Submitted:
13 October 2024
Posted:
14 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Hydrolytic activities of the PON1 enzyme
3. Association of PON1 with Human Disease
3.1. PON1, Oxidative Stress, Inflammation, and CVD
3.2. PON1, Lipid Oxidation, Hcy-thiolactone, and Alzheimer’s Disease
3.2.1. Mice
3.2.2. Humans
3.3. PON1 Depletion, Dysregulation of Signaling Pathways, and Cancer
4. PON1 Has no Intrinsic Anti-oxidant Activity: Don’t Waste Clean Thoughts on Dirty Enzymes
5. Mechanistic Bases of PON1 Involvement in Human Disease
5.1. Low PON1 Activity in Dysfunctional HDL Is Associated with Impaired Nitric Oxide Production in Endothelial Cells
5.2. Pon1 Depletion Affects Expression of Genes Involved in Inflammation, Oxidative Stress, and Blood Clotting
5.3. Pon1 Depletion Increases Expression of Liver Oxidative Stress Genes and Accelerates Atherosclerosis
5.4. Pon1 Depletion Increases Expression of Oxidative Stress Genes in Liver, Kidney, and Brain
5.5. Pon1 Depletion in Scarb1-/- Mice Is Associated with Upregulated Expression of Oxidative Stress Genes
5.6. Pon1 Depletion in Scarb1-/- Mice Affects Expression of Oxidative Stress and Inflammation-Related Liver Genes
5.7. PON1 Regulates the Expression of Hepatic Genes Involved in HDL Metabolism, Oxidative Stress, and Inflammation.
5.8. Pon1 1 Ameliorates Renal Lipotoxicity by Regulating Genes Involved in Activating Lipophagy and Inhibiting Pyroptosis
5.9. PON1-Q192R Polymorphism Influences Oxidative Stress and Inflammation Proteins in Human Plasma
5.10. Pon1−/− Genotype Influences Oxidative Stress and Inflammation Proteins in Mouse Plasma
5.11. Metabolic Stress Amplifies Pro-Inflammatory, Pro-Oxidative, and Pro-Atherogenic Changes in Mouse Plasma Proteome Induced by Pon1 Depletion
6. Conclusions
Data Availability Statement
Acknowledgments
Disclosures
References
- Libby, P., Inflammation in atherosclerosis. Nature 2002, 420, (6917), 868-74.
- Hopkins, P. N., Molecular biology of atherosclerosis. Physiol Rev 2013, 93, (3), 1317-542. [CrossRef]
- Witztum, J. L.; Steinberg, D., The oxidative modification hypothesis of atherosclerosis: does it hold for humans? Trends Cardiovasc Med 2001, 11, (3-4), 93-102.
- Navab, M.; Ananthramaiah, G. M.; Reddy, S. T.; Van Lenten, B. J.; Ansell, B. J.; Fonarow, G. C.; Vahabzadeh, K.; Hama, S.; Hough, G.; Kamranpour, N.; Berliner, J. A.; Lusis, A. J.; Fogelman, A. M., The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res 2004, 45, (6), 993-1007. [CrossRef]
- Libby, P.; Ridker, P. M.; Hansson, G. K., Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, (7347), 317-25. [CrossRef]
- Besler, C.; Heinrich, K.; Rohrer, L.; Doerries, C.; Riwanto, M.; Shih, D. M.; Chroni, A.; Yonekawa, K.; Stein, S.; Schaefer, N.; Mueller, M.; Akhmedov, A.; Daniil, G.; Manes, C.; Templin, C.; Wyss, C.; Maier, W.; Tanner, F. C.; Matter, C. M.; Corti, R.; Furlong, C.; Lusis, A. J.; von Eckardstein, A.; Fogelman, A. M.; Luscher, T. F.; Landmesser, U., Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest 2011, 121, (7), 2693-708.
- Barter, P. J.; Nicholls, S.; Rye, K. A.; Anantharamaiah, G. M.; Navab, M.; Fogelman, A. M., Antiinflammatory properties of HDL. Circ Res 2004, 95, (8), 764-72. [CrossRef]
- Zerrad-Saadi, A.; Therond, P.; Chantepie, S.; Couturier, M.; Rye, K. A.; Chapman, M. J.; Kontush, A., HDL3-mediated inactivation of LDL-associated phospholipid hydroperoxides is determined by the redox status of apolipoprotein A-I and HDL particle surface lipid rigidity: relevance to inflammation and atherogenesis. Arterioscler Thromb Vasc Biol 2009, 29, (12), 2169-75.
- Navab, M.; Hama, S. Y.; Anantharamaiah, G. M.; Hassan, K.; Hough, G. P.; Watson, A. D.; Reddy, S. T.; Sevanian, A.; Fonarow, G. C.; Fogelman, A. M., Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res 2000, 41, (9), 1495-508. [CrossRef]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A., Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin 2017, 8, 66-77. [CrossRef]
- Soran, H.; Schofield, J. D.; Liu, Y.; Durrington, P. N., How HDL protects LDL against atherogenic modification: paraoxonase 1 and other dramatis personae. Curr Opin Lipidol 2015, 26, (4), 247-56.
- Mackness, B.; Beltran-Debon, R.; Aragones, G.; Joven, J.; Camps, J.; Mackness, M., Human tissue distribution of paraoxonases 1 and 2 mRNA. IUBMB Life 2010, 62, (6), 480-2. [CrossRef]
- Marsillach, J.; Mackness, B.; Mackness, M.; Riu, F.; Beltran, R.; Joven, J.; Camps, J., Immunohistochemical analysis of paraoxonases-1, 2, and 3 expression in normal mouse tissues. Free radical biology & medicine 2008, 45, (2), 146-57.
- Leduc, V.; Legault, V.; Dea, D.; Poirier, J., Normalization of gene expression using SYBR green qPCR: a case for paraoxonase 1 and 2 in Alzheimer's disease brains. J Neurosci Methods 2011, 200, (1), 14-9.
- Witucki, L.; Jakubowski, H., Depletion of Paraoxonase 1 (Pon1) Dysregulates mTOR, Autophagy, and Accelerates Amyloid Beta Accumulation in Mice. Cells 2023, 12, (5). [CrossRef]
- Moren, X.; Lhomme, M.; Bulla, A.; Sanchez, J. C.; Kontush, A.; James, R. W., Proteomic and lipidomic analyses of paraoxonase defined high density lipoprotein particles: Association of paraoxonase with the anti-coagulant, protein S. Proteomics Clin Appl 2016, 10, (3), 230-8. [CrossRef]
- Humbert, R.; Adler, D. A.; Disteche, C. M.; Hassett, C.; Omiecinski, C. J.; Furlong, C. E., The molecular basis of the human serum paraoxonase activity polymorphism. Nat Genet 1993, 3, (1), 73-6. [CrossRef]
- Gan, K. N.; Smolen, A.; Eckerson, H. W.; La Du, B. N., Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos 1991, 19, (1), 100-6.
- Jakubowski, H., Calcium-dependent human serum homocysteine thiolactone hydrolase. A protective mechanism against protein N-homocysteinylation. J Biol Chem 2000, 275, (6), 3957-62. [CrossRef]
- Jakubowski, H.; Goldman, E., Synthesis of homocysteine thiolactone by methionyl-tRNA synthetase in cultured mammalian cells. FEBS Lett 1993, 317, (3), 237-40.
- Jakubowski, H., Quality control in tRNA charging. Wiley Interdiscip Rev RNA 2012, 3, (3), 295-310. [CrossRef]
- Jakubowski, H., Homocysteine Modification in Protein Structure/Function and Human Disease. Physiol Rev 2019, 99, (1), 555-604.
- Jakubowski, H.; Ambrosius, W. T.; Pratt, J. H., Genetic determinants of homocysteine thiolactonase activity in humans: implications for atherosclerosis. FEBS Lett 2001, 491, (1-2), 35-9. [CrossRef]
- Jakubowski, H., Homocysteine thiolactone: metabolic origin and protein homocysteinylation in humans. J Nutr 2000, 130, (2S Suppl), 377S-381S. [CrossRef]
- Perla-Kajan, J.; Borowczyk, K.; Glowacki, R.; Nygard, O.; Jakubowski, H., Paraoxonase 1 Q192R genotype and activity affect homocysteine thiolactone levels in humans. FASEB J 2018, fj201800346R. [CrossRef]
- Costa, L. G.; Cole, T. B.; Jarvik, G. P.; Furlong, C. E., Functional genomic of the paraoxonase (PON1) polymorphisms: effects on pesticide sensitivity, cardiovascular disease, and drug metabolism. Annu Rev Med 2003, 54, 371-92.
- Bhattacharyya, T.; Nicholls, S. J.; Topol, E. J.; Zhang, R.; Yang, X.; Schmitt, D.; Fu, X.; Shao, M.; Brennan, D. M.; Ellis, S. G.; Brennan, M. L.; Allayee, H.; Lusis, A. J.; Hazen, S. L., Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008, 299, (11), 1265-76. [CrossRef]
- Tang, W. H.; Hartiala, J.; Fan, Y.; Wu, Y.; Stewart, A. F.; Erdmann, J.; Kathiresan, S.; Consortium, C. A.; Roberts, R.; McPherson, R.; Allayee, H.; Hazen, S. L., Clinical and genetic association of serum paraoxonase and arylesterase activities with cardiovascular risk. Arterioscler Thromb Vasc Biol 2012, 32, (11), 2803-12. [CrossRef]
- Mackness, B.; Durrington, P.; McElduff, P.; Yarnell, J.; Azam, N.; Watt, M.; Mackness, M., Low paraoxonase activity predicts coronary events in the Caerphilly Prospective Study. Circulation 2003, 107, (22), 2775-9. [CrossRef]
- Dube, P.; Khalaf, F. K.; DeRiso, A.; Mohammed, C. J.; Connolly, J. A.; Battepati, D.; Lad, A.; Breidenbach, J. D.; Kleinhenz, A. L.; Khatib-Shahidi, B.; Patel, M.; Tassavvor, I.; Gohara, A. F.; Malhotra, D.; Morgan, E. E.; Haller, S. T.; Kennedy, D. J., Cardioprotective Role for Paraoxonase-1 in Chronic Kidney Disease. Biomedicines 2022, 10, (9). [CrossRef]
- Meneses, M. J.; Silvestre, R.; Sousa-Lima, I.; Macedo, M. P., Paraoxonase-1 as a Regulator of Glucose and Lipid Homeostasis: Impact on the Onset and Progression of Metabolic Disorders. Int J Mol Sci 2019, 20, (16). [CrossRef]
- Cervellati, C.; Trentini, A.; Romani, A.; Bellini, T.; Bosi, C.; Ortolani, B.; Zurlo, A.; Passaro, A.; Seripa, D.; Zuliani, G., Serum paraoxonase and arylesterase activities of paraoxonase-1 (PON-1), mild cognitive impairment, and 2-year conversion to dementia: A pilot study. J Neurochem 2015, 135, (2), 395-401.
