1. Introduction
By 2050, the world population is expected to approach 10 billion, and food production will need to increase to meet this growing demand [
1]. In response to this challenge, there is an urgent need to explore methods that enable efficient food production while reducing the use of resources and space. Hydroponics, a method of cultivating plants without soil, presents a promising solution. In hydroponics, plant roots are directly immersed in a nutrient solution composed of water and essential nutrients for plant growth [
2], offering significant advantages in terms of resource efficiency [
3]. Hydroponics has gained global attention for its potential to minimize environmental impacts by improving the efficient use of water and fertilizers while reducing or even eliminating the need for certain agricultural pesticides [
4]. However, despite these advantages, hydroponic systems are not immune to contamination, particularly when nutrient solutions are reused. Throughout the cultivation cycle, the composition of the nutrient solution can vary depending on the plants' nutritional needs. Producers are encouraged to recycle nutrient solutions in hydroponic systems for both environmental and economic reasons. However, reusing nutrient solutions introduces the risk of contamination, particularly by pathogens that can easily spread through the system, leading to plant diseases and reduced productivity (Scarlett et al., 2015).
Among the microorganisms of concern is Escherichia coli, a bacterium commonly found in the intestines of humans and animals. While many strains of E. coli are harmless, some pathogenic strains, such as E. coli O157, can cause severe foodborne illnesses (Martins et al., 2010). The incidence of E. coli contamination in hydroponic systems has been documented in several studies, particularly in leafy green crops such as lettuce and spinach, which are commonly grown hydroponically. These crops are especially vulnerable because they are often consumed raw, increasing the risk of transmission of foodborne pathogens (Franz et al., 2005). Regions with warmer climates or suboptimal sanitation practices in greenhouses tend to report higher incidences of contamination (Danyluk et al., 2012). The presence of E. coli in hydroponic systems can result from several factors, including contaminated water sources, poor handling of nutrient solutions, or cross-contamination from workers or equipment. Studies have shown that once E. coli enters a hydroponic system, it can quickly proliferate due to the continuous recirculation of nutrient solutions, particularly in systems like Nutrient Film Technique (NFT) and Deep-Water Culture (DWC), where the same nutrient solution is reused over extended periods (Fisher & Sutton, 2007). Research by Moura et al. (2019) confirmed that this recirculation, especially under static conditions, increases the likelihood of bacterial sedimentation, reducing exposure to disinfection methods such as UV-C. Different strains of E. coli can affect the performance of crops in distinct ways. Pathogenic strains, such as E. coli O157, not only pose a risk to human health but can also negatively impact plant physiology. Research has shown that pathogenic E. coli can adhere to plant roots, forming biofilms that impede nutrient uptake and stunt plant growth (Cooley et al., 2003). In contrast, non-pathogenic strains may not cause direct harm to the plants but can still pose significant food safety risks if consumed. The ability of E. coli to survive and persist in hydroponic systems depends on several factors, including the composition of the nutrient solution, temperature, and the presence of other competing microorganisms.
The potential for contamination, particularly when nutrient solutions are reused, represents a critical threat to hydroponic systems. Pathogens such as E. coli can compromise plant health, reduce productivity, and pose food safety risks. In light of these challenges, the present study aims to evaluate the effectiveness of UV-C light in inactivating Escherichia coli in hydroponic nutrient solutions used in indoor cultivation systems. We hypothesize that UV-C radiation will significantly reduce bacterial contamination, thereby enhancing food safety and maintaining the overall quality of the hydroponic system. Various decontamination methods, including the use of oxidizing agents, filtration, ultraviolet (UV) radiation, and heat treatments, have been proposed to mitigate contamination in nutrient solutions (Scarlett et al., 2015). UV-C radiation, in particular, has shown promise as an effective disinfection technique. UV-C light at 254 nm damages the DNA of microorganisms, creating thymine dimers that disrupt replication and biological functions, thereby inactivating bacteria like E. coli (Kowalski, 2010). Unlike chemical treatments, UV-C disinfection does not leave harmful residues, making it a more environmentally friendly option (Beauvais et al., 2021).
The use of UV-C radiation for disinfection in hydroponic systems has been explored in several studies. Kowalski (2010) demonstrated the ability of UV-C to inactivate a variety of microorganisms in water, including E. coli, through photochemical damage to DNA. García et al. (2014) applied UV-C in hydroponic systems for lettuce production, achieving significant reductions in microbial load. However, the effectiveness of UV-C treatment varied depending on factors such as fluid dynamics and the presence of organic matter, which can hinder light penetration. Moura et al. (2019) further investigated the role of fluid movement, showing that dynamic conditions, such as continuous fluid circulation, significantly improve UV-C effectiveness by ensuring uniform exposure to microorganisms. In contrast, under static conditions, the bacterial inactivation rate was lower due to the sedimentation of bacteria, which limited exposure to UV-C radiation. These findings align with earlier studies by Kowalski (2010) and García et al. (2014), which highlight the importance of solution movement for effective UV-C treatment in hydroponic systems. This study builds upon these findings by comparing UV-C efficacy under both static and dynamic fluid conditions, focusing on bacterial inactivation in nutrient solutions contaminated with Escherichia coli. By evaluating the interaction between UV-C treatment and fluid conditions, this study aims to provide a clearer understanding of the practical implementation of UV-C in hydroponic systems to improve food safety and nutrient solution quality.
