
- Li, Q.; Roberts, A.C.; Glanzman, D.L. Synaptic Facilitation and Behavioral Dishabituation in Aplysia: Dependence on Release of Ca2+ from Postsynaptic Intracellular Stores, Postsynaptic Exocytosis, and Modulation of Postsynaptic AMPA Receptor Efficacy. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 5623–5637. [CrossRef]
- Miller, C.A.; Sweatt, J.D. Covalent Modification of DNA Regulates Memory Formation. Neuron 2007, 53, 857–869. [CrossRef]
- Martinowich, K.; Hattori, D.; Wu, H.; Fouse, S.; He, F.; Hu, Y.; Fan, G.; Sun, Y.E. DNA Methylation-Related Chromatin Remodeling in Activity-Dependent Bdnf Gene Regulation. Science 2003, 302, 890–893. [CrossRef]
- Lubin, F.D.; Roth, T.L.; Sweatt, J.D. Epigenetic Regulation of bdnf Gene Transcription in the Consolidation of Fear Memory. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 10576–10586. [CrossRef]
- Guan, Z.; Giustetto, M.; Lomvardas, S.; Kim, J.-H.; Miniaci, M.C.; Schwartz, J.H.; Thanos, D.; Kandel, E.R. Integration of Long-Term-Memory-Related Synaptic Plasticity Involves Bidirectional Regulation of Gene Expression and Chromatin Structure. Cell 2002, 111, 483–493. [CrossRef]
- Vecsey, C.G.; Hawk, J.D.; Lattal, K.M.; Stein, J.M.; Fabian, S.A.; Attner, M.A.; Cabrera, S.M.; McDonough, C.B.; Brindle, P.K.; Abel, T.; et al. Histone Deacetylase Inhibitors Enhance Memory and Synaptic Plasticity via CREB: CBP-Dependent Transcriptional Activation. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 6128–6140. [CrossRef]
- Chen, S.; Cai, D.; Pearce, K.; Sun, P.Y.-W.; Roberts, A.C.; Glanzman, D.L. Reinstatement of long-term memory following erasure of its behavioral and synaptic expression in Aplysia. eLife 2014, 3, e03896. [CrossRef]
- Maddox, S.A.; Schafe, G.E. Epigenetic alterations in the lateral amygdala are required for reconsolidation of a Pavlovian fear memory. Learn. Mem. Cold Spring Harb. N 2011, 18, 579–593. [CrossRef]
- Roth, T.L.; Sweatt, J.D. Regulation of chromatin structure in memory formation. Curr. Opin. Neurobiol. 2009, 19, 336–342. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