- Dong, L.; Dong, C.; Yu, Y.; Jiao, X.; Zhang, X.; Zhang, X.; Li, Z., Transcriptomic analysis of Paraoxonase 1 expression in hepatocellular carcinoma and its potential impact on tumor immunity. Clin Transl Oncol 2024. [CrossRef]
- Zhang, Q.; Jiang, Z.; Xu, Y., HDL and Oxidation. Adv Exp Med Biol 2022, 1377, 63-77.
- Marsillach, J.; Adorni, M. P.; Zimetti, F.; Papotti, B.; Zuliani, G.; Cervellati, C., HDL Proteome and Alzheimer's Disease: Evidence of a Link. Antioxidants (Basel) 2020, 9, (12). [CrossRef]
- Davidson, W. S.; Silva, R. A.; Chantepie, S.; Lagor, W. R.; Chapman, M. J.; Kontush, A., Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler Thromb Vasc Biol 2009, 29, (6), 870-6. [CrossRef]
- de Souza, J. A.; Vindis, C.; Negre-Salvayre, A.; Rye, K. A.; Couturier, M.; Therond, P.; Chantepie, S.; Salvayre, R.; Chapman, M. J.; Kontush, A., Small, dense HDL 3 particles attenuate apoptosis in endothelial cells: pivotal role of apolipoprotein A-I. J Cell Mol Med 2010, 14, (3), 608-20. [CrossRef]
- Jakubowski, H., Proteomic Exploration of Paraoxonase 1 Function in Health and Disease. Int J Mol Sci 2023, 24, (9). [CrossRef]
- Borowczyk, K.; Shih, D. M.; Jakubowski, H., Metabolism and neurotoxicity of homocysteine thiolactone in mice: evidence for a protective role of paraoxonase 1. Journal of Alzheimer's disease : JAD 2012, 30, (2), 225-31. [CrossRef]
- Borowczyk, K.; Piechocka, J.; Glowacki, R.; Dhar, I.; Midtun, O.; Tell, G. S.; Ueland, P. M.; Nygard, O.; Jakubowski, H., Urinary excretion of homocysteine thiolactone and the risk of acute myocardial infarction in coronary artery disease patients: the WENBIT trial. J Intern Med 2019, 285, (2), 232-244. [CrossRef]
- Sauls, D. L.; Lockhart, E.; Warren, M. E.; Lenkowski, A.; Wilhelm, S. E.; Hoffman, M., Modification of fibrinogen by homocysteine thiolactone increases resistance to fibrinolysis: a potential mechanism of the thrombotic tendency in hyperhomocysteinemia. Biochemistry 2006, 45, (8), 2480-7. [CrossRef]
- Sikora, M.; Skrzydlewski, P.; Perla-Kajan, J.; Jakubowski, H., Homocysteine thiolactone contributes to the prognostic value of fibrin clot structure/function in coronary artery disease. PLoS One 2022, 17, (10), e0275956. [CrossRef]
- Khersonsky, O.; Tawfik, D. S., Structure-reactivity studies of serum paraoxonase PON1 suggest that its native activity is lactonase. Biochemistry 2005, 44, (16), 6371-82.
- Draganov, D. I.; Teiber, J. F.; Speelman, A.; Osawa, Y.; Sunahara, R.; La Du, B. N., Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res 2005, 46, (6), 1239-47. [CrossRef]
- Mohammed, C. J.; Lamichhane, S.; Connolly, J. A.; Soehnlen, S. M.; Khalaf, F. K.; Malhotra, D.; Haller, S. T.; Isailovic, D.; Kennedy, D. J., A PON for All Seasons: Comparing Paraoxonase Enzyme Substrates, Activity and Action including the Role of PON3 in Health and Disease. Antioxidants (Basel) 2022, 11, (3). [CrossRef]
- Slutsky Smith, E. A.; Khatib, S.; Szuchman-Sapir, A., Fishing for lipid lactones using selective reaction and characteristic fragmentation pattern. J Chromatogr B Analyt Technol Biomed Life Sci 2022, 1197, 123201. [CrossRef]
- Momma, T. Y.; Kuhnle, G. G. C.; Fong, R. Y.; Ensunsa, J. L.; Crozier, A.; Schroeter, H.; Ottaviani, J. I., 5-(3',4'-Dihydroxyphenyl)-gamma-Valerolactone Is a Substrate for Human Paraoxonase: A Novel Pathway in Flavan-3-ol Metabolism. Mol Nutr Food Res 2023, 67, (17), e2300281.
- Sesso, H. D.; Manson, J. E.; Aragaki, A. K.; Rist, P. M.; Johnson, L. G.; Friedenberg, G.; Copeland, T.; Clar, A.; Mora, S.; Moorthy, M. V.; Sarkissian, A.; Carrick, W. R.; Anderson, G. L.; Group, C. R., Effect of cocoa flavanol supplementation for the prevention of cardiovascular disease events: the COcoa Supplement and Multivitamin Outcomes Study (COSMOS) randomized clinical trial. Am J Clin Nutr 2022, 115, (6), 1490-1500. [CrossRef]
- Baker, L. D.; Manson, J. E.; Rapp, S. R.; Sesso, H. D.; Gaussoin, S. A.; Shumaker, S. A.; Espeland, M. A., Effects of cocoa extract and a multivitamin on cognitive function: A randomized clinical trial. Alzheimers Dement 2023, 19, (4), 1308-1319. [CrossRef]
- Mena, P.; Bresciani, L.; Brindani, N.; Ludwig, I. A.; Pereira-Caro, G.; Angelino, D.; Llorach, R.; Calani, L.; Brighenti, F.; Clifford, M. N.; Gill, C. I. R.; Crozier, A.; Curti, C.; Del Rio, D., Phenyl-gamma-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: synthesis, analysis, bioavailability, and bioactivity. Nat Prod Rep 2019, 36, (5), 714-752. [CrossRef]
- Angelino, D.; Carregosa, D.; Domenech-Coca, C.; Savi, M.; Figueira, I.; Brindani, N.; Jang, S.; Lakshman, S.; Molokin, A.; Urban, J. F., Jr.; Davis, C. D.; Brito, M. A.; Kim, K. S.; Brighenti, F.; Curti, C.; Blade, C.; Del Bas, J. M.; Stilli, D.; Solano-Aguilar, G. I.; Santos, C. N. D.; Del Rio, D.; Mena, P., 5-(Hydroxyphenyl)-gamma-Valerolactone-Sulfate, a Key Microbial Metabolite of Flavan-3-ols, Is Able to Reach the Brain: Evidence from Different in Silico, In Vitro and In Vivo Experimental Models. Nutrients 2019, 11, (11). [CrossRef]
- Corral-Jara, K. F.; Nuthikattu, S.; Rutledge, J.; Villablanca, A.; Morand, C.; Schroeter, H.; Milenkovic, D., Integrated Multi-Omic Analyses of the Genomic Modifications by Gut Microbiome-Derived Metabolites of Epicatechin, 5-(4'-Hydroxyphenyl)-gamma-Valerolactone, in TNFalpha-Stimulated Primary Human Brain Microvascular Endothelial Cells. Front Neurosci 2021, 15, 622640. [CrossRef]
- Costa, L. G.; Giordano, G.; Cole, T. B.; Marsillach, J.; Furlong, C. E., Paraoxonase 1 (PON1) as a genetic determinant of susceptibility to organophosphate toxicity. Toxicology 2013, 307, 115-22. [CrossRef]
- Shih, D. M.; Gu, L.; Xia, Y. R.; Navab, M.; Li, W. F.; Hama, S.; Castellani, L. W.; Furlong, C. E.; Costa, L. G.; Fogelman, A. M.; Lusis, A. J., Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature 1998, 394, (6690), 284-7.