The practical implications of this study are substantial, particularly for large-scale hydroponic farming operations. The application of UV-C technology in these systems could offer a reliable, eco-friendly solution for controlling microbial contamination without the need for chemical disinfectants, which can affect the composition of nutrient solutions and harm the environment. In economic terms, UV-C disinfection has the potential to reduce costs associated with plant losses and food safety recalls due to microbial contamination, making it a cost-effective approach for long-term hydroponic cultivation. Moreover, in ecological terms, the use of UV-C aligns with the growing demand for sustainable agriculture by minimizing the use of harmful chemicals and lowering the environmental footprint of food production systems. As hydroponics continues to expand globally, particularly in urban agriculture settings, integrating UV-C technology could be a key factor in enhancing food safety and production efficiency at a commercial scale. To assess this, hydroponic nutrient solutions contaminated with E. coli will be treated with UV-C light, and bacterial inactivation will be monitored under static and dynamic fluid conditions. The impact of UV-C treatment on both microbial load and nutrient solution quality will be evaluated.
2. Materials and Methods
Hydroponic solution. The nutrient solution was maintained at an electrical conductivity of 1.8 μS/cm, equivalent to the environmental temperature, with a pH range of 5.5-6.3. The nutrient solution used in this study was composed of 7.30% w/w Nitrogen (N), 12.62% w/w Potassium (K₂O), 3.97% w/w Calcium (Ca), 2.39% w/w Sulfur (S), 0.01% w/w Boron (B), 0.01% w/w Copper (Cu), 0.05% w/w Iron (Fe), 0.01% w/w Manganese (Mn), 0.06% w/w Zinc (Zn), 0.05% w/w Nickel (Ni), 1.80% w/w Magnesium (Mg), and 7.19% w/w Phosphorus (P₂O₅) (Plenan Ferti®). Iron was supplemented at 6% w/w (Biolchim®). This composition corresponds to the electrical conductivity (EC) of a hydroponic solution suitable for plant development (HELBEL JUNIOR et al., 2008). The quality of the nutrient solution, balanced in its purity, ensures adequate plant growth, which is measured by electrical conductivity (EC, s/cm). The electrical conductivity of the hydroponic solution was monitored with a portable conductivity meter AK52 (ASKO) for six days to evaluate the influence of UV-C radiation on the solution.
Bacteria. Escherichia coli (ATCC 25922) was used to contaminate the hydroponic fluid. The pre-inoculum was prepared by suspending 1 mL of the microorganism in 9 mL of Brain Heart Infusion (BHI) liquid culture medium and incubating at 37°C with 150 rpm agitation for 16 hours. Five milliliters of this culture were then inoculated into 45 mL of fresh BHI medium under the same conditions in a rotary incubator (Quimis®), which was repeated for 12 tubes. The bacterial culture was centrifuged at 3000 rpm for 15 minutes (Centrifuge 5702 Eppendorf®) and re-suspended in sterile distilled water. The optical density (OD) method was used to quantify the bacteria using a Cary 50 Bio UV-Vis Spectrophotometer (Varian, Australia) at 600 nm, adjusted to an initial concentration of 10⁶-10⁸ CFU/mL. The standard colony count was performed to quantify E. coli colonies. According to Silva (2017), the CFU/mL values are presented on a logarithmic scale (base 10). The objective of the study is to verify the effectiveness of the treatment in reducing E. coli in the nutrient solution.
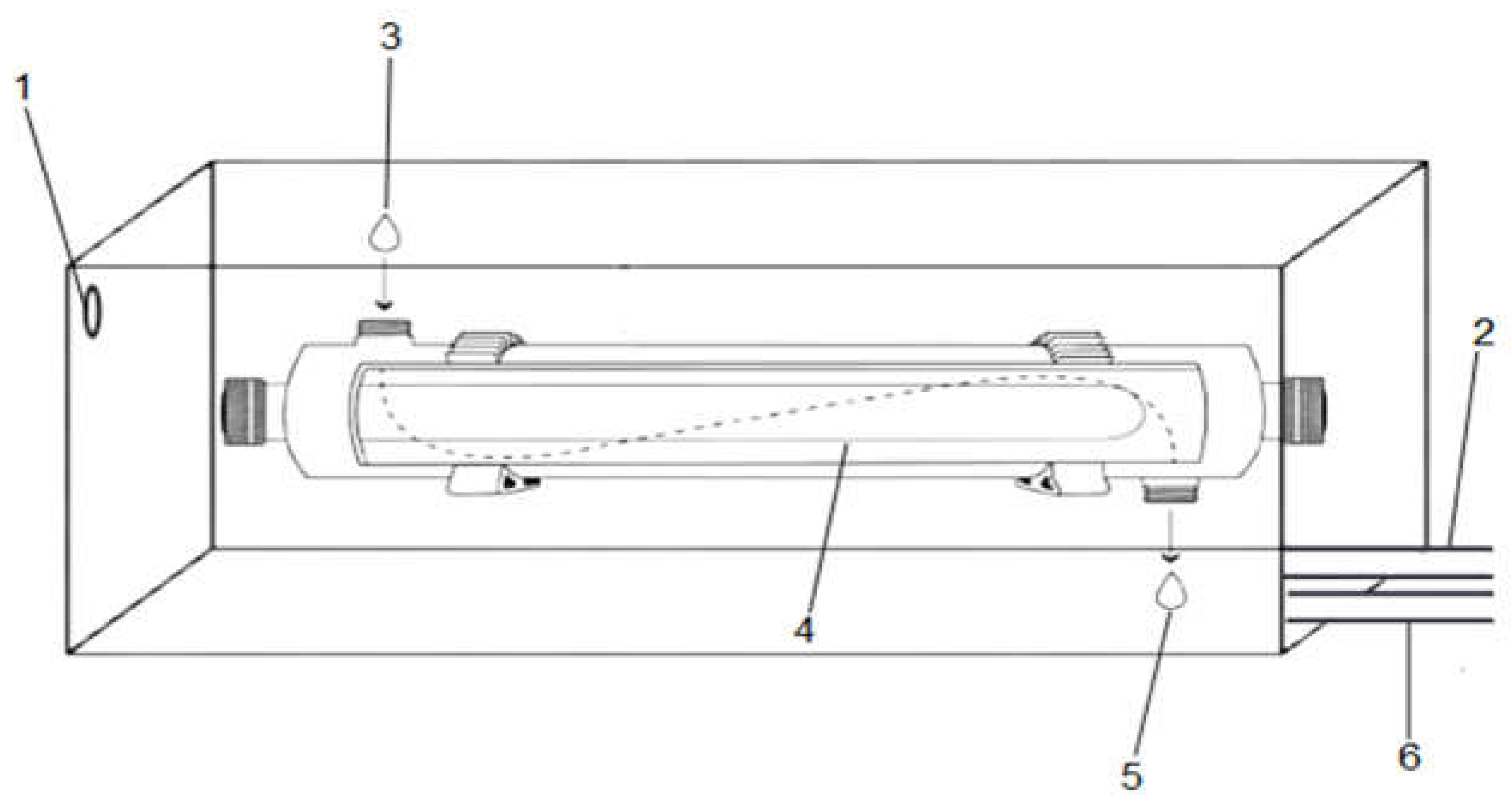
UV-C Decontamination System. The decontamination system (
Figure 1) consisted of a UV-C lamp with a luminous power of 1.6 W, as specified by the manufacturer (OSRAM). The lamp had a diameter of 0.6 cm and was integrated into a reactor system with an influent connection for the hydroponic solution, an access point for fluid exposure to UV-C light, and an outlet for the treated solution. This system had two openings: one for water inflow and another for fluid outflow, both connected to hoses that facilitated circulation of 11 liters of hydroponic solution inside a 20-liter container.
The power of this lamp in mW/cm² was measured using a COHERENT electronic power meter (model LABMAX-TOPO). The power was measured at the ends and along the length of the lamp, and from these values, the average radiation intensity was calculated, corresponding to 5.66 mW/cm². The light dose was determined based on the exposure time, with the values used in this study being 20.37 mJ/cm² and 489.02 mJ/cm².
The recommended EC in hydroponic systems so as not to cause any problems in the plant's development varies from 1.5 to 2.5 S/cm (DALASTRA, 2017). Based on this recommendation, we adjusted the EC of the solution to 1.8 EC (S/cm). The circulation of the solution from the reservoir to the system was carried out through a submersion pump with a flow rate of 200 L/h. The flow of the hydroponics volume to be decontaminated was 55 mL/s.