- Shih, D. M.; Xia, Y. R.; Wang, X. P.; Miller, E.; Castellani, L. W.; Subbanagounder, G.; Cheroutre, H.; Faull, K. F.; Berliner, J. A.; Witztum, J. L.; Lusis, A. J., Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J Biol Chem 2000, 275, (23), 17527-35. [CrossRef]
- Hong, C. G.; Florida, E.; Li, H.; Parel, P. M.; Mehta, N. N.; Sorokin, A. V., Oxidized low-density lipoprotein associates with cardiovascular disease by a vicious cycle of atherosclerosis and inflammation: A systematic review and meta-analysis. Front Cardiovasc Med 2022, 9, 1023651. [CrossRef]
- Kotani, K.; Watanabe, Y.; Miura, K.; Gugliucci, A., Paraoxonase 1 and Non-Alcoholic Fatty Liver Disease: A Meta-Analysis. Biomolecules 2021, 26(8), 2323. [CrossRef]
- Suszynska-Zajczyk, J.; Luczak, M.; Marczak, L.; Jakubowski, H., Inactivation of the paraoxonase 1 gene affects the expression of mouse brain proteins involved in neurodegeneration. J Alzheimers Dis 2014, 42, (1), 247-60. [CrossRef]
- Bednarz-Misa, I.; Berdowska, I.; Zboch, M.; Misiak, B.; Zielinski, B.; Placzkowska, S.; Fleszar, M.; Wisniewski, J.; Gamian, A.; Krzystek-Korpacka, M., Paraoxonase 1 decline and lipid peroxidation rise reflect a degree of brain atrophy and vascular impairment in dementia. Adv Clin Exp Med 2020, 29, (1), 71-78. [CrossRef]
- Bade, J. D.; Veeramalla, V.; Naidu, M. B. R.; Lalitha, D. L.; Ponnada, S. C.; Kandi, V., Serum Activities of Paraoxonase 1 (PON1) in Predicting Liver Damage Among Patients Diagnosed With Hepatocellular Carcinoma: A Case-Control Study. Cureus 2023, 15, (9), e46234. [CrossRef]
- Tward, A.; Xia, Y. R.; Wang, X. P.; Shi, Y. S.; Park, C.; Castellani, L. W.; Lusis, A. J.; Shih, D. M., Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation 2002, 106, (4), 484-90. [CrossRef]
- Mackness, B.; Quarck, R.; Verreth, W.; Mackness, M.; Holvoet, P., Human paraoxonase-1 overexpression inhibits atherosclerosis in a mouse model of metabolic syndrome. Arterioscler Thromb Vasc Biol 2006, 26, (7), 1545-50. [CrossRef]
- She, Z. G.; Zheng, W.; Wei, Y. S.; Chen, H. Z.; Wang, A. B.; Li, H. L.; Liu, G.; Zhang, R.; Liu, J. J.; Stallcup, W. B.; Zhou, Z.; Liu, D. P.; Liang, C. C., Human paraoxonase gene cluster transgenic overexpression represses atherogenesis and promotes atherosclerotic plaque stability in ApoE-null mice. Circ Res 2009, 104, (10), 1160-8. [CrossRef]
- Brophy, V. H.; Jampsa, R. L.; Clendenning, J. B.; McKinstry, L. A.; Jarvik, G. P.; Furlong, C. E., Effects of 5' regulatory-region polymorphisms on paraoxonase-gene (PON1) expression. American journal of human genetics 2001, 68, (6), 1428-36. [CrossRef]
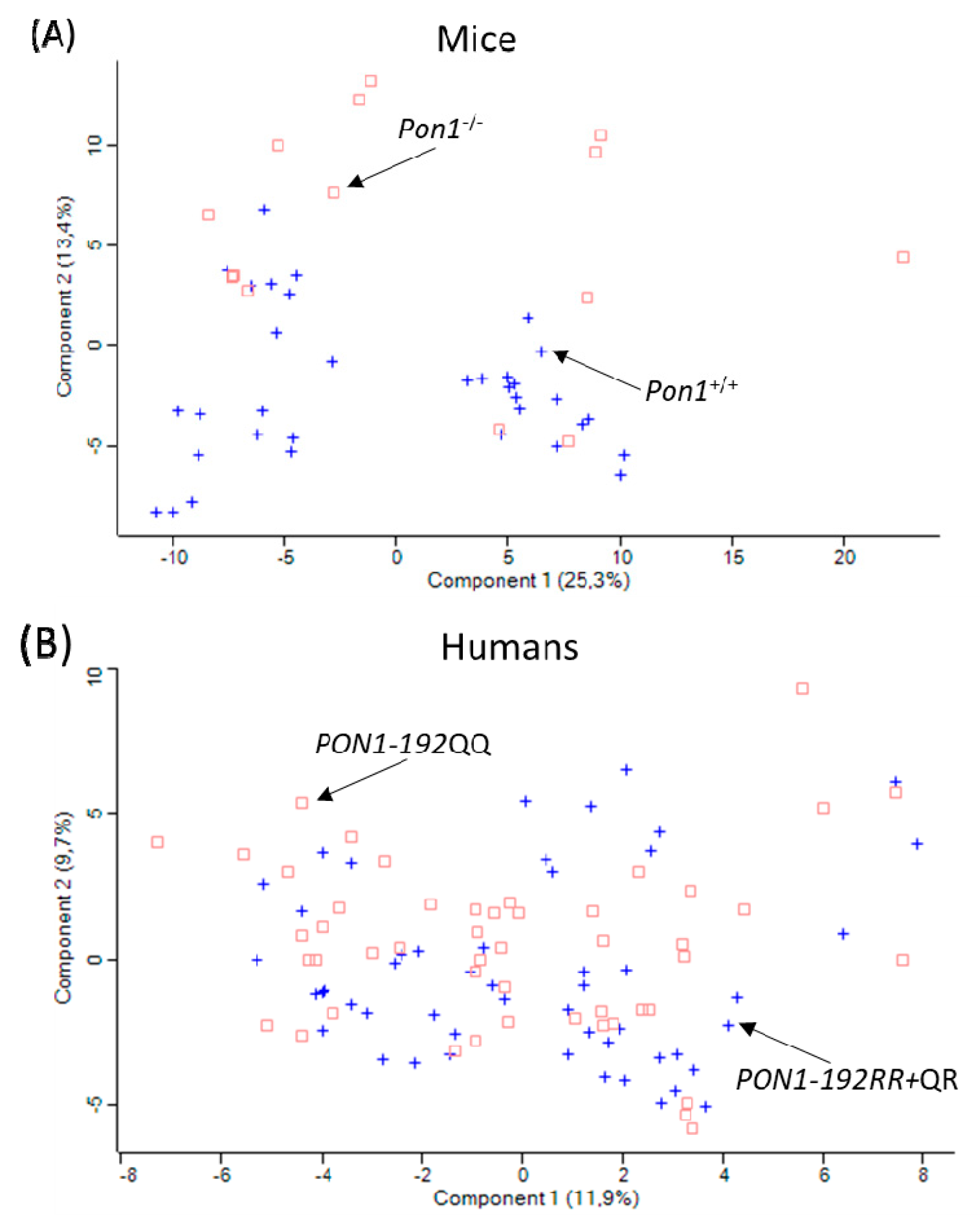
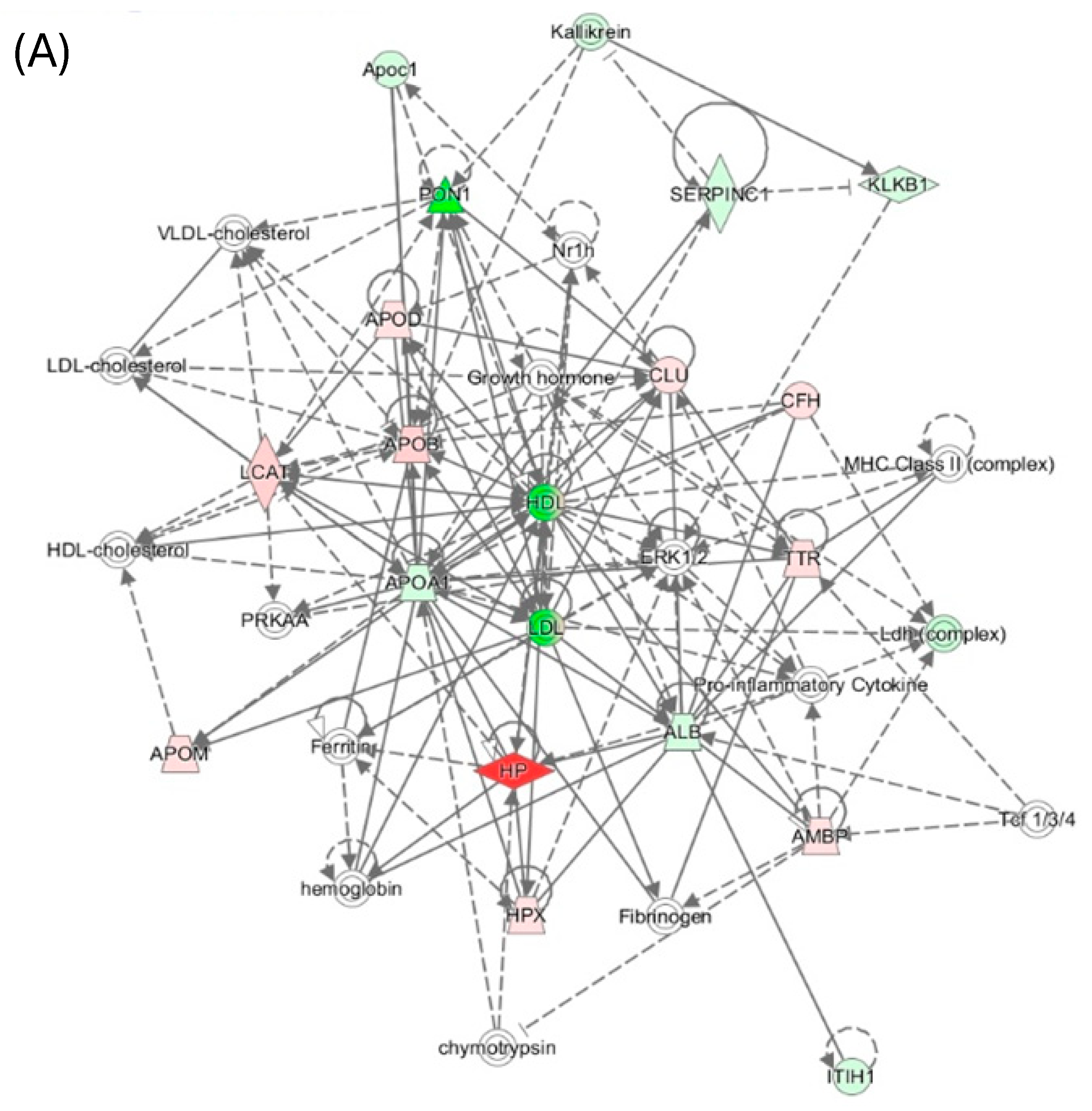
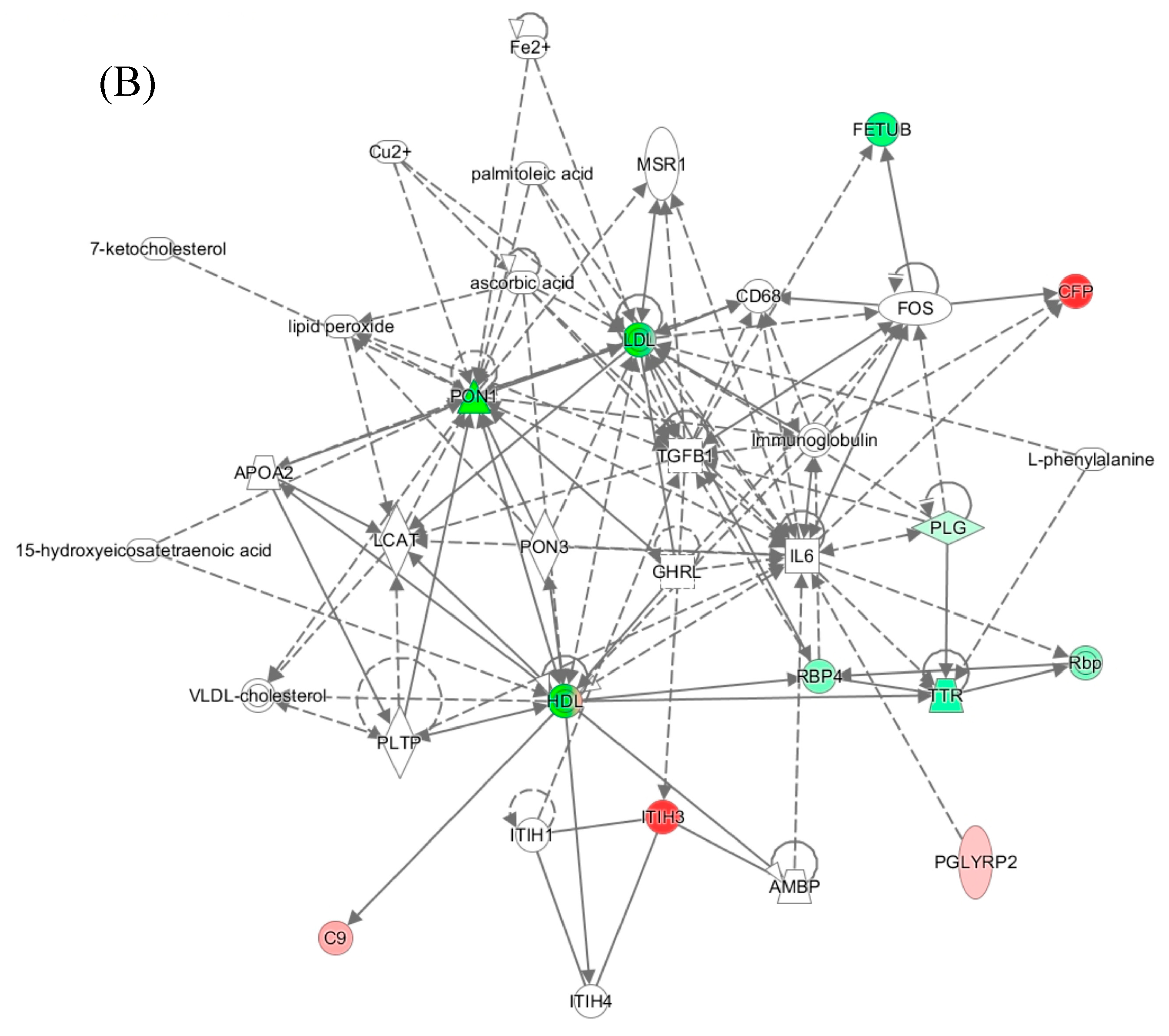
- Sikora, M.; Bretes, E.; Perla-Kajan, J.; Lewandowska, I.; Marczak, L.; Jakubowski, H., Genetic Attenuation of Paraoxonase 1 Activity Induces Proatherogenic Changes in Plasma Proteomes of Mice and Humans. Antioxidants (Basel) 2020, 9, (12). [CrossRef]
- Kresanov, P.; Vasankari, T.; Ahotupa, M.; Kaikkonen, J.; Hutri-Kahonen, N.; Juonala, M.; Kahonen, M.; Lehtimaki, T.; Viikari, J.; Raitakari, O. T., Paraoxonase-1 and oxidized lipoprotein lipids. The Cardiovascular Risk in Young Finns Study. Atherosclerosis 2015, 241, (2), 502-6. [CrossRef]
- Ahotupa, M.; Marniemi, J.; Lehtimaki, T.; Talvinen, K.; Raitakari, O. T.; Vasankari, T.; Viikari, J.; Luoma, J.; Yla-Herttuala, S., Baseline diene conjugation in LDL lipids as a direct measure of in vivo LDL oxidation. Clin Biochem 1998, 31, (4), 257-61. [CrossRef]
- Esterbauer, H.; Striegl, G.; Puhl, H.; Rotheneder, M., Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun 1989, 6, (1), 67-75.