3. Results
3.1. Effects of UV-C on Hydroponics Solution Components
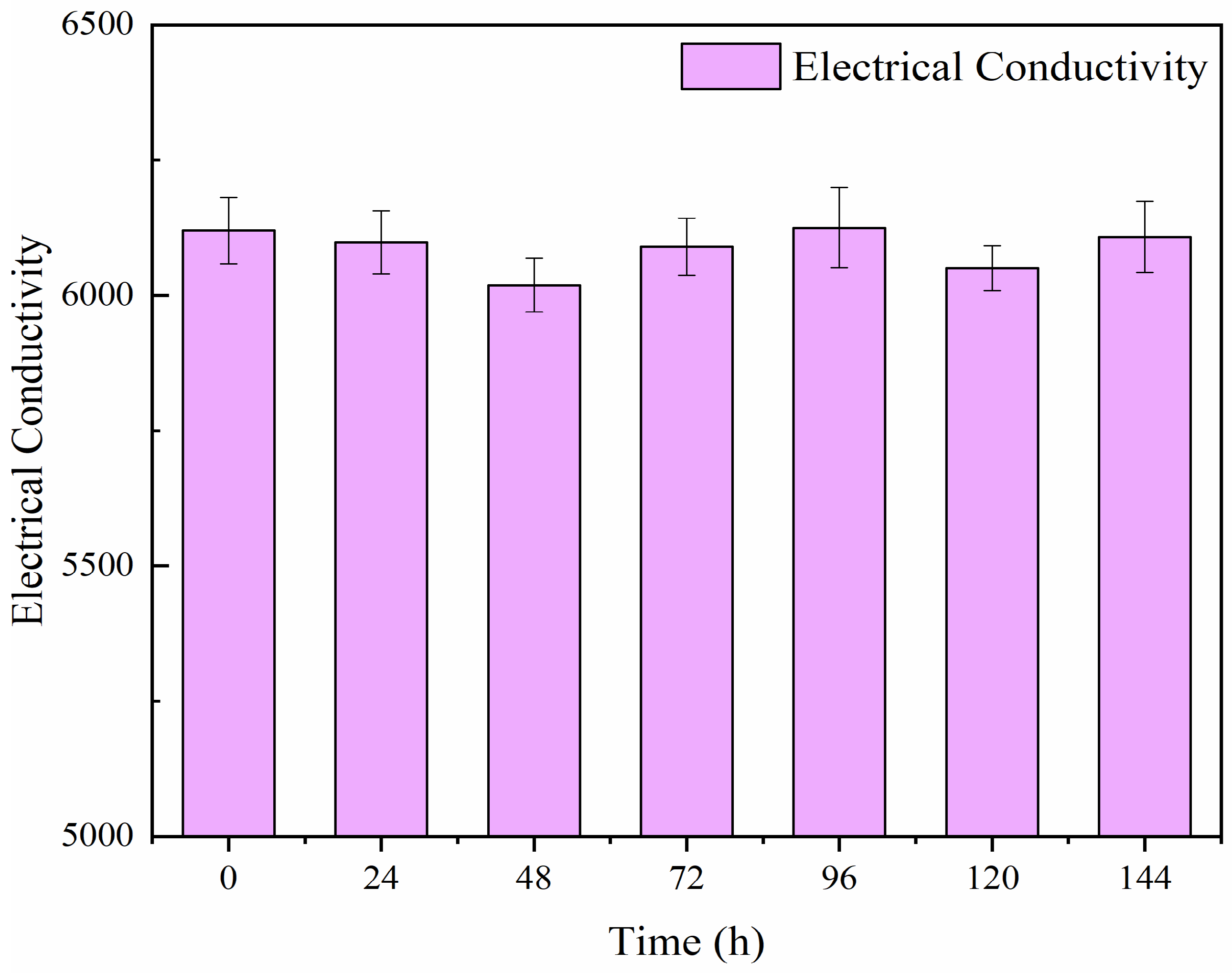
The results presented in
Figure 2 illustrate the exposure of hydroponic fluid components to UV-C radiation, aimed at evaluating potential degradation. The initial electrical conductivity (EC) of the nutrient solution on the first day was 6256 μS/cm (0 h), and after six days of UV-C exposure, the EC increased slightly to 6457 μS/cm. Over the 144-hour exposure period, the results indicated that there was no significant change in the electrical conductivity of the solution. Any notable change in EC could suggest degradation of the substances within the fluid, which might negatively impact the efficiency of microbial inactivation by UV-C.
3.2. Decontamination Efficiency
During the microbial inactivation tests, the concentration of microorganisms remained stable, likely due to sedimentation in the reservoir storing the hydroponic solution. To address this, a submersion pump (described in item 3.4) was introduced to create a reactional environment under dynamic conditions, ensuring continuous fluid circulation during treatment.
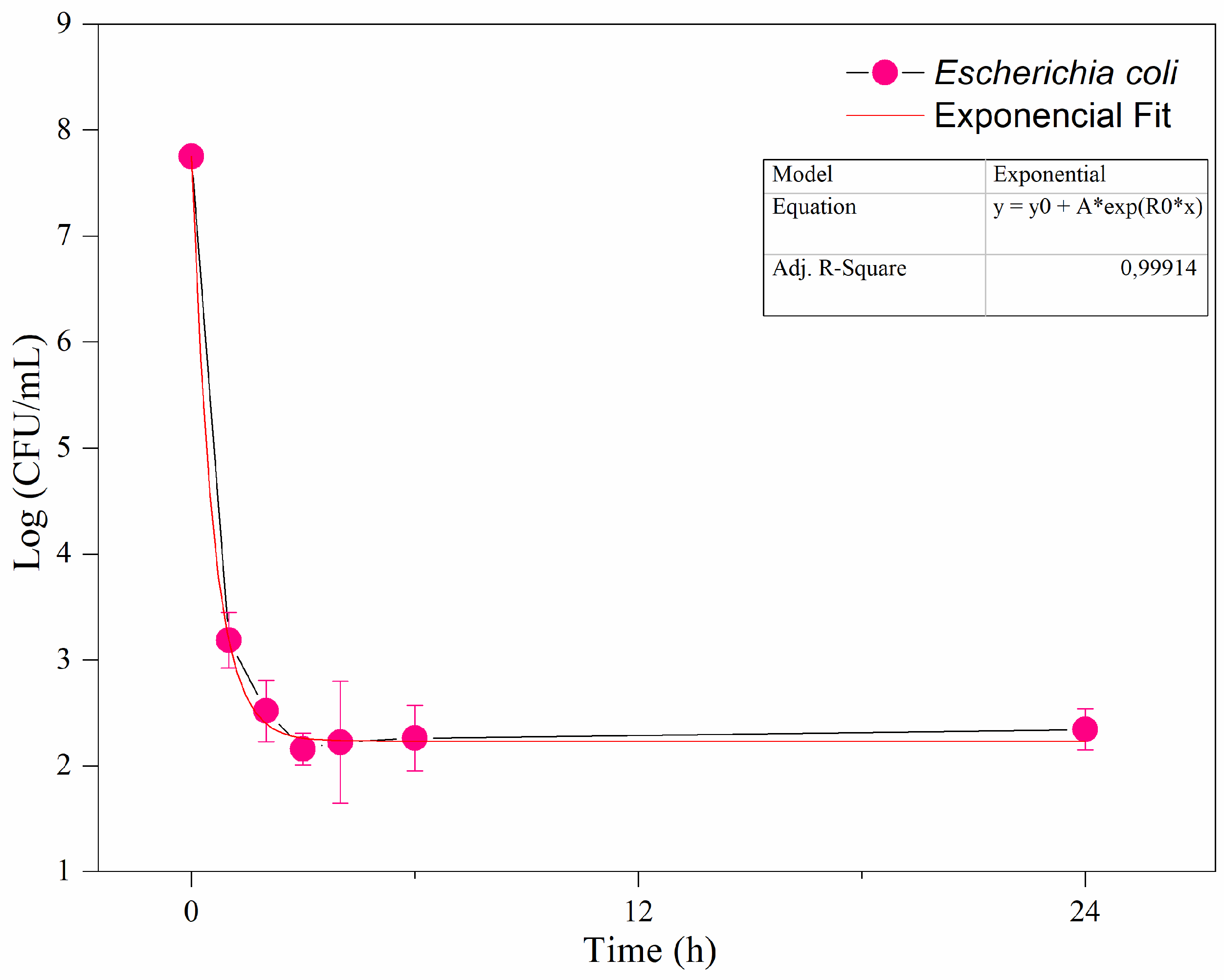
Figure 3 shows the inactivation of E. coli in the hydroponic solution after 24 hours of exposure to UV-C under dynamic conditions (with the pump active). The results demonstrate an exponential reduction, with the most significant inactivation occurring during the initial hours. Within 3 hours, there was a reduction of approximately 5.2 log CFU/mL. A statistical analysis was performed, fitting the data to an exponential model, which yielded an R² value of 0.99, indicating a strong fit and reinforcing the novelty of the findings. After 24 hours of UV-C exposure, the concentration of remaining microorganisms in the hydroponic solution stabilized (
Figure 3).