- Kuchta, A.; Strzelecki, A.; Cwiklinska, A.; Toton, M.; Gruchala, M.; Zdrojewski, Z.; Kortas-Stempak, B.; Gliwinska, A.; Dabkowski, K.; Jankowski, M., PON-1 Activity and Plasma 8-Isoprostane Concentration in Patients with Angiographically Proven Coronary Artery Disease. Oxid Med Cell Longev 2015, 2015, 5136937. [CrossRef]
- Carlson, C. S.; Heagerty, P. J.; Hatsukami, T. S.; Richter, R. J.; Ranchalis, J.; Lewis, J.; Bacus, T. J.; McKinstry, L. A.; Schellenberg, G. D.; Rieder, M.; Nickerson, D.; Furlong, C. E.; Chait, A.; Jarvik, G. P., TagSNP analyses of the PON gene cluster: effects on PON1 activity, LDL oxidative susceptibility, and vascular disease. J Lipid Res 2006, 47, (5), 1014-24. [CrossRef]
- Hayden, K. M.; Norton, M. C.; Darcey, D.; Ostbye, T.; Zandi, P. P.; Breitner, J. C.; Welsh-Bohmer, K. A.; Cache County Study, I., Occupational exposure to pesticides increases the risk of incident AD: the Cache County study. Neurology 2010, 74, (19), 1524-30.
- Jones, N., Alzheimer disease: Risk of dementia and Alzheimer disease increases with occupational pesticide exposure. Nat Rev Neurol 2010, 6, (7), 353.
- Tanzi, R. E., The genetics of Alzheimer disease. Cold Spring Harb Perspect Med 2012, 2, (10).
- Blatter, M. C.; James, R. W.; Messmer, S.; Barja, F.; Pometta, D., Identification of a distinct human high-density lipoprotein subspecies defined by a lipoprotein-associated protein, K-45. Identity of K-45 with paraoxonase. Eur J Biochem 1993, 211, (3), 871-9.
- Oakley, H.; Cole, S. L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; Berry, R.; Vassar, R., Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci 2006, 26, (40), 10129-40.
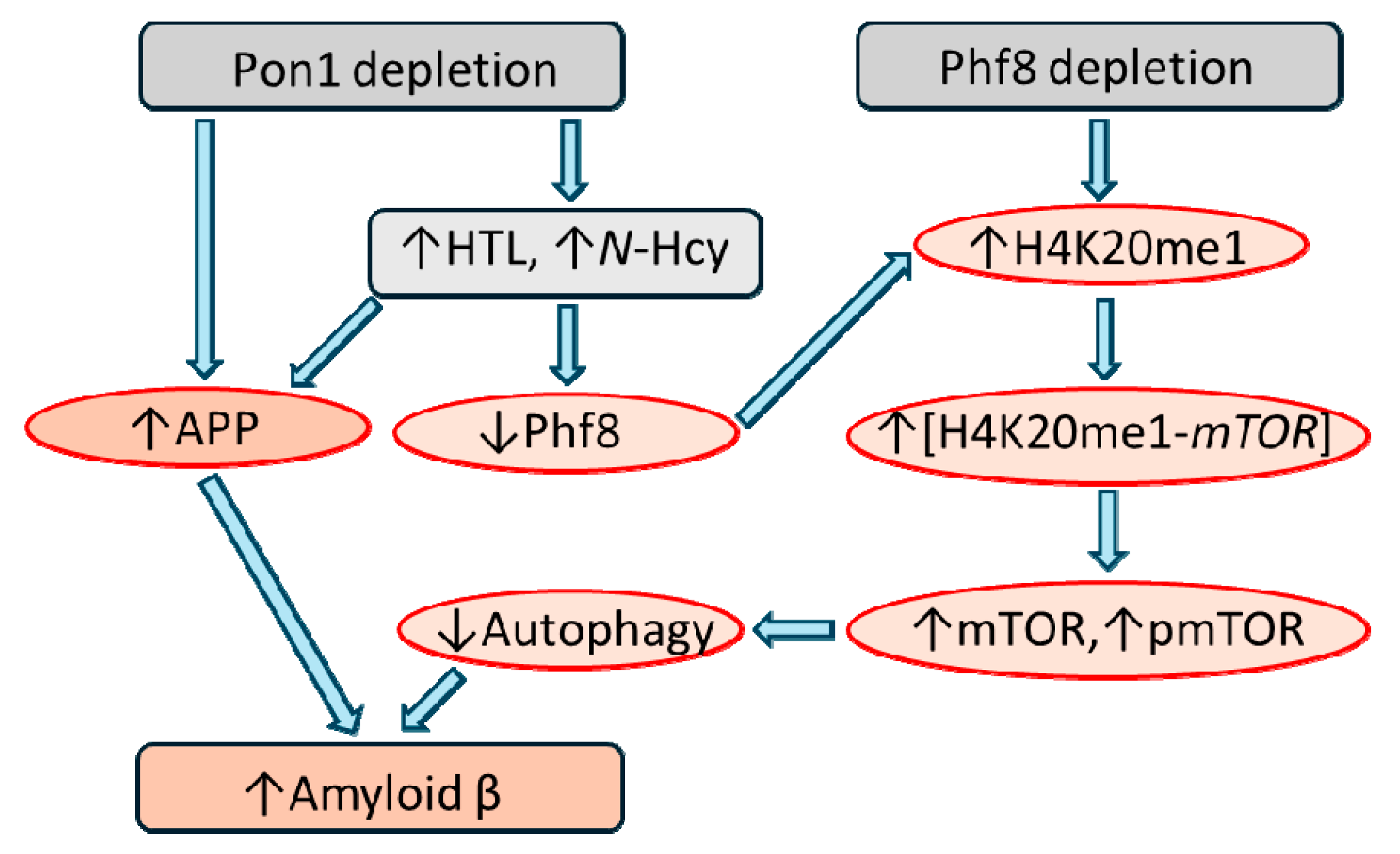
- Khayati, K.; Antikainen, H.; Bonder, E. M.; Weber, G. F.; Kruger, W. D.; Jakubowski, H.; Dobrowolski, R., The amino acid metabolite homocysteine activates mTORC1 to inhibit autophagy and form abnormal proteins in human neurons and mice. FASEB J 2017, 31, (2), 598-609. [CrossRef]
- Yates, S. C.; Zafar, A.; Hubbard, P.; Nagy, S.; Durant, S.; Bicknell, R.; Wilcock, G.; Christie, S.; Esiri, M. M.; Smith, A. D.; Nagy, Z., Dysfunction of the mTOR pathway is a risk factor for Alzheimer's disease. Acta Neuropathol Commun 2013, 1, 3. [CrossRef]
- Chen, X.; Wang, S.; Zhou, Y.; Han, Y.; Li, S.; Xu, Q.; Xu, L.; Zhu, Z.; Deng, Y.; Yu, L.; Song, L.; Chen, A. P.; Song, J.; Takahashi, E.; He, G.; He, L.; Li, W.; Chen, C. D., Phf8 histone demethylase deficiency causes cognitive impairments through the mTOR pathway. Nat Commun 2018, 9, (1), 114. [CrossRef]
- Witucki, L., Jakubowski, H.,, Homocysteine Metabolites Inhibit Autophagy and Elevate Amyloid Beta by Impairing Phf8/H4K20me1-dependent Epigenetic Regulation of mTOR in Cystathionine β-Synthase-Deficient Mice. 2023 bioRxiv. [CrossRef]
- Jakubowski, H., Homocysteine Thiolactone Detoxifying Enzymes and Alzheimer's Disease. Int J Mol Sci 2024, 25, (15). [CrossRef]
- Perla-Kajan, J.; Jakubowski, H., Paraoxonase 1 protects against protein N-homocysteinylation in humans. FASEB J 2010, 24, (3), 931-6.