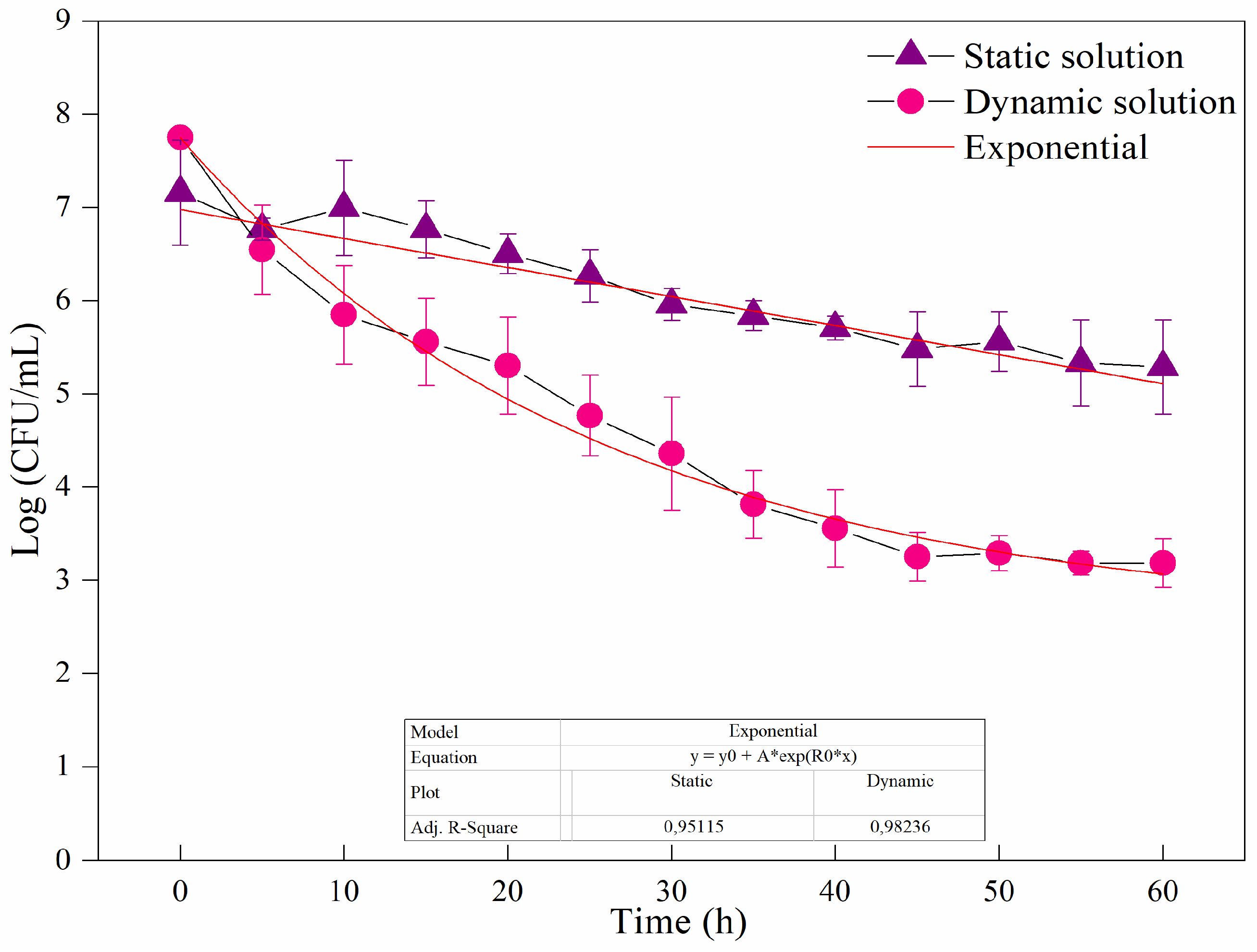
After observing bacterial sedimentation in the nutrient solution, we analyzed the efficiency of bacterial inactivation in the fluid stored in a reservoir, which was later sent for decontamination in the reactor under the two conditions described in item 3.4. When exposing the nutrient solution to UV-C for one hour at a dose of 20.37 mJ/cm², two distinct bacterial inactivation curves were observed depending on whether the fluid was static or dynamic during exposure (Figure 4). In the static condition, after 60 minutes, there was a reduction of approximately 2 log CFU/mL, whereas in the dynamic condition, a more significant inactivation of approximately 4.5 log CFU/mL occurred. Statistical fitting was performed again, yielding an R² of 0.95 for the static condition and an R² of 0.99 for the dynamic condition, further corroborating the findings presented in Figure 4. These results highlight the importance of fluid movement in hydroponic systems for improving UV-C decontamination efficiency. Even in the absence of a pump to induce dynamic conditions, a notable reduction in microbial load was still achieved.
Figure 5.
Microbial reduction of Escherichia Coli in Log (CFU/mL) in hydroponic fluid with UV-C light exposed for 1 h, the nutrient solution is maintained in two different conditions. The results are presented through the media and the registry.
Figure 5.
Microbial reduction of Escherichia Coli in Log (CFU/mL) in hydroponic fluid with UV-C light exposed for 1 h, the nutrient solution is maintained in two different conditions. The results are presented through the media and the registry.
4. Discussion
This study standardized protocols to determine the optimal conditions for bacterial inactivation in hydroponic fluids using ultraviolet (UV-C) light, testing different light doses and flow behaviors (dynamic and static). Bacterial inactivation occurs when UV-C light is absorbed by compounds or microorganisms in the fluid, leading to the formation of reactive oxygen species (Acher et al., 2015). Previous studies have investigated decontamination using UV-C light, particularly in sewage treatment for agricultural reuse, where it has been observed that decontamination efficiency decreases, and electrical conductivity (EC) values increase significantly with specific doses of UV-C (Voltolini, 2021). Other methods, such as the use of cold atmospheric plasma, have also shown an increase in EC, highlighting the advantage of UV-C in decontamination, as no significant changes in EC were observed after days of UV-C exposure (Fernandes, 2020). Several studies have reported reductions of one log less with half the UV-C dose used in this study to treat water with lower turbidity (Beck et al., 2017; Chang et al., 1985; Oliveira, 2003). Sommer et al. (1998) observed a reduction of approximately 4 logs with a dose of 7 mJ/cm², while more complex fluids, such as cabbage juice with high turbidity, required doses up to 108.3 mJ/cm² to achieve a 5.8 log reduction of E. coli P36. This study demonstrated that fluid turbidity, as well as the presence of suspended particles, can impair the UV-C inactivation mechanism (Pierscianowski et al., 2021).
In this work, E. coli inactivation occurred at 20 mJ/cm², resulting in a reduction of approximately 2 logs under static conditions. When compared with Oliveira (2003) and Chang et al. (1985), who achieved a 3 log reduction with 10 mJ/cm², it was observed that under static conditions, bacteria tend to settle at the bottom, reducing their exposure to UV-C. However, dynamic flow conditions demonstrated significantly greater bacterial inactivation due to the continuous fluid movement, which prevents sedimentation and ensures greater exposure of microorganisms to UV-C light.
The nutrient solution used in hydroponic systems is a complex mixture of minerals, salts, and water. The oxidation of some of these minerals can alter their charge and degrade elements such as iron, which, when oxidized, can destabilize Fe chelates and lead to Fe³⁺ formation (Archer, Heuer et al., 1997). Additionally, these minerals can absorb UV-C light, interfering with the transmission of radiation to bacteria. Despite these challenges, this study demonstrated bacterial reductions ranging from 2 to 4.5 logs within one hour, depending on the fluid flow conditions. When comparing this study with others that applied UV-C to fluids of different compositions and turbidity levels, it is clear that this work contributes to a new understanding of UV-C efficacy in complex solutions, such as those used in hydroponic systems. In previous studies, such as Sommer et al. (1998) and Oliveira (2003), greater inactivation efficiency was observed in fluids with lower turbidity, reinforcing the idea that turbidity impairs UV-C transmission. However, this study demonstrated that, even in more complex and highly turbid fluids, UV-C can still be effective, particularly when the fluid is kept in continuous motion.
Moreover, this study differs from previous ones by focusing on hydroponic solutions, which have unique characteristics such as high levels of salts and minerals. These characteristics affect UV-C absorption differently when compared to simpler fluids like drinking water or wastewater. The analysis of flow conditions also presents a significant difference, as dynamic fluid movement proved crucial in improving UV-C efficacy by preventing bacterial sedimentation, which was not extensively explored in previous works. Although the efficacy of UV-C disinfection has been demonstrated, practical challenges remain in large-scale applications in commercial hydroponic systems. The results of this study indicate that UV-C technology can be an effective tool for controlling bacterial contamination in nutrient solutions without altering electrical conductivity, an essential parameter for plant growth. The adoption of UV-C can reduce the need for chemical disinfectants, which is beneficial both for food safety and environmental sustainability.
However, the large-scale application of UV-C faces challenges, such as limited light penetration in systems with high turbidity and the need to ensure continuous fluid movement to optimize exposure. Considering these factors, it is crucial to design hydroponic systems that maximize UV-C efficacy while maintaining low energy and maintenance costs, such as the regular replacement of UV-C lamps. UV-C technology also aligns with the growing demand for more sustainable agricultural practices, as it reduces the need for chemical products and lowers the environmental impact of production systems. As hydroponic systems continue to expand globally, particularly in urban areas, UV-C can play a key role in increasing the efficiency and safety of food production.
This study also presents some limitations that must be considered. Variability in the composition of hydroponic fluids, such as mineral concentration and turbidity, can affect the efficacy of UV-C disinfection. Additionally, the sedimentation of bacteria in static systems limits their exposure to UV-C radiation, highlighting the importance of dynamic systems. Fluid composition variability across different types of hydroponic systems may also influence the applicability of the results in broader contexts. The study focused on the inactivation of E. coli, which may not fully represent the diversity of microorganisms present in hydroponic systems. Therefore, future studies should investigate the impact of UV-C on other common pathogens in hydroponic environments. Furthermore, the long-term impact of continuous UV-C exposure on the nutrient solution and plant development has yet to be explored.
This study addresses a significant gap in the application of UV-C technology to hydroponic systems, a field that has garnered increasing attention in recent years. While UV-C has been widely studied in applications such as water purification and wastewater treatment, its use in complex hydroponic fluids, which exhibit high turbidity and variable composition, remains an emerging area of research. This work provides evidence that UV-C disinfection is a viable option for these systems, especially when there is continuous fluid movement, something that has been underexplored in previous research. The contribution of this study helps consolidate the use of UV-C in commercial hydroponic farming contexts, providing critical data for developing more efficient and safer systems.