- Jakubowski, H.; Perla-Kajan, J.; Finnell, R. H.; Cabrera, R. M.; Wang, H.; Gupta, S.; Kruger, W. D.; Kraus, J. P.; Shih, D. M., Genetic or nutritional disorders in homocysteine or folate metabolism increase protein N-homocysteinylation in mice. Faseb J 2009, 23, (6), 1721-7. [CrossRef]
- Smith, A. D.; Refsum, H., Homocysteine - from disease biomarker to disease prevention. J Intern Med 2021, 290, (4), 826-854. [CrossRef]
- Bacchetti, T.; Vignini, A.; Giulietti, A.; Nanetti, L.; Provinciali, L.; Luzzi, S.; Mazzanti, L.; Ferretti, G., Higher Levels of Oxidized Low Density Lipoproteins in Alzheimer's Disease Patients: Roles for Platelet Activating Factor Acetyl Hydrolase and Paraoxonase-1. J Alzheimers Dis 2015, 46, (1), 179-86.
- Cagnin, A.; Leon, A.; Vianello, D.; Colavito, D.; Favaretto, S.; Zarantonello, G.; Stecca, A.; Ermani, M.; Zambon, A., LDL density and oxidation are modulated by PON1 promoter genotype in patients with Alzheimer's disease. J Alzheimers Dis 2013, 34, (2), 377-85.
- Forner, A.; Reig, M.; Bruix, J., Hepatocellular carcinoma. Lancet 2018, 391, (10127), 1301-1314.
- Mackness, M. I.; Durrington, P. N.; Mackness, B., The role of paraoxonase 1 activity in cardiovascular disease: potential for therapeutic intervention. Am J Cardiovasc Drugs 2004, 4, (4), 211-7.
- Mackness, M. I.; Arrol, S.; Durrington, P. N., Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett 1991, 286, (1-2), 152-4. [CrossRef]
- Watson, A. D.; Berliner, J. A.; Hama, S. Y.; La Du, B. N.; Faull, K. F.; Fogelman, A. M.; Navab, M., Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest 1995, 96, (6), 2882-91.
- Aviram, M.; Rosenblat, M.; Bisgaier, C. L.; Newton, R. S.; Primo-Parmo, S. L.; La Du, B. N., Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest 1998, 101, (8), 1581-90. [CrossRef]
- Aviram, M.; Billecke, S.; Sorenson, R.; Bisgaier, C.; Newton, R.; Rosenblat, M.; Erogul, J.; Hsu, C.; Dunlop, C.; La Du, B., Paraoxonase active site required for protection against LDL oxidation involves its free sulfhydryl group and is different from that required for its arylesterase/paraoxonase activities: selective action of human paraoxonase allozymes Q and R. Arterioscler Thromb Vasc Biol 1998, 18, (10), 1617-24.
- Bayrak, A.; Bayrak, T.; Bodur, E.; Kilinc, K.; Demirpence, E., The effect of HDL-bound and free PON1 on copper-induced LDL oxidation. Chem Biol Interact 2016, 257, 141-6. [CrossRef]
- Aviram, M.; Rosenblat, M., Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med 2004, 37, (9), 1304-16. [CrossRef]
- Liu, Y.; Mackness, B.; Mackness, M., Comparison of the ability of paraoxonases 1 and 3 to attenuate the in vitro oxidation of low-density lipoprotein and reduce macrophage oxidative stress. Free Radic Biol Med 2008, 45, (6), 743-8. [CrossRef]
- Kornberg, A., Ten commandments of enzymology, amended. Trends Biochem Sci 2003, 28, (10), 515-7. [CrossRef]
- Kresge, N.; Simoni, R. D.; Hill, R. L., Unraveling the Enzymology of Oxidative Phosphorylation: the Work of Efraim Racker. Journal of Biological Chemistry 2006, 281, (4), e4-e6. [CrossRef]
- Marathe, G. K.; Zimmerman, G. A.; McIntyre, T. M., Platelet-activating factor acetylhydrolase, and not paraoxonase-1, is the oxidized phospholipid hydrolase of high density lipoprotein particles. J Biol Chem 2003, 278, (6), 3937-47. [CrossRef]
- Ahmed, Z.; Ravandi, A.; Maguire, G. F.; Emili, A.; Draganov, D.; La Du, B. N.; Kuksis, A.; Connelly, P. W., Apolipoprotein A-I promotes the formation of phosphatidylcholine core aldehydes that are hydrolyzed by paraoxonase (PON-1) during high density lipoprotein oxidation with a peroxynitrite donor. J Biol Chem 2001, 276, (27), 24473-81. [CrossRef]
- Ahmed, Z.; Ravandi, A.; Maguire, G. F.; Emili, A.; Draganov, D.; La Du, B. N.; Kuksis, A.; Connelly, P. W., Multiple substrates for paraoxonase-1 during oxidation of phosphatidylcholine by peroxynitrite. Biochem Biophys Res Commun 2002, 290, (1), 391-6. [CrossRef]
- Ahmed, Z.; Babaei, S.; Maguire, G. F.; Draganov, D.; Kuksis, A.; La Du, B. N.; Connelly, P. W., Paraoxonase-1 reduces monocyte chemotaxis and adhesion to endothelial cells due to oxidation of palmitoyl, linoleoyl glycerophosphorylcholine. Cardiovasc Res 2003, 57, (1), 225-31. [CrossRef]
- Teiber, J. F.; Draganov, D. I.; La Du, B. N., Purified human serum PON1 does not protect LDL against oxidation in the in vitro assays initiated with copper or AAPH. J Lipid Res 2004, 45, (12), 2260-8. [CrossRef]
- Connelly, P. W.; Draganov, D.; Maguire, G. F., Paraoxonase-1 does not reduce or modify oxidation of phospholipids by peroxynitrite. Free Radic Biol Med 2005, 38, (2), 164-74. [CrossRef]
- Rodrigo, L.; Mackness, B.; Durrington, P. N.; Hernandez, A.; Mackness, M. I., Hydrolysis of platelet-activating factor by human serum paraoxonase. Biochem J 2001, 354, (Pt 1), 1-7.
- Mashima, R.; Yamamoto, Y.; Yoshimura, S., Reduction of phosphatidylcholine hydroperoxide by apolipoprotein A-I: purification of the hydroperoxide-reducing proteins from human blood plasma. J Lipid Res 1998, 39, (6), 1133-40. [CrossRef]
- Rye, K. A.; Barter, P. J., Cardioprotective functions of HDLs. J Lipid Res 2014, 55, (2), 168-79. [CrossRef]
- Garner, B.; Waldeck, A. R.; Witting, P. K.; Rye, K. A.; Stocker, R., Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J Biol Chem 1998, 273, (11), 6088-95.
- Panzenbock, U.; Stocker, R., Formation of methionine sulfoxide-containing specific forms of oxidized high-density lipoproteins. Biochim Biophys Acta 2005, 1703, (2), 171-81. [CrossRef]
- Graham, A.; Hassall, D. G.; Rafique, S.; Owen, J. S., Evidence for a paraoxonase-independent inhibition of low-density lipoprotein oxidation by high-density lipoprotein. Atherosclerosis 1997, 135, (2), 193-204. [CrossRef]
- Suszynska-Zajczyk, J.; Luczak, M.; Marczak, L.; Jakubowski, H., Hyperhomocysteinemia and bleomycin hydrolase modulate the expression of mouse brain proteins involved in neurodegeneration. J Alzheimers Dis 2014, 40, (3), 713-26. [CrossRef]
- Suszynska-Zajczyk, J.; Jakubowski, H., Paraoxonase 1 and dietary hyperhomocysteinemia modulate the expression of mouse proteins involved in liver homeostasis. Acta Biochim Pol 2014, 61, (4), 815-23. [CrossRef]
- Suszynska-Zajczyk, J.; Sikora, M.; Jakubowski, H., Paraoxonase 1 deficiency and hyperhomocysteinemia alter the expression of mouse kidney proteins involved in renal disease. Mol Genet Metab 2014, 113, (3), 200-6. [CrossRef]
- Sikora, M.; Jakubowski, H., Changes in redox plasma proteome of Pon1-/- mice are exacerbated by a hyperhomocysteinemic diet. Free Radic Biol Med 2021, 169, 169-180.
- Cao, J.; Xu, Y.; Li, F.; Shang, L.; Fan, D.; Yu, H., Protein markers of dysfunctional HDL in scavenger receptor class B type I deficient mice. J Transl Med 2018, 16, (1), 155. [CrossRef]
- Liu, Q.; Xiao, J. J.; Wang, S.; Li, Y.; Yang, L. J.; Lu, Q. Y.; Wu, X. Y.; Cao, J.; Yu, H.; Zhang, B. F., Paraoxonase 1 Ameliorates Renal Lipotoxicity by Activating Lipophagy and Inhibiting Pyroptosis. Am J Pathol 2022, 192, (11), 1531-1545. [CrossRef]
- Zhao, X. J.; Liu, L. C.; Guo, C.; Shen, W. W.; Cao, J.; Du, F.; Wu, D. F.; Yu, H., Hepatic paraoxonase 1 ameliorates dysfunctional high-density lipoprotein and atherosclerosis in scavenger receptor class B type I deficient mice. Ann Transl Med 2021, 9, (13), 1063. [CrossRef]
- Yuhanna, I. S.; Zhu, Y.; Cox, B. E.; Hahner, L. D.; Osborne-Lawrence, S.; Lu, P.; Marcel, Y. L.; Anderson, R. G.; Mendelsohn, M. E.; Hobbs, H. H.; Shaul, P. W., High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med 2001, 7, (7), 853-7.
- Trigatti, B. L.; Fuller, M., HDL signaling and protection against coronary artery atherosclerosis in mice. J Biomed Res 2016, 30, (2), 94-100.
- Seetharam, D.; Mineo, C.; Gormley, A. K.; Gibson, L. L.; Vongpatanasin, W.; Chambliss, K. L.; Hahner, L. D.; Cummings, M. L.; Kitchens, R. L.; Marcel, Y. L.; Rader, D. J.; Shaul, P. W., High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ Res 2006, 98, (1), 63-72.