5. Conclusion
This study demonstrated that UV-C technology is an effective tool for E. coli inactivation in hydroponic systems, with bacterial reductions ranging from 2 to 4.5 logs, depending on fluid movement. The process was more efficient under dynamic fluid conditions, highlighting the importance of continuous circulation systems to optimize UV-C efficacy. Crucially, UV-C treatment did not alter the nutrient composition or electrical conductivity of the hydroponic solution, both critical factors for plant growth. These findings emphasize the potential of UV-C light for microbiological control in hydroponic systems, offering a sustainable and chemical-free method to enhance food safety while maintaining the nutrient quality of the solution. Given these results, UV-C presents itself as a promising solution for large-scale hydroponic disinfection, contributing to both the efficiency and safety of innovative agricultural systems.
Author Contributions
Conceptualization, B.C., S.L.P., V.S B. and K.C. methodology, B.C., S.L.P., V.S B. and K.C.; validation, B.C., S.L.P., formal analysis, B.C.; investigation, B.C..; resources, V.S B.; data curation, B.C., S.L.P.; writing—original draft preparation, B.C., and K.C.; writing—review and editing, B.C., and K.C.; visualization, B.C., S.L.P., V.S B. and K.C.; supervision, V.S B. and K.C.; project administration, V.S B. and K.C.; funding acquisition, V.S B. All authors have read and agreed to the published version of the manuscript. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This research was funded by the São Paulo Research Foundation (FAPESP) through the Research Center for Optics and Photonics (CEPOF), grant number 2013/07276-1.
Acknowledgments
The authors would like to thank the University of São Paulo (USP), the Institute of Physics of São Carlos (IFSC-USP), the National Council for Scientific and Technological Development (CNPq), the São Paulo Research Foundation (FAPESP), and the Research Center for Optics and Photonics (CEPOF) for their invaluable support. This research would not have been possible without their contributions, including financial support, administrative assistance, and access to essential resources and infrastructure.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Khan, M.; Zhang, S.; Xia, J. Global food security: Perspectives, challenges, and solutions. Food Sci. Technol. Int. 2020, 26, 219–236. [Google Scholar]
- Lazzarotto, A. Nutrient dynamics in hydroponic systems: Theory and practice. Hydroponic Agric. Rev. 2019, 4, 29–46. [Google Scholar]
- Braga, L. Efficient resource management in hydroponics. Hydroponic Syst. J. 2015, 12, 45–58. [Google Scholar]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Environmental impacts of hydroponic systems: Waste management and water usage. Environ. Technol. Innov. 2017, 6, 31–41. [Google Scholar]
- Cooley, M.B.; Chao, D.; Mandrell, R.E. Escherichia coli O157: Survival and growth on lettuce is altered by the presence of epiphytic bacteria. J. Food Prot. 2003, 66, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Danyluk, M.D.; Friedrich, L.M.; Schaffner, D.W. Modeling the growth of Escherichia coli O157 in lettuce and spinach as a function of temperature. J. Food Prot. 2012, 75, 469–476. [Google Scholar]
- Fisher, P.R.; Sutton, B.G. Management of recirculating nutrient solutions in hydroponics. Plant Soil 2007, 298, 67–82. [Google Scholar]
- Franz, E.; van Bruggen, A.H.C.; Semenov, A.M. Modeling the contamination of lettuce with Escherichia coli O157 from manure-amended soil. Appl. Environ. Microbiol. 2005, 71, 3738–3746. [Google Scholar]
- Martins, C.; Batista, C.; Andrade, A. Escherichia coli O157:H7: Characteristics, detection methods, and incidence in foodborne outbreaks. Food Microbiol. J. 2010, 23, 112–121. [Google Scholar]
- Moura, P.; Santos, H.; Silva, R. Fluid dynamics in hydroponic systems and its effect on UV-C efficacy for microbial control. J. Hydroponics Aquaponics 2019, 8, 63–72. [Google Scholar]
- Scarlett, K.S.; McCauley, R.C.; Parker, M.L. Pathogen control in recirculating hydroponic nutrient systems: A review of techniques. Plant Pathol. J. 2015, 15, 72–86. [Google Scholar]
- Beauvais, W.; Glover, B.; Pruvot, M.; Arsenault, J.; Dubé, C. Ultraviolet (UV) disinfection of drinking water: Current practices and research needs. J. Water Supply Res. Technol. AQUA 2021, 70, 281–293. [Google Scholar]
- Kowalski, W. Ultraviolet germicidal irradiation (UVGI) for water and wastewater treatment. In Disinfection and Decontamination: Principles, Applications, and Design; Kowalski, W., Ed.; Springer: Berlin, Germany, 2010; pp. 233–246. [Google Scholar]
- García, J.L.; Iglesias, S.; Alcaraz, D.; Martinez, S. Application of UV-C radiation in hydroponic lettuce production systems. J. Hortic. Sci. 2014, 89, 17–25. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).