- Ng, D. S.; Chu, T.; Esposito, B.; Hui, P.; Connelly, P. W.; Gross, P. L., Paraoxonase-1 deficiency in mice predisposes to vascular inflammation, oxidative stress, and thrombogenicity in the absence of hyperlipidemia. Cardiovasc Pathol 2008, 17, (4), 226-32. [CrossRef]
- Acton, S.; Rigotti, A.; Landschulz, K. T.; Xu, S.; Hobbs, H. H.; Krieger, M., Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996, 271, (5248), 518-20. [CrossRef]
- Zanoni, P.; Khetarpal, S. A.; Larach, D. B.; Hancock-Cerutti, W. F.; Millar, J. S.; Cuchel, M.; DerOhannessian, S.; Kontush, A.; Surendran, P.; Saleheen, D.; Trompet, S.; Jukema, J. W.; De Craen, A.; Deloukas, P.; Sattar, N.; Ford, I.; Packard, C.; Majumder, A.; Alam, D. S.; Di Angelantonio, E.; Abecasis, G.; Chowdhury, R.; Erdmann, J.; Nordestgaard, B. G.; Nielsen, S. F.; Tybjaerg-Hansen, A.; Schmidt, R. F.; Kuulasmaa, K.; Liu, D. J.; Perola, M.; Blankenberg, S.; Salomaa, V.; Mannisto, S.; Amouyel, P.; Arveiler, D.; Ferrieres, J.; Muller-Nurasyid, M.; Ferrario, M.; Kee, F.; Willer, C. J.; Samani, N.; Schunkert, H.; Butterworth, A. S.; Howson, J. M.; Peloso, G. M.; Stitziel, N. O.; Danesh, J.; Kathiresan, S.; Rader, D. J.; Consortium, C. H. D. E.; Consortium, C. A. E.; Global Lipids Genetics, C., Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science 2016, 351, (6278), 1166-71.
- Zhang, Y.; Da Silva, J. R.; Reilly, M.; Billheimer, J. T.; Rothblat, G. H.; Rader, D. J., Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest 2005, 115, (10), 2870-4. [CrossRef]
- Leiva, A.; Verdejo, H.; Benitez, M. L.; Martinez, A.; Busso, D.; Rigotti, A., Mechanisms regulating hepatic SR-BI expression and their impact on HDL metabolism. Atherosclerosis 2011, 217, (2), 299-307. [CrossRef]
- Van Eck, M.; Hoekstra, M.; Hildebrand, R. B.; Yaong, Y.; Stengel, D.; Kruijt, J. K.; Sattler, W.; Tietge, U. J.; Ninio, E.; Van Berkel, T. J.; Pratico, D., Increased oxidative stress in scavenger receptor BI knockout mice with dysfunctional HDL. Arterioscler Thromb Vasc Biol 2007, 27, (11), 2413-9. [CrossRef]
- Chester, J.; Johnston, E.; Walker, D.; Jones, M.; Ionescu, C. M.; Wagle, S. R.; Kovacevic, B.; Brown, D.; Mikov, M.; Mooranian, A.; Al-Salami, H., A Review on Recent Advancement on Age-Related Hearing Loss: The Applications of Nanotechnology, Drug Pharmacology, and Biotechnology. Pharmaceutics 2021, 13, (7). [CrossRef]
- Miettinen, H. E.; Rayburn, H.; Krieger, M., Abnormal lipoprotein metabolism and reversible female infertility in HDL receptor (SR-BI)-deficient mice. J Clin Invest 2001, 108, (11), 1717-22.
- Ruan, X. Z.; Varghese, Z.; Moorhead, J. F., An update on the lipid nephrotoxicity hypothesis. Nat Rev Nephrol 2009, 5, (12), 713-21. [CrossRef]
- Rodrigo, L.; Hernandez, A. F.; Lopez-Caballero, J. J.; Gil, F.; Pla, A., Immunohistochemical evidence for the expression and induction of paraoxonase in rat liver, kidney, lung and brain tissue. Implications for its physiological role. Chem Biol Interact 2001, 137, (2), 123-37. [CrossRef]
- Miljkovic, M.; Stefanovic, A.; Vekic, J.; Zeljkovic, A.; Gojkovic, T.; Simic-Ogrizovic, S.; Bogavac-Stanojevic, N.; Cerne, D.; Ilic, J.; Stefanovic, I.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V.; Kotur-Stevuljevic, J., Activity of paraoxonase 1 (PON1) on HDL(2) and HDL(3) subclasses in renal disease. Clin Biochem 2018, 60, 52-58. [CrossRef]
- Kontush, A.; Lindahl, M.; Lhomme, M.; Calabresi, L.; Chapman, M. J.; Davidson, W. S., Structure of HDL: particle subclasses and molecular components. Handb Exp Pharmacol 2015, 224, 3-51.
- Huuskonen, J.; Olkkonen, V. M.; Jauhiainen, M.; Ehnholm, C., The impact of phospholipid transfer protein (PLTP) on HDL metabolism. Atherosclerosis 2001, 155, (2), 269-81. [CrossRef]
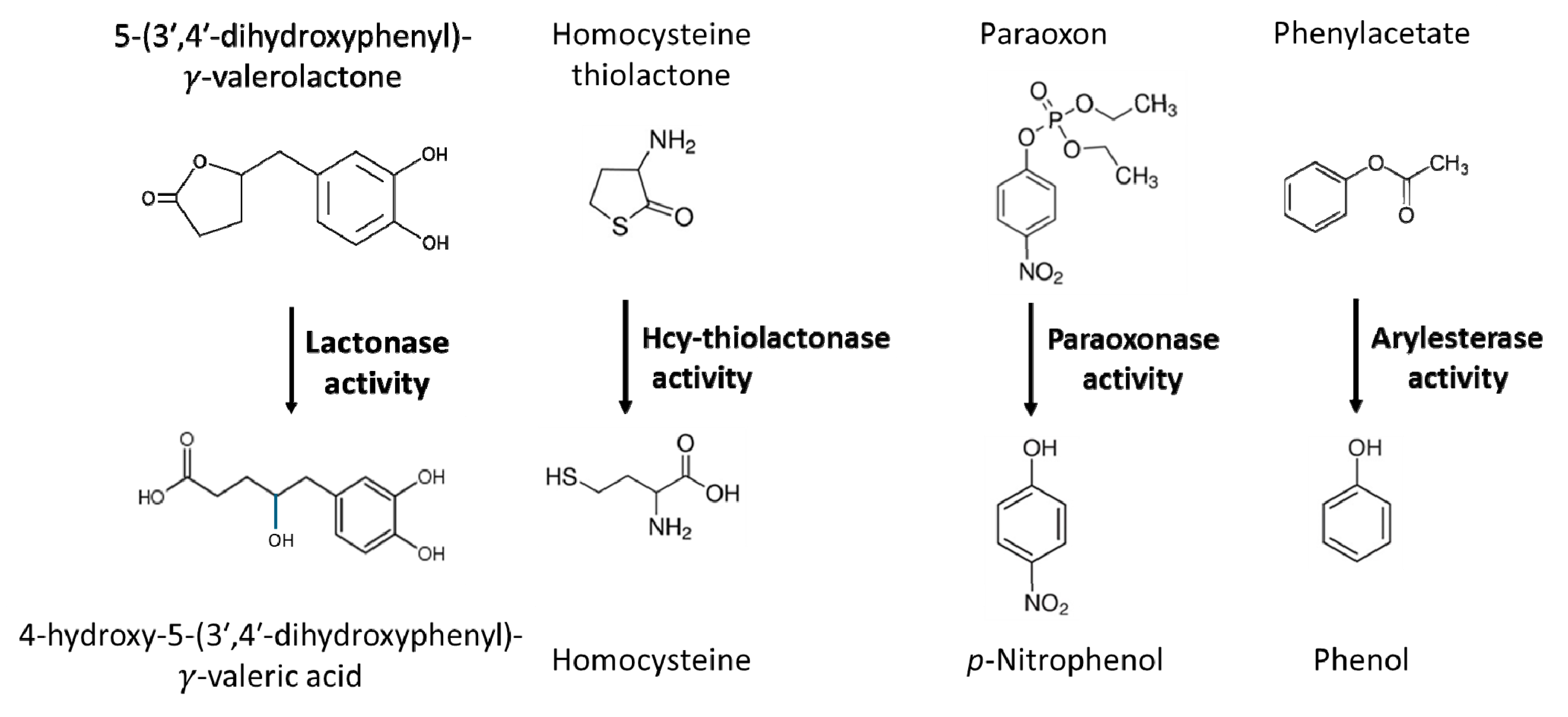
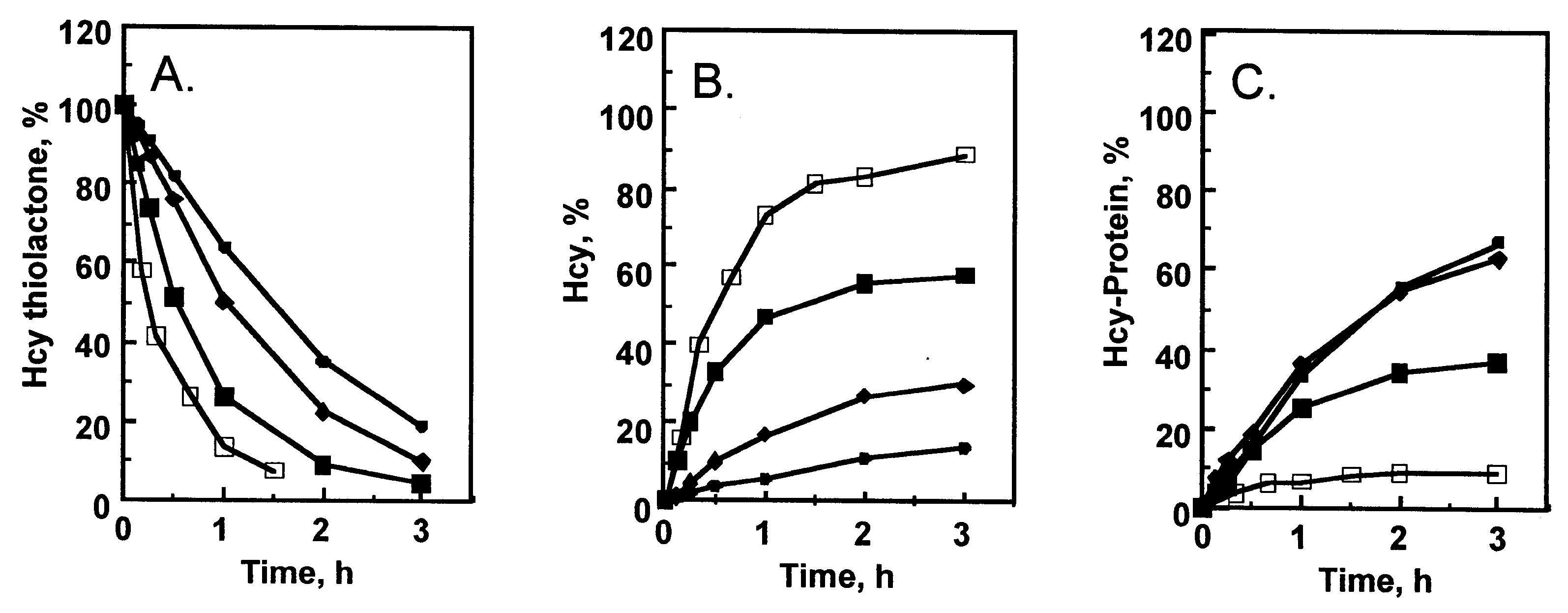
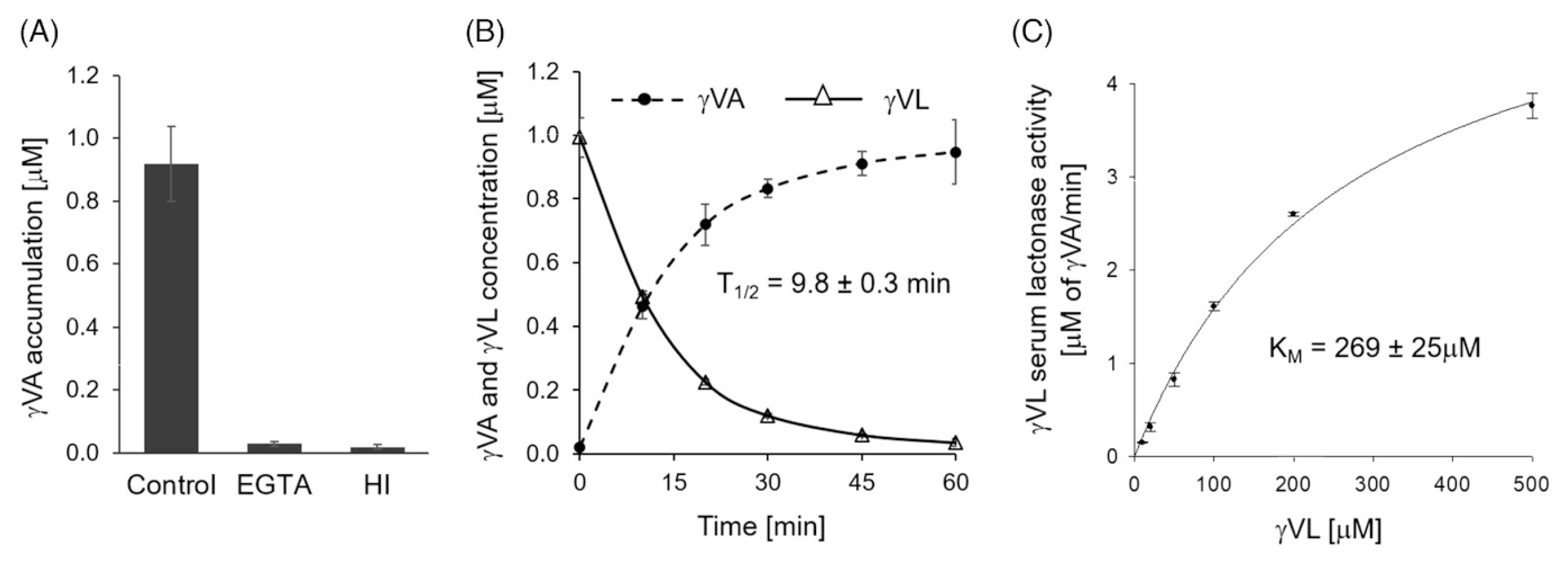
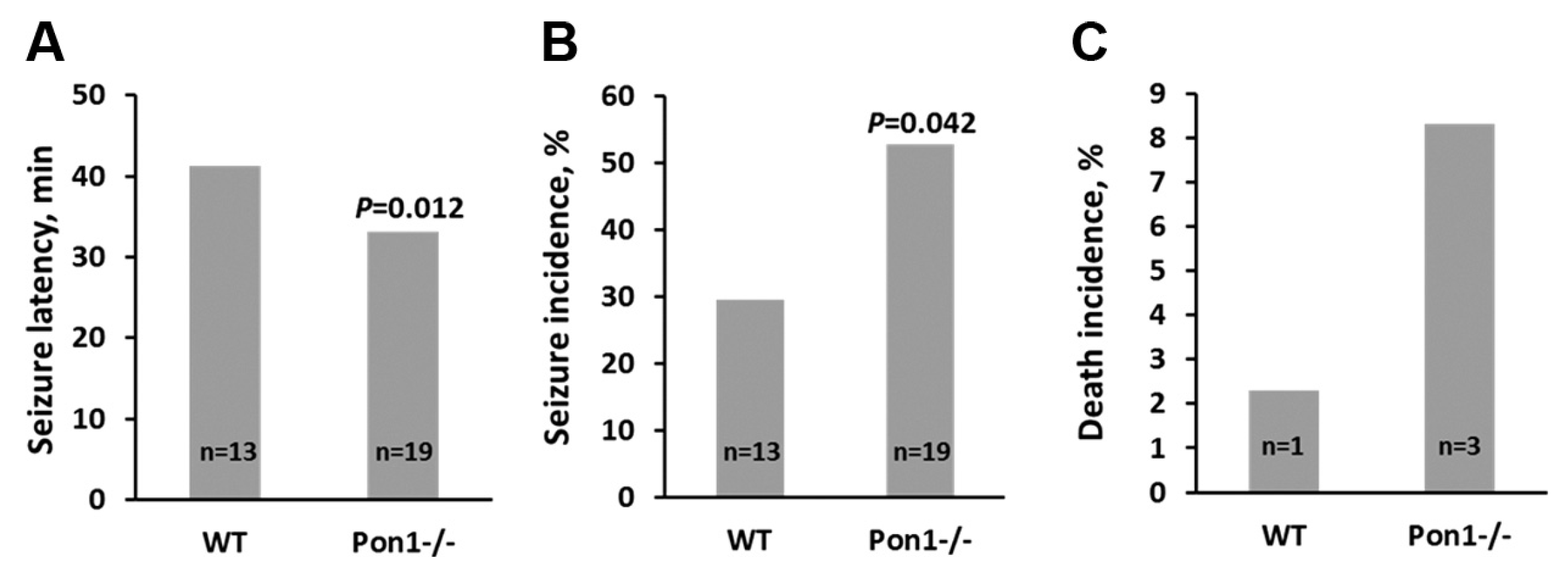
| Disease | PON1 activity | PON1 genotype | Oxidative stress | Inflammation | References |
| Cardiovascular disease | ↓ | PON1-192QQ | ↑ | ↑ | [27,29,56,69] |
| Kidney disease | ↓ | ND | ND | ↑ | [30,31] |
| Fatty liver disease | ↓ | ND | ↑ | ↑ | [57] |
| Alzheimer’s disease | ↓ | PON1-107TT | ↑ | ND | [32,58,59,84] |
| Hepatocellular carcinoma | ↓ | ND | ↑ | ↑ | [33,60] |
| *ND, not determined. Up and down arrows indicate the direction of change. | |||||
| Protein name | Change in Pon1-/- vs. Pon1+/+ brain* |
Change in human AD brain (another neuropathy or animal model) ** | |
|---|---|---|---|
| Std. diet | 1%-Met diet | ||
| Brain-specific | |||
| Ncald | – | ↑ | ↓, (↓ Gls-/- mouse) |
| Nrgn | ↓ | ↑ | ↓ |
| Stmn1 | – | ↑ | ↓, (↑ MS, TLE, SMA, schizophrenia), (↑ HD4 mouse model) |
| Anti-oxidant defense | |||
| Sod1 | ↓ | – | (↑ ALS) |
| Prdx2 | – | ↑ | ↑ |
| DJ-1 (Park7) | ↓ | ↑ | ↑ |
| Energy metabolism | |||
| Ak1 | – | ↑ | ↑ |
| Cell cycle | |||
| GDI1 | – | ↑ | (↑ rat ischemic brain) |
| Ran | – | ↑ | ↑ |
| Cytoskeleton assembly | |||
| Tbcb | ↓ | ↑ | (↑ GAN) |
| CapZa2 | ↑ | – | ↑ CapZb2# |
| Other proteins | |||
| Hdhd2 | – | ↑ | |
| Model | Treatment | Gene expression assessment | Outcome | Referencee | ||
|---|---|---|---|---|---|---|
| Methods | Tissue | In vivo | In vitro | |||
| Pon1-/- vs Pon1+/+mice, high fat diet | Pon1 deletion in WT mice | Enzymatic activity assay, northern blot, RT-qPCR, western blot |
Liver, plasma | Pon1 protein absent in Pon1-/- mice, ↑lipid peroxides in HDL, no lipid peroxides in LDL, ↑atherosclerosis; no atherosclerosis in Pon1-/- mice on a chow diet | ↑Lipid peroxides in hLDL, ↑MCP1, ↑monocyte migration ameliorated by Pon1+/+ HDL in a cell co-culture model of the arterial wall; Pon1-/- HDL does not prevent hLDL oxidation | Shih DM et al. [54] |
| Pon1-/-ApoE-/- vs. Pon1+/+ApoE-/- mice, std chow diet | Pon1 deletion in ApoE-/- mice | Enzymatic activity assay, northern blot, RT-qPCR |
Liver, plasma | ↑Lipid peroxides in LDL, ↑oxidized phospholipid epitopes in plasma; ↑HO1, PPARγ, ↑oxLDL receptors (SRA, CD36, macrosialin), but not HDL receptor SR-BI, in the liver; no change in anti-oxLDL and anti-MDA-LDL autoantibody levels; ↑atherosclerosis | LDL from Pon1-/- mice elevated lipid hydroperoxide and monocyte transmigration | Shih DM et al. [55] |
| Human PON cluster transgenic mice | PON1, PON2, PON3 overexpression | Enzymatic activity assay, RT-qPCR, western blot, ELISA |
↑PON1 expression, ↓atherosclerosis ↓Icam-1, ↓Mcp-1, ↓TNF-α, ↓IL-6, ↓Mmp-9 |
PON Tg HDL ameliorated Cu-induced LDL oxidation | She ZG et al. [63] | |
| Pon1-/- vs. Pon1+/+mice, std chow diet | Pon1 deletion | 2D-SDS-PAGE gel electrophoresis, MALDI-TOF mass spectrometry | Brain | ↓Sod1, ↓DJ-1 | [109] Brain | |
| Liver | ↑Prdx2, ↑Ftl, ↑ApoE | [110] Liver | ||||
| Kidney | ↑Prdx2, ↓ApoA1 | [111] Kidney | ||||
| Enzymatic activity assay, Label-free nanoLC-MS/MS mass spectrometry |
Plasma | ↓Alb, ↓Blvrb, ↑Ambp, ↑(Hpx, ↑ApoD, ↑ApoM, ↑Hp), ↑Ttr | Sikora M et al. [65] | |||
| Pon1-/- vs. Pon1+/+mice, high methionine diet | Pon1 deletion | Enzymatic activity assay, Label-free nanoLC-MS/MS mass spectrometry |
Plasma | ↓Alb$, ↑Hp, ↑Hpx ,↓Alad, ↑Cp, ↓Gclm, ↓Cat, ↑Ctsb, ↓Gsn, ↑Grn, ↓Prdx2#, ↓Prdx6, ↓Txn, ↓Igfbp3, ↓Park7#, ↓Pebp1#, ↓Ppia, ↓Serpina3k | Sikora M et al. [112] | |
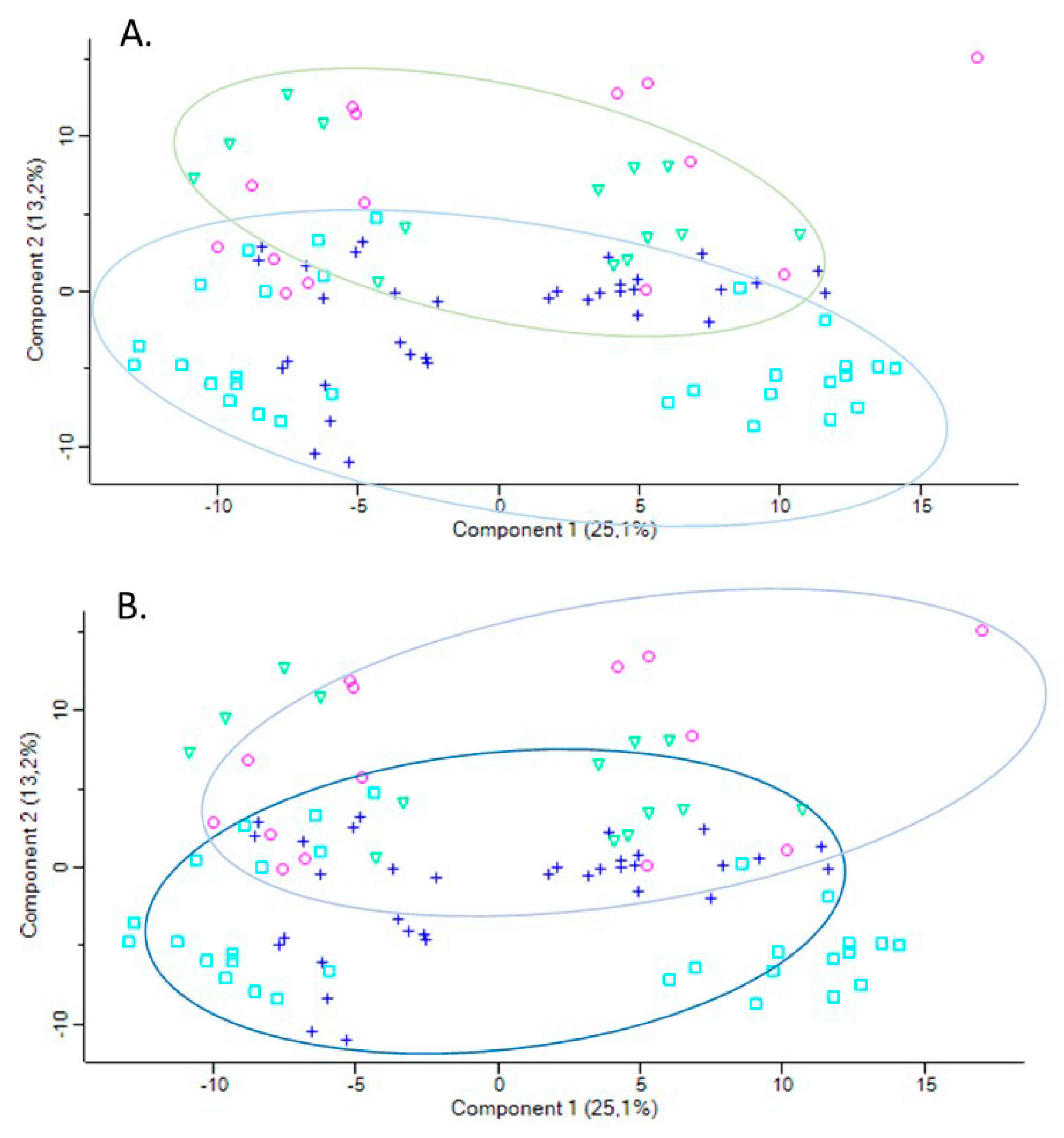
| PON1-192QQ vs. PON1-192RR+QR humans | none | Enzymatic activity assay, Label-free nanoLC-MS/MS mass spectrometry |
plasma | APOA1↓, PON1↓, APOD↑, APOM↑, HP↓, GPX3↑ | Sikora M et al. [65] | |
| SR-BI-/- vs SR-BI-/- mice | SR-BI deletion, probucol treatment | Enzymatic activity assay, 2D-SDS-PAGE gel electrophoresis, LC-MS/MS mass spectrometry, western blot, RT-qPCR | Plasma, HDL | ↓HDL protein, ↓ApoA1, ↓Pon1 protein and activity, ↑Saa, ↑ApoA4, ↑A1AT, ↑Mpo Probucol upregulated Pon1, downregulated Saa, ApoA4, A1AT, and Mpo thereby improving HDL function |
↑Mcp1, Tnf-α in oxLDL treated macrophages; SR-BI+/+ HDL reduced Mcp1, Tnf-α while SR-BI−/− HDL had no effect |
Cao J et al. [113] |
| Pon1+SR-BI-/- mice | Pon1 overexpression SR-BI-/- mice using lentivirus vector | Enzymatic activity assay, RT-qPCR, western blot |
Kidney |
Pon1+SR-BI-/- mice: ↓renal Pon1 expression & plasma activity, ↑ expression of redox (p47phox, Nox1, Nox4) and inflammation related (Il1b, Il6) genes, ↓anti-inflammatory cytokine Il10. Pon1+SR-BI-/- mice fed with a high-fat diet : ↑plasma & renal Pon1 expression & activity, ↓renal redox (p47phox, Nox1, Nox4, Sod) and inflammation related (Tnfα, Il6) genes, ↑anti-inflammatory cytokine Il10 |
Liu Q et al. [114] | |
| Liver | Pon1+SR-BI-/- mice: ↑hepatic/plasma Pon1, ↑ApoE, ↑Lcat, ↓plasma ALT activity, ↓ROS levels & MPO activity, ↓acute-phase & pro-inflammatory plasma proteins (ApoA4, A1AT, Saa), ↑hepatic ApoA1, Ldlr, Lxrα, Abca, Abcg5, Abcg8, ↓Tnf-α, ↓Il6, ↓ atherosclerosis | Macrophages treated with Pon1+SR-BI-/- HDL: ↓mRNA for inflammatory cytokines IL-6, TNFα, NOX1, ↑mRNA for anti-inflammatory cytokines IL-4, IL-10; mRNA for cholesterol transport ↓Scarb1, ↑Abca1 | Zhao X J et al. [115] | |||
| Human PON1 | ||
|---|---|---|
| Genotype (n) | Activity a | Protein b |
| PON1-192RR (19) | 100 | 100 |
| PON1-192QR (30) | 20.6 | 63.0 |
| PON1-192QQ (51) | 14.3 | 60.0 |
| Mouse Pon1 | ||
|---|---|---|
| Genotype (n) | Activity a | Protein b |
| Pon1+/+ (17) | 100 | 100 |
| Pon1-/- (8) | 0.0 | 2.0 |
| Unique to HHcy diet mice (n = 66) | Unique to control diet mice (n = 27) |
Proteins affected both in HHcy and control diet mice (n = 23) |
|---|---|---|
| Oxidative stress (n = 15): ↓Alad, ↑Cp, ↓Gclm, ↓Cat, ↑Ctsb, ↓Gsn, ↑Grn, ↓Prdx2#, ↓Prdx6, ↓Txn, ↓Igfbp3, ↓Park7#, ↓Pebp1#, ↓Ppia, ↓Serpina3k |
Oxida tive stress (n = 1): ↓Blvrb |
Oxidative stress (n = 3): ↓Alb$, ↑Hp, ↑Hpx |
| Immune response (n = 15): ↓Il1rap, Igh (n=10↑, 1↓), ↑Igk (n = 3), | Immune response (n = 10): ↑Clu$, ↑Igh (n=3↑, 1↓), ↑Igk (n=3), ↑Igl, ↑Igm | Immune response (n = 9): ↑Igh (n = 4), ↑Igj, ↑Igk (n = 3), ↑Igl |
| Acute phase response (n = 6): ↑A2m$, ↑Ahsg, ↑Orm1, ↑Orm2, ↑Saa1, ↑Saa2 |
Acute phase response (n = 1): ↑Ttr |
Acute phase response (n = 1): ↑Ambp |
| Complement/coagulation (n = 5): ↑Apcs, ↓F13a1, ↑C3, ↑Cfb, ↑Cfhr1 |
Complement/coagulation (n = 4): ↑AI182371, ↑F2, ↓F13b, ↓Mbl1 | Complement/coagulation (n = 3): ↑Cfh, ↓Klkb1, ↓Serpinc1 |
| Blood coagulation (n = 6): ↑Serpina10, ↓Gp1ba, ↑Gp5, ↑Itih3, ↑Pros1$, ↓Proz | Blood coagulation (n = 3): ↓Hgfac, ↑Hrg‡, ↓Itih1 | |
| Lipoprotein/lipid metabolism (n = 5): ↓ApoA2, ↓ApoC2, ↓Azgp1, ↓Pgp, ↑Pltp |
Lipoprotein metabolism (n=4): ↓Afm, ↑ApoD, ↑ApoM, ↑Lcat |
Lipoprotein metabolism (n = 4): ↓ApoA1, ↑ApoB, ↓ApoC1, ↓Pon1 |
| Protein turnover (n = 5): ↓Apeh, ↓Mug2, ↓Serpina3m, ↓Uba1, ↓Uba52 | Protein turnover (n = 1): ↑Fetub | Protein turnover (n=1): ↓Mug1; |
| Other proteins (n = 8): ↓Atic, ↓Nme1, ↓Pnp (purine metabolism), ↓Tpi, ↓Tkt (glucose metabolism), ↓Ran# (nucleoplasmic transport), ↓Rbp4 (retinol transport), ↓Spp2 (bone remodeling) |
Other proteins (n = 3): ↓Aldoa, ↓Ldha (glucose metabolism), ↓Lifr (tissue regeneration) |
Other proteins (n = 2): ↓Bpgm (glucose metabolism), ↓Ica (carbonic anhydrase inhibitor) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